Hierarchical supramolecular assembly based on metal coordination of asymmetric ligand pairs and host–guest recognition†
Hui
Li
 *a,
Yan
Dong
a,
RiQiang
Li
a,
Yan
Zhang
a,
Shengyong
Liu
a and
Wei
Tian
*a,
Yan
Dong
a,
RiQiang
Li
a,
Yan
Zhang
a,
Shengyong
Liu
a and
Wei
Tian
 *b
*b
aJiangxi Province Key Laboratory of Functional Crystalline Materials Chemistry, School of Chemistry and Chemical Engineering, Jiangxi University of Science and Technology, Ganzhou 341000, Jiangxi Province, P.R. China. E-mail: lh@jxust.edu.cn
bShaanxi Key Laboratory of Chemical Additives for Industry, College of Chemistry and Chemical Engineering, Shaanxi University of Science and Technology, Xi'an 710021, P. R. China. E-mail: baiyang@sust.edu.cn
First published on 15th November 2024
Abstract
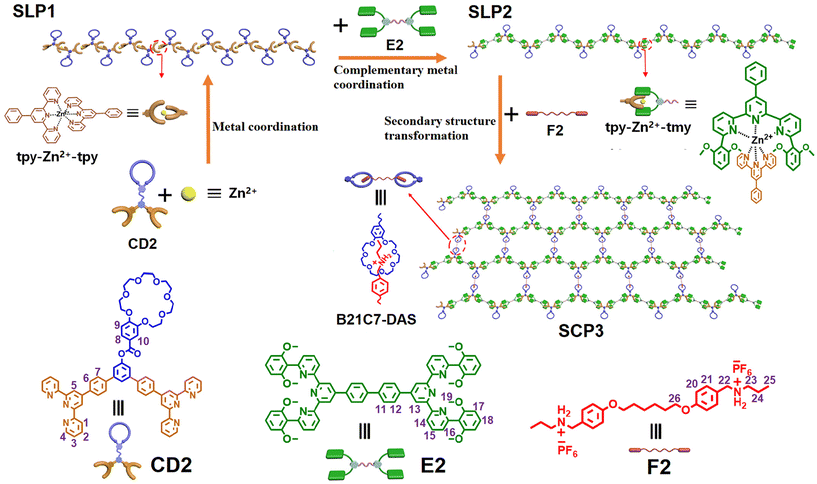
Three distinct monomers were synthesized, namely CD2, E2, and F2. Monomer CD2 is composed of a crown ether group (B21C7) and two terpyridine (tpy) groups, monomer E2 contains two 6,6′′-di(2,6-dimethoxylphenyl)-substituted terpyridine (tmy) groups, and the homoditopic F2 comprises two ammonium salts (DAS). By combining monomer CD2 with Zn(OTf)2, a supramolecular linear polymer (SLP1) was successfully obtained through metal coordination based on terpyridyl units. Upon introducing monomer E2 into the solution containing SLP1, a novel supramolecular linear polymer (SLP2) was formed in the solution via complementary metal coordination. Furthermore, by adding another monomer F2 to the solution of SLP2, we observed a further structural transformation of SLP2 into a supramolecular crosslinked polymer (SCP3). This transformation was facilitated by double noncovalent interactions involving both complementary metal coordination and host–guest recognition. Notably, all resulting supramolecular polymers, SLP1, SLP2, and SCP3, exhibited remarkable sensitivity towards OH− along with noticeable changes in their fluorescence emission properties. Moreover, at high concentrations, SCP3 demonstrated the ability to form a supramolecular gel while also exhibiting excellent self-healing properties.
Introduction
The exploration of new materials has led to the emergence of a fascinating class of chemical species known as supramolecular polymers.1 These polymers serve as a unique bridge between traditional polymers and supramolecular chemistry, offering unprecedented opportunities for the design and synthesis of materials with tailored functionalities and responsive behaviors. Supramolecular polymers are characterized by their assembly from low molecular units or macromolecular building blocks through noncovalent interactions.2–4 This distinguishes them from conventional polymers, which are typically formed through covalent bonds between repeating monomeric units. The noncovalent nature of supramolecular polymers endows them with dynamic and reversible properties, enabling them to respond to stimuli.5–10 Supramolecular polymers can self-assemble into well-defined structures, often with high selectivity and specificity, which makes them ideal candidates for application in the development of advanced functional materials including biomaterials,11 separation techniques,12 and electronics.13 Studies on supramolecular polymers have garnered significant attention and progressed rapidly due to the potential applications of supramolecular polymers in a wide range of areas; however, challenges persist in achieving precise control over the structure of supramolecular polymers and in understanding the relationship between their structure and function.The types of non-covalent interactions employed in supramolecular polymers include metal coordination,14 host–guest recognition,15 and hydrogen bonding,16 among others. Metal coordination, as a type of non-covalent interaction, exhibits high directional specificity and strong binding forces.17–21 Terpyridine (tpy), an important type of ligand, has been used in the preparation of supramolecular assemblies.22,23 However, when applied to the construction of complex supramolecular structures, unexpected assembly errors often occur in multi-component systems, leading to outcomes that deviate from the intended design.24,25 Challenges remain in realizing controllable supramolecular assembly in multi-component assembly systems. Compared to the symmetric tpy-M-tpy metal coordination, some asymmetric terpyridine-based ligand pairs have been found to form complementary coordination structures (tpy-M-tmy) with metal ions through a highly self-selective process,24,25 where the sterically hindered substituents at the terpyridyl 6,6′′-positions strongly restrain the formation of homoleptic complexes, while the π–π stacking interactions between the substituents and pyridine rings facilitate the high-fidelity heteroleptic complexation, providing easy access to quantitative self-assembly. Given the promising advantages of these asymmetric complementary ligand pairs and their assembly properties, it is desirable to explore novel supramolecular systems with well-defined structures and study their interesting properties. Herein, we report new supramolecular polymers constructed based on the symmetric tpy-Zn2+-tpy metal coordination and demonstrate the interesting structural transformation achieved by the asymmetric complementary metal coordination of tpy-Zn2+-tmy (Scheme 1). Furthermore, these supramolecular polymers could undergo secondary structural transformation to form supramolecular crosslinked polymers through double noncovalent interactions involving metal coordination and host–guest interaction.
Results and discussion
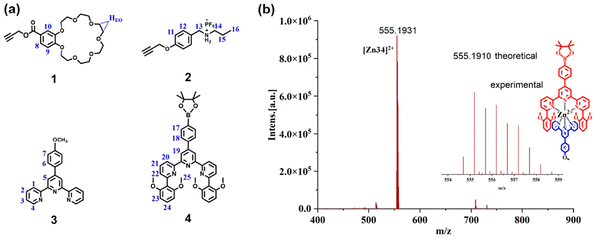
Three distinct monomers were synthesized, the heterotritopic CD2 is composed of a crown ether group (B21C7) and two tpy groups, the homoditopic E2 contains two 6,6′′-di(2,6-dimethoxylphenyl)-substituted terpyridine (tmy) groups, and the homoditopic F2 comprises two ammonium salts (DAS). To study the self-assembly behaviour of monomers, four model compounds, 1–4, were synthesized (Fig. 2a). Subsequently, mixtures of these model compounds were prepared in deuterated chloroform and deuterated acetonitrile (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), and their 1H NMR spectra were recorded. The 1H NMR analysis of the model compounds 1 + 2 in CDCl3–CD3CN (2
1, v/v), and their 1H NMR spectra were recorded. The 1H NMR analysis of the model compounds 1 + 2 in CDCl3–CD3CN (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) revealed the binding between the crown ether and ammonium salt in solution (Fig. S1†); the measured binding constant of B21C7-DAS was about 698 M−1.26 The chemical shift changes in the 1H NMR of 3 + Zn2+ indicated that terpyridyl could form a coordination structure with Zn2+ (Fig. S2e†).27,28 When a solution containing 4 + Zn(OTf)2 was prepared, it was observed that the formation rate of tmy-Zn2+-tmy significantly decreased compared to that of tpy-Zn2+-tpy, and only reached an incomplete conversion (74%) after 2 days (Fig. S2a†). It is hypothesized that the presence of two bulky substituents created steric hindrance that prevented the formation of the tmy-Zn2+-tmy coordination complex.24 However, upon mixing 3 + 4 + Zn(OTf)2 in solvent, new NMR chemical shifts were observed, indicating the formation of the tpy-Zn2+-tmy heteroleptic complex (Fig. S2c†).24 The measured binding constants of 3 + Zn(OTf)2 and 3 + 4 + Zn(OTf)2 were 5.23 × 106 M−1 and 2.96 × 107 M−1, respectively. The higher binding constant of tpy-Zn2+-tmy supported the formation of the high-fidelity heteroleptic complex. The high-resolution ESI-MS spectrum further gave evidence of the formation of tpy-Zn2+-tmy (Fig. 1b). Finally, the 1H NMR of the solution containing 1, 2, 3, and 4 along with Zn(OTf)2 gave clear evidence of the occurrence of a self-sorting process (Fig. S3†). Specifically, in CDCl3–CD3CN, the crown ether effectively binds the ammonium salt, while tpy selectively combines with tmy in the presence of Zn2+.
1, v/v) revealed the binding between the crown ether and ammonium salt in solution (Fig. S1†); the measured binding constant of B21C7-DAS was about 698 M−1.26 The chemical shift changes in the 1H NMR of 3 + Zn2+ indicated that terpyridyl could form a coordination structure with Zn2+ (Fig. S2e†).27,28 When a solution containing 4 + Zn(OTf)2 was prepared, it was observed that the formation rate of tmy-Zn2+-tmy significantly decreased compared to that of tpy-Zn2+-tpy, and only reached an incomplete conversion (74%) after 2 days (Fig. S2a†). It is hypothesized that the presence of two bulky substituents created steric hindrance that prevented the formation of the tmy-Zn2+-tmy coordination complex.24 However, upon mixing 3 + 4 + Zn(OTf)2 in solvent, new NMR chemical shifts were observed, indicating the formation of the tpy-Zn2+-tmy heteroleptic complex (Fig. S2c†).24 The measured binding constants of 3 + Zn(OTf)2 and 3 + 4 + Zn(OTf)2 were 5.23 × 106 M−1 and 2.96 × 107 M−1, respectively. The higher binding constant of tpy-Zn2+-tmy supported the formation of the high-fidelity heteroleptic complex. The high-resolution ESI-MS spectrum further gave evidence of the formation of tpy-Zn2+-tmy (Fig. 1b). Finally, the 1H NMR of the solution containing 1, 2, 3, and 4 along with Zn(OTf)2 gave clear evidence of the occurrence of a self-sorting process (Fig. S3†). Specifically, in CDCl3–CD3CN, the crown ether effectively binds the ammonium salt, while tpy selectively combines with tmy in the presence of Zn2+.
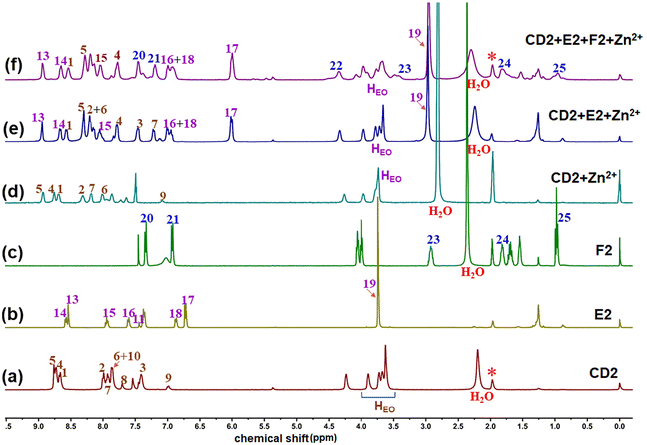
The self-assembly behavior of monomers was then examined using 1H NMR analysis. When a solution of CD2 + Zn(OTf)2 was prepared, the 1H NMR of CD2 + Zn(OTf)2 showed noticeable upfield shifts for the protons H2, H5, and H7 on CD2, signifying the formation of tpy-Zn2+-tpy coordination.22 Upon adding monomer E2 into the solution of CD2 + Zn(OTf)2, the original 1H NMR of CD2 + Zn(OTf)2 completely transformed into a more intricate 1H NMR of CD2 + E2 + Zn(OTf)2 within 4 minutes. By analyzing the 1H-1H COSY NMR of CD2 + E2 + Zn(OTf)2 and comparing it with the 1H NMR of model molecules (Fig. S5†), the complex 1H NMR of CD2 + E2 + Zn(OTf)2 was identified. As shown in Fig. 2e, the chemical shifts of protons H2, H3, and H6 on CD2 moved downfield, while the complex protons H4, H5, and H7 on CD2 shifted upfield. Meanwhile, the chemical shifts of protons H13, H14, and H15 on E2 moved downfield and the protons H16 and H17 on E2 shifted upfield. The new chemical shifts signified that the tpy-Zn2+-tpy coordination was destroyed and a complementary tpy-Zn2+-tmy coordination occurred.24 On this basis, the introduction of the monomer F2 into the CD2 + E2 + Zn(OTf)2 solution resulted in the occurrence of new chemical shifts (Fig. 2f), the chemical shifts of protons H20 and H21 on the monomer F2 moved downfield, confirming the occurrence of the B21C7-DAS host–guest interaction.26 The proton HEO on monomer CD2 correlated with the proton H21 on monomer F2 in the 2D NOESY spectrum (Fig. S9†), further verifying that the crown ether bound to the ammonium salt and that CD2 + E2 + F2 + Zn(OTf)2 self-assembled to form a supramolecular crosslinked polymer SCP3. The influence of monomer concentrations on supramolecular polymerization was then investigated. With increasing monomer concentration, all 1H NMR spectra of CD2 + Zn(OTf)2, CD2 + E2 + Zn(OTf)2, and CD2 + E2 + F2 + Zn(OTf)2 became broader (Fig. S10–S12†), indicating that CD2 + Zn(OTf)2, CD2 + E2 + Zn(OTf)2, and CD2 + E2 + F2 + Zn(OTf)2 could self-assemble to form the supramolecular polymers SLP1, SLP2, and SCP3 at high concentrations, respectively.
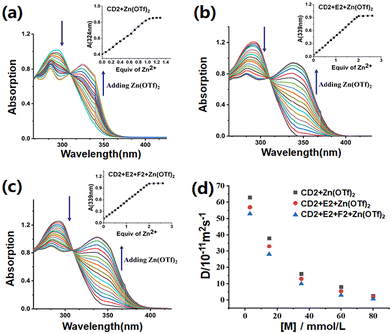
The self-assembly of monomers was investigated through UV-Vis absorption experiments. Fig. 3a shows the UV-Vis absorption spectrum of CD2, revealing an absorption band at 295 nm, attributed to the π–π* transition of terpyridine groups. Upon the addition of zinc ions to the CD2 solution, a new absorption band emerged at 324 nm and an isoabsorptive point was observed at 309 nm. These findings suggested that the free tpy gradually formed a metal coordination structure with Zn2+. When the molar ratio of CD2 to Zn2+ reached 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, we observed maximum absorption at 324 nm, which provided evidence for the formation of tpy-Zn2+-tpy. The solutions of CD2 + E2 and CD2 + E2 + F2 were subsequently titrated with Zn(OTf)2. Upon the addition of zinc ions to the system of CD2 + E2, a new absorption band appeared at 339 nm and an isoabsorptive point was observed at 310 nm in the UV-Vis absorption spectrum (Fig. 3b). When the stoichiometric ratio of CD2
1, we observed maximum absorption at 324 nm, which provided evidence for the formation of tpy-Zn2+-tpy. The solutions of CD2 + E2 and CD2 + E2 + F2 were subsequently titrated with Zn(OTf)2. Upon the addition of zinc ions to the system of CD2 + E2, a new absorption band appeared at 339 nm and an isoabsorptive point was observed at 310 nm in the UV-Vis absorption spectrum (Fig. 3b). When the stoichiometric ratio of CD2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E2
E2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn2+ was equilibrated at 1
Zn2+ was equilibrated at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, maximum absorption detected was 339 nm, which further confirmed the formation of tpy-Zn2+-tmy in the system. A similar absorption curve was observed in the CD2 + E2 + F2 + Zn(OTf)2 system, and the curve also showed that the B21C7-DAS reaction did not influence the formation of tpy-Zn2+-tmy (Fig. 3c).
2, maximum absorption detected was 339 nm, which further confirmed the formation of tpy-Zn2+-tmy in the system. A similar absorption curve was observed in the CD2 + E2 + F2 + Zn(OTf)2 system, and the curve also showed that the B21C7-DAS reaction did not influence the formation of tpy-Zn2+-tmy (Fig. 3c).
The self-assembly was further investigated using two-dimensional DOSY spectroscopy. Increasing the concentration of CD2 from 2 to 80 mM resulted in a significant reduction in the diffusion coefficient (D) for the CD2 + Zn(OTf)2 system, from 6.24 × 10−10 to 2.51 × 10−11 m2 s−1 (Fig. 3d and Fig. S13†). This observation suggested that CD2 + Zn(OTf)2 self-assembled by metal coordination to form SLP1 at relatively high concentrations. When an equimolar amount of E2 was subsequently added to the SLP1 system (with both CD2 and E2 at a concentration of 80 mM), the D value decreases to 1.87 × 10−11 m2 s−1 (Fig. 3d and Fig. S14†). The experiment indicated that after adding monomer E2 into the solution of CD2 + Zn(OTf)2, SLP1 transformed into a supramolecular linear polymer SLP2 with a higher molecular weight.29,30 When F2 was further introduced into the solution of SLP2 (CD2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E2
E2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F2
F2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn2+=2
Zn2+=2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), the resulting D value was found to be 6.68 × 10−12 m2 s−1, indicating that the addition of monomer F2 led to the formation of a higher molecular weight SCP3 from SLP2 (Fig. 3d and Fig. S15†). When the monomer concentrations were diluted, all D values exhibited an increase, confirming the concentration dependence of supramolecular polymerization.
4), the resulting D value was found to be 6.68 × 10−12 m2 s−1, indicating that the addition of monomer F2 led to the formation of a higher molecular weight SCP3 from SLP2 (Fig. 3d and Fig. S15†). When the monomer concentrations were diluted, all D values exhibited an increase, confirming the concentration dependence of supramolecular polymerization.
The viscosity experiment was conducted to further explore the supramolecular polymerization. Fig. 4a shows the specific viscosity of CD2 + Zn(OTf)2 and CD2 + E2 + Zn(OTf)2 as a function of CD2 concentration (plotted on a logarithmic scale). In the low concentration region, the slopes of the viscosity curves for CD2 + Zn(OTf)2 and CD2 + E2 + Zn(OTf)2 are found to be approximately 0.91 and 0.96, respectively. However, the viscosity curves for CD2 + Zn(OTf)2 and CD2 + E2 + Zn(OTf)2 exhibited slopes of 1.88 and 1.94, respectively, at relatively high concentrations, signifying a conversion from low molecular weight oligomers to larger supramolecular polymers.31 Furthermore, the reduced viscosity of CD2 + E2 + F2 + Zn(OTf)2 displayed a significant exponential increase as monomer concentration rose (Fig. 4b), indicating the formation of a crosslinked SCP network with larger dimensions.
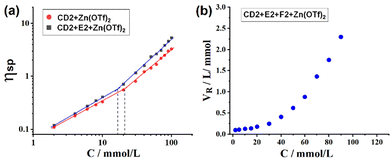 | ||
| Fig. 4 (a) Specific viscosity of CD2 + Zn(OTf)2 and CD2 + E2 + Zn(OTf)2 and (b) reduced viscosity of CD2 + E2 + F2 + Zn(OTf)2 as a function of CD2 concentration. | ||
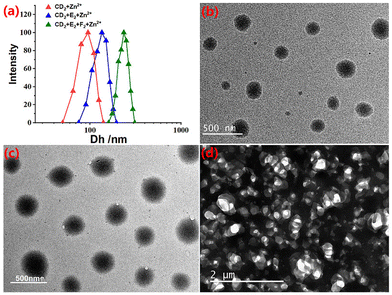
Dynamic light scattering (DLS) was then performed to investigate the sizes of the supramolecular polymers. SLP1, constructed through CD2 + Zn(OTf)2 (CD2 = 65 mM) in CDCl3–CD3CN, had an average hydrodynamic diameter (Dh) of 95 nm (Fig. 5a). The Dh of SLP2, constructed through CD2 + E2 + Zn(OTf)2 at the concentration of 65 mM, was 134 nm. SLP2 exhibited a larger size compared to the SLP1 system, which may be attributed to the higher binding constants of tpy-Zn2+-tmy on SLP2 compared to that of tpy-Zn2+-tpy on SLP1.24 SCP3, constructed through CD2 + E2 + F2 + Zn(OTf)2, displayed the largest size of 236 nm at the same concentrations due to the formation of a crosslinked net structure (Fig. 5a). The morphologies of supramolecular polymers were observed using TEM, and global nanostructures were observed for both SLP1 and SLP2 in the representative TEM images (Fig. 5b and c). These spherical shapes were presumably caused by the bending and folding of supramolecular polymer chains. In contrast to these global morphologies in SLP1 and SLP2 systems, a net structure was observed for SCP3 in the representative TEM image (Fig. 5d). It was speculated that this net morphology arose from the crosslinked structure present in SCP3.
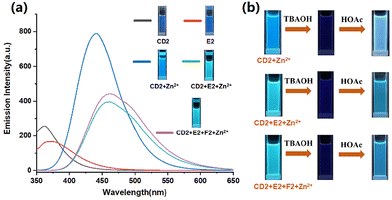
Subsequently, we examined the fluorescence emission characteristics of supramolecular polymers. The maximum fluorescence emission of the CD2 solution appeared at 361 nm (Fig. 6a). After adding zinc ions into the CD2 solution, the solution exhibited an increase in fluorescence emission due to the occurrence of tpy-Zn2+-tpy coordination,32 resulting in a redshift of the maximum fluorescence emission band from 361 nm to 443 nm. The mixed solution of CD2 + Zn(OTf)2 indicated blue emission under UV lamp irradiation. Furthermore, upon further addition of E2 into the solution of CD2 + Zn(OTf)2, there was a slight redshift in the maximum emission band from 441 nm to 458 nm (Fig. 6a), and the fluorescence emission intensity of the solution decayed due to the formation of tpy-Zn2+-tmy. Furthermore, the fluorescence emission spectrum of CD2 + E2 + F2 + Zn(OTf)2 solution also exhibited a slight redshift and decreased fluorescence intensity compared to that of CD2 + Zn(OTf)2 (Fig. 6a). Compared to the homoleptic tpy-Zn2+-tpy, the heteroleptic tpy-Zn2+-tmy coordination formed donor–acceptor–donor π–π stacking, which provided thermodynamic stabilization and also induced fluorescence emission decay due to the non-radiative transition. The lower fluorescence quantum yields of CD2 + E2 + Zn(OTf)2 and CD2 + E2 + F2 + Zn(OTf)2 verified the increase of the non-radiative decay process (Fig. S20†).
The stimuli–response of supramolecular polymers was subsequently explored. The fluorescence emission of SLP1, SLP2, and SCP3 could be regulated by disrupting the metal coordination. As OH− could react with zinc ions, resulting in the formation of Zn(OH)2,33 the tpy-Zn2+-tpy on the backbone of supramolecular polymers could be destroyed by adding OH−. When organic bases (TBAOH) were added to the solutions of SLP1, SLP2, and SCP3, significant changes in the fluorescence emissions of SLP1, SLP2, and SCP3 were observed due to the removal of Zn2+ (Fig. 6b and Fig. S21†). All fluorescence intensities decreased due to the destruction of metal coordination. However, when acetic acid was subsequently added to the solutions, the fluorescence emission recovered in all three solutions due to acid–base neutralization and the reformation of metal coordination. Since SCP3 not only contains tpy-Zn2+-tmy metal coordination but also comprises host–guest interaction of B21C7-DAS, it should be noted that the change in pH could also affect the host–guest interaction of B21C7-DAS in the SCP3 system,34 which would induce the disassembly/reassembly of SCP3.
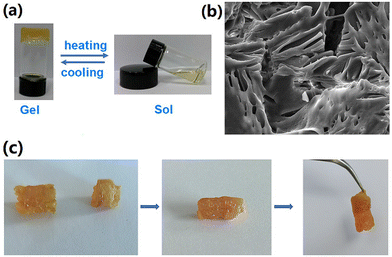
The transformation of the supramolecular crosslinked polymer SCP3 into a gel was observed when the monomer concentration exceeded 115 mM (Fig. 7a). Upon heating the gel to 50 °C, the gel transformed into a sol, but upon cooling, it reverted back to a gel in a completely reversible transformation process. The SEM image revealed the net morphology of the xerogel prepared by freeze drying technique (Fig. 7b). An investigation of the self-repairing properties of the SCP gel was subsequently conducted. As shown in Fig. 7c, the macroscopic self-healing phenomenon was observed by cutting the gel into two pieces followed by connecting them back to the original shape. Self-healing started immediately after connecting the cut faces. After 8 min, the combined gel was strong enough to be held vertically. The self-healing characteristic of the gel primarily originated from its dynamic and reversible noncovalent bonds within the network of the gel. When the gel was cut, it resulted in disruption of noncovalent interactions along the cut face. Nevertheless, due to the reversible nature of these noncovalent bonds, they swiftly reformed, launching the self-healing process.
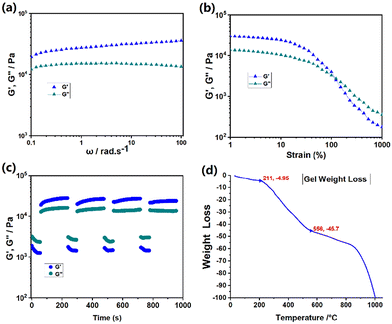
Rheological experiments were performed to analyze gel properties. A sample of the gel was initially prepared at a concentration of 150 mM by heating the mixture of CD2 + E2 + F2 + Zn(OTf)2 to 55 °C and then cooling it to ambient temperature. Rheological characterization demonstrated that the storage modulus consistently exhibited higher values compared to the loss modulus during the frequency sweep (Fig. 8a). The experiment result indicated that stable supramolecular gel network was formed by the B21C7-DAS recognition and tpy-Zn2+-tmy coordination interaction. The strain sweep experiment demonstrated that G′′ surpassed G′ when the strain exceeded 133% (Fig. 8b), indicating the destruction of the crosslinking network under a large strain, where the viscous property of the sample became dominant when damaged under high strain conditions. The step strain tests were performed to analyze the self-repairing phenomenon of the gel. During the process of step strain sweep, a range of strains from 300% to 1% were applied to the gel sample. At high strains, damage was observed as G′′ surpassed G′ (Fig. 8c). However, when the strain was reduced from 300% to 1%, there was a rapid restoration in both G′ and G′′ values, signifying that the gel's network structure could be quickly repaired due to its dynamic reversibility of noncovalent interactions. This consistent trend was observed in multiple cyclic strain scan experiments, confirming the exceptional self-repairing ability possessed by the SCP gel. Finally, the TGA experiment was performed to analyze the thermostability of the SCP gel. Before the experiment, the solvent of the gel was removed at ambient temperature. The TGA curve revealed a slight degradation in the range of 30–211 °C (Fig. 8d). After a temperature transition from 211 to 566 °C, notable decomposition occurred, resulting in a substantial weight reduction ranging between 4.95% and 45.7%. The primary cause of this significant weight loss could be the breakdown of crown ether and ammonium salt.
Conclusions
In summary, three distinct monomers, CD2, E2, and F2, were synthesized. CD2 + Zn(OTf)2 could self-assemble into the supramolecular polymer SLP1 via symmetric tpy-Zn2+-tpy metal coordination. Upon introducing E2 into the solution containing SLP1, the interesting structural transformation occurred from SLP1 to SLP2 through the complementary tpy-Zn2+-tmy metal coordination. Furthermore, these supramolecular polymer SLP2 could undergo secondary structure transformation to form supramolecular crosslinked polymer SCP3 through double noncovalent interactions involving complementary metal coordination and host–guest interaction. Importantly, all resulting supramolecular polymers SLP1, SLP2, and SCP3 showed high sensitivity towards pH along with significant changes in their fluorescence emission properties. Furthermore, at high concentrations, SCP3 demonstrated its ability to form a gel while also exhibiting exceptional self-healing capabilities. The assembly methodology employed in this study may be utilized for fabricating tailored polymers with potential applications in the fields of fluorescence and self-healing materials.Author contributions
Writing – original draft & editing: H. L.; synthetic experiments: Y. D.; data curation and software: R. Q. L.; methodology: Y. Z.; drawing: S. Y. L.; and writing – review & editing: W. T.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 22161020 and 21801100), the Natural Science Foundation of Jiangxi Province (20212BAB203014), and the Program of Qingjiang Excellent Young Talents, Jiangxi University of Science and Technology.References
- T. Aida, E. W. Meijer and S. L. Stupp, Functional supramolecular polymers, Science, 2012, 335, 813–817 CrossRef CAS.
- Y. X. Liu, L. L. Wang, L. Zhao, Y. G. Zhang, Z. T. Li and F. H. Huang, Multiple hydrogen bonding driven supramolecular architectures and their biomedical applications, Chem. Soc. Rev., 2024, 53, 1592–1623 RSC.
- H. W. Gibson, N. Yamaguchi and J. W. Jones, Supramolecular Pseudorotaxane Polymers from Complementary Pairs of Homoditopic Molecules, J. Am. Chem. Soc., 2003, 125, 3522–3533 CrossRef CAS PubMed.
- R. P. Sijbesma, F. H. Beijer, L. Brunsveld, B. J. B. Folmer, J. H. Hirschberg, R. F. Lange, J. K. Lowe and E. W. Meijer, Reversible Polymers Formed from Self-Complementary Monomers Using Quadruple Hydrogen Bonding, Science, 1997, 278, 1601–1604 CrossRef CAS.
- T. Hirao, H. Kudo, T. Amimoto and T. Haino, Sequence-Controlled Supramolecular Terpolymerization Directed by Specific Molecular Recognitions, Nat. Commun., 2017, 8, 634 CrossRef.
- Y. F. Han, Y. K. Tian, Z. J. Li and F. Wang, Donor−acceptor-type supramolecular polymers on the basis of preorganized molecular tweezers/guest complexation, Chem. Soc. Rev., 2018, 47, 5165–5176 RSC.
- L. Wang, L. Cheng, G. Li, K. Liu, Z. Zhang, P. Li, S. Dong, W. Yu, F. Huang and X. Yan, A Self-Cross-Linking Supramolecular Polymer Network Enabled by Crown-Ether-Based Molecular Recognition, J. Am. Chem. Soc., 2020, 142, 2051–2058 CrossRef CAS.
- C. J. Lu, M. M. Zhang, D. T. Tang, X. Z. Yan, Z. Y. Zhang, Z. X. Zhou, B. Song, H. Wang, X. P. Li, S. C. Yin, H. Sepehrpour and P. J. Stang, Fluorescent Metallacage-Core Supramolecular Polymer Gel Formed by Orthogonal Metal Coordination and Host–Guest Interactions, J. Am. Chem. Soc., 2018, 140, 7674–7680 CrossRef CAS PubMed.
- K. Kinjo, T. Hirao, S. Kihara, Y. Katsumoto and T. Haino, Supramolecular Porphyrin Copolymer Assembled through Host–Guest Interactions and Metal–Ligand Coordination, Angew. Chem., Int. Ed., 2015, 54, 14830–14834 CrossRef CAS PubMed.
- A. Lavrenova, D. W. R. Balkenende, Y. Sagara, S. Schrettl, Y. C. Simon and C. Weder, Mechano- and Thermoresponsive Photoluminescent Supramolecular Polymer, J. Am. Chem. Soc., 2017, 139, 4302–4305 CrossRef CAS.
- Z. Y. Wang, C. Sun, K. Yang, X. Y. Chen and R. B. Wang, Cucurbituril-Based Supramolecular Polymers for Biomedical Applications, Angew. Chem., Int. Ed., 2022, 61, e202206763 CrossRef CAS.
- Y. F. Li, X. Y. Lou, C. Y. Wang, Y. Wang, Y. Jia, Q. Lin and Y. W. Yang, Synthesis of stimuli-responsive pillararene based supramolecular polymer materials for the detection and separation of metal ions, Chin. Chem. Lett., 2023, 34, 107877 CrossRef CAS.
- X. Y. Yang, W. Q. Cai, S. Dong, K. Zhang, J. Zhang, F. H. Huang, F. Huang and Y. Cai, Fluorescent Supramolecular Polymers Based on Pillar[5]arene for OLED Device Fabrication, ACS Macro. Lett., 2017, 6, 647–651 CrossRef CAS PubMed.
- K. C. Bentz and S. M. Cohen, Supramolecular Metallopolymers: From Linear Materials to Infinite Networks, Angew. Chem., Int. Ed., 2018, 57, 14992–15001 CrossRef CAS PubMed.
- D. Liu, D. P. Wang, M. Wang, Y. J. Zheng, K. Koynov, G. K. Auernhammer, H. J. Butt and T. Ikeda, Supramolecular Organogel Based on Crown Ether and Secondary Ammoniumion Functionalized Glycidyl Triazole Polymers, Macromolecules, 2013, 46, 4617–4625 CrossRef CAS.
- C. Schmuck and W. Wienand, Self-Complementary Quadruple Hydrogen-Bonding Motifs as a Functional Principle: From Dimeric Supramolecules to Supramolecular Polymers, Angew. Chem., Int. Ed., 2001, 40, 4363–4369 CrossRef CAS.
- S. Chakraborty, W. Hong, K. J. Endres, T. Z. Xie, L. Wojtas, C. N. Moorefield, C. Wesdemiotis and G. R. Newkome, Terpyridine-Based, Flexible Tripods: From a Highly Symmetric Nanosphere to Temperature-Dependent, Irreversible, 3D Isomeric Macromolecular Nanocages, J. Am. Chem. Soc., 2017, 139, 3012–3020 CrossRef CAS.
- B. Zheng, Y. L. Hou, L. Y. Gao and M. M. Zhang, Luminescent Metallo-Supramolecular Polymers, Chin. J. Chem., 2019, 37, 843–854 CrossRef CAS.
- A. Winter and U. S. Schubert, Synthesis and characterization of metallo-supramolecular polymers, Chem. Soc. Rev., 2016, 45, 5311–5357 RSC.
- S. Datta, M. L. Saha and P. J. Stang, Hierarchical Assemblies of Supramolecular Coordination Complexes, Acc. Chem. Res., 2018, 51, 2047–2063 CrossRef CAS.
- S. Chakraborty and G. R. Newkome, Terpyridine-based metallosupramolecular constructs: tailored monomers to precise 2D-motifs and 3D-metallocages, Chem. Soc. Rev., 2018, 47, 3991–4016 RSC.
- Y. Ding, P. Wang, Y. K. Tian, Y. J. Tian and F. Wang, Formation of stimuli-responsive supramolecular polymeric assemblies via orthogonal metal–ligand and host–guest interactions, Chem. Commun., 2013, 49, 5951–5953 RSC.
- S. Bhowmik, B. N. Ghosh, V. Marjomäki and K. Rissanen, Nanomolar Pyrophosphate Detection in Water and in a Self-Assembled Hydrogel of a Simple Terpyridine-Zn2+ Complex, J. Am. Chem. Soc., 2014, 136, 5543–5546 CrossRef CAS PubMed.
- S. Y. Wang, J. H. Fu, Y. P. Liang, Y. J. He, Y. S. Chen and Y. T. Chan, Metallo-Supramolecular Self-Assembly of a Multicomponent Ditrigon Based on Complementary Terpyridine Ligand Pairing, J. Am. Chem. Soc., 2016, 138, 3651–3654 CrossRef CAS PubMed.
- Y. J. He, T. H. Tu, M. K. Su, C. W. Yang, K. V. Kong and Y. T. Chan, Facile Construction of Metallo-Supramolecular P3HTb-PEO Diblock Copolymers via Complementary Coordination and Their Self-Assembled Nanostructures, J. Am. Chem. Soc., 2017, 139, 4218–4224 CrossRef CAS PubMed.
- C. J. Zhang, S. J. Li, J. Q. Zhang, K. L. Zhu, N. Li and F. H. Huang, Benzo-21-Crown-7/Secondary Dialkylammonium Salt [2]Pseudorotaxane- and [2]Rotaxane-Type Threaded Structures, Org. Lett., 2007, 9, 5553–5556 CrossRef CAS.
- P. Verma, A. Singh, F. A. Rahimi, P. Sarkar, S. Nath, S. K. Pati and T. K. Maji, Charge-transfer regulated visible light driven photocatalytic H2 production and CO2 reduction in tetrathiafulvalene based coordination polymer gel, Nat. Commun., 2021, 12, 7313 CrossRef CAS.
- H. Hofmeiera and U. S. Schubert, Recent developments in the supramolecular chemistry of terpyridine–metal complexes, Chem. Soc. Rev., 2004, 33, 373–399 RSC.
- S. Dong, Y. Luo, X. Yan, B. Zheng, X. Ding, Y. Yu, Z. Ma, Q. Zhao and F. H. Huang, A Dual-Responsive Supramolecular Polymer Gel Formed by Crown Ether Based Molecular Recognition, Angew. Chem., Int. Ed., 2011, 50, 1905–1909 CrossRef CAS PubMed.
- T. F. A. Greef, G. Ercolani, G. B. Ligthart, E. W. Meijer and R. P. Sijbesma, Influence of Selectivity on the Supramolecular Polymerization of AB-Type Polymers Capable of Both A·A and A·B Interactions, J. Am. Chem. Soc., 2008, 130, 13755–13764 CrossRef PubMed.
- T. Xiao, X. Feng, Q. Wang, C. Lin, L. Wang and Y. Pan, Switchable supramolecular polymers from the orthogonal self-assembly of quadruple hydrogen bonding and benzo-21-crown-7−secondary ammonium salt recognition, Chem. Commun., 2013, 49, 8329–8331 RSC.
- C. J. Li, K. Han, J. Li, Y. Y. Zhang, W. Chen, Y. H. Yu and X. S. Jia, Supramolecular Polymers Based on Efficient Pillar[5]arene-Neutral Guest Motifs, Chem. – Eur. J., 2013, 19, 11892–11897 CrossRef CAS.
- B. B. Shi, K. C. Jie, Y. J. Zhou, D. Y. Xia and Y. Yao, Formation of fluorescent supramolecular polymeric assemblies via orthogonal pillar[5]arene-based molecular recognition and metal ion coordination, Chem. Commun., 2015, 51, 4503–4506 RSC.
- X. Z. Yan, D. H. Xu, X. D. Chi, J. Z. Chen, S. Y. Dong, X. Ding, Y. H. Yu and F. H. Huang, A Multiresponsive, Shape-Persistent, and Elastic Supramolecular Polymer Network Gel Constructed by Orthogonal Self-Assembly, Adv. Mater., 2012, 24, 362–369 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qo01926a |
| This journal is © the Partner Organisations 2025 |