Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Green synthesis of new adamantane-containing dihydropyrimidine derivatives as potential anticancer agents†
Adeleh Moshtaghi Zonouz *a,
Mina Abkar Arasa,
Nahideh Jafarib,
Zahra Rezaeia and
Hamed Hamishehkar
*a,
Mina Abkar Arasa,
Nahideh Jafarib,
Zahra Rezaeia and
Hamed Hamishehkar *c
*c
aDepartment of Chemistry, Faculty of Science, Azarbaijan Shahid Madani University, Tabriz, Iran. E-mail: adelehmz@yahoo.com; ac.moshtaghi@azaruniv.ac.ir
bFaculty of Chemical and Petroleum Engineering, University of Tabriz, Tabriz, Iran
cDrug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran. E-mail: hamishehkarh@tbzmed.ac.ir; Hamishehkar.hamed@gmail.com
First published on 13th March 2025
Abstract
Despite significant progress in cancer treatment, cancer remains a major focus of research due to medication resistance and side effects. In this study, bioactive adamantane-containing dihydropyrimidine (DHPM) derivatives were synthesized through the multi-component Biginelli reaction of N-(adamant-1-yl)acetoacetamide, benzaldehyde derivatives, and thiourea in the presence of trifluoroacetic acid (TFA, 2 mol%) as catalyst under solvent free conditions. This method provides an effective and significantly improved modification of the original Biginelli reaction in terms of yield and reaction time. The synthesized DHPM derivatives were subjected to cytotoxicity screening against the A-549 human non-small cell lung cancer (NSCLC) cell line to evaluate their effects on cell growth inhibition. MTT cytotoxicity assay was used to determine IC50 values. Among the target analogs, IIb, IIj, IId, and IIg demonstrated the best activity with IC50 values of 1.03, 8.36, 10.38, and 16.04 μg mL−1, respectively. Additionally, we assessed the possible mechanisms for cell growth inhibition and induction of apoptotic cell death using the DAPI and Annexin V-FITC staining. The average percentages of apoptotic cells were 21.35%, 28.35%, 32.73, and 43.33% for IIg, IId, IIj, and IIb treatment groups, respectively. These results suggest that the synthesized adamantane-containing dihydropyrimidines can be considered as encouraging molecules for further drug development as anticancer agents.
1. Introduction
Cancer is a serious condition associated with complex biological processes in which a group of cells proliferates abnormally. The disease has been recognized as an important global health problem.1 The number of cancer-related deaths worldwide is expected to increase to 12 million annually by 2030. Despite the enormous progress in cancer treatment over the past few decades, it remains a major area of research due to medication resistance and drug side effects. Hence, it is essential to conduct research on drug development for cancer treatment. Heterocyclic structures remain interesting synthesis candidates due to their significant biological properties. Particularly, dihydropyrimidine (DHPM) derivatives are well recognized for their extensive biological functions.2 The DHPM molecules are structural analogs of Monastrol with important biological characteristics such as antiviral,3 anticancer,4 antibacterial,5 and calcium channel function modulation.6 Pharmaceutical scientists have used Monastrol to formulate new chemotherapy drugs based on the DHPM structure or its modification with other substitutions. Numerous investigations have shown that DHPM derivatives like Monastrol inhibit human kinesin Eg5, leading to apoptosis.7 However, some studies showed other targets, such as centrin, calcium channels, and topoisomerase I for DHPM molecules. In nucleic acids, three types of nucleobases are pyrimidine derivatives, which include cytosine in DNA and RNA, uracil in RNA, and thymine in DNA.8 Hence, designing and developing DHPM derivatives is a stimulating focus for synthetic organic chemistry. Solvent-free and catalyst-free green techniques have been reported as a method to produce DHPM derivatives and their synthesis. In a study, Pramanik et al. used common fruit juice to develop an efficient, sustainable, and environmentally friendly method for preparing DHPM derivatives at room temperature.9 Bhatewara et al. produced DHPM using microwave irradiation and assessed their antibacterial characteristics.10 Also Saleem Farooq et al. developed a simple, efficient, cost-effective, and green approach for the synthesis of dihydropyrimidine derivatives via the Biginelli reaction.11 In another study, Morvarid Najjar et al. synthesized dihydropyrimidinone derivatives by a GQDs-based magnetically nanocatalyst under solvent-free conditions.12 A comparable study recognized the adamantane moiety as a crucial pharmacophore in several pharmacologically active drugs. The incorporation of the lipophilic adamantyl moiety into several bioactive molecules enhanced lipophilicity, improving bioavailability and controlling therapeutic efficiency.13 In our previous study, some adamantane-containing DHPM derivatives have been synthesized using a three-component Biginelli reaction of N-(adamant-1 yl)acetoacetamide, benzaldehyde derivatives and thiourea in acetonitrile in the presence of trifluoroacetic acid (TFA, 10 mol%) as a catalyst.14Accordingly, this study aimed to report a green synthesis for adamantane-containing DHPM derivatives under solvent-free conditions. To this end, we synthesized DHPM derivatives through the Biginelli reaction of N-(adamant-1-yl)acetoacetamide, benzaldehyde derivatives, thiourea, and TFA (2 mol%) as a catalyst. This technique provides an effective improved modification of the original Biginelli reaction in terms of yield and short reaction times under solvent-free conditions. The prepared compounds have been characterized using FT-IR, 1H and 13C NMR spectra, and elementary analyses. Finally, the cytotoxic activity of the DHPM derivatives was assessed against the A-549 lung cancer cells.
2. Results and discussion
2.1 Chemistry
Recently, we reported the one-pot three-component Biginelli procedure for the preparation of adamantane-containing DHPM derivatives using N-(adamantan-1-yl)acetoacetamide, benzaldehyde derivatives, and thiourea in CH3CN under reflux conditions for 36–42 h in the presence of TFA (10 mol%).14 Nowadays, one of the ultimate goals and challenges in ecofriendly synthetic methodologies is the green process for the preparation of biologically active substances from small and commercially available compounds. Many organic solvents and catalysts are potentially toxic to the ecosystem. Therefore, their use should be minimized or even be replaced with green alternative solvents and catalysts. Accordingly, in this research we prepared the potentially valuable nitrogen-containing heterocyclic molecules and described Biginelli-type synthesis of adamantane-containing DHPMs through one-pot three-component condensation of N-(adamant-1-yl)acetoacetamide (I), benzaldehyde derivatives, and thiourea in the presence of a catalytic amount of TFA (2 mol%) under solvent-free conditions (Scheme 1). N-(Adamantan-1-yl)acetoacetamide (I) was prepared by nucleophilic amidation of ethyl acetoacetate with 1-adamantylamine in the presence of a catalytic amount of triethylamine (5 mol%) in refluxing toluene under an argon atmosphere. First, the reaction of benzaldehyde (1 equiv.), N-(adamantan-1-yl)acetoacetamide (1 equiv.), and thiourea (1 equiv.) was investigated under solvent-free conditions without any catalyst at room temperature. This condition did not afford the desired product. Then, reaction was performed in the presence of catalyst. However, using catalyst such as CF3COOH at room temperature did not afford the desired product. Therefore, the reaction was performed at different temperatures. The best result was obtained when the reaction was carried out at 50 °C. This process was improved by modulating reaction temperature, reaction time, and chemical proportions under solvent-free conditions. | ||
| Scheme 1 Synthesis of 5-(1-adamantylcarbamoyl)-6-methyl-4-aryl-3,4-dihydropyrimidin-2(1H)-thiones II via Biginelli reaction. | ||
Upon further evaluation, the enhanced yields and optimal reaction times were achieved when using TFA (2 mol%), N-(adamant-1-yl)acetoacetamide (1 mmol), 2-methyl benzaldehyde (1 mmol), and thiourea (1 mmol) under solvent-free conditions at 50 °C, resulting in the synthesis of DHPM IIb in 85% yield within 60 minutes. A series of different DHPM analogs IIa–k were then synthesized using these optimized conditions (Scheme 1 and Table 1).
| Entry | R | Product | Time (min) | Yield (%) | MP (°C) |
|---|---|---|---|---|---|
| a IIf is 8-oxa-10,12-diazatricyclo [7.3.1.02,7] tridecatriene derivative. | |||||
| 1 | H | IIa | 60 | 83 | 238 |
| 2 | 2-Me | IIb | 60 | 85 | 258 |
| 3 | 3-Me | IIc | 85 | 81 | 256 |
| 4 | 4-Me | IId | 85 | 70 | 230 |
| 5 | 3-OMe | IIe | 70 | 74 | 256–259 |
| 6 | 2-OH | IIf a | 90 | 75 | >250 |
| 7 | 3-OH | IIh | 90 | 80 | 181–185 |
| 8 | 3-NO2 | IIi | 90 | 60 | >270 |
| 9 | 4-NO2 | IIg | 90 | 68 | 216–218 |
| 10 | 2-Cl | IIj | 60 | 67 | 184–186 |
| 11 | 3-Cl | IIk | 90 | 50 | 238 |
The obtained products were characterized by IR, 1H NMR and 13C NMR. For example, the FT-IR spectrum of 5-(1-adamantylcarbamoyl)-6-methyl-4-nitrophenyl-3,4-dihydropyrimidin-2(1H)-thione (IIg) confirmed the presence of the functional groups NH at 3369 cm−1 and 3185 cm−1, and the amide carbonyl (NH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) as a strong absorption band around 1619 cm−1 and C
O) as a strong absorption band around 1619 cm−1 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S at 1250 cm−1. The 1H NMR spectrum of IIg displayed adamantane protons at 1.55 (s, 6H, Ad), 1.83 (s, 6H, Ad), 1.93 (s, 3H, Ad) ppm, the CH-4 proton at 5.29 ppm, and the three NH protons at 7.23, 9.31, and 9.89 ppm. The 13C NMR spectrum further confirmed the product by the presence of an amide carbonyl signal at 165.6 and C
S at 1250 cm−1. The 1H NMR spectrum of IIg displayed adamantane protons at 1.55 (s, 6H, Ad), 1.83 (s, 6H, Ad), 1.93 (s, 3H, Ad) ppm, the CH-4 proton at 5.29 ppm, and the three NH protons at 7.23, 9.31, and 9.89 ppm. The 13C NMR spectrum further confirmed the product by the presence of an amide carbonyl signal at 165.6 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S at 174.8 ppm. The presence of C
S at 174.8 ppm. The presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C signals at 107.8 and 150.6 ppm was another reason for confirming the formation of this product. Similar to the our previous research,14 with ortho hydroxy-substituted aromatic aldehyde, salicylaldehyde, the expected product is not a simple DHPM. However, after the domino Biginelli condensation/Michael addition reaction, the 13-(1-adamantylcarbamoyl)-9-methyl-11-thioxo-8-oxa-10,12-diazatricyclo[7.3.1.02,7]trideca-2,4,6-triene derivative (IIf) was obtained. The intramolecular Michael addition might be due to the steric proximity of the ortho OH substituent of the aromatic ring to the C-6 carbon of the pyrimidine ring.
C signals at 107.8 and 150.6 ppm was another reason for confirming the formation of this product. Similar to the our previous research,14 with ortho hydroxy-substituted aromatic aldehyde, salicylaldehyde, the expected product is not a simple DHPM. However, after the domino Biginelli condensation/Michael addition reaction, the 13-(1-adamantylcarbamoyl)-9-methyl-11-thioxo-8-oxa-10,12-diazatricyclo[7.3.1.02,7]trideca-2,4,6-triene derivative (IIf) was obtained. The intramolecular Michael addition might be due to the steric proximity of the ortho OH substituent of the aromatic ring to the C-6 carbon of the pyrimidine ring.
2.2 Biological evaluation
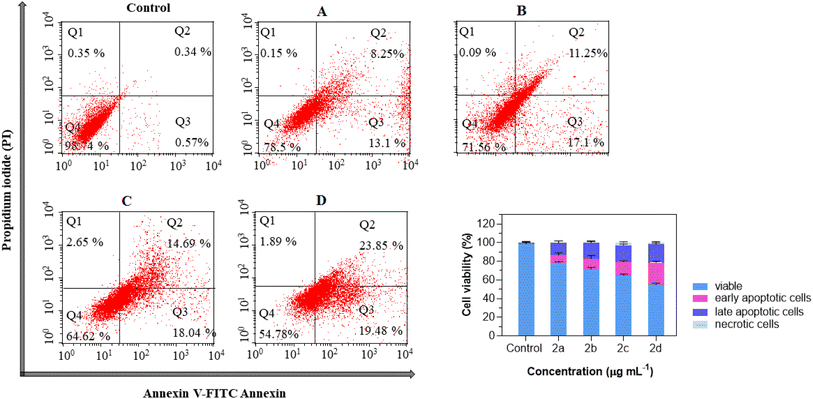
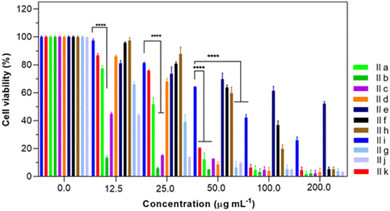 | ||
| Fig. 1 Cell viabilities of non-small-cell lung cancer cell lines A-549 with different treatment groups for 48 h. ****P < 0.0001 (n = 3). | ||
| Compounds | IIa | IIb | IIc | IId | IIe | IIf | IIg | IIh | IIi | IIj | IIk |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (μg mL−1) | 19.82 | 1.03 | 16.94 | 10.38 | 41.80 | 91.56 | 16.04 | 64.89 | 79.59 | 8.36 | 24.23 |
Substitution of a methyl group on the phenyl ring displayed significant cytotoxicity against A-549 cancer cell lines with IC50 values of 1.03 and 10.38 μg mL−1 for ortho and para positions, respectively. Accordingly, it can be inferred that the position of the methyl substituent on the phenyl ring greatly influences cytotoxicity. Methyl substitution at the ortho position, in particular, displayed remarkable cytotoxicity against the tested cancer cell lines. Also, the effect of the nitro functional group on the phenyl-substituted derivatives showed that these electron-poor substations were active at the para position in A-549 cancer cell lines with an IC50 value of 16.04 μg mL−1. In addition, substitution at the para position displayed remarkable cytotoxicity compared to the meta-analog substitutions. All these results suggest that the structural features had a profound effect on the biological profile of the compound.
Overall, the selected DHPM derivatives treated with A-549 cancer cell lines increased their sensitivity to apoptosis, with varying percentages in different phases, thereby boosting the overall apoptosis rate. Recent studies have shown that a series of substituted dihydropyrimidinone treatment groups effectively induced apoptosis in various cancer cell lines.17
3. Experimental section
3.1 Chemistry
3.1.2.1 5-(1-Adamantylcarbamoyl)-6-methyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-thione (IIa). White solid; yield: 83%; mp 238 °C; IR (KBr):
![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) = 3396, 3244, 3173 (NH), 2906, 2848 (C–H), 1619 (C
= 3396, 3244, 3173 (NH), 2906, 2848 (C–H), 1619 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1559 (C
O), 1559 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1331 (C–N), 1203 (C
C), 1331 (C–N), 1203 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) cm−1; 1H NMR (DMSO-d6, 500 MHz): δ = 1.57 (s br, 6H, Ad, C
S) cm−1; 1H NMR (DMSO-d6, 500 MHz): δ = 1.57 (s br, 6H, Ad, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3), 1.84 (s, 6H, Ad), 1.94 (s, 6H, Ad), 5.21 (s, 1H, C(4)-H), 7.10 (s, 1H, NH–C
C–CH3), 1.84 (s, 6H, Ad), 1.94 (s, 6H, Ad), 5.21 (s, 1H, C(4)-H), 7.10 (s, 1H, NH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 7.20 (d, J = 8 Hz, 2H, Ar-H), 7.25 (t, J = 7.5 Hz, 1H, Ar-H), 7.33 (t, J = 7.5 Hz, 2H, Ar-H), 9.18 (s, 1H, NH), 9.73 (s, 1H, NH) ppm; 13C NMR (DMSO-d6, 125 MHz): δ = 16.2 (C
O), 7.20 (d, J = 8 Hz, 2H, Ar-H), 7.25 (t, J = 7.5 Hz, 1H, Ar-H), 7.33 (t, J = 7.5 Hz, 2H, Ar-H), 9.18 (s, 1H, NH), 9.73 (s, 1H, NH) ppm; 13C NMR (DMSO-d6, 125 MHz): δ = 16.2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3), 28.8 (3C-Ad), 36.0 (3C-Ad), 40.9 (3C-Ad), 51.2 (C-Ad), 55.4 (CH), 108.5 (
C–CH3), 28.8 (3C-Ad), 36.0 (3C-Ad), 40.9 (3C-Ad), 51.2 (C-Ad), 55.4 (CH), 108.5 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 143.2 (
C), 143.2 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 126.3 (C2, C6), 127.5 (C4), 128.4 (C3, C5), 132.63 (C1), 165.5 (C
C), 126.3 (C2, C6), 127.5 (C4), 128.4 (C3, C5), 132.63 (C1), 165.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 174.1 (C
O), 174.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) ppm; anal. calcd for C22H27N3OS: C, 69.26; H, 7.13; N, 11.01. Found: C, 69.29; H, 7.27; N, 10.98.
S) ppm; anal. calcd for C22H27N3OS: C, 69.26; H, 7.13; N, 11.01. Found: C, 69.29; H, 7.27; N, 10.98.
3.1.2.2 5-(1-Adamantylcarbamoyl)-6-methyl-4-(2-methylphenyl)-3,4-dihydropyrimidin-2(1H)-thione (IIb). White solid; yield: 85%; mp 258 °C; IR (KBr):
![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) = 3382, 3244, 3187 (NH), 2907, 2845 (C–H), 1627 (C
= 3382, 3244, 3187 (NH), 2907, 2845 (C–H), 1627 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1597 (C
O), 1597 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1332 (C–N), 1197 (C
C), 1332 (C–N), 1197 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) cm−1; 1H NMR (DMSO-d6, 500 MHz): δ = 1.51 (s, 3H, C
S) cm−1; 1H NMR (DMSO-d6, 500 MHz): δ = 1.51 (s, 3H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3), 1.54 (s, 3H, Ad), 1.75 (s, 6H, Ad), 1.92 (s, 6H, Ad), 2.34 (s, 3H, Ar-CH3), 5.42 (s, 1H, C(4)-H), 7.01 (s, 1H, NH–C
C–CH3), 1.54 (s, 3H, Ad), 1.75 (s, 6H, Ad), 1.92 (s, 6H, Ad), 2.34 (s, 3H, Ar-CH3), 5.42 (s, 1H, C(4)-H), 7.01 (s, 1H, NH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 7.11 (t, J = 7.5 Hz, 1H, Ar-H), 7.15 (d, J = 7 Hz, 1H, Ar-H), 7.18 (d, J = 7.5 Hz, 1H, Ar-H), 7.21 (t, J = 7.5 Hz, 1H, Ar-H), 8.99 (s, 1H, NH), 9.66 (s, 1H, NH) ppm; 13C NMR (DMSO-d6, 125 MHz): δ = 16.0 (C
O), 7.11 (t, J = 7.5 Hz, 1H, Ar-H), 7.15 (d, J = 7 Hz, 1H, Ar-H), 7.18 (d, J = 7.5 Hz, 1H, Ar-H), 7.21 (t, J = 7.5 Hz, 1H, Ar-H), 8.99 (s, 1H, NH), 9.66 (s, 1H, NH) ppm; 13C NMR (DMSO-d6, 125 MHz): δ = 16.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3), 18.9 (Ar-CH3), 28.7 (3C-Ad), 36.0 (3C-Ad), 40.8 (3C-Ad), 51.0 (C-Ad), 52.7 (C(4)-H), 108.5 (
C–CH3), 18.9 (Ar-CH3), 28.7 (3C-Ad), 36.0 (3C-Ad), 40.8 (3C-Ad), 51.0 (C-Ad), 52.7 (C(4)-H), 108.5 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 141.6 (
C), 141.6 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 126.3 (C5), 127.4 (C3), 128.3 (C4), 130.1 (C6), 132.0 (C2), 134.9 (C1-phenyl), 165.4 (C
C), 126.3 (C5), 127.4 (C3), 128.3 (C4), 130.1 (C6), 132.0 (C2), 134.9 (C1-phenyl), 165.4 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 173.3 (C
O), 173.3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) ppm; anal. calcd for C23H29N3OS: C, 69.84; H, 7.39; N, 10.62. Found: C, 69.78; H, 7.09; N, 10.88.
S) ppm; anal. calcd for C23H29N3OS: C, 69.84; H, 7.39; N, 10.62. Found: C, 69.78; H, 7.09; N, 10.88.
3.1.2.3 5-(1-Adamantylcarbamoyl)-6-methyl-4-(3-methylphenyl)-3,4-dihydropyrimidin-2(1H)-thione (IIc). White solid; yield: 81%; mp 256 °C; IR (KBr):
![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) = 3400, 3195, 3088 (NH), 2905, 2851 (C–H), 1617 (C
= 3400, 3195, 3088 (NH), 2905, 2851 (C–H), 1617 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1559 (C
O), 1559 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1197 (C
C), 1197 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) cm−1; 1H NMR (DMSO-d6, 500 MHz): δ = 1.57 (s, 6H, Ad), 1.85 (s, 6H, Ad), 1.92 (s, 3H, C
S) cm−1; 1H NMR (DMSO-d6, 500 MHz): δ = 1.57 (s, 6H, Ad), 1.85 (s, 6H, Ad), 1.92 (s, 3H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3), 1.96 (s, 3H, Ad), 2.28 (s, 3H, Ar-CH3), 5.18 (s, 1H, C(4)-H), 6.98–7.00 (m, 2H, Ar-H), 7.07 (d, J = 7.5 Hz, 1H, Ar-H), 7.10 (s, 1H, NH–C
C–CH3), 1.96 (s, 3H, Ad), 2.28 (s, 3H, Ar-CH3), 5.18 (s, 1H, C(4)-H), 6.98–7.00 (m, 2H, Ar-H), 7.07 (d, J = 7.5 Hz, 1H, Ar-H), 7.10 (s, 1H, NH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 7.22 (t, J = 9 Hz, 1H, Ar-H), 9.13 (s, 1H, NH), 9.69 (s, 1H, NH) ppm; 13C NMR (DMSO-d6, 125 MHz): δ = 16.2 (C
O), 7.22 (t, J = 9 Hz, 1H, Ar-H), 9.13 (s, 1H, NH), 9.69 (s, 1H, NH) ppm; 13C NMR (DMSO-d6, 125 MHz): δ = 16.2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3), 21.15 (Ar-CH3), 28.8 (3C-Ad), 36.0 (3C-Ad), 40.9 (3C-Ad), 51.2 (C-Ad), 55.3 (CH), 108.6 (
C–CH3), 21.15 (Ar-CH3), 28.8 (3C-Ad), 36.0 (3C-Ad), 40.9 (3C-Ad), 51.2 (C-Ad), 55.3 (CH), 108.6 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 143.1 (
C), 143.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 123.3 (C6), 126.8 (C4), 127.9 (C2), 128.3 (C5), 132.3 (C3), 137.3 (C1), 165.5 (C
C), 123.3 (C6), 126.8 (C4), 127.9 (C2), 128.3 (C5), 132.3 (C3), 137.3 (C1), 165.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 174.1 (C
O), 174.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) ppm; anal. calcd for C23H29N3OS: C, 69.84; H, 7.39; N, 10.62. Found: C, 69.98; H, 7.19; N, 10.78.
S) ppm; anal. calcd for C23H29N3OS: C, 69.84; H, 7.39; N, 10.62. Found: C, 69.98; H, 7.19; N, 10.78.
3.1.2.4 5-(1-Adamantylcarbamoyl)-6-methyl-4-(4-methylphenyl)-3,4-dihydropyrimidin-2(1H)-thione (IId). White solid; yield: 70%; mp 230 °C; IR (KBr):
![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) = 3411, 3241, 3187 (NH), 2917, 2850 (C–H), 1616 (C
= 3411, 3241, 3187 (NH), 2917, 2850 (C–H), 1616 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1555 (C
O), 1555 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1385 (C–N), 1201 (C
C), 1385 (C–N), 1201 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) cm−1; 1H NMR (DMSO-d6, 500 MHz): δ = 1.57 (s, 6H, Ad), 1.85 (s, 6H, Ad), 1.93 (s, 3H, C
S) cm−1; 1H NMR (DMSO-d6, 500 MHz): δ = 1.57 (s, 6H, Ad), 1.85 (s, 6H, Ad), 1.93 (s, 3H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3), 1.96 (s, 3H, Ad), 2.26 (s, 3H, Ar-CH3), 5.16 (s, 1H, C(4)-H), 7.09–7.14 (m, 4H, Ar-H), 7.09 (s, 1H, NH–C
C–CH3), 1.96 (s, 3H, Ad), 2.26 (s, 3H, Ar-CH3), 5.16 (s, 1H, C(4)-H), 7.09–7.14 (m, 4H, Ar-H), 7.09 (s, 1H, NH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 9.14 (s, 1H, NH), 9.69 (s, 1H, NH) ppm; 13C NMR (DMSO-d6, 125 MHz): δ = 16.2 (C
O), 9.14 (s, 1H, NH), 9.69 (s, 1H, NH) ppm; 13C NMR (DMSO-d6, 125 MHz): δ = 16.2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3), 20.6 (Ar-CH3), 28.8 (3C-Ad), 36.0 (3C-Ad), 40.9 (3C-Ad), 51.2 (C-Ad), 55.0 (CH), 108.6 (
C–CH3), 20.6 (Ar-CH3), 28.8 (3C-Ad), 36.0 (3C-Ad), 40.9 (3C-Ad), 51.2 (C-Ad), 55.0 (CH), 108.6 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 140.3 (
C), 140.3 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 126.2 (C2, C6), 128.9 (C3, C5), 132.5 (C4), 136.5 (C1), 165.5 (C
C), 126.2 (C2, C6), 128.9 (C3, C5), 132.5 (C4), 136.5 (C1), 165.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 173.9 (C
O), 173.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) ppm; anal. calcd for C23H29N3OS: C, 69.84; H, 7.39; N, 10.62. Found: C, 69.68; H, 7.59; N, 10.98.
S) ppm; anal. calcd for C23H29N3OS: C, 69.84; H, 7.39; N, 10.62. Found: C, 69.68; H, 7.59; N, 10.98.
3.1.2.5 5-(1-Adamantylcarbamoyl)-6-methyl-4-(3-methoxyphenyl)-3,4-dihydropyrimidin-2(1H)-thione (IIe). White solid; yield: 74%; mp 256–259 °C; IR (KBr):
![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) = 3392, 3180, 3085 (NH), 2901, 2850 (C–H), 1615 (C
= 3392, 3180, 3085 (NH), 2901, 2850 (C–H), 1615 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1557 (C
O), 1557 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1198 (C
C), 1198 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) cm−1; 1H NMR (DMSO-d6, 500 MHz): δ = 1.60 (s, 6H, Ad), 1.89 (s, 6H, Ad), 1.96 (s, 3H, C
S) cm−1; 1H NMR (DMSO-d6, 500 MHz): δ = 1.60 (s, 6H, Ad), 1.89 (s, 6H, Ad), 1.96 (s, 3H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3), 1.99 (s, 3H, Ad), 3.75 (s, 3H, Ar-OCH3), 5.21 (s, 1H, C(4)-H), 6.77 (s, 1H, Ar-H), 6.81 (d, J = 7.5 Hz, 1H, Ar-H), 6.85 (d, J = 8 Hz, 1H, Ar-H), 7.16 (s, 1H, NH–C
C–CH3), 1.99 (s, 3H, Ad), 3.75 (s, 3H, Ar-OCH3), 5.21 (s, 1H, C(4)-H), 6.77 (s, 1H, Ar-H), 6.81 (d, J = 7.5 Hz, 1H, Ar-H), 6.85 (d, J = 8 Hz, 1H, Ar-H), 7.16 (s, 1H, NH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 7.28 (t, J = 7.5 Hz, 1H, Ar-H), 9.19 (s, 1H, NH), 9.76 (s, 1H, NH) ppm; 13C NMR (DMSO-d6, 125 MHz): δ = 16.2 (C
O), 7.28 (t, J = 7.5 Hz, 1H, Ar-H), 9.19 (s, 1H, NH), 9.76 (s, 1H, NH) ppm; 13C NMR (DMSO-d6, 125 MHz): δ = 16.2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3), 28.8 (3C-Ad), 36.0 (3C-Ad), 40.9 (3C-Ad), 51.2 (C-Ad), 54.9 (CH), 55.1 (Ar-OCH3), 108.5 (
C–CH3), 28.8 (3C-Ad), 36.0 (3C-Ad), 40.9 (3C-Ad), 51.2 (C-Ad), 54.9 (CH), 55.1 (Ar-OCH3), 108.5 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 159.3 (
C), 159.3 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 112.1 (C4), 112.4 (C2), 118.3 (C6), 129.5 (C5), 132.7 (C1), 144.7 (C3), 165.4 (C
C), 112.1 (C4), 112.4 (C2), 118.3 (C6), 129.5 (C5), 132.7 (C1), 144.7 (C3), 165.4 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 174.2 (C
O), 174.2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) ppm; anal. calcd for C23H29N3O2S: C, 67.12; H, 7.10; N, 10.21. Found: C, 67.44; H, 7.03; N, 10.35.
S) ppm; anal. calcd for C23H29N3O2S: C, 67.12; H, 7.10; N, 10.21. Found: C, 67.44; H, 7.03; N, 10.35.
3.1.2.6 13-(1-Adamantylcarbamoyl)-9-methyl-11-thioxo-8-oxa-10,12-diazatricyclo [7.3.1.02,7] trideca-2,4,6-triene (IIf). Yellow solid; yield: 75%; mp > 250 °C; IR (KBr):
![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) = 3352, 3277 (NH), 2914, 2850 (C–H), 1633 (C
= 3352, 3277 (NH), 2914, 2850 (C–H), 1633 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1229 (C
O), 1229 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S), 1154 (C–O) cm−1; 1H NMR (DMSO-d6, 500 MHz): δ = 1.62 (s, 6H, Ad), 1.66 (s, 3H, C–C–CH3), 1.92 (s, 6H, Ad), 2.01 (s, 3H, Ad), 2.91 (s, 1H, CH-13), 4.40 (d, J = 4 Hz, 1H, CH-1), 6.80 (d, J = 8 Hz, 1H, Ar-H), 6.91 (t, J = 7.5 Hz, 1H, Ar-H), 7.15 (d, J = 7.5 Hz, 1H, Ar-H), 7.19 (t, J = 7.5 Hz, 1H, Ar-H), 7.54 (s, 1H, NH–C
S), 1154 (C–O) cm−1; 1H NMR (DMSO-d6, 500 MHz): δ = 1.62 (s, 6H, Ad), 1.66 (s, 3H, C–C–CH3), 1.92 (s, 6H, Ad), 2.01 (s, 3H, Ad), 2.91 (s, 1H, CH-13), 4.40 (d, J = 4 Hz, 1H, CH-1), 6.80 (d, J = 8 Hz, 1H, Ar-H), 6.91 (t, J = 7.5 Hz, 1H, Ar-H), 7.15 (d, J = 7.5 Hz, 1H, Ar-H), 7.19 (t, J = 7.5 Hz, 1H, Ar-H), 7.54 (s, 1H, NH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 8.86 (s, 1H, NH), 8.93 (d, J = 4 Hz, 1H, NH) ppm; 13C NMR (DMSO-d6, 125 MHz): δ = 22.8 (CH3), 28.8 (3C-Ad), 36.0 (3C-Ad), 40.8 (3C-Ad), 42.0 (CH-13), 49.1 (C-Ad), 50.9 (CH-1), 81.8 (C-9), 116.4 (C3), 120.5 (C5), 124.9 (C4), 128.6 (C6), 129.4 (C2), 150.7 (C7), 166.6 (C
O), 8.86 (s, 1H, NH), 8.93 (d, J = 4 Hz, 1H, NH) ppm; 13C NMR (DMSO-d6, 125 MHz): δ = 22.8 (CH3), 28.8 (3C-Ad), 36.0 (3C-Ad), 40.8 (3C-Ad), 42.0 (CH-13), 49.1 (C-Ad), 50.9 (CH-1), 81.8 (C-9), 116.4 (C3), 120.5 (C5), 124.9 (C4), 128.6 (C6), 129.4 (C2), 150.7 (C7), 166.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 176.1 (C
O), 176.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) ppm; anal. calcd for C22H27N3O2S: C, 66.47; H, 6.85; N, 10.57. Found: C, 66.87; H, 7.08; N, 10.37.
S) ppm; anal. calcd for C22H27N3O2S: C, 66.47; H, 6.85; N, 10.57. Found: C, 66.87; H, 7.08; N, 10.37.
3.1.2.7 5-(1-Adamantylcarbamoyl)-6-methyl-4-(3-hydroxyphenyl)-3,4-dihydropyrimidin-2(1H)-thione (IIh). White solid; yield: 80%; mp 183–185 °C; IR (KBr):
![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) = 3380, 3323, 3217 (NH, OH, broad), 2908, 2852 (C–H), 1619 (C
= 3380, 3323, 3217 (NH, OH, broad), 2908, 2852 (C–H), 1619 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1197 (C
O), 1197 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) cm−1; 1H NMR (DMSO-d6, 500 MHz): δ = 1.57 (s, 6H, Ad), 1.86 (s, 6H, Ad), 1.92 (s, 3H, Ad), 1.98 (s, 3H, CH3), 5.12 (s, 1H, C(4)-H), 6.61–6.62 (m, 3H, Ar-H), 7.04 (s, 1H, NH–C
S) cm−1; 1H NMR (DMSO-d6, 500 MHz): δ = 1.57 (s, 6H, Ad), 1.86 (s, 6H, Ad), 1.92 (s, 3H, Ad), 1.98 (s, 3H, CH3), 5.12 (s, 1H, C(4)-H), 6.61–6.62 (m, 3H, Ar-H), 7.04 (s, 1H, NH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 7.10 (t, J = 7.5 Hz, 1H, Ar-H), 9.12 (s, O–H), 9.38 (s, 1H, NH), 9.68 (s, 1H, NH) ppm; 13C NMR (DMSO-d6, 125 MHz): δ = 16.2 (C
O), 7.10 (t, J = 7.5 Hz, 1H, Ar-H), 9.12 (s, O–H), 9.38 (s, 1H, NH), 9.68 (s, 1H, NH) ppm; 13C NMR (DMSO-d6, 125 MHz): δ = 16.2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3), 28.8 (3C-Ad), 36.1 (3C-Ad), 40.9 (3C-Ad), 51.2 (C-Ad), 55.4 (CH), 108.6 (
C–CH3), 28.8 (3C-Ad), 36.1 (3C-Ad), 40.9 (3C-Ad), 51.2 (C-Ad), 55.4 (CH), 108.6 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 157.5 (
C), 157.5 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 113.3 (C4), 114.4 (C2), 116.9 (C6), 129.2 (C5), 132.5 (C1), 144.7 (C3), 165.5 (C
C), 113.3 (C4), 114.4 (C2), 116.9 (C6), 129.2 (C5), 132.5 (C1), 144.7 (C3), 165.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 174.0 (C
O), 174.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) ppm; anal. calcd for C22H27N3O2S: C, 66.47; H, 6.85; N, 10.57. Found: C, 66.69; H, 7.12; N, 10.37.
S) ppm; anal. calcd for C22H27N3O2S: C, 66.47; H, 6.85; N, 10.57. Found: C, 66.69; H, 7.12; N, 10.37.
3.1.2.8 5-(1-Adamantylcarbamoyl)-6-methyl-4-(3-nithrophenyl)-3,4-dihydropyrimidin-2(1H)-thione (IIi). Yellow solid; yield: 60%; mp > 270 °C; IR (KBr):
![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) = 3373, 3163 (br. s, NH), 2906, 2848 (C–H), 1642 (C
= 3373, 3163 (br. s, NH), 2906, 2848 (C–H), 1642 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1566 (C
O), 1566 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1529, 1331 (NO2), 1202 (C
C), 1529, 1331 (NO2), 1202 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) cm−1; 1H NMR (DMSO-d6, 500 MHz): δ = 1.56 (s br, 6H, Ad, C
S) cm−1; 1H NMR (DMSO-d6, 500 MHz): δ = 1.56 (s br, 6H, Ad, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3), 1.83 (s, 6H, Ad), 1.95 (s, 6H, Ad), 5.36 (s, 1H, C(4)-H), 7.27 (s, 1H, Ar-H), 7.64–7.69 (m, 2H, Ar-H), 8.06 (s, 1H, NH–C
C–CH3), 1.83 (s, 6H, Ad), 1.95 (s, 6H, Ad), 5.36 (s, 1H, C(4)-H), 7.27 (s, 1H, Ar-H), 7.64–7.69 (m, 2H, Ar-H), 8.06 (s, 1H, NH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 8.15 (d, J = 8 Hz, 1H, Ar-H), 9.34 (s, 1H, NH), 9.93 (s, 1H, NH) ppm; 13C NMR (DMSO-d6, 62.5 MHz): δ = 16.0 (C
O), 8.15 (d, J = 8 Hz, 1H, Ar-H), 9.34 (s, 1H, NH), 9.93 (s, 1H, NH) ppm; 13C NMR (DMSO-d6, 62.5 MHz): δ = 16.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3), 28.7 (3C-Ad), 36.0 (3C-Ad), 40.8 (3C-Ad), 51.3 (C-Ad), 54.8 (CH), 107.5 (
C–CH3), 28.7 (3C-Ad), 36.0 (3C-Ad), 40.8 (3C-Ad), 51.3 (C-Ad), 54.8 (CH), 107.5 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 147.8 (
C), 147.8 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 121.0 (C5), 121.5 (C4), 122.5 (C2), 130.1 (C6), 133.5 (C1), 145.1 (C3), 165.2 (C
C), 121.0 (C5), 121.5 (C4), 122.5 (C2), 130.1 (C6), 133.5 (C1), 145.1 (C3), 165.2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 174.4 (C
O), 174.4 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) ppm; anal. calcd for C22H26N4O3S: C, 61.95; H, 6.14; N, 13.14. Found: C, 61.69; H, 6.46; N, 13.18.
S) ppm; anal. calcd for C22H26N4O3S: C, 61.95; H, 6.14; N, 13.14. Found: C, 61.69; H, 6.46; N, 13.18.
3.1.2.9 5-(1-Adamantylcarbamoyl)-6-methyl-4-(4-nithrophenyl)-3,4-dihydropyrimidin-2(1H)-thione (IIg). Yellow solid; yield: 68%; mp 216–218 °C; IR (KBr):
![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) = 3369, 3185 (NH), 2912, 2853 (CH), 1619 (C
= 3369, 3185 (NH), 2912, 2853 (CH), 1619 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1530, 1346 (NO2), 1250 (C
O), 1530, 1346 (NO2), 1250 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) cm−1; 1H NMR (DMSO-d6, 250 MHz): δ = 1.55 (s br, 6H, Ad, C
S) cm−1; 1H NMR (DMSO-d6, 250 MHz): δ = 1.55 (s br, 6H, Ad, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3), 1.83 (s, 6H, Ad), 1.93 (s, 6H, Ad), 5.29 (s, 1H, C(4)-H), 7.23 (s, 1H, NH–C
C–CH3), 1.83 (s, 6H, Ad), 1.93 (s, 6H, Ad), 5.29 (s, 1H, C(4)-H), 7.23 (s, 1H, NH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 7.43 (d, J = 8 Hz, 2H, Ar-H), 8.22 (d, J = 8 Hz, 2H, Ar-H), 9.31 (s, 1H, NH), 9.89 (s, 1H, NH) ppm; 13C NMR (DMSO-d6, 62.5 MHz):16.75 (C
O), 7.43 (d, J = 8 Hz, 2H, Ar-H), 8.22 (d, J = 8 Hz, 2H, Ar-H), 9.31 (s, 1H, NH), 9.89 (s, 1H, NH) ppm; 13C NMR (DMSO-d6, 62.5 MHz):16.75 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3), 29.20, 36.42, 41.25, 51.76 (10C-Ad), 55.39 (CH), 107.81, 150.59 (
C–CH3), 29.20, 36.42, 41.25, 51.76 (10C-Ad), 55.39 (CH), 107.81, 150.59 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 124.18 (C2, C6, Ar), 128.09 (C3, C5, Ar), 133.99 (C4), 147.27 (C1, Ar), 165.64 (C
C), 124.18 (C2, C6, Ar), 128.09 (C3, C5, Ar), 133.99 (C4), 147.27 (C1, Ar), 165.64 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 174.81 (C
O), 174.81 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) ppm; anal. calcd for C22H26N4O3S: C, 61.95; H, 6.14; N, 13.14. Found: C, 61.89; H, 6.23; N, 13.33.
S) ppm; anal. calcd for C22H26N4O3S: C, 61.95; H, 6.14; N, 13.14. Found: C, 61.89; H, 6.23; N, 13.33.
3.1.2.10 5-(1-Adamantylcarbamoyl)-6-methyl-4-(2-chlorophenyl)-3,4-dihydropyrimidin-2(1H)-thione (IIj). White solid; yield: 67%; mp 184–186 °C; IR (KBr):
![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) = 3405, 3297, 3185 (NH), 2906, 2847 (C–H), 1618 (C
= 3405, 3297, 3185 (NH), 2906, 2847 (C–H), 1618 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1566 (C
O), 1566 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1362 (C–N), 1260 (C
C), 1362 (C–N), 1260 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S), 750 (C–Cl) cm−1; 1H NMR (DMSO-d6, 500 MHz): δ = 1.51 (s, 6H, Ad), 1.75 (s, 3H, C
S), 750 (C–Cl) cm−1; 1H NMR (DMSO-d6, 500 MHz): δ = 1.51 (s, 6H, Ad), 1.75 (s, 3H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3), 1.79 (s, 3H, Ad), 1.91 (s, 6H, Ad), 5.60 (s, 1H, C(4)-H), 7.16 (s, 1H, NH–C
C–CH3), 1.79 (s, 3H, Ad), 1.91 (s, 6H, Ad), 5.60 (s, 1H, C(4)-H), 7.16 (s, 1H, NH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 7.26–7.29 (m, 1H, Ar-H), 7.34–7.38 (m, 3H, Ar-H), 9.07 (s, 1H, NH), 9.78 (s, 1H, NH) ppm; 13C NMR (DMSO-d6, 125 MHz): δ = 15.9 (C
O), 7.26–7.29 (m, 1H, Ar-H), 7.34–7.38 (m, 3H, Ar-H), 9.07 (s, 1H, NH), 9.78 (s, 1H, NH) ppm; 13C NMR (DMSO-d6, 125 MHz): δ = 15.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3), 28.7 (3C-Ad), 36.0 (3C-Ad), 40.7 (3C-Ad), 51.0 (C-Ad), 53.0 (CH), 107.8 (
C–CH3), 28.7 (3C-Ad), 36.0 (3C-Ad), 40.7 (3C-Ad), 51.0 (C-Ad), 53.0 (CH), 107.8 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 140.3 (
C), 140.3 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 127.5 (C6), 129.2 (C3, C5), 129.8 (C2), 131.4 (C4), 132.6 (C1), 164.9 (C
C), 127.5 (C6), 129.2 (C3, C5), 129.8 (C2), 131.4 (C4), 132.6 (C1), 164.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 173.9 (C
O), 173.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) ppm; anal. calcd for C22H26N3ClOS: C, 63.52; H, 6.30; N, 10.10. Found: C, 67.29; H, 6.13; N, 10.40.
S) ppm; anal. calcd for C22H26N3ClOS: C, 63.52; H, 6.30; N, 10.10. Found: C, 67.29; H, 6.13; N, 10.40.
3.1.2.11 5-(1-Adamantylcarbamoyl)-6-methyl-4-(3-chlorophenyl)-3,4-dihydropyrimidin-2(1H)-thione (IIk). White solid; yield: 50%; mp 238 °C; IR (KBr)
![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) = 3394, 3238, 3174 (NH), 2912, 2849 (CH), 1619 (C
= 3394, 3238, 3174 (NH), 2912, 2849 (CH), 1619 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1559 (C
O), 1559 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1203 (C
C), 1203 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) cm−1; 1H NMR (DMSO-d6, 250 MHz): δ = 1.55 (s br, 6H, Ad, C
S) cm−1; 1H NMR (DMSO-d6, 250 MHz): δ = 1.55 (s br, 6H, Ad, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3), 1.83 (s, 6H, Ad), 1.91 (s, 6H, Ad), 5.197 (s, 1H, C(4)-H), 7.19 (s, 1H, NH), 7.12–7.36 (m, 4H, Ar-H), 9.204 (s, 1H, NH), 9.805 (s, 1H, NH) ppm; 13C NMR (DMSO-d6, 62.5 MHz): δ = 16.73 (C
C–CH3), 1.83 (s, 6H, Ad), 1.91 (s, 6H, Ad), 5.197 (s, 1H, C(4)-H), 7.19 (s, 1H, NH), 7.12–7.36 (m, 4H, Ar-H), 9.204 (s, 1H, NH), 9.805 (s, 1H, NH) ppm; 13C NMR (DMSO-d6, 62.5 MHz): δ = 16.73 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3), 29.20, 36.45, 40, 51.73 (Ad), 55.32 (CH), 108.36, 159 (C
C–CH3), 29.20, 36.45, 40, 51.73 (Ad), 55.32 (CH), 108.36, 159 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 125.41 (C2, Ar),126.64 (C6, Ar), 127.82 (C4, Ar), 130.82 (C5, Ar), 133.45 (C3, Ar), 145.86 (C1, Ar), 165.81 (C
C), 125.41 (C2, Ar),126.64 (C6, Ar), 127.82 (C4, Ar), 130.82 (C5, Ar), 133.45 (C3, Ar), 145.86 (C1, Ar), 165.81 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 174.67 (C
O), 174.67 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) ppm; anal. calcd for C22H26N3ClOS: C, 63.52; H, 6.30; N, 10.10. Found: C, 67.19; H, 6.36; N, 10.24.
S) ppm; anal. calcd for C22H26N3ClOS: C, 63.52; H, 6.30; N, 10.10. Found: C, 67.19; H, 6.36; N, 10.24.
3.2 Biological activity of IIa–k
RPMI-1640 medium, penicillin, streptomycin, fetal calf serum, sodium bicarbonate, phosphate-buffered saline, trypsin, gentamycin sulfate, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), paraformaldehyde, and 4,6-diamidino-2-phenylindole (DAPI) were obtained from Sigma Chemicals Co. Cells undergoing early and late apoptosis were easily distinguished using the eBioscience Annexin V Apoptosis Detection Kit (Annexin V-FITC kit, eBioscience, Vienna, Austria).4. Conclusion
In this study, we developed a solvent-free method for synthesizing DHPMs using TFA as an efficient catalyst for the Biginelli-type reaction. This method enables the one-pot, three-component condensation of N-(adamant-1-yl)acetoacetamide, benzaldehyde derivatives, and thiourea. The technique improves upon the original Biginelli reaction by enhancing yields and reducing reaction times under solvent-free conditions. A diverse library of DHPM analogs was synthesized using this approach, and many derivatives exhibited significant anti-proliferative activity against A-549 lung cancer cells. Notably, compounds IIb, IIj, IId, and IIg demonstrated particularly high cytotoxic activity. As can be seen, the compound 5-(1-adamantylcarbamoyl)-6-methyl-4-(2-methylphenyl)-3,4-dihydropyrimidin-2(1H)-thione (IIb) showed even better cytotoxicity manner than the common anticancer drug (doxorubicin). Further analysis with DAPI staining and Annexin V-FITC apoptosis assays revealed that these compounds induced apoptosis in A-549 cells. Overall, the synthesized DHPM derivatives show promising potential for development into novel cancer therapeutics with appropriate structural modifications.Data availability
The data supporting the findings of this study are included in the ESI† file. Additional data will be available upon the request.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors acknowledge Iran National Science Foundation (INSF) and the Research Office of Azarbaijan Shahid Madani University for financial support with funding grant numbers: 98003283 and 1401/60, respectively. This work has also been supported financially by the Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran (Grant number: 66672).References
- R. L. Siegel, K. D. Miller and A. Jemal, Ca-Cancer J. Clin., 2019, 69, 7 CrossRef PubMed.
- T. U. Mayer, T. M. Kapoor, S. J. Haggarty, R. W. King, S. L. Schreiber and T. J. Mitchison, Sci., 1999, 286, 971 CrossRef CAS PubMed.
- E. W. Hurst and R. Hull, J. Med. Chem., 1960, 3, 215 CrossRef PubMed.
- E. Klein, S. DeBonis, B. Thiede, D. A. Skoufias, F. Kozielski and L. Lebeau, Bioorg. Med. Chem., 2007, 15, 6474 CrossRef CAS PubMed.
- B. Ramesh and C. M. Bhalgat, Eur. J. Med. Chem., 2011, 46, 1882 CrossRef CAS PubMed.
- G. C. Rovnyak, K. S. Atwal, A. Hedberg, S. D. Kimball, S. Moreland, J. Z. Gougoutas, B. C. O'Reilly, J. Schwartz and M. F. Malley, J. Med. Chem., 1992, 35, 3254 CrossRef CAS PubMed.
- T. M. Kapoor, T. U. Mayer, M. L. Coughlin and T. J. Mitchison, J. Cell Biol., 2000, 150, 975 CrossRef CAS PubMed.
- R. Kaur, P. Kaur, S. Sharma, G. Singh, S. Mehndiratta, P. MS Bedi and K. Nepali, Recent Pat. Anti-Cancer Drug Discovery, 2015, 10, 23 CrossRef CAS PubMed.
- T. Pramanik and A. H. Pathan, Res. J. Pharm., Biol. Chem. Sci., 2014, 5, 444 CAS.
- A. Bhatewara, S. R. Jetti, T. Kadre, P. Paliwal and S. Jain, Int. J. Med. Chem., 2013, 2013(1), 197612 Search PubMed.
- S. Farooq, F. A. Alharthi, A. Alsalme, A. Hussain, A. A. Dar, A. Hamid and S. Koul, RSC Adv., 2020, 10, 42221 RSC.
- M. Najjar, M. A. Nasseri, M. Darroudi and A. Allahresani, J. Environ. Chem. Eng., 2022, 10, 108854 CrossRef CAS.
- L. H. Al-Wahaibi, H. M. Hassan, A. M. Abo-Kamar, H. A. Ghabbour and A. A. El-Emam, Mol, 2017, 22, 710 CrossRef PubMed.
- M. Abkar Aras and A. Moshtaghi Zonouz, J. Sulfur Chem., 2023, 44, 361 CrossRef CAS.
- H. Mousazadeh, M. Milani, N. Zarghami, E. Alizadeh and K. D. Safa, Basic Clin. Pharmacol. Toxicol., 2017, 121, 390 CrossRef CAS PubMed.
- (a) B. I. Tarnowski, F. G. Spinale and J. H. Nicholson, Biotech. Histochem., 1991, 66, 296 CrossRef; (b) L. Li, L. Dong, J. Zhang, F. Gao, J. Hui and J. Yan, Int. J. Mol. Med., 2019, 43, 1241 CAS.
- (a) S. Sana, R. Tokala, D. M. Bajaj, N. Nagesh, K. K. Bokara, G. Kiranmai, U. J. Lakshmi, S. Vadlamani, V. Talla and N. Shankaraiah, Bioorg. Chem., 2019, 93, 103317 CrossRef PubMed; (b) J. Dowarah, D. Patel, B. N. Marak, U. C. S. Yadav, P. K. Shah, P. K. Shukla and V. P. Singh, RSC Adv., 2021, 11, 35737 RSC.
- M. A. Aras, N. Jafari, A. M. Zonouz and H. Hamishehkar, J. Mol. Struct., 2025, 1321, 139708 CrossRef.
- N. Jafari, H. Hamishehkar and M. Mohammadpourfard, Algal Res., 2024, 103560 CrossRef.
- N. Jafari, M. Mohammadpourfard and H. Hamishehkar, J. Drug Deliv. Sci. Technol., 2024, 100, 106133 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00284b |
| This journal is © The Royal Society of Chemistry 2025 |