Multiscale engineering of anode catalyst layers in proton exchange membrane water electrolyzers
Qianqian
Liu
ab,
Yanfei
Wang
*c,
Xiao
Liang
a,
Hui
Chen
*a and
Xiaoxin
Zou
 *a
*a
aState Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, P. R. China. E-mail: xxzou@jlu.edu.cn; chenhui@jlu.edu.cn
bSchool of Materials Science and Engineering, Xi’an University of Science and Technology, Xi’an 710054, China
cPetrochina Petrochemical Research Institute, Beijing 102206, China. E-mail: wangyanfei010@petrochina.com.cn
First published on 19th November 2024
Abstract
Proton exchange membrane water electrolyzers (PEMWEs) play a key role in promoting the development of the clean hydrogen energy industry and accelerating the achievement of carbon neutrality goals due to their advantages of high efficiency, low energy consumption, ease of integration and fast response. In PEMWEs, the water oxidation reaction in the anode catalytic layer is the core process, and its catalytic efficiency directly determines the performance and stability of the electrolyzers. Therefore, enhancement of reactant transport, electron/proton transfer, and oxygen release by cross-scale optimisation of the anode catalytic layer is crucial for improving the efficiency of PEMWEs. This article highlights recent advances in optimizing the anode catalytic layer of PEMWEs through multi-scale engineering strategies. We first introduce the basic structure of PEMWEs and the importance of the anode catalyst. Subsequently, we discuss in detail the multiscale optimisation strategy of the anode catalyst layer, including the design of active sites at the atomic scale, the morphology regulation at the nano/micro scale, the catalytic layer optimization at the macroscopic scale and the comprehensive synergistic effect of multiscale engineering. Finally, we conclude and look forward to the existing challenges and future research directions for optimising anode catalyst layers by multiscale engineering.
1. Introduction
Hydrogen, a clean and sustainable energy carrier, is of irreplaceable importance in promoting energy transformation and realising a low-carbon economy.1–6 Proton exchange membrane water electrolyzers (PEMWEs) for hydrogen generation have the advantages of compact size, large current density (>1 A cm−2), high conversion efficiency (80–90%) and high hydrogen purity (>99.99%) over other conventional technologies (e.g., alkaline water electrolyzers).7–12 In particular, the high flexibility and functional tunability of PEMWEs allow them to be coupled with fluctuating and intermittent renewable energy sources such as wind and solar power, and they are thus regarded as promising technology for large-scale green hydrogen production.13–15A catalyst coated membrane (CCM) is the central component of PEMWEs and consists of a solid electrolyte membrane, and anodic and cathodic catalyst layers located on both sides of the membrane.16 In PEMWEs, the oxygen evolution reaction (OER) occurs at the anode in a strongly acidic environment, and its slow kinetics and harsh four-electron transfer process require OER catalysts with high activity and stability.17,18 Currently, IrO2 is considered to be the most advanced anode catalyst in PEMWEs and the only catalytic material that can achieve high efficiency and stability in acidic media.19–21 In order to ensure the performance and lifetime of PEMWEs, the iridium loading of the anode catalyst layer in CCMs is usually as high as 2–4 mgIr cm−2.22–24 However, iridium resources are extremely scarce globally, with a crustal abundance of only 4 × 10−8%.25–27 And it is very expensive (US $ 175 g−1 in August 2024), almost five times the price of platinum, which limits the large-scale application of PEMWEs. Therefore, it is important to reduce the iridium load in the anode catalytic layer.
Researchers have developed a series of novel Ir-based catalysts to increase the activity of iridium-based catalysts and decrease the iridium loading, including perovskite oxides (e.g. SrIrO328), pyrochlorite oxides (e.g. Y2Ir2O729), mixed-metal oxides (e.g. W1−xIrxO3−δ30), Ir alloys (e.g. IrY31), and supported Ir-based catalysts (e.g. IrOx/TiO232), among others. These new catalysts generally outperform commercial IrO2 in three-electrode cells, but their potential for use in actual PEMWEs has not been demonstrated. Although some catalysts have been tested in PEMWEs, their performance in terms of activity and/or stability has not been satisfactory. Rarely, some catalysts show high activity, which is also usually achieved with high iridium loading.
The key reason for this is that the CCM test imposes much more strict requirements for anode catalysts which work at an ampere-level current density under a strong acidic, oxidizing environment. Besides high intrinsic activity and structural stability, a competent catalyst in CCMs should fulfill more criteria simultaneously, such as good electrical conductivity to deliver ampere-level current density and high corrosion resistance to protect the catalysts serving in severe environments. Moreover, the catalysts should possess suitable geometry and size to present high catalyst layer porosity for effective mass transport in CCMs. Therefore, multiscale optimisation of the anode catalyst layer is necessary to achieve high performance at low iridium loadings. To the best of our knowledge, researchers have summarised some optimisation strategies to improve the performance of iridium-based catalysts under acidic conditions, but there are almost no summarised reports on the multi-scale synergistic optimisation of iridium-based anode catalyst layers based on PEMWEs.
In this paper, the basic structure of a PEMWE and the importance of the anode catalyst are first introduced. Subsequently, the multiscale optimisation strategy of the anode catalyst layer is discussed in detail, including the design of active sites at the atomic scale, the modulation of morphology and structure at the nano/micro scale, the optimal integration of the catalyst layer at the macroscopic scale, and the comprehensive synergistic effect of multiscale engineering. Finally, this paper provides an outlook on the future research direction of multiscale engineering in anode catalyst layer design. This thesis not only provides a comprehensive multiscale engineering perspective to understand and design the anode catalyst layer of PEMWEs, but also anticipates that through continuous material innovation and structural optimisation, combined with advanced characterisation techniques, a more efficient, stable and economical PEMWE system will be realised.
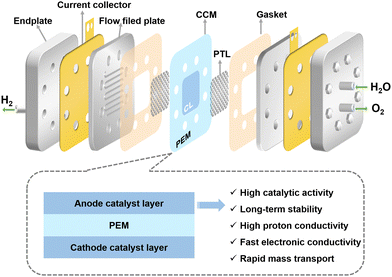
2. Structure and principle of a PEMWE
Fig. 1 shows the construction of the main components of a PEMWE, which are, from inside to outside, a PEM, catalyst layers (CLs), porous transport layers (PTLs), bipolar plates (BPs), end plates, and some sealing elements. The function of the PEM is to allow the passage of protons (H+) to take part in electrolytic reactions, while preventing the direct passage of electrons and gases. The CLs are usually applied on both sides of the membrane, where the anodic catalyst promotes the oxidation of water molecules to O2 and H+, and the cathodic catalyst promotes the reduction of H+ to H2. The CCM, consisting of the PEM and the CL at the cathode and anode, is the central component of the PEMWE. In addition, the PTLs act as an electronic conductor between the CCM and the BPs to ensure efficient mass transfer of liquids (H2O) and gases (H2 and O2). The functions of the BP include the transport of water and gaseous products, electrical conductivity, and heat transfer. The BPs are usually inscribed with flow channels to optimise the flow of reactants and products. End plates act as fixed electrolyzer components to ensure the integrity and stability of the PEMWE.PEMWEs usually adopt the anode water supply mode, the reactants (usually pure H2O) flow from the anode inlet to the anode CL through BP and PTL, catalysing the OER of the anode under the action of potential (eqn (1)):
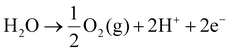 | (1) |
| 2H+ + 2e− → H2(g) | (2) |
The generated H2 reaches the outlet of the cathode end via PTL and BP and is dried and collected through a gas–liquid separation system to obtain high-purity H2.33,34
Due to the rapid proton conductivity of the PEM, PEMWEs can respond quickly to current changes and are suitable for coupling with renewable energy sources. At the same time, the PEM can effectively separate H2 and O2, avoiding their mixing in the tank and improving safety. In addition, PEMWEs use pure H2O as reactants and do not require the addition of strong electrolyte solutions to improve conductivity, reducing corrosion and contamination problems.
3. Challenges for achieving low Ir loading CCMs
The PEMWE voltage (Ecell, eqn (3)) consists of the reversible potential (Erev) and three main overpotentials, namely the kinetic overpotential (ηkin), the ohmic overpotential (ηΩ), and the mass transfer polarisation overpotential (ηmt).| Ecell = Erev + ηkin + ηmt | (3) |
The anode CL is composed of an iridium-based catalyst material and ionomers. The anode CL of commercial PEMWEs usually relies on a high loading of IrO2 (≥2 mgIr cm−2), with a catalytic layer thickness of approximately 10 μm.20,22,36 This elevated iridium loading is essential for ensuring both the activity and stability of the CL. The ionomers play a critical role by forming a proton transport channel within the anode CL, facilitating the movement of H+ ions from the anode side through the CL to the membrane. Additionally, ionomers contribute to the stabilization of the catalysts, by preventing their detachment or agglomeration during electrolysis, thus preserving the integrity and catalytic activity of the CL. Furthermore, ionomers affect the transport of H2O and O2 within the CL. Therefore, optimizing the content and structure of ionomers can enhance the transport channels for H2O and O2 in the CL, ultimately improving the efficiency and stability of the PEMWE. In summary, both the catalysts and the ionomers in the anode CL significantly impact the performance of the PEMWE.
If the iridium load is reduced, the catalytic layer will become uneven and discontinuous, which will not only reduce the number of active sites, but also lead to an increase in the resistance inside the CL, so that the current density and energy conversion efficiency of the PEMWE will be reduced. Therefore, an excellent anode catalyst layer with low Ir loading should have the following characteristics: (1) high catalytic activity. High catalytic activity can accelerate the OER and increase the H2 production rate of the PEMWE. (2) Chemical stability. Under an environment of acidity and high potential, the anode catalyst layer needs to have sufficient chemical stability to resist corrosion and deactivation to ensure the long-term stable operation of the electrolysis tank. (3) Excellent electronic and proton conductivity. This allows for the rapid transfer of electrons from the external circuit to the catalytic active sites during electrolysis, reducing the polarization of the electrode and reducing energy loss. (4) Efficient mass transfer capability. This not only ensures that H2O can reach the catalyst surface efficiently to maximise the accessibility and reaction efficiency of the active sites, but also enables the generated O2 to be released smoothly from the CL, avoiding the increase in mass transfer resistance caused by the accumulation of O2 on the surface of the CL.
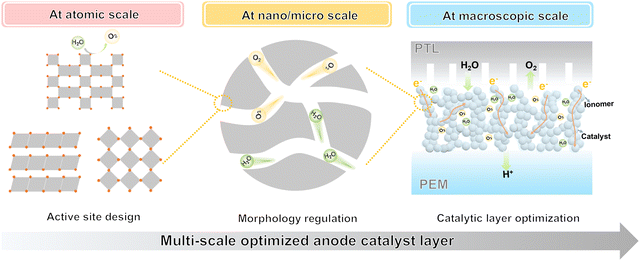
From the above, it can be seen that a multi-scale synergistic optimisation of the anode catalyst layer is required to achieve the optimal performance of the PEMWE (Fig. 2). Specifically, this includes (1) active site design on the atomic scale. At the atomic scale, the design of active sites is the key to improving the performance of the OER. The intrinsic activity of the catalyst is enhanced by adjusting the chemical composition, crystal structure and electronic structure of the material. (2) Structure modulation on the nano-microscale. At the nanoscale, the size, shape and structure of the catalyst are tuned to increase the number of active sites and improve the diffusion of reactants and products. (3) Optimal integration of the catalyst layer scale. The mass and charge transfer efficiency of the CL and the long-term stability of the PEMWE are balanced by adjusting the thickness, shape and content of the ionomer of the anode CL. Therefore, through the multi-scale synergistic optimisation, the overall improvement of the anode catalyst layer performance can be achieved, promoting the commercialisation and scale-up application of the PEMWE.
4. Strategies for designing OER catalysts
4.1 Active site design at the atomic scale
During the OER process, the catalyst surface reacts with multiple intermediates (such as OH*, O*, HOO*). As can be seen from the volcano plot, an ideal OER catalyst should neither bind too strongly nor too weakly to oxygen-containing intermediates. Rutile iridium oxide exhibits structural stability under acidic OER conditions, but the activity of iridium sites is still not ideal, mainly due to its strong adsorption capacity for oxygen intermediates.37 Therefore, optimizing the intrinsic activity of Ir sites at the atomic scale is crucial for enhancing its catalytic efficiency.The introduction of other components into iridium oxide is an important strategy to optimize the electronic structure of the Ir sites. The multicomponent effects can effectively control the adsorption/desorption of the reaction intermediates during the catalytic reaction, thereby improving the catalytic performance. The Zou group constructed theoretical models of IrO2–ZrO2 and IrO2–TiO2 solid solutions.38,39 The Ti and Zr atoms were found to weaken the oxygen binding ability of Ir sites and ensure that the theoretical activity reaches the highest quality. The authors further prepared the highly active IrO2–ZrO2 and IrO2–TiO2 solid solutions by etching Sr atoms from SrTiO3–SrIrO3 and SrZrO3–SrIrO3 solid solutions during the OER in acid. For SrZrO3–SrIrO3 solid solution, iridium content was 46% less than that of IrO2, but its iridium mass activity for the OER was thirty-nine times higher than that of IrO2 (Fig. 3(a)–(c)). Up to now, a variety of alloying and solid solution catalysts (e.g., IrRu alloy,40 Ir1−xRuxO2,41 and Ir0.5Ni0.5Ox42) have been explored to significantly lower iridium content and maintain high catalytic activity. However, the widespread occurrence of chemical dissolution and surface reconstruction presents significant challenges for a precise understanding of their active sites.
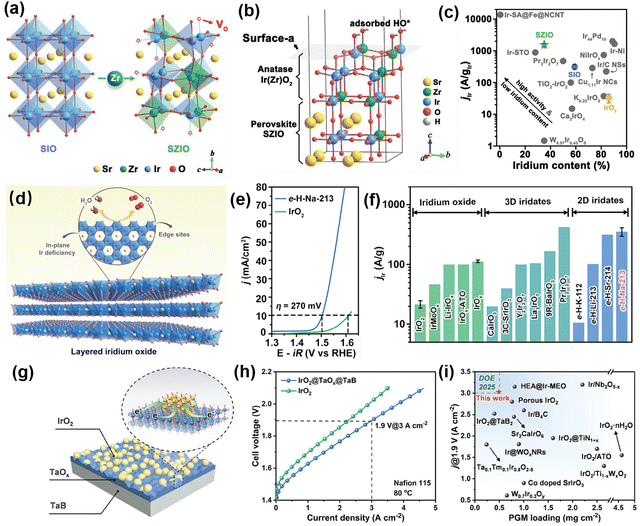 | ||
| Fig. 3 (a) Theoretical model of A-Ti(Ir)O2/P. (b) A volcano plot showing the theoretical overpotentials of OER over different materials as a function of their ΔGO*–ΔGHO* values. (c) Comparison of iridium mass activities (jIr) of iridium-based catalysts at a potential of 1.53 V. Copyright 2020, WILEY-VCH.39 (d) Crystal structure of e-H-Na-213. (e) Polarization curves for OER with e-H-Na-213 and IrO2. (f) Comparison of Ir mass activity (jIr) of e-H-Na-213 and the reported catalysts including iridium oxides, 3D iridates, and 2D iridates. Copyright 2024, WILEY-VCH.34 (g) Schematic description of interfacial electronic interaction for IrO2@TaOx@TaB. (h) Polarization curves of PEMWEs using IrO2@TaOx@TaB and IrO2 anodes. (i) Comparison of PEMWE performances and CCM PGM loadings of different anode catalysts. Copyright 2024, WILEY-VCH.43 | ||
The crystal structure design of Ir-based oxides allows precise control of the distribution and local environment of Ir sites. In recent years, iridates (e.g. Ir-based perovskites,44–48 Ir-based double perovskite compounds49–51 and pyrochlore iridates52–55) have attracted great attention for the acidic OER, because their various crystal structures provide diverse connectivity of IrO6 octahedra. For example, pseudo-cubic perovskite SrIrO3 (3C-SrIrO3) is a typical representative of Ir-based perovskites, in which all IrO6 octahedrons are corner-shared, and its catalytic activity is higher than that of rutile iridium oxide. The leaching of Sr from the surface layers during the OER process results in amorphous IrOx as the real catalytically active phases. The IrO3 or anatase IrO2 motifs are proposed to be highly active sites for the perovskite-derived amorphous IrOx phase.56,57 Layered iridates (such as SrIr2O6,58 Sr2IrO4,8 Na2IrO314) are considered to be able to restrain the uncontrollable surface amorphization. The reversibility of interlayer ion exchange provides a dynamic balance for the stabilization of iridium sites in layered iridates. The edge Ir sites of these layered iridates are theoretically identified to be active sites with higher intrinsic activity than IrO2. For example, the Zou group34 treated Na2IrO3 in an acidic solution at room temperature and subsequently exfoliated and obtained the 2D iridium oxide nanosheets with intrinsic in-plane iridium deficiency. The edge Ir site can reach the theoretical optimal value, and its iridium mass activity is 16.5 times higher than that of IrO2 (Fig. 3(d)–(f)).
The crystal structure not only affects the activity of the catalyst but also has a direct relationship with the stability of its catalytic sites.59–61 Our group62 reported another new type of monoclinic strontium iridium (6H-SrIrO3), which has both the corner-sharing IrO6 octahedra and the face-sharing IrO6 octahedral dimers. Theoretical calculations show that the existence of face-sharing IrO6 octahedron dimers weakens the Ir–O bond on the catalyst surface during the reaction, thus promoting the rate-determination step in OER and improving the stability of iridium active sites. The success of the 6H-SrIrO3 catalyst encouraged our group to explore a series of iridates with face-sharing connection modes, such as Ba4PrIr3O12,63 Ba3LnIr2O964 and Ba3TiIr2O9,65 to enhance the structural stability. Recently, Chung et al.66 studied the performance of eleven AxIryOz-type oxides (A = Ca, Sr, Ba, Y, Pr, Nd) as OER electrocatalysts in an acidic environment. It is found that these iridium oxides can be divided into three groups according to the connection strength between [IrO6] octahedral and show different catalytic activity and stability. Especially, the [IrO6] octahedrons of iridium oxides with strong connectivity (such as BaIrO3 and Ca2IrO4) are closely connected by face sharing or edge sharing, showing excellent cycle stability and activity improvement. In contrast, iridium oxides with weak connectivity show a rapid decline in catalytic activity. The research reveals the direct correlation between the crystal structure and the stability of the iridium-based OER catalyst and emphasizes the importance of edge sharing or face sharing [IrO6] geometry.
Optimizing the Ir active sites can be significantly achieved through the use of supported catalysts, which facilitate the regulation of interactions between iridium oxide and the support materials. This interaction includes electronic and geometric effects, which can affect the adsorption capacity of the catalyst surface, the activation energy, and the stability of the reaction intermediates.67–71 However, the harsh acid and oxidizing conditions of the anodic OER make it difficult for traditional support materials (carbon,72–74 metal oxides,69,70,75–78etc.) to achieve ideal results. At present, the design and synthesis of new supporting materials (TiN,79 TiONx,80 Nb4N5,81 TaB2,82etc.) with high conductivity, strong corrosion resistance and large surfaces for obtaining supported iridium catalysts are one of the research hotspots of PEMWEs. Zou et al.43 obtained a supported low-iridium catalyst (IrO2@TaOx@TaB) with high activity and stability through a combination of carrier and support technology innovation. IrO2@TaOx@TaB exhibits a dual-interface structure: (i) IrO2/TaOx interfacial electron interaction can enhance the catalytic activity; (ii) the compact TaOx layer can inhibit the further oxidation of TaB and stabilize the IrO2 catalytic layer, thus improving the structural stability. Integrating IrO2@TaOx@TaB into the CCM, the resulting PEMWEs attain a current density of 3.06 A cm−2 at 2 V and retain 2 A cm−2 current density for 1500 h. Notably, this PEMWE performance and iridium-loading are ahead of the U.S. DOE 2025 target (Fig. 3(g)–(i)).
In conclusion, the performance of the catalyst can be significantly improved by regulating the electronic structure and coordination environment of the catalyst at the atomic scale. However, iridium-based catalysts generally face the problems of structural reconstruction and dynamic change in an acidic environment. Therefore, it is still a great challenge to recognize and stabilize the active sites at the atomic scale.
4.2 Morphology regulation at the nano/micro scale
Owing to the high surface energy, rutile IrO2 nanoparticles, as the anode catalyst layer (ACL) of the PEMWE, often form tightly packed aggregates on the membrane surface, which leads to large diffusion losses in the electrolyzer.83 In order to solve this problem, the researchers optimized the pore scale structure (nano/micro scale) of the ACL by adjusting the morphology of the catalysts, thus improving the mass transfer.84–87 Such optimization can not only increase the surface area of the catalyst and provide more active sites, but also facilitate the improvement of mass transfer, reduce bubble aggregation and enhance the desorption and release of oxygen, thus playing a key role in improving energy efficiency and reducing costs in the OER process.Recently, the synthesis of porous iridium-based catalysts has attracted much attention because of its remarkable improvement in catalytic performance. Among many methods, the template method stands out because of its unique spatial confinement and becomes a model for regulating the morphology and structure of nano-materials. Through the template method, researchers successfully synthesized innovative materials such as 2D mesoporous metallic Ir nanosheets (Fig. 4(a) and (b))88 and mesoporous iridium oxide films,89 which showed their advantages of flexible and simple operation. Then, the dealloying method, as another powerful means, also showed great application potential in the field of preparing porous iridium-based catalysts. Different from the template method, the dealloying method skillfully constructs porous structures by selectively dissolving specific components in the alloy, such as a 3D nano-porous IrNi alloy,90 highly porous Ir–Cu nanocrystals (Fig. 4(c))91 and a nano-porous IrxOs(1−x) alloy.92 These achievements are not only simple in the preparation process, but also easy to control, which indicates its broad prospects in mass production. In addition, selective etching also plays an important role in preparing iridium-based catalysts with special porous structures.91,93 For example, Guo et al. synthesized a series of Ir-based metal porous hollow nanocrystals by Fe3+ selective etching of Ir-based metal core–shell nanocrystal precursors. Especially, IrCoNi ternary porous hollow nanocrystals show better acidic OER activity and stability than commercial Ir/C catalysts (Fig. 4(d)).94 In order to solve the problem of low efficiency of water transmission and oxygen bubble removal in the OER process caused by high-density accumulated IrOx-ionomer agglomerates. Zhao et al.83 prepared IrOx-ionomer agglomerates (ODT-1.0) with cavity structure using the sacrificial template method, aiming at improving the transmission of oxygen bubbles in the ACL, thus reducing the mass transfer overpotential and improving the overall performance of PEMWEs (Fig. 4(e)–(g)). The results show that the optimized aggregate structure has significantly improved the OER performance, and the current density is as high as 7.0 A cm−2@2.07 V in the actual CCM under 0.72 mgIr cm−2.
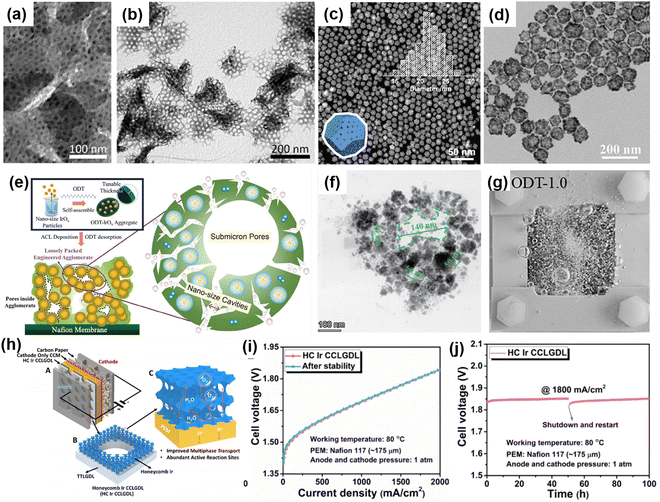 | ||
| Fig. 4 (a) SEM and (b) TEM images of the mesoporous Ir nanosheets. Copyright 2018, American Chemical Society.88 (c) TEM image of P-IrCu1.4. Copyright 2018, American Chemical Society.91 (d) TEM image of IrCoNi. Copyright 2017, WILEY-VCH.94 (e) Schematic diagram of the fabrication process for the electrode using agglomerate engineering. (f) Structure of IrOx-ionomer agglomerate (sample ODT-1.0) after removal of ODT. (g) Bubble visualization for ODT-1.0 in the transparent three-electrode system with the utilization of a high-speed camera from the front side and DSLR from the upper side. Copyright 2024, WILEY-VCH.83 (h) Schematic of the PEMEC incorporated with the HC Ir CCLGDL and HC CCLGDL. (i) Cell performance comparison before and after the stability test. (j) Stability test. Copyright 2023, American Chemical Society.95 | ||
In the process of exploring and improving the performance of PEMWE, porous Ir-based catalysts have emerged as a focal point of application research in electrolyzers due to their unique structural advantages.96 These materials characterized by high specific surface area and improved mass transfer properties not only optimize the kinetics of electrochemical reactions but also significantly improve the efficiency and stability of the overall water electrolysis system. Peron et al.97 reported a macroscopically porous particle with approximately 75% porosity composed of IrOx nanoneedle networks. This high porosity structure promotes gas transmission and reactant contact, thus achieving higher current density and voltage efficiency, and its PEMWE performance is 1.68 V@1 A cm−2. As the activity increases, the stability of porous structures in long-term operation has also been concerned. Kuai et al.98 prepared a porous hollow IrO1−x microsphere (IrO1−x-PHM) by the microdrop-confined fusion/blasting (MCFB) strategy. It was shown that the catalyst with a porous hollow structure could significantly improve the catalytic active site utilisation and mass transfer efficiency, which led to excellent performance in the PEMWE. Peron et al.99 deeply analyzed the role of porosity as a morphological marker in the degradation process of the anode catalyst layer. By observing the morphological evolution of anode layer under different pore structures, this study reveals that the CL/PTL interface of anode is the key area of performance degradation and emphasizes the importance of optimizing pore structure to slow down degradation and improve durability. In order to further realize the economical and efficient technology of hydrogen production by PEMWEs, Zhang et al.95 prepared a novel type of highly porous iridium-coated thin film electrode by combining a rapid electroplating process with an efficient template removal technology (Fig. 4(h)). This electrode features a honeycomb catalyst layer, achieving a low PEMWE voltage (1.84 V@2 A cm−2) and demonstrating high quality activity (4.2 A mgIr−2@1.7 V) at a low iridium loading of 0.27 mg cm−2 (Fig. 4(i) and (j)). Its unique structural design not only reduces the cost, but also significantly improves the performance and stability of PEMWEs.
Nano/micro scale optimisation of iridium-based catalysts can significantly enhance the exposure of active sites and the transport efficiency of reactants by adjusting the catalyst size, morphology, pore structure, and morphology of the support carrier, which plays a crucial role in enhancing its catalytic activity and stability in oxygen generation reactions. Despite substantial progress, the use of porous iridium oxide in PEMWEs remains limited. The impact of the porous structure on the material transport properties and the performance of the electrolytic cell in relation to the CCM are yet to be thoroughly investigated.
4.3 Catalytic layer optimization at the macroscopic scale
PEMWEs have a high current density of >1 A cm−2 and frequent gas–liquid transfer. The factors affecting its performance include not only the reaction kinetics of the catalyst, but also mass transport, electronic conductivity and proton conductivity properties of CLs, which are often ignored in the three-electrode cell. These properties can be optimized by regulating the structure of the catalyst layer in terms of the thickness of the CL, porosity, ion transport channels, etc.CL thickness directly affects reaction kinetics, mass transfer efficiency and overall electrolytic performance. Therefore, the appropriate thickness of the catalytic layer is crucial to achieve efficient and stable catalytic performance. Gasteiger et al.100 investigated the effect of IrO2/TiO2 catalysts with different loadings on PEMWE performance. The researchers indirectly controlled the thickness of the electrode by changing the catalyst loading. The results showed that an optimal Ir loading of 1 to 2 mgIr cm−2, corresponding to an electrode thickness of about 4 to 8 μm, provided the best performance (Fig. 5(a) and (b)). The CL is too thin, resulting in discontinuity of the catalytic layer, reducing the active site and increasing the internal resistance. While too thick CLs will increase the internal diffusion resistance. Whereas very low or very high Ir loading leads to increased cell voltage and performance loss due to water transport limitations or catalyst layer discontinuities.
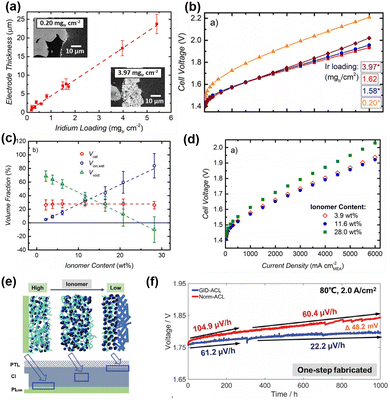 | ||
| Fig. 5 (a) Electrode thickness vs. iridium loading determined from the areal weight of the anode electrodes. (b) Polarization curves for different Ir anode loadings. Copyright 2018, Electrochemical Society.100 (c) Electrode volume fractions versus ionomer content for the IrO2/TiO2 catalyst. (d) Polarization curves for MEAs with different anode ionomer loadings. Copyright 2016, Electrochemical Society.101 (e) The designed GID-ACL structure with enriched ionomer at the ACL/PEM interface and reduced ionomer at the ACL/PTL interface. (f) Durability test of Norm-ACL and GID-ACL at 2.0 A cm−2. Copyright 2024, WILEY-VCH.102 | ||
The ionomer content also plays a crucial role in PEMWEs, affecting the conductivity, porosity and catalyst utilization efficiency within the CL. Appropriate ionomer content can improve proton conductivity and reduce PEMWE voltage loss. Too low ionomer content may lead to insufficient proton conductivity and increase PEMWE voltage loss. However, too high ionomer content may fill the pores between the catalysts, limit the mass transfer of O2 and H2O, increase the electronic contact resistance, insulate part of the catalysts, and thus reduce the efficiency of the PEMWE. Therefore, optimizing the ionomer content is critical to achieving an efficient and stable PEMWE. For example, Gasteiger et al.101 investigated the effect of ionomer content in the anode catalyst layer of IrO2/TiO2 on the performance of PEMWEs. The researchers found that the optimal ionomer content was 11.6 wt%, which corresponded to an electrode porosity of about 35% (Fig. 5(c) and (d)). Lower or higher than this optimum ionomer content will result in reduced PEM cell performance, mainly in the form of increased cell voltage and reduced electrolytic efficiency.
The OER process inside the ACL is a completely coupled reaction-diffusion process. Due to the difference in reaction space and mass transfer balance of the ACL, the gradient hierarchical structure of the ACL is constructed by adjusting the ionomer and catalyst composition parameters of the anode CL, which is an effective strategy to optimize the anode CL structure. For example, Zhang et al.103 effectively reduced the internal voltage loss of the ACL by coordinately regulating the ratio of IrO2/TiNx and the gradient distribution of ionomer content. It was found that the ratio of IrO2/TiNx and the content of ionomer in gradient design increased and decreased with increasing distance from the membrane, respectively. This structure showed significant advantages in improving the utilization rate of active sites, proton conductivity and reaction kinetics. At the current density of 3 A cm−2, the optimal design of the membrane electrode (MEA-6) shows the lowest electrolyzer voltage (2.118 V), which indicates that this cooperative gradient design is an effective strategy to optimize the performance of PEMWEs. Moreover, due to the local expansion and migration of ionomers, the PEMWE performance deteriorates rapidly and its stability deteriorates. Tao et al.102 proposed a strategy to enhance the stability of PEMWEs by optimizing the distribution of ionomers (Fig. 5(e)). Specifically, by adjusting the composition of the catalyst ink, the ionomer between the ACL/PTL interface is reduced and the ionomer between the ACL/PEM interface is increased, thus optimizing the distribution of ionomers in the anode CL. The experimental results show that the CL with gradient distribution exhibits a lower decay rate than traditional CL when operating at high temperatures and high current density, and can maintain stable electron, proton and water–gas transport channels more effectively (Fig. 5(f)).
The conductivity in the CL is also one of the key factors affecting the efficiency and energy consumption of the PEMWE. The high conductivity of the CL can provide lower electron transport resistance, ensuring that electrons can be quickly transferred from the electrode to the catalytic active site during electrolysis, thus accelerating the generation of O2. Generally, the conductivity is closely related to the composition, loading capacity and dispersion of the catalysts. Rohlfing et al.104 loaded 30 wt% and 45 wt% content of IrO2 on TiO2 carriers using molten salt oxidation, respectively. It was found that by optimising the oxidation temperature and the loading amount of Ir, uniform distribution and interconnections of IrO2 nanoparticles could be achieved to improve the conductivity and activity of the catalyst. Millet et al.105 selected micron Ti particles as the IrO2 carrier to prepare the IrO2/Ti catalyst. The results show that IrO2/Ti has a larger electrochemically active surface area than the pure IrO2, mainly because the introduction of Ti not only improves the dispersion of the Ir active site, but also helps to establish a close electronic contact between the catalyst layer and the current collector, reducing the ohmic resistance within the catalyst layer.
Therefore, optimization from the catalyst layer scale can reduce the mass transfer resistance, enabling more efficient transport of reactants and products through the CL. In addition, due to the improved mass transfer efficiency, the contact time between the reactants and the catalyst is shortened, and the reaction rate can be enhanced, which improves the electrolysis efficiency and voltage of PEMWEs. Although some progress has been made in optimizing the spatial structure design of the anode catalytic layer, the research on solving the spatial reaction differentiation in the catalytic layer and realizing the high utilization rate and mass transfer balance of the iridium catalyst is still relatively scarce.
5. Multi-scale synergistic optimization
Integrating multi-scale synergistic optimization CLs from the atomic scale, the nano/micro scale to the macro scale has obvious advantages in PEMWEs. The synthesis considers the optimization of the intrinsic activity, morphology, and macro configuration of CLs, aiming to enhance the dispersibility of the catalyst, improve mass transfer efficiency, and optimize the transfer path of electrons and protons. This multiscale synergistic optimization strategy significantly reduces the overpotential, thereby improving the overall performance and long-term stability of PEMWEs.In order to cooperatively improve the in-plane conductivity, gas/water transport capacity and mechanical stability of the anode CL, some researchers have begun to pay attention to the multi-scale collaborative optimization of the catalytic layer. The hierarchical structure can build a more complex pore structure, which can not only provide more mass transfer channels, enabling the reactants to reach the catalytically active sites more rapidly, but also move the products out of the catalytically active sites more efficiently. From the perspective of mass transfer, the hierarchical structure can significantly reduce the mass transfer resistance and improve the mass transfer efficiency of PEMWEs. For example, Guo et al.4 constructed an efficient CL with a fast electron transport network and an unhindered mass transport channel by assembling IrRu sub-nanosheets (IrRu HNWs) on Pd nanowires with a high aspect ratio. Such a hierarchical structure enables the construction of more complex pore structures, which not only improves the accessibility of active sites, but also optimises the diffusion path of substances in the catalyst. The integration of IrRu HNWs into CCM enables efficient PEMWEs at low Ir loading (0.35 mgIr cm−2), demonstrating excellent activity of 2.44 V@6.0 A cm−2 and stable operation for at least 240 h at a current density of 2.0 A cm−2 (Fig. 6(a)–(c)).
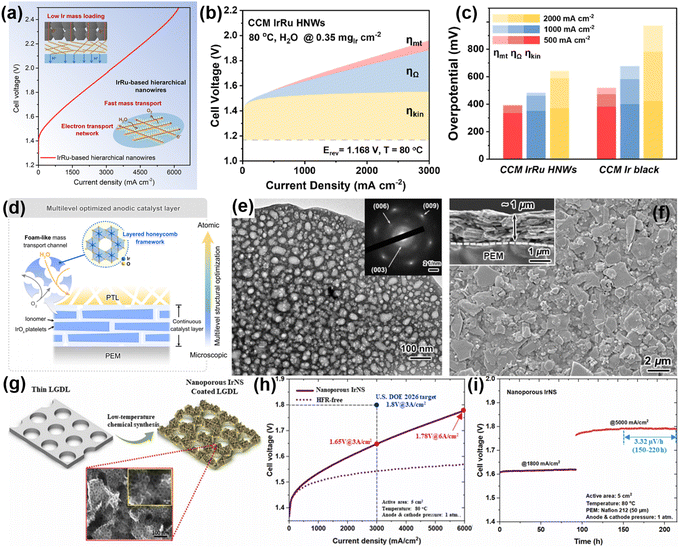 | ||
| Fig. 6 (a) Catalyst layers made of hierarchical IrRu sub nanosheets on nanowires. (b) and (c) Various overpotential contributions to the polarization curves of different catalysts at various current densities. Copyright 2024, Cell Press.4 (d) Schematic representation of multilevel structural optimization for the anodic catalyst layer with H-IrOx FPs as the anode catalyst. (e) TEM image and SAED pattern (inset) of H-IrOx FPs. (f) SEM images of the H-IrOx FP-based anodic catalytic layer, with an inset showing the cross-sectional view. Copyright 2024, WILEY-VCH.106 (g) Schematic illustrating the design of the nanoporous Ir NS integrated electrode. (h) Polarization curves of a nanoporous Ir NS integrated electrode. (i) Cell stability tests. Copyright 2024, Elsevier.107 | ||
The oxygen transport efficiency of PEMWEs is low at high current density, and the number and activity of intrinsic active sites of the catalyst are also limited. These two factors together lead to the increase of mass transfer overpotential, which further limits the improvement in the overall performance of PEMWEs. Recently, our group started to focus on multiscale synergistic optimisation to improve the performance and efficiency of PEMWEs.106,108 Based on the previous understanding of the characteristics of the active site of layered iridium, our group106 prepared IrOx foam platelets (H-IrOx FPs) with an edge-sharing IrO6 octahedral honeycomb framework by the low-temperature synthesis method. This structure optimizes the ACL from the atomic to microscopic scale, significantly improves the catalytic activity and stability, and reduces the iridium loading. Therefore, its integration into CCM can achieve an efficient PEMWE (2 V@3.4 A cm−2), and it can maintain stability for at least 2000 h (Fig. 6(d)–(f)).
From the perspective of multi-scale synergistic optimization, porous transmission electrode (PTE) has obvious advantages in PEMWEs. At the microscopic scale, the utilization rate and reactivity of the catalyst were improved by fine adjusting the arrangement and morphology of the catalyst. At the mesoscopic scale, optimizing the aggregate and pore structure promotes the effective transmission and distribution of gas and reduces the mass transmission resistance; at the macro scale, the reasonable design of the electrode structure enhances the contact area between gas and electrolyte and the interfacial reaction kinetics, thus realizing the multi-level synergistic improvement of the overall performance. In recent years, researchers have reported a series of PTEs (e.g., IrO2/Ti,109–111 Ir/Ti,57,112–114 IrO2/TiO2/Ti,115–117 IrOx/Ti118–121etc.) and proved their advantages. In particular, Zhang et al.107 reported a novel porous iridium nanosheet (Ir NS) electrode without ionomer. The Ir NS electrode has high catalytic activity, good electrode conductivity and excellent liquid/gas transport properties. At low catalyst loadings (≤0.3 mg cm−2), the Ir NS requires only a low voltage of 1.65 V and 1.78 V at current densities of 3 and 6 A cm−2, respectively, and exhibits stable performance at high current densities of 5 A cm−2 (Fig. 6(g)–(i)). Compared to conventional ionic polymer-based electrodes, this ionomer-free Ir NS electrode significantly reduces the activation loss and ohmic resistance of PEMWEs and improves the mass specific activity.
At present, there are still some challenges in the multi-scale synergistic optimization of ACL in PEMWEs, including the low utilization rate of active sites caused by uneven catalyst dispersion, the limited mass transfer channels in the catalytic layer, and the mismatch between the structural design of the catalytic layer and the performance of PEMWEs. The future direction should prioritize deeply understanding the intricate interaction mechanism of multi-scale structures within the CL. Additionally, efforts should be made to develop innovative catalysts and support materials, as well as optimize the preparation process of the CL. These advancements will aim to achieve more efficient active site exposure, smoother material transport paths, and a more rational structural layout, ultimately enhancing the electrolytic efficiency and stability of PEMWEs.
6. Summary
This review provides a comprehensive, multi-scale engineering perspective to understand and design the anode CL of PEMWEs. Specifically, it includes: (i) at the atomic scale, the rational construction and stabilization of highly active iridium sites; (ii) at the nano/micro scale, morphology regulation for the improvement of mass transfer; (iii) at the macro scale, the preparation of low-iridium, uniform and continuous CLs. The multi-scale engineering can realize the efficient transport of protons, electrons and molecules at the solid–liquid–gas three-phase reaction interface. In order to stimulate more attractive research works, we propose the following potential directions for future exploration:(i) Bridging the gap between highly active OER catalysts and effective anode CLs. Over the past decade, remarkable achievements have been made in the research and development of Ir-based OER catalysts with high activity. However, it is worth noting that these excellent high-activity performances are almost limited to the three-electrode cell test, and few of them have been successfully translated into the excellent performance of CCM in the actual PEMWEs. It is one of the biggest challenges to effectively transform the catalyst with high activity and suitable for mass production into CLs which shows excellent performance in CCM. In CCM, CLs are not only the core area of electrochemical reaction, but also carry the task of efficient transport of O2, protons, electrons and water molecules, which are very important for the OER process. In order to bridge the gap between academic research and industrial application, researchers should not only optimize the activity and stability of catalysts, but also pay special attention to their conductivity, dispersibility, mass transfer properties and hydrophilicity. These properties are very important for improving the utilization efficiency of catalysts in electrodes, promoting proton/electron and water/gas transport, and enhancing electrode reaction kinetics. At the same time, in the CCM design stage, factors such as the interaction between catalyst and ionomer, catalyst loading, distribution uniformity and microstructure of catalyst layer need to be considered comprehensively to maximize catalyst performance. In addition, it is necessary to develop a catalyst preparation technology suitable for industrial production to realize efficient, low-cost and large-scale production of catalysts.
(ii) In situ characterization of the electrochemical reaction of anode CLs. In order to optimize the performance of PEMWEs, it is particularly important to deeply understand the basic principle of electrochemical reaction on its internal three-phase interface and its dynamic performance in actual operation. However, this task is extremely challenging, because the catalyst/reaction site is located on the CL, and the CL is covered by PTL, BP and end plate, which makes direct observation and monitoring extremely inconvenient. Therefore, advanced characterization techniques and analytical methods are needed to indirectly reveal the electrochemical processes on these hidden interfaces. In 2016, the in situ visualization system constructed by Zhang et al. initially demonstrated its potential to directly observe and discover the real electrochemical reaction and bubble evolution process.122 Looking forward to the future, in situ characterizations of PEMWEs (e.g. electrochemical impedance, in situ Raman spectroscopy, in situ high-speed camera imaging, etc.) will provide a powerful means to solve the problem of unclear internal mechanisms of traditional PEMWEs under complex working conditions. The combination of these methods can not only break the limitation of the membrane electrode as a “black box”, but also capture the dynamic information of the surface/interface of CLs and the reaction process from the microscopic scale to guide the optimal design of catalysts and membrane electrodes.
(iii) Exploration of the degradation mechanism of anode CLs. PEMWEs are operated in extremely complex environments, such as high potential operating conditions, strong acidic medium and frequent gas–liquid alternating flushing, and its catalyst has undergone various degradation mechanisms, which significantly affect its performance. These mechanisms include the dissolution of the catalyst, morphological changes of the catalyst (agglomeration, size changes, etc.), redeposition and covering of active sites on the surface of the catalyst, corrosion and deformation of carrier materials, and electrochemical and stress concentration at the interface between the CL and the PTL. It is particularly noteworthy that when the iridium content in the catalyst is low, these unfavorable evolution trends in microstructure are more obvious, which further aggravates the attenuation of catalyst performance. Researchers should focus on and strengthen the research on the failure mechanism of the CL in PEMWEs. This includes, but is not limited to, observing the change of the microstructure of the CL by high-resolution microscopic imaging technology, monitoring the dynamic evolution of catalytic activity and stability by in situ electrochemical testing, and combining theoretical calculation to simulate the interaction between the catalytic reaction path and the interface. Through in-depth analysis of the key mechanisms such as material degradation, active site inactivation and interface interaction changes in the CL during long-term operation, it can not only provide a theoretical basis for designing more durable and efficient catalysts, but also promote the optimization and upgrading of the overall electrolytic cell system.
Data availability
In the preparation of this comprehensive review article titled “Multiscale engineering of anode catalyst layers in proton exchange membrane water electrolyzers” we have meticulously gathered and analyzed data from a wide range of sources to ensure the accuracy and comprehensiveness of our findings. The data utilized in this study encompasses both primary and secondary research materials including but not limited to published articles, scholarly books, government reports, industry analyses, and online databases. All data used in this review was obtained in accordance with ethical guidelines for research integrity and responsible data sharing. We respect the privacy and confidentiality of individuals and institutions whose data has been referenced, ensuring that no sensitive information is disclosed without appropriate permissions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 22179046 and 22279040), the Natural Science Foundation of Shaanxi Province (2023-JC-QN-0497), and the Jilin Province Science and Technology Development Plan (no. 20220402006GH).Notes and references
- A. Li, P. Zhang, E. Kan and J. Gong, Wettability adjustment to enhance mass transfer for heterogeneous electrocatalysis and photocatalysis, eScience, 2024, 4, 100157 CrossRef.
- Y. Liu, X. Liang, H. Chen, R. Gao, L. Shi, L. Yang and X. Zou, Iridium-containing water-oxidation catalysts in acidic electrolyte, Chin. J. Catal., 2021, 42, 1054–1077 CrossRef CAS.
- Q. Wu, Y. Wang, K. Zhang, Z. Xie, K. Sun, W. An, X. Liang and X. Zou, Advances and status of anode catalysts for proton exchange membrane water electrolysis technology, Mater. Chem. Front., 2023, 7, 1025–1045 RSC.
- L. Tao, F. Lv, D. Wang, H. Luo, F. Lin, H. Gong, H. Mi, S. Wang, Q. Zhang, L. Gu, M. Luo and S. Guo, Mass-efficient catalyst layer of hierarchical sub-nanosheets on nanowire for practical proton exchange membrane electrolyzer, Joule, 2024, 8, 450–460 CrossRef CAS.
- X. Wang, M. Yu and X. Feng, Electronic structure regulation of noble metal-free materials toward alkaline oxygen electrocatalysis, eScience, 2023, 3, 100141 CrossRef.
- D. Yang, L. Cao, J. Huang, G. Jiao, D. Wang, Q. Liu, G. Li, C. He and L. Feng, Reversible active bridging sulfur sites grafted on Ni3S2 nanobelt arrays for efficient hydrogen evolution reaction, J. Colloid Interface Sci., 2023, 649, 194–202 CrossRef CAS PubMed.
- Q. Liu, K. Liu, J. Huang, C. Hui, X. Li and L. Feng, A review of modulation strategies for improving the catalytic performance of transition metal sulfide self-supported electrodes for the hydrogen evolution reaction, Dalton Trans., 2024, 53, 3959–3969 RSC.
- H. Chen, L. Shi, K. Sun, K. Zhang, Q. Liu, J. Ge, X. Liang, B. Tian, Y. Huang, Z. Shi, Z. Wang, W. Zhang, M. Liu and X. Zou, Protonated Iridate Nanosheets with a Highly Active and Stable Layered Perovskite Framework for Acidic Oxygen Evolution, ACS Catal., 2022, 12, 8658–8666 CrossRef CAS.
- M. Carmo, D. L. Fritz, J. Mergel and D. Stolten, A comprehensive review on PEM water electrolysis, Int. J. Hydrogen Energy, 2013, 38, 4901–4934 CrossRef CAS.
- H. Gao, Z. Xiao, S. Du, T. Liu, Y. C. Huang, J. Shi, Y. Zhu, G. Huang, B. Zhou, Y. He, C. L. Dong, Y. Li, R. Chen and S. Wang, Reducing the Ir–O Coordination Number in Anodic Catalysts based on IrOx Nanoparticles towards Enhanced Proton-exchange-membrane Water Electrolysis, Angew. Chem., Int. Ed., 2023, 62, e202313954 CrossRef CAS PubMed.
- Q. Liu, J. Huang, Y. Zhao, L. Cao, K. Li, N. Zhang, D. Yang, L. Feng and L. Feng, Tuning the coupling interface of ultrathin Ni3S2@NiV-LDH heterogeneous nanosheet electrocatalysts for improved overall water splitting, Nanoscale, 2019, 11, 8855–8863 RSC.
- Q. Liu, J. Huang, L. Cao, K. Kajiyoshi, K. Li, Y. Feng, C. Fu, L. Kou and L. Feng, V-Doping Triggered Formation and Structural Evolution of Dendritic Ni3S2@NiO Core–Shell Nanoarrays for Accelerating Alkaline Water Splitting, ACS Sustainable Chem. Eng., 2020, 8, 6222–6233 CrossRef CAS.
- X. Zou, L. Wang, Q. Pan and X. Liang, Ensuring Stability of Anode Catalysts in PEMWE: From Material Design to Practical Application, ChemSusChem, 2024, e202401220 Search PubMed.
- T. Yang, X. Yang, N. Gao, M. Tang, X. Wang, C. Liu, W. Xing and J. Ge, High density iridium synergistic sites boosting CO-tolerate performance for PEMFC anode, eScience, 2024, 4, 100230 CrossRef.
- M. Fan, X. Liang, H. Chen and X. Zou, Low-iridium electrocatalysts for acidic oxygen evolution, Dalton Trans., 2020, 49, 15568–15573 RSC.
- L. An, C. Wei, M. Lu, H. Liu, Y. Chen, G. G. Scherer, A. C. Fisher, P. Xi, Z. J. Xu and C.-H. Yan, Recent Development of Oxygen Evolution Electrocatalysts in Acidic Environment, Adv. Mater., 2021, 33, 2006328 CrossRef CAS PubMed.
- Z. Lin, T. Wang and Q. Li, Designing active and stable Ir-based catalysts for the acidic oxygen evolution reaction, Ind. Chem. Mater., 2023, 1, 299–311 RSC.
- M. Chen, N. Kitiphatpiboon, C. Feng, A. Abudula, Y. Ma and G. Guan, Recent progress in transition-metal-oxide-based electrocatalysts for the oxygen evolution reaction in natural seawater splitting: A critical review, eScience, 2023, 3, 100111 CrossRef.
- H. Su, C. Yang, M. Liu, X. Zhang, W. Zhou, Y. Zhang, K. Zheng, S. Lian and Q. Liu, Tensile straining of iridium sites in manganese oxides for proton-exchange membrane water electrolysers, Nat. Commun., 2024, 15, 95 CrossRef PubMed.
- S. Wang, T. Shen, C. Yang, G. Luo and D. Wang, Engineering Iridium-Based Oxygen Evolution Reaction Electrocatalysts for Proton Exchange Membrane Water Electrolyzers, ACS Catal., 2023, 13, 8670–8691 CrossRef CAS.
- L. She, G. Zhao, T. Ma, J. Chen, W. Sun and H. Pan, On the Durability of Iridium-Based Electrocatalysts toward the Oxygen Evolution Reaction under Acid Environment, Adv. Funct. Mater., 2022, 32, 2108465 CrossRef CAS.
- H. Yu, J. Ke and Q. Shao, Two Dimensional Ir-Based Catalysts for Acidic OER, Small, 2023, 19, 2304307 CrossRef CAS PubMed.
- H. Li, Y. Xu, N. Lv, Q. Zhang, X. Zhang, Z. Wei, Y. Wang, H. Tang and H. Pan, Ti-Doped SnO2 Supports IrO2 Electrocatalysts for the Oxygen Evolution Reaction (OER) in PEM Water Electrolysis, ACS Sustainable Chem. Eng., 2023, 11, 1121–1132 CrossRef CAS.
- H. Wu, Y. Wang, Z. Shi, X. Wang, J. Yang, M. Xiao, J. Ge, W. Xing and C. Liu, Recent developments of iridium-based catalysts for the oxygen evolution reaction in acidic water electrolysis, J. Mater. Chem. A, 2022, 10, 13170–13189 RSC.
- G. Gao, Z. Sun, X. Chen, G. Zhu, B. Sun, Y. Yamauchi and S. Liu, Recent advances in Ru/Ir-based electrocatalysts for acidic oxygen evolution reaction, Appl. Catal., B, 2024, 343, 123584 CrossRef CAS.
- A. Li, S. Kong, K. Adachi, H. Ooka, K. Fushimi, Q. Jiang, H. Ofuchi, S. Hamamoto, M. Oura, K. Higashi, T. Kaneko, T. Uruga, N. Kawamura, D. Hashizume and R. Nakamura, Atomically dispersed hexavalent iridium oxide from MnO2 reduction for oxygen evolution catalysis, Science, 2024, 384, 666–670 CrossRef CAS PubMed.
- J. Kibsgaard and I. Chorkendorff, Considerations for the scaling-up of water splitting catalysts, Nat. Energy, 2019, 4, 430–433 CrossRef.
- S. Shin, T. Kwon, K. Kim, M. Kim, M. H. Kim and Y. Lee, Single-Phase Perovskite SrIrO3 Nanofibers as a Highly Efficient Electrocatalyst for a pH-Universal Oxygen Evolution Reaction, ACS Appl. Energy Mater., 2022, 5, 6146–6154 CrossRef CAS.
- P. C. Shih, J. Kim, C. J. Sun and H. Yang, Single-Phase Pyrochlore Y2Ir2O7 Electrocatalyst on the Activity of Oxygen Evolution Reaction, ACS Appl. Energy Mater., 2018, 1, 3992–3998 CrossRef CAS.
- S. Kumari, B. P. Ajayi, B. Kumar, J. B. Jasinski, M. K. Sunkara and J. M. Spurgeon, A low-noble-metal W1−xIrxO3−δ water oxidation electrocatalyst for acidic media via rapid plasma synthesis, Energy Environ. Sci., 2017, 10, 2432–2440 RSC.
- X. Xiong, J. Tang, Y. Ji, W. Xue, H. Wang, C. Liu, H. Zeng, Y. Dai, H. J. Peng, T. Zheng, C. Xia, X. Liu and Q. Jiang, High-Efficiency Iridium-Yttrium Alloy Catalyst for Acidic Water Electrolysis, Adv. Energy Mater., 2024, 14, 2304479 CrossRef CAS.
- C. E. Moore, J. Eastcott, M. Cimenti, N. Kremliakova and E. L. Gyenge, Novel methodology for ex situ characterization of iridium oxide catalysts in voltage reversal tolerant proton exchange membrane fuel cell anodes, J. Power Sources, 2019, 417, 53–60 CrossRef CAS.
- T. Liu, C. Chen, Z. Pu, Q. Huang, X. Zhang, A. M. Al-Enizi, A. Nafady, S. Huang, D. Chen and S. Mu, Non-Noble-Metal-Based Electrocatalysts for Acidic Oxygen Evolution Reaction: Recent Progress, Challenges, and Perspectives, Small, 2024, 2405399 CrossRef PubMed.
- L. Wang, R. Du, X. Liang, Y. Zou, X. Zhao, H. Chen and X. Zou, Optimizing Edge Active Sites via Intrinsic In-Plane Iridium Deficiency in Layered Iridium Oxides for Oxygen Evolution Electrocatalysis, Adv. Mater., 2024, 36, 2312608 CrossRef CAS PubMed.
- C. Yang, W. Ling, Y. Zhu, Y. Yang, S. Dong, C. Wu, Z. Wang, S. Yang, J. Li, G. Wang, Y. Huang, B. Yang, Q. Cheng, Z. Liu and H. Yang, Surface hydroxylation engineering to boost oxygen evolution reaction on IrO2/TiO2 for PEM water electrolyzer, Appl. Catal., B, 2024, 358, 124462 CrossRef CAS.
- L. Chen, W. Zhao, J. Zhang, M. Liu, Y. Jia, R. Wang and M. Chai, Recent Research on Iridium-Based Electrocatalysts for Acidic Oxygen Evolution Reaction from the Origin of Reaction Mechanism, Small, 2024, 2403845 CrossRef CAS PubMed.
- I. C. Man, H.-Y. Su, F. Calle-Vallejo, H. A. Hansen, J. I. Martínez, N. G. Inoglu, J. Kitchin, T. F. Jaramillo, J. K. Nørskov and J. Rossmeisl, Universality in Oxygen Evolution Electrocatalysis on Oxide Surfaces, ChemCatChem, 2011, 3, 1159–1165 CrossRef CAS.
- H. Chen, L. Shi, X. Liang, L. Wang, T. Asefa and X. Zou, Optimization of Active Sites via Crystal Phase, Composition, and Morphology for Efficient Low-Iridium Oxygen Evolution Catalysts, Angew. Chem., Int. Ed., 2020, 59, 19654–19658 CrossRef CAS PubMed.
- X. Liang, L. Shi, R. Cao, G. Wan, W. Yan, H. Chen, Y. Liu and X. Zou, Perovskite-Type Solid Solution Nano-Electrocatalysts Enable Simultaneously Enhanced Activity and Stability for Oxygen Evolution, Adv. Mater., 2020, 32, 2001430 CrossRef CAS PubMed.
- J. Zhang, X. Cao, Y. F. Jiang, S. F. Hung, W. Liu, H. B. Yang, C. Q. Xu, D. S. Li, T. Zhang, Y. Li, J. Li and B. Liu, Surface enrichment of Ir on the IrRu alloy for efficient and stable water oxidation catalysis in acid, Chem. Sci., 2022, 13, 12114–12121 RSC.
- A. D. Bertelsen, M. Kløve, N. L. N. Broge, M. Bondesgaard, R. B. Stubkjær, A.-C. Dippel, Q. Li, R. Tilley, M. R. Vogel Jørgensen and B. B. Iversen, Formation Mechanism and Hydrothermal Synthesis of Highly Active Ir1−xRuxO2 Nanoparticles for the Oxygen Evolution Reaction, J. Am. Chem. Soc., 2024, 146, 23729–23740 CrossRef CAS PubMed.
- J. Ruiz Esquius, G. Algara-Siller, I. Spanos, S. J. Freakley, R. Schlögl and G. J. Hutchings, Preparation of Solid Solution and Layered IrOx–Ni(OH)2 Oxygen Evolution Catalysts: Toward Optimizing Iridium Efficiency for OER, ACS Catal., 2020, 10, 14640–14648 CrossRef CAS.
- Y. Wang, Z. Zhao, X. Liang, X. Zhao, X. Wang, S. Jana, Y. A. Wu, Y. Zou, L. Li, H. Chen and X. Zou, Supported IrO2 Nanocatalyst with Multilayered Structure for Proton Exchange Membrane Water Electrolysis, Adv. Mater., 2024, 2407717 CrossRef CAS PubMed.
- R. Zhang, N. Dubouis, M. Ben Osman, W. Yin, M. T. Sougrati, D. A. D. Corte, D. Giaume and A. Grimaud, A Dissolution/Precipitation Equilibrium on the Surface of Iridium-Based Perovskites Controls Their Activity as Oxygen Evolution Reaction Catalysts in Acidic Media, Angew. Chem., Int. Ed., 2019, 58, 4571–4575 CrossRef CAS PubMed.
- J. Edgington, N. Schweitzer, S. Alayoglu and L. C. Seitz, Constant Change: Exploring Dynamic Oxygen Evolution Reaction Catalysis and Material Transformations in Strontium Zinc Iridate Perovskite in Acid, J. Am. Chem. Soc., 2021, 143, 9961–9971 CrossRef CAS PubMed.
- H. J. Song, H. Yoon, B. Ju and D.-W. Kim, Highly Efficient Perovskite-Based Electrocatalysts for Water Oxidation in Acidic Environments: A Mini Review, Adv. Energy Mater., 2021, 11, 2002428 CrossRef CAS.
- J. Li, L. Zheng, B. Huang, Y. Hu, L. An, Y. Yao, M. Lu, J. Jin, N. Zhang, P. Xi and C. H. Yan, Activated Ni–O–Ir Enhanced Electron Transfer for Boosting Oxygen Evolution Reaction Activity of LaNi1−xIrxO3, Small, 2022, 18, 2204723 CrossRef CAS PubMed.
- L. Zhang, H. Jang, Z. Li, H. Liu, M. G. Kim, X. Liu and J. Cho, SrIrO3 modified with laminar Sr2IrO4 as a robust bifunctional electrocatalyst for overall water splitting in acidic media, Chem. Eng. J., 2021, 419, 129604 CrossRef CAS.
- M. Retuerto, L. Pascual, O. Piqué, P. Kayser, M. A. Salam, M. Mokhtar, J. A. Alonso, M. Peña, F. Calle-Vallejo and S. Rojas, How oxidation state and lattice distortion influence the oxygen evolution activity in acid of iridium double perovskites, J. Mater. Chem. A, 2021, 9, 2980–2990 RSC.
- M. Retuerto, L. Pascual, J. Torrero, M. A. Salam, Á. Tolosana-Moranchel, D. Gianolio, P. Ferrer, P. Kayser, V. Wilke, S. Stiber, V. Celorrio, M. Mokthar, D. G. Sanchez, A. S. Gago, K. A. Friedrich, M. A. Peña, J. A. Alonso and S. Rojas, Highly active and stable OER electrocatalysts derived from Sr2MIrO6 for proton exchange membrane water electrolyzers, Nat. Commun., 2022, 13, 7935 CrossRef CAS PubMed.
- S. Ganguly, R. Datta, P. Basera, S. K. De, K. K. Mistry, C. Loha and S. Ghosh, A-Site Modulation of Co–Ir Based Double Perovskite Oxides (A2CoIrO6, A = Sr, Nd, Pr, and Sm) for Maximization of Water Oxidation and Hybrid Electrolysis Derived Isopropanol Upconversion in Acid Medium, ACS Sustainable Chem. Eng., 2024, 12, 849–859 CrossRef CAS.
- C. Shang, C. Cao, D. Yu, Y. Yan, Y. Lin, H. Li, T. Zheng, X. Yan, W. Yu, S. Zhou and J. Zeng, Electron Correlations Engineer Catalytic Activity of Pyrochlore Iridates for Acidic Water Oxidation, Adv. Mater., 2019, 31, 1805104 CrossRef PubMed.
- L. Zhu, C. Ma, Z. Wang, X. Gong, L. Cao and J. Yang, Tuning the hybridization state of Ir–O to improve the OER activity and stability of iridium pyrochlore via Zn doping, Appl. Surf. Sci., 2022, 576, 151840 CrossRef CAS.
- D. Lebedev, M. Povia, K. Waltar, P. M. Abdala, I. E. Castelli, E. Fabbri, M. V. Blanco, A. Fedorov, C. Copéret, N. Marzari and T. J. Schmidt, Highly Active and Stable Iridium Pyrochlores for Oxygen Evolution Reaction, Chem. Mater., 2017, 29, 5182–5191 CrossRef CAS.
- Q. Zhang, T. Liu, H. Guo, Y. Chen, Y. Di, Z. Zhang and F. Wang, Lead-Induced Microstrain in Synthesis and Manipulation of Porous Pyrochlore for Boosting Oxygen Evolution Reaction, Adv. Funct. Mater., 2024, 34, 2306176 CrossRef CAS.
- L. C. Seitz, C. F. Dickens, K. Nishio, Y. Hikita, J. Montoya, A. Doyle, C. Kirk, A. Vojvodic, H. Y. Hwang, J. K. Norskov and T. F. Jaramillo, A highly active and stable IrOx/SrIrO3 catalyst for the oxygen evolution reaction, Science, 2016, 353, 1011–1014 CrossRef CAS PubMed.
- C. Liu, M. Carmo, G. Bender, A. Everwand, T. Lickert, J. L. Young, T. Smolinka, D. Stolten and W. Lehnert, Performance enhancement of PEM electrolyzers through iridium-coated titanium porous transport layers, Electrochem. Commun., 2018, 97, 96–99 CrossRef CAS.
- Y. Lin, Y. Dong, X. Wang and L. Chen, Electrocatalysts for the Oxygen Evolution Reaction in Acidic Media, Adv. Mater., 2023, 35, 2210565 CrossRef CAS PubMed.
- M. Li, J. Qi, H. Zeng, J. Chen, Z. Liu, L. Gu, J. Wang, Y. Zhang, M. Wang, Y. Zhang, X. Lu and C. Yang, Structural impacts on the degradation behaviors of Ir-based electrocatalysts during water oxidation in acid, J. Colloid Interface Sci., 2024, 674, 108–117 CrossRef CAS PubMed.
- J. Edgington, R. Vicente, S. Vispute, R. Li, M. E. Sweers, S. R. Sullivan, P. S. Fernandez and L. C. Seitz, Dynamics of Highly Active Ln3IrO7 Catalysts for the Oxygen Evolution Reaction in Acid, Adv. Energy Mater., 2024, 2402333 CrossRef.
- L. Zhu, C. Ma, D. Li, X. Shao, L. Cao and J. Yang, Designing of Hexagonal Nanosheets with Edge-Sharing [IrO6] Octahedral Crystals for Efficient and Stable Acidic Water Splitting, Adv. Funct. Mater., 2024, 34, 2313375 CrossRef CAS.
- L. Yang, G. Yu, X. Ai, W. Yan, H. Duan, W. Chen, X. Li, T. Wang, C. Zhang, X. Huang, J. S. Chen and X. Zou, Efficient oxygen evolution electrocatalysis in acid by a perovskite with face-sharing IrO6 octahedral dimers, Nat. Commun., 2018, 9, 5236 CrossRef CAS PubMed.
- R. Gao, Q. Zhang, H. Chen, X. Chu, G. D. Li and X. Zou, Efficient acidic oxygen evolution reaction electrocatalyzed by iridium-based 12L-perovskites comprising trinuclear face-shared IrO6 octahedral strings, J. Energy Chem., 2020, 47, 291–298 CrossRef.
- W. Feng, H. Chen, Q. Zhang, R. Gao and X. Zou, Lanthanide-regulated oxygen evolution activity of face-sharing IrO6 dimers in 6H-perovskite electrocatalysts, Chin. J. Catal., 2020, 41, 1692–1697 CrossRef CAS.
- Q. Zhang, X. Liang, H. Chen, W. Yan, L. Shi, Y. Liu, J. Li and X. Zou, Identifying Key Structural Subunits and Their Synergism in Low-Iridium Triple Perovskites for Oxygen Evolution in Acidic Media, Chem. Mater., 2020, 32, 3904–3910 CrossRef CAS.
- C. W. Song, J. Lim, H. B. Bae and S.-Y. Chung, Discovery of crystal structure–stability correlation in iridates for oxygen evolution electrocatalysis in acid, Energy Environ. Sci., 2020, 13, 4178–4188 RSC.
- M. Kost, M. Kornherr, P. Zehetmaier, H. Illner, D. S. Jeon, H. Gasteiger, M. Döblinger, D. Fattakhova-Rohlfing and T. Bein, Chemical Epitaxy of Iridium Oxide on Tin Oxide Enhances Stability of Supported OER Catalyst, Small, 2024, 20, 2404118 CrossRef CAS PubMed.
- J. Islam, S. K. Kim, M. M. Rahman, P. T. Thien, M. J. Kim, H. S. Cho, C. Lee, J. H. Lee and S. Lee, The effect of iridium content in boron carbide-supported iridium catalyst on the activity and stability of proton exchange membrane water electrolyzer, Mater. Today Energy, 2023, 32, 101237 CrossRef CAS.
- J. Park, E. Liu, S. Angizi, A. Abdellah, E. Y. Kirici and D. Higgins, Formation of Core-Shell Ir@TiO2 Nanoparticles through Hydrogen Treatment as Acidic Oxygen Evolution Reaction Catalysts, Adv. Funct. Mater., 2024, 2408848 CrossRef CAS.
- Y. Qin, Y. Wang, R. Wen, L. Wang, M. Dou and F. Wang, Interface-Engineering Strategy for Boosting Low-Ir Catalytic Water Oxidation Using a Conductive Ti4O7 Support, ACS Catal., 2024, 14, 13915–13926 CrossRef CAS.
- W. Gou, Z. Xia, X. Tan, Q. Xue, F. Ye, S. Dai, M. Zhang, R. Si, Y. Zou, Y. Ma, J. C. Ho and Y. Qu, Highly active and stable amorphous IrOx/CeO2 nanowires for acidic oxygen evolution, Nano Energy, 2022, 104, 107960 CrossRef CAS.
- F. Bizzotto, J. Quinson, J. Schröder, A. Zana and M. Arenz, Surfactant-free colloidal strategies for highly dispersed and active supported IrO2 catalysts: Synthesis and performance evaluation for the oxygen evolution reaction, J. Catal., 2021, 401, 54–62 CrossRef CAS.
- J. Zhang, G. Wang, Z. Liao, P. Zhang, F. Wang, X. Zhuang, E. Zschech and X. Feng, Iridium nanoparticles anchored on 3D graphite foam as a bifunctional electrocatalyst for excellent overall water splitting in acidic solution, Nano Energy, 2017, 40, 27–33 CrossRef CAS.
- X. Wu, B. Feng, W. Li, Y. Niu, Y. Yu, S. Lu, C. Zhong, P. Liu, Z. Tian, L. Chen, W. Hu and C. M. Li, Metal-support interaction boosted electrocatalysis of ultrasmall iridium nanoparticles supported on nitrogen doped graphene for highly efficient water electrolysis in acidic and alkaline media, Nano Energy, 2019, 62, 117–126 CrossRef CAS.
- M. Kost, M. Kornherr, P. Zehetmaier, H. Illner, D. S. Jeon, H. Gasteiger, M. Döblinger, D. Fattakhova-Rohlfing and T. Bein, Chemical Epitaxy of Iridium Oxide on Tin Oxide Enhances Stability of Supported OER Catalyst, Small, 2024, 2404118 CrossRef CAS PubMed.
- L. Y. Chueh, C. H. Kuo, R. H. Yang, D.-H. Tsai, M. H. Tsai, C. C. Yang, H. Y. Chen, C. H. Wang and Y. T. Pan, WOx nanowire supported ultra-fine Ir-IrOx nanocatalyst with compelling OER activity and durability, Chem. Eng. J., 2023, 464, 142613 CrossRef CAS.
- Y. Wu, C. Guo, R. Yao, K. Zhang, J. Li and G. Liu, Modulating Carrier Oxygen Vacancies to Enhance Strong Oxide-Support Interaction in IrO2/Nb2O5−x Catalysts for Promoting Acidic Oxygen Evolution Reaction, Adv. Funct. Mater., 2024, 2410193 CrossRef.
- Q. Huang, S. Zhuang, Y. Zheng, X. Peng, Z. Ye and D. Li, Boron-incorporated IrO2-Ta2O5 coating as an efficient electrocatalyst for acidic oxygen evolution reaction, Chem. Eng. J., 2024, 491, 152040 CrossRef CAS.
- H. Lv, H. Yao, Y. Sun, D. Hu, Y. Gao, J. Chen and C. Zhang, Ultrafine IrNi nanoparticles supported onto titanium nitride as low-iridium and highly active OER electrocatalysts for proton exchange membrane water electrolysis, Int. J. Hydrogen Energy, 2024, 84, 634–640 CrossRef CAS.
- L. Moriau, M. Bele, Ž. Marinko, F. Ruiz-Zepeda, G. Koderman Podboršek, M. Šala, A. K. Šurca, J. Kovač, I. Arčon, P. Jovanovič, N. Hodnik and L. Suhadolnik, Effect of the Morphology of the High-Surface-Area Support on the Performance of the Oxygen-Evolution Reaction for Iridium Nanoparticles, ACS Catal., 2021, 11, 670–681 CrossRef CAS PubMed.
- X. Duan, H. Liu, W. Zhang, Q. Ma, Q. Xu, L. Khotseng and H. Su, Boosting the efficiency of IrOx nanoparticles with micron-flower Nb4N5 support for oxygen evolution reaction in polymer electrolyte membrane water electrolyzer, Electrochim. Acta, 2023, 470, 143271 CrossRef CAS.
- Y. Wang, M. Zhang, Z. Kang, L. Shi, Y. Shen, B. Tian, Y. Zou, H. Chen and X. Zou, Nano-metal diborides-supported anode catalyst with strongly coupled TaOx/IrO2 catalytic layer for low-iridium-loading proton exchange membrane electrolyzer, Nat. Commun., 2023, 14, 5119 CrossRef CAS PubMed.
- C. Zhao, S. Yuan, X. Cheng, S. Shen, N. Zhan, R. Wu, X. Mei, Q. Wang, L. An, X. Yan and J. Zhang, Agglomerate Engineering to Boost PEM Water Electrolyzer Performance, Adv. Energy Mater., 2024, 2401588 CrossRef CAS.
- W. Liang, C. Wang, J. Li, J. Yin, Z. Wu, S. Li and Y. Du, Ir-Doped Bilayer Heterojunction Hollow Nanoboxes for Electrocatalytic Oxygen Evolution, Inorg. Chem., 2023, 62, 20072–20079 CrossRef CAS PubMed.
- Z. Zhu, W. Hu, X. Wu, Q. Zhang, Y. Hu, Q. Yan, X. Wang and W. Yuan, In situ self-assembled macroporous interconnected nanosheet arrays of Ni-1,3,5-benzenetricarboxylate metal–organic framework on Ti mesh as high-performance oxygen evolution electrodes, J. Colloid Interface Sci., 2023, 639, 274–283 CrossRef CAS PubMed.
- J. Qiao, D. Dong, Q. Lin, L. Kong, W. Jiang, W. He and Z. Sun, Zr and Ir Codoped Perovskite Nanosheets for Efficient and Stable Oxygen Evolution, ACS Appl. Energy Mater., 2023, 6, 10593–10599 CrossRef CAS.
- S. Liu, Y. Zhang, X. Mao, L. Li, Y. Zhang, L. Li, Y. Pan, X. Li, L. Wang, Q. Shao, Y. Xu and X. Huang, Ultrathin perovskite derived Ir-based nanosheets for high-performance electrocatalytic water splitting, Energy Environ. Sci., 2022, 15, 1672–1681 RSC.
- B. Jiang, Y. Guo, J. Kim, A. E. Whitten, K. Wood, K. Kani, A. E. Rowan, J. Henzie and Y. Yamauchi, Mesoporous Metallic Iridium Nanosheets, J. Am. Chem. Soc., 2018, 140, 12434–12441 CrossRef CAS PubMed.
- E. Ortel, T. Reier, P. Strasser and R. Kraehnert, Mesoporous IrO2 Films Templated by PEO-PB-PEO Block-Copolymers: Self-Assembly, Crystallization Behavior, and Electrocatalytic Performance, Chem. Mater., 2011, 23, 3201–3209 CrossRef CAS.
- S. Chatterjee, S. Intikhab, L. Profitt, Y. Li, V. Natu, R. Gawas and J. Snyder, Nanoporous multimetallic Ir alloys as efficient and stable electrocatalysts for acidic oxygen evolution reactions, J. Catal., 2021, 393, 303–312 CrossRef CAS.
- Y. Pi, J. Guo, Q. Shao and X. Huang, Highly Efficient Acidic Oxygen Evolution Electrocatalysis Enabled by Porous Ir–Cu Nanocrystals with Three-Dimensional Electrocatalytic Surfaces, Chem. Mater., 2018, 30, 8571–8578 CrossRef CAS.
- Y.-T. Kim, P. P. Lopes, S.-A. Park, A. Y. Lee, J. Lim, H. Lee, S. Back, Y. Jung, N. Danilovic, V. Stamenkovic, J. Erlebacher, J. Snyder and N. M. Markovic, Balancing activity, stability and conductivity of nanoporous core-shell iridium/iridium oxide oxygen evolution catalysts, Nat. Commun., 2017, 8, 1449 CrossRef PubMed.
- Z. Zhuang, Y. Wang, C. Q. Xu, S. Liu, C. Chen, Q. Peng, Z. Zhuang, H. Xiao, Y. Pan, S. Lu, R. Yu, W. C. Cheong, X. Cao, K. Wu, K. Sun, Y. Wang, D. Wang, J. Li and Y. Li, Three-dimensional open nano-netcage electrocatalysts for efficient pH-universal overall water splitting, Nat. Commun., 2019, 10, 4875 CrossRef CAS PubMed.
- J. Feng, F. Lv, W. Zhang, P. Li, K. Wang, C. Yang, B. Wang, Y. Yang, J. Zhou, F. Lin, G. C. Wang and S. Guo, Iridium-Based Multimetallic Porous Hollow Nanocrystals for Efficient Overall-Water-Splitting Catalysis, Adv. Mater., 2017, 29, 1703798 CrossRef PubMed.
- L. Ding, W. Wang, Z. Xie, K. Li, S. Yu, C. B. Capuano, A. Keane, K. Ayers and F.-Y. Zhang, Highly Porous Iridium Thin Electrodes with Low Loading and Improved Reaction Kinetics for Hydrogen Generation in PEM Electrolyzer Cells, ACS Appl. Mater. Interfaces, 2023, 15, 24284–24295 CrossRef CAS PubMed.
- Y. Wang, X. Guo, X. Wang, J. Huang, L. Yin, W. Zhu and Z. Zhuang, Construction of steady-active self-supported porous Ir-based electrocatalysts for the oxygen evolution reaction, Chem. Commun., 2023, 59, 1813–1816 RSC.
- M. Faustini, M. Giraud, D. Jones, J. Rozière, M. Dupont, T. R. Porter, S. Nowak, M. Bahri, O. Ersen, C. Sanchez, C. Boissière, C. Tard and J. Peron, Hierarchically Structured Ultraporous Iridium-Based Materials: A Novel Catalyst Architecture for Proton Exchange Membrane Water Electrolyzers, Adv. Energy Mater., 2019, 9, 1802136 CrossRef.
- L. Liu, T. Huang, X. Yang, S. Liu, S. Wang, L. Xiang, G. Wang and L. Kuai, Microdrop-confined synthesis and regulation of porous hollow Ir-based catalysts for the mass transfer-enhanced electrolysis of pure water, Sci. Bull., 2024, 69, 1081–1090 CrossRef CAS PubMed.
- S. Duran, A. Grimaud, M. Faustini and J. Peron, Porosity as a Morphology Marker to Probe the Degradation of IrO2 Anode Catalyst Layers in Proton Exchange Membrane Water Electrolyzers, Chem. Mater., 2023, 35, 8590–8598 CrossRef CAS.
- M. Bernt, A. Siebel and H. A. Gasteiger, Analysis of Voltage Losses in PEM Water Electrolyzers with Low Platinum Group Metal Loadings, J. Electrochem. Soc., 2018, 165, F305 CrossRef CAS.
- M. Bernt and H. A. Gasteiger, Influence of Ionomer Content in IrO2/TiO2 Electrodes on PEM Water Electrolyzer Performance, J. Electrochem. Soc., 2016, 163, F3179 CrossRef CAS.
- H. Liu, X. Wang, K. Lao, L. Wen, M. Huang, J. Liu, T. Hu, B. Hu, S. Xie, S. Li, X. Fang, N. Zheng and H. B. Tao, Optimizing Ionomer Distribution in Anode Catalyst Layer for Stable Proton Exchange Membrane Water Electrolysis, Adv. Mater., 2024, 36, 2402780 CrossRef CAS PubMed.
- H. Lv, Y. Sun, S. Wang, J. Chen, Y. Gao, D. Hu, H. Yao and C. Zhang, Synergistic gradient distribution of IrO2/TiNx ratio and ionomer content reduces the internal voltage loss of the anode catalytic layer in PEM water electrolysis, Appl. Energy, 2024, 363, 123012 CrossRef CAS.
- D. Böhm, M. Beetz, C. Gebauer, M. Bernt, J. Schröter, M. Kornherr, F. Zoller, T. Bein and D. Fattakhova-Rohlfing, Highly conductive titania supported iridium oxide nanoparticles with low overall iridium density as OER catalyst for large-scale PEM electrolysis, Appl. Mater. Today, 2021, 24, 101134 CrossRef.
- C. Rozain, E. Mayousse, N. Guillet and P. Millet, Influence of iridium oxide loadings on the performance of PEM water electrolysis cells: Part II – Advanced oxygen electrodes, Appl. Catal., B, 2016, 182, 123–131 CrossRef CAS.
- Z. Xie, H. Chen, X. Wang, Y. A. Wu, Z. Wang, S. Jana, Y. Zou, X. Liang, X. Zhao and X. Zou, Honeycomb-Structured IrOx Foam Platelets as the Building Block of Anode Catalyst Layer in PEM Water Electrolyzer, Angew. Chem., Int. Ed., 2024, e202415032 Search PubMed.
- Z. Xie, L. Ding, S. Yu, W. Wang, C. B. Capuano, A. Keane, K. Ayers, D. A. Cullen, H. M. Meyer and F. Y. Zhang, Ionomer-free nanoporous iridium nanosheet electrodes with boosted performance and catalyst utilization for high-efficiency water electrolyzers, Appl. Catal., B, 2024, 341, 123298 CrossRef CAS.
- Z. Xie, X. Liang, Z. Kang, Y. Zou, X. Wang, Y. A. Wu, G. King, Q. Liu, Y. Huang, X. Zhao, H. Chen and X. Zou, High-Porosity, Layered Iridium Oxide as an Efficient, Durable Anode Catalyst for Water Splitting, CCS Chem., 2024, 1–13 Search PubMed.
- Y. J. Park, J. Lee, Y. S. Park, J. Yang, M. J. Jang, J. Jeong, S. Choe, J. W. Lee, J. D. Kwon and S. M. Choi, Electrodeposition of High-Surface-Area IrO2 Films on Ti Felt as an Efficient Catalyst for the Oxygen Evolution Reaction, Front. Chem., 2020, 8, 593272 CrossRef CAS PubMed.
- M. Bühler, F. Hegge, P. Holzapfel, M. Bierling, M. Suermann, S. Vierrath and S. Thiele, Optimization of anodic porous transport electrodes for proton exchange membrane water electrolyzers, J. Mater. Chem. A, 2019, 7, 26984–26995 RSC.
- R. Shan, Z. Zhang, M. Kan, T. Zhang, Q. Zan and Y. Zhao, A novel highly active nanostructured IrO2/Ti anode for water oxidation, Int. J. Hydrogen Energy, 2015, 40, 14279–14283 CrossRef CAS.
- S. Yu, K. Li, W. Wang, Z. Xie, L. Ding, Z. Kang, J. Wrubel, Z. Ma, G. Bender, H. Yu, J. Baxter, D. A. Cullen, A. Keane, K. Ayers, C. B. Capuano and F. Y. Zhang, Tuning Catalyst Activation and Utilization Via Controlled Electrode Patterning for Low-Loading and High-Efficiency Water Electrolyzers, Small, 2022, 18, 2107745 CrossRef CAS PubMed.
- S. Yu, Z. Xie, K. Li, L. Ding, W. Wang, G. Yang and F.-Y. Zhang, Morphology engineering of iridium electrodes via modifying titanium substrates with controllable pillar structures for highly efficient oxygen evolution reaction, Electrochim. Acta, 2022, 405, 139797 CrossRef CAS.
- A. Laube, A. Hofer, S. Ressel, A. Chica, J. Bachmann and T. Struckmann, PEM water electrolysis cells with catalyst coating by atomic layer deposition, Int. J. Hydrogen Energy, 2021, 46, 38972–38982 CrossRef CAS.
- T. L. Doan, H. E. Lee, M. Kim, W. C. Cho, H. S. Cho and T. Kim, Influence of IrO2/TiO2 coated titanium porous transport layer on the performance of PEM water electrolysis, J. Power Sources, 2022, 533, 231370 CrossRef CAS.
- J. Han, H. Yoo, M. Kim, G. Lee and J. Choi, High-performance bipolar plate of thin IrOx-coated TiO2 nanotubes in vanadium redox flow batteries, Catal. Today, 2017, 295, 132–139 CrossRef CAS.
- M. Geuß, M. Milosevic, M. Bierling, L. Löttert, D. Abbas, D. Escalera-López, V. Lloret, K. Ehelebe, K. J. J. Mayrhofer, S. Thiele and S. Cherevko, Investigation of Iridium-Based OER Catalyst Layers in a GDE Half-Cell Setup: Opportunities and Challenges, J. Electrochem. Soc., 2023, 170, 114510 CrossRef.
- Y. Wang, R. Ma, Z. Shi, H. Wu, S. Hou, Y. Wang, C. Liu, J. Ge and W. Xing, Inverse doping IrOx/Ti with weakened Ir–O interaction toward stable and efficient acidic oxygen evolution, Chem, 2023, 9, 2931–2942 CAS.
- D. Wu, X. Wang, Z. Wang and X. Wu, Two-step electrodeposition to achieve durable IrOx electrode with a semi-crystalline structure for efficient OER, Mater. Today Sustainability, 2023, 24, 100533 CrossRef.
- R. Zhao, Z. Wang, Q. Xu, X. Niu, Y. Han, Y. Qin and Q. Wang, Self-supported amorphous iridium oxide catalysts for highly efficient and durable oxygen evolution reaction in acidic media, Electrochim. Acta, 2021, 391, 138955 CrossRef CAS.
- G. Doo, J. Park, J. Park, J. Heo, J. Jung, D. W. Lee, H. Bae, J. Hyun, E. Oh, J. Kwen, K. M. Kim and H. T. Kim, Contact Problems of IrOx Anodes in Polymer Electrolyte Membrane Water Electrolysis, ACS Energy Lett., 2023, 8, 2214–2220 CrossRef CAS.
- J. Mo, Z. Kang, S. T. Retterer, D. A. Cullen, T. J. Toops, J. B. Green, M. M. Mench and F. Y. Zhang, Discovery of true electrochemical reactions for ultrahigh catalyst mass activity in water splitting, Sci. Adv., 2016, 2, e1600690 CrossRef PubMed.
| This journal is © the Partner Organisations 2025 |