Open Access Article
Open Access ArticleChitosan immunomodulation: insights into mechanisms of action on immune cells and signaling pathways
Majed Ghattas ab,
Garima Dwivedi
ab,
Garima Dwivedi c,
Anik Chevrier
c,
Anik Chevrier a,
Delano Horn-Bourque
a,
Delano Horn-Bourque ab,
Mohamad-Gabriel Alameh
ab,
Mohamad-Gabriel Alameh cd and
Marc Lavertu
cd and
Marc Lavertu *ab
*ab
aDepartment of Chemical Engineering, Polytechnique Montreal, Montreal, QC, Canada. E-mail: marc.lavertu@polymtl.ca
bInstitute of Biomedical Engineering, Polytechnique Montreal, Montreal, QC, Canada
cPerelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA
dPenn Institute for RNA Innovation, University of Pennsylvania, Philadelphia, PA, USA
First published on 10th January 2025
Abstract
Chitosan, a biodegradable and biocompatible natural polymer composed of β-(1–4)-linked N-acetyl glucosamine (GlcNAc) and D-glucosamine (GlcN) and derived from crustacean shells, has been widely studied for various biomedical applications, including drug delivery, cartilage repair, wound healing, and tissue engineering, because of its unique physicochemical properties. One of the most promising areas of research is the investigation of the immunomodulatory properties of chitosan, since the biopolymer has been shown to modulate the maturation, activation, cytokine production, and polarization of dendritic cells and macrophages, two key immune cells involved in the initiation and regulation of innate and adaptive immune responses, leading to enhanced immune responses. Several signaling pathways, including the cGAS–STING, STAT-1, and NLRP3 inflammasomes, are involved in chitosan-induced immunomodulation. This review provides a comprehensive overview of the current understanding of the in vitro immunomodulatory effects of chitosan. This information may facilitate the development of chitosan-based therapies and vaccine adjuvants for various immune-related diseases.
1. Introduction
Chitosan (CS) has attracted significant attention owing to its immunomodulatory and biodegradable properties. Upon introduction into the immune system, it stimulates the secretion of various growth factors, chemokines, bioactive lipids, and pro- and anti-inflammatory cytokines by the innate immune cells.1 CS is a naturally derived, biocompatible, biodegradable, and non-toxic cationic polymer composed of β-(1–4)-linked N-acetyl glucosamine (GlcNAc) and D-glucosamine (GlcN) (Fig. 1). It is most often defined as chitin with a degree of deacetylation (DDA) >50%.2 CS is obtained by the hydrolysis of acetyl groups (deacetylation, typically performed under strong alkaline conditions) of chitin (Fig. 1), a component of the exoskeleton of shrimps, crabs, and fungi, and is the second most abundant polysaccharide on earth after cellulose. The intrinsic properties of CS, such as its DDA and average molecular weight (MW), have been reported to potentially affect its immunostimulatory properties.3 Nevertheless, the exact influence of the physicochemical properties of CS, particularly its DDA and MW, remains uncertain, as conflicting data have been reported.4–12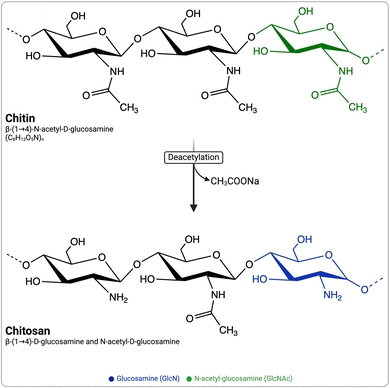 | ||
| Fig. 1 Schematic representation of chitin and chitosan structures and the common alkaline deacetylation process for converting chitin to chitosan. | ||
Moreover, investigations into the immunostimulatory aspect of this polymer often overlook crucial factors such as accurate measurement of DDA, MW, levels of endotoxin contamination and appropriate dosage, and other pertinent considerations (e.g., insolubles, heavy metal content, and residue on ignition). Consequently, these discrepancies can potentially yield varied outcomes.
This review aims to offer a comprehensive understanding of how CS and its intrinsic parameters affect specific types of immune cells, known as myeloid antigen-presenting cells (APCs), including dendritic cells and macrophages. Additionally, this review seeks to delve into the intracellular and biomolecular mechanisms of action of CS and to examine its signaling cascades and immune activation abilities. The goal is to provide an in-depth overview of the complex interplay between chitosan and myeloid APCs at the molecular and cellular levels. In vivo studies are beyond the scope of this review.
1.1. Influence of chitosan's physicochemical properties on immunomodulation
CS can be produced from chitin through partial deacetylation in an alkaline solution, either under heterogeneous conditions (high NaOH concentration, high temperature, short reaction time) or homogeneous conditions (lower NaOH, moderate temperature, longer reaction time).13,14 A recent method used enzymatic digestion followed by mass spectrometry, leveraging the specific action of chitinosanase from Alternaria alternata.15 Different production methods may result in distinct deacetylation patterns.13 The distribution of acetyl groups can lead to variable chemical properties along the chitosan chain, which can affect its solubility, thermal stability, and biodegradability.16,17DDA is a crucial physicochemical characteristic of CS and refers to the proportion of GlcN units with respect to the total number of monomers. DDA can be quantified using different methods, including infrared and ultraviolet spectrophotometry and 1H NMR.3,18 DDA can influence various properties of CS, including its solubility, degree of crystallinity, hydrophilicity, chemical reactivity, and cellular responses.3,19 DDA strongly influences the biodegradability, with high-DDA CS being more difficult to degrade.20,21 Lysozyme, an enzyme present in the lysosomes of cells, has the ability to degrade bacterial cell walls and extracellular materials that have been internalized by cells. It can target GlcNAc sequences containing more than three residues.20–22 However, it is incapable of breaking down GlcN or CS that have a relatively small proportion of randomly distributed GlcNAc residues.20,21 In a study investigating the impact of the DDA on cytokine release, endotoxin-free chitosans with 80% and 97% DDAs were incubated with mouse macrophages. No notable difference was detected in the release of Tumor Necrosis Factor α (TNF-α) between the two samples, and the levels were comparable to those of the control group, which was only exposed to media.10 However, in gene therapy applications, CS–plasmid DNA nanoparticles with a lower DDA (80% vs. 90%) resulted in higher antibody levels. Histological analysis confirmed this finding, showing increased inflammatory infiltrates in lower DDA CS, which correlated with higher antibody titers.9 In other reports, Nishimura et al.6 found a link between CS immunological activity and its DDA. They observed that CS with a 70% DDA exhibited optimal adjuvanticity, whereas CS with a 30% DDA resulted in lower immunogenicity. However, recent studies have presented conflicting results. Scherließ et al.7 demonstrated that CS with 76% DDA elicited higher immune responses compared to CS with 81% DDA.
Another key parameter of CS is its molecular weight (Mw), which represents the average number of monomers per chain and influences the viscosity and solubility of CS.23 The MW of CS should be carefully controlled and monitored during the production process. In addition to affecting the physical properties, the MW also may influence its biological activity. Studies have shown that low-MW CS at 1–10 μg mL−1 doses (MW: 50–190 kDa; DDA: 75–85%) can induce proinflammatory cytokine and chemokine (IL-6, Ifnb, and Cxcl10) production in bone marrow-derived dendritic cells (BMDCs) earlier than high-MW CS (MW: 310–375 kDa; DDA >75%).11 Additionally, high-MW CS elicits higher expression of CD80, CD86, and MHC class II in BMDCs than low-MW CS.11 However, another study found that at 2.5 and 10 μg mL−1 doses, higher MW CS (50 kDa) significantly increased COX-2 and MCP-1 expression, while lower MW CS (3 kDa) significantly boosted anti-inflammatory cytokine IL-10 expression.12 This suggests that low-MW CS promotes the expression of genes associated with key molecules in the NF-κB and AP-1 pathways.12
CS can be chemically modified through its reactive –NH2 and –OH groups. Common modifications include N,N,N-trimethylation,24 glycol conjugation,25 mannose conjugation,26 carboxymethyl conjugation,27 sulfate conjugation28 among others. While these modifications may, in certain cases, influence CS's immunogenicity, this aspect is beyond the scope of this review.
In summary, while it has been suggested that the physicochemical properties of CS, particularly its DDA and MW, may influence the immune response to this polymer, the precise effect of these parameters on immune reactions remains ambiguous with contradictory findings reported in the literature. Nevertheless, understanding the impact of CS's physicochemical properties on immune cells is crucial for the development of CS-based immunomodulatory compounds and requires further investigation.
2. Exploring the interactions between immune cells and chitosan
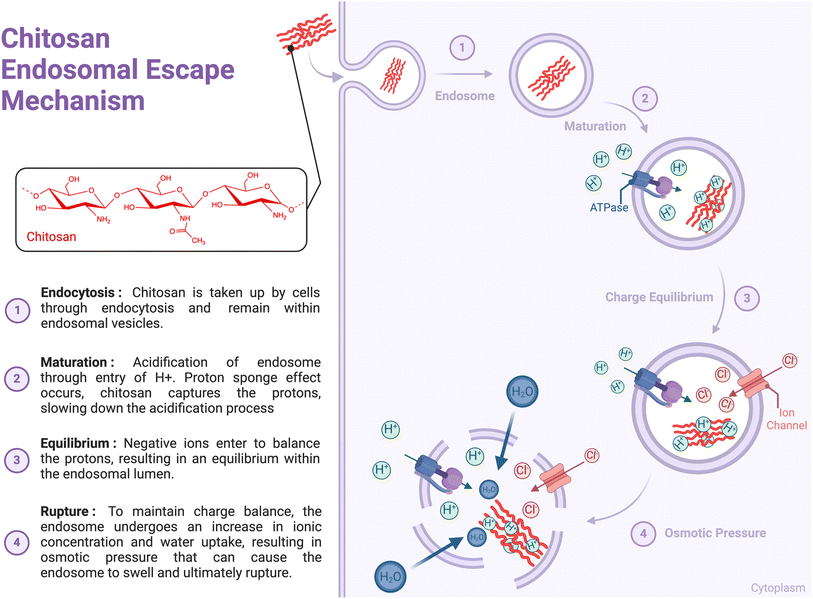
Several studies have investigated the interaction between CS and immune cells; however, the lack of information on endotoxin levels or other contaminants in CS preparations has hindered data interpretation.10 These studies have highlighted the involvement of various immune sensors and receptors, including Toll-Like Receptor 4 (TLR-4),29,30 TLR-2,31,32 CD14, CR3,33 mannose receptor,34 NKR-P1,35 megalin36 and Dectin-1,37 which can lead to receptor-mediated phagocytosis. However, a specific cell surface receptor for CS has not yet been identified. Additionally, CS can interact with cells through electrostatic interactions, as its positive charge (pKa ∼ 6.5) can trigger receptor-independent endocytosis, such as clathrin-mediated endocytosis or micropinocytosis.38 Upon entering cells, CS has been reported to rely on the proton sponge mechanism to burst the endosomal membrane that surrounds the polymer. The mechanism involves sponging protons delivered by vacuolar-ATPase, causing cationic polymers to become protonated as endosomal pH changes (around the pKa of polymers). This leads to endosomal swelling and leakage, eventually resulting in membrane rupture and allowing the polymer to escape into the cytoplasm (Fig. 2).39 It has been proposed that high-MW CS-based complexes can escape endosomes owing to enzymatic CS degradation, which results in the production of oligo- and monosaccharides that increase endosome osmolarity and cause membrane rupture.40 However, high-DDA CS are less sensitive to enzymatic degradation.40 The physicochemical characteristics of CS may influence cellular interactions with chitosan, including endosomal escape.2.1. Chitosan and dendritic cells (DCs)
The importance of dendritic cells (DCs) in innate and adaptive immune responses is well known.41 They are strategically located in tissues, such as the skin, respiratory tract, and gut, where they identify and process antigens from pathogens. DCs can activate T cells to initiate an immune response or induce tolerance to prevent autoimmunity.42 They also secrete cytokines that modulate the immune response and influence the fate of T-cells.43 DCs mediate innate and adaptive immunity by activating myeloid and lymphoid cells. Immature DCs have a high phagocytic capacity and present antigens to lymphocytes in distal lymphoid organs. Mature DCs activate T cells via direct contact with antigenic peptides and co-stimulatory signals.DCs originate from hematopoietic stem cells and differentiate into various subsets, such as cDC1, cDC2, and pDC. Each subset contains distinct transcription factors and surface markers.44 cDC1 can cross-present antigens on MHC class I to CD8+ T cells and plays a crucial role in capturing antigens and inducing cytotoxic T-cell responses.45 cDC2s preferentially express antigens on MHC class II molecules and activate CD4+ T cells. All DC subsets produce cytokines and chemokines that activate other innate immune cells, including natural killer and NKT cells.31,46
When biomaterials are introduced into the body, dendritic cells (DCs) interact with them, potentially leading to tolerance or immune activation. This interaction occurs via pattern recognition receptors (PRRs), phagocytosis, and endocytosis. Biomaterials can modify DC function, affecting their phenotype, cytokine production, and antigen-presenting capacity.47 Understanding DC-biomaterial interactions is vital for designing biomaterials for immune activation or tolerance for applications such as prophylactic and therapeutic vaccines against infectious pathogens and cancer.48
CS has been studied for its potential impact on DC activation, which is a crucial step in initiating the immune response. Evidence has shown that CS (MW: 612 kDa) promotes DC maturation which is accompanied by an upregulation in costimulatory molecules (CD80, CD83 and CD86) and pro-inflammatory cytokines (interferon-gamma, IFN-γ).49 In addition, CS-treated DCs have been shown to exhibit enhanced antigen presentation and T-cell activation in vitro.49 In a study by Oliveira et al.,50 human monocyte-derived DCs differentiated on CS films (DDA: 87–88%, Mw: 324 ± 27 kDa) exhibited significant upregulation of CD86, a co-stimulatory molecule, and partial, non-significant upregulation of CD83, a DC activation marker. The use of CS also reduced IL-10 production and increased TGF-β1, TNF-α, and interleukin-1 beta (IL-1β) (p < 0.001) levels. These results suggest that CS modulates immune response by influencing DC activation.
Nod-like receptor pyrin domain-containing 3 (NLRP3) is an intracellular protein that functions as a sensor for diverse classes of stimuli, such as pathogens, internal stress signals, and environmental irritants. NLRP3 can be activated by multiple signals, such as the efflux of potassium ions (K+) or chloride ions (Cl−), influx of calcium ions (Ca2+), lysosomal disruption, mitochondrial dysfunction, metabolic changes, and trans-Golgi disassembly.51 Its activation triggers the formation and stimulation of the NLRP3 inflammasome, which in turn elicits the release of inflammatory cytokines, including IL-1β and IL-18, as well as a mode of gasdermin D regulated programmed cell death called pyroptosis.51 The inflammasome can be primed by the recognition of pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) that bind to pattern recognition receptors (PRRs) and by cytokines such as TNF, which stimulate the transcription factor NF-κB and gene expression. Priming also stimulates a metabolic shift from oxidative phosphorylation to glycolysis, which indirectly upregulates the transcription of IL-1β. An important revelation by Bueter et al. in the context of CS immunoactivation was the confirmation that the strong IL-1β response elicited in murine BMDCs relies on the NLRP3 pathway explained above.39
In their work, Mori et al.52 compared the inhibitory effects of various adjuvants on TLR agonist-induced IL-12 production from DCs. Among the adjuvants tested, alum was found to strongly inhibit IL-12 secretion, whereas CS (DDA of 75–90% and MW of 150–400 kDa) did not exhibit such inhibitory effects. The authors proposed that the alum-driven PI3 kinase signaling pathway may be responsible for the observed inhibition, which could explain why alum is not efficient in promoting Th1 responses. When combined with the TLR9 agonist CpG, CS enhanced the secretion of Th1 (IL-12) and Th17 (IL-6 and IL-23) cell-polarizing cytokines by DCs. Notably, CpG–chitosan demonstrated superior efficacy in promoting these immune responses compared with CpG-alum when co-administered with OVA.52 This study underscores the significance of selecting the right adjuvant in vaccine development, as it can greatly impact the nature and strength of the immune responses generated.
In another study, Carroll et al.53 investigated the effect of CS (DDA-75–90% and MW-150–400 kDa) on BMDCs. When exposed to increasing doses of CS (2, 4, or 8 μg mL−1), the expression of activation markers and co-stimulatory molecules, such as CD40 and CD86, was elevated on the surface of BMDCs. Interestingly, activation was found to be dependent on type I IFN receptor (IFNAR). High levels of IFN-α and IFN-β were detected, whereas pro-inflammatory cytokines such as IL-12p40 and IL-6 were absent.
Villiers et al.29 and Zhang et al.30 demonstrated that CS can activate DCs through a TLR4-dependent mechanism at the membrane level indicated by the upregulation of membrane proteins, including MHC class II molecules and co-receptor molecules, CD80 and CD86, comparable to Lipopolysaccharide (LPS) stimulation. The authors showed that CS-induced DC activation was impaired in TLR4−/− DCs, further demonstrating a TLR4-dependent mechanism. However, CS was unable to induce the production of cytokines, such as IL-12, IL-10, IL-1β, TNF-α, and IL-6, indicating that DCs activated by CS were unable to stimulate T cells.
Challenging the findings of Villiers et al.29 and Zhang et al.,30 Carroll et al.53 showed that CS was capable of inducing the upregulation of CD40 in cells with defective TLR4 signaling, indicating that CS can promote DC activation through a TLR-4 independent pathway.53 In their study,53 CS was found to promote the transcription and secretion of type I interferons. Interestingly, when exposed to CS, IFNAR-deficient mice (Ifnar−/−) showed a remarkable reduction in serum antibody, cytokine, and chemokine release (IFN-γ and CXCL10), IgG2c antigen-specific antibody responses, and decreased expression of co-stimulatory markers.53 These findings highlight the importance of the IFNAR signaling pathway in regulating the immune response to CS exposure.
To assess the performance of CS-stimulated DCs, Villiers et al.29 used a mixed lymphocyte reaction and showed that both CS-stimulated and immature DCs were unable to activate T cells, whereas LPS-activated DCs were able to do so. The researchers also found that CS activation of DCs does not lead to the production of cytokines, such as IL-10, IL-12, IL-1β, TNF, and IL-6. Further investigation revealed that T cell activation by CS-stimulated DCs did not produce TGF-β, IL-10, IL-4, IL-12, and IL-6, and very low IFN-γ and IL-2 secretion. Overall, this study suggests that CS-induced DC activation has limited functional effects on T-cell activation. Based on their results, CS's TLR4-dependent mechanism may explain the lack of cytokine production and limited T-cell activation, indicating that further research is required to fully understand its potential as an immunomodulatory agent.
When DCs were treated with multiple doses of CS for 24 h, followed by LPS treatment to verify the effect of CS on cytokine production or secretion, CS did not block cytokine production. However, a CS-induced alteration in the IL-10/IL-12 balance was observed in the cytokine profile of stimulated cells, indicating that although CS does not impede the restimulation of DCs, it reorients their cytokine profiles.29
Collectively, these results provide important insights into the mechanisms underlying CS-induced DC activation and maturation. Although Villiers et al. suggested that CS activates DCs through a TLR-4-dependent mechanism, Carroll et al. showed that CS promotes DC activation through a TLR-4-independent pathway. Furthermore, Carroll et al. demonstrated that CS-induced DC activation is dependent on IFNAR signaling via the IFN 1 pathway, which is a more unique mechanism than other conventional mechanisms.11 These findings highlight conflicting results in the literature and underscore the need for further research to understand the precise mechanism of CS on DCs. Such investigations are crucial for the development of chitosan-based immunostimulatory biomaterial.
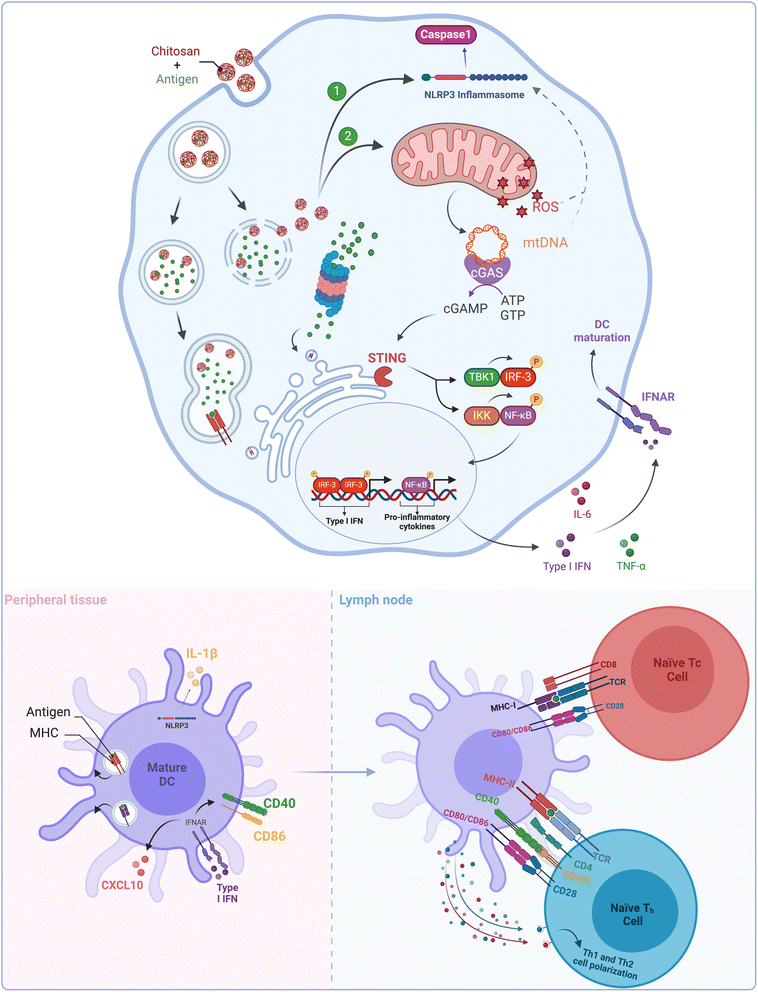
The ability of different organisms to recognize foreign DNA is crucial for their immunity. This critical function is predominantly carried out via the cyclic GMP–AMP synthase (cGAS)–stimulator of interferon genes (STING) pathway in mammalian cells. Upon binding to cytosolic double-stranded DNA, cGAS undergoes structural changes leading to its activation. Once activated, cGAS converts ATP and GTP into cyclic dinucleotides known as 2′,3′-cyclic GMP–AMP (cGAMP), which is then detected by a cyclic dinucleotide sensor located on the endoplasmic reticulum called the Stimulator of Interferon Genes (STING). Once cGAMP binds to STING, it is transported to the Golgi complex where it activates TANK-binding kinase 1 (TBK1) and the IκB kinase (IKK) complex. These kinases then phosphorylate two transcription factors, namely, interferon 3 regulatory factor (IRF3) and nuclear factor (NF)-κB. IRF3 and NF-κB then form dimers and are transported to the nucleus, where they initiate the transcription of type I interferon and proinflammatory genes, respectively.54 In summary, upon recognition of intracellular DNA, cGAMP is produced from cGAS, leading to the activation of TBK1 and IKK, which in turn activates IRF3 and NF-κB to initiate type I interferon and proinflammatory gene expression (Fig. 3).
The study from Carroll et al.53 found that CS activates the cGAS–STING signaling pathway because CS-induced type I IFN response and the expression of costimulatory molecules were significantly diminished in DCs derived from cGAS− or STING− mice. This study further found that CS internalization in DCs caused mitochondrial stress and led to the production of reactive oxygen species (ROS).55 It was hypothesized that the release of mtDNA (mitochondrial DNA) was due to the opening of the mitochondrial permeability transition pores, which were triggered by mitochondrial stress (Fig. 3). This hypothesis was validated when the response was found to be inhibited in cells treated with DNase I.
Taken together, these findings highlight the significant involvement of the cGAS–STING pathway and mtDNA release in CS-triggered maturation of DCs. This pathway has been recognized as a vital mediator for regulating immune responses, with its agonists demonstrating robust immune defenses against infections and cancer.56 These findings suggest promising possibilities for the development of potent CS-based immunostimulatory agents (Table 1).
| Chitosan | Cell type | Dose | Main outcomes | Ref. |
|---|---|---|---|---|
| DDA: 87–88%, Mw: 324 kDa | Human monocyte-derived DCs | N/A | - Upregulation of CD86 | 50 |
| - Increased TGF-β1, TNF-α, IL-1β | ||||
| - Reduced IL-10 production | ||||
| DDA: 75–90%, Mw: 150–400 kDa | Bone marrow-derived DCs (BMDCs) | 2, 4, or 8 μg mL | - Increased expression of CD40 and CD86 | 53 |
| - High levels of IFN-α and IFN-β | ||||
| - Dependent on IFNAR signaling | ||||
| - Activate cGas–STING pathway | ||||
| DDA: 76%, Mw: high | BMDCs | 0.1 mg mL−1 | - Strong IL-1β response observed | 39 |
| - NLRP3 pathway is essential for IL-1β release | ||||
| DDA: 75–90%, Mw: 150–400 kDa + CpG | BMDCs | 2 μg mL−1 | - Enhanced secretion of IL-12 (Th1) and IL-6, IL-23 (Th17) | 52 |
| - Superior immune response with CpG | ||||
| - No inhibition of IL-12 | ||||
| DDA: >85% Mw: >40 kDa | BMDCs | 5, 10, 20,40 μg mL | - Activation via TLR4 mechanism | 29 |
| - Upregulation of MHC II, CD80, CD86 | ||||
| - Altered IL-10/IL-12 balance | ||||
| - CS reorients cytokine profiles by IL-10/IL-12 balance |
• CS induces DCs activation via TLR4-independent pathways, with IFNAR and cGAS–STING signaling playing significant roles.
• CS enhances costimulatory molecules (e.g., CD80, CD86) and selectively affects cytokine profiles, such as increasing IFN-γ, TNF-α, and IL-1β while limiting IL-12 production.
• CS activates DCs through mitochondrial stress, triggering the release of reactive oxygen species and mitochondrial DNA, which drives immune signaling pathways.
2.2. Chitosan and macrophages
Leukocytes, also known as white blood cells, are crucial components of the human immune system that protect the body against foreign invaders, such as pathogens. Leukocytes are classified into monocytes, neutrophils, eosinophils, basophils, and lymphocytes, based on their morphology and function. Monocytes, which are key players in the early stages of the immune response, are recruited in response to biomaterials and biopolymers, such as implants and scaffolds, and undergo differentiation into macrophages and DCs.Macrophages are phagocytic cells that engulf and digest foreign particles including biomaterials and pathogens. They are involved in the primary (acute) inflammatory response triggered by the release of proinflammatory cytokines and chemokines.57 Macrophages release cytokines and chemokines such as TNF-α, IL-1β, and IL-6, which activate other immune cells and alter their activity and metabolism, leading to the recruitment of additional immune cells, such as neutrophils and lymphocytes, to the site of injury or infection.58 Macrophages also play a key role in the clearance of exogenous antigens via phagocytosis.
Macrophages can adjust their phenotype in response to their microenvironment, with three major polarized macrophage subsets: M0, M1, and M2. This phenotypic plasticity allows macrophages to exhibit either pro-inflammatory or anti-inflammatory features depending on the cues they receive.59 M0 macrophages are precursors of M1 and M2 macrophages, have a noninflammatory phenotype, and are capable of phagocytosis and antigen presentation.
M1 macrophages are pro-inflammatory and triggered by pathogens. They secrete pro-inflammatory cytokines, such as IL-6 and TNF-α, which aid in the elimination of pathogens and infections.60 M2 macrophages, on the other hand, are associated with an anti-inflammatory response and are usually encountered in wound healing to resolve inflammation. M2 macrophages are initiated by various cytokines and immune complexes, releasing high concentrations of anti-inflammatory cytokines such as IL-1ra and low concentrations of pro-inflammatory cytokines.50,61 There are multiple subtypes of M2 macrophages including M2a, M2b, M2c, and M2d.
The interaction between biomaterials and macrophages involves various receptors (e.g., TLRs, scavenger receptors, and complement receptors), leading to signaling events, such as cytokine release, ROS production, and surface marker expression (CD80 and CD86).62 Understanding these interactions is crucial for developing immunomodulatory biomaterials, and immunoengineering strategies, such as surface modifications and bioactive molecule incorporation, can modulate macrophage responses to biomaterials and provide insights into inflammation-associated diseases.63–65
Bueter and colleagues39 found that CS (DDA of 76%) could stimulate the secretion of IL-1β in macrophages at all stages of differentiation (M1, M2, and intermediate). However, the response was found to be more significant in M1 macrophages, which were differentiated in vitro using granulocyte-macrophage colony-stimulating factor (GM-CSF) and IFN-γ, compared to M2 macrophages, which were differentiated using macrophage colony-stimulating factor (M-CSF).54 This suggests that CS has a stronger effect on pro-inflammatory M1 macrophages than on anti-inflammatory M2 macrophages, with a potential implication on the modulation of the immune response under certain conditions.
Researchers have investigated the process of inflammasome activation by CS and found that it is dependent on two factors namely, cellular K+ efflux and acidification-dependent lysosomal destabilization.39 Furthermore, they found that the activation of the NLRP3 inflammasome by CS requires the presence of mitochondrial ROS. The NLRP3 inflammasome, once activated, cleaves pro-IL1β into its bioactive form using caspase 1. In summary, CS stimulates the NLRP3 inflammasome through a process that involves K+ efflux, lysosomal destabilization, and mitochondrial ROS, resulting in the activation of caspase 1 and cleavage of pro-IL1β into its bioactive form.
Fong et al.66 investigated whether CS (81.5% DDA, 132 kDa, 2.02 PDI, block-acetylated) could stimulate macrophages in different polarization states to release functional mesenchymal stem cell (MSCs) chemokines, primarily anabolic factors (e.g., MCP-1, IP-10 and MIP-1β). The latter invokes a chemoattractive response that leads to MSC migration to the affected area, where they can facilitate tissue regeneration and repair in response to injury or inflammation. In their study, conditioned medium from M0 and M2a macrophages stimulated with CS induced 2-fold more chemotaxis from MSC than low-serum control medium, whereas conditioned medium from CS-induced M1 macrophages failed to induce MSC chemotaxis. Furthermore, their findings showed that CS activated the NLRP3 inflammasome, resulting in IL-1β and IL-18 responses in murine bone marrow-derived macrophages, however when used as scaffold (85% and 96% DDA) in Vasconcelos et al.67 CS did not trigger the NLRP3 inflammasome activation in macrophages. CS was highly phagocytosed, leading to an increase in the release of anabolic factors from M0 and M2a macrophages, including MCP-1, IP-10, MIP-1β, IL-1ra, IL-10, and platelet-derived growth factor (PDGF). It also induced IL-1β release, with a much higher amount of IL-1ra than that of IL-1β. CS stimulation of M1 macrophages increased the expression of pro-inflammatory cytokines, such as IL-1β, IL-6, IL-8, and IP-10, as well as decreased levels of vascular endothelial growth factor (VEGF) and MCP-1, relative to M0 and M2a cells. Furthermore, M1 macrophages showed increased secretion of TNF-α compared to M2a cells, but a lower level of TNF-α secretion compared to M0 cells. The secretion of IP-10 was induced by the STAT-1 signaling pathway and delayed when triggered by 82% DDA–chitosan. The activation of STAT-1 relies on the activation of protein kinase-C agonists, which have not yet been identified. One crucial factor is DDA, as it was observed that 80% DDA CS activated the STAT-1 pathway, whereas 98% DDA did not, leading to unpaired release of IP-10; the reason for this is not yet clear.
They also ruled out the possibility that 80% DDA CS triggered the release of IL-1ra via the IL-4/STAT-6 signaling axis in experiments conducted on PMA-differentiated U937 macrophages (M0). Moreover, they found that the enhanced release of IL-1ra caused by 82% DDA CS was not dependent on paracrine IL-4 and IL-10 signaling or IL-1β release, indicating that this effect is mediated by signaling pathways that require further investigation. These findings imply that CS can be tailored to induce specific cytokine responses while avoiding others, including IL-1ra release while inhibiting IL-1β release (Fig. 4).
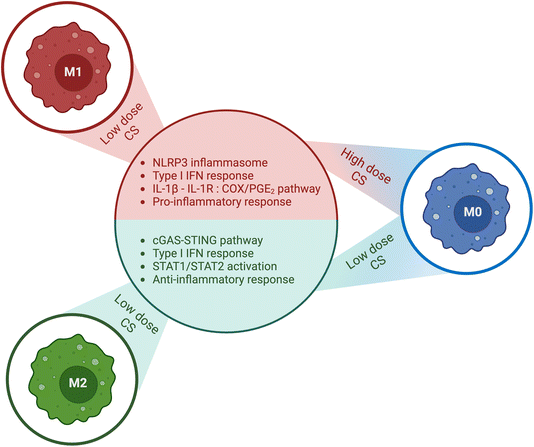 | ||
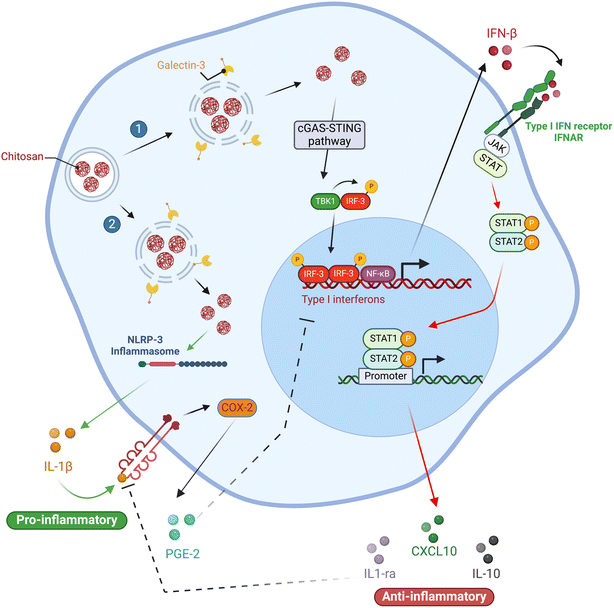
| Fig. 4 Summary of the impact of chitosan (CS) dose and macrophage activation state. Chitosan exhibits distinct effects on macrophages, which are contingent on their activation state and the dose of chitosan administered. In M0 macrophages, low doses of chitosan stimulated the production of type 1 IFN, resulting in augmented release of IL-1ra and CXCL10/IP-10, and decreased levels of IL-1β and PGE2. In contrast, high doses of chitosan activated the NLRP3 inflammasome, leading to enhanced release of IL-1β and PGE2, and suppressed the release of IL-1ra and CXCL10/IP-10. Furthermore, chitosan amplifies the secretion of proinflammatory cytokines in M1 polarized macrophages. Conversely, in M2 state macrophages, chitosan amplifies the secretion of anti-inflammatory cytokines. The corresponding figure has been adapted from ref. 68. | ||
Another study by Vasconcelos et al.61 aimed to explore how the DDA in 3D porous CS scaffolds influences the macrophage response in vivo, particularly with respect to M1/M2 phenotypic polarization profiles. Using a rodent air pouch model, the DDA of the CS scaffolds was found to exert a significant effect on macrophage activation and polarization where CS scaffolds with 95% DDA showed decreased adhesion of inflammatory cells. Furthermore, the predominant phenotype of the adherent macrophages was M2. The exudates had a higher number of F4/80+/CD206+ cells (M2 macrophages) than F4/80+/CCR7+ cells (M1 macrophages).61 Additionally, lower levels of pro-inflammatory cytokines and higher levels of anti-inflammatory cytokines were detected in inflammatory exudates.61 Conversely, when CS scaffolds with a DDA of 85% were utilized, opposite results were obtained. In this instance, M1 macrophages were the principal macrophages present in the adherent scaffold and exudates, and elevated levels of proinflammatory cytokines were observed. Overall, this study emphasizes the significance of considering the deacetylation property of CS scaffolds when designing biomaterials for biomedical applications because it has the potential to significantly influence the macrophage response and, consequently, the overall success of the biomaterial.
Building on what was previously done, Fong et al.69 examined a range of CS doses and discovered that the effect of CS is influenced not only by the DDA but also by the dose and polydispersity of the polymer. It was observed that chitosan can elicit two distinct cytokine responses that are mutually exclusive, which is attributable to varying levels of lysosomal disruption. Certain chitosan preparations (10 or 190 kDa with 80% DDA, and 3, 5, or 10 kDa with 98% DDA) induced macrophages to release CXCL10 and IL-1ra at 5–50 mg mL−1, or activated the inflammasome to release IL-1β and PGE2 at 50–150 mg mL−1.69
At low doses, CS causes mild lysosomal disruption, which in turn triggers a type 1 IFN response and phosphorylation of STAT-1/STAT-2. This cascade of events ultimately leads to the release of IL-1ra, which counteracts the effects of IL-1β and CXCL10/IP-10, leading to an anti-inflammatory response accompanied by the suppression of the pro-inflammatory response. In another study, stimulation with ≤100 μg mL−1 of 80% DDA CS caused unprimed primary macrophages to release anti-inflammatory IL-1ra without triggering inflammasome activation or IL-1β release.50,70 However, increasing the dose to 500 μg mL−1 resulted in the secretion of IL-1β and CCL5/RANTES suggesting a role of dose of CS in its influence on inflammatory and immune responses.70 These findings were confirmed by Chou et al.,71 who used CS with a high DDA (>90%) and varying molecular weights (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 150
000, 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, or 300
000, or 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) at doses of 1, 2.5, 12.5, or 62.5 μg mL−1. They also observed that this anti-inflammatory response was achieved by suppressing cyclooxygenase-2 (COX-2), which in turn inhibits the production of PGE2. Yoon et al.72 also reported similar outcomes using CS oligosaccharides with a MW of less than 10
000 Da) at doses of 1, 2.5, 12.5, or 62.5 μg mL−1. They also observed that this anti-inflammatory response was achieved by suppressing cyclooxygenase-2 (COX-2), which in turn inhibits the production of PGE2. Yoon et al.72 also reported similar outcomes using CS oligosaccharides with a MW of less than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da and a DDA of 90–95%. When administered at higher doses, CS caused greater lysosomal disruption, activating the inflammasome and the subsequent release of IL-1β and PGE2. The release of IL-1β and PGE2 suppresses the type 1 IFN response, resulting in a pro-inflammatory response.69 Additionally, lysosomal disruption caused galectin-3 (a marker for vacuole lysis) recruitment to vesicles containing CS, which in turn led to slowed autophagy flux. Overall, these findings suggest that different CS doses have varying effects on immune responses, promoting anti-inflammatory responses at low doses and a proinflammatory response at higher doses. These effects are likely due to the different levels of lysosomal disruption induced by the different doses of CS.
000 Da and a DDA of 90–95%. When administered at higher doses, CS caused greater lysosomal disruption, activating the inflammasome and the subsequent release of IL-1β and PGE2. The release of IL-1β and PGE2 suppresses the type 1 IFN response, resulting in a pro-inflammatory response.69 Additionally, lysosomal disruption caused galectin-3 (a marker for vacuole lysis) recruitment to vesicles containing CS, which in turn led to slowed autophagy flux. Overall, these findings suggest that different CS doses have varying effects on immune responses, promoting anti-inflammatory responses at low doses and a proinflammatory response at higher doses. These effects are likely due to the different levels of lysosomal disruption induced by the different doses of CS.
The cGAS–STING pathway described by Carroll et al.53 is also relevant to macrophages, where it is responsible for activating the type 1 IFN response to release CXCL10/IP-10. However, it is worth noting that when exposed to high doses of CS, M1 polarized macrophages are at a greater risk of pyknosis, a state characterized by chromatin condensation, ultimately resulting in apoptosis.68
When Oliveira and colleagues50 differentiated human monocyte-derived macrophages on CS films without exogenous cytokines, CS (DDA 88–89%, MW 324 kDa) induced phenotypic polarization of macrophages, similar to the M2 state. This was evidenced by the significant percentage of cells displaying CD206 (an M2 phenotype cell surface marker) and reduced expression of MHC-II (HLA-DR) and CD86, which are traditional M1 phenotypic markers. During the initial three days of incubation with CS, pro-inflammatory cytokines (TNF-α and IL-1β) were induced by macrophages. However, between days 3 and 10, there was a decrease in these cytokines, with a concurrent upregulation of anti-inflammatory cytokines (IL-10 and TGF-β1), compared to the control. Additionally, CS enhanced MMP9 activity in macrophages, but not in DCs, which is believed to increase the migratory behavior of macrophages but does not affect DCs. Overall, these findings highlight the dynamic cytokine profile induced by CS films on macrophages, which is characterized by a gradual shift from pro-inflammatory to anti-inflammatory cytokine expression. The selective enhancement of MMP9 activity in macrophages further suggests a role of CS in regulating macrophage migration. Proposed mechanisms of chitosan's effects on macrophage activation are summarized in (Fig. 5).
According to Wu et al.,73 the viability of RAW264.7 macrophages remained unaffected at concentrations of 2.5, 10, and 40 μg mL−1 of low-MW CS (3 kDa and 50 kDa). However, low-MW CS significantly increased the pinocytic activity of cells in a dose-dependent manner. Interestingly, 3 kDa CS at 10 and 40 μg mL−1 doses prompted stronger pinocytic activity compared to 50 kDa chitosan at the same dose, suggesting that CS induces pinocytic activity of macrophages in a MW and dose-dependent manner. Cytokine profiling showed that low-MW CS led to a dose-dependent increase in TNF-α secretion. Additionally, 3 kDa chitosan significantly increased IFN-γ and IL-6 levels, whereas 50 kDa CS did not. Furthermore, the addition of low-MW CS resulted in a concentration-dependent increase in inducible nitric oxide synthase (iNOS) secretion, indicating a potential M1 polarizing effect. In another study,55 the GlcNAc unit of the CS molecule was found to be responsible for macrophage nitric oxide (NO) secretion, as opposed to the GlcN residue. In summary, these studies show that low-MW CS has a greater stimulatory effect and pinocytic activity on macrophages, and leads to increased secretion of pro-inflammatory cytokines and iNOS, potentially promoting M1 polarization via the GlcNAc unit of CS. These results provide important insights into the potential immunomodulatory interactions between CS and macrophages. Findings from key studies on the effects of chitosan on macrophages are summarized in (Table 2).
| Chitosan | Cell type | Dose | Main outcomes | Ref. |
|---|---|---|---|---|
| DDA 76% | M1, M2 macrophage | N/A | - IL-1β secretion stimulated in all macrophage types, more in M1 | 39 |
| - NLRP3 inflammasome activation dependent on K+ efflux, lysosomal destabilization, and mitochondrial ROS | ||||
| - Pro-inflammatory M1 response stronger than M2 | ||||
| DDA 81.5%, Mw 132 kDa | M0, M1, M2a Macrophage | 5, 50 μg mL−1 | - CS-induced conditioned media increased MSC chemotaxis for M0 and M2a, but not for M1 | 66 |
| - Activation of NLRP3 inflammasome led to IL-1β, IL-18 responses | ||||
| - Higher IL-1ra compared to IL-1β; M1 produced pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) | ||||
| DDA 95%, 85% | Rodent macrophage | N/A | - DDA 95% scaffold led to M2 polarization, anti-inflammatory cytokines | 61 |
| - DDA 85% scaffold led to M1 polarization, pro-inflammatory cytokines | ||||
| - DDA in scaffolds significantly influences M1/M2 responses | ||||
| DDA 80%, Mw 10/190 kDa | Macrophage | 5–150 mg mL−1 | - Low dose (5–50 mg mL−1) led to anti-inflammatory cytokine (IL-1ra) release | 69 |
| - High dose (50–150 mg mL−1) activated inflammasome, released IL-1β | ||||
| - Higher dose triggered pro-inflammatory response due to lysosomal disruption | ||||
| DDA 88–89%, Mw 324 kDa | Human monocytes | N/A | - Initial pro-inflammatory cytokine release (TNF-α, IL-1β), switched to anti-inflammatory (IL-10, TGF-β1) | 50 |
| - CS induced M2 polarization in monocytes without external cytokines | ||||
| - Increased MMP9 activity for macrophage migration | ||||
| Mw 3, 50 kDa | RAW264.7 macrophage | 2.5, 10, 40 μg mL−1 | - CS increased pinocytosis in a dose-dependent manner | 73 |
| - 3 kDa CS induced higher pro-inflammatory cytokines (TNF-α, IFN-γ) than 50 kDa | ||||
| - GlcNAc unit responsible for nitric oxide secretion, promoting M1 polarization |
• CS stimulates NLRP3 inflammasome activation in macrophages through mechanisms like K+ efflux, lysosomal destabilization, and mitochondrial ROS, leading to IL-1β secretion.
• CS activates the STAT-1 pathway, which is involved in the regulation of pro-inflammatory cytokines such as IP-10.
• The GlcNAc component of CS is implicated in stimulating nitric oxide (NO) secretion from macrophages.
3. Conclusion
In conclusion, although chitosan has been extensively studied for its biomedical potential, there is still a lack of consensus on the nature and strength of its immune responses. CS is a family of polymers with a range of DDA and MW, which complicates the comparison of studies; the impact of these properties on the immune response remains to be fully clarified. Further research is needed to fully understand the mechanisms underlying the complex effects of CS on the immune system.It is necessary to highlight the following features of this research to date:
Firstly, it is essential to address the characterization of CS in research. Often, properties such as the DDA and MW are only partially characterized or presented as ranges, leading to potential biases and errors. To ensure the suitability of CS for biomedical studies, thorough characterization of these properties, as well as other relevant properties,74,75 should be a minimum requirement.
Secondly, there is potential for enhancing interdisciplinary coordination within CS research. Involving disciplines such as biomaterial chemical engineering, biomedical/medical engineering, immunology, vaccinology, and polymer chemistry could improve the quality and relevance of studies in medical and biomedical fields.
Thirdly, attention to the degree of purity of CS is crucial, with special attention to endotoxin content. Overlooking the potential for cross-contamination with endotoxins can lead to distorted conclusions and significant errors in the research outcomes. Recognizing and accounting for endotoxin content will help to ensure the integrity and accuracy of the findings.
Lastly, when conducting in vitro studies with CS, careful consideration should be given to factors such as the cell line used, exposure to stimuli for cell differentiation, and the phenotype of the cells. The immune response to CS can vary based on these cellular characteristics, making it essential to account for these characteristics to ensure accurate and reliable research results.
This review highlights the significance of CS dosage in biomedical applications. It is essential to carefully consider the appropriate CS dose for a given application because the efficacy, activation, and immune cell polarization of the treatment may depend on the administered dose. Therefore, proper attention to the chitosan dosage is critical for successful biomedical outcomes.
In a broader context, the use of CS in biomedical and immune-engineering applications has the potential to influence these fields. However, to realize this potential fully, it is critical to address the issues outlined above. To fully unlock the potential of CS as an effective tool for the development of novel immunostimulatory biomaterials, more research is needed to systematically understand its immunomodulatory capacity. The ability to finely tune chitosan polymers to elicit specific types of immune responses could be highly valuable but requires a deeper understanding of the underlying mechanisms involved. Therefore, continued investigation of the immunomodulatory properties of chitosan is essential for the development of innovative biomedical applications.
Abbreviations
| APCs | Antigen-presenting cells |
| ATP | Adenosine triphosphate |
| BMDCs | Bone marrow-derived dendritic cells |
| CD | Cluster of differentiation |
| cGAMP | 2′,3′-Cyclic GMP–AMP |
| cGAS | Cyclic GMP–AMP synthase |
| CS | Chitosan |
| DAMPs | Danger-associated molecular patterns |
| DCs | Dendritic cells |
| DDA | Degree of deacetylation |
| DNA | Deoxyribonucleic acid |
| GlcN | D-Glucosamine |
| GlcNAc | N-Acetyl glucosamine |
| IFN | Interferon |
| IFNAR | Interferon-alpha/beta receptor |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| MHC | Major histocompatibility complex |
| MW | Average molecular weight |
| NaOH | Sodium hydroxide |
| NF-κB | Nuclear factor-kappa B |
| NLRP3 | Nod-like receptor pyrin domain-containing |
| NMR | Nuclear magnetic resonance |
| NO | Nitric oxide |
| PAMPs | Pathogen-associated molecular patterns |
| PDI | Polydispersity index |
| PRRs | Pattern recognition receptors |
| ROS | Reactive oxygen species |
| STAT | Signal transducer and activator of transcription |
| STING | Stimulator of interferon genes |
| TBK1 | TANK-binding kinase 1 |
| TLR | Toll-like receptor |
| TNF | Tumor necrosis factor |
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review paper.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Majed Ghattas received a doctoral scholarship from the Arbour Foundation (Québec, Canada), the Natural Sciences and Engineering Research Council of Canada (NSERC) graduate scholarship, the NSERC CREATE PrEEmiuM program and the Fonds de recherche du Québec – Nature et Technologies (FRQNT). This work was supported by the NSERC Discovery grant program RGPIN-2019-07243. Figures were created with https://BioRender.com.References
- D. Fong and C. D. Hoemann, Chitosan immunomodulatory properties: perspectives on the impact of structural properties and dosage, Future Sci. OA, 2017, 4(1), FSO225 CrossRef PubMed.
- M. Rinaudo, Chitin and chitosan: Properties and applications, Prog. Polym. Sci., 2006, 31(7), 603–632 CrossRef CAS.
- M. D. Buschmann, et al., Chitosans for delivery of nucleic acids, Adv. Drug Delivery Rev., 2013, 65(9), 1234–1270 CrossRef CAS PubMed.
- N. A. Dzung, et al., Chitosan nanoparticle as a novel delivery system for A/H1n1 influenza vaccine: safe property and immunogenicity in mice, International Journal of Biotechnology and Bioengineering, 2011, 5(12), 915–922 Search PubMed.
- Y. Ghendon, et al., Evaluation of properties of chitosan as an adjuvant for inactivated influenza vaccines administered parenterally, J. Med. Virol., 2009, 81(3), 494–506 CrossRef CAS PubMed.
- K. Nishimura, et al., Adjuvant activity of chitin derivatives in mice and guinea-pigs, Vaccine, 1985, 3(5), 379–384 CrossRef CAS PubMed.
- R. Scherließ, et al., In vivo evaluation of chitosan as an adjuvant in subcutaneous vaccine formulations, Vaccine, 2013, 31(42), 4812–4819 CrossRef PubMed.
- A. Vila, et al., Low molecular weight chitosan nanoparticles as new carriers for nasal vaccine delivery in mice, Eur. J. Pharm. Biopharm., 2004, 57(1), 123–131 CrossRef CAS PubMed.
- M. Jean, et al., Chitosan–plasmid nanoparticle formulations for IM and SC delivery of recombinant FGF-2 and PDGF-BB or generation of antibodies, Gene Ther., 2009, 16(9), 1097–1110 CrossRef CAS PubMed.
- S. Ravindranathan, et al., Effect of Chitosan Properties on Immunoreactivity, Mar. Drugs, 2016, 14(5), 91 CrossRef PubMed.
- A. T. Lampe, et al., High- and low-molecular-weight chitosan act as adjuvants during single-dose influenza A virus protein vaccination through distinct mechanisms, Biotechnol. Bioeng., 2021, 118(3), 1224–1243 CrossRef CAS PubMed.
- B. Zheng, et al., Molecular weight-dependent immunostimulative activity of low molecular weight chitosan via regulating NF-κB and AP-1 signaling pathways in RAW264. 7 macrophages, Mar. Drugs, 2016, 14(9), 169 CrossRef PubMed.
- K. Kurita, T. Sannan and Y. Iwakura, Studies on chitin, 4. Evidence for formation of block and random copolymers of N-acetyl-D-glucosamine and D-glucosamine by hetero- and homogeneous hydrolyses, Macromol. Chem. Phys., 1977, 178, 3197–3202 CrossRef CAS.
- M. J. Hellmann, et al., Heterogeneously deacetylated chitosans possess an unexpected regular pattern favoring acetylation at every third position, Nat. Commun., 2024, 15(1), 6695 CrossRef CAS PubMed.
- M. Kohlhoff, et al., Chitinosanase: A fungal chitosan hydrolyzing enzyme with a new and unusually specific cleavage pattern, Carbohydr. Polym., 2017, 174, 1121–1128 CrossRef CAS PubMed.
- K. Kurita, Chitin and Chitosan: Functional Biopolymers from Marine Crustaceans, Mar. Biotechnol., 2006, 8(3), 203–226 CrossRef CAS PubMed.
- H. Sashiwa and S.-i. Aiba, Chemically modified chitin and chitosan as biomaterials, Prog. Polym. Sci., 2004, 29(9), 887–908 CrossRef CAS.
- B. Farhadihosseinabadi, et al., Crosstalk between chitosan and cell signaling pathways, Cell. Mol. Life Sci., 2019, 76(14), 2697–2718 CrossRef CAS PubMed.
- H. V. Avilez, et al., Production of chitosan coatings on metal and ceramic biomaterials, in Chitosan Based Biomaterials: Fundamentals, 2017, pp. 255–293 Search PubMed.
- S.-i. Aiba, Studies on chitosan: 4. Lysozymic hydrolysis of partially N-acetylated chitosans, Int. J. Biol. Macromol., 1992, 14(4), 225–228 CrossRef CAS PubMed.
- K.-i. Amano and E. Ito, The Action of Lysozyme on Partially Deacetylated Chitin, Eur. J. Biochem., 1978, 85(1), 97–104 CrossRef CAS PubMed.
- A. Kristiansen, K. M. Vårum and H. Grasdalen, The interactions between highly de-N-acetylated chitosans and lysozyme from chicken egg white studied by 1H-NMR spectroscopy, Eur. J. Biochem., 1998, 251(1–2), 335–342 CrossRef CAS PubMed.
- M. R. Kasaai, J. Arul and G. Charlet, Intrinsic viscosity–molecular weight relationship for chitosan, J. Polym. Sci., Part B:Polym. Phys., 2000, 38(19), 2591–2598 CrossRef CAS.
- E. D. Freitas, et al., An overview of current knowledge on the properties, synthesis and applications of quaternary chitosan derivatives, Polymers, 2020, 12(12), 2878 CrossRef CAS PubMed.
- J. Feng, et al., Synthesis, characterization and in vitro evaluation of a novel glycol chitosan-EDTA conjugate to inhibit aminopeptidase-mediated degradation of thymopoietin oligopeptides, Molecules, 2017, 22(8), 1253 CrossRef PubMed.
- Z. Zhang, et al., Mannosylated chitosan nanoparticles loaded with ABP antigen server as a novel nucleic acid vaccine against Nocardia Seriolae infection in Micropterus Salmoides, Aquaculture, 2023, 574, 739635 CrossRef CAS.
- J.-J. Kim, et al., Immunoadjuvant efficacy of N-carboxymethyl chitosan for vaccination via dendritic cell activation, J. Med. Food, 2014, 17(2), 268–277 CrossRef CAS PubMed.
- C. Xu, et al., In vivo immunological activity of chitosan-derived nanoparticles, Int. J. Biol. Macromol., 2024, 262, 130105 CrossRef CAS PubMed.
- C. Villiers, et al., From Secretome Analysis to Immunology chitosan induces major alterations in the activation of dendritic cells via a TLR-4 dependent mechanism, Mol. Cell. Proteomics, 2009, 8, 1252–1264 CrossRef CAS PubMed.
- P. Zhang, et al., Toll like receptor 4 (TLR4) mediates the stimulating activities of chitosan oligosaccharide on macrophages, Int. Immunopharmacol., 2014, 23(1), 254–261 CrossRef CAS PubMed.
- K. Fuchs, et al., The fungal ligand chitin directly binds TLR2 and triggers inflammation dependent on oligomer size, EMBO Rep., 2018, 19(12), e46065 CrossRef PubMed.
- S. Shim, et al., Induction of Th2 response through TLR2-mediated MyD88-dependent pathway in human microfold cells stimulated with chitosan nanoparticles loaded with Brucella abortus Mdh, Microb. Pathog., 2020, 142, 104040 CrossRef CAS PubMed.
- S. H. Chang, et al., Effect of chitosan molecular weight on anti-inflammatory activity in the RAW 264.7 macrophage model, Int. J. Biol. Macromol., 2019, 131, 167–175 CrossRef CAS PubMed.
- Y. Han, et al., Role of mannose receptor in oligochitosan-mediated stimulation of macrophage function, Int. Immunopharmacol., 2005, 5(10), 1533–1542 CrossRef CAS PubMed.
- T. Semenuk, et al., Synthesis of chitooligomer-based glycoconjugates and their binding to the rat natural killer cell activation receptor NKR-P1, Glycoconjugate J., 2001, 18(10), 817–826 CrossRef CAS PubMed.
- S. Gao, et al., Megalin-mediated specific uptake of chitosan/siRNA nanoparticles in mouse kidney proximal tubule epithelial cells enables AQP1 gene silencing, Theranostics, 2014, 4(10), 1039–1051 CrossRef PubMed.
- C. L. Bueter, C. A. Specht and S. M. Levitz, Innate sensing of chitin and chitosan, PLoS Pathog., 2013, 9(1), e1003080 CrossRef CAS PubMed.
- M. Huang, E. Khor and L.-Y. Lim, Uptake and Cytotoxicity of Chitosan Molecules and Nanoparticles: Effects of Molecular Weight and Degree of Deacetylation, Pharm. Res., 2004, 21(2), 344–353 CrossRef CAS PubMed.
- C. L. Bueter, et al., Spectrum and mechanisms of inflammasome activation by chitosan, J. Immunol., 2014, 192(12), 5943–5951 CrossRef CAS PubMed.
- M. Köping-Höggård, et al., Chitosan as a nonviral gene delivery system. Structure-property relationships and characteristics compared with polyethylenimine in vitro and after lung administration in vivo, Gene Ther., 2001, 8(14), 1108–1121 CrossRef PubMed.
- S. K. Wculek, et al., Dendritic cells in cancer immunology and immunotherapy, Nat. Rev. Immunol., 2020, 20(1), 7–24 CrossRef CAS PubMed.
- P. Bousso, T-cell activation by dendritic cells in the lymph node: lessons from the movies, Nat. Rev. Immunol., 2008, 8(9), 675–684 CrossRef CAS PubMed.
- J. Banchereau and R. M. Steinman, Dendritic cells and the control of immunity, Nature, 1998, 392(6673), 245–252 CrossRef CAS PubMed.
- A. Gardner and B. Ruffell, Dendritic Cells and Cancer Immunity, Trends Immunol., 2016, 37(12), 855–865 CrossRef CAS PubMed.
- E. W. Roberts, et al., Critical Role for CD103(+)/CD141(+) Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma, Cancer Cell, 2016, 30(2), 324–336 CrossRef CAS PubMed.
- K. Shimizu, M. Asakura and S.-i. Fujii, Prolonged Antitumor NK Cell Reactivity Elicited by CXCL10-Expressing Dendritic Cells Licensed by CD40L+CD4+ Memory T Cells, J. Immunol., 2011, 186(10), 5927–5937 CrossRef CAS PubMed.
- F. J. Zhu, et al., Role of dendritic cells in the host response to biomaterials and their signaling pathways, Acta Biomater., 2019, 94, 132–144 CrossRef CAS PubMed.
- M. Ghattas, et al., Vaccine Technologies and Platforms for Infectious Diseases: Current Progress, Challenges, and Opportunities, Vaccines, 2021, 9(12), 1490 CrossRef CAS PubMed.
- Y.-C. Lin, P.-J. Lou and T.-H. Young, Chitosan as an adjuvant-like substrate for dendritic cell culture to enhance antitumor effects, Biomaterials, 2014, 35(31), 8867–8875 CrossRef CAS PubMed.
- M. I. Oliveira, et al., Chitosan drives anti-inflammatory macrophage polarisation and pro-inflammatory dendritic cell stimulation, Eur. Cells Mater., 2012, 24, 136–152 CrossRef CAS PubMed.
- K. V. Swanson, M. Deng and J. P. Y. Ting, The NLRP3 inflammasome: molecular activation and regulation to therapeutics, Nat. Rev. Immunol., 2019, 19(8), 477–489 CrossRef CAS PubMed.
- A. Mori, et al., The vaccine adjuvant alum inhibits IL-12 by promoting PI3 kinase signaling while chitosan does not inhibit IL-12 and enhances Th1 and Th17 responses, Eur. J. Immunol., 2012, 42(10), 2709–2719 CrossRef CAS PubMed.
- E. C. Carroll, et al., The Vaccine Adjuvant Chitosan Promotes Cellular Immunity via DNA Sensor cGAS-STING-Dependent Induction of Type I Interferons, Immunity, 2016, 44(3), 597–608 CrossRef CAS PubMed.
- K.-P. Hopfner and V. Hornung, Molecular mechanisms and cellular functions of cGAS–STING signalling, Nat. Rev. Mol. Cell Biol., 2020, 21(9), 501–521 CrossRef CAS PubMed.
- G. Peluso, et al., Chitosan-mediated stimulation of macrophage function, Biomaterials, 1994, 15(15), 1215–1220 CrossRef CAS PubMed.
- S. N. Kulikov, et al., Effect of the molecular weight of chitosan on its antiviral activity in plants, Appl. Biochem. Microbiol., 2006, 42(2), 200–203 CrossRef CAS.
- D. Hirayama, T. Iida and H. Nakase, The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis, Int. J. Mol. Sci., 2017, 19(1), 92 CrossRef PubMed.
- T. A. Wynn, Fibrotic disease and the T(H)1/T(H)2 paradigm, Nat. Rev. Immunol., 2004, 4(8), 583–594 CrossRef CAS PubMed.
- S. J. Galli, N. Borregaard and T. A. Wynn, Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils, Nat. Immunol., 2011, 12(11), 1035–1044 CrossRef CAS PubMed.
- P. J. Murray, et al., Macrophage activation and polarization: nomenclature and experimental guidelines, Immunity, 2014, 41(1), 14–20 CrossRef CAS PubMed.
- D. P. Vasconcelos, et al., Macrophage polarization following chitosan implantation, Biomaterials, 2013, 34(38), 9952–9959 CrossRef CAS PubMed.
- Y. Liu and T. Segura, Biomaterials-Mediated Regulation of Macrophage Cell Fate, Front. Bioeng. Biotechnol., 2020, 8, 609297 CrossRef PubMed.
- D. Hachim, et al., Shifts in macrophage phenotype at the biomaterial interface via IL-4 eluting coatings are associated with improved implant integration, Biomaterials, 2017, 112, 95–107 CrossRef CAS PubMed.
- J. Li, et al., Tailoring Materials for Modulation of Macrophage Fate, Adv. Mater., 2021, 33(12), e2004172 CrossRef PubMed.
- J. M. Anderson, A. Rodriguez and D. T. Chang, Foreign body reaction to biomaterials, Semin. Immunol., 2008, 20(2), 86–100 CrossRef CAS PubMed.
- D. Fong, et al., Biodegradable chitosan microparticles induce delayed STAT-1 activation and lead to distinct cytokine responses in differentially polarized human macrophages in vitro, Acta Biomater., 2015, 12, 183–194 CrossRef CAS PubMed.
- D. P. Vasconcelos, et al., 3D chitosan scaffolds impair NLRP3 inflammasome response in macrophages, Acta Biomater., 2019, 91, 123–134 CrossRef CAS PubMed.
- D. Fong and C. D. Hoemann, Chitosan immunomodulatory properties: perspectives on the impact of structural properties and dosage, Future Sci. OA, 2018, 4(1), Fso225 CrossRef CAS PubMed.
- D. Fong, et al., Lysosomal rupture induced by structurally distinct chitosans either promotes a type 1 IFN response or activates the inflammasome in macrophages, Biomaterials, 2017, 129, 127–138 CrossRef CAS PubMed.
- J. Guzmán-Morales, et al., Biodegradable chitosan particles induce chemokine release and negligible arginase-1 activity compared to IL-4 in murine bone marrow-derived macrophages, Biochem. Biophys. Res. Commun., 2011, 405(4), 538–544 CrossRef PubMed.
- T. C. Chou, E. Fu and E. C. Shen, Chitosan inhibits prostaglandin E2 formation and cyclooxygenase-2 induction in lipopolysaccharide-treated RAW 264.7 macrophages, Biochem. Biophys. Res. Commun., 2003, 308(2), 403–407 CrossRef CAS PubMed.
- H. J. Yoon, et al., Chitosan oligosaccharide (COS) inhibits LPS-induced inflammatory effects in RAW 264.7 macrophage cells, Biochem. Biophys. Res. Commun., 2007, 358(3), 954–959 CrossRef CAS PubMed.
- N. Wu, et al., Immunostimulative Activity of Low Molecular Weight Chitosans in RAW264.7 Macrophages, Mar. Drugs, 2015, 13(10), 6210–6225 CrossRef CAS PubMed.
- P. L. Ma, et al., New Insights into Chitosan−DNA Interactions Using Isothermal Titration Microcalorimetry, Biomacromolecules, 2009, 10(6), 1490–1499 CrossRef CAS PubMed.
- A. Chevrier, et al., Injectable chitosan-platelet-rich plasma implants to promote tissue regeneration: in vitro properties, in vivo residence, degradation, cell recruitment and vascularization, J. Tissue Eng. Regener. Med., 2018, 12(1), 217–228 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |