DOI:
10.1039/D4RA08840A
(Paper)
RSC Adv., 2025,
15, 4546-4552
Ratiometric discrimination of Th4+ ions by a fluorogenic quinoline appended phenanthridine sensor and its applications†
Received
17th December 2024
, Accepted 3rd February 2025
First published on 10th February 2025
Abstract
A new phenanthridine appended quinoline-based chemoreceptor 5-(5-(quinolin-8-yl)thiophen-2-yl)-tetrahydrodibenzo[a,i]phenanthridine (PHQBA) was successfully synthesized and characterized by 1H, 13C, and HRMS spectral analyses. The promising chelation-induced process in PHQBA was accelerated by Th4+ ions, which impart robust ratiometric green fluorescence at 515 nm. The host–guest complex formed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry between Th4+ ions and PHQBA was demonstrated by Jobs plot experiments. The Benesi–Hildebrand (BH) plot was employed to compute the binding constant for the complexation of PHQBA + Th4+, which was determined to be 3.77 × 105 M−1. To comprehend the detection mechanism of PHQBA, DFT, and TDDFT, eased computational studies were conducted and well supported by the experimental results. Moreover, the limit of detection (LOD) of PHQBA was determined as 223 nM, which defines the remarkable optical sensitivity of the sensor PHQBA. Further, portable paper strip detection and real-time determination of Th4+ ions in real water samples ensure the practical applications of PHQBA.
1 binding stoichiometry between Th4+ ions and PHQBA was demonstrated by Jobs plot experiments. The Benesi–Hildebrand (BH) plot was employed to compute the binding constant for the complexation of PHQBA + Th4+, which was determined to be 3.77 × 105 M−1. To comprehend the detection mechanism of PHQBA, DFT, and TDDFT, eased computational studies were conducted and well supported by the experimental results. Moreover, the limit of detection (LOD) of PHQBA was determined as 223 nM, which defines the remarkable optical sensitivity of the sensor PHQBA. Further, portable paper strip detection and real-time determination of Th4+ ions in real water samples ensure the practical applications of PHQBA.
Introduction
Thorium is a potentially important radioactive metal in the earth's crust that is extensively used in modern industrial platforms.1 It has plenty of applications in the formulation of lantern mantles, lenses, carbon lamps, ceramics, and welding appliances.2 Since thorium exists in a +4-oxidation state, most nuclear fuel recycling is accomplished in a medium with high acidity.3,4 Undesirably, thorium ions create serious contamination in waterbodies such as seas, lakes, and rivers which immensely affects living creatures. Also, the toxicity of thorium ions can cause renal injury and dermatitis.5 According to the reports of the World Health Organisation (WHO), the permissible limit of thorium is 0.6 μg per kg per day.6 Therefore, there is a need to develop a precise method for the quantification of thorium ions. Many spectroanalytical techniques are available, but it can be challenging to identify target analytes due to their complex operating procedures. Examples of these techniques include electrothermal atomic absorption spectroscopy (ETASS),7 inductively coupled plasma emission spectroscopy (ICP-AES),8 accelerator mass spectrometry (AMS),9 laser ablation microprobe mass analyzer (LAAMA),10 atomic absorption spectroscopy (AAS)11 and neutron activation analysis (NAA).12 While comparing these approaches, fluorescent sensing is an emerging alternative to conventional methods13–18 for the detection of analytes due to their selectivity, sensitivity, rapid response, and ease of access. Detection of heavy metal ions follows various photochemical mechanisms including Forster resonance energy transfer (FRET),19 intramolecular charge transfer (ICT),20 exited state intramolecular charge transfer (ESIPT),21 and twisted intramolecular charge transfer (TICT).22 Among all these photochemical processes, chelation enhanced fluorescence process predominantly produces a well-defined spectral response that accelerates sensitive ratiometric detection of analytes.23–30 Due to the well-functionalized design31,32 of phenanthridine-based chemosensing systems, it has gained significant attention, which plays a vital role in spectroscopic properties. The advantages of these sensors include bright emission,33 large Stokes shift,34 and ease of emission wavelength modulation35,36 upon analyte interaction. These features are necessary for the rational construction of ratiometric sensors. While comparing turn-on and turn-off fluorescent probes, ratiometric chemosensors could provide precise calibration by decoding dual emission ratio signals, which could improve the sensitivity with great accuracy during the determination of target analytes.37–41 Hence, designing a ratiometric fluorescent sensor to identify Th4+ ions with greater sensitivity and selectivity is crucial. In our group, phenanthridine-derived chemosensors have recently been employed as effective tools for the selective detection of thorium ions. These sensors exhibit a turn-on fluorescence response42–44 upon interaction with thorium ions, enabling low detection limits. Ratiometric fluorescent probes are particularly advantageous as they provide signal ratios, enhancing the dynamic range and offering built-in correction for environmental effects. The observed emission color change is valuable for ratiometric fluorescence detection. To improve the optical performance of the sensor, we have rationally incorporated quinoline conjugation into the phenanthridine system. In our endeavor to develop a novel phenanthridine-derived ratiometric thorium ion sensor, PHQBA has been constructed. In the detection process, the chelation of heavy metal (Th4+) to the electron-rich PHQBA ligand, results in the formation of a new PHQBA + Th4+ complex. As a result, ratiometric fluorescence has been accomplished. Fascinatingly, the sensor PHQBA exclusively detects Th4+ ions even in the presence of other cations, thereby ensuring anti-interference properties. The detection mechanism of PHQBA is well strengthened by DFT, HRMS, and NMR titration experiments. Further, the Jobs plot and Benesi–Hildebrand (B–H) plot depict the binding stoichiometry and association constant of Th4+ ions. On account of their greater emission properties, portable fluorescent paper strips were exploited for the selective and sensitive discrimination of Th4+ ions. In addition, Th4+ ions were successfully detected in environmental water samples with excellent recovery.
Results and discussion
Design and synthesis of PHQBA
In general, phenanthridine-based fluorescent sensors possess well-defined spectral responses, which is highly advantageous for analyte detection owing to their ability to hinder the self-absorption and inner filtering effects.45–47 The designing of a thiophene-appended quinoline moiety to the phenanthridine system facilitates the chelation-enhanced fluorescence (CHEF) process with Th4+ ions. As shown in Scheme 1, the design of the chemoreceptor PHQBA includes two steps. In step one, compound 1a was successfully synthesized by the reaction of 5-bromo-2-thiophenecarboxaldehyde and 2-tetralone. Finally, quinoline moiety was introduced to the phenanthridine system by a Suzuki coupling reaction to extend the conjugation of the phenanthridine system (PHQBA) for achieving ratiometric fluorescence detection (Scheme 1). The compounds were characterized by 1H, 13C NMR, and HRMS analyses (Fig. S1–S5†).
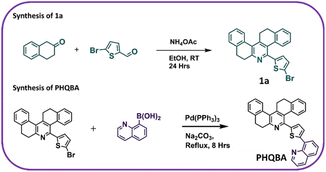 |
| | Scheme 1 Synthesis of sensor PHQBA. | |
Absorption studies of PHQBA
Optical selectivity is considered to be a crucial tool for the evaluation of a chemosensor. In this regard, the influence of absorption on the detection properties of PHQBA was initially inspected in the presence of various cation solutions such as Ca2+, Ba2+, Al3+, Cd2+, Cu2+, Th4+, Co2+, Fe2+, Fe3+, Hg2+, Ni2+, Cr3+, Pb2+, Pd2+ Zn2+ La3+, and Yb3+ in ACN/H2O (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v). As illustrated in Fig. 1a, PHQBA displayed an explicit absorption band at 278 and 380 nm, which could be accredited to π–π* and n–π* electronic transition respectively. Competitive cations have no significant impact on the absorption of PHQBA. However, the introduction of the Th4+ ion to the PHQBA solution resulted in the appearance of a new hypsochromic absorption band at 312 nm. The resulting absorption band with a hypsochromic shift48 demonstrated the active chelation of Th4+ ions with the sensor PHQBA. Further scrutiny of PHQBA's detection properties with Th4+ ions was carried out through the emission titration experiments (Fig. 1b). The gradual addition of thorium ions (0–11 equiv.) to the PHQBA solution resulted in the decreased absorption intensity at 380 nm and the subsequent enhancement aroused at 312 nm. The shifts in the absorption bands to produce isosbestic points at 290 and 342 nm revealed the formation of a new complex PHQBA + Th4+ These findings prove that the sensor PHQBA has selective and sensitive detection capability with Th4+ ions. To investigate the effect of pH on the detection properties of PHQBA, absorption experiments were performed in the presence and absence of Th4+ ions. As shown in Fig. S12,† the absorption intensity of PHQBA is nearly stable over a broad pH range of 6–12 and shows an enhanced intensity at pH 2–5, likely due to the protonation of the PHQBA. Upon the addition of Th4+ ions, the absorption intensity of the PHQBA + Th4+ complex persists stable within the pH range of 6–9 and began to decrease beyond pH 9, due to the hydrolysis of thorium ions. These results indicate that the sensor is capable of responding to Th4+ ions across a pH range of 2–12.
2 v/v). As illustrated in Fig. 1a, PHQBA displayed an explicit absorption band at 278 and 380 nm, which could be accredited to π–π* and n–π* electronic transition respectively. Competitive cations have no significant impact on the absorption of PHQBA. However, the introduction of the Th4+ ion to the PHQBA solution resulted in the appearance of a new hypsochromic absorption band at 312 nm. The resulting absorption band with a hypsochromic shift48 demonstrated the active chelation of Th4+ ions with the sensor PHQBA. Further scrutiny of PHQBA's detection properties with Th4+ ions was carried out through the emission titration experiments (Fig. 1b). The gradual addition of thorium ions (0–11 equiv.) to the PHQBA solution resulted in the decreased absorption intensity at 380 nm and the subsequent enhancement aroused at 312 nm. The shifts in the absorption bands to produce isosbestic points at 290 and 342 nm revealed the formation of a new complex PHQBA + Th4+ These findings prove that the sensor PHQBA has selective and sensitive detection capability with Th4+ ions. To investigate the effect of pH on the detection properties of PHQBA, absorption experiments were performed in the presence and absence of Th4+ ions. As shown in Fig. S12,† the absorption intensity of PHQBA is nearly stable over a broad pH range of 6–12 and shows an enhanced intensity at pH 2–5, likely due to the protonation of the PHQBA. Upon the addition of Th4+ ions, the absorption intensity of the PHQBA + Th4+ complex persists stable within the pH range of 6–9 and began to decrease beyond pH 9, due to the hydrolysis of thorium ions. These results indicate that the sensor is capable of responding to Th4+ ions across a pH range of 2–12.
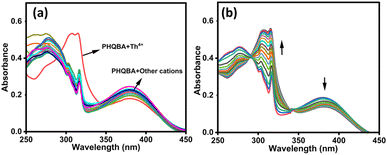 |
| | Fig. 1 (a) Absorption spectra of PHQBA (2 × 10−5 M) in the presence of competing cations (1 × 10−4 M); (b) absorption spectra of PHQBA in the presence of varying concentrations of thorium ions (0–11 equiv.). | |
Emission studies of PHQBA
Inspired by the absorption experiments, the detection ability of sensor PHQBA was examined in ACN/H2O (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) by treating with competing metal ion solutions including Ca2+, Ba2+, Al3+, Cd2+, Cu2+, Th4+, Co2+, Fe2+, Fe3+, Hg2+, Ni2+, Cr3+, Pb2+, Pd2+ Zn2+, La3+, and Yb3+. As illustrated in Fig. 2a, the free sensor PHQBA showed a maximum emission intensity of 478 nm. After the addition of Th4+ ions, the emission band at 478 nm red-shifted to 515 nm, which could be attributed to the synergic binding of Th4+ ion with the sensor PHQBA. Notably, other cations did not cause any significant spectral alterations as they led to no discernible binding interactions with the sensor PHQBA. To explore the sensitive detection properties of PHQBA with Th4+ ions, emission titration experiments were performed in ACN/H2O (8
2, v/v) by treating with competing metal ion solutions including Ca2+, Ba2+, Al3+, Cd2+, Cu2+, Th4+, Co2+, Fe2+, Fe3+, Hg2+, Ni2+, Cr3+, Pb2+, Pd2+ Zn2+, La3+, and Yb3+. As illustrated in Fig. 2a, the free sensor PHQBA showed a maximum emission intensity of 478 nm. After the addition of Th4+ ions, the emission band at 478 nm red-shifted to 515 nm, which could be attributed to the synergic binding of Th4+ ion with the sensor PHQBA. Notably, other cations did not cause any significant spectral alterations as they led to no discernible binding interactions with the sensor PHQBA. To explore the sensitive detection properties of PHQBA with Th4+ ions, emission titration experiments were performed in ACN/H2O (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) medium. Thereby, the gradual addition of Th4+ ions (0–11 equiv.) to the PHQBA solution resulted in diminished emission intensity at 478 nm, and the successive emission enhancement was attained at 515 nm (Fig. 2b). The emergence of a clear iso-emissive point at 496 nm indicated the formation of a new complex PHQBA + Th4+. As a result, the emission color of PHQBA transformed from blue to green (Fig. 2c). The calculated quantum yield values of PHQBA and its complex PHQBA + Th4+ were found to be 0.12 and 0.14 respectively. The slight enhancement in the quantum yield corresponds to the redshifted ratiometric fluorescence of PHQBA on chelating with Th4+ ions. These findings provided further evidence that sensor PHQBA had strong fluorescence detection abilities towards Th4+ ions. To clearly distinguish the emission of PHQBA and its complex PHQBA + Th4+, the chromaticity plot (CIE) was plotted49 using the emission spectra. As demonstrated in Fig. S8,† the color coordinates of the free probe (PHQBA) were found to be in a blue region with the coordinates of X = 0.145, Y = 0.258, further, the introduction of Th4+ the color coordinates of PHQBA were shifted towards the green region with the coordinates of X = 0.215, Y = 0.499. These results denote that PHQBA acts as a versatile sensor for the ratiometric detection of Th4+ ions. The limit of detection is an essential parameter for evaluating the performance of the chemosensor. The limit of detection was determined by the standard formula 3σ/slope50 where σ is the standard deviation of blank measurements of PHQBA (n = 10). The corresponding slope value was obtained from the concentration-dependent linear plot (Fig. S6†). The limit of detection was calculated to be 223 nM, which is comparatively lower than the previously reported thorium sensors (Table S1†). Thus, sensor PHQBA can be used as a versatile sensitive tool for the quantitative ratiometric determination of Th4+ ions with outstanding sensitivity. Interestingly, the present work relies on evaluating the ratio of fluorescence intensities at two distinct wavelengths (515 and 478 nm). This self-calibration eliminates variations caused by external factors like light source intensity, detector sensitivity, and sample concentration, making the results more robust and reliable.
2, v/v) medium. Thereby, the gradual addition of Th4+ ions (0–11 equiv.) to the PHQBA solution resulted in diminished emission intensity at 478 nm, and the successive emission enhancement was attained at 515 nm (Fig. 2b). The emergence of a clear iso-emissive point at 496 nm indicated the formation of a new complex PHQBA + Th4+. As a result, the emission color of PHQBA transformed from blue to green (Fig. 2c). The calculated quantum yield values of PHQBA and its complex PHQBA + Th4+ were found to be 0.12 and 0.14 respectively. The slight enhancement in the quantum yield corresponds to the redshifted ratiometric fluorescence of PHQBA on chelating with Th4+ ions. These findings provided further evidence that sensor PHQBA had strong fluorescence detection abilities towards Th4+ ions. To clearly distinguish the emission of PHQBA and its complex PHQBA + Th4+, the chromaticity plot (CIE) was plotted49 using the emission spectra. As demonstrated in Fig. S8,† the color coordinates of the free probe (PHQBA) were found to be in a blue region with the coordinates of X = 0.145, Y = 0.258, further, the introduction of Th4+ the color coordinates of PHQBA were shifted towards the green region with the coordinates of X = 0.215, Y = 0.499. These results denote that PHQBA acts as a versatile sensor for the ratiometric detection of Th4+ ions. The limit of detection is an essential parameter for evaluating the performance of the chemosensor. The limit of detection was determined by the standard formula 3σ/slope50 where σ is the standard deviation of blank measurements of PHQBA (n = 10). The corresponding slope value was obtained from the concentration-dependent linear plot (Fig. S6†). The limit of detection was calculated to be 223 nM, which is comparatively lower than the previously reported thorium sensors (Table S1†). Thus, sensor PHQBA can be used as a versatile sensitive tool for the quantitative ratiometric determination of Th4+ ions with outstanding sensitivity. Interestingly, the present work relies on evaluating the ratio of fluorescence intensities at two distinct wavelengths (515 and 478 nm). This self-calibration eliminates variations caused by external factors like light source intensity, detector sensitivity, and sample concentration, making the results more robust and reliable.
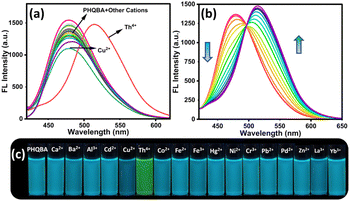 |
| | Fig. 2 (a) Fluorescence spectra of PHQBA (2 × 10−5 M) in the presence of various cations (1 × 10−4 M); (b) fluorescence spectra of PHQBA with the gradual addition of Th4+ (0–11 equiv.); (c) emission color changes of PHQBA in the presence of series of cations (λex = 389 nm). | |
Interference, reaction kinetics, and pH effect of PHQBA on detecting Th4+
To explore the anti-interference capabilities of PHQBA (2 × 10−5), interference studies were fluorometrically carried out based on the emission ratio of F515/F478 nm. The effect of interference was investigated in the presence of various competing cations (1 × 10−4) such as Ca2+, Ba2+, Al3+, Cd2+, Cu2+, Th4+, Co2+, Fe2+, Fe3+, Hg2+, Ni2+, Cr3+, Pb2+, Pd2+, Zn2+, La3+, and Yb3+. No significant interference was observed. Further, the addition of Th4+ ion to the PHQBA containing other analyte solutions exhibited enhanced emission intensity in the emission ratio of F515/F478 nm, which designates that the sensor PHQBA's sensing capability was sufficient to distinguish Th4+ ions from other competing cations and supports the potential identification of Th4+ ions coexistence with other cations (Fig. 3a). Further, to explore the influence of pH on the detection properties of PHQBA, pH experiments were conducted in the range of 2–12. According to Fig. S11,† the emission of PHQBA is consistent across a broad pH range of 6–12 and is observed to be enhanced in an acidic medium spanning from 2–5. The protonation of the N atoms in quinoline and pyridine within the PHQBA system may cause increased emission intensity in the acidic medium. On the other hand, thorium(IV) nitrate has the potential to undergo hydrolysis and breakdown when subjected to higher alkaline conditions (11–12). When Th4+ ions were added to the PHQBA solution, the emission intensity began to increase in the pH range of 6–10 and decreased at pH 11–12. Therefore, the ideal pH range for Th4+ ion detection ranges between 6 and 10. The pH-responsive characteristic of the PHQBA sensor makes it perfect for detecting Th4+ under optimal physiological conditions. Rapid detection of analytes is one of the remarkable characteristics of an ideal chemosensor.51 For this, time-dependent emission studies were explored. According to Fig. 3b, the free sensor PHQBA showed feeble emission in the ratio of F515/F478 nm. Further, on the addition of Th4+ ion to the PHQBA solution, the emission intensity significantly elevated within five seconds. Hence, the sensor PHQBA was employed as an inevitable tool for the rapid detection of Th4+ ions.
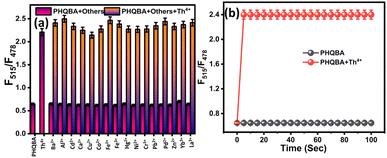 |
| | Fig. 3 (a) Interference of PHQBA (2 × 10−5 M) in the presence of other cations (1 × 10−4 M) and coexisting with Th4+; (b) time-dependent emission response of PHQBA with Th4+ (λex = 389 nm). | |
Inspection of the detection mechanism
The sensor PHQBA encompasses a phenanthridine fluorophore, which is extensively conjugated with thiophene and quinoline moiety. Thus, it produces a blue emissive band. Since the Th4+ is electron deficient, it will have a binding interaction with electron-rich heteroatoms [N, N, S] in the PHQBA system. By the effect of synergic chelation, sensor PHQBA generates distinctive hypsochromic absorption and ratiometric green emission signals with Th4+ ions. Moreover, the binding stoichiometry of PHQBA with Th4+ ions was successively assessed by jobs plot experiments. As depicted in Fig. 4a, the maximum fluorescence intensity was attained when the molar fraction reached 0.5, revealing that the most likely binding mode was a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio for the PHQBA + Th4+ complex. To authenticate the stoichiometric binding of the sensor PHQBA with Th4+ ions, the Benesi–Hildebrand plot was linearly fitted between [1/(F0 − F)] and 1/[Th4+]. The association constant of the formed complex was calculated using the formula: Ka = intercept/slope.52 The Ka value for PHQBA was determined as 3.77 × 105 M−1. Such a significant value denotes the robust binding of sensor PHQBA with Th4+ ions (Fig. 4b).53 To further understand the coordination mode of PHQBA with Th4+, a series of 1H NMR titration experiments were conducted in DMSO-d6. As shown in Fig. 4c, the base spectrum represents the free sensor. Upon the sequential addition of Th4+ ions (0–11 equivalents), significant changes were observed in the pyridyl proton of PHQBA (a) at 9.59 ppm, including a marked decrease in intensity. Furthermore, the incremental addition of Th4+ ions induced slight upfield shifts at 8.97 ppm (b), 7.06 ppm (d), and 6.97 ppm (e). A minor upfield shift was also noted for the c proton, which shifted from 8.29 ppm to 8.28 ppm. Notably, the aliphatic protons of PHQBA (g, h, and f) were significantly impacted, with their intensities progressively decreasing as the Th4+ concentration increased. These observations collectively indicate the effective chelation of Th4+ ions by PHQBA. Additionally, the mass of the complex was confirmed by high-resolution mass spectroscopy. According to the mass spectral data, the observed mass of the complex PHQBA + Th(NO3)4 was 973.1636, which matched its calculated mass (973.1633) (Fig. S7†). To confirm nitrate binding, we have recorded FTIR spectra. As depicted in Fig. S13,† the observed stretching frequency at 1025 cm−1 confirms the presence of NO3− in the PHQBA + Th4+ system.54
1 stoichiometric ratio for the PHQBA + Th4+ complex. To authenticate the stoichiometric binding of the sensor PHQBA with Th4+ ions, the Benesi–Hildebrand plot was linearly fitted between [1/(F0 − F)] and 1/[Th4+]. The association constant of the formed complex was calculated using the formula: Ka = intercept/slope.52 The Ka value for PHQBA was determined as 3.77 × 105 M−1. Such a significant value denotes the robust binding of sensor PHQBA with Th4+ ions (Fig. 4b).53 To further understand the coordination mode of PHQBA with Th4+, a series of 1H NMR titration experiments were conducted in DMSO-d6. As shown in Fig. 4c, the base spectrum represents the free sensor. Upon the sequential addition of Th4+ ions (0–11 equivalents), significant changes were observed in the pyridyl proton of PHQBA (a) at 9.59 ppm, including a marked decrease in intensity. Furthermore, the incremental addition of Th4+ ions induced slight upfield shifts at 8.97 ppm (b), 7.06 ppm (d), and 6.97 ppm (e). A minor upfield shift was also noted for the c proton, which shifted from 8.29 ppm to 8.28 ppm. Notably, the aliphatic protons of PHQBA (g, h, and f) were significantly impacted, with their intensities progressively decreasing as the Th4+ concentration increased. These observations collectively indicate the effective chelation of Th4+ ions by PHQBA. Additionally, the mass of the complex was confirmed by high-resolution mass spectroscopy. According to the mass spectral data, the observed mass of the complex PHQBA + Th(NO3)4 was 973.1636, which matched its calculated mass (973.1633) (Fig. S7†). To confirm nitrate binding, we have recorded FTIR spectra. As depicted in Fig. S13,† the observed stretching frequency at 1025 cm−1 confirms the presence of NO3− in the PHQBA + Th4+ system.54
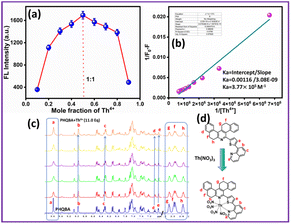 |
| | Fig. 4 (a) Jobs plot of PHQBA + Th4+; (b) Benesi–Hildebrand plot of PHQBA + Th4+; (c) series of 1H NMR titration spectra of PHQBA with Th4+ ions (0–11 equiv.). | |
DFT calculations
To gather clear insights into the theoretical mechanistic aspects of Th4+ binding, DFT calculations were performed by employing B3LYP/6-31G*(d,p) levels in Gaussian 16 software.55–58 For the thorium atom, the SDD basis set was employed59 as an effective core potential. The optimized electronic structures of PHQBA and its complex PHQBA + Th4+ are depicted in Fig. S10.† In the PHQBA + Th4+ complex, the receptor PHQBA utilizes N atoms of pyridine, quinoline, and sulfur of thiophene to enable efficient chelation with Th4+. The oscillatory strength, Z-matrix, calculated energy gap, and frontier molecular orbitals of PHQBA and its complex PHQBA + Th4+ are tabulated in Tables S2–S4.† The frontier molecular orbital images of PHQBA and its complex PHQBA + Th4+ are illustrated in Fig. 5. In the HOMO (highest occupied molecular orbitals) of PHQBA, the electron density predominately resided throughout the system, which denotes the higher electron density of the PHQBA ligand. In LUMO (lowest unoccupied molecular orbitals) the electron density was located at the phenanthridine skeleton, implying the adequate charge transfer effect in the PHQBA system. The calculated energy gap between the HOMO and LUMO orbitals of PHQBA was 3.3753 eV. Moreover, in the HOMO of the PHQBA + Th4+ complex, the phenanthridine thiophene moiety had the enriched electron density, whereas, in the LUMO orbitals, the electron density was predominately found in the quinoline moiety. Further, the calculated energy gap between the HOMO and LUMO of the PHQBA + Th4+ complex was found to be 3.723 eV, suggesting that the chelation of the Th4+ ion effectively enhances the energy gap of the Th4+ complex. The introduction of Th4+ ions into the detection medium has the potential to alter the local vicinity of PHQBA molecules. Such alterations can exert a significant influence on the electronic structure and optical characteristics of the material. Perturbations in the environmental conditions can lead to shifts in the energy levels associated with electronic transitions, thereby inducing a red shift in emission.60,61 Concurrently, the overall band gap of the PHQBA + Th4+ complex may increase as a consequence of the perturbed electronic structure induced by the surrounding medium. Based on this ratiometric fluorescence sensing mechanism, the frontier molecular orbitals are mainly distributed at quinoline moiety in the Th4+ complex, while the excited electron of the nitrogen unit is back to the ground state resulting in ratiometric fluorescence. These findings denote that sensor PHQBA could synergically coordinate with the Th4+ atom to form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complex with the sensor PHQBA.
1 stoichiometric complex with the sensor PHQBA.
 |
| | Fig. 5 The frontier molecular orbitals of PHQBA and PHQBA + Th4+ complex. | |
Portable test strips aided detection
Portable test strip facilitated detection is a facile and intellectual approach for the visual discrimination of target analytes.62 In our endeavor to assess the practical value of PHQBA, portable test strip experiments have been conducted in our laboratory. The selectivity of PHQBA (2 × 10−5) in the presence of various ion solutions (1 × 10−4) such as Ca2+, Ba2+, Al3+, Cd2+, Cu2+, Co2+, Fe2+, Fe3+, Hg2+, Ni2+, Cr3+, Pb2+, Pd2+, Zn2+, La3+, and Yb3+ were tested appropriately. When seen under the illumination of UV-365 nm, PHQBA showed a remarkable selectivity to Th4+ ions over other cations. Only after the exposure of Th4+ ions, the blue emission of PHQBA altered to green, as shown in Fig. 6. However, other cation solutions did not show appreciable emission changes in the PHQBA-impregnated strips. Hence, it can be used as a portable sensor for the ratiometric discrimination of Th4+ ions.
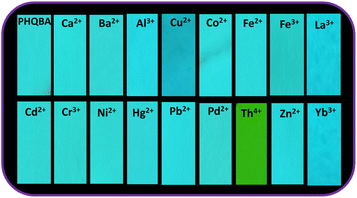 |
| | Fig. 6 PHQBA (2 × 10−5) impregnated test strips for Th4+ ion (1 × 10−4) detection. | |
Reproducible nature of PHQBA
Reversibility and reusability are characteristic features of a noble chemosensor. In this regard, the reproducible properties of PHQBA were explored in ACN/H2O (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). Sensor PHQBA demonstrated an intensive emission band at 478 nm, which produced a characteristic blue fluorescence. Then, the addition of Th4+ ions to the PHQBA solution resulted in ratiometric green fluorescence. Since ethylenediaminetetraacetic acid (EDTA) is a potential chelating agent,63 Th4+ ion-facilitated ratiometric fluorescence was substantially reversed by the subsequent additions of EDTA. As depicted in Fig. 7, the emission changes were incredibly imitated in fluorescence spectra, in which the introduction of EDTA solution in the PHQBA + Th4+ complex, resulted in the regeneration of the probe's emission at 478 nm. These outcomes imply that the Th4+ ion has been significantly eradicated from the sensor and that it finely achieves the reversibility process induced by the efficient interaction of EDTA with the PHQBA + Th4+ complex. These reproducible and reversible processes (blue to green fluorescence) were repeated for 4 cycles (Fig. S9†) with successive alternative additions of Th4+ and EDTA. The outcomes revealed that the sensor PHQBA can be reused and also sensitively employed for the detection of Th4+ ions.
2, v/v). Sensor PHQBA demonstrated an intensive emission band at 478 nm, which produced a characteristic blue fluorescence. Then, the addition of Th4+ ions to the PHQBA solution resulted in ratiometric green fluorescence. Since ethylenediaminetetraacetic acid (EDTA) is a potential chelating agent,63 Th4+ ion-facilitated ratiometric fluorescence was substantially reversed by the subsequent additions of EDTA. As depicted in Fig. 7, the emission changes were incredibly imitated in fluorescence spectra, in which the introduction of EDTA solution in the PHQBA + Th4+ complex, resulted in the regeneration of the probe's emission at 478 nm. These outcomes imply that the Th4+ ion has been significantly eradicated from the sensor and that it finely achieves the reversibility process induced by the efficient interaction of EDTA with the PHQBA + Th4+ complex. These reproducible and reversible processes (blue to green fluorescence) were repeated for 4 cycles (Fig. S9†) with successive alternative additions of Th4+ and EDTA. The outcomes revealed that the sensor PHQBA can be reused and also sensitively employed for the detection of Th4+ ions.
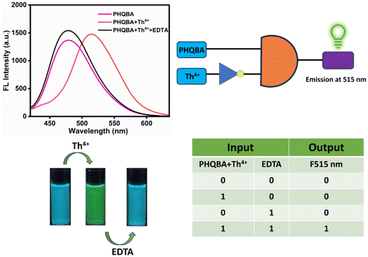 |
| | Fig. 7 Reversibility studies of PHQBA in the presence of Th4+ with EDTA and their logic gates representation. | |
Real-time applications
The versatile sensing properties of PHQBA inspired us to explore its practical efficacy in various environmental water samples.64 The known concentration of Th4+ ion solution was spiked with realistic water samples (drinking water, lake water, and tap water collected from Vellore). Then the experiment was repeated three times with each sample. The average recoveries and relative standard deviations (R. S. D.) of the corresponding spiked samples are tabulated in Table 1. As represented in Table 1, the average recoveries of various water samples ranged from 90.1 to 98.5%, and the corresponding relative standard deviation values were found to be 0.97 to 1.19%.
Table 1 Tabulation of real water sample analysisa
| Sample |
Added (μM) |
Found (μM) |
Recovery (%) |
RSD (%) |
| Samples were collected in and around Vellore. |
| Lake water |
0.5 |
0.46 |
92.2 |
1.01 |
| 1.0 |
0.90 |
90.1 |
0.97 |
| Tap water |
0.5 |
0.47 |
94.3 |
0.99 |
| 1.0 |
0.96 |
96.5 |
1.19 |
| Drinking water |
0.5 |
0.49 |
98.5 |
1.09 |
| 1.0 |
0.97 |
97.6 |
1.11 |
Based on these analytical findings, sensor PHQBA was effectively applied to determine Th4+ ion in real water samples with reliability and accuracy. Hence, the sensor PHQBA emerged as a valuable candidate for the real-time analysis of Th4+ ions in real water samples.
Conclusion
A new phenanthridine-derived quinoline-based fluorescent sensor was successfully developed for the ratiometric discrimination of Th4+ ions. On interaction with Th4+ ions, the emission of PHQBA ratiometrically changed from blue to green with the facilitation of chelation enhanced fluorescence process. The versatile sensing properties of PHQBA endorse hypsochromic absorption, and the redshifted emission bands during the interaction with Th4+ were thoroughly investigated by absorption and emission experiments. The binding interaction of Th4+ ions with PHQBA was explored by 1H NMR, HRMS, and DFT analysis. The portable test strips confirmed that the sensor PHQBA can selectively detect Th4+ ions without the aid of any sophisticated instrumental facility. Also, the sensor PHQBA was analyzed for the real-time detection of Th4+ ions in real water samples. The versatile features of PHQBA such as reversibility/reusability, selectivity, and practical utility will accelerate the advancement of fluorescent sensors in adaptable analytical applications. Developing ultrasensitive chemosensors for the detection of radioactive ions is a critical research area with extensive implications in environmental monitoring, nuclear safety, and non-proliferation initiatives. Systematic monitoring of thorium ions is particularly essential to ensure compliance with nuclear regulations and to safeguard public health and safety. Advanced tools capable of detecting ultra-low concentrations of thorium in soil, water, and air are crucial for identifying contamination and assessing its environmental impact. In our laboratory, ongoing research focuses on innovative structural modifications to develop highly sensitive detection methods capable of identifying radioactive elements at extremely low concentrations. These advanced analytical techniques aim to significantly enhance the accuracy, sensitivity, and efficiency of analyte detection, even in complex and challenging environments, thereby paving the way for more effective and reliable monitoring solutions in the future.
Data availability
The data supporting this article have been included as part of the ESI.†
Conflicts of interest
The authors declare no financial interests.
Acknowledgements
Selin Manoj Kumar sincerely thanks Vellore Institute of Technology for providing a Teaching Cum Research Assistant (Project No.: VIT/HR/2022/19062). The DST-FIST NMR facility at VIT University is duly acknowledged. Finally, the authors thank Dr R. Srinivasan, SSL-VIT, for language editing.
References
- M. Cuney, Non-Renewable Resource Issues: Geoscientific and Societal Challenges, 2012, pp. 91–129 Search PubMed.
- M. Corn, Handbook of Hazardous Materials, Academic Press, 2012 Search PubMed.
- C. S. K. Raju and M. Subramanian, J. Hazard. Mater., 2007, 145, 315–322 Search PubMed.
- S. Yang, N. Tan, X. Yan, F. Chen, W. Long and Y. Lin, Mar. Pollut. Bull., 2013, 74, 213–219 CrossRef CAS PubMed.
- S. Yang, N. Tan, X. Yan, F. Chen, W. Long and Y. Lin, Mar. Pollut. Bull., 2013, 74, 213–219 Search PubMed.
- D. Brugge, J. L. deLemos and B. Oldmixon, Rev. Environ. Health, 2005, 20, 177–194 CAS.
- E. Zambrzycka-Szelewa and B. Godlewska-Zylkiewicz, Spectrochim. Acta, Part B, 2024, 213, 106859 CrossRef CAS.
- M. Ioannidou, G. Zachariadis, A. Anthemidis and J. Stratis, Talanta, 2005, 65, 92–97 CAS.
- A. M. Clark, A. D. Nelson, T. L. Bailey, D. Blankstein, C. Boomershine, G. M. Brown, P. C. Burns, S. Carmichael, L. K. Callahan and J. Koros, Nucl. Instrum. Methods Phys. Res., B, 2024, 548, 165253 CrossRef CAS.
- X. Qiu, Z. Hu, T. He, T. Luo, W. Zhang, M. Li, K. Zong, Z. Wang and Y. Liu, J. Anal. At. Spectrom., 2024, 39, 545–557 RSC.
- J. Sardans, F. Montes and J. Peñuelas, Spectrochim. Acta, Part B, 2010, 65, 97–112 CrossRef.
- M. Ghosh, M. Sarma, S. Dagupta, J. Datta and K. Swain, Anal. Chem. Lett., 2024, 14, 382–394 CrossRef CAS.
- K. P. Carter, A. M. Young and A. E. Palmer, Chem. Rev., 2014, 114, 4564–4601 CrossRef CAS PubMed.
- A. P. De Silva, H. N. Gunaratne, T. Gunnlaugsson, A. J. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515–1566 CrossRef CAS PubMed.
- M. Dutta and D. Das, TrAC, Trends Anal. Chem., 2012, 32, 113–132 CrossRef CAS.
- T. Rasheed, M. Bilal, F. Nabeel, H. M. Iqbal, C. Li and Y. Zhou, Sci. Total Environ., 2018, 615, 476–485 CrossRef CAS PubMed.
- D. Jothi, S. Munusamy, S. Enbanathan and S. K. Iyer, RSC Adv., 2022, 12, 8570–8577 RSC.
- S. M. Kumar, D. Jothi, S. Munusamy, S. Enbanathan and S. K. Iyer, J. Photochem. Photobiol. Chem., 2023, 434, 114269 CrossRef.
- Z. Aydin, J. Turk. Chem. Soc., Sect. A, 2020, 7, 277–286 Search PubMed.
- X. M. Wang, H. Yan, Y. Chen and H. B. Bao, Adv. Mater. Res., 2011, 239, 1105–1108 Search PubMed.
- D. Mohanasundaram, R. Bhaskar, G. G. V. Kumar, J. Rajesh and G. Rajagopal, Microchem. J., 2021, 164, 106030 CrossRef CAS.
- H.-I. Un, C.-B. Huang, C. Huang, T. Jia, X.-L. Zhao, C.-H. Wang, L. Xu and H.-B. Yang, Org. Chem. Front., 2014, 1, 1083–1090 RSC.
- L. Gao, C. Deng, J. Xiong, P. Zhu, Q. Chen and K. Tan, Microchem. J., 2019, 150, 104096 CrossRef CAS.
- Z. Zhang, J. Feng, P. Huang, S. Li and F.-Y. Wu, Sens. Actuators, B, 2019, 298, 126891 Search PubMed.
- G. Lu, Z. Jia, M. Yu, M. Zhang and C. Xu, Molecules, 2023, 28, 7818 CrossRef CAS PubMed.
- M. Royzen, Z. Dai and J. W. Canary, J. Am. Chem. Soc., 2005, 127, 1612–1613 CrossRef CAS PubMed.
- H. Wang, H. Xing, W. Liu, Y. Hao, L. Zhang, Z. Yang, Q. Hu, S. Shuang, C. Dong and X. Gong, Sens. Actuators, B, 2022, 352, 130991 CrossRef CAS.
- N. Tohora, S. Ahamed, M. Mahato, J. Chourasia, S. Ali and S. K. Das, J. Photochem. Photobiol. Chem., 2024, 457, 115921 CrossRef CAS.
- Y. Li, L. Li, X. Pu, G. Ma, E. Wang, J. Kong, Z. Liu and Y. Liu, Bioorg. Med. Chem. Lett., 2012, 22, 4014–4017 CrossRef CAS PubMed.
- M. Gao, P. Xie, L. Wang, X. Miao and F. Guo, Res. Chem. Intermed., 2015, 41, 9673–9685 CrossRef CAS.
- Y. Chen, F. Li and Z. Bo, Macromolecules, 2010, 43, 1349–1355 CrossRef CAS.
- S. Yashmin, S. Mondal, R. Das, P. Banerjee and A. T. Khan, Org. Biomol. Chem., 2022, 20, 7302–7315 RSC.
- D. Wu, B. Fang, M. Zhang, W. Du, J. Zhang, X. Tian, Q. Zhang, H. Zhou, J. Wu and Y. Tian, Dyes Pigm., 2018, 159, 142–150 CrossRef CAS.
- S. Karthik, J. Ajantha, S. Easwaramoorthi and T. Gandhi, New J. Chem., 2020, 44, 9530–9539 RSC.
- R. M. Gadirov, L. G. Samsonova, K. M. Degtyarenko, A. E. Kurtsevich, I. K. Yakushchenko and T. N. Kopylova, J. Fluoresc., 2021, 31, 1333–1342 CrossRef CAS PubMed.
- S. Swaminathan, S. Munusamy, D. Jothi and S. K. Iyer, ChemistrySelect, 2021, 6, 858–864 CrossRef.
- L. Liu, L. Ga and J. Ai, Biosens. Bioelectron., 2022, 213, 114456 CrossRef CAS PubMed.
- S. M. Kumar, S. Munusamy, S. Manickam, D. Jothi, S. Enbanathan and S. K. Iyer, J. Mol. Liq., 2023, 381, 121828 CrossRef CAS.
- X. Pei, Y. Pan, L. Zhang and Y. Lv, Appl. Spectrosc. Rev., 2021, 56, 324–345 CrossRef CAS.
- S. Manoj Kumar and S. Kulathu Iyer, J. Org. Chem., 2024, 89, 5392–5400 CrossRef CAS PubMed.
- S. Enbanathan, S. Munusamy, D. Jothi, S. Manojkumar, S. Manickam and S. K. Iyer, RSC Adv., 2022, 12, 27839–27845 RSC.
- S. Seenan and K. I. Sathiyanarayanan, Inorg. Chem. Commun., 2021, 132, 108825 CrossRef CAS.
- S. Sawminathan and S. K. Iyer, Spectrochim. Acta, Part A, 2022, 265, 120403 CrossRef CAS PubMed.
- D. Jothi, S. Manickam, S. Sawminathan, S. Munusamy, S. A. Kumar and S. K. Iyer, Dyes Pigm., 2022, 197, 109826 CrossRef CAS.
- W. Li, Y. Fu, T. Liu, H. Li and M. Huang, Spectrochim. Acta, Part A, 2023, 288, 122147 CrossRef CAS.
- N. N. Zhadin and R. R. Alfano, J. Biomed. Opt., 1998, 3, 171–186 CrossRef CAS.
- S. Srinivas and R. Mutharasan, Biotechnol. Bioeng., 1987, 30, 769–774 CrossRef CAS PubMed.
- A. Elabd, RSC Adv., 2016, 6, 45525–45532 RSC.
- R. Yao, Z. Li, G. Liu, C. Fan and S. Pu, Talanta, 2021, 234, 122612 CrossRef CAS PubMed.
- S. M. Kumar, D. Jothi, S. Munusamy, S. Enbanathan and S. K. Iyer, J. Photochem. Photobiol. Chem., 2023, 434, 114269 CrossRef.
- N. Duan, S. Yang, H. Tian and B. Sun, Food Chem., 2021, 358, 129839 CrossRef CAS PubMed.
- S. M. Kumar, S. Munusamy, S. Manickam, D. Jothi, S. Enbanathan and S. K. Iyer, J. Mol. Liq., 2023, 381, 121828 CrossRef CAS.
- R. Wang and Z. Yu, Acta Phys.-Chim. Sin., 2007, 23, 1353–1359 CrossRef CAS.
- A. Knyazev, G. Fukin, R. Rumyantcev, M. Komshina, I. Savushkin and A. Paranyuk, Polyhedron, 2016, 117, 600–603 CrossRef CAS.
- B. Pavankumar, P. Ranjan, R. R. Panicker, P. C. Jha, C. Brahmananda Rao, R. Desikan and A. Sivaramakrishna, ChemistrySelect, 2024, 9, e202304509 CrossRef CAS.
- J. E. Del Bene, W. B. Person and K. Szczepaniak, J. Phys. Chem., 1995, 99, 10705–10707 CrossRef CAS.
- H. Kruse, L. Goerigk and S. Grimme, J. Org. Chem., 2012, 77, 10824–10834 CrossRef CAS PubMed.
- M. P. Andersson and P. Uvdal, J. Phys. Chem., 2005, 109, 2937–2941 CrossRef CAS PubMed.
- R. Sk, S. A. Kumar, K. Vijayakrishna, A. Sivaramakrishna, C. V. S. B. Rao, N. Sivaraman and S. K. Sahoo, Inorg. Chem., 2018, 57, 15270–15279 CrossRef PubMed.
- S. Seenan, S. Manickam, S. Sawminathan, D. Jothi and S. K. Iyer, J. Photochem. Photobiol. Chem., 2022, 430, 113952 CrossRef CAS.
- S. Sawminathan and S. K. Iyer, New J. Chem., 2021, 45, 6033–6041 RSC.
- Y. Shen, Y. Wei, C. Zhu, J. Cao and D.-M. Han, Coord. Chem. Rev., 2022, 458, 214442 CrossRef CAS.
- S. M. Kumar, S. Munusamy, D. Jothi, S. Enbanathan and S. K. I. Iyer, J. Mol. Liq., 2023, 373, 121235 CrossRef CAS.
- S. M. Kumar, S. Munusamy, D. Jothi, S. Enbanathan, J. Haribabu and S. K. Iyer, Opt. Mater., 2023, 144, 114382 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article a,
Sivaranjani Ranganadina and
Sathiyanarayanan Kulathu Iyer
a,
Sivaranjani Ranganadina and
Sathiyanarayanan Kulathu Iyer *a
*a
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry between Th4+ ions and PHQBA was demonstrated by Jobs plot experiments. The Benesi–Hildebrand (BH) plot was employed to compute the binding constant for the complexation of PHQBA + Th4+, which was determined to be 3.77 × 105 M−1. To comprehend the detection mechanism of PHQBA, DFT, and TDDFT, eased computational studies were conducted and well supported by the experimental results. Moreover, the limit of detection (LOD) of PHQBA was determined as 223 nM, which defines the remarkable optical sensitivity of the sensor PHQBA. Further, portable paper strip detection and real-time determination of Th4+ ions in real water samples ensure the practical applications of PHQBA.
1 binding stoichiometry between Th4+ ions and PHQBA was demonstrated by Jobs plot experiments. The Benesi–Hildebrand (BH) plot was employed to compute the binding constant for the complexation of PHQBA + Th4+, which was determined to be 3.77 × 105 M−1. To comprehend the detection mechanism of PHQBA, DFT, and TDDFT, eased computational studies were conducted and well supported by the experimental results. Moreover, the limit of detection (LOD) of PHQBA was determined as 223 nM, which defines the remarkable optical sensitivity of the sensor PHQBA. Further, portable paper strip detection and real-time determination of Th4+ ions in real water samples ensure the practical applications of PHQBA.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v). As illustrated in Fig. 1a, PHQBA displayed an explicit absorption band at 278 and 380 nm, which could be accredited to π–π* and n–π* electronic transition respectively. Competitive cations have no significant impact on the absorption of PHQBA. However, the introduction of the Th4+ ion to the PHQBA solution resulted in the appearance of a new hypsochromic absorption band at 312 nm. The resulting absorption band with a hypsochromic shift48 demonstrated the active chelation of Th4+ ions with the sensor PHQBA. Further scrutiny of PHQBA's detection properties with Th4+ ions was carried out through the emission titration experiments (Fig. 1b). The gradual addition of thorium ions (0–11 equiv.) to the PHQBA solution resulted in the decreased absorption intensity at 380 nm and the subsequent enhancement aroused at 312 nm. The shifts in the absorption bands to produce isosbestic points at 290 and 342 nm revealed the formation of a new complex PHQBA + Th4+ These findings prove that the sensor PHQBA has selective and sensitive detection capability with Th4+ ions. To investigate the effect of pH on the detection properties of PHQBA, absorption experiments were performed in the presence and absence of Th4+ ions. As shown in Fig. S12,† the absorption intensity of PHQBA is nearly stable over a broad pH range of 6–12 and shows an enhanced intensity at pH 2–5, likely due to the protonation of the PHQBA. Upon the addition of Th4+ ions, the absorption intensity of the PHQBA + Th4+ complex persists stable within the pH range of 6–9 and began to decrease beyond pH 9, due to the hydrolysis of thorium ions. These results indicate that the sensor is capable of responding to Th4+ ions across a pH range of 2–12.
2 v/v). As illustrated in Fig. 1a, PHQBA displayed an explicit absorption band at 278 and 380 nm, which could be accredited to π–π* and n–π* electronic transition respectively. Competitive cations have no significant impact on the absorption of PHQBA. However, the introduction of the Th4+ ion to the PHQBA solution resulted in the appearance of a new hypsochromic absorption band at 312 nm. The resulting absorption band with a hypsochromic shift48 demonstrated the active chelation of Th4+ ions with the sensor PHQBA. Further scrutiny of PHQBA's detection properties with Th4+ ions was carried out through the emission titration experiments (Fig. 1b). The gradual addition of thorium ions (0–11 equiv.) to the PHQBA solution resulted in the decreased absorption intensity at 380 nm and the subsequent enhancement aroused at 312 nm. The shifts in the absorption bands to produce isosbestic points at 290 and 342 nm revealed the formation of a new complex PHQBA + Th4+ These findings prove that the sensor PHQBA has selective and sensitive detection capability with Th4+ ions. To investigate the effect of pH on the detection properties of PHQBA, absorption experiments were performed in the presence and absence of Th4+ ions. As shown in Fig. S12,† the absorption intensity of PHQBA is nearly stable over a broad pH range of 6–12 and shows an enhanced intensity at pH 2–5, likely due to the protonation of the PHQBA. Upon the addition of Th4+ ions, the absorption intensity of the PHQBA + Th4+ complex persists stable within the pH range of 6–9 and began to decrease beyond pH 9, due to the hydrolysis of thorium ions. These results indicate that the sensor is capable of responding to Th4+ ions across a pH range of 2–12.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) by treating with competing metal ion solutions including Ca2+, Ba2+, Al3+, Cd2+, Cu2+, Th4+, Co2+, Fe2+, Fe3+, Hg2+, Ni2+, Cr3+, Pb2+, Pd2+ Zn2+, La3+, and Yb3+. As illustrated in Fig. 2a, the free sensor PHQBA showed a maximum emission intensity of 478 nm. After the addition of Th4+ ions, the emission band at 478 nm red-shifted to 515 nm, which could be attributed to the synergic binding of Th4+ ion with the sensor PHQBA. Notably, other cations did not cause any significant spectral alterations as they led to no discernible binding interactions with the sensor PHQBA. To explore the sensitive detection properties of PHQBA with Th4+ ions, emission titration experiments were performed in ACN/H2O (8
2, v/v) by treating with competing metal ion solutions including Ca2+, Ba2+, Al3+, Cd2+, Cu2+, Th4+, Co2+, Fe2+, Fe3+, Hg2+, Ni2+, Cr3+, Pb2+, Pd2+ Zn2+, La3+, and Yb3+. As illustrated in Fig. 2a, the free sensor PHQBA showed a maximum emission intensity of 478 nm. After the addition of Th4+ ions, the emission band at 478 nm red-shifted to 515 nm, which could be attributed to the synergic binding of Th4+ ion with the sensor PHQBA. Notably, other cations did not cause any significant spectral alterations as they led to no discernible binding interactions with the sensor PHQBA. To explore the sensitive detection properties of PHQBA with Th4+ ions, emission titration experiments were performed in ACN/H2O (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) medium. Thereby, the gradual addition of Th4+ ions (0–11 equiv.) to the PHQBA solution resulted in diminished emission intensity at 478 nm, and the successive emission enhancement was attained at 515 nm (Fig. 2b). The emergence of a clear iso-emissive point at 496 nm indicated the formation of a new complex PHQBA + Th4+. As a result, the emission color of PHQBA transformed from blue to green (Fig. 2c). The calculated quantum yield values of PHQBA and its complex PHQBA + Th4+ were found to be 0.12 and 0.14 respectively. The slight enhancement in the quantum yield corresponds to the redshifted ratiometric fluorescence of PHQBA on chelating with Th4+ ions. These findings provided further evidence that sensor PHQBA had strong fluorescence detection abilities towards Th4+ ions. To clearly distinguish the emission of PHQBA and its complex PHQBA + Th4+, the chromaticity plot (CIE) was plotted49 using the emission spectra. As demonstrated in Fig. S8,† the color coordinates of the free probe (PHQBA) were found to be in a blue region with the coordinates of X = 0.145, Y = 0.258, further, the introduction of Th4+ the color coordinates of PHQBA were shifted towards the green region with the coordinates of X = 0.215, Y = 0.499. These results denote that PHQBA acts as a versatile sensor for the ratiometric detection of Th4+ ions. The limit of detection is an essential parameter for evaluating the performance of the chemosensor. The limit of detection was determined by the standard formula 3σ/slope50 where σ is the standard deviation of blank measurements of PHQBA (n = 10). The corresponding slope value was obtained from the concentration-dependent linear plot (Fig. S6†). The limit of detection was calculated to be 223 nM, which is comparatively lower than the previously reported thorium sensors (Table S1†). Thus, sensor PHQBA can be used as a versatile sensitive tool for the quantitative ratiometric determination of Th4+ ions with outstanding sensitivity. Interestingly, the present work relies on evaluating the ratio of fluorescence intensities at two distinct wavelengths (515 and 478 nm). This self-calibration eliminates variations caused by external factors like light source intensity, detector sensitivity, and sample concentration, making the results more robust and reliable.
2, v/v) medium. Thereby, the gradual addition of Th4+ ions (0–11 equiv.) to the PHQBA solution resulted in diminished emission intensity at 478 nm, and the successive emission enhancement was attained at 515 nm (Fig. 2b). The emergence of a clear iso-emissive point at 496 nm indicated the formation of a new complex PHQBA + Th4+. As a result, the emission color of PHQBA transformed from blue to green (Fig. 2c). The calculated quantum yield values of PHQBA and its complex PHQBA + Th4+ were found to be 0.12 and 0.14 respectively. The slight enhancement in the quantum yield corresponds to the redshifted ratiometric fluorescence of PHQBA on chelating with Th4+ ions. These findings provided further evidence that sensor PHQBA had strong fluorescence detection abilities towards Th4+ ions. To clearly distinguish the emission of PHQBA and its complex PHQBA + Th4+, the chromaticity plot (CIE) was plotted49 using the emission spectra. As demonstrated in Fig. S8,† the color coordinates of the free probe (PHQBA) were found to be in a blue region with the coordinates of X = 0.145, Y = 0.258, further, the introduction of Th4+ the color coordinates of PHQBA were shifted towards the green region with the coordinates of X = 0.215, Y = 0.499. These results denote that PHQBA acts as a versatile sensor for the ratiometric detection of Th4+ ions. The limit of detection is an essential parameter for evaluating the performance of the chemosensor. The limit of detection was determined by the standard formula 3σ/slope50 where σ is the standard deviation of blank measurements of PHQBA (n = 10). The corresponding slope value was obtained from the concentration-dependent linear plot (Fig. S6†). The limit of detection was calculated to be 223 nM, which is comparatively lower than the previously reported thorium sensors (Table S1†). Thus, sensor PHQBA can be used as a versatile sensitive tool for the quantitative ratiometric determination of Th4+ ions with outstanding sensitivity. Interestingly, the present work relies on evaluating the ratio of fluorescence intensities at two distinct wavelengths (515 and 478 nm). This self-calibration eliminates variations caused by external factors like light source intensity, detector sensitivity, and sample concentration, making the results more robust and reliable.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio for the PHQBA + Th4+ complex. To authenticate the stoichiometric binding of the sensor PHQBA with Th4+ ions, the Benesi–Hildebrand plot was linearly fitted between [1/(F0 − F)] and 1/[Th4+]. The association constant of the formed complex was calculated using the formula: Ka = intercept/slope.52 The Ka value for PHQBA was determined as 3.77 × 105 M−1. Such a significant value denotes the robust binding of sensor PHQBA with Th4+ ions (Fig. 4b).53 To further understand the coordination mode of PHQBA with Th4+, a series of 1H NMR titration experiments were conducted in DMSO-d6. As shown in Fig. 4c, the base spectrum represents the free sensor. Upon the sequential addition of Th4+ ions (0–11 equivalents), significant changes were observed in the pyridyl proton of PHQBA (a) at 9.59 ppm, including a marked decrease in intensity. Furthermore, the incremental addition of Th4+ ions induced slight upfield shifts at 8.97 ppm (b), 7.06 ppm (d), and 6.97 ppm (e). A minor upfield shift was also noted for the c proton, which shifted from 8.29 ppm to 8.28 ppm. Notably, the aliphatic protons of PHQBA (g, h, and f) were significantly impacted, with their intensities progressively decreasing as the Th4+ concentration increased. These observations collectively indicate the effective chelation of Th4+ ions by PHQBA. Additionally, the mass of the complex was confirmed by high-resolution mass spectroscopy. According to the mass spectral data, the observed mass of the complex PHQBA + Th(NO3)4 was 973.1636, which matched its calculated mass (973.1633) (Fig. S7†). To confirm nitrate binding, we have recorded FTIR spectra. As depicted in Fig. S13,† the observed stretching frequency at 1025 cm−1 confirms the presence of NO3− in the PHQBA + Th4+ system.54
1 stoichiometric ratio for the PHQBA + Th4+ complex. To authenticate the stoichiometric binding of the sensor PHQBA with Th4+ ions, the Benesi–Hildebrand plot was linearly fitted between [1/(F0 − F)] and 1/[Th4+]. The association constant of the formed complex was calculated using the formula: Ka = intercept/slope.52 The Ka value for PHQBA was determined as 3.77 × 105 M−1. Such a significant value denotes the robust binding of sensor PHQBA with Th4+ ions (Fig. 4b).53 To further understand the coordination mode of PHQBA with Th4+, a series of 1H NMR titration experiments were conducted in DMSO-d6. As shown in Fig. 4c, the base spectrum represents the free sensor. Upon the sequential addition of Th4+ ions (0–11 equivalents), significant changes were observed in the pyridyl proton of PHQBA (a) at 9.59 ppm, including a marked decrease in intensity. Furthermore, the incremental addition of Th4+ ions induced slight upfield shifts at 8.97 ppm (b), 7.06 ppm (d), and 6.97 ppm (e). A minor upfield shift was also noted for the c proton, which shifted from 8.29 ppm to 8.28 ppm. Notably, the aliphatic protons of PHQBA (g, h, and f) were significantly impacted, with their intensities progressively decreasing as the Th4+ concentration increased. These observations collectively indicate the effective chelation of Th4+ ions by PHQBA. Additionally, the mass of the complex was confirmed by high-resolution mass spectroscopy. According to the mass spectral data, the observed mass of the complex PHQBA + Th(NO3)4 was 973.1636, which matched its calculated mass (973.1633) (Fig. S7†). To confirm nitrate binding, we have recorded FTIR spectra. As depicted in Fig. S13,† the observed stretching frequency at 1025 cm−1 confirms the presence of NO3− in the PHQBA + Th4+ system.54

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complex with the sensor PHQBA.
1 stoichiometric complex with the sensor PHQBA.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). Sensor PHQBA demonstrated an intensive emission band at 478 nm, which produced a characteristic blue fluorescence. Then, the addition of Th4+ ions to the PHQBA solution resulted in ratiometric green fluorescence. Since ethylenediaminetetraacetic acid (EDTA) is a potential chelating agent,63 Th4+ ion-facilitated ratiometric fluorescence was substantially reversed by the subsequent additions of EDTA. As depicted in Fig. 7, the emission changes were incredibly imitated in fluorescence spectra, in which the introduction of EDTA solution in the PHQBA + Th4+ complex, resulted in the regeneration of the probe's emission at 478 nm. These outcomes imply that the Th4+ ion has been significantly eradicated from the sensor and that it finely achieves the reversibility process induced by the efficient interaction of EDTA with the PHQBA + Th4+ complex. These reproducible and reversible processes (blue to green fluorescence) were repeated for 4 cycles (Fig. S9†) with successive alternative additions of Th4+ and EDTA. The outcomes revealed that the sensor PHQBA can be reused and also sensitively employed for the detection of Th4+ ions.
2, v/v). Sensor PHQBA demonstrated an intensive emission band at 478 nm, which produced a characteristic blue fluorescence. Then, the addition of Th4+ ions to the PHQBA solution resulted in ratiometric green fluorescence. Since ethylenediaminetetraacetic acid (EDTA) is a potential chelating agent,63 Th4+ ion-facilitated ratiometric fluorescence was substantially reversed by the subsequent additions of EDTA. As depicted in Fig. 7, the emission changes were incredibly imitated in fluorescence spectra, in which the introduction of EDTA solution in the PHQBA + Th4+ complex, resulted in the regeneration of the probe's emission at 478 nm. These outcomes imply that the Th4+ ion has been significantly eradicated from the sensor and that it finely achieves the reversibility process induced by the efficient interaction of EDTA with the PHQBA + Th4+ complex. These reproducible and reversible processes (blue to green fluorescence) were repeated for 4 cycles (Fig. S9†) with successive alternative additions of Th4+ and EDTA. The outcomes revealed that the sensor PHQBA can be reused and also sensitively employed for the detection of Th4+ ions.






