 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Underpotentially-deposited silver substrates reverse the odd–even interfacial properties of CF3-terminated SAMs†
Maria D. Marquez,
Ruwanthi Amarasekara,
Daniela Rodriguez,
Oussama Zenasni,
Han Ju Lee,
Tianlang Yu,
Siwakorn Sakunkaewkasem,
Steven Baldelli and
T. Randall Lee *
*
Department of Chemistry and the Texas Center for Superconductivity, University of Houston, Houston, Texas 77204-5003, USA. E-mail: trlee@uh.edu
First published on 9th June 2025
Abstract
Modifications to the metal substrate used in alkanethiol self-assembled monolayer (SAM) formation impact the structural characteristics of the thin films and their macroscopic interfacial properties. In this study, evaporated gold surfaces underwent electrochemical modification, where a monolayer of silver was deposited through underpotential deposition (UPD). The effects of such a modification of the gold substrate on the structural and interfacial properties of n-alkanethiol and CF3-terminated SAMs, wherein the latter bears an interfacial dipole at the CF3–CH2 transition, were explored. Structural analysis of the films revealed well-ordered monolayers on both gold and UPD Ag surfaces. Ellipsometric thickness assessment and X-ray photoelectron spectroscopy (XPS) of UPD Ag surfaces showed that the adsorbates formed densely packed monolayers that were ∼4 Å thicker than their counterparts on gold. These variations were attributed to the different binding geometries adopted by the sulfur atoms on the respective metals, which in turn dictates the tilt angles and the orientation of the terminal moiety. Polarization modulation infrared reflection−absorption spectroscopy (PM-IRRAS) revealed a shift in the orientation of the chain termini, likely due to differences in the mobility of underlying methylene units between substrates. Moreover, odd–even effects in the contact angle data of both polar and nonpolar liquids show changes in interfacial wettability further highlighting the impact of the subtle change to the substrate on the film structure.
Introduction
The ability to tailor the properties of metal surfaces in a controlled manner at the nanoscale is of paramount importance to material engineers and surface scientists. Among the methods developed to achieve such control is the use of self-assembled monolayers (SAMs). In particular, SAMs have seen vast usage in a variety of applications ranging from surfaces pertinent to biological applications,1,2 lubricants for microelectromechanical systems (MEMs),3,4 corrosion inhibitors,5,6 nanoparticle protectants/stabilizers,7,8 antiadhesive films for surfaces,9,10 and catalyst modifiers in hydrogenation reactions due to its simplicity to develop the monolayer thin film.11,12 Among the several types of SAMs, perhaps the most studied systems involve the use of alkanethiols on noble metals, with gold being the most widely used metal. This system continues to be widely studied due to ease of preparation of the films, inertness of the Au substrate, strong Au–S bond of ∼50 kcal mol−1, well defined structural features, and the ability to manipulate the interfacial properties of the films via synthetic tailoring of the organic adsorbates.13,14 SAMs generated from thiol adsorbates as well as other headgroups (i.e., carboxylic acids and phosphonic acids) have also been generated on Ag15 as well as Cu surfaces.16,17 However, the rapid oxidation of the aforementioned substrates in air make it difficult to work with alkanethiol-based SAM systems.13Consequently, investigating custom metal substrates, where either a SAM deposited substrate or a bare substrate is adorned with a foreign metal species, has been undertaken as an alternative approach to broaden the application of easily oxidizable metals (i.e., silver and copper).18,19 Such substrates are obtained via the electrochemical deposition of the metal species, such as Cd2+,20,21 Pb2+,21–23 Ag+,24,25 and Cu2+,26,27 at a potential less negative than the equilibrium (Nernst) potential for the bulk deposition of the metal.28,29 The aforementioned phenomenon, known as underpotential deposition (UPD), is dictated by a stronger adatom–substrate interaction than an adatom–adatom interaction, which occurs during bulk material deposition, leading to strong adsorption to the dissimilar metal.30 The UPD of metals has emerged as a method for designing and precisely controlling metal surfaces due to the reproducible control of the surface coverage.29 The use of UPD has led to understanding nucleation and growth mechanisms of film deposition,31 measuring the electroactive surface area of metal-based materials,32 the development of fuel cells via the oxidation of glucose,33 and catalyst preparation.34 The use of SAMs developed atop UPD metals are limited but has been explored for the enhancement of thermal stability of SAMs on Au,35–37 the use of non-sulfur adsorbates on Au,38,39 the post modification of SAM surfaces,40 as well as SAM templating.41 Furthermore, the aforementioned studies found that the structural and interfacial properties of the SAMs on UPD Ag differ from those of SAMs on bare Au. Specifically, the observed differences can be attributed to the adlayer structure the SAMs of alkanethiols adopt on the metal surfaces, (√3 × √3)R30° on Au(111)14,15 and (√7 × √7)R19° on bulk Ag.13,42 Thus, our aim is to exploit this inherent difference in adopted structure of the alkanethiols on the metals to tune the interfacial properties of SAM-functionalized surfaces, specifically oriented dipoles.
The ability to tune surface and interfacial properties by generating an array of oriented dipoles on thin films is of significant interest in modern nanotechnology.43–45 Such surfaces are used in aligning energy levels to achieve work-function modifications in organic field transistors46,47 and solar cells,48,49 generating surfaces for the immobilization of biomolecules and biolabeling applications,50,51 and patterning for photo-responsive surfaces.52,53 The plethora of SAM-related literature points toward the properties of the generated SAMs as being dictated by the functionality of the organic molecules adsorbed on the surface.54–56 Altering the distribution of electron density of a film has proven an effective strategy since the order of magnitude and direction of the generated dipole has a profound effect on the interfacial properties of the generated films.44 In SAMs, interfacial dipoles can be generated by modification of the organic constituent via the introduction of electron withdrawing moieties, more specifically highly electronegative fluorine atoms.57–59 Numerous reports have shown that SAMs generated from selectively fluorinated adsorbates (FSAMs) exhibit extraordinary interfacial properties in the resulting films.10,60,61 Further, targeting different degrees of fluorination in the terminus of the adsorbate allows for tuning the interfacial properties (i.e., wettability, friction, and adhesion). For instance, minimal fluorination in an alkanethiol (i.e., CF3-terminated) led to the first example of a class of SAMs with oriented dipoles at the fluorocarbon–hydrocarbon junction (FC–HC), which gave monolayer thin films with a more hydrophilic nature than corresponding alkanethiol films.62–64 Upon increasing the amount of fluorination in the adsorbate, the influence of the FC–HC dipole diminishes, leading to surfaces that exhibit interfacial properties resembling those of polytetrafluoroethylene.59,62,65 Further research led to the development of FSAMs with an inverted dipole (i.e., HC–FC representing the hydrocarbon–fluorocarbon junction) as well as alkyl-terminated FSAMs where the HC–FC dipole is systematically buried.66,67 The impact of the former study lies in the wetting behavior that is governed by the interactions between the interfacial dipole and restrained H-bonded liquids, as highlighted by a reversal in the wettability of the HC–FC-terminated films from the FC–HC-terminated films when in contact with polar protic liquids.67 The latter study highlights the role both the HC–FC dipole as well as the structure of the adsorbate play on the interfacial properties of the film. To further manipulate surface properties via oriented dipoles, herein, we aim to modify evaporated Au substrates by the underpotential deposition of Ag metal in efforts to manipulate the interfacial energetics of monolayers using a series of CF3-terminated alkanethiols of the form CF3(CH2)mSH (F1HmSH), where m = 16–19, shown in Fig. 1. Our goal is to not only modulate the FC–HC dipole but also elucidate the effect the UPD Ag plays on the structural properties of the films. As a comparison, the analogous alkanethiols, CH3(CH2)nSH (HnSH), where n = 18–20, were included in the study.
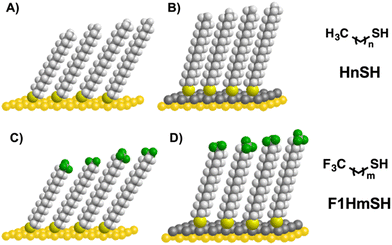 | ||
| Fig. 1 Molecules used in this study along with an illustration showing the SAMs on Au (A) and (C) and UPD Ag (B) and (D). Hydrogen atoms are denoted as white spheres while fluorine atoms are green. | ||
All SAMs were characterized by ellipsometry to determine the thickness of the films and X-ray photoelectron spectroscopy (XPS) to determine the chemical composition of the films. Polarization-modulation infrared reflection–absorption spectroscopy (PM-IRRAS) was used to determine the conformational order of the films. Finally, contact angle goniometry was used to probe the differences in the wetting properties of the molecules on the different metal substrates.
Experimental
Materials and methods
Gold Shot (99.999%) was bought from Kamis Inc. Chromium rods (99.9%) were purchased from R. D. Mathis Company. Silicon(100) wafers (polished, single crystal) were bought from University Wafer and used as received. Tetrahydrofuran (Sigma-Aldrich) and ethanol (Aaper Alcohol and Chemical Co) used on the SAMs were used as received. The adsorbate 1-octadecanethiol (H18SH) was purchased from Sigma-Aldrich. 1-heptadecanethiol (H17SH), 1-nonadecanethiol (H19SH), 1-eicosanethiol (H20SH), 17,17,17-trifluoroheptadecane-1-thiol (F1H16SH), 18,18,18-trifluorooctadecane-1-thiol (F1H17SH), 19,19,19-trifluorononadecane-1-thiol (F1H18SH), and 20,20,20-trifluoroicosane-1-thiol (F1H19SH) were synthesized according to procedures found in the literature.67,68Substrate preparation
Gold slides were prepared by the thermal evaporation of 1000 Å of gold atop 100 Å of chromium on Si(100) wafers under vacuum (pressure ≤ 6 × 10−5 torr) at a rate of 0.5 Å s−1. The wafers were then cut into slides and stored in milliQ water until used for electrochemical measurements.Underpotential deposition of silver (UPD Ag)
For cyclic voltammetry (CV), a Princeton Applied Research potentiostat/galvanostat model 263A was used to modulate the potential applied to and measure the current of the electrochemical systems. A homemade glass cell contained the three electrodes used for electrochemical measurements using gold slides as the working electrodes, a platinum wire as the counter electrode, and mercury/mercurous sulfate in saturated K2SO4 as the reference electrode. The reference electrode is +0.64 V relative to the normal hydrogen electrode (NHE). The electrolyte for all CVs was 0.1 M sulfuric acid (Ultrex II Ultrapure Reagent from J. T. Baker) with 0.6 mM Ag2SO4 (99.999% trace metals basis from Aldrich) added for the silver voltammetry. Gold slides were cycled ten times in sulfuric acid, rinsed with plenty of milliQ water and stored in milliQ water until measurement of their optical properties with ellipsometry. For the deposition of silver, the cycled gold slides were cycled in deaerated silver solution ten times at a scan rate of 15 mV s−1, then held at a potential of 0.15 V vs MSE for the underpotential deposition of silver,69 pulled out of the cell while held at potential, rinsed with copious amounts of milliQ water, and stored in milliQ water until analysis with ellipsometry. Representative cyclic voltammograms for gold and silver are shown in Fig. S1 in the ESI.† Using stripping voltammetry, we were able to determine that we have a 0.46 ± 0.03 monolayer coverage of silver on gold. This value was compared to the 0.81 ± 0.04 monolayer value derived from XPS calculations36 that include silver oxides in the final coverage. A detailed discussion regarding monolayer coverage of Ag and representative voltammograms (Fig. S2, ESI†) are included in the ESI.†Monolayer formation and characterization
To minimize exposure to the lab environment, immediately after collecting ellipsometric measurements on the bare substrates, the slides were immersed in a 1 mM ethanol solution of the corresponding thiol in a 40 mL vial, previously cleaned with piranha. The self-assembled monolayers were allowed to form for 48 h in the dark at ambient temperature. During this equilibration time, factors such as the high affinity of thiols to Au and Ag substrates, and stabilization of the SAMs by van der Waals forces (∼1 to 2 kcal mol−1 per methylene unit) lead to the formation of densely packed monolayers.14,68,70,71 Prior to characterization of the SAMs, the slides were rinsed with THF followed by ethanol and dried with ultra-pure nitrogen.A Rudolph Auto El III ellipsometer equipped with a He–Ne laser (632.8 nm) set at an incidence angle of 70° and a refractive index of 1.45, a value typical for organic thin films,72 was used to obtain thickness measurements for the monolayer films. An average of three measurements per slide was used as the reported thickness.
X-ray photoelectron spectroscopy (XPS) was performed on a PHI 5700 X-ray photoelectron spectrometer with a monochromatic Al Kα X-ray source (1486.7 eV) incident at 90° relative to the axis of the hemispherical analyzer with a takeoff angle of 45° from the surface and a pass energy of 23.5 eV. The Au 4f7/2 peak was referenced to 84.0 eV in all the spectra.
Polarization-modulation infrared reflectance-absorption spectroscopy (PM-IRRAS) was performed using a Nicolet Nexus 670 Fourier transform equipped with a mercury–cadmium–telluride (MCT) detector and a Hinds Instrument PEM-90 photoelastic modulator. The surfaces were mounted at an incident angle of 80° for the p-polarized light with respect to the surface normal. The spectra were collected using 512 scans at a resolution of 2 cm−1.
Contact angle data were obtained using a ramé-hart model 100 contact angle goniometer set up with a Matrix Technologies micro-Electrapette 25 to dispense probe liquids. The advancing contact angles (θa) and receding contact angles (θr) were obtained at a speed of 1 μL s−1. The reported data were reproducible to ±1° and are an average of six measurements with readings being made from each side of three droplets on different locations along the slides (12 total measurements).
The contacting liquids used in the study include a variety of nonpolar, polar protic, and polar aprotic liquids: bromonaphthalene (BNP – Sigma Aldrich); decalin (DC – Acros Organics); hexadecane (HD – Aldrich); perfluorodecalin (FDC – Synquest Labs); acetonitrile (MeCN – Sigma Aldrich); nitro-benzene (NB – Acros); dimethylformamide (DMF – Sigma Aldrich); dimethyl sulfoxide (DMSO – Sigma Aldrich); formamide (FA – Sigma Aldrich); glycerol (GY – Sigma Aldrich); and water (H2O – Millipore water with resistivity of 18.2 Ω).
Results and discussion
Ellipsometric thickness assessment
In this study, the series of CF3-terminated SAMs were compared to their hydrocarbon analogs formed on the same batch of vapor-deposited gold that was treated electrochemically prior to formation of the SAMs. Accounts in the literature have fully characterized these hydrocarbon SAMs, which serve as a point of reference in the analysis of the SAMs on UPD Ag and the FSAMs on both substrates36,67 Fig. 2 and Table 1 depict the average thickness measurements for the SAMs used in the study. The thickness values of the H17SH, H18SH, H19SH, and H20SH SAMs on gold exhibit thicknesses of 21, 22, 23, and 25 Å, respectively, and are in accordance with literature values.67 Additionally, the observed increase in the thickness values with increasing chain length, ∼1 Å, is consistent with the increase in the methylene units of the hydrocarbon backbone.68,73 Notably, the hydrocarbon SAMs on UPD Ag are much thicker than the corresponding SAMs on Au by ∼3 Å, which is in accordance to the difference in thickness for a hydrocarbon SAM on Au and Ag in the literature.36 Moreover, the F1HmSH analogs exhibited SAM thicknesses on gold that agree with literature values, 18, 20, 21, and 22 Å for the F1H16SH, F1H17SH, F1H18SH, and F1H19SH SAMs, respectively.67 Following a similar trend as the HnSH SAMs, the F1HmSH adsorbates produced films that were 4 Å thicker on UPD Ag compared to their counterparts on gold. | ||
Fig. 2 Average thickness measurements obtained for the (A) HnSH SAMs and the (B) F1HmSH SAMs on Au (■) and UPD Ag ( ). ). | ||
| Adsorbate | Au thickness (Å) | UPD Ag thickness (Å) | Adsorbate | Au thickness (Å) | UPD Ag thickness (Å) |
|---|---|---|---|---|---|
| H17SH | 21 | 24 | F1H16SH | 18 | 22 |
| H18SH | 22 | 26 | F1H17SH | 20 | 24 |
| H19SH | 23 | 27 | F1H18SH | 21 | 25 |
| H20SH | 25 | 29 | F1H19SH | 22 | 26 |
The conformation of alkanethiols SAMs on bulk Ag have been described in the literature as having an orientation different from that of alkanethiols on Au.13 For our study, we anticipate that the molecules on UPD Ag will behave similarly to adsorbates on bulk Ag. In terms of orientation and structure, alkanethiols on an Au surface adopt a twist angle of ∼55° and an overall tilt of ∼33°, with respect to the surface normal.13,74,75 While on Ag surfaces, the same molecule will have a twist angle of ∼45° and a tilt of ∼10°.13,74,75 The underlying difference in the orientation the adsorbates adopt on the metal substrates lies in the different binding geometry the sulfur atom adopts on the respective metals. Specifically, the Au–S–C bond angle is ∼104°, while the Ag–S–C bond angle is ∼180°.76 Thus, the difference in the binding geometries dictates the orientation of the carbon backbone on the metals. Consequently, a more densely packed monolayer is formed on the UPD Ag surface as compared to the Au surfaces, a scenario that might lead to a thicker film. Given the similarities in structure between the alkanethiols and the CF3-terminated alkanethiols (i.e., the only difference is the fluorocarbon at the terminus), the CF3-terminated alkanethiols are expected to adopt the same orientation as the former. However, we note that the CF3-terminated alkanethiols yielded films on both gold and UPD Ag surfaces with slightly lower thickness values than the alkanethiol analogs. Previous research on CF3-terminated alkanethiol SAMs determined the thicknesses for these types of SAMs are ∼1 Å shorter than the hydrocarbon analogs, in agreement with our observations.67
Analysis of the monolayer films by XPS
XPS is a surface-sensitive technique that yields qualitative and quantitative information on most elements present on a sample. In the analysis of SAMs, XPS can also give insight into the structural features of the films.77 For the hydrocarbon SAMs, a survey scan detected the presence of Au, C and S, as well as Ag for the SAMs on UPD Ag. Fig. S3 (ESI†) shows XPS spectra of Au 4f and Ag 3d regions for the HnSH SAMs on UPD Ag surfaces. Fig. 3 and 4 depict high resolution spectra of the C 1s and S 2p regions for the hydrocarbon SAMs on Au and the Ag 3d, O 1s, C 1s, and S 2p regions for the hydrocarbon SAMs on UPD Ag, respectively. Tables S1 and S2 (ESI†) list the peak positions for the photoelectrons of the HnSH and F1HmSH SAMs on the metal substrates respectively. The high-resolution spectra for the regions of interest for the F1HmSH SAMs on Au (C 1s, F 1s, and S 2p) and UPD Ag (Ag 3d, O 1s, C 1s, F 1s, and S 2p) are shown in Fig. 5 and Fig. 6, respectively. All of the SAMs in the study, on Au and UPD Ag, exhibit a characteristic doublet with a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the S 2p region, with a binding energy of ∼162.0 eV for the S 2p3/2, which is indicative of a bound thiolate.78 Furthermore, the lack of a peak at higher binding energies, ∼164 and ∼168 eV, indicates the absence of highly oxidized sulfur species or unbound thiol on the surface. More importantly, XPS spectra of the SAMs on UPD Ag show no signals corresponding to the O 1s core level region (binding energy ∼535 eV),77 thus confirming the absence of silver oxides. (see Fig. 4(A) and (B) as well as Fig. 6(A) and (B)).
1 in the S 2p region, with a binding energy of ∼162.0 eV for the S 2p3/2, which is indicative of a bound thiolate.78 Furthermore, the lack of a peak at higher binding energies, ∼164 and ∼168 eV, indicates the absence of highly oxidized sulfur species or unbound thiol on the surface. More importantly, XPS spectra of the SAMs on UPD Ag show no signals corresponding to the O 1s core level region (binding energy ∼535 eV),77 thus confirming the absence of silver oxides. (see Fig. 4(A) and (B) as well as Fig. 6(A) and (B)).
 | ||
| Fig. 4 XPS spectra for the (A) Ag 3d, (B) O 1s, (C) C 1s, and (D) S 2p region for the HnSH SAMs on UPD Ag. | ||
 | ||
| Fig. 6 XPS spectra for the (A) Ag 3d, (B) O 1s, (C) C 1s, (D) F 1s, and (E) S 2p region for the F1HmSH SAMs on UPD Ag. | ||
In addition to obtaining the elemental composition and oxidation state of elements present in a SAM, a qualitative examination of the packing density of the chains can be obtained from the binding energy of the C 1s peak. For the hydrocarbon SAMs on Au, the C 1s peak appears at ∼284.9 ± 0.1 eV while on UPD Ag the same peak is at ∼285.2 ± 0.1 eV. The shifts to a higher binding energies for the hydrocarbon SAMs on UPD Ag are indicative of a more densely packed film compared to the comparative films on Au.79 Specifically, in the present study, the observed increase in binding energy can be attributed to the chains being more upright on the UPD Ag surface.80,81 On the other hand, the binding energy of C 1s for the FSAMs on Au, shows a slight decrease (∼284.8 eV) in comparison to the hydrocarbon SAMs on Au, which suggests a lower chain packing in the former films plausibly due to the larger chain termini. A similar decrease in the binding energy is seen when comparing the FSAMs on UPD Ag to the hydrocarbon SAMs on UPD Ag, 285.1 eV and ∼285.2 eV, respectively. However, it should be noted that between the two metal substrates, the FSAMs on UPD Ag exhibit a shift to higher binding energy for the C 1s electrons, which is in accordance with the trend observed in the hydrocarbon SAMs on the two metals. Nevertheless, the trends observed in the XPS spectra of the SAMs suggest that the methylene chains on Ag are more upright and therefore allow for a more densely packed film, as previously concluded in a friction-force microscopy study of SAMs on Au and Ag which saw increased stability in SAMs on Ag due to their greater packing density compared to the monolayers on Au.82
Conformational order by PM-IRRAS
SAMs were characterized using PM-IRRAS to gain insight into the relative crystallinity and conformational order of the alkyl chains in the organic monolayer thin films. The conformational order of the SAMs is determined by the position of the C–H antisymmetric stretch of the methylene units . Appearance of this band at 2918 cm−1 indicates that the hydrocarbon chains are well ordered and exhibit a trans-extended conformation, making crystalline-like surfaces.83,84 On the other hand, a shift to a higher wavenumber is characteristic of a disordered, or liquid-like film. For the hydrocarbon SAMs on Au, all of the SAMs in the series are well ordered, as shown in Fig. 7, displaying their
. Appearance of this band at 2918 cm−1 indicates that the hydrocarbon chains are well ordered and exhibit a trans-extended conformation, making crystalline-like surfaces.83,84 On the other hand, a shift to a higher wavenumber is characteristic of a disordered, or liquid-like film. For the hydrocarbon SAMs on Au, all of the SAMs in the series are well ordered, as shown in Fig. 7, displaying their  at 2918 cm−1. A similar observation can be seen with the hydrocarbon SAMs on UPD Ag with the
at 2918 cm−1. A similar observation can be seen with the hydrocarbon SAMs on UPD Ag with the  at 2918/2917 cm−1.
at 2918/2917 cm−1.
Another aspect to note from the analysis of the SAMs on both substrates from the PM-IRRAS spectra involves the difference in intensity of the methyl antisymmetric to symmetric C–H stretches,  at ∼2964 cm−1 and
at ∼2964 cm−1 and  at ∼2878 cm−1. A discernable “odd–even” effect is observed with the ratio of the intensities of the aforementioned bands. For the SAMs on Au, the intensity of the symmetric and antisymmetric stretches on the even SAMs, H18SH and H20SH, is roughly the same, ∼1
at ∼2878 cm−1. A discernable “odd–even” effect is observed with the ratio of the intensities of the aforementioned bands. For the SAMs on Au, the intensity of the symmetric and antisymmetric stretches on the even SAMs, H18SH and H20SH, is roughly the same, ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. On the other hand, in the odd SAMs, H17SH and H19SH, the intensity of the symmetric C–H stretch is weaker than the intensity of the antisymmetric stretch, leading to a higher antisymmetric to symmetric stretching ratio, ∼2
1 ratio. On the other hand, in the odd SAMs, H17SH and H19SH, the intensity of the symmetric C–H stretch is weaker than the intensity of the antisymmetric stretch, leading to a higher antisymmetric to symmetric stretching ratio, ∼2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Interestingly, for these hydrocarbon SAMs on UPD Ag, the trends are the opposite. For the even-numbered chains, H18SH and H20SH, the intensity of the antisymmetric stretching band is higher than that of the symmetric stretching, while for the odd-numbered chains, H17SH and H19SH, the intensity of the two stretching bands is roughly the same. The reason for the change in the intensities can be attributed to the orientation of the methyl termini in the films on the two metal surfaces, as illustrated in Fig. 8. According to metal-surface selection rules governed by the PM-IRRAS technique, the intensity of vibrations whose transition dipole moments lie parallel to the surface normal are enhanced, while the intensity diminishes for vibrations with a transition dipole moment more perpendicular to the surface normal.79 For the hydrocarbon SAMs on Au, the terminal methyl group in the odd-numbered chains is tilted away from the surface normal, giving a transition dipole moment for the symmetric stretch that is slightly perpendicular to the surface normal; whereas in the even-numbered chains, the transition dipole moment is parallel to the surface normal, and vice versa for the antisymmetric stretch. However, the direction of the terminal methyl group is opposite in hydrocarbon SAMs on UPD Ag; the odd-numbered chains have a more upward orientation, while the even numbered chains have a tilted direction.
1. Interestingly, for these hydrocarbon SAMs on UPD Ag, the trends are the opposite. For the even-numbered chains, H18SH and H20SH, the intensity of the antisymmetric stretching band is higher than that of the symmetric stretching, while for the odd-numbered chains, H17SH and H19SH, the intensity of the two stretching bands is roughly the same. The reason for the change in the intensities can be attributed to the orientation of the methyl termini in the films on the two metal surfaces, as illustrated in Fig. 8. According to metal-surface selection rules governed by the PM-IRRAS technique, the intensity of vibrations whose transition dipole moments lie parallel to the surface normal are enhanced, while the intensity diminishes for vibrations with a transition dipole moment more perpendicular to the surface normal.79 For the hydrocarbon SAMs on Au, the terminal methyl group in the odd-numbered chains is tilted away from the surface normal, giving a transition dipole moment for the symmetric stretch that is slightly perpendicular to the surface normal; whereas in the even-numbered chains, the transition dipole moment is parallel to the surface normal, and vice versa for the antisymmetric stretch. However, the direction of the terminal methyl group is opposite in hydrocarbon SAMs on UPD Ag; the odd-numbered chains have a more upward orientation, while the even numbered chains have a tilted direction.
 | ||
| Fig. 8 Illustration of the HnSH SAMs on (A) Au and (B) UPD Ag surfaces with the orientation of the terminal methyl group for odd and even numbered chains. | ||
In a similar fashion, the relative ratio between the methylene antisymmetric to symmetric stretches changes depending on the substrate; the effect is apparent in the spectra of the FSAMs rather than in the spectra of the HSAMs (vide infra). The ratio between the methylene peaks is decidedly lower for the SAMs on UPD Ag than Au. This can also be explained according to the surface selection rule. As the chains tilt upright with respect to the surface normal, the methylene antisymmetric transition dipole moment must be getting progressively perpendicular to the surface normal, causing a lower ratio between the methylene stretching mode intensities.76
The PM-IRRAS spectra of the CF3-terminated FSAMs in Fig. 9 shows that the F1HmSH SAMs on the Au and UPD Ag surfaces are well-ordered, with the alkyl chains having a trans-extended configuration. Previous studies on these types of FSAMs have shown that the carbon backbones have similar structural features as the hydrocarbon analogs.85 Interesting to note in the spectra of the FSAMs, is the differences in the relative ratios of the intensities of the methylene symmetric and antisymmetric stretches between the two metal surfaces. For the FSAMs on UPD Ag, the relative ratio of the antisymmetric to symmetric stretch appears to be lower than that for the FSAMs on Au. This phenomenon might be due to either an increase in the intensity of the symmetric stretch or a decrease in the intensity of the antisymmetric stretch; both likely arise from a decrease in chain tilt on the UPD Ag surface.86
In contrast to the alkanethiol SAMs, the spectra of the FSAMs show a discernable “odd–even” effect in the relative ratios of the methylene stretches between odd and even chain-length molecules. The lack of odd–even effects in the C–H stretching vibrations of the methylene moieties in the HnSH SAMs suggests a uniform chain orientation on the surface for these films.77 Previous studies on SAMs have shown that an additional degree of freedom in the terminal group can exhibit changes in the extent of the odd–even effects in relation to molecular orientation.87–90 Alkanethiol SAMs terminated with rigid aryl groups with odd numbered chains show a higher packing density due to the upright molecular orientation compared to the inclined orientation of terminal groups in even numbered alkyl chains.87,88 However, Dauselt et al. observed a diminished odd–even effect for anthracene terminated SAMs,89 a behavior resulting from the rotation of the terminal moiety leading to greater packing of even numbered alkyl chains.89,90 In contrast, in this study, the presence of a bulky fluorinated moiety allows the methylene units beneath it to rotate about their chain axis due to a lower packing density compared to non-fluorinated alkanes.91 Therefore, it can be inferred that, owing to the bulkiness of the CF3 terminus, the adjacent methylene group is likely to demonstrate a certain degree of molecular mobility, giving rise to a shift in the surface orientation of the chains.85 An intriguing observation is the apparent reversal of the “odd–even” effect in the relative ratios of the methylene peaks when the substrate is changed from Au to UPD Ag. For the odd FSAMs (F1H16SH and F1H18SH) on Au, the relative ratio between the antisymmetric and symmetric stretches appears to be lower than the even FSAMs (F1H17SH and F1H19SH). The opposite trend is observable for the FSAMs on UPD Ag. This reversed trend suggests that the termini of the chains might be rearranging to attain a lower surface energy, leading to alterations in the intensity of the peaks.67
Wettability
The 'odd–even effect' is a well-known characteristic of n-alkanes. Physical parameters such as melting points, densities, and thermodynamic properties of fusion and sublimation of n-alkanes have exhibited odd–even alterations.92,93 The molecular origin of these variations arise from the ‘packing effect’ where in odd-numbered alkyl chains, the terminal methyl moieties are oriented to minimize interactions with neighboring terminal groups, reducing packing efficiency and leading to lower melting points and densities compared to the more tightly packed even-numbered n-alkane chains.92,93 Similarly, alkanethiol assemblies have exhibited odd–even effects in electronic properties such as current–voltage response,94 charge transport, capacitance, chemical reactivity, and tribological properties among others.42,75 In this study, n-alkanethiol SAMs on Au and UPD Ag were probed with a variety of liquids ranging in polarity to evaluate the odd–even effect on SAM wettability on the two different metals. The probe liquids used were: water – H2O; glycerol – GL; formamide – FA; dimethyl sulfoxide – DMSO; dimethylformamide – DMF; nitrobenzene – NB; acetonitrile – ACN; bromonaphthalene – BNP; decalin – DC; hexadecane – HD; and perfluorodecalin – FDC. Surface tension values of the contacting liquids are presented in Table S3 (ESI†).95–97Fig. 10 shows the advancing contact angle values for the hydrocarbon SAMs on Au and UPD Ag for the nonpolar liquids: BNP, DC, HD, and FDC. Separately, Fig. 11 shows the contact angles for the polar contacting liquids: H2O, GL, FA, DMSO, DMF, ACN, and NB. (see also Tables S4 and S5, ESI†). For the hydrocarbon SAMs on Au, there is an odd–even effect in which the odd chains (total number of carbon atoms in the chain) are more wettable than the even chains. This phenomenon can be attributed to the orientation of the terminal methyl group: in the even SAMs, the methyl group is oriented more perpendicular to the surface, while in the odd SAMs, it is tilted away (see Fig. 8) allowing for an increase in molecular contact with the underlying CH2 unit.98 For the hydrocarbon SAMs on UPD Ag, the odd–even effect is the opposite; the even SAMs are more wettable than the odd ones. The inversion of the odd even effect for the UPD Ag surfaces can also be attributed to the orientation of the terminal methyl group: in the odd SAMs, the methyl group is more perpendicular to the surface, while in the even SAMs, it is tilted as illustrated in Fig. 8. Moreover, the odd–even effect observed in the wettability of the hydrocarbon SAMs on both substrates is in accordance with the interpretation made above in the PM-IRRAS section for these SAMs (vide supra).
 | ||
| Fig. 10 Advancing contact angles for BNP, DC, HD, and FDC on HnSH SAMs on (A) Au and (B) UPD surfaces. Error bars fall within the symbols. | ||
 | ||
| Fig. 11 Advancing contact angles for H2O, GL, FA, DMSO, DMF, NB, and ACN on HnSH SAMs on (A) Au and (B) UPD surfaces. Error bars fall within the symbols. | ||
The advancing contact angles for nonpolar liquids on the FSAMs are presented in Fig. 12 and those of polar liquids in Fig. 13. (see also Tables S5 and S6, ESI†). With the nonpolar liquids, the contact angle values for the FSAMs are higher than those on the hydrocarbon SAMs. On the hydrocarbon SAMs, there are favorable dispersive interactions between the nonpolar liquids and the hydrocarbon surfaces. On the FSAMS, there are unfavorable non-ideal interactions between the fluorinated surface and the hydrocarbon liquids. These observations follow the phenomena of “like dissolves like”. Moreover, the contact angles of the polar liquids are lower on the FSAMs when compared to the hydrocarbon SAMs. This observation has been attributed to the presence of an interfacial dipole in the FSAMs.64
 | ||
| Fig. 12 Advancing contact angles for BNP, DC, HD, and FDC on F1HmSH SAMs on (A) Au and (B) UPD Ag surfaces. Error bars fall within the symbols. | ||
 | ||
| Fig. 13 Advancing contact angles for H2O, GL, FA, DMSO, DMF, NB, and ACN on F1HmSH SAMs on (A) Au and (B) UPD surfaces. Error bars fall within the symbols. | ||
The FSAMs on Au show an odd–even effect in which the even chains are more wettable than the odd ones for both sets of liquids tested. Important to note is the inversion of the odd–even effect observed for the FSAMs from what is observed with the hydrocarbon SAMs. The trends in the wettability of the hydrocarbon SAMs can be explained in terms of atomic contact between the surface and the contacting liquid.67 However, the same argument cannot be made with the FSAMs. Previous research with CF3-terminated alkanethiols has demonstrated that the presence of a permanent dipole at the interface of these types of SAMs has a profound effect on the wettability of the films.62–64 For the FSAMs on Au, the dipole is oriented more along the surface normal in even chains (∼17° with respect to the surface normal) while for the odd chains it is tilted away (∼58° with respect to the surface normal).67 When the dipoles are aligned along the surface (i.e., the even chains) there are greater dipole–dipole interactions when in contact with the polar liquids, but only dipole-induced dipole interactions when in contact with the nonpolar liquids. On the other hand, when the dipoles are canted (i.e. the odd chains) there is a compensation between the dipoles which leads to a reduced favorable interaction between the surface and the liquid.
In the case of FDC, the odd–even effect that is observed cannot be attributed to a dipole effect, but is more likely due to van der Waals (induced dipole-induced dipole) interactions. In the even-numbered chains, the CF3 group is pointed more along the surface normal, exclusively exposing fluorine atoms, whereas in the odd chains, the CF3 group is canted, exposing the underlying CH2. Exposure of the CH2 in the odd chains causes an unfavorable dispersive interaction with the fluorinated liquid and a higher contact angle.
For the FSAMs on UPD Ag, the odd–even effect is opposite to that observed on Au. Previous research has shown that the CF3-terminated SAMs have similar structural properties as their hydrocarbon analogs.85 Correspondingly, it is reasonable to assume that the CF3-terminated alkanethiols on the UPD Ag surfaces will have similar structural properties as the alkanethiols on UPD Ag. Taking the twist and tilt angle, ∼45° and ∼11° respectively, that an alkanethiol adopts on a silver surface13,75 gives the model featured in Fig. 14. It is apparent from the wettability data and the model in Fig. 14, that the CF3 termini on the films have the opposite orientation on the silver surface.
 | ||
| Fig. 14 Illustration of the F1HmSH SAMs on (A) Au and (B) UPD Ag surfaces with the orientation of the dipole for odd and even numbered chains. | ||
The crystallographic structure and properties of alkanethiol SAM systems are known to differ depending on whether the molecules contain an odd or even number of carbon atoms.99 A simulation study by Ramin et al. showed the relationship between carbon parity to structural characteristics of the films.99 However, considering the number of methylene units of SAMs used in our study, results in literature showed that for molecules where n > C15, structural features such as the tilt angle, tilt angle orientation, gauche defects and trans percentage has no dependence on the odd–even effect or on the chain length. These SAM structures exhibit a similarly strong and uniform structural order, confirming that the significant differences in wettability observed in this study as an ‘odd–even effect’ stem from the interfacial dipole induced by the orientation of the terminal moiety.
Conclusions
The alkanethiols and CF3-terminated alkanethiols in this study were used to form SAMs on Au and UPD Ag with distinct properties. Analysis of the films by ellipsometry showed that the thickness of the SAMs on UPD Ag were thicker by ∼4 Å than the corresponding SAMS on Au. The thicker film was attributed to the inherent differences in the binding geometry of the sulfur anchor to the metal substrate, which leads to changes in tilt angles adopted by the chains. Analysis by XPS confirmed the composition of the films and presence of an unoxidized Ag layer. Further analysis revealed a more densely packed monolayer on UPD Ag than on Au. The increase in packing density of the SAMs on UPD Ag likely arises from the molecules being more upright, due to the (√7 × √7)R19° adlayer structure, allowing them to pack more densely and thus contributing to the formation of thicker films. Further, analysis by PM-IRRAS revealed well-ordered films with the chains adopting a trans-extended conformation on both substrates. Moreover, the wettability data obtained from the SAMs generated on both substrates allowed us to probe the direction of the dipole, further highlighting the likely changes in the chain orientation between the two substrates.Author contributions
Conceptualization: T. R. L.; formal analysis: M. M., D. R., R. A., T. R. L. and S. B.; funding acquisition: T. R. L. and S. B.; investigation: M. M., D. R., R. A., O. Z., H. J. L., T. Y. and S. S.; methodology: M. M., D. R., T. R. L. and S. B.; project administration: T. R. L. and S. B.; resources: T. R. L. and S. B.; supervision: T. R. L. and S. B.; validation: M. M., D. R., R. A., T. R. L. and S. B.; writing – original draft: M. M. and R. A.; writing – review & editing: T. R. L.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for financial support from the NSF Division of Chemistry (CHE-2109174 and CHE-2246583).References
- G. V. Latag, T. Nakamura, D. Palai, E. A. Q. Mondarte and T. Hayashi, Investigation of three-dimensional bacterial adhesion manner on model organic surfaces using quartz crystal microbalance with energy dissipation monitoring, ACS Appl. Bio. Mater., 2023, 6, 1185–1194 CrossRef CAS.
- K. Son, T. Uzawa, Y. Ito, T. Kippin, K. W. Plaxco and T. Fujie, Survey of oligoethylene glycol-based self-assembled monolayers on electrochemical aptamer-based sensor in biological fluids, Biochem. Biophys. Res. Commun., 2023, 668, 1–7 CrossRef CAS PubMed.
- J. Yang, M. Zhou, J. Liu, H. Wang and C. Weng, Fabrication and tribological properties of self-assembled monolayers of alkanethiols on nickel substrates, Appl. Surf. Sci., 2021, 559, 149963 CrossRef CAS.
- O. Cooper, H.-P. Phan, B. Wang, S. Lowe, C. J. Day, N.-T. Nguyen and J. Tiralongo, Functional microarray platform with self-assembled monolayers on 3C-silicon carbide, Langmuir, 2020, 36, 13181–13192 CrossRef CAS PubMed.
- L. Feng, S. Zheng, H. Zhu, X. Ma and Z. Hu, Corrosion inhibition of novel dithiane self-assembled monolayers (SAMs) on copper, J. Taiwan Inst. Chem. Eng., 2023, 142, 104610 CrossRef CAS.
- J. Telegdi, Formation of self-assembled anticorrosion films on different metals, Materials, 2020, 13, 5089 CrossRef CAS PubMed.
- C. S. Park, H. J. Lee, A. C. Jamison and T. R. Lee, Robust maleimide-functionalized gold surfaces and nanoparticles generated using custom-designed bidentate adsorbates, Langmuir, 2016, 32, 7306–7315 CrossRef CAS.
- H. J. Lee, A. C. Jamison, Y. Yuan, C.-H. Li, S. Rittikulsittichai, I. Rusakova and T. R. Lee, Robust carboxylic acid-terminated organic thin films and nanoparticle protectants generated from bidentate alkanethiols, Langmuir, 2013, 29, 10432–10439 CrossRef CAS PubMed.
- Y. Choi, H.-V. Tran and T. R. Lee, Self-assembled monolayer coatings on gold and silica surfaces for antifouling applications: A review, Coatings, 2022, 12, 1462 CrossRef CAS.
- L. R. Hill, J. W. Craft, P. Chinwangso, H.-V. Tran, M. D. Marquez and T. R. Lee, Antifouling coatings generated from unsymmetrical partially fluorinated spiroalkanedithiols, ACS Appl. Bio. Mater., 2021, 4, 1563–1572 CrossRef.
- K. R. Kahsar, D. K. Schwartz and J. W. Medlin, Control of metal catalyst selectivity through specific noncovalent molecular interactions, J. Am. Chem. Soc., 2014, 136, 520–526 CrossRef CAS PubMed.
- P. D. Coan, C. A. Farberow, M. B. Griffin and J. W. Medlin, Organic modifiers promote furfuryl alcohol ring hydrogenation via surface hydrogen-bonding interactions, ACS Catal., 2021, 11, 3730–3739 CrossRef CAS.
- J. C. Love, L. A. Estroff, J. K. Kriebel, R. G. Nuzzo and G. M. Whitesides, Self-assembled monolayers of thiolates on metals as a form of nanotechnology, Chem. Rev., 2005, 105, 1103–1170 CrossRef CAS.
- C. Vericat, M. E. Vela, G. Benitez, P. Carro and R. C. Salvarezza, Self-assembled monolayers of thiols and dithiols on gold: New challenges for a well-known system, Chem. Soc. Rev., 2010, 39, 1805–1834 RSC.
- P. N. Floriano, O. Schlieben, E. E. Doomes, I. Klein, J. Janssen, J. Hormes, E. D. Poliakoff and R. L. McCarley, A grazing incidence surface X-ray absorption fine structure (GIXAFS) study of alkanethiols adsorbed on Au, Ag, and Cu, Chem. Phys. Lett., 2000, 321, 175–181 CrossRef CAS.
- I. Martinovic, G. Zlatic, Z. Pilic, L. Susie, O. Kowalska, D. Petrovic, F. Falak and J. Miskovic, Self-assembled monolayers of alkanethiol as inhibitors against copper corrosion in synthetic acid rain, Int. J. Electrochem. Sci., 2019, 14, 4206–4215 CrossRef CAS.
- W. Zhao, M. Göthelid, S. Hosseinpour, M. B. Johansson, G. Li, C. Leygraf and C. M. Johnson, The nature of self-assembled octadecylphosphonic acid (ODPA) layers on copper substrates, J. Colloid Interface Sci., 2021, 581, 816–825 CrossRef CAS PubMed.
- P. He, A. H. S. Daaoub, S. Sangtarash, H. Sadeghi and H. J. Yoon, Thermopower in underpotential deposition-based molecular junctions, Nano Lett., 2024, 24, 1988–1995 CrossRef CAS PubMed.
- M. Hong, Y. Yokota, R. A. Wong, N. Hayazawa, E. Kazuma and Y. Kim, Underpotential deposition of silver on gold for surface catalysis of plasmon-enhanced reduction of 4-nitrothiophenol, J. Phys. Chem. C, 2021, 125, 16569–16575 CrossRef CAS.
- Z. Dursun, Ş. U. Karabiberoğlu, B. Gelmez and A. Başaran, Electrocatalytic oxidation of ethanol on various metal ad-layer modified Au(111) electrodes in alkaline solution, Turk. J. Chem., 2011, 35, 349–359 CAS.
- J. W. F. Robertson, D. J. Tiani and J. E. Pemberton, Underpotential deposition of thallium, lead, and cadmium at silver electrodes modified with self-assembled monolayers of (3-mercaptopropyl)trimethoxysilane, Langmuir, 2007, 23, 4651–4661 CrossRef CAS.
- P. Sebastián-Pascual and M. Escudero-Escribano, Surface characterization of copper electrocatalysts by lead underpotential deposition, J. Electroanal. Chem., 2021, 896, 115446 CrossRef.
- F. W. S. Lucas, N. C. Ramos, D. K. Schwartz, J. W. Medlin and A. Holewinski, Understanding reactivity of self-assembled monolayer-coated electrodes: SAM-induced surface reconstruction, Electrochim. Acta, 2023, 459, 142586 CrossRef CAS.
- M. R. Ehrenburg, E. B. Molodkina, P. Broekmann and A. V. Rudnev, Underpotential deposition of silver on Au(111) from an air- and water-stable ionic liquid visualized by in-situ STM, ChemElectroChem, 2019, 6, 1149–1156 CrossRef CAS.
- I. Park and H. Baltruschat, In situ friction study of Ag underpotential deposition (UPD) on Au(111) in aqueous electrolyte, Chem. Phys. Chem., 2021, 22, 952–959 CrossRef CAS.
- M. Yang, H. Zhang and Q. Deng, Understanding the copper underpotential deposition process at strained gold surface, Electrochem. Commun., 2017, 82, 125–128 CrossRef CAS.
- Z. Tan, K. Li, Y. Gu, Z. Nan, W. Wang, L. Sun, B. Mao and J. Yan, Unconventional electrochemical behaviors of Cu underpotential deposition in a chloride-based deep eutectic solvent: High underpotential shift and low coverage, Anal. Chem., 2023, 95, 6458–6466 CrossRef CAS PubMed.
- N. Mayet, K. Servat, K. B. Kokoh and T. W. Napporn, Probing the surface of noble metals electrochemically by underpotential deposition of transition metals, Surfaces, 2019, 2, 257–276 CrossRef CAS.
- E. Herrero, L. J. Buller and H. D. Abruña, Underpotential deposition at single crystal surfaces of Au, Pt, Ag and other materials, Chem. Rev., 2001, 101, 1897–1930 CrossRef CAS PubMed.
- D. M. Kolb, M. Przasnyski and H. Gerischer, Underpotential deposition of metals and work function differences, J. Electroanal. Chem. Interfacial Electrochem., 1974, 54, 25–38 CrossRef CAS.
- C. Cai, X. Ma, Y. Li, R. Zhou, C. An and J. Li, Investigation of copper underpotential deposition on polycrystalline gold electrodes, Int. J. Electrochem. Sci., 2023, 18, 100317 CrossRef CAS.
- G. A. Ragoisha, Y. M. Aniskevich, A. S. Bakavets and E. A. Streltsov, Electrochemistry of metal adlayers on metal chalcogenides, J. Solid State Electrochem., 2020, 24, 2585–2594 CrossRef CAS.
- C. Jin and I. Taniguchi, Electrocatalytic activity of silver modified gold film for glucose oxidation and its potential application to fuel cells, Mater. Lett., 2007, 61, 2365–2367 CrossRef CAS.
- A. F. Meese, C. Napier, D. J. Kim, K. Rigby, T. Hedtke, D. Leshchev, E. Stavitski, L. R. Parent and J. Kim, Underpotential deposition of 3D transition metals: Versatile electrosynthesis of single-atom catalysts on oxidized carbon supports, Adv. Mater., 2024, 36, 2311341 CrossRef CAS PubMed.
- M.-H. Hsieh and C. Chen, Scanning tunneling microscopy observations of butanethiol self-assembled monolayers on Ag underpotential deposition modified Au(111), Langmuir, 2000, 16, 1729–1733 CrossRef CAS.
- G. K. Jennings and P. E. Laibinis, Self-assembled n-alkanethiolate monolayers on underpotentially deposited adlayers of silver and copper on gold, J. Am. Chem. Soc., 1997, 119, 5208–5214 CrossRef CAS.
- G. K. Jennings and P. E. Laibinis, Underpotentially deposited metal layers of silver provide enhanced stability to self-assembled alkanethiol monolayers on gold, Langmuir, 1996, 12, 6173–6175 CrossRef CAS.
- S.-Y. Lin, T.-K. Tsai, C.-M. Lin, C. Chen, Y.-C. Chan and H.-W. Chen, Structures of self-assembled monolayers of n-alkanoic acids on gold surfaces modified by underpotential deposition of silver and copper: Odd−even effect, Langmuir, 2002, 18, 5473–5478 CrossRef CAS.
- H. Aitchison, H. Lu, S. W. L. Hogan, H. Früchtl, I. Cebula, M. Zharnikov and M. Buck, Self-assembled monolayers of oligophenylenecarboxylic acids on silver formed at the liquid–solid interface, Langmuir, 2016, 32, 9397–9409 CrossRef CAS PubMed.
- M. DeLeon and S. Baldelli, Electroreductive desorption of alkanethiols on gold and UPD copper/gold surfaces using in situ second harmonic generation, J. Electrochem. Soc., 2020, 167, 166519 CrossRef CAS.
- R. Ortiz de la Morena, A. Asyuda, H. Lu, H. Aitchison, K. Turner, S. M. Francis, M. Zharnikov and M. Buck, Shape controlled assembly of carboxylic acids: Formation of a binary monolayer by intercalation into molecular nanotunnels, Phys. Chem. Chem. Phys., 2020, 22, 4205–4215 RSC.
- Z. Wang, J. Chen, S. Oyola-Reynoso and M. Thuo, The Porter-Whitesides discrepancy: Revisiting odd-even effects in wetting properties of n-alkanethiolate SAMs, Coatings, 2015, 5, 1034–1055 CrossRef CAS.
- J. Wang, V. Gadenne, L. Patrone and J.-M. Raimundo, Self-assembled monolayers of push–pull chromophores as active layers and their applications, Molecules, 2024, 29, 559 CrossRef CAS.
- H. J. Lee, A. C. Jamison and T. R. Lee, Surface dipoles: A growing body of evidence supports their impact and importance, Acc. Chem. Res., 2015, 48, 3007–3015 CrossRef CAS PubMed.
- E. Zojer, A. Terfort and M. Zharnikov, Concept of embedded dipoles as a versatile tool for surface engineering, Acc. Chem. Res., 2022, 55, 1857–1867 CrossRef CAS PubMed.
- M. Li, Y. Cao, K. Xie and J. Tang, Molecular engineering on kinetics-driven self-assembled monolayers working as auxiliary layers on dielectrics in organic field-effect transistors, Adv. Electron. Mater., 2024, 10, 2300712 CrossRef CAS.
- H. Ren, M. Ouyang, J. Wang, L. Zhang and Y. Fu, Direct self-assembled monolayers treatment on the polar polymer dielectric towards ultra-flexible organic field-effect transistors, Mater. Lett., 2022, 324, 132495 CrossRef CAS.
- W. Li, E. Martínez-Ferrero and E. Palomares, Self-assembled molecules as selective contacts for efficient and stable perovskite solar cells, Mater. Chem. Front., 2024, 8, 681–699 RSC.
- W. Jiang, M. Liu, Y. Li, F. R. Lin and A. K.-Y. Jen, Rational molecular design of multifunctional self-assembled monolayers for efficient hole selection and buried interface passivation in inverted perovskite solar cells, Chem. Sci., 2024, 15, 2778–2785 RSC.
- J. Koc, L. Schardt, K. Nolte, C. Beyer, T. Eckhard, P. Schwiderowski, J. L. Clarke, J. A. Finlay, A. S. Clare, M. Muhler, A. Laschewsky and A. Rosenhahn, Effect of dipole orientation in mixed, charge-equilibrated self-assembled monolayers on protein adsorption and marine biofouling, ACS Appl. Mater. Interfaces, 2020, 12, 50953–50961 CrossRef CAS.
- D. Blasi, L. Sarcina, A. Tricase, A. Stefanachi, F. Leonetti, D. Alberga, G. F. Mangiatordi, K. Manoli, G. Scamarcio, R. A. Picca and L. Torsi, Enhancing the sensitivity of biotinylated surfaces by tailoring the design of the mixed self-assembled monolayer synthesis, ACS Omega, 2020, 5, 16762–16771 CrossRef CAS PubMed.
- M. Singh, N. Kaur and E. Comini, The role of self-assembled monolayers in electronic devices, J. Mater. Chem. C, 2020, 8, 3938–3955 RSC.
- D. Zhang, C. Li, G. Zhang, J. Tian and Z. Liu, Phototunable and photopatternable polymer semiconductors, Acc. Chem. Res., 2024, 57, 625–635 CAS.
- L. Wang, U. S. Schubert and S. Hoeppener, Surface chemical reactions on self-assembled silane-based monolayers, Chem. Soc. Rev., 2021, 50, 6507–6540 RSC.
- A. Batdelger, S.-G. Lee and S.-G. Park, The effect of terminal group of hole injection self-assembled monolayers on the performance of optoelectronic devices on ITO anodes, Mater. Chem. Phys., 2023, 301, 127666 CrossRef CAS.
- E. Arkan, E. Yalcin, M. Unal, M. Z. Y. Arkan, M. Can, C. Tozlu and S. Demic, Effect of functional groups of self-assembled monolayer molecules on the performance of inverted perovskite solar cell, Mater. Chem. Phys., 2020, 254, 123435 CrossRef CAS.
- D. Rodriguez, M. D. Marquez, O. Zenasni, L. T. Han, S. Baldelli and T. R. Lee, Surface dipoles induce uniform orientation in contacting polar liquids, Chem. Mater., 2020, 32, 7832–7841 CrossRef CAS.
- C. Fischer, S. Das, Q. Zhang, Y. Liu, L. Weinhardt, D. O’Hagan, M. Zharnikov and A. Terfort, Lateral dipole moments induced by all-cis-pentafluorocyclohexyl groups cause unanticipated effects in self-assembled monolayers, Nano Res., 2023, 16, 11030–11041 CrossRef CAS.
- O. Zenasni, A. C. Jamison and T. R. Lee, The impact of fluorination on the structure and properties of self-assembled monolayer films, Soft Matter, 2013, 9, 6356 RSC.
- J. Li, W. Cao, J. Li and M. Ma, Fluorination to enhance superlubricity performance between self-assembled monolayer and graphite in water, J. Colloid Interface Sci., 2021, 596, 44–53 CrossRef CAS.
- C.-W. Chang, H.-H. Hsu, C.-S. Hsu and J.-T. Chen, Achieving area-selective atomic layer deposition with fluorinated self-assembled monolayers, J. Mater. Chem. C, 2021, 9, 14589–14595 RSC.
- R. Colorado and T. R. Lee, Physical organic probes of interfacial wettability reveal the importance of surface dipole effects, J. Phys. Org. Chem., 2000, 13, 796–807 CrossRef CAS.
- M. Graupe, T. Koini, H. I. Kim, N. Garg, Y. F. Miura, M. Takenaga, S. S. Perry and T. R. Lee, Wettability and friction of CF3-terminated monolayer films on gold, Mater. Res. Bull., 1999, 34, 447–453 CrossRef CAS.
- M. Graupe, M. Takenaga, T. Koini, R. Colorado and T. R. Lee, Oriented surface dipoles strongly influence interfacial wettabilities, J. Am. Chem. Soc., 1999, 121, 3222–3223 CrossRef CAS.
- R. Colorado and T. R. Lee, Wettabilities of self-assembled monolayers on gold generated from progressively fluorinated alkanethiols, Langmuir, 2003, 19, 3288–3296 CrossRef CAS.
- M. D. Marquez, O. Zenasni, D. Rodriguez, T. Yu, S. Sakunkaewkasem, F. Toro Figueira, A. Czader, S. Baldelli and T. R. Lee, Burying the inverted surface dipole: Self-assembled monolayers derived from alkyl-terminated partially fluorinated alkanethiols, Chem. Mater., 2020, 32, 953–968 CrossRef CAS.
- O. Zenasni, M. D. Marquez, A. C. Jamison, H. J. Lee, A. Czader and T. R. Lee, Inverted surface dipoles in fluorinated self-assembled monolayers, Chem. Mater., 2015, 27, 7433–7446 CrossRef CAS.
- C. D. Bain, E. B. Troughton, Y. T. Tao, J. Evall, G. M. Whitesides and R. G. Nuzzo, Formation of monolayer films by the spontaneous assembly of organic thiols from solution onto gold, J. Am. Chem. Soc., 1989, 111, 321–335 CrossRef CAS.
- M. Azhagurajan, T. Itoh and K. Itaya, Ultra-high-resolution differential interference microscopy of Ag deposition on an ultraflat Au(111), J. Phys. Chem., 2016, 120, 16221–16227 CAS.
- L. Srisombat, A. C. Jamison and T. R. Lee, Stability: A key issue for self-assembled monolayers on gold as thin-film coatings and nanoparticle protectants, Colloids Surf., A, 2011, 390, 1–19 CrossRef CAS.
- C. Vericat, M. E. Vela and R. C. Salvarezza, Self-assembled monolayers of alkanethiols on Au(111): surface structures, defects and dynamics, Phys. Chem. Chem. Phys., 2005, 7, 3258–3268 RSC.
- M. D. Porter, T. B. Bright, D. L. Allara and C. E. D. Chidsey, Spontaneously organized molecular assemblies. 4. structural characterization of n-alkyl thiol monolayers on gold by optical ellipsometry, infrared spectroscopy, and electrochemistry, J. Am. Chem. Soc., 1987, 109, 3559–3568 CrossRef CAS.
- C. D. Bain and G. M. Whitesides, Attenuation lengths of photoelectrons in hydrocarbon films, J. Phys. Chem., 1989, 93, 1670–1673 CrossRef CAS.
- C. Du, R. S. Andino, M. C. Rotondaro, S. W. Devlin, S. Erramilli, L. D. Ziegler and M. M. Thuo, Substrate roughness and tilt angle dependence of sum-frequency generation odd–even effects in self-assembled monolayers, J. Phys. Chem., 2022, 126, 7294–7306 CAS.
- F. Tao and S. L. Bernasek, Understanding odd−even effects in organic self-assembled monolayers, Chem. Rev., 2007, 107, 1408–1453 CrossRef CAS.
- I.-W. P. Chen, C.-C. Chen, S.-Y. Lin and C. Chen, Effect of underpotentially deposited adlayers on sulfur bonding schemes of organothiols self-assembled on polycrystalline gold: sp or sp3 hybridization, J. Phys. Chem. B, 2004, 108, 17497–17504 CrossRef CAS.
- P. E. Laibinis, G. M. Whitesides, D. L. Allara, Y. T. Tao, A. N. Parikh and R. G. Nuzzo, Comparison of the structures and wetting properties of self-assembled monolayers of n-alkanethiols on the coinage metal surfaces, copper, silver, and gold, J. Am. Chem. Soc., 1991, 113, 2370–7167 CrossRef.
- D. G. Castner, K. Hinds and D. W. Grainger, X-ray photoelectron spectroscopy sulfur 2p study of organic thiol and disulfide binding interactions with gold surfaces, Langmuir, 1996, 12, 5083–5086 CrossRef CAS.
- J. C. Vickerman and I. S. Gilmore, Surface analysis: The principal techniques, Wiley, 2009 Search PubMed.
- K. Heister, L. S. O. Johansson, M. Grunze and M. Zharnikov, A detailed analysis of the C 1s photoemission of n-alkanethiolate films on noble metal substrates, Surf. Sci., 2003, 529, 36–46 CrossRef CAS.
- J. Jia, A. Giglia, M. Flores, O. Grizzi, L. Pasquali and V. A. Esaulov, 1,4-Benzenedimethanethiol interaction with Au(110), Ag(111), Cu(100), and Cu(111) surfaces: Self-assembly and dissociation processes, J. Phys. Chem. C, 2014, 118, 26866–26876 CrossRef CAS.
- N. J. Brewer, T. T. Foster, G. J. Leggett, M. R. Alexander and E. McAlpine, Comparative investigations of the packing and ambient stability of self-assembled monolayers of alkanethiols on gold and silver by friction force microscopy, J. Phys. Chem. B, 2004, 108, 4723–4728 CrossRef CAS.
- R. A. MacPhail, H. L. Strauss, R. G. Snyder and C. A. Elliger, Carbon-hydrogen stretching modes and the structure of n-alkyl chains. 2. Long, all-trans chains, J. Phys. Chem., 1984, 88, 334–341 CrossRef CAS.
- R. G. Snyder, H. L. Strauss and C. A. Elliger, Carbon-hydrogen stretching modes and the structure of n-alkyl chains. 1. Long, disordered chains, J. Phys. Chem., 1982, 86, 5145–5150 CrossRef CAS.
- J. Pflaum, G. Bracco, F. Schreiber, R. Colorado, O. E. Shmakova, T. R. Lee, G. Scoles and A. Kahn, Structure and electronic properties of CH3- and CF3-terminated alkanethiol monolayers on Au(111): A scanning tunneling microscopy, surface X-ray and helium scattering study, Surf. Sci., 2002, 498, 89–104 CrossRef CAS.
- S. Frey, K. Heister, M. Zharnikov, M. Grunze, K. Tamada, R. Colorado, M. Graupe, O. E. Shmakova and T. R. Lee, Structure of self-assembled monolayers of semifluorinated alkanethiols on gold and silver substrates, Isr. J. Chem., 2000, 40, 81–97 CrossRef CAS.
- S. Lee, A. Puck, M. Graupe, R. ColoradoJr, Y.-S. Shon, T. R. Lee and S. S. Perry, Structure, wettability, and frictional properties of phenyl-terminated SAMs, Langmuir, 2001, 17, 7364–7370 CrossRef CAS.
- F. Chesneau, B. Schüpbach, K. Szelągowska-Kunstman, N. Ballav, P. Cyganik, A. Terfort and M. Zharnikov, Self-assembled monolayers of perfluoroterphenyl-substituted alkanethiols: specific characteristics and odd–even effects, Phys. Chem. Chem. Phys., 2010, 12, 12123–12137 RSC.
- J. Dauselt, J. Zhao, M. Kind, R. Binder, A. Bashir, A. Terfort and M. Zharnikov, Compensation of the odd−even effects in araliphatic self-assembled monolayers by nonsymmetric attachment of the aromatic part, J. Phys. Chem. C, 2011, 115, 2841–2854 CrossRef CAS.
- W. Azzam, A. Subaihi, M. Rohwerder, A. Bashir, A. Terfort and M. Zharnikov, Odd–even effects in aryl-substituted alkanethiolate SAMs: Nonsymmetrical attachment of aryl unit and its impact on the SAM structure, Phys. Chem. Chem. Phys., 2024, 26, 7563–7572 RSC.
- J. F. Rabolt, T. P. Russell and R. J. Twieg, Structural studies of semifluorinated n-alkanes. 1. Synthesis and characterization of F(CF2)n(CH2)mH in the solid state, Macromolecules, 1984, 17, 2786–2794 CrossRef CAS.
- J. C. S. Costa, A. Mendes and L. M. N. B. F. Santos, Chain length dependence of the thermodynamic properties of n-alkanes and their monosubstituted derivatives, J. Chem. Eng. Data, 2018, 63, 1–20 CrossRef CAS.
- K. Yang, Z. Cai, A. Jaiswal, M. Tyagi, J. S. Moore and Y. Zhang, Dynamic odd–even effect in liquid n-alkanes near their melting points, Angew. Chem., Int. Ed., 2016, 55, 14090–14095 CrossRef CAS PubMed.
- H. Cabrera-Tinoco, A. C. L. Moreira, R. Valencia-Bedregal, L. Borja-Castro, A. Perez-Carreño, A. Lalupu-García, C. Mendoza-Alejo, C. H. W. Barnes, J. W. Seo and L. D. L. S. Valladares, Effective coupling model to treat the odd−even effect on the current−voltage response of saturated linear carbon chains single-molecule junctions, ACS Omega, 2024, 9, 35323–35331 CrossRef CAS.
- F. M. Fowkes, F. L. Riddle, W. E. Pastore and A. A. Weber, Interfacial interactions between self-associated polar liquids and squalane used to test equations for solid—liquid interfacial interactions, Colloids Surf., 1990, 43, 367–387 CrossRef CAS.
- I. Mc. N. Smallwood, Handbook of organic solvent properties, Arnold, 1996 Search PubMed.
- B. Jańczuk and T. Białlopiotrowicz, Surface free-energy components of liquids and low energy solids and contact angles, J. Colloid Interface Sci., 1989, 127, 189–204 CrossRef.
- D. Barriet, P. Chinwangso and T. R. Lee, Can cyclopropyl-terminated self-assembled monolayers on gold be used to mimic the surface of polyethylene?, ACS Appl. Mater. Interfaces, 2010, 2, 1254–1265 CrossRef CAS PubMed.
- L. Ramin and A. Jabbarzadeh, Odd–even effects on the structure, stability, and phase transition of alkanethiol self-assembled monolayers, Langmuir, 2011, 27, 9748–9759 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qm00541d |
| This journal is © the Partner Organisations 2025 |




