In situ polymerization-driven quasi-solid electrolytes for Li-metal batteries
Jinxuan
Liu
ab,
Yinglei
Wu
*a,
Zhenying
Chen
bc,
Zhixin
Xu
b,
Jinhui
Zhu
 *b and
Xiaodong
Zhuang
*b and
Xiaodong
Zhuang
 *bd
*bd
aSchool of Chemistry and Chemical Engineering, Shanghai University of Engineering Science, Shanghai 201620, China. E-mail: wuyl@sues.edu.cn
bThe Soft2D Lab, State Key Laboratory of Metal Matrix Composites, Shanghai Key Laboratory of Electrical Insulation and Thermal Ageing, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai 200240, China. E-mail: zhujinhui1109@sjtu.edu.cn; zhuang@sjtu.edu.cn
cCollege of Chemistry, Zhengzhou University, Zhengzhou 450001, Henan, China
dFrontiers Science Center for Transformative Molecules, Zhang Jiang Institute for Advanced Study, Shanghai Jiao Tong University, Shanghai 201203, China
First published on 6th May 2025
Abstract
Li-metal batteries (LMBs) have emerged as the most promising energy storage technology to achieve high energy density exceeding 400 W h kg−1. However, the conventional liquid electrolytes in LMBs present significant challenges, including severe reactivity with Li metal anodes and substantial safety risks. Quasi-solid electrolytes (QSEs), which combine the advantages of both solid and liquid electrolytes, have been identified as an ideal alternative for practical LMB applications. In particular, QSEs fabricated through in situ polymerization strategies offer enhanced interfacial compatibility, simplified manufacturing processes, reduced production costs, and represent one of the most commercially viable pathways for QSE development. This review provides a comprehensive analysis of in situ polymerization-driven QSEs and their application in LMBs. QSEs are systematically categorized based on the polymerization mechanisms of the polymer matrix, including free radical, cationic, anionic, and electrochemical polymerizations. Furthermore, the strategies for improving the performance of QSE-based LMBs are thoroughly discussed, with a focus on enhancing flame-retardant properties, high-voltage compatibility, and low-temperature operation. Finally, the review concludes with a critical assessment of the current state and future prospects of QSE-based LMBs, providing valuable insights into the future development of safer and more efficient energy storage systems.
1. Introduction
The global electrified energy revolution has spurred an unprecedented demand for advanced rechargeable batteries. While commercial Li-ion batteries (LIBs) based on graphite anodes and liquid electrolytes (LEs) have dominated the market, their limited energy density and inherent safety concerns hinder their ability to meet the growing requirements of high-energy-density and safe energy storage systems, particularly for applications such as electric vehicles and electric aircrafts.1,2 To address these challenges, the development of next-generation anode materials and electrolytes with enhanced safety, higher energy density, longer cycle life, and cost-effectiveness has become imperative. Among potential anode materials, Li metal anodes (LMAs) stand out as the “holy grail” due to their exceptional theoretical specific capacity (3860 mA h g−1) and the lowest electrochemical potential (−3.04 V vs. SHE), enabling Li-metal batteries (LMBs) to achieve energy densities exceeding 400 W h kg−1.3,4 However, the practical application of LMBs remains hindered by several critical issues, including dendrite formation, significant volume changes, low coulombic efficiency (CE), and severe interfacial side reactions between LMAs and LEs. These side reactions often lead to heat generation and safety hazards such as LE volatilization, combustion, or even explosion.5To mitigate these challenges, replacing LEs with solid electrolytes (SEs) has emerged as a promising strategy. SEs not only address safety concerns but also alleviate some intrinsic issues of LMAs through tailored electrolyte design.6,7 SEs can be broadly classified into two categories: quasi-solid electrolytes (QSEs) and all-solid electrolytes (ASEs). While ASE-based LMBs offer enhanced safety by eliminating liquid components entirely, they face significant hurdles, including severe side reactions between LMAs and inorganic ASEs (e.g., sulfides8,9 and halides10), poor ionic conductivity in polymer-type ASEs,11 and inadequate solid–solid interfacial contact.12 In contrast, QSEs, which consist of a polymer matrix infused with minimal LEs, combine the advantages of both LEs and SEs. The polymer matrix provides mechanical stability and confines the LE, reducing the risk of flammable solvent leakage, while the residual liquid ensures effective electrode wetting and facilitates ionic conduction.13,14 This unique combination enables QSEs to achieve a balance between mechanical robustness and electrochemical performance, making them a compelling alternative for next-generation LMBs.15
QSEs can be fabricated via two primary approaches: ex situ and in situ methods.16 The ex situ approach involves preparing QSE membranes by dissolving polymer matrices, Li salts, and plasticizers into solvents to form a homogeneous solution, which is then cast into membranes using conventional techniques. However, this method often results in significant interfacial gaps and reduced contact between the QSE membrane and electrodes, leading to high interfacial impedance and suboptimal cell performance.17 In contrast, the in situ approach involves assembling the cell with a precursor solution containing polymer monomers, Li salts, and other components, followed by triggering polymerization under specific conditions (e.g., heat or chemical initiation). This method eliminates the need for laborious out-of-cell membrane preparation, enabling the direct formation of QSEs within the cell. The in situ approach not only enhances interfacial compatibility but also simplifies the manufacturing process, reduces production costs, and represents one of the most commercially viable pathways for QSE development.
In situ polymerization typically involves impregnating a separator with a precursor solution composed of monomers, Li salts, initiators, and optional additives (e.g., plasticizers or fillers), followed by polymerization under controlled conditions.18 Depending on the monomer and initiation mechanism, in situ polymerization patterns can be categorized into free radical polymerization,19 cationic polymerization,20 anionic polymerization,21 and electrochemical polymerization.22 These methods utilize monomers with unsaturated functional groups (e.g., vinyl, allyl, epoxide) to form either linear or cross-linked polymer structures.
Currently, significant research efforts are focused on elucidating the mechanisms of in situ polymerization for QSE fabrication and advancing the electrochemical performance of QSE-based LMBs. This review aims to provide a comprehensive analysis of in situ polymerization techniques for quasi-solid-state (QSS) LMBs, systematically evaluating the advantages and limitations of different polymerization strategies, including free radical, cationic, anionic, and electrochemical polymerizations. Additionally, this review explores strategies to enhance specific performance metrics of in situ polymerization-based QSSLMBs, such as flame retardancy, high-voltage stability, and low-temperature operation. Finally, we offer a forward-looking perspective on the future of QSEs and their role in the development of high-performance LMBs, providing insights to guide further research and innovation in this rapidly evolving field.
2. Mechanisms of polymerizations
The polymer matrix is a critical component of QSEs, and its design must satisfy several key requirements: (1) incorporation of polar functional groups to enhance compatibility with Li salts and improve ionic conduction efficiency;23 (2) low glass transition temperature and low crystallinity to facilitate LE absorption and promote ion mobility; and (3) high mechanical strength, thermal stability, and chemical stability to broaden the electrochemical stability window (ESW).24 Consequently, the selection of polymer monomers for in situ polymerization is pivotal, as the resulting polymers must adhere to these principles. Furthermore, a deep understanding of the polymerization mechanisms is essential for monomer functionalization and the optimization of polymerization conditions. Based on the types of monomers, four primary polymerization mechanisms are employed: free radical polymerization, cationic polymerization, anionic polymerization, and electrochemical polymerization. Each mechanism is discussed in detail below.2.1 Free radical polymerization mechanism
Free radical polymerization is one of the most widely used methods for constructing polymer matrices in QSEs. This process involves three key stages: initiation, propagation, and termination of free radicals. Olefinic monomers containing unsaturated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, such as vinyl carbonates and acrylates, are commonly used as raw materials. The polymerization begins with the decomposition of an initiator (e.g., peroxides, azo compounds, or redox systems) to generate free radicals (R˙). These radicals, possessing unpaired electrons, attack the π-electron cloud of the monomer's unsaturated bonds, forming reactive radical intermediates. The double bond breaks, allowing the radicals to react with monomers, initiating chain growth. The polymer chain continues to propagate by reacting with additional monomers until termination occurs, typically through radical collision or reaction with a chemical agent, halting further growth. Free radicals can be generated via thermal decomposition of initiators, UV irradiation, high-energy radiation, electrochemical methods, or plasma techniques. However, thermal initiation remains the most cost-effective, safe, and commercially viable approach for constructing QSE matrices in LMBs. For instance, as illustrated in Fig. 1a, the initiator azobisisobutyronitrile (AIBN) undergoes thermal decomposition to generate free radicals. These radicals then react with ethylene glycol diacrylate (EGDA) monomers, forming EGDA radical intermediates. These intermediates undergo further cross-linking and polycondensation, ultimately yielding a PEGDA polymer matrix.25
C bonds, such as vinyl carbonates and acrylates, are commonly used as raw materials. The polymerization begins with the decomposition of an initiator (e.g., peroxides, azo compounds, or redox systems) to generate free radicals (R˙). These radicals, possessing unpaired electrons, attack the π-electron cloud of the monomer's unsaturated bonds, forming reactive radical intermediates. The double bond breaks, allowing the radicals to react with monomers, initiating chain growth. The polymer chain continues to propagate by reacting with additional monomers until termination occurs, typically through radical collision or reaction with a chemical agent, halting further growth. Free radicals can be generated via thermal decomposition of initiators, UV irradiation, high-energy radiation, electrochemical methods, or plasma techniques. However, thermal initiation remains the most cost-effective, safe, and commercially viable approach for constructing QSE matrices in LMBs. For instance, as illustrated in Fig. 1a, the initiator azobisisobutyronitrile (AIBN) undergoes thermal decomposition to generate free radicals. These radicals then react with ethylene glycol diacrylate (EGDA) monomers, forming EGDA radical intermediates. These intermediates undergo further cross-linking and polycondensation, ultimately yielding a PEGDA polymer matrix.25
 | ||
| Fig. 1 Representative polymerization mechanisms: (a) free radical polymerization, (b) cationic polymerization, (c) anionic polymerization, and (d) electrochemical polymerization. | ||
The ring size of monomers plays a significant role in the kinetics of free radical polymerization. For vinyl monomers bearing cyclic substituents (e.g., methyl methacrylate derivatives), the ring structure can influence the stability of propagating radicals. For example, cyclohexyl methacrylate exhibits a slower polymerization rate than its linear analogs due to steric hindrance imposed by the cyclic group, which restricts radical rotation and chain propagation. This steric effect reduces the monomer's reactivity during radical addition steps, impacting both molecular weight distribution and overall polymerization efficiency.26
The limitations of the free radical polymerization method include the following: (1) the high-temperature reaction conditions can lead to heat accumulation, gas evolution, and degradation of plasticizers. (2) Compared to other polymerization techniques, free radical polymerization typically exhibits lower monomer conversion rates, which may result in an inhomogeneous polymer matrix. (3) The use of radical initiators can introduce non-electrolyte components, which may react with the LMA and adversely affect battery cycling performance.
To address these challenges, recent research has proposed several strategies: (1) initiator development: the use of novel initiators, such as low-temperature initiators (e.g., azo compounds) and photoinitiators with high selectivity and conversion efficiency (e.g., 2,2-dimethoxy-2-phenylacetophenone), helps lower the polymerization temperature, minimizing thermal degradation and improving matrix uniformity. (2) Monomer design: the development of multifunctional monomers compatible with existing initiation systems can enhance both the monomer conversion rate and the homogeneity of the resulting polymer matrix. (3) Initiator optimization: selecting initiators that are chemically compatible with LMA or modifying existing initiators to reduce side reactions can improve the overall stability and performance of the QSEs.
2.2 Cationic polymerization mechanism
Cationic polymerization differs from free radical polymerization in that the growing polymer chain carries a positive charge, balanced by a counter-anion.27 This mechanism enables the preparation of QSE matrices under mild conditions without introducing impurities, making it suitable for conventional electrolyte materials. The process begins with the generation of cations from strong acids or Lewis acid initiators (e.g., H+, PF5, AlCl3, or BF3). These cations react with the double bonds of monomers or epoxide structures, forming positively charged intermediates. The intermediates propagate the polymerization chain by reacting with additional monomers. Termination occurs when the cation combines with a solvent molecule, impurity, or counter-anion.28A prominent example is the cationic polymerization of 1,3-dioxolane (DOL), an ether solvent widely used in electrolyte preparation. DOL exhibits a low dielectric constant (ε ≈ 7), good ionic conductivity (≈1.0 mS cm−1), and low interfacial resistance post-polymerization.29 As shown in Fig. 1b, the initiator LiPF6 decomposes into LiF and PF5, with PF5 reacting with trace water to form the strong Lewis acid H+(PF5OH)−. This acid initiates the ring-opening polymerization (ROP) of DOL, resulting in a linear long-chain polymer.
Similar to free radical polymerization, monomer ring size has a pronounced effect on the kinetics of cationic polymerization. This is primarily due to the influence of ring strain on the ease of ring-opening. Monomers with smaller rings exhibit higher ring strain, resulting in lower energy barriers for ring-opening and faster polymerization rates. Five-membered rings such as DOL are commonly used in cationic polymerization due to their moderate ring strain and ease of activation by Lewis acids (e.g., AlCl3, BF3) or Li salts (e.g., LiPF6). In contrast, six-membered rings like 1,3-dioxane (DOX) require stronger initiators or elevated temperatures due to reduced ring strain. Nevertheless, polymers derived from larger rings often exhibit improved ionic conduction anisotropy due to their more rigid polymer backbones.
Therefore, small-ring monomers like DOL offer advantages in terms of rapid polymerization and high ionic conductivity but may require optimization to ensure oxidative stability. Conversely, macrocyclic monomers such as DOX are better suited for high-voltage applications but demand tailored polymerization conditions. Future studies may explore the tuning of monomer properties—through fluorination, silanization, copolymerization, or nano-confinement—to further balance ring size effects and optimize battery performance.
The cationic polymerization approach also presents several limitations: (1) it is highly sensitive to moisture and impurities, which may cause premature termination or unwanted side reactions. (2) The oxidative stability of the resulting QSEs is typically poor, making them susceptible to degradation under high-voltage conditions. (3) The ionic conductivity of cationically polymerized QSEs is often lower than that of those produced via free radical polymerization.
To mitigate these issues, the following solutions have been explored: (1) purity control: employing high-purity raw materials can minimize the negative impact of moisture and contaminants. (2) Chemical modification: introducing fluorinated or halogenated groups into the polymer matrix can significantly enhance oxidative stability. (3) Composite design: incorporating high-conductivity additives such as ionic liquids or nano-fillers can improve the overall ionic conductivity of the QSEs.
2.3 Anionic polymerization mechanism
Anionic polymerization is another chain-growth polymerization mechanism, where the growing polymer chain carries a negative charge balanced by counter-cations.30 This method is particularly effective for monomers with electron-withdrawing substituents, which render the double bonds electrophilic. Initiators such as alkali metals, their hydrides, amides, and metal–organic compounds generate nucleophilic species that initiate polymerization. Anionic polymerization is often referred to as “living polymerization” because the propagation reaction continues without termination until all monomers are consumed. This characteristic allows for precise control over polymer architecture, enabling the synthesis of well-defined block copolymers and other complex structures.31 Additionally, anionic polymerization produces polymers with narrow molecular weight distributions due to the high stability of the anionic active centers, which do not terminate spontaneously.For example, ethyl cyanoacrylate (ECA), a highly electrophilic monomer, undergoes anionic polymerization initiated by Li+ from Li salt decomposition. As illustrated in Fig. 1c, the generated anions react with downstream monomers, achieving controlled chain growth.21,32 Polymers containing C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –C
O and –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N functional groups exhibit strong hydrogen bonding interactions with the aluminum-plastic film in pouch cells, effectively immobilizing the electrolyte and enhancing leakage prevention, thereby improving battery safety.
N functional groups exhibit strong hydrogen bonding interactions with the aluminum-plastic film in pouch cells, effectively immobilizing the electrolyte and enhancing leakage prevention, thereby improving battery safety.
In anionic polymerization, the impact of monomer ring size on polymerization kinetics mirrors that observed in cationic systems. Specifically, under comparable conditions, small-ring monomers (e.g., ethylene oxide) exhibit polymerization rates that are one to two orders of magnitude faster than those of larger-ring counterparts (e.g., propylene oxide), due to the higher ring strain that facilitates faster ring-opening reactions.33
Anionic polymerization is associated with a complex process, stringent reaction conditions, and QSEs with generally low ionic conductivity. To overcome these drawbacks, researchers have proposed the following strategies: (1) conducting polymerization in an inert atmosphere to stabilize reactive species. (2) Designing monomers with functional groups that promote high ionic conductivity and oxidative stability. (3) Enhancing the mechanical and electrochemical properties of the resulting QSEs through heat treatment or chemical crosslinking.
2.4 Electrochemical polymerization mechanism
Electrochemical polymerization, depicted in Fig. 1d, involves the application of an electric field (constant potential or current) to trigger redox reactions of monomers at the electrode surface. This process generates highly reactive intermediates, such as free radicals or cations, which combine with other monomers to form cross-linked networks or linear polymers. Unlike the other polymerization methods, electrochemical polymerization does not require an initiator. Instead, it relies on the oxidation of monomers to form reactive intermediates, which propagate the polymerization chain. Termination occurs when the reactive intermediates are exhausted.34 This method enables the rapid and controllable synthesis of conductive polymers, making it a promising approach for QSE fabrication in LMBs.Although electrochemical polymerization offers precise control over QSE formation, it suffers from several drawbacks, including non-uniform film formation, high production costs, limited material compatibility, and inferior mechanical strength. These limitations currently hinder its scalability and practical application compared to other polymerization methods.
2.5 Summary and comparison
As discussed in the preceding sections, the polymerization mechanism critically determines the structural and compositional properties of QSEs, thereby governing the performance and safety of QSSLMBs. To provide readers with a clear and systematic overview, we have summarized the key advantages, disadvantages, and potential applications of each polymerization approach in Table 1. This comparative analysis offers an intuitive reference for selecting the most suitable synthesis strategy based on specific battery requirements.| Mechanisms | Core advantages | Major limitations | Typical application scenario |
|---|---|---|---|
| Free radical | (1) Wide range of monomers (styrene, acrylates, etc.) | (1) Wide molecular weight distribution (dispersity D > 1.5) | Composite QSEs, interfacial modification |
| (2) Simple operation, low cost (heat initiation) | (2) Large amounts of initiators (e.g., peroxides, azo compounds) are required and harmful by-products may remain | ||
| (3) The triggering method is more flexible | |||
| Cationic | (1) Suitable for low polarity monomers (e.g., ethers, epoxy resins) | (1) Harsh reaction conditions, sensitive to impurities (H2O, O2, etc., terminate the reaction) | High ionic conductivity QSEs (e.g., PDOL) |
| (2) High reactivity (can be performed at low temperatures) | (2) High toxicity of initiators (e.g., PF5, BF3) | ||
| (3) Non-terminating “active” features | |||
| Anionic | (1) Narrow molecular weight distribution (D ≈ 1.05–1.2) | (1) Strict anaerobic conditions (complex operation) | Wide ESW QSEs (e.g., PAN-based) |
| (2) Controllable “activity” (designable block copolymers) | (2) High catalyst cost (e.g., alkyl Li) | ||
| Electro-chemical | (1) No chemical initiators required (green) | (1) High requirements for conductive substrate | Interface protection layer, flexible batteries |
| (2) High spatiotemporal control (electrode surface-oriented polymerization) | (2) Demanding monomers (need redox active groups) | ||
| (3) Fast film formation (suitable for battery interface modification) | (3) High equipment complexity (requires precise potential control) |
3. Categories of QSEs
The QSE matrices fabricated via the in situ polymerization method exhibit significant diversity, primarily influenced by the choice of monomers, initiators, and reaction conditions. In this section, the various types of QSEs and their corresponding LMBs are systematically categorized and discussed based on the polymerization mechanisms employed for the QSE substrates.3.1 Free radical polymerization pattern
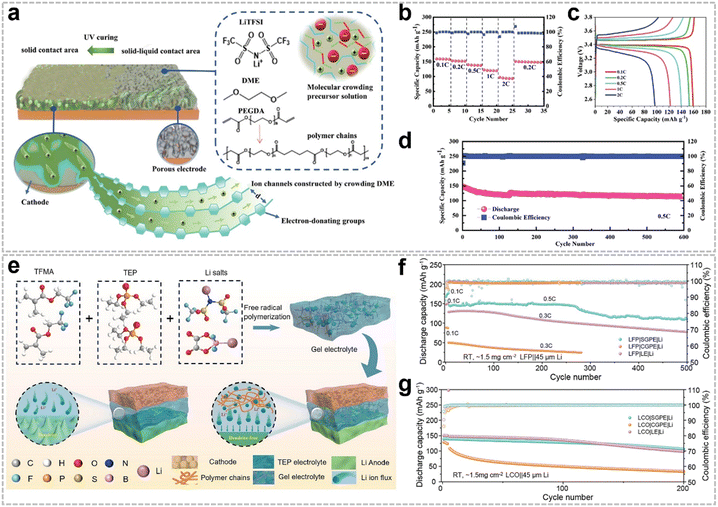
Free radical polymerization is a pivotal technique for the in situ preparation of QSE matrices, offering a versatile approach to address critical challenges in LMBs, such as Li dendrite growth and interfacial instability. QSEs synthesized via this method exhibit excellent mechanical properties, high ionic conductivity, and robust stability, making them highly suitable for advanced battery applications. The primary monomers used in free radical polymerization include acrylates (e.g., methacrylate, ethylene glycol acrylates, and pentaerythritol acrylates) and carbonates (e.g., vinyl ethylene carbonate (VEC) and vinyl carbonate (VC)). Common initiators for this process include azo compounds, peroxides, and redox systems.35 Free radicals are typically generated through thermal decomposition of initiators, but alternative methods such as UV irradiation, high-energy radiation, electrolysis, and plasma technology are also employed, providing flexibility for diverse application scenarios and process requirements.Functionalized acrylate monomers further enhance the properties of QSEs. For example, trifluoromethyl methacrylate (TFMA), a F-rich monomer, was used to prepare a QSE with a dual-salt system (Li bis(trifluoromethane)sulfonimide (LiTFSI) and Li difluoro-oxalate-boronate (LiDFOB)) (Fig. 2e).39 The F-rich groups facilitated the formation of an inorganic-rich solid electrolyte interphase (SEI) and cathode electrolyte interphase (CEI), improving electrochemical performance. Additionally, the incorporation of triethyl phosphonate (TEP) enhanced flame retardancy through polar interactions with TFMA. The resulting Li|Li cell achieved stable cycling for over 1000 h at 0.1 mA cm−2, while the Li|LFP full cell retained a specific capacity of 110.4 mA h g−1 after 500 cycles at 0.5C (Fig. 2f). Notably, this QSE demonstrated compatibility with high-voltage LiCoO2 (LCO) cathodes, maintaining a specific capacity of 120 mA h g−1 after 200 cycles at 4.2 V (Fig. 2g).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C functional groups, exhibits weak coordination with Li+ and facile polymerization, making it a safe and stable choice for LMBs.40 Similarly, VC, an unsaturated additive, features a (–O(C
C functional groups, exhibits weak coordination with Li+ and facile polymerization, making it a safe and stable choice for LMBs.40 Similarly, VC, an unsaturated additive, features a (–O(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–O–) structure that strongly interacts with Li+, enhancing SEI formation.41,42
O)–O–) structure that strongly interacts with Li+, enhancing SEI formation.41,42
VEC-based QSEs have demonstrated high ionic conductivity (2.1 mS cm−1 at RT), a wide ESW (up to 4.5 V vs. Li/Li+), and excellent interfacial stability.43 The Li+ transport mechanism in these QSEs involves complex coupling/decoupling interactions between Li+ and O atoms in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O groups, as well as segmental polymer motions. Consequently, Li|LFP full cells achieved a specific capacity of ∼165 mA h g−1 at RT and ∼104 mA h g−1 at −15 °C at 0.1C.
O and C–O groups, as well as segmental polymer motions. Consequently, Li|LFP full cells achieved a specific capacity of ∼165 mA h g−1 at RT and ∼104 mA h g−1 at −15 °C at 0.1C.
To further enhance their performance, copolymer matrices have been developed by combining VEC with linear diallyl carbonate (DAC) and N,N′-methylenebis-acrylamide (MBA) (Fig. 3a).44 The weakly solvating DAC weakened Li+ coordination, while MBA cross-linking improved mechanical strength and restricted anion migration through hydrogen bonding with DFOB−. This design, based on an enthalpy–entropy manipulation strategy (Fig. 3b), resulted in a QSE with an ionic conductivity of 0.66 mS cm−1, a Li+ transference number of 0.76, and high oxidation stability. The Li|LiNi0.8Co0.1Mn0.1O2 (NCM811) full cell retained 82% capacity after 800 cycles, attributed to the robust cross-linked network that mitigated intergranular cracking (Fig. 3c and d).
VC-based QSEs, inspired by their role in SEI stabilization, have also been explored.45,46 These QSEs exhibited an ESW of up to 4.5 V vs. Li/Li+ and compatibility with high-voltage LCO cathodes. However, their low ionic conductivity (9.82 × 10−5 S cm−1 at 50 °C) remains a limitation.
Another innovative QSE was prepared by incorporating 2,2,3,4,4,4-hexafluorobutyl methacrylate and EGDA into VC-based polymer chains (Fig. 4c and d).48 This QSE demonstrated an ionic conductivity of 0.63 mS cm−1, a Li+ transference number of 0.82, and an ESW of up to 5.1 V vs. Li/Li+. The loose coordination of polymer chains and efficient ion transport through oligomers facilitated rapid Li+ migration, ensuring compatibility with both LMA and high-voltage cathodes.
Despite its widespread use, free radical polymerization faces several challenges. First, the monomer conversion rate is relatively low compared to other polymerization methods, such as cationic ROP. The observed differences in monomer conversion efficiency arise primarily from the inherent distinctions between free radical polymerization and ionic ROP, particularly in their propagation and termination mechanisms. Free radical polymerization proceeds via a three-step process: chain initiation → chain propagation → chain termination. Due to the high reactivity of free radicals, termination reactions (such as radical coupling or disproportionation) readily occur, often leading to premature chain termination and thus lower monomer conversion. For instance, Kwok et al. reported that when EGDA was polymerized using AIBN as the initiator, the monomer conversion reached only about 70% in the absence of inhibitors. Moreover, residual free radicals were found to cause interfacial side reaction.49 In contrast, ionic ROP involves the nucleophilic or electrophilic opening of cyclic ether rings by an initiator, resulting in the formation of a stable active center that supports continuous chain growth without an inherent termination step (unless a chain transfer agent is present). This stability typically leads to higher monomer conversion rates. For example, in the case of LiPF6-initiated polymerization of DOL, a monomer conversion of 85.2% was achieved, and the resultant linear polymer exhibited a dense structure with ESW up to 4.6 V vs. Li/Li+.50 Similarly, Li et al. demonstrated that ROP-derived copolymers achieved approximately 90% monomer conversion, compared to 50–80% for those produced via free radical polymerization under similar conditions.51
Initiator selection also significantly influences the polymerization efficiency in free radical systems. The two primary classes of initiators are azo compounds and peroxides. Azo compounds, such as AIBN, typically decompose at 40–60 °C, while peroxides require higher temperatures (>80 °C) to generate radicals, which may not be compatible with the thermal constraints of LMB environments. Consequently, peroxide-initiated systems often exhibit slower reaction rates and lower monomer conversions. For instance, the AIBN-initiated polymerization of EGDA in an ethylene carbonate (EC)/DMC solvent system yielded monomer conversion rates of approximately 45–55%.52 Meanwhile, benzoyl peroxide-initiated DOL polymerization in liquid electrolyte systems resulted in lower conversions of 30–40.53
Furthermore, in LMB systems, conventional initiators may exhibit reduced activity due to potential side reactions with electrolyte components or electrode materials. This challenge underscores the need for continued innovation. Future research could explore co-initiator strategies, polymer architecture design, and the use of tailored catalysts or additives to enhance radical generation and promote higher conversion rates in free radical polymerization processes.
Second, the introduction of non-electrolyte components (e.g., initiators) may adversely affect QSE performance, including potential reactions with LMA. Third, the requirement for high-temperature thermal initiation raises concerns about heat dissipation, gas formation, and Li salt solubility in large-capacity pouch cells. Addressing these issues through further research and optimization is essential for advancing QSSLMB technology.
3.2 Cationic polymerization pattern
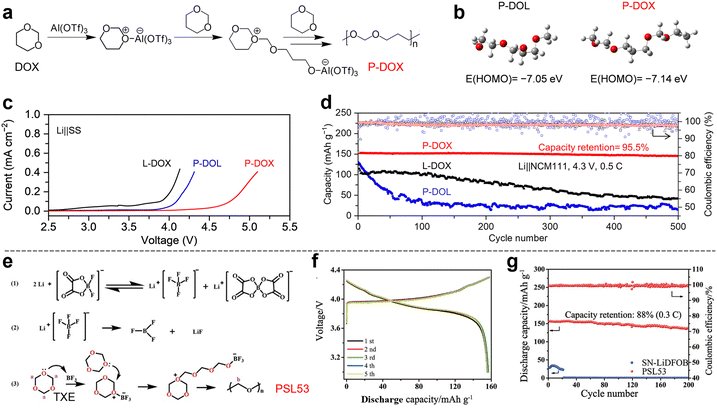
Cationic polymerization is a highly efficient strategy for the in situ synthesis of QSE matrices. This method typically relies on protonic acids or Lewis acids as initiators, targeting O atoms in O-containing heterocyclic rings (e.g., cyclic ethers, cyclic carbonates) or N-containing heterocyclic monomers. These monomers are susceptible to cationic attack, enabling ROP.54,55 This approach is particularly suitable for epoxy alkane electrolyte materials, as it can initiate polymerization at double bonds or ring structures. A key advantage of cationic polymerization is its rapid chain growth; however, the high reactivity of carbocations can lead to side reactions or reduced product stability, necessitating careful control of the polymerization process. Among the monomers used in cationic polymerization, cyclic ethers have garnered significant attention due to their excellent compatibility with LMA, low viscosity, and high ionic conductivity. Representative cyclic ether monomers include DOL,56 DOX,57 and 1,3,5-trioxane (TXE).58 This section also explores the in situ polymerization of DOL in the presence of mechanical supports to enhance QSE performance.DOL monomer. DOL, a five-membered cyclic ether, is widely used as an electrolyte solvent due to its low viscosity and high ionic conductivity. However, its low ESW (4 V vs. Li/Li+) limits its application. Cationic ROP of DOL offers an effective strategy to improve both the electrochemical stability of the electrolyte and the cycling performance of corresponding LMBs. This polymerization can be initiated by positively charged intermediates, such as LiPF6, Al(OTf)3, and BF3.
Early studies explored the ROP of DOL,59,60 but recent advancements have focused on LiPF6 as an initiator due to its dual role as an electrolyte component, eliminating the need for additional additives.29 For example, a novel QSE was synthesized by in situ ROP of DOL initiated by LiPF6 in the presence of DME at ambient temperature (Fig. 5a and b). During the reaction, LiPF6 decomposes into LiF and PF5, with PF5 reacting with trace water to form the strong Lewis acid H+(PF5OH)−, which triggers DOL polymerization into a linear long-chain polymer. The resulting QSE exhibited an ionic conductivity of 3.8 mS cm−1 and excellent compatibility with LMA. The Li|Li symmetric cell demonstrated stable cycling for over 800 h at 0.5 mA cm−2 with minimal overpotential. Additionally, the QSE was compatible with various cathodes, including S, LFP, and LiNi0.6Co0.2Mn0.2O2 (NCM622), delivering excellent cycling performance (1000, 700, and 100 cycles for Li|S, Li|LFP, and Li|NCM622 cells, respectively). This simple, efficient, and impurity-free strategy represents a promising direction for advanced QSE systems in LMBs.
To further enhance DOL-derived QSEs, plasticizers such as EC,61 fluoroethylene carbonate (FEC),62 and methyl ethyl carbonate (MEC)63 have been incorporated. These carbonate plasticizers improve thermal stability, dielectric properties, and reduce volatility. For instance, Archer et al.61 developed a QSE by blending DOL with EC, achieving a solid-like QSE even at low DOL concentrations (<40%). The resulting QSE exhibited superior electrochemical performance and oxidative stability, enabling compatibility with high-voltage NCM622 cathodes. Similarly, a QSE prepared from DOL, FEC, and hexamethylene diisocyanate (HDI) demonstrated enhanced compatibility with LCO cathodes, attributed to the synergistic effects of FEC and HDI in forming stable interfacial layers.62
Al(OTf)3 has also been employed as an initiator for DOL polymerization (Fig. 5c and d).64 The Al-based cation attaches to the O atom in DOL, initiating ROP. The resulting QSE exhibited moderate ionic conductivity (>1 mS cm−1 at RT) and excellent interfacial compatibility. The Li|Cu cell achieved a Li plating/stripping efficiency of >98% over 300 cycles, while full cells with S, LFP, and NCM622 cathodes demonstrated stable cycling for over 700 cycles with >99% CE. This work highlights the potential of cationic polymerization to meet both bulk and interfacial conductivity requirements for practical QSSLMBs. Despite their advantages, DOL-derived QSEs suffer from poor oxidative stability, limiting their compatibility with high-voltage cathodes.
DOX monomer. To address the oxidative stability limitations of DOL, DOX, a six-membered cyclic ether, has been explored as a monomer for QSE synthesis.57 Like DOL, DOX undergoes cationic ROP initiated by Lewis acids such as Al(OTf)3 (Fig. 6a). The lower highest occupied molecular orbital (HOMO) energy level of DOX-based polymers compared to DOL-based polymers (Fig. 6b) results in a wider ESW (up to 4.7 V vs. Li/Li+) (Fig. 6c). Additionally, DOX-derived QSEs facilitate the formation of robust, inorganic-rich SEI layers, enhancing Li metal compatibility. The Li|Li symmetric cell exhibited a high Li+ transference number (0.75) and stable cycling (over 1300 h). The DOX-derived QSE also demonstrated excellent compatibility with high-voltage LiNi1/3Co1/3Mn1/3O2 (NCM111) cathodes, retaining 84% capacity after 100 cycles at 1C and 4.5 V vs. Li/Li+ (Fig. 6d). This study underscores the importance of monomer molecular design in improving QSE performance for high-energy-density QSSLMBs.
TXE monomer. TXE has also been investigated as a monomer for QSE synthesis. For example, a eutectic QSE was prepared by ROP of TXE initiated by LiDFOB in the presence of succinonitrile (SN) (Fig. 6e).58 The interaction between the O atoms in TXE and the cyano groups in SN formed a deep eutectic solution, which polymerized at 80 °C to yield a QSE with an ionic conductivity of 0.114 mS cm−1 and an ESW of 4.5 V vs. Li/Li+. The QSE formed protective layers on both the LCO cathode and LMA, enabling the 4.3 V Li|LCO cell to retain 88% capacity after 200 cycles (Fig. 6f and g).
Similarly, a heat-resistant porous polyimide nanofiber (PI NF) film was developed via sol–gel electrospinning and heat treatment (Fig. 7a–d).66 The PI NF-supported QSE, prepared by LiPF6-initiated DOL polymerization, demonstrated high ionic conductivity (2.9 mS cm−1), a Li+ transference number of 0.61, and an ESW of 4.8 V vs. Li/Li+. The Li|LFP full cell achieved a specific capacity of 151.8 mA h g−1 at 0.5C, retaining 91.8% capacity after 200 cycles. This QSE is also suitable for large-capacity pouch cells, highlighting its practical potential.
Another innovative approach involved modifying PVDF-HFP with polydopamine (PDA) to create a composite nanofibrous skeleton (Fig. 7e–h).67 The PDA-enhanced QSE exhibited improved mechanical strength, ionic conductivity, and Li+ transference number due to hydrogen bonding with the polymer matrix and TFSI anions. The Li|LFP full cell demonstrated stable cycling for over 800 cycles at 2C, with 83.2% capacity retention, effectively suppressing Li dendrite growth. This strategy offers a promising pathway for high-performance, safe QSSLMBs compatible with existing battery production processes.
Cationic ROP offers several advantages, including rapid reaction rates, high molecular weight polymers, and compatibility with epoxy monomers. However, challenges such as sensitivity to moisture and impurities, difficulty in termination, and suboptimal electrochemical stability remain. Ether-based QSEs, in particular, exhibit low oxidative stability, limiting their compatibility with high-voltage cathodes and the energy density of QSSLMBs. Future research should focus on enhancing the oxidative stability of cyclic ether-based QSEs through solvent/salt ratio optimization, cross-linking copolymerization, plasticizer incorporation, and high-voltage additives. These advancements will be critical for developing QSEs capable of meeting the demands of next-generation high-energy-density batteries.
3.3 Anionic polymerization pattern
Anionic polymerization is another effective strategy for the in situ synthesis of QSE matrices. This method is initiated by nucleophilic reagents, such as alkali metals, their hydrides, amides, and metal–organic compounds.28,30 Often referred to as “living polymerization,” anionic polymerization can proceed without termination under appropriate conditions, enabling precise control over polymer architecture. This characteristic allows for the synthesis of well-defined copolymers, such as block copolymers, by sequentially adding different monomers until complete conversion to macromolecules is achieved. Additionally, anionic polymerization produces polymers with narrow molecular weight distributions and high reproducibility, as the active anionic centers do not terminate spontaneously. However, the process is highly sensitive to moisture and O2, requiring stringent reaction conditions to ensure reproducibility. While QSEs prepared via anionic polymerization are less extensively studied compared to other methods, they have shown promise, particularly with monomers containing cyano and acrylate groups, such as ECA. Furthermore, cyclic ethers (e.g., DOL) and carbonates can also be polymerized through anionic ring-opening strategies.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) attached to the C
N) attached to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond. The cyano group's low-energy lowest unoccupied molecular orbital (LUMO) enhances thermal stability, while its strong interaction with transition metal ions prevents their diffusion from the cathode to the anode, mitigating capacity loss—a critical advantage for high-voltage cathodes (>5 V vs. Li/Li+).21 These properties make cyanoacrylate-based polymers highly suitable for QSEs paired with high-voltage cathodes, offering both performance and safety benefits.
C bond. The cyano group's low-energy lowest unoccupied molecular orbital (LUMO) enhances thermal stability, while its strong interaction with transition metal ions prevents their diffusion from the cathode to the anode, mitigating capacity loss—a critical advantage for high-voltage cathodes (>5 V vs. Li/Li+).21 These properties make cyanoacrylate-based polymers highly suitable for QSEs paired with high-voltage cathodes, offering both performance and safety benefits.
For example, the ECA monomer has been used to in situ prepare QSEs through anionic polymerization (Fig. 8a).68 The resulting QSE, composed of an ECA polymer matrix and LiClO4/carbonate electrolyte, achieved an ionic conductivity of 2.7 mS cm−1 at RT and an ESW of up to 4.8 V vs. Li/Li+. When paired with LFP and LiNi1.5Mn0.5O4 cathodes, the QSE demonstrated stable charge–discharge cycling and excellent rate capability (Fig. 8b and c). Additionally, a flexible thin-film Li|LFP cell incorporating this QSE successfully powered an LED and delivered a specific capacity of ∼130 mA h g−1 after 20 cycles with high CE (Fig. 8d). Beyond QSEs, ECA polymer nanocoatings have also been employed to protect high-voltage LiNi0.5Mn1.5O4 cathodes69 and LMA,70 further highlighting their versatility.
Anionic polymerization has significant potential for QSSLMBs, not only for in situ QSE preparation but also for fabricating protective polymer layers for LMA and cathode materials. By precisely controlling polymerization conditions, polymers with excellent ionic conductivity, mechanical strength, and electrochemical stability can be synthesized. Future research should focus on developing new functional monomers with enhanced properties, such as high electrochemical stability and high-temperature resistance, to further improve the performance of QSEs in QSSLMBs. Additionally, addressing the sensitivity of anionic polymerization to moisture and O2 will be critical for scaling up production and ensuring reproducibility. These advancements will pave the way for next-generation high-performance and safe energy storage systems.
3.4 Electrochemical polymerization pattern
Electrochemical polymerization is an effective technique for the in situ construction of QSE matrices. This method can be initiated by an electrochemical reaction without the need for additional thermal or chemical initiators, offering advantages such as simplified processing, reduced solvent consumption, and enhanced material properties.74,75 However, electropolymerization faces challenges including the formation of high-molecular-weight polymers and thick films due to the limited diffusion of monomers to the substrate electrode surface. Additionally, if the resulting polymer layer exhibits low electronic conductivity, the rate and continuity of subsequent polymerization reactions may be hindered.A well-studied monomer, DOL, can undergo ring-opening chain growth through electrochemical polymerization (Fig. 9a).76 It had been observed that after several cycles in a Li|LCO cell using a DOL-based electrolyte at a relatively high current density (100 mA g−1), the LE (1 M LiTFSI in DOL/DME with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio) undergoes irreversible polymerization, resulting in improved cycling stability. Cyclic voltammetry (CV) tests confirm the irreversibility of this polymerization process, which does not negatively impact the electrochemical performance of LCO. The proposed mechanism involves an electronic attack on the O atom of the DOL ring, leading to C–O bond cleavage and the formation of a negatively charged O species along with a –CH2 radical. This radical then interacts with another DOL monomer, triggering successive polymerization reactions.
1 weight ratio) undergoes irreversible polymerization, resulting in improved cycling stability. Cyclic voltammetry (CV) tests confirm the irreversibility of this polymerization process, which does not negatively impact the electrochemical performance of LCO. The proposed mechanism involves an electronic attack on the O atom of the DOL ring, leading to C–O bond cleavage and the formation of a negatively charged O species along with a –CH2 radical. This radical then interacts with another DOL monomer, triggering successive polymerization reactions.
Another ether-based monomer, dipropylene glycol dimethyl ether (DPGDME), has also been explored for in situ QSE fabrication via electrochemical polymerization (Fig. 9b).34 The polymerization of DPGDME was confirmed through cycling tests in a Li|Li cell, where the initial voltage–capacity curve exhibited a characteristic crescent shape but gradually evolved into a profile resembling that of other polymer electrolytes. The proposed polymerization mechanism involves Li+ attacking the DPGDME molecule, leading to the cleavage of a single C–O bond. The resulting DPGDME radicals then attack the hypomethyl group, where the active α-H is abstracted, leading to the formation of di-DPGDME. Concurrently, the dimer loses a –CH3 group, ultimately forming the DPGDME polymer. As a result, the QSE-based Li|Li cell demonstrated exceptional stability for over 3000 h, as well as robust performance under high-temperature conditions (50 °C) and high areal capacities (10 mA h cm−2). Furthermore, the assembled Li|SPAN cell achieved high sulfur utilization (98.4%) and rapid charging capability (10C). Notably, this system exhibited remarkable cycling stability, with 99.5% capacity retention over 400 cycles and an average CE exceeding 99.9995%, indicating negligible irreversible Li consumption.
Despite its potential, electrochemical polymerization faces challenges such as the insufficient ionic conductivity of the resulting polymers and the complexity of the underlying chemical reactions, making precise control difficult. Future research directions should focus on: (1) developing novel functional monomers to enhance ionic conductivity and stability, and (2) optimizing polymerization conditions to achieve efficient and uniform film formation.
4. Characters of QSSLMBs
QSE represents an intermediate state between LE and ASE, combining the advantages of both. It exhibits relatively high ionic conductivity, low liquid content, and good mechanical strength. These properties enable QSSLMBs to achieve high energy density while maintaining enhanced safety. However, to broaden their potential applications, certain aspects of QSSLMBs, such as safety performance and cell performance under extreme conditions (e.g., high voltage and low temperature), still require further improvement.Compared to traditional LMBs that use highly flammable LEs, QSE-based QSSLMBs significantly reduce the risk of severe accidents, such as fires or explosions, in cases of thermal runaway, short circuits, or external impacts. Nevertheless, since QSEs still contain trace amounts of flammable solvents, these safety risks cannot be entirely eliminated. Therefore, further enhancing the safety of QSSLMBs remains a critical area of research. Additionally, to increase the energy density of QSSLMBs, cathode active materials with high voltage plateaus are desirable. However, these materials are often incompatible with organic QSEs. Furthermore, the ionic conductivity of organic QSEs decreases significantly at low temperatures due to the freezing of their liquid components. As a result, modifying organic QSEs is essential to improve cell performance under extreme conditions, including high voltage and low temperature environments. The mechanical properties of in situ polymerized QSEs, along with the energy density and safety performance of the corresponding QSSLMBs, are summarized in Table 2. Detailed characterizations and discussions are presented in the following subsections.
| QSEs | Monomer | Polymerization method | Mechanical strength | Pouch cell | Safety thresholds | Ref. |
|---|---|---|---|---|---|---|
| F-GPE | PEGDA | Free radical | — | 1.35 A h | −40 to 60 °C | 25 |
| ESW 4.7 V vs. Li/Li+ | ||||||
| DOL–EC | DOL/EC | Cationic | 15.31 MPa | 1.0 A h | ESW 5.0 V vs. Li/Li+ | 29 |
| VEC–DAC–MBA | VEC/DAC/MBA | Free radical | 1.0 MPa | — | ESW 4.5 V vs. Li/Li+ | 44 |
| PFVS | VC/PEGDA | Free radical | — | 2.6 A h | ESW 5.1 V vs. Li/Li+ | 77 |
| SGPE | TFMA/TEP | Cationic | Good mechanical strength and flexibility | — | 0–80 °C | 39 |
| ESW 5.1 V vs. Li/Li+ | ||||||
| P-ECA | ECA | Anionic | Good flexibility and strength | — | ESW 4.8 V vs. Li/Li+ | 68 |
| P-DPGDME | DPGDME | Electrochemical | — | — | Cycling at 50 °C for 400 cycles | 34 |
| FRSE | VC/TEP | Cationic | 12.4 GPa | 2.4 A h | Stable operation at 50 °C passed the pinprick test | 78 |
| FGPE | HFBA/PETEA | Free radical | — | 402.5 W h kg−1 | High temperature | 79 |
| Self-extinguishing | ||||||
| ESW 5.0 V vs. Li/Li+ |
4.1 Flame-retardant properties
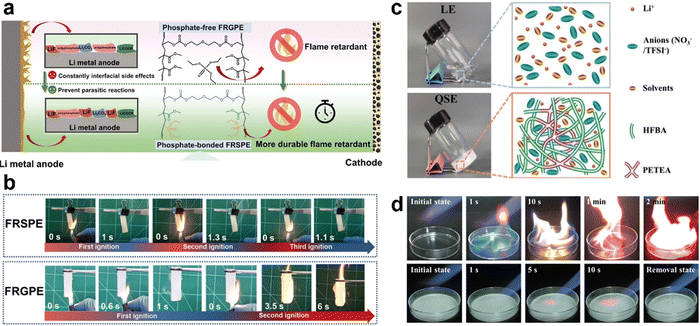
In LIBs, thermal runaway typically progresses through three critical stages: heat generation, heat accumulation, and thermal runaway. However, LMBs face even more severe thermal runaway challenges due to the exceptionally high chemical reactivity of LMA with LEs.80 Studies have demonstrated that electrolytes play a pivotal role in the thermal runaway process. Therefore, developing electrolytes with high thermal stability and flame retardancy is essential for enhancing battery safety. QSEs have shown significant potential in improving the safety performance of LMBs, primarily due to their excellent leakage prevention properties and enhanced interfacial compatibility with electrode materials through the incorporation of plasticizers. Nevertheless, the flammability of residual LE components in QSEs remains a safety concern that requires further attention. To address this issue, researchers are exploring strategies such as incorporating highly effective flame retardants into electrolytes or chemically grafting flame-retardant functional groups onto the polymer backbone to create molecular-level fire barriers.For instance, a non-flammable QSE was developed by encapsulating the flame-retardant liquid TEP into an in situ solidified polycarbonate (VC polymer) matrix (Fig. 10a–c).78 This optimized QSE demonstrated a high ionic conductivity of 4.4 mS cm−1 (Fig. 10d), a large Li+ transference number of 0.76, a Young's modulus of 12.4 GPa, and a wide ESW of 4.9 V vs. Li/Li+. These properties enabled the QSE to effectively suppress Li dendrite growth and undesirable side reactions at the LMA while maintaining compatibility with high-voltage cathodes. The assembled Li|NCM811 prototype cell achieved 87.7% capacity retention after 200 cycles (Fig. 10e), and an A h-level pouch cell exhibited impressive safety by passing the nail penetration test. This study provides valuable insights into the design of non-flammable QSEs for the practical realization of safe and stable QSSLMBs (Fig. 10f).
To address the drawbacks of physically mixed flame retardants, Cao and Yang et al.85 developed a P-containing flame-retardant QSE through the in situ polymerization of methyl methacrylate (MMA), allyl diethylene glycol carbonate (ADC), and the flame-retardant monomer diethyl vinyl phosphate (DEVP) (Fig. 11a). The QSE with chemically grafted flame retardants exhibited more durable non-flammability compared to QSEs with physically mixed flame retardants (Fig. 11b). Additionally, the grafted QSE demonstrated a high ionic conductivity of 0.18 mS cm−1 at RT, a wide ESW of 4.4 V vs. Li/Li+, excellent thermal stability (up to 190 °C), and superior compatibility with LMAs. As a result, the assembled Li|LFP cell delivered remarkable cycling stability, retaining 70.9% of its capacity after 1000 cycles at 25 °C and 69.1% after 200 cycles at 80 °C.
Another innovative approach involved the fabrication of a F-containing flame-retardant QSE through the in situ radical polymerization of hexafluorobutyl acrylate (HFBA) with a PETEA crosslinker (Fig. 11c).86 This method integrates fluorinated solvents into the polymer backbone of the QSE, eliminating the need for additional flame retardants. The HFBA-based QSE leverages the gas-phase radical scavenging effect of F radicals, which trap hydrogen radicals generated during combustion, thereby interrupting the chain reaction and extinguishing flames. In ignition tests, this QSE demonstrated exceptional non-flammability (Fig. 11d). When overcharged to 4.5 V, pouch cells with LEs experienced severe thermal runaway, while those with the QSE showed only a slight temperature increase. Furthermore, the QSE-based pouch cells exhibited minimal temperature changes during overheating and open flame exposure tests, highlighting their superior safety performance under abusive conditions. These results underscore the potential of this innovative QSE for real-world applications, particularly in electric vehicles, power tools, and energy storage systems.
The incorporation of flame retardants into QSEs significantly enhances the safety of QSSLMBs, reducing the risks of fire or explosion during overcharging, over-discharging, thermal runaway, or external impacts. While physical mixing of flame retardants offers a straightforward approach, chemical grafting is more advantageous and represents the future direction for QSE development, despite its complexity and higher cost. Additionally, flame retardants must be precisely designed to minimize adverse effects on battery performance. As safety requirements for batteries continue to rise, the development of non-flammable QSSLMBs will play a critical role in meeting global safety standards and environmental regulations, particularly in high-demand applications such as electric vehicles and grid-scale energy storage systems.
4.2 High-voltage compatibility
To maximize the energy density of QSSLMBs, cathode active materials with high voltage plateaus are essential. However, high-voltage QSSLMBs face challenges due to the instability of QSEs. The organic components of QSEs are prone to oxidative decomposition under high voltage, leading to poor electrochemical stability and safety concerns. To ensure compatibility with commercial high-voltage cathode materials, QSEs must exhibit an ESW exceeding 4.3 V (vs. Li/Li+). Therefore, developing QSEs with high-voltage stability is crucial. This can be achieved through chemical modifications of the polymer matrix, such as copolymerization, cross-linking, and halogenation, or by incorporating functional fillers, including Li+-insulating and Li+-conductive materials.Fluorination of the polymer matrix is a widely used method to enhance oxidation resistance. The introduction of F atoms reduces the regularity of the polymer structure and lowers the energy levels due to F's high electronegativity.87 For example, a novel QSE with a fluorinated polymer matrix was developed using PETEA and hexafluoroacetone (HFA) monomers (Fig. 12a).79 The HFA monomer exhibits a high HOMO energy level (−7.0241 eV), enabling it to participate in the formation of a CEI layer, significantly enhancing the QSE's compatibility with high-voltage cathodes (Fig. 12b). Additionally, the strong interaction between the F-containing structure in the QSE matrix and the PF6− anion suppresses anodic currents, resulting in a wide ESW of 5.0 V (vs. Li/Li+) (Fig. 12c). The assembled Li|NCM811 full cell with this fluorinated QSE delivered a specific capacity of 224 mA h g−1 at 0.1C (Fig. 12d) and retained 58.6% of its capacity after 200 cycles at 1C (Fig. 12e). The fluorinated QSE not only benefits the cathode side but also facilitates the formation of a LiF-rich interfacial layer on the LMA, suppressing side reactions and Li dendrite growth due to the low LUMO energy level (−2.8938 eV) of the HFA segment.
Copolymerization and cross-linking are also effective strategies to enhance the high-voltage stability of QSEs. Monomers containing polar groups are particularly suitable for copolymerization and cross-linking, as polar groups readily form covalent bonds. For instance, a QSE with a fluorinated and cross-linked polymer matrix was prepared via the in situ polymerization of 3,3,3-trifluoropropene oxide (TFPO) and pentaerythritol glycidyl ether (PEE) monomers (Fig. 12f).88 This QSE exhibited a high oxidation potential of 5.1 V (vs. Li/Li+) (Fig. 12g), attributed to the fluorinated and cross-linked polyether matrix. The QSSLMB with an NCM622 cathode maintained 78% capacity retention after 1000 cycles at 0.5C and a cutoff voltage of 4.3 V (Fig. 12h). Even at an ultrahigh cutoff voltage of 4.7 V, the cell achieved 74.2% capacity retention after 100 cycles at 0.5C (Fig. 12i). The electron-withdrawing –CF3 group in the QSE matrix contributed to the formation of a LiF-rich SEI/CEI layer, enhancing compatibility with the LMA.
For example, yttria-stabilized zirconia (YSZ), a Li+-insulating filler, was introduced into a QSE prepared via the in situ polymerization of DOL (Fig. 13a).92 The Lewis acidic properties of YSZ (Zr4+, Y3+, and O vacancies) increased the polymerization efficiency of DOL to 98.5%, minimized by-product formation, and enhanced high-voltage stability, resulting in an ESW exceeding 4.9 V (vs. Li/Li+). The QSE-based Li|NCM622 cell exhibited stable cycling for over 800 cycles, with the formation of a Li2ZrO3-rich SEI layer that promoted uniform Li deposition and suppressed dendrite growth.
 | ||
| Fig. 13 (a) Schematic of DOL-derived QSE with YSZ filler. In situ preparation of PEGDA-based QSEs with LLAZO filler (b) and LLZTO filler (c). | ||
Li+-conductive fillers, such as garnet-type materials, are particularly advantageous due to their high ionic conductivity, wide ESWs (>5 V vs. Li/Li+), and compatibility with LMAs.11 For instance, a QSE was prepared by in situ polymerization of EGDA with one-dimensional LLAZO nanofibers (Fig. 13b).93 The LLAZO nanofibers were chemically functionalized with silane and covalently grafted to the polymer matrix, preventing filler aggregation and enhancing interfacial compatibility. The strong Lewis acid–base interactions between LLAZO and Li salts facilitated Li+ migration, reducing interfacial impedance and forming a stable interfacial layer. This QSE achieved an impressive ESW of 5.3 V (vs. Li/Li+) and enabled the Li|NCM111 cell to deliver an excellent rate performance up to 10C.
Another QSE, fabricated via in situ polymerization of EGDA with asymmetric LLZTO fillers (Fig. 13c),94 demonstrated enhanced high-voltage stability. The LLZTO fillers, enriched on the QSE surface, promoted uniform Li deposition, inhibited dendrite formation, and improved the ESW to 5.13 V (vs. Li/Li+) due to the filler's high oxidative stability.
QSEs are inherently unstable under high-voltage conditions due to the high HOMO energy levels of the polymer matrix (susceptible to reactive O species) and various reactive functional groups (susceptible to free electrons). High-voltage stability can be improved through chemical modifications of the polymer matrix to enhance oxidation resistance or by incorporating high-voltage-resistant inorganic fillers. While chemical modification offers superior performance, it is more complex and costly. In contrast, filler incorporation is simpler and more cost-effective, though less impactful. Therefore, the ideal approach may involve combining both strategies to develop QSEs with excellent high-voltage stability, paving the way for their application in high-energy-density batteries for electric vehicles, power tools, and grid-scale energy storage systems.
4.3 Low-temperature operation
The performance of LIBs at low temperatures (below −20 °C) is a critical factor in evaluating their practicality, especially given the wide range of applications in cold environments, such as winter conditions, polar regions, and outer space. Conventional LIBs often fail to operate effectively at low temperatures due to the sharp decline in the ionic conductivity of LEs. Similarly, QSEs suffer from significantly reduced ionic conductivity at low temperatures, primarily due to the “freezing” of the polymer matrix and the limited mobility of residual LEs.95 At low temperatures, the mobility of polymer chain segments decreases, and the viscosity of the solvent increases, both of which hinder ionic migration. Additionally, the polymer matrix becomes glassy, increasing its rigidity and potentially leading to brittleness. This reduced mechanical strength can cause cracks or fractures in the polymer film, compromising the integrity of the QSE and ultimately resulting in battery failure or shortened lifespan. To address these challenges, researchers have explored various strategies to enhance the low-temperature performance of QSEs, including the introduction of liquid plasticizers (e.g., ionic liquids, carbonate solvents, or oligomer molecules), interfacial regulation (e.g., optimizing the SEI and CEI), and chemical modifications of the polymer matrix (e.g., copolymerization, cross-linking, or the introduction of ionic side groups).In another study, a novel QSE was prepared via the in situ polymerization of DOL with a single-solute LiBF4 and a high-dielectric-constant solvent, FEC.96 The synergistic effect of the DOL polymer, BF4− anions, and FEC enabled the formation of a robust and dynamically stable SEI layer containing LiF and Li+-conducting LixBOyFz. This resulted in a Li|LCO cell that exhibited stable cycling performance across a wide temperature range, from −60 to −20 °C.
Improving the low-temperature performance of QSSLMBs is critical for their practical application in cold environments. This can be achieved through the optimization of the polymer matrix, contained LEs, and additives. Nevertheless, achieving reliable performance at extremely low temperatures remains a major challenge, particularly in maintaining both conductivity and mechanical integrity. Future research should focus on advancing existing methods and developing new strategies to enhance the low-temperature performance of QSEs. Such advancements will drive the widespread adoption of electric vehicles and portable electronic devices in cold regions and provide innovative solutions for grid energy storage. With continued research, we anticipate the development of more efficient, stable, and safe low-temperature QSSLMBs, contributing to the realization of green energy and sustainable development goals.
4.4 Impact of optimization strategies
To meet the growing demands for high energy density, enhanced safety, and broad temperature adaptability in QSSLMBs, the optimization of QSEs must consider material innovation, interfacial regulation, and process engineering. In this section, we systematically review key strategies used to enhance QSE performance, clarify their synergistic effects on Li+ transport, ESW, and mechanical properties, and highlight promising future research directions.5. Summary and outlook
5.1 Summary
QSEs, which combine the advantages of ASEs and LEs, represent one of the most promising candidates for practical LMB applications. Current research on in situ polymerization-derived QSEs primarily focuses on two key directions: (1) developing novel polymer matrices through innovative monomers, initiators, and polymerization reactions to achieve enhanced mechanical strength and electrochemical stability. (2) Tailoring QSE properties by optimizing the polymer matrix, Li salts, plasticizers, and additives to enable specific functionalities such as flame retardancy, high-voltage compatibility, and low-temperature performance.Despite these advancements, several critical challenges remain: (1) interfacial instability with LMAs: the significant volume expansion/contraction of LMAs during cycling leads to poor interfacial contact, promoting Li dendrite formation. QSEs must therefore balance high mechanical strength (to suppress dendrites) with sufficient ionic conductivity. (2) Limited ESW: high-voltage cathodes are essential for high-energy-density QSSLMBs, yet most QSEs exhibit insufficient oxidative stability due to residual monomers and plasticizers. (3) Complex optimization of preparation conditions: QSE fabrication involves numerous interdependent parameters (e.g., component selection, concentrations, polymerization temperature/time, infiltration duration), requiring precise control to ensure electrolyte homogeneity and process reproducibility. (4) Reduced energy density: the incorporation of additives (e.g., flame retardants, plasticizers) often lowers the energy density of QSSLMBs compared to LE-based systems.
Notable progress has been made in addressing these challenges. For instance: a fluorinated polymer matrix (derived from PETEA and VEC monomers) achieved an ultra-wide ESW (up to 5.3 V vs. Li/Li+).47 A non-flammable QSE was developed by encapsulating liquid TEP within an in situ-formed polycarbonate (VC polymer) matrix.78 A fluorinated polyether-based QSE enabled a QSSLMB with an energy density exceeding 400 W h kg−1.6 Future research should prioritize: (1) streamlining QSE preparation processes. (2) Enhancing mechanical robustness and electrochemical stability. (3) Minimizing trade-offs between functionality and energy density. These efforts will be critical to advancing the practical deployment of QSEs in next-generation LMBs.
In this review, the in situ fabricated QSEs were classified from the aspects of polymerization patterns of the polymer matrix, as follows:
Free radical polymerization is one of the most widely used methods for synthesizing the QSE matrix. This method involves the initiation of polymerization through free radicals, which can be generated thermally, photochemically, or via redox reactions. QSEs produced via free radical polymerization typically exhibit good ionic conductivity and mechanical strength. However, they often suffer from limited electrochemical stability at high voltages, which restricts their use in high-energy-density batteries.
Cationic polymerization is another approach to fabricate the QSE matrix, where the polymerization is initiated by cationic species. This method is particularly useful for producing QSEs with high molecular weights and good thermal stability. However, the ionic conductivity of QSEs obtained through cationic polymerization is generally lower compared to those produced by free radical polymerization. Additionally, the process is sensitive to moisture and other impurities, which can hinder the polymerization process.
Anionic polymerization involves the initiation of polymerization by anionic species. This method allows for precise control over the molecular weight and architecture of the polymer, leading to a QSE matrix with well-defined structures and properties. QSEs produced via anionic polymerization often exhibit excellent mechanical properties and thermal stability. However, similar to cationic polymerization, the ionic conductivity of these QSEs is relatively low, and the process is highly sensitive to impurities.
Electrochemical polymerization is a unique method where the polymerization is initiated by an applied electric field. This technique allows for the direct formation of QSEs on the electrode surface, leading to excellent interfacial contact between the electrolyte and the electrode. QSEs produced via electrochemical polymerization typically exhibit high ionic conductivity and good electrochemical stability. However, the process is complex and requires precise control over the polymerization conditions.
The characteristics of QSSLMBs were also introduced, as follows:
Flame retardancy is a critical safety feature for QSEs in LMBs. Several strategies have been explored to enhance the flame retardancy of QSEs, including the incorporation of flame-retardant additives and the use of inherently flame-retardant polymers. While significant progress has been made, achieving a balance between flame retardancy and other performance metrics, such as ionic conductivity and mechanical strength, remains a challenge.
High-voltage performance is essential for the development of high-energy-density LMBs. QSEs with high electrochemical stability are required to withstand the high voltages encountered in these batteries. While some QSEs have demonstrated good high-voltage performance, further improvements are needed to enhance their stability and prevent degradation at high voltages.
Low-temperature performance is another critical aspect for the practical application of LMBs. QSEs with high ionic conductivity at low temperatures are essential to ensure the efficient operation of batteries in cold environments. Several approaches, such as the use of low-temperature plasticizers and the optimization of polymer structures, have been explored to improve the low-temperature performance of QSEs. However, achieving high ionic conductivity at low temperatures without compromising other properties remains a significant challenge.
5.2 Outlook
Despite the substantial progress achieved in the development of QSEs for LMBs, several key challenges remain. These include the need to further enhance ionic conductivity—particularly under low-temperature conditions—improve electrochemical stability at high voltages, and boost flame-retardant performance. Additionally, issues related to the scalability, cost-efficiency, and practicality of polymerization methods must be addressed to enable the widespread commercial application of QSEs.Future research should focus on the following directions:
Development of novel functional monomers: emphasis should be placed on designing new monomers with properties such as self-healing capabilities, high oxidative stability, and inherent flame retardancy. This may be achieved through chemical modifications such as fluorination, phosphorization, or sulfurization of existing monomers. Such developments aim to produce QSEs with superior Li dendrite suppression, high-voltage compatibility, and improved safety profiles.
Optimization of polymerization strategies: the polymerization process plays a critical role in determining the performance of the polymer matrix. Combining theoretical modeling with experimental data can help in precisely controlling the molecular weight and segment distribution of polymer chains. Additionally, hybrid polymerization techniques—such as the integration of photoinitiated and thermally initiated processes—can reduce energy consumption while enhancing control. Innovative in situ polymerization methods based on microfluidic technology also show promise for achieving uniform electrolyte thickness and improved interfacial compatibility.
Incorporation of advanced functional materials: introducing nanostructured fillers (e.g., MXenes, MOF-derived materials) and ionic liquids can significantly enhance ion transport efficiency and flame retardancy. These materials offer multifunctional benefits and open new possibilities for tuning the mechanical and electrochemical properties of QSEs.
Interdisciplinary and technological integration: cross-disciplinary approaches are essential for accelerating innovation in this field. The application of machine learning algorithms can assist in optimizing molecular design and predicting polymer properties. Coupling these insights with advances in battery system integration—such as the development of compatible electrode materials and optimized cell architectures—can facilitate the practical deployment of QSEs. Furthermore, expanding research into emerging applications such as flexible electronics, wearable devices, and grid-level energy storage systems will broaden the impact of QSE technology.
In summary, while considerable advancements have been made in the field of QSEs for LMBs, further innovation in materials science, polymer chemistry, and system integration is essential. The future success of QSEs hinges on the development of advanced materials and scalable polymerization methods that can meet the performance demands of next-generation QSSLMBs.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (NSFC: 22209107 and 52173205).References
- A. Innocenti, S. Beringer and S. Passerini, Cost and performance analysis as a valuable tool for battery material research, Nat. Rev. Mater., 2024, 9, 347–357 CrossRef CAS
.
- V. Viswanathan, A. H. Epstein, Y. M. Chiang, E. Takeuchi, M. Bradley, J. Langford and M. Winter, The challenges and opportunities of battery-powered flight, Nature, 2022, 601, 519–525 CrossRef CAS PubMed
.
- Q. Xu, X. Lai, G. Zhang, T. Li and Y. Qiu, Tailoring the passivation layer on lithium metal anode and aluminum collector by regulating the solvation structure to enhance the long-term cycling of lithium metal batteries, Chem. Eng. J., 2024, 480, 148021 CrossRef CAS
.
- S. Zou, Y. Yang, J. Wang, X. Zhou, X. Wan, M. Zhu and J. Liu, In-situ polymerization of solid-state polymer electrolytes for lithium metal batteries: a review, Energy Environ. Sci., 2024, 17, 4426–4460 RSC
.
- K. Hatzell, W. Chang, W. Bao, M. Cai, T. Glossmann, S. Kalnaus, B. Liaw, Y. S. Meng, R. Mohtadi and Y. Wang, Aligning lithium metal battery research and development across academia and industry, Joule, 2024, 8, 1550–1555 CrossRef CAS
.
- J. Zhu, R. Zhao, J. Zhang, X. Song, J. Liu, N. Xu, H. Zhang, X. Wan, X. Ji, Y. Ma, C. Li and Y. Chen, Long-cycling and High-voltage Solid State Lithium Metal Batteries Enabled by Fluorinated and Crosslinked Polyether Electrolytes, Angew. Chem., Int. Ed., 2024, 63, e202400303 CrossRef CAS PubMed
.
- K. Jun, Y. Chen, G. Wei, X. Yang and G. Ceder, Diffusion mechanisms of fast lithium-ion conductors, Nat. Rev. Mater., 2024, 9, 887–905 CrossRef CAS
.
- K. J. Kim, M. Balaish, M. Wadaguchi, L. Kong and J. L. Rupp, Solid-state Li–metal batteries: challenges and horizons of oxide and sulfide solid electrolytes and their interfaces, Adv. Energy Mater., 2021, 11, 2002689 CrossRef CAS
.
- C. Wang, Y. Wu, S. Wang, E. van der Heide and X. Zhuang, Interface issues between cathode and electrolyte in sulfide-based all-solid-state lithium batteries and improvement strategies of interface performance through cathode modification, Mater. Res. Bull., 2025, 181, 113078 CrossRef CAS
.
- Q. Wang, Y. Zhou, X. Wang, H. Guo, S. Gong, Z. Yao, F. Wu, J. Wang, S. Ganapathy and X. Bai, Designing lithium halide solid electrolytes, Nat. Commun., 2024, 15, 1050 CrossRef CAS PubMed
.
- S. Liu, W. Liu, D. Ba, Y. Zhao, Y. Ye, Y. Li and J. Liu, Filler-integrated composite polymer electrolyte for solid-state lithium batteries, Adv. Mater., 2023, 35, 2110423 CrossRef CAS
.
- Q. Huang, Y. Wu, Z. He, S. Wang, J. Zhu and X. Zhuang, Doping Strategies for Improving Performance of Li-Argyrodite Solid-State Electrolyte, Energy Technol., 2025, 13, 2401420 CrossRef CAS
.
- X. Yang, Q. Yin, C. Wang, K. Doyle-Davis, X. Sun and X. Li, Towards practically accessible high-voltage solid-state lithium batteries: From fundamental understanding to engineering design, Prog. Mater. Sci., 2023, 140, 101193 CrossRef CAS
.
- T. Schmaltz, F. Hartmann, T. Wicke, L. Weymann, C. Neef and J. Janek, A Roadmap for Solid-State Batteries, Adv. Energy Mater., 2023, 13, 2301886 CrossRef CAS
.
- J. Song, Y. Wang and C. C. Wan, Review of gel-type polymer electrolytes for lithium-ion batteries, J. Power Sources, 1999, 77, 183–197 CrossRef CAS
.
- C. Han, X. Shui, G. Chen, G. Xu, J. Ma, S. Dong, S. Wang, X. Zhou, Z. Cui, L. Qiao and G. Cui, Recent progress in gel polymer electrolyte for lithium metal batteries, Giant, 2024, 20, 100337 CrossRef CAS
.
- V. Vijayakumar, B. Anothumakkool, S. Kurungot, M. Winter and J. R. Nair, In situ polymerization process: an essential design tool for lithium polymer batteries, Energy Environ. Sci., 2021, 14, 2708–2788 RSC
.
- M. Sun, Z. Zeng, W. Zhong, Z. Han, L. Peng, S. Cheng and J. Xie, In-situ polymerization methods for polymer-based solid-state lithium batteries, Batteries Supercaps, 2022, 5, e202200338 CrossRef CAS
.
- Z. Shen, J. Zhong, S. Jiang, W. Xie, S. Zhan, K. Lin, L. Zeng, H. Hu, G. Lin and Y. Lin, Polyacrylonitrile porous membrane-based gel polymer electrolyte by in situ free-radical polymerization for stable Li metal batteries, ACS Appl. Mater. Interfaces, 2022, 14, 41022–41036 CrossRef CAS
.
- F. Chen, X. Wang, M. Armand and M. Forsyth, Cationic polymer-in-salt electrolytes for fast metal ion conduction and solid-state battery applications, Nat. Mater., 2022, 21, 1175–1182 CrossRef CAS PubMed
.
- P. Hu, J. Chai, Y. Duan, Z. Liu, G. Cui and L. Chen, Progress in nitrile-based polymer electrolytes for high performance lithium batteries, J. Mater. Chem. A, 2016, 4, 10070–10083 RSC
.
- T. Liu, J. Zhang, W. Han, J. Zhang, G. Ding, S. Dong and G. Cui, In situ polymerization for integration and interfacial protection towards solid state lithium batteries, J. Electrochem. Soc., 2020, 167, 070527 CrossRef CAS
.
- S. Azmi, A. Klimek and E. Frackowiak, Why electrochemical capacitor electrolytes should not be ignored?, Electrochim. Acta, 2023, 452, 142347 CrossRef CAS
.
- M. Chen, Z. Yue, Y. Wu, Y. Wang, Y. Li and Z. Chen, Thermal stable polymer-based solid electrolytes: Design strategies and corresponding stable mechanisms for solid-state Li metal batteries, Sustainable Mater. Technol., 2023, 36, e00587 CrossRef CAS
.
- H. He, Y. Wang, M. Li, J. Qiu, Y. Wen and J. Chen, In situ cross-linked fluorinated gel polymer electrolyte based on PEGDA-enabled lithium-ion batteries with a wide temperature operating range, Chem. Eng. J., 2023, 467, 143311 CrossRef CAS
.
- K. S. Pedersen and H. P. Rønningsen, Influence of Wax Inhibitors on Wax Appearance Temperature, Pour Point, and Viscosity of Waxy Crude Oils, Energy Fuels, 2003, 17, 321–328 CrossRef CAS
.
- S. Aoshima and S. Kanaoka, A renaissance in living cationic polymerization, Chem. Rev., 2009, 109, 5245–5287 CrossRef CAS
.
-
S. Kobayashi and K. Müllen, Encyclopedia of polymeric nanomaterials, Springer Berlin Heidelberg, Berlin Heidelberg, 2015 Search PubMed
.
- F.-Q. Liu, W.-P. Wang, Y.-X. Yin, S.-F. Zhang, J.-L. Shi, L. Wang, X.-D. Zhang, Y. Zheng, J.-J. Zhou and L. Li, Upgrading traditional liquid electrolyte via in situ gelation for future lithium metal batteries, Sci. Adv., 2018, 4, eaat5383 CrossRef CAS PubMed
.
-
B. M. Mandal, Fundamentals of polymerization, World Scientific, 2012 Search PubMed
.
- D. Luo, L. Zheng, Z. Zhang, M. Li, Z. Chen, R. Cui, Y. Shen, G. Li, R. Feng and S. Zhang, Constructing multifunctional solid electrolyte interface via in-situ polymerization for dendrite-free and low N/P ratio lithium metal batteries, Nat. Commun., 2021, 12, 186 CrossRef CAS
.
- J. Ju, S. Dong, Y. Cui, Y. Zhang, B. Tang, F. Jiang, Z. Cui, H. Zhang, X. Du and T. Lu, Leakage-proof electrolyte chemistry for a high-performance lithium–sulfur battery, Angew. Chem., Int. Ed., 2021, 60, 16487–16491 CrossRef CAS
.
- E. Muresan, S. Oprea, V. Hulea, T. Malutan and M. Vata, Kinetic studies for the esterification of acetic acid with epichlorohydrin over an anion exchange resin catalyst, Open Chem., 2008, 6, 419–428 CrossRef CAS
.
- J. Chen, H. Lu, X. Zhang, Y. Zhang, J. Yang, Y. Nuli, Y. Huang and J. Wang, Electrochemical polymerization of nonflammable electrolyte enabling fast-charging lithium-sulfur battery, Energy Storage Mater., 2022, 50, 387–394 CrossRef
.
- Y. Xie, K. Zhang, Y. Yamauchi, K. Oyaizu and Z. Jia, Nitroxide radical polymers for emerging plastic energy storage and organic electronics: fundamentals, materials, and applications, Mater. Horiz., 2021, 8, 803–829 RSC
.
- W. Min, L. Li, M. Wang, S. Ma, H. Feng, W. Wang, H. Ding, T. Cheng, Z. Li, T. Saito, H. Yang and P.-F. Cao, Mastering the Copolymerization Behavior of Ethyl Cyanoacrylate as Gel Polymer Electrolyte for Lithium-metal Battery Application, Angew. Chem., Int. Ed., 2025, 64, e202422510 CrossRef CAS
.
- L. Sun, K. Higaki and R. C. McDonald, Performance characteristics of lithium-ion cells using in situ polymerized electrolytes, J. Power Sources, 1997, 68, 352–356 CrossRef CAS
.
- M. Zhou, W. Chen, H. Yang, Y. Hu, T. Lei, D. Chen, S. Wang, Y. Zhang and J. Xiong, Molecular Crowding Solid Polymer Electrolytes for Lithium Metal Battery by In Situ Polymerization, Adv. Energy Mater., 2025, 15, 2403082 CrossRef CAS
.
- Q. Sun, Z. Gong, T. Zhang, J. Li, X. Zhu, R. Zhu, L. Wang, L. Ma, X. Li and M. Yuan, Molecule-Level Multiscale Design of Nonflammable Gel Polymer Electrolyte to Build Stable SEI/CEI for Lithium Metal Battery, Nano-Micro Lett., 2025, 17, 18 CrossRef CAS PubMed
.
- F. Pfeiffer, A. Griggio, M. Weiling, J. F. Wang, F. Reißig, C. Peschel, L. Pillatsch, S. Warrington, S. Nowak and V. Grimaudo, Tracing the Cross-Talk Phenomenon of Vinylethylene Carbonate to Unveil its Counterintuitive Influence as an Electrolyte Additive on High-Voltage Lithium-Ion Batteries, Adv. Energy Mater., 2024, 14, 2402187 CrossRef CAS
.
- X. Zhang, G. Gao, W. Wang, J. Wang, L. Wang and T. Liu, Synergy of an In Situ-Polymerized Electrolyte and a Li3N–LiF-Reinforced Interface Enables Long-Term Operation of Li-Metal Batteries, ACS Appl. Mater. Interfaces, 2022, 14, 49811–49819 CrossRef CAS
.
- G. Xiao, H. Xu, C. Bai, M. Liu and Y. B. He, Progress and perspectives of in situ polymerization method for lithium-based batteries, Interdiscip. Mater., 2023, 2, 609–634 CAS
.
- Z. Lin, X. Guo, Z. Wang, B. Wang, S. He, L. A. O'Dell, J. Huang, H. Li, H. Yu and L. Chen, A wide-temperature superior ionic conductive polymer electrolyte for lithium metal battery, Nano Energy, 2020, 73, 104786 CrossRef CAS
.
- G. Ye, L. Zhu, Y. Ma, M. He, C. Zheng, K. Shen, X. Hong, Z. Xiao, Y. Jia, P. Gao and Q. Pang, Molecular Design of Solid Polymer
Electrolytes with Enthalpy–Entropy Manipulation for Li Metal Batteries with Aggressive Cathode Chemistry, J. Am. Chem. Soc., 2024, 146, 27668–27678 CrossRef CAS
.
- C. Korepp, H. Santner, T. Fujii, M. Ue, J. Besenhard, K.-C. Möller and M. Winter, 2-Cyanofuran—A novel vinylene electrolyte additive for PC-based electrolytes in lithium-ion batteries, J. Power Sources, 2006, 158, 578–582 CrossRef CAS
.
- J. Chai, Z. Liu, J. Ma, J. Wang, X. Liu, H. Liu, J. Zhang, G. Cui and L. Chen, In situ generation of poly (vinylene carbonate) based solid electrolyte with interfacial stability for LiCoO2 lithium batteries, Adv. Sci., 2017, 4, 1600377 CrossRef PubMed
.
- Q. Zhou, C. Fu, R. Li, X. Zhang, B. Xie, Y. Gao, G. Yin and P. Zuo, Poly (vinyl ethylene carbonate)-based dual-salt gel polymer electrolyte enabling high voltage lithium metal batteries, Chem. Eng. J., 2022, 437, 135419 CrossRef CAS
.
- H. Peng, T. Long, J. Peng, H. Chen, L. Ji, H. Sun, L. Huang and S. G. Sun, Molecular Design for In-Situ Polymerized Solid Polymer Electrolytes Enabling Stable Cycling of Lithium Metal Batteries, Adv. Energy Mater., 2024, 14, 2400428 CrossRef CAS
.
- C. Y. Kwok, Q. Pang, A. Worku, X. Liang, M. Gauthier and L. F. Nazar, Impact of the Mechanical Properties of a Functionalized Cross-Linked Binder on the Longevity of Li–S Batteries, ACS Appl. Mater. Interfaces, 2019, 11, 22481–22491 CrossRef CAS
.
- X. Yang, H. Deng, J. Xu, D. Ye, X. Jiang, Y. Chen, K. Sun and Z. Liu, Control the explosive polymerization of 1,3-dioxolane in LiPF6 electrolyte by Lewis acid-base interactions, J. Energy Storage, 2024, 101, 113793 CrossRef
.
- P. Li, S. Wang, J. Hao, X. Wang, S.-M. Hao, Y. Lu, H. Li, W. Zhou and Y. Li, Efficiencies of Various in situ Polymerizations of Liquid Electrolytes and the Practical Implications for Quasi Solid-state Batteries, Angew. Chem., Int. Ed., 2023, 62, e202309613 CrossRef CAS
.
- C. Yuan, L. Yang, L. Hou, J. Li, Y. Sun, X. Zhang, L. Shen, X. Lu, S. Xiong and X. W. Lou, Flexible Hybrid Paper Made of Monolayer Co3O4 Microsphere Arrays on rGO/CNTs and Their Application in Electrochemical Capacitors, Adv. Funct. Mater., 2012, 22, 2560–2566 CrossRef CAS
.
- H. Duan, Y.-X. Yin, Y. Shi, P.-F. Wang, X.-D. Zhang, C.-P. Yang, J.-L. Shi, R. Wen, Y.-G. Guo and L.-J. Wan, Dendrite-Free Li-Metal Battery Enabled by a Thin Asymmetric Solid Electrolyte with Engineered Layers, J. Am. Chem. Soc., 2018, 140, 82–85 CrossRef CAS PubMed
.
- G. Cui, J. Zhang, L. Huang, S. Yao, P. Han, Z. Yuan, H. Zhang, Y. Zhang, H. Cui, D. Wang and S. Zhang, Research Progress on Room-temperature Solid-state Lithium Metal Batteries with Poly(ethylene oxide)-based Solid Polymer Electrolytes, Acta Chim. Sin., 2024, 82, 849–855 CrossRef
.
- Y. Ma, X. Lin, J. Li, X. Zhou, X. Liu and Q. Gu, Recent Progress on Polymer Solid Electrolytes for Lithium Metal Batteries, Acta Chim. Sin., 2024, 82, 449–457 CrossRef
.
- H. Yang, M. Jing, L. Wang, H. Xu, X. Yan and X. He, PDOL-based solid electrolyte toward Practical application: Opportunities and challenges, Nano-Micro Lett., 2024, 16, 127 CrossRef
.
- Y. Liu, H. Zou, Z. Huang, Q. Wen, J. Lai, Y. Zhang, J. Li, K. Ding, J. Wang and Y.-Q. Lan, In situ polymerization of 1,3-dioxane as a highly compatible polymer electrolyte to enable the stable operation of 4.5 V Li-metal batteries, Energy Environ. Sci., 2023, 16, 6110–6119 RSC
.
- H. Wu, B. Tang, X. Du, J. Zhang, X. Yu, Y. Wang, J. Ma, Q. Zhou, J. Zhao and S. Dong, LiDFOB initiated in situ polymerization of novel eutectic solution enables room-temperature solid lithium metal batteries, Adv. Sci., 2020, 7, 2003370 CrossRef CAS
.
- M. Okada, Y. Yamashita and Y. Ishii, Polymerization of 1, 3-dioxolane, Die Makromol. Chem.: Macromol. Chem. Phys., 1964, 80, 196–207 CrossRef CAS
.
- D. Aurbach, O. Youngman and P. Dan, The electrochemical behavior of 1, 3-dioxolane—LiClO4 solutions—II. Contaminated solutions, Electrochim. Acta, 1990, 35, 639–655 CrossRef CAS
.
- K. Khan, Z. Tu, Q. Zhao, C. Zhao and L. A. Archer, Synthesis and properties of poly-ether/ethylene carbonate electrolytes with high oxidative stability, Chem. Mater., 2019, 31, 8466–8472 CrossRef CAS
.
- Z. Geng, Y. Huang, G. Sun, R. Chen, W. Cao, J. Zheng and H. Li, In-situ polymerized solid-state electrolytes with stable cycling for Li/LiCoO2 batteries, Nano Energy, 2022, 91, 106679 CrossRef CAS
.
- S. Song, W. Gao, G. Yang, Y. Zhai, J. Yao, L. Lin, W. Tang, N. Hu and L. Lu, Hybrid poly-ether/carbonate ester electrolyte engineering enables high oxidative stability for quasi-solid-state lithium metal batteries, Mater. Today Energy, 2022, 23, 100893 CrossRef CAS
.
- Q. Zhao, X. Liu, S. Stalin, K. Khan and L. A. Archer, Solid-state polymer electrolytes with in-built fast interfacial transport for secondary lithium batteries, Nat. Energy, 2019, 4, 365–373 CrossRef CAS
.
- J. Ma, Y. Wu, H. Jiang, X. Yao, F. Zhang, X. Hou, X. Feng and H. Xiang, In situ directional polymerization of poly (1, 3-dioxolane) solid electrolyte induced by cellulose paper-based composite separator for lithium metal batteries, Energy Environ. Mater., 2023, 6, e12370 CrossRef CAS
.
- Y. Huang, S. Liu, Q. Chen, K. Jiao, B. Ding and J. Yan, Constructing highly conductive and thermomechanical stable quasi-solid electrolytes by self-polymerization of liquid electrolytes within porous polyimide nanofiber films, Adv. Funct. Mater., 2022, 32, 2201496 CrossRef CAS
.
- D. Chen, M. Zhu, P. Kang, T. Zhu, H. Yuan, J. Lan, X. Yang and G. Sui, Self-enhancing gel polymer electrolyte by in situ construction for enabling safe lithium metal battery, Adv. Sci., 2022, 9, 2103663 CrossRef CAS
.
- Y. Cui, J. Chai, H. Du, Y. Duan, G. Xie, Z. Liu and G. Cui, Facile and reliable in situ polymerization of poly (ethyl cyanoacrylate)-based polymer electrolytes toward flexible lithium batteries, ACS Appl. Mater. Interfaces, 2017, 9, 8737–8741 CrossRef CAS PubMed
.
- Z. Liu, P. Hu, J. Ma, B. Qin, Z. Zhang, C. Mou, Y. Yao and G. Cui, Conformal poly (ethyl α-cyanoacrylate) nano-coating for improving the interface stability of LiNi0.5Mn1.5O4, Electrochim. Acta, 2017, 236, 221–227 CrossRef CAS
.
- Z. Hu, S. Zhang, S. Dong, W. Li, H. Li, G. Cui and L. Chen, Poly (ethyl α-cyanoacrylate)-based artificial solid electrolyte interphase layer for enhanced interface stability of Li metal anodes, Chem. Mater., 2017, 29, 4682–4689 CrossRef CAS
.
- J. Wei, H. Yue, Z. Shi, Z. Li, X. Li, Y. Yin and S. Yang, In situ gel polymer electrolyte with inhibited lithium dendrite growth and enhanced interfacial stability for lithium-metal batteries, ACS Appl. Mater. Interfaces, 2021, 13, 32486–32494 CrossRef CAS
.
- W. Zhang, S. Zhang, L. Fan, L. Gao, X. Kong, S. Li, J. Li, X. Hong and Y. Lu, Tuning the LUMO energy of an organic interphase to stabilize lithium metal batteries, ACS Energy Lett., 2019, 4, 644–650 CrossRef CAS
.
- G. Zeng, S. Dai, X. Chen, L. Qiu, X. Kong, M. Huang and T. Wen, Solid-State Graft Polymer Electrolytes with Conductive Backbones and Side Chains for Lithium Batteries, Macromolecules, 2024, 57, 1258–1265 CrossRef CAS
.
- H. D. Abruña, P. Denisevich, M. Umana, T. J. Meyer and R. W. Murray, Rectifying interfaces using two-layer films of electrochemically polymerized vinylpyridine and vinylbipyridine complexes of ruthenium and iron on electrodes, J. Am. Chem. Soc., 1981, 103, 1–5 CrossRef
.
- R. Nölle, K. Beltrop, F. Holtstiege, J. Kasnatscheew, T. Placke and M. Winter, A reality check and tutorial on electrochemical characterization of battery cell materials: How to choose the appropriate cell setup, Mater. Today, 2020, 32, 131–146 CrossRef
.
- L. Kong, H. Zhan, Y. Li and Y. Zhou, In situ fabrication of lithium polymer battery basing on a novel electro-polymerization technique, Electrochem. Commun., 2007, 9, 2557–2563 CrossRef CAS
.
- H. Peng, T. Long, J. Peng, H. Chen, L. Ji, H. Sun, L. Huang and S. G. Sun, Molecular Design for In-Situ Polymerized Solid Polymer Electrolytes Enabling Stable Cycling of Lithium Metal Batteries, Adv. Energy Mater., 2024, 14, 2400428 CrossRef CAS
.
- S.-J. Tan, J. Yue, Y.-F. Tian, Q. Ma, J. Wan, Y. Xiao, J. Zhang, Y.-X. Yin, R. Wen and S. Xin, In-situ encapsulating flame-retardant phosphate into robust polymer matrix for safe and stable quasi-solid-state lithium metal batteries, Energy Storage Mater., 2021, 39, 186–193 CrossRef
.
- F. Wang, J. Zhong, Y. Guo, Q. Han, H. Liu, J. Du, J. Tian, S. Tang and Y. Cao, Fluorinated nonflammable in situ gel polymer electrolyte for high-voltage lithium metal batteries, ACS Appl. Mater. Interfaces, 2023, 15, 39265–39275 CrossRef CAS
.
- B. Yang, T. Li, Y. Pan, L. Yang, K. Li, J. Chen, Z. Yan, A. Hu and J. Long, Design strategy towards flame-retardant gel polymer electrolytes for safe lithium metal batteries, Energy Mater., 2024, 4, 400061 CAS
.
- Y. Meng, D. Zhou, R. Liu, Y. Tian, Y. Gao, Y. Wang, B. Sun, F. Kang, M. Armand, B. Li, G. Wang and D. Aurbach, Designing phosphazene-derivative electrolyte matrices to enable high-voltage lithium metal batteries for extreme working conditions, Nat. Energy, 2023, 8, 1023–1033 CrossRef CAS
.
- T.-L. Chen, M. Liu, X.-Y. Fan, Y.-H. Feng, Q. Liu, X.-R. Liu, H. Xin and P.-F. Wang, Nonflammable Sulfone-Based Electrolytes with Mechanically and Thermally Stable Interfaces Enabling LiNi0.5Mn1.5O4 to Operate at High Temperature, ACS Energy Lett., 2024, 9, 5452–5460 CrossRef CAS
.
- S. Das and A. J. Bhattacharyya, Influence of water and thermal history on ion transport in lithium salt-succinonitrile plastic crystalline electrolytes, Solid State Ionics, 2010, 181, 1732–1739 CrossRef CAS
.
- P. Jaumaux, Q. Liu, D. Zhou, X. Xu, T. Wang, Y. Wang, F. Kang, B. Li and G. Wang, Deep-eutectic-solvent-based self-healing polymer electrolyte for safe and long-life lithium-metal batteries, Angew. Chem., Int. Ed., 2020, 59, 9134–9142 CrossRef CAS
.
- Z. Li, S. Zhu, S. Gao, Y. He, H. Ding, D. Yang, H. Yang and P. F. Cao, Fireproof Solid Polymer Electrolyte with Chemically Bonded Phosphorus Toward Stable and Safe Lithium-Metal Battery, Adv. Funct. Mater., 2024, 34, 2409836 CrossRef CAS
.
- A. Hu, W. Chen, F. Li, M. He, D. Chen, Y. Li, J. Zhu, Y. Yan, J. Long and Y. Hu, Nonflammable polyfluorides-anchored quasi-solid electrolytes for ultra-safe anode-free lithium pouch cells without thermal runaway, Adv. Mater., 2023, 35, 2304762 CrossRef CAS
.
- L. Su, Y. Zhu, X. Zhan, K. Yu, T. Guo, K. Gu, H. Wu, L. Wang, Y. Wang and X. Wang, Pentafluorobenzene boronic acid with strong Lewis acidity for the modification of PEO-based polymer electrolytes, J. Mater. Chem. A, 2024, 12, 5768–5777 RSC
.
- J. Zhu, R. Zhao, J. Zhang, X. Song, J. Liu, N. Xu, H. Zhang, X. Wan, X. Ji and Y. Ma, Long-cycling and High-voltage Solid State Lithium Metal Batteries Enabled by Fluorinated and Crosslinked Polyether Electrolytes, Angew. Chem., Int. Ed., 2024, 63, e202400303 CrossRef CAS PubMed
.
- M. V. Reddy, C. M. Julien, A. Mauger and K. Zaghib, Sulfide and oxide inorganic solid electrolytes for all-solid-state Li batteries: a review, Nanomaterials, 2020, 10, 1606 CrossRef CAS
.
- S. Zhang, Z. Li, Y. Guo, L. Cai, P. Manikandan, K. Zhao, Y. Li and V. G. Pol, Room-temperature, high-voltage solid-state lithium battery with composite solid polymer electrolyte with in-situ thermal safety study, Chem. Eng. J., 2020, 400, 125996 CrossRef CAS
.
- G. Ge, Y. Wu, E. van der Heide, Z. Chen, J. Zhu and X. Zhuang, Carbon nanomaterials-constructed electrodes for rechargeable metal-ion batteries, J. Energy Storage, 2024, 90, 111900 CrossRef
.
- H. Yang, B. Zhang, M. Jing, X. Shen, L. Wang, H. Xu, X. Yan and X. He, In situ catalytic polymerization of a highly homogeneous PDOL composite electrolyte for long-cycle high-voltage solid-state lithium batteries, Adv. Energy Mater., 2022, 12, 2201762 CrossRef CAS
.
- C. Yan, P. Zhu, H. Jia, Z. Du, J. Zhu, R. Orenstein, H. Cheng, N. Wu, M. Dirican and X. Zhang, Garnet-rich composite solid electrolytes for dendrite-free, high-rate, solid-state lithium-metal batteries, Energy Storage Mater., 2020, 26, 448–456 CrossRef
.
- D. Cai, X. Qi, J. Xiang, X. Wu, Z. Li, X. Luo, X. Wang, X. Xia, C. Gu and J. Tu, A cleverly designed asymmetrical composite electrolyte via in-situ polymerization for high-performance, dendrite-free solid state lithium metal battery, Chem. Eng. J., 2022, 435, 135030 CrossRef CAS
.
- X. Zhou, Y. Zhou, L. Yu, L. Qi, K.-S. Oh, P. Hu, S.-Y. Lee and C. Chen, Gel polymer electrolytes for rechargeable batteries toward wide-temperature applications, Chem. Soc. Rev., 2024, 53, 5291–5337 RSC
.
- X. Chen, C. Qin, F. Chu, F. Li, J. Liu and F. Wu, Contriving a gel polymer electrolyte to drive quasi-solid-state high-voltage Li metal batteries at ultralow temperatures, Energy Environ. Sci., 2025, 18, 910–922 RSC
.
- J. Yu, X. Lin, J. Liu, J. T. Yu, M. J. Robson, G. Zhou, H. M. Law, H. Wang, B. Z. Tang and F. Ciucci, In situ fabricated quasi-solid polymer electrolyte for high-energy-density lithium metal battery capable of subzero operation, Adv. Energy Mater., 2022, 12, 2102932 CrossRef CAS
.
- Z. Li, R. Yu, S. Weng, Q. Zhang, X. Wang and X. Guo, Tailoring polymer electrolyte ionic conductivity for production of low-temperature operating quasi-all-solid-state lithium metal batteries, Nat. Commun., 2023, 14, 482 CrossRef PubMed
.
- W. Ma, X. Cui, Y. Chen, S. Wan, S. Zhao, J. Gong, G. Wang and S. Chen, Designing a Refined Multi-Structural Polymer Electrolyte Framework for Highly Stable Lithium-Metal Batteries, Angew. Chem., 2025, 137, e202415617 CrossRef
.
| This journal is © the Partner Organisations 2025 |