 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enhanced flexible supercapacitors with boron-doped graphene electrodes and carbon quantum dot gel electrolytes†
Dilara Koroglu a,
Haluk Bingolbc and
Betul Uralcan
a,
Haluk Bingolbc and
Betul Uralcan *a
*a
aDepartment of Chemical Engineering, Bogazici University, Bebek, Istanbul, 34342, Turkey. E-mail: betul.uralcan@boun.edu.tr; Tel: +90(212)359-6871
bScience and Technology Research and Application Center (BITAM), Necmettin Erbakan University, Konya 42090, Turkey
cDepartment of Basic Science, Faculty of Engineering, Necmettin Erbakan University, Konya 42090, Turkey
First published on 14th February 2025
Abstract
Flexible solid state supercapacitors have gained significant importance in energy storage device technology. In this work, flexible solid-state supercapacitors are designed with enhanced capacitance, bending cycle stability and energy density. Activated carbon (AC) is synthesized from cabbage leaves and boron doped reduced graphene oxide (BRGO) is incorporated into AC to improve mechanical flexibility. On the other hand, carbon quantum dots (CQDs) and acetonitrile (ACN) as solvent are incorporated into a gel electrolyte. We investigate the concentration of boron in the electrode material and that of CQDs in the gel electrolyte and reveal that the capacitance, bending properties and energy density of the solid-state supercapacitor are simultaneously improved with the optimum composition of AC/BRGO in the CQD/gel electrolyte. This demonstration of composite electrode and electrolyte materials could substantially improve the capacitance, cycle stability and energy density of solid-state supercapacitors.
1 Introduction
As global energy demands rise with population growth, research efforts are increasingly directed towards optimizing energy storage devices. Among these, flexible solid-state supercapacitors stand out for their excellent bending properties, cycling stability, and high power density. Obtaining enhanced performance in these devices relies on the rigorous selection and design of electrode and electrolyte materials.1–8Activated carbons (ACs) are widely used in supercapacitors due to their large surface area, high porosity, commercial availability, and cost-effectiveness.9 ACs are typically produced through physical or chemical activation processes, offering versatility for various applications.10 However, ACs tend to be brittle, necessitating the incorporation of carbon additives to enhance flexibility while maintaining cycling stability.11 Among these additives, reduced graphene oxide (RGO) stands out for its tunable flexibility and electrochemical stability.11–13 Recent advancements have seen RGO being doped with heteroatoms like boron, nitrogen, phosphorous, and sulfur, enhancing its electronic properties.14–17
Boron doping introduces boron atoms into the graphene lattice, replacing carbon atoms. This substitution can lead to lattice distortions, potentially affecting the material's mechanical properties. However, the strong boron–carbon bonds can help maintain structural integrity, and the specific impact on mechanical properties depends on factors such as doping concentration and distribution.18 Incorporating boron-doped reduced graphene oxide (BRGO) into an activated carbon (AC) matrix can enhance the composite's mechanical stability. The interaction between BRGO sheets and the AC matrix facilitates stress distribution during mechanical loading, reducing the likelihood of fracture. Additionally, boron doping can improve the adhesion between graphene sheets and the matrix, further enhancing mechanical stability. For example, Wang et al. demonstrated that boron doping at high concentrations (>1%) could decrease the material's tensile strength by inducing defects19 via the molecular dynamic (MD) simulations. Dai et al. reported that graphene can maintain a large fraction of its pristine strength and stiffness after substituting boron for carbon atoms by MD simulations.20 Further, Peng et al. explored boron-doped porous graphene as an electrode material for flexible devices, achieving three times the areal capacitance compared to non-doped counterparts and maintaining 90% capacitance retention over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles under bending conditions.21 Similarly, Pandian et al.18 synthesized boron-doped reduced graphene for flexible solid-state supercapacitors, reporting a capacitance of 266 F g−1 at a current density of 1 A g−1 and 98% capacitance retention over 5000 cycles at 5 A g−1. Addition to those studies, boron doping leads to a significant improvement in the material's capacitance, which is a critical property for supercapacitors and energy storage devices. For example, Niu et al. reported that 4% boron-doped graphene exhibited an 86% improvement in capacitance compared to pristine graphene.22 Similarly, Zuo et al. observed a gravimetric capacitance of up to 281 F g−1 for porous boron-doped graphene F g−1.23 Han et al. demonstrated a capacitance of 200 F g−1 for BRGO in 6 M KOH,24 while Yeom et al. reported an outstanding capacitance of 448 F g−1 for BRGO under the same conditions.25 Thirumal et al. observed a capacitance increase from 53 F g−1 to 113 F g−1 upon boron doping of graphene oxide.26 Boron, being electron-deficient, introduces holes into the graphene structure, enhancing hole concentration and facilitating charge transport.27 This p-doping effect improves electrical conductivity, which, combined with the mechanical properties, makes BRGO a promising material for energy storage applications.28
000 cycles under bending conditions.21 Similarly, Pandian et al.18 synthesized boron-doped reduced graphene for flexible solid-state supercapacitors, reporting a capacitance of 266 F g−1 at a current density of 1 A g−1 and 98% capacitance retention over 5000 cycles at 5 A g−1. Addition to those studies, boron doping leads to a significant improvement in the material's capacitance, which is a critical property for supercapacitors and energy storage devices. For example, Niu et al. reported that 4% boron-doped graphene exhibited an 86% improvement in capacitance compared to pristine graphene.22 Similarly, Zuo et al. observed a gravimetric capacitance of up to 281 F g−1 for porous boron-doped graphene F g−1.23 Han et al. demonstrated a capacitance of 200 F g−1 for BRGO in 6 M KOH,24 while Yeom et al. reported an outstanding capacitance of 448 F g−1 for BRGO under the same conditions.25 Thirumal et al. observed a capacitance increase from 53 F g−1 to 113 F g−1 upon boron doping of graphene oxide.26 Boron, being electron-deficient, introduces holes into the graphene structure, enhancing hole concentration and facilitating charge transport.27 This p-doping effect improves electrical conductivity, which, combined with the mechanical properties, makes BRGO a promising material for energy storage applications.28
Electrolytes also play a crucial role in determining the energy density and safety of supercapacitors.29–35 Gel polymer electrolytes (GPEs) have emerged as key components in solid-state supercapacitors due to their high conductivity and enhanced flexibility.36,37 These GPEs serve dual roles as both electrolyte and separator in flexible supercapacitor configurations.38 Optimizing GPE performance involves selecting the appropriate combination of host polymer, solvent, and electrolytic salt. Poly(vinyl alcohol) (PVA) is widely used for preparing gel polymer electrolytes, owing to its high hydrophilicity, affordability, and safety.39 The preparation of gel electrolytes involves mixing PVA with various aqueous solutions of electrolytic salts, such as H2SO4, H3PO4, KOH, NaOH, KCl, NaCl, and LiCl, to facilitate charge transfer and improve capacitance.40 Since water is used to dissolve PVA/KOH mixture, potential window is restricted to 1 V. Herein, we incorporate acetonitrile (ACN) into water as solvent for the gel polymer electrolyte to widen the operating potential window; thereby, enhancing energy density. The incorporation of ACN into water for gel electrolyte preparation represents a novel approach in the literature. Previous studies have explored various additives in gel polymer electrolytes. For instance, Guofu and colleagues demonstrated that the addition of K3Fe(CN)6 to PVA/KOH gel electrolyte improved specific capacitance to 431 F g−1.41 Haijun et al.42 investigated KI incorporation into PVA/KOH gel electrolyte with activated carbon electrodes, achieving a specific capacitance of 237 F g−1.42 As compared to other carbon materials, CQDs have ultra small sizes (less than 10 nm) and abundant functional groups on their surface, which donate them with uniform dispersion and excellent electron transfer/reservoir properties.43,44 The incorporation of CQDs into the pores of activated carbon was explored by Kumar et al. and this electrode material showed high capacitance and very stable electrochemical behavior.45 Kumar et al. explored the performance of CQDs as aqueous electrolytes, achieving a specific capacitance of 155 F g−1 for graphene-based electrodes.46 Herein, carbon quantum dots were chosen to be added to the electrolyte due to their unique properties. Their incorporation can increase the overall performance by facilitating better ion transport, improving the interface between the electrolyte and electrode leading to better charge transfer, contributing to the stability of the device. CQDS with exceptional conducting properties arise from the quantum confinement and edge effect, which is related to the motion of charge carriers (like electrons) in such small structures. The motion of charges becomes restricted in one or more dimensions, leading to discrete energy levels, which alters the electronic properties such as increased conductivity.47,48 Further, the presence of diverse heteroatom-containing functional groups (such as oxygen and nitrogen) on carbon nanomaterials provides numerous active sites that improve electrochemical performance.46,49
The rest of this paper is organized as follows. In Section 2, we describe the material synthesis process, device fabrication and give details on structural and electrochemical characterization techniques. In Section 3, we report the structural and electrochemical characterization results, and discuss our findings on the fabricated solid-state supercapacitors. In Section 4, we provide concluding remarks and suggest some possible directions for future inquiry.
2 Experimental section
2.1 Synthesis of activated carbon from cabbage leaves
Cabbage leaves (4 g) were cut, washed, and dehydrated at 110 °C for 12 hours. The dehydrated cabbage leaves were carbonized at 600 °C for 2 hours in a tubular furnace (Protherm) under argon (Ar) protection. Subsequently, the carbonized cabbage leaves underwent chemical activation with potassium hydroxide (KOH, Merck–pellets for analysis) solution (Cabbage leaves![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH ratio of 1
KOH ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) at room temperature for 10 hours on a magnetic stirrer. The resulting solution was dried at 80 °C and annealed at 800 °C (with a heating rate of 5 °C min−1) for 2 hours under Ar atmosphere. Finally, the products were washed with 1 M hydrochloric acid (HCl, Merck, 37% for anaylsis) and distilled water via centrifugation (1500 rpm, 20 min, 25 cycles) until the solution was neutral. The resulting solution was dried at 80 °C overnight to obtain activated carbon (AC). The overall AC production procedure is given in our previous work.50
4) at room temperature for 10 hours on a magnetic stirrer. The resulting solution was dried at 80 °C and annealed at 800 °C (with a heating rate of 5 °C min−1) for 2 hours under Ar atmosphere. Finally, the products were washed with 1 M hydrochloric acid (HCl, Merck, 37% for anaylsis) and distilled water via centrifugation (1500 rpm, 20 min, 25 cycles) until the solution was neutral. The resulting solution was dried at 80 °C overnight to obtain activated carbon (AC). The overall AC production procedure is given in our previous work.50
2.2 Preparation of CQD
CQDs were synthesized using citric acid (Sigma-Aldrich) and urea (Sigma-Aldrich) via solvothermal processes, as depicted in our previous work.50 Briefly, 0.384 g citric acid and 0.060 g urea were dissolved in 10 mL dimethylformamide (DMF, Sigma-Aldrich) and then sonicated to ensure uniform mixing. The mixture underwent a solvothermal reaction at 180 °C for 6 h in a teflon-lined stainless autoclave (50 mL). The resulting brown solution was purified using a dialysis bag (MWCO: 0.5 kDa, Spectrum Laboratories Inc. (USA)) for 15 minutes. Ultra-pure water were freshly prepared using a Millipore Direct-Q3 water purification system. The obtained CQD solution was stored at +4 °C.2.3 Structural characterization
(N2) adsorption–desorption analyses were conducted using a Micromeritics ASAP 2020 Plus Chemi automated instrument to determine the micropore structure of the samples (AC and AC-10BRGO). The materials were degassed at 240 °C for 6 hours and the specific surface area was calculated using the Brauner Emmett Teller (BET) method. Raman analysis was performed on powder AC and AC-10BRGO (Reinshaw inVia, λ = 532 nm). Surface chemistry was investigated using X-ray photoelectron spectroscopy (XPS) with a Thermo KAlpha X-ray Photoelectron Spectrometer. The gel electrolytes were characterized by thermal gravimetric analysis (TGA) using a TA Instruments SDT 650, X-ray diffraction (XRD) using a PANalytical X'Pert PRO with a wavelength of λ = 1.54059 Å, and Fourier-transform infrared (FTIR) spectroscopy using a Bruker Vertex 80v spectrometer.2.4 Fabrication and characterization of solid-state supercapacitors
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. N-Methyl-2-pyrrolidone (NMP) was added to the mixture at a ratio of 0.15 g mL−1, followed by mixing at 300 rpm for 2 hours at 70 °C. The obtained slurry was further stirred using a magnetic stirrer at room temperature for 12 hours to ensure homogeneity. Silver nanoparticles ink (Sigma-Aldrich), was printed on a flexible polyethylene terephthalate (PET) film with a surface area of 1 cm2 and heated at 130 °C for 5 hours. The AC/BRGO/PTFE slurry was uniformly spread onto the silver current collector using a doctor blade and dried at 95 °C for 1 hour.
1. N-Methyl-2-pyrrolidone (NMP) was added to the mixture at a ratio of 0.15 g mL−1, followed by mixing at 300 rpm for 2 hours at 70 °C. The obtained slurry was further stirred using a magnetic stirrer at room temperature for 12 hours to ensure homogeneity. Silver nanoparticles ink (Sigma-Aldrich), was printed on a flexible polyethylene terephthalate (PET) film with a surface area of 1 cm2 and heated at 130 °C for 5 hours. The AC/BRGO/PTFE slurry was uniformly spread onto the silver current collector using a doctor blade and dried at 95 °C for 1 hour.3 Results and discussion
3.1 Structural characterization of active electrode and electrolyte materials
Fig. 1a and b display the N2 adsorption–desorption isotherms for AC and AC-10BRGO, respectively. The AC exhibits a type I isotherm characteristic of a microporous structure dominated by micropores, as per the IUPAC classification. In contrast, AC-10BRGO demonstrates a hybrid type I–IV isotherm, indicating a bimodal micro/mesoporous pore structure. The nitrogen is adsorbed by the AC-10BRGO, mainly, at low relative pressures which is typical of microporous structure. At higher relative pressures, with continued adsorption, mesoporosity is present in the structure of AC-10BRGO. The pore size distribution curves, shown in Fig. 1c and d for AC and AC-10BRGO respectively, further elucidate these findings. The specific surface area and cumulative pore volume of AC are measured as 3038 m2 g−1 and 1.02 cm3 g−1, respectively, while AC-10BRGO possesses a smaller BET surface area (1724 m2 g−1) and cumulative pore volume (0.98 cm3 g−1).
The Raman and XPS analyses of AC were given in our previous work.50 The Raman spectrum of AC reveals D- and G-peaks centered at 1360 and 1570 cm−1, respectively (Fig. 2a).50 These peaks are fit using the Lorentzian and Breit–Wigner–Fano functions for D- and G-peaks, respectively. The D-band is attributed to the defect of highly ordered carbonaceous materials while the G-band is at-tributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C strecthing vibrations. The peak intensity ratio of the D-band and the G-band indicates the carbon-containing defects. The ratio of the intensities of the peaks (ID/IG) is obtained as 0.92, indicating a defective nature of AC.
C strecthing vibrations. The peak intensity ratio of the D-band and the G-band indicates the carbon-containing defects. The ratio of the intensities of the peaks (ID/IG) is obtained as 0.92, indicating a defective nature of AC.
 | ||
| Fig. 2 Characterization of AC (a) Raman spectroscopy analysis of AC, fitting curves. Red line represents the Lorentzian fit to the D-peak and blue line represents the BWF fit to the fl-peak, (b)the total XPS spectra, (c) deconvoluted C 1s XPS Spectra, and (d) deconvoluted O 1s XPS Spectra.50 | ||
XPS spectra of AC, depicted in Fig. 2b, depict a C/O ratio of ≃11.50 To further investigate the oxygen functional groups on the surface of AC, the deconvoluted C 1s and O 1s XPS spectra are presented in Fig. 2c and d, respectively.50 The C 1s peak is deconvoluted into four peaks, indicating various carbon functionalities. Meanwhile, the O 1s spectra suggest that C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O components dominate the surface functionalities of AC, followed by carboxylic functional groups (O
O components dominate the surface functionalities of AC, followed by carboxylic functional groups (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH). The presence of N2 doping into the carbon structure is confirmed by the peak at 536.0 eV.
C–OH). The presence of N2 doping into the carbon structure is confirmed by the peak at 536.0 eV.
The composite material, prepared by incorporating BRGO into AC, was also characterized by Raman, XPS, and FTIR. Raman spectra of RGO and BRGO show that the ratio of the D-band to G-band (ID/IG) in the Raman spectra of the RGO samples is 1.1 and that of BRGO is 2.2 (Fig. S2†). ID/IG ratio of 1.1 indicates a relatively moderate defect density in RGO. This suggests that a significant amount of oxygenated functional groups have been removed and some of the graphitic structure have been restored. The moderate ratio indicates that while the material is not pristine graphene, it has good potential for conductivity. On the other hand, ID/IG ratio for BRGO (2.2) indicates that BRGO has a significantly higher defect density compared to RGO. This may be attributed to additional defects or functional group modifications introduced into the graphene lattice during the synthesis of BRGO. Specifically, some reduction processes can lead to over-reduction, disrupting the graphene network and increasing disorder. The high level of disorder in the material creates defects that can act as active sites, enhancing electrochemical performance. Furthermore, the presence of additional oxygenated groups in the BRGO structure could improve electrolyte wettability, contributing to its overall performance. This relatively high ID/IG ratio is consistent with the introduction of defects and functional groups, which is typically expected in the presence of dopants, such as boron. The ID/IG ratio of AC-10BRGO, determined from Raman spectroscopy (Fig. 3a), was found to be 3, much higher than that of AC (0.92), suggesting successful boron doping on the reduced graphene oxide sheet. This increase in the ID/IG ratio indicates the presence of defects, potentially acting as active sites, may have enhanced capacitance.
In order to further explore the type and composition of functional groups on the surface of AC-10BRGO, the deconvoluted C 1s and the O 1s XPS spectra are presented in Fig. (Fig. 3c and d). C 1s peak is deconvoluted into three peaks, i.e. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C at 284.5 eV, C–O at 285.9 eV, and C
C at 284.5 eV, C–O at 285.9 eV, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 288.6 eV. The O 1s peak is deconvoluted into three peaks, i.e. C
O at 288.6 eV. The O 1s peak is deconvoluted into three peaks, i.e. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 531.6 eV, C–O at 533.2 eV and O–C
O at 531.6 eV, C–O at 533.2 eV and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 535.8 eV, indicating oxygen surface functionalities that may improve the wettability of the electrodes. XPS analysis also confirms the presence of boron in the AC-10BRGO structure. A peak found at 192.3 eV is a characteristic of B–O bond in B2O3, which proves boron incorporation into AC structure.
O at 535.8 eV, indicating oxygen surface functionalities that may improve the wettability of the electrodes. XPS analysis also confirms the presence of boron in the AC-10BRGO structure. A peak found at 192.3 eV is a characteristic of B–O bond in B2O3, which proves boron incorporation into AC structure.
Additionally, XRD patterns of AC and AC-10BRGO (Fig. S3†) display peaks at 24.5° and 42.8°, with AC-10BRGO showing more intense peaks, confirming successful boron doping. These findings are consistent with the results obtained from Raman, XPS, and FTIR analyses.
Scanning electron microscopy (SEM) was used to gather information on the morphology of the powdered activated carbon and activated carbon-boron doped reduced graphene oxide (Fig. S6†). The image (Fig. S6a†) shows irregularly shaped particles with rough and porous surfaces, characteristic of activated carbon. The particle size distribution from Fig. 1c appears heterogeneous, with some larger and smaller aggregates. The porous structure suggests a high surface area (Fig. 1c). The composite (Fig. S6b†) exhibits a layered and sheet-like morphology, typical of reduced graphene oxide (RGO). The boron doping may have caused defect formation and wrinkling, leading to the observed folded and stacked layers. The layers appear to be interconnected, which can enhance electrical conductivity and ion transport pathways. AC is not distinctly visible as separate particles but the layered morphology of the BRGO likely encapsulates or intercalates the AC particles. This can occur during the synthesis process, where AC becomes embedded in or coated by the BRGO sheets. AC may contribute to the overall roughness or slight irregularities seen in the BRGO layers, as it provides a porous and textured substrate for the graphene sheets to anchor onto. While the BRGO layers dominate the visual morphology, AC likely maintains its porous structure within the composite as depicted by Fig. 1c and d. These pores might not be directly visible due to the overlapping graphene layers but still contribute to the composite's overall surface area and ion accessibility. In this material, AC could serve as a structural backbone, providing mechanical stability to the composite. The layered BRGO sheets alone might be prone to agglomeration or collapse, but the dispersed AC particles may help maintain separation between layers and improve the structural integrity of the composite. While AC is not really visible, the wrinkles, folds, and roughness in the BRGO layers could be influenced by the underlying AC particles, indicating a potential interaction between the two materials. This interaction could also enhance the composite's conductivity and ion transport properties.
 | ||
| Fig. 4 Structural characterizations of solo gel-electrolytes (a) TflA curves, (b) FTIR spectrum and (c) XRD spectrum. | ||
FTIR spectra were obtained for CQD, as well as as-prepared and CQD-added gel electrolyte samples. The presence of bands at 3330 and 2900 cm−1 is attributed to O–H stretching and bending, and CH2 asymmetric stretching, respectively. 51 Additionally, bands at 1650 and 1100 cm−1 (as indicated by the red circle in Fig. 4b) correspond to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O stretching, respectively, indicating the incorporation of CQDs into the gel electrolyte.
O and C–O stretching, respectively, indicating the incorporation of CQDs into the gel electrolyte.
XRD patterns of pure PVA, as-prepared and CQD-added gel electrolyte films are illustrated in Fig. 4c. The XRD spectrum of PVA exhibits a peak at 20°, indicative of a semi-crystalline structure. 52 However, in the prepared gel electrolyte, addition of KOH to PVA results in an amorphous structure. Upon incorporation of CQDs, a broad peak is observed in the range of 20–40°, suggesting an increase in the amorphous domain of the material. 53 Although the mechanism of ion transportation in gel electrolytes remains unclear, it is speculated that polymer chain motion exists within the amorphous structure. 51,53
3.2 Electrochemical characterization
 | (1) |
The electrochemical measurements conducted at various boron concentrations (AC-5BRGO, AC-10BRGO, AC-15BRGO) provide insights into the performance of the electrodes. Fig. 5a–d depict the cyclic voltammograms of the samples at scan rates ranging from 50 to 500 mV s−1 within a 1.4 V potential window, demonstrating their electrochemical stability. Furthermore, Fig. 5e presents the specific capacitance as a function of scan rate for each boron concentration. The optimal boron concentration is determined to be 10 wt%, resulting in an areal capacitance of 140 mF cm−2 at 50 mV s−1. The comparison of structural character-izations of AC and AC-10BRGO are given in Section 3.1. Based on N2 adsorption–desorption isotherms, both AC and AC-10BRGO has a microporous structure. The pore size distribution of AC is centered at 0.74 nm and that of AC-10BRGO is centered at 1.3 nm. The specific surface area of AC-10BRGO (1724 m2 g−1) is smaller than that of AC (3038 m2 g−1), suggesting that although specific surface area is significant for ion adsorption, the accessibility of electrolyte ions to the electrode surface outweighs for a better electrochemical performance.55 The defective structure observed in AC-10BRGO, indicated by the threefold higher ID/IG ratio (3) compared to AC (0.92) from Raman analysis, suggests a significant role in ion adsorption due to the presence of defects, serving as active sites for electrolyte ions.56,57 Further confirmation is provided by XPS spectra, revealing a dominance of oxygen surface functionalities in AC-10BRGO, as evidenced by the lower C/O ratio (8.96) compared to AC (11). The enhanced wettability resulting from the decoration of oxygen functional groups58 on the electrode surface is supported by both Raman and XPS analyses. Additionally, FTIR results corroborate these findings, suggesting successful boron doping into the AC structure due to B–O bond at 1300 cm−1 and the presence of oxygen functionalities on the electrode surface.
Fig. 6a, c and e display the cyclic voltammograms of the materials at various scan rates ranging from 50 to 500 mV s−1. Additionally, charge–discharge curves spanning from 0.5 to 5 A g−1 are depicted in Fig. 6b, d and f.
The capacitance from charging–discharging cycle is calculated as a function of current density using
 | (2) |
Cyclic voltammograms and GCD curves indicate that charge storage is primarily through physical adsorption of ions. 1.5 wt% CQD addition to gel electrolyte has an adverse impact on capacitance (14.2 F g−1 at 0.5 A g−1) and one plausible explanation for the reduced capacitance at the highest CQD concentration is that CQDs may agglomerate and cause blockage within polymeric network. On the other hand, 1 wt% CQD loading in gel polymer electrolyte enhances capacitance (107 F g−1 at 0.5 A g−1), energy density (29 W h kg−1 at 1.4 V) and scan rate dependence, which may be attributed to the fast charging properties of the composite materials. This composite material exhibits enhanced energy density performance compared to the metrics reported by other researchers for symmetric solid-state supercapacitors (Table S1†). The electrochemical characterizations of CQD added gel electrolyte confirm the results obtained from the structural characterizations which indicate the successful incorporation of CQD into gel electrolyte, as discussed in Section 3.1.2.
Ionic conductivity can be calculated by the equation below:
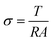 | (3) |
4 Conclusion
In this study, we have successfully fabricated flexible solid-state supercapacitors using cabbage-derived activated carbon (AC) and boron-doped reduced graphene oxide (BRGO) composites. We have elucidated the structural and electrochemical properties of the materials using structural and electrochemical characterization techniques including N2 adsorption–desorption measurements, XPS, Raman spectroscopy, FTIR spectroscopy and cyclic voltammetry.The electrochemical measurements conducted at various boron concentrations (AC-5BRGO, AC-10BRGO, AC-15BRGO) provided insights into the performance of the electrodes. Specifically, AC-10BRGO exhibited superior electrochemical stability and specific capacitance, with an optimal boron concentration of 10 wt%. Moreover, the flexibility of the assembled supercapacitors was evaluated by subjecting them to bending at different angles (90° and 120°). AC-10BRGO, 0.5CQD, and 1CQD exhibited excellent flexibility, maintaining structural integrity with capacitance retention exceeding 90% over 1000 cycles under various bending angles. Overall, our findings highlight the promising potential of cabbage-derived AC and BRGO composites for flexible solid-state supercapacitor applications. The combination of superior electrochemical performance and flexibility makes these materials attractive candidates for various portable and wearable electronics, energy storage systems, and flexible electronic devices. Further optimization and exploration of these materials could lead to significant advancements in the field of flexible energy storage technologies.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Dilara Koroglu: investigation, validation, data curation, writing – review and editing. Haluk Bingöl: resources. Betul Uralcan: conceptualization, resources, supervision, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by grants from The Scientific and Technological Research Council of Turkey (TUBITAK) under BIDEB 2232-A International Fellowship for Outstanding Researchers (Project Number T118C220) and Istanbul Kalkınma Ajansı, Turkey (Project Number TR10/21/YEP/0001).References
- D. P. Dubal, N. R. Chodankar, D.-H. Kim and P. Gomez Romero, Chem. Soc. Rev., 2018, 47, 2065–2129 RSC.
- J. V. Vaghasiya, C. C. Mayorga-Martinez and M. Pumera, Chem. Soc. Rev., 2020, 49, 7819–7844 RSC.
- G. A. Tafete, M. K. Abera and G. Thothadri, J. Energy Storage, 2022, 48, 103938 CrossRef.
- G. Jiang, R. A. Senthil, Y. Sun, T. R. Kumar and J. Pan, J. Power Sources, 2022, 520, 230886 CrossRef CAS.
- M. Addicoat, R. Atkin, J. N. C. Lopes, M. C. Gomes, M. Firestone, R. Gardas and J. Wishart, et al., Faraday Discuss., 2018, 206, 291–337 RSC.
- A. Abbott, L. Aldous, N. Borisenko, S. Coles, O. Fontaine, J. D. G. Garcia, R. Gardas, O. Hammond, L. J. Hardwick and P.-H. Haumesser, et al., Faraday Discuss., 2018, 206, 405–426 RSC.
- D. Kaya, D. Koroglu, E. Aydın and B. Uralcan, J. Power Sources, 2023, 568, 232987 CrossRef CAS.
- A. Abbott, H. Abe, L. Aldous, R. Atkin, M. Bendová, M. Busato, J. N. C. Lopes, M. C. Gomes, B. Cross and C. Dietz, et al., Faraday Discuss., 2018, 206, 113–139 RSC.
- C. Schütter, S. Pohlmann and A. Balducci, Adv. Energy Mater., 2019, 1900334 CrossRef.
- A. Ahmad, M. A. Gondal, M. Hassan, R. Iqbal, S. Ullah, A. S. Alzahrani, W. A. Memon, F. Mabood and S. Melhi, ACS Omega, 2023, 8, 21653–21663 CrossRef CAS PubMed.
- M. Soni, P. Kumar, J. Pandey, S. K. Sharma and A. Soni, Carbon, 2018, 128, 172–178 CrossRef CAS.
- M. T. U. Malik, A. Sarker, S. S. Mahmud Rahat and S. B. Shuchi, Mater. Today Commun., 2021, 28, 102685 CrossRef CAS.
- P. Zhang, K. Wang, X. Liu, L. Wang and W. Gao, J. Mater. Sci., 2022, 57, 20242–20258 CrossRef.
- L. S. Panchakarla, K. S. Subrahmanyam, S. K. Saha, A. Govindaraj, H. R. Krishnamurthy, U. V. Waghmare and C. N. R. Rao, Adv. Mater., 2009, 21, 4726–4730 CrossRef CAS.
- D. Wei, Y. Liu, Y. Wang, H. Zhang, L. Huang and G. Yu, Nano Lett., 2009, 9, 1752–1758 CrossRef CAS PubMed.
- C. Zhang, N. Mahmood, H. Yin, F. Liu and Y. Hou, Adv. Mater., 2013, 25, 4932–4937 CrossRef CAS PubMed.
- N. H. A. Rosli, K. S. Lau, T. Winie, S. X. Chin and C. H. Chia, Diamond Relat. Mater., 2021, 120, 108696 CrossRef CAS.
- P. M. Pandian and A. Pandurangan, Diamond Relat. Mater., 2021, 118, 108495 CrossRef CAS.
- G. Wang, X. Li, Y. Wang, Z. Zheng, Z. Dai, X. Qi, L. Liu, Z. Cheng, Z. Xu and P. Tan, et al., J. Phys. Chem. C, 2017, 121, 26034–26043 CrossRef CAS.
- Z. Dai, G. Wang, Z. Zheng, Y. Wang, S. Zhang, X. Qi, P. Tan, L. Liu, Z. Xu and Q. Li, et al., Carbon, 2019, 147, 594–601 CrossRef CAS.
- Z. Peng, R. Ye, J. A. Mann, D. Zakhidov, Y. Li, P. R. Smalley, J. Lin and J. M. Tour, ACS Nano, 2015, 9, 5868–5875 CrossRef CAS PubMed.
- L. Niu, Z. Li, W. Hong, J. Sun, Z. Wang, L. Ma, J. Wang and S. Yang, Electrochim. Acta, 2013, 108, 666–673 CrossRef CAS.
- Z. Zuo, Z. Jiang and A. Manthiram, J. Mater. Chem. A, 2013, 1, 13476–13483 RSC.
- J. Han, L. L. Zhang, S. Lee, J. Oh, K.-S. Lee, J. R. Potts, J. Ji, X. Zhao, R. S. Ruoff and S. Park, ACS Nano, 2013, 7, 19–26 CrossRef CAS PubMed.
- D.-Y. Yeom, W. Jeon, N. D. K. Tu, S. Y. Yeo, S.-S. Lee, B. J. Sung, H. Chang, J. A. Lim and H. Kim, Sci. Rep., 2015, 5, 9817 CrossRef CAS PubMed.
- V. Thirumal, A. Pandurangan, R. Jayavel and R. Ilangovan, Synth. Met., 2016, 220, 524–532 CrossRef CAS.
- P. M. Pandian and A. Pandurangan, Diamond Relat. Mater., 2021, 118, 108495 CrossRef CAS.
- S. Li, Z. Wang, H. Jiang, L. Zhang, J. Ren, M. Zheng, L. Dong and L. Sun, Chem. Commun., 2016, 52, 10988–10991 RSC.
- V. Lockett, R. Sedev, J. Ralston, M. Horne and T. Rodopoulos, J. Phys. Chem. C, 2008, 112, 7486–7495 CrossRef CAS.
- G. Feng and P. T. Cummings, J. Phys. Chem. Lett., 2011, 2, 2859–2864 CrossRef CAS.
- R. Burt, K. Breitsprecher, B. Daffos, P.-L. Taberna, P. Simon, G. Birkett, X. S. Zhao, C. Holm and M. Salanne, J. Phys. Chem. Lett., 2016, 7, 4015–4021 CrossRef CAS PubMed.
- A. S. Korkut and B. Uralcan, Nanotechnology, 2023, 34, 235402 CrossRef CAS PubMed.
- D. J. Bozym, B. Uralcan, D. T. Limmer, M. A. Pope, N. J. Szamreta, P. G. Debenedetti and I. A. Aksay, J. Phys. Chem. Lett., 2015, 6, 2644–2648 CrossRef CAS PubMed.
- B. Uralcan, I. A. Aksay, P. G. Debenedetti and D. T. Limmer, J. Phys. Chem. Lett., 2016, 7, 2333–2338 CrossRef CAS PubMed.
- B. Uralcan and I. B. Uralcan, ACS Appl. Mater. Interfaces, 2022, 14, 16800–16808 CrossRef CAS PubMed.
- M. Maksoud, R. Amer Fahim, A. Shalan, M. Abd El-Kodous, O. Samuel Oluwaseun, A. Osman, C. Farrell, A. Al-Muhtaseb, A. Awed, A. Ashour and D. Rooney, Environ. Chem. Lett., 2020, 375–439 Search PubMed.
- C. Zhong, D. Yida, W. Hu, J. Qiao, L. Zhang and J. Zhang, Chem. Soc. Rev., 2015, 44, 7431–7920 RSC.
- A. Arya and A. Sharma, Ionics, 2017, 23, 497–540 CrossRef CAS.
- S. T. Senthilkumar, R. K. Selvan, N. Ponpandian and J. S. Melo, RSC Adv., 2012, 2, 8937–8940 RSC.
- B. Jinisha, K. Anilkumar, M. Manoj, C. M. Ashraf, V. Pradeep and S. Jayalekshmi, J. Solid State Electrochem., 2019, 23, 3343–3353 CrossRef CAS.
- G. Ma, J. Li, K. Sun, H. Peng, J. Mu and Z. Lei, J. Power Sources, 2014, 256, 281–287 CrossRef CAS.
- H. Yu, J. Wu, L. Fan, K. Xu, X. Zhong, Y. Lin and J. Lin, Electrochim. Acta, 2011, 56, 6881–6886 CrossRef CAS.
- Y. Qing, Y. Jiang, H. Lin, L. Wang, A. Liu, Y. Cao, R. Sheng, Y. Guo, C. Fan, S. Zhang, D. Jia and Z. Fan, J. Mater. Chem. A, 2019, 7, 6021–6027 RSC.
- J. Xiao, R. Momen and C. Liu, Electrochem. Commun., 2021, 132, 107143 CrossRef CAS.
- V. B. Kumar, A. Borenstein, B. Markovsky, D. Aurbach, A. Gedanken, M. Talianker and Z. Porat, J. Phys. Chem. C, 2016, 120, 13406–13413 CrossRef CAS.
- S. Kumar, M. Goswami, N. Singh, N. Sathish, M. Reddy and S. Kumar, J. Energy Storage, 2022, 55, 105522 CrossRef.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726–6744 CrossRef CAS PubMed.
- Z. Zhao and Y. Xie, J. Power Sources, 2017, 337, 54–64 CrossRef CAS.
- K. Dave and V. G. Gomes, et al., Nano Energy, 2019, 66, 104093 CrossRef.
- D. Koroglu, H. Bingol and B. Uralcan, Nanotechnology, 2024, 36, 105402 CrossRef PubMed.
- F. Santos, J. P. Tafur, J. Abad and A. J. Fernández Romero, J. Electroanal. Chem., 2019, 850, 113380 CrossRef CAS.
- M. Jiang, J. Zhu, C. Chen, Y. Lu, Y. Ge and X. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 3473–3481 CrossRef CAS PubMed.
- C.-C. Yang, Mater. Lett., 2004, 58, 33–38 CrossRef CAS.
- B. Qi, K. Ren, Y. Lin, S. Zhang, T. Wei and Z. Fan, Particuology, 2022, 60, 1–13 CrossRef CAS.
- H. Zhong, F. Xu, Z. Li, R. Fu and D. Wu, Nanoscale, 2013, 5, 4678–4682 RSC.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558–1565 CrossRef CAS.
- N. Dwivedi, S. Kumar, H. Malik, G. Gupta, C. M. Rauthan and O. Panwar, Appl. Surf. Sci., 2011, 257, 6804–6810 CrossRef CAS.
- X. ran Li, Y. hui Jiang, P. zhi Wang, Y. Mo, W. de Lai, Z. jiong Li, R. ji Yu, Y. ting Du, X. ren Zhang and Y. Chen, New Carbon Mater., 2020, 35, 232–243 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Flexible supercapacitor set-up. Raman analysis of BRGO. XRD spectrum and SEM images of AC and AC-10BRGO. Impedance spectroscopy of AC-10BRGO and AC-10BRGO/1CQD. See DOI: https://doi.org/10.1039/d4ra06990k |
| This journal is © The Royal Society of Chemistry 2025 |





