Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Polyhedral oligomeric silsesquioxane difluoroboron complexes as cooperative octo-site catalysts for the photooxidation of sulfides to sulfoxides†
Mateusz Janeta * and
Sławomir Szafert
* and
Sławomir Szafert
Faculty of Chemistry, University of Wrocław, F. Joliot-Curie 14, 50-383 Wrocław, Poland. E-mail: mateusz.janeta@uwr.edu.pl
First published on 17th April 2025
Abstract
The incorporation of difluoroboron into the side arms of polyhedral oligomeric silsesquioxanes (POSSs) opens up new possibilities for the construction of metal-free photocatalysts with tailored properties. Herein, we report the design and synthesis of novel difluoroboron complexes of POSSs (POSS-tert-BF2, POSS-sal-BF2 and POSS-npht-BF2) derived from imine-functionalized POSSs, which were utilized as efficient photocatalysts. The complexes demonstrated exceptional photocatalytic performance in the aerobic oxidation of sulfides to sulfoxides, significantly outperforming their silsesquioxane-free counterparts. POSS-tert-BF2 demonstrated a high singlet oxygen quantum yield of 48%. This study highlights the feasibility of intramolecular cooperative activity in catalytic reactions and identifies key factors influencing its effectiveness. Furthermore, it underscores the potential of POSS as a versatile building block for the development of advanced photocatalytic materials.
Introduction
Polyhedral oligomeric silsesquioxanes (POSSs) have garnered significant attention in materials science due to their unique hybrid inorganic–organic structure and broad range of applications. POSS molecules with the general formula (RSiO3/2)n (where n = 6, 8, 10 or 12 and R can be H, alkyl or aryl) are composed of an inorganic silicon–oxygen cage core (Si–O–Si framework) and organic substituents attached to the silicon atoms. This combination endows POSS-based materials with exceptional thermal stability, mechanical strength, and chemical resistance, making them highly suitable for a wide range of applications including catalysis,1–5 nanocomposites,6,7 polymer fillers,8,9 porous materials,10,11 and drug delivery systems.12,13 Beyond the cubic structures, other unique types, including lantern,14 butterfly,15 Janus16 or double-decker17 shaped cages, have also gained attention for their potential applications.In a cooperative catalysis system, multiple catalytic moieties operate synergistically, often achieving enhanced catalytic efficiency or specificity compared to noncooperative catalytic systems.18 Chen et al. reported an amphiphilic (salen)Co complex that leverages hydrophobic interactions to enhance the efficiency of a cooperative catalyst.19
In the field of silsesquioxanes, we have observed increasing interest in POSS-based systems which could serve as homogenous catalysts. We recently showed that the tetranuclear zinc(II) POSS complex, Zn4@POSS-1, has demonstrated high efficiency as a quattro-site catalyst for the synthesis of cyclic carbonates from epoxides and CO2 under low-pressure conditions, where the zinc(II) centers in Zn4@POSS-1 functioned cooperatively.20 Furthermore, POSS molecules with imine linkages have been successfully employed in designing porous frameworks, making them highly suitable for applications such as gas storage, adsorption and separation processes, and demonstrating a cooperative effect in iodine capture.21
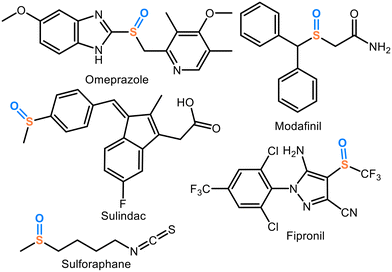
Sulfoxide-containing compounds play a vital role in medicinal chemistry and organic synthesis. They appear in numerous pharmaceuticals (Chart 1) such as omeprazole (a proton-pump inhibitor), modafinil (used for narcolepsy), sulindac (an anti-inflammatory prodrug), and sulforaphane (a natural anti-cancer agent). Sulfoxides are also present in non-medicinal products like the insecticide fipronil. Consequently, the development of sustainable and selective methods for oxidation of sulfides to sulfoxides is therefore essential in advancing these applications.22 Oxidation of thioethers has enabled their use in chemical warfare agent (CWA) neutralization.
However, traditional methods for oxidizing organic compounds to synthesize sulfoxides often rely on stoichiometric amounts of toxic heavy metals23 or highly reactive oxidants, such as m-CPBA, TBHP, UHP, 2-iodobenzoic acid, and oxone or hydrogen peroxide activated by metal catalysts based on tungsten, manganese, copper, iron, molybdenum or gold.22 These methods require in many cases over-stoichiometric amounts of oxidant and catalysts or operate under high temperatures.
These conventional approaches not only raise serious environmental concerns due to the generation of substantial quantities of toxic byproducts, but they also pose safety risks associated with the handling of hazardous materials. To address these challenges, the photocatalytic activation of molecular oxygen has emerged as a promising green alternative.
BODIPY dyes have proved to be effective in the oxidation of thioanisole.24–26 Li et al. demonstrated that thioanisole can be successfully oxidized to the corresponding sulfoxide within 24 hours, using methanol as the solvent.24 Later, in 2014, a dimeric BODIPY dye was introduced, significantly enhancing the efficiency of this oxidation reaction and marking a notable advancement in the application of boron-containing dyes for sulfide photooxidation.27
Inspired by these findings, we have designed and are presenting herein the synthesis, characterization, and structural investigation of new difluoroboron complexes with POSS as a catalyst for the photooxidation of sulfides to sulfoxides, examining the presence of a cooperative effect.
Results and discussion
Synthesis and characterization of POSS-imine-BF2
The synthetic pathways for fluoroborate POSS complexes POSS-imine-BF2 (where imine = tert, sal or npht) are shown in Scheme 1. Imine ligands bearing a POSS moiety (POSS-1, POSS-2, POSS-3, Chart S1, ESI†) were conveniently synthesized by reacting octa(3-aminopropyl)silsesquioxane hydrochloride with the corresponding aldehyde derivative in the presence of triethylamine as a deprotonation agent. Boron complexation was accomplished using boron trifluoride etherate and dry diisopropylethylamine (DIPEA) in dichloromethane at 40 °C, yielding POSS-imine-BF2 complexes in high yields. By-products were removed by washing with water. The final products were purified by filtration through a short plug of silica gel using dichloromethane (DCM) and subsequently recrystallized from a DCM/MeOH mixture. It is worth noting that POSS-imine-BF2 complexes exhibit excellent stability under ambient conditions and can be stored for several weeks without showing any signs of decomposition. The structures of the complexes were confirmed by (1H, 11B, 13C, 19F, 29Si) NMR, DRIFT, UV-vis spectroscopy, MALDI spectrometry and elemental and TG-DTA analysis.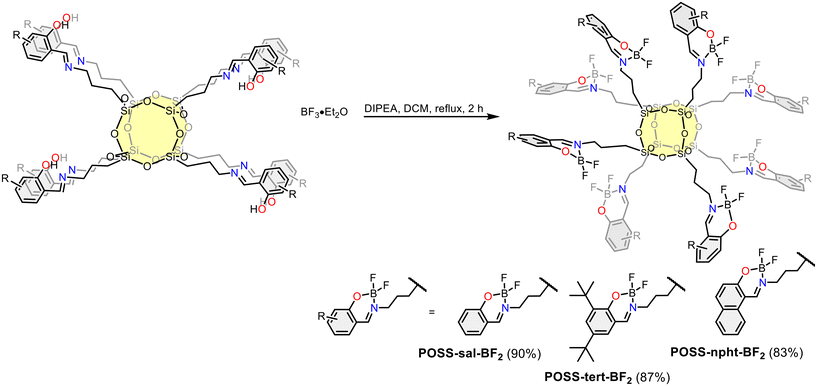 | ||
| Scheme 1 Synthesis of POSS-sal-BF2, POSS-tert-BF2 and POSS-npht-BF2. Isolated yields in parentheses. | ||
In the 1H NMR spectrum of POSS-tert-BF2 (Fig. 1a), the main indication of complexation is the disappearance of the OH signal at 13.95 ppm which is observed for the free ligand,20 and a downfield shift of the two doublets attributed to the protons of the phenyl ring, from 7.35 and 7.06 to 7.62 and 7.33 ppm. The 1H NMR spectrum also showed a sharp distinct signal at 8.38 ppm corresponding to the imine group and three signals in the alkyl region at 3.72–3.69, 2.01–1.94, 0.69–0.66 ppm attributed to iminopropyl functional groups. Additionally, two signals at 1.44 and 1.29 were observed, corresponding to the protons of the tert-butyl groups (Fig. 1a and Fig. S22, ESI†). Compared to the spectra of the pure ligand, the higher chemical shifts observed for the imine moiety, along with the absence of the broad OH signal, suggest coordination through both the imine and phenoxo groups. This was further corroborated by 11B NMR (Fig. 1b and Fig. S22, ESI†), which showed a peak at 0.49 ppm, which is consistent with the sp3 boron range and indicates N,O-coordination.28
 | ||
| Fig. 1 (a) 1H NMR (500 MHz, CDCl3) spectrum, (b) 11B NMR (160 MHz, CDCl3) spectrum, and (c) 29Si NMR (99 MHz, CDCl3) spectrum of POSS-tert-BF2. | ||
The 19F NMR spectrum (Fig. S25, ESI†) revealed a doublet of doublets at δ = −136.8 ppm, indicating the presence of two diastereotopic fluorine atoms split by a boron atom. The 13C{1H} NMR spectrum (Fig. S23, ESI†) matched well with the expected structures, showing a set of symmetry-equivalent signals for the organic side groups. In the DRIFT spectrum (Fig. S30, ESI†) of POSS-tert-BF2, new bands that were absent in the free ligand spectrum appeared at 1308 and 1055 cm−1, attributed to the νB–O and νB–N modes, respectively. Bands at 1570, 1150 and 939 cm−1 were assigned to the B–F vibrations. The νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N band shifted from 1633 cm−1 in the free ligand to 1644 cm−1 in POSS-tert-BF2, while the νO–H stretching vibrations disappeared, indicating that the ligand coordinates via the phenol and imine groups. These findings align with the NMR spectroscopy observations (see above). Characteristic vibrations of the Si–O–Si moieties were observed at 1100 cm−1, confirming the presence of a closed silsesquioxane cage structure. The substitution of all side arms in POSS-tert-BF2 was unambiguously verified by mass spectrometry. The MALDI mass spectrum (Fig. S31, ESI†) showed a signal at m/z 3017.4677 [M + Na]+ (calcd 3017.4762), which matched well with the theoretical value, confirming its octametallic structure. Elemental analysis further supported the proposed formulations, and the presence of eight boron atoms per POSS cage was verified using inductively coupled plasma optical emission spectroscopy (ICP-OES).
N band shifted from 1633 cm−1 in the free ligand to 1644 cm−1 in POSS-tert-BF2, while the νO–H stretching vibrations disappeared, indicating that the ligand coordinates via the phenol and imine groups. These findings align with the NMR spectroscopy observations (see above). Characteristic vibrations of the Si–O–Si moieties were observed at 1100 cm−1, confirming the presence of a closed silsesquioxane cage structure. The substitution of all side arms in POSS-tert-BF2 was unambiguously verified by mass spectrometry. The MALDI mass spectrum (Fig. S31, ESI†) showed a signal at m/z 3017.4677 [M + Na]+ (calcd 3017.4762), which matched well with the theoretical value, confirming its octametallic structure. Elemental analysis further supported the proposed formulations, and the presence of eight boron atoms per POSS cage was verified using inductively coupled plasma optical emission spectroscopy (ICP-OES).
It is worth noting that reorganization of the POSS cage can occur in the presence of F− ions, a phenomenon previously observed by Laine et al.29 However, in this study, the integrity of the nanocage was confirmed by the presence of a single resonance in the 29Si NMR spectrum, characteristic of a T8R8 structure with T3 silicon units. The 29Si NMR spectrum of POSS-tert-BF2 exhibited a resonance at −67.1 ppm (Fig. 1c, Fig. S26, ESI†), attributed to all eight magnetically equivalent silicon atoms. This observation confirms that no cage rearrangement had occurred and that only a T8 cage was present in the resulting structure. Further confirmation was provided by diffusion-ordered spectroscopy (DOSY), which supported the nanometer-scale size of the formed structures. The hydrodynamic radii of POSS-tert-BF2, POSS-sal-BF2 and POSS-npht-BF2 were calculated using the Stokes–Einstein Gierer–Wirtz Estimation (SEGWE) model,30 yielding approximate values of 1.15, 1.06, and 1.16 nm, respectively, with diffusion coefficients (D) of 1.13 × 10−10, 5.10 × 10−10, and 1.12 × 10−10 m2 s−1, respectively. Notably, the DOSY spectra exhibited a single, well-defined band for all proton signals, confirming that the POSS structures maintained consistent sizes (Fig. 2, Fig. S27, S40 and S53, ESI†).
Thermal properties
The inorganic framework of polyhedral oligomeric silsesquioxanes (POSSs), specifically the Si–O–Si bonds, enables the exceptional chemical and thermal resistance of these compounds. Compared to purely organic compounds, hybrid organic–inorganic silsesquioxanes exhibit significantly enhanced thermal stability, a critical property for their diverse applications across various fields.To assess the thermal stability of the difluoroboron complexes POSS-tert-BF2, POSS-sal-BF2, and POSS-npht-BF2, thermogravimetric analysis (TGA) was conducted under oxidative conditions (O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2 = 40
N2 = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60). The TGA results showed the high thermal resistance of POSSs, depicting 5% weight loss at 350, 335, and 339 °C for POSS-tert-BF2, POSS-sal-BF2, POSS-npht-BF2, respectively (Fig. 3). This remarkable stability can be attributed to the presence of the rigid silsesquioxane cage structure.31 These findings highlight the excellent thermal stability of the difluoroboron-functionalized POSS compounds and confirm their potential for use in high-temperature applications. The oxidative decomposition of POSS-tert-BF2, and of POSS-sal-BF2 and POSS-npht-BF2, proceeded in two distinct steps. The initial weight loss was associated with the decomposition of the organic side chains, while the subsequent weight loss corresponded to degradation of the silsesquioxane cage. Extending the analysis up to 1000 °C under oxidative conditions allowed for determination of the materials’ compositions based on their ceramic yields. Notably, pyrolysis of the POSS compounds in air resulted in the formation of silicon oxide (SiO2), as confirmed by DRIFT analysis. The TGA curve for POSS-tert-BF2 exhibited a total weight loss of 83.6%, corresponding to a residual SiO2 content of 16.40%. This value is in close agreement with the theoretical prediction of 15.97%. For POSS-sal-BF2, the ceramic yield at 900 °C was found to be 26.17%, exceeding the theoretical value of 22.93%. Similarly, POSS-npht-BF2 demonstrated a ceramic yield of 22.84%, aligning well with its theoretical value of 19.25%.
60). The TGA results showed the high thermal resistance of POSSs, depicting 5% weight loss at 350, 335, and 339 °C for POSS-tert-BF2, POSS-sal-BF2, POSS-npht-BF2, respectively (Fig. 3). This remarkable stability can be attributed to the presence of the rigid silsesquioxane cage structure.31 These findings highlight the excellent thermal stability of the difluoroboron-functionalized POSS compounds and confirm their potential for use in high-temperature applications. The oxidative decomposition of POSS-tert-BF2, and of POSS-sal-BF2 and POSS-npht-BF2, proceeded in two distinct steps. The initial weight loss was associated with the decomposition of the organic side chains, while the subsequent weight loss corresponded to degradation of the silsesquioxane cage. Extending the analysis up to 1000 °C under oxidative conditions allowed for determination of the materials’ compositions based on their ceramic yields. Notably, pyrolysis of the POSS compounds in air resulted in the formation of silicon oxide (SiO2), as confirmed by DRIFT analysis. The TGA curve for POSS-tert-BF2 exhibited a total weight loss of 83.6%, corresponding to a residual SiO2 content of 16.40%. This value is in close agreement with the theoretical prediction of 15.97%. For POSS-sal-BF2, the ceramic yield at 900 °C was found to be 26.17%, exceeding the theoretical value of 22.93%. Similarly, POSS-npht-BF2 demonstrated a ceramic yield of 22.84%, aligning well with its theoretical value of 19.25%.
 | ||
| Fig. 3 TGA trace graph (under oxidative conditions) for POSS-tert-BF2, POSS-sal-BF2 and POSS-npht-BF2. Theoretical values of ceramic yields are given in parentheses. | ||
Synthesis prop-imine-BF2
To investigate the role of the silsesquioxane cage in photocatalytic activity, we synthesized difluoroboron complexes without the silsesquioxane core, designated as prop-imine-BF2 (prop-tert-BF2, prop-sal-BF2 and prop-npht-BF2, Scheme 2). These compounds were obtained similarly to the POSS-based complexes, using boron trifluoride etherate and dry diisopropylethylamine in dichloromethane at 40 °C. By-products were removed through column chromatography. The structures of the resulting complexes were confirmed by 1H, 11B, 13C and 19F NMR, DRIFT, and UV-vis spectroscopy. | ||
| Scheme 2 Synthesis of prop-tert-BF2, prop-sal-BF2 and prop-npht-BF2. Isolated yields in parentheses. | ||
Photophysical properties
In DCM solution, POSS-tert-BF2, POSS-sal-BF2, and POSS-npht-BF2 exhibited strong π–π* transition with absorption maxima at 270 nm, 280 nm, and 230 nm, respectively (Fig. 4a). Additional absorption peaks were observed at 367 nm for POSS-tert-BF2, 348 for POSS-sal-BF2, and 325, 355 and 372 for POSS-npht-BF2, which can be attributed to intramolecular charge transfer (ICT) transition (Fig. 4a, Table 1). The formation of the B–N bond, through the donation of a lone pair from the nitrogen atom to the boron atom, reduces the energy gap between the π* and π orbitals of the ligand, resulting in a bathochromic shift in the UV-vis spectra of POSS-tert-BF2, POSS-sal-BF2 and POSS-npht-BF2 relative to their respective ligands.| Compound | λabs (nm) | ε (M−1 cm−1) | λem (nm) | Stokes shift (nm) | Φfa (%) |
|---|---|---|---|---|---|
| a Determined by comparison with quinine sulfate in 0.1 M H2SO4 (Φf = 57.7%). Excitation wavelength, 360 nm.b Excitation wavelength, 330 nm. | |||||
| POSS-tert-BF2 | 367 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
463 | 98 | 27.0 |
| prop-tert-BF2 | 367 | 4300 | 463 | 98 | 30.9 |
| POSS-sal-BF2 | 348 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
430 | 82 | 10.0 |
| prop-sal-BF2 | 348 | 4700 | 426 | 78 | 12.0 |
| POSS-npht-BF2 | 325/372 | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200/63 200/63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
429 | 100/57b | 3.6/4.0b |
| prop-npht-BF2 | 372 | 7900 | 428 | 56 | 12.0 |
Upon photoexcitation, POSS-tert-BF2 emitted a bright blue light with a maximum at 463 nm, a quantum yield (Φf) of 27%, and a Stokes shift of 98 nm (Fig. 4b, Table 1). In contrast, POSS-npht-BF2 displayed a faint emission centered at 429 nm with Φf = 4%, which is typical of BF2 complexes with naphthalene ligands.32 POSS-sal-BF2 emitted light at 430 nm with a quantum yield of 10%. The incorporation of bulky tert-butyl groups in POSS-tert-BF2 at the periphery of the POSS molecule induced bathochromic shifts in both absorption and emission wavelengths, as well as an increase in the quantum yield compared to POSS-sal-BF2. These bulky groups help effectively restrict intramolecular rotations within the organic POSS framework, thereby reducing fluorescence quenching. Interestingly, POSS-tert-BF2, POSS-sal-BF2, and POSS-npht-BF2 exhibited lower emission intensities compared to their non-POSS counterparts, prop-tert-BF2, prop-sal-BF2, and prop-npht-BF2 (Fig. 4b, Fig. S72 in the ESI,† and Table 1), despite having a larger number of chromophores. This suggests an increased contribution of nonradiative decay pathways, such as self-absorption, due to the higher chromophore density. A similar phenomenon has been previously observed in stilbenevinylsilsesquioxanes.33 For example, a lower emission intensity (Φ = 11%) was noted for stilbenevinyl-T12 compared to stilbenevinyl-T8 (Φf = 36%) and p-triethoxysilyl-VS (Φf = 38%).
Photocatalytic activity and ROS generation
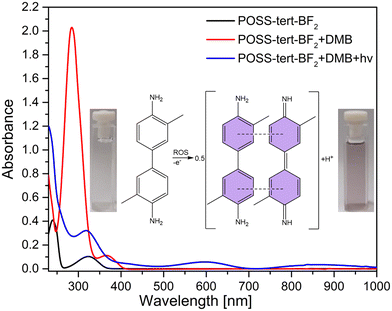
Having established the efficiency of POSS-tert-BF2, we proceeded to evaluate its photocatalytic activity. To investigate the activation effect of POSS-tert-BF2 on molecular oxygen, 3,3′-dimethylbenzidine (DMB) was used as an indicator for reactive oxygen species (ROS) generation. Upon subjecting a dichloromethane solution of POSS-tert-BF2 and DMB to constant light irradiation (Herolab NU-15, λirr = 365 nm, 90 s, intensity 1.30 mW cm−2), characteristic absorption bands of the DMB cation radical34 appeared at 370 and 650 nm (Fig. 5, Fig. S1, Scheme S1, ESI†). Notably, the oxidation rates of DMB varied significantly under different atmospheric conditions (N2 and O2), providing compelling evidence that the reactive oxygen species generated in the system under irradiation indeed originated from molecular oxygen.35 A similar phenomenon was observed for POSS-sal-BF2, POSS-npht-BF2, prop-tert-BF2, prop-sal-BF2 and prop-npht-BF2 confirming their ability to generate ROS during light irradiation (Fig. S2–S6, ESI†).Singlet oxygen quantum yields
To evaluate the ability of POSS-tert-BF2 to generate singlet oxygen (1O2), 9,10-diphenylanthracene (DPA) was employed as a trapping agent. DPA selectively reacts with 1O2, leading to a decrease in its absorbance. Upon irradiating a methanol solution containing DPA and POSS-tert-BF2, a reduction in the DPA absorption peak was observed (Fig. 6a), indicating the formation of 1O2. To further confirm this, DPA oxidation experiments were conducted in deuterated methanol (MeOD-d4), as the extended lifetime of 1O2 in a deuterated solvent should enhance DPA oxidation if 1O2 generation occurs. This effect arises because the longer 1O2 lifetime increases the likelihood of its reaction with DPA before relaxing to the ground state. In contrast, when experiments were conducted in the presence of NaN3, a known 1O2 quencher, no decrease in DPA absorbance was observed, further validating singlet oxygen generation upon POSS-tert-BF2 irradiation.The singlet oxygen quantum yield (SOQY, Φ(1O2)) was determined by monitoring the photooxidation of DPA in methanol in the presence of POSS derivatives (Fig. S7a–S12a, ESI†). Changes in DPA absorbance at 391 nm were measured over time using low concentrations of photosensitizer and DPA to minimize potential 1O2 quenching by the photocatalyst. SOQYs were calculated by plotting the change in DPA absorbance against irradiation time (see Fig. S7b–S12b, ESI† for details). The calculated Φ(1O2) values for POSS-tert-BF2, POSS-sal-BF2, POSS-npht-BF2, prop-tert-BF2, prop-sal-BF2 and prop-npht-BF2 were 48%, 35%, 46%, 27%, 26%, and 18%, respectively, highlighting the high efficiency of POSS-tert-BF2 in generating singlet oxygen. Higher singlet oxygen quantum yield and therefore higher DPA oxidation were obtained for the octametallic POSS-tert-BF2, POSS-sal-BF2, and POSS-npht-BF2 than with the monometallic analogues prop-tert-BF2, prop-sal-BF2 and prop-npht-BF2 under the same reaction conditions, which can be explained by the occurrence of the intramolecular cooperative effect for compounds bearing the POSS moiety, which was further investigated.
Photocatalytic oxidation of sulfides to sulfoxides
Since POSS-tert-BF2 exhibits a significant singlet oxygen quantum yield and generates other reactive oxygen species, we conducted experiments on photocatalytic oxidation of thioanisole by POSS-tert-BF2 using medium-pressure mercury lamp. We first inspected the photooxidation of 0.425 mmol of thioanisole in 12 mL of CH3OH solution with 0.5 mol% POSS-tert-BF2 (loading based on acceptor sites) as a sensitizer (entry 4 Table 2) at room temperature. Following irradiation with a 150 W TQ lamp (radiation flux ∅ 300–600 nm: 24.8 W), full conversion was achieved within 40 minutes, resulting in a turnover number (TON) of 1582 and a turnover frequency (TOF) of 2373 h−1. The reaction carried out at the mmol scale gave a yield of 98% (entry 7 Table 2). The external quantum efficiency of thioanisole photooxidation, determined relative to ferrioxalate actinometry in the presence of the POSS-tert-BF2 system, reached 59%. Control experiments conducted without the photosensitizer, without light, or under anaerobic conditions using a distilled solvent resulted in negligible conversion of thioanisole (entries 1–3, Table 2).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Photosensitizers | Yield [%] | TONf | TOFg [h−1] |
|---|---|---|---|---|
| a Reaction conditions: thioanisole (0.425 mmol), POSS-imine-BF2 (0.266 μmol, 0.0625 mol%, 0.5 mol% based on acceptor sites), MeOH (12 mL), O2, 40 min, medium pressure mercury vapor lamp (radiation flux ∅ 300–600 nm: 24.8 W), room temperature.b DCM as a solvent.c Reaction conditions: thioanisole (0.085 mmol), POSS-tert-BF2 (0.106 μmol, 0.125 mol%, 1 mol% based on acceptor sites), MeOH (2.4 mL), O2, 25 min, medium pressure mercury vapor lamp (radiation flux ∅ 300–600 nm: 24.8 W), room temperature.d Thioanisole (1.0 mmol), POSS-tert-BF2 (0.63 μmol, 0.0625 mol%, 0.5 mol% based on acceptor sites), MeOH (29 mL), O2, 40 min.e prop-imine-BF2 (2.13 μmol, 0.5%mol).f Turnover number (TON) = total number of moles of thioanisole consumed/mol of catalyst.g Turnover frequency (TOF) = TON/time (hours). | ||||
| 1 | None | 0 | ||
| 2 | No light | 0 | ||
| 3 | Anaerobic conditions | 0 | ||
| 4 | POSS-tert-BF2 | 99 | 1582 | 2373 |
| 5 | POSS-tert-BF2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
30 | 479 | 719 |
| 6 | POSS-tert-BF2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
99 | 794 | 1905 |
| 7 | POSS-tert-BF2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
98 | 1566 | 2349 |
| 8 | prop-tert-BF2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e e |
65 | 130 | 195 |
| 9 | POSS-sal-BF2 | 70 | 1118 | 1678 |
| 10 | prop-sal-BF2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e e |
59 | 118 | 177 |
| 11 | POSS-npht-BF2 | 90 | 1438 | 2157 |
| 12 | prop-npht-BF2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e e |
60 | 120 | 180 |
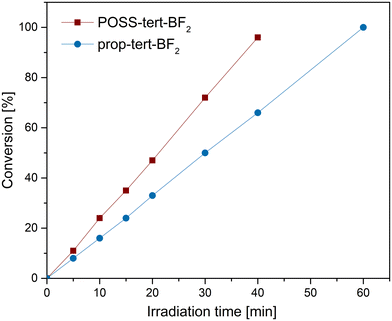
The conversion of thioanisole was only 30% in pure DCM (entry 5, Table 2). However, under the same reaction conditions using MeOH, the conversion increased to 99% (entry 4, Table 2). Protonic solvents, such as MeOH or H2O, are known to stabilize the intermediate for the formation of 1O2,36 and, in turn, accelerate photooxidation. These differences in conversion also suggest that 1O2 can be identified as one of the ROS involved in the photooxidation of thioanisole. Comparing the activity of different POSS compounds with various substitutions on the phenyl ring, we observed that introducing steric hindrance, such as with tert-butyl groups at the 3- and 5-positions of the phenyl ring in POSS-tert-BF2, significantly increased the reaction efficiency (99% in 40 minutes, entry 4, Table 2) compared to POSS-sal-BF2, which lacks bulky substituents (70% in 40 minutes, entry 9, Table 2). Furthermore, introducing steric hindrance at the 5,6-positions of the phenyl ring in POSS-npht-BF2 (entry 11, Table 2) by replacing the phenyl ring with a naphthalene ring also improved efficiency relative to POSS-sal-BF2. However, the efficiency remained lower than that of POSS-tert-BF2, underscoring the critical role of steric hindrance at the 3-position of the phenyl ring in promoting the conversion of thioanisole. The results indicate that POSS-tert-BF2 exhibits a high efficiency in photocatalytic oxidation of thioanisole and it was further studied in detail.
The reaction progress was monitored in real time using NMR spectroscopy (Fig. S14, ESI†), which revealed that the conversion of thioanisole to methyl phenyl sulfoxide with POSS-tert-BF2 as a photosensitizer increased steadily over time, achieving complete conversion within 40 minutes and following zero-order kinetics (Fig. 7). In comparison, the use of prop-tert-BF2 resulted in only 65% substrate conversion after 40 minutes, with full conversion achieved after 60 minutes. These findings clearly highlight the superior reaction efficiency and faster catalytic performance of POSS-tert-BF2 compared to prop-tert-BF2. Higher conversion (99%) of thioanisole was obtained with the octametallic POSS-tert-BF2 than with the monometallic analogue prop-tert-BF2 (65%) under the same reaction conditions, supporting the occurrence of intramolecular cooperative catalysis with POSS-tert-BF2. This observation aligns with the observed enhancement in the efficiency of singlet oxygen quantum yields (see above). A similar intramolecular cooperative effect was previously reported for Zn4@POSS-1, which contains the POSS-1 ligand, in the formation of cyclic carbonates from epoxides.37 A similar enhancement in efficiency was observed for POSS-sal-BF2 and POSS-npht-BF2. Comparing the activity of POSS-npht-BF2 with prop-npht-BF2, a higher yield of 90% versus 60% (entries 11 and 12, Table 2), respectively, was obtained for POSS-npht-BF2. The activity of POSS-sal-BF2 is also higher than that of prop-sal-BF2, 70% versus 59% (entries 9 and 10, Table 2).
Recycling of photocatalyst
The recyclability of POSS-tert-BF2 was evaluated by performing consecutive photocatalytic reactions. POSS-tert-BF2 retained the majority of its catalytic activity even after five cycles, with conversion rates only slightly decreasing from 99% to 96% (Fig. 8). The post-reaction 1H and 11B NMR spectra (Fig. S73 and S74, ESI†) of POSS-tert-BF2 were identical to those obtained before the reactions, suggesting that the structural integrity of the catalyst is preserved over multiple cycles. Additionally, ICP-OES analysis of the recovered POSS-tert-BF2 confirmed that the octametallic structure remained intact, with no detectable boron leaching into the reaction mixture. These findings indicate the robustness and reusability of POSS-tert-BF2 as a stable photocatalyst under the tested conditions.Substrate scope
To assess the versatility of POSS-tert-BF2 as a photocatalyst in the photooxidation of thioanisole derivatives in methanol, a series of substrates with various substituents were evaluated. As illustrated in Scheme 3, thioanisole derivatives containing electron-donating (–CH3) and electron-withdrawing (–CN, –CHO, –C(O)CH3) groups achieved high yields of 99%, 82%, 78%, and 96%, respectively. Bromine-substituted derivatives at ortho and meta positions also exhibited high yields (99%). The low activity of biphenyl sulfide (10%) might be attributed to its steric effect, which prohibits sulfoxide formation and is commonly observed in the literature.38These results underscore the potential of POSS-tert-BF2 in facilitating thioanisole photooxidation reactions under mild conditions. Notably, nearly complete substrate conversion was achieved within 40 minutes for most derivatives, highlighting the efficiency of POSS-tert-BF2. Compared to traditional photocatalysts such as rose bengal,39 tetra-o-acetylriboflavin,36 and flavin dibromide,40 which often require extended reaction times or additional promoters, POSS-tert-BF2 demonstrates superior or comparable performance.
Quenching experiments
To investigate the process and the reactive species involved in the photocatalytic oxidation of sulfides to sulfoxides, we conducted separate experiments using individual free radical or singlet oxygen scavengers. They included KI, NaN3, DABCO, and 1,4-benzoquinone (BQ), which are used to eliminate holes (h+), singlet oxygen (1O2), and superoxide anion radicals (O2˙−), respectively. As depicted in Fig. 9, the incorporation of KI, NaN3, and DABCO led to a significant reduction in the yield of the oxidation reaction, resulting in yields of 11%, 11%, and 19% respectively. The inclusion of BQ under the same reaction conditions reduced conversion to 56%. This observation implies that 1O2 and h+ function as the principal active species in the photocatalytic oxidation of sulfides to sulfoxides. Therefore, POSS-tert-BF2 acts as both a type I and a type II photocatalyst. A plausible mechanism for oxidation of sulfides is provided based on the findings from the aforementioned studies and relevant literature sources.38,41 With the results of quenching experiments, conducted to determine the impact of ROS, the likely reaction mechanisms proposed are shown in Scheme 4. POSS-tert-BF2 is first photoexcited, generating photogenerated electron–hole pairs upon light irradiation, which then oxidize sulfides into cationic free radicals. The separated electrons reduce O2 to form reactive oxygen species (O2˙−). Then sulfide cation free radicals can react with O2˙− to form a persulfoxide intermediate. Simultaneously, the sulfide can react with singlet oxygen (1O2) to form a thiadioxirane intermediate. In the final step, both of these reaction intermediates react with another sulfide molecule to yield sulfoxide. | ||
| Scheme 4 Proposed reaction mechanisms of sulfide-selective oxidation by POSS-tert-BF2 in the presence of O2. | ||
Conclusion
In summary, a new type of octanuclear difluoroboron coordination compound was designed and synthesized through a reaction of boron trifluoride etherate with imine functionalized polyhedral silsesquioxanes. For the first time, multifunctional imine-POSS-based difluoroboron complexes were synthesized and characterized. POSS-tert-BF2, POSS-sal-BF2, and POSS-npht-BF2 were successfully tested in the photooxidation of sulfides to sulfoxide, demonstrating high activity and selectivity of the tested catalytic system. Thioanisole was converted under mild conditions (room temperature) and with a short reaction time (40 minutes) into sulfoxide in high yield (99%). High yields were also obtained for thioanisole derivatives containing both electron-donating and electron-withdrawing groups, showcasing the versatility of the obtained system.Moreover, we have demonstrated the first example of an intramolecular cooperative catalytic system for the photooxidation of sulfides to sulfoxides. This was achieved through the unique properties of the silsesquioxane core in POSS compounds. Our study highlights several advantages of POSS-based photocatalysts, which make them particularly suitable for the photooxidation of sulfides to sulfoxides.
• High photostability: the silsesquioxane core provides durability against oxidation, which enhances the longevity of the catalyst.
• High singlet oxygen 1O2 generation ability: a high singlet oxygen quantum yield of 48% in MeOH was achieved for POSS-tert-BF2.
• High efficiency: the higher TON in relation to non-POSS analogues was caused by the intramolecular cooperative effect.
• Environmentally friendly system: the photocatalyst does not contain heavy metals and does not require highly reactive oxidants, toxic byproducts are not produced, and oxidation occurs under oxygen.
Materials and methods
All information regarding the synthesis, chemical characterization, experimental details and materials used in this study have been included as part of the ESI.†Author contributions
Conceptualization, M. J. and S. S. Data curation, M. J. Validation, M. J. Formal analysis, M. J. Funding acquisition, M. J. and S. S. Investigation, M. J. Methodology, M. J. Visualization, M. J. Writing – original draft, M. J. Writing – review & editing, M. J.Data availability
The data supporting this article have been included as part of the ESI.† It contains materials, structures of POSS ligands, characterization methods, syntheses, description of experiments. UV-vis spectra of DPA upon light irradiation in presence of photocatalysts, UV-vis-NIR spectra of the cationic radical of DMB generated upon light irradiation in the presence of photocatalysts, 1H NMR spectra of crude mixtures for oxidation of thioanisole using POSS-tert-BF2, 1H NMR spectra of photooxidation products, 1H, 13C, 11B, 19F, 29Si, DOSY, NOESY and HSQC NMR, DRIFT, UV-vis spectra, MALDI mass spectra, and TG-DTA data.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the National Science Centre 2022/45/B/ST4/01511 (S. S.) and University of Wrocław for the BPIDUB.12.2024 (M. J.) grant under the ‘The Excellence Initiative – Research University’ programme for support of this research. M. J. gratefully acknowledges the financial support by the Ministry of Education and Science through the scholarship for outstanding young scientists (SMN/16/1767/2020/3).References
- V. Ervithayasuporn, K. Kwanplod, J. Boonmak, S. Youngme and P. Sangtrirutnugul, Homogeneous and heterogeneous catalysts of organopalladium functionalized-polyhedral oligomeric silsesquioxanes for Suzuki–Miyaura reaction, J. Catal., 2015, 332, 62–69 CrossRef CAS.
- Y. Liu, K. Koizumi, N. Takeda, M. Unno and A. Ouali, Synthesis of octachloro- and octaazido-functionalized T8-cages and application to recyclable palladium catalyst, Inorg. Chem., 2022, 61, 1495–1503 CrossRef CAS.
- C. Calabrese, C. Aprile, M. Gruttadauria and F. Giacalone, POSS nanostructures in catalysis, Catal. Sci. Technol., 2020, 10, 7415–7447 RSC.
- C. Sun and H. Liu, Highly selective oxidation of styrene to styrene oxide over a tetraphenylporphyrin-bridged silsesquioxane-based hybrid porous polymer, ACS Appl. Polym. Mater., 2022, 4, 5471–5481 CrossRef.
- A. B. Soni, G. Satishkumar and M. W. C. Man, Palladium nanoparticles stabilized in ionic liquid-based periodic mesoporous organosilica: An efficient heterogeneous catalyst for oxidative acylation of ketones, ChemistrySelect, 2024, 9, e202403464 CrossRef CAS.
- Y. Rodriguez Herrero and A. Ullah, Hydrophobic polyhedral oligomeric silsesquioxane support enhanced methanol production from CO2 hydrogenation, ACS Appl. Mater. Interfaces, 2023, 15, 14399–14414 CAS.
- M. Mansourian-Tabaei, M. Majdoub, D. Sengottuvelu, D. L. Stoddard, R. V. K. G. Thirumalai, M. G. Ucak-Astarlioglu, A. Al-Ostaz and S. Nouranian, Polyurea/aminopropyl isobutyl polyhedral oligomeric silsesquioxane-functionalized graphene nanoplatelet nanocomposites for force protection applications, ACS Appl. Mater. Interfaces, 2024, 16, 19625–19641 CrossRef CAS.
- T. Hamada, Y. Nakanishi, K. Okada, S. Tsukada, A. Uedono and J. Ohshita, Thermal insulating property of silsesquioxane hybrid film induced by intramolecular void spaces, ACS Appl. Polym. Mater., 2021, 3, 3383–3391 CrossRef CAS.
- X. Meng, Y. Liu, S. Wang, J. Du, Y. Ye, X. Song and Z. Liang, Silsesquioxane–carbazole-corbelled hybrid porous polymers with flexible nanopores for efficient CO2 conversion and luminescence sensing, ACS Appl. Polym. Mater., 2020, 2, 189–197 CrossRef CAS.
- H. Yang and H. Liu, Pyrene-functionalized silsesquioxane as fluorescent nanoporous material for antibiotics detection and removal, Microporous Mesoporous Mater., 2020, 300, 110135 CrossRef CAS.
- Q. Wang, M. Unno and H. Liu, Ultrafast and highly selective gold recovery with high capture capacity from electronic waste by upconversion of a silsesquioxane-based hybrid luminescent aerogel, J. Mater. Chem. A, 2024, 12, 5679–5691 RSC.
- G. Wei, K. Zhang, Y. Gu, S. Guang, J. Feng and H. Xu, Novel multifunctional nano-hybrid polyhedral oligomeric silsesquioxane-based molecules with high cell permeability: molecular design and application for diagnosis and treatment of tumors, Nanoscale, 2021, 13, 2982–2994 RSC.
- L. Fan, X. Wang, Q. Cao, Y. Yang and D. Wu, POSS-based supramolecular amphiphilic zwitterionic complexes for drug delivery, Biomater. Sci., 2019, 7, 1984–1994 RSC.
- T. Uchida, Y. Egawa, T. Adachi, N. Oguri, M. Kobayashi, T. Kudo, N. Takeda, M. Unno and R. Tanaka, Synthesis, structures, and thermal properties of symmetric and Janus “lantern cage” siloxanes, Chem. – Eur. J., 2019, 25, 1683–1686 CrossRef CAS.
- N. Oguri, Y. Egawa, N. Takeda and M. Unno, Janus-cube octasilsesquioxane: Facile synthesis and structure elucidation, Angew. Chem., Int. Ed., 2016, 55, 9336–9339 CrossRef CAS PubMed.
- A. Blázquez-Moraleja, M. E. Pérez-Ojeda, J. R. Suárez, M. L. Jimeno and J. L. Chiara, Efficient multi-click approach to well-defined two-faced octasilsesquioxanes: the first perfect Janus nanocube, Chem. Commun., 2016, 52, 5792–5795 RSC.
- A. Mrzygłód, M. P. García Armada, M. Rzonsowska, B. Dudziec and M. Nowicki, Metallodendrimers unveiled: Investigating the formation and features of double-decker silsesquioxane-based silylferrocene dendrimers, Inorg. Chem., 2023, 62, 16932–16942 CrossRef PubMed.
- M. A. Stevens and A. L. Colebatch, Cooperative approaches in catalytic hydrogenation and dehydrogenation, Chem. Soc. Rev., 2022, 51, 1881–1898 RSC.
- P. Solís-Muñana, J. Salam, C. Z.-J. Ren, B. Carr, A. E. Whitten, G. G. Warr and J. L.-Y. Chen, An amphiphilic (salen)Co complex – Utilizing hydrophobic interactions to enhance the efficiency of a cooperative catalyst, Adv. Synth. Catal., 2021, 363, 3207–3213 CrossRef.
- M. Janeta, T. Lis and S. Szafert, Zinc imine polyhedral oligomeric silsesquioxane as a quattro-site catalyst for the synthesis of cyclic carbonates from epoxides and low-pressure CO2, Chem. – Eur. J., 2020, 26, 13686–13697 CrossRef CAS.
- M. Janeta, W. Bury and S. Szafert, Porous silsesquioxane–imine frameworks as highly efficient adsorbents for cooperative iodine capture, ACS Appl. Mater. Interfaces, 2018, 10, 19964–19973 CrossRef CAS.
- E. Skolia, P. L. Gkizis and C. G. Kokotos, Aerobic photocatalysis: Oxidation of sulfides to sulfoxides, ChemPlusChem, 2022, 87, e202200008 CrossRef CAS PubMed.
- K. Kamata, K. Sugahara, Y. Kato, S. Muratsugu, Y. Kumagai, F. Oba and M. Hara, Heterogeneously catalyzed aerobic oxidation of sulfides with a BaRuO3 nanoperovskite, ACS Appl. Mater. Interfaces, 2018, 10, 23792–23801 CrossRef CAS.
- W. Li, Z. Xie and X. Jing, BODIPY photocatalyzed oxidation of thioanisole under visible light, Catal. Commun., 2011, 16, 94–97 CrossRef CAS.
- H. Wang, G. W. Wagner, A. X. Lu, D. L. Nguyen, J. H. Buchanan, P. M. McNutt and C. J. Karwacki, Photocatalytic oxidation of sulfur mustard and its simulant on BODIPY-incorporated polymer coatings and fabrics, ACS Appl. Mater. Interfaces, 2018, 10, 18771–18777 CrossRef CAS.
- X.-L. Li, N. Han, R.-Z. Zhang, K.-K. Niu, R.-Z. Dong, H. Liu, S. Yu, Y.-B. Wang and L.-B. Xing, Host–guest photosensitizer of a cationic BODIPY derivative and cucurbit[7]uril for high-efficiency visible light-induced photooxidation reactions, ACS Appl. Mater. Interfaces, 2023, 15, 55803–55812 CrossRef CAS PubMed.
- L. Wang, J. Cao, J. Wang, Q. Chen, A. Cui and M. He, Facile synthesis of dimeric BODIPY and its catalytic activity for sulfide oxidation under visible light, RSC Adv., 2014, 4, 14786–14790 RSC.
- R. Kumar Yadav, D. Parveen, B. Mondal and D. Kumar Roy, The role of spacers as a probe in variation of photoluminescence properties of mono- and bi-nuclear boron compounds, Chem. – Asian J., 2025, 20, e202401113 CrossRef CAS.
- J. C. Furgal, T. G. Iii and R. M. Laine, D5h [PhSiO1.5]10 synthesis via F− catalyzed rearrangement of [PhSiO1.5]n. An experimental/computational analysis of likely reaction pathways, Dalton Trans., 2016, 45, 1025–1039 RSC.
- R. Evans, G. Dal Poggetto, M. Nilsson and G. A. Morris, Improving the interpretation of small molecule diffusion coefficients, Anal. Chem., 2018, 90, 3987–3994 CrossRef CAS.
- M. Janeta and S. Szafert, Synthesis, characterization and thermal properties of T8 type amido-POSS with p-halophenyl end-group, J. Organomet. Chem., 2017, 847, 173–183 CrossRef CAS.
- R. P. Nandi, P. Sudhakar, N. K. Kalluvettukuzhy and P. Thilagar, Triarylborane-appended anils and boranils: solid-state emission, mechanofluorochromism, and phosphorescence, Chem. – Eur. J., 2020, 26, 16306–16317 CrossRef CAS PubMed.
- J. C. Furgal, J. H. Jung, T. Goodson and R. M. Laine, Analyzing structure–photophysical property relationships for isolated T8, T10, and T12 stilbenevinylsilsesquioxanes, J. Am. Chem. Soc., 2013, 135, 12259–12269 CrossRef CAS PubMed.
- P. D. Josephy, Oxidative activation of benzidine and its derivatives by peroxidases., Environ. Health Perspect., 1985, 64, 171–178 CrossRef CAS.
- H. Zhai, Z. Wei, X. Jing and C. Duan, A porphyrin-faced Zn8L6 cage for selective oxidation of C(sp3)–H bonds and sulfides, Inorg. Chem., 2024, 63, 14375–14382 CrossRef CAS.
- J. Dad'ová, E. Svobodová, M. Sikorski, B. König and R. Cibulka, Photooxidation of sulfides to sulfoxides mediated by tetra-O-acetylriboflavin and visible light, ChemCatChem, 2012, 4, 620–623 CrossRef.
- S. Akine, T. Taniguchi and T. Nabeshima, Cooperative formation of trinuclear zinc(II) complexes via complexation of a tetradentate oxime chelate ligand, salamo, and zinc(II) acetate, Inorg. Chem., 2004, 43, 6142–6144 CrossRef CAS PubMed.
- W. Gong, X. Deng, K. Dong, L. Liu and G. Ning, A boranil-based conjugated microporous polymer for efficient visible-light-driven heterogeneous photocatalysis, Polym. Chem., 2021, 12, 3153–3159 RSC.
- X. Gu, X. Li, Y. Chai, Q. Yang, P. Li and Y. Yao, A simple metal-free catalytic sulfoxidation under visible light and air, Green Chem., 2013, 15, 357–361 RSC.
- C. Dang, L. Zhu, H. Guo, H. Xia, J. Zhao and B. Dick, Flavin dibromide as an efficient sensitizer for photooxidation of sulfides, ACS Sustainable Chem. Eng., 2018, 6, 15254–15263 CrossRef CAS.
- Y. Liu, Z. Zhao, W. Xu and W. Gong, Extending 2D covalent organic frameworks by inserting anthracene for promoted white-light-mediated photocatalysis, Catal. Sci. Technol., 2024, 14, 3211–3218 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5qi00323g |
| This journal is © the Partner Organisations 2025 |