Open Access Article
Open Access Article1,2 Wagner–Meerwein shift in aza-Nazarov cyclization: Bi(III)-catalyzed substrate-dependent divergent synthesis of highly substituted pyrroles and indenes†
Deepak Datta Gaonkara,
Shon Gangaia,
Krishna Mhaskea and
Rishikesh Narayan *ab
*ab
aSchool of Chemical and Materials Sciences (SCMS), Indian Institute of Technology Goa, Farmagudi, Goa 403401, India. E-mail: rishikesh.narayan@iitgoa.ac.in; Web: https://sites.google.com/iitgoa.ac.in/narayan-lab/home
bSchool of Interdisciplinary Life Sciences (SILS), Indian Institute of Technology Goa, Farmagudi, Goa 403401, India
First published on 26th April 2025
Abstract
The Nazarov reaction and its variants such as aza-Nazarov and iso-Nazarov cyclizations are versatile methods for the synthesis of five-membered ring systems including pyrroles and indenes. The 1,2-Wagner Meerwein shift has been combined in a domino sequence with both Nazarov and aza-Nazarov-like reactions for the synthesis of cyclopentenone and indole derivatives, respectively. However, the same sequence has not been applied for the synthesis of pyrroles, possibly due to the high reactivity of 1-azapentadienyl cation intermediates. In this report, we present the first example of an aza-Nazarov/1,2-Wagner Meerwein shift domino sequence for the synthesis of highly substituted pyrroles. The use of Bi(III) as a mild main group metal catalyst was found to be crucial to control the high reactivity of the intermediate. The substrate demonstrated substituent-dependent divergence in product formation to selectively give indenes through iso-Nazarov cyclization. Detailed mechanistic investigations reveal the electrocyclization nature of the reaction involving a cationic intermediate generated under Lewis acid and/or ‘hidden Brǿnsted acid’ catalysis conditions.
Introduction
Pyrroles represent a pre-eminent heterocyclic molecular scaffold with a vast array of uses. They are ubiquitously found in natural products of both terrestrial and marine origins with a wide bioactivity profile including antibiotic, antitumor, antiviral activities etc.1 Besides their natural occurrence, pyrroles are also a useful class of pharmacophores and agrochemicals (Fig. 1).2 Atorvastatin, the cholesterol-lowering penta-substituted pyrrole drug, is one of the most successful FDA-approved drugs ever. Inspired by the rich chemistry of pyrroles in biochemical systems owing to their electron-rich nature, pyrroles have been used as synthons or advanced intermediates in organic synthesis especially within the realm of challenging natural product total synthesis.3 The importance of pyrroles in organic materials applications has been growing steadily due to their excellent electronic properties such as a high-lying HOMO, high electrical conductivity etc.4 The utility profile of indenes mirrors that of pyrroles quite well. They are naturally abundant and are also found as structural cores in FDA-approved drugs such as sulindac, dimetindene etc. (Fig. 1).5 Besides these, both natural and synthetic indene analogues have been found to display a wide range of bioactivities including anti-cancer,6a anti-microbial,6b anti-allergic,6c and fungicidal activities.6d Given their importance, the synthesis of pyrroles7 as well indenes8 has continued to attract attention from synthetic chemists.Nazarov cyclization,9 a 4π-thermal conrotatory electrocyclization, and its highly useful variants such as aza-Nazarov,10 iso-Nazarov,11 and interrupted Nazarov cyclization12 have been a cornerstone for the synthesis of five-membered carbocyclic as well as heterocyclic systems. While Nazarov and iso-Nazarov reactions lead to the formation of cyclopentenone derivatives, the aza-Nazarov reaction gives nitrogen-containing five-membered heterocycles such as pyrroles as products.13 Sustained and ingenious efforts from various research groups have resulted in expanding the scope of these reactions.14 The Frontier group merged the highly versatile 1,2-Wagner–Meerwein rearrangement as a subsequent step of the Nazarov cyclization to access carbocyclic spiro-systems.15 Based on some early work by Moody and Rees,16 the Driver group devised a strategy to merge C–N bond forming aza-Nazarov electrocyclization with the Wagner–Meerwein shift using a variety of styryl azide substrates under Rh(II) catalysis to obtain various types of indoles (Scheme 1a).17 They proposed the involvement of a benzannulated 1-aza pentadienyl cation as the key intermediate for the electrocyclization followed by cationic rearrangement. Despite substantial mechanistic understanding, a similar domino sequence consisting of aza-Nazarov cyclization with Wagner–Meerwein rearrangement has not yet been applied for the synthesis of pyrroles. This would entail controlled generation and reactivity of the isolated 1-azapentadienyl cation, which is obviously challenging given the highly reactive nature of this cationic intermediate. Driver et al. have reported a synthesis of pyrroles from dienyl azide along the lines of indole synthesis, which involves cyclization of the aza-pentadienyl cation intermediate but it doesn't involve the 1,2-shift (Scheme 1b).18 Their efforts to force the 1,2-shift by di-substitution on terminal carbon (δ) didn't yield any product, thus, underscoring the challenge of combining these two steps in a domino sequence with the non-annulated or isolated aza-pentadienyl cation. In our design, we hypothesized that the required non-annulated 1-azapentadienyl cation could be generated under mild acidic conditions from the 1-aza-1,3-pentadien-5-ol precursor (Scheme 1c). The cation could undergo facile electrocyclization to generate a pyrrolium cation with tetra-substituted carbon to trigger the 1,2-Wagner Meerwein rearrangement, which should eventually form a pyrrole upon aromatization.
 | ||
| Scheme 1 (a) State of the art for the electrocyclization–Wagner Meerwein domino sequence for the synthesis of indoles; (b) synthesis of pyrroles from dienyl azide; (c) our reaction design. | ||
Herein, we report the first example of a domino 4π electrocyclization/1,2-Wagner Meerwein rearrangement sequence for the synthesis of highly substituted pyrroles. The reaction was found to work optimally using BiCl3, an inexpensive, mild and non-toxic main group metal catalyst, in DCE under ambient conditions.19 Moreover, the reaction showed substrate-dependent divergent behaviour leading to highly efficient and selective synthesis of indenes through an alternative iso-Nazarov reaction pathway.
Optimization of the reaction conditions
In order to test our hypothesis, we synthesized tertiary alcohol 1a and subjected it to various Lewis and Brønsted acidic conditions in DCE as the solvent. It was found to be highly reactive in the presence of most catalysts such as Sc(OTf)3, Cu(OTf)2, InCl3, SnCl4, AlCl3, BF3·OEt2 etc. leading to decomposition (Table 1, entries 1–3 and S1†). Among the catalysts attempted, BiCl3 proved to be the most amenable. However, it gave penta-substituted pyrrole 3a as the sole product in a good 55% yield instead of the expected pyrrole 2a (Table 1, entry 4). Pyrrole 3a seems to form from the electrophilic aromatic substitution reaction of pyrrole 2a with the 1-azapentadienyl cation Int-1 generated in the medium under catalytic conditions. The exclusive formation of 3a also indicates that the rate of electrophilic aromatic substitution is at least comparable to that of the formation of pyrrole 2a. At this point, we reasoned that increasing the amount of catalyst might ensure near simultaneous conversion of all 3° alcohol 1a into the azapentadienyl cation intermediate, which will stop the reaction at pyrrole 2a instead of 3a. Hence, when the catalyst loading was increased to 0.2 equiv. and eventually to 1.2 equivalents, the selectivity switched in favour of the formation of pyrrole 2a as the sole product, albeit in a modest yield of 33% (entries 5 and 6). Other Bi(III) catalysts such as Bi(OTf)3 and acids such TFA and TfOH proved to be too harsh and gave much inferior results, predominantly leading to decomposition (entries 7 and 8). Except DCM, other solvents such as MeOH, THF, acetone, HFIP, DMF, DMSO etc. couldn't furnish any product, whereas toluene and acetonitrile showed slow reactivity and gave 3a in a poor yield of 15% (entries 9 and 10 and S1†). Further increasing the amount of BiCl3 to 2 equivalents led to lower yields (entry 11). The high reactivity of 1a reinforced the need to block the C-3 position to protect the aza-pentadienyl cation intermediate (Int-1) from reacting with any potential nucleophile in the medium as well as preventing pyrrole 2a from reacting further through electrophilic aromatic substitution. Hence, in the case of alcohol 1b with a C-3 methyl substituent, the yield increased to 40%, which is not a significant increase. This result indicated that other factors might be responsible for the modest yields of the domino sequence (entry 12). Hence, we turned our attention to gauging the impact of the efficiency of the 1,2-Wagner–Meerwein shift, the second step of the domino sequence, on the yield of the reaction. It was presumed that an inefficient 1,2-shift might lead to decomposition of the reactive cationic intermediate. Indeed, when relatively more electron-rich o-tolyl was used as an aryl substituent in substrate 1c, compared to the phenyl in 1b, the yield of the corresponding pyrrole 2g increased to an excellent 90% (entry 13). These two modifications i.e. blocking the C-3 position and using relatively electron-rich aryls allowed the reaction to be carried out successfully under catalytic conditions (20 mol% and 5 mol%) with an excellent yield of 87% (entries 14 and 15). Hence, we decided to proceed with an appropriate amount of BiCl3 in DCE under ambient conditions as the optimal reaction conditions for the exploration of the scope of the aza-Nazarov/1,2-shift domino sequence.| Entry | Substrate | Catalyst | Loading (x equiv.) | Solvent | Yieldb (%) | |
|---|---|---|---|---|---|---|
| 2a/2e/2g | 3a | |||||
| a Reaction conditions: 1 (0.1 mmol, 1 equiv.), BiCl3 (as indicated), solvent (2 mL), rt.b Isolated yields after column chromatography. | ||||||
| 1 | 1a | Sc(OTf)3 | 0.1 | DCE | — | <5 |
| 2 | 1a | Cu(OTf)2 | 0.2 | DCE | — | <5 |
| 3 | 1a | InCl3 | 0.2 | DCE | — | <5 |
| 4 | 1a | BiCl3 | 0.1 | DCE | — | 55 |
| 5 | 1a | BiCl3 | 0.2 | DCE | — | 68 |
| 6 | 1a | BiCl3 | 1.2 | DCE | 33 | — |
| 7 | 1a | Bi(OTf)3 | 1.2 | DCE | 9 | — |
| 8 | 1a | TfOH/TFA | 1.2 | DCE | Trace | — |
| 9 | 1a | BiCl3 | 1.2 | DCM | 30 | — |
| 10 | 1a | BiCl3 | 1.2 | MeOH/THF/acetone/DMF/DMSO | Trace | — |
| 11 | 1a | BiCl3 | 2.0 | DCE | 29 | |
| 12 | 1b | BiCl3 | 1.2 | DCE | 40 | — |
| 13 | 1c | BiCl3 | 1.2 | DCE | 90 | — |
| 14 | 1c | BiCl3 | 0.2 | DCE | 87 | — |
| 15 | 1c | BiCl3 | 0.05 | DCE | 87 | — |
Scope of the reaction for the synthesis of pyrroles
After optimizing various aspects of the reaction conditions for the electrocyclization–cationic rearrangement cascade, we proceeded to explore the scope of this reaction. At first, the scope with respect to the synthesis of tri-substituted pyrroles (R3,4 = H) was explored. As expected, the reactions were found to proceed with moderate efficiency and gave the expected products 2a–2d in relatively modest yields (28–38%) (Scheme 2). Moreover, the products 2b–2d were isolated as an inseparable mixture of two possible regioisomers emanating from migration of both the aryl groups (R5, R6) during the 1,2-shift. We then turned our attention to the synthesis of tetra-substituted pyrroles, beginning with identical aryl groups (R5 = R6) at the terminal carbon in 1. The substrates with phenyl and 4-tolyl as aryl groups were found to give the corresponding pyrroles 2e and 2f in modest yields of 40% and 34%, respectively. Surprisingly, the substrates with 2-tolyl and 2,4-dimethylphenyl groups demonstrated much better reactivity and gave the corresponding pyrroles 2g and 2h in excellent 90% and 79% yields, respectively. Interestingly, these two pyrroles, owing to the presence of ortho methyl substituents and the resulting stereogenic axes, were obtained as a mixture of two atropisomers in ∼0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Relatively more electron-rich 4-methoxyphenyl gave the product 2i in a diminished 51% yield, due to its high reactivity.
1 ratio. Relatively more electron-rich 4-methoxyphenyl gave the product 2i in a diminished 51% yield, due to its high reactivity.
 | ||
Scheme 2 Scope of the pyrrole synthesis. Reaction conditions: 1 (0.1 mmol, 1 equiv.), BiCl3 (0.12 mmol, 1.2 equiv.), 1,2-DCE (2 mL), rt for the indicated time; a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 equiv. of BiCl3 were used. 0.2 equiv. of BiCl3 were used. | ||
We proceeded to explore the reaction with non-identical aryl groups (R5 ≠ R6) at the terminal carbon, which could potentially give a mixture of regioisomeric pyrroles as the products. Towards this, a variety of alcohols with R5 as phenyl and R6 as variably substituted aryl groups were subjected to the reaction conditions. Expectedly, electron-donating substituents such as methoxy and methyl at the 4-position of R6 demonstrated better reactivity and gave the corresponding products in good yields but as a mixture of regioisomers. In the case of p-methoxyphenyl, pyrroles 2k and 2k′ were obtained as an inseparable mixture of regioisomers in the ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in 65% combined yield. Similarly, p-tolyl gave a 3
1 in 65% combined yield. Similarly, p-tolyl gave a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of regioisomeric pyrroles 2l/2l′ in 64% yield. 4-Fluorophenyl as R6 led to pyrroles 2j and 2j′ as a mixture of regioisomers in the ratio of 4.4
1 mixture of regioisomeric pyrroles 2l/2l′ in 64% yield. 4-Fluorophenyl as R6 led to pyrroles 2j and 2j′ as a mixture of regioisomers in the ratio of 4.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 in 26% yield. The strongly electron-withdrawing CF3 group showed diminished reactivity and gave a mixture of products 2m/2m′ in a 1.6
10 in 26% yield. The strongly electron-withdrawing CF3 group showed diminished reactivity and gave a mixture of products 2m/2m′ in a 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratio in 20% yield. In contrast to p-substitution, electron-donating substituents at the m- and o-position displayed much better selectivity generally in 1,2-migration and gave the corresponding pyrroles 2n–2q, predominantly as a single regioisomer in moderate to good yields (41–65%). We explored multiple other combinations of variably substituted aryls at R5 and R6 to obtain a range of pyrroles (2r–2z) in good to excellent yields. In all cases, the methoxy-substituted phenyl displayed distinct preference over other aryl groups in terms of their migrating tendency. Interestingly, combinations of o-tolyl with p-fluoro and p-CF3 phenyls led to separable mixtures of regioisomeric pyrroles. Pyrroles 2aa/2aa′ and 2ac/2ac′ were obtained in an approximately 1
10 ratio in 20% yield. In contrast to p-substitution, electron-donating substituents at the m- and o-position displayed much better selectivity generally in 1,2-migration and gave the corresponding pyrroles 2n–2q, predominantly as a single regioisomer in moderate to good yields (41–65%). We explored multiple other combinations of variably substituted aryls at R5 and R6 to obtain a range of pyrroles (2r–2z) in good to excellent yields. In all cases, the methoxy-substituted phenyl displayed distinct preference over other aryl groups in terms of their migrating tendency. Interestingly, combinations of o-tolyl with p-fluoro and p-CF3 phenyls led to separable mixtures of regioisomeric pyrroles. Pyrroles 2aa/2aa′ and 2ac/2ac′ were obtained in an approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, which could be a combined effect of the electronic and steric impact on the rate of 1,2-migration.15 However, 2ab was obtained predominantly as a single regioisomer in 53% yield with a small amount of the other isomer. The structures of the products were assigned based on NOE analysis of the regioisomers (Fig. S6–8†). The synthesis of pyrrole 2ad in a good yield of 73% demonstrates that R2 and R3 are amenable for other alkyl substitutions. Heteroaryl substituents like furan and thiophene could not be tolerated in the reaction and led to complex reaction mixtures.
1 ratio, which could be a combined effect of the electronic and steric impact on the rate of 1,2-migration.15 However, 2ab was obtained predominantly as a single regioisomer in 53% yield with a small amount of the other isomer. The structures of the products were assigned based on NOE analysis of the regioisomers (Fig. S6–8†). The synthesis of pyrrole 2ad in a good yield of 73% demonstrates that R2 and R3 are amenable for other alkyl substitutions. Heteroaryl substituents like furan and thiophene could not be tolerated in the reaction and led to complex reaction mixtures.
The scalability of a reaction is a good measure of its practicality. Pyrrole 2h was obtained in an excellent 80% yield when the reaction was performed at 0.6 mmol scale (0.26 g), without any significant deviation from that of the small-scale reaction. Besides scalability, in general, the scope of pyrrole synthesis proved to be broad with the possibility of varying all the available positions on the pyrrole ring especially with aryl substituents.
Iso-Nazarov reaction for the synthesis of indenes 4
Interestingly, when the R4 substituent in substrate 1 was changed to methyl instead of H, the reaction adopted a completely different path and proceeded through the iso-Nazarov pathway to give substituted indenes 4 as the sole products of the reaction under catalytic conditions and in very good yields generally (Scheme 3). The generality of this transformation was explored by varying different substituents in alcohol 1. The oxime substituent R2 as methyl and n-octyl worked very well and gave indenes 4a and 4b as the corresponding products in excellent yields of 76% and 84%, respectively. When two electronically different aryl groups were used as R5 and R6 substituents, the indenes were obtained as an inseparable mixture of regioisomeric products due to the possibility of cyclization through both the aryl groups. With R5/R6 as p-methoxyphenyl and phenyl, indenes 4c/4c′ were obtained in 67% yield with a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 ratio, which remained the same for indenes 4d/4d′ with R2 being n-octyl. Similarly, when one of the aryl groups was p-tolyl, indenes 4e/4e′ were obtained in 53% yield in a 10
1.3 ratio, which remained the same for indenes 4d/4d′ with R2 being n-octyl. Similarly, when one of the aryl groups was p-tolyl, indenes 4e/4e′ were obtained in 53% yield in a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.9 ratio. A combination of 2,4-mesityl and p-tolyl substituents led to an almost equimolar ratio of 4g/4g′ in an excellent 80% combined yield. Electronically different p-fluoro phenyl and p-tolyl gave indene 4h as a single regioisomer in 71% yield. Interestingly, when n-butyl was used as one of the R5/R6 substituents, the reaction still proceeded efficiently and gave indene 4i in a very good yield of 72%. Notably, the possible highly conjugated elimination product 4i′ was not observed in the reaction, which indicates the selective nature of the reaction for iso-Nazarov cyclization over facile elimination. The position of the double bond in the indene ring was confirmed through 1H–15N HMBC correlation in indene 4a, which showed strong three-bond correlation of the oxime nitrogen with the methine proton (Scheme 3, top right and Fig. S9, ESI†).
2.9 ratio. A combination of 2,4-mesityl and p-tolyl substituents led to an almost equimolar ratio of 4g/4g′ in an excellent 80% combined yield. Electronically different p-fluoro phenyl and p-tolyl gave indene 4h as a single regioisomer in 71% yield. Interestingly, when n-butyl was used as one of the R5/R6 substituents, the reaction still proceeded efficiently and gave indene 4i in a very good yield of 72%. Notably, the possible highly conjugated elimination product 4i′ was not observed in the reaction, which indicates the selective nature of the reaction for iso-Nazarov cyclization over facile elimination. The position of the double bond in the indene ring was confirmed through 1H–15N HMBC correlation in indene 4a, which showed strong three-bond correlation of the oxime nitrogen with the methine proton (Scheme 3, top right and Fig. S9, ESI†).
 | ||
| Scheme 3 Scope of the formation of indenes 4 through iso-Nazarov cyclization. Reaction conditions: 1 (0.08 mmol, 1 equiv.), BiCl3 (0.016 mmol, 0.2 equiv.), 1,2-DCE (2 mL), rt. | ||
N–O bond cleavage for the synthesis of NH-pyrroles
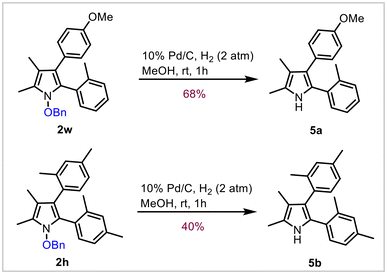
In order to demonstrate the suitability of this methodology to obtain NH-pyrroles, we carried out N–O bond hydrogenation in pyrroles 2w and 2h under Pd/C conditions to obtain the corresponding NH-pyrroles 5a and 5b in 68% and 40% yields, respectively (Scheme 4). This further expands the scope of the reaction to obtain N-unsubstituted pyrroles, which are amenable for further functionalization.Crystal structures of the pyrroles
The structures of the product pyrroles were confirmed through single crystal X-ray structural analysis of compounds 2w (left) and 2x (right) (Fig. 2 and S1, 2†).Mechanistic experiments
Impact of the double-bond geometry
In order to gain insights into the possible mechanism of the reaction, we conducted a range of relevant experiments. The efficiency of 4π-electrocyclization reactions is known to be impacted by the shape or conformation of the reactive intermediate and the ensuing transition states.13a,20 In order to gauge any such potential impact of the C3–C4 double bond geometry on the efficiency of this reaction, pure E- and Z-diastereomers were reacted separately under the reaction conditions. The Z-isomer 1n reacted more efficiently than the E-isomer 1m and gave pyrrole 2i in 57% versus 51% yield, respectively, which hints at the electrocyclization nature of the pyrrole formation (Scheme 5 and Fig. S3–5†).Potential role of hidden Brǿnsted acid catalysis
The role of the so-called ‘hidden Brǿnsted acid catalysis’ in the case of Lewis acid catalysed reactions is well documented.21 Hence, it is important to delineate the possible mechanistic pathways as well as the catalytic species in such reactions. In the light of such literature precedence, we investigated this transformation through a series of experiments under suitable conditions with alcohol 1ac as the substrate to obtain pyrrole 2w. Initially, the reaction was carried out using varying amounts of BiCl3 in normal DCE under ambient conditions to gauge the efficiency and the rate of the product formation. The reaction was found to be equally effective under 20 mol% and 2 mol% catalyst loading (Table 2, entries 1 and 2). However, the rate of the reaction decreased significantly from 20 min with 20 mol% to 5 h with 2 mol%. 1 mol% catalyst loading led to a highly inefficient reaction with incomplete conversion and a poor yield of 12% (entry 3). In order to study the impact of any hidden Brǿnsted acid, the reaction was carried out under dry conditions (dry DCE and N2 atmosphere), which didn't result in any significant difference in the yield of the reaction (72%). However, it did prolong the reaction time to 2 h from 20 min (entry 4 vs. 1), possibly due to the slow generation of HCl during the cyclization under these conditions. In another experiment, water was used as a co-solvent with DCE (10% v/v) which resulted in a significantly reduced yield of only 37% after a substantially prolonged reaction time of 50 h (entry 5). This clearly indicates the inhibitory effect of excess water on the reaction. In order to further support this observation, we carried out the reaction in the presence of 1N HCl instead of BiCl3, which gave the product in a comparable 42% yield, thereby, confirming the earlier observation (entry 6). Based on these experiments, it may be concluded that the reaction is catalyzed either by BiCl3 alone or cooperatively with a hidden Brǿnsted acid resulting from the trace amount of moisture in the solvent.Hammett relationships
Hammett equations have been traditionally employed to gain mechanistic insights into organic reactions especially with respect to the effect of substituents. Since we presumed the generation of cationic intermediates in the reaction, analysing from the perspective of Hammett relationships seemed pertinent. We used the regioisomeric ratios of the pyrroles (2j/2j′, 2k/2k′, 2l/2l′, 2m/2m′) obtained in the case of non-identical aryl substituents (R5, R6) on the terminal carbon of the 1-azapentadienyl system to plot the reaction rates against substituent constants σpara and σ+ to gain insights into the nature of the 1,2-shift (Fig. 3A and B). In both cases, a linear relationship was observed with the reaction rate. The linear plot of the rate constant against σpara gives a value of −2.049 for the reaction constant ρ for this reaction. ρ being a non-zero value suggests that the reaction is sensitive to the nature of substituents and it being a negative value indicates a build-up of positive charges or cationic intermediates during the course of the reaction.Proposed mechanism
Based on the mechanistic insights gained above and literature precedence,13,17,18 the following plausible mechanism may be proposed for the synthesis of the primary products pyrrole 2 and indene 4 as well as the secondary product pyrrole 3 (Scheme 6). The Bi(III) catalyst and/or the hidden Brǿnsted acid mediated dehydration to generate the aza-pentadienyl cation A is the common first step in the formation of all three products. The reaction shows divergence in product formation depending primarily on the nature of the R3 substituent which gets eliminated in the last step of the domino sequence and enables aromatization in pyrrole 2. Hence, when R3 is a hydrogen, the cation A generated likely in the ‘W’-conformation13a (W-A) has to undergo two C–C bond rotations – along C2–C3 and C3–C4 – to attain the U-conformation (U-A), which is required for electrocyclization. Based on DFT calculations,13a the C–C bond rotations have been shown to be energy-intensive steps in these systems which is also reflected in this reaction through the difference in the efficiency of product formation from E- and Z-isomers of the substrate (Scheme 5). Attainment of the U-conformation leads to the formation of the pyrrolium cation intermediate B through 4π-electrocyclization. The 1,2-aryl shift in B through the cationic transition state TSB–C leads to the formation of the 3H-pyrrolium cation C, which undergoes aromatization through the loss of the 3H-proton to form pyrrole 2 as the final product. When both R2,3 are hydrogens, penta-substituted pyrrole 3 is obtained as the product in the presence of a catalytic amount of the catalyst. Under catalytic conditions only a limited amount of the aza-pentadienyl cation A is generated at any time, which leads to a build-up of pyrrole 2′ in the presence of cation A. This results in a facile electrophilic aromatic substitution reaction between 2′ and A, thus, giving per-substituted pyrrole 3 as the final product. The simultaneous and exclusive formation of 3 under these conditions indicates a comparable or faster rate of electrophilic aromatic substitution compared to the formation of pyrrole 2.The substrates with a non-hydrogen R3 substituent such as methyl, on account of their inability to undergo aromatization to form a pyrrole, undergo the competing iso-Nazarov pathway through the cationic intermediate D. D possibly undergoes cyclization through the TSD–E to form E, which undergoes re-aromatization of the aryl ring through the loss of a proton to form indene 4 as the final product. This reaction pathway is well-precedented for the synthesis of indenes.11 However, the reaction may also proceed through a simple electrophilic aromatic substitution to give the same product.
Conclusion
In conclusion, we have developed a mechanistically novel synthesis of highly substituted pyrroles through a domino sequence of aza-Nazarov cyclization followed by a 1,2-Wagner Meerwein shift of the aryl groups. The substrate demonstrated substituent-dependent divergence in product formation to give indenes through iso-Nazarov cyclization, besides pyrroles. The highly reactive nature of the 1-azapentadienyl cation intermediate necessitated using Bi(III) as a mild catalyst under ambient conditions for the successful synthesis of pyrroles as well as indenes. The reactions were found to generally work better with electron-rich substituents on the aryl ring. The methodology was found to possess wide scope and efficient scalability. Mechanistic investigations indicate the electrocyclization nature of the reaction involving a cationic intermediate generated cooperatively under Lewis and Hidden Brǿnsted acid catalysis. Hopefully, the structural as well as mechanistic determinants of the domino sequence presented in this manuscript will spur further explorations in this domain along the lines of explorations for indole synthesis.Experimental section
General procedure for the synthesis of pyrroles 2
To a clean and dried vial containing a stir bar were added BiCl3 (0.02–1.2 equiv.) and alcohol 1 (1.0 equiv.). The mixture was dissolved in 2 mL of DCE. The reaction mixture was stirred at room temperature and the progress of the reaction was monitored using TLC (EtOAc/hexane 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) under UV exposure. After completion, as demonstrated by the full consumption of alcohol 1 on TLC, the reaction mixture was quenched with saturated aq. NaHCO3, and the layers were separated. The aqueous layer was washed with DCM (3 × 10 mL) and the combined organic layers were dried over anhydrous Na2SO4. The solvent was removed in vacuo to obtain the crude mixture. The pure product pyrrole 2 was obtained by silica gel column chromatography using 0–2% diethyl ether in hexane as the mobile phase (eluent).
10) under UV exposure. After completion, as demonstrated by the full consumption of alcohol 1 on TLC, the reaction mixture was quenched with saturated aq. NaHCO3, and the layers were separated. The aqueous layer was washed with DCM (3 × 10 mL) and the combined organic layers were dried over anhydrous Na2SO4. The solvent was removed in vacuo to obtain the crude mixture. The pure product pyrrole 2 was obtained by silica gel column chromatography using 0–2% diethyl ether in hexane as the mobile phase (eluent).
General procedure for the synthesis of indenes 4
To a clean and dried vial containing a stir bar were added BiCl3 (0.02 mmol, 0.2 equiv.) and alcohol 1aj (0.11 mmol, 1.0 equiv.). The mixture was dissolved in 2 mL of DCE. The reaction mixture was stirred at room temperature and the progress of the reaction was monitored using TLC (EtOAc/hexane 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) under UV exposure. After completion, as demonstrated by the full consumption of alcohol 1aj on TLC, the reaction mixture was quenched with saturated aq. NaHCO3, and the layers were separated. The aqueous layer was washed with DCM (3 × 10 mL) and the combined organic layer was dried over anhydrous Na2SO4. The solvent was removed in vacuo to obtain the crude mixture. The pure product 4a was obtained by column chromatography using 0–2% diethyl ether in hexane as the mobile phase (eluent).
10) under UV exposure. After completion, as demonstrated by the full consumption of alcohol 1aj on TLC, the reaction mixture was quenched with saturated aq. NaHCO3, and the layers were separated. The aqueous layer was washed with DCM (3 × 10 mL) and the combined organic layer was dried over anhydrous Na2SO4. The solvent was removed in vacuo to obtain the crude mixture. The pure product 4a was obtained by column chromatography using 0–2% diethyl ether in hexane as the mobile phase (eluent).
Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for compounds 2w and 2x have been deposited at the CCDC under accession numbers 2413039 and 2413038, respectively.†Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
R. N. would like to acknowledge the support from DST-SERB through a grant (SRG/2019/000728). D. D. G. would like to thank the CSIR for a fellowship and Prof. Santosh G. Tilve, Madhuri Gaikwad (School of Chemical Sciences, Goa University) and Dr Rushil Fernandes (Syngenta Biosiences Ltd, Goa) for help in performing some reactions. S. G. and K. M. would like to acknowledge the fellowship support from IIT Goa. All the authors acknowledge the infrastructural support provided by IIT Goa.References
- A. Domagala, T. Jarosz and M. Lapkowski, Living on pyrrolic foundations – Advances in natural and artificial bioactive pyrrole derivatives, Eur. J. Med. Chem., 2015, 100, 176–187 CrossRef CAS.
- (a) V. Bhardwaj, D. Gumber, V. Abbot, S. Dhimana and P. Sharma, Pyrrole: a resourceful small molecule in key medicinal hetero-aromatics, RSC Adv., 2015, 5, 15233–15266 RSC; (b) B. H. Ganesh, A. G. Raj, B. Aruchamy, P. Nanjan, C. Drago and P. Ramani, Pyrrole: A Decisive Scaffold for the Development of Therapeutic Agents and Structure-Activity Relationship, ChemMedChem, 2023, 19, e202300447 CrossRef PubMed.
- (a) W. J. Olivier, J. A. Smith and A. C. Bissember, Synthesis of Pyrrolidine- and γ-Lactam-Containing Natural Products and Related Compounds from Pyrrole Scaffolds, Chem. Rec., 2021, 22, e202100277 CrossRef PubMed; (b) T. J. Donohoe and R. E. Thomas, Partial reduction of pyrroles: application to natural product synthesis, Chem. Rec., 2007, 7, 180–190 CrossRef CAS PubMed.
- C. Bulumulla, R. Gunawardhana, P. L. Gamage, J. T. Miller, R. N. Kularatne, M. C. Biewer and M. C. Stefan, Pyrrole-Containing Semiconducting Materials: Synthesis and Applications in Organic Photovoltaics and Organic Field-Effect Transistors, ACS Appl. Mater. Interfaces, 2020, 12, 32209–32232 CrossRef CAS.
- (a) G. Majetich and J. M. Shimkus, The Taiwaniaquinoids: A Review, J. Nat. Prod., 2010, 73, 284–298 CrossRef CAS; (b) J. L. Wood, B. G. Pujanauski and R. Sarpong, Synthesis of the Tetracyclic Core of the Neomangicols Using a Late-Stage Indene Alkylation, Org. Lett., 2009, 11, 3128–3131 CrossRef CAS PubMed.
- (a) C. A. Herdman, T. E. Strecker, R. P. Tanpure, Z. Chen, A. Winters, J. Gerberich, L. Liu, E. Hamel, R. P. Mason, D. J. Chaplin, M. L. Trawick and K. G. Pinney, Synthesis and Biological Evaluation of Benzocyclooctene-based and Indene-based Anticancer Agents that Function as Inhibitors of Tubulin Polymerization, Med. Chem. Commun., 2016, 7, 2418–2427 RSC; (b) A. K. Kahlon, A. S. Negi, R. Kumari, K. K. Srivastava, S. Kumar, M. P. Darokar and A. Sharma, Identification of 1-chloro-2-formyl indenes and tetralenes as novel antistaphylococcal agents exhibiting sortase A inhibition, Appl. Microbiol. Biotechnol., 2014, 98, 2041–2051 CrossRef CAS; (c) W. J. Moree, B. F. Li, K. S. Zamani, J. Yu, T. Coon, C. Huang, D. Marinkovic, F. C. Tucci, S. Malany, M. J. Bradbury, L. M. Hernandez, J. Wen, H. Wang, S. R. Hoare, R. E. Petroski, K. Jalali, C. Yang, A. Sacaan, A. Madan, P. D. Crowe and G. Beaton, Identification of a novel selective H1-antihistamine with optimized pharmacokinetic properties for clinical evaluation in the treatment of insomnia, Bioorg. Med. Chem. Lett., 2010, 20, 5874–5878 CrossRef CAS PubMed; (d) S. Tu, L.-H. Xu, L.-Y. Ye, X. Wang, Y. Sha and Z.-Y. Xiao, Synthesis and Fungicidal Activities of Novel Indene-Substituted Oxime Ether Strobilurins, J. Agric. Food Chem., 2008, 56, 5247–5253 CrossRef CAS.
- S. C. Philkhana, F. O. Badmus, I. C. Dos Reis and R. Kartika, Recent Advancements in Pyrrole Synthesis, Synthesis, 2021, 1531–1555 CrossRef CAS.
- A. Rinaldi, D. Scarpi and E. G. Occhiato, Recent Advances in the Synthesis of Indenes, Eur. J. Org. Chem., 2019, 7401–7419 CrossRef CAS.
- (a) M. G. Vinogradov, O. V. Turova and S. G. Zlotin, Nazarov reaction: current trends and recent advances in the synthesis of natural compounds and their analogs, Org. Biomol. Chem., 2017, 15, 8245–8269 RSC; (b) D. R. Wenz and J. R. de Alaniz, The Nazarov Cyclization: A Valuable Method to Synthesize Fully Substituted Carbon Stereocenters, Eur. J. Org. Chem., 2015, 23–37 CrossRef CAS.
- M. J. Di Grandi, Nazarov-like cyclization reactions, Org. Biomol. Chem., 2014, 12, 5331–5345 RSC.
- M. J. Riveira, L. A. Marsili and M. P. Mischne, The iso-Nazarov reaction, Org. Biomol. Chem., 2017, 15, 9255–9274 RSC.
- A. V. Yadykov and V. Z. Shirinian, Recent Advances in the Interrupted Nazarov Reaction, Adv. Synth. Catal., 2020, 362, 702–723 CrossRef CAS.
- (a) R. Narayan, R. Fröhlich and E.-U. Würthwein, Synthesis of Pyrroles through a 4π-Electrocyclic Ring-Closure Reaction of 1-Azapentadienyl Cations, J. Org. Chem., 2012, 77, 1868–1879 CrossRef CAS; (b) R. Narayan, C.-G. Daniliuc and E.-U. Würthwein, Preparation of NH-Pyrroles under Super electrophilic Conditions by an Aza-Nazarov Reaction Cascade with Indole as Neutral Leaving Group: Experiment and Theory, Eur. J. Org. Chem., 2012, 6021–6032 CrossRef CAS; (c) D. Allegue, J. Santamaría and A. Ballesteros, Gold(I)-Catalyzed Indole Synthesis through Aza-Nazarov-Type Cyclization of α-Imino Gold Carbene Complexes and Arenes, Adv. Synth. Catal., 2021, 363, 5272–5278 CrossRef CAS; (d) S. E. Donmez, E. Soydaş, G. Aydın, O. Şahin, U. Bozkaya and Y. E. Türkmen, Aza-Nazarov Cyclization Reactions via Anion Exchange Catalysis, Org. Lett., 2019, 21, 554–558 CrossRef CAS PubMed; (e) B. B. Yagci, S. E. Donmez, O. Şahin and Y. E. Türkmen, Catalytic aza-Nazarov cyclization reactions to access α-methylene-γ-lactam heterocycles, Beilstein J. Org. Chem., 2023, 19, 66–77 CrossRef CAS PubMed; (f) J. Dieker, R. Fröhlich and E.-U. Würthwein, Substituted 3-Hydroxypyrroles from 1-Azapenta-1,4-dien-3-ones:The Aza-Nazarov Reaction – Synthesis and Quantum Chemical Calculations, Eur. J. Org. Chem., 2006, 5339–5356 CrossRef CAS; (g) N. Ghavtadze, R. Fröhlich and E.-U. Würthwein, 2H-Pyrrole Derivatives from an Aza-Nazarov Reaction Cascade Involving Indole as the Neutral Leaving Group, Eur. J. Org. Chem., 2008, 3656–3667 CrossRef CAS.
- A. J. Frontier and J. J. Hernandez, New Twists in Nazarov Cyclization Chemistry, Acc. Chem. Res., 2020, 53, 1822–1832 CrossRef CAS PubMed.
- (a) J. Huang, D. Lebœuf and A. J. Frontier, Understanding the Fate of the Oxyallyl Cation following Nazarov Electrocyclization: Sequential Wagner-Meerwein Migrations and the Synthesis of Spirocyclic Cyclopentenones, J. Am. Chem. Soc., 2011, 133, 6307–6317 CrossRef CAS; (b) D. Lebœuf, J. Huang, V. Gandon and A. J. Frontier, Using Nazarov Electrocyclization to Stage Chemoselective [1,2]-Migrations: Stereoselective Synthesis of Functionalized Cyclopentenones, Angew. Chem., Int. Ed., 2011, 50, 10981–10985 CrossRef PubMed; (c) D. Lebœuf, J. Ciesielski, V. Gandon and A. J. Frontier, Experimental and Theoretical Studies on the Nazarov Cyclization/Wagner–Meerwein Rearrangement Sequence, J. Am. Chem. Soc., 2012, 134, 6296–6308 CrossRef.
- (a) R. S. Gairns, C. J. Moody and C. W. Rees, Rapid migration of sulphur groups in the photochemical conversion of 3-azido-2-vinylthiophenes into thienopyrroles, J. Chem. Soc., Chem. Commun., 1985, 1818–1819 RSC; (b) R. S. Gairns, C. J. Moody, C. W. Rees and S. C. Tsoi, Photochemical rearrangement of fused 1λ4,2-thiazines (2-azathiabenzenes); rapid migration of methylthio and phenylthio groups, J. Chem. Soc., Perkin Trans. 1, 1986, 497–498 RSC; (c) Z. Shi, Y. Ren, B. Li, S. Lu and W. Zhang, CuI-catalyzed photochemical or thermal reactions of 3-(2-azidobenzylidene) lactams. Application to the synthesis of fused indoles, Chem. Commun., 2010, 46, 3973–3975 RSC.
- (a) K. Sun, S. Liu, P. M. Bec and T. G. Driver, Rhodium-Catalyzed Synthesis of 2,3-Disubstituted Indoles from β,β-Disubstituted Stryryl Azides, Angew. Chem., Int. Ed., 2011, 50, 1702–1706 CrossRef CAS; (b) B. J. Stokes, S. Liu and T. G. Driver, Rh2(II)-Catalyzed Nitro-Group Migration Reactions: Selective Synthesis of 3-Nitroindoles from β-Nitro Styryl Azides, J. Am. Chem. Soc., 2011, 133, 4702–4705 CrossRef CAS PubMed; (c) C. Kong, N. Jana and T. G. Driver, Rh2(II)-Catalyzed Selective Aminomethylene Migration from Styryl Azides, Org. Lett., 2013, 15, 824–827 CrossRef CAS; (d) B. J. Stokes and T. G. Driver, Transition Metal-Catalyzed Formation of N-Heterocycles via Aryl- or Vinyl C–H Bond Amination, Eur. J. Org. Chem., 2011, 4071–4088 CrossRef CAS; (e) N. Jana and T. G. Driver, Assembly of functionalized carbocycles or N-heterocycles through a domino electrocyclization–[1,2] migration reaction sequence, Org. Biomol. Chem., 2015, 13, 9720–9741 RSC.
- H. Dong, M. Shen, J. E. Redford, B. J. Stokes, A. L. Pumphrey and T. G. Driver, Transition Metal-Catalyzed Synthesis of Pyrroles from Dienyl Azides, Org. Lett., 2007, 9, 5191–5194 CrossRef CAS.
- E. Lopez, S. C. Thorp and R. S. Mohan, Bismuth(III) compounds as catalysts in organic synthesis: A mini review, Polyhedron, 2022, 222, 115765 CrossRef CAS.
- C. J. Hastings, R. G. Bergman and K. N. Raymond, Origins of Large Rate Enhancements in the Nazarov Cyclization Catalyzed by Supramolecular Encapsulation, Chem. – Eur. J., 2014, 20, 3966–3973 CrossRef CAS PubMed.
- (a) T. T. Dang, F. Boeck and L. Hintermann, Hidden Brønsted Acid Catalysis: Pathways of Accidental or Deliberate Generation of Triflic Acid from Metal Triflates, J. Org. Chem., 2011, 76, 9353–9361 CrossRef CAS PubMed; (b) R. K. Schmidt, K. Muther, C. Mück-Lichtenfeld, S. Grimme and M. Oestreich, Silylium Ion-Catalyzed Challenging Diels–Alder Reactions: The Danger of Hidden Proton Catalysis with Strong Lewis Acids, J. Am. Chem. Soc., 2012, 134, 4421–4428 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2413039 and 2413038. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5qo00168d |
| This journal is © the Partner Organisations 2025 |