Open Access Article
Open Access ArticleDesign, analysis, and application of metal–organic framework derived carbons
Joshua A.
Powell
 *ab,
Yihao
Yang
*ab,
Yihao
Yang
 a and
Hong-Cai
Zhou
a and
Hong-Cai
Zhou
 *a
*a
aDepartment of Chemistry, Texas A&M University, College Station, TX 77843, USA. E-mail: j.powell1@uq.edu.au; zhou@chem.tamu.edu
bSchool of Chemical Engineering, The University of Queensland, St Lucia, QLD 4072, Australia
First published on 6th February 2025
Abstract
Metal–organic framework-derived carbons (MOFdCs) have emerged as a rapidly growing class of porous materials over the past 15 years. Inspired by the principles of templated synthesis used in organic chemistry and nanomaterials, MOFdCs combine the highly tunable features of metal–organic frameworks with the versatility and stability of traditional porous carbon materials. Although many advances in MOFdC applications have been driven by an exploratory approach, there is a growing body of systematic, hypothesis-driven studies of the underlying chemistry and materials science of MOFdC structure, design, and structure–property relationships. This review summarizes developments in the systematic design and characterization of MOFdCs and strategies developed to control the structure of these materials. The role of variables such as pyrolysis temperature, gas environment, and the atomic- and nanoscale structure of the template MOF are described, with particular emphasis on their relationship to MOFdC structure and applications. By focusing on the fundamental principles underpinning MOFdC design, this work will lead to more rationally designed MOFdC materials and greater efficiencies in the development of MOFdCs for a host of applications.
Introduction
Porous materials are of growing importance in our society, as we seek highly efficient catalysts and adsorbents for energy storage and environmental remediation. Charcoal, a porous carbon material, has been described in sources from cultures across the world from as early as 1500 BC and has been used for medicinal applications and water purification for centuries.1–4 In the late 20th and early 21st centuries, chemists such as Robson, Yaghi, and Kitagawa introduced a new type of porous material, known as porous coordination polymers or metal–organic frameworks (MOFs).5–10 These materials exhibited both permanent porosity and highly tunable structures and represented a significant advance in obtaining a high level of control over the structure of porous materials. Research over the last 25–30 years has expanded greatly on these early reports to produce MOFs that are more stable11–17 and have various functionalities apportioned throughout the framework structure.8,17–22 However, despite the sophistication of these materials, their stability is often poor compared to more established porous materials, such as porous carbon or zeolites, thus limiting their applicability.23–27 Consequently, the commercialization of MOFs has been limited to a small number of niche applications and the need for a highly controllable porous material remains.26–28Templated synthesis is a key strategy by which structural control has been achieved in fields such as organic chemistry, biochemistry, and nanomaterials.29–37 As their cost has decreased and their ease of synthesis has improved, MOFs have begun to be used as templates for the design and synthesis of new MOF-derived materials. One example of this is the advent of MOF-derived carbon (MOFdC) materials, which are porous carbons derived from the thermal decomposition of MOFs.37–40 Some MOFdCs are also decorated with metal species derived from the inorganic components of the MOFs. Compared to their template MOFs, MOFdCs are typically much more thermally and chemically robust,37,38 which provides greater opportunities for their application under working conditions for electrocatalysis and other industrial chemical processes.41–44 Most importantly, the structure of the template MOF can be tuned or altered to impart specific properties into the MOFdC. By encapsulating guests inside the framework, doping the structure with additional metal ions to form bimetallic MOFs, or altering the connectivity or composition of the inorganic or organic building units, the structure of MOFdCs can be tuned in a fashion that is unusually controllable for an amorphous material.
Early MOFdC development
Many early MOFdCs required the incorporation of additional carbon sources into the template framework to circumvent excessive loss of carbon through combustion. Xu and coworkers reported one of the first MOFdCs, which was synthesized via carbonization of a polyfurfuryl alcohol@MOF-5 composite at 1000 °C under an argon atmosphere. Under these conditions, the organic linkers and polyfurfuryl alcohol were transformed into a porous carbon material with a high surface area.39 Due to the high carbonization temperature and the low boiling point of zinc, no metal was retained in the MOFdC structure, although zinc oxide could be observed at lower carbonization temperatures. Notably, the carbon structure contained micropores, mesopores, and macropores. Later research has shown that the micropores in MOFdC structures are typically derived directly from the micropores of the template MOF.45 Xu later extended this research to test the application of these materials as electrodes for supercapacitors.46Following this seminal work, other researchers applied similar strategies to generate MOFdCs from other template MOFs. Furfuryl alcohol remained a popular additive and was later applied in the carbonization of zeolitic imidazolate frameworks (ZIFs) by several researchers (Fig. 1a).47,48 Gao and coworkers studied the incorporation of various different carbon additives, including a phenolic resin, carbon tetrachloride, and ethylenediamine, finding that carbonization of additive-free MOF-5 produced a MOFdC with high porosity, while carbonization of MOF-5 with all three additives produced a more graphitic carbon.49
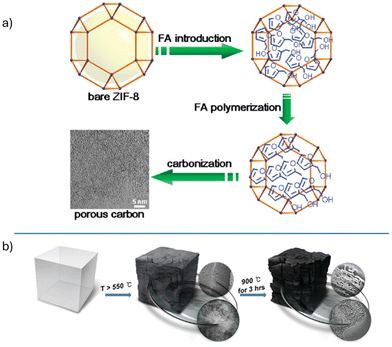 | ||
| Fig. 1 (a) Incorporation of furfuryl alcohol prior to carbonization increases carbon content in the final MOFdC material. Reprinted with permission from ref. 47. Copyright 2011 American Chemical Society; (b) direct carbonization of MOF crystals can also be achieved. Reprinted with permission from ref. 54. Copyright 2012 American Chemical Society. | ||
Eventually, MOF carbonization strategies that did not require the inclusion of an additional carbon source were developed (Fig. 1b). Around the time that Xu and coworkers published their MOFdC study, other researchers reported the synthesis of MOF-derived multiwalled carbon nanotubes, where the metal center of the MOF was used to catalyze the formation of the carbon structure.50 However, examples of direct MOF carbonizations where the porous structure of the template MOF was retained did not arise until the early 2010s.
One of the earliest examples was the direct carbonization of an aluminium-based MOF by Yamauchi and coworkers.51 The MOF in question had previously been carbonized with the addition of furfuryl alcohol,52 however Yamauchi and coworkers found that the carbon content of the 1,4-naphthalene dicarboxylic acid linker was in fact sufficient to produce a carbon matrix with high porosity after the material was treated to remove the alumina nanoparticles derived from the inorganic building units (IBUs).51 Notably, the carbonization temperature was a key variable that affected the porosity of the MOFdC, with carbonization at 800 °C producing the MOFdC with the highest surface area. At temperatures above 900 °C, the MOFdCs exhibited a drastic reduction in porosity due to graphitization and collapse of the carbon pore structure.
In contrast to the Al-MOF template described above, many MOFdCs are formed from Zn-MOFs, as the low boiling point of zinc metal leads to the removal of the metal species without any subsequent processing. Banerjee and Kurungot directly carbonized several zinc-based MOFs and coordination polymers to produce porous carbons that required no post-synthetic treatment due to the evaporation of the zinc. In an effort to maximize carbon content and surface area, this study explored a range of different linkers with varied lengths and aromaticity.53 Park and coworkers also reported the direct carbonization of IRMOFs 1, 3, and 8 (IRMOF = isoreticular MOF). They were able to tune the pore size of the MOFdCs and optimize them for hydrogen uptake by altering both the structure of the template MOF and the pyrolysis temperature.54
One of the most popular MOF templates for the formation of MOFdCs is ZIF-8. This framework is comprised of zinc ions as IBUs and 2-methylimidazolate linkers that provide sufficient carbon content to form highly porous MOFdCs without the incorporation of additional carbon sources. Furthermore, ZIF-8 is both inexpensive and synthetically simple to produce in the quantities required for carbonization, as cost is a key limitation to the commercialization of MOFs and MOFdCs.47,55–59
As the field of MOFdCs has begun to mature, more complex MOFdC structures are being generated. While some MOFdC research remains focused on simple porous carbon structures, many researchers have begun to synthesize MOFdCs with N- or S-doped carbons or containing metal species derived from the template MOF.47,60 These more complex structures represent a significant advance in the versatility of MOFdCs, as they provide several additional structural variables that can be tailored to form materials with specific structures or functionalities.
Structural features of MOFdCs
There is a great deal of diversity in the structure of MOFdCs, however there are several features that are common to many MOFdCs (Fig. 2). First is the presence of a porous carbon matrix, which is a universal feature in these materials.46,49,52,61–63 Originally derived from a combination of the organic linker and additional carbon-containing guests, and later from the organic linkers alone, the structure of this porous carbon is strongly related to the structure of the template MOF. The carbon typically contains a mixture of micropores and mesopores, the sizes of which are derived from the pores of the original MOF template.45,49,52,64 Pore size distributions of MOFdCs obtained via gas sorption analysis show that the micropores in the carbon are of the same size as the micropores in the MOF. The mesopores are generated by a combination of combustion of the organic components and in situ defect formation leading to partial structural collapse during the carbonization.45,64–66 The relative concentrations of micro- versus mesopores can be tuned based on the pyrolysis conditions. Harsher carbonization conditions, such as high temperatures or oxidizing gas environments, lead to the formation of greater mesoporosity, as the harsh conditions generate more defects in the MOF during the pre-carbonization heating and encourage a greater degree of combustion of the organic components. Depending on the composition of the organic linker or organic additives, the carbon matrix can also have additional elements doped into the non-graphitic carbon structure. Most common is nitrogen-doping, which is achieved by either including a nitrogen-rich guest like urea in the MOF prior to carbonization60,67 or by designing a MOF structure with nitrogen-rich linkers, such as the ZIF-8/ZIF-67 system.47 Carbonization of these nitrogen-rich templates produces N-doped carbon, which enhances the energy storage properties and the electrochemical activity of the material.40,47,61,62,68 MOFdCs with sulfur-doped and phosphorus-doped carbons have also been reported, however these are less common.69 MOF-derived materials that do not contain a carbon matrix can also be generated,70–72 however these materials are outside the scope of this review.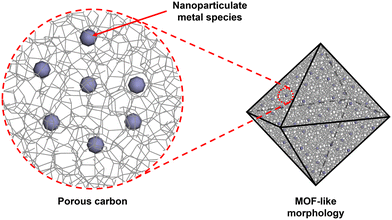 | ||
| Fig. 2 Schematic of MOFdC structural features showing MOF-like morphology, porous carbon, and nanoparticulate metal species. | ||
On a macro scale, MOFdCs also inherit structural features from the template MOFs. As highly ordered crystalline materials, MOFs generally grow in well-defined morphologies, such as octahedra, cubes, or rods. Upon carbonization, these morphologies are well-maintained, with MOFdCs usually exhibiting the same morphology as the template MOF crystals.40,73,74 Due to the loss of material through partial combustion or evaporation of the metal species, the faces of octahedral or cubic MOFdCs are often sunken in.75 Furthermore, due to the loss of relatively strong coordination bonds in three dimensions and the generation of additional grain boundaries, MOFdCs are generally more brittle than the template MOFs.76
Another feature that is common, though not universal, in MOFdCs is the presence of inorganic components. While some MOFdC research focuses on the structure of the carbon components and will deliberately remove the inorganic components,51,52 the field of metal-containing MOFdCs is growing rapidly.37,68,77–79 Inorganic components typically exhibit nanosized domains, as the temperatures of MOF pyrolysis are often not high enough to melt or sinter the metal species into larger domains.80,81 In some cases, the inorganic species are single atoms supported on the carbon,62,77,82 however this is often difficult to achieve due to the high temperatures of the carbonization and the close proximity of the metal ions in the template MOF structure. A variety of metal species can be formed in a MOFdC, including metals, metal oxides, metal nitrides, metal carbides, and metal sulfides.45,79,83–87 In cases where more than one metal ion is contained within the IBU or hosted in the pores of the MOF, multimetallic inorganic species or alloys are often formed.88–92 Due to the small size of the domains and inorganic node distortions that occur upon heating, metal species in MOFdCs are often poorly ordered, which makes traditional bulk scale or average structure analysis challenging or poorly representative of the true structure of the species.83,84,93–95 Consequently, many researchers in the field have relied on local structure analysis techniques, such as X-ray or neutron total scattering or extended X-ray absorption fine structure (EXAFS) to complement average structure analysis.45,83,84,94–96
Effect of carbonization conditions on MOFdC structure
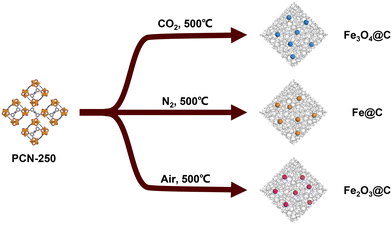
More recently, research into the influence of carbonization conditions on the MOFdC structure and composition has begun to be studied. An early work in this area was performed by Banerjee, Poddar, and coworkers, who compared carbonization of a range of MOFs in both air and nitrogen environments. They found that the inorganic species produced from carbonization in air exclusively formed metal oxides, regardless of the identity of the metal (M = Mg, Mn, Co, Cu, Zn, Cd). However, carbonization in nitrogen produced metallic nanoparticles of cobalt and copper, while the other metals were not reduced under these conditions due to their lower oxidation potential.87In another such study, Zhou and coworkers conducted a detailed investigation of the iron MOF PCN-250 (PCN = porous coordination network) and the effects of pyrolysis temperature and gas environment on the structure of the resulting iron species and carbon matrix in the MOFdC (Fig. 3).45 They found that pyrolysis in oxidizing gases produced MOFdCs with low surface areas and low carbon content, suggesting that the oxidative conditions caused combustion of the linker before it could be carbonized. In contrast, non-oxidizing atmospheres typically produced much higher surface areas and higher carbon content, suggesting that a non-oxidizing environment is key for retaining the porous structure of the MOF after carbonization. Chapman and coworkers similarly investigated the pyrolysis of PCN-250 under reducing atmospheres, corroborating the results reported by Zhou and expanding the scope of the research to consider the structure of metal oxide nanoparticles derived from multimetallic PCN-250.94 Zhou and coworkers also demonstrated that the linker decomposition process involves an oxidative decarboxylation, which in turn reduces the iron of the MOF cluster to iron(0) before later being oxidized, while Chapman and coworkers identified metal node distortions prior to structural collapse, suggesting that both node and linker dynamics contribute to the MOF decomposition.45,65,66,94 This process also shed light on differences in the iron oxide phase present following pyrolysis in different gas environments. While carbonization in oxidizing gases produced a single phase of iron oxide due to uniform oxidation of the iron(0) intermediate during the carbonization, carbonization in non-oxidizing gases produced multiple iron oxide phases due to the CO2 produced during the carbonization and post-carbonization oxidation upon exposure to ambient air.45 Carbonization in more strongly reducing gas environments produced iron(II) oxide, perhaps by inhibiting the oxidative decarboxylation of the linker.94 Notably, this mechanistic understanding of the carbonization process has subsequently been applied to target the formation of MOFdCs with specific structural features.97 Cheetham, Wang, and coworkers also observed the effect of gas environment on the metal species of a MOFdC. Carbonization of a manganese triazolate framework under rigorously oxygen-free conditions produced nanoparticulate manganese nitride that was air-stable, a property not found in bulk manganese nitrides. In contrast, carbonization in air produced manganese oxide.83 As the nitrogen of the manganese nitride was derived directly from the triazolate linker, this suggests that different reaction mechanisms may be at play depending on the gas environment.
Zhou and coworkers also noted some significant differences in MOFdC structure based on pyrolysis temperature. When Zn-MOF-74 was carbonized below 600 °C, ZnO nanoparticles were produced, while carbonization above 600 °C caused sintering of the ZnO into a bulk domain.84 This sintering causes an increase in mesoporosity as the ZnO sinters to the exterior of the MOFdC, leaving mesoporous voids on the interior of the material. While this specific sintering behavior had not been previously observed in MOFdCs, other temperature dependent effects have been described. The first MOFdC reported was calcined at 1000 °C to ensure evaporation of the zinc species derived from the IBU, however it was also possible to obtain zinc oxide-containing MOFdCs by carbonization at lower temperatures, albeit at the expense of high porosity.39 The rate of heating can also influence this sintering behavior. More rapid heating ramps typically produce larger, more crystalline metal species, as the activation energy for grain growth can more readily be overcome.98,99 Notably, the effect of heating ramp is generally negligible on the decomposition pathways or the identity of the final metal species, as demonstrated by pyrolysis of HKUST-1 (HKUST = Hong Kong University of Science and Technology) at heating ramps from 2–5 °C min−1 (Fig. 4).96 By conducting in situ powder X-ray diffraction (PXRD), X-ray absorption spectroscopy (XAS), and X-ray total scattering pair distribution function (PDF) experiments, the authors observed that the MOF decomposed at 300–325 °C into a mixture of Cu(0) and Cu(I) oxide, the latter of which was an intermediate that was subsequently reduced to Cu(0) at higher temperatures (above 350 °C). While the Cu(I) oxide intermediate was not observed upon rapid heating (over 2500 °C min−1), this difference was ascribed to the limited time/temperature resolution that could be achieved in the analysis and does not necessarily indicate a change to the mechanism of Cu(0) formation.96
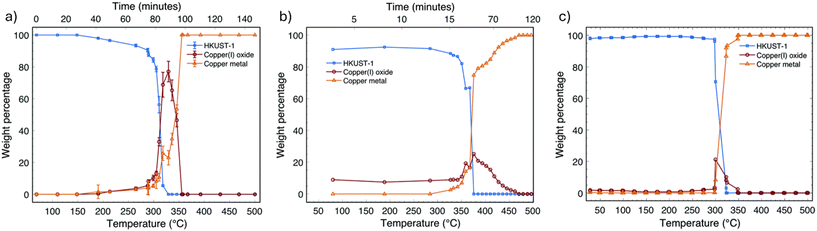 | ||
| Fig. 4 Evolution of MOF, Cu(I) oxide, and Cu(0) phases during carbonization of HKUST-1, as measured by XAS (a), X-ray PDF (b), and PXRD (c). Reproduced from ref. 96 with permission from the Royal Society of Chemistry. | ||
Effect of MOF template structure on MOFdC structure
In addition to the carbonization conditions, features of the MOF template structure such as dimensionality, IBU structure, and connectivity also play a role in determining the structure of the MOFdC. When comparing the carbonization products of copper MOFs HKUST-1 and F-MOF-4 (F-MOF = fluorinated metal–organic framework), the 3-dimensional HKUST-1 produces larger copper particles than the 2-dimensional F-MOF-4.87 This enhanced growth was hypothesized to be a consequence of the dimensionality, as the HKUST-1-derived copper particles are agglomerated from all three dimensions. This hypothesis also held for the comparison between the 2-dimensional cobalt MOF MOF-CJ4 and the 3-dimensional cobalt MOF Co-HFMOF-D (HFMOF = partially fluorinated metal–organic framework).87The effect of linker structure on the pore structure of the carbon matrix of the MOFdC has also been investigated. In another study, several zinc MOFs with a range of linkers were carbonized to produce metal-free MOFdCs of varied structure. Carbonization of MOF templates with more rigid and aromatic linkers produced porous carbons that maintained the morphology and pore structure of the template MOF, while more flexible linkers produced carbons with much less well-ordered pore structures.53
The influence of MOF structure on the resulting MOFdC was further explored by Zhou and coworkers. When comparing pyrolysis of Zn-MOF-74 and UiO-66 (UiO = University of Oslo), they found that carbonization of Zn-MOF-74 and its higher connected and more thermally stable linker produced MOFdCs with higher surface area by preventing pre-carbonization pore collapse.84 Furthermore, they found that carbonization of UiO-66 produced primarily cubic zirconia due to the templating effect of the cubic Zr6-oxo clusters, despite this phase typically being considered unstable at particle sizes above 2 nm.100
Cheetham, Wang and coworkers note that while oxygen-free carbonization of a manganese triazolate framework produces manganese nitride, carbonization of the analogous iron and cobalt triazolates under the same conditions produce iron carbide and cobalt metal respectively.83 While this was not explored in depth, it further emphasizes that different inorganic components in the MOF can result in different reaction pathways and different inorganic products. Likewise, Zhou and Chapman have both demonstrated that incorporation of a heterometal into the cluster of PCN-250 can produce different metal phases following carbonization, with PCN-250-Fe2Ni in particular favoring the formation of fcc-Ni due to the favorability of FeNi alloy formation and selective Ni extraction from the cluster during the pyrolysis process.94,97
One of the most significant advances in MOFdCs in the last decade is the development of the metal ion “fence” strategy to form MOF-derived single atom catalysts (SACs) supported on a porous carbon matrix (Fig. 5). Following reports of Co SACs formed from bimetallic ZIF structures,101,102 this strategy was more systematically developed by Wu, Li, and coworkers, who carbonized a mixed cobalt/zinc ZIF to produce single cobalt atoms anchored to a N-doped carbon matrix.77 By incorporating zinc ions in the ZIF structure as a “fence” between cobalt ions, the cobalt ions were too spatially separated to undergo significant aggregation and thus remained embedded as single atoms in the carbon with a porphyrinic planar structure. Wang and coworkers further investigated the relative activity of MOF-derived Co-SACs and MOF-derived cobalt nanoparticles for nitroarene hydrogenation, showing that the SACs were significantly more active.103 This fencing strategy has since been used with other metal dopants, including iron,62,104–107 manganese,104 nickel,107–110 and copper.110,111
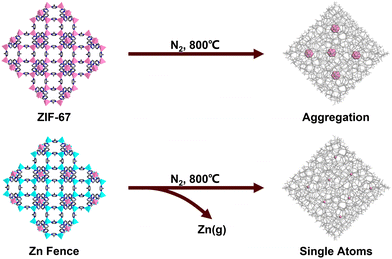 | ||
| Fig. 5 “Fencing” strategy for the generation of ZIF-derived Co-SACs. The procedure is generalizable to other metals. | ||
An alternative strategy that relies on the same principle is that described by Zhou, Li and Fischer, in which the porphyrin center of a porphyrinic zirconium-based MOF was partially metalated with nickel and several other transition metal ions.82 As with the fence strategy, the incomplete metalation of the porphyrin center resulted in significant distance between the nickel ions and prevented agglomeration or sintering into larger particles upon carbonization of the MOF, instead producing SACs on a porous carbon support. However, this strategy is not universally applicable. When a zinc-porphyrin MOF metalated with palladium is used in place of the zirconium-porphyrin MOF metalated with nickel, the final material contained a palladium-zinc alloy instead of single palladium atoms dispersed on the carbon.112
More complex metal species can be produced by designing MOF templates with more than one metal ion in the IBU. Prussian blue, a coordination polymer known since the early 1700s, can be calcined to form porous multimetallic iron oxide structures, with partial substitution of the iron atoms by heterometals such as manganese or cobalt.90,91 Another popular MOF template for the formation of alloys is M-btc (H3btc = benzene 1,3,5-tricarboxylic acid), as multimetallic MOFs of this structure can be readily synthesized in one-pot reactions simply by varying the concentration of each respective metal ion in the reaction mixture.88,89
It is also possible to incorporate secondary metals by impregnating the MOF with the metal or metal precursor prior to carbonization. This strategy has commonly been performed with the ZIF-8/ZIF-67 system, as techniques for impregnation of metal nanoparticles in this MOF are well-established. This is a particularly desirable strategy when the metal of interest is too expensive to be used as the backbone of the MOF, for example noble metals such as gold or platinum.113,114 In many cases, this strategy is used to form alloys or multimetallic species that are more active than the individual metals alone.92,114–117
Structure–function relationships in MOFdCs
Gas storage
As with most materials, the functionality of MOFdCs is dictated by their structure. Inspired by the promise of MOFs in gas storage applications, early work with MOFdCs similarly focused heavily on the gas storage properties of the materials. The first report of a MOFdC involved a discussion of the hydrogen uptake properties of the material,39 which remained a popular application for MOFdCs throughout the early 2010s.47,54,87 Crucially, MOFdCs outperformed porous carbons produced by other methods due to the high concentration of ultramicropores.54,118 Furthermore, some highly graphitic MOFdCs are excellent materials for sensing aromatic compounds due to their high surface area and the affinity of the graphitic sp2-hybridized carbons for aromatic molecules.51 MOFdCs have also been used as sorbents for liquids and some dyes due to the hydrophobicity of the carbon and their high porosity.75,119–122 In addition to their high uptake of oil and dyes, the presence of magnetic cobalt- or iron-based nanoparticles derived from the MOF IBUs can significantly improve recovery and recyclability of the sorbent.75,119Catalysis
MOF-derived bimetallic metal species have also been used as Fenton catalysts, where peroxide and hydroxide radicals are generated to degrade organic pollutants.123 Similarly, MOF-derived SACs, often formed using the fencing strategy, have also been applied as Fenton or Fenton-like catalysts,106,124 as have MOF-derived cobalt nanoparticles125 and partially carbonized Ce-TCPP nanocomposites (H4TCPP = tetrakis(4-carboxyphenyl)porphyrin).126 Even metal-free MOFdCs have been applied as catalysts for peroxydisulfate activation due to the high activity of nitrogen-doped carbon structure.55,56MOFdCs are also strong candidates for large-scale industrial catalysis at elevated temperatures and pressures.41,42,127,128 Fischer–Tropsch synthesis of hydrocarbon chains from syngas is an important industrial reaction which is typically performed at high temperatures and pressures.41 While MOFs may not be stable under these conditions, MOFdCs are significantly more resistant to high temperatures.37 A variety of iron- and cobalt-based MOFs have been carbonized and applied for Fischer–Tropsch catalysis, with either ultrasmall metal nanoparticles or SACs as the active species. Gascon and coworkers have produced several iron-based MOFdCs that equal or outperform commercial Fischer–Tropsch catalysts.42,127
Electrocatalysis
Due to the high concentration and good dispersion of metal species and the conductivity of the porous carbon matrix, MOFdCs have also shown significant promise as electrocatalysts for a variety of reactions. This review will briefly summarize several key reactions for which MOFdCs have been used as catalysts and the types of MOFdCs that have been applied in this way. Readers are directed to other recent reviews for a more detailed discussion of the design and application of MOFdCs for electrocatalysis.61,68,129–132Electrochemical nitrogen reduction is an important reaction for energy storage as it presents a key avenue for hydrogen fixation and ammonia production. MOFdCs have recently been applied in this process.132,133 In one such example, Cu-btc was calcined at several different temperatures to produce either a Cu-SAC or copper nanoparticles. The Cu-SACs were highly effective at promoting nitrate reduction while suppressing the accumulation of toxic nitrite ions.134 Li and coworkers similarly found cobalt-doped iron nanoparticles derived from MOF-74 were effective nitrate reduction catalysts.135 Nitrate is not the only substrate that has been converted to ammonia by MOF-derived catalysts. A bismuth-based MOFdC was used to fix nitrogen under ambient conditions with very high faradaic efficiency.136
Carbon dioxide utilization is another key process for which MOFdCs have been applied as electrocatalysts. The ZIF-8 system is a popular template for this reaction due to the propensity to form SACs upon carbonization. Both monometallic Ni-SACs and tandem systems that include Ni-SACs and either Cu- or Fe-SACs within the same porous carbon have been generated.107,108,110 Other nickel-based MOFs have also been carbonized to produce Ni-SACs with promising results.88,137 Similarly, HKUST-1-derived Cu–C composites have been applied for CO2 electroreductions to both methanol and ethanol with high faradaic efficiencies and very low overpotentials (Fig. 6).138 Titanium-based MOFs have also been applied as templates for the generation of carbon dioxide reduction reaction (CO2-RR) catalysts. In one example, MIL-125-derived Mg-doped titania (MIL = Materials Institute Lavoisier) was used to photocatalytically reduce carbon dioxide, with the magnesium dopant promoting the generation of surface Ti3+ ions to improve charge separation. Furthermore, the Mg-doping increased the surface area of the titania by discouraging sintering.139 In another, a mixed titanium-iron MOF was used to generate titanomaghemite nanoparticles with a high Fe/Ti ratio that cannot be achieved via traditional inorganic synthetic methods. This MOFdC was also an effective electrocatalyst for CO2-RR.140
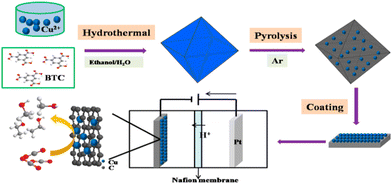 | ||
| Fig. 6 Synthesis and utilization of HKUST-1-derived Cu–C composites for CO2 electroreduction to C1 and C2 alcohols. Reprinted with permission from ref. 138. Copyright 2017 American Chemical Society. | ||
Electrochemical water splitting reactions are also an important target reaction for MOFdC catalysts. Oxygen reduction reactions (ORR) are one of the most common applications in the literature for metal-containing MOFdCs.44,61 While early work in this area focused on the generation of iron nanoparticles,141 the ZIF-8/ZIF-67 system later became a popular template for the synthesis of these catalysts due to its propensity to form SACs, a consequence of the high nitrogen content in the carbon matrix and the ability to “fence” the Co ions with zinc in a bimetallic framework.77,105,142–144 Cobalt is generally the most active SAC for these reactions, however iron and copper sites have also been employed.105,145 Other MOFs have also been used as templates for generating SACs as ORR catalysts, such as Fe-doped NH2-MIL-101(Al).146 These materials have been found to exhibit higher ORR activity than commercial Pt/C catalysts, representing an important step towards reducing society's reliance on noble metals and increasing the sustainability of ORR.105,147
In addition to ORR, MOFdCs have been employed as multifunctional catalysts that are active towards both ORR and oxygen evolution reactions (OER), the latter of which is significantly more challenging due to its slow kinetics.43,44,68,148 A diverse range of template MOFs have been used to generate such catalysts. In one example, a Zn-MOF was used as a host for copper or platinum nanoparticles and was carbonized to form a nanoparticle@porous carbon material without the inclusion of IBU-derived nanoparticles.149 More common, however, is the generation of IBU-derived nanoparticles, such as through the carbonization of thiourea@Fe-btc to form an FeS/Fe3C@C composite or the carbonization of a cobalt-vanadium mixed-metal MOF to form cobalt and vanadium nitride nanoparticles.85,86
Energy storage
Finally, energy storage has recently emerged as an application for MOFdCs due to their high specific capacity, high surface area, and robustness towards volume expansion of guest materials.79,150,151 Importantly, the porous carbon of MOFdCs is often graphitic in nature, thus the materials can be engineered to exhibit high capacities and enhanced ion transport compared to non-porous graphite.84,152,153 These properties mean that MOFdCs often excel as anode materials for lithium, potassium, and sodium ion batteries.151–157The conductive nature of the carbon also allows MOFdCs to be used as matrices for other high-capacity anode materials. Silicon anode lithium ion batteries are highly desired due to the extremely high theoretical capacity (4200 mA h g−1), however challenges such as volumetric expansion upon lithiation limit the amount of silicon that can be incorporated without experiencing severe capacity fade.158–160 Several studies have demonstrated the utility of MOFdCs as a porous carbon host for nanoparticulate silicon (Fig. 7).150,160–162 The high porosity of the carbon structure enables highly efficient lithium ion diffusion, leading to high specific capacity and high capacity retention.161 Furthermore, the mechanically flexible porous carbon structure of the MOFdC mitigates the detrimental effects of silicon expansion, due to the porous carbon acting as a buffer to reduce strain or induce in situ formation of ultrasmall silicon nanodots.150,160
 | ||
| Fig. 7 Synthesis of Si@MOFdC composite anode materials. Reprinted with permission from ref. 150. Copyright 2023 American Chemical Society. | ||
Conclusions and outlook
MOFdCs are an attractive alternative to MOFs due to their enhanced stability and conductivity, and an improvement upon traditional porous carbons due to their microporosity and tunability. As demonstrated in this review, a vast array of MOFdC structures can be selectively synthesized through strategies such as judicious choice of carbonization conditions, tuning the MOF structure, and incorporating guests in the MOF prior to carbonization. As the field of MOFdCs continues to grow, there are a number of areas ripe for future research (Fig. 8).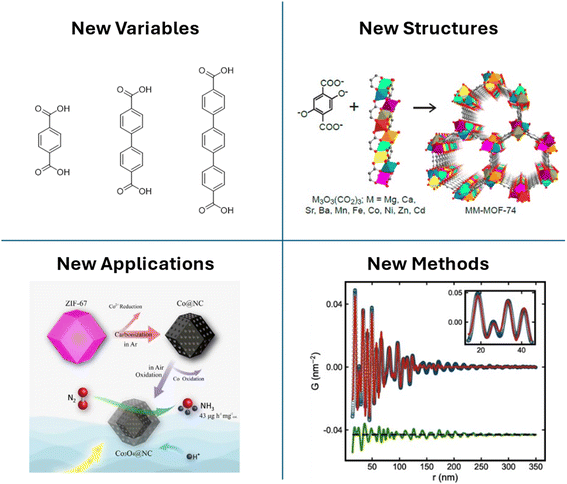 | ||
| Fig. 8 Future directions for MOFdC research include investigation of previously unstudied structural variables (e.g. linker length, top left), targeted design of multimetallic inorganic structures using multimetallic MOF platforms (top right, reprinted with permission from ref. 167. Copyright American Chemical Society 2014), targeted design of electrocatalysts at commercial scale (bottom left, reprinted with permission from ref. 133. Copyright American Chemical Society 2019) and use as a model system for developing advanced analytical techniques (e.g. sasPDF, bottom right, reproduced from ref. 169 with permission from the International Union of Crystallography). | ||
While the literature to date has focused primarily on the templating effect of the IBU and framework structure and the impact of pyrolysis conditions, there are many other variables that have yet to be considered in the design of MOFdCs. Variables such as linker aromaticity and rigidity have been studied for their influence on the carbon structure,53 however their impact on the structure of the metal species has not been considered. Other factors, such as linker length and connectivity, have not yet been systematically studied for closely related MOF templates, such as isoreticular expansions of a template MOF.11,163 The inclusion of additional functional groups on linkers also provides a pathway for altering the structure of the metal species in the MOFdC, as they may interact differently with various chemical moieties throughout the carbonization process.93,164
MOFdCs also represent a step forward in the applicability of MOF-based materials due to their improved stability over the template MOFs. To this end, future research should consider the design of MOFdCs tailored to specific applications by using these principles. As electrocatalytic applications are widely investigated, future studies should employ systematic approaches to develop highly active, but inexpensive MOF-derived electrocatalysts, for example using bimetallic MIL-100(Fe) as a template instead of the more costly PCN-250.44,97,165,166 Additional developments, such as the design of trimetallic (or more) MOFs as precursors for new and unusual inorganic materials, should also be pursued.140,167
Finally, MOFdCs provide a fascinating platform for the development of new analytical techniques and the advancement and extension of existing techniques. The nature of MOFdCs as poorly ordered solid materials that may contain regions of greater structuring, such as metal nanoparticles, challenges our existing conceptions on how to study the structure of materials. A swathe of advanced complementary techniques is required to fully describe the structure of MOFdCs and new techniques may be able to extract information from unexpected places. Given the often small size of the inorganic nanoparticles in MOFdCs, the combination of small- and wide-angle X-ray scattering (SAXS and WAXS) would provide an avenue to study the coevolution of X-ray scattering and Bragg diffraction peaks in situ, thus supporting models derived from other advanced in situ techniques.96 Due to the differences in intensity of the scattering and diffraction features and the challenges of simultaneous detection across wide q-ranges, this type of analysis will inspire novel approaches in detector design and data collection methods that can be similarly applied in characterization of other advanced materials. Other cutting-edge techniques, particularly in the realm of local structure analysis, can also be further developed using MOFdCs as a model system. Due to their poor crystallinity, proper characterization of MOFdCs often requires the use of local structure analysis techniques, such as PDF or far-infrared (terahertz) spectroscopy.45,94,168 While it is possible to collect this type of data using conventional instrumentation, the cost and time required to obtain high quality data typically necessitates the use of synchrotron radiation. As the field of MOFdCs continues to grow, it will spur further advances in high-intensity X-ray sources and high-resolution detectors to enable greater accessibility, as well as the development of other advanced local structure analysis techniques, such as small angle scattering PDF (sas-PDF) and single-atom-sensitive electron energy loss spectroscopy (EELS).169,170
Author contributions
J. A. P. and H.-C. Z. conceptualized the manuscript, J. A. P. and Y. Y. prepared the manuscript, H.-C. Z. conducted supervision.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Welch Foundation through the Welch Endowed Chair to H. C. Z. A-0030. Elements of the TOC graphic were generated with the assistance of Google Gemini.References
- C. P. Bryan and G. E. Smith, Ancient Egyptian medicine: the Papyrus Ebers, Ares Publishers, Chicago, 1974 Search PubMed.
- J. R. Partington, Origins and Development of Applied Chemistry, Longmans, Green & Co, London, 1935 Search PubMed.
- F. Çeçen and Ö. Aktaş, in Activated Carbon for Water and Wastewater Treatment, 2011, pp. 1–11. DOI:10.1002/9783527639441.ch1.
- E. M. Diamond and K. T. H. Farrer, Watering The Fleet And The Introduction Of Distillation, The Mariner's Mirror, 2005, 91, 548–553 CrossRef.
- H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Design and synthesis of an exceptionally stable and highly porous metal-organic framework, Nature, 1999, 402, 276 CrossRef CAS.
- S. Kitagawa and M. Kondo, Functional Micropore Chemistry of Crystalline Metal Complex-Assembled Compounds, Bull. Chem. Soc. Jpn., 1998, 71, 1739–1753 CrossRef CAS.
- M. Kondo, T. Yoshitomi, H. Matsuzaka, S. Kitagawa and K. Seki, Three-Dimensional Framework with Channeling Cavities for Small Molecules: {[M2(4, 4′-bpy)3(NO3)4]·xH2O}n (M = Co, Ni, Zn), Angew. Chem., Int. Ed. Engl., 1997, 36, 1725–1727 CrossRef CAS.
- M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. Keeffe and O. M. Yaghi, Systematic Design of Pore Size and Functionality in Isoreticular MOFs and Their Application in Methane Storage, Science, 2002, 295, 469 CrossRef CAS PubMed.
- R. Robson, B. F. Abrahams, S. R. Batten, R. W. Gable, B. F. Hoskins and J. Liu, in Supramolecular Architecture, American Chemical Society, 1992, vol. 499, ch. 19, pp. 256–273 Search PubMed.
- B. F. Hoskins and R. Robson, Infinite polymeric frameworks consisting of three dimensionally linked rod-like segments, J. Am. Chem. Soc., 1989, 111, 5962–5964 CrossRef CAS.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed.
- C. Serre, F. Millange, S. Surblé and G. Férey, A Route to the Synthesis of Trivalent Transition-Metal Porous Carboxylates with Trimeric Secondary Building Units, Angew. Chem., Int. Ed., 2004, 43, 6285–6289 CrossRef PubMed.
- C. T. Lollar, J.-S. Qin, J. Pang, S. Yuan, B. Becker and H.-C. Zhou, Interior Decoration of Stable Metal–Organic Frameworks, Langmuir, 2018, 34, 13795–13807 CrossRef CAS PubMed.
- S. Yuan, L. Feng, K. Wang, J. Pang, M. Bosch, C. Lollar, Y. Sun, J. Qin, X. Yang, P. Zhang, Q. Wang, L. Zou, Y. Zhang, L. Zhang, Y. Fang, J. Li and H.-C. Zhou, Stable Metal–Organic Frameworks: Design, Synthesis, and Applications, Adv. Mater., 2018, 30, 1704303 CrossRef PubMed.
- S. Yuan, J. S. Qin, C. T. Lollar and H. C. Zhou, Stable Metal-Organic Frameworks with Group 4 Metals: Current Status and Trends, ACS Cent. Sci., 2018, 4, 440–450 CrossRef CAS PubMed.
- S. Ahn, N. E. Thornburg, Z. Li, T. C. Wang, L. C. Gallington, K. W. Chapman, J. M. Notestein, J. T. Hupp and O. K. Farha, Stable Metal–Organic Framework-Supported Niobium Catalysts, Inorg. Chem., 2016, 55, 11954–11961 CrossRef CAS PubMed.
- D. Feng, Z.-Y. Gu, J.-R. Li, H.-L. Jiang, Z. Wei and H.-C. Zhou, Zirconium-Metalloporphyrin PCN-222: Mesoporous Metal–Organic Frameworks with Ultrahigh Stability as Biomimetic Catalysts, Angew. Chem., Int. Ed., 2012, 51, 10307–10310 CrossRef CAS PubMed.
- L. Liu and S. G. Telfer, Systematic Ligand Modulation Enhances the Moisture Stability and Gas Sorption Characteristics of Quaternary Metal–Organic Frameworks, J. Am. Chem. Soc., 2015, 137, 3901–3909 CrossRef CAS PubMed.
- L. Feng, K.-Y. Wang, X.-L. Lv, J. A. Powell, T.-H. Yan, J. Willman and H.-C. Zhou, Imprinted Apportionment of Functional Groups in Multivariate Metal–Organic Frameworks, J. Am. Chem. Soc., 2019, 141, 14524–14529 CrossRef CAS PubMed.
- T.-T. Li, Y.-M. Liu, T. Wang, Y.-L. Wu, Y.-L. He, R. Yang and S.-R. Zheng, Regulation of the surface area and surface charge property of MOFs by multivariate strategy: Synthesis, characterization, selective dye adsorption and separation, Microporous Mesoporous Mater., 2018, 272, 101–108 CrossRef CAS.
- X.-J. Yu, Y.-M. Xian, C. Wang, H.-L. Mao, M. Kind, T. Abu-Husein, Z. Chen, S.-B. Zhu, B. Ren, A. Terfort and J.-L. Zhuang, Liquid-Phase Epitaxial Growth of Highly Oriented and Multivariate Surface-Attached Metal–Organic Frameworks, J. Am. Chem. Soc., 2019, 141, 18984–18993 CrossRef CAS PubMed.
- S. Yuan, W. Lu, Y.-P. Chen, Q. Zhang, T.-F. Liu, D. Feng, X. Wang, J. Qin and H.-C. Zhou, Sequential Linker Installation: Precise Placement of Functional Groups in Multivariate Metal–Organic Frameworks, J. Am. Chem. Soc., 2015, 137, 3177–3180 CrossRef CAS PubMed.
- G. Mouchaham, S. Wang and C. Serre, in Metal–Organic Frameworks: Applications in Separations and Catalysis, ed. H. Garcia and S. Navalon, Wiley-VCH, Weinheim, 2018, pp. 1–28 Search PubMed.
- M. Ding, X. Cai and H.-L. Jiang, Improving MOF stability: approaches and applications, Chem. Sci., 2019, 10, 10209–10230 RSC.
- N. C. Burtch, H. Jasuja and K. S. Walton, Water Stability and Adsorption in Metal–Organic Frameworks, Chem. Rev., 2014, 114, 10575–10612 CrossRef CAS PubMed.
- Frameworks for commercial success, Nat. Chem., 2016, 8, 987–987 CrossRef PubMed.
- S. Bose, D. Sengupta, T. M. Rayder, X. Wang, K. O. Kirlikovali, A. K. Sekizkardes, T. Islamoglu and O. K. Farha, Challenges and Opportunities: Metal–Organic Frameworks for Direct Air Capture, Adv. Funct. Mater., 2023, 2307478, DOI:10.1002/adfm.202307478.
- K. Hoffman, BASF becomes first company to successfully produce metal-organic frameworks on a commercial scale for carbon capture, 2023, https://www.basf.com/global/en/media/news-releases/2023/10/p-23-327.html Search PubMed.
- Templated Organic Synthesis, ed. F. Diederich and P. J. Stang, Wiley-VCH, Weinheim, 2000 Search PubMed.
- S. Begum, Z. Hassan, S. Bräse and M. Tsotsalas, Polymerization in MOF-Confined Nanospaces: Tailored Architectures, Functions, and Applications, Langmuir, 2020, 36, 10657–10673 CrossRef CAS PubMed.
- S. Dasgupta and J. Wu, in Comprehensive Supramolecular Chemistry II, ed. J. L. Atwood, Elsevier, Oxford, 2017, pp. 251–268. DOI:10.1016/B978-0-12-409547-2.12588-0.
- Y. Liu, J. Goebl and Y. Yin, Templated synthesis of nanostructured materials, Chem. Soc. Rev., 2013, 42, 2610–2653 RSC.
- R. R. Poolakkandy and M. M. Menamparambath, Soft-template-assisted synthesis: a promising approach for the fabrication of transition metal oxides, Nanoscale Adv., 2020, 2, 5015–5045 RSC.
- J. Wang, Y. Wang, H. Hu, Q. Yang and J. Cai, From metal–organic frameworks to porous carbon materials: recent progress and prospects from energy and environmental perspectives, Nanoscale, 2020, 12, 4238–4268 RSC.
- F. Aricó, J. D. Badjic, S. J. Cantrill, A. H. Flood, K. C. F. Leung, Y. Liu and J. F. Stoddart, in Templates in Chemistry II, ed. C. A. Schalley, F. Vögtle and K. H. Dötz, Springer Berlin Heidelberg, Berlin, Heidelberg, 2005, pp. 203–259. DOI:10.1007/b104330.
- R. K. O'Reilly, A. J. Turberfield and T. R. Wilks, The Evolution of DNA-Templated Synthesis as a Tool for Materials Discovery, Acc. Chem. Res., 2017, 50, 2496–2509 CrossRef PubMed.
- L. Oar-Arteta, T. Wezendonk, X. Sun, F. Kapteijn and J. Gascon, Metal organic frameworks as precursors for the manufacture of advanced catalytic materials, Mater. Chem. Front., 2017, 1, 1709–1745 RSC.
- C. Healy, K. M. Patil, B. H. Wilson, L. Hermanspahn, N. C. Harvey-Reid, B. I. Howard, C. Kleinjan, J. Kolien, F. Payet, S. G. Telfer, P. E. Kruger and T. D. Bennett, The thermal stability of metal-organic frameworks, Coord. Chem. Rev., 2020, 419, 213388 CrossRef CAS.
- B. Liu, H. Shioyama, T. Akita and Q. Xu, Metal-Organic Framework as a Template for Porous Carbon Synthesis, J. Am. Chem. Soc., 2008, 130, 5390–5391 Search PubMed.
- W. Chaikittisilp, K. Ariga and Y. Yamauchi, A new family of carbon materials: synthesis of MOF-derived nanoporous carbons and their promising applications, J. Mater. Chem. A, 2013, 1, 14–19 RSC.
- K. O. Otun, X. Liu and D. Hildebrandt, Metal-organic framework (MOF)-derived catalysts for Fischer-Tropsch synthesis: Recent progress and future perspectives, J. Energy Chem., 2020, 51, 230–245 CrossRef.
- V. P. Santos, T. A. Wezendonk, J. J. D. Jaén, A. I. Dugulan, M. A. Nasalevich, H.-U. Islam, A. Chojecki, S. Sartipi, X. Sun, A. A. Hakeem, A. C. J. Koeken, M. Ruitenbeek, T. Davidian, G. R. Meima, G. Sankar, F. Kapteijn, M. Makkee and J. Gascon, Metal organic framework-mediated synthesis of highly active and stable Fischer-Tropsch catalysts, Nat. Commun., 2015, 6, 6451 CrossRef CAS PubMed.
- J. Du, F. Li and L. Sun, Metal–organic frameworks and their derivatives as electrocatalysts for the oxygen evolution reaction, Chem. Soc. Rev., 2021, 50, 2663–2695 RSC.
- H.-F. Wang, L. Chen, H. Pang, S. Kaskel and Q. Xu, MOF-derived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions, Chem. Soc. Rev., 2020, 49, 1414–1448 RSC.
- G. S. Day, J. Li, E. A. Joseph, P. Metz, Z. Perry, M. R. Ryder, K. Page and H.-C. Zhou, Metal oxide decorated porous carbons from controlled calcination of a metal-organic framework, Nanoscale Adv., 2020, 2, 2758–2767 RSC.
- B. Liu, H. Shioyama, H. Jiang, X. Zhang and Q. Xu, Metal–organic framework (MOF) as a template for syntheses of nanoporous carbons as electrode materials for supercapacitor, Carbon, 2010, 48, 456–463 CrossRef CAS.
- H.-L. Jiang, B. Liu, Y.-Q. Lan, K. Kuratani, T. Akita, H. Shioyama, F. Zong and Q. Xu, From Metal–Organic Framework to Nanoporous Carbon: Toward a Very High Surface Area and Hydrogen Uptake, J. Am. Chem. Soc., 2011, 133, 11854–11857 CrossRef CAS PubMed.
- P. Pachfule, B. P. Biswal and R. Banerjee, Control of Porosity by Using Isoreticular Zeolitic Imidazolate Frameworks (IRZIFs) as a Template for Porous Carbon Synthesis, Chem. – Eur. J., 2012, 18, 11399–11408 CrossRef CAS PubMed.
- J. Hu, H. Wang, Q. Gao and H. Guo, Porous carbons prepared by using metal–organic framework as the precursor for supercapacitors, Carbon, 2010, 48, 3599–3606 CrossRef CAS.
- L. Chen, J. Bai, C. Wang, Y. Pan, M. Scheer and X. You, One-step solid-state thermolysis of a metal–organic framework: a simple and facile route to large-scale of multiwalled carbon nanotubes, Chem. Commun., 2008, 1581–1583, 10.1039/B718476J.
- M. Hu, J. Reboul, S. Furukawa, N. L. Torad, Q. Ji, P. Srinivasu, K. Ariga, S. Kitagawa and Y. Yamauchi, Direct Carbonization of Al-Based Porous Coordination Polymer for Synthesis of Nanoporous Carbon, J. Am. Chem. Soc., 2012, 134, 2864–2867 CrossRef CAS PubMed.
- L. Radhakrishnan, J. Reboul, S. Furukawa, P. Srinivasu, S. Kitagawa and Y. Yamauchi, Preparation of Microporous Carbon Fibers through Carbonization of Al-Based Porous Coordination Polymer (Al-PCP) with Furfuryl Alcohol, Chem. Mater., 2011, 23, 1225–1231 CrossRef CAS.
- H. B. Aiyappa, P. Pachfule, R. Banerjee and S. Kurungot, Porous Carbons from Nonporous MOFs: Influence of Ligand Characteristics on Intrinsic Properties of End Carbon, Cryst. Growth Des., 2013, 13, 4195–4199 CrossRef CAS.
- S. J. Yang, T. Kim, J. H. Im, Y. S. Kim, K. Lee, H. Jung and C. R. Park, MOF-Derived Hierarchically Porous Carbon with Exceptional Porosity and Hydrogen Storage Capacity, Chem. Mater., 2012, 24, 464–470 CrossRef CAS.
- Y. Liu, W. Miao, X. Fang, Y. Tang, D. Wu and S. Mao, MOF-derived metal-free N-doped porous carbon mediated peroxydisulfate activation via radical and non-radical pathways: Role of graphitic N and CO, Chem. Eng. J., 2020, 380, 122584 CrossRef CAS.
- R. Luo, J. Wu, J. Zhao, D. Fang, Z. Liu and L. Hu, ZIF-8 derived defect-rich nitrogen-doped carbon with enhanced catalytic activity for efficient non-radical activation of peroxydisulfate, Environ. Res., 2022, 204, 112060 CrossRef CAS PubMed.
- M. Kim, X. Xu, R. Xin, J. Earnshaw, A. Ashok, J. Kim, T. Park, A. K. Nanjundan, W. A. El-Said, J. W. Yi, J. Na and Y. Yamauchi, KOH-Activated Hollow ZIF-8 Derived Porous Carbon: Nanoarchitectured Control for Upgraded Capacitive Deionization and Supercapacitor, ACS Appl. Mater. Interfaces, 2021, 13(44), 52034–52043 CrossRef CAS PubMed.
- K. S. Park, Z. Ni, A. P. Côté, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. M. Yaghi, Exceptional chemical and thermal stability of zeolitic imidazolate frameworks, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef CAS PubMed.
- Y.-R. Lee, M.-S. Jang, H.-Y. Cho, H.-J. Kwon, S. Kim and W.-S. Ahn, ZIF-8: A comparison of synthesis methods, Chem. Eng. J., 2015, 271, 276–280 CrossRef CAS.
- J. Li, Y. Chen, Y. Tang, S. Li, H. Dong, K. Li, M. Han, Y.-Q. Lan, J. Bao and Z. Dai, Metal–organic framework templated nitrogen and sulfur co-doped porous carbons as highly efficient metal-free electrocatalysts for oxygen reduction reactions, J. Mater. Chem. A, 2014, 2, 6316–6319 RSC.
- J. Zhang, C. Xu, Y. Zhang, Y. Li, B. Liu, P. Huo, D. Liu and J. Gui, Structural and compositional analysis of MOF-derived carbon nanomaterials for the oxygen reduction reaction, Chem. Commun., 2024, 60, 2572–2590 RSC.
- D. Wang, H. Liu, Z. Cao, T. Cai, P. Han, J. Song, L. Kong and C. Liu, Ordered porous nitrogen-doped carbon with atomically dispersed FeN4 for efficient oxygen reduction reaction in microbial fuel cell, Sci. Total Environ., 2022, 838, 156186 CrossRef CAS PubMed.
- S. Xu, A. Dong, Y. Hu, Z. Yang, S. Huang and J. Qian, Multidimensional MOF-derived carbon nanomaterials for multifunctional applications, J. Mater. Chem. A, 2023, 11, 9721–9747 RSC.
- L. Chai, X. Wang, Y. Hu, X. Li, S. Huang, J. Pan, J. Qian and X. Sun, In-MOF-Derived Hierarchically Hollow Carbon Nanostraws for Advanced Zinc-Iodine Batteries, Adv. Sci., 2022, 9, 2105063 CrossRef CAS PubMed.
- H. F. Drake, G. S. Day, S. W. Vali, Z. Xiao, S. Banerjee, J. Li, E. A. Joseph, J. E. Kuszynski, Z. T. Perry, A. Kirchon, O. K. Ozdemir, P. A. Lindahl and H.-C. Zhou, The thermally induced decarboxylation mechanism of a mixed-oxidation state carboxylate-based iron metal–organic framework, Chem. Commun., 2019, 55, 12769–12772 RSC.
- H. F. Drake, Z. Xiao, G. S. Day, S. W. Vali, W. Chen, Q. Wang, Y. Huang, T.-H. Yan, J. E. Kuszynski, P. A. Lindahl, M. R. Ryder and H.-C. Zhou, Thermal decarboxylation for the generation of hierarchical porosity in isostructural metal–organic frameworks containing open metal sites, Mater. Adv., 2021, 2, 5487–5493 RSC.
- Y. Tan, K. Zhu, D. Li, F. Bai, Y. Wei and P. Zhang, N-doped graphene/Fe–Fe3C nano-composite synthesized by a Fe-based metal organic framework and its anode performance in lithium ion batteries, Chem. Eng. J., 2014, 258, 93–100 CrossRef CAS.
- J. Chen and J. Qian, Insights on MOF-derived metal–carbon nanostructures for oxygen evolution, Dalton Trans., 2024, 53, 2903–2916 RSC.
- Q. Ren, H. Wang, X.-F. Lu, Y.-X. Tong and G.-R. Li, Recent Progress on MOF-Derived Heteroatom-Doped Carbon-Based Electrocatalysts for Oxygen Reduction Reaction, Adv. Sci., 2018, 5, 1700515 CrossRef PubMed.
- N. Lu, X. Zhang, X. Yan, D. Pan, B. Fan and R. Li, Synthesis of novel mesoporous sulfated zirconia nanosheets derived from Zr-based metal–organic frameworks, CrystEngComm, 2020, 22, 44–51 RSC.
- X. Yan, N. Lu, B. Fan, J. Bao, D. Pan, M. Wang and R. Li, Synthesis of mesoporous and tetragonal zirconia with inherited morphology from metal–organic frameworks, CrystEngComm, 2015, 17, 6426–6433 RSC.
- S. Ni and Y. Li, t-ZrO2 prepared by a novel zirconium oxalate synthesised solvothermally, Micro–Nano Lett., 2018, 13, 919–922 CrossRef CAS.
- C. Wang, J. Kim, J. Tang, M. Kim, H. Lim, V. Malgras, J. You, Q. Xu, J. Li and Y. Yamauchi, New Strategies for Novel MOF-Derived Carbon Materials Based on Nanoarchitectures, Chem, 2020, 6, 19–40 CAS.
- J. Ren, Y. Huang, H. Zhu, B. Zhang, H. Zhu, S. Shen, G. Tan, F. Wu, H. He, S. Lan, X. Xia and Q. Liu, Recent progress on MOF-derived carbon materials for energy storage, Carbon Energy, 2020, 2, 176–202 CrossRef CAS.
- N. L. Torad, M. Hu, S. Ishihara, H. Sukegawa, A. A. Belik, M. Imura, K. Ariga, Y. Sakka and Y. Yamauchi, Direct Synthesis of MOF-Derived Nanoporous Carbon with Magnetic Co Nanoparticles toward Efficient Water Treatment, Small, 2014, 10, 2096–2107 CrossRef CAS PubMed.
- A. I. Cherevko, I. A. Nikovskiy, Y. V. Nelyubina, K. M. Skupov, N. N. Efimov and V. V. Novikov, 3D-Printed Porous Magnetic Carbon Materials Derived from Metal–Organic Frameworks, Polymers, 2021, 13(22), 3881, DOI:10.3390/polym13223881.
- P. Yin, T. Yao, Y. Wu, L. Zheng, Y. Lin, W. Liu, H. Ju, J. Zhu, X. Hong, Z. Deng, G. Zhou, S. Wei and Y. Li, Single Cobalt Atoms with Precise N-Coordination as Superior Oxygen Reduction Reaction Catalysts, Angew. Chem., Int. Ed., 2016, 55, 10800–10805 CrossRef CAS PubMed.
- J. Hwang, A. Ejsmont, R. Freund, J. Goscianska, B. V. K. J. Schmidt and S. Wuttke, Controlling the morphology of metal–organic frameworks and porous carbon materials: metal oxides as primary architecture-directing agents, Chem. Soc. Rev., 2020, 49, 3348–3422 RSC.
- N. Kitchamsetti and J. S. Cho, A roadmap of MOFs derived porous carbon, oxides, chalcogenides, and phosphides of metals: Synthesis, properties, parameter modulation and their utilization as an electrode for Li/Na/K-ion batteries, J. Energy Storage, 2024, 84, 110947 CrossRef.
- Y. Dai, P. Lu, Z. Cao, C. T. Campbell and Y. Xia, The physical chemistry and materials science behind sinter-resistant catalysts, Chem. Soc. Rev., 2018, 47, 4314–4331 RSC.
- G. I. Finch and K. P. Sinha, On Reaction in the Solid State, Proc. R. Soc. London, Ser. A, 1957, 239, 145–153 CAS.
- Z. Zhou, J. Zhang, S. Mukherjee, S. Hou, R. Khare, M. Döblinger, O. Tomanec, M. Otyepka, M. Koch, P. Gao, L. Zhou, W. Li and R. A. Fischer, Porphyrinic MOF derived Single-atom electrocatalyst enables methanol oxidation, Chem. Eng. J., 2022, 449, 137888 CrossRef CAS.
- Y. Hu, C. Li, S. Xi, Z. Deng, X. Liu, A. K. Cheetham and J. Wang, Direct Pyrolysis of a Manganese-Triazolate Metal–Organic Framework into Air-Stable Manganese Nitride Nanoparticles, Adv. Sci., 2021, 8, 2003212 CrossRef CAS PubMed.
- G. S. Day, H. F. Drake, A. Contreras-Ramirez, M. R. Ryder, K. Page and H.-C. Zhou, Controlled Metal Oxide and Porous Carbon Templation Using Metal-Organic Frameworks, Cryst. Growth Des., 2021, 21, 4249–4258 CrossRef CAS.
- Y.-W. Li, W.-J. Zhang, J. Li, H.-Y. Ma, H.-M. Du, D.-C. Li, S.-N. Wang, J.-S. Zhao, J.-M. Dou and L. Xu, Fe-MOF-Derived Efficient ORR/OER Bifunctional Electrocatalyst for Rechargeable Zinc–Air Batteries, ACS Appl. Mater. Interfaces, 2020, 12, 44710–44719 CrossRef CAS PubMed.
- H. Zheng, N. Xu, B. Hou, X. Zhao, M. Dong, C. Sun, X.-L. Wang and Z.-M. Su, Bimetallic Metal–Organic Framework-Derived Graphitic Carbon-Coated Small Co/VN Nanoparticles as Advanced Trifunctional Electrocatalysts, ACS Appl. Mater. Interfaces, 2021, 13, 2462–2471 CrossRef CAS PubMed.
- R. Das, P. Pachfule, R. Banerjee and P. Poddar, Metal and metal oxide nanoparticle synthesis from metal organic frameworks (MOFs): finding the border of metal and metal oxides, Nanoscale, 2012, 4, 591–599 RSC.
- S. Payra, N. Devaraj, K. Tarafder and S. Roy, Unprecedented Electroreduction of CO2 over Metal Organic Framework-Derived Intermetallic Nano-Alloy Cu0.85Ni0.15/C, ACS Appl. Energy Mater., 2022, 5, 4945–4955 CrossRef CAS.
- Q. Zhang, X. Zhang, D. Lei, S. Qiao, Q. Wang, X. Shi, C. Huang, G. He and F. Zhang, MOF-Derived Hollow Carbon Supported Nickel-Cobalt Alloy Catalysts Driving Fast Polysulfide Conversion for Lithium-Sulfur Batteries, ACS Appl. Mater. Interfaces, 2023, 15, 15377–15386 CrossRef CAS PubMed.
- F. Zheng, D. Zhu, X. Shi and Q. Chen, Metal–organic framework-derived porous Mn1.8Fe1.2O4 nanocubes with an interconnected channel structure as high-performance anodes for lithium ion batteries, J. Mater. Chem. A, 2015, 3, 2815–2824 RSC.
- H. Guo, T. Li, W. Chen, L. Liu, X. Yang, Y. Wang and Y. Guo, General design of hollow porous CoFe2O4 nanocubes from metal–organic frameworks with extraordinary lithium storage, Nanoscale, 2014, 6, 15168–15174 RSC.
- Z. Du, F. Chen, S. Fang, X. Yang, Y. Ge, K. Shurtz, H.-C. Zhou, Y. H. Hu and Y. Li, Engineering Bimetallic Ni-Cu Nanoparticles Confined in MOF-Derived Nanocomposite for Efficient Dry Reforming of Methane, ES Energy Environ., 2024, 23, 1097 CAS.
- J. B. DeCoste, G. W. Peterson, H. Jasuja, T. G. Glover, Y.-G. Huang and K. S. Walton, Stability and degradation mechanisms of metal–organic frameworks containing the Zr6O4(OH)4 secondary building unit, J. Mater. Chem. A, 2013, 1, 5642–5650 RSC.
- Z. Chen, Z. Chen, O. K. Farha and K. W. Chapman, Mechanistic Insights into Nanoparticle Formation from Bimetallic Metal–Organic Frameworks, J. Am. Chem. Soc., 2021, 143, 8976–8980 CrossRef CAS PubMed.
- A. E. Platero-Prats, A. Mavrandonakis, L. C. Gallington, Y. Liu, J. T. Hupp, O. K. Farha, C. J. Cramer and K. W. Chapman, Structural Transitions of the Metal-Oxide Nodes within Metal–Organic Frameworks: On the Local Structures of NU-1000 and UiO-66, J. Am. Chem. Soc., 2016, 138, 4178–4185 CrossRef CAS PubMed.
- M. Folkjær, L. F. Lundegaard, H. S. Jeppesen, M. J. Marks, M. S. Hvid, S. Frank, G. Cibin and N. Lock, Pyrolysis of a metal–organic framework followed by in situ X-ray absorption spectroscopy, powder diffraction and pair distribution function analysis, Dalton Trans., 2022, 51, 10740–10750 RSC.
- J. A. Powell, H. Lin, Y.-C. Hsu, D. E. Young and H.-C. Zhou, Exploitation of MOF decomposition mechanisms to tailor MOF-derived carbon structure in mono- and multimetallic PCN-250, Cryst. Growth Des., 2025 DOI:10.1021/acs.cgd.4c01690.
- P. Yang, X. Song, C. Jia and H.-S. Chen, Metal-organic framework-derived hierarchical ZnO/NiO composites: Morphology, microstructure and electrochemical performance, J. Ind. Eng. Chem., 2018, 62, 250–257 CrossRef CAS.
- P. Ge, H. Hou, S. Li, L. Yang and X. Ji, Tailoring Rod-Like FeSe2 Coated with Nitrogen-Doped Carbon for High-Performance Sodium Storage, Adv. Funct. Mater., 2018, 28, 1801765 CrossRef.
- S. Tsunekawa, S. Ito, Y. Kawazoe and J. T. Wang, Critical Size of the Phase Transition from Cubic to Tetragonal in Pure Zirconia Nanoparticles, Nano Lett., 2003, 3, 871–875 CrossRef CAS.
- Y.-Z. Chen, C. Wang, Z.-Y. Wu, Y. Xiong, Q. Xu, S.-H. Yu and H.-L. Jiang, From Bimetallic Metal-Organic Framework to Porous Carbon: High Surface Area and Multicomponent Active Dopants for Excellent Electrocatalysis, Adv. Mater., 2015, 27, 5010–5016 CrossRef CAS PubMed.
- B. You, N. Jiang, M. Sheng, W. S. Drisdell, J. Yano and Y. Sun, Bimetal–Organic Framework Self-Adjusted Synthesis of Support-Free Nonprecious Electrocatalysts for Efficient Oxygen Reduction, ACS Catal., 2015, 5, 7068–7076 CrossRef CAS.
- H. Wang, Y. Wang, Y. Li, X. Lan, B. Ali and T. Wang, Highly Efficient Hydrogenation of Nitroarenes by N-Doped Carbon-Supported Cobalt Single-Atom Catalyst in Ethanol/Water Mixed Solvent, ACS Appl. Mater. Interfaces, 2020, 12, 34021–34031 CrossRef CAS PubMed.
- S. Gong, C. Wang, P. Jiang, L. Hu, H. Lei and Q. Chen, Designing highly efficient dual-metal single-atom electrocatalysts for the oxygen reduction reaction inspired by biological enzyme systems, J. Mater. Chem. A, 2018, 6, 13254–13262 RSC.
- H. Li, X. Chen, J. Chen, K. Shen and Y. Li, Hierarchically porous Fe,N-doped carbon nanorods derived from 1D Fe-doped MOFs as highly efficient oxygen reduction electrocatalysts in both alkaline and acidic media, Nanoscale, 2021, 13, 10500–10508 RSC.
- W. Yang, P. Hong, D. Yang, Y. Yang, Z. Wu, C. Xie, J. He, K. Zhang, L. Kong and J. Liu, Enhanced Fenton-like degradation of sulfadiazine by single atom iron materials fixed on nitrogen-doped porous carbon, J. Colloid Interface Sci., 2021, 597, 56–65 CrossRef CAS PubMed.
- L. Jiao, J. Zhu, Y. Zhang, W. Yang, S. Zhou, A. Li, C. Xie, X. Zheng, W. Zhou, S.-H. Yu and H.-L. Jiang, Non-Bonding Interaction of Neighboring Fe and Ni Single-Atom Pairs on MOF-Derived N-Doped Carbon for Enhanced CO2 Electroreduction, J. Am. Chem. Soc., 2021, 143, 19417–19424 CrossRef CAS PubMed.
- F. Pan, H. Zhang, Z. Liu, D. Cullen, K. Liu, K. More, G. Wu, G. Wang and Y. Li, Atomic-level active sites of efficient imidazolate framework-derived nickel catalysts for CO2 reduction, J. Mater. Chem. A, 2019, 7, 26231–26237 RSC.
- P. Yue, K. Xiong, L. Ma, J. Li, L. Zhang, X. Zhu, Q. Fu and Q. Liao, MOF-Derived Ni Single-Atom Catalyst with Abundant Mesopores for Efficient Mass Transport in Electrolytic Bicarbonate Conversion, ACS Appl. Mater. Interfaces, 2022, 14, 54840–54847 CrossRef CAS PubMed.
- H. Cheng, X. Wu, M. Feng, X. Li, G. Lei, Z. Fan, D. Pan, F. Cui and G. He, Atomically Dispersed Ni/Cu Dual Sites for Boosting the CO2 Reduction Reaction, ACS Catal., 2021, 11, 12673–12681 CrossRef CAS.
- S.-M. Li, Y. Shi, J.-J. Zhang, Y. Wang, H. Wang and J.-X. Lu, Atomically Dispersed Copper on N-Doped Carbon Nanosheets for Electrocatalytic Synthesis of Carbamates from CO2 as a C1 Source, ChemSusChem, 2021, 14, 2050–2055 CrossRef CAS PubMed.
- X. Cao, R. Tong, S. Tang, B. W. L. Jang, A. Mirjalili, J. Li, X. Guo, J. Zhang, J. Hu and X. Meng, Design of Pd-Zn Bimetal MOF Nanosheets and MOF-Derived Pd3.9Zn6.1/CNS Catalyst for Selective Hydrogenation of Acetylene under Simulated Front-End Conditions, Molecules, 2022, 27(17), 5736 CrossRef CAS PubMed.
- T.-H. Yang, P.-C. Han, I. T. Wang, C. H. Chen, M. A. M. Khan, C.-Y. Wen and K. C. W. Wu, Water-Based Synthesis of Gold Single Atoms-Embedded, Metal–Organic Frameworks-Derived Nanoporous Carbon Nanoparticles with Enhanced Reduction Ability, Adv. Mater. Interfaces, 2021, 8, 2001638 CrossRef CAS.
- N. Du, C. Wang, R. Long and Y. Xiong, N-doped carbon-stabilized PtCo nanoparticles derived from Pt@ZIF-67: Highly active and durable catalysts for oxygen reduction reaction, Nano Res., 2017, 10, 3228–3237 CrossRef CAS.
- Z. Qi, Y. Pei, T. W. Goh, Z. Wang, X. Li, M. Lowe, R. V. Maligal-Ganesh and W. Huang, Conversion of confined metal@ZIF-8 structures to intermetallic nanoparticles supported on nitrogen-doped carbon for electrocatalysis, Nano Res., 2018, 11, 3469–3479 CrossRef CAS.
- X. X. Wang, S. Hwang, Y.-T. Pan, K. Chen, Y. He, S. Karakalos, H. Zhang, J. S. Spendelow, D. Su and G. Wu, Ordered Pt3Co Intermetallic Nanoparticles Derived from Metal–Organic Frameworks for Oxygen Reduction, Nano Lett., 2018, 18, 4163–4171 CrossRef CAS PubMed.
- D. B. Christensen, R. L. Mortensen, S. Kramer and S. Kegnæs, Study of CoCu Alloy Nanoparticles Supported on MOF-Derived Carbon for Hydrosilylation of Ketones, Catal. Lett., 2020, 150, 1537–1545 CrossRef CAS.
- A. Almasoudi and R. Mokaya, Preparation and hydrogen storage capacity of templated and activated carbons nanocast from commercially available zeolitic imidazolate framework, J. Mater. Chem., 2012, 22, 146–152 RSC.
- A. Banerjee, R. Gokhale, S. Bhatnagar, J. Jog, M. Bhardwaj, B. Lefez, B. Hannoyer and S. Ogale, MOF derived porous carbon–Fe3O4 nanocomposite as a high performance, recyclable environmental superadsorbent, J. Mater. Chem., 2012, 22, 19694–19699 RSC.
- Z. Hasan, D.-W. Cho, I.-H. Nam, C.-M. Chon and H. Song, Preparation of calcined zirconia-carbon composite from metal organic frameworks and its application to adsorption of crystal violet and salicylic acid, Materials, 2016, 9, 261 CrossRef PubMed.
- B. Chen, X. Zhang, Y. Liu, X. Ma, X. Wang, X. Cao and L. Lian, Magnetic porous carbons derived from iron-based metal-organic framework loaded with glucose for effective extraction of synthetic organic dyes in drinks, J. Chromatogr. A, 2022, 1661, 462716 CrossRef CAS PubMed.
- M. Yu, H. Dong, Y. Zheng and W. Liu, Ternary metal oxide embedded carbon derived from metal organic frameworks for adsorption of methylene blue and acid red 73, Chemosphere, 2021, 280, 130567 CrossRef CAS PubMed.
- A. Fu, Z. Liu and Z. Sun, Cu/Fe oxide integrated on graphite felt for degradation of sulfamethoxazole in the heterogeneous electro-Fenton process under near-neutral conditions, Chemosphere, 2022, 297, 134257 CrossRef CAS PubMed.
- B. N. Bhadra, N. A. Khan and S. H. Jhung, Co supported on N-doped carbon, derived from bimetallic azolate framework-6: a highly effective oxidative desulfurization catalyst, J. Mater. Chem. A, 2019, 7, 17823–17833 RSC.
- J. Cao, Z. Yang, W. Xiong, Y. Zhou, Y. Wu, M. Jia, S. Sun, C. Zhou, Y. Zhang and R. Zhong, Peroxymonosulfate activation of magnetic Co nanoparticles relative to an N-doped porous carbon under confinement: Boosting stability and performance, Sep. Purif. Technol., 2020, 250, 117237 CrossRef CAS.
- S. Zhao, S. Li, Y. Long, X. Shen, Z. Zhao, Q. Wei, S. Wang, Z. Zhang, X. Zhang and Z. Zhang, Ce-based Heterogeneous Catalysts by Partial Thermal Decomposition of Ce-MOFs in Activation of Peroxymonosulfate for the Removal of Organic Pollutants under Visible Light, Chemosphere, 2021, 130637, DOI:10.1016/j.chemosphere.2021.130637.
- T. A. Wezendonk, Q. S. E. Warringa, V. P. Santos, A. Chojecki, M. Ruitenbeek, G. Meima, M. Makkee, F. Kapteijn and J. Gascon, Structural and elemental influence from various MOFs on the performance of Fe@C catalysts for Fischer–Tropsch synthesis, Faraday Discuss., 2017, 197, 225–242 RSC.
- Q. Zhao, S. Huang, X. Han, J. Chen, J. Wang, A. Rykov, Y. Wang, M. Wang, J. Lv and X. Ma, Highly active and controllable MOF-derived carbon nanosheets supported iron catalysts for Fischer-Tropsch synthesis, Carbon, 2021, 173, 364–375 CrossRef CAS.
- A. Shahzad, F. Zulfiqar and M. Arif Nadeem, Cobalt containing bimetallic ZIFs and their derivatives as OER electrocatalysts: A critical review, Coord. Chem. Rev., 2023, 477, 214925 CrossRef CAS.
- Y. Song, C. Yu, D. Ma and K. Liu, Recent progress on ZIF-8 based MOF derivatives for electrocatalysis, Coord. Chem. Rev., 2024, 499, 215492 CrossRef CAS.
- L. Chai, R. Li, Y. Sun, K. Zhou and J. Pan, MOF-derived Carbon-Based Materials for Energy-Related Applications, Adv. Mater., 2025, 2413658 CrossRef CAS PubMed.
- B. Han, J. Liu, C. Lee, C. Lv and Q. Yan, Recent Advances in Metal-Organic Framework-Based Nanomaterials for Electrocatalytic Nitrogen Reduction, Small Methods, 2023, 7, 2300277 CrossRef CAS PubMed.
- S. Luo, X. Li, B. Zhang, Z. Luo and M. Luo, MOF-Derived Co3O4@NC with Core–Shell Structures for N2 Electrochemical Reduction under Ambient Conditions, ACS Appl. Mater. Interfaces, 2019, 11, 26891–26897 CrossRef CAS PubMed.
- T. Zhu, Q. Chen, P. Liao, W. Duan, S. Liang, Z. Yan and C. Feng, Single-Atom Cu Catalysts for Enhanced Electrocatalytic Nitrate Reduction with Significant Alleviation of Nitrite Production, Small, 2020, 16, 2004526 CrossRef CAS PubMed.
- S. Zhang, M. Li, J. Li, Q. Song and X. Liu, High-ammonia selective metal–organic framework–derived Co-doped Fe/Fe2O3 catalysts for electrochemical nitrate reduction, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2115504119 CrossRef CAS PubMed.
- F. Wang, L. Zhang, T. Wang, F. Zhang, Q. Liu, H. Zhao, B. Zheng, J. Du and X. Sun, In Situ Derived Bi Nanoparticles Confined in Carbon Rods as an Efficient Electrocatalyst for Ambient N2 Reduction to NH3, Inorg. Chem., 2021, 60, 7584–7589 CrossRef CAS PubMed.
- S. Yang, J. Zhang, L. Peng, M. Asgari, D. Stoian, I. Kochetygov, W. Luo, E. Oveisi, O. Trukhina, A. H. Clark, D. T. Sun and W. L. Queen, A metal–organic framework/polymer derived catalyst containing single-atom nickel species for electrocatalysis, Chem. Sci., 2020, 11, 10991–10997 RSC.
- K. Zhao, Y. Liu, X. Quan, S. Chen and H. Yu, CO2 Electroreduction at Low Overpotential on Oxide-Derived Cu/Carbons Fabricated from Metal Organic Framework, ACS Appl. Mater. Interfaces, 2017, 9, 5302–5311 CrossRef CAS PubMed.
- X. Feng, F. Pan, P. Zhang, X. Wang, H.-C. Zhou, Y. Huang and Y. Li, Metal-Organic Framework MIL-125 Derived Mg2+-Doped Mesoporous TiO2 for Photocatalytic CO2 Reduction, ChemPhotoChem, 2021, 5, 79–89 CrossRef CAS.
- J. Castells-Gil, S. Ould-Chikh, A. Ramírez, R. Ahmad, G. Prieto, A. R. Gómez, L. Garzón-Tovar, S. Telalovic, L. Liu, A. Genovese, N. M. Padial, A. Aguilar-Tapia, P. Bordet, L. Cavallo, C. Martí-Gastaldo and J. Gascon, Unlocking mixed oxides with unprecedented stoichiometries from heterometallic metal-organic frameworks for the catalytic hydrogenation of CO2, Chem. Catal., 2021, 1, 364–382 CrossRef CAS.
- S. Zhao, H. Yin, L. Du, L. He, K. Zhao, L. Chang, G. Yin, H. Zhao, S. Liu and Z. Tang, Carbonized Nanoscale Metal–Organic Frameworks as High Performance Electrocatalyst for Oxygen Reduction Reaction, ACS Nano, 2014, 8, 12660–12668 CrossRef CAS PubMed.
- Y. Chen, R. Gao, S. Ji, H. Li, K. Tang, P. Jiang, H. Hu, Z. Zhang, H. Hao, Q. Qu, X. Liang, W. Chen, J. Dong, D. Wang and Y. Li, Atomic-Level Modulation of Electronic Density at Cobalt Single-Atom Sites Derived from Metal–Organic Frameworks: Enhanced Oxygen Reduction Performance, Angew. Chem., Int. Ed., 2021, 60, 3212–3221 CrossRef CAS PubMed.
- Y. Yin, J. Wang, T. Li, J. P. Hill, A. Rowan, Y. Sugahara and Y. Yamauchi, Nanoarchitecturing Carbon Nanodot Arrays on Zeolitic Imidazolate Framework-Derived Cobalt–Nitrogen-Doped Carbon Nanoflakes toward Oxygen Reduction Electrocatalysts, ACS Nano, 2021, 15, 13240–13248 CrossRef CAS PubMed.
- W. Zhang, X. Yao, S. Zhou, X. Li, L. Li, Z. Yu and L. Gu, ZIF-8/ZIF-67-Derived Co-N-Embedded 1D Porous Carbon Nanofibers with Graphitic Carbon-Encased Co Nanoparticles as an Efficient Bifunctional Electrocatalyst, Small, 2018, 14, 1800423 CrossRef PubMed.
- Z. Wang, H. Jin, T. Meng, K. Liao, W. Meng, J. Yang, D. He, Y. Xiong and S. Mu, Fe, Cu-Coordinated ZIF-Derived Carbon Framework for Efficient Oxygen Reduction Reaction and Zinc–Air Batteries, Adv. Funct. Mater., 2018, 28, 1802596 CrossRef.
- X. Xie, L. Peng, H. Yang, G. I. N. Waterhouse, L. Shang and T. Zhang, MIL-101-Derived Mesoporous Carbon Supporting Highly Exposed Fe Single-Atom Sites as Efficient Oxygen Reduction Reaction Catalysts, Adv. Mater., 2021, 33, 2101038 CrossRef CAS PubMed.
- S. Yuan, J. Zhang, L. Hu, J. Li, S. Li, Y. Gao, Q. Zhang, L. Gu, W. Yang, X. Feng and B. Wang, Decarboxylation-Induced Defects in MOF-Derived Single Cobalt Atom@Carbon Electrocatalysts for Efficient Oxygen Reduction, Angew. Chem., Int. Ed., 2021, 60, 21685–21690 CrossRef CAS PubMed.
- J. Song, C. Wei, Z.-F. Huang, C. Liu, L. Zeng, X. Wang and Z. J. Xu, A review on fundamentals for designing oxygen evolution electrocatalysts, Chem. Soc. Rev., 2020, 49, 2196–2214 RSC.
- A. Parkash, Metal-organic framework derived ultralow-loading platinum-copper catalyst: a highly active and durable bifunctional electrocatalyst for oxygen-reduction and evolution reactions, Nanotechnology, 2021, 32, 325703 CrossRef CAS PubMed.
- J. W. Sturman, M. S. E. Houache, W. D. do Pim, E. A. Baranova, M. Murugesu and Y. Abu-Lebdeh, Critical Investigation of Metal–Organic-Frameworks to Improve the Silicon Anode of Lithium-Ion Batteries, ACS Appl. Energy Mater., 2024, 7, 21–30 CrossRef CAS.
- R. Kaur, V. A. Chhabra, V. Chaudhary, K. Vikrant, S. K. Tripathi, Y. Su, P. Kumar, K.-H. Kim and A. Deep, Metal-organic frameworks and their derivatives as anode material in lithium-ion batteries: Recent advances towards novel configurations, Int. J. Energy Res., 2022, 46, 13178–13204 CrossRef CAS.
- G. Chu, C. Wang, Z. Yang, L. Qin and X. Fan, MOF-derived porous graphitic carbon with optimized plateau capacity and rate capability for high performance lithium-ion capacitors, Int. J. Miner., Metall. Mater., 2024, 31, 395–404 CrossRef CAS.
- K. Xue, Y. Si, S. Xie, J. Yang, Y. Mo, B. Long, W. Wei, P. Cao, H. Wei, H. Guan, E. G. Michaelis, G. Guo, Y. Yue and C. Shan, Free-Standing N-Doped Porous Carbon Fiber Membrane Derived From Zn–MOF-74: Synthesis and Application as Anode for Sodium-Ion Battery With an Excellent Performance, Front. Chem., 2021, 9, 647545 CrossRef CAS PubMed.
- R. Tian, S.-H. Park, P. J. King, G. Cunningham, J. Coelho, V. Nicolosi and J. N. Coleman, Quantifying the factors limiting rate performance in battery electrodes, Nat. Commun., 2019, 10, 1933 CrossRef PubMed.
- A. Li, Y. Tong, B. Cao, H. Song, Z. Li, X. Chen, J. Zhou, G. Chen and H. Luo, MOF-derived multifractal porous carbon with ultrahigh lithium-ion storage performance, Sci. Rep., 2017, 7, 40574 CrossRef CAS PubMed.
- Y. Li, J. Zhang and M. Chen, MOF-derived carbon and composites as advanced anode materials for potassium ion batteries: A review, Sustainable Mater. Technol., 2020, 26, e00217 CrossRef CAS.
- Y. Zhang, M. Sha, Q. Fu, H. Zhao and Y. Lei, An overview of metal-organic frameworks-derived carbon as anode materials for sodium- and potassium-ion batteries, Mater. Today Sustain., 2022, 18, 100156 Search PubMed.
- C. K. Chan, H. Peng, G. Liu, K. McIlwrath, X. F. Zhang, R. A. Huggins and Y. Cui, High-performance lithium battery anodes using silicon nanowires, Nat. Nanotechnol., 2008, 3, 31–35 CrossRef CAS PubMed.
- M. Ko, S. Chae, J. Ma, N. Kim, H.-W. Lee, Y. Cui and J. Cho, Scalable synthesis of silicon-nanolayer-embedded graphite for high-energy lithium-ion batteries, Nat. Energy, 2016, 1, 1–8 Search PubMed.
- B. Chen, L. Chen, L. Zu, Y. Feng, Q. Su, C. Zhang and J. Yang, Zero-Strain High-Capacity Silicon/Carbon Anode Enabled by a MOF-Derived Space-Confined Single-Atom Catalytic Strategy for Lithium-Ion Batteries, Adv. Mater., 2022, 34, 2200894 CrossRef CAS PubMed.
- R. Gao, J. Tang, X. Yu, S. Tang, K. Ozawa, T. Sasaki and L.-C. Qin, In situ synthesis of MOF-derived carbon shells for silicon anode with improved lithium-ion storage, Nano Energy, 2020, 70, 104444 CrossRef CAS.
- Y.-J. Qiao, H. Zhang, Y.-X. Hu, W.-P. Li, W.-J. Liu, H.-M. Shang, M.-Z. Qu, G.-C. Peng and Z.-W. Xie, A chain-like compound of Si@CNT nanostructures and MOF-derived porous carbon as an anode for Li-ion batteries, Int. J. Miner., Metall. Mater., 2021, 28, 1611 CrossRef CAS.
- H. Deng, S. Grunder, K. E. Cordova, C. Valente, H. Furukawa, M. Hmadeh, F. Gándara, A. C. Whalley, Z. Liu, S. Asahina, H. Kazumori, M. O'Keeffe, O. Terasaki, J. F. Stoddart and O. M. Yaghi, Large-Pore Apertures in a Series of Metal-Organic Frameworks, Science, 2012, 336, 1018 CrossRef CAS PubMed.
- J. Hou, M. L. Ríos Gómez, A. Krajnc, A. McCaul, S. Li, A. M. Bumstead, A. F. Sapnik, Z. Deng, R. Lin, P. A. Chater, D. S. Keeble, D. A. Keen, D. Appadoo, B. Chan, V. Chen, G. Mali and T. D. Bennett, Halogenated Metal–Organic Framework Glasses and Liquids, J. Am. Chem. Soc., 2020, 142, 3880–3890 CrossRef CAS PubMed.
- M. A. Sayeed and A. P. O'Mullane, Electrodeposition at Highly Negative Potentials of an Iron-Cobalt Oxide Catalyst for Use in Electrochemical Water Splitting, ChemPhysChem, 2019, 20, 3112–3119 CrossRef CAS PubMed.
- M. Giménez-Marqués, A. Santiago-Portillo, S. Navalón, M. Álvaro, V. Briois, F. Nouar, H. Garcia and C. Serre, Exploring the catalytic performance of a series of bimetallic MIL-100(Fe, Ni) MOFs, J. Mater. Chem. A, 2019, 7, 20285–20292 RSC.
- L. J. Wang, H. Deng, H. Furukawa, F. Gándara, K. E. Cordova, D. Peri and O. M. Yaghi, Synthesis and Characterization of Metal–Organic Framework-74 Containing 2, 4, 6, 8, and 10 Different Metals, Inorg. Chem., 2014, 53, 5881–5883 CrossRef CAS PubMed.
- D. Capková, T. Kazda, O. Čech, N. Király, T. Zelenka, P. Čudek, A. Sharma, V. Hornebecq, A. S. Fedorková and M. Almáši, Influence of metal-organic framework MOF-76(Gd) activation/carbonization on the cycle performance stability in Li-S battery, J. Energy Storage, 2022, 51, 104419 CrossRef.
- C.-H. Liu, E. M. Janke, R. Li, P. Juhás, O. Gang, D. V. Talapin and S. J. Billinge, sasPDF: pair distribution function analysis of nanoparticle assemblies from small-angle scattering data, J. Appl. Crystallogr., 2020, 53, 699–709 CrossRef CAS PubMed.
- N. Allasia, S. M. Collins, Q. M. Ramasse and G. Vilé, Hidden Impurities Generate False Positives in Single Atom Catalyst Imaging, Angew. Chem., Int. Ed., 2024, 63, e202404883 CrossRef CAS PubMed.
| This journal is © the Partner Organisations 2025 |