Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Silagermylenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/h2_char_e001.gif) O bonds and radical fragmentation of CO2-expanded bis(germylene) by a cyclic (alkyl)(amino)carbene†
O bonds and radical fragmentation of CO2-expanded bis(germylene) by a cyclic (alkyl)(amino)carbene†
Anna-Lena
Thömmes
 a,
Robin
Völker
a,
Robin
Völker
 a,
Bernd
Morgenstern
b,
Michael
Zimmer
a,
Dominik
Munz
a,
Bernd
Morgenstern
b,
Michael
Zimmer
a,
Dominik
Munz
 c,
Christopher W. M.
Kay
c,
Christopher W. M.
Kay
 de and
David
Scheschkewitz
de and
David
Scheschkewitz
 *a
*a
aKrupp-Chair for General and Inorganic Chemistry, Saarland University, 66123 Saarbrücken, Germany. E-mail: scheschkewitz@mx.uni-saarland.de
bInorganic Solid-State Chemistry, Saarland University, 66123 Saarbrücken, Germany
cCoordination Chemistry, Saarland University, 66123 Saarbrücken, Germany
dPhysical Chemistry and Chemistry Education, Saarland University, 66123 Saarbrücken, Germany
eLondon Centre for Nanotechnology, University College London, London WC1H 0AH, UK
First published on 15th April 2025
Abstract
The transformation of the greenhouse gas CO2 into value-added products represents a major contemporary challenge. Low-valent p-block compounds typically react at the oxygen termini of CO2 due to the oxophilicity of the metal centers. We now report on the selective activation of CO2 and ethyl isocyanate at the central carbon atom by an N-heterocyclic carbene (NHC)-stabilized para-silylenephenylene-bridged bis(germylene). During the net silagermylenation, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) X (X = O, NEt) bonds are inserted into the Ge–Si bonds through cooperativity of the low-valent metal center and the electrophilic silyl backbone. The germanium(II) centers are retained in the products, as is confirmed by multinuclear NMR data, IR spectroscopy and X-ray analysis and supported by DFT calculations. Attempts to substitute the NHCs by cyclic (alkyl)(amino)carbenes (CAACs) resulted in a germylene-CAAC radical by homolytic cleavage of the Si–O bonds as evidenced by single crystal X-ray diffraction and continuous-wave EPR spectroscopy.
X (X = O, NEt) bonds are inserted into the Ge–Si bonds through cooperativity of the low-valent metal center and the electrophilic silyl backbone. The germanium(II) centers are retained in the products, as is confirmed by multinuclear NMR data, IR spectroscopy and X-ray analysis and supported by DFT calculations. Attempts to substitute the NHCs by cyclic (alkyl)(amino)carbenes (CAACs) resulted in a germylene-CAAC radical by homolytic cleavage of the Si–O bonds as evidenced by single crystal X-ray diffraction and continuous-wave EPR spectroscopy.
Introduction
Small molecules are elementary, easily accessible and inexpensive potential chemical feedstocks, and as such their transformation into value-added products is extensively investigated, particularly in the case of the greenhouse gas CO2. In recent years, heavier p-block compounds have been demonstrated to activate small molecules without the support of the typically applied transition metals.1 In low-valent Group 14 chemistry, heavier alkene and alkyne analogues have been applied to CO2 activation2,3 as well as various silylenes and even bis(silylene)s.4 Whereas silylenes exclusively react under participation of the lone-pair and the vacant p orbital at the low-valent silicon in these reactions, the few reports on CO2 reactivity of the heavier congeners5,6 suggest a pronounced dependency on the nature of the substituents.Despite the plethora of stable germylenes,7 reports on their reactivity towards CO2 are surprisingly scarce. Sita et al. have reported the metathesis of a homoleptic amino-substituted germylene and CO2 to provide bis(siloxy)germylene dimer I (Scheme 1).5a Only very recently, a gallyl-substituted germylene has been shown to reduce CO2 to CO through insertion of an oxygen atom into the Ge–Ga bond to give galloxy derivative II.5b In contrast, in the reaction of an N-heterocyclic imino (NHI) di-substituted germylene, the carbonyl group adds in a [2 + 2]-cycloaddition across the Ge–N bonds to provide four-coordinate germylene III.5c The only known examples for reactions of the germanium center at the CO2 carbon atom are hydrogermylenation reactions with heteroleptic hydrido germylenes, in which carboxy germylenes with Ge–C bonds, such as IV, are obtained instead.5d,e Such germylenes afford methanol and formic acid derivatives in stoichiometric reactions with suitable reactions5e,f and have also been proposed as intermediates of the rare examples of catalytic CO2 derivatization by main-group systems.5g Certain digermynes undergo related insertion reactions of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, forming germylenyl esters Va–c.3
O bond, forming germylenyl esters Va–c.3
 | ||
| Scheme 1 Selected products of reactions of germylenes and digermynes with CO2 (Ar1 = 2,6-(2,6-iPr2C6H3)2C6H3, Dip = 2,6-iPr2C6H3, Mes = 2,4,6-Me3C6H2). | ||
Bis(tetrylene)s with linking units, spatially separating the two tetrylene centers, are of particular interest, for instance, as bidentate ligands or for the activation of small molecules.1d,8 In our heavier acyclic diene metathesis (HADMET) polymerization, bis(germylene)s even constitute transient intermediates to σ,π-conjugated Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ge-containing polymers.9 A corresponding stabilized NHC-adduct has been isolated and employed as alternative precursor to poly(digermene)s.10 Hence, we became interested in the derivatization of this NHC-bis(germylene). While simple substitution of the NHC occurs with a nucleophilic and sterically demanding CAAC,10 we anticipated a fundamentally different reactivity with small electrophiles. Here we report on the activation of carbon dioxide and ethyl isocyanate through selective insertion reactions into the Ge–Si bonds of the silylenearylene-bridged NHC-bis(germylene) under retention of the germanium(II) functionalities. The CO2 insertion product is shown to undergo CAAC-induced radical fragmentation.
Ge-containing polymers.9 A corresponding stabilized NHC-adduct has been isolated and employed as alternative precursor to poly(digermene)s.10 Hence, we became interested in the derivatization of this NHC-bis(germylene). While simple substitution of the NHC occurs with a nucleophilic and sterically demanding CAAC,10 we anticipated a fundamentally different reactivity with small electrophiles. Here we report on the activation of carbon dioxide and ethyl isocyanate through selective insertion reactions into the Ge–Si bonds of the silylenearylene-bridged NHC-bis(germylene) under retention of the germanium(II) functionalities. The CO2 insertion product is shown to undergo CAAC-induced radical fragmentation.
Results and discussion
Exposure of a benzene suspension of yellow NHC-bis(germylene) 1 to CO2 at room temperature results in immediate dissolution and decolorization. Multinuclear NMR spectroscopy of the crude reaction mixture confirms quantitative conversion to a new product to which we tentatively assign the constitution of silyl ester 2a (Scheme 2), where the carboxy groups have been inserted into the Ge–Si bonds, based on NMR spectroscopic analysis. Notably, the silagermylenation of CS2 has very recently been reported to result in the corresponding silyl dithioester.11 Silyl ester 2a is isolated as an off-white powder by removing the solvent under vacuum. | ||
| Scheme 2 Reactions of NHC-bis(germylene) 1 with CO2 and ethyl isocyanate, respectively, to carboxy- and amide-functionalized NHC-bis(germylene)s 2a,b (Tip = 2,4,6-triisopropylphenyl). | ||
A characteristic 13C{1H} NMR low-field resonance at 173.2 ppm due to the carbenic carbon atoms as well as a broad septet in the 1H NMR spectrum at 5.69 ppm arising from the N-bonded isopropyl groups suggest NHC-coordination to the Ge(II) centers.10,12,13 In comparison to organic esters (158 to 177 ppm),14 the carbonyl carbon resonance at 204.9 ppm of 2a is similarly downfield-shifted as oxycarbonyl germylenes Va–c (206 to 209 ppm).3 Furthermore, the considerable deviation from the corresponding shifts of organic germylenyl esters (∼177 ppm)15 and oxycarbonyl silanes (184 to 192 ppm)16 speaks against the formation of the corresponding product with reversed regioselectivity, i.e. with Ge–O and Si–C bonds (Scheme S1, ESI†), and corroborates the suggested constitution of 2a.
In line with a more electronegative environment, the 29Si{1H} NMR peaks at 2.09 and 2.05 ppm are considerably downfield shifted compared to the germylenyl substituted silicon atoms of the starting material 1 (−11.07, −11.19 ppm).10 Such low-field shifts are indeed characteristic for silanoles, silyl ethers and silyl esters with Si–O bonds (−3 to 34 ppm)17 in contrast to the corresponding alkyl silanes (−11 to −3 ppm)18 and in particular to acyl silanes, including esters (−30 to −7 ppm).16a,19 The two 29Si{1H} NMR peaks with equal intensities in addition to two sets of signals for the phenylene, the NMe2 and SiMe2 groups in the 13C{1H} and 1H NMR spectra suggest the presence of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereomeric mixture of the racemate and the meso-form. Similar results have been obtained for NHC-bis(germylene) 1
1 diastereomeric mixture of the racemate and the meso-form. Similar results have been obtained for NHC-bis(germylene) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and corroborate NHC-coordination to the germylene centers in a pyramidal manner, thus giving rise to Ge-centered chirality. The peak separations are slightly smaller in the case of 2a, in line with the larger spatial separation of the respective nuclei from the germanium stereocenters. Further evidence for the presence of the ester functionality is provided by a characteristic band in the IR spectrum at 1613 cm−1, just between the C
10 and corroborate NHC-coordination to the germylene centers in a pyramidal manner, thus giving rise to Ge-centered chirality. The peak separations are slightly smaller in the case of 2a, in line with the larger spatial separation of the respective nuclei from the germanium stereocenters. Further evidence for the presence of the ester functionality is provided by a characteristic band in the IR spectrum at 1613 cm−1, just between the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations in germylenyl esters Vb,c (1581, 1593 cm−1)3b and acyl digermenes (1649 to 1653 cm−1).20
O stretching vibrations in germylenyl esters Vb,c (1581, 1593 cm−1)3b and acyl digermenes (1649 to 1653 cm−1).20
The formation of silyl ester 2avs. the alternative germylenyl ester (Scheme S1, ESI†) is rationalized by the formation of Si–O bonds (BDE ∼ 191 kcal mol−1),21 which are substantially stronger than Ge–O bonds (BDE ∼ 158 kcal mol−1).21 Accordingly, DFT-calculations for both isomers confirm the silyl ester 2a to be favored by ΔG = 11.4 kcal mol−1 (see ESI†).
Unfortunately, crystallization attempts from pentane, hexane, benzene and thf remained unsuccessful, in part presumably due to the limited stability of 2a in solution. In particular in thf, precipitation of NHC–CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 concomitant with the formation of a mixture of unidentified products is already observed after a few hours. We therefore turned our attention to ethyl isocyanate as an isolobal imine analogue of CO2 in the anticipation that the additional organic substituent could facilitate crystallization without compromising the reactivity.
22 concomitant with the formation of a mixture of unidentified products is already observed after a few hours. We therefore turned our attention to ethyl isocyanate as an isolobal imine analogue of CO2 in the anticipation that the additional organic substituent could facilitate crystallization without compromising the reactivity.
The reaction of NHC-bis(germylene) 1 with two equivalents of EtNCO in benzene at room temperature (Scheme 2) indeed gives rise to very similar NMR spectroscopic data, in line with the formation of the corresponding bis(amide) 2b. The characteristic 13C{1H} and 1H NMR peaks of the coordinated NHCs are observed at 172.6 ppm and 5.73 ppm, and the carbonyl carbon resonances at 212.2 and 211.9 ppm. The latter peaks are slightly downfield-shifted with respect to 2a (204.9 ppm), as is typically observed for amides and imides in comparison to the corresponding esters.14b,23 The low-field shift of the 29Si{1H} NMR resonances at −6.3 and −6.4 ppm in comparison to germylenyl silane 1 (−11.07, −11.19 ppm)10 is considerably less pronounced than in the case of 2a (2.09, 2.05 ppm). This is in perfect agreement with the substitution of the O- for an N-substituent at silicon as previously observed for silylamines (PhMe2SiNR2: −5 to −1 ppm)17e in comparison to the corresponding silanole (PhMe2SiOH: 7 ppm), for instance.17d
The appearance of two 13C{1H} carbonyl carbon resonances for 2b in addition to the somewhat larger separation of the two 29Si{1H} NMR peaks in comparison to 2a hints towards the presence of rotational isomers due to the newly introduced amide moieties. Hindered rotation about the C–N bond is further corroborated by low-temperature NMR spectra: the 29Si{1H} peak ratio changes from 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at room temperature to 2.4
1 at room temperature to 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at −60 °C, suggesting one of the conformations of the zwitterionic form of the amide functionalities in 2b (Scheme 3) to be thermodynamically favored.
1 at −60 °C, suggesting one of the conformations of the zwitterionic form of the amide functionalities in 2b (Scheme 3) to be thermodynamically favored.
 | ||
| Scheme 3 Schematic representation of the E- and Z-conformations of the zwitterionic form of the amide moieties in 2b. | ||
Most probably, the interaction of the SiMe2 group with the formally negatively charged oxygen center in the E-conformer is favorable compared to the germylene lone-pair in the Z-conformer, also on steric grounds. Equally characteristic for the presence of rotamers is the distinct increase of the peak separation at low temperature (40 Hz at −60 °C, 9.3 Hz at rt), corresponding to an estimated rotational barrier of ΔG‡ ∼ 60 kJ mol−1 (see ESI†). Similar to dimethylbenzamide (62 kJ mol−1),24a the rotation in 2b is slightly less hindered than in N,N-dialkyl formamides and acetamides (68 to 88 kJ mol−1),24 which suggests competing interactions of the lone-pairs at the nitrogen and the germanium centers with the carbonyl group. Even the SiMe2, the aryl protons of the Tip groups and the NHCs’ isopropyl methine groups give rise to peak splitting in the 1H NMR spectrum at −60 °C, despite a larger spatial separation from the C–N bonds (see ESI†).
In stark contrast to this and as expected for a diastereomeric mixture, 2a does not show coalescence behavior. Notably, in the 1H NMR spectrum of 2a accidental isochrony of the diastereomers’ peaks is exhibited in thf-d8 as opposed to C6D6. It is therefore not surprising, that the thf-d8 spectra of 2b show no additional peak splitting either. Given the structural similarities and the common synthetic origin, however, the presence of different diastereomers is assumed for 2b as well. Corresponding investigations in C6D6 are nonetheless prohibited by its poor solubility.
As in the case of organic amides,25 the diminished C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond strength due to the interaction with the nitrogen lone-pair is reflected in the IR spectrum: the C
O bond strength due to the interaction with the nitrogen lone-pair is reflected in the IR spectrum: the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching frequency of 2b (1548 cm−1) is considerably red-shifted compared to ester 2a (1613 cm−1).
O stretching frequency of 2b (1548 cm−1) is considerably red-shifted compared to ester 2a (1613 cm−1).
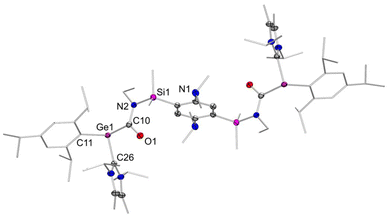
The molecular structure of the meso-form of 2b, obtained by X-ray diffraction on a single crystal (Fig. 1), indeed confirms coordination of the carbene to the germylene center with a characteristically elongated Ge–C single bond (2.078(2) Å). While this is in line with silyl–aryl and diaryl NHC-germylenes (2.067 to 2.078 Å;  ),10,12 the pyramidalization in 2b (
),10,12 the pyramidalization in 2b ( ) is slightly increased in comparison. In contrast, the angles are considerably less acute than in intramolecularly stabilized oxycarbonyl germylenes Va,b and the related CS2 insertion product of a silyl germylene (
) is slightly increased in comparison. In contrast, the angles are considerably less acute than in intramolecularly stabilized oxycarbonyl germylenes Va,b and the related CS2 insertion product of a silyl germylene ( ).3,11 With the smaller pyramidalization, enhanced interaction of the germanium lone-pair with the carbonyl moiety is possible, thus diminishing the donation of the nitrogen lone-pairs to the carbonyl groups: the N2–C10 bond (1.382(3) Å) lies at the upper end of the range constituted by considerably conjugated amides and anilines (1.31 to 1.38 Å).26 The partial zwitterionic character in amides as opposed to esters results in an elongated C
).3,11 With the smaller pyramidalization, enhanced interaction of the germanium lone-pair with the carbonyl moiety is possible, thus diminishing the donation of the nitrogen lone-pairs to the carbonyl groups: the N2–C10 bond (1.382(3) Å) lies at the upper end of the range constituted by considerably conjugated amides and anilines (1.31 to 1.38 Å).26 The partial zwitterionic character in amides as opposed to esters results in an elongated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond length of 1.239(2) Å compared to germylenyl oxycarbonyl germylenes Va,b (1.19, 1.20 Å).3 Notably, the coordination environment of the nitrogen centers are perfectly planar (
O double bond length of 1.239(2) Å compared to germylenyl oxycarbonyl germylenes Va,b (1.19, 1.20 Å).3 Notably, the coordination environment of the nitrogen centers are perfectly planar ( ) and the carbonyl group only slightly deviates (Si1–N2–C10–O1 9.1(2)°) from the presumably favored E-conformation (vide supra).
) and the carbonyl group only slightly deviates (Si1–N2–C10–O1 9.1(2)°) from the presumably favored E-conformation (vide supra).
According to DFT calculations for 2b at the BP86-D3(BJ)/def2-TZVPP//BP86-D3(BJ)/def2-SVP level of theory, the HOMO and HOMO−1 (Fig. 2) are best represented by lone-pairs at the germanium atoms with π-contributions from the amide functionalities. In line with pronounced interaction between the germylene lone-pairs and the carbonyl groups, HOMO−4 and HOMO−5 exhibit contributions from the carbonyl groups as well as from the germanium and the nitrogen atoms (see ESI†). The LUMO is located at the arylene linker with little admixture from the adjacent silylene moieties. The unoccupied π orbitals at higher energy (LUMO+1 and LUMO+2) are predominantly located at the NHC ligands, suggesting σ,π-interaction only between the π-system of the linker and the SiMe2 groups. In fact, the in-phase and out-of-phase combinations of the pairwise occurring orbitals at both sides of the bridging unit are degenerate, confirming the absence of extended conjugation between the germylene centers.
 | ||
| Fig. 2 Selected frontier orbitals of amide-functionalized NHC-bis(germylene) 2b (contour value 0.036). Hydrogen atoms omitted for clarity. | ||
The activation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) X (X = N, O) bonds is presumably achieved through cooperativity of the nucleophilic germylene lone-pair and the electrophilic silyl backbone in bis(germylene) 1, driven by the formation of strong Si–X bonds. This has been confirmed theoretically by Gonnade and Sen et al. for the silagermylenation of CS2.11 Notably, neither the CAAC-stabilized bis(germene) analogue of 1 with Ge
X (X = N, O) bonds is presumably achieved through cooperativity of the nucleophilic germylene lone-pair and the electrophilic silyl backbone in bis(germylene) 1, driven by the formation of strong Si–X bonds. This has been confirmed theoretically by Gonnade and Sen et al. for the silagermylenation of CS2.11 Notably, neither the CAAC-stabilized bis(germene) analogue of 1 with Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds10 nor the corresponding bis(digermene) with Ge
C bonds10 nor the corresponding bis(digermene) with Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ge bonds9a react similarly with CO2, corroborating the importance of the nucleophilicity-enhancing NHC-donor.
Ge bonds9a react similarly with CO2, corroborating the importance of the nucleophilicity-enhancing NHC-donor.
Since the reaction of NHC-bis(germylene) 1 with CAACMe, as the stronger σ-donor and π-acceptor, yields the corresponding bis(germene),10 we attempted the generation of Ge![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds in the corresponding reaction of bis(carboxygermylene) 2a as well. Surprisingly, however, treatment of 2a with two equivalents of CAACMe affords NHC-germylene 3 comprising a tethered radical center, albeit in a very low yield of 11% (Scheme 4). The constitution of 3 is confirmed by single crystal X-ray crystallography and continuous-wave EPR spectroscopy (Fig. 3). The obtained g-factor (2.0035) is slightly smaller than in germylenyl substituted CAAC-radicals (2.0070, 2.0075; A(14N) = 5.0, 5.3 G),27 in line with a larger distance of the unpaired electron from the germanium. Furthermore, no 73Ge coupling is observed and the 14N coupling (A(14N) = 6.0 G), manifest in a triplet splitting, is only slightly increased, hence suggesting the spin-density to be predominantly located on the substituent.
C double bonds in the corresponding reaction of bis(carboxygermylene) 2a as well. Surprisingly, however, treatment of 2a with two equivalents of CAACMe affords NHC-germylene 3 comprising a tethered radical center, albeit in a very low yield of 11% (Scheme 4). The constitution of 3 is confirmed by single crystal X-ray crystallography and continuous-wave EPR spectroscopy (Fig. 3). The obtained g-factor (2.0035) is slightly smaller than in germylenyl substituted CAAC-radicals (2.0070, 2.0075; A(14N) = 5.0, 5.3 G),27 in line with a larger distance of the unpaired electron from the germanium. Furthermore, no 73Ge coupling is observed and the 14N coupling (A(14N) = 6.0 G), manifest in a triplet splitting, is only slightly increased, hence suggesting the spin-density to be predominantly located on the substituent.
 | ||
| Scheme 4 Synthesis of paramagnetic NHC-germylene 3 (NHC = 1,3-diisopropylimidazol-4,5-dimethyl-2-ylidene, CAACMe = 1-(2,6-diisopropylphenyl)-3,3,5,5-tetramethyl-pyrrolidin-2-ylidene). | ||
Accordingly, the molecular structure of 3 (Fig. 3) exhibits an elongated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond (1.238(1) Å) compared to oxycarbonyl germylenes Va,b (1.19, 1.20 Å)3 and even similar to amide 2b (1.239(2) Å). The short C1–C9 bond length of 1.441(1) Å confirms partial double bond character, in stark contrast to CAACMe–CO2 (1.52 Å),28 but in agreement with the delocalization of the unpaired electron across the carbonyl group and the CAAC substituent. The C1–N1 distance (1.376(1) Å) is similar to reported germylenyl substituted CAAC-radicals (1.37 Å).27 Considerable pyramidalization (
O bond (1.238(1) Å) compared to oxycarbonyl germylenes Va,b (1.19, 1.20 Å)3 and even similar to amide 2b (1.239(2) Å). The short C1–C9 bond length of 1.441(1) Å confirms partial double bond character, in stark contrast to CAACMe–CO2 (1.52 Å),28 but in agreement with the delocalization of the unpaired electron across the carbonyl group and the CAAC substituent. The C1–N1 distance (1.376(1) Å) is similar to reported germylenyl substituted CAAC-radicals (1.37 Å).27 Considerable pyramidalization (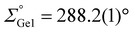 ), comparable to a reported siloxy aryl NHC-germylene (
), comparable to a reported siloxy aryl NHC-germylene ( ),29 suggests the presence of a lone-pair at germanium and no significant contribution from the unpaired electron. This is further confirmed by the Ge1–O1 (1.9528(7) Å) and C9–O1 distances (1.339(1) Å), which are comparable to germylenyl oxycarbonyl germylenes Va,b (Ge–O: 1.96 Å, C–O: 1.33 to 1.34 Å).3
),29 suggests the presence of a lone-pair at germanium and no significant contribution from the unpaired electron. This is further confirmed by the Ge1–O1 (1.9528(7) Å) and C9–O1 distances (1.339(1) Å), which are comparable to germylenyl oxycarbonyl germylenes Va,b (Ge–O: 1.96 Å, C–O: 1.33 to 1.34 Å).3
In line with the experimental data, the Mulliken spin densities obtained at the UB3LYP-D3(BJ)/def2-TZVPP//UB3LYP-D3(BJ)/def2-SVP level of theory are highest at the carbene fragment (Fig. 3). The computed g-value of 2.0033 and the 14N coupling constant of A(14N) = 4.2 G validate the accuracy of the computational model.
Apparently, germylene 3 is formed via CAAC-induced homolytic cleavage of the Si–O bonds in 2a. Hence, most likely, a highly reactive silicon centered diradical by-product is formed, whose unselective decomposition and/or polymerization prevent its identification (Scheme 4). While CAACs are known to stabilize main group radicals,30 they are, however, typically obtained through reduction or hydrogen abstraction. Substitution induced synthesis has only been reported in two cases for silicon and diborane centered diradicals.31 With the carboxy group as an additional spacer separating the radical center from the Ge(II) moiety in 3, as opposed to the directly connected CAAC-germylenes,27 analogous diradicals could be envisaged. We are currently investigating more economic pathways towards such (poly-)radical germylenes, avoiding the use of the relatively complicated linking unit of 2a.
Conclusions
In conclusion, the silagermylenation reactions of CO2 and EtNCO with a para-silylenephenylene-bridged NHC-bis(germylene) are reported. These highly atom-economic transformations constitute a convenient strategy for the selective backbone functionalization with acyl groups under retention of the low-valent germylenes’ integrity. In particular, this may facilitate the synthesis and characterization of structurally derived functionalized poly(digermene)s. In a reaction of the obtained carboxy germylene with CAACMe, structural evidence for an unprecedented germylene with a tethered radical is provided, which may serve as a prototype for related (poly-)radicals in future investigations.Author contributions
A.-L. T. and D. S. conceptualized the project. D. S. held the project administration and supervision. A.-L. T. (lead) and R. V. (supporting) synthesized and isolated the compounds and collected the NMR, IR, and EPR spectroscopic data. A.-L. T. performed the quantum chemical calculations and carried out the visualization and analysis of the data. B. M. performed the single crystal X-ray analyses and the refinement of the structures. M. Z. performed the VT-NMR spectroscopic measurements. D. S., D. M. and A.-L. T. acquired funding for the project. A.-L. T. (original draft + review and editing) and D. S. (review and editing) wrote the manuscript. C. W. M. K. and D. M. validated the spectroscopic data and the quantum chemical calculations and contributed to the revision and editing of the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Fonds der Chemischen Industrie for a Kekulé fellowship for A.-L. T. and the German Research Foundation for funding (“Solar-Driven Chemistry: Light-Induced Small Molecule Fixation by Diradicals”; DFG SCHE 906/9-1). We acknowledge the instrumentation facilities provided by the service center for X-ray analysis established with the financial support from Saarland University and the German Research Foundation (INST 256/506-1 and INST 256/582-1). We thank Prof. Stella Stopkowicz for access to the computational cluster.References
- Reviews: (a) R. Akhtar, K. Gaurav and S. Khan, Applications of low-valent compounds with heavy group-14 elements, Chem. Soc. Rev., 2024, 53, 6150–6243 RSC; (b) S. Sinha and J. Jiang, Main group elements in electrochemical hydrogen evolution and carbon dioxide reduction, Chem. Commun., 2023, 59, 11767–11779 RSC; (c) M. Pérez-Jiménez, H. Corona, F. de la Cruz-Martínez and J. Campos, Donor-Acceptor Activation of Carbon Dioxide, Chem. – Eur. J., 2023, 29, e202301428 CrossRef PubMed; (d) A. Saddington, S. Yao and M. Driess, Recent advances in low-valent silicon chemistry, in Advances in Inorganic Chemistry, ed. K. Meyer and R. van Eldik, Academic Press, 2023, vol. 82, pp. 119–156 Search PubMed; (e) F. Dankert and C. Hering-Junghans, Heavier group 13/15 multiple bond systems: synthesis, structure and chemical bond activation, Chem. Commun., 2022, 58, 1242–1262 RSC; (f) S. Fujimori and S. Inoue, Small Molecule Activation by Two-Coordinate Acyclic Silylenes, Eur. J. Inorg. Chem., 2020, 3131–3142 CrossRef CAS PubMed; (g) C. Shan, S. Yao and M. Driess, Where silylene–silicon centres matter in the activation of small molecules, Chem. Soc. Rev., 2020, 49, 6733–6754 RSC; (h) Y. Su and R. Kinjo, Small molecule activation by boron-containing Heterocycles, Chem. Soc. Rev., 2019, 48, 3613–3659 RSC; (i) R. L. Melen, Frontiers in molecular p-block chemistry: From structure to reactivity, Science, 2019, 363, 479–484 CrossRef CAS PubMed; (j) T. J. Hadlington, M. Driess and C. Jones, Low-valent group 14 element hydride chemistry: towards catalysis, Chem. Soc. Rev., 2018, 47, 4176–4197 RSC; (k) C. Weetman and S. Inoue, The Road Travelled: After Main-Group Elements as Transition Metals, ChemCatChem, 2018, 10, 4213–4228 CrossRef CAS; (l) T. Chu and G. I. Nikonov, Oxidative Addition and Reductive Elimination at Main-Group Element Centers, Chem. Rev., 2018, 118, 3608–3680 CrossRef CAS PubMed; (m) X. Wang, C. Xia and L. Wu, Homogeneous carbon dioxide reduction with p-block element-containing reductants, Green Chem., 2018, 20, 5415–5426 RSC; (n) S. Yadav, S. Saha and S. S. Sen, Compounds with Low-Valent p-Block Elements for Small Molecule Activation and Catalysis, ChemCatChem, 2016, 8, 486–501 CrossRef CAS; (o) B. Blom and M. Driess, Recent Advances in Silylene Chemistry: Small Molecule Activation En-Route Towards Metal-Free Catalysis, in Functional Molecular Silicon Compounds II. Structure and Bonding, ed. D. Scheschkewitz, Springer International Publishing, 2014, vol. 156, pp. 85–123 Search PubMed.
- (a) N. Wiberg, W. Niedermayer, K. Polborn and P. Mayer, Reactivity of the Isolable Disilene R*PhSi=SiPhR* (R*=Sit,Bu3), Chem. – Eur. J., 2002, 8, 2730–2739 CrossRef CAS PubMed; (b) D. Gau, R. Rodriguez, T. Kato, N. Saffon-Merceron, A. de Cózar, F. P. Cossío and A. Baceiredo, Synthesis of a Stable Disilyne Bisphosphine Adduct and Its Non-Metal-Mediated CO2 Reduction to CO, Angew. Chem., Int. Ed., 2011, 50, 1092–1096 ( Angew. Chem. , 2011 , 123 , 1124–1128 ) CrossRef CAS PubMed; (c) J. Li, M. Hermann, G. Frenking and C. Jones, The Facile Reduction of Carbon Dioxide to Carbon Monoxide with an Amido-Digermyne, Angew. Chem., Int. Ed., 2012, 51, 8611–8614 ( Angew. Chem. , 2012 , 124 , 8739–8742 ) CrossRef CAS PubMed; (d) D. Wendel, T. Szilválsi, D. Henschel, P. J. Altmann, C. Jandl, S. Inoue and B. Rieger, Precise Activation of Ammonia and Carbon Dioxide by an Iminodisilene, Angew. Chem., Int. Ed., 2018, 57, 14575–14579 ( Angew. Chem. , 2018 , 130 , 14783–14787 ) CrossRef CAS PubMed; (e) A. Kostenko and M. Driess, Geometrically Compelled Disilene with λ4-Coordinate SiII Atoms, J. Am. Chem. Soc., 2018, 140, 16962–16966 CrossRef CAS PubMed.
- (a) A. Caise, L. P. Griffin, C. McManus, A. Heilmann and S. Aldridge, Reversible Uptake of CO2 by Pincer Ligand Supported Dimetallynes, Angew. Chem., Int. Ed., 2022, 61, e202117496 ( Angew. Chem. , 2022 , 134 , e202117496 ) CrossRef CAS PubMed; (b) J. Fan, S. Quek, M.-C. Yang, Z.-F. Zhang, M.-D. Su and C.-W. So, Reversible CO2 activation by a N-phosphinoamidinato digermyne, Chem. Commun., 2022, 58, 1033–1036 RSC.
- (a) P. Jutzi, D. Eikenberg, A. Möhrke, B. Neumann and H.-G. Stammler, Decamethylsilicocene Chemistry: Unprecedented Multistep Reactions of a Silicon(II) Compound with the Heterocumulenes CO2, COS, CS2, and RNCS (R = Methyl, Phenyl), Organometallics, 1996, 15, 753–759 CrossRef CAS; (b) S. Yao, Y. Xiong, M. Brym and M. Driess, An Isolable Silanoic Ester by Oxygenation of a Stable Silylene, J. Am. Chem. Soc., 2007, 129, 7268–7269 CrossRef CAS PubMed; (c) X. Liu, X.-Q. Xiao, Z. Xu, X. Yang, Z. Li, Z. Dong, C. Yan, G. Lai and M. Kira, Reactions of an Isolable Dialkylsilylene with Carbon Dioxide and Related Heterocumulenes, Organometallics, 2014, 33, 5434–5439 CrossRef CAS; (d) K. Junold, M. Nutz, J. A. Baus, C. Burschka, C. Fonseca Guerra, F. M. Bickelhaupt and R. Tacke, The Donor-Stabilized Silylene Bis[N,N′-diisopropylbenzamidinato(−)]silicon(II): Synthesis, Electronic Structure, and Reactivity, Chem. – Eur. J., 2014, 20, 9319–9329 CrossRef CAS PubMed; (e) F. M. Mick, J. A. Baus, M. Nutz, C. Burschka, J. Poater, F. M. Bickelhaupt and R. Tacke, Reactivity of the Donor-Stabilized Silylenes [iPrNC(Ph)NiPr]2Si and [iPrNC(Ni,Pr2)NiPr]2Si: Activation of CO2 and CS2, Chem. – Eur. J., 2015, 21, 16665–16672 CrossRef PubMed; (f) D. Wendel, A. Porzelt, F. A. D. Herz, D. Sarkar, C. Jandl, S. Inoue and B. Rieger, From Si(II) to Si(IV) and Back: Reversible Intramolecular Carbon–Carbon Bond Activation by an Acyclic Iminosilylene, J. Am. Chem. Soc., 2017, 139, 8134–8137 CrossRef CAS PubMed; (g) A. V. Protchenko, P. Vasko, D. C. Huan Do, J. Hicks, M. Á. Fuentes, C. Jones and S. Aldridge, Reduction of Carbon Oxides by an Acyclic Silylene: Reductive Coupling of CO, Angew. Chem., Int. Ed., 2019, 58, 1808–1812 ( Angew. Chem. , 2019 , 131 , 1822–1826 ) CrossRef CAS PubMed; (h) M.-P. Luecke, E. Pens, S. Yao and M. Driess, An Isolable Bis(Silanone-Borane) Adduct, Chem. – Eur. J., 2020, 26, 4500–4504 CrossRef CAS PubMed; (i) Y. Xiong, S. Yao, A. Ruzicka and M. Driess, Distinctly different reactivity of bis(silylenyl)- versus phosphanyl-silylenyl-substituted o-dicarborane towards O2, N2O and CO2, Chem. Commun., 2021, 57, 5965–5968 RSC.
- (a) L. R. Sita, J. R. Babcock and R. Xi, Facile Metathetical Exchange between Carbon Dioxide and the Divalent Group 14 Bisamides M[N(SiMe3)2]2 (M = Ge and Sn), J. Am. Chem. Soc., 1996, 118, 10912–10913 CrossRef CAS; (b) A. Bücker, C. Wölper and S. Schulz, Activation of heteroallenes by metal-substituted electron-rich tetrylenes, Polyhedron, 2024, 247, 116702 CrossRef; (c) L. Groll, J. A. Kelly and S. Inoue, Reactivity of NHI-Stabilized Heavier Tetrylenes towards CO2 and N2O, Chem. – Asian J., 2024, 19, e202300941 CrossRef CAS PubMed; (d) A. Jana, D. Ghoshal, H. W. Roesky, I. Objartel, G. Schwab and D. A. Stalke, A Germanium(II) Hydride as an Effective Reagent for Hydrogermylation Reactions, J. Am. Chem. Soc., 2009, 131, 1288–1293 CrossRef CAS PubMed; (e) G. Tan, W. Wang, B. Bloma and M. Driess, Mechanistic studies of CO2 reduction to methanol mediated by an N-heterocyclic germylene hydride, Dalton Trans., 2014, 43, 6006–6011 RSC; (f) A. Jana, G. Tavčar, H. W. Roesky and M. John, Germanium(II) hydride mediated reduction of carbon dioxide to formic acid and methanol with ammonia borane as the hydrogen source, Dalton Trans., 2010, 39, 9487–9489 RSC; (g) T. J. Hadlington, C. E. Kefalidis, L. Maron and C. Jones, Efficient Reduction of Carbon Dioxide to Methanol Equivalents Catalyzed by Two-Coordinate Amido-Germanium(II) and -Tin(II) Hydride Complexes, ACS Catal., 2017, 7, 1853–1859 CrossRef CAS.
- (a) J. R. Babcock, L. Liable-Sands, A. L. Rheingold and L. R. Sita, Syntheses, Structural Characterizations, and Heterocumulene Metathesis Studies of New Monomeric Bis(triorganosilylamido)tin(II) Derivatives, Organometallics, 1999, 18, 4437–4441 CrossRef CAS; (b) A. Jana, H. W. Roesky, C. Schulzke and A. Döring, Reactions of Tin(II) Hydride Species with Unsaturated Molecules, Angew. Chem., Int. Ed., 2009, 48, 1106–1109 ( Angew. Chem. , 2009 , 121 , 1126–1129 ) CrossRef CAS PubMed; (c) D. A. Dickie, E. N. Coker and R. A. Kemp, Formation of a Reversible, Intramolecular Main-Group Metal–CO2 Adduct, Inorg. Chem., 2011, 50, 11288–11290 CrossRef CAS PubMed; (d) S. Weiß, M. Widemann, K. Eichele, H. Schubert and L. Wesemann, Low valent lead and tin hydrides in reactions with heteroallenes, Dalton Trans., 2021, 50, 4952–4958 RSC; (e) A. V. Protchenko, M. Á. Fuentes, J. Hicks, C. McManus, R. Tirfoin and S. Aldridge, Reactions of a diborylstannylene with CO2 and N2O: diboration of carbon dioxide by a main group bis(boryl) complex, Dalton Trans., 2021, 50, 9059–9067 RSC; (f) D. Sarkar, L. Groll, D. Munz, F. Hanusch and S. Inoue, Ligand Assisted CO2 Activation and Catalytic Valorization by an NHI-Stabilized Stannylene, ChemCatChem, 2022, 14, e202201048 CrossRef CAS.
- Reviews: (a) W. P. Neumann, Germylenes and Stannylenes, Chem. Rev., 1991, 91, 311–334 CrossRef CAS; (b) N. N. Zemlyanskii, I. V. Borisova, M. S. Nechaev, V. N. Khrustalev, V. V. Lunin, M. Y. Antipin and Y. A. Ustynyuk, Divalent silicon, germanium, and tin compounds with element-heteroatom bonds, Russ. Chem. Bull. Int. Ed., 2004, 53, 980–1006 CrossRef CAS; (c) Y. Mizuhata, T. Sasamori and N. Tokitoh, Stable Heavier Carbene Analogues, Chem. Rev., 2009, 109, 3479–3511 CrossRef CAS PubMed; (d) M. Asay, C. Jones and M. Driess, N-Heterocyclic, Carbene Analogues with Low-Valent Group 13 and Group 14 Elements: Syntheses, Structures, and Reactivities of a New Generation of Multitalented Ligands, Chem. Rev., 2011, 111, 354–396 CrossRef CAS PubMed; (e) J. A. Cabeza and P. García-Álvarez, Polydentate Amidinato-Silylenes, -Germylenes and -Stannylenes, Chem. – Eur. J., 2024, 30, e202400786 CrossRef CAS PubMed.
- Reviews: (a) N. Mukherjee and M. Majumdar, Diverse Functionality of Molecular Germanium: Emerging Opportunities as Catalysts, J. Am. Chem. Soc., 2024, 146, 24209–24232 CrossRef CAS PubMed; (b) X. Gao, Y. He and J. Cui, Charting the frontiers of Bis-germylene chemistry, J. Organomet. Chem., 2024, 1012, 123146 CrossRef CAS; (c) S. Yao, A. Saddington, Y. Xiong and M. Driess, Chelating Bis-silylenes As Powerful Ligands To Enable Unusual Low-Valent Main-Group Element Functions, Acc. Chem. Res., 2023, 56, 475–488 CrossRef CAS PubMed.
- (a) L. Klemmer, A.-L. Thömmes, M. Zimmer, V. Huch, B. Morgenstern and D. Scheschkewitz, Metathesis of Ge=Ge double bonds, Nat. Chem., 2020, 13, 373–377 CrossRef PubMed; (b) A.-L. Thömmes, T. Büttner, B. Morgenstern, O. Janka, G. Kickelbick, B.-J. Niebuur, T. Kraus, M. Gallei and D. Scheschkewitz, Near-Infinite-Chain Polymers with Ge=Ge Double Bonds, Angew. Chem., Int. Ed., 2024, 63, e202415103 ( Nahezu unendlich lange Polymere mit Ge=Ge-Doppelbindungen, Angew. Chem. , 2024 , 136 , e202415103 ) CrossRef PubMed.
- A.-L. Thömmes, B. Morgenstern, M. Zimmer, D. M. Andrada and D. Scheschkewitz, σ,π-Conjugated Bis(germylene) Adducts with NHC and CAACs, Chem. – Eur. J., 2023, 29, e202301273 CrossRef PubMed.
- V. S. Ajithkumar, N. Khilari, P. B. Ghanwat, G. Venugopal, D. Koley and S. S. Sen, Activation of carbon disulfide by a hypersilyl germylene, Dalton Trans., 2024, 53, 10814–10818 RSC.
- (a) K. L. Hurni, P. A. Rupar, N. C. Payne and K. M. Baines, Stabilization of a Transient Diorganogermylene by an N-Heterocyclic Carbene, Organometallics, 2007, 26, 4109–4111 CrossRef; (b) A. J. Ruddy, P. A. Rupar, K. J. Bladek, C. J. Allan, J. C. Avery and K. M. Baines, On the Bonding in N-Heterocyclic Carbene Complexes of Germanium(II), Organometallics, 2010, 29, 1362–1367 CrossRef CAS.
- (a) A. Jana, V. Huch and D. Scheschkewitz, NHC-Stabilized Silagermenylidene: A Heavier Analogue of Vinylidene, Angew. Chem., Int. Ed., 2013, 52, 12179–12182 ( NHC-Stabilisiertes Silagermenyliden: ein schweres Analogon von Vinyliden, Angew. Chem. , 2013 , 125 , 12401–12404 ) CrossRef CAS PubMed; (b) A. Jana, V. Huch, H. S. Rzepa and D. Scheschkewitz, A Multiply Functionalized Base-Coordinated GeII Compound and Its Reversible Dimerization to the Digermene, Angew. Chem., Int. Ed., 2015, 54, 289–292 ( Eine mehrfach funktionalisierte Basen-koordinierte GeII-Verbindung und ihre reversible Dimerisierung zum Digermen, Angew. Chem. , 2015 , 127 , 291–295 ) CrossRef CAS PubMed; (c) D. Nieder, C. B. Yildiz, A. Jana, M. Zimmer, V. Huch and D. Scheschkewitz, Dimerization of a marginally stable disilenyl germylene to tricyclic systems: evidence for reversible NHC-coordination, Chem. Commun., 2016, 52, 2799–2802 RSC; (d) D. Nieder, V. Huch, C. B. Yildiz and D. Scheschkewitz, Regiodiscriminating Reactivity of Isolable NHC-Coordinated Disilenyl Germylene and Its Cyclic Isomer, J. Am. Chem. Soc., 2016, 138, 13996–14005 CrossRef CAS PubMed.
- (a) A. B. Terent'ev, V. I. Dostovalova and R. K. Freidlina, Carbon-13 N.m.r. Spectra of Branched Carboxylic Acids and their Derivatives, Org. Magn. Reson., 1977, 9, 301–307 CrossRef; (b) K. L. Williamson, M. U. Hasan and D. R. Clutter, Conformational Analysis by NMR. 13C Nuclear Magnetic Resonance Spectra of Saturated and Unsaturated Carboxylic Acids and Their Corresponding Esters and Anhydrides, J. Magn. Reson., 1978, 30, 367–383 CAS.
- N. N. Zemlyansky, I. V. Borisova, V. N. Khrustalev, M. Y. Antipin, Y. A. Ustynyuk, M. S. Nechaev and V. V. Lunin, New Stable Germylenes, Stannylenes, and Related Compounds. 3. Stable Monomers XGeOCH2CH2NMe2 (X = Cl, OCOMe) with Only One Intramolecular Coordination Metal–Nitrogen Bond: Synthesis and Structure, Organometallics, 2003, 22, 5441–5446 CrossRef CAS.
- (a) K. Igawa, N. Kokan and K. Tomooka, Asymmetric Synthesis of Chiral Silacarboxylic Acids and Their Ester Derivatives, Angew. Chem., Int. Ed., 2010, 49, 728–731 CrossRef CAS PubMed; (b) K. Igawa, Y. Kawasaki, S. Nozaki, N. Kokan and K. Tomooka, Ozone Oxidation of Silylalkene: Mechanistic Study and Application for the Synthesis of Silacarboxylic Acid Derivatives, J. Org. Chem., 2020, 85, 4165–4171 CrossRef CAS PubMed.
- Ph3SiOH (−13 ppm)17d constitutes an exception to the general trend and was omitted from the discussion for clarity; (a) J. Schraml, V. Chvalovský, M. Mägi and E. Lippmaa, The role of electronic and steric effects in 29Si-NMR spectra of compounds with Si–O–C group, Collect. Czech. Chem. Commun., 1981, 46, 377–390 CrossRef CAS; (b) I. Zicmane, E. Liepins, L. M. Ignatovich and E. Lukevics, A 13C, 17O, 19Si NMR study of some silicon, germanium and tin acyloxyderivatives, J. Organomet. Chem., 1991, 417, 355–362 CrossRef CAS; (c) P. Lassacher, A. G. Brook and A. J. Lough, Reactions of Silenes: A New Silene to Silene Thermal Rearrangement, Organometallics, 1995, 14, 4359–4365 CrossRef CAS; (d) Y. Okada, M. Oba, A. Arai, K. Tanaka, K. Nishiyama and W. Ando, Diorganotelluride-Catalyzed Oxidation of Silanes to Silanols under Atmospheric Oxygen, Inorg. Chem., 2010, 49, 383–385 CrossRef CAS PubMed; (e) A. I. Ojeda-Amador, J. Munarriz, P. Alamán-Valtierra, V. Polo, R. Puerta-Oteo, M. V. Jiménez, F. J. Fernández-Alvarez and J. J. Pérez-Torrente, Mechanistic Insights on the Functionalization of CO2 with Amines and Hydrosilanes Catalyzed by a Zwitterionic Iridium Carboxylate-Functionalized Bis-NHC Catalyst, ChemCatChem, 2019, 11, 5524–5535 CrossRef CAS.
- S. Fortier, Y. Zhang, H. K. Sharma and K. H. Pannell, Formation of Silicon–Carbon Bonds by Photochemical Irradiation of (η5-C5H5)Fe(CO)2SiR3 and (η5-C5H5)Fe(CO)2Me to Obtain R3SiMe, Organometallics, 2010, 29, 1041–1044 CrossRef CAS PubMed.
- P. C. Bulman Page, S. S. Klair and S. Rosenthal, Synthesis and Chemistry of Acyl Silanes, Chem. Soc. Rev., 1990, 19, 147–195 RSC.
- L. Klemmer, Y. Kaiser, V. Huch, M. Zimmer and D. Scheschkewitz, Persistent Digermenes with Acyl and α-Chlorosilyl Functionalities, Chem. – Eur. J., 2019, 25, 12187–12195 CrossRef CAS PubMed.
- Y.-R. Luo, Comprehensive Handbook of Chemical Bond Energies, Taylor & Francis, Boca Raton, 2007, vol. 1, pp. 455–480 Search PubMed.
- X. Wang, J. Zhang, L. Wang and L. Deng, High-Spin Iron(II) Alkynyl Complexes with N-Heterocyclic Carbene Ligation: Synthesis, Characterization, and Reactivity Study, Organometallics, 2015, 34, 2775–2782 CrossRef CAS.
- M. U. Hasan, 13C NMR Spectra of some Amides and Imides. Effect of Inductive and Mesomeric Interactions, Cyclization and Hydrogen Bonding on 13C NMR Chemical Shifts, Org. Magn. Reson., 1980, 14, 447–450 CrossRef.
- (a) L. W. Reeves, R. C. Shaddick and K. N. Shaw, Nuclear Magnetic Resonance Studies of Multi-site Chemical Exchange. III. Hindered Rotation in Dimethylacetamide, Dimethyl Trifluoroacetamide, and Dimethyl Benzamide, Can. J. Chem., 1971, 49, 3683–3691 CrossRef CAS; (b) M. Feigel, Rotation Barriers of Amides in the Gas Phase, J. Phys. Chem., 1983, 87, 3054–3058 CrossRef CAS; (c) V. S. Dimitrov and J. A. Ladd, Dynamic NMR: comparison of the experimental barriers to internal rotation in N,N-dimethylformamide with those calculated by the ab initio SCF MO method, J. Mol. Struct., 1987, 159, 107–112 CrossRef CAS.
- (a) T. Threlfall, The infrared spectra of amides. Part 1. The stretching vibrations of primary carboxamides, Vib. Spectrosc., 2022, 121, 103386 CrossRef CAS; (b) H. F. Shurvell, Spectra-Structure Correlations in the Mid- and Far-infrared, in Handbook of Vibrational Spectroscopy, ed. P. Griffiths and J. M. Chalmers, John Wiley & Sons, Chichester, 2006, vol. 1, pp. 1783–1816 Search PubMed.
- (a) M. Chen, B. Lei, X. Wang, H. Rong, H. Song and Z. Mo, A Silylene-Stabilized Germanium Analogue of Alkynylaluminum, Angew. Chem., Int. Ed., 2022, 61, e202204495 ( Angew. Chem. , 2022 , 134 , e202204495 ) CrossRef CAS PubMed; (b) R. S. C. Charman, N. J. Evans, L. E. English, S. E. Neale, P. Vasko, M. F. Mahon and D. J. Liptrot, The structures and reactivity of NHC-supported copper(I) triphenylgermyls, Chem. Sci., 2024, 15, 584–593 RSC; (c) L. K. Saunders, H. Nowell, L. E. Hatcher, H. J. Shepherd, S. J. Teat, D. R. Allan, P. R. Raithby and C. C. Wilson, Exploring short strong hydrogen bonds engineered in organic acid molecular crystals for temperature dependent proton migration behaviour using single crystal synchrotron X-ray diffraction (SCSXRD), CrystEngComm, 2019, 21, 5249–5260 RSC; (d) J. R. Holden, C. Dickinson and C. M. Bock, The Crystal Structure of 2,4,6-Trinitroaniline, J. Phys. Chem., 1972, 76, 3597–3602 CrossRef CAS.
- A. Pratap Singh, P. P. Samuel, H. W. Roesky, M. C. Schwarzer, G. Frenking, N. S. Sidhu and B. A. Dittrich, A Singlet Biradicaloid Zinc Compound and Its Nonradical Counterpart, J. Am. Chem. Soc., 2013, 135, 7324–7329 CrossRef PubMed.
- M. M. Siddiqui, S. K. Sarkar, S. Sinhababu, P. N. Ruth, R. Herbst-Irmer, D. Stalke, M. Ghosh, M. Fu, L. Zhao, D. Casanova, G. Frenking, B. Schwederski, W. Kaim and H. W. Roesky, Isolation of Transient Acyclic Germanium(I) Radicals Stabilized by Cyclic Alkyl(amino) Carbenes, J. Am. Chem. Soc., 2019, 141, 1908–1912 CrossRef CAS PubMed.
- D. Sarkar, C. Weetman, S. Dutta, E. Schubert, C. Jandl, D. Koley and S. Inoue, N-Heterocyclic Carbene-Stabilized Germa-acylium Ion: Reactivity and Utility in Catalytic CO2 Functionalizations, J. Am. Chem. Soc., 2020, 142, 15403–15411 CrossRef CAS PubMed.
- (a) C. D. Martin, M. Soleilhavoup and G. Bertrand, Carbene-stabilized main group radicals and radical ions, Chem. Sci., 2013, 4, 3020–3030 RSC; (b) S. Kundu, S. Sinhababu, V. Chandrasekhar and H. W. Roesky, Stable cyclic (alkyl)(amino)carbene (cAAC) radicals with main group substituents, Chem. Sci., 2019, 10, 4727–4741 RSC; (c) K. Breitwieser, H. Bahmann, R. Weiss and D. Munz, Gauging Radical Stabilization with Carbenes, Angew. Chem., Int. Ed., 2022, 61, e202206390 ( Angew. Chem. , 2022 , 134 , e202206390 ) CrossRef CAS PubMed.
- (a) K. C. Mondal, H. W. Roesky, M. C. Schwarzer, G. Frenking, I. Tkach, H. Wolf, D. Kratzert, R. Herbst-Irmer, B. Niepötter and D. Stalke, Conversion of a Singlet Silylene to a stable Biradical, Angew. Chem., Int. Ed., 2013, 52, 1801–1805 ( Umwandlung eines Singulett-Silylens in ein stabiles Biradikal, Angew. Chem. , 2013 , 125 , 1845–1850 ) CrossRef CAS PubMed; (b) J. Böhnke, T. Dellermann, M. A. Celik, I. Krummenacher, R. D. Dewhurst, S. Demeshko, W. C. Ewing, K. Hammond, M. Heß, E. Bill, E. Welz, M. I. S. Röhr, R. Mitrić, B. Engels, F. Meyer and H. Braunschweig, Isolation of diborenes and their 90°-twisted diradical congeners, Nat. Commun., 2018, 9, 1197 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details on the synthetic procedures and analyses, SC-XRD, NMR and IR spectroscopic data and DFT calculations. CCDC 2427550 (2b) and 2427551 (3). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5qi00678c |
| This journal is © the Partner Organisations 2025 |