Open Access Article
Open Access ArticleDesign strategies for organelle-selective fluorescent probes: where to start?
Samira Husen Alamudi *a and
Yong-An Lee*b
*a and
Yong-An Lee*b
aDepartment of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Indonesia, Depok, Indonesia 16424. E-mail: samirahusenalamudi@gmail.com; Tel: +6221-7270027
bGenome Institute of Singapore (GIS), Agency for Science, Technological, and Research (A*STAR), 60 Biopolis Street, Genome, Singapore 138672
First published on 22nd January 2025
Abstract
Monitoring physiological changes within cells is crucial for understanding their biological aspects and pathological activities. Fluorescent probes serve as powerful tools for this purpose, offering advantageous characteristics over genetically encoded probes. While numerous organelle-selective probes have been developed in the past decades, several challenges persist. This review explores the strategies and key factors contributing to the successful rationale design of these probes. We systematically discuss the typical mode of cellular uptake generally adopted by fluorescent probes and provide a detailed examination of the key factors to consider in design rationale from two perspectives: the properties of the target organelle and the physicochemical properties of the probe itself. Additionally, recent examples of organelle-targeted probes are presented, along with a discussion of the current challenges faced by fluorescent probes in the field.
1. Introduction
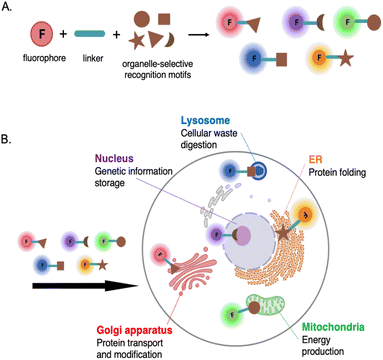
One of the most popular techniques in bioimaging is fluorescence-based imaging with the use of small fluorescent probes. This technique has revolutionized the study of molecular structures and subcellular dynamics in living cells, offering valuable insights into the mechanisms governing cellular processes, potential therapeutic targets, and other interventions on biological systems.Fluorescent probes offer several advantages for imaging cellular systems: they are relatively small, exhibit high specificity and sensitivity, and cause minimal disruption and negligible perturbation to the native functions of cells. Importantly, they can enable real-time imaging and multiplexing, which is particularly valuable for subcellular applications within organelles. To achieve this, the probes must be meticulously designed not only for specific targeting of organelles but also to possess the capability of penetrating cell membranes. These probes are commonly designed on the basis of having: a fluorophore, linker, and recognition moiety.1 This recognition moiety allows the probes to bind to and track specific organelles or other biological targets within cells.
Intracellular organelles, such as nucleus, mitochondria, endoplasmic reticulum (ER), and lysosomes, are critical for maintaining the proper functioning of cells. Mitochondria are responsible for producing cellular energy; nucleus for the storage and processing of genetic information; ER and Golgi apparatus for transporting proteins and other molecules within cells; and lysosome for breaking down and digesting cellular waste. Probing these specific organelles could further provide insights into the dynamic behavior of organelles inside the cellular complex system, their interactions with other cellular components, and their role in cellular functions and diseases. For instance, the contact of mitochondria and ER plays an important role in intracellular calcium homeostasis and other metabolic processes.2,3
Despite challenges, a good number of organelle-selective fluorescent probes have been successfully developed and have shown excellent selectivity and sensitivity in living cells and even to the level of nanoscale imaging.4 Some comprehensive reviews on this subject have been published elsewhere recently.5–10 These reviews highlight the recent progress and summarize the reported fluorescent probes targeting intracellular organelles in the last decade. However, a systematic review that outlines a step-by-step design rationale and key factors contributing to the successful development of small fluorescent probes for intracellular organelle imaging is currently still lacking. Therefore, this review aims to address this knowledge gap by presenting an overview of the important factors that should be considered in the design of such probes.
We hope that this review could serve as a stepping stone for researchers working on the development of such probes, providing insights that would contribute to the advancement of design strategies for the successful development of cell-permeable probes tailored for intracellular organelle imaging.
2. Targeting subcellular organelles
Organelles are membrane-bound structures with unique functions that are essential for the overall function of cells. To highlight the complexity and organization of cells, they have been likened to a ‘microscopic city,’ where organelles perform specialized functions that collectively contribute to maintaining cell health and functionality.The development of fluorescent probes targeting specific organelles is important for several reasons. First, it allows researchers to study the function and behavior of specific organelles in living cells in real-time, providing important insights into the role of organelles in cellular processes and disease.11 Second, it enables the monitoring of changes in organelle function in response to various stimuli or interventions. For instance, hypoxia and ROS can trigger mitophagy, the targeted degradation of mitochondria through autophagy, which is linked to tumor progression.12,13 Third, it holds promise for various applications in both diagnostics and therapeutics. An example of this is the development of mitochondria-targeting photosensitizer agents, such as MitDt, for photodynamic therapy in cancer treatment.14
Given their specialized functions, organelles need to maintain an optimum chemical environment, including factors such as oxidation state, pH, and cation concentrations, as well as require the transport of enzymes and metabolites to their appropriate locations. Any alteration in the chemical composition inside the organelle, whether due to abnormality or disease, can adversely affect its function, leading to a disruption in overall cell health.15 Therefore, it is essential to also take into account the microenvironments of organelles while designing probes and studying their physiology and pathology.
Schematic representation of a typical fluorescent probe design for targeting organelles is depicted in Fig. 1.
3. Cellular uptake of the fluorescent probes
The initial stage of directing fluorescent probes to intracellular organelles involves the uptake of these probes by the cells. This process is mainly determined firstly by the interactions between the probe and the plasma membrane. The membrane, which is the outer boundary of cells, is composed of multiple lipid species and membrane proteins that are also often conjugated to polysaccharides. In general, lipids form a negatively charged bilayer, providing a morphological role to the membrane, while proteins regulate the exchange of substances and provide cellular signaling, serving a functional role. The membrane is not a static body; it is continuously in motion, creating a highly dynamic structure.16 Typically, the transport mechanism of probe molecules can be divided into two main groups: passive or equilibrative diffusion and carrier-mediated transport.3.1 Passive or equilibrative diffusion
Passive or also called equilibrative diffusion is a mechanism where molecules move across the cell membrane. This process is driven by a concentration or electric potential gradient and does not require energy or intermediary protein transporters.16 Polarity and size of the crossing molecules are key factors influencing this transmembrane diffusion. Small nonpolar gases like O2, CO2, and N2, as well as small polar molecules such as ethanol, can easily pass through lipid membranes.While small size and adequate lipophilicity are usually required for this type of diffusion, interesting exceptions have been observed with certain exogenous natural products, such as the cyclic peptide drug Cyclosporin A (CsA).17 CsA is an outlier phenomenon. Despite its relatively large size (MW = 1200 Da), CsA can passively cross the plasma membrane. This is attributed to its reversible formation of intramolecular hydrogen bonds and the shielding of polarity through lipophilic side chains.18 Similar logic design may accordingly be applicable for other “oversized” fluorescent probes (MW >1000 Da) that exceed the size limit suggested by Lipinski's Rule of Five, which prefers molecules with good absorption to be under 500 Da.19
Generally, molecules with a negative charge have low cellular uptake efficiency due to the inside of the cell being more negatively charged relative to the outside.20 This creates an electric potential gradient that hinders the movement of negatively charged molecules against it.
3.2 Carrier-mediated transport
Cells utilize membrane carrier or transporter proteins to assist in the entry or export of molecules that are not sufficiently permeable. These transporters are embedded in the cell membrane and have specific binding sites that can recognize and bind to particular molecules. The expression of these transporters may also vary depending on the cell type.Carrier-mediated transport can be classified into two main types: facilitated diffusion and active transport.21 Facilitated diffusion involves the passive movement of molecules along their concentration gradient. In this process, carrier proteins facilitate the transport of molecules that would otherwise have difficulty crossing the membrane due to their size, charge, or hydrophobicity. On the other hand, active transport requires the expenditure of energy in the form of ATP. It enables the transport of molecules against their concentration gradient, moving from an area of lower concentration to an area of higher concentration. This process is crucial for maintaining concentration gradient across cellular membranes and for the selective uptake or extrusion of specific molecules or ions.
In the cellular transport system, the uptake of molecules by cells is usually facilitated by solute carriers (SLCs) transporters, while the efflux of molecules from the cell is mediated by ATP-binding cassette (ABC) family transporters.22,23 Fluorescent probes are typically trapped inside cells either by binding to target molecules or by being retained by transporter gates. A recent study by Chang group investigated whether the staining and high permeability property of probe CO-1 (MW = ∼494 Da),24 a cyclooctyne-containing BODIPY probe, is mediated by protein transporters. Through ABC-CRISPRi-based screening and inhibitor tests in mammalian cells, they found that CO-1 can freely penetrate cells, while its efflux out of the cell membrane is facilitated by ABCB1 and DIRC2 transporters.25 This type of staining or entry mechanism that relies on transporters has also recently introduced as to gating-oriented live cell distinction (GOLD).26
An illustration of the transport mechanisms of fluorescent probes in cells can be seen in Fig. 2.
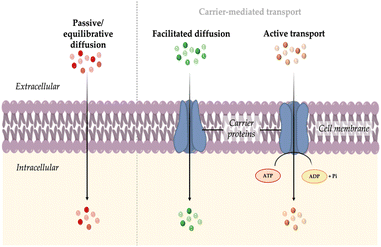 | ||
| Fig. 2 Transport mechanisms of probes into cell: passive or equilibrative diffusion, facilitated diffusion, and active transport. | ||
4. Design rationale for organelle-targeting fluorescent probes
Designing fluorescent probes typically begins by identifying a target analyte, which is then combined with a known recognition motif and a fluorophore using common strategies.27 Probes targeting intracellular organelles are designed to not only specifically and selectively bind to the target but also to be compatible with the cellular environment, enabling detection and analysis in living cells. However, the plasma membrane is highly selective and impermeable to most molecules, posing a significant challenge in developing probes that can efficiently penetrate the membrane.The design rationale for these probes is based on several factors: (i) properties of the target organelle and (ii) physicochemical properties of the probe.
4.1 Properties of target organelles
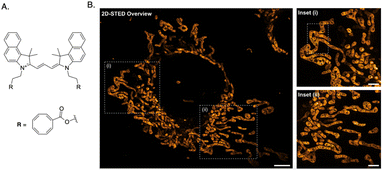
The structure and physicochemical properties of organelles play a crucial role, as each organelle possesses unique molecular constituents that can be selectively targeted by specific probes. For example, lysosomes possess an acidic vesicular structure that allows the accumulation of weakly basic substances, while negative mitochondrial membrane potential attracts cationic amphipathic molecules to accumulate in the mitochondria. In this section, we will explore the diverse properties of organelles, highlighting their significance in ensuring the efficacy and success of probe design.For instance, mitochondria have highly convoluted inner membranes called cristae, which typically adopt tubular or lamellar shapes and extend into the matrix space. The cristae architecture adapts to different cellular conditions and undergoes changes in response to various stimuli.28 Additionally, studying cristae dynamics is challenging due to the small size of mitochondria: the size of mitochondrial tubules ranges between 200 to 700 nm, while the distance from one crista to another can be below 100 nm. A recent study reported an orthogonal labeling strategy where a benzo-fused cyanine dye was conjugated to cyclooctatetraene (COT) for live-cell stimulated emission depletion (STED) microscopy of mitochondria. The dye, PK Mito Orange (PKMO), serves as a mitochondrial inner membrane (IM) fluorescent marker, enabling prolonged super-resolution imaging of IM dynamics in mammalian cell lines and primary cells (Fig. 3).29,30
Cell nuclei are enclosed by a double layer nuclear envelope, with numerous nuclear pores distributed across its surface. The pores establish a connection between the interior of the nucleus and the cytoplasm and are part of large protein structures known as nuclear pore complexes (NPCs). The nuclear pores serve as selective hydrophilic channels, posing a challenge for molecular probes attempting to enter the nucleus through them.31 Although small molecules, such as ions and metabolites, can diffuse through the pores via passive diffusion, larger macromolecules require active transport facilitated by nuclear translocation signals and transport carriers. Hence, when designing a nucleus-selective probe, careful consideration must be given to the hydrophilicity, lipophilicity, and charge density of the probe to ensure it can penetrate the nuclear envelope effectively.
Mitochondria have a unique own phospholipid, the cardiolipin (CL), that is located exclusively in mitochondrial IM. CL-specific fluorescent probe, which is sensitive to lipid peroxidation, hence can be developed to accumulate in mitochondria. Probe MitoCLox was recently developed for not only targeting mitochondria, but also for tracing CL oxidation.33 This probe incorporates a BODIPY (581/591) fluorophore conjugated with a triphenylphosphonium (TPP) residue using a long and flexible linker. The linker, containing two peptide bonds, mimics the SS-20 peptide and serves as a scaffold for introducing additional positive charges. MitoCLox reported a lipid-mediated response to oxidative stress by exogenous prooxidants or by internal redox changes.34
The nucleus houses the genetic material DNA, and currently, the primary focus in the design of nucleus-targeting probes is centered around the abundant presence of negatively charged DNA molecules within this organelle. This strategy is done by incorporating hydrophilic cations into the structure of the probes and then exploiting the capability of hydrophilic cations in the probes to strongly interact with negatively charged DNA through electrostatic interactions.35 These probes feature short hydrophobic chains and planar aromatic cationic structures. These features are later extensively adopted by the widely used nucleus commercial probes, such as Hoechst, DAPI, and SYBR Green 1, which selectively bind to double-stranded DNA (dsDNA) at the minor groove.36
Galactosyltransferase and protein kinase D have the ability to localize to the Golgi apparatus by utilizing cysteine residues or cysteine-rich domains for anchoring. This observation has led to the demonstration that fluorescent molecules can specifically target the Golgi when modified with L-cysteine. As a result, several probes have been developed using this strategy.37,38
Popularly used lysosomal probes, like LysoTracker or LysoSensor, are designed based on a fluorophore linked to a weak base, which remains uncharged in neutral pH but becomes protonated in the acidic interior of lysosomes, leading to enhanced fluorescence upon entry to the lysosome.39
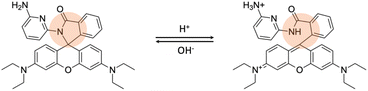
Generally, fluorescent probes for detecting lysosomal pH rely on rhodamine, morpholine, pyridine, and quinoline derivatives.7 Rhodamine-based probes with a spirocyclic structure are well-suited as fluorophores for “off–on” applications. These molecules do not exhibit fluorescence in basic and neutral environments because the spirocyclic structure remains in the “closed” state (Fig. 4). However, the ring opens under acidic conditions, resulting in a significant increase in fluorescence intensity. Some reported examples are probe RS1–3 and probe A–B.40,41
Mitochondria are known to have a highly negative membrane potential. Lipophilic and positively charged molecules can exploit the membrane potential of mitochondria, leading to their accumulation within the organelle.42,43 For instance, TPP, cyanine, rhodamine, pyridinium, and quinoline derivatives are popular scaffolds for designing mitochondria selective probes.44 One widely-used example is tetramethylrhodamine ethyl ester (TMRE) or MitoTracker Red, which contains lipophilic cations and has a large multi-aromatic structure with positively charged nitrogen atoms, accumulating specifically in the mitochondria of viable cells due to their affinity for the negatively charged mitochondrial membrane potential.39
Mitochondria are involved in various redox reactions, particularly in energy production through oxidative phosphorylation. This is influenced by the activity of the mitochondrial electron transport chain and the generation of reactive oxygen species (ROS), and may vary based on the cell's metabolic state and the equilibrium between oxidized and reduced species. The mitochondria-targeting probe MitoSOX, which has a dihydroethidium-based scaffold with a targeting moiety TPP, interacts with various reactive oxygen species (ROS).45 Selective excitation of the 2-hydroxylethidium product allows for differentiation between the product generated from superoxide oxidation and other potentially formed oxidized products.
Mitochondrial glutathione (mGSH) plays a crucial role in antioxidant defense systems by protecting against oxidative damage resulting from normal aerobic metabolism. The fluorescent probe MitoRT was reported to effectively track the dynamics of mitochondrial glutathione (mGSH) in live cells (Fig. 5A).46 The probe demonstrates selective targeting of mitochondria through the mitochondrial membrane potential (MMP). Using structured illumination microscopy (SIM) imaging, MitoRT unveiled the cells' tendency to maintain higher levels of mitochondrial glutathione (mGSH) compared to cytosolic levels across various physiological conditions.
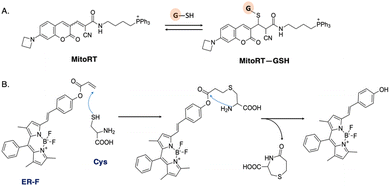 | ||
| Fig. 5 (A) Reversible Michael addition reaction between MitoRT and GSH. TPP is used as mitochondria-directing moiety. (B) Proposed mechanism of reaction between probe ER-F and cysteine (Cys). | ||
The endoplasmic reticulum is another organelle with a significant redox potential. It is involved in protein folding, modification, and quality control processes. The ER contains enzymes such as protein disulfide isomerases (PDIs) and glutathione, which play crucial roles in maintaining the redox balance and facilitating protein folding through the formation and reduction of disulfide bonds.47 Probe ER-F exhibits specificity towards cysteine (Cys), which serves as the precursor for glutathione biosynthesis (Fig. 5B).48 ER-F demonstrates the capability to detect Cys specifically localized in the ER region of HCT116 cells. It is designed based on a BODIPY scaffold, employing the nucleophilic addition of both thiol and amino groups, followed by a cyclization reaction, to achieve targeted recognition of Cys.49
For example, in the case of lysosomes, there is a high concentration of hydrogen ions (H+) maintained by the action of proton pumps located in the lysosomal membrane. This high H+ concentration serves as a target for probes that are sensitive to pH changes. In another case, the lumen of the endoplasmic/sarcoplasmic reticulum (ER/SR), which makes up less than 10% of the cell volume, is responsible for storing over 90% of the intracellular Ca2+ and plays a crucial role in regulating Ca2+ signaling.50
Each organelle possesses a unique lipid composition, leading to variations in their lipophilic properties. For example, the plasma membrane mainly consists of phospholipids, while the mitochondrial inner membrane (IM) contains a high proportion of cardiolipin (CL).51 These differences in lipid composition contribute to variations in the lipophilicity of organelles. Lipophilic probes have a higher tendency to enter and accumulate in organelles with greater lipophilicity. Modifying the probe's structure by incorporating lipophilic components or adjusting the balance between hydrophobicity and hydrophilicity can then enhance its ability to target specific organelles.
4.2 Physicochemical properties of the probe
The physicochemical properties of a probe, such as its size, charge, and lipophilicity, are crucial for its ability to penetrate cell membranes and selectively target specific organelles. In many cases, modifications to the probe's chemical structure or the utilization of specialized delivery techniques are often necessary to achieve optimal penetration and targeting efficiency. Furthermore, it is crucial for the probe to exhibit non-toxic characteristics, maintain stability under physiological conditions, and resist degradation by intracellular enzymes or other cellular components.Fine-tuning these characteristics facilitates effective cellular uptake and intracellular delivery. For example, a relatively small probe size is desired for sufficient cellular permeability, and careful evaluation of the probe's charge is important to avoid repulsion by the negatively charged cell membrane. Moreover, an appropriate level of lipophilicity aids in navigating the hydrophobic cell membrane environment.
In this section, we will explore the physicochemical characteristics of fluorescent probes to gain insights into their significance in the effective design of organelle-targeting probes.
This principle aligns with a study conducted by Sung et al. involving the development of mitochondria-targeting near-infrared (NIR) probes based on silicon-rhodamine. In the study, it was found that the optimized library design, which is based on lipophilic cationic fluorochrome, exhibited a molecular weight range from 470 to 555 Da.52
Passive entry into cells becomes more challenging for larger probes. Pye et al. observed that cyclic peptide-based probes with molecular weights ranging from approximately 800 Da to 1200 Da exhibit a notable decrease in passive cell permeability as their size increases (Fig. 6A).53 This decline in permeability, especially beyond a molecular weight of 1000 Da, aligns with previous research highlighting the scarcity of orally administered drugs and clinical candidates with higher molecular weights.54 These findings indicate that the observations made by Pye et al.53 extend beyond the specific cyclic peptides studied and are applicable to various types of cell-permeable molecules.
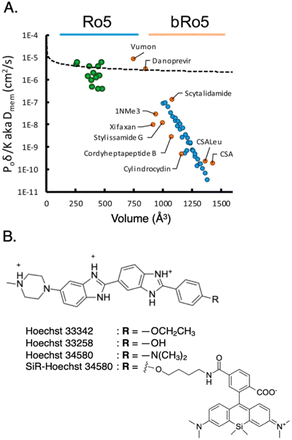 | ||
| Fig. 6 (A) Membrane diffusion vs. molecular volume. Small molecules conforming to the Ro5 rule (green dots) approximately follow an exponential trend, while larger molecules beyond the Ro5 rule (bRo5, blue dots) experience steep decline in permeability. To ensure the generality of these trends, a variety of unrelated synthetic and natural products were subjected to the same analysis (orange dots). Reproduced with permission from ref. 53, Copyright 2017 American Chemical Society. (B) Structure of nucleus-targeting probe Hoechst and its derivatives. Typical nucleus-targeting probe features hydrophilic cations, hydrophobic chains, and planar aromatic structure, to bind to minor groove of dsDNA and/or intercalate between the base pairs of DNA helix. | ||
It is worth noting that the analysis of cell permeability in traditional small-molecule drugs reveals an interesting trend: larger molecules tend to encounter enhanced efflux mediated by transporters. This effect is observed in macrocyclic compounds within the size range of 400–800 Da, where increasing molecular size leads to an escalation in efflux. ATP-binding cassette transporters, such as P-glycoprotein (e.g., ABCB1), breast cancer resistance protein (ABCG2), and other multidrug resistance-associated proteins, are responsible for this phenomenon.55
Organelles may exhibit specific binding sites or accumulation sites that are more effectively recognized by certain shapes or structures. It is essential to customize the shape of the probe according to the specific organelle being targeted. In the context of DNA targeting in the nucleus, it is commonly preferred to use probes with a planar structure, as this planar shape enables efficient intercalation between the base pairs of the DNA helix. One notable example is Hoechst and its derivatives, such as hoeBDP and hoeTMR for application as turn-on probes56 and SiR-Hoechst for use in super-resolution microscopy (Fig. 6B).57
Probes with favorable water solubility offer several advantages. They can minimize nonspecific interactions with other cellular components, thereby reducing off-target effects.58 Additionally, these water-soluble probes demonstrate higher biocompatibility and are less susceptible to aggregation or precipitation, ensuring prolonged stability and functionality within the cell. These characteristics make them more reliable as sensors or imaging tools in various cellular applications.
To modulate the water solubility, one approach is to incorporate hydrophilic functional groups, such as hydroxyl (–OH), amino (–NH2), carboxyl (–COOH), and others, into the probes' chemical structure. This would promote the rapid dissolution of probes in aqueous environments due to the strong affinity between the hydrophilic groups and water molecules. Ji et al. reported that the addition of a sulfonated alkyl chain not only can increase the water solubility of probes but also lead to reduced fluorescence background, enhanced signal-to-noise ratio, and improved sensitivity.59 Covalently attaching water-soluble polymers, like the commonly used polyethylene glycol (PEG), to the probe can create a hydrophilic coating, enhancing its solubility and stability in aqueous solutions. This strategy has been employed, e.g., with coumarin and rhodamine scaffolds, as previously reported.60,61
Lipophilic probes have an advantage in crossing these lipid membranes due to their affinity for the hydrophobic environment, eventually gaining access to the interior of cells. Lipophilic characteristics are purposefully incorporated into the structure of mitochondria targeting probe, allowing the probes to be selectively enriched in the inner mitochondrial membrane, known for its higher lipid content.62 An example of such a probe is Rhodamine 123 (RH123). RH123 has the ability to diffuse across the mitochondrial membrane driven by the potential and concentration gradients.
However, RH123 is less lipophilic than other lipophilic cation dyes,62 resulting in minimal impact on the membrane surface potential and exhibiting similar kinetic constants for both influx and efflux from the mitochondrial matrix. This feature makes RH123 a suitable candidate for measuring the mitochondrial membrane potential more accurately.63
(Horobin et al.,64 2013; Horobin & Rashid-Doubell,65 2013) In quantitative structure–activity relationship (QSAR) studies, one major property used is log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P, which measures lipophilicity or hydrophilicity. By comparing the properties of a historical training set of probes, structure–property relationships can be established to assist in designing probes with desirable characteristics. One approach is to utilize the amphiphilic index (AI), developed by Horobin,64 which calculates the impact of amphiphilicity using the log P of the lipophilic domain to predict the cellular localization of fluorescent probes. This can also be a basis for the prediction of the probe's cellular localization (Fig. 7A).64,65
P, which measures lipophilicity or hydrophilicity. By comparing the properties of a historical training set of probes, structure–property relationships can be established to assist in designing probes with desirable characteristics. One approach is to utilize the amphiphilic index (AI), developed by Horobin,64 which calculates the impact of amphiphilicity using the log P of the lipophilic domain to predict the cellular localization of fluorescent probes. This can also be a basis for the prediction of the probe's cellular localization (Fig. 7A).64,65
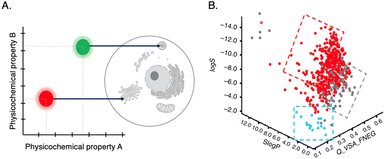 | ||
Fig. 7 (A) QSAR model: mapping probe physicochemical properties onto preferred probe's intracellular localization. (B) 3D scatter plot of QSAR analysis for three properties: lipophilicy (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P), molecular charge (Q_VSA_FNEG) and solubility (log P), molecular charge (Q_VSA_FNEG) and solubility (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S). Blue and red dots represent probes that are cell permeable. Reproduced with permission from ref. 58, copyright 2016 Nature Publishing. S). Blue and red dots represent probes that are cell permeable. Reproduced with permission from ref. 58, copyright 2016 Nature Publishing. | ||
To achieve suitable lipophilicity of a probe, commonly used approaches include incorporating hydrophobic functional groups (e.g., alkyl chains, aromatic rings, or long fatty acid chains) or attaching lipid tails or lipid-like structures to the probe's chemical structure. It is important to find the right balance in terms of lipophilicity to achieve optimal cell permeability and aqueous solubility, particularly for larger molecules. As the molecular weight increases, the acceptable range of lipophilicity becomes narrower, imposing a maximum limit on the size of molecules that can effectively penetrate cell membranes.53
A recent study highlighted the importance of molecular charge in determining cell permeability and non-specific intracellular retention of small fluorescent probes.58 By employing cheminformatics and cellular high-throughput screening (HTS) of a probe library and through QSAR analysis, it was discovered that desirable probes should possess a slight positive charged (preferred fractional negative van der Waals surface area, Q_VSA_FNEG, is 0.15–0.35) to facilitate efficient penetration into cells. This study yielded a predictive model that aids in designing highly permeable background-free probes for intracellular imaging applications (Fig. 7B).58
The choice of probe charge depends on the target organelle. Positively charged probes are often needed to target organelles with membrane potential, such as lipophilic cations for targeting mitochondria44 or hydrophilic cations for the nucleus.31 Conversely, negatively charged probes may be employed to target positively charged organelles, such as lysosomes.
5. Recent examples of fluorescent probes for intracellular organelles
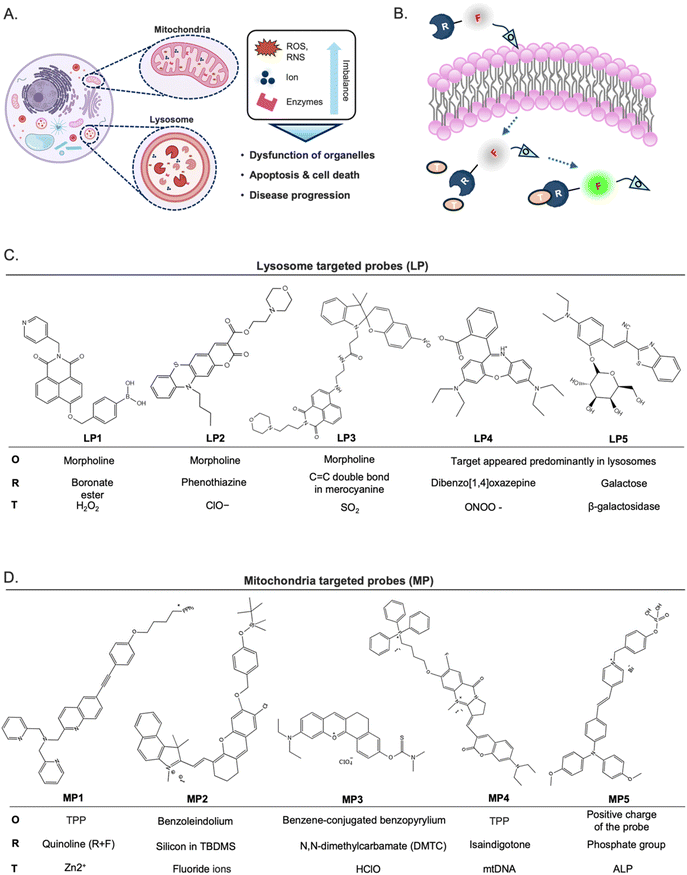
Recent advancements in fluorescence imaging techniques have significantly broadened the utility of fluorescent probes in investigating diverse cellular processes at the molecular level. Notably, probes targeted toward intracellular organelles have emerged as prominent tools for the precise detection of cellular metabolites and transient chemical messengers, thereby facilitating the study of biochemical pathways. Furthermore, recent literature has highlighted various imaging strategies and innovative chemical frameworks aimed at improving target intracellular organelle specificity. Mitochondria and lysosomes are key organelles that play pivotal roles in various organisms, with their functions closely linked to the onset of numerous diseases (Fig. 8A).66–70 Turn-on or ratiometric fluorescent probes are generally preferred over turn-off types due to their superior sensitivity.71 Organelle-targeting ratiometric fluorescent probes typically consist of a fluorophore, recognition units, and organelle-targeting moieties (Fig. 8B). In this section, we provide representative examples of recently developed fluorescent probes for intracellular organelles.5.1 Lysosome-targeted fluorescent probe development
Lysosomes play a crucial role as weakly acidic vesicles housing numerous housekeeping proteins and enzymes responsible for the degradation of cellular proteins, lipids, and nucleic acids. The intricate process of lysosomal processing involves multiple endocytic pathways and autophagy regulated by lysosomal signaling mechanisms.72 Lysosomal biological species, such as reactive oxygen species (ROS), including hypochlorite (ClO−), peroxynitrite (ONOO−), and hydrogen peroxide (H2O2), and reactive sulfur species (RSS), such as sulfur dioxide (SO2), hold pivotal importance in physiological processes, particularly in the regulation of lysosomal functions, and are implicated in various diseases.70,73 Therefore, there has been a pressing need to develop highly sensitive and selective molecular tools for detecting biological species within lysosomes.The predominant approach for enhancing the delivery of fluorescent probes into lysosomes involves modifying them with lipophilic amines. This strategy capitalizes on the selective entrapment of probes within lysosomes due to the membrane impermeability of protonated amines found in these organelles. Among the various groups utilized for constructing lysosome-targeted fluorescent probes, 4-(2-hydroxyethyl) morpholine and N,N-dimethylethylenediamine stand out as particularly popular structures with a fluorophore due to its alkalinity.74
Hydrogen peroxide (H2O2) stands as one of the foremost ROS, playing a pivotal role in redox signaling and oxidative stress within lysosomes. Nonetheless, dysregulated H2O2 levels are implicated in the onset of various diseases, including neurodegenerative disorders and cancer.75,76 Consequently, the detection and imaging of physiological H2O2 within lysosomes have garnered significant interest. A boronate ester has been incorporated as a typical sensing moiety of H2O2 for lysosome-targeted fluorescent H2O2 probes. Notably, Lin et al. recently introduced a two-photon probe, LP1, designed for detecting H2O2 in lysosomes (Fig. 8C).77 LP1 boasts an “acceptor–π–acceptor” electronic structure, initially displaying weak fluorescence. Upon encountering H2O2, the boronate ester group within P1 undergoes oxidation, converting it into an electron-rich hydroxyl group. This transformation triggers a substantial fluorescence enhancement around 550 nm owing to intramolecular charge transfer (ICT) from the hydroxyl group donor to the naphthalimide acceptor. Crucially, LP1 exhibits remarkable selectivity for H2O2, remaining unaffected in the presence of other analytes. It has been effectively utilized for visualizing lysosomal H2O2 in both cellular and tissue samples via two-photon fluorescence imaging techniques.
Liu and colleagues synthesized LP2 by combining phenothiazine coumarin with a morpholine unit for detecting lysosomal ClO− (Fig. 8C).78 The fluorescence titration of LP2 with ClO− revealed a blue shift in emission, shifting from 610 to 535 nm. In contrast to the aforementioned example, this shift indicated that the ICT process was inhibited owing to the oxidation of the phenothiazine moiety. Fluorescence imaging experiments successfully demonstrated the effectiveness of LP2 in detecting ClO− within living cells and zebrafish.
SO2 is a crucial indicator of lysosomal function and oxidative stress,79 prompting the development of numerous probes for detecting these redox-active species. Zhang et al. presented a fluorescent probe, LP3, by developing a strategy for light-controlled detection of lysosomal SO2.80 The LP3 features a morpholine moiety that is easily protonated, facilitating its accumulation in lysosomes. However, its ability to respond to SO2 is initially inactive due to the UV-controlled sensing mechanism. Upon UV irradiation, the spiropyran (SP) structure converts to the merocyanine (MR) state, resulting in a significant “red-shift” in fluorescence—from green to red—through a Förster resonance energy transfer (FRET) process from the naphthalimide donor to the MR acceptor. The MR state, activated by UV, includes a distinctive C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond, serving as a specific recognition site for SO2. This UV-activated approach allows for precise ratiometric monitoring of SO2 in lysosomes, significantly reducing the risk of false positive signals during probe transit within cells. Additionally, this method revealed for the first time that lysosomal SO2 levels increase with rising temperatures. Tissue imaging further indicated that SO2 may play a protective role against small intestinal injury caused by heat shock, offering potential insights into the pathological mechanisms and mortality associated with heatstroke. This combined light-controlled detection method and lysosome-specific targeting strategies are valuable for exploring potential disease markers and pathologies.
C double bond, serving as a specific recognition site for SO2. This UV-activated approach allows for precise ratiometric monitoring of SO2 in lysosomes, significantly reducing the risk of false positive signals during probe transit within cells. Additionally, this method revealed for the first time that lysosomal SO2 levels increase with rising temperatures. Tissue imaging further indicated that SO2 may play a protective role against small intestinal injury caused by heat shock, offering potential insights into the pathological mechanisms and mortality associated with heatstroke. This combined light-controlled detection method and lysosome-specific targeting strategies are valuable for exploring potential disease markers and pathologies.
While lysosomotropic amine has been dominated as a lysosome targeting core, it is worth noting that fluorescent probes lacking lysosomotropic groups also exhibit efficacy in detecting species within lysosomes, albeit through a different mechanism. These probes typically function as logic molecules, emitting fluorescence signals exclusively in lysosomes due to the presence of recognition elements, which appear predominantly in lysosomes, without the recognition unit on the probe.
LP4, which Zhang and colleagues presented, is a representative example recently developed as a novel class of rhodamine derivatives, featuring the dibenzo[1,4]oxazepine core, which selectively reacts with ONOO−, yielding oxazines with significantly longer analytical wavelengths.81 Notably, LP4 releases the oxazine moiety emitting in the near-infrared wavelength region upon reaction with ONOO−. The innovative chemistry involved in dibenzo[1,4] oxazepine ring formation, coupled with its specific reactivity toward ONOO−, presented a promising approach for developing rhodamine-based bioimaging probes with NIR wavelengths.
Enzymes, specifically or mainly produced in lysosomes, could be targeted to develop lysosome-specific fluorescent probes. β-Galactosidase (β-gal) is predominantly produced and presented in lysosomes and is notably associated with senescence. The abnormal accumulation of β-gal activity, which is referred to as senescence-associated (SA) β-gal, has been widely recognized as a key biomarker in the study of cellular senescence.82,83 Liu and colleagues recently introduced a novel ratiometric fluorescent probe, LP5, which utilizes benzothiazolyl acetonitrile dye as its fluorophore.84 LP5 exhibited a pronounced blue-shifted emission, serving as a sensitive indicator of β-galactosidase (β-gal) activity. Upon interaction with β-gal, the excimer emission of P1 at 620 nm decreased, while the emission at 533 nm significantly increased, resulting in a highly sensitive ratiometric probe with a low detection limit. LP5 also demonstrated precise lysosomal localization, allowing for real-time monitoring of senescent cell emergence. The probe produced strong green fluorescence in senescent cells and red fluorescence in normal cells, enabling the detection of endogenous SA β-gal in living cells. Additionally, in an in vivo model of drug-induced senescence, LP5 showed significant fluorescence enhancement corresponding to β-gal activity. This allowed for the detection of senescence in specific organs and skin tissues, revealing that drug-induced senescence was most pronounced in the brain, skin, and muscle tissues. These findings underscored the significant potential of LP5 in advancing biomedical research related to senescence.
5.2 Mitochondria-targeted fluorescent probe development
Mitochondria, termed the “powerhouse” of cells, are vital organelles responsible for generating adenosine triphosphate (ATP), the primary energy source for various biological processes. Beyond energy production, mitochondria regulate the homeostasis of cellular small molecules such as ROS, active nitrogen, metal cations, protons, and anions and participate in cellular processes like autophagy and protein degradation. Additionally, mitochondria play crucial roles in programmed cell death mechanisms such as apoptosis.85,86 Given their multifaceted functions, it is not surprising that mitochondria are implicated in various diseases.87 Consequently, fluorescent probes designed to target mitochondria offer a valuable means of facilitating the detection of their dynamic localization and morphological alterations, as well as the observation of their physiological processes.Notably, mitochondria consist of a double lipid bilayer structure comprising the outer membrane, inner membrane, matrix, and intermembrane space (IMS). Within the matrix, oxidative phosphorylation and the tricarboxylic acid cycle occur, facilitated by proteins in the matrix and inner mitochondrial membrane (IMM). Proton transfer during these processes leads to a membrane potential across the IMM.87 Leveraging this potential difference enables the accumulation of positively charged molecules, such as membrane-permeable or lipophilic cationic compounds, on the matrix side of the IMM, which is crucial for designing specific probes targeting mitochondria. One widely used commercial probe for targeting mitochondria is MitoTracker Green, which possesses a positive charge, ensuring selective mitochondrial targeting.88
Detecting mitochondrial contents can also develop mitochondrial-targeting fluorescent probes. Research has shown the presence of several reactive small molecules within mitochondria. Consequently, various researchers have endeavored to visualize the distribution and elucidate the action mechanisms of these small molecules within mitochondria through the design and synthesis of prominent fluorescent probes targeting specific mitochondrial contents.
Metal ions play crucial roles in various aspects of mitochondrial physiology. Among these, zinc ion (Zn2+) is particularly involved in key biological processes such as DNA synthesis, enzyme catalysis, and gene transcription, where fluctuations in Zn2+ levels play a significant role.89 Consequently, the detection of Zn2+ levels has emerged as a prominent focus in the development of mitochondrial fluorescent probes. To understand the role of Zn2+ in biological processes, especially autophagy and signal transduction, Ning et al. developed a two-photon ratio probe, MP1, specifically targeting mitochondria to detect Zn2+ levels.90 By utilizing triphenylphosphine as the targeting group, the probe was successfully localized within the mitochondria, enhancing the sensitivity of two-photon signal detection for Zn2+.
While the investigation of cations within mitochondria has been extensively explored, the role of anions remains equally crucial. Fluoride ions (F−), in particular, can lead to metabolic disorders upon their mitochondrial accumulation. Zhang et al. synthesized the mitochondrial fluorescent probe MP2 for detecting fluorine in mitochondria.91 Fluoride ions triggered the removal of the silyl-protecting group, enabling the phenoxide to react with the –CN group, transforming non-fluorescence into highly fluorescent MP2. Consequently, MP2 proved effective in detecting and imaging fluorine within mitochondria, garnering significant attention in the realms of cell biology and medical science.
Mitochondrial reactive oxygen species (ROS) play a critical role in regulating redox homeostasis within mitochondria and are also involved in various physiological and pathological processes, particularly in cancer. Increased ROS levels have been observed in several types of cancer, where they contribute to the activation of multiple tumorigenic signaling pathways.92,93 Clearly, assessing these mitochondrial bioactive species levels is pivotal for elucidating the underlying mechanisms of tumor development and progression. Consequently, researchers have endeavored to develop probes specifically designed to monitor ROS levels within mitochondria.
Hypochlorous acid (HClO) is one of the ROS generated as metabolic by-products within the mitochondria. To expand the repertoire of available HClO probes and broaden their biological applications, numerous “turn-on” fluorescence probes relying on hydrolysis reactions have been developed. Examples include MP3, which utilized N,N-dimethylcarbamate as the recognition group.94 Initially, MP3 exhibited minimal fluorescence signals. However, upon encountering HClO, the thiocarbamate moiety undergoes hydrolysis, leading to the liberation of the probes. Consequently, the fluorescence of the resultant fluorophores is restored, facilitated by the enhanced ICT process. These probes boast high selectivity, rapid response, and low detection limits.
Mitochondria are semi-autonomous organelles that contain their own genetic system, comprising mitochondrial DNA (mtDNA) and the associated transcription enzymes. Human mtDNA, comprising 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 569 base pairs, encodes RNAs and peptides crucial for mitochondrial function. Emerging research suggests associations between mtDNA and various physiological processes such as neurodegenerative diseases, aging, and cancer,95 sparking interest in mtDNA imaging. However, distinguishing mtDNA from more abundant nuclear DNA remained the first challenge. Tan's group introduced MP4, building on their experience with isaindigotone derivatives,96–98 which exhibit excellent selectivity for DNA G-quadruplexes (G4), which are secondary structures of DNA by guanine-rich sequences. Utilizing TPP as a mitochondrial targeting moiety, MP4 selectively responds to the GQ structure in mtDNA, shedding light on its impact on cellular energy metabolism. Using MP4 to investigate the previously elusive dynamics of mtDNA G-quadruplexes (G4s), the researchers unexpectedly found that these structures primarily form in cancer and endothelial cells that depend on glycolysis for their energy production.
569 base pairs, encodes RNAs and peptides crucial for mitochondrial function. Emerging research suggests associations between mtDNA and various physiological processes such as neurodegenerative diseases, aging, and cancer,95 sparking interest in mtDNA imaging. However, distinguishing mtDNA from more abundant nuclear DNA remained the first challenge. Tan's group introduced MP4, building on their experience with isaindigotone derivatives,96–98 which exhibit excellent selectivity for DNA G-quadruplexes (G4), which are secondary structures of DNA by guanine-rich sequences. Utilizing TPP as a mitochondrial targeting moiety, MP4 selectively responds to the GQ structure in mtDNA, shedding light on its impact on cellular energy metabolism. Using MP4 to investigate the previously elusive dynamics of mtDNA G-quadruplexes (G4s), the researchers unexpectedly found that these structures primarily form in cancer and endothelial cells that depend on glycolysis for their energy production.
Enzymes highly expressed in mitochondria are potential target molecules for the development of mitochondria-targeted fluorescent probes. Alkaline phosphatase (ALP) is an enzyme that plays a crucial role in various metabolic processes by transferring phosphate groups to proteins, nucleic acids, and other molecules. It is exclusively active within mitochondria, where it serves as a catalyst for dephosphorylation reactions.99 Perturbations in ALP levels have been associated with various diseases, including various cancers.100,101 Consequently, ALP serves as a crucial biomarker in medical diagnostics. Exploiting the ability of ALP to remove phosphate groups from chemical structures provides an effective strategy for constructing fluorescent probes capable of detecting ALP levels within living tissue. Tang's group recently developed an ALP-responsive fluorescent probe, MP5, specifically designed for imaging and photodynamic therapy (PDT) in cancer cells.102 MP5 utilizes a phosphate group as its recognition site, paired with the photosensitizer TPAPy, which demonstrates aggregation-induced emission properties. The elevated ALP expression in cancer cells promotes the enzymatic hydrolysis of MP5's phosphate group, releasing the hydroxyproline residue TPAPy. This process enhances the fluorescence response through aggregation, allowing for effective differentiation between cancerous and normal cells. Structures of these mitochondria-targeted probes can be seen in Fig. 8D.
5.3 Other organelles-targeted fluorescent probe development
Recent advancements in the development of intracellular fluorescent probes have significantly contributed to the better study of organelles' functions and dynamics in living cells.103,104 Dysfunctions in other organelles, including Golgi apparatus and nucleus as well as the plasma membrane, have also been implicated in a wide range of pathological conditions.105–107The Golgi apparatus, cell membrane, and nucleus are critical organelles, and their dysfunctions have been implicated in a wide range of pathological conditions. The Golgi apparatus, in particular, serves as a central station for protein processing and trafficking within cells, playing an essential role in maintaining cellular function. Its involvement in various physiological processes and diseases has made it a prominent target for the development of fluorescent probes aimed at visualizing and understanding its dynamics. Recent advancements in Golgi-targeted fluorescent probe design have focused on improving specificity, photostability, and functionality. These probes generally consist of a fluorophore conjugated to a Golgi-targeting moiety, ensuring precise localization and minimal interference with Golgi function. For instance, researchers have developed probes that utilize specific enzymes localized to the Golgi apparatus. Such targeting moieties guide the attached fluorophores to the Golgi, enabling real-time imaging of their structure and function. Additionally, modifications to fluorophore structures have been implemented to enhance photostability and brightness, facilitating long-term imaging studies.108
Cyclooxygenase-2 (COX-2), an enzyme localized in the Golgi apparatus, plays a pivotal role in converting arachidonic acid into prostaglandins. Given its elevated expression in inflammatory conditions and various cancers, COX-2 serves as a valuable biomarker for these diseases. Consequently, several COX-2-specific fluorescent probes have been developed based on COX-2 inhibitors. In 2013, X. Peng and colleagues introduced an innovative “off–on” two-photon (TP) fluorescent probe, GP1, for Golgi apparatus localization in cancer cells. This probe combines acenaphtho[1,2-b]quinoxaline (ANQ) as the fluorophore with indomethacin (IMC) as the COX-2 recognition moiety. In aqueous buffer, GP1 adopts a folded configuration, resulting in weak fluorescence due to a photoinduced electron transfer (PET) effect between ANQ and IMC. Upon interaction with COX-2, IMC binds to the enzyme, triggering a significant fluorescence enhancement. Notably, the probe's maximum emission wavelength shifts from 457 nm to 547 nm upon COX-2 binding. GP1 demonstrates exceptional Golgi localization in cancer cells, achieving a Pearson's co-localization coefficient of 0.97. Its high specificity makes it a powerful tool for investigating morphological changes in the Golgi apparatus during cancer cell apoptosis.109
Building on this work, X. Peng's team developed a near-infrared (NIR) Golgi-localized fluorescent probe, GP2, replacing the ANQ fluorophore with a NIR-emitting Nile blue dye to achieve longer excitation and emission wavelengths (630 nm and 670 nm, respectively). Compared to GP1, GP2 offers reduced photodamage to biological samples, an improved signal-to-noise ratio, and deeper tissue penetration. These features make it particularly suitable for in vivo bioimaging applications.110
Recognizing the superior COX-2 targeting ability of celecoxib (CCB) derivatives, X. Peng's group in 2021 developed a probe named GP3. This probe conjugates a Nile blue unit to CCB via a six-carbon linker. The mechanism of action mirrors that of GP2, exhibiting selective binding to COX-2. Subcellular localization studies reveal that GP3 preferentially accumulates in the Golgi apparatus, leading to increased fluorescence intensity upon COX-2 binding. GP3 has been successfully applied to distinguish cancer cells from normal cells, demonstrating its potential as a diagnostic tool for identifying COX-2-expressing cancer cells. These probes exemplify the strategic design of COX-2-specific fluorescent markers for targeted imaging of the Golgi apparatus in cancer cells, facilitating the study of Golgi dynamics and COX-2-related pathological processes.111
The plasma membrane, while not an intracellular organelle, plays a crucial role in various cellular processes. Recent studies have highlighted the significance of membrane microdomains, or lipid rafts, in human diseases, such as cancer progression and their potential contribution to amyloid formation in neurodegenerative diseases.112,113 This underscores the importance of investigating cell membrane dynamics and morphology.
Fluorescent probes targeting the plasma membrane have been instrumental in these investigations. Traditionally, these probes consist of an environment-sensitive fluorophore conjugated with a membrane-anchoring moiety, ensuring membrane specificity and minimizing diffusion. Recent advancements have introduced probes with additional functionalities, such as multiple fluorescence signals for high-resolution imaging or the capability to detect intracellular signaling molecules. For instance, Xu and colleagues developed a plasma membrane probe, PP1, designed to self-assemble in the plasma membrane upon activation by GPI-anchored ectophosphatase.114 The probe comprises three components: an environmentally sensitive 4-nitro-2,1,3-benzoxadiazole (NBD) fluorophore, a membrane-anchoring cholesterol unit, and a phospho-D-tyrosine group that triggers self-assembly. Upon dephosphorylation by ectophosphatases, the probe self-assembles within the plasma membrane, allowing visualization of lipid raft distribution. Notably, this probe has been used to observe changes in membrane dynamics of cancer cells following treatment with an anticancer drug candidate, demonstrating its potential application in drug screening.
In another study, researchers developed a series of N-BPM-Cn probes, with PP2 being a notable example.115 These probes are designed for high-contrast imaging of the plasma membrane in live cells. N-PP2 consists of a benzothiazole-based fluorophore-conjugated with a C10 alkyl chain, facilitating its integration into the lipid bilayer. This design enables the probe to provide bright and stable fluorescence specific to the plasma membrane, enhancing imaging capabilities. Additionally, the development of a PP2 has been reported for imaging the plasma membrane in live cells. This probe features a benzothiazole-based fluorophore linked to a C10 alkyl chain, allowing it to anchor effectively to the plasma membrane. N-BPM-C10 exhibits high fluorescence intensity and photostability, making it suitable for long-term imaging applications. These advancements in plasma membrane-targeted fluorescent probes have significantly enhanced our ability to study membrane dynamics, organization, and associated cellular processes, providing valuable tools for biomedical research.
With respect to nucleus-targeting probes, those targeting DNA with a planar structure are commonly preferred, as their shape allows for efficient intercalation between the base pairs of the DNA helix. Noteworthy examples include Hoechst probes and their derivatives (e.g., hoeBDP and hoeTMR), as well as SiR-Hoechst, the latter of which is specifically designed for applications in super-resolution microscopy.56,57 Recent advancements in the development of these probes have significantly contributed to the study of nuclear functions and dynamics in living cells. New probes have been designed to achieve more targeted functionalities, including those that can distinguish between different chromatin states or track nuclear processes. For instance, SYTO probes can be excited by Vis-NIR radiation, which reduces cell damage during prolonged imaging, unlike Hoechst probes that require UV light for excitation. Additionally, a number of probes based on cyanine scaffolds have been developed for nuclear targeting. In 2020, Kurutos et al. designed a series of Cl-TO probes that exhibit lower cytotoxicity and stronger fluorescence enhancement compared to commercially available thiazole orange.116 In 2021, the same group developed monomethyl cyanine probes (AK-C1, AK-C2, and AK-C3), which are capable of staining nucleoli with high sensitivity to AU-rich RNA, showing a 379-fold increase in emission signal.117 In 2022, Aristova and colleagues reported T-4, a symmetric trimethylcyanine probe suitable for nucleolar imaging due to its strong sensitivity to nucleic acids, along with good biocompatibility and photostability, making it well-suited for long-term cell imaging.118
6. Summary and outlook
Organelle-targeting fluorescent probes have gained increasing attention in the past decade due to their proven potential in monitoring diverse physiological and pathological activities and unraveling the molecular mechanisms of biological events. These probes, designed with various fluorophores, recognition moieties, and targeting ligands, have been meticulously prepared and have now successfully been applied in monitoring cancer, photodynamic therapy (PDT), diabetes, drug-induced oxidative/nitrative stress, inflammation, and others, making them powerful tools for bioanalysis.This review provides an overview of strategies and factors to consider in designing organelle-targeting probes. The mode of cellular uptake adopted by the fluorescent probes and key factors contributing to the successful design of such probes, including properties of the target organelle and physicochemical properties of the probes themselves, are carefully discussed. While a number of well-performing organelle-targeting fluorescent probes have been developed in recent years, some aspects in the field still face some challenges, including the following:
(1) Fluorescent probes are often prone to photobleaching, posing challenges in meeting the requirement for prolonged and dynamic observation, such as in STED imaging, of specific biotarget within organelles. Therefore, developing probes with excellent light stability and minimal phototoxicity is essential to improve probes' photostability and reduce photodamage to living cells.
(2) Some available probes still face poor targeting efficiency. For instance, efficient targeting strategies for important organelles like the Golgi apparatus and lipid droplets are still scarce, hindering the investigation of crucial biological processes in these subcellular structures. Therefore, exploration on expanding chemical toolbox for novel targeting and/or recognition ligands towards these organelles is especially required.
(3) Efficient cellular uptake and membrane permeability remain important hurdles to tackle, as probes need to penetrate cell membranes to reach their intracellular target organelles. As cellular uptake is influenced by the probe's physicochemical properties, it is crucial to optimize these parameters.
(4) Though QSAR analysis could help in ‘simplifying’ the journey in discovering desirable probes, it has some challenges on its own. QSAR models rely on large datasets of fluorescent probes with known properties and cellular localizations. Obtaining comprehensive and diverse datasets for various organelles is a challenge due to limited experimental data availability. Developing robust QSAR models that accurately predict the localization of fluorescent probes requires handling the complexity of multiple physicochemical properties and their interdependencies.
We hope that this review helps in better understanding of some key properties, facilitates the rational design of the probes, and enables the development of probes that can specifically and selectively target organelles for detection and analysis in living cells. Future research in this area may focus on further refining probe design, optimizing delivery mechanisms, and exploring new imaging techniques to push the boundaries of bioimaging and its applications in biomedical research and clinical settings.
We hope that insights from this review helps in better understanding some key properties, facilitates the rational design of the probes, and enables the development of probes that can specifically and selectively target organelles for detection and analysis in living cells. Future research in this area may focus on further refining probe design, optimizing delivery mechanisms, and exploring new imaging techniques to push the boundaries of bioimaging and its applications in biomedical research and clinical settings.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
S. H. A.: funding acquisition, conceptualizing, writing—original draft, writing—review, and editing. Y-A. L.: writing—original draft, writing—review, and editing.Conflicts of interest
Authors declare no conflict of interest.Acknowledgements
This research is funded by Directorate of Research and Development, Universitas Indonesia under Hibah PUTI Q1 [NKB-487/UN2.RST/HKP.05.00/2023].Notes and references
- D. Wu, A. C. Sedgwick, T. Gunnlaugsson, E. U. Akkaya, J. Yoon and T. D. James, Fluorescent chemosensors: The past, present and future, Chem. Soc. Rev., 2017, 46(23), 7105–7123, 10.1039/c7cs00240h.
- I. C. M. Simoes, G. Morciano and M. Lebiedzinska-Arciszewska, et al., The mystery of mitochondria-ER contact sites in physiology and pathology: A cancer perspective, Biochim. Biophys. Acta, Mol. Basis Dis., 2020, 1866(10), 165834, DOI:10.1016/j.bbadis.2020.165834.
- D. V. Ziegler, D. Vindrieux and D. Goehrig, et al., Calcium channel ITPR2 and mitochondria–ER contacts promote cellular senescence and aging, Nat. Commun., 2021, 12(1), 720, DOI:10.1038/s41467-021-20993-z.
- R. Zhai, B. Fang and Y. Lai, et al., Small-molecule fluorogenic probes for mitochondrial nanoscale imaging, Chem. Soc. Rev., 2022, 52(3), 942–972, 10.1039/d2cs00562j.
- S. Lu, Z. Dai, Y. Cui and D. M. Kong, Recent Development of Advanced Fluorescent Molecular Probes for Organelle-Targeted Cell Imaging, Biosensors, 2023, 13(3), 360, DOI:10.3390/bios13030360.
- N. E. Choi, J. Y. Lee, E. C. Park, J. H. Lee and J. Lee, Recent Advances in Organelle-Targeted Fluorescent Probes, Molecules, 2021, 26(1), 217, DOI:10.3390/molecules26010217.
- J. Yin, L. Huang, L. Wu, J. Li, T. D. James and W. Lin, Small molecule based fluorescent chemosensors for imaging the microenvironment within specific cellular regions, Chem. Soc. Rev., 2021, 50(21), 12098–12150, 10.1039/d1cs00645b.
- D. Singh, D. Rajput and S. Kanvah, Fluorescent probes for targeting endoplasmic reticulum: Design strategies and their applications, Chem. Commun., 2022, 58(15), 2413–2429, 10.1039/d1cc06944f.
- J. Lin, K. Yang and E. J. New, Strategies for organelle targeting of fluorescent probes, Org. Biomol. Chem., 2021, 19(43), 9339–9357, 10.1039/d1ob01447a.
- S. H. Alamudi and Y. T. Chang, Advances in the design of cell-permeable fluorescent probes for applications in live cell imaging, Chem. Commun., 2018, 54(97), 13641–13653, 10.1039/c8cc08107g.
- E. Burbridge and C. Adrain, Organelle homeostasis: from cellular mechanisms to disease, FEBS J., 2022, 289(22), 6822–6831, DOI:10.1111/febs.16667.
- M. Frank, S. Duvezin-Caubet and S. Koob, et al., Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner, Biochim. Biophys. Acta, Mol. Cell Res., 2012, 1823(12), 2297–2310, DOI:10.1016/j.bbamcr.2012.08.007.
- G. Bellot, R. Garcia-Medina and P. Gounon, et al., Hypoxia-Induced Autophagy Is Mediated through Hypoxia-Inducible Factor Induction of BNIP3 and BNIP3L via Their BH3 Domains, Mol. Cell. Biol., 2009, 29(10), 2570–2581, DOI:10.1128/mcb.00166-09.
- I. Noh, D. Y. Lee and H. Kim, et al., Enhanced Photodynamic Cancer Treatment by Mitochondria-Targeting and Brominated Near-Infrared Fluorophores, Adv. Sci., 2018, 5(3), 1700481, DOI:10.1002/advs.201700481.
- O. A. Weisz, Organelle acidification and disease, Traffic, 2003, 4(2), 57–64, DOI:10.1034/j.1600-0854.2003.40201.x.
- N. J. Yang and M. J. Hinner, Getting across the cell membrane: an overview for small molecules, peptides, and proteins, Methods Mol. Biol., 2015, 1266, 29–53, DOI:10.1007/978-1-4939-2272-7_3.
- C. L. Ahlbach, K. W. Lexa and A. T. Bockus, et al., Beyond cyclosporine A: Conformation-dependent passive membrane permeabilities of cyclic peptide natural products, Future Med. Chem., 2015, 7(16), 2121–2130, DOI:10.4155/fmc.15.78.
- A. Whitty, M. Zhong, L. Viarengo, D. Beglov, D. R. Hall and S. Vajda, Quantifying the chameleonic properties of macrocycles and other high-molecular-weight drugs, Drug Discovery Today, 2016, 21(5), 712–717, DOI:10.1016/j.drudis.2016.02.005.
- C. A. Lipinski, Lead- and drug-like compounds: The rule-of-five revolution, Drug Discovery Today:Technol., 2004, 1(4), 337–341, DOI:10.1016/j.ddtec.2004.11.007.
- Y. Ma, K. Poole, J. Goyette and K. Gaus, Introducing membrane charge and membrane potential to T cell signaling, Front. Immunol., 2017, 8, 1513, DOI:10.3389/fimmu.2017.01513.
- D. B. Kell, The transporter-mediated cellular uptake and efflux of pharmaceutical drugs and biotechnology products: How and why phospholipid bilayer transport is negligible in real biomembranes, Molecules, 2021, 26(18), 5629, DOI:10.3390/molecules26185629.
- M. E. Morris, V. Rodriguez-Cruz and M. A. Felmlee, SLC and ABC Transporters: Expression, Localization, and Species Differences at the Blood–Brain and the Blood-Cerebrospinal Fluid Barriers, AAPS J., 2017, 19(5), 1317–1331, DOI:10.1208/s12248-017-0110-8.
- S. H. Alamudi, M. Kimoto and I. Hirao, Uptake mechanisms of cell-internalizing nucleic acid aptamers for applications as pharmacological agents, RSC Med. Chem., 2021, 12(10), 1640–1649, 10.1039/d1md00199j.
- S. H. Alamudi, D. Su, K. J. Lee, J. Y. Lee, J. L. Belmonte-Vázquez, H.-S. Park, E. Peña-Cabrera and Y.-T. Chang, A palette of background-free tame fluorescent probes for intracellular multi-color labelling in live cells, Chem. Sci., 2018, 9(8), 2376–2383, 10.1039/c7sc04716a.
- C. L. Miasiro, H. Seok Kim, S. Husen Alamudi and Y. T. Chang, ABCB1 Can Actively Pump-out the Background-Free Tame Fluorescent Probe CO-1 From Live Cells, Chem.–Asian J., 2022, 17(12), e202200229, DOI:10.1002/asia.202200229.
- X. Liu and Y. T. Chang, Fluorescent probe strategy for live cell distinction, Chem. Soc. Rev., 2022, 51(5), 1573–1591, 10.1039/d1cs00388g.
- J. S. Lee, Y. K. Kim, M. Vendrell and Y. T. Chang, Diversity-oriented fluorescence library approach for the discovery of sensors and probes, Mol. BioSyst., 2009, 5(5), 411–421, 10.1039/b821766c.
- S. Cogliati, J. A. Enriquez and L. Scorrano, Mitochondrial Cristae: Where Beauty Meets Functionality, Trends Biochem. Sci., 2016, 41(3), 261–273, DOI:10.1016/j.tibs.2016.01.001.
- T. Liu, T. Stephan and P. Chen, et al., Multi-color live-cell STED nanoscopy of mitochondria with a gentle inner membrane stain, Proc. Natl. Acad. Sci. U. S. A., 2022, 119(52), e2215799119, DOI:10.1073/pnas.2215799119.
- C. Wang, M. Taki and Y. Sato, et al., A photostable fluorescent marker for the superresolution live imaging of the dynamic structure of the mitochondrial cristae, Proc. Natl. Acad. Sci. U. S. A., 2019, 116(32), 15817–15822, DOI:10.1073/pnas.1905924116.
- C. Hu, S. Xu, Z. Song, H. Li and H. Liu, Recent Advance in Nucleus-Targeted Fluorescent Probes for Bioimaging, Detection and Therapy, Chemosensors, 2023, 11(2), 125, DOI:10.3390/chemosensors11020125.
- S. D. Wiedner, L. N. Anderson and N. C. Sadler, et al., Live Cell Organelle-Specific Activity-Based Protein Profiling, Angew Chem. Int. Ed. Engl., 2014, 53(11), 2919–2922, DOI:10.1002/anie.201309135.
- K. G. Lyamzaev, N. V. Sumbatyan and A. M. Nesterenko, et al., MitoCLox: A Novel Mitochondria-Targeted Fluorescent Probe for Tracing Lipid Peroxidation, Oxid. Med. Cell. Longevity, 2019, 1–11 Search PubMed.
- K. G. Lyamzaev, A. A. Panteleeva and A. A. Karpukhina, et al., Novel Fluorescent Mitochondria-Targeted Probe MitoCLox Reports Lipid Peroxidation in Response to Oxidative Stress in Vivo, Oxid. Med. Cell. Longevity, 2020, 2020, 3631272, DOI:10.1155/2020/3631272.
- R. W. Horobin, J. C. Stockert and F. Rashid-Doubell, Fluorescent cationic probes for nuclei of living cells: Why are they selective? A quantitative structure-activity relations analysis, Histochem. Cell Biol., 2006, 126(2), 165–175, DOI:10.1007/s00418-006-0156-7.
- J. J. Welch, F. J. Rauscher and T. A. Beerman, Targeting DNA-binding drugs to sequence-specific transcription factor-DNA complexes. Differential effects of intercalating and minor groove binding drugs, J. Biol. Chem., 1994, 269(49), 31051–31058, DOI:10.1016/s0021-9258(18)47389-9.
- R. S. Li, P. F. Gao and H. Z. Zhang, et al., Chiral nanoprobes for targeting and long-term imaging of the Golgi apparatus, Chem. Sci., 2017, 8(10), 6829–6835, 10.1039/c7sc01316g.
- W. Zhang, J. Zhang, P. Li, J. Liu, D. Su and B. Tang, Two-photon fluorescence imaging reveals a Golgi apparatus superoxide anion-mediated hepatic ischaemia-reperfusion signalling pathway, Chem. Sci., 2019, 10(3), 879–883, 10.1039/c8sc03917h.
- B. Zhitomirsky, H. Farber and Y. G. Assaraf, LysoTracker and MitoTracker Red are transport substrates of P-glycoprotein: implications for anticancer drug design evading multidrug resistance, J. Cell. Mol. Med., 2018, 22(4), 2131–2141, DOI:10.1111/jcmm.13485.
- H. Li, C. Wang and M. She, et al., Two rhodamine lactam modulated lysosome-targetable fluorescence probes for sensitively and selectively monitoring subcellular organelle pH change, Anal. Chim. Acta, 2015, 900, 97–102, DOI:10.1016/j.aca.2015.10.021.
- S. Zhang, T. H. Chen and H. M. Lee, et al., Luminescent probes for sensitive detection of ph changes in live cells through two near-infrared luminescence channels, ACS Sens., 2017, 2(7), 924–931, DOI:10.1021/acssensors.7b00137.
- K. K. Gaddale Devanna, J. M. Gawel and T. A. Prime, et al., Tetra-arylborate lipophilic anions as targeting groups, Chem. Commun., 2021, 57(25), 3147–3150, 10.1039/d0cc07924c.
- L. D. Zorova, V. A. Popkov and E. Y. Plotnikov, et al., Mitochondrial membrane potential, Anal. Biochem., 2018, 552, 50–59, DOI:10.1016/j.ab.2017.07.009.
- J. Zielonka, J. Joseph and A. Sikora, et al., Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications, Chem. Rev., 2017, 117(15), 10043–10120, DOI:10.1021/acs.chemrev.7b00042.
- B. C. Dickinson, D. Srikun and C. J. Chang, Mitochondrial-targeted fluorescent probes for reactive oxygen species, Curr. Opin. Chem. Biol., 2010, 14(1), 50–56, DOI:10.1016/j.cbpa.2009.10.014.
- J. Chen, X. Jiang and C. Zhang, et al., Reversible Reaction-Based Fluorescent Probe for Real-Time Imaging of Glutathione Dynamics in Mitochondria, ACS Sens., 2017, 2(9), 1257–1261, DOI:10.1021/acssensors.7b00425.
- D. B. Medinas, P. Rozas and C. Hetz, Critical roles of protein disulfide isomerases in balancing proteostasis in the nervous system, J. Biol. Chem., 2022, 298(7), 102087, DOI:10.1016/j.jbc.2022.102087.
- F. Ali, H. A. Anila, N. Taye, R. G. Gonnade, S. Chattopadhyay and A. Das, A fluorescent probe for specific detection of cysteine in the lipid dense region of cells, Chem. Commun., 2015, 51(95), 16932–16935, 10.1039/c5cc07450a.
- S. K. Pramanik and A. Das, Fluorescent probes for imaging bioactive species in subcellular organelles, Chem. Commun., 2021, 57(91), 12058–12073, 10.1039/d1cc04273d.
- S. Tang, H. C. Wong and Z. M. Wang, et al., Design and application of a class of sensors to monitor Ca 2+ dynamics in high Ca 2+ concentration cellular compartments, Proc. Natl. Acad. Sci. U. S. A., 2011, 108(39), 16265–16270, DOI:10.1073/pnas.1103015108.
- W. Basu Ball, J. K. Neff and V. M. Gohil, The role of nonbilayer phospholipids in mitochondrial structure and function, FEBS Lett., 2018, 592(8), 1273–1290, DOI:10.1002/1873-3468.12887.
- J. Sung, J. G. Rho and G. G. Jeon, et al., A New Infrared Probe Targeting Mitochondria via Regulation of Molecular Hydrophobicity, Bioconjugate Chem., 2019, 30(1), 210–217, DOI:10.1021/acs.bioconjchem.8b00845.
- C. R. Pye, W. M. Hewitt and J. Schwochert, et al., Nonclassical Size Dependence of Permeation Defines Bounds for Passive Adsorption of Large Drug Molecules, J. Med. Chem., 2017, 60(5), 1665–1672, DOI:10.1021/acs.jmedchem.6b01483.
- B. C. Doak, B. Over, F. Giordanetto and J. Kihlberg, Oral druggable space beyond the rule of 5: Insights from drugs and clinical candidates, Chem. Biol., 2014, 21(9), 1115–1142, DOI:10.1016/j.chembiol.2014.08.013.
- P. Matsson and J. Kihlberg, How Big Is Too Big for Cell Permeability?, J. Med. Chem., 2017, 60(5), 1662–1664, DOI:10.1021/acs.jmedchem.7b00237.
- A. Nakamura, K. Takigawa and Y. Kurishita, et al., Hoechst tagging: A modular strategy to design synthetic fluorescent probes for live-cell nucleus imaging, Chem. Commun., 2014, 50(46), 6149–6152, 10.1039/c4cc01753f.
- G. Lukinavičius, C. Blaukopf and E. Pershagen, et al., SiR-Hoechst is a far-red DNA stain for live-cell nanoscopy, Nat. Commun., 2015, 6, 8497, DOI:10.1038/ncomms9497.
- S. H. Alamudi, R. Satapathy and J. Kim, et al., Development of background-free tame fluorescent probes for intracellular live cell imaging, Nat. Commun., 2016, 7(11964), 1–9, DOI:10.1038/ncomms11964.
- L. Ji, X. Zhou and R. Liu, et al., Design of a selective and water-soluble fluorescent probe targeting Tau fibrils for intracellular and in vivo imaging, Sens. Actuators, B, 2023, 380, 133415, DOI:10.1016/j.snb.2023.133415.
- G. Li, F. Tao and Q. Liu, et al., A highly selective and reversible water-soluble polymer based-colorimetric chemosensor for rapid detection of Cu2+ in pure aqueous solution, New J. Chem., 2016, 40(5), 4513–4518, 10.1039/c5nj03526k.
- W. L. Cui, M. H. Wang and Y. H. Yang, et al., A water-soluble polymer fluorescent probe via RAFT polymerization for dynamic monitoring of cellular lipid droplet levels and zebrafish imaging, New J. Chem., 2022, 46(34), 16539–16546, 10.1039/d2nj03226k.
- H. Wang, B. Fang and B. Peng, et al., Recent Advances in Chemical Biology of Mitochondria Targeting, Front. Chem., 2021, 9, 683220, DOI:10.3389/fchem.2021.683220.
- A. Baracca, G. Sgarbi, G. Solaini and G. Lenaz, Rhodamine 123 as a probe of mitochondrial membrane potential: Evaluation of proton flux through F0 during ATP synthesis, Biochim. Biophys. Acta, Bioenerg., 2003, 1606(1–3), 137–146, DOI:10.1016/S0005-2728(03)00110-5.
- R. W. Horobin, F. Rashid-Doubell, J. D. Pediani and G. Milligan, Predicting small molecule fluorescent probe localization in living cells using QSAR modeling. 1. Overview and models for probes of structure, properties and function in single cells, Biotech. Histochem., 2013, 88(8), 440–460, DOI:10.3109/10520295.2013.780634.
- R. W. Horobin and F. Rashid-Doubell, Predicting small molecule fluorescent probe localization in living cells using QSAR modeling. 2. Specifying probe, protocol and cell factors; selecting QSAR models; predicting entry and localization, Biotech. Histochem., 2013, 88(8), 461–476, DOI:10.3109/10520295.2013.780635.
- J. Chow, J. Rahman, J. C. Achermann, M. T. Dattani and S. Rahman, Mitochondrial Disease and Endocrine Dysfunction, Nat. Rev. Endocrinol., 2017, 13, 92–104, DOI:10.1038/nrendo.2016.151.
- S. Vyas, E. Zaganjor and M. C. Haigis, Mitochondria and Cancer, Cell, 2016, 166, 555–566, DOI:10.1016/j.cell.2016.07.002.
- A. Johri and M. F. Beal, Mitochondrial Dysfunction in Neurodegenerative Diseases, J. Pharmacol. Exp. Ther., 2012, 342, 619–630, DOI:10.1124/jpet.112.192138.
- H. Xu and D. Ren, Lysosomal Physiology, Annu. Rev. Physiol., 2015, 77, 57–80, DOI:10.1146/annurev-physiol-021014-071649.
- S. R. Bonam, F. Wang and S. Muller, Lysosomes as a Therapeutic Target, Nat. Rev. Drug Discov., 2019, 18, 923–948, DOI:10.1038/s41573-019-0036-1.
- D. Wu, L. Chen, Q. Xu, X. Chen and J. Yoon, Design Principles, Sensing Mechanisms, and Applications of Highly Specific Fluorescent Probes for HOCl/OCl, Acc. Chem. Res., 2019, 52, 2158–2168, DOI:10.1021/acs.accounts.9b00307.
- R. M. Perera and R. Zoncu, The Lysosome as a Regulatory Hub, Annu. Rev. Cell Dev. Biol., 2016, 32, 223–253, DOI:10.1146/annurev-cellbio-111315-125125.
- S. Li, Y. Xiao, C. Chen and L. Jia, Recent Progress in Organic Small-Molecule Fluorescent Probe Detection of Hydrogen Peroxide, ACS Omega, 2022, 7, 15267–15274, DOI:10.1021/acsomega.2c00117.
- P. Gao, W. Pan, N. Li and B. Tang, Fluorescent Probes for Organelle-Targeted Bioactive Species Imaging, Chem. Sci., 2019, 10, 6035–6071, 10.1039/c9sc01652j.
- Y. Geng, Z. Wang, J. Zhou, M. Zhu, J. Liu and T. D. James, Recent Progress in the Development of Fluorescent Probes for Imaging Pathological Oxidative Stress, Chem. Soc. Rev., 2023, 52, 3873–3926, 10.1039/d2cs00172a.
- P. Gao, W. Pan, N. Li and B. Tang, Fluorescent Probes for Organelle-Targeted Bioactive Species Imaging, Chem. Sci., 2019, 10, 6035–6071, 10.1039/C9SC01652J.
- M. Ren, B. Deng, J.-Y. Wang, X. Kong, Z.-R. Liu, K. Zhou, L. He and W. Lin, A Fast Responsive Two-Photon Fluorescent Probe for Imaging H2O2 in Lysosomes with a Large Turn-on Fluorescence Signal, Biosens. Bioelectron., 2016, 79, 237–243, DOI:10.1016/j.bios.2015.12.046.
- J. Liu, P. Niu, Y. Rong, W. Chen, X. Liu, L. Wei and X. Song, A Phenothiazine Coumarin Based Ratiometric Fluorescent Probe for Real-Time Detection of Lysosomal Hypochlorite in Living Cell and Zebra Fish, Spectrochim. Acta, Part A, 2021, 261, 120024, DOI:10.1016/j.saa.2021.120024.
- H. Chen, Z. Yu, S. Ren and Y. Qiu, Fluorescent Probes Design Strategies for Imaging Mitochondria and Lysosomes, Front. Pharmacol, 2022, 13, 915609, DOI:10.3389/fphar.2022.915609.
- W. Zhang, F. Huo, Y. Yue, Y. Zhang, J. Chao, F. Cheng and C. Yin, Heat Stroke in Cell Tissues Related to Sulfur Dioxide Level Is Precisely Monitored by Light-Controlled Fluorescent Probes, J. Am. Chem. Soc., 2020, 142, 3262–3268, DOI:10.1021/jacs.9b13936.
- H. Zhang, Y. Xu, H. Li, W. Shi, X. Li and H. Ma, New Rhodamines with Changeable π-Conjugation for Lengthening Fluorescence Wavelengths and Imaging Peroxynitrite, Chem, 2022, 8, 287–295, DOI:10.1016/j.chempr.2021.11.023.
- B. Y. Lee, J. A. Han, J. S. Im, A. Morrone, K. Johung, E. C. Goodwin, W. J. Kleijer, D. DiMaio and E. S. Hwang, Senescence-Associated Beta-Galactosidase Is Lysosomal Beta-Galactosidase, Aging Cell, 2006, 5, 187–195, DOI:10.1111/j.1474-9726.2006.00199.x.
- Y. Gao, Y. Hu, Q. Liu, X. Li, X. Li, C.-Y. Kim, T. D. James, J. Li, X. Chen and Y. Guo, Two-Dimensional Design Strategy to Construct Smart Fluorescent Probes for the Precise Tracking of Senescence, Angew Chem. Int. Ed. Engl., 2021, 60, 10756–10765, DOI:10.1002/anie.202101278.
- C. Liu, Y. Mei, H. Yang, Q. Zhang, K. Zheng, P. Zhang and C. Ding, Ratiometric Fluorescent Probe for Real-Time Detection of β-Galactosidase Activity in Lysosomes and Its Application in Drug-Induced Senescence Imaging, Anal. Chem., 2024, 96, 3223–3232, DOI:10.1021/acs.analchem.3c05896.
- D. R. Green and J. C. Reed, Mitochondria and Apoptosis, Science, 1998, 281, 1309–1312, DOI:10.1126/science.281.5381.1309.
- S. W. G. Tait and D. R. Green, Mitochondria and Cell Death: Outer Membrane Permeabilization and Beyond, Nat. Rev. Mol. Cell Biol., 2010, 11, 621–632, DOI:10.1038/nrm2952.
- Quantitative Analysis of Mitochondrial Membrane Potential Heterogeneity in Unsynchronized and Synchronized Cancer Cells, DOI:10.1096/fj.202001693R.
- K. Neikirk, A. G. Marshall, B. Kula, N. Smith, S. LeBlanc and A. Hinton, MitoTracker: A Useful Tool in Need of Better Alternatives, Eur. J. Cell Biol., 2023, 102, 151371, DOI:10.1016/j.ejcb.2023.151371.
- T. Ma, L. Zhao, J. Zhang, R. Tang, X. Wang, N. Liu, Q. Zhang, F. Wang, M. Li and Q. Shan, et al., A Pair of Transporters Controls Mitochondrial Zn2+ Levels to Maintain Mitochondrial Homeostasis, Protein Cell, 2022, 13, 180–202, DOI:10.1007/s13238-021-00881-4.
- P. Ning, J. Jiang, L. Li, S. Wang, H. Yu, Y. Feng, M. Zhu, B. Zhang, H. Yin and Q. Guo, et al., A Mitochondria-Targeted Ratiometric Two-Photon Fluorescent Probe for Biological Zinc Ions Detection, Biosens. Bioelectron., 2016, 77, 921–927, DOI:10.1016/j.bios.2015.10.061.
- S. Zhang, J. Fan, S. Zhang, J. Wang, X. Wang, J. Du and X. Peng, Lighting up Fluoride Ions in Cellular Mitochondria Using a Highly Selective and Sensitive Fluorescent Probe, Chem. Commun., 2014, 50, 14021–14024, 10.1039/c4cc05094k.
- S. S. Sabharwal and P. T. Schumacker, Mitochondrial ROS in Cancer: Initiators, Amplifiers or an Achilles' Heel?, Nat. Rev. Cancer, 2014, 14, 709–721, DOI:10.1038/nrc3803.
- M. P. Murphy, How Mitochondria Produce Reactive Oxygen Species, Biochem. J., 2009, 417, 1–13, DOI:10.1042/BJ20081386.
- Q. Pang, T. Li, C. Yin, K. Ma and F. Huo, Comparing the Abundance of HClO in Cancer/Normal Cells and Visualizing in Vivo Using a Mitochondria-Targeted Ultra-Fast Fluorescent Probe, Analyst, 2021, 146, 3361–3367, 10.1039/D1AN00375E.
- L. C. Greaves, A. K. Reeve, R. W. Taylor and D. M. Turnbull, Mitochondrial DNA and Disease, J. Pathol., 2012, 226, 274–286, DOI:10.1002/path.3028.
- J.-W. Yan, W.-J. Ye, S.-B. Chen, W.-B. Wu, J.-Q. Hou, T.-M. Ou, J.-H. Tan, D. Li, L.-Q. Gu and Z.-S. Huang, Development of a Universal Colorimetric Indicator for G-Quadruplex Structures by the Fusion of Thiazole Orange and Isaindigotone Skeleton, Anal. Chem., 2012, 84, 6288–6292, DOI:10.1021/ac300207r.
- X.-C. Chen, G.-X. Tang, W.-H. Luo, W. Shao, J. Dai, S.-T. Zeng, Z.-S. Huang, S.-B. Chen and J.-H. Tan, Monitoring and Modulating mtDNA G-Quadruplex Dynamics Reveal Its Close Relationship to Cell Glycolysis, J. Am. Chem. Soc., 2021, 143, 20779–20791, DOI:10.1021/jacs.1c08860.
- K. G. Zyner, A. Simeone, S. M. Flynn, C. Doyle, G. Marsico, S. Adhikari, G. Portella, D. Tannahill and S. Balasubramanian, G-Quadruplex DNA Structures in Human Stem Cells and Differentiation, Nat. Commun., 2022, 13, 142, DOI:10.1038/s41467-021-27719-1.
- P. D. Wilson, L. M. Franks, D. C. Cottell and F. Benham, Alkaline Phosphatase in Mitochondria, Cell Biol. Int. Rep., 1977, 1, 85–92, DOI:10.1016/0309-1651(77)90014-5.
- L. An, W. Yin and D. Sun, Albumin-to-Alkaline Phosphatase Ratio as a Promising Indicator of Prognosis in Human Cancers: Is It Possible?, BMC Cancer, 2021, 21, 247, DOI:10.1186/s12885-021-07921-6.
- X. Guo, Q. Zou, J. Yan, X. Zhen and H. Gu, Prognostic Effect of Pretreatment Albumin-to-Alkaline Phosphatase Ratio in Human Cancers: A Meta-Analysis, PLoS One, 2020, 15, e0237793, DOI:10.1371/journal.pone.0237793.
- K. W. K. Lam, J. H. C. Chau, E. Y. Yu, F. Sun, J. W. Y. Lam, D. Ding, R. T. K. Kwok, J. Sun, X. He and B. Z. Tang, An Alkaline Phosphatase-Responsive Aggregation-Induced Emission Photosensitizer for Selective Imaging and Photodynamic Therapy of Cancer Cells, ACS Nano, 2023, 17, 7145–7156, DOI:10.1021/acsnano.2c08855.
- S. Chakraborty, M. M. Joseph and S. Varughese, et al., A new pentacyclic pyrylium fluorescent probe that responds to pH imbalance during apoptosis, Chem. Sci., 2020, 11(47), 12695–12700, 10.1039/d0sc02623a.
- S. Chakraborty, A. K. Bindra, A. Thomas, Y. Zhao and A. Ajayaghosh, pH-Assisted multichannel heat shock monitoring in the endoplasmic reticulum with a pyridinium fluorophore, Chem. Sci., 2024, 15(28), 10851–10857, 10.1039/d4sc01977f.
- J. Liu, Y. Huang, T. Li, Z. Jiang, L. Zeng and Z. Hu, The Role of the Golgi Apparatus in Disease (Review), Int. J. Mol. Med., 2021, 47, 1, DOI:10.3892/ijmm.2021.4871.
- Y.-H. Chi, Z.-J. Chen and K.-T. Jeang, The Nuclear Envelopathies and Human Diseases, J. Biomed. Sci., 2009, 16, 96, DOI:10.1186/1423-0127-16-96.
- C. Dias and J. Nylandsted, Plasma Membrane Integrity in Health and Disease: Significance and Therapeutic Potential, Cell Discovery, 2021, 7, 1–18, DOI:10.1038/s41421-020-00233-2.
- S. Xu, K.-C. Yan, Z.-H. Xu, Y. Wang, T. D. James and T. James, Fluorescent Probes for Targeting the Golgi Apparatus: Design Strategies and Applications, Chem. Soc. Rev., 2024, 53, 7590–7631, 10.1039/D3CS00171G.
- H. Zhang, J. Fan, J. Wang, S. Zhang, B. Dou and X. Peng, An Off-on COX-2-Specific Fluorescent Probe: Targeting the Golgi Apparatus of Cancer Cells, J. Am. Chem. Soc., 2013, 135, 11663–11669, DOI:10.1021/ja4056905.
- B. Wang, J. Fan, X. Wang, H. Zhu, J. Wang, H. Mu and X. Peng, A Nile Blue Based Infrared Fluorescent Probe: Imaging Tumors That over-Express Cyclooxygenase-2, Chem. Commun., 2014, 51, 792–795, 10.1039/C4CC08915D.
- B. Gurram, M. Li, J. Fan, J. Wang and X. Peng, Near-Infrared Fluorescent Probe for Fast Track of Cyclooxygenase-2 in Golgi Apparatus in Cancer Cells, Front. Chem. Sci. Eng., 2020, 14, 41–52, DOI:10.1007/s11705-019-1796-1.
- S. Staubach and F.-G. Hanisch, Lipid Rafts: Signaling and Sorting Platforms of Cells and Their Roles in Cancer, Expert Rev. Proteomics, 2011, 8, 263–277, DOI:10.1586/epr.11.2.
- G. Di Paolo and T.-W. Kim, Linking Lipids to Alzheimer's Disease: Cholesterol and Beyond, Nat. Rev. Neurosci., 2011, 12, 284–296, DOI:10.1038/nrn3012.
- H. Wang, Z. Feng, S. J. Del Signore, A. A. Rodal and B. Xu, Active Probes for Imaging Membrane Dynamics of Live Cells with High Spatial and Temporal Resolution over Extended Time Scales and Areas, J. Am. Chem. Soc., 2018, 140, 3505–3509, DOI:10.1021/jacs.7b13307.
- B. Zheng, Y. Tian, S. Liu, J. Yang, F. Wu and H. Xiong, Non-Solvatochromic Cell Membrane-Targeted NIR Fluorescent Probe for Visualization of Polarity Abnormality in Drug-Induced Liver Injury Mice, Anal. Chem., 2023, 95, 12054–12061, DOI:10.1021/acs.analchem.3c02005.
- A. Kurutos, T. Ilic-Tomic, F. S. Kamounah, A. A. Vasilev and J. Nikodinovic-Runic, Non-cytotoxic photostable monomethine cyanine platforms: Combined paradigm of nucleic acid staining and in vivo imaging, J. Photochem. Photobiol., A, 2020, 397, 112598, DOI:10.1016/j.jphotochem.2020.112598.
- A. Kurutos, J. Nikodinovic-Runic, A. Veselinovic, J. B. Veselinovic, F. S. Kamounah and T. Ilic-Tomic, RNA-targeting low-molecular-weight fluorophores for nucleoli staining: Synthesis, in silico modelling and cellular imaging, New J. Chem., 2021, 45, 12818–12829, 10.1039/D1NJ01659H.
- D. Aristova, R. Selin, H. S. Heil, V. Kosach, Y. Slominsky, S. Yarmoluk, V. Pekhnyo, V. Kovalska, R. Henriques and A. Mokhir, et al., Trimethine cyanine dyes as NA-sensitive probes for visualization of cell compartments in fluorescence microscopy, ACS Omega, 2022, 7, 47734–47746, DOI:10.1021/acsomega.2c05231.
| This journal is © The Royal Society of Chemistry 2025 |