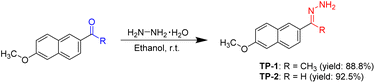Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Selective fluorescent probe for Tl3+ ions through metal-induced hydrolysis and its application for direct assay of artificial urine†
Myung Gil Choi ,
Yerin Jang,
Mi Gang Kim,
Sangdoo Ahn
,
Yerin Jang,
Mi Gang Kim,
Sangdoo Ahn * and
Suk-Kyu Chang
* and
Suk-Kyu Chang *
*
Department of Chemistry, Chung-Ang University, Seoul 06974, Republic of Korea. E-mail: sangdoo@cau.ac.kr; skchang@cau.ac.kr; Fax: +82 2 825 4736; Tel: +82 2 820 5230
First published on 15th January 2025
Abstract
In this research, we report a simple fluorescent probe designed to detect thallium(III) ions (Tl3+) in artificial urine samples. The Tl3+ signaling probe (TP-1) was readily prepared from 2-acetyl-6-methoxynaphthalene and hydrazine. In a pH 4.8 acetate buffer solution containing 1% (v/v) N,N-dimethylformamide as a solubilizer, probe TP-1 exhibited turn-on fluorescence signaling behavior in the presence of Tl3+. Other metal ions, anions, and major urine components such as uric acid, urea, and creatinine did not produce any noticeable fluorescence changes. The Tl3+ signaling of TP-1 was attributed to the hydrolysis of the hydrazone moiety, yielding the parent fluorophore 2-acetyl-6-methoxynaphthalene. The detection limit of TP-1 for Tl3+ sensing was 19 nM, and the signaling was completed within 2 min. Additionally, to further optimize the Tl3+ signaling of the hydrazone derivatives, we compared the effect of structural variations between the closely related ketone-hydrazone (TP-1) and aldehyde-hydrazone (TP-2) derivatives. We confirmed that the ketone-hydrazone (TP-1) demonstrated rapid and stable Tl3+ signaling behavior with satisfactory stability under the measurement conditions. Finally, as a practical application, a Tl3+ assay in artificial urine samples was performed using a smartphone as a portable signaling measurement and data analysis device.
1. Introduction
Thallium, a Group 13 (boron group) post-transition metal, is well-known for its notorious toxicity in various environments.1 Despite its dangerous nature, thallium is used in several modern industrial fields, including the electronics industry for photoelectric cells and infrared detectors, and the manufacturing of glass with a high refractive index and low melting point.2 Additionally, thallium serves as an agent in nuclear medical scans and is utilized in medical products due to its resistance to acid and corrosion, as well as its antifriction properties.3 However, the widespread presence of thallium in the environment poses significant health risks,4 even at low concentrations, and causes severe neurological and gastrointestinal disorders.5 For these reasons, the United States Environmental Protection Agency (EPA) classifies thallium as a significant pollutant.6 Consequently, there is ongoing research interest in developing convenient and sensitive analytical methods specifically for detecting thallium.Thallium primarily exists in two oxidation states: thallous (Tl+) and thallic (Tl3+) ions. It is known to be more toxic than other representative heavy metal ions, such as cadmium and mercury.7 Thallium(I) is more stable and prevalent than thallium(III), but the latter is four times more toxic to humans and animals than the former.8 Traditionally, the determination of thallium has relied on standard instrument-based methods such as atomic absorption spectroscopy,9,10 inductively coupled plasma mass spectrometry,11,12 voltammetry/potentiometry,13 spectrophotometry,14,15 and fluorimetry,16 all of which are known for their high sensitivity and accuracy. However, these techniques require sophisticated instrumentation and are often costly, making them unsuitable for on-site, real-time monitoring of thallium levels. This limitation is particularly challenging in settings where rapid, accessible detection is crucial, such as in field-based or point-of-care scenarios. Therefore, the development of convenient and practical detection methods for thallium ions without resorting to complicated heavy instruments is necessary.
Various sensors and reaction-based molecular probes utilizing colorimetric and fluorometric responses have been actively investigated for the easy determination of toxic metal ions due to their selective complex formation ability and specific reactivity toward target species.17,18 These sensors and probes offer several advantages, such as simplicity, cost-effectiveness, portability, and the potential for real-time detection. Several thallium signaling sensors have been reported, employing the formation of selective host-guest type thallium complexes with hydroxamic acid and bis-pyridine ligands of the host system.14,19 Among the various exceptional thallium sensing approaches, reaction-based probes have recently emerged as a particularly attractive option due to their high sensitivity, remarkable selectivity, and rapid response times.20 For instance, the oxidation of trifluoperazine and arsenoxylphenylazo rhodanine derivatives has been investigated for the determination of Tl3+ ions in alloys, minerals, and urine samples.21,22 The oxidative coupling reaction between 3-methyl-2-benzothiazolinone hydrazone (MBTH) and 10,11-dihydro-5H-dibenzo[b,f]azepine (IDB) has also been reported for Tl3+ sensing in practical water samples and urine.23 Furthermore, oxidative hydrolysis of rhodamine sulfonhydrazide and hydroxamic acid has been identified as effective means for the colorimetric and fluorescent signaling for Tl3+ ions.24,25 The properties, signaling mechanisms, and practical applications of these reported Tl3+ signaling systems are summarized in Table S1 (ESI).†
A thallium-selective probe was designed by using well-established 2-acetyl-6-methoxynaphthalene as a signaling chromo-fluorophore and a hydrazone moiety as a signaling trigger. Hydrazones are highly useful and versatile compounds in organic and medicinal chemistry,26,27 and they are extensively utilized in the construction of metal–organic frameworks (MOFs), covalent organic frameworks (COFs), dynamic combinatorial chemistry, and as hole-transporting materials.28 Notably, numerous chemical sensors and probes based on hydrazone-containing molecules have been developed for detecting and visualizing chemically and environmentally significant metal ions, anions, and biologically important species.29 Hydrazone-based chemosensors for the determination of cyanide,30 fluoride,31 and acetate ions32 have been exploited through selective addition of the analyte or deprotonation of the hydrazone subunit. Additionally, several sensors incorporating hydrazone function as a binding site have been created for metal ion sensing through metal-hydrazone complex formation.33–35 In parallel to these, a number of hydrazone-based, reaction-based probes have been investigated for the determination of metal ions such as Cu2+ and Hg2+,36,37 as well as common oxidants like hypochlorite38 and peroxynitrite.39,40
In this research, we aimed to develop an easy and rapid method for the convenient measurement of urinary thallium levels of suspected acute thallium poisoning in the field. Initial clinical tests for the screening of thallium poisoning include urine tests, blood tests, and electrolyte tests that can easily check the patient's metal pollution status. Among these, analyzing thallium ions using urine with spectroscopic methods is more rapid and convenient due to the ease of sample collection and preparation as well as the simplicity of analysis without using complex equipment. We report the results obtained for a simple fluorescent signaling probe exhibiting useful fluorescence signaling for Tl3+ ions via the metal-assisted oxidative hydrolysis of a hydrazone moiety. We comparatively investigated the Tl3+ signaling behavior using two similar-structured ketone-hydrazone (TP-1) and aldehyde-hydrazone (TP-2) candidates. The thallium signaling of probe TP-1 ensures the rapid and convenient detection of the thallium level, without interference from common metal ions, anions, and major components of urine solution. The unique design of TP-1 offers not only a high selectivity for Tl3+ ions, even amidst common components in artificial urine, but also a rapid fluorescence response and compatibility with smartphone-based analysis, making it particularly suitable for field-based diagnostics. The practical application of the developed probe was ascertained by the successful determination of Tl3+ levels in artificial urine samples using merely a smartphone as a signaling measurement and analysis device. This advancement could be transformative in clinical and environmental settings where rapid, accessible detection of toxic metals is essential for timely intervention, using artificial urine as a readily available analyte for clinical testing.
2. Experimental
2.1 Preparation of Tl3+ signaling probes (TP-1 and TP-2)
Hydrazones of 2-acetyl-6-methoxynaphthalene and 6-methoxy-2-naphthaldehyde were synthesized following a modified procedure from the literature.37 For TP-1, 2-acetyl-6-methoxynaphthalene (0.40 g, 2.0 mmol) was placed in a 50 mL round-bottom flask and dissolved in 10 mL of ethanol. To this solution, an excess amount of hydrazine monohydrate (0.46 mL, 10.0 mmol) was added dropwise. The reaction mixture was stirred at room temperature for 12 hours. The resulting precipitate was then filtered and washed three times with 5 mL portions of ethanol. The product was purified by recrystallization from ethanol, yielding TP-1 as a white-yellow powder (0.38 g, 88.8% yield). The purity of TP-1 was verified to be >99% by high performance liquid chromatography.Similarly, to prepare TP-2, 6-methoxy-2-naphthaldehyde (0.37 g, 2.0 mmol) was added to a 50 mL round-bottom flask and dissolved in 10 mL of ethanol. Hydrazine monohydrate (0.46 mL, 10.0 mmol) was then slowly added to the solution. The reaction was allowed to proceed with stirring at room temperature for 12 hours. Afterward, the precipitate was collected by filtration, washed thoroughly with ethanol, and purified by recrystallization from ethanol, yielding TP-2 as a white-yellow powder (0.37 g, 92.5% yield). The purity of TP-2 was determined to be >99% by high performance liquid chromatography.
The spectral data for both compounds are as follows:
TP-1: 1H NMR (600 MHz, DMSO-d6) δ 7.93 (d, J = 1.8 Hz, 1H), 7.91 (dd, J = 8.6, 1.9 Hz, 1H), 7.82 (d, J = 8.9 Hz, 1H), 7.71 (d, J = 8.6 Hz, 1H), 7.27 (d, J = 2.6 Hz, 1H), 7.13 (dd, J = 8.9, 2.5 Hz, 1H), 6.38 (s, 2H), 3.86 (s, 3H), 2.11 (s, 3H); 13C NMR (150 MHz, DMSO-d6): δ157.65, 142.61, 135.54, 133.97, 130.04, 128.82, 126.75, 124.13, 123.72, 118.86, 106.39, 55.62, 11.59; HRMS (EI+, m/z): calcd for C13H14N2O+ [M]+: 214.1106, found 214.1106.
TP-2: 1H NMR (600 MHz, DMSO-d6) δ 7.82 (s, 1H), 7.79 (d, J = 8.9 Hz, 1H), 7.74 (br s, 3H), 7.28 (d, J = 2.5 Hz, 1H), 7.14 (dd, J = 8.9, 2.6 Hz, 1H), 6.76 (s, 2H), 3.86 (s, 3H); 13C NMR (150 MHz, DMSO-d6): δ 157.71, 139.11, 134.33, 132.45, 129.74, 128.92, 127.42, 125.29, 123.44, 119.14, 106.69, 55.65; HRMS (EI+, m/z): calcd for C12H12N2O+ [M]+: 200.0950, found 200.0947.
2.2 Preparation of stock solutions for Tl3+ detection
A 500 μM stock solution of probes TP-1 and TP-2 was prepared by dissolving in N,N-dimethylformamide (DMF). Stock solutions of metal ions and anions, each at a concentration of 10.0 mM, were prepared using deionized water (DI water). Tl3+ stock solution was prepared in 0.05 M HCl solution and standardized by iodometric titration.41 The acetate buffer solution, with a pH of 4.8, was formulated according to a procedure described in the literature.422.3 Preparation of samples for Tl3+ sensing
The sample solutions for Tl3+ signaling using TP-1 and TP-2 were prepared under optimized conditions using a pH 4.8 acetate buffer solution containing 1% (v/v) DMF. In a 15 mL sample vial, 15 μL of the 10.0 mM analyte stock solution was added and diluted with 2.80 mL of DI water and 150 μL of 200 mM acetate buffer solution at pH 4.8. Subsequently, 30 μL of a 0.50 mM solution of either probe TP-1 or TP-2 was added to the vial and gently mixed. The final concentrations of the probe, analyte, and buffer in the sample solution were 5.0 μM, 50 μM, and 10 mM, respectively. All samples were prepared and measured in triplicate, and the error bars were calculated based on the standard deviation of these measurements.2.4 Exploring the mechanism of Tl3+ sensing of TP-1
To obtain the purified signaling product for Tl3+ sensing, probe TP-1 (0.021 g, 0.10 mmol) was dissolved in 10 mL of DMF. Thallium nitrate trihydrate (0.088 g, 0.20 mmol) was then dissolved in 0.50 mL of a 50 mM HCl solution. This thallium solution was subsequently diluted with 9.0 mL of DI water and 0.50 mL of 100 mM acetate buffer solution at pH 4.8. The thallium solution was carefully added to the probe TP-1 solution and mixed for 1 h. The reaction progression of the Tl3+ signaling was monitored using thin-layer chromatography (TLC) measurement. The resulting product was extracted with dichloromethane (DCM) and purified using column chromatography (silica gel, eluent: DCM). The purified product of Tl3+ sensing was then characterized by 1H and 13C NMR spectroscopy and mass spectrometry.2.5 Tl3+ analysis of artificial urine samples
Tl3+ analysis of artificial urine samples using a smartphone as an all-in-one platform for signal acquisition and data processing was performed following a modified procedure described in the literature.433. Results and discussion
The Tl3+-selective probes have been developed using the unique oxidative properties of thallic ions, among other chemical reactions. For instance, oxidation of trifluoperazine derivative to its sulfoxide form,21 as well as the oxidative hydrolysis of sulfonhydrazine24 and rhodamine hydroxamate25 have been employed for successful Tl3+ analysis. In this study, we explored the Tl3+ signaling behavior of hydrazone derivatives of the methoxynaphthalene fluorophore, leveraging the unique Tl3+ metal ion-selective hydrolysis characteristic of the hydrazone moiety. The hydrolysis of hydrazones has previously been utilized for the construction of selective signaling probes targeting Cu2+ and Hg2+ ions.34,37,44 The designed probes, TP-1 and TP-2, were synthesized through the reaction of 2-acetyl-6-methoxynaphthalene (for ketone hydrazone) and 6-methoxy-2-naphthaldehyde (for aldehyde hydrazone) with hydrazine monohydrate in ethanol, yielding 88.8% and 92.5%, respectively (Scheme 1). The structures of probes TP-1 and TP-2 were confirmed by 1H and 13C NMR spectroscopy and high-resolution mass spectrometry (ESI†). Additionally, the photophysical properties of TP-1 and TP-2 in the presence and absence of Tl3+ ions are summarized in Table S2 (ESI).†To investigate the Tl3+ signaling behaviors of TP-1 and TP-2, we initially examined the UV-vis spectra of both probes in the presence and absence of Tl3+ ions. As illustrated in Fig. S1 (ESI),† the absorbance changes of TP-1 and TP-2 were not significant. Consequently, we conducted the fluorescence-based Tl3+ signaling experiments. Ketone and aldehyde hydrazones have been reported to exhibit different stabilities and reactivities toward the target material.37 Therefore, we explored the time-dependent Tl3+ signaling behaviors of TP-1 and TP-2 and their stability under measurement conditions. As demonstrated in Fig. S2 (ESI),† the ketone-based hydrazone TP-1 exhibited rapid signaling in response to Tl3+ ions, and it remained stable without decomposing under the measurement conditions. In contrast, the aldehyde-based hydrazone TP-2 itself underwent hydrolysis under the experimental conditions (Fig. S3, ESI†). Consequently, the thallium signaling experiments were primarily conducted using the ketone-based hydrazone TP-1.
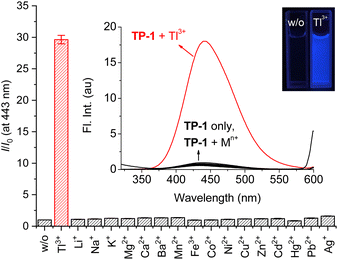
Initially, we confirmed changes in the fluorescence emission of probe TP-1 in the presence of Tl3+ ions and other representative metal ions (Fig. 1). Probe TP-1 exhibited weak fluorescence emission around 435 nm. However, upon treatment with Tl3+ ion, the probe displayed a significant enhancement in fluorescence emission at 443 nm, along with blue fluorescence under UV-lamp irradiation. Conversely, the other tested metal ions did not exhibit any noticeable changes. We quantified these fluorescence changes of TP-1 with metal ions by measuring the fluorescence enhancement at 443 nm (I/I0 at 443 nm). As depicted in Fig. 1, the fluorescence enhancement (I/I0) for Tl3+ ions was 29.6. Meanwhile, the I/I0 values for other metal ions varied within a narrow range, from 0.86 for Hg2+ ions to 1.61 for Ag+ ions. Additionally, we investigated the fluorescence signaling behavior of TP-1 toward common anions. As shown in Fig. S4 (ESI),† probe TP-1 demonstrated a significant fluorescence response toward Tl3+ ions over the tested anions, with the fluorescence enhancement (I/I0) ranging narrowly from 0.93 for SO32− to 1.81 for P2O74−.
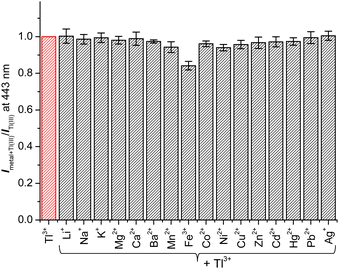
Next, to assess the practical applicability of TP-1 for sensing Tl3+ ions in urine, we evaluated the effect of coexisting ions on the Tl3+ signaling behavior of the probe. As illustrated in Fig. 2, the signaling of the probe for Tl3+ was unaffected by the presence of other tested metal ions serving as background. The fluorescence intensity enhancement ratio (Imetal+Tl(III)/ITl(III)) of TP-1 in the presence of common metal ions varied slightly, ranging from 84.2% for Fe3+ to 100.5% for Ag+. Additionally, we verified that the Tl3+ signaling of the probe was not impacted by common background anionic species (Fig. S5, ESI†). The fluorescence intensity enhancement ratio (Ianion+Tl(III)/ITl(III)) for the tested anions fluctuated minimally, from 93.2% for I− to 100.1% for F−. Furthermore, we tested the fluorescence response of the probe toward representative urine components such as uric acid, urea, and creatinine for its application to the urinary thallium determination.45 As shown in Fig. S6 (ESI),† in the presence of these urine components, the Tl3+ signaling behavior was not affected noticeably. Furthermore, we confirmed that the Tl3+ signaling of TP-1 remained stable in their presence.
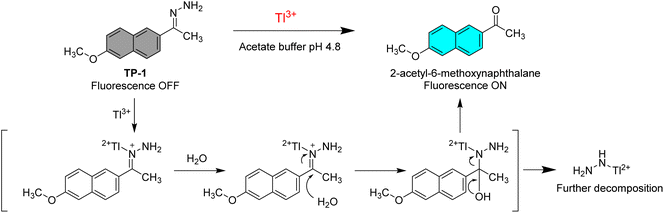
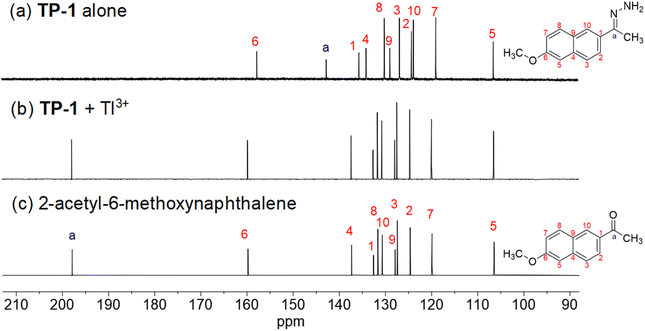
The Tl3+ signaling mechanism of TP-1 can be explained by the Tl3+-assisted hydrolysis of the hydrazone moiety of the probe, yielding the strongly fluorescent 2-acetyl-6-methoxynaphthalene fluorophore (Scheme 2). This proposed sensing mechanism was investigated through 1H/13C NMR and mass measurements, as well as TLC monitoring of the Tl3+ sensing process. In the 13C NMR spectra, probe TP-1 displayed a hydrazone C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N carbon peak at 142.6 ppm. However, after treatment with Tl3+ ions, the C
N carbon peak at 142.6 ppm. However, after treatment with Tl3+ ions, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N carbon peak disappeared, and a new carbonyl C
N carbon peak disappeared, and a new carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O carbon peak at 197.9 ppm appeared (Fig. 3). Additionally, as shown in the 1H NMR spectra (Fig. S7, ESI†), we confirmed that the hydrazone NH2 protons of the probe at 6.38 ppm completely disappeared following treatment with Tl3+ ions. We also confirmed that the 1H and 13C NMR spectra of the signaling product of the probe were identical to those of the expected signaling product, 2-acetyl-6-methoxynaphthalene. Furthermore, from the mass analysis, we confirmed that the Tl3+ signaling product of the probe exhibited a peak with m/z = 200.1, consistent with 2-acetyl-6-methoxynaphthalene (calculated m/z = 200.08) (Fig. S8, ESI†). In addition, TLC monitoring also ascertained that probe TP-1 yielded the strongly fluorescent 2-acetyl-6-methoxynaphthalene as a signaling product (Fig. S9, ESI†). Moreover, to further investigate the sensing mechanism, we conducted pH-dependent Tl3+ signaling studies. As shown in Fig. S10 (ESI),† the fluorescence signaling of TP-1 for Tl3+ ions decreased dramatically below pH 3.0. This result is attributed to the protonation of the hydrazone moiety, which disrupts Tl3+ binding and inhibits the hydrolysis mechanism of the signaling process.
O carbon peak at 197.9 ppm appeared (Fig. 3). Additionally, as shown in the 1H NMR spectra (Fig. S7, ESI†), we confirmed that the hydrazone NH2 protons of the probe at 6.38 ppm completely disappeared following treatment with Tl3+ ions. We also confirmed that the 1H and 13C NMR spectra of the signaling product of the probe were identical to those of the expected signaling product, 2-acetyl-6-methoxynaphthalene. Furthermore, from the mass analysis, we confirmed that the Tl3+ signaling product of the probe exhibited a peak with m/z = 200.1, consistent with 2-acetyl-6-methoxynaphthalene (calculated m/z = 200.08) (Fig. S8, ESI†). In addition, TLC monitoring also ascertained that probe TP-1 yielded the strongly fluorescent 2-acetyl-6-methoxynaphthalene as a signaling product (Fig. S9, ESI†). Moreover, to further investigate the sensing mechanism, we conducted pH-dependent Tl3+ signaling studies. As shown in Fig. S10 (ESI),† the fluorescence signaling of TP-1 for Tl3+ ions decreased dramatically below pH 3.0. This result is attributed to the protonation of the hydrazone moiety, which disrupts Tl3+ binding and inhibits the hydrolysis mechanism of the signaling process.
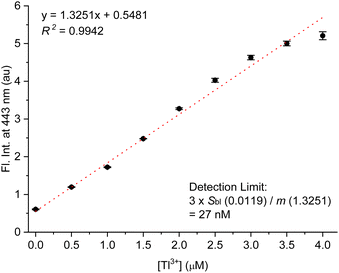
To estimate the detection limit of TP-1 for Tl3+ ions, we conducted experiments to observe the Tl3+ concentration-dependent fluorescence signaling behavior of the probe (Fig. 4). The fluorescence emission at 443 nm linearly increased with increasing concentrations of Tl3+ (R2 = 0.9864). The detection limit for Tl3+ was determined following the IUPAC recommendation, using the equation LOD = 3sblk/m, where sblk represents the standard deviation of the blank signal and m denotes the slope of the calibration curve.46 From Fig. 4, the standard deviation of the blank signal (sblk) was calculated to be 0.0105, and the slope of the titration curve (m) was determined to be 1.7003. Using these values, the detection limit for Tl3+ was calculated to be 19 nM. This low detection limit highlights the high sensitivity of TP-1 for Tl3+ detection, making it suitable for practical applications such as clinical diagnostics and environmental monitoring. Next, we confirmed the pH profile of the Tl3+ signaling across a pH range from 1.3 to 7.0. As shown in Fig. S10 (ESI),† Tl3+ signaling of the probe was effective in the pH range from 4.8 to 7.0. In acidic conditions, TP-1 exhibited an increase in fluorescence due to the protonation of the hydrazone moiety. However, the Tl3+ signaling of TP-1 decreased markedly under highly acidic conditions because the protonation disrupted Tl3+ binding at the hydrazone moiety, thereby interfering the signaling process. Meanwhile, in basic conditions, Tl3+ signaling slightly decreased because Tl3+ ions can be converted to Tl2O3.47 Additionally, we evaluated the stability of TP-1 stock solutions under different storage conditions. The Tl3+ response of TP-1 was tested using stock solutions stored for immediate use, 1 day, 3 days, 5 days, and 1 week at room temperature in the dark. As shown in Fig. S11 (ESI),† the results confirmed that TP-1 retains its fluorescence signaling ability throughout these storage periods, demonstrating its stability and suitability for practical applications.
 | ||
| Fig. 4 Calibration curve for fluorescence titration of TP-1 with Tl3+. [TP-1] = 5.0 μM, [Tl3+] = 0–4.0 μM, in a pH 4.8 acetate buffer solution (10 mM) containing 1% (v/v) DMF. λex = 309 nm. | ||
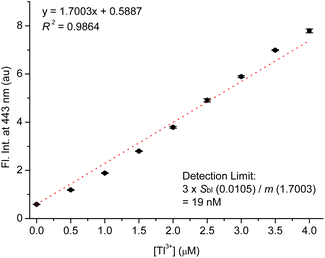
As mentioned in the introduction, urinary thallium is one of the most convenient indicators for diagnosing thallium poisoning, alongside testing thallium ions in hair and blood samples. For this reason, we investigated the Tl3+ signaling of TP-1 in artificial urine samples. As shown in the previous results, we evaluated the interference effects of representative urine components, including urea, creatinine, uric acid, common metal ions and anions, ammonia, and glucose. These tests confirmed that the assay components do not produce background fluorescence or interfere with the Tl3+ signaling of TP-1. Therefore, we examined the Tl3+ concentration-based fluorescence signaling behavior in artificial urine solutions using the fluorescence spectroscopy. As shown in Fig. 5, the fluorescence intensity at 443 nm quantitatively increased with the Tl3+ concentration up to 4.0 μM (R2 = 0.9942). This result demonstrates that TP-1 can reliably determine Tl3+ ions in artificial urine samples.
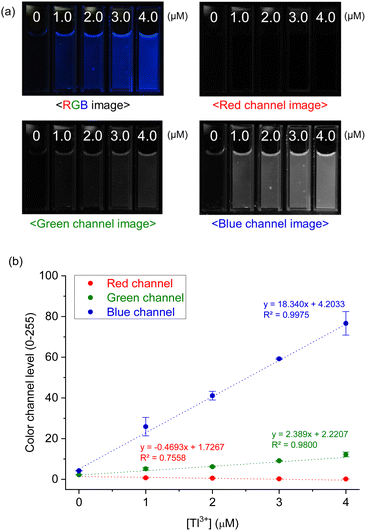
Next, to evaluate the practical applicability of the probe, a Tl3+ assay in artificial urine samples was conducted using a smartphone as an easily accessible device for image capture and data analysis.48 As demonstrated in Fig. 6a, TP-1 exhibited enhanced blue fluorescence with increasing Tl3+ concentrations. The RGB color channel levels of the fluorescence images were analyzed using a smartphone-based color analysis application. A calibration curve based on the blue channel showed satisfactory linearity for Tl3+ ions in artificial urine samples (Fig. 6b). Although the assay showed a relatively high error of 18.0% at a low concentration of 1.0 μM, the smartphone-based results were in good agreement with those obtained from fluorescence method (Table 1). While traditional methods using a fluorescence spectroscopy are generally recognized for their superior accuracy and precision, the smartphone-based method provides a practical and rapid alternative for detecting Tl3+ ions. This approach is particularly advantageous for field settings and point-of-care applications, where accessibility and simplicity are critical. The smartphone-based assay demonstrated reliable performance useful for rapid screening in suspected cases of acute thallium poisoning, making it a valuable tool for on-site diagnostics. From these findings, we conclude that TP-1 is a robust probe for detecting Tl3+ ions in artificial urine, offering a rapid, cost-effective, and accessible method for on-site Tl3+ detection. While this study focused on artificial urine to mimic real-world conditions due to ethical constraints, the results strongly support the practical applicability of TP-1 for diagnosing acute Tl3+ poisoning in complex biological matrices.
| [Tl3+] (μM)b | Using smartphone (μM) | Relative error (%) | Using fluorescence spectroscopy (μM) | Relative error (%) |
|---|---|---|---|---|
| a Reported values are given as mean ± standard deviation, n = 3.b [Tl3+] was standardized using iodometric titration. | ||||
| 0 | Not detected | — | Not detected | — |
| 1.0 | 1.18 ± 0.26 | 18.0 | 0.89 ± 0.09 | −11.3 |
| 2.0 | 2.01 ± 0.12 | −0.5 | 2.05 ± 0.02 | 2.8 |
| 3.0 | 2.99 ± 0.02 | −0.5 | 3.07 ± 0.05 | 2.6 |
4. Conclusions
We have developed a convenient Tl3+-selective fluorescent signaling probe, which is simple to prepare and effective for the early diagnosis of acute thallium poisoning using easily available urine samples. Probe TP-1, a hydrazone derivative of 2-acetyl-6-methoxynaphthalene, demonstrated pronounced turn-on type fluorescence signaling behavior specifically in response to Tl3+ ions. The mechanism of Tl3+ signaling of the probe involves the unique Tl3+-assisted hydrolysis of the hydrazone moiety, leading to the generation of the parent fluorophore. Notably, the Tl3+ signaling was unaffected by the presence of common metal ions, anions, and major urine components such as uric acid, urea, and creatinine. Furthermore, structural improvement through comparative testing of ketone-hydrazone (TP-1) and aldehyde-hydrazone (TP-2) derivatives confirmed that probe TP-1 exhibited rapid and stable Tl3+ signaling behavior with sufficient stability under the measurement conditions. The utility of the probe was further highlighted by successfully conducting a Tl3+ assay in artificial urine samples using a smartphone for signal capture and analysis. These results affirm the practical potential of probe TP-1 for diagnosing acute thallium poisoning in readily obtainable human urine samples.Data availability
Data will be made available upon request.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This research was supported by the Chung-Ang University Graduate Research Scholarship in 2023.Notes and references
- L. Manzo and E. Sabbioni, in Handbook on Toxicity of Inorganic Compounds, ed. H. G. Seiler, H. Sigel and A. Sigel, Marcel Dekker, New York, 1988 Search PubMed
.
- A. Nolan, D. Schaumloffel, E. Lombi, L. Ouerdane, R. Lobinski and M. McLaughlin, J. Anal. At. Spectrom., 2004, 19, 757–761 RSC
.
- H. Micke and H. U. Wolf, Thallium and Thallium Compounds, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co., KGaA, Weinheim, 2012 Search PubMed
.
- American Public Health Association, Standard Methods for the Examination of Water and Wastewater, Washington, D.C., 19th edn, 1995, pp. 3–113 Search PubMed
.
- H. Liu and G. Liao, Brain Behav., 2021, 11, e02032 CrossRef PubMed
.
- EPA, Effluent Guidelines, Toxic and Priority Pollutants Under the Clean Water Act, https://www.epa.gov/eg/toxic-and-priority-pollutants-under-clean-water-act Search PubMed.
- L. Ralph and M. R. Twiss, Bull. Environ. Contam. Toxicol., 2002, 68, 261–268 CAS
.
- M. Méndez-Armenta, C. Nava-Ruiz, F. Fernández-Valverde, A. Sánchez-García and C. Rios, Environ. Toxicol. Pharmacol., 2011, 32, 107–112 CrossRef PubMed
.
- D. Zendelovska and T. Stafflov, Anal. Sci., 2001, 17, 425–428 CrossRef CAS PubMed
.
- A. F. Silva, D. L. G. Borges, B. Welz, M. G. R. Vale, M. M. Silva, A. Klassen and U. Heitman, Spectrochim. Acta, Part B, 2004, 59, 841–846 CrossRef
.
- B. Krasnodebska-Ostrega, M. Sadowska, K. Piotrowska and M. Wojda, Talanta, 2013, 112, 73–79 CrossRef CAS PubMed
.
- U. Karlsson, A. Duker and S. Karlsson, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2006, 41, 1155–1167 CrossRef CAS PubMed
.
- M. M. Hassanien, Kh. S. Abou-El-Sherbini and G. A. E. Mostafa, Talanta, 2003, 59, 383–392 CrossRef CAS PubMed
.
- Y. K. Agrawal and V. J. Bhatt, Analyst, 1986, 111, 761–765 RSC
.
- A. S. Amin, A. A. M. El-Sharjawy and M. A. Kassem, Spectrochim. Acta, Part A, 2013, 110, 262–268 CrossRef CAS PubMed
.
- D. Mihajlovic and T. Stafilov, X-Ray Spectrom., 1998, 27, 397–400 CrossRef CAS
.
- P. Ghosh and P. Roy, Chem. Commun., 2023, 59, 5174–5200 RSC
.
- D. K. Iyer, A. Shaji, S. P. Singh, A. Tripathi, A. Hazra, S. Mandal and P. Ghosh, Coord. Chem. Rev., 2023, 495, 215371 CrossRef CAS
.
- B. Rezaei, S. Meghdadi and N. Majidi, Spectrochim. Acta, Part A, 2007, 67, 92–97 CrossRef CAS PubMed
.
- M. E. Jun, B. Roy and K. H. Ahn, Chem. Commun., 2011, 47, 7583–7601 RSC
.
- H. D. Revanasiddappa and T. N. K. Kumar, Anal. Sci., 2002, 18, 1131–1135 CrossRef CAS PubMed
.
- S. Ge, P. Dai, J. Yu, Y. Zhu, J. Huang, C. Zhang, L. Ge and F. Wan, Int. J. Environ. Anal. Chem., 2010, 90, 1139–1147 CrossRef CAS
.
- P. Nagaraja, N. G. S. Al-Tayar, A. Shivakumar, A. K. Shresta and A. K. Gowda, J. Mex. Chem. Soc., 2009, 53, 201–208 CAS
.
- Y. J. Lee, M. G. Choi, J. H. Yoo, T. J. Park, S. Ahn and S.-K. Chang, J. Photochem. Photobiol., A, 2020, 394, 112471 CrossRef CAS
.
- J. H. Yoo, Y. J. Lee, K. M. Lee, M. G. Choi, T. J. Park and S.-K. Chang, New J. Chem., 2021, 45, 603–609 RSC
.
- R. Lazny and A. Nodzewska, Chem. Rev., 2010, 110, 1386–1434 CrossRef CAS PubMed
.
- S. Kobayashi, Y. Mori, J. S. Fossey and M. M. Salter, Chem. Rev., 2011, 111, 2626–2704 CrossRef CAS PubMed
.
- X. Su and I. Aprahamian, Chem. Soc. Rev., 2014, 43, 1963–1981 RSC
.
- T. M. Pereira and A. E. Kümmerle, Hydrazone-Based Small-Molecule Chemosensors, in Computational Biology and Chemistry, ed. P. Behzadi and N. Bernabò, IntechOpen, UK, 2020, ch. 6 Search PubMed
.
- Q. Lin, X. Liu, T.-B. Wei and Y.-M. Zhang, Chem.–Asian J., 2013, 8, 3015–3021 CrossRef CAS PubMed
.
- F. Han, Y. Bao, Z. Yang, T. M. Fyles, J. Zhao, X. Peng, J. Fan, Y. Wu and S. Sun, Chem.–Eur. J., 2007, 13, 2880–2892 CrossRef CAS PubMed
.
- Y.-H. Qiao, H. Lin, J. Shao and H.-K. Lin, Spectrochim. Acta, Part A, 2009, 72, 378–381 CrossRef PubMed
.
- S. K. Ramasamy, A. Chinnathambi, S. A. Alharbi, G. Venkatesan, A. Pugazhendhi and G. Sathiyan, J. Mol. Struct., 2024, 1302, 137411 CrossRef CAS
.
- H. Hosseinjani-Pirdehi, N. O. A. Mahmoodi and A. Taheri, J. Photochem. Photobiol., A, 2021, 421, 113524 CrossRef CAS
.
- S. Kumar, S. Mahata and V. Manivannan, J. Photochem. Photobiol., A, 2024, 450, 115436 CrossRef CAS
.
- M. H. Kim, H. H. Jang, S. Yi, S.-K. Chang and M. S. Han, Chem. Commun., 2009, 4838–4840 RSC
.
- M. G. Choi, H. Ryu, M. S. Han and S.-K. Chang, Tetrahedron Lett., 2016, 57, 4360–4363 CrossRef CAS
.
- C. Zhang, X. Li, Y. Wang, S. Nie and C. Liu, Luminescence, 2024, 39, e4613 Search PubMed
.
- S. Kim, C. W. Ko, T. Lim, S. Yoo, H. J. Ham, S.-Y. Kang, S. Kang, S. K. Cho and M. S. Han, Dyes Pigment, 2019, 171, 107762 CrossRef CAS
.
- B. Gu, C. Wu, C. Zhang, S. He, S. Tang, H. Li and Y. Shen, Spectrochim. Acta, Part A, 2021, 262, 120100 CrossRef CAS PubMed
.
- P. D. Sharma and Y. K. Gupta, Talanta, 1973, 20, 903–905 CrossRef CAS PubMed
.
- D. D. Perin and B. Dempsey, Buffers for pH and Metal Ion Control, Chapman and Hall Ltd, London, UK, 1979 Search PubMed
.
- M. G. Choi, H. Ryu, M. J. Cho, S. K. Lee and S.-K. Chang, Sens. Actuators, B, 2017, 244, 307–313 CrossRef CAS
.
- R. Zhu, H. Li, A. Guo, Y. Zhao, S. Wang and J. Dong, Inorg. Chim. Acta, 2023, 556, 121652 CrossRef CAS
.
- K. Tuantet, M. Janssen, H. Temmink, G. Zeeman, R. H. Wijffels and C. J. N. Buisman, J. Appl. Phycol., 2014, 26, 287–297 CrossRef CAS
.
- D. C. Harris, Quantitative Chemical Analysis, W. H. Freeman and Company, New York, 8th edn, 2010, pp. 103–105 Search PubMed
.
- J. A. Switzer, in Electrochemistry of Nanomaterials, ed. G. Hodes, Wiley-VCH, Verlag GmbH, Weinheim, 2001, p. 82 Search PubMed
.
- M. J. Cho, H. Ryu, H. J. Lee and S.-K. Chang, Sens. Actuators, B, 2017, 241, 285–291 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: UV-vis, fluorescence, and Tl3+ assay in artificial urine sample, 1H and 13C NMR spectra and high-resolution mass result of probes TP-1 and TP-2. See DOI: https://doi.org/10.1039/d4ra06726f |
| This journal is © The Royal Society of Chemistry 2025 |