Open Access Article
Open Access ArticleDevelopment of AlN-loaded PET separators from waste water bottle plastics with superior thermal characteristics for next-generation lithium-ion batteries†
Alpha Chi Him Tsanga,
Marco Yu Lam Wongb,
Chi-Wing Tsang a,
Dawson Wai-Shun Suena and
Xiao-Ying Lu
a,
Dawson Wai-Shun Suena and
Xiao-Ying Lu *a
*a
aDepartment of Construction, Environment and Engineering, Technological and Higher Education Institute of Hong Kong, Hong Kong. E-mail: xylu@thei.edu.hk; Tel: +852 2176 1453
bDepartment of Civil and Environmental Engineering, School of Engineering, The Hong Kong University of Science and Technology, Hong Kong
First published on 26th February 2025
Abstract
Preventing short circuit hazard due to lithium (Li) dendrite formation across a separator from the anode of a lithium-ion battery (LIB) throughout operation is important; however, conventional separator materials cannot fulfil the increasing safety standards of next-generation LIBs. Thus, developing separator materials with high Li dendrite suppression ability in order to prevent short circuit is of paramount importance for realising next-generation LIBs. In this study, aluminum nitride-loaded polyethylene terephthalate (PET/AlN) composites with micro-/nanoarchitecture were synthesized using PET that was recycled from commercial waste bottles via an electrospinning strategy. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) suggested that AlN nanoparticles were encapsulated in PET micro-/nanoarchitecture fibres. Thermogravimetric analysis indicated that the AlN content in the composite materials was about 4–5 wt%. X-ray photoelectron spectroscopy (XPS) and Fourier-transform infrared (FTIR) spectroscopy confirmed the PET polymer structure of PET/AlN composites. The PET/AlN 4 wt% separator exhibited a porosity of 69.23%, according to the n-butanol uptake test, and a high electrolyte uptake of 521.69%. Most importantly, electrochemical results revealed that when evaluated at a current density of 0.5C, PET/AlN 4 wt% composites could deliver a reversible specific capacity of 238.2 mA h g−1 after 100 cycles. When C-rate capability tests were conducted at high charge–discharge densities of 0.2, 0.5, 1, 2, and 4C, the PET/AlN 4 wt% composite manifested average specific capacities of about 225.3, 218.4, 191.0, 127.5, and 28.1 mA h g−1, respectively. The excellent electrochemical performance of the PET/AlN 4 wt% composite could probably be attributed to the combined benefits of AlN nanoparticles and the micro-/nanoarchitecture. These unique features of PET/AlN were advantageous for effective Li ion transport in repeated charge–discharge cycles and strong hydrothermal stability, thereby resulting in safety, high capacity and excellent C-rate performance. Overall, this study demonstrated the excellent electrochemical performance of PET/AlN composites as stable separator materials for advanced LIBs.
Introduction
High performance batteries, including lithium-ion batteries (LIBs), are important for various applications, including portable devices and electric vehicles (EVs), in the modern world to fight against the energy crisis.1–3 However, the problem of uncontrolled dendrite growth due to Li plating/stripping and ‘dead Li’ formation2 results in battery hazard due to overheating caused by short circuit and reduction of coulombic efficiency.4 As a result, the use of separators with suitable protection is important to ensure battery safety.2Polyethylene terephthalate (PET) has recently become an attractive separator material for LIBs owing to its excellent thermal stability, mechanical properties, tensile strength, good thermal shrinkage, and electronic isolation.2,5 Furthermore, plastic waste is one of the major sources of PET. Moreover, huge amount of plastic waste is one of the environmental problems due to their almost non-biodegradable nature, especially that of simple molecular polymers (polyethene, PVC, PET, etc.). Effective recycling or recovery of plastics can relax the overloading of the landfill site and reduce the secondary land pollution associated from the landfill. Drinking water bottle is a major source of PET, and the best way to extract PET for LIB separator manufacturing is via the effective recycling of waste drinking water bottles. Electrospinning is an effective and low-cost method compared to the conventional methodology for producing a thin porous polymer matrix with a large specific surface area,5–24 ionic conductivity,13–15,25 and mechanical strength.14,26
Conventional polymeric separators still face the problem of inevitable dendritic penetration growth of Li from the anode to the cathode via the porous array separator with large pore size, which may result in the short circuit of LIBs with consequent fire hazard; thus, the employment of a suitable coating to modify the separator becomes a solution to the hazard.27 Modifying the polymeric separator is an important direction to enhance the operation safety of LIBs. Inorganic coatings such as Al2O3 can enhance the ionic conductivity,28–30 mechanical strength,28,29,31 thermal stability and wettability of the polymer-based separator,2,28,29,31–43 which makes them widely applicable in the lithium-ion battery separator. However, owing to the limitation of oxide coatings (Al2O3) separator, including insoluble properties of the metal oxide precursors in non-aqueous solvent for coating, the resulting layer may be eventually damaged by interfacial stress during Li plating/stripping process, which can lead to loss of functionality.1,44 Finally, fatal safety hazards will be induced.1 Alternatively, conductive nitride-based compounds (e.g., boron nitride (BN)45 and aluminum nitride (AlN)1,46) have stronger electrochemical and chemical stability, thermal conductivity towards metallic Li, and mechanical robustness compared to metal oxides. Furthermore, the porous network of AlN has better shielding effect for separators to protect the Li anode1,46 by suppressing sharp Li dendritic growth over widely investigated metal oxide separator coatings. Such strength makes AlN an alternative separator coating for short-circuit prevention in LIBs.
To date, the excellent performance of electrospun AlN-modified PP and PET-based separators is not fully explored compared to conventional metal oxide-modified polymer-based separators. Furthermore, waste drinking water bottles are regarded as the best source of PET for separator manufacturing if the proper recycling process of commercial PET bottles is applied. Such proposed works are beneficial to achieve a reduction in disposal of plastic waste and simultaneous development of novel environment-friendly LIB separators. Herein, we demonstrate a facile construction of AlN nanoparticles-loaded PET separator for LIBs via large scale and low-cost electrospinning method and PET obtained by recycling waste plastic from drinking water bottles. The synthesized separator showed enhanced properties in coin-type LIBs with electrochemical stability up to 100 cycles from galvanostatic cyclic performance studies, excellent thermal stability, high electrolyte wettability according to the solvent uptake test, and water contact angle. All are beneficial to the development of next-generation LIBs separators (enhanced operation safety) in an environmental and low-cost way.
Experimental
Materials
Spent commercial PET drinking water bottle (430 mL, made by recycled PET, Watsons HK), aluminium nitride (AlN nanoparticles, 500 nm), trifluoroacetic acid (TFA, Sigma-Aldrich), dichloromethane (DCM, Sigma-Aldrich), commercial NCM 523 powder (Sigma-Aldrich), Super P powder (Thermo Scientific Chemicals), polyvinylidene fluoride (PVDF), N-methyl-2-pyrrolidone (NMP, Sigma-Aldrich), and lithium hexafluorophosphate in ethylene carbonate/ethylmethanecarbonate (LiPF6 in EC + EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v), Sigma-Aldrich) were used as received.
1 v/v), Sigma-Aldrich) were used as received.
PET separator synthesis
The PET composite fibre-based nonwoven membranes were synthesized by the electrospinning method at room temperature (Fig. S1a†). First of all, the PET-based solution for electrospinning was synthesized by dissolving small pieces (5 mm × 5 mm) of cut recycled PET bottle piece (Fig. S2a†), which were washed, followed by drying at 60 °C for 6 h as the first step. A desired amount of purified PET piece was then dissolved into TFA and DCM binary solvent with a weight ratio of 2.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (TFA
1 (TFA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DCM = 70 g
DCM = 70 g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 g) to form 15 wt% PET solution after stirring them all for 24 h to ensure complete dissolution of recycled PET (Fig. S2b†). PET/AlN was synthesized by mixing the desired amount of AlN nanoparticle composites (Fig. S1b†) into the pure PET solution mentioned above in order to produce 20 g PET/AlN solutions (PET/AlN 1 wt%, PET/AlN 2 wt%, PET/AlN 3 wt%, PET/AlN 4 wt%, PET/AlN 5 wt%) with different AlN loading (1 wt%, 2 wt%, 3 wt%, 4 wt%, 5 wt%).
30 g) to form 15 wt% PET solution after stirring them all for 24 h to ensure complete dissolution of recycled PET (Fig. S2b†). PET/AlN was synthesized by mixing the desired amount of AlN nanoparticle composites (Fig. S1b†) into the pure PET solution mentioned above in order to produce 20 g PET/AlN solutions (PET/AlN 1 wt%, PET/AlN 2 wt%, PET/AlN 3 wt%, PET/AlN 4 wt%, PET/AlN 5 wt%) with different AlN loading (1 wt%, 2 wt%, 3 wt%, 4 wt%, 5 wt%).
The prepared solutions (pure PET and PET/AlN) were injected into syringes of 10 mL with G20 metal needles (Fig. S1c†). Voltages of +15.00 kV and −0.00 kV were applied to the needle and rotary collector, respectively. Electrospinning was performed with a dosing distance of about 60 mm and dosing rate (solution feeding rate) of 0.15 mm min−1 (0.05 g min−1) for 6.6 h. The resulting PET-based composite was then dried in ambient air before use (Fig. S2c†). The overall flow is summarized in Fig. 1.
 | ||
| Fig. 1 Flow of recycled PET-based battery separator synthesis from waste plastic bottles via the electrospinning process. | ||
Battery activity analysis
The ionic conductivity (σ) of PET-based separators was evaluated via electrochemical impedance spectroscopy (EIS) analysis using an electrochemical workstation (CHI660E, CHI), which was modified from other reports.47,48 Briefly, CR2032 coin cell was assembled by stacking the liquid electrolyte (1 M LiPF6 in EC + EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v))-soaked AlN/PET-based separators (diameter: 16 mm, thickness: 0.21 mm, area: 2.01 cm2), which was sandwiched between two stainless-steel electrode plates in the battery casing. EIS experiments were conducted with AC amplitude of 5 mV at room temperature and frequency varied from 0.1 Hz to 1 MHz. The ionic conductivity was calculated by the equation
1 v/v))-soaked AlN/PET-based separators (diameter: 16 mm, thickness: 0.21 mm, area: 2.01 cm2), which was sandwiched between two stainless-steel electrode plates in the battery casing. EIS experiments were conducted with AC amplitude of 5 mV at room temperature and frequency varied from 0.1 Hz to 1 MHz. The ionic conductivity was calculated by the equationwhere d is the membrane thickness, S refers to the cross-sectional area, and Rb is the bulk resistance obtained at the high frequency intercept of AC impedance on the real axis.
Coin cells (CR 2032) were assembled in an argon-filled glove box at room temperature. Briefly, uniform cathode ink was synthesized by dispersing a mixture of commercial NCM 523 powder, conductive carbon (Super P) powder and conductive binder of polyvinylidene fluoride (PVDF) (dry weight ratio (wt%) = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with N-methyl-2-pyrrolidinone (NMP) solvent. The synthesized cathode ink was then fabricated on the current collector of aluminum (Al) sheet with cathode thickness of 15 μm, followed by overnight drying in an oven (60 °C) to remove all NMP solvent. Cathode (diameter: 12 mm, active material (NCM 523) loading: 4 mg cm−2) and lithium metal of the anode were employed with a liquid electrolyte-soaked separator. The CR2032 battery performance was analyzed by a LAND battery tester (CT2001A) at room temperature. Cycling performance was analyzed at a current density of 0.5C in the voltage window of 3.0–4.2 V vs. Li+/Li, where the 2nd operation cycle was counted as the first testing cycle as the 1st operation cycle was start-up operation in nature. Meanwhile, capacity rate measurement was carried out at current density of 0.2C, 0.5C, 1C, 2C and 4C. A commercial separator (Celgard® 2400) was used for control coin cell analysis. Cyclic voltammetry (CV) analysis of coin cells was carried out with the method modified from elsewhere.49,50 Briefly, the PET-based separator-equipped NCM 523 coin cell was tested in the voltage range of 3.0–4.2 V vs. Li/Li+ and scanning rate of 0.05 mV s−1 for 4 cycles by an electrochemical workstation (CHI660E, CHI). All measurements were carried out at room temperature.
1) with N-methyl-2-pyrrolidinone (NMP) solvent. The synthesized cathode ink was then fabricated on the current collector of aluminum (Al) sheet with cathode thickness of 15 μm, followed by overnight drying in an oven (60 °C) to remove all NMP solvent. Cathode (diameter: 12 mm, active material (NCM 523) loading: 4 mg cm−2) and lithium metal of the anode were employed with a liquid electrolyte-soaked separator. The CR2032 battery performance was analyzed by a LAND battery tester (CT2001A) at room temperature. Cycling performance was analyzed at a current density of 0.5C in the voltage window of 3.0–4.2 V vs. Li+/Li, where the 2nd operation cycle was counted as the first testing cycle as the 1st operation cycle was start-up operation in nature. Meanwhile, capacity rate measurement was carried out at current density of 0.2C, 0.5C, 1C, 2C and 4C. A commercial separator (Celgard® 2400) was used for control coin cell analysis. Cyclic voltammetry (CV) analysis of coin cells was carried out with the method modified from elsewhere.49,50 Briefly, the PET-based separator-equipped NCM 523 coin cell was tested in the voltage range of 3.0–4.2 V vs. Li/Li+ and scanning rate of 0.05 mV s−1 for 4 cycles by an electrochemical workstation (CHI660E, CHI). All measurements were carried out at room temperature.
Materials characterization
The morphology and microstructure of separators was observed by a scanning electron microscope (SEM, LEO-1530) and transmission electron microscope (TEM, Philips FEI Tecnai G2 20 S-Twin Scanning TEM), respectively. The chemical composition of the AlN/PET-based composite was analyzed by energy dispersive spectroscopy (EDS equipped in LEO-1530 SEM), Fourier-transform infrared (FTIR) spectroscopy and X-ray photoelectron spectroscopy (XPS), respectively. The crystal structure of the composite was analysed by an X-ray diffractometer (XRD) equipped with Cu-Kα radiation. The accelerating voltage and current used were 40 kV and 20 mA, respectively. The scanning range of 2θ was set between 5 and 95°, with a step size of 0.02° and 0.01° s−1. The electrolyte wettability was evaluated via contact angle measurement, with the electrolyte droplet on the separator measured by a Drop Shape Analyzer (DSA25, Kruss). Thermal dimensional stability was checked by heating the separators in an oven at 100 °C for 120 min and degree of shrinkage was investigated at every 30 min interval. Differential scanning calorimetry was conducted to measure the thermal performance of separators. The size (area) of each separator for characterization was 2.01 cm2 (Fig. S2d†). The porosity of separators was evaluated by conducting n-butanol uptake test, where 6 mL of n-butanol was added to each sample and 2 hours for soaking was required at room temperature. Dry weight (0.011 g) and wet weight of the membranes were measured, for which excess solutions on the surface should be removed after soaking. The porosity was calculated by the following equationwhere ww and wd represent the weight of wet and dry membranes respectively, ρb is the density of n-butanol, and V refers to the geometric volume of membranes.
Electrolyte uptake test was carried out by soaking the weighed membranes in the liquid electrolyte of 1 M LiPF6 in EC + EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume) for 1 hour at room temperature. Excess electrolyte solution on the membrane surface was removed with wipes. Electrolyte uptake (EU) was calculated by the following equation
1 in volume) for 1 hour at room temperature. Excess electrolyte solution on the membrane surface was removed with wipes. Electrolyte uptake (EU) was calculated by the following equation
The change in the surface morphology of the separators against the fresh separators (PET/AlN 4 wt% and PET/AlN 5 wt%) throughout the cycling performance test was analyzed by SEM analysis after extracting spent separators from the mechanically scrapped CR2032.
Results and discussion
Characterization of the electrospun PET/AlN membrane
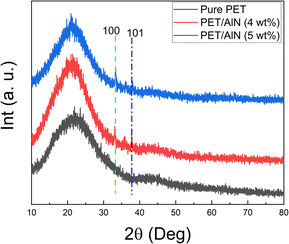
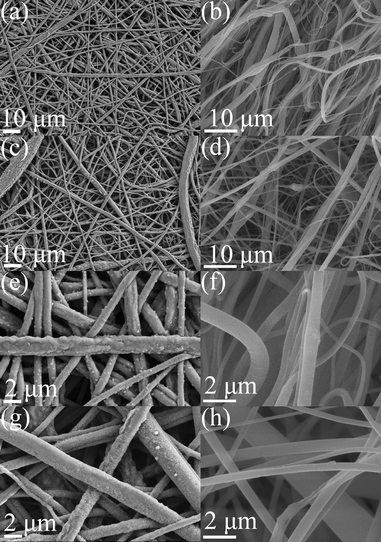
The morphological properties of electrospun PET-based membrane are shown in the SEM image of Fig. 2 and S3,† which reflected the random oriented nanowire pattern of PET network array in the electrospun products and similar to the reported products.5 AlN nanoparticles was observed on the PET nanowire network in the high magnification SEM image of PET samples upon modification in the raw PET/AlN gel ink, as illustrated in Fig. 2a–d and S3† by comparison of smooth PET nanowire in the pure PET sample (Fig. 2c–f). The successful loading of AlN nanoparticles was reflected from the obvious Al EDS peak in the EDS survey scan spectrum (Fig. S4a†), and the C and O (polymer skeleton) and the dot pattern of Al (loaded AlN nanoparticles) observed in the EDS mapping of PET/AlN 4 wt% (Fig. S4b–e†), while the N signal (survey scan N peak in Fig. S4a,† N Kα1 mapping pattern in Fig. S4f†) was not as obvious as the other three elements in the electrospun sample. It primarily showed that AlN nanoparticles were not loaded on the surface of the nanofiber but embedded instead. A similar finding was observed from the TEM and HR-TEM images of the PET/AlN 4 wt% nanofibers (Fig. S5†) where the AlN nanoparticles were not loaded on the nanofiber surface but inside the fibre since the crystal pattern was observed in the HR-TEM at high magnification (Fig. S5d†), which was located in the interior region of the nanofibre pattern when compared to the corresponding SEM (Fig. 2) and EDS results (Fig. S4†). Further analysis of XRD (Fig. 3 and S6†) revealed that PET-based composites are generally amorphous in nature by comparison to the commercial Celgard® 2400 membrane. However, among PET-based membrane samples, the appearance of weak sharp peaks observed at 33.3° (PET/AlN 4 wt% and PET/AlN 5 wt%) and 37.8° (PET/AlN 4 wt%) in AlN-loaded PET membrane (Fig. 3) compared to pure PET stand for (100) and (101) phase of AlN (JCPDS card no. 25-1133), respectively. It is similar to the reference data of pure AlN and some AlN-modified separators.1,46,51 These are strong evidence of the successful loading of AlN nanoparticles in the PET array. The weak XRD signal of AlN further confirmed the successful encapsulation of AlN nanoparticles into PET nanofibers to form composite separators.Chemical composition analysis of the PET-based membrane is important for understanding the chemical structures, including bonding (functional group) and elemental composition. FTIR results (Fig. 4) showed that the FTIR spectrum of PET/AlN is almost identical to that of pure PET. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C group (1504 and 1582 cm−1), –CH2– group (2961 cm−1), –OH group (3443 cm−1), C
C group (1504 and 1582 cm−1), –CH2– group (2961 cm−1), –OH group (3443 cm−1), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (keto) group (1715 cm−1), and C–O group due to deformation of the –OH group (1410 cm−1) were observed in the spectra of PET/AlN 4 wt%, which closely matched with the FTIR spectrum of pure PET, as reported elsewhere.52 In contrast, stretching shift due to the presence of AlN nanoparticles was not observed in the PET/AlN samples. From further analysis by XPS (Fig. 5), the survey (Fig. 5a and b) and HR-XPS spectra (C 1s: Fig. 5c and d, N 1s: Fig. S7a and b,† Al 2p: Fig. S7c and d†) were almost similar with each other (PET/AlN 4 wt% and PET/AlN 5 wt%). One clear peak at 284.45–284.8 eV with one shoulder peak at 287.75–288.2 eV was observed in the C 1s patterns of both PET/AlN 4 wt% (Fig. 5c) and PET/AlN 5 wt% (Fig. 5d). These peaks were contributed by sp2 C–C (284.45–284.8 eV), –C
O (keto) group (1715 cm−1), and C–O group due to deformation of the –OH group (1410 cm−1) were observed in the spectra of PET/AlN 4 wt%, which closely matched with the FTIR spectrum of pure PET, as reported elsewhere.52 In contrast, stretching shift due to the presence of AlN nanoparticles was not observed in the PET/AlN samples. From further analysis by XPS (Fig. 5), the survey (Fig. 5a and b) and HR-XPS spectra (C 1s: Fig. 5c and d, N 1s: Fig. S7a and b,† Al 2p: Fig. S7c and d†) were almost similar with each other (PET/AlN 4 wt% and PET/AlN 5 wt%). One clear peak at 284.45–284.8 eV with one shoulder peak at 287.75–288.2 eV was observed in the C 1s patterns of both PET/AlN 4 wt% (Fig. 5c) and PET/AlN 5 wt% (Fig. 5d). These peaks were contributed by sp2 C–C (284.45–284.8 eV), –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (287.75–288.2 eV) in PET/AlN 4 wt% and PET/AlN 5 wt%, which matched with the reference result of pure PET composites.53 In contrast, very weak signal peaks were observed in both the N 1s and Al 2p spectra of AlN (Fig. S7†) by comparison with reported AlN-modified separator samples.54 It possibly resulted from the embedding of the loaded AlN nanoparticles inside the PET nanowires array rather than deposition on the separator surface during the PET/AlN ink electrospinning process. This is primarily supported by the appearance of the Al EDS peaks (Fig. S4a†), EDS element mapping spectrum of Al (Fig. S4e†), and XRD pattern of AlN (Fig. 3).
O (287.75–288.2 eV) in PET/AlN 4 wt% and PET/AlN 5 wt%, which matched with the reference result of pure PET composites.53 In contrast, very weak signal peaks were observed in both the N 1s and Al 2p spectra of AlN (Fig. S7†) by comparison with reported AlN-modified separator samples.54 It possibly resulted from the embedding of the loaded AlN nanoparticles inside the PET nanowires array rather than deposition on the separator surface during the PET/AlN ink electrospinning process. This is primarily supported by the appearance of the Al EDS peaks (Fig. S4a†), EDS element mapping spectrum of Al (Fig. S4e†), and XRD pattern of AlN (Fig. 3).
 | ||
| Fig. 5 Survey XPS of (a) PET/AlN 4 wt% and (b) PET/AlN 5 wt%. HR-XPS of C 1s of (c) PET/AlN 4 wt% and (d) PET/AlN 5 wt%. | ||
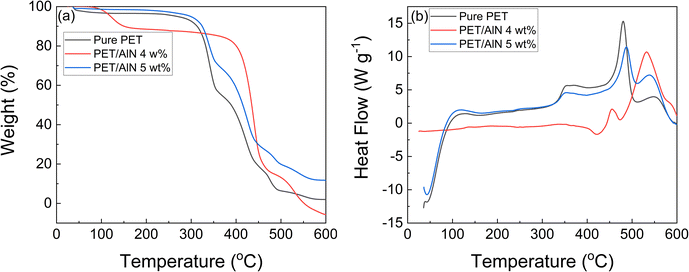
Prevention of battery burning hazard caused by overheating during prolonged service is an important criterion for a polymer-based separator; thus, thermal stability becomes an important parameter to be analyzed. From the heat shrinkage test, as shown in Fig. 6, obvious shrinkage of the PET/AlN membrane was observed in low AlN content range (≤2 wt%) (Fig. 6a–c) after 15 min of the thermal stability test. The lower the AlN percentage, the more obvious the shrinkage of the PET/AlN membrane in the same time duration as they shrink into a roll rather than a sheet. Even though slight membrane shrinkage under heat was still observed at an AlN content of 3 wt% (Fig. 6d), such a PET/AlN membrane was still regarded as unstable. In contrast, the shape of the PET/AlN membrane remained unchanged after 120 min when the wt% of AlN is larger than 3 (Fig. 6e and f). Serious shrinking also took place in a commercial Celgard® 2400 membrane after 15 min of the thermal testing (Fig. 6g). Furthermore, TGA and DSC results of PET-based membranes (Fig. 7) showed that 10% weight loss was observed in PET/AlN 4 wt% compared to that of PET/AlN 5 wt% and pure PET at 400 °C. The melting point of PET/AlN 4 wt%, PET/AlN 5 wt%, and pure was 100 °C, 200 °C and 200 °C, respectively. Such results showed that PET/AlN 4 wt% and PET/AlN 5 wt% separator membranes had higher thermal stability than pure PET membrane and commercial Celgard® 2400 membrane for LIBs.
Material properties including the surface area are also important to understand the electrolyte uptake and water proofing properties of the PET-based separator in order to prevent liquid leakage after heavy duty and hence improve the safety level of LIBs. The nanoporous structure of the PET/AlN-based composite (Fig. 2 and S3†) was the possible reason of exhibiting high porosity and large electrolyte uptake. It was reflected from the n-butane uptake test (Table 1) where the PET-based membrane exhibited higher porosity in the range of 67–74% with electrolyte uptake percentage of 499.56–629.65% compared to that of commercial Celgard® 2400 separator (porosity = 62.3%; electrolyte uptake = 201.59%). In addition, the higher the AlN content in the PET-based membrane, the lower the porosity of the PET-based membrane. It may be partially contributed by the tight pore structure in the PET-based composite at different AlN loading percentages (Fig. 2 and S3†). Another possible reason may be the superhydrophobicity of PET composites, as reflected from the contact angle measurement illustrated in Fig. 8 and S8,† where the contact angle increased from 122° (pure PET) and 119° (PET/AlN 1 wt%) to >135° in PET/AlN (2–5 wt%) with a water droplet. These are much larger that of commercial Celgard® 2400 (107°). Furthermore, the contact angle test results between the electrolyte in the PET-based membrane showed that no droplet (contact angle ∼ 0°) was observed, while small electrolyte drop with a contact angle of 51° was recorded when samples were replaced with commercial Celgard® 2400 membrane. Such observation showed strong electrolyte uptake ability with superhydrophobicity in PET-based membranes, which was enhanced upon AlN modification with higher AlN wt% compared to commercial lithium-ion battery separator. It is similar to the reported works on AlN or conductive nitride-modified polymer membranes in contact angle tests with the electrolyte,1,55 which is associated with the surface chemical polarity of AlN with unique micro/nanoporous structure in the AlN-modified separator membrane. As a result, it promoted effective diffusion/transport of ions between interfaces.1
| Separator | Porosity (%) | Electrolyte uptake (%) |
|---|---|---|
| Pure PET | 73.56 | 629.65 |
| PET/AlN 1 wt% | 71.08 | 558.81 |
| PET/AlN 2 wt% | 70.56 | 521.26 |
| PET/AlN 3 wt% | 70.12 | 555.94 |
| PET/AlN 4 wt% | 69.23 | 521.69 |
| PET/AlN 5 wt% | 67.01 | 499.56 |
| Celgard® 2400 | 62.26 | 201.59 |
Electrochemical activity of electrospun PET/AlN-based separator
In order to investigate the influence of AlN nanostructures towards the electrochemical activity of the PET-based separator batteries (ionic conductivity and effect towards cell redox), comparison studies on the PET separator-based batteries against a commercial PP separator (Celgard® 2400) were carried out. The Nyquist plots (EIS analysis) illustrated in Fig. S9a† showed that the modification of PET by AlN nanoparticles has very little difference on batteries equipped with both pure PET and commercial PP separators in terms of the shape of the curve, while higher impedance was recorded in both PET/AlN 4 wt% and PET/AlN 5 wt% compared to pure PET, which confirmed that the addition of AlN nanoparticles to the PET ink improved the stability of the electrospun PET-based composite against metallic Li in the coin battery. The CV curve shown in Fig. S9b† showed that the redox behavior of the electrospun PET/AlN separator equipped coin cell was similar to that of commercial PP (Celgard® 2400) or pure PET separator-equipped coin cell. This is reflected in the similar shape of the CV curves of the NCM 523 coin batteries with redox peak positions in the range of 3.783–3.833 V (reduction) and 3.708–3.718 V (oxidation), and similar to those reported for Celgard® 2400-equipped NCM 523 coin batteries.56,57 The redox peak of forward scan (reduction: 3.803 V) and backward scan (oxidation: 3.713 V) in a Celgard® 2400 separator was laid in the range of that in electrospun PET separators (reduction: 3.783–3.833 V, oxidation: 3.708–3.718 V). This showed that electrospun PET separator did not alternate the redox behavior of the commercial NCM 523 cathode. The output current of forward scan peak in electrospun PET separators (pure PET, PET/AlN 4 and 5 wt%: 0.174–0.182 mA) was comparable to that of Celgard® 2400 (0.182 mA). The output current of the forward scan peak of electrospun PET/AlN 2 wt% and PET/AlN 3 wt% separators was 0.137 and 0.165 mA, respectively, which may be owing to the fluctuation of the cell performance equipped with the above mentioned separators. Overall, the performance of the electrospun PET/AlN-based separators was comparable to the commercial PP separators. Such a finding was supported by similar results in the reported polymer-based separator-equipped lithium-ion batteries (lithium cobalt oxide (LiCoO2: LCO)50 or lithium-iron-phosphate (LiFePO4: LFP)49). Only difference is that the battery in the current work is NCM 523 type.Cell performance is an indicator of the PET-based separator quality determination when are equipped in commercial LIBs for commercialization. Only pure PET, PET/AlN 4 wt% and PET/AlN 5 wt% were chosen as potential candidates for LIBs applications due to the fact that poor thermal stability of the PET/AlN separator with AlN wt% ≤ 3 was confirmed by the thermal stability test (Fig. 6). Such candidates show that the safety problem by the short circuit between the NCM 523 cathode and Li anode eventually takes place. The reversible capacity and cycling performance of LIBs with AlN/PETs in CR2032 coin cells were further investigated to demonstrate the potential applications in LIBs. Fig. 9a, b and S10a† show the specific capacities of CR2032 cells between 3.0 and 4.2 V at 0.5C. For the first cycle (2nd operation cycle), the capacity for charging and discharging was approximately 256.8 (PET/AlN 5 wt%) > 250.4 (PP) > 237.2 (PET/AlN 4 wt%) mA h g−1; and 230.9 (PP) > 225.8 (PET/AlN 5 wt%) > 200.1 (PET/AlN 4 wt%) mA h g−1, respectively. The corresponding coulombic efficiency was 92.2% (PP) > 87.9% (PET/AlN 5 wt%) > 84.4% (PET/AlN 4 wt%), which indicates high electrochemical reversibility. As a result of the formation of irreversible solid–electrolyte interphase (SEI) layers on the active material surface, capacity loss occurred in the first cycle. The better cycling performance was attributed to the increases in electrolyte uptake and conductivity of lithium ions, which provided an easier path for ionic transport and better electrolyte retention.58 Reversible charging capacity and discharging capacity in descending order were 241.7 (PET/AlN 4 wt%) > 239.5 (PET/AlN 5 wt%) > 0225.7 (PP) mA h g−1 and 240.4 (PET/AlN 5 wt%) > 236.8 (PET/AlN 4 wt%) > 225.6 (PP) mA h g−1 after 100 cycles (101th operating cycle), the descending discharge capacity retention was 100.35% (PET/AlN 5 wt%) > 99.97% (PP) > 97.97% (PET/AlN 4 wt%), indicating no obvious decline in repeated charge–discharge cycles. The recorded discharge specific capacity of the PP separator equipped coin battery (225.6–230.9 mA h g−1, Fig. S10a†) was similar to some of the reported PP separator-equipped pristine NCM 523 coin batteries where the discharge specific capacity was 202 to 225 mA h g−1 in the cyclic performance test.59,60 Overall, the recorded specific capacity of the electrospun PET/AlN separator was slightly higher than that compared to the one from commercial PP separators with lower capacity retention rate, as mentioned previously (Fig. 9a, b and S10a†). Such a finding was similar to the overall tendency of some synthesized polymer separators reported elsewhere, even though graphite47 or LFP48,49 cathode was used.
Various current densities were used in repeated charge–discharge cycles to evaluate the rate capability (C-rate) of the commercial PP separator and PET/AlN-equipped coin cells. Fig. 9c, d and S9b† showed the 50-cycle C-rate performance of CR2032 cells with commercialized PP separator and PET/AlN (4 and 5 wt%). The reversible capacity of the battery equipped with the commercialized PP separator was about 238.5 mA h g−1 at 0.2C and decreased to 134.6 mA h g−1 at 4C, which showed relatively low discharge capacity and rapid decline. For the cell with 5 wt% PET/AlN, the capacities were 246.5, 225.1, 208.2, 179.2, and 116.5 mA h g−1 at current densities of 0.2C, 0.5C, 1C, 2C and 4C, respectively. In contrast, the cell with 4 wt% PET/AlN exhibited a capacity of 225.3, 218.4, 191.0, 127.5, 28.1 mA h g−1 in the identical range of current densities mentioned previously.
During the cell cycle charging/discharging process, the capacity attenuation was due to active material dissolution, electrolyte decomposition and the formation of resistive layers on electrodes, leading to polarization between electrolyte–electrode interfaces.61,62 Resistance between the separator and metallic lithium in the anode with excellent battery performance is key to the success of the high-performance battery with high safety. The improvement in the impedance and comparable battery redox properties (Fig. S9†) in the AlN nanoparticles-modified PET separator with stronger battery performance (cycling and C-rate test (Fig. 9)) to the commercial PP separator-equipped battery was recorded in the current study. Furthermore, the cycling performances of lithium-ion battery separators are influenced by the high wettability, porosity and electrolyte uptake due to the great migration of the lithium ion between the electrode–electrolyte interface. The stronger electrolyte uptake and lower pore size (Table 1 and Fig. 8), and strong thermal stability (Fig. 6) of the electrospun PET/AlN 4 wt% and 5 wt% separators showed that the electrospun PET/AlN 4 wt% and PET/AlN 5 wt% separator, same as that of the reported polymer separators, enhanced output cycling performance under fixed C-rate and varying C-rate due to (1) the enhanced Li+ transfer between electrodes by the low interfacial impedance according to the EIS test (Fig. S9a†), (2) stronger electrolyte uptake, and (3) effective Li dendrite prevention due to the relatively low pore size in the PET/AlN 4 wt% and 5 wt% polymer (Table 1), which have been explored elsewhere with other cathodic materials (graphite47 or LFP48,49).
In order to understand the stability of the separator after prolonged battery service to estimate the potential value of the electrospun membrane produced from recycled PET bottles, further characterization of the spent separator was carried out by comparison with fresh separators. The SEM result illustrated in Fig. 10 under different magnifications showed that the morphology of the electrospun PET/AlN 4 wt% and 5 wt% separators was changed from smooth (PET/AlN 4 wt%: Fig. 10b and f; PET/AlN 5 wt%: Fig. 10d and h) into rough (PET/AlN 4 wt%: Fig. 10a and e; PET/AlN 5 wt%: Fig. 10c and g) after the cycling performance test (0.5C, 101 cycles). The rough surface might be due to the deposition of LiPF6 onto PET/AlN fibers. Even though the rough surface observed in spent PET/AlN 5 wt% (Fig. 10e and g) may be the reason of the relatively strong fluctuation in the discharge capacity in the cyclic performance at 0.5C (Fig. 9b) and C-rate test (Fig. 9d) of PET/AlN 5 wt% compared to those of PET/AlN 4 wt% (Fig. 9a and c) correspondingly.
The summarized results in Tables 1, 2 and Fig. 6, 8–10 and S10† show that PET/AlN 4 wt% separator-equipped cell were regarded as the best candidate due to relatively lower specific capacity fading and fluctuation upon fixed (cycling performance at 0.5C: Fig. 9a and b) or increased current density (C-rate: Fig. 9c and d) compared to the PET/AlN 5 wt% separator. Such a finding provided a new potential for developing next-generation separators via the recycling of low-cost waste commercial PET bottles with suitable modifications in industrial scale.
| Separator | PET/AlN 4 wt% | PET/AlN 5 wt% |
|---|---|---|
| Charging capacity (mA h g−1) | 240.5 | 241.7 |
| Discharging capacity (mA h g−1) | 236.8 | 238.2 |
| Coulombic efficiency (%) | 98.0 | 100.4 |
| Capacity (0.5C) (mA h g−1) | 225.3 | 246.5 |
| Capacity (0.2C) (mA h g−1) | 218.4 | 225.1 |
| Capacity (1C) (mA h g−1) | 191.0 | 208.2 |
| Capacity (2C) (mA h g−1) | 127.5 | 179.2 |
| Capacity (4C) (mA h g−1) | 28.1 | 115.6 |
Conclusions
PET/AlN nanostructures obtained from waste drinking water bottles were successfully synthesized via electrospinning in this study. The synthesized PET/AlN separator composites exhibited excellent hydrothermal stability, superhydrophobicity and electrolyte uptake properties in comparison to the commercial Celgard® 2400 separator and pure PET composites, where PET/AlN 4 wt% is regarded as the best one in above mentioned tests. With regards to the electrochemical performance of the PET/AlN composite as the separator, which is equipped in the coin cell, it could deliver a reversible specific capacity of 240.5 mA h g−1 after 100 cycles at a current density 0.5C without significant capacity fading. PET/AlN 4 wt% also exhibited relatively higher stability in both cycling and C-rate analysis. This could be attributed to the unique morphology of PET/AlN in PET/AlN 4 wt%. Overall, this study demonstrated the excellent electrochemical performance of the PET/AlN composite as a high-stability separator material for next-generation LIBs.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There is no conflicts to declare.Acknowledgements
This work is financially supported by Hong Kong Competitive Research Funding Schemes for the Local self-financing Degree Sector Faculty Development Scheme (No. UGC/FDS25/E06/19).Notes and references
- Y. Guo, Q. Wu, L. W. Liu, G. C. Li, L. J. Yang, X. Z. Wang, Y. W. Ma and Z. Hu, Adv. Sci., 2022, 9, 2200411 CrossRef CAS PubMed
.
- B. R. Cai, J. H. Cao, W. H. Liang, L. Y. Yang, T. Liang and D. Y. Wu, ACS Appl. Energy Mater., 2021, 4, 5293–5303 CrossRef CAS
.
- Q. Q. Lu, C. C. Liu, Y. R. Zhao, W. A. Pan, K. Xie, P. F. Yue, G. S. Zhang, A. Omar, L. X. Liu, M. H. Yu and D. Mikhailova, SusMat, 2023, 3, 471–497 CrossRef CAS
.
- Y. Q. Feng, H. Liu and Q. Q. Lu, Nano Res., 2024, 17, 1337–1365 CrossRef CAS
.
- J. L. Hao, G. T. Lei, Z. H. Li, L. J. Wu, Q. Z. Xiao and L. Wang, J. Membr. Sci., 2013, 428, 11–16 CrossRef CAS
.
- X. Y. Jiang, L. F. Xiao, X. P. Ai, H. X. Yang and Y. L. Cao, J. Mater. Chem. A, 2017, 5, 23238–23242 RSC
.
- R. P. Luo, C. Wang, Z. X. Zhang, W. Q. Lv, Z. H. Wei, Y. N. Zhang, X. Y. Luo and W. D. He, ACS Appl. Energy Mater., 2018, 1, 921–927 CrossRef CAS
.
- C. J. Fang, S. L. Yang, X. F. Zhao, P. F. Du and J. Xiong, Mater. Res. Bull., 2016, 79, 1–7 CrossRef CAS
.
- Y. Ma, J. P. Hu, Z. T. Wang, Y. Q. Zhu, X. L. Ma and C. B. Cao, J. Power Sources, 2020, 451, 227759 CrossRef CAS
.
- M. Yanilmaz, M. Dirican and X. W. Zhang, Electrochim. Acta, 2014, 133, 501–508 CrossRef CAS
.
- Y. E. Miao, G. N. Zhu, H. Q. Hou, Y. Y. Xia and T. X. Liu, J. Power Sources, 2013, 226, 82–86 CrossRef CAS
.
- D. Z. Wu, C. Shi, S. H. Huang, X. C. Qiu, H. Wang, Z. Zhan, P. Zhang, J. B. Zhao, D. H. Sun and L. W. Lin, Electrochim. Acta, 2015, 176, 727–734 CrossRef CAS
.
- W. Ul Arifeen, M. Kim, D. Ting, R. Kurniawan, J. Choi, K. Yoo and T. J. Ko, Mater. Chem. Phys., 2020, 245, 122780 CrossRef CAS
.
- G. Zainab, X. F. Wang, J. Y. Yu, Y. Y. Zhai, A. A. Babar, K. Xiao and B. Ding, Mater. Chem. Phys., 2016, 182, 308–314 CrossRef CAS
.
- L. J. Wang, Z. H. Wang, Y. Sun, X. Liang and H. F. Xiang, J. Membr. Sci., 2019, 572, 512–519 CrossRef CAS
.
- J. Y. Tan, L. Y. Kong, Z. M. Qiu and Y. R. Yan, J. Solid State Electrochem., 2018, 22, 3363–3373 CrossRef CAS
.
- M. Yanilmaz, Y. Lu, J. D. Zhu and X. W. Zhang, J. Power Sources, 2016, 313, 205–212 CrossRef CAS
.
- W. X. Yang, Y. B. Liu, X. M. Hu, J. B. Yao, Z. J. Chen, M. Hao, W. J. Tian, Z. Huang and F. Y. Li, Polymers, 2019, 11, 1671 CrossRef CAS PubMed
.
- L. Y. Cao, P. An, Z. W. Xu and J. F. Huang, J. Electroanal. Chem., 2016, 767, 34–39 CrossRef CAS
.
- M. Cai, D. Yuan, X. Zhang, Y. Pu, X. F. Liu, H. W. He, L. X. Zhang and X. Ning, J. Power Sources, 2020, 461, 228123 CrossRef CAS
.
- C. Y. Cao, L. Tan, W. W. Liu, J. Q. Ma and L. Li, J. Power Sources, 2014, 248, 224–229 CrossRef CAS
.
- J. Shayapat, O. H. Chung and J. S. Park, Electrochim. Acta, 2015, 170, 110–121 CrossRef CAS
.
- F. L. Huang, W. T. Liu, P. Y. Li, J. X. Ning and Q. F. Wei, Materials, 2016, 9, 75 CrossRef PubMed
.
- Q. F. Wang, Y. Yu, J. Ma, N. Zhang, J. J. Zhang, Z. H. Liu and G. L. Cui, J. Power Sources, 2016, 327, 196–203 CrossRef CAS
.
- D. Boriboon, T. Vongsetskul, P. Limthongkul, W. Kobsiriphat and P. Tammawat, Carbohydr. Polym., 2018, 189, 145–151 CrossRef CAS PubMed
.
- J. Ding, Y. Kong, P. Li and J. R. Yang, J. Electrochem. Soc., 2012, 159, A1474–A1480 CrossRef CAS
.
- P. J. Kim, Nanomaterials, 2021, 11, 2275 CrossRef CAS PubMed
.
- W. K. Shin and D. W. Kim, J. Power Sources, 2013, 226, 54–60 CrossRef CAS
.
- K. Bicy, S. Suriyakumar, P. A. Paul, A. S. Anu, N. Kalarikkal, A. M. Stephen, V. G. Geethamma, D. Rouxel and S. Thomas, New J. Chem., 2018, 42, 19505–19520 RSC
.
- N. X. Wei, J. N. Hu, M. Z. Zhang, J. L. He and P. H. Ni, Electrochim. Acta, 2019, 307, 495–502 CrossRef CAS
.
- X. L. Dong, W. L. Mi, L. H. Yu, Y. Jin and Y. S. Lin, Microporous Mesoporous Mater., 2016, 226, 406–414 CrossRef CAS
.
- W. Wang, Y. Yuan, J. L. Wang, Y. Zhang, C. Liao, X. W. Mu, H. B. Sheng, Y. C. Kan, L. Song and Y. Hu, ACS Appl. Energy Mater., 2019, 2, 4167–4174 CrossRef CAS
.
- J. C. Liu, K. Yang, Y. D. Mo, S. J. Wang, D. M. Han, M. Xiao and Y. Z. Meng, J. Power Sources, 2018, 400, 502–510 CrossRef CAS
.
- Y. Wang, Q. L. Wang, Y. Lan, Z. L. Song, J. P. Luo, X. Q. Wei, F. G. Sun, Z. H. Yue, C. Q. Yin, L. Zhou and X. M. Li, Solid State Ionics, 2020, 345, 115188 CrossRef CAS
.
- H. Li, F. Lin, H. Wang, H. H. Wu, Y. X. Yang, L. Yu, W. Liu and D. W. Luo, New J. Chem., 2020, 44, 3838–3846 RSC
.
- J. Q. Cui, J. Q. Liu, C. F. He, J. Li and X. F. Wu, J. Membr. Sci., 2017, 541, 661–667 CrossRef CAS
.
- J. D. Xie, C. C. Fu, C. C. Liao, R. S. Juang and Y. A. Gandomi, Asia-Pac. J. Chem. Eng., 2019, 14, e2335 CrossRef
.
- G. H. Sun, J. C. Guo, H. Q. Niu, N. J. Chen, M. Y. Zhang, G. F. Tian, S. L. Qi and D. Z. Wu, RSC Adv., 2019, 9, 40084–40091 RSC
.
- G. H. Feng, Z. H. Li, L. W. Mi, J. Y. Zheng, X. M. Feng and W. H. Chen, J. Power Sources, 2018, 376, 177–183 CrossRef CAS
.
- K. Peng, B. Wang, Y. M. Li and C. C. Ji, RSC Adv., 2015, 5, 81468–81473 RSC
.
- K. L. Xu, Y. Qin, T. Xu, X. H. Xie, J. X. Deng, J. L. Qi and C. Huang, J. Membr. Sci., 2019, 592, 117364 CrossRef CAS
.
- X. K. Zhang, N. Li, Z. M. Hu, J. R. Yu, Y. Wang and J. Zhu, J. Membr. Sci., 2019, 581, 355–361 CrossRef CAS
.
- D. Li, D. Q. Shi, Y. G. Xia, L. Qiao, X. F. Li and H. M. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 8742–8750 CrossRef CAS PubMed
.
- Z. J. Wang, Y. Y. Wang, C. Wu, W. K. Pang, J. F. Mao and Z. P. Guo, Chem. Sci., 2021, 12, 8945–8966 RSC
.
- A. C. M. de Moraes, W. J. Hyun, N. S. Luu, J. M. Lim, K. Y. Park and M. C. Hersam, ACS Appl. Mater. Interfaces, 2020, 12, 8107–8114 CrossRef CAS PubMed
.
- P. J. H. Kim and V. G. Pol, ACS Appl. Mater. Interfaces, 2019, 11, 3917–3924 CrossRef CAS PubMed
.
- R. Badrnezhad, A. Fathollahi Zonouz and H. Pourfarzad, Anal. Bioanal. Electrochem., 2022, 14, 160–178 CAS
.
- H. P. Cai, X. Tong, K. Chen, Y. F. Shen, J. S. Wu, Y. Y. Xiang, Z. Wang and J. S. Li, Polymers, 2018, 10, 574 CrossRef PubMed
.
- D. N. Li, H. Xu, Y. Liu, Y. S. Jiang, F. F. Li and B. Xue, Ionics, 2019, 25, 5341–5351 CrossRef CAS
.
- L. Sheng, R. Xu, H. Zhang, Y. Z. Bai, S. J. Song, G. J. Liu, T. Wang, X. L. Huang and J. P. He, J. Electroanal. Chem., 2020, 873, 114391 CrossRef CAS
.
- B. R. Li, B. Wen, H. Z. Chen, W. Zhang, X. Meng, M. Y. Jia and F. Chen, Corros. Sci., 2021, 178, 109058 CrossRef CAS
.
- A. P. D. Pereira, M. H. P. da Silva, E. P. Lima, A. D. Paula and F. J. Tommasini, Mater. Res., 2017, 20, 411–420 CrossRef
.
- J. H. Oh, T. J. Ko, M. W. Moon and C. H. Park, RSC Adv., 2017, 7, 25597–25604 RSC
.
- T. Zhang, H. C. Lu, J. Yang, Z. X. Xu, J. L. Wang, S. I. Hirano, Y. S. Guo and C. D. Liang, ACS Nano, 2020, 14, 5618–5627 CrossRef CAS PubMed
.
- Z. F. Liu, Q. M. Hu, S. T. Guo, L. Yu and X. L. Hu, Adv. Mater., 2021, 33, 2008088 CrossRef CAS PubMed
.
- G. H. Jiang, Y. N. Zhang, Q. Meng, Y. J. Zhang, P. Dong, M. Y. Zhang and X. Yang, ACS Sustain. Chem. Eng., 2020, 8, 18138–18147 CrossRef CAS
.
- X. Q. Chen, C. F. Yang, Y. B. Yang, H. M. Ji and G. Yang, J. Alloys Compd., 2022, 909, 164691 CrossRef CAS
.
- D. Z. Wu, J. L. He, M. Z. Zhang, P. H. Ni, X. F. Li and J. K. Hu, J. Power Sources, 2015, 290, 53–60 CrossRef CAS
.
- J. A. Gilbert, J. Bareño, T. Spila, S. E. Trask, D. J. Miller, B. J. Polzin, A. N. Jansen and D. P. Abraham, J. Electrochem. Soc., 2017, 164, A6054–A6065 CrossRef CAS
.
- Q. Yang, W. Wang, K. Qian and B. Li, Adv. Mater. Interfaces, 2019, 6, 1801764 CrossRef
.
- J. H. Cho, J. H. Park, M. H. Lee, H. K. Song and S. Y. Lee, Energy Environ. Sci., 2012, 5, 7124–7131 RSC
.
- S. M. Oh, S. T. Myung, J. B. Park, B. Scrosati, K. Amine and Y. K. Sun, Angew. Chem., Int. Ed., 2012, 51, 1853–1856 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra06478j |
| This journal is © The Royal Society of Chemistry 2025 |