Open Access Article
Open Access ArticlePreparation and characterization of Ru-TiO2/PC/Fe3O4 composite catalyst with enhanced photocatalytic performance and magnetic recoverability under simulated solar light
Ruixiang Wu *ab,
Hanyan Zhangc,
Wenhua Liub,
Renao Baia,
Delun Zhenga,
Xiufang Tian*a,
Weikai Linb,
Lejian Lia and
Qianwei Kea
*ab,
Hanyan Zhangc,
Wenhua Liub,
Renao Baia,
Delun Zhenga,
Xiufang Tian*a,
Weikai Linb,
Lejian Lia and
Qianwei Kea
aCollege of Construction and Ecology, Shantou Polytechnic, Shantou 515078, Guangdong, China. E-mail: rxwu@stpt.edu.cn; xftian@stpt.edu.cn
bGuangdong Provincial Laboratory of Marine Biotechnology, Institute of Marine Sciences, Shantou University, Shantou 515063, Guangdong, China
cShantou Goworld Display Co. Ltd, LongJiang Road No. 12, ShanTou 515043, Guangdong, China
First published on 10th January 2025
Abstract
This research focuses on the development of a novel Ru-doped TiO2/grapefruit peel biochar/Fe3O4 (Ru-TiO2/PC/Fe3O4) composite catalyst, which exhibits exceptional photocatalytic efficacy under simulated solar light irradiation. The catalyst is highly effective in the degradation of rhodamine B (RhB), methylene blue (MB), methyl orange (MO), as well as actual industrial dye wastewater (IDW), and can be recovered magnetically for multiple reuse cycles. Significantly, the PCTRF-100 sample exhibited degradation efficiencies of 99.4% for RhB and 99.8% for MB within 60 min, and 98.04% for MO within 120 min. In the case of actual dye wastewater, a reduction in chemical oxygen demand from 1540 mg L−1 to 784 mg L−1 was achieved within 300 min, corresponding to a degradation rate of 46.81%. The remarkable photocatalytic activity observed is primarily attributed to the synergistic interactions among Ru-TiO2, biochar, and Fe3O4, which effectively facilitate the separation and migration of electron–hole pairs in TiO2.
1 Introduction
The swift advancement of modern agriculture and industry has led to an enhancement in living standards. Nevertheless, this progress has concurrently exacerbated environmental pollution, particularly water pollution, which has emerged as a significant environmental and social challenge.1–3 Wastewater containing colored dyes, released from industries such as textiles, printing, papermaking, and coatings, encompasses toxic, carcinogenic, and teratogenic substances. Inadequate treatment of such wastewater can result in profound ecological damage.4,5 As a result, the development of efficient and environmentally sustainable technologies to reduce these pollutants has become a major research focus within the environmental field. Presently, methods such as adsorption, Fenton technology, advanced oxidation processes, and photocatalysis are extensively employed for the treatment of dye wastewater.6–8 Among these methods, photocatalytic technology is regarded as the most promising green technology for environmental protection due to its ecological compatibility, high efficiency, and cost-effectiveness.9,10The foundation of photocatalytic technology is the development of catalysts that are both efficient and environmentally benign. At present, materials such as TiO2, ZnO, and CdS are extensively utilized as photocatalysts,11–13 with TiO2 being regarded as the most promising due to its non-toxic nature, low cost, superior physicochemical properties, and high stability.14 Nonetheless, TiO2 suffers from limitations, including low visible light utilization,15 a high rate of electron–hole recombination,16 and challenges associated with separation and recovery,17,18 which constrain its practical applications. To address these drawbacks, our research team has successfully synthesized gradient porous TiO2 (ref. 19) and TiO2/grapefruit peel (GP) biochar composite materials,20 employing GP as a biological template and carbon source, which has significantly enhanced photocatalytic performance. However, the issues of limited visible light utilization and difficulty in separation and recovery persist. Research indicates that doping with transition metals, such as Ru, can substantially improve the visible light activity of TiO2. For instance, Kulkarni et al.21 synthesized Ru/TiO2 composites using an impregnation technique, which demonstrated considerable activity in the catalytic degradation of benzyl butyl phthalate. Elsalamony et al.22 prepared Ru/TiO2 photocatalysts through impregnation, markedly enhancing the catalytic degradation performance of 2-chlorophenol under visible light. Additionally, Zhang et al.23 developed Ru-doped TiO2 spheres, which exhibited strong photocatalytic hydrogen production performance under visible light conditions. Nevertheless, these studies did not resolve the challenge of separation and recovery. It has been established that TiO2 composite catalysts, when combined with magnetic materials, can be swiftly separated and recovered using an external magnetic field, thereby improving their practicality.24,25 For example, Niu et al.26 designed a core–shell structured Fe3O4@TiO2 photocatalyst, which displayed high catalytic activity for the degradation of neutral red in aqueous solution under visible light and could be easily recovered with a magnet. Similarly, Zhang et al.27 synthesized Fe3O4@C@Ru composites via a three-step method, and Rajabathar et al.28 prepared Fe3O4/TiO2 composites using a hydrothermal synthesis method. Both catalysts achieved degradation rates exceeding 90% for MB under simulated solar light and could be effectively reused through magnetic separation.
Building upon prior research, a Ru-TiO2/PC/Fe3O4 composite catalyst was successfully synthesized utilizing a low-temperature hydrothermal method. This composite exhibited high efficiency in the degradation of RhB, MB, MO, and IDW under simulated solar light conditions, while also offering magnetic separation capabilities. A comprehensive examination of its synthesis process, photocatalytic efficiency, underlying mechanisms, and facile separation and recovery methods is crucial for advancing its practical applications.
2 Materials and methods
2.1 Materials
Grapefruit peels were purchased from Shantou Agricultural and Trade Market, Shantou, China. The following chemicals were utilized: tetrabutyl titanate(TBOT)(500 mL, AR), RhB (100 g, AR), RuCl3 (1 g, AR), FeCl2·4H2O (5 g, AR), FeCl3·6H2O (5 g, AR), MB (100 g, AR) and MO (100 g, AR) acquired from Shanghai Macklin Company Limited; as well as potassium hydroxide (500 g, AR), anhydrous ethanol (500 mL, AR) and acetic acid (500 mL, AR), NH3·H2O (500 mL, AR) both procured from Sinopharm Chemical Reagent Co., Ltd.2.2 Preparation of Ru-TiO2/PC/Fe3O4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio and dissolved in 100 mL of ultrapure water, to which 2.0 g of pretreated GP powder was added in a rotary evaporator. The system was maintained under a vacuum of −0.09 MPa, with a rotation speed of 180 rpm, for a vacuum rotary impregnation period of 12 h. Subsequently, 100 mL of ammonia solution was introduced to the mixture to promote the formation of Fe3O4. The Fe3O4-loaded GP powder was then separated by magnetic means and washed until the pH reached neutral (pH 7), followed by vacuum drying at 60 °C for 4 h. Finally, the dried material was placed in a tube furnace and subjected to heating at 400 °C under a nitrogen atmosphere for 2 h, after which it was allowed to cool naturally to room temperature, resulting in the formation of the Fe3O4/PC composite material.
1 molar ratio and dissolved in 100 mL of ultrapure water, to which 2.0 g of pretreated GP powder was added in a rotary evaporator. The system was maintained under a vacuum of −0.09 MPa, with a rotation speed of 180 rpm, for a vacuum rotary impregnation period of 12 h. Subsequently, 100 mL of ammonia solution was introduced to the mixture to promote the formation of Fe3O4. The Fe3O4-loaded GP powder was then separated by magnetic means and washed until the pH reached neutral (pH 7), followed by vacuum drying at 60 °C for 4 h. Finally, the dried material was placed in a tube furnace and subjected to heating at 400 °C under a nitrogen atmosphere for 2 h, after which it was allowed to cool naturally to room temperature, resulting in the formation of the Fe3O4/PC composite material.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 1
50, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, 1
20, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1
10, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1
5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 1
2, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, various amounts of Fe3O4/PC, ranging from 10 mg to 500 mg, were introduced into beaker B and ultrasonically dispersed for 1 h to form suspension B. Suspension B was then combined with suspension A, thoroughly mixed, and subjected to an additional hour of ultrasonic treatment. The resulting mixture was transferred to a reaction vessel and heated at 140 °C for 6 h in a forced-air drying oven. Following the reaction, the mixture was allowed to cool naturally, then subjected to centrifugation, filtration, and washing. The final product was vacuum-dried at 60 °C for 4 h, yielding a series of Ru-TiO2/PC/Fe3O4 composite materials. These were designated as PCTRF-x, where x corresponds to the mass of Fe3O4/PC incorporated. The resulting samples were labeled as PCTRF-10, PCTRF-25, PCTRF-50, PCTRF-100, PCTRF-250, and PCTRF-500, while the Ru-TiO2/PC sample without Fe3O4/PC addition was labeled as PCTRF-0.
1, various amounts of Fe3O4/PC, ranging from 10 mg to 500 mg, were introduced into beaker B and ultrasonically dispersed for 1 h to form suspension B. Suspension B was then combined with suspension A, thoroughly mixed, and subjected to an additional hour of ultrasonic treatment. The resulting mixture was transferred to a reaction vessel and heated at 140 °C for 6 h in a forced-air drying oven. Following the reaction, the mixture was allowed to cool naturally, then subjected to centrifugation, filtration, and washing. The final product was vacuum-dried at 60 °C for 4 h, yielding a series of Ru-TiO2/PC/Fe3O4 composite materials. These were designated as PCTRF-x, where x corresponds to the mass of Fe3O4/PC incorporated. The resulting samples were labeled as PCTRF-10, PCTRF-25, PCTRF-50, PCTRF-100, PCTRF-250, and PCTRF-500, while the Ru-TiO2/PC sample without Fe3O4/PC addition was labeled as PCTRF-0.2.3 Photocatalytic performance evaluation
The photocatalytic performance of the Ru-TiO2/PC/Fe3O4 composite material was rigorously assessed using a 600 W xenon lamp (equipped with a solar light filter) as the illumination source, with RhB, MB, and MO solutions serving as simulants for organic dye wastewater. The capability of the composite in the treatment of actual IDW was also investigated. The experimental procedure was as follows: 100 mL of a dye solution at a concentration of 10 mg L−1 and 50 mg of the composite catalyst were introduced into a quartz reactor. The mixture was stirred in darkness for 30 to 60 min to achieve adsorption equilibrium. Subsequently, the xenon lamp and a circulating water cooling system were activated, with a maintained distance of 15 cm between the light source and the liquid surface, allowing the photocatalytic reaction to proceed under continuous stirring. Throughout the experiment, 5 mL of the supernatant was periodically withdrawn from the solution, filtered through a 0.45-micron membrane, and its absorbance was measured to determine the concentration until the experiment was completed. The relationship between C/C0 and time (t) was plotted, and the pollutant removal efficiency as well as the catalyst recovery rate were calculated according to eqn (1) and (2):
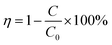 | (1) |
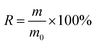 | (2) |
To evaluate the recyclability performance of the catalyst, the used catalyst is initially separated and recovered through magnetic methods, followed by subsequent washing and drying procedures. The treated catalyst is then reutilized in the subsequent photocatalytic experiments. This cycle is repeated multiple times to thoroughly assess the recyclability performance of the catalyst.
To ensure the reliability and reproducibility of the experimental results, each experiment was performed in triplicate (n = 3). The results were presented as mean ± standard deviation (SD) to clearly demonstrate the data distribution characteristics. One-way analysis of variance (ANOVA) was conducted to analyze the results of different treatment groups, with the significance level set at p < 0.05. Tukey's post hoc test was employed for mean separation to identify significant differences among treatment groups.
3 Results and discussion
3.1 Structural analysis of Ru-TiO2/PC/Fe3O4
To examine the crystal structure and phase composition of the Fe3O4/PC and Ru-TiO2/PC/Fe3O4 series samples, X-ray diffraction (XRD) analysis was performed, as depicted in Fig. 1a. The XRD pattern of the Fe3O4/PC sample reveals diffraction peaks at 2θ = 30.08°, 35.63°, 43.05°, 53.41°, 56.94°, 62.52°, and 73.96°, which correspond to the tetragonal crystal system of magnetite (PDF #88-0866). These peaks align with the (220), (311), (400), (442), (511), (440), and (533) crystal planes.30,31 The Ru-TiO2/PC/Fe3O4 series samples exhibit diffraction peaks at 2θ = 25.26°, 37.82°, 48.03°, 53.91°, 55.03°, 62.68°, 68.83°, 70.25°, and 75.14°, matching the standard diffraction peaks of anatase TiO2 (PDF #86-1157), specifically the (101), (004), (200), (105), (211), (204), (116), (220), and (215) crystal planes.32,33 This observation confirms that the titanium dioxide in the Ru-TiO2/PC/Fe3O4 samples retains its anatase structure. Additionally, no diffraction peaks for Ru are detected, which could be due to the homogeneous distribution of Ru within TiO2 at low concentrations, resulting in the formation of discrete compounds.34,35 As the Fe3O4/PC content increases, the characteristic diffraction peaks of Fe3O4 become more pronounced in the XRD patterns of Ru-TiO2/PC/Fe3O4. Furthermore, the absence of characteristic peaks associated with biochar may be attributed to its amorphous nature or the potential masking of its peaks by the prominent peaks of TiO2 and Fe3O4. This phenomenon will be further investigated using Raman spectroscopy in subsequent analyse.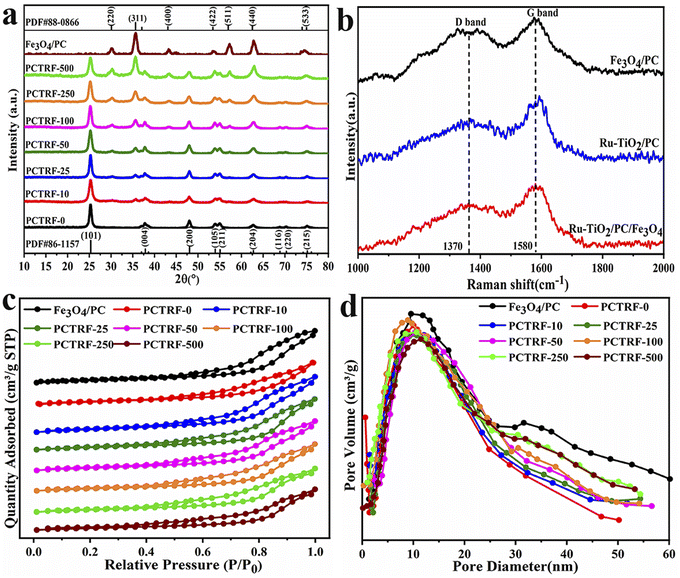 | ||
| Fig. 1 (a) XRD patterns, (b) Raman spectra, (c) nitrogen adsorption–desorption isotherms, and (d) pore size distribution curves of Fe3O4/PC and Ru-TiO2/PC/Fe3O4 series samples. | ||
Raman spectroscopy is considered an optimal technique for characterizing the structural properties of carbon materials. In this study, Raman spectroscopic analysis was carried out on Fe3O4/PC, Ru-TiO2/PC (PCTRF-0), and Ru-TiO2/PC/Fe3O4 (PCTRF-100) samples. The results, as illustrated in Fig. 1b, demonstrate that all three samples displayed the characteristic peaks of graphitic carbon at approximately 1580 cm−1 and 1370 cm−1, corresponding to the G-band and D-band, respectively.36 The G-band is indicative of the degree of graphitization, whereas the D-band signifies the presence of defects and structural disorder within the material.37,38 The degree of disorder and graphitization of the samples was assessed by fitting and calculating the peak area ratio R (ID/IG ratio). A higher R-value suggests a greater degree of disorder in the material, while a lower R-value signifies a higher degree of graphitization.39 The calculated R values for Fe3O4/PC, Ru-TiO2/PC, and Ru-TiO2/PC/Fe3O4 were 1.03, 0.95, and 0.98, respectively, confirming the presence of graphitic carbon in all three samples.
To gain a more profound understanding of the porous structure of the material, nitrogen adsorption–desorption isotherms were measured using an automatic specific surface area analyzer, allowing for the estimation of key parameters such as surface area, pore volume, and pore size. The results are presented in Fig. 1c, d and Table 1. As illustrated in Fig. 1c, the N2 adsorption–desorption isotherms of Fe3O4/PC and Ru-TiO2/PC/Fe3O4 series samples conform to the International Union of Pure and Applied Chemistry type IV curve, which suggests a mesoporous structure with pore diameters ranging from 2 nm to 50 nm.39 In the relative pressure range of P/P0 = 0.4–1.0, H3-type hysteresis loops were observed, which are typically associated with slit-like or interlayer pores,29 indicating the possible presence of such pores in the samples. Fig. 1d reveals that the Fe3O4/PC sample exhibits a bimodal Barrett–Joyner–Halenda pore size distribution, with a primary peak around 14 nm and a secondary peak between 30–60 nm. This suggests that the pores in the Fe3O4/PC sample are predominantly mesopores, with a minor presence of macropores. The pore sizes of the Ru-TiO2/PC/Fe3O4 series samples are mainly distributed between 10–30 nm. Table 1 provides detailed information on the specific surface area, pore volume, and average pore diameter of these samples. The data indicate that the Fe3O4/PC sample possesses a relatively large specific surface area, pore volume, and average pore diameter. For the Ru-TiO2/PC/Fe3O4 composite materials, the specific surface area and pore volume initially increase and then decrease with the addition of more Fe3O4/PC. This finding implies that an optimal proportion of Fe3O4/PC promotes the formation of materials with larger specific surface areas, whereas excessive content causes aggregation and pore blockage, reducing pore structure and volume.
| Materials | Surface area (m2 g−1) | Pore volume (cm3 g−1) | Average pore diameter (nm) |
|---|---|---|---|
| Fe3O4/PC | 123.72 | 0.26 | 16.48 |
| PCTRF-0 | 110.03 | 0.20 | 13.42 |
| PCTRF-10 | 111.21 | 0.22 | 13.73 |
| PCTRF-25 | 113.46 | 0.23 | 14.14 |
| PCTRF-50 | 115.61 | 0.24 | 14.87 |
| PCTRF-100 | 117.86 | 0.25 | 15.29 |
| PCTRF-250 | 112.24 | 0.21 | 13.81 |
| PCTRF-500 | 108.62 | 0.19 | 13.07 |
From the data in Table 1, it is evident that among the Ru-TiO2/PC/Fe3O4 series samples, PCTRF-100 exhibits the largest specific surface area, pore volume, and average pore diameter. It has been reported that a larger surface area in photocatalytic materials correlates with more active sites, which is essential for enhancing photocatalytic performance.35 Therefore, the PCTRF-100 sample shows potential as an effective photocatalyst.
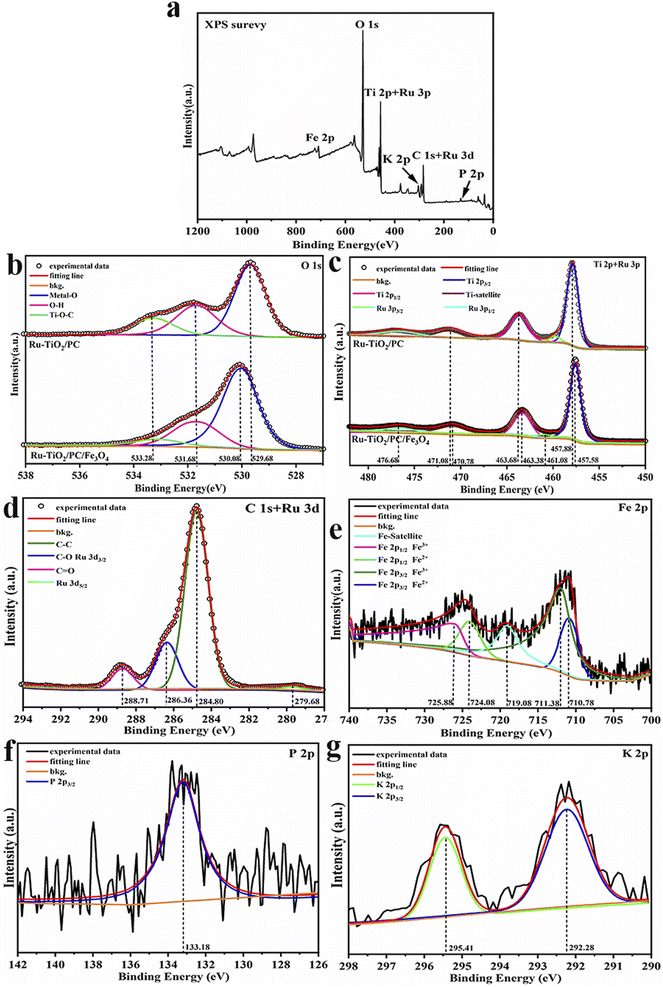
3.2 Surface elemental analysis of Ru-TiO2/PC/Fe3O4
The chemical composition, elemental chemical states, and surface electron distribution of the Ru-TiO2/PC/Fe3O4 sample were analyzed using X-ray photoelectron spectroscopy (XPS). The findings are presented in Fig. 2 and Table 2.| Element | BE/eV | Group | At. % | |
|---|---|---|---|---|
| Ti 2p | 457.92 | Ti–O | 10.22 | 14.19 |
| 463.61 | Ti–O | 3.97 | ||
| Ru 3p | 461.08 | Ru4+ | 0.19 | 0.72 |
| 476.68 | Ru4+ | 0.53 | ||
| O 1s | 530.29 | Ti–O/Fe–O/Ru-O | 29.52 | 45.92 |
| 531.98 | O–H | 11.65 | ||
| 533.40 | C–O/P–O/O–H | 4.75 | ||
| C 1s | 284.80 | C–C/H | 20.37 | 34.94 |
| 286.36 | C–O | 10.29 | ||
| 288.71 | C–O–Ti, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
4.28 | ||
| Ru 3d | 279.61 | Ru4+ | 0.43 | 0.43 |
| P 2p | 133.18 | P–O | 0.48 | 0.48 |
| K 2p | 292.28 | K+ | 0.24 | 0.41 |
| Fe 2p | 295.41 | K+ | 0.17 | 2.91 |
| 710.78 | Fe2+ | 0.52 | ||
| 711.38 | Fe3+ | 1.37 | ||
| 724.08 | Fe2+ | 0.43 | ||
| 725.88 | Fe3+ | 0.59 | ||
Fig. 2a illustrates the XPS survey spectrum of Ru-TiO2/PC/Fe3O4. Observed at 530.60 eV, 458.44 eV, 285.04 eV, 710.99 eV, 293.36 eV, and 133.19 eV are the characteristic peaks of O 1s, Ti 2p and Ru 3p, C 1s and Ru 3d, Fe 2p, K 2p, and P 2p, respectively, which signify the presence of O, C, Ti, Fe, Ru, K, and P elements within the composite material. To further scrutinize these characteristic elements, high-resolution XPS spectra were acquired (Fig. 2b–g). The high-resolution O 1s spectrum (Fig. 2b) was deconvoluted into three distinct peaks. The peak located at 530.08 eV is ascribed to lattice oxygen,40 which is associated with O–Ti bonds in Ru-TiO2, Ru–O bonds, and O–Fe bonds in Fe3O4. The binding energy of lattice oxygen in Ru-TiO2/PC/Fe3O4 (530.08 eV) exhibits a marked blue shift when compared to Ru-TiO2/PC (529.68 eV), suggesting an enhanced interfacial interaction between TiO2 and Fe3O4, which may indicate the formation of a heterojunction40,41. The peaks at 533.28 eV and 531.68 eV are assigned to O–H bonds and Ti–O–C bonds, respectively.42,43 The presence of O–H bonds is advantageous for the generation of ·OH radicals, thereby enhancing catalytic activity, whereas the Ti–O–C bonds imply the incorporation of a minor amount of C atoms into the TiO2 lattice during calcination.44 The high-resolution Ti 2p and Ru 3p spectrum (Fig. 2c) was resolved into five peaks. The characteristic peaks at 469.98 eV, 463.38 eV, and 457.58 eV correspond to the satellite peak of Ti, Ti 2p1/2, and Ti 2p3/2, respectively, which are indicative of Ti4+ species.45 These peaks, when compared to Ru-TiO2/PC, exhibit a red shift of approximately 0.3 eV, suggesting a reduction in the binding energy of TiO2 as a consequence of the heterojunction between Fe3O4 and TiO2.46 Additionally, the peaks at 476.68 eV and 461.08 eV are attributed to Ru 3p1/2 and Ru 3p3/2 characteristic peaks, respectively.47 The high-resolution C 1s + Ru 3d spectrum (Fig. 2d) was deconvoluted into four distinct peaks. The peaks at 288.71 eV and 284.80 eV correspond to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–C bonds, respectively.48 The peak at 286.36 eV represents an overlap of C–O bonds and Ru 3d3/2, while the peak at 279.68 eV is assigned to Ru 3d5/2.49 The high-resolution Fe 2p spectrum (Fig. 2e) reveals five peaks. The characteristic peak at 719.08 eV is identified as the satellite peak of Fe. The peaks located at 725.88 eV, 724.08 eV, 711.38 eV, and 710.78 eV correspond to Fe 2p1/2 (Fe3+), Fe 2p1/2 (Fe2+), Fe 2p3/2 (Fe3+), and Fe 2p3/2 (Fe2+) characteristic peaks, respectively.50 The atomic ratio of Fe3+ to Fe2+ is estimated to be approximately 2
O and C–C bonds, respectively.48 The peak at 286.36 eV represents an overlap of C–O bonds and Ru 3d3/2, while the peak at 279.68 eV is assigned to Ru 3d5/2.49 The high-resolution Fe 2p spectrum (Fig. 2e) reveals five peaks. The characteristic peak at 719.08 eV is identified as the satellite peak of Fe. The peaks located at 725.88 eV, 724.08 eV, 711.38 eV, and 710.78 eV correspond to Fe 2p1/2 (Fe3+), Fe 2p1/2 (Fe2+), Fe 2p3/2 (Fe3+), and Fe 2p3/2 (Fe2+) characteristic peaks, respectively.50 The atomic ratio of Fe3+ to Fe2+ is estimated to be approximately 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which is consistent with the properties of Fe3O4.51 The high-resolution P 2p and K 2p spectra (Fig. 2f and g) confirm the presence of P and K elements.52,53 Grapefruit peel, as a biomass source, naturally contains minerals such as phosphorus and potassium, which may exist in the form of inorganic salts or organic compounds. During the catalyst preparation process, these elements might be retained and incorporated into the final material.
1, which is consistent with the properties of Fe3O4.51 The high-resolution P 2p and K 2p spectra (Fig. 2f and g) confirm the presence of P and K elements.52,53 Grapefruit peel, as a biomass source, naturally contains minerals such as phosphorus and potassium, which may exist in the form of inorganic salts or organic compounds. During the catalyst preparation process, these elements might be retained and incorporated into the final material.
Table 2 provides an overview of the XPS binding energy and atomic percentage evaluation results for the Ru-TiO2/PC/Fe3O4 sample. The findings from the XPS analysis suggest that the synthesis of the Ru-TiO2/PC/Fe3O4 composite with heteroatoms of P and K, was successfully achieved.
3.3 Morphological analysis of Ru-TiO2/PC/Fe3O4
To explore the morphological structure of the samples, field emission scanning electron microscopy (SEM) was utilized to characterize Fe3O4/PC, Ru-TiO2/PC, and Ru-TiO2/PC/Fe3O4. The corresponding results are depicted in Fig. 3. Fig. 3a1–a3 provide the SEM images and particle size distribution of the Fe3O4/PC sample. It can be observed that the Fe3O4/PC sample effectively mirrors the morphological structure of the GP powder (Fig. 3a1). At higher magnification (Fig. 3a2), the wrinkled and porous architecture of the GP biochar is distinctly visible, with spherical Fe3O4 nanoparticles uniformly distributed across its surface, exhibiting no signs of agglomeration. The particle size distribution graph (Fig. 3a3) reveals that the diameter of the Fe3O4 particles is predominantly within the range of 10–20 nm. Fig. 3b1–b3 display SEM images of the Ru-TiO2/PC sample at various magnifications, alongside its particle size distribution. It is evident that Ru-TiO2/PC primarily consists of spherical nanoparticles with diameters ranging from 10–14 nm, which are uniformly aligned along the GP fibers, resulting in a well-defined porous structure. Fig. 3c1–c3 showcase SEM images of the Ru-TiO2/PC/Fe3O4 sample at different magnifications. The sample exhibits pronounced porosity and a layered structure, with its surface covered by nanoparticles. Upon further magnification (Fig. 3c2), it is revealed that spherical nanoparticles are evenly dispersed on the biochar, with porous structures evident in the composite material. The particle size distribution graph (Fig. 3c3) indicates that the particle sizes within the composite material are primarily concentrated between 10–20 nm.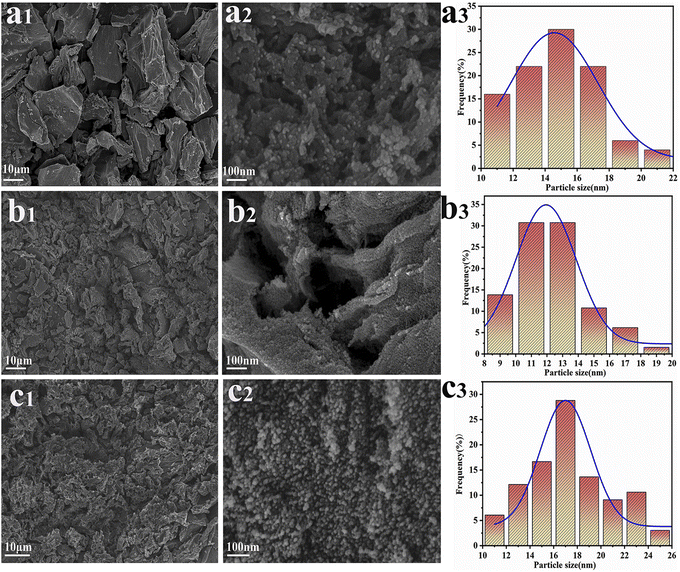 | ||
| Fig. 3 SEM images and particle size distribution diagrams of Fe3O4/PC (a1–a3), Ru-TiO2/PC (b1–b3), and Ru-TiO2/PC/Fe3O4 (c1–c3) samples. | ||
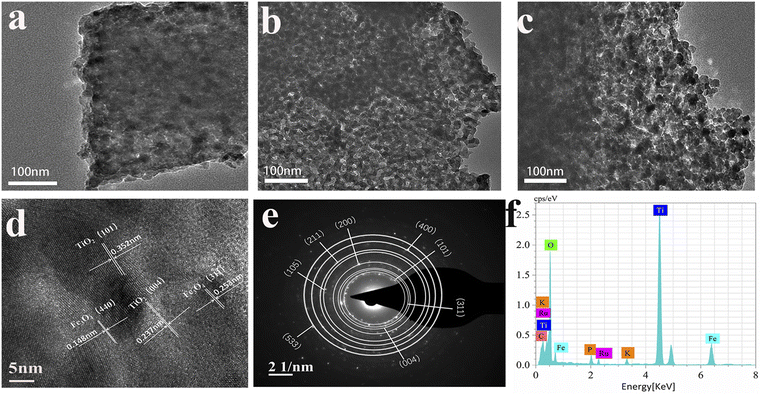
To further examine the microstructure of the samples, field emission transmission electron microscopy was utilized to characterize the Fe3O4/PC, Ru-TiO2/PC, and Ru-TiO2/PC/Fe3O4 samples, as depicted in Fig. 4. In Fig. 4a, it is evident that quasi-spherical Fe3O4 nanoparticles are uniformly distributed on the biochar within the Fe3O4/PC sample, with no evidence of agglomeration and particle diameters ranging from 10 to 20 nm. Fig. 4b illustrates that the Ru-TiO2/PC sample is composed of nanoparticles integrated with thin carbon layers, where quasi-spherical Ru-TiO2 nanoparticles are systematically aligned along the biochar fibers, with particle diameters between 10 and 14 nm. Fig. 4c demonstrates that both Fe3O4 and Ru-TiO2 nanoparticles are uniformly dispersed on the GP biochar in the Ru-TiO2/PC/Fe3O4 sample, with particle sizes approximately between 10 and 20 nm, which aligns with the SEM observations. Fig. 4d presents the high-resolution transmission electron microscopy (HRTEM) image of the Ru-TiO2/PC/Fe3O4 sample, revealing four distinct lattice fringes. The lattice spacings of 0.253 nm and 0.148 nm are associated with the (311) and (440) crystal planes of Fe3O4,39 while the spacings of 0.352 nm and 0.237 nm correspond to the (101) and (004) crystal planes of TiO2,35 corroborating the XRD results. Due to the similar ionic radii of Ru4+ and Ti4+, and the minimal doping concentration of Ru, the incorporation of Ru did not significantly alter the lattice structure of TiO2.49 Fig. 4e displays the selected area electron diffraction (SAED) pattern of the Ru-TiO2/PC/Fe3O4 sample, showing diffraction rings corresponding to the (101) and (004) crystal planes of TiO2 and the (311) crystal plane of Fe3O4, indicative of a polycrystalline structure. Fig. 4f presents the energy dispersive X-ray spectroscopy (EDS) spectrum of the Ru-TiO2/PC/Fe3O4 sample, which further verifies the presence of Ti, O, C, Fe, Ru, P, K, and other elements in the sample.
Fig. 5 illustrates the transmission electron microscopy (TEM) elemental mapping of the Ru-TiO2/PC/Fe3O4 sample. The mapping delineates the distribution of Ti, O, C, Fe, Ru, P, and K elements. Ti, O, and C are observed to be extensively and uniformly dispersed, maintaining shapes consistent with the TEM mapping of the sample. Fe is primarily present in the form of spots and clusters, indicative of an uneven distribution of iron. Ru is sparsely but evenly distributed, exhibiting a shape akin to Ti, which suggests uniform doping of Ru into TiO2. P and K are distributed as uniform small dots, indicating that despite their low concentrations, their distribution is homogeneous, likely originating from the biochar-based material. Correlating these findings with the results from XRD, Raman, and XPS analyses, it can be concluded that the Ru-TiO2/PC/Fe3O4 composite material, comprising Ru-doped TiO2, GP biochar, and Fe3O4 with P and K elements, has been successfully synthesized.
 | ||
| Fig. 5 TEM image (a) and the corresponding elemental mapping images of (b) Ti, (c) O, (d) C, (e) Fe, (f) Ru, (g) P, and (h) K for the Ru-TiO2/PC/Fe3O4 sample. | ||
3.4 Mechanism of formation of the Ru-TiO2/PC/Fe3O4 composite
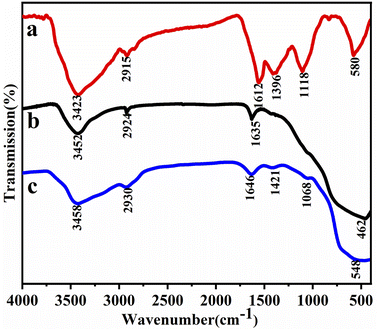
To elucidate the formation mechanism of the Ru-TiO2/PC/Fe3O4 composite, Fourier-transform infrared (FTIR) spectroscopy was performed on Fe3O4/PC, Ru-TiO2/PC, and Ru-TiO2/PC/Fe3O4. The results are presented in Fig. 6.As depicted in the figure, three samples exhibit a prominent and broad peak around 3400 cm−1, which is attributed to the stretching vibrations of –OH groups.54 Furthermore, distinct stretching vibration peaks corresponding to C–H bonds, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C–O–C are observed near 2900 cm−1 and 1600 cm−1. These findings indicate the presence of abundant functional groups, such as hydroxyl and carboxyl groups, in the samples.55 In the FTIR spectrum of Fe3O4/PC (Fig. 6a), the peaks observed at 1396 cm−1 and 1118 cm−1 are attributed to the stretching vibrations of C–H and C–O, respectively. Additionally, the characteristic peak located around 580 cm−1 corresponds to the Fe–O bond.56 Combined with the XRD analysis, these results further confirm the presence of Fe3O4. In the FTIR spectrum of the Ru-TiO2/PC sample (Fig. 6b), a prominent and broad peak at 462 cm−1 is observed, corresponding to Ti–O–Ti bonds, which are characteristic of the TiO2 structure.57 In the FTIR spectrum of Ru-TiO2/PC/Fe3O4 (Fig. 6c), alongside the characteristic peaks of biochar, additional peaks corresponding to Ti–O, Fe–O, and Ti–O–C bonds are observed. These results not only confirm the successful synthesis of the Ru-TiO2/PC/Fe3O4 composite but also highlight its intricate chemical structure.
O, and C–O–C are observed near 2900 cm−1 and 1600 cm−1. These findings indicate the presence of abundant functional groups, such as hydroxyl and carboxyl groups, in the samples.55 In the FTIR spectrum of Fe3O4/PC (Fig. 6a), the peaks observed at 1396 cm−1 and 1118 cm−1 are attributed to the stretching vibrations of C–H and C–O, respectively. Additionally, the characteristic peak located around 580 cm−1 corresponds to the Fe–O bond.56 Combined with the XRD analysis, these results further confirm the presence of Fe3O4. In the FTIR spectrum of the Ru-TiO2/PC sample (Fig. 6b), a prominent and broad peak at 462 cm−1 is observed, corresponding to Ti–O–Ti bonds, which are characteristic of the TiO2 structure.57 In the FTIR spectrum of Ru-TiO2/PC/Fe3O4 (Fig. 6c), alongside the characteristic peaks of biochar, additional peaks corresponding to Ti–O, Fe–O, and Ti–O–C bonds are observed. These results not only confirm the successful synthesis of the Ru-TiO2/PC/Fe3O4 composite but also highlight its intricate chemical structure.
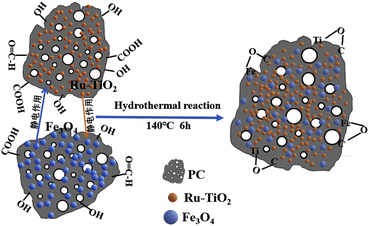
Based on the comprehensive analysis results from XRD, XPS, Raman, SEM, and FTIR, a formation mechanism model for the Ru-TiO2/PC/Fe3O4 composite material was proposed, as illustrated in Fig. 7. The surface of biochar is abundant in functional groups, including –OH and –COOH, which interact with TiO2 and Fe3O4 nanoparticles through hydrogen bonding and van der Waals forces. These interactions strengthen the adhesion between the nanoparticles and the biochar, contributing to the composite's structural stability. Furthermore, the –OH and –COOH groups on the biochar surface confer a negative charge, whereas the surfaces of the metal oxide particles exhibit a positive charge. This electrostatic interaction facilitates the binding of biochar in Ru-TiO2/PC with Fe3O4 particles, as well as the interaction of biochar in Fe3O4/PC with TiO2 particles, leading to the formation of primary composite structures. During the hydrothermal reaction, suitable temperature and pressure conditions promote coordination and condensation reactions between the carboxyl and hydroxyl groups on the biochar surface and the TiO2 and Fe3O4 nanoparticles. These interactions result in the formation of stable oxygen bridges (e.g., Ti–O–C and Fe–O–C), which significantly enhance the structural stability of the composite material. This effective microscopic bonding between the metal oxide particles and the biochar ultimately leads to the formation of a porous Ru-TiO2/PC/Fe3O4 composite.
3.5 Magnetic properties of Ru-TiO2/PC/Fe3O4
The magnetic hysteresis loops of Fe3O4/PC and the Ru-TiO2/PC/Fe3O4 series samples were evaluated through the application of a vibrating sample magnetometer (VSM), with the results illustrated in Fig. 8.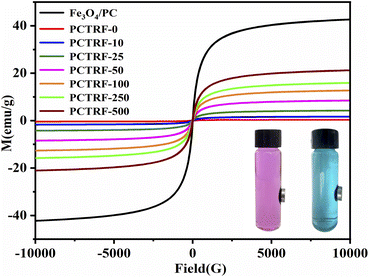 | ||
| Fig. 8 Magnetic hysteresis loops of Fe3O4/PC and PCTRF-x (x = 0, 10, 25, 50, 100, 250, 500) samples. | ||
As depicted in the figure, all samples are characterized by “S”-shaped magnetization curves. The saturation magnetization (Ms) of Fe3O4/PC is recorded at 43.86 emu g−1, whereas the Ms values of the Ru-TiO2/PC/Fe3O4 series samples are observed to be lower than this benchmark, progressively increasing as the Fe3O4/PC content rises. Notably, the Ms of the PCTRF-0 sample approximates zero, signifying non-magnetic characteristics. In contrast, the Ms of the PCTRF-500 sample is measured at 22.01 emu g−1, which indicates pronounced magnetic properties. The inset in Fig. 8 demonstrates the separation effect of the PCTRF-100 sample in RhB and MB solutions after exposure to an external magnetic field for 5 min. This observation confirms that the Ru-TiO2/PC/Fe3O4 sample can be effectively segregated from the solution under the influence of an external magnetic field, thereby enhancing its recyclability.
3.6 Photoelectric performance of Ru-TiO2/PC/Fe3O4
To explore the light absorption characteristics of the materials and determine their band gap energies, UV-visible diffuse reflectance spectroscopy (UV-vis DRS) was employed on the Fe3O4/PC and Ru-TiO2/PC/Fe3O4 series samples, with the findings depicted in Fig. 9a. It can be seen from the figure that the Fe3O4/PC sample demonstrated a distinct absorption peak around 480 nm, which indicates strong absorption within the visible light spectrum. With the increasing content of Fe3O4/PC, the Ru-TiO2/PC/Fe3O4 series samples progressively exhibited the characteristic absorption peak of Fe3O4, and their light absorption intensity in the visible region was significantly augmented.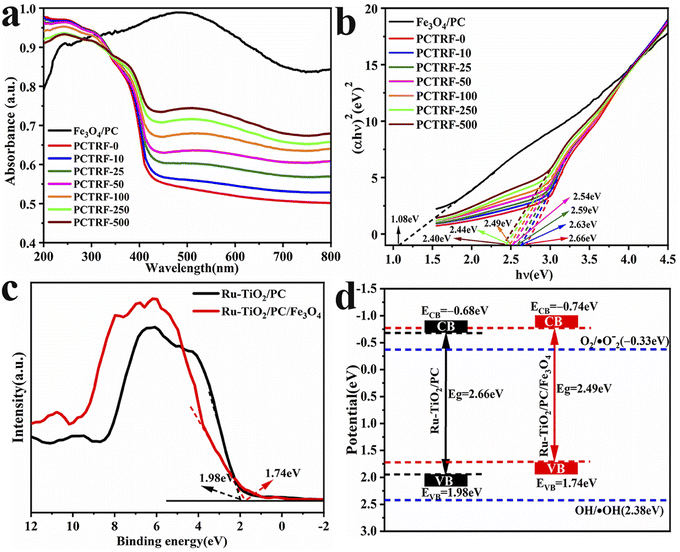 | ||
| Fig. 9 (a) UV-vis DRS spectra of Fe3O4/PC and Ru-TiO2/PC/Fe3O4 series samples, (b) Tauc plot curves, (c) VB-XPS spectra of Ru-TiO2/PC and Ru-TiO2/PC/Fe3O4 samples, and (d) band gap structure. | ||
The Kubelka–Munk formula and Tauc plots method were combined to plot the Tauc plot curve,58,59 as depicted in Fig. 9b. The band gap energy (Eg) was determined by extrapolating the linear segment to its intersection with the x-axis. The calculated Eg for Fe3O4/PC was approximately 1.08 eV, while that for Ru-TiO2/PC was around 2.66 eV. For the Ru-TiO2/PC/Fe3O4 series samples, the band gap energy Eg decreased from 2.63 eV to 2.40 eV, suggesting that Ru-TiO2/PC/Fe3O4 exhibits a narrower band gap, facilitating the easier excitation of electrons.
Valence band X-ray photoelectron spectroscopy (VB-XPS) tests were performed on Ru-TiO2/PC (PCTRF-0) and Ru-TiO2/PC/Fe3O4 (PCTRF-100) samples. The valence band potentials (EVB) were estimated at 1.98 eV and 1.74 eV, respectively, using the linear extrapolation method (Fig. 9c). Utilizing the formula ECB = EVB − Eg,60 the conduction band potentials (ECB) for the two samples were calculated as −0.68 eV and −0.74 eV, respectively. From these data, the band structure diagram of the samples was constructed (Fig. 9d). As illustrated in the figure, both the EVB and ECB of Ru-TiO2/PC/Fe3O4 exhibit a shift towards the negative direction compared to Ru-TiO2/PC. This shift indicates that the Ru-TiO2/PC/Fe3O4 composite material possesses a higher electron transfer efficiency, facilitating the effective separation of photogenerated electron–hole pairs and thereby enhancing its photocatalytic performance.61 Therefore, the Ru-TiO2/PC/Fe3O4 composite material shows considerable potential for applications in photocatalysis.
To assess the photogenerated electron–hole separation and transport efficiency of the samples, photocurrent measurements, electrochemical impedance spectroscopy (EIS), and photoluminescence (PL) tests were performed on Fe3O4/PC and Ru-TiO2/PC/Fe3O4 series samples. The results are presented in Fig. 10. The photocurrent–time response curves (Fig. 10a) reveal that all samples display rapid and stable responses to the light source, thereby demonstrating their capability to generate photoinduced carriers. Notably, the PCTRF-100 sample exhibits the highest photocurrent, which signifies superior carrier separation and transport efficiency. The EIS data (Fig. 10b) indicate that the Ru-TiO2/PC/Fe3O4 sample possesses the lowest impedance, implying an optimal carrier transport rate. The PL spectra (Fig. 10c) indicate that the Fe3O4/PC sample shows the highest PL peak intensity, whereas the PCTRF-100 sample displays the lowest PL peak intensity. A reduced PL peak intensity suggests a lower electron–hole recombination efficiency,62 indicating that the PCTRF-100 sample achieves the lowest photogenerated electron–hole recombination rate. In conclusion, the PCTRF-100 sample demonstrates superior carrier separation and transport efficiency, as well as the lowest electron–hole recombination rate, underscoring its potential as a highly efficient photocatalyst.
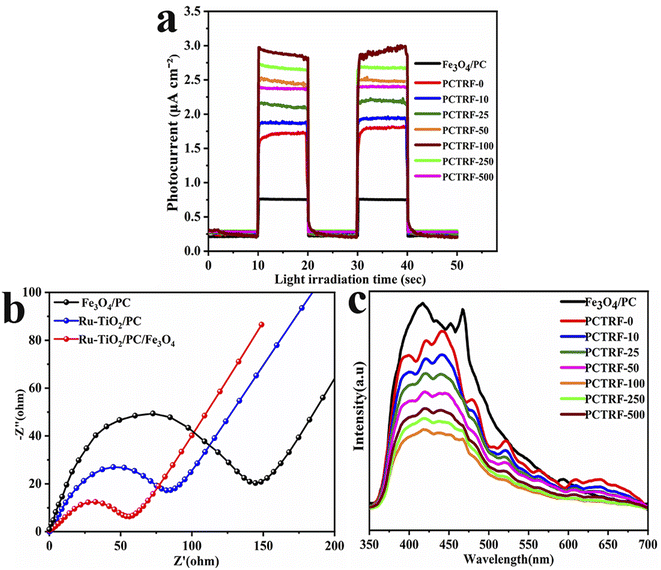 | ||
| Fig. 10 (a) Time-dependent photocurrent response curves; (b) EIS plots; and (c) PL spectra of Fe3O4/PC and Ru-TiO2/PC/Fe3O4 series samples. | ||
3.7 Photocatalytic performance of Ru-TiO2/PC/Fe3O4
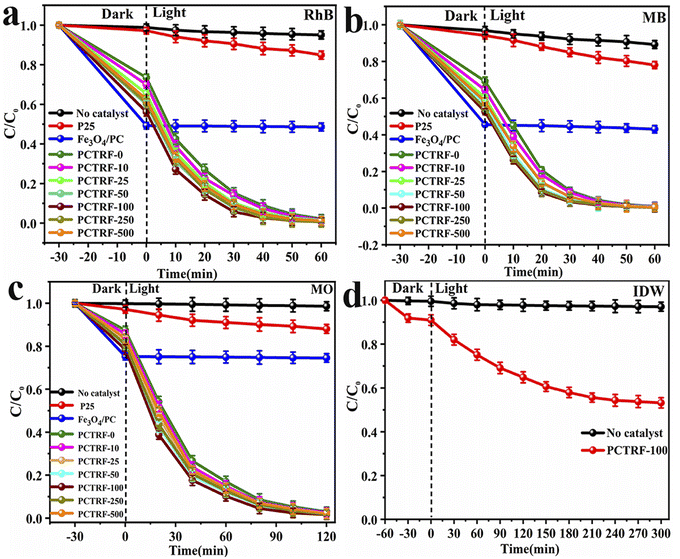
The photocatalytic degradation efficiency of the Ru-TiO2/PC/Fe3O4 composite material for RhB, MB, MO, and actual IDW under simulated solar light was assessed, with the corresponding results presented in Fig. 11. Fig. 11a–c illustrate that, in the absence of photocatalysts, the degradation efficiencies of RhB, MO, and MB remain extremely low, suggesting that these dyes are relatively stable under simulated solar light, making them suitable as target pollutants for photodegradation experiments. During the dark adsorption phase, the Fe3O4/PC sample exhibits adsorption rates of 50.78%, 54.46%, and 24.78% for RhB, MB, and MO, respectively, which significantly exceed those of the Ru-TiO2/PC/Fe3O4 series samples. This enhanced performance can be attributed to its higher biochar content and larger specific surface area. For the Ru-TiO2/PC/Fe3O4 series samples, the adsorption capacity for RhB, MB, and MO initially increases and subsequently decreases as the Fe3O4/PC content rises, with the PCTRF-100 sample demonstrating the most effective adsorption performance. During the photocatalytic degradation phase, Fe3O4/PC shows low degradation efficiency for RhB, MB, and MO, indicating inadequate photocatalytic activity. Under simulated solar light, the Ru-TiO2/PC/Fe3O4 series samples rapidly enhance the degradation efficiencies of RhB and MB within the first 30 min, reaching equilibrium after 60 min. For MO, a rapid decrease in degradation efficiency is observed within 80 min, followed by equilibrium after 120 min. As the Fe3O4/PC content increases, the photocatalytic performance of the composite catalyst initially improves and then declines. Among the samples, PCTRF-100 exhibits the most superior photocatalytic performance, achieving degradation rates of 99.4% and 99.8% for RhB and MB within 60 min, respectively, and 98.04% for MO within 120 min. These findings clearly indicate that an optimal mass ratio of Fe3O4/PC to Ru-TiO2/PC in the composite material is essential for maximizing photocatalytic performance. However, an excessive amount of Fe3O4 can cover the active sites of TiO2, thereby diminishing its surface activity and light transmittance, which in turn negatively impacts its photocatalytic efficiency.To assess the degradation effectiveness of the Ru-TiO2/PC/Fe3O4 composite catalyst on actual IDW, the best-performing PCTRF-100 sample was selected for experimentation. The IDW used in the experiment was obtained from a dyeing factory in Shantou City, Guangdong Province, and was characterized by an initial pH of approximately 8.0 and a chemical oxygen demand (COD) of about 1540 mg L−1. Due to the complex composition and high content of solid suspended particles in the dye wastewater, pretreatment was required prior to the experiment, involving multiple filtrations and high-speed centrifugation to eliminate solid suspended particles. Following pretreatment, 5 mL of the supernatant was diluted to 100 mL, and 50 mg of the PCTRF-100 sample was added. The photocatalytic experiment was carried out according to the procedure described in Section 2.3, with absorbance measured at a wavelength of 240 nm. The C/C0–t relationship curve was plotted, as depicted in Fig. 11d. The results showed that under simulated solar light, the PCTRF-100 sample exhibited photocatalytic activity toward actual IDW, achieving a degradation efficiency of 46.82% after 300 min of irradiation. This efficiency was significantly lower than that observed for single dye solutions, reflecting the complexity of actual IDW. Although the photocatalytic degradation effect of the PCTRF-100 sample on actual IDW did not fully meet expectations, it was able to reduce the COD value of IDW from 1540 mg L−1 to 784 mg L−1, demonstrating its potential value in treating actual IDW.
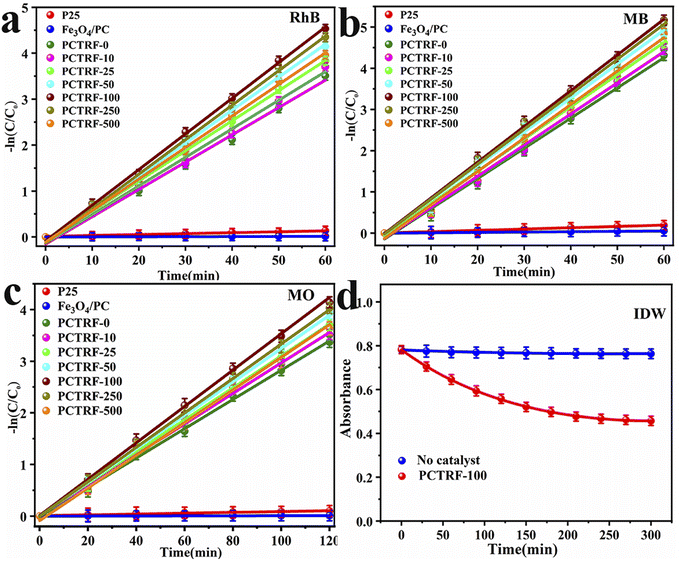
Furthermore, kinetic analysis was performed on the photocatalytic degradation processes of RhB, MB, MO, and IDW by the Ru-TiO2/PC/Fe3O4 series samples, with the results presented in Fig. 12 and Table 3. As depicted in Fig. 12a–c, the photocatalytic degradation of RhB, MB, and MO by the Ru-TiO2/PC/Fe3O4 series samples conforms well to a linear first-order kinetic model, with R2 values exceeding 0.99, which indicates a high degree of correlation. The data in Table 3 show that the rate constants (k) for the P25 and Fe3O4/PC samples are relatively low. The k values of the Ru-TiO2/PC/Fe3O4 series samples initially increase and then decrease as the Fe3O4/PC content is increased. Among these samples, the PCTRF-100 sample exhibits the highest k values, with kRhB, kMB, and kMO measured at 0.07667 min−1, 0.08671 min−1, and 0.03458 min−1, respectively, confirming its superior photocatalytic performance. Due to its complexity, a non-linear kinetic model was employed to fit the actual IDW. The model equation is given by At = Xe−kt + E, where At represents the absorbance of residual pollutants, X is the reaction amplitude, k denotes the rate constant, and E is the reaction endpoint.63 After fitting, an R2 value of 0.998 was obtained, indicating that the process of the PCTRF-100 sample in treating actual IDW aligns well with the non-linear least squares kinetic model (Fig. 12d). The rate constant was determined to be 0.001673 min−1, demonstrating its effectiveness in treating actual dye wastewater.
| Samples | kRhB (min−1) | RRhB2 | kMB (min−1) | RMB2 | kMO (min−1) | RMO2 | kIDW (min−1) | RIDW2 |
|---|---|---|---|---|---|---|---|---|
| P25 | 0.00215 | 0.9872 | 0.00320 | 0.9903 | 0.00077 | 0.9885 | — | — |
| Fe3O4/PC | 0.00022 | 0.9819 | 0.00089 | 0.9837 | 0.000076 | 0.9820 | — | — |
| PCTRF-0 | 0.05763 | 0.9949 | 0.07420 | 0.9950 | 0.02843 | 0.9980 | — | — |
| PCTRF-10 | 0.06062 | 0.9947 | 0.07644 | 0.9958 | 0.02954 | 0.9982 | — | — |
| PCTRF-25 | 0.06454 | 0.9989 | 0.07853 | 0.9978 | 0.03081 | 0.9993 | — | — |
| PCTRF-50 | 0.07046 | 0.9986 | 0.08266 | 0.9977 | 0.03241 | 0.9992 | — | — |
| PCTRF-100 | 0.07667 | 0.9987 | 0.08671 | 0.9970 | 0.03458 | 0.9992 | 0.006515 | 0.9982 |
| PCTRF-250 | 0.07358 | 0.9983 | 0.08500 | 0.9972 | 0.03327 | 0.9997 | — | — |
| PCTRF-500 | 0.06735 | 0.9979 | 0.08199 | 0.9963 | 0.03029 | 0.9974 | — | — |
The PCTRF-100 sample, which exhibited the highest photocatalytic performance, was compared with other magnetic catalysts reported in the literature, as summarized in Table 4. It can be seen that PCTRF-100 demonstrated superior degradation efficiency for RhB, MB, and MO under simulated solar light when compared to previously reported magnetic composite catalysts. To date, there have been no reports in the literature on the use of magnetic composite photocatalysts for the degradation of actual dye wastewater. However, the Ru-TiO2/PC/Fe3O4 composite catalyst investigated in this study also exhibited photocatalytic activity in treating actual IDW.
| Photocatalyst | Dosage (mg/100 mL) | Light source | Irradiation time (min) | Pollutant (conc.) | Removal efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| Fe3O4@Ru-TiO2 | 30 | 300 W Xe lamp | 120 | MB (15 mg L−1) | 70.20 | 2 |
| TiO2/SiO2@Fe3O4 | 100 | 1.5 kW Xe lamp | 60 | Ketamine (76 μg L−1) | 100 | 64 |
| C–TiO2@Fe3O4/AC | 100 | 300 W Xe lamp | 30 | Congo red (100 mg L−1) | 92.92 | 65 |
| Fe3O4/TiO2 | 100 | Direct sunlight | 40 | MB (5 ppm) | 97.00 | 28 |
| g-C3N4/Fe3O4/TiO2 | 30 | 500 W Xe lamp | 120 | MB (10 mg L−1) | 98.10 | 66 |
| Fe3O4–TiO2 | 100 | 500 W Xe lamp | 40 | RhB (5 mg L−1) | 99.20 | 67 |
| Fe3O4/C/TiO2 | 100 | 500 W Xe lamp | 120 | Phenol (10 mg L−1) | 82.46 | 68 |
| C–TiO2/Fe3O4 | 20 | Natural sunlight | 150 | MO (5 mg L−1) | 99.68 | 69 |
| Ru-TiO2/PC/Fe3O4 | 50 | 600 W Xe lamp | 60 | RhB (10 mg L−1) | 99.21 | This work |
| Ru-TiO2/PC/Fe3O4 | 50 | 600 W Xe lamp | 60 | MB (10 mg L−1) | 99.70 | This work |
| Ru-TiO2/PC/Fe3O4 | 50 | 600 W Xe lamp | 120 | MO (10 mg L−1) | 98.31 | This work |
| Ru-TiO2/PC/Fe3O4 | 50 | 600 W Xe lamp | 300 | Industrial dye wastewater | 46.81 | This work |
3.8 Recyclability of Ru-TiO2/PC/Fe3O4
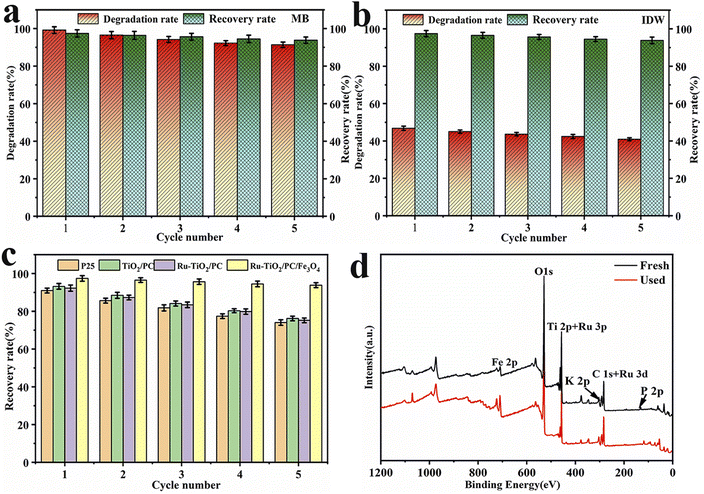
The recyclability and cyclic stability of catalysts are essential factors for their practical application and commercial viability. Therefore, the Ru-TiO2/PC/Fe3O4 sample with the highest photocatalytic performance was selected for five cycles of degradation experiments on MB solution and actual dye wastewater to assess its cyclic stability. In these experiments, after each reaction, the catalyst was separated and recovered using an external magnetic field, then washed, dried, and reused. Each cycle was performed under identical conditions, and the recovery rate and degradation rate were calculated. The results are depicted in Fig. 13.Fig. 13a and b indicate that after five cycles, Ru-TiO2/PC/Fe3O4 maintained good recovery and photocatalytic activity for both MB solution and actual IDW. The recovery rate remained above 93%, while the degradation rate of MB reached 91.38%, with only a slight decrease observed in the degradation rate for IDW. ANOVA and Tukey's tests confirmed significant differences in degradation rates across cycles (p < 0.05). This decline is likely due to dye molecules blocking or damaging the catalyst's porous structure, reducing active sites. Fig. 13c illustrates that the recovery rate of Ru-TiO2/PC/Fe3O4 is significantly higher than that of other catalysts, being 18.62%, 17.62%, and 20.76% higher than Ru-TiO2/PC, TiO2/PC, and P25, respectively. Fig. 13d presents a comparison of XPS spectra for samples before and after cyclic reactions. Although the intensity of some characteristic peaks weakened after five rounds of photodegradation, no new diffraction peaks were observed, suggesting that while some active sites of the catalyst may have been partially passivated, its chemical composition remained unchanged. This stability could be attributed to the advantages conferred by the composite structure of Ru-TiO2/PC and Fe3O4/PC.
3.9 Photocatalytic mechanism of Ru-TiO2/PC/Fe3O4 composite
To elucidate the mechanism underlying the photocatalytic degradation of dye wastewater by Ru-TiO2/PC/Fe3O4, electron paramagnetic resonance (EPR) tests were conducted to analyze the active free radicals generated during the photocatalytic reaction. Under xenon lamp irradiation, DMPO was utilized as a spin-trapping agent to capture ·OH and ·O2−, while TEMPO was employed to verify the presence of h+. The results (Fig. 14a) indicated that no EPR signal was detected in the absence of light irradiation. However, after 5 min of exposure to light, the EPR spectrum of the methanol system exhibited distinct six-line spectra, whereas the aqueous system showed four-line signal peaks with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. These peaks corresponded to ·O2− and ·OH trapped by DMPO, respectively.70 In Fig. 14b, three 1
1 ratio. These peaks corresponded to ·O2− and ·OH trapped by DMPO, respectively.70 In Fig. 14b, three 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 TEMPO signal peaks were observed, and upon light irradiation, a significant reduction in peak intensity was noted. This confirmed the generation of h+ under light irradiation, as h+ reacted with TEMPO, leading to a decrease in TEMPO signal intensity. This phenomenon aligns with previous research findings.71 In summary, the Ru-TiO2/PC/Fe3O4 composite catalyst is capable of generating active free radicals, such as ·O2−, ·OH, and h+, under light irradiation, which are essential in the photocatalytic reaction process.
1 TEMPO signal peaks were observed, and upon light irradiation, a significant reduction in peak intensity was noted. This confirmed the generation of h+ under light irradiation, as h+ reacted with TEMPO, leading to a decrease in TEMPO signal intensity. This phenomenon aligns with previous research findings.71 In summary, the Ru-TiO2/PC/Fe3O4 composite catalyst is capable of generating active free radicals, such as ·O2−, ·OH, and h+, under light irradiation, which are essential in the photocatalytic reaction process.
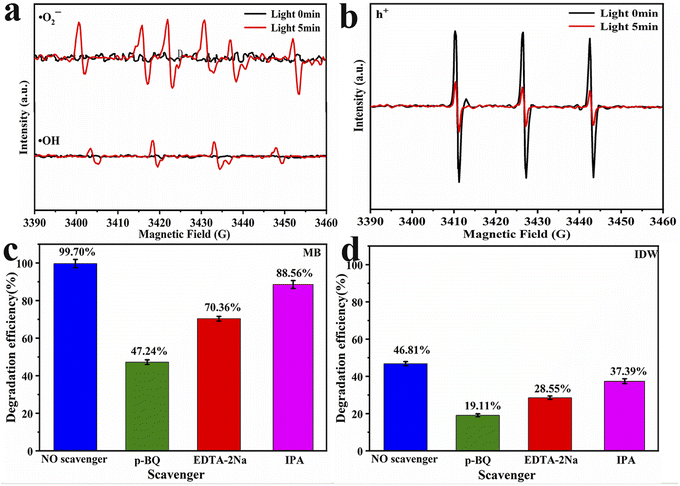 | ||
| Fig. 14 EPR spectra of Ru-TiO2/PC/Fe3O4 sample: (a) ·O2− and ·OH; (b) h+. Results of free radical trapping experiments with error bars: (c) MB; (d) IDW. | ||
To further investigate the role of various free radicals in the photocatalytic process, radical trapping experiments were conducted in MB solution and IDW. p-Benzoquinone (p-BQ), ethylenediaminetetraacetic acid disodium salt (EDTA-2Na), and isopropanol (IPA) were employed as scavengers for ·O2−, h+, and ·OH, respectively.72,73 The results are presented in Fig. 14c and d. As depicted in the figures, the addition of p-BQ resulted in a reduction in the degradation rates of MB and IDW by 52.46% and 27.70%, respectively. Similarly, the introduction of EDTA-2Na led to decreases in degradation rates by 29.34% and 18.26%, respectively. When IPA was added, the degradation rates declined by 10.14% and 9.42%, respectively. These findings suggest that ·O2− plays the most significant role in the photocatalytic process, followed by h+, while the influence of ·OH is comparatively minor.
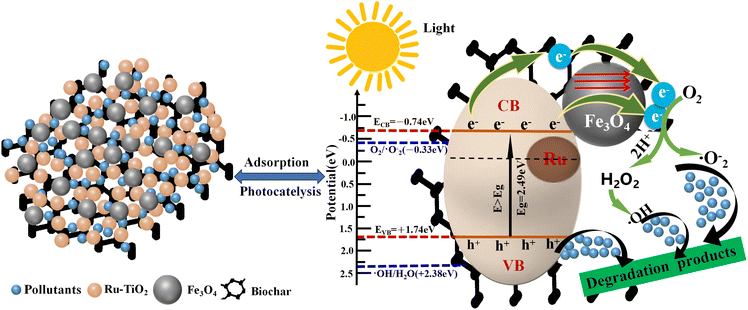
Based on the EPR analysis and the results of free radical trapping experiments, the mechanism of photocatalytic degradation of pollutants by the Ru-TiO2/PC/Fe3O4 composite material is elucidated (Fig. 15). The superior photocatalytic performance of this composite material can be attributed to the synergistic effects of Ru-doped TiO2, biochar, and Fe3O4. The doping of Ru reduces the bandgap energy of TiO2, enabling its excitation under simulated solar light to produce electron–hole pairs. Furthermore, Ru doping introduces intermediate energy levels that facilitate electron transition and transport. Biochar, which contains graphitic carbon, exhibits good conductivity and functions as an electron acceptor, capturing electrons from the conduction band and intermediate energy levels, thereby promoting effective electron–hole separation. Additionally, the internal magnetic field of Fe3O4 nanoparticles modulates the electron transport pathways, effectively suppressing the recombination of electrons and holes. These synergistic interactions result in the retention of more e− and h+ within the reaction system.74 The conduction band potential of Ru-TiO2/PC/Fe3O4 (−0.74 eV) is lower than the reduction potential of O2/·O2− (−0.33 eV), allowing e− to react with O2 on the material's surface to generate strongly oxidizing ·O2−. However, its valence band potential (+1.74 eV) is higher than the oxidation potential of ·OH/H2O (+2.38 eV), preventing h+ from directly oxidizing H2O to ·OH. Both EPR tests and free radical trapping experiments confirm that ·OH is also one of the active species. It is speculated that ·OH is formed through the reaction of ·O2− with H2O, initially producing H2O2, which subsequently decomposes.75 The combined action of ·O2−, ·OH, and h+ degrades dye pollutants into colorless small molecules. The entire process can be described in detail through chemical eqn (1)–(7).76
| Ru-TiO2/PC/Fe3O4 + hv → h+ + e−(TiO2) | (1) |
| e−(TiO2) → e−(PC, Ru) | (2) |
| e−(Ru) → e−(PC) | (3) |
| O2 + e−(TiO2, PC) → ·O2− | (4) |
| O2 + 2H+ + 2e− → H2O2 | (5) |
| H2O2 + e− → OH− + ·OH | (6) |
| (·O2−, h+ and ·OH) + pollutants → degraded products | (7) |
 | ||
| Fig. 15 Schematic diagram of the mechanism for photocatalytic degradation of organic pollutants by Ru-TiO2/PC/Fe3O4 composite material. | ||
4 Conclusion
In this study, a series of Ru-TiO2/PC/Fe3O4 composite materials with varying mass ratios was successfully synthesized, and their structures and physicochemical properties were thoroughly characterized using XRD, XPS, SEM, and TEM. The analysis revealed that Fe3O4 within the composite adopted a cubic spinel structure, characteristic of magnetite, while TiO2 predominantly exhibited the anatase phase. It was found that Ru-TiO2 and Fe3O4 nanoparticles were uniformly distributed on the GP biochar. The composite material demonstrated a large specific surface area, a narrow band gap, high photocurrent, and low electrochemical impedance. Photocatalytic studies demonstrate that the Ru-TiO2/PC/Fe3O4 composite catalyst outperforms P25, Fe3O4/PC, and Ru-TiO2/PC in degrading dye pollutants. The photocatalytic efficiency increases with the Fe3O4/PC content up to an optimal level, after which it declines, with PCTRF-100 exhibiting the highest performance. Under 600 W xenon lamp irradiation, PCTRF-100 achieved degradation rates of 99.4% and 99.8% for RhB and MB within 60 minutes, respectively, and 98.04% for MO within 120 minutes. Additionally, it demonstrated significant effectiveness in treating industrial dyeing wastewater, achieving a degradation rate of 46.82% within 300 minutes and reducing the COD value from 1540 mg L−1 to 784 mg L−1. Furthermore, the composite material is easily separable and recyclable under a magnetic field, exhibiting excellent cycling stability. Analysis of the photocatalytic mechanism revealed that ·O2− and h+ were the primary active species generated during the photocatalytic process, while the synergistic interactions among Ru-TiO2, biochar, and Fe3O4 effectively enhanced the separation efficiency of photogenerated electron–hole pairs, significantly improving photocatalytic performance. This study developed an efficient and recyclable photocatalyst from waste biomass, providing significant insights into its application in environmental remediation and related fields. Future research should focus on expanding the range of target pollutants, studying long-term stability, evaluating performance under natural sunlight, and integrating with advanced treatment technologies to further enhance its industrial application value.Data availability
Data are available upon request from the authors.Author contributions
Ruixiang wu (conceptualization; data curation; formal analysis; investigation; methodology; software; supervision; validation; writing – original draft; writing – review & editing). Hanyan Zhang (data curation; investigation; methodology; resources; validation). Wenhua Liu (conceptualization; funding acquisition; project administration; resources; supervision; writing – review & editing). Renao Bai (formal analysis; investigation). Delun Zheng (data curation; investigation; software). Xiufang Tian (data curation; investigation; methodology). Weikai Lin (data curation; investigation; software). Lejian Li (data curation; investigation; software). Qianwei Ke (data curation; investigation; software).Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support from the Special Fund Project for Science and Technology Innovation Strategy of Guangdong Province (No. pdjh2021b0916).References
- S. S. Neetu, P. Srivastava and L. Bahadur, Sol. Energy, 2020, 208, 173–181 CrossRef CAS.
- Q. Zhang, L. Y. Yu, B. R. Yang, C. C. Xu, W. Zhang, Q. Xu and G. W. Diao, Chem. Phys. Lett., 2021, 774, 138609 CrossRef CAS.
- R. Singh, M. Kumar, H. Khajuria, J. Ladol and H. N. Sheikh, Chem. Pap., 2018, 72, 1181–1192 CrossRef CAS.
- N. Soltani, E. Saion, M. Z. Hussein, M. Erfani, A. Abedini, G. Bahmanrokh, M. Navasery and P. Vaziri, Int. J. Mol. Sci., 2012, 13(10), 12242–12258 CrossRef CAS PubMed.
- C. A. D'Amato, R. Giovannetti, M. Zannotti, E. Rommozzi, S. Ferraro, C. Seghetti, M. Minicucci, R. Gunnella and A. Di Cicco, Appl. Surf. Sci., 2018, 441, 575–587 CrossRef.
- R. Taourati, M. Khaddor, A. Laghzal and A. E. Kasmi, Environ. Sci. Technol., 2020, 8, e00305 CAS.
- S. Q Chen, H. D. Liang, M. Shen and Y. X. Jin, Appl. Phys. A: Mater. Sci. Process., 2018, 124, 305 CrossRef.
- H. R. Mardani, Res. Chem. Intermed., 2017, 43, 5795–5810 CrossRef CAS.
- P. K. Boruah, P. Borthakur, G. Darabdhara, C. K. Kamaja, I. Karbhal, M. V. Shelke, P. Phukan, D. Saikia and M. R. Das, RSC Adv., 2016, 6, 11049–11063 RSC.
- C. Chen, Z. H. Ma, S. Y. Zhou, T. Q. Li and X. L. Sun, Catal. Lett., 2017, 147, 2399–2409 CrossRef CAS.
- K. S. Ranjith, P. Manivel, R. T. Rajendrakumar and T. Uyar, Chem. Eng. J., 2017, 325, 588–600 CrossRef CAS.
- H. R. Chen, K. Shen, J. Y. Chen, X. D. Chen and Y. W. Li, J. Mater. Chem. A, 2017, 5, 9937–9945 RSC.
- B. Uzunbayir, U. Kartal, E. C. Doluel, M. Yurddaskal and M. Erol, J. Sol-Gel Sci. Technol., 2020, 96(2), 441–451 CrossRef CAS.
- T. T. Qian, X. P. Yin, J. H. Li, H. E. Nian, H. Xu, Y. Deng and X. Wang, J. Mater. Sci. Technol., 2017, 33(11), 1314–1322 CrossRef CAS.
- T. Hariff, M. Montazer, R. Dillert and D. W. Bahnemann, J. Clean. Prod., 2018, 196, 688–697 CrossRef.
- Z. Y. Zhang, C. C. Zhao, Y. S. Duan, C. Wang, Z. C. Zhao, H. J. Wang and Y. J. Gao, Appl. Surf. Sci., 2020, 527, 146693 CrossRef CAS.
- S. Khammar, N. Bahramifar and H. Younesi, J. Hazard. Mater., 2020, 394, 122422 CrossRef CAS PubMed.
- P. P. Chen, Y. Y. Cai, J. Wang, K. W. Wang, Y. S. Tao, J. D. Xue and H. G. Wang, Powder Technol., 2018, 326, 272–280 CrossRef CAS.
- R. X. Wu, W. H. Liu, R. A. Bai, X. F. Tian, W. K. Lin, L. J. Li and Q. W. Ke, RSC Adv., 2024, 14, 15604–15618 RSC.
- R. X. Wu, W. H. Liu, R. A. Bai, D. L. Zheng, X. F. Tian, W. K. Lin, Q. W. Ke and L. J. Li, Molecules, 2024, 29(9), 2090 CrossRef CAS PubMed.
- R. M. Kulkami, R. S. Malladi, M. S. Hanagadakar, M. R. Doddamani, B. Santhakumari and S. D. Kulkarni, J. Mater. Sci.: Mater. Electron., 2016, 27(12), 13065–13074 CrossRef.
- R. A. Elsalamony and S. A. Mahmoud, Arab. J. Chem., 2017, 10(2), 194–205 CrossRef CAS.
- H. B Zhang, S. W. Zuo, M. Qiu, S. B. Wang, Y. F. Zhang and X. W. Lou, Sci. Adv., 2020, 6(39), abb9823 CrossRef PubMed.
- N. I. Mohd Razip, K. M. Lee, C. W. Lai and B. H. Ong, Mater. Res. Express, 2019, 6(7), 075517 CrossRef.
- A. C. Sun, Adv. Powder Technol., 2018, 29(3), 719–725 CrossRef CAS.
- H. L. Niu, Q. M. Wang, H. X. Liang, M. Chen, C. J. Mao, J. M. Song, S. Y. Zhang, Y. H. Gao and C. Chen, Materials, 2014, 7(5), 4034–4044 CrossRef CAS PubMed.
- Q. Zhang, L. Y. Yu, C. C. Xu, J. Y. Zhao, H. Y. Pan, M. Chen, Q. Xu and G. W. Diao, Appl. Surf. Sci., 2019, 483, 241–251 CrossRef CAS.
- J. R. Rajabathar, R. Thankappan, A. Sutha, H. Al-Lohedan, A. M. Karami, S. A. Kumar, G. Devendrapandi, S. R. Marjorie and R. Balu, Opt. Mater., 2024, 148, 114820 CrossRef CAS.
- H. X. Huang, T. Guo, K. Wang, Y. Li and G. K. Zhang, Sci. Total Environ., 2021, 758, 143957 CrossRef CAS PubMed.
- Z. Y. Zhang and J. L. Kong, J. Hazard. Mater., 2011, 193, 325–329 CrossRef CAS PubMed.
- B. Y. Wang, M. Zhang, W. Z. Li, L. L. Wang, J. Zheng, W. J. Gan and J. L. Xu, Dalton Trans., 2015, 44, 17020–17025 RSC.
- Z. Y. Zhang, Z. Y. Xiong, C. C. Zhao, P. J. Guo, H. J. Wang and Y. J. Gao, Appl. Surf. Sci., 2021, 565, 150554 CrossRef CAS.
- R. Djellabi, B. Yang, Y. Wang, X. Q. Cui and X. Zhao, Chem. Eng. J., 2019, 366, 172–180 CrossRef CAS.
- T. Rajaramanan, M. Natarajan, P. Ravirajan, M. Senthilnanthanan and D. Velauthapillai, Energies, 2020, 13(7), 1532 CrossRef CAS.
- M. Ismael, New J. Chem., 2019, 43, 9596–9605 RSC.
- M. M. Shi, D. Bao, S. J. Li, B. R. Wulan, J. M. Yan and Q. Jiang, Adv. Energy Mater., 2018, 8(21), 1800124 CrossRef.
- Y. Y. Cao, G. H. Shen, Y. Zhang, C. F. Gao, Y. F. Li, P. Z. Zhang, W. H. Xiao and L. J. Han, Sci. Total Environ., 2019, 692, 479–489 CrossRef CAS PubMed.
- Y. Ling, M. Zhang, X. K Li, J. Zheng and J. L. Xu, Dalton Trans., 2018, 47, 10093–10101 RSC.
- H. F. Zhuang, H. Zhu, J. Zhang, S. D. Shan, C. G. Fang, H. J Tang and Q. N. Xie, Bioresour. Technol., 2020, 296, 122306 CrossRef CAS PubMed.
- B. Sun, N. Lu, Y. Su, H. T. Yu, X. Y. Meng and Z. M. Gao, Appl. Surf. Sci., 2017, 394, 479–487 CrossRef CAS.
- Y. G. Tan, Z. Shu, J. Zhou, T. T. Li, W. B. Wang and Z. L. Zhao, Appl. Catal., B, 2018, 230, 260–268 CrossRef CAS.
- A. A. Gonçalves, P. C. Siciliano, O. C. Alves, D. V. Cesar and C. A. Henriques, Top. Catal., 2020, 63(14), 1017–1029 CrossRef.
- D. L. Qian, D. Y. Chen, N. J. Li, Q. F. Xu, H. Li, J. H. He and J. M. Lu, J. Membr. Sci., 2018, 554, 16–25 CrossRef CAS.
- D. Li, X. L. Guo, H. R. Song, T. Y. Sun and J. F. Wan, J. Hazard. Mater., 2018, 351, 250–259 CrossRef CAS PubMed.
- X. H. Jia, R. R. Dai, D. D. Lian, S. Han, X. Y. Wu and H. J. Song, Appl. Surf. Sci., 2017, 392, 268–276 CrossRef CAS.
- L. J. Duan, N. Jiang, N. Lu, K. F. Shang, J. Li and Y. Wu, Appl. Catal., B, 2018, 221, 521–529 CrossRef CAS.
- J. N. Li, Q. C. Zhang, J. M. Liu, M. R. Yu, H. Y. Ma, J. C. Yang, S. Ye, T. R. Reina and J. Liu, J. Colloid Interface Sci., 2020, 577, 512–522 CrossRef CAS PubMed.
- S. Banerjee, P. Benjwal, M. Singh and K. K. Kar, Appl. Surf. Sci., 2018, 439, 560–568 CrossRef CAS.
- S. Wang, B. Liu, Y. Zhu, Z. R. Ma, B. B. Liu, X. Miao, R. X. Ma and C. Y. Wang, Sol. Energy, 2018, 169, 335–342 CrossRef CAS.
- H. H. Li, Z. Q. Jin, N. Lu, J. M. Pan, J. L. Xu, X. B. Yin and M. Zhang, Dalton Trans., 2024, 53, 6974 RSC.
- X. L. Wang, M. W. Zhang, J. M. Zhao, G. Y. Huang and H. Y. Sun, Appl. Surf. Sci., 2018, 427, 1054–1063 CrossRef CAS.
- C. Y. Kuo, C. H. Wu, J. T. Wu and Y. R. Chen, React. Kinet. Mech. Catal., 2015, 114, 753–766 CrossRef CAS.
- S. F. Zhao, Y. J. Ji, M. Jeong, H. Choe, J. W. Lee, S. Y. Kim, S. Saqlain, Z. K. Peng, Z. Y. Liu and Y. D. Kim, Chem. Eng. J., 2022, 444, 136500 CrossRef CAS.
- W. B. Chai, X. Y. Liu, J. C. Zou, X. Y. Zhang, B. B. Li and T. T. Yin, Pomelo peel modified with acetic anhydride and styrene as new sorbents for removal of oil pollution, Carbohydr. Polym., 2015, 132, 245–251 CrossRef CAS PubMed.
- S. O. Dan and S. H. Khan, An Overview on Green Titanium Nanoparticles Based on Plants: Synthesis, Potential Applications and Eco-Friendly Approach, Int. J. Life Sci. Biotechnol. Pharma Res., 2022, 12(5), 215–P233 Search PubMed.
- Y. Zhou, S. R. Cao, C. X. Xi, X. L. Li, L. Zhang, G. M. Wang and Z. Q. Chen, A novel Fe3O4/graphene oxide/citrus peel-derived bio-char based nanocomposite with enhanced adsorption affinity and sensitivity of ciprofloxacin and sparfloxacin, Bioresour. Technol., 2019, 292, 121951 CrossRef CAS PubMed.
- E. Grabowskaa, M. Diak, T. Klimczuk, W. Lisowski and A. Z. Medynska, Novel decahedral TiO2 photocatalysts modified with Ru or Rh NPs: Insight into the mechanism, Mol. Catal., 2017, 434, 154–166 CrossRef CAS.
- Y. Zhou, S. R. Cao, C. X. Xi, X. L. Li, L. Zhang, G. M. Wang and Z. Q. Chen, Bioresour. Technol., 2019, 292, 121951 CrossRef CAS PubMed.
- E. Grabowskaa, M. Diak, T. Klimczuk, W. Lisowski and A. Z. Medynska, Mol. Catal., 2017, 434, 154–166 CrossRef CAS.
- Y. F. Liu, L. J. Wang, X. W. Dai, J. Zhang, J. Li and Y. B. Ma, J. Alloys Compd., 2024, 976, 172985 CrossRef CAS.
- B. Mercyrani, R. H. Maya, M. S. López, C. T. Th and S. Velumani, J. Mater. Sci.: Mater. Electron., 2018, 29(18), 15436–15444 CrossRef CAS.
- Y. M. Hunge, A. A. Yadav, S. Khan, K. Takagi, N. Suzuki, K. Teshima and C. Terashima, J. Colloid Interface Sci., 2021, 582, 1058–1066 CrossRef CAS PubMed.
- Y. Jiang and A. Liu, Environ. Sci. Pollut. Res. Int., 2023, 30, 31923–31934 CrossRef CAS PubMed.
- Y. J. Yang, Y. Yao, L. He, Y. T. Zhong, Y. Ma and J. N. Yao, J. Mater. Chem. A, 2015, 3, 10060–10068 RSC.
- J. T. Zhang, Y. H. Cao, P. Zhao, T. F. Xie, Y. H. Lin and Z. Mu, Colloids Surf., A, 2020, 601, 125019 CrossRef CAS.
- Z. Y. Chen, W. W. P. Lai, H. H. H. Lin, J. X. Tan, K. C. W. Wu and A. Y. C. Lin, J. Environ. Chem. Eng., 2022, 10, 108637 CrossRef CAS.
- L. Zhu, X. Q. Kong, C. W. Yang, B. X. Ren and Q. Tang, J. Hazard. Mater., 2020, 381, 120910 CrossRef CAS PubMed.
- X. N. Wei and H. L. Wang, J. Alloys Compd., 2018, 763, 844–853 CrossRef CAS.
- Q. Z. Mu, Y. F. Sun, A. Y. Guo, X. Z. Yu, X. Y. Xu, A. J. Cai and X. P. Wang, Mater. Res. Express, 2019, 6, 0950c3 CrossRef CAS.
- Y. Liu, J. F. Wan, C. T. Liu and Y. B. Li, Mater. Sci. Technol., 2016, 32(8), 1743284715y CrossRef.
- M. Gebrezgiabher, G. Gebreslassie, T. Gebretsadik, G. Yeabyo, F. Elemo, Y. Bayeh, M. Thomas and W. Linert, J. Compos. Sci., 2019, 3(3), 75 CrossRef CAS.
- S. N. Zhuo, H. Y. Ren, G. L. Cao, G. J. Xie, D. F. Xing, N. Q. Ren and B. F. Liu, Chem. Eng. J., 2022, 440, 135947 CrossRef CAS.
- Z. H. Li, H. Bai, J. L. Wei, J. J. Wang, L. L. Kong, X. Wang, X. D. Xia and J. Y. Duan, J. Colloid Interface Sci., 2022, 610, 893–904 CrossRef CAS PubMed.
- W. D. Dai, L. Jiang, J. Wang, Y. J. Pu, Y. F. Zhu, Y. X. Wang and B. B. Xiao, Chem. Eng. J., 2020, 397, 125476 CrossRef CAS.
- S. Q. Wu, X. Y. Li, Y. Q. Tian, Y. Lin and Y. H. Hu, Eng. J., 2021, 406, 126747 CrossRef CAS.
- J. H. Tan, X. Wang, W. S. Hou, X. L. Zhang, L. Q. Liu, J. H. Ye and D. F. Wang, J. Alloys Compd., 2019, 792, 918e927 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |