Unveiling thermal treatment effects on the thermomechanical and IR optical properties of chalcogenide hybrid inorganic/organic polymers†
Wonmoo
Byun‡
ab,
Jae Hyuk
Hwang‡
 a,
Jiseok
Han
ac,
Juntae
Joo
ac,
Sangmin
Park
ad,
Woohwa
Lee
a,
Hyun
Kim
a,
Jiseok
Han
ac,
Juntae
Joo
ac,
Sangmin
Park
ad,
Woohwa
Lee
a,
Hyun
Kim
 ad,
Chang-Geun
Chae
ad,
Sungmin
Park
ad,
Chang-Geun
Chae
ad,
Sungmin
Park
 *ad and
Dong-Gyun
Kim
*ad and
Dong-Gyun
Kim
 *ad
*ad
aAdvanced Materials Division, Korea Research Institute of Chemical Technology, 141 Gajeong-ro, Yuseong-gu, Daejeon 34114, Republic of Korea. E-mail: parks@krict.re.kr; dgkim@krict.re.kr
bDepartment of Chemical and Biological Engineering, Korea University, 145 Anam-ro, Seongbuk-gu, Seoul 02841, Republic of Korea
cDepartment of Materials Science and Engineering, Korea University, 145 Anam-ro, Seongbuk-gu, Seoul, 02841, Republic of Korea
dAdvanced Materials and Chemical Engineering, KRICT School, University of Science and Technology, 217 Gajeong-ro, Yuseong-gu, Daejeon 34114, Republic of Korea
First published on 4th December 2024
Abstract
We report the influence of post-thermal treatment on the thermomechanical and infrared (IR) optical properties of chalcogenide hybrid inorganic/organic polymers. Using 20 wt% of the tricyclopentadiene (TCPD) crosslinker, elemental sulfur was inverse vulcanized into as-synthesized poly(sulfur80-random-TCPD20) (S80T20) and then thermally treated under different conditions. 140 °C for 12 h was found to be optimal for improving both the thermomechanical and IR optical properties. It is due to the increase in crosslinking density after the reduction of unreacted ES and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in the crosslinker, while thermal degradation and oxidation were controlled. Glass transition temperature, storage modulus (at 25 °C), and mid-IR transmittance (1 mm-thick) values of S80T20 increased from 6.5 to 29.2 °C, 1.5 to 2.0 GPa, and 38.5 to 41.2%, respectively. Such a strategy could also be applied to S/Se chalcogen mixture-based CHIPs, endowing them with potential for IR optical applications.
C bonds in the crosslinker, while thermal degradation and oxidation were controlled. Glass transition temperature, storage modulus (at 25 °C), and mid-IR transmittance (1 mm-thick) values of S80T20 increased from 6.5 to 29.2 °C, 1.5 to 2.0 GPa, and 38.5 to 41.2%, respectively. Such a strategy could also be applied to S/Se chalcogen mixture-based CHIPs, endowing them with potential for IR optical applications.
1. Introduction
Infrared (IR) imaging is a crucial technology for surveillance,1 medicine,2 and autonomous vehicles.3 For designing high performance IR optical devices, materials with high IR transparency and refractive index (n) are essential.4 Inorganic materials, such as germanium and chalcogenide glasses, have been utilized as traditional IR optical materials due to their high refractive index (n > 2) and excellent IR transparency.4 However, these materials are toxic, brittle, and difficult to process, thus leading to high manufacturing costs. On the other hand, replacing them with low-cost polymeric materials still remains a challenge due to high IR absorption and limited n values (1.6–1.8), originating from hydrocarbon-rich chains and low molar refractions, respectively.5Since Pyun et al. reported a seminal research study on inverse vulcanization of elemental sulfur (ES) in 2013,6 various chalcogenide hybrid inorganic/organic polymers (CHIPs) for IR optical applications have been reported.7,8 With the abundant sulfur content (>50 wt%), CHIPs show high IR transparency and refractive index (n).9–12 Among the chalcogen elements, selenium (Se), which reacts well with molten sulfur, has also been incorporated into sulfur-based CHIPs to improve the refractive indices.11,13 Moreover, through the dynamic polysulfide bond exchange reaction,10 CHIPs possess self-healable and reprocessable properties, which cannot be achieved in conventional inorganic transmissive materials.7 The thermomechanical and IR optical properties of CHIPs can be appropriately tuned by controlling the content of sulfur and crosslinkers; however, the trade-off relationship between such properties due to different sulfur contents restricts the replacement of conventional inorganic materials with CHIPs.14–16 When the sulfur content in CHIPs increases, IR transmittance can be improved, but a corresponding decrease in the crosslinker content results in a lower Tg value. To overcome such a trade-off relationship, studies have been conducted to improve the thermomechanical properties of CHIPs by adjusting the chemical structure of the crosslinker. To improve IR transmittance while maintaining good thermomechanical properties, molecularly designed norbornadiene-dimer (NBD2) crosslinker-based CHIPs with 70 wt% sulfur content have been reported.17 Meanwhile, the formation of self-crosslinked domains between 1,3,5-trivinylbenzene crosslinkers in CHIPs was found to improve thermomechanical properties even at a high sulfur content (>70 wt%).18 Moreover, a simultaneous improvement of the thermomechanical and IR optical properties by using an IR inactive and highly crosslinkable symmetric 1,3,5-benzenetrithiol crosslinker has been reported.12
Thermal treatment is known as a common technique to enhance the thermomechanical properties of polymers,19 and can also be applied to CHIPs.10,20 Basically, products that are immediately vitrified after inverse vulcanization may not completely react with CHIPs. Residual ES may be kinetically trapped within the vitrified products or unreacted C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds may also exist possibly due to the differences in the reactivity of asymmetric crosslinkers.21 The thermal treatment enables the scission of polysulfide chains and recombination of the generated sulfur radicals, resulting in the structural rearrangement of CHIPs.22 Therefore, studies have been reported on changes in the mechanical and thermomechanical properties of vitrified products following thermal treatment. Pyun et al. investigated the mechanical and thermomechanical properties of CHIPs by thermally treating unreacted sulfur-containing prepolymers in a glass vial or a polydimethylsiloxane (PDMS) mold, confirming that mechanical properties were improved when cured in the PDMS mold.10 By reacting residual vinyl groups of divinylbenzene (DVB), Lim et al. improved the thermal properties of poly(S-r-DVB) via thermal treatment at 120 °C.14 Recently, Wie et al. conducted a systematic investigation under various post-thermal treatment conditions to enhance the thermomechanical properties of CHIPs.23 However, previous studies have been limited to focusing only on changes in the mechanical, thermal, and thermomechanical properties of CHIPs after thermal treatment. Practical IR optical applications of CHIPs still require a more systematic and in-depth investigation of the effect of post-thermal treatment conditions on both the IR optical and thermomechanical properties of CHIPs.
C bonds may also exist possibly due to the differences in the reactivity of asymmetric crosslinkers.21 The thermal treatment enables the scission of polysulfide chains and recombination of the generated sulfur radicals, resulting in the structural rearrangement of CHIPs.22 Therefore, studies have been reported on changes in the mechanical and thermomechanical properties of vitrified products following thermal treatment. Pyun et al. investigated the mechanical and thermomechanical properties of CHIPs by thermally treating unreacted sulfur-containing prepolymers in a glass vial or a polydimethylsiloxane (PDMS) mold, confirming that mechanical properties were improved when cured in the PDMS mold.10 By reacting residual vinyl groups of divinylbenzene (DVB), Lim et al. improved the thermal properties of poly(S-r-DVB) via thermal treatment at 120 °C.14 Recently, Wie et al. conducted a systematic investigation under various post-thermal treatment conditions to enhance the thermomechanical properties of CHIPs.23 However, previous studies have been limited to focusing only on changes in the mechanical, thermal, and thermomechanical properties of CHIPs after thermal treatment. Practical IR optical applications of CHIPs still require a more systematic and in-depth investigation of the effect of post-thermal treatment conditions on both the IR optical and thermomechanical properties of CHIPs.
Herein, we report a systematic thermal treatment strategy to control both the thermomechanical and IR optical properties of CHIPs. Tricyclopentadiene (TCPD), with a rigid multi-cyclic olefin structure containing relatively fewer C–H bonds compared to typical hydrocarbon materials,17,21 was used as a crosslinker to enhance the thermomechanical properties and IR transmittance of inverse vulcanized CHIPs. To verify the property tunability of CHIPs using a controlled thermal treatment strategy, poly(sulfur-random-tricyclopentadiene) (poly(S-r-TCPD)) copolymers were firstly synthesized (Fig. 1a). As-synthesized poly(S-r-TCPD) was thermally treated at temperatures of 110, 140, and 170 °C for 3, 6, 12, 24, and 48 h, respectively (Fig. 1b), and the trends of thermomechanical and IR optical properties were investigated. To confirm whether such a thermal treatment strategy also applies to CHIPs containing S/Se chalcogen mixtures, we synthesized poly(sulfur-random-selenium-random-tricyclopentadiene) poly(S-r-Se-r-TCPD)s with different S/Se contents. These CHIPs exhibited similar trends in the thermomechanical and IR optical properties compared to poly(S-r-TCPD) depending on the thermal treatment conditions. The thermal treatment strategy reported in this study can provide valuable guidelines for the preparation of CHIPs for IR optical applications.
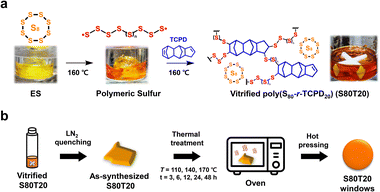 | ||
| Fig. 1 Schematic illustrations of (a) inverse vulcanization of ES to S80T20 using the TCPD crosslinker and (b) post-processing of the vitrified S80T20. | ||
2. Results and discussion
2.1. Thermomechanical properties of S80T20 under different thermal treatment conditions
As shown in Fig. 1a, ES was inverse vulcanized by using a low-cost commercial TCPD crosslinker. Since a sulfur content lower than 80 wt% reduces the IR optical properties and a sulfur content higher than 80 wt% significantly reduces the thermomechanical properties,6,18 we fixed the sulfur and TCPD contents as 80 and 20 wt% (96 and 4 mol%), respectively. ES was reacted with TCPD through solvent-free inverse vulcanization at 160 °C referring the dicyclopentadiene-based reaction,21,24 and the vitrified poly(S80-r-TCPD20) (S80T20) was obtained after 20 min (Fig. S1, ESI†). To verify the reaction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in TCPD, we conducted Fourier transform infrared (FT-IR) analysis. The norbornene C
C bonds in TCPD, we conducted Fourier transform infrared (FT-IR) analysis. The norbornene C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond peak at 1567 cm−1 in TCPD disappears while the cyclopentene C
C bond peak at 1567 cm−1 in TCPD disappears while the cyclopentene C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond peak at 1609 cm−1 remains (Fig. S2, ESI†).21 In addition, through differential scanning calorimetry (DSC) analysis, the melting peak of residual ES is clearly observed (Fig. S3, ESI†). Immediately after vitrification, a certain amount of unreacted sulfur is trapped due to increased viscosity during the inverse vulcanization reaction.14 Thus, additional thermal treatment is required for a complete reaction between the cyclopentene C
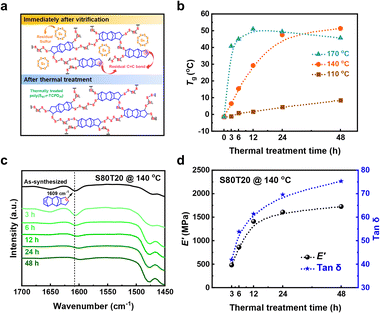
C bond peak at 1609 cm−1 remains (Fig. S2, ESI†).21 In addition, through differential scanning calorimetry (DSC) analysis, the melting peak of residual ES is clearly observed (Fig. S3, ESI†). Immediately after vitrification, a certain amount of unreacted sulfur is trapped due to increased viscosity during the inverse vulcanization reaction.14 Thus, additional thermal treatment is required for a complete reaction between the cyclopentene C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and residual sulfur. We applied post-thermal treatment on the vitrified S80T20 at different temperatures and monitored the changes in Tg values over 3, 6, 12, 24, and 48 h, respectively. As the structural rearrangement of the CHIPs through dynamic polysulfide bond exchanges activates above 100 °C,10 we conducted thermal treatment at 110 and 170 °C, as well as a general curing temperature of 140 °C. When the vitrified samples inside the glass vial were thermally treated directly, auto-acceleration occurred due to the Trommsdorff–Norrish effect (Fig. S4, ESI†).21 Therefore, vitrified samples were quenched in liquid nitrogen (LN2) and then the chunk samples were thermally treated in an oven (Fig. 1b).
C bond and residual sulfur. We applied post-thermal treatment on the vitrified S80T20 at different temperatures and monitored the changes in Tg values over 3, 6, 12, 24, and 48 h, respectively. As the structural rearrangement of the CHIPs through dynamic polysulfide bond exchanges activates above 100 °C,10 we conducted thermal treatment at 110 and 170 °C, as well as a general curing temperature of 140 °C. When the vitrified samples inside the glass vial were thermally treated directly, auto-acceleration occurred due to the Trommsdorff–Norrish effect (Fig. S4, ESI†).21 Therefore, vitrified samples were quenched in liquid nitrogen (LN2) and then the chunk samples were thermally treated in an oven (Fig. 1b).
Fig. 2a represents the schematic chemical structures of S80T20 immediately after vitrification and after thermal treatment. As shown in Fig. 2b, the Tg values of S80T20 gradually increase with the thermal treatment time at 110 °C, facilitating additional crosslinking of the cyclopentene C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and residual sulfur. However, the melting peak of ES is still observed for S80T20 even after the 24 h of thermal treatment (Fig. S5, ESI†), suggesting that 110 °C is relatively low to achieve sufficient additional crosslinking. Meanwhile, residual sulfur is not observed after 3 h of thermal treatment at 140 and 170 °C (Fig. S6 and S7, ESI†). These results are consistent with the lower gel fraction values of the samples thermally treated at 110 °C compared to those of the samples thermally treated at 140 and 170 °C (Table S1, ESI†). The Tg values of each sample gradually increase during the first 24 h of thermal treatment at 140 °C, reaching a saturation point thereafter (Fig. 2b). The mortar-milled powdered S80T20 sample was analyzed using FT-IR in ATR mode. In the FT-IR spectra, the C
C bond and residual sulfur. However, the melting peak of ES is still observed for S80T20 even after the 24 h of thermal treatment (Fig. S5, ESI†), suggesting that 110 °C is relatively low to achieve sufficient additional crosslinking. Meanwhile, residual sulfur is not observed after 3 h of thermal treatment at 140 and 170 °C (Fig. S6 and S7, ESI†). These results are consistent with the lower gel fraction values of the samples thermally treated at 110 °C compared to those of the samples thermally treated at 140 and 170 °C (Table S1, ESI†). The Tg values of each sample gradually increase during the first 24 h of thermal treatment at 140 °C, reaching a saturation point thereafter (Fig. 2b). The mortar-milled powdered S80T20 sample was analyzed using FT-IR in ATR mode. In the FT-IR spectra, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond peak of the cyclopentene moiety of TCPD disappears after a thermal treatment time of 24 h at 140 °C (Fig. 2c). Solid-state NMR analysis and reductive degradation methods are commonly employed for precise structural characterization. Accordingly, we conducted solid-state 13C cross-polarization magic-angle spinning solid-state nuclear magnetic resonance spectroscopy (13C CP-MAS NMR) analysis for the structural characterization of CHIPs.25–27 As shown in Fig. S8 (ESI†), C–S and C–C bond peaks of S80T20 are observed. Meanwhile, the C
C bond peak of the cyclopentene moiety of TCPD disappears after a thermal treatment time of 24 h at 140 °C (Fig. 2c). Solid-state NMR analysis and reductive degradation methods are commonly employed for precise structural characterization. Accordingly, we conducted solid-state 13C cross-polarization magic-angle spinning solid-state nuclear magnetic resonance spectroscopy (13C CP-MAS NMR) analysis for the structural characterization of CHIPs.25–27 As shown in Fig. S8 (ESI†), C–S and C–C bond peaks of S80T20 are observed. Meanwhile, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C peaks gradually decrease with increasing thermal treatment time, disappearing entirely after 24 h, consistent with the results from the IR analysis. From the DMA, storage modulus (E′) at 25 °C and Tg values from the tan
C peaks gradually decrease with increasing thermal treatment time, disappearing entirely after 24 h, consistent with the results from the IR analysis. From the DMA, storage modulus (E′) at 25 °C and Tg values from the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ maximum peak also increase with thermal treatment time, followed by saturation after 24 h (Fig. 2d and Fig. S9, S10, ESI†). It is inferred that the dominant influence of the increased Tg upon thermal treatment is the increased crosslinking density due to the decrease in unreacted sulfur and C
δ maximum peak also increase with thermal treatment time, followed by saturation after 24 h (Fig. 2d and Fig. S9, S10, ESI†). It is inferred that the dominant influence of the increased Tg upon thermal treatment is the increased crosslinking density due to the decrease in unreacted sulfur and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds of the TCPD crosslinker (Table S2, ESI†). In contrast, for the samples thermally treated at 170 °C, the Tg values start to decrease after 12 h (Fig. 2b and Fig. S11, ESI†). From thermogravimetric analysis (TGA), the weight loss due to the thermal degradation of S80T20 at 170 °C is 0.21%, whereas the weight loss at 140 °C is 0.02% (Fig. S12, ESI†). Thus, we assumed that the harsh temperature of 170 °C accelerates the cumulative thermal degradation and leads to an increase in the free volume of S80T20, followed by the decreased Tg.28 From the elemental analysis (EA) of thermally treated S80T20, the sulfur content is found to be steeply declined when thermally treated at 170 °C, whereas the sample thermally treated at 140 °C shows only a slight decrease in the sulfur content over time (Table S3, S4, and Fig. S13, ESI†). Hence, we determined that a thermal treatment temperature of 140 °C is optimal for inducing gradual enhancement of the thermomechanical properties of CHIPs.
C bonds of the TCPD crosslinker (Table S2, ESI†). In contrast, for the samples thermally treated at 170 °C, the Tg values start to decrease after 12 h (Fig. 2b and Fig. S11, ESI†). From thermogravimetric analysis (TGA), the weight loss due to the thermal degradation of S80T20 at 170 °C is 0.21%, whereas the weight loss at 140 °C is 0.02% (Fig. S12, ESI†). Thus, we assumed that the harsh temperature of 170 °C accelerates the cumulative thermal degradation and leads to an increase in the free volume of S80T20, followed by the decreased Tg.28 From the elemental analysis (EA) of thermally treated S80T20, the sulfur content is found to be steeply declined when thermally treated at 170 °C, whereas the sample thermally treated at 140 °C shows only a slight decrease in the sulfur content over time (Table S3, S4, and Fig. S13, ESI†). Hence, we determined that a thermal treatment temperature of 140 °C is optimal for inducing gradual enhancement of the thermomechanical properties of CHIPs.
2.2. IR optical properties of S80T20 under various thermal treatment conditions
After analyzing the thermomechanical properties of S80T20, we investigated the influence of thermal treatment conditions on the IR optical properties. This time, we conducted the experiment by focusing on thermal treatments at 140 and 170 °C, because the thermal treatment at 110 °C was found to be insufficient to complete inverse vulcanization (Fig. S5, ESI†). Each sample was hot-pressed under 10 MPa for 20 minutes to fabricate 1 mm-thick windows at the same temperature as that used for the thermal treatment (Fig. S14 and S15, ESI†). As thermal treatment time increases, transparent windows become darker (Fig. 3a). The transmittance in the visible region decreases with thermal treatment time, particularly in the green wavelength range (Fig. S16, ESI†). This decrease in transmittance may be responsible for the gradual color change of the S80T20 window from yellow to dark red, which is attributed to thermal degradation and oxidation. The transmittance of each window in the mid-wavelength infrared region (MWIR, 3–5 μm) was measured through transmission mode of FT-IR, and the transmitted region was calculated by the integration (Fig. 3b). Overall, the windows thermally treated at 140 °C exhibit higher IR transmittance compared to those thermally treated at 170 °C. For the samples thermally treated at 140 °C, IR transmittance gradually increases with thermal treatment time and then decreases after 12 h (Fig. S17 and Table S5, ESI†). However, IR transmittance of the windows thermally treated at 170 °C rapidly decreases over time (Fig. S18 and Table S6, ESI†). We attribute the decreasing IR transmittance to the acceleration of degradation and oxidation under prolonged thermal treatment at elevated temperatures. As the thermal treatment time increases, the oxygen-to-carbon ratio tends to increase due to the thermal degradation and oxidation of S80T20 (Fig. S19, ESI†). The refractive indices depending on the thermal treatment time also show a similar trend to the IR transmittance (Fig. 3c, Fig. S20 and Table S7, S8, ESI†). The molar refractivity improves because of the increased crosslinking density after 12 h at 140 °C; however, at 170 °C or after 24 h at 140 °C, the molar refractivity decreases owing to thermal degradation and oxidation.29 Considering the successive decrement in the IR optical properties with thermal degradation and oxidation at 170 °C, which is consistent with that after 12 h at 140 °C, we concluded that a thermal treatment condition of 12 h at 140 °C is optimal, which is also the point at which the increase in thermomechanical properties becomes slight.2.3. Applicable systematic thermal treatment of S/Se chalcogen mixture-based CHIPs
Finally, we verified whether such a post-thermal treatment strategy is also applicable to mixed chalcogen-based CHIPs. Mixed S/Se chalcogens (a S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se ratio of up to 70
Se ratio of up to 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
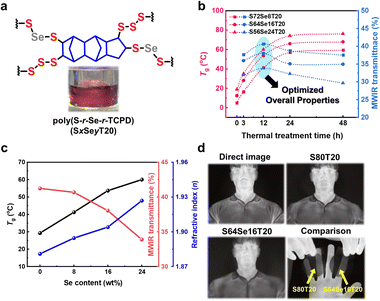
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) were inverse vulcanized using 20 wt% of TCPD to obtain poly(Sx-r-Sey-r-TCPD20)s (SxSeyT20)s, where x, y, and 20 indicate the average weight percentage (wt%) of S, Se, and TCPD, respectively (Fig. 4a, Fig. S21–23, and Table S9, ESI†). Se, which exhibits a high refractive index (n ∼ 2.74), can form an S–Se bond by reacting with ring-opened sulfur radicals in its gray selenium (hexagonal Se) precursor state.11,30 From the FT-IR spectra and DSC thermograms, the cyclopentene C
30) were inverse vulcanized using 20 wt% of TCPD to obtain poly(Sx-r-Sey-r-TCPD20)s (SxSeyT20)s, where x, y, and 20 indicate the average weight percentage (wt%) of S, Se, and TCPD, respectively (Fig. 4a, Fig. S21–23, and Table S9, ESI†). Se, which exhibits a high refractive index (n ∼ 2.74), can form an S–Se bond by reacting with ring-opened sulfur radicals in its gray selenium (hexagonal Se) precursor state.11,30 From the FT-IR spectra and DSC thermograms, the cyclopentene C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in TCPD and residual sulfur exist in SxSeyT20s immediately after vitrification the same as the S80T20 (Fig. S24 and S25, ESI†). We also monitored the changes in Tg values of SxSeyT20s under the same thermal treatment conditions as those of S80T20 (Fig. S26, ESI†). As shown in Fig. 4b, Tgs of SxSeyT20s increase with thermal treatment time at 140 °C. From the DMA analysis, E′ at 25 °C and the Tg measured by the maximum value of tan
C bond in TCPD and residual sulfur exist in SxSeyT20s immediately after vitrification the same as the S80T20 (Fig. S24 and S25, ESI†). We also monitored the changes in Tg values of SxSeyT20s under the same thermal treatment conditions as those of S80T20 (Fig. S26, ESI†). As shown in Fig. 4b, Tgs of SxSeyT20s increase with thermal treatment time at 140 °C. From the DMA analysis, E′ at 25 °C and the Tg measured by the maximum value of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ also increase gradually (Fig. S27 and Table S10, ESI†). However, the MWIR transmittance of SxSeyT20s begins to decrease after 12 h (Fig. 4b), which is consistent with the trend of declining IR optical properties observed in S80T20 due to thermal oxidation and degradation (Fig. S28 and Table S11, ESI†). Therefore, we confirmed that the thermomechanical and IR optical properties of SxSeyT20s can also be optimized when thermally treated at 140 °C for 12 h. The S–Se bonds are still observed in all samples after such thermal treatment (Fig. S29, ESI†).31 From the perspective of limitations in adopting crosslinkers and increasing the chalcogen content in the synthesis of CHIPs,32 it is believed that a well-established thermal treatment process can improve their overall performance for IR optical applications.
δ also increase gradually (Fig. S27 and Table S10, ESI†). However, the MWIR transmittance of SxSeyT20s begins to decrease after 12 h (Fig. 4b), which is consistent with the trend of declining IR optical properties observed in S80T20 due to thermal oxidation and degradation (Fig. S28 and Table S11, ESI†). Therefore, we confirmed that the thermomechanical and IR optical properties of SxSeyT20s can also be optimized when thermally treated at 140 °C for 12 h. The S–Se bonds are still observed in all samples after such thermal treatment (Fig. S29, ESI†).31 From the perspective of limitations in adopting crosslinkers and increasing the chalcogen content in the synthesis of CHIPs,32 it is believed that a well-established thermal treatment process can improve their overall performance for IR optical applications.
For SxSeyT20s thermally treated under the optimally tailored conditions, the Tg value is found to increase from 29.2 °C to 60.0 °C when the Se content increases from 0 to 24 wt% (Fig. 4c). Since the atomic weight of Se is approximately 2.46 times higher than that of sulfur, an increase in the Se content in SxSeyT20s results in shorter polysulfide/selenide chains, thus leading to an increase in Tg due to a higher crosslinking density. On the other hand, the MWIR transmittance of SxSeyT20 gradually decreases as the Se content increases (Fig. 4b). This is because the MWIR transmittance of selenide is lower than that of sulfide chains. Moreover, as the Se content increases, the mole ratio of overall chalcogen decreases, thereby increasing the unit volume of TCPD in SxSeyT20.32 Since the refractive index of Se (n ∼ 2.74) is 37% higher than that of sulfur (n ∼ 2.0),11,30 the refractive index of SxSeyT20s increases from 1.88 to 1.93 (at 829 nm) with the Se content from 0 to 24, regardless of the relatively lower mol % (17.8%) of overall chalcogens compared to that of S80T20 (Fig. 4c, Fig. S30, and Table S12, ESI†). To evaluate the thermal imaging performance of the CHIPs after the thermal treatment, we conducted an MWIR thermal imaging (Fig. 4d). IR thermal imaging experiments were performed by placing 1 mm-thick S80T20 and SxSeyT20s windows in front of the lens of an MWIR camera (3–5 μm) (Fig. S31 and S32, ESI†). Among these windows, the imaging resolution of the S64Se16T20 is not significantly different by from that of the S80T20 (Fig. S33, ESI†), but possesses improved Tg (from 29.2 °C to 53.6 °C) and refractive index values (from 1.88 to 1.90 at 829 nm), respectively (Fig. 4c).
3. Conclusions
In summary, we synthesized CHIPs through the inverse vulcanization of chalcogens (sulfur and selenium) with the TCPD crosslinker and investigated the influence of post-thermal treatment on their thermomechanical and IR optical properties. The as-synthesized vitrified products with unreacted sulfur and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds of TCPD were thermally treated under conditions of different temperatures and times. When thermally treated at a high temperature of 170 °C or over 12 h at 140 °C, the thermomechanical and IR optical properties of CHIPs are deteriorated due to accumulative thermal degradation and oxidation, attesting that the thermal treatment condition of 140 °C for 12 h is optimal. Moreover, upon thermal treatment, SxSeyT20s show the same behavior as S80T20, suggesting that such a thermal treatment strategy can also be applied to S/Se chalcogen mixture-based CHIPs. This study provides insight into improving the overall properties of CHIPs from a post-thermal treatment perspective for diverse applications, including IR optic lenses, composite materials, and reliable electronic devices.
C bonds of TCPD were thermally treated under conditions of different temperatures and times. When thermally treated at a high temperature of 170 °C or over 12 h at 140 °C, the thermomechanical and IR optical properties of CHIPs are deteriorated due to accumulative thermal degradation and oxidation, attesting that the thermal treatment condition of 140 °C for 12 h is optimal. Moreover, upon thermal treatment, SxSeyT20s show the same behavior as S80T20, suggesting that such a thermal treatment strategy can also be applied to S/Se chalcogen mixture-based CHIPs. This study provides insight into improving the overall properties of CHIPs from a post-thermal treatment perspective for diverse applications, including IR optic lenses, composite materials, and reliable electronic devices.
Author contributions
W. Byun: writing – original draft, visualization, methodology, and data curation. J. H. Hwang: writing – original draft, visualization, methodology, and investigation. J. Han: validation and investigation. J. Joo: validation and investigation. S. Park: validation and methodology. W. Lee: validation and methodology. H. Kim: methodology and investigation. C.-G. Chae: methodology and investigation. S. Park: writing – review and editing, supervision, and resources. D.-G. Kim: writing – review and editing, supervision, and project administration. All authors have given approval to the final version of the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts of interests to declare.Acknowledgements
This work was supported by the Nano Material Technology Development Program (2021M3H4A1A0304142622) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT, Ministry of Trade, Industry and Energy (20011153), and the Korea Research Institute of Chemical Technology (KRICT) core project (KS2421-20).Notes and references
- H.-J. Kwon and S.-H. Lee, Visible and near-infrared image acquisition and fusion for night surveillance, Chemosensors, 2021, 9, 75 CrossRef
.
- E. Ring and K. Ammer, Infrared thermal imaging in medicine, Phys. Meas., 2012, 33, 33 CrossRef PubMed
.
- B. Miethig, A. Liu, S. Habibi and M. V. Mohrenschildt, 2019 IEEE Transportation Electrification Conference and Expo (ITEC), 2019, 1–5.
-
M. Vollmer, Infrared thermal imaging. Computer Vision: A Reference Guide, Springer, 2020, 1–4 Search PubMed
.
- T. Higashihara and M. Ueda, Recent progress in high refractive index polymers, Macromolecules, 2015, 48, 1915–1929 CrossRef CAS
.
- W. J. Chung, J. J. Griebel, E. T. Kim, H. Yoon, A. G. Simmonds, H. J. Ji, P. T. Dirlam, R. S. Glass, J. J. Wie, N. A. Nguyen, B. W. Guralnick, J. Park, Á. Somogyi, P. Theato, M. E. Mackay, Y.-E. Sung, K. Char and J. Pyun, The use of elemental sulfur as an alternative feedstock for polymeric materials, Nat. Chem., 2013, 5, 518–524 CrossRef CAS PubMed
.
- T. S. Kleine, R. S. Glass, D. L. Lichtenberger, M. E. Mackay, K. Char, R. A. Norwood and J. Pyun, 100th Anniversary of Macromolecular Science Viewpoint: High Refractive Index Polymers from Elemental Sulfur for Infrared Thermal Imaging and Optics, ACS Macro Lett., 2020, 9, 245–259 CrossRef CAS PubMed
.
- J. Pyun and R. A. Norwood, Infrared plastic optics and photonic devices using chalcogenide hybrid inorganic/organic polymers via inverse vulcanization of elemental sulfur, Prog. Polym. Sci., 2024, 156, 101865 CrossRef CAS
.
- J. J. Griebel, S. Namnabat, E. T. Kim, R. Himmelhuber, D. H. Moronta, W. J. Chung, A. G. Simmonds, K.-J. Kim, J. Van Der Laan, N. A. Nguyen, E. L. Dereniak, M. E. Mackay, K. Char, R. S. Glass, R. A. Norwood and J. Pyun, New infrared transmitting material via inverse vulcanization of elemental sulfur to prepare high refractive index polymers, Adv. Mater., 2014, 26, 3014–3018 CrossRef CAS
.
- J. J. Griebel, N. A. Nguyen, S. Namnabat, L. E. Anderson, R. S. Glass, R. A. Norwood, M. E. Mackay, K. Char and J. Pyun, Dynamic covalent polymers via inverse vulcanization of elemental sulfur for healable infrared optical materials, ACS Macro Lett., 2015, 4, 862–866 CrossRef CAS PubMed
.
- L. E. Anderson, T. S. Kleine, Y. Zhang, D. D. Phan, S. Namnabat, E. A. LaVilla, K. M. Konopka, L. Ruiz Diaz, M. S. Manchester, J. Schwiegerling, R. S. Glass, M. E. Mackay, K. Char, R. A. Norwood and J. Pyun, Chalcogenide hybrid inorganic/organic polymers: Ultrahigh refractive index polymers for infrared imaging, ACS Macro Lett., 2017, 6, 500–504 CrossRef CAS PubMed
.
- M. Lee, Y. Oh, J. Yu, S. G. Jang, H. Yeo, J.-J. Park and N.-H. You, Long-wave infrared transparent sulfur polymers enabled by symmetric thiol cross-linker, Nat. Commun., 2023, 14, 2866 CrossRef CAS PubMed
.
- D. A. Boyd, C. C. Baker, J. D. Myers, V. Q. Nguyen, G. A. Drake, C. C. McClain, F. H. Kung, S. R. Bowman, W. Kim and J. S. Sanghera, ORMOCHALCs: organically modified chalcogenide polymers for infrared optics, Chem. Commun., 2017, 53, 259–262 RSC
.
- S. Park, D. Lee, H. Cho, J. Lim and K. Char, Inverse vulcanization polymers with enhanced thermal properties via divinylbenzene homopolymerization-assisted
cross-linking, ACS Macro Lett., 2019, 8, 1670–1675 CrossRef CAS PubMed
.
- D. A. Boyd, V. Q. Nguyen, C. C. McClain, F. H. Kung, C. C. Baker, J. D. Myers, M. P. Hunt, W. Kim and J. S. Sanghera, Optical properties of a sulfur-rich organically modified chalcogenide polymer synthesized via inverse vulcanization and containing an organometallic comonomer, ACS Macro Lett., 2019, 8, 113–116 CrossRef CAS
.
- K. W. Park, E. A. Tafili, F. Fan, Z. Zujovic and E. M. Leitao, Synthesis and characterization of polysulfides formed by the inverse vulcanisation of cyclosiloxanes with sulfur, Polym. Chem., 2022, 13, 4717–4726 RSC
.
- T. S. Kleine, T. Lee, K. J. Carothers, M. O. Hamilton, L. E. Anderson, L. Ruiz Diaz, N. P. Lyons, K. R. Coasey, W. O. ParkerJr, L. Borghi, M. E. Mackay, K. Char, R. S. Glass, D. L. Lichtenberger, R. A. Norwood and J. Pyun, Infrared fingerprint engineering: a molecular-design approach to long-wave infrared transparency with polymeric materials, Angew. Chem., Int. Ed., 2019, 58, 17656–17660 CrossRef CAS PubMed
.
- J. H. Hwang, S. H. Kim, W. Cho, W. Lee, S. Park, Y. S. Kim, J. C. Lee, K. J. Lee, J. J. Wie and D. G. Kim Adv, A Microphase Separation Strategy for the Infrared Transparency-Thermomechanical Property Conundrum in Sulfur-Rich Copolymers, Adv. Opt. Mater., 2023, 11, 2202432 CrossRef CAS
.
- N. Saba and M. A. Jawaid, A review on thermomechanical properties of polymers and fibers reinforced polymer composites, J. Ind. Eng. Chem., 2018, 67, 1–11 CrossRef CAS
.
- B. T. Michal, C. A. Jaye, E. J. Spencer and S. J. Rowan, Inherently photohealable and thermal shape-memory polydisulfide networks, ACS Macro Lett., 2013, 2, 694–699 CrossRef CAS PubMed
.
- D. Parker, H. Jones, S. Petcher, L. Cervini, J. Griffin, R. Akhtar and T. Hasell, Low cost and renewable sulfur-polymers by inverse vulcanisation, and their potential for mercury capture, J. Mater. Chem. A, 2017, 5, 11682–11692 RSC
.
- J. J. Griebel, N. A. Nguyen, A. V. Astashkin, R. S. Glass, M. E. Mackay, K. Char and J. Pyun, Preparation of dynamic covalent polymers via inverse vulcanization of elemental sulfur, ACS Macro Lett., 2014, 3, 1258–1261 CrossRef CAS PubMed
.
- N. Han, W. Cho, J. H. Hwang, S. Won, D.-G. Kim and J. J. Wie, Enhancement of thermomechanical properties of sulfur-rich polymers by post-thermal treatment, Polym. Chem., 2023, 14, 943–951 RSC
.
- L. Blight, B. Currell, B. Nash, R. Scott and C. Stillo, New Uses of Sulfur-II, Adv. Chem. Ser., 1978, 165, 13 CrossRef CAS
.
- C. M. Marshall, J. Molineux, K.-S. Kang, V. Kumirov, K.-J. Kim, R. A. Norwood, J. T. Njardarson and J. Pyun, Synthesis of Polycyclic Olefinic Monomers from Norbornadiene for Inverse Vulcanization: Structural and Mechanistic Consequences, J. Am. Chem. Soc., 2024, 146, 24061–24074 CrossRef CAS PubMed
.
- J. Bao, K. P. Martin, E. Cho, K.-S. Kang, R. S. Glass, V. Coropceanu, J.-L. Bredas, W. O’Neil ParkerJr, J. T. Njardarson and J. Pyun, On the Mechanism of the Inverse Vulcanization of Elemental Sulfur: Structural Characterization of Poly(sulfur-random-(1,3-diisopropenylbenzene)), J. Am. Chem. Soc., 2023, 145, 12386–12397 CrossRef CAS PubMed
.
- M. H. Qureshi, J. Bao, T. S. Kleine, K.-J. Kim, K. J. Carothers, J. Molineux, E. Cho, K.-S. Kang, N. P. Godman, V. Coropceanu, J.-L. Bredas, R. A. Norwood, J. T. Njardarson and J. Pyun, Synthesis of Deuterated and Sulfurated Polymers by Inverse Vulcanization: Engineering Infrared Transparency via Deuteration, J. Am. Chem. Soc., 2023, 145, 27821–27829 CrossRef CAS PubMed
.
- R. P. White and J. E. Lipson, Polymer Free Volume and Its Connection to the Glass Transition, Macromolecules, 2016, 49, 3987–4007 CrossRef CAS
.
- Y. Su, E. B. D. S. Filho, N. Peek, B. Chen and A. E. Stiegman, High refractive index polymers (n > 1.7), based on thiol–ene cross-linking of polarizable P = S and P = Se organic/inorganic monomers, Macromolecules, 2019, 52, 9012–9022 CrossRef CAS
.
- J. Molineux, T. Lee, K. J. Kim, K.-S. Kang, N. P. Lyons, A. Nishant, T. S. Kleine, S. W. Durfee, J. Pyun and R. A. Norwood, Fabrication of Plastic Optics from Chalcogenide Hybrid Inorganic/Organic Polymers for Infrared Thermal Imaging, Adv. Opt. Mater., 2023, 12, 2301971 CrossRef
.
- S. N. Yannopoulos, Structure and photo-induced effects in elemental chalcogens: a review on Raman scattering, J. Mater. Sci.: Mater. Electron., 2020, 31, 7565–7595 CrossRef CAS
.
- S. Cui, R. Chahal, C. Boussard-Plédel, V. Nazabal, J.-L. Doualan, J. Troles, J. Lucas and B. Bureau, From Selenium- to Tellurium-Based Glass Optical Fibers for Infrared Spectroscopies, Molecules, 2013, 18, 5373–5388 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qm00785a |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2025 |