Dynamic supramolecular nanosheet structures formed by aromatic amphiphiles and their functions
Yanqiu Wang
 ab and
Myongsoo Lee
ab and
Myongsoo Lee
 *b
*b
aEngineering Research Center of Optoelectronic Functional Materials, Ministry of Education, School of Materials Science and Engineering, Changchun University of Science and Technology, Changchun, 130022, China
bDepartment of Chemistry, Fudan University, Shanghai, 200438, China. E-mail: mslee@fudan.edu.cn
First published on 28th May 2025
Abstract
Supramolecular nanostructures based on the self-assembly of aromatic amphiphiles have received considerable attention because these structures based on non-covalent interactions can be dynamic, leading to switchable nanomaterials in response to external stimuli. The self-assembled materials combine the simplicity of small molecules with the versatility of self-assembly, with a wide range of applications proposed in biomedicine, nanotechnology, etc. Among the diverse self-assembled nanostructures, two-dimensional (2D) nanosheet structures are promising objects in the materials science field due to their ultrathin, large surface areas. Much research effort has been devoted to the study of 2D nanosheet structures based on diverse shapes and conformations of the aromatic segments. This review describes recent progress in the development of nanosheet structures through the self-assembly of rationally designed π-conjugated aromatic amphiphile building blocks. Potential applications, such as chiral separation and biological application, are also discussed. Various views on 2D nanosheet structures have been proposed in the literature, and in this respect, we have attempted to provide a systematic account based on our research progress in recent years. We hope that this will provide a useful reference for 2D nanosheet structures formed by the rational design of aromatic amphiphile self-assembly. We also anticipate that this strategy will provide an opportunity for broadening the application potential of 2D nanosheet structures.
Yanqiu Wang received her BS degree from Jilin Normal University in 2012 and PhD degree in chemistry from Jilin University in 2017. After completion of her PhD, she joined as an Assistant Professor at the College of Materials Science and Engineering at Changchun University of Science and Technology, Changchun, China. Her research interests are supramolecular assembly and functionalized supramolecular structures. The focus of her current research is the development of supramolecular chiral separation materials. |
Myongsoo Lee is a Professor of Chemistry at Fudan University. He received his Ph.D. degree from the Case Western Reserve University in 1992 in liquid crystal polymerization. After postdoctoral research work at the University of Illinois at Urbana, he was made a professor at Yonsei University in 1994, Seoul National University in 2009, Jilin University in 2013, and Fudan University in 2019, where he continues to work on self-assembling systems and dynamic supramolecular nanomaterials. |
1. Introduction
Two-dimensional (2D) sheet-like nanostructures are promising objects in materials science and have been widely utilized in many fields including electronics, optoelectronics, catalysts, energy storage, energy generation, sensors, separation, and biomedicines owing to their unique physical and chemical properties, such as high anisotropy, large specific surface area, and high surface energy.1–4 For instance, anisotropy provides unique structural design strategies for flexible electronics and high-efficiency catalytic systems; a large specific surface area and a layered architecture synergistically reduce charge transfer resistance and enhance ion diffusion kinetics. Over the past decades, extensive research efforts of 2D sheet-like nanostructures has been widely reported, such as sheet stacking films5 and layer-by-layer (LBL) assembly.6 Considering the unique structure of two-dimensional nanosheets, molecular self-assembly systems of amphiphilic molecules through weak noncovalent interactions including hydrogen bonding, electrostatic interactions, and charge transfer interactions are the most progressive candidates among various well-ordered nanostructures, including fibers, ribbons, tubes, cylindrical micelles, vesicles, sheets, and toroids.7–13Much research effort has been devoted to the study of supramolecular self-assembly structures based on various building blocks.14–19 Among diverse nanostructures, self-assembled nanosheets based on aromatic building blocks have attracted considerable attention because their interactions are robust, which makes it possible to control the size and shape of the self-assembled structures through environmental conditions.20–23 It is remarkable that an individual layered sheet structure has high potential to drive 3D stacking owing to the huge surface energy. Compared to 3D materials, 2D nanosheet structures have extremely high surface areas and many potential applications. Therefore, creating a single layered 2D nanosheet structure through supramolecular self-assembly is highly valuable. Several research groups have designed and constructed self-assembled single layered nanosheets. For example, Fernández and co-workers exploited halogen bonding (XB) as a reversible network element to regulate the photoresponsive and adaptive behavior of supramolecular 2D sheet structures.24 Liu and co-workers reported a self-assembly strategy to fabricate 2D nanosheets using imidazolium functionalized pillar[6]arene (P6) as the cationic bridging monomer and phosphotungstic acid.25,26 Tamiaki and co-workers also greatly advanced the study of self-assembled sheet structures by developing artificial chromosomal supramolecular nanosheets prepared by the self-assembly of a synthetic zinc 31-methoxy-chlorophyll derivative having amide and urea groups as the substituents at the 17-position.27 These successful examples greatly broadened the application areas of self-assembled nanosheet structures.
Typical examples of basic building blocks for self-assembly including lipid molecules,28,29 surfactants,30–33 aromatic amphiphiles,34,35 block polymers,36–38 DNA-π-conjugated amphiphiles,39–41 and peptide derivatives42–46 have been reported. Among them, amphiphile molecules, containing both hydrophobic and hydrophilic segments and the intrinsic potential of self-assembly in aqueous environments, are ideally suitable for fabricating responsive self-assembled nanostructures because of their dynamic and reversible conformational changes, which are easily triggered by different external stimuli, such as guest molecules,47–51 pH,52,53 ions,54–56 light,57,58 and temperature.59,60 In this review, we describe the recent progress in the development of 2D nanosheet structures through the self-assembly of rationally designed π-conjugated aromatic amphiphile building blocks. The nanosheet structures originate from either the lateral association of fibers or tubules, monolayer packing of aromatic amphiphiles, or transformation from scrolls. Herein, we have classified 2D nanosheet structures based on the shapes of the hydrophobic aromatic building blocks and associated them with their self-assembly behavior in aqueous solutions. Our classification will provide a better understanding of self-assembly behavior, which is key to constructing supramolecular 2D nanosheet structures. We have shown that the combination of hydrophilic chains into aromatic segments generates dynamic 2D nanosheet structures and how these self-assembled nanosheet structures exhibit switchable motion in response to external stimuli, such as temperature, guests, and pH, towards the aim of constructing smart materials. We highlight some potential applications of supramolecular nanosheet structures, such as chiral separation and biological applications.
2. Nanosheet structures based on the self-assembly of macrocycle amphiphiles
Among various self-assembly building blocks, macrocycles, ring-like molecular entities, have received great attention owing to their well-defined intrinsic pore structures which are useful for molecular recognition, sensing, catalysis, etc.,61,62 as well as owing to many emergent functional properties which could not be achieved from linear molecules. Macrocycle amphiphiles consisting of hydrophobic macrocyclic aromatic segments and hydrophilic oligoether dendrons grafted on both sides have been extensively studied because of their shape persistence. Therefore, geometric macrocycle isomers based on anthracene units were rationally designed and synthesized (Fig. 1a). HPLC was used to separate the isomers (Fig. 1b), and the self-assembled nanostructures in aqueous solution were investigated.63 The self-assembly of the cis-isomer, 1, generated static planar sheets with in-plane parallel arrangements of the primary nanofibers (Fig. 1c). The formation of flat nanostructures was also confirmed using cryogenic TEM (Cryo-TEM), which showed sheet-like objects with straight edges indicating that the sheets are robust and free-standing in bulk solution. Additional structural information on the sheets was obtained by fluorescence optical microscopy (FOM) and atomic force microscopy (AFM) measurements on a hydrophilic mica substrate in the completely dried state. The AFM image of 1 revealed planar sheets with a thickness of ∼3.2 nm, which is consistent with the expected thickness of single layers. Circular dichroism (CD) spectroscopy measurements revealed apparent CD signals in the spectral range of the aromatic segment, indicating that the in-plane nanofibers of the 2D sheets are based on a helical structure with a chiral bias (Fig. 1d).64 To understand the chiral primary structure of the 2D assembly, we performed molecular dynamics simulations using Desmond from Schrodinger Suites (Fig. 1e). The simulations of 1 showed that the aromatic macrocycle adopts a concave configuration with the upward positioning of the two oligoether dendrons where the anthracene units at both ends of the concave macrocycle are twisted with respect to each other, generating intrinsic molecular chirality.65 | ||
| Fig. 1 (a) Molecular structures of cis-1 and trans-2 macrocycle isomers. (b) HPLC traces of 1 and 2 after separation. (c) Negatively stained TEM image of 1 from 0.01 wt% aqueous solution. Scale bar: 100 nm. The inset shows a Cryo-TEM image of 1 from 0.01 wt% aqueous solution. Scale bar: 200 nm. (d) Time-dependent CD spectra of 1 from 0.01 wt% aqueous solution. (e) Molecular dynamics simulation results of 1 in a water environment. Dendrimers are omitted for clarity. Time-dependent TEM images of 2 from 0.01 wt% aqueous solution after aging for (f) 30 min and (g) 3 h. Scale bars: 50 nm. The inset image shows the top view of the tubular scroll. Inset scale bar: 30 nm. (h) A representation of dynamic rolled sheets. Reproduced with permission from ref. 63. Copyright 2016 Wiley-VCH. | ||
However, the self-assembly of the trans-isomer 2 generates dynamic rolled sheets that are reversibly unrolled in response to a thermal signal. To investigate dynamic motion, time-dependent transmission electron microscopy (TEM) experiments were performed. Time-dependent TEM revealed that the trans-isomer 2 self-assembles into flexible nanofibers at the initial stages of self-assembly (10 min), which were arranged parallel to each other to form flat sheets after 30 min (Fig. 1f). Subsequently, the sheets spontaneously roll-up perpendicular to the fiber long axis into closely-packed scrolls with an external diameter of approximately 40 nm (Fig. 1g), and the inset image of Fig. 1g indicates that the scrolls consist of multilayer walls. CD spectroscopy measurements revealed that the signals appeared after 30 min of aging under ambient conditions and became more apparent with increasing aging time up to 3 h. These results, together with the time-dependent TEM data, suggest that the adjacent aromatic macrocycles are twisted with respect to each other in one direction, driving the planar sheets to fold perpendicular to the fiber axis into closely packed scrolls. Interestingly, the scrolls undergo reversible unfolding at 40 °C at which the oligoether chains underwent dehydration.66–69 Upon heating to 37 °C, the closely-packed scrolls gradually loosened and subsequently transformed into open sheets (Fig. 1h). In contrast to the dynamic rolled sheet structures of 2, the planar structures were maintained even at higher temperatures without any noticeable structural changes, indicating that the 2D structures were static within the temperature range of our investigations.
The above results prompted us to investigate the switchable self-assembly behavior of planar aromatic macrocycle amphiphiles. The planar aromatic macrocycle amphiphile isomers 3 and 4 were further constructed.70 The macrocycle amphiphiles self-assemble into stable, single-layered 2D sheet structures through lateral association of primary fibrils in aqueous solution (Fig. 2a). The TEM image in Fig. 2b shows the formation of flat 2D sheet structures with uniform sheet thickness (Fig. 2b). Closer examination of the images showed that the sheet consisted of longitudinal stripes with a regular spacing of ∼3.5 nm, demonstrating that the sheet assembly originated from the lateral association of preformed fibrils (Fig. 2b, inset). The formation of the planar sheet nanostructures was further confirmed using Cryo-TEM and AFM. The formation of single layered 2D sheets covered by oligoether surfaces suggests that the aromatic sheets would have thermo-responsive characteristics in aqueous solution due to the thermal dehydration behavior of the oligoether chains.65,66,71 TEM experiments were performed on the aqueous solution of cis-3 at 40 °C. In contrast to the image taken at room temperature, the image revealed thin fibrils with a uniform diameter of 4 nm (Fig. 2c), which was further confirmed by AFM measurements. This result demonstrates that the sheets can split into uniform nanofibers in response to heating. The splitting of the 2D sheets upon heating was attributed to the thermal dehydration of the dendritic ethylene oxide chains in aqueous media. Upon cooling to room temperature and then resting for 24 h, complete recovery of the original sheets was observed, indicating that the sheets underwent reversible splitting between the sheet and nanofiber states (Fig. 2a). The thermal dynamic behavior of trans-4 was similar to that of cis-3. In short, we successfully constructed thermally dynamic 2D nanosheet structures by self-assembly of geometric macrocycle amphiphile isomers.
 | ||
| Fig. 2 (a) Molecular structures and schematic representation of the formation of a 2D sheet that undergoes reversible break-up into nanofibers. (b) Negatively stained TEM image of 2D sheets from 0.01 wt% aqueous solution of cis-3. The inset shows a magnified image, scale bar: 20 nm. (c) Negatively stained TEM image of cis-3 from 0.01 wt% aqueous solution at 40 °C. Reproduced with permission from ref. 70. Copyright 2021 Royal Society of Chemistry. | ||
3. Nanosheet structures based on self-assembly of rod amphiphiles
Rod amphiphiles consisting of rigid aromatic rods as the hydrophobic part and flexible dendron chains as the hydrophilic part exhibit typical amphiphilic characteristic and are promising candidates for fabricating self-assembled structures. The rigid nature and strong π–π stacking interactions of the conjugated rod amphiphiles make them favorable for stacking leading to the formation of sheet structures. In this section, we introduce several rod-shaped amphiphilic building blocks that exhibit 2D supramolecular nanosheet structures in solution.Supramolecular chirality can be induced by chiral transfer from chiral moieties to self-assembled nanostructures.72 Therefore, chiral dendrimers were grafted on the side faces of tetraphenylethylene (TPE) segments which have great potential for generating 2D chiral supramolecular structures due to presence of the flat rod aromatic segment.73 Dynamic light scattering (DLS) experiments were performed on tetra-substituted molecule 5 and di-substituted molecule 6 in MeOH. The DLS data of 5 and 6 showed a diameter of ∼4 nm for both molecules, which is similar to the size of the single molecules, and fluorescence shows very weak emission, indicating that both 5 and 6 exist as molecularly dissolved states in pure MeOH, considering that TPE-based molecules are non-emissive in the dissolved state but highly enhanced emission could be seen in both the aggregated form and the solid-state.74,75 To investigate the aggregation behavior of 5 and 6, salt water (including 50 mM KF) was added to MeOH solution (0.01 wt%). With increasing water content up to 40% and with 50 mM KF salt, both the diameter and fluorescence intensity increased remarkably, indicating that addition of water to the methanol solution induced molecular assembly (Fig. 3a). The aggregation behavior of 5 and 6 at a concentration of 0.01 wt% in aqueous salt solution had been investigated using TEM and Cryo-TEM, and the images of both molecules showed 2D flat sheet structures which was further confirmed by TEM (Fig. 3b). AFM experiments were performed to obtain further information on the nanosheet structures of 5 and 6. The AFM images revealed 2D sheet structures with a thickness of ∼3.8 nm and ∼4.6 nm formed by the self-assembly of 5 and 6, respectively. Considering the thickness of 2D sheets formed by molecule 5, which is in agreement with the molecular dimension of 5 (∼3.5 nm from CPK), it is suggested that the sheet structures of 5 are based on monolayer packing. Nevertheless, the molecular dimension of 6 is ∼2.4 nm from CPK, and the observed thickness implies that the sheet structures consist of interdigitated bimolecular packing. Interestingly, when CD spectroscopy experiments were performed on the sheet structures, the sheet solution of 6 revealed a strong Cotton effect, in sharp contrast to that of 5 which is CD silent (Fig. 3c). This indicates that molecule 5 generates nonchiral 2D nanosheet structures, whereas di-substituted molecule 6 generates chiral 2D nanosheet structures. The formation mechanism of the 2D sheet structures of 5 and 6 was studied under highly diluted conditions (0.002 wt%). TEM images revealed that the solution of 5 showed small sheet-like aggregates at lower concentrations, demonstrating that the 2D sheet structures of 5 were formed by a 2D array of aromatic segments with top and bottom surfaces covered by hydrophilic dendritic segments (Fig. 3a). The diluted solution of 6 showed uniform nanofiber structures with a diameter of ∼4.6 nm together with a Cotton effect in the spectral range of the aromatic segments, which was further confirmed by AFM (Fig. 3d), demonstrating that the sheet structures of 6 originated from the lateral assembly of the helical nanofibers (Fig. 3a). The results described herein demonstrate that molecule 5 self-assembles into nonchiral 2D nanosheet structures formed by a 2D array of monomer molecules with top and bottom surfaces covered by hydrophilic dendritic segments. Molecule 6 formed a chiral sheet structure originating from the lateral assembly of helical nanofibers, suggesting that the aromatic segments were stacked on top of each other with mutual rotation.
 | ||
| Fig. 3 (a) Molecular structures of tetra-substituted molecule 5 and disubstituted molecule 6, with schematic representations of their self-assembly. (b) TEM image of 6 in aqueous solution (including 50 mM KF) at 0.01 wt%, inset: Cryo-TEM image of 6 in aqueous solution (including 50 mM KF) at 0.01 wt%. (c) CD experiments of 5 and 6 in aqueous solution (including 50 mM KF) at 0.01 wt%. (d) AFM image of 6 in aqueous solution (including 50 mM KF) at 0.002 wt%. Reproduced with permission from ref. 73. Copyright 2020 Wiley-VCH. | ||
Although 2D nanosheet structures have been extensively studied, reports on supramolecular switching between tubules and sheets through reversible lateral association are rare. Recently, the unique self-assembly of a seesaw-shaped aromatic amphiphile structure (molecule 7) was reported (Fig. 4a), which formed unique supramolecular tubular bamboo-culm structures.76 The tubular structure possessed a hydrophobic cavity that could capture a hydrophobic guest (Fig. 4b). The addition of hydrophobic trans-azobenzene enabled the tubular culm to transform into a hierarchical sheet assembly via enhanced interactions between tubules (Fig. 4c). This transformation between tubules and sheets was also confirmed by TEM, which showed lateral connection of the tubular structures to form flat sheets. Interestingly, the transformation was reversible when UV irradiation was performed due to the conversion of trans-azobenzene to its cis-isomer. The chiral sheet structures were disassembled into their original tubular structures due to the shorter length of cis-azobenzene after 8 h of UV irradiation which decreased the lateral interactions. The full recovery of the sheet structures was achieved by resting in a dark environment, which demonstrated the reversible transformation between tubules and sheets triggered by UV irradiation due to the trans–cis isomerization of azobenzene. Such a unique switch motion between tubules and 2D nanosheet structures provides great potential for fabricating functionally reversible materials by photo-irradiation.
 | ||
| Fig. 4 (a) Molecular structure of 7 and the schematic of the reversible switching between a supramolecular tubular culm and a hierarchical sheet-assembly, based on a seesaw-shaped amphiphile. (b) Negatively stained TEM image of molecule 7 (53 μM) in MeOH–H2O (40/60, v/v). The inset image shows the separated hollow-tubular interior by nodes per 1.3 nm. (c) Negatively stained TEM image and Cryo-TEM image (inset) of 7 (53 μM) with two equivalents of azobenzene in MeOH–H2O (40/60, v/v) solution. Reproduced with permission from ref. 76. Copyright 2020 Wiley-VCH. | ||
Synthetic supramolecules can form sophisticated nanostructures depending on their constituent molecular units. Therefore, controlled self-assembly of synthetic aromatic amphiphiles has emerged as a pivotal tool for constructing functional nanostructures. The lateral association of aromatic rods in amphiphilic molecules leading to supramolecular 2D membrane structures has been reported.77 Herein, charged rod amphiphiles 8 and 9 were synthesized, and the fluorescence of the aggregation-induced emission (AIE) luminogen 10 was modulated based on their self-assembly (Fig. 5a).78 Rod amphiphile 8 self-assembled into flat sheet structures surrounded by negatively-charged carboxylates at neutral pH (Fig. 5b). The sheets are free standing in bulk solution with a thickness of 2.8 nm, demonstrating that the aromatic rods of 8 are packed in a monolayer arrangement in which the rods are aligned parallel to the sheet plane. Then, the aggregation behavior of cationic distyrylanthracene (DSA) derivative 10 with AIE properties was investigated in the presence of self-assembled sheets of 8 in aqueous solution at pH 7.4. Upon addition of dye 10 to the negatively-charged sheets of 8, the fluorescence intensity of 10 was significantly increased, while pure 10 in pH 7.4 did not show any noticeable fluorescence emission due to the inhibition of aggregation by electrostatic repulsion between the positive charges of N,N,N-trimethylaniline groups of 10 (Fig. 5c). These results demonstrated that the negative charges on the sheet surfaces of 8 neutralized the positive charges of 10 to induce the aggregation of 10 (Fig. 5d). Amphiphile 9 with two amino groups was synthesized to confirm the electrostatic interactions between carboxylates of 8 and the ammonium groups of 10 rather than intercalation of the aromatic segments of 10 into the self-assembled rods of 8. The self-assembly of amphiphile 9 also showed a flat 2D structure in aqueous solution, which was surrounded by positively-charged ammonium groups at pH 7.4. The emission spectrum of 10 did not increase even after the addition of 9, and the NMR spectrum of 10 with the positively-charged sheet of 9 was identical to that of 10 (Fig. 5e). These results demonstrate that the aromatic segments of 10 did not intercalate into the rod segments of the sheets. Taken together, it can be considered that the fluorescence turn-on nanodomains of the positively-charged DSA dye 10 are formed by self-assembly on the negatively-charged sheet surfaces of 8 in bulk solution by electrostatic interactions.
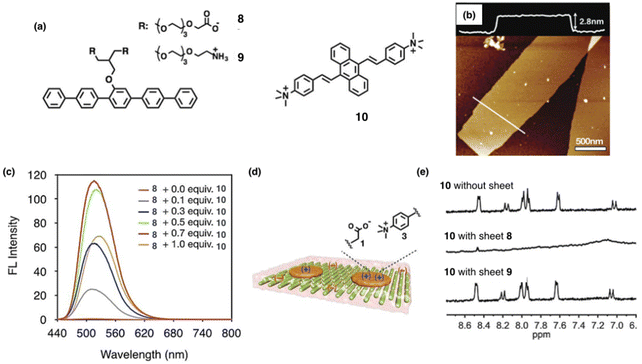 | ||
| Fig. 5 (a) Molecular structures of 8 and 9 for self-assembled sheets with charges, and cationic distyrylanthracene derivative 10 for fluorescent synthetic rafts. (b) Negatively stained TEM image of 8 (60 mM). Reproduced with permission from ref. 77. Copyright 2018 Royal Society of Chemistry. (c) Emission spectra of different equivalents of 10 with 8 (115 mM) in 10 mM phosphate buffered saline (PBS) solutions at 421 nm excitation wavelength. (d) Representation of aggregates of 10 for the synthetic rafts on the negatively-charged sheet of 8 through electrostatic interaction. (e) 1H-NMR (500 MHz) spectra of 10 without and with sheets in D2O at 25 °C. Reproduced with permission from ref. 78. Copyright 2019 Wiley-VCH. | ||
4. Nanosheet structures based on the self-assembly of pyrene amphiphiles
Pyrene, a classical fluorophore, has attracted considerable attention due to its unique optical and electronic properties. Owing to its diverse luminescence behavior, depending on the solution state, molecular packing pattern, and morphology, pyrene is often used as a fluorophore to construct various semiconductor devices. Pyrene can easily form dynamic and static excimers in solutions with long wavelength fluorescence emission, resulting in wide potential applications. In this section, we introduce several nanosheet structures that are based on the self-assembly of pyrene amphiphiles.As an extension of our efforts to create flat 2D sheet structures, pyrene-based aromatic amphiphiles have been synthesized, which can be triggered by environmental changes. We designed the pyrene as an optically-active aromatic segment to construct fluorescence-switching 2D materials.79 The molecule 11 consisting of an aromatic segment and oligoether dendrons grafted at opposite sides of the aromatic plane was synthesized (Fig. 6a), which formed single-layer planar sheet structures at neutral pH (pH = 7) as observed by TEM. Interestingly, the sheet structures based on pyrene units are sensitive to a small change in pH, showing strong excimer emission at pH 7, but quenching of the fluorescence emission at pH 5 without sacrificing its intact 2D self-assembled sheet structure (Fig. 6b and c). The dynamic switching behavior of the sheet structures was due to the changing packing mode of the pyrene aromatic segments. The hydrophobic and conjugated pyrene moieties showed close stacking through strong π–π interactions at pH 7, which caused the strong emission of the pyrene excimer. However, the pyridine segments were protonated at pH 5, which increased the repulsive interactions between adjacent pyridinium cations, loosening pyrene packing. We attributed the fluorescence quenching of the sheet solution to the loose packing of pyrene units (Fig. 6d). Such fluorescence emission enhancement and quenching were reversible between pH 7 and 5. We successfully constructed reversible fluorescence sheet structures by adjusting the intermolecular interactions of pyrene segments, which are promising candidates for use in fluorescence sensors.
 | ||
Fig. 6 (a) Molecular structure of amphiphile 11. (b) Fluorescence spectra of 11 (50 mM) in MeOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v) with pH = 5 and pH = 7. (c) TEM image of 11 (25 mM) in MeOH/H2O (3 9 v/v) with pH = 5 and pH = 7. (c) TEM image of 11 (25 mM) in MeOH/H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 v/v) with pH = 7. (d) Schematic representation of the fluorescence emission on/off switching mechanism. Reproduced with permission from ref. 79. Copyright 2025 Royal Society of Chemistry. 17 v/v) with pH = 7. (d) Schematic representation of the fluorescence emission on/off switching mechanism. Reproduced with permission from ref. 79. Copyright 2025 Royal Society of Chemistry. | ||
Supramolecular self-assembly materials formed by noncovalent interactions are switchable when triggered by external stimuli such as guest molecules, pH, solvent, and temperature. With this idea in mind, π-electron rich pyrene-based aromatic amphiphiles were synthesized to facilitate charge transfer complexation with π-electron deficient 7,7,8,8-tetracyanoquinodimethane (TCNQ), so that stimuli-responsive supramolecular materials were constructed and the self-assembled nanostructures in aqueous solution were investigated (Fig. 7a).80 Pyrene amphiphile 12 was composed of a hydrophobic pyrene segment and a hydrophilic D-mannose segment, which presented micelle structures as confirmed by TEM experiments. However, after the addition of TCNQ to the aqueous solution of amphiphile 12, a color change from colorless to yellow was observed. A characteristic broad absorption band arising from intermolecular charge transfer interactions was detected at approximately 441 nm, indicating the formation of a charge transfer interaction between π-electron rich pyrene and π-electron deficient TCNQ. The maximum amount of TCNQ loading per amphiphilic molecule was 1.0 equivalent as determined by optical microscopy. The optical microscopy image of 12 complexed with 1.0 equivalent of TCNQ revealed that isolated 2D sheet structures were formed in the bulk solution (Fig. 7b). The negative staining TEM and AFM experiments revealed that the sheets were flat and uniform, consistent with the Cryo-TEM results. The small-angle X-ray diffraction analysis performed on the films of aqueous solution provided a reflection of 4.55 nm as the d-spacing between sheets (Fig. 7c), which is consistent with the thickness (4.4 nm) obtained from the AFM. The close packing of pyrene and TCNQ was confirmed by the wide-angle X-ray scattering (WAXS) pattern which showed a strong reflection at 3.4 Å associated with the π–π stacking distance. 1H-NMR spectroscopy and density functional theory (DFT) calculations further indicated parallel stacking interactions between pyrene and TCNQ. When CD measurement was performed on the charge transfer complex solution, apparent CD signals were detected in the spectral range of the aromatic segments (Fig. 7d), which indicated that the supramolecular sheet structures derived using TCNQ were chiral due to the asymmetric stacking of the aromatic segments. Interestingly, the resulting supramolecular chiral sheets showed disassembly when TCNQ was irreversibly reduced to its anion by 2.0 equivalents of sodium sulfide (Fig. 7e). The molecular orbital energy levels of pyrene, TCNQ, and the TCNQ dianion indicate that the sheet disassembly is due to the lack of charge-transfer interactions between pyrene and the TCNQ dianion. Therefore, reduction-responsive supramolecular sheets were constructed using TCNQ successfully (Fig. 7f).
 | ||
| Fig. 7 (a) Molecular structures of amphiphile 12 and TCNQ. (b) Phase-contrast optical microscopy image of 12 (314 × 10−6 M) in an aqueous solution with one equivalent of TCNQ. (c) SAXS pattern of 12 with 1.0 equivalent of TCNQ after freeze-drying. (d) CD spectra of amphiphile 12 (314 μM) with different equivalents of TCNQ in an aqueous solution. (e) Negatively stained TEM image of 12 (314 × 10−6 M) with one equivalent of TCNQ sequentially treated with 2.0 equivalents of sodium sulfide in an aqueous solution. (f) Schematic representation of sheet assembly via charge-transfer interactions between 12 and TCNQ and sheet disassembly upon hydrogen-sulfide-triggered TCNQ reduction. Reproduced with permission from ref. 80. Copyright 2023 Wiley-VCH. | ||
Peptides, which share amino acid monomers with proteins, serve as fundamental structural frameworks in biological systems. Artificially designed β-sheet peptides have attracted considerable attention in various research areas because they are composed of biocompatible amino acids. To endow a peptide chain with the self-assembling feature, an amphiphilic peptide was synthesized by grafting the pyrene group to the peptide chain by a click reaction (Fig. 8a).81 To gain insight into the self-assembly behavior of the pyrene-based peptide 13, TEM experiments were performed under different dilution conditions. ∼3 nm sized micelle structures were obtained at 0.001 wt% and laterally-associated small fragments of flat structures were obtained at 0.003 wt% (Fig. 8b). Molecular dynamics simulations revealed that the dimer height of 13 was 3 nm, which indicated that the hydrophilic peptide helices were aligned in one direction and parallel to the flat membrane surface to form peptide bilayers with hydrophobic pyrene units (Fig. 8c). Interestingly, the flat sheet structures formed by 13 were dynamic. With an increase in the concentration of 13, the flat sheet structures transformed into vesicles, decreasing the surface energy. Therefore, 2D nanosheet structures were constructed using a pyrene-based peptide self-assembly.
 | ||
| Fig. 8 (a) Chemical structure of 13. (b) TEM images of 13 at different concentrations. (c) Energy-minimized packing structure of the dimeric association of 13. Reproduced with permission from ref. 81. Copyright 2017 Royal Society of Chemistry. | ||
5. The functions of 2D supramolecular nanosheet structures
Supramolecular systems formed by noncovalent interactions such as hydrogen bonding, electrostatic, π–π, and charge transfer interactions are reversible and dynamic. These noncovalent interactions play important roles in the formation of dynamic supramolecular structures. Among supramolecular nanostructures, 2D nanosheet structures have received great attention owing to their large surface area, which has many potential functions. For example, we have reported a chiral sheet structure consisting of parallel arrangements of helical fibers.73 Considering that the void spaces formed between the helical fiber arrangements would be chiral with hydrophobic aromatic walls,82,83 the chiral sheet structures were functional as enantioselective membranes that were able to preferentially absorb one enantiomer over the other from a racemic amino acid solution (Fig. 9a). When we increased the concentration of chiral fragments formed by molecule 13, the chiral fragment transformed into vesicles owing to the surface energy of enantioselective membranes.81 The vesicles originated from the budding of the flat membranes consisting of a planar nematic-like alignment of rod-like α-helical peptides on the external surfaces and hydrophobic pyrene inside the membranes. When racemic amino acids were added to the self-assembly solution of 13, only a single enantiomer was encapsulated through selective diffusion across the peptide membranes. The encapsulated enantiomer underwent further chemical reactions inside the vesicles upon its separation from the opposite enantiomer in the environment (Fig. 9b). This function can be used as a chiral selector serving as a nanoreactor. In addition to the above functions, the 2D sheets can also control the protein aggregation size.78 Synthetic rafts were constructed by floating 10 on the surface of the negatively-charged sheets of 8. After adding negatively-charged Con A to the rafts at neutral pH, discrete aggregates of Con A on the rafts in sheets of 8 with a uniform size were observed (Fig. 9c). The positively-charged sheets of 9 without synthetic rafts were constructed for comparison. However, the TEM experiment showed that Con A aggregates with random sizes (Fig. 9d), indicating that synthetic rafts could play an important role in the formation of discrete Con A aggregates with a uniform size. It is well known that D-mannose plays several important roles, such as binding to FimH, an adhesive subunit of type-1 fimbriae expressed in almost all bacteria. Inspired by this selective binding capability, the sheets formed by molecule 12 with D-mannose moieties on the sheet surface were considered to prevent infection by pathogenic bacteria and regulate bacterial proliferation.80 With this idea in mind, the supramolecular sheets were co-incubated with E. coli ORN 178, which expresses FimH in its wild-type pili. TEM, SEM, and fluorescence microscopy images showed agglutinated bacteria on the supramolecular sheets, which noticeably increased in size owing to the interaction between D-mannose and FimH (Fig. 9e). These results indicate that the supramolecular sheets induce agglutination of ORN 178 via specific binding between FimH and D-mannose on the sheet surface. In short, supramolecular self-assembled 2D sheet structures formed by aromatic amphiphiles have great potential for application in chiral separation and biological fields. We can also extend the chiral separation function to include a chiral selector serving as a nanoreactor. | ||
| Fig. 9 (a) Schematic representation of the capture and chiral separation of racemic phenylalanine with a chiral sheet. Reproduced with permission from ref. 73. Copyright 2020 Wiley-VCH. (b) Schematic representation of enantioselective encapsulation and further chemical transformation inside the vesicle nanoreactor. A; dendritic alkyne, D; D-enantiomer of G4, L; L-enantiomer of G4, D–A; reaction product. Reproduced with permission from ref. 81. Copyright 2017 Royal Society of Chemistry. Negatively stained TEM images of Con A on (c) synthetic rafts and (d) sheets of 9 at pH 7.4. The bottom image shows the size profiles of Con A aggregates in the range of 10 to 50 nm. Reproduced with permission from ref. 78. Copyright 2019 Wiley-VCH. (e) Specific binding of ORN 178 GFP FimH to the supramolecular sheets. TEM, SEM, and optical microscopy (phase-contrast and fluorescence) images with the supramolecular sheets. Green fluorescence is due to the green fluorescence protein (GFP) of ORN 178. The scale bar of phase-contrast optical microscopy is 20 μm. Reproduced with permission from ref. 80. Copyright 2023 Wiley-VCH. | ||
6. Conclusions and perspective
The self-assembly of aromatic amphiphiles consisting of hydrophilic and hydrophobic segments is an interesting research field because of their dynamic behavior in combination with various functions arising from π-conjugated systems. Supramolecular nanostructures exhibit significantly different properties depending on the structure and topology of their building blocks, and thus their properties and functions can be readily controlled by molecular designs. The studies described in this review suggest that self-assembly of aromatic amphiphiles is attractive for generating ultrathin nanosheet structures. Herein, we have focused on the construction of two dimensional nanosheet structures, dynamic stimuli responsive behavior, and potential applications of self-assembly formed through rationally designed aromatic amphiphiles. From our studies, we can draw the following three general strategies for the preparation of sheets: (1) lateral association of primary fibers or tubules, (2) monolayer packing of aromatic amphiphiles, and (3) transformation from scrolls. We believe that the above strategies provide a guiding principle and new insight into the formation of ultrathin nanosheet structures by self-assembly using rationally designed amphiphiles.In particular, the dynamic and reversible nature of supramolecular interactions (such as hydrogen bonding and π–π stacking) endows nanomaterials with self-healing and stimuli-responsive capabilities. However, these properties also make nanosheets susceptible to disassembly in complex environments (such as high temperatures, extreme pH, or ionic strength), thereby limiting their long-term application under harsh conditions. Although the above research progress provides significant insights for the understanding of the formation of 2D nanosheet structures by self-assembly, more research efforts are still necessary to construct stable functional materials using aromatic amphiphiles by rational design. For example, the incorporation of dynamic covalent bonds (such as imine/disulfide bonds) and supramolecular interactions allows the construction of adaptive smart nanosheets, which exhibit both high stability and reconfigurability, enabling environmentally responsive functional switching.84–86 We anticipate that this contribution will help researchers to make further efforts to design unique aromatic amphiphiles with more functions, which will generate novel properties of supramolecular 2D nanosheet structures.
Author contributions
Conceptualization, funding acquisition, methodology, project administration, supervision, writing – review & editing: M. L.; formal analysis, funding acquisition, investigation, writing – original draft: Y. W.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Jilin Province Science and Technology Development Project, grant number YDZJ202301ZYTS298, the Science and Technology Research Project of the Education Department of Jilin Province, grant number JJKH20210806KJ, and the National Natural Science Foundation of China (grant no. 92156023 and 92356306).References
- B. Shen, Y. Kim and M. Lee, Supramolecular Chiral 2D Materials and Emerging Functions, Adv. Mater., 2020, 32, 1505669 Search PubMed.
- C. Tan, X. Cao, X. Wu, Q. He, J. Yang, X. Zhang, J. Chen, W. Zhao, S. Han, G.-H. Nam, M. Sindoro and H. Zhang, Recent Advances in Ultrathin Two-Dimensional Nanomaterials, Chem. Rev., 2017, 117, 6225–6331 CrossRef CAS PubMed.
- M. S. Lohse and T. Bein, Covalent Organic Frameworks: Structures, Synthesis, and Applications, Adv. Funct. Mater., 2018, 28, 1705553 CrossRef.
- M. Vybornyi, A. V. Rudnev, S. M. Langenegger, T. Wandlowski, G. Calzaferri and R. Ghäner, Formation of Two-dimensional Supramolecular Polymers by Amphiphilic Pyrene oligomers, Angew. Chem., Int. Ed., 2013, 52, 11488–11493 CrossRef CAS PubMed.
- W. Wei, M. Zhang, J. Ren and K. Wang, Two-Dimensional Nanosheet Stacking Structure Films for Li/Na/K-Ion Batteries and Supercapacitors, ACS Appl. Nano Mater., 2024, 7, 14844–14864 CrossRef CAS.
- K. Ariga, J. P. Hill and Q. Ji, Layer-by-layer Assembly as a Versatile Bottom-up Nanofabrication Technique for Exploratory Research and Realistic Application, Chem. Phys., 2007, 9, 2319–2340 CAS.
- J.-K. Kim, E. Lee, M.-C. Kim, E. Sim and M. Lee, Reversible Transformation of Helical Coils and Straight Rods in Cylindrical Assembly of Elliptical Macrocycles, J. Am. Chem. Soc., 2009, 131, 17768–17770 CrossRef CAS PubMed.
- J. D. Hartgerink, E. Beniash and S. I. Stupp, Self-assembly and Mineralization of Peptide-amphiphile Nanofibers, Science, 2001, 294, 1684–1688 CrossRef CAS PubMed.
- Z. Huang, H. Lee, E. Lee, S.-K. Kang, J.-M. Nam and M. Lee, Responsive Nematic Gels from the Self-assembly of Aqueous Nanofibers, Nat. Commun., 2011, 2, 459 CrossRef PubMed.
- E. Lee, J.-K. Kim and M. Lee, Reversible Scrolling of Two-dimensional Sheets from the Self-assembly of Laterally Grafted Amphiphilic Rods, Angew. Chem., Int. Ed., 2009, 48, 3657–3660 CrossRef CAS PubMed.
- M.-S. Kim, M. Kim, S. Son, S.-Y. Cho, S. Lee, D.-K. Won, J. Ryu, I. Bae, H.-M. Kim and K.-B. Kim, Sheet Resistance Analysis of Interface-Engineered Multilayer Graphene: Mobility Versus Sheet Carrier Concentration, ACS Appl. Mater. Interfaces, 2020, 12, 30932–30940 CrossRef CAS PubMed.
- J. Sakamoto, J. Heijst, O. Lukin and A. D. Schlüter, Two-dimensional Polymers: Just a Dream of Synthetic Chemists?, Angew. Chem., Int. Ed., 2009, 48, 1030–1069 CrossRef CAS PubMed.
- T. Fukui, S. Kawai, S. Fujinuma, Y. Matsushita, T. Yasuda, T. Sakurai, S. Seki, M. Takeuchi and K. Sugiyasu, Control over Diferentiation of Metastable Supramolecular Assembly in One and Two Dimensions, Nat. Chem., 2017, 9, 493–499 CrossRef CAS PubMed.
- C. Wang, Z. Wang and X. Zhang, Amphiphilic Building Blocks for Self-Assembly: From Amphiphiles to Supra-amphiphiles, Acc. Chem. Res., 2012, 45(4), 608–618 CrossRef CAS PubMed.
- Y.-B. Lim, K. S. Moon and M. Lee, Recent Advances in Functional Supramolecular Nanostructures Assembled from Bioactive Building Blocks, Chem. Soc. Rev., 2009, 38, 925–934 RSC.
- J. Bohlin, A. J. Turberfield, A. A. Louis and P. Šulc, Designing the Self-Assembly of Arbitrary Shapes Using Minimal Complexity Building Blocks, ACS Nano, 2023, 17, 5387–5398 CrossRef CAS PubMed.
- L. C. Palmer and S. I. Stupp, Molecular Self-assembly into One-Dimensional Nanostructures, Acc. Chem. Res., 2008, 41, 1674–1684 CrossRef CAS PubMed.
- C. Wang, Z. Wang and X. Zhang, Amphiphilic Building Blocks for Self-assembly: From Amphiphiles to Supra-amphiphiles, Acc. Chem. Res., 2012, 45, 608–618 CrossRef CAS PubMed.
- J. Pfeuffer-Rooschüz, L. Schmid, A. Prescimone and K. Tiefenbacher, Xanthene[n]arenes: Exceptionally Large, Bowl-Shaped Macrocyclic Building Blocks Suitable for Self-Assembly, JACS Au, 2021, 1, 1885–1891 CrossRef PubMed.
- T. Govindaraju and M. B. Avinash, Two-dimensional Nanoarchitectonics: Organic and Hybrid Materials, Nanoscale, 2012, 4, 6102–6117 RSC.
- B. Shen, Y. Kim and M. Lee, Supramolecular Chiral 2D Materials and Emerging Functions, Adv. Mater., 2020, 32, 1505669 Search PubMed.
- J. Zhang, X. Fan, X. Meng, J. Zhou, M. Wang, S. Chen, Y. Cao, Y. Chen, C. W. Bielawski and J. Geng, Ice-Templated Large-Scale Preparation of Two-Dimensional Sheets of Conjugated Polymers: Thickness-Independent Flexible Supercapacitance, ACS Nano, 2021, 15, 8870–8882 CrossRef CAS PubMed.
- K. Tahara, A. Noguchi, R. Nakayama, E. Ghijsens, S. De Feyter and Y. Tobe, Reversing the Handedness of Self-Assembled Porous Molecular Networks through the Number of Identical Chiral Centres, Angew. Chem., Int. Ed., 2019, 58, 7733–7738 CrossRef CAS PubMed.
- A. M. Manjarres, A. Albers and G. Fernández, Photoregulated Supramolecular Polymerization through Halogen Bonding, Angew. Chem., Int. Ed., 2024, 64, e202419720 CrossRef PubMed.
- N. Cheng, Y. Chen, X. Wu and Y. Liu, 2D Organic–inorganic Nanosheets via Self-assembly of a Pillar[6]arene and Polyoxometalate for Enhanced Degradation Efficiency, Chem. Commun., 2018, 54, 6284–6287 RSC.
- K.-D. Zhang, J. Tian, D. Hanifi, Y. Zhang, A. C.-H. Sue, T.-Y. Zhou, L. Zhang, X. Zhao, Y. Liu and Z.-T. Li, A Single-layer 2D Supramolecular Organic Framework (SOF) by the Tunable Complexation between CB[8]- and BP-containing Aromatic Guests, J. Am. Chem. Soc., 2013, 135, 17913–17918 CrossRef CAS PubMed.
- S. Shoji, T. Ogawa, S. Matsubara and H. Tamiaki, Bioinspired Supramolecular Nanosheets of Zinc cChlorophyll Assemblies, Sci. Rep., 2019, 9, 14006 CrossRef PubMed.
- Y. Zhou and T. Shimizu, Lipid Nanotubes: A Unique Template To Create Diverse One-Dimensional Nanostructures, Chem. Mater., 2008, 20, 625–633 CrossRef CAS.
- N. Han, Q. Zhao, L. Wan, Y. Wang, Y. Gao, P. Wang, Z. Wang, J. Zhang, T. Jiang and S. Wang, Hybrid Lipid-Capped Mesoporous Silica for Stimuli-Responsive Drug Release and Overcoming Multidrug Resistance, ACS Appl. Mater. Interfaces, 2015, 7, 3342–3351 CrossRef CAS PubMed.
- M. Poorsargol, B. Sohrabi and M. Dehestani, Study of the Gemini Surfactant’ Self-Assembly on Graphene Nanosheets: Insights from Molecular Dynamic Simulation, J. Phys. Chem. A, 2018, 122, 3873–3885 CrossRef CAS PubMed.
- J. S. Kłos and J. Paturej, Complexation between Dendritic Polyelectrolytes and Amphiphilic Surfactants: The Impact of Surfactant Concentration and Hydrophobicity, Macromolecules, 2023, 56, 5022–5032 CrossRef.
- C. Nie, P. Pouyan, D. Lauster, J. Trimpert, Y. Kerkhoff, G. P. Szekeres, M. Wallert, S. Block, A. K. Sahoo, J. Dernedde, K. Pagel, B. B. Kaufer, R. R. Netz, M. Ballauff and R. Haag, Polysulfates Block SARS-CoV-2 Uptake through Electrostatic Interactions, Angew. Chem., Int. Ed., 2021, 60, 15870–15878 CrossRef CAS PubMed.
- Y. Fan and Y. Wang, Self-Assembly and Functions of Star-Shaped Oligomeric Surfactants, Langmuir, 2018, 34, 11220–11241 CrossRef CAS PubMed.
- S. Fu, N. Niu, S. Song, D. Yan, J. Ge, J. Li, Z. Peng, L. Li, Y. Xiong, L. Wang, D. Wang and B. Z. Tang, Facile Construction of Dendritic Amphiphiles with AggregationInduced Emission Characteristics for Supramolecular Self-Assembly, Macromolecules, 2022, 55, 4742–4751 CrossRef CAS.
- S. Li, B. Xia, B. Javed, W. D. Hasley, A. Melendez-Davila, M. Liu, M. Kerzner, S. Agarwal, Q. Xiao, P. Torre, J. G. Bermudez, K. Rahimi, N. Y. Kostina, M. Möller, C. Rodriguez-Emmenegger, M. L. Klein, V. Percec and M. C. Good, Direct Visualization of Vesicle Disassembly and Reassembly Using Photocleavable Dendrimers Elucidates Cargo Release Mechanisms, ACS Nano, 2020, 14, 7398–7411 CrossRef CAS PubMed.
- T.-Y. Kim, S.-M. Hur and A. Ramírez-Hernández, Effect of Block Sequence on the Solution Self-Assembly of Symmetric ABCBA Pentablock Polymers in a Selective Solvent, J. Phys. Chem. B, 2023, 127, 2575–2586 CrossRef CAS PubMed.
- A. L. Liberman-Martin, A. B. Chang, C. K. Chu, R. H. Siddique, B. Lee and R. H. Grubbs, Processing Effects on the Self-Assembly of Brush Block Polymer Photonic Crystals, ACS Macro Lett., 2021, 10, 1480–1486 CrossRef CAS PubMed.
- F. Xu, J. Zhang, P. Zhang, X. Luan and Y. Mai, “Rod-coil” Copolymers Get Self-assembled in solution, Mater. Chem. Front., 2019, 3, 2283–2307 RSC.
- Q. Li, K. J. Lambright, M. G. Taylor, K. Kirschbaum, T.-Y. Luo, J. Zhao, G. Mpourmpakis, S. Mokashi-Punekar, N. L. Rosi and R. Jin, Reconstructing the Surface of Gold Nanoclusters by Cadmium Doping, J. Am. Chem. Soc., 2017, 139, 17779–17782 CrossRef CAS PubMed.
- N. Krishnan, M. Golla, H. V. P. Thelu, S. K. Albert, S. Atchimnaidu, D. Perumal and R. Varghese, Self-assembly of DNA-tetraphenylethylene Amphiphiles into DNA-grafted Nanosheets as a Support for the Immobilization of Gold Nanoparticles: a Recyclable Catalyst with Anhanced Activity, Nanoscale, 2018, 10, 17174–17181 RSC.
- T. Du, W. Yuan, Z. Zhao and S. Liu, Reversible Morphological Tuning of DNA-perylenebisdiimide Assemblies through Host-guest Interaction, Chem. Commun., 2019, 55, 3658–3661 RSC.
- L. Dong, H. Chen, T. Liu, J. Zhu, M. Yu and Q. Yuan, Poly(l-cysteine) Peptide Amphiphile Derivatives Containing Disulfide Bonds: Synthesis, Self-Assembly-Induced β-Sheet Nanostructures, pH/Reduction Dual Response, and Drug Release, Biomacromolecules, 2021, 22, 5374–5381 CrossRef CAS PubMed.
- R. Chang, C. Yuan, P. Zhou, R. Xing and X. Yan, Peptide Self-assembly: From Ordered to Disordered, Acc. Chem. Res., 2024, 57, 289–301 CrossRef CAS PubMed.
- W. Du, X. Hu, W. Wei and G. Liang, Intracellular Peptide Self-Assembly: A Biomimetic Approach for in Situ Nanodrug Preparation, Bioconjugate Chem., 2018, 29, 826–837 CrossRef CAS PubMed.
- I. W. Hamley, Self-Assembly, Bioactivity, and Nanomaterials Applications of Peptide Conjugates with Bulky Aromatic Terminal Groups, ACS Appl. Bio Mater., 2023, 6, 384–409 CrossRef CAS PubMed.
- J.-W. Chang, R. D. Chakravarthy, N.-T. Chu, J.-C. Liu, M.-Y. Yeh and H.-C. Lin, Self-Assembly of the Tetraphenylethylene-Capped Diserine through a Hierarchical Assembly Process, Bioconjugate Chem., 2023, 34, 562–571 CrossRef CAS PubMed.
- Y. Wang, Z. Huang, Y. Kim, Y. He and M. Lee, Guest-Driven Inflation of Self-Assembled Nanofibers through Hollow Channel Formation, J. Am. Chem. Soc., 2014, 136, 16152–16155 CrossRef CAS PubMed.
- Y. Kim and M. Lee, Supramolecular Capsules from Bilayer Membrane Scission Driven by Corannulene, Chem. – Eur. J., 2015, 21, 5736–5740 CrossRef CAS PubMed.
- B. Shen, Y. He, Y. Kim, Y. Wang and M. Lee, Spontaneous Capture of Carbohydrate Guests through Folding and Zipping of Self-Assembled Ribbons, Angew. Chem., Int. Ed., 2016, 55, 2382–2386 CrossRef CAS PubMed.
- K. Liu, Y. Yao, Y. Liu, C. Wang, Z. Li and X. Zhang, Self-Assembly of Supra-amphiphiles Based on Dual Charge-Transfer Interactions: From Nanosheets to Nanofibers, Langmuir, 2012, 28, 10697–10702 CrossRef CAS PubMed.
- S. Fu, A. Pang, X. Guo, Y. He, S. Song, J. Ge, J. Li, W. Li, Y. Xiong, L. Wang, D. Wang and B. Z. Tang, Bioinspired Supramolecular Nanotoroids with Aggregation-Induced Emission Characteristics, ACS Nano, 2022, 16, 12720–12726 CrossRef CAS PubMed.
- Y. Kim, H. Li, Y. He, X. Chen, X. Ma and M. Lee, Collective Helicity Switching of a DNA-coat Assembly, Nat. Nanotechnol., 2017, 12, 551–556 CrossRef CAS PubMed.
- S. Bao, S. Wu, L. Huang, X. Xu, R. Xu, Y. Li, Y. Liang, M. Yang, D. K. Yoon, M. Lee and Z. Huang, Supramolecular Nanopumps with Chiral Recognition for Moving Organic Pollutants from Water, ACS Appl. Mater. Interfaces, 2019, 11, 31220–31226 CrossRef CAS PubMed.
- B. J. Richardson, C. Zhang, P. Rauthe, A.-N. Unterreiner, D. V. Golberg, B. L. J. Poad and H. Frisch, Peptide Self-Assembly Controlled Photoligation of Polymers, J. Am. Chem. Soc., 2023, 145, 15981–15989 CrossRef CAS PubMed.
- X. Liu, L. Du, J. Liu, Y. Shi, Q. Liu, Y. Xu, Y. Xia, X. Wang, D. Ding, X. Li and D. Lin, NaCl-Responsive Ultrashort Peptide to Trigger Self-Assembly of TPE-Capped Supramolecular Hydrogelator, Biomacromolecules, 2025, 26, 258–265 CrossRef CAS PubMed.
- R. Xue, Y. Han, Y. Xiao, J. Huang, B. Z. Tang and Y. Yan, Lithium Ion Nanocarriers Self-Assembled from Amphiphiles with Aggregation-Induced Emission Activity, ACS Appl. Nano Mater., 2018, 1, 122–131 CrossRef CAS.
- H. Chen, Y. Fan, X. Yu, V. Semetey, S. Trépout and M.-H. Li, Light-Gated Nano-Porous Capsules from Stereoisomer-Directed Self-Assemblies, ACS Nano, 2021, 15, 884–893 CrossRef CAS PubMed.
- C. Liu, Z. Cheng, X. Xu, N. Wang, X. Luo and X. Xin, Fluorescent Nanocomposite Prepared through Supramolecular Self-Assembly of a Tetraphenylethene Derivative and Polyoxometalate for Light-Emitting Diodes, ACS Appl. Nano Mater., 2024, 7, 11455–11464 CrossRef CAS.
- L. Tan, T. Zheng, Y. Li and M. Lee, Stereodivergent Macrocyclization in Dynamic Chiral Confinement, Chem. – Eur. J., 2024, e202404207 Search PubMed.
- Y. Yang, Q. Han, Y.-R. Pei, S. Yu, Z. Huang and L. Y. Jin, Stimuli-Responsive Supramolecular Chirality Switching and Nanoassembly Constructed by n-Shaped Amphiphilic Molecules in Aqueous Solution, Langmuir, 2021, 37, 1215–1224 CrossRef CAS PubMed.
- A. K. Yudin, Introduction: Macrocycles, Chem. Rev., 2019, 119, 9723–9723 CrossRef CAS PubMed.
- Z. Li and Y.-W. Yang, Macrocycle-Based Porous Organic Polymers for Separation, Sensing, and Catalysis, Adv. Mater., 2022, 34, 2107401 CrossRef CAS PubMed.
- Y. Wang, Y. Kim and M. Lee, Static and Dynamic Nanosheets from Selective Assembly of Geometric Macrocycle Isomers, Angew. Chem., Int. Ed., 2016, 55, 13122–13126 CrossRef CAS PubMed.
- J. Kumaki, S.-I. Sakurai and E. Yashima, Visualization of Synthetic Helical Polymers by High-resolution Atomic Force Microscopy, Chem. Soc. Rev., 2009, 38, 737–746 RSC.
- M. M. Safont-Sempere, P. Osswald, K. Radacki and F. Würthner, Chiral Self-Recognition and Self-Discrimination of Strapped Perylene Bisimides by π-Stackong Dimerization, Chem. – Eur. J., 2010, 16, 7380–7384 CrossRef CAS PubMed.
- Y. Kim, J. Kang, B. Shen, Y. Wang, Y. He and M. Lee, Open-Closed Switching of Synthetic Tubular Pores, Nat. Commun., 2015, 6, 8650 CrossRef CAS PubMed.
- W. Li, Y. Kim, J. Li and M. Lee, Dynamic Self-assembly of Coordination Polymers in Aqueous Solution, Soft Mater., 2014, 10, 5231–5242 RSC.
- W. Li, Y. Kim and M. Lee, Intelligent Supramolecular Assembly of Aromatic Block Molecules in Aqueous Solution, Nanoscale, 2013, 5, 7711–7723 RSC.
- Z. Huang, H. Lee, E. Lee, S.-K. Kang, J.-M. Nam and M. Lee, Responsive Nematic Gels from the Self-Assembly of Aqueous Nanofibers, Nat. Commun., 2011, 2, 459 CrossRef PubMed.
- Y. Wang, X. Feng and M. Lee, Self Division of 2-D Sheets in Aromatic Macrocycle Assembly, Org. Chem. Front., 2021, 8, 3681–3685 RSC.
- E. Lee, J. K. Kim and M. Lee, Reversible Scrolling of Two-Dimensional Sheets from Self-Assembly of Laterally-Grafted Amphiphilic Rods, Angew. Chem., Int. Ed., 2009, 48, 3657–3660 CrossRef CAS PubMed.
- H.-J. Kim, T. Kim and M. Lee, Responsive Nanostructures from Aqueous Assembly of Rigid-Flexible Block Molecules, Acc. Chem. Res., 2011, 44, 72–82 CrossRef CAS PubMed.
- Y. Wang and M. Lee, Self-Assembly of Tetraphenylethylene-based Amphiphiles into 2-Dimensional Chiral Sheets for Enantioselective Sorption, ChemPlusChem, 2020, 85, 711–714 CrossRef CAS PubMed.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Aggregation-induced Emission, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC.
- J. B. Zhang, B. Xu, J. L. Chen, S. Q. Ma, Y. J. Dong, L. J. Wang, B. Li, L. Ye and W. J. Tian, An Organic Luminescent Molecule: What Will Happen When the “Butterflies” Come Together?, Adv. Mater., 2014, 26, 739–745 CrossRef PubMed.
- H. Sun, L. Dong, Y. Kim and M. Lee, Supramolecular Tubule from Seesaw Shaped Amphiphile and Its Hierarchical Evolution into Sheet, Chem. – Asian J., 2020, 15, 2470–2474 CrossRef CAS PubMed.
- X. Liu, H. Li, Y. Kim and M. Lee, Assembly-Disassembly Switching of Self-Sorted Nanotubules Forming Dynamic 2-D Porous Heterostructure, Chem. Commun., 2018, 54, 3102–3105 RSC.
- Y. Kim, X. Liu, H. Li and M. Lee, Fluorescence Turn-on Synthetic Lipid Rafts on Supramolecular Sheets and Hierarchical Concanavalin A Assembly, Chem. – Asian J., 2019, 14, 952–957 CrossRef CAS PubMed.
- W. Zhang, Y. Li, T. Zheng, Y. Xie, X.-Y. Dai and M. Lee, Fluorescence Switching 2-D Sheet structure Formed by Self-Assembly of Cruciform Amphiphiles, Soft Matter, 2025, 21, 1451–1454 RSC.
- D. Lee, L. Dong, Y. R. Kim, J. Kim, M. Lee and Y. Kim, Reduction-Responsive Supramolecular Sheets for Selective Regulation of Facultative Anaerobe Agglutination, Adv. Healthcare Mater., 2023, 12, 2203136 CrossRef CAS PubMed.
- X. Chen, Y. Wang, H. Wang, Y. Kim and M. Lee, α-Helical Peptide Vesicles with Chiral Membranes as Enantioselective Nanoreactors, Chem. Commun., 2017, 53, 10958–10961 RSC.
- J. Shen and Y. Okamoto, Efficient Separation of Enantiomers Using Stereoregular Chiral Polymers, Chem. Rev., 2016, 116, 1094–1138 CrossRef CAS PubMed.
- X. Chen, Y. He, Y. Kim and M. Lee, Reversible Short α-Helical Peptide Assembly for Controlled Capture and Selective Release of Enantiomers, J. Am. Chem. Soc., 2016, 138, 5773–5776 CrossRef CAS PubMed.
- Y. Wang, P. Xing, W. An, M. Ma, M. Yang, T. Luan, R. Tang, B. Wang and A. Hao, pH-Responsive Dipeptide-Based Dynamic Covalent Chemistry Systems Whose Products and Self-Assemblies Depend on the Structure of Isomeric Aromatic Dialdehydes, Langmuir, 2018, 34, 13725–13734 CrossRef CAS PubMed.
- Y. Chen, H. Tang, H. Chen and H. Li, Self-Assembly via Condensation of Imine or Its N-Substituted Derivatives, Acc. Chem. Res., 2023, 56, 2838–2850 CrossRef CAS PubMed.
- Q. Zhang, D.-H. Qu, B. L. Feringa and H. Tian, Disulfide-Mediated Reversible Polymerization toward Intrinsically Dynamic Smart Materials, J. Am. Chem. Soc., 2022, 144, 2022–2033 CrossRef CAS PubMed.
| This journal is © the Partner Organisations 2025 |
