Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Benzobischalcogeno[3,2-c]quinolines: tuning electronic and structural properties with group 16 elements†
Christopher
Hüßler
 a,
Martin C.
Dietl‡
a,
Martin C.
Dietl‡
 a,
Matthias
Scherr‡
a,
Matthias
Scherr‡
 a,
Emma
Butigan
a,
Robin
Heckershoff
a,
Eric F.
Lopes
a,
Emma
Butigan
a,
Robin
Heckershoff
a,
Eric F.
Lopes
 a,
Justin
Kahle
a,
Justin
Kahle
 a,
Petra
Krämer
a,
Frank
Rominger
a,
Matthias
Rudolph
a and
A. Stephen K.
Hashmi
a,
Petra
Krämer
a,
Frank
Rominger
a,
Matthias
Rudolph
a and
A. Stephen K.
Hashmi
 *ab
*ab
aOrganisch-Chemisches Institut (OCI), Heidelberg University, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany. E-mail: hashmi@hashmi.de
bChemistry Department, Faculty of Science, King Abdulaziz University, Jeddah, 21589, Saudi Arabia
First published on 29th November 2024
Abstract
Chalcogen-substituted π-extended indolocarbazoles were synthesized through a nucleophilic cyclization of tethered diynes with sulfur, selenium or tellurium sources, followed by a Pictet–Spengler reaction. These combined synthetic strategies enabled the facile access to heptacyclic π-extended molecules, offering a broad modularity at various stages of the synthesis route. In total, twenty-six novel heptacyclic compounds were synthesized. Their photophysical and structural properties were investigated experimentally as well as theoretically.
Introduction
Over the past 70 years, various synthetic procedures for indolocarbazoles (ICZs) have been developed.1–6 In 2020, our group presented a gold-catalyzed7–10 approach towards π-extended benzo[a]benzo[6,7]indolo[2,3-h]carbazoles (BBICZs).11 These BBICZs show hole transport mobilities of up to 1 cm2 V−1 s−1 in organic thin-film transistors (TFT).12 Such π-conjugated N-heteropolycyclic molecules are promising compounds for applications in organic light-emitting diodes (OLEDs),13–16 organic field-effect transistors (OFETs),11,12,17–19 or organic photovoltaics (OPV).20,21 By isosteric replacements of CH-units by nitrogen atoms, the electronic properties of these molecules can be modified, for example in azaacenes,22,23 tetraazaperopyrenes,24 or dipyrrolopyrazines.25,26 We applied this technique to our previously synthesized BBICZs for the synthesis of benzobispyrrolo[3,2-c]quinolines (BBPQs) via a bidirectional Pictet–Spengler reaction.27Now we envisioned the replacement of the indole NH groups of BBPQs with chalcogen atoms to obtain benzobischalcogeno[3,2-c]quinolines (BBCQs) (Scheme 1), which we report here. Thiophene-fused π-conjugated molecules show greater stability against degradation, making them appealing for the fabrication of electronic and optical devices.28 Moreover, the incorporation of heavy chalcogen atoms is associated with an enhanced orbital overlap, therefore increasing charge mobility, particularly in organic photovoltaics.29–32 In addition, the synthesis of the central benzodichalcogenophen-core is achieved through a short sequence.
 | ||
| Scheme 1 Previous Pictet–Spengler-approach towards benzobispyrrolo[3,2-c]quinolines27 and our procedure to obtain the chalcogenated congeners. Overall yields are presented. | ||
Prior to its application in the syntheses of BBPQs, the use of the Pictet–Spengler reaction in materials science was scarce with only a few examples documented.33–35 Its original application more than 100 years ago primarily involved the synthesis of alkaloid skeletons, such as tetrahydroisoquinoline derivatives, which depict a pharmacological importance, for instance in the preparation of antihypertensive drugs, hence highlighting it as one of the fundamental reactions in natural product and pharmaceutical synthesis.36–39
Results and discussion
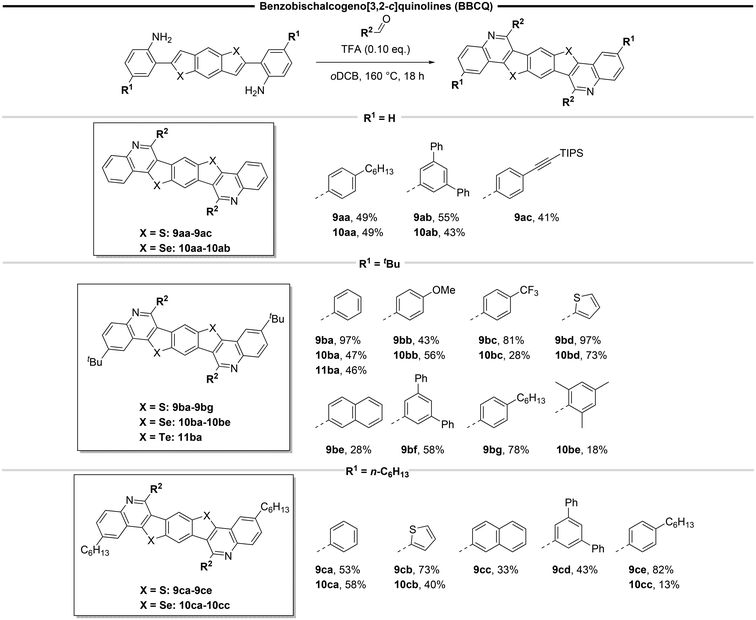
Syntheses of BBCQs
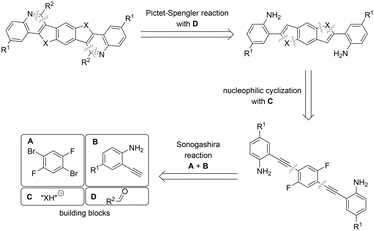
To synthesize the target heptacyclic BBCQs, we envisioned a convergent modular strategy which has proven successful in our previous work, allowing a quick and variable synthesis by combining building blocks (Scheme 2).11 Firstly, building blocks A and B were combined in a Sonogashira coupling, introducing variability by the utilization of different alkyne components. Subsequently, the obtained diyne moieties underwent cyclization using nucleophilic chalcogen sources C. Finally, a Pictet–Spengler reaction with various aldehydes D was conducted to access BBCQs. This approach results in three reactions where a modular procedure is possible, facilitating the incorporation of various substituents, enabling the synthesis of a wide range of potentially semiconducting molecules.The central precursor for the target compounds, 1,4-dibromo-2,5-difluorobenzene 4 (building block A in Scheme 2) serves as a cheap and commercially available starting material.
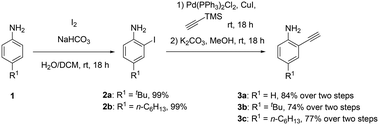
The synthesis of building block B consists of an ortho-iodination of para-alkylated (tert-butyl and n-hexyl) anilines 1 through a modified literature procedure according to Flynn et al., resulting in 2a and 2b in quantitative yield.40 The choice of alkyl substituents was made to avoid poor solubilities of the addressed large planar systems. Subsequently, these compounds underwent a Sonogashira coupling with the non-alkylated, commercially available, 2-iodoaniline, followed by a deprotection. The resulting 4-alkylated or unsubstituted ortho-ethynyl-anilines 3a–c were obtained in high yields ranging from 74 to 84% over two steps (Scheme 3).
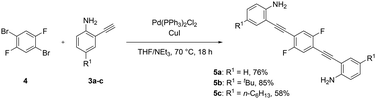
By reacting building block A and Bvia a Sonogashira reaction, diynes 5a–c were synthesized in good yields of 58 to 85% (Scheme 4). These compounds might serve as promising blue emitters based on their optical properties, with quantum yields of up to 74% and emission wavelengths ranging from 450 to 469 nm, suggesting potential applications in organic devices. For 5a and 5b, crystals suitable for single crystal X-ray structure analysis were obtained (see the ESI†).
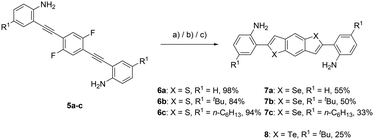
In the next step, the diynes were subjected to bidirectional nucleophilic substitution and subsequent hydrochalcogenation with building block C (Scheme 5). The use of N-methyl-2-pyrrolidone (NMP) as a high boiling solvent was essential for the successful two-fold reaction. This choice of an aprotic polar solvent is in agreement with known procedures for synthesising benzodichalcogenophenes.41,42 Another notable aspect within this step is the direct precipitation of the products, simplifying the work up, only decantation or filtration followed by washing are necessary. The synthesis of the thiophene ring of the benzodithiophenes 6a–c proceeded smoothly under open flask conditions when using sodium sulfide, yielding the desired products in excellent yields of up to 98%. For generation of the selenophene subunit, the reactive hydrogen selenide was prepared in situ by using LiBEt3H and elemental selenium. After the subsequent addition of the reactant and overnight heating, the benzodiselenophenes 7a–c were formed in moderate yields of up to 55%. The benzoditellurophene 8 was prepared following a similar procedure as for selenium in a moderate yield of 25%. Additionally, single crystals suitable for single crystal X-ray structure analysis were obtained for 8 (see the ESI†). Only the tert-butyl-substituted derivative of this benzoditellurophene was synthesized, demonstrating the ability to prepare the telluracycles.
To obtain the target molecules, compounds 6–8 were subjected to a Pictet–Spengler reaction, employing various aldehydes (building block D) to yield BBCQs 9–11 (Scheme 6). Initially, the known procedure for synthesizing BBPQs was used.27 However, these reaction conditions were optimized for the activated nucleophilic C3-site43 of the indole moieties. When applied to our system, no reaction occurred. To our delight, this issue was overcome by applying elevated temperatures of 160 °C in ortho-dichlorobenzene (oDCB) as solvent. Additionally, trifluoroacetic acid (TFA) was employed instead of para-toluenesulfonic acid as a more acidic catalyst to ensure a complete conversion.
It is noteworthy that in the case of the non-alkylated bischalcogenophenes 6a and 7a, only substituents exhibiting high solubility (9aa, 9ac and 10aa) or interference with the π-stacking (9ab, 10ab) could be introduced. When aldehydes lacking these properties were used, due to low solubility the product could not be fully characterized. Products 9aa–9ac and 10aa–10ab were obtained in satisfactory yields of 41 to 55%. Examining the tert-butyl substituted examples 9b, 10b and 11b, a wide range of target molecules was obtained. Multiple substituents with various properties, including electron-neutral (9ba, 9bf, 9bg, 10ba, 10be, 11ba), electron-donating (9bb, 10bb), electron-withdrawing (9bc, 10bc), heterocyclic (9bd, 10bd) or polycyclic (9be) examples were introduced successfully thanks to the tert-butyl group inducing solubility and therefore simplifying the workup process. However, the scope is limited to the use of aryl aldehydes, since aliphatic aldehydes undergo prior aldol condensation under our conditions, resulting in multiple side products (depending on the grade of oligomerization of the aldehyde) that cannot be separated due to similar polarities and solubilities. This effect was also observed in the synthesis of smaller systems under milder conditions.44 Additionally, no product formation is observed when using 2,2-disubstituted alkyl aldehydes.
Usually, for thiophenes the best yields are obtained, followed by selenophenes and tellurophenes. The yield of benzobisthieno[3,2-c]quinolines 9b is significantly higher compared to the selenium-substituted counterparts 10b in most instances. Moreover, aldehydes bearing sterically demanding groups R2 could not be implemented. In the case of benzobistelluro[3,2-c]quinoline 11ba, a successful conversion was accomplished with a yield of 46%. While in theory, additional examples could be obtained, only 11ba was synthesized for the sake of illustrating the reaction principle.
Finally, n-hexyl substituted BBCQs 9c and 10c were synthesized in low to good yields of 13–82%. It is noteworthy that, apart from the presented substituents R2, other aldehydes can be used for the synthesis of 9b, 10b and 11b.
Structural properties
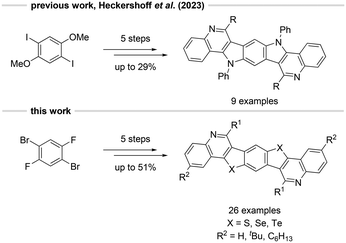
For numerous BBCQs, specifically 9aa, 9ba, 9bc, 10ba, 10be, 10cc and 11ba, single crystal X-ray structure analyses were conducted. In the following paragraph, the solid-state molecular structures of 9ba, 10ba and 11ba will be discussed (Fig. 1; for all obtained molecular structures, see the ESI†).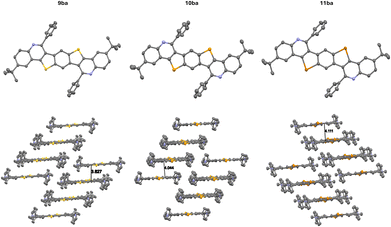 | ||
| Fig. 1 Solid state molecular structures (top) and packing motifs (bottom) of 9ba (CCDC 2380041), 10ba (CCDC 2380043) and 11ba (CCDC 2380046).† The thermal ellipsoids are set at a 50% probability level and hydrogen atoms are omitted for clarity. | ||
All three compounds bear a tert-butyl substituent at the R1 position, while the chalcogen units vary between sulfur, selenium and tellurium. The triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group is present in all examples, with one molecule per unit cell, and are located on crystallographic inversion centres. The central conjugated π-system remains fully planar in each instance. In the subsequent section, selected bond lengths, bond angles and packing motifs will be discussed by direct comparison of 9ba, 10ba and 11ba, as the chalcogen atom represents the only difference among them (Table 1).
space group is present in all examples, with one molecule per unit cell, and are located on crystallographic inversion centres. The central conjugated π-system remains fully planar in each instance. In the subsequent section, selected bond lengths, bond angles and packing motifs will be discussed by direct comparison of 9ba, 10ba and 11ba, as the chalcogen atom represents the only difference among them (Table 1).
Significant disparities are observed in the lengths of the C2–X3 bond and the C4–X3 bond, elongating by approximately 0.13 Å when comparing 10ba with 9ba. In the case of tellurium, this elongation increases to about 0.20–0.22 Å in comparison to the selenium-substituted congener. This observation probably results from the increasing van der Waals-radii in group 16. Consequently, the bond angles C2–X3–C4 decrease, and C2–C10–C9 increase with higher atomic number. All other bond lengths and angles do not differ significantly from each other. Despite being substituted with a bulky tert-butyl group which would typically hinder an ordered orientation, all examples are stacked in a planar fashion in the packing motifs. Furthermore, strong π–π interactions are observed in all crystal structures with intermolecular distances between molecule planes of 3.827 Å for 9ba, 4.044 Å for 10ba and 4.111 Å for 11ba. This trend is also likely attributed to the increasing van der Waals radii of the chalcogens.
Another interesting observation is the dependence of the incorporated substituent R2 on the packing motif (Fig. 2).
 | ||
| Fig. 2 Comparison of the solid state molecular structures (top) and packing motifs (bottom) of 9ba (CCDC 2380041) and 9bc (CCDC 2380042).† The thermal ellipsoids are set at a 50% probability level and hydrogen atoms are omitted for clarity. | ||
When 4-(trifluoromethyl)benzaldehyde is used, the packing motif of the BBCQ changes drastically, from a layer structure to a herringbone-type motif, with a herringbone angle of 79°. Therefore, the molecular packing motif can be influenced by the choice of building block D (Scheme 2), emphasizing the importance of modularity of the synthetic approach towards BBCQs.
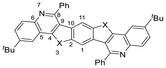
Optoelectronic properties
Precursors 5–8 and BBCQs 9–11 were studied by UV-Vis and fluorescence spectroscopy in DCM (Fig. 3 and Table 2). Compounds 5a–c exhibit comparable absorption and emission properties, with absorption maxima ranging from 376 to 382 nm and emission maxima of 450 to 469 nm, respectively. These compounds show high quantum yields of 61–74%. For 6a–c, 7a–c and 8, a trend in quantum yields is observed. Quantum yields for sulfur-containing compounds range from 16 to 17% but decrease significantly for selenium-containing counterparts to 2–3% and drop below 1% for the respective ditellurophene. Benzodichalcogenophen precursors 6 and 7 exhibit similar values for the absorption maxima of 352 to 357 nm, while tellurophene 8, shows an absorption maximum at 404 nm. Emission maxima range from 376 to 450 nm, with optical bandgaps calculated based on the absorption onset ranging from 2.87 to 3.10 eV.| Compound | λ max,abs [nm] | λ max,em [nm] | Stokes shift [cm−1] | λ onset,abs [nm] | E g(opt) [eV] | QYd [%] | HOMOe [eV] | LUMOe [eV] | E g(calc) [eV] |
|---|---|---|---|---|---|---|---|---|---|
| a Maximum of the longest absorption wavelength. b Maximum of the shortest emission wavelength. c Optical gap estimated from λonset,abs: Eg(opt) = 1239.8/λonset,abs. d Fluorescence quantum yield. e Data derived from DFT-calculations. f HOMO–LUMO gap. g Data from an earlier publication.27 | |||||||||
| 5a | 376 | 450 | 4374 | 413 | 3.00 | 66 | — | — | — |
| 5b | 382 | 468 | 4810 | 423 | 2.93 | 74 | — | — | — |
| 5c | 382 | 469 | 4856 | 427 | 2.90 | 61 | — | — | — |
| 6a | 352 | 429 | 5099 | 400 | 3.10 | 16 | — | — | — |
| 6b | 355 | 441 | 5493 | 403 | 3.07 | 16 | — | — | — |
| 6c | 356 | 438 | 5259 | 407 | 3.05 | 17 | — | — | — |
| 7a | 353 | 376 | 1733 | 406 | 3.05 | 2 | — | — | — |
| 7b | 356 | 439 | 5311 | 416 | 2.98 | 2 | — | — | — |
| 7c | 357 | 446 | 5590 | 417 | 2.98 | 3 | — | — | — |
| 8 | 404 | 450 | 2530 | 432 | 2.87 | <1 | — | — | — |
| 9aa | 353 | 383 | 2219 | 367 | 3.38 | 4 | −5.88 | −2.00 | 3.88 |
| 9ab | 354 | 383 | 2139 | 371 | 3.34 | 3 | −5.99 | −2.11 | 3.88 |
| 9ac | 354 | 407 | 3679 | 371 | 3.34 | 2 | −6.02 | −2.17 | 3.85 |
| 9ba | 371 | 380 | 638 | 382 | 3.24 | 5 | −5.85 | −1.97 | 3.88 |
| 9bb | 372 | 390 | 1241 | 395 | 3.14 | 4 | −5.91 | −2.04 | 3.86 |
| 9bc | 370 | 386 | 1120 | 403 | 3.07 | 4 | −6.00 | −2.14 | 3.86 |
| 9bd | 356 | 466 | 6631 | 380 | 3.26 | 4 | −5.91 | −2.03 | 3.87 |
| 9be | 373 | 405 | 2118 | 391 | 3.17 | 1 | −5.84 | −1.98 | 3.87 |
| 9bf | 377 | 390 | 884 | 395 | 3.14 | 3 | −5.77 | −2.14 | 3.63 |
| 9bg | 375 | 385 | 693 | 401 | 3.09 | 4 | −5.93 | −2.05 | 3.88 |
| 9ca | 371 | 382 | 776 | 391 | 3.17 | 4 | −5.92 | −2.04 | 3.88 |
| 9cb | 355 | 468 | 6801 | 382 | 3.24 | 8 | −5.96 | −2.07 | 3.89 |
| 9cc | 375 | 390 | 1026 | 392 | 3.16 | 1 | −5.92 | −2.09 | 3.83 |
| 9cd | 373 | 383 | 700 | 387 | 3.20 | 2 | −5.94 | −2.11 | 3.83 |
| 9ce | 380 | 391 | 740 | 395 | 3.14 | 3 | −5.92 | −2.07 | 3.85 |
| 10aa | 379 | 450 | 4163 | 400 | 3.10 | <1 | −5.94 | −2.13 | 3.81 |
| 10ab | 380 | 450 | 4094 | 405 | 3.06 | <1 | −5.92 | −2.15 | 3.77 |
| 10ba | 380 | 391 | 740 | 397 | 3.12 | 1 | −5.87 | −2.11 | 3.77 |
| 10bb | 379 | 393 | 940 | 395 | 3.14 | <1 | −5.84 | −2.08 | 3.77 |
| 10bc | 377 | 393 | 1080 | 402 | 3.08 | <1 | −5.93 | −2.17 | 3.76 |
| 10bd | 364 | 435 | 4484 | 386 | 3.21 | <1 | −5.90 | −2.14 | 3.76 |
| 10be | 378 | 389 | 748 | 394 | 3.14 | 1 | −5.86 | −2.07 | 3.79 |
| 10ca | 381 | 433 | 3152 | 403 | 3.07 | 1 | −5.87 | −2.11 | 3.75 |
| 10cb | 364 | 408 | 2963 | 391 | 3.17 | <1 | −5.89 | −2.16 | 3.73 |
| 10cc | 381 | 392 | 737 | 395 | 3.14 | 1 | −5.85 | −2.10 | 3.75 |
| 11ba | 409 | 468 | 3082 | 430 | 2.88 | <1 | −5.66 | −2.14 | 3.52 |
| BBPQ-Ph | 364 | 395 | 2156 | 388 | 3.20 | 10 | −5.62 | −1.74 | 3.88 |
Examining BBCQs 9, 10 and 11, multiple trends emerge. Firstly, minor influence of different substituents R2 is observed. Negligible differences in absorption maxima are noted when comparing unsubstituted sulfur-containing examples 9a, as well as tert-butyl- or n-hexyl-substituted examples 9b and 9c. However, for 9bd and 9cb (R2 = 2-thiophene), a remarkable blue shift of the absorption maximum is observed. Comparison of thiophen- and selenophen-annulated BBCQs indicates, that the absorption maxima are redshifted with heavier chalcogens. This trend is also reflected in the optical bandgaps, with slightly smaller values of 3.06–3.21 eV for examples 10, compared to 3.07–3.38 eV for examples 9. The direct influence of the chalcogen used on the optical bandgap is apparent when comparing the absorption spectra of 9ba, 10ba and 11ba, all having the same substituents R1 and R2.
The general substitution of alkyl substituents R1 has a remarkable impact on the photophysical properties of BBCQs. Comparing 9aa, 9bg and 9ce reveals a redshift of 22–27 nm for the absorption maxima. However, discrepancies between tert-butyl and n-hexyl substituents are marginal. These alkyl substituents R1 have a higher impact on the fluorescence spectra than the implemented aldehydes R2 when comparing 9a and 10a (R1 = H) to their alkyl substituted counterparts. In this case, emission maxima range from 383 to 450 nm, resulting in Stokes shifts of 2139–4163 cm−1, except for n-hexyl substituted 10ca and 10cb, which exhibit emission maxima of 408–433 nm, resulting in Stokes shifts of 2963–3152 cm−1. Moreover, compounds 9bd, 9cb, 10bd and 10cb, all with R2 as 2-thiophen, show high emission maxima (408–468 nm) and large Stokes shifts (2963–6801 cm−1). Fluorescence quantum yields (QY) are in the low range of 1–8% for sulfur-substituted compounds 9. For selenium- and tellurium-substituted counterparts 10 and 11, the QY barely reaches the 1%-mark. This observation is most likely contributed to the heavy-atom-effect,45 which is induced by the presence of selenium and tellurium atoms.
Quantum chemical calculations
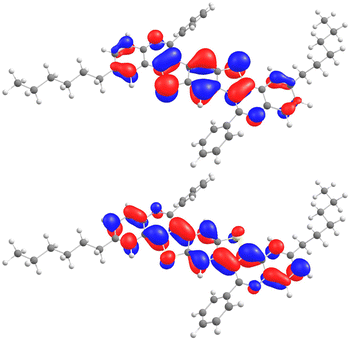
For the frontier orbital energies of 9–11, DFT calculations were performed using Orca 5.0.3.46 The B3LYP/G functional and the def2-TZVP basis set were employed for the calculations (Table 2, see the ESI for more details†).47–50 For the sulfur-substituted compounds 9, the HOMO-energies are located between −6.02 and −5.77 eV, while the LUMO-energies range from −2.17 to −1.97 eV, resulting in theoretical band gaps of 3.63–3.89 eV. For the selenium-substituted compounds 10, the HOMO-energies are located between −5.94 and −5.84 eV, and the LUMO-energies range from −2.17 to −2.07 eV, leading to theoretical band gaps of 3.73–3.85 eV. 11ba exhibits the smallest theoretical band gap of 3.52 eV with a HOMO-energy of −5.66 eV and a LUMO-energy of −2.14 eV. It is observable that the variation of the band gap among each group of BBCQs is minimal. This can be attributed to the fact that, for all examples 9–11, the HOMO and LUMO are located within the π-conjugated heptacycle (Fig. 4, see the ESI for more details†).Additionally, it becomes apparent that the substituents R1 and R2 only exhibit a marginal electron density in the HOMO and a node in the LUMO, leading to the conclusion that they are not part of the conjugated π-system, which explains the small differences in both the theoretical and optical band gaps. This was also observed for the BBPQs we reported previously.27 When compared to the optical band gaps, the theoretical gaps show a consistent discrepancy of 0.49 to 0.79 eV due to the inherent tendency of the calculations to overestimate the LUMO energies.
Comparison to structurally related analogues
To examine the impact of isosteric replacements of heteroatoms within the 5-membered ring of the heptacycles, 9aa, 10aa and 11ba were compared to structurally related BBPQ-Ph, previously discussed in an earlier publication (Fig. 5).27 It is noteworthy that the exact pattern of BBPQ-Ph (R1 = H, R2 = Ph) could not be replicated with the benzodichalcogenophenes used in this work. To address this, the closest related examples for each chalcogen were employed for comparison, since quantum chemical calculations indicated that the choice of R1 and R2 does not affect the π-conjugated heptacyclic system (see Fig. 4 and the ESI for more details†). Substitution of the NH-group by sulfur, selenium or tellurium resulted in modified photophysical properties. Compound 9aa showed blue-shifted absorption and emission maxima by 11–12 nm, leading to a similar Stokes shift of around 2200 cm−1, compared with BBPQ-Ph. Its optical gap of 3.38 eV was larger than that for BBPQ-Ph by 0.18 eV. A reverse trend was observed for selenium and tellurium substitutions. For 10aa, the absorption and emission maxima were red-shifted by 15 nm and 55 nm, respectively, consequently reducing the optical gap by 0.1 eV, compared to BBPQ-Ph. This observation was even more significant for 11ba, with absorption and emission maxima at 409 nm and 468 nm, respectively, resulting in a redshift of 45 nm and 73 nm and an optical gap of 2.88 eV, being 0.32 eV lower if compared to the NH-substituted counterpart. BBPQ-Ph exhibited the highest quantum yield among its counterparts, with 10%, compared to 1–4%. Despite structural discrepancies, the combination of enabling an ordered layered crystal packing motif through appropriate choice of R1 and R2, along with choosing a chalcogen atom X as a substitute for NH, improves the potential for possible fabrication in organic devices, as discussed previously.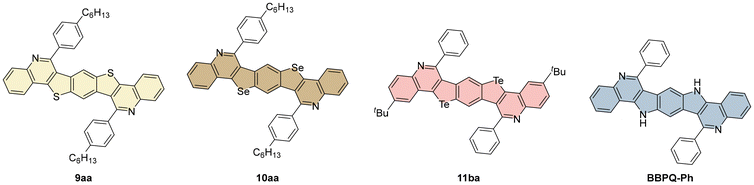 | ||
| Fig. 5 BBCQs 9aa, 10aa, 11ba and structurally related BBPQ-Ph,27 which are used to compare the difference in the photophysical properties caused by isosteric replacement of pyrrolo-NH moieties by chalcogen atoms. | ||
Stability-measurements
Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) were performed on 9bd, 10bc and 11ba, each representing one distinct chalcogen within the BBCQs, under a nitrogen atmosphere at a heating rate of 10 °C min−1. For the sulfur- and selenium-containing examples, no significant mass loss was observed at temperatures up to 400 °C (for TGA & DSC-Plots see the ESI†). However, for the tellurium-doped sample, a slight mass loss of 5% was noted starting from 366 °C. Overall, all three compounds exhibit a high thermal stability, enabling for potential vapor deposition onto device substrates.Conclusions
We present an efficient method for synthesizing chalcogen-substituted indolocarbazole-derivatives with a broad substrate scope of twenty-six examples. The isosteric replacement of the NH group with a chalcogen atom significantly influences the optical and theoretical band gaps. Moreover, we showed that while the introduction of peripheral substituents does not affect the band gap significantly, it does impact the structural properties. We extended the scope of previously reported benzobispyrrolo[3,2-c]quinolines (BBPQs) with the new class of benzobischalcogeno[3,2-c]quinolines BBCQs, allowing for more precise tailoring of electronic and structural properties in such heptacyclic conjugated π-systems. While oxygen nucleophiles can also be utilized to obtain benzofuran-moieties,42 the synthesis of furan-substituted derivatives of BBCQs remains challenging. The reason lies in the presence of the ortho-aminoalkynyl units in our precursor molecules, favouring the formation of an indole when adding a strong oxygen-containing base, such as KOH. A high thermal stability was observed for some examples of the synthesized BBCQs, making these compounds promising candidates for incorporation into organic devices.Data availability
The authors declare that all the data used for this manuscript can be found in its ESI. The single crystal structures used in this manuscript have been assigned the CCDC 2380037–2380046.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
C. H., J. K. and R. H. are grateful for the funding by the Deutsche Forschungsgemeinschaft (DFG; SFB 1249 – N-Heteropolyzyklen als Funktionsmaterialien). M. S. and A. S. K. H. are grateful for the funding by the Hector Fellow Academy. The authors acknowledge the support from the state of Baden-Württemberg through bwHPC and the German Research Foundation (DFG) through grant no INST 40/575-1 FUGG (JUSTUS 2 cluster). We thank Fabian Jester and Prof. Uwe Bunz (Heidelberg University) for TGA/DSC measurements.References
- M. L. Tomlinson, 177. Experiments on the preparation of indolocarbazoles. Part IV. The preparation of indolo(3′ : 2′-1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)carbazole, J. Chem. Soc., 1951, 809–811 RSC.
2)carbazole, J. Chem. Soc., 1951, 809–811 RSC. - H. M. Grotta, C. J. Riggle and A. E. Bearse, Preparation of Some Condensed Ring Carbazole Derivatives, J. Org. Chem., 1961, 26, 1509–1511 CrossRef CAS.
- W. Lamm, W. Jugelt and F. Pragst, Photochemische Synthese von Carbazolen und Indolo[3, 2−b]carbazolen, J. Prakt. Chem., 1975, 317, 284–292 CrossRef CAS.
- J. Tholander and J. Bergman, Syntheses of 6,12-disubstituted 5,11-dihydroindolo[3,2-b]carbazoles, including 5,11-dihydroindolo[3,2-b]carbazole-6,12-dicarbaldehyde, an extremely efficient ligand for the TCDD (Ah) receptor, Tetrahedron, 1999, 55, 12577–12594 CrossRef CAS.
- K. Kawaguchi, K. Nakano and K. Nozaki, Synthesis of ladder-type pi-conjugated heteroacenes via palladium-catalyzed double N-arylation and intramolecular O-arylation, J. Org. Chem., 2007, 72, 5119–5128 CrossRef CAS.
- M. Vlasselaer and W. Dehaen, Synthesis of Linearly Fused Benzodipyrrole Based Organic Materials, Molecules, 2016, 21, 785 CrossRef.
- A. S. K. Hashmi, Gold-catalyzed organic reactions, Chem. Rev., 2007, 107, 3180–3211 CrossRef CAS PubMed.
- R. J. Harris and R. A. Widenhoefer, Gold carbenes, gold-stabilized carbocations, and cationic intermediates relevant to gold-catalysed enyne cycloaddition, Chem. Soc. Rev., 2016, 45, 4533–4551 RSC.
- A. S. K. Hashmi, Introduction: Gold Chemistry, Chem. Rev., 2021, 121, 8309–8310 CrossRef CAS PubMed.
- C. M. Hendrich, K. Sekine, T. Koshikawa, K. Tanaka and A. S. K. Hashmi, Homogeneous and Heterogeneous Gold Catalysis for Materials Science, Chem. Rev., 2021, 121, 9113–9163 CrossRef CAS.
- C. M. Hendrich, L. M. Bongartz, M. T. Hoffmann, U. Zschieschang, J. W. Borchert, D. Sauter, P. Krämer, F. Rominger, F. F. Mulks, M. Rudolph, A. Dreuw, H. Klauk and A. S. K. Hashmi, Gold Catalysis Meets Materials Science – A New Approach to π–Extended Indolocarbazoles, Adv. Synth. Catal., 2021, 363, 549–557 CrossRef.
- C. Wang, H. Dong, W. Hu, Y. Liu and D. Zhu, Semiconducting π-conjugated systems in field-effect transistors: a material odyssey of organic electronics, Chem. Rev., 2012, 112, 2208–2267 CrossRef CAS PubMed.
- H. Shi, J. Dai, X. Wu, L. Shi, J. Yuan, L. Fang, Y. Miao, X. Du, H. Wang and C. Dong, A novel dimesitylboron-substituted indolo[3,2-b]carbazole derivative: Synthesis, electrochemical, photoluminescent and electroluminescent properties, Org. Electron., 2013, 14, 868–874 CrossRef CAS.
- H.-C. Ting, Y.-M. Chen, H.-W. You, W.-Y. Hung, S.-H. Lin, A. Chaskar, S.-H. Chou, Y. Chi, R.-H. Liu and K.-T. Wong, Indolo[3,2-b]carbazole/benzimidazole hybrid bipolar host materials for highly efficient red, yellow, and green phosphorescent organic light emitting diodes, J. Mater. Chem., 2012, 22, 8399–8407 RSC.
- D. Chen, S.-J. Su and Y. Cao, Nitrogen heterocycle-containing materials for highly efficient phosphorescent OLEDs with low operating voltage, J. Mater. Chem. C, 2014, 2, 9565–9578 RSC.
- K. Brunner, A. van Dijken, H. Börner, J. J. A. M. Bastiaansen, N. M. M. Kiggen and B. M. W. Langeveld, Carbazole compounds as host materials for triplet emitters in organic light-emitting diodes: tuning the HOMO level without influencing the triplet energy in small molecules, J. Am. Chem. Soc., 2004, 126, 6035–6042 CrossRef CAS.
- Z. Liang, Q. Tang, J. Xu and Q. Miao, Soluble and stable N-heteropentacenes with high field-effect mobility, Adv. Mater., 2011, 23, 1535–1539 CrossRef CAS.
- Y. Li, Y. Wu, S. Gardner and B. S. Ong, Novel Peripherally Substituted Indolo[3,2− b ]carbazoles for High–Mobility Organic Thin–Film Transistors, Adv. Mater., 2005, 17, 849–853 CrossRef CAS.
- A. Facchetti, Semiconductors for organic transistors, Mater. Today, 2007, 10, 28–37 CrossRef CAS.
- A. Mishra and P. Bäuerle, Small molecule organic semiconductors on the move: promises for future solar energy technology, Angew. Chem., Int. Ed., 2012, 51, 2020–2067 CrossRef CAS PubMed.
- P. R. Nitha, S. Soman and J. John, Indole fused heterocycles as sensitizers in dye-sensitized solar cells: an overview, Mater. Adv., 2021, 2, 6136–6168 RSC.
- Q. Miao, T.-Q. Nguyen, T. Someya, G. B. Blanchet and C. Nuckolls, Synthesis, assembly, and thin film transistors of dihydrodiazapentacene: an isostructural motif for pentacene, J. Am. Chem. Soc., 2003, 125, 10284–10287 CrossRef CAS PubMed.
- U. H. F. Bunz, N-heteroacenes, Chem. – Eur. J., 2009, 15, 6780–6789 CrossRef CAS PubMed.
- T. Riehm, G. de Paoli, A. E. Konradsson, L. de Cola, H. Wadepohl and L. H. Gade, Tetraazaperopyrenes: a new class of multifunctional chromophores, Chem. – Eur. J., 2007, 13, 7317–7329 CrossRef CAS.
- R. Heckershoff, T. Schnitzer, T. Diederich, L. Eberle, P. Krämer, F. Rominger, M. Rudolph and A. S. K. Hashmi, Efficient Synthesis of Dipyrrolobenzenes and Dipyrrolopyrazines via Bidirectional Gold Catalysis: a Combined Synthetic and Photophysical Study, J. Am. Chem. Soc., 2022, 144, 8306–8316 CrossRef CAS.
- J. Kahle, A. V. Mackenroth, C. Hüßler, P. D. Römgens, P. Schimanski, P. Krämer, M. Brückner, T. Oeser, F. Rominger, M. Rudolph and A. S. K. Hashmi, Modular synthetic strategies for dipyrrolopyrazines, Org. Chem. Front., 2024, 11, 2996–3003 RSC.
- R. Heckershoff, L. Eberle, N. Richert, C. Delavier, M. Bruckschlegel, M. R. Schäfer, P. Krämer, F. Rominger, M. Rudolph and A. S. K. Hashmi, Versatile access to nitrogen-rich π-extended indolocarbazoles via a Pictet–Spengler approach, Org. Chem. Front., 2022, 10, 12–21 RSC.
- W. Wu, Y. Liu and D. Zhu, Pi-conjugated molecules with fused rings for organic field-effect transistors: design, synthesis and applications, Chem. Soc. Rev., 2010, 39, 1489–1502 RSC.
- S. Duhović and M. Dincă, Synthesis and Electrical Properties of Covalent Organic Frameworks with Heavy Chalcogens, Chem. Mater., 2015, 27, 5487–5490 CrossRef.
- A. A. Jahnke and D. S. Seferos, Polytellurophenes, Macromol. Rapid Commun., 2011, 32, 943–951 CrossRef CAS PubMed.
- A. A. Jahnke, G. W. Howe and D. S. Seferos, Polytellurophenes with properties controlled by tellurium-coordination, Angew. Chem., Int. Ed., 2010, 49, 10140–10144 CrossRef CAS PubMed.
- P. Gao, D. Beckmann, H. N. Tsao, X. Feng, V. Enkelmann, W. Pisula and K. Müllen, Benzo1,2-b:4,5-b'bisbbenzothiophene as solution processible organic semiconductor for field-effect transistors, Chem. Commun., 2008, 1548–1550 RSC.
- S. Guo, J. Wang, X. Fan, X. Zhang and D. Guo, Synthesis of pyrazolo1,5-cquinazoline derivatives through copper-catalyzed tandem reaction of 5-(2-bromoaryl)-1H-pyrazoles with carbonyl compounds and aqueous ammonia, J. Org. Chem., 2013, 78, 3262–3270 CrossRef CAS.
- S. Guo, L. Tao, W. Zhang, X. Zhang and X. Fan, Regioselective Synthesis of Indolo1,2-cquinazolines and 11H-Indolo3,2-cquinolines via Copper-Catalyzed Cascade Reactions of 2-(2-Bromoaryl)-1H-indoles with Aldehydes and Aqueous Ammonia, J. Org. Chem., 2015, 80, 10955–10964 CrossRef CAS.
- G. Abbiati, A. Arcadi, M. Chiarini, F. Marinelli, E. Pietropaolo and E. Rossi, An alternative one-pot gold-catalyzed approach to the assembly of 11H-indolo3,2-cquinolines, Org. Biomol. Chem., 2012, 10, 7801–7808 RSC.
- A. Pictet and T. Spengler, Über die Bildung von Isochinolin–derivaten durch Einwirkung von Methylal auf Phenyl–äthylamin, Phenyl–alanin und Tyrosin, Ber. Dtsch. Chem. Ges., 1911, 44, 2030–2036 CrossRef CAS.
- E. D. Cox and J. M. Cook, The Pictet-Spengler condensation: a new direction for an old reaction, Chem. Rev., 1995, 95, 1797–1842 CrossRef CAS.
- S. Watanuki, K. Matsuura, Y. Tomura, M. Okada, T. Okazaki, M. Ohta and S.-I. Tsukamoto, Synthesis and pharmacological evaluation of 1-isopropyl-1,2,3,4-tetrahydroisoquinoline derivatives as novel antihypertensive agents, Chem. Pharm. Bull., 2011, 59, 1029–1037 CrossRef CAS.
- G. Tatsui, Über die Synthese von Carbolinderivaten, J. Pharm. Soc. Jpn., 1928, 48, 453–459 CrossRef CAS.
- A. S. Dillon and B. L. Flynn, Polyynes to Polycycles: Domino Reactions Forming Polyfused Chalcogenophenes, Org. Lett., 2020, 22, 2987–2990 CrossRef CAS PubMed.
- M. Nakano and K. Takimiya, Sodium Sulfide-Promoted Thiophene-Annulations: Powerful Tools for Elaborating Organic Semiconducting Materials, Chem. Mater., 2017, 29, 256–264 CrossRef CAS.
- R. Hudson, N. P. Bizier, K. N. Esdale and J. L. Katz, Synthesis of indoles, benzofurans, and related heterocycles via an acetylene-activated SNAr/intramolecular cyclization cascade sequence in water or DMSO, Org. Biomol. Chem., 2015, 13, 2273–2284 RSC.
- R. J. Sundberg, in Heterocyclic Scaffolds II. ed. G. W. Gribble, Springer, Berlin, Heidelberg, 2010, pp. 47–115 Search PubMed.
- J. Seayad, A. M. Seayad and B. List, Catalytic asymmetric Pictet-Spengler reaction, J. Am. Chem. Soc., 2006, 128, 1086–1087 CrossRef CAS PubMed.
- D. S. McClure, Triplet-Singlet Transitions in Organic Molecules. Lifetime Measurements of the Triplet State, J. Chem. Phys., 1949, 17, 905–913 CrossRef CAS.
- F. Neese, Software update: The ORCA program system—Version 5.0, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2022, 12, e1606 Search PubMed.
- A. D. Becke, Density-functional thermochemistry. III. The role of exact exchange, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- P. J. Stephens, F. J. Devlin, C. F. Chabalowski and M. J. Frisch, Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields, J. Phys. Chem., 1994, 98, 11623–11627 CrossRef CAS.
- F. Weigend and R. Ahlrichs, Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- F. Weigend, Accurate Coulomb-fitting basis sets for H to Rn, Phys. Chem. Chem. Phys., 2006, 8, 1057–1065 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, analytical data and spectra. CCDC 2380037–2380046. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qo01921k |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2025 |