Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fabrication and electrochemical study of copper doped zinc sulfide/graphene nanocomposites for supercapacitors†
Shumaila Saleemab,
Sadia Khalid *b,
Aalia Nazira,
Yaqoob Khanb,
Imtiaz Ahmadbc and
Rameeshabd
*b,
Aalia Nazira,
Yaqoob Khanb,
Imtiaz Ahmadbc and
Rameeshabd
aInstitute of Physics, The Islamia University of Bahawalpur, Bahawalpur, 63100, Pakistan
bNanosciences & Technology Department, National Centre for Physics, Quaid-e-Azam University Campus, Shahdra Valley Road, Islamabad, 45320, Pakistan. E-mail: sadiabzu@gmail.com; sadia.khalid@ncp.edu.pk
cDepartment of Physics, University of Science and Technology (UET), Lahore, 54890, Pakistan
dDepartment of Physics, Faculty of Science, University of Sialkot, Sialkot, 50700, Pakistan
First published on 11th July 2025
Abstract
Transition metal sulfides exhibit excellent electrochemical performance and electrochemical energy storage capacity. Herein, we present high-capacity supercapacitor electrode based on copper doped zinc sulfide/graphene (ZCG) synthesized by co-precipitation method. Various techniques have been employed to characterize the ZCG nanocomposite including electrochemical measurements. The ZCG nanocomposites exhibit high crystallinity and phase purity. In three-electrode system and 1 M aqueous KOH solution, the prepared ZCG electrodes are evaluated using galvanostatic charge–discharge cycles (GCD), cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS). The ZCG electrode exhibits an ultrahigh specific capacitance of 2295 F g−1 at a relatively low scan rate of 5 mV s−1 from CV and 743 F g−1 at 100 A g−1 from GCD, with exceptional cycling stability (93% capacity retention after 1000 cycles). Furthermore, the ZCG10 symmetric coin cell exhibits a specific capacitance, energy density, power density of 130.8 F g−1, 18 W h kg−1, 2400 W kg−1 at current density of 1.2 A g−1 in a 1 M KOH solution from GCD. The ZCG hybrid electrode material can be predicted a potential hybrid electrode material for the future development of energy storage devices.
Introduction
For decades, scientists have been working hard to develop electrochemical energy storage systems with ideal capacitance values (in the hundreds of F g−1). The initial investigations have shown the unexpected potential of supercapacitors and established a novel way for the advancement of electrochemical energy storage devices.1 Renewable energy technologies are considered as significant opportunities for the future and require further advancement in this century to assume a major role in energy production. Numerous energy storage technologies are now available, each with varying levels of development.2 Energy storage systems involve a range of energy storage technologies such as redox flow batteries, rechargeable batteries, fuel cells, and supercapacitors.3,4Supercapacitors, also known as electrochemical capacitors, store electric charges through static adsorption (electric double-layer capacitance) or redox reaction (pseudocapacitance) mechanisms. Supercapacitors possess distinctive features including high power density, rapid charging or discharging rates, extended lifespan, and secure operation. Because of environmentally friendly electrode materials, supercapacitors are extensively employed in many commercial applications such as braking systems for automobiles and elevators, electrical grid stability, electric buses, computer memory backup, and consumer electronics like Samsung's stylus pen.5,6
Transition metal-based electrode materials are very favourable electrodes for supercapacitors (SCs) due to their remarkable electrochemical stability, specific power, and rapid charging/discharging rates.7,8 Transition metal sulfides (TMSs) are gaining recognition due to their unique electrical and electrochemical characteristics. Ongoing research has prioritized the use of readily available and non-harmful materials for electrode production, with the aim of achieving cost-effectiveness. TMSs have improved electrochemical performance compared to transition metal oxides (TMOs) due to the substitution of oxygen atoms with sulfur atoms. The characteristics and effectiveness of TMSs are strongly linked to their configurations, dimensions, and surface morphologies.9,10 However, these metal sulfides encounter challenges such as limited electrochemical stability, short lifespan, and low energy density.11
Graphene, an extremely thin sheet consisting of carbon atoms connected in a planar arrangement with sp2 bonds, has garnered significant interest because of its exceptional electrical, mechanical, thermal, and optical characteristics. The integration of graphene with inorganic materials including metals, metal oxides, and metal sulfides has garnered significant attention in recent years due to their versatile capabilities. Various synthesis methods have been developed and implemented to achieve graphene-based nanocomposites with controlled features.12 It has acquired significant attention because to its non-toxicity, cost-effectiveness, environmental friendliness, and absence of heavy metals.6,12,13 Graphene exhibits a significant ability to transport charge and is compatible with living organisms, enabling effective separation of charges in composite structures.14 Graphene, due to its extensive surface area, can serve as a conductive for the separation and transmission of electrons and holes.
ZnS possesses a wide band gap (3.5–3.8 eV), making it a suitable host for metal doping, including substitutional doping at the zinc sites. Recent reports indicate that the cubic form of ZnS is the most stable crystallographic form at room temperature. However, it undergoes a transformation to the hexagonal form when subjected to heat treatment at temperatures over 1020 °C.15 Moreover, doping with transition metals can significantly enhance electrochemical performance in binary metal sulfides, resulting the superior electrochemical performance compared to single transition metal sulfides. Therefore, it is more important to enhance the electrochemical efficiency of pseudocapacitors by the enhancement of their electrode internal structure, morphology and surface area.16,17
In recent years, there has been significant interest in weakly magnetic and paramagnetic ion-doped semiconductors.18 Doping improves semiconductor properties by giving a better way for controlling their optical, electrical, and ion transport properties.19,20 Cu2+ is a favourable metallic option for introducing impurities into zinc metal, beside chalcogenide. This is due to the almost same ionic radii of both cations, which is a crucial feature in the doping process.21–24 By adding the small amount of Cu into ZnS lattice, both the elemental composition and morphology can be modified. Furthermore, the material attains exceptionally high electrochemical performance, which offers a promising direction for development of future high performance electrode materials.25 Asfaram et al.26 studied the synthesis and analysis of Cu2+ doped ZnS on activated carbon for the purpose of simultaneous ternary adsorption of dyes. Hussain et al.16 studied the improved electrochemical performance and cycle stability of Cu doped ZnS nanostructures, which was due to interconnecting polyhedron-like structures, and binder-free electrode design.
Electrochemical performance of a supercapacitor electrode has stayed a challenge. Aim of this work is to improve electrochemical performance of electrode material by rational designing of electrode material. This can be achieved using strategies like doping and introduction of carbon nanomaterial. The combination of Cu doped ZnS and graphene creates a synergistic effect with enhanced electrochemical properties. Graphene, known for its high surface area, electrical conductivity, and mechanical strength, can improve the overall performance of the supercapacitor electrode when combined with Cu doped ZnS. It has been observed that a high specific capacitance and longer cyclic stability can be attained using graphene.27,28
The other challenge is agglomeration of metal sulfide nanoparticles that lowers the specific capacitance of the electrode material by reducing active surface area.29 In present work, Triton X-100 (TX-100), a non-ionic surfactant has been used that played an indispensable role in controlling the particle size of the synthesized ZnS nanoparticles, acting as a surfactant that limited agglomeration and promoted uniform nucleation.30 The presence of TX-100 during the coprecipitation process led to smaller and more uniform ZnS particles, likely due to its ability to stabilize the growing crystals and prevent uncontrolled aggregation.31
In this work, pure ZnS, copper-doped ZnS (ZCS) nanoparticles, and copper-doped ZnS/graphene (ZCG) are fabricated via the simple and economic coprecipitation method. In this study, the optimal Cu doping concentration in ZnS has been evaluated in order to improve its properties in energy storage applications such as supercapacitor. In the nanoscale regime, graphene assisted in the manipulation of electronic energy states and surface area. Due to these qualities, ZCS and ZCG are viable materials for energy applications. To validate the formation of ZCS and ZCG nanoparticles, structural, morphological, optical, and thermal properties, specific surface area and chemical state of samples have been conducted, using powder XRD, FE-SEM, DRS, FTIR, TGA, BET and XPS. Using CV, GCD and EIS, electrochemical behaviour has been investigated. Furthermore, effect of doping concentration on the electrochemical performance of nanocomposites has been comprehensively studied. The overall findings revealed improved electrochemical performance of composites as compared to pure ZnS. This valuable insights about the ZCG nanocomposite opens the economic avenues towards facile synthesis of highly efficient reproducible supercapacitor electrode materials.
Experiment
Material and methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05 ratio of ZS or ZCS to G suspensions in ultrasonic bath (Fig. 3). The samples were labelled as ZG, ZCG2, ZCG4, ZCG6, ZCG8, ZCG10.
0.05 ratio of ZS or ZCS to G suspensions in ultrasonic bath (Fig. 3). The samples were labelled as ZG, ZCG2, ZCG4, ZCG6, ZCG8, ZCG10.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and then dried in the air. The fabrication of working electrode was done using a simple one-step sonication procedure. The suspension of ZCG nanocomposite, carbon black (conductive agent), and PVA as binder was prepared by using sonication method for 1 hour and deposited onto a GCE. The mass loading of working electrode was 0.5 μg. Working electrode of ZCG nanocomposite was dried and used for electrochemical measurements.
1) and then dried in the air. The fabrication of working electrode was done using a simple one-step sonication procedure. The suspension of ZCG nanocomposite, carbon black (conductive agent), and PVA as binder was prepared by using sonication method for 1 hour and deposited onto a GCE. The mass loading of working electrode was 0.5 μg. Working electrode of ZCG nanocomposite was dried and used for electrochemical measurements.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and then dried in oven. For ZCG10 coin cell fabrication, active material (90%), carbon black (5%) and binder (5%) were used to fabricate electrodes.
1) and then dried in oven. For ZCG10 coin cell fabrication, active material (90%), carbon black (5%) and binder (5%) were used to fabricate electrodes.Characterizations
Bragg's equation was used to calculate interplanar spacing (d) between diffracting planes in pure ZnS, ZCS, and ZCG nanocomposites:
2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = nλ θ = nλ
| (1) |
By using Scherrer eqn (2) the crystallite size was calculated.
 | (2) |
The band gap energy was evaluated from diffuse reflectance spectra.
 | (3) |
The relationship between band gap and the absorption coefficient of a semiconductor material is as follows:
| (αhν)n = A(hν − Eg) | (4) |
 | (5) |
Using the eqn (6), the Cs was calculated from GCD curves.40
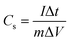 | (6) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dt” is area under the discharge curve, and “ΔV” is a potential window (V). Coulombic efficiency of ZCG nanocomposites were calculated using the eqn (7).41
dt” is area under the discharge curve, and “ΔV” is a potential window (V). Coulombic efficiency of ZCG nanocomposites were calculated using the eqn (7).41
 | (7) |
Bode plots, on other hand, help to figure out how fast the electrode discharge at a phase angle 45°. This is called the relaxation time (τ).42 The relation between frequency response (knee frequency, fo) and the response time constant τ of supercapacitor can be expressed by eqn (8).43
 | (8) |
 | (9) |
Cs from GCD data was calculated using eqn (10).
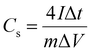 | (10) |
The power density (W kg−1) and energy density (W h kg−1) were calculated according to following eqn (11) and (12).
 | (11) |
 | (12) |
Results and discussion
XRD
XRD analysis was used to investigate crystallographic information of synthesized ZS, ZCS and ZCG (Fig. 4a and b). Cubic crystal system observed in accordance with the sphalerite ZnS. The diffraction peaks at 28.29°, 47.81°, and 57.16°, correspond to the (111), (022), and (131) crystal planes, similar to diffraction peaks of face-centered cubic sphalerite ZnS structure (JCPDS 96-500-0089). The crystallite size of prepared nanomaterials at high intensity peak are shown in (Table 1). Further, the un-doped ZnS powder had stronger peaks than the doped ones, and as the Cu-doping concertation increases, the peaks become broader. This suggests that the addition of Cu-doping lessens the nanocrystalline nature of the powder. The lack of extra peaks indicated that the prepared powders had no impurity phases.45 | ||
| Fig. 4 XRD patterns of (a) standard ZnS, ZS, ZCS2, ZCS4, ZCS6, ZCS8, ZCS10 and (b) standard ZnS, ZG. ZCG2, ZCG4, ZCG6, ZCG8, ZCG10. | ||
| Sample | 2θ (°) | FWHM (°) | Crystallite size (nm) |
|---|---|---|---|
| ZS | 28.441 | 0.7085 | 11.57 |
| ZCS2 | 28.8953 | 1.152 | 7.12 |
| ZCS4 | 28.8984 | 2.9023 | 2.82 |
| ZCS6 | 29.1472 | 3.03753 | 2.70 |
| ZCS8 | 29.2441 | 3.11455 | 2.65 |
| ZCS10 | 29.3217 | 3.16545 | 2.59 |
| ZG | 28.7209 | 1.152 | 7.12 |
| ZCG2 | 28.9728 | 3.00881 | 2.73 |
| ZCG4 | 28.9728 | 3.7574 | 2.18 |
| ZCG6 | 29.0697 | 4.17287 | 1.97 |
| ZCG8 | 29.1473 | 4.20025 | 1.96 |
| ZCG10 | 29.1473 | 4.2994 | 1.91 |
However, due to the presence of graphene in the synthesized nanocomposite, no additional diffraction peaks were found. This might be attributed to the addition of minute quantities of graphene during the synthesis procedure.46
As the copper content increased, the as-prepared crystallite size of nanomaterials decreased from 7.12 nm to 2.65 nm. This could be variation in ionic radius between Cu2+ and Zn2+ ions. When Cu2+ ions are added to ZnS, gap between Cu2+ and Zn2+ ions in the ZnS lattice decreases because Cu2+ (73 pm) has a lower ionic radius than Zn2+ (74 pm). With copper doping of the ZCS particles, a reduction in crystallite size was observed due to the substitutional impurity behaviour of the dopant ion. A comparable trend was also documented in literature regarding ZnS thin films sprayed with Cu and dip-coated with Cu and Al-doping.45,47
The XRD patterns show that diffraction peaks shift towards higher 2θ angle due to Cu doping. This right shift usually causes the expansion of crystal lattice (Table 1). Consequently, the lattice parameter changes confirming the substitution of Cu2+ for Zn2+ in the crystal lattice.48
FE-SEM
The FE-SEM was used to analyse surface morphology of both pure and doped ZnS, ZCS and ZCG samples (Fig. 5). The FE-SEM demonstrates that ZCS samples exhibit a spherical morphology with a random size distribution for different Cu concentrations. The addition of Cu resulted in a decrease in particle size compared to pure ZnS nanoparticles. The particle size of as-prepared samples decreased from 55 nm to 22 nm with increase of Cu concentration. When Cu is doped into ZnS, Cu ions typically replace some Zn ions in the crystal lattice. Since Cu2+ ions (ionic radius 73 pm) are smaller than Zn2+ ions (ionic radius 74 pm), this substitution introduces lattice distortions or strain.45,47 Therefore, particle size of ZCS nanomaterials decrease with the increase of Cu concentration.The morphological study of the ZnS nanoparticles is uniformly attached to surface of graphene sheets (Fig. 6). The absence of graphene, no uniform distribution of ZnS were formed using the similar conditions used for synthesis of ZCG nanocomposites. These results clearly suggests that existence of graphene play an important role in formation of homogeneous ZnS aggregates. ZG and ZCG nanocomposites showed the sheets like structure.
TGA/DSC
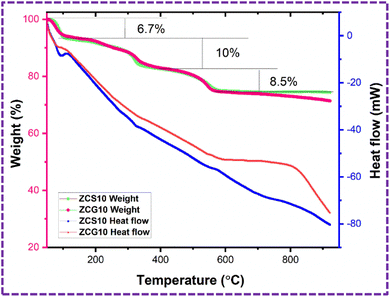
TGA/DSC was performed to assess thermal stability of ZCS10 and ZCG10 nanocomposite. TGA curve of ZCS10 and ZCG10 exhibited three distinct weight loss segments between 74–100% and 71–100% representing only 26% and 29% of the total weight (Fig. 7). According to the analysis, the residual solvents evaporate at below 100 °C49 6.7% of the sample's weight loss at temperature 71–105 °C was ascribed to the loss or evaporation of bound water molecules.50 The second weight loss, which occurs at temperature 105–345 °C, is 10% which attributed to structure changes of Cu-doped ZnS.51 The weight loss recorded in third region at 345–570 °C is 7.9% due to the thermal degradation of the carbon-chain framework52 and unstable components in the presence of samples.49 There is no weight loss after 570 °C. The final weight of the ZCS10 and ZCG10 nanocomposite is 74.3% and 71.3%. The weight loss in ZCG10 is more than ZCS10 which is attributed to the removal of carbon containing groups.53The DSC associated with TGA of ZCS10 and ZCG10 nanocomposite exhibited heat flow modify with temperature and was used to determine crystallization and melting temperature. The crystallization temperatures of ZCS10 were determined at 326 °C and 576 °C and crystallization temperature of ZCG10 were at 347 °C and 557 °C.
XPS
Fig. 8 presents the XPS analysis of chemical compositions and metal oxidation states of ZCS10. The survey spectrum depicted in Fig. 8a presents the existence of C 1s, Zn 2p, Cu 2p3/2, and S 2p atoms in the ZCS10 composition. The C 1s exhibits three different signals, with the primary peak located at 284.1 eV attributed to sp2 and sp3 C–H/C–C carbon atoms. A secondary component is mostly attributed to C–O and C–N chemical environments at ∼286 eV peak. The peak component centred at 288.1 eV is finally attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (Fig. 8b).54 The presence of Zn2+ oxidation states is indicated by binding energy of Zn 2p3/2 and Zn 2p1/2 at 1021 and 1043 eV (Fig. 8c). There is no visible peak shift after Cu doping.55 For the copper dopant, binding energies of Cu 2p3/2 and Cu 2p1/2 are 932 eV and 952 eV, as seen in Fig. 8d. These values can be attributed to either metallic or cationic Cu species according to previous studies.55,56 The spin–orbit interaction divides the S 2p level into two states with an energy difference of 1.2 eV. The S 2p1/2 and S 2p3/2 levels were detected at 162.2 eV and 161.0 eV, respectively, corresponding to ZnS. At 169.0 eV, which corresponds to SO4 of metal ions, oxidized sulfur was detected (Fig. 11e).57 All spectra confirm the chemical composition of the ZCS10.58 The XPS results further demonstrated the successful formation of composite.
O (Fig. 8b).54 The presence of Zn2+ oxidation states is indicated by binding energy of Zn 2p3/2 and Zn 2p1/2 at 1021 and 1043 eV (Fig. 8c). There is no visible peak shift after Cu doping.55 For the copper dopant, binding energies of Cu 2p3/2 and Cu 2p1/2 are 932 eV and 952 eV, as seen in Fig. 8d. These values can be attributed to either metallic or cationic Cu species according to previous studies.55,56 The spin–orbit interaction divides the S 2p level into two states with an energy difference of 1.2 eV. The S 2p1/2 and S 2p3/2 levels were detected at 162.2 eV and 161.0 eV, respectively, corresponding to ZnS. At 169.0 eV, which corresponds to SO4 of metal ions, oxidized sulfur was detected (Fig. 11e).57 All spectra confirm the chemical composition of the ZCS10.58 The XPS results further demonstrated the successful formation of composite.
 | ||
| Fig. 8 (a) XPS survey spectra of ZCS10 (b) spectra of C 1s (c) spectra of Zn 2p, (d) spectra of Cu 2p3/2, (e) spectra of S 2p. | ||
Fig. 9 presents the XPS analysis of chemical compositions and metal oxidation states of ZCG10. The survey spectrum depicted in Fig. 9a presents the existence of C 1s, Zn 2p, Cu 2p3/2, and S 2p atoms in the ZCS10 composition. The C1s exhibits three different signals, with the primary peak located at 284.6 eV attributed to sp2 and sp3 C–H/C–C carbon atoms. A secondary component is mostly attributed to C–O and C–N chemical environments at ∼286 eV peak. The peak component cantered at 288.5 eV is finally attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (Fig. 9b) The presence of Zn2+ oxidation states is indicated by binding energy of Zn 2p3/2 and Zn 2p1/2 at 1021 and 1043 eV (Fig. 9c). There is no visible peak shift after Cu doping.55 For the copper dopant, binding energies of Cu 2p3/2 and Cu 2p1/2 are 932 eV and 952 eV, as seen in Fig. 9d. These values can be attributed to either metallic or cationic Cu species according to previous studies.55,56 The S 2p1/2 and S 2p3/2 levels were detected at 162.5 eV, 161.7 eV and 161.7 eV, respectively, corresponding to ZnS. At 168.5 eV, which corresponds to SO4 of metal ions, oxidized sulfur was detected (Fig. 9e).57 All spectra confirm the chemical composition of the ZCS10.58 The XPS results further demonstrated the successful formation of composite.
O (Fig. 9b) The presence of Zn2+ oxidation states is indicated by binding energy of Zn 2p3/2 and Zn 2p1/2 at 1021 and 1043 eV (Fig. 9c). There is no visible peak shift after Cu doping.55 For the copper dopant, binding energies of Cu 2p3/2 and Cu 2p1/2 are 932 eV and 952 eV, as seen in Fig. 9d. These values can be attributed to either metallic or cationic Cu species according to previous studies.55,56 The S 2p1/2 and S 2p3/2 levels were detected at 162.5 eV, 161.7 eV and 161.7 eV, respectively, corresponding to ZnS. At 168.5 eV, which corresponds to SO4 of metal ions, oxidized sulfur was detected (Fig. 9e).57 All spectra confirm the chemical composition of the ZCS10.58 The XPS results further demonstrated the successful formation of composite.
 | ||
| Fig. 9 (a) XPS survey spectra of ZCG10 (b) spectra of C 1s (c) spectra of Zn 2p, (d) spectra of Cu 2p3/2, (e) spectra of S 2p. | ||
BET
The BET method is a standard approach used for measuring specific surface area, pore size and pore volume. The large surface area is a primary parameter for supercapacitors.59 The efficiency of supercapacitors can be improved by the enhanced transport of electrons and ions at the interface, as indicated by the BET analysis of ZCG10 nanocomposites with a large surface area. Additionally, the electrode storage capacity is increased by the presence of larger surface interfaces for charge (electrolyte ions) storage, which is facilitated by the large surface area.The BET specific surface area, pore size and pore volume of the prepared sample was determined to be 104.3 m2 g−1, 1.99 nm and 0.052 cm3 g−1 for ZCG10 nanocomposite. The BET analysis indicates that ZCG10 nanocomposites with large surface area can enhance transport of electrons and ions at the interface, thereby improving the efficiency of supercapacitors.60
Electrochemical measurements
The electrochemical performance of ZCG nanocomposites was thoroughly evaluated by CV and GCD measurements at room temperature in three electrode system. When ZCG nanocomposites prepared for supercapacitor applications in 1 M KOH electrolyte, the electrochemical reactions can involve various processes including ion adsorption/desorption and redox reactions. Here's a representation of the electrochemical reactions:Adsorption of K+ ions onto graphene surface:
| Graphene + KOH → graphene_K + OH− | (13) |
Adsorption of OH− ions onto graphene surface:
| Graphene + OH− → graphene_OH | (14) |
Redox reactions involving Cu dopants and ZnS during charging and discharging:61
| Cu+ + 2OH− → Cu(OH)2 + 2e− | (15) |
The non-faradaic process may be due to the development of double layer at electrode/electrolyte interfaces during disinsertion/insertion of positive ions (K+) on ZnS nanoparticles.
| ZnS(surface) + K+ + e−(ZnS–K+)surface | (16) |
The faradaic reaction process is also detected in ZnS causes slight deviation of rectangular shape in CV curve at all the scan rates. The possible faradaic process can be explained as follows,62,63
| ZnS + OH− ⇌ ZnSOH + e− | (17) |
| ZnSOH + OH− ⇌ H2O + ZnSO + e− | (18) |
A three-electrode system was used to investigate electrochemical measurements. Fig. 10 displays the comparative CV curves of ZG, ZCG2, ZCG4, ZCG6, ZCG8, and ZCG10 nanocomposites. The curves were obtained using a constant scan rate of 5–350 mV s−1 and within the potential window −0.6 to 0.6, CV curves demonstrate that the Cu-doped ZnS nanocomposites exhibit larger areas under their CV curves compared to the undoped ZnS, therefore emphasizing the significant contribution of Cu in enhancing the electrochemical performance. The Cs of the ZCG nanocomposites curves increases with the increase of Cu concentration.16 The CV curves exhibit a capacitive behaviour with nearly a rectangular shape, denoting a reversible system.64 The calculated values are given in Table 2. The enhanced electrochemical characteristics of ZCG10 can be attributed to presence of redox-peaks and electrochemically active nature of the doped electrode materials compared to the ZG nanocomposite.16 The redox peaks become higher with increase of scan rate. The specific capacitance of ZCG10 nanocomposite is higher than other nanocomposites (Fig. 11). Furthermore, the CV curves exhibited excellent stability even at a rapid scan rate of 350 mV s−1, indicating a fast electrochemical response.
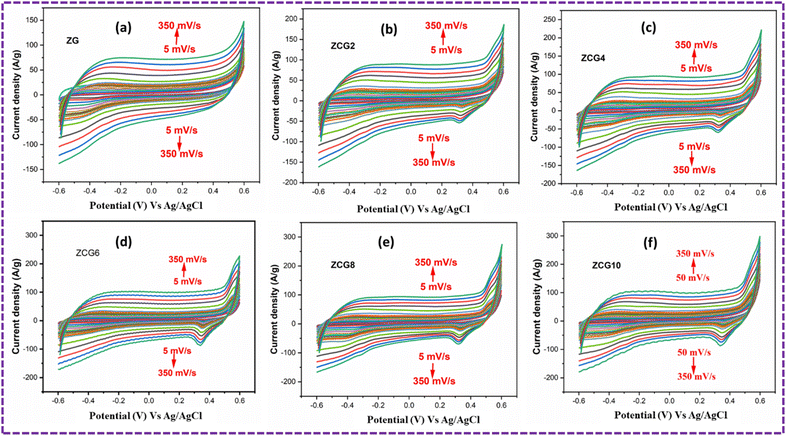 | ||
| Fig. 10 CV curves of all samples at 5–350 mV s−1 scan rates in three electrode system. (a) ZG, (b) ZCG2, (c) ZCG4, (d) ZCG6, (e) ZCG8 and (f) ZCG10. | ||
| Samples | Electrolyte (M KOH) | Scan rate (mV s−1) | Specific capacitance (F g−1) | Efficiency (%) |
|---|---|---|---|---|
| ZG | 1 | 5 | 1312 | 99 |
| ZCG2 | 1 | 5 | 1381 | 94.7 |
| ZCG4 | 1 | 5 | 1749 | 89.1 |
| ZCG6 | 1 | 5 | 1813 | 88.9 |
| ZCG8 | 1 | 5 | 1848 | 88.8 |
| ZCG10 | 1 | 5 | 2295 | 85.8 |
The kinetics of the charge storage mechanism of ZCG10 nanocomposites electrodes were analysed from CV curves (Fig. 12). The graph between the scan rate (mV s−1) and peak current densities (A g−1) is shown (Fig. 12a) which corresponds to a linear relationship and indicates that the charge storage is hybrid (Fig. 12b). for further confirmation of this hypothesis, we used the slopes of the log(i) vs. log(v) plot to derive b values (Fig. 12d) via equations.65
| i = avb | (19) |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i = log i = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a + b a + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v v
| (20) |
The ZCG electrode has a EDLC behavior, as indicated by b values in the range. It is known that diffusion-controlled mechanism occurs when b = 0.5, while capacitive contribution occurs when b = 1. The percentage of charge contributed by diffusion and capacitance may also be estimated by equations.65,66
Eqn (15) has been used to evaluate percentage of diffusion and capacitive charge contribution.65
| i(V) = k1v + k2v1/2 | (21) |
| Samples | Electrolyte (M KOH) | Specific capacitance (F g−1) | Energy density (W h kg−1) | Power density (W kg−1) | Capacitive retention (%) | Columbic efficiency (%) |
|---|---|---|---|---|---|---|
| ZG | 1 | 473.4 | 23.66 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 657.3 657.3 |
98 after 1000 cycles | 96 after 1000 cycles |
| ZCG2 | 1 | 507.4 | 25.36 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 355.59 355.59 |
94 after 1000 cycles | 99 after 1000 cycles |
| ZCG4 | 1 | 517.3 | 25.85 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 109.53 109.53 |
91 after 1000 cycles | 99 after 1000 cycles |
| ZCG6 | 1 | 528.2 | 26.40 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 077.65 077.65 |
90 after 1000 cycles | 93 after 1000 cycles |
| ZCG8 | 1 | 589.4 | 29.46 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 989.43 989.43 |
90 after 1000 cycles | 93 after 1000 cycles |
| ZCG10 | 1 | 669.9 | 33.48 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 782.19 782.19 |
89 after 1000 cycles | 93 after 1000 cycles |
Fig. 13a shows the 1st and 100th cycle of CV curves at 50 mV s−1 which is used to calculated the efficiency of nanocomposites.
 | ||
| Fig. 13 Graph of efficiency comparison, (a) ZG, (b) ZCG2, (c) ZCG4, (d) ZCG6, (e) ZCG8 and (f) ZCG10. | ||
GCD curves of optimal ZCG electrodes with various current densities were also evaluated at potential range 0f 0.0–0.6 V in Fig. 14. It was evident that at various current densities, the electrodes of nanocomposites exhibited almost similar curves at different current densities. GCD curves have a isosceles triangular shape,67 indicating good electrochemically capacitive behaviour with less faradaic reaction.68,69 Furthermore, IR drop at different current densities is quite small. This confirms the capacitive characteristics of the system, which demonstrate excellent electrochemical reversibility. The electrodes specific capacitance was determined using eqn (6). The ZCG10 electrode has the greatest specific capacitance compared to all other electrodes under the given current densities. The findings suggest that charge transfer occurs at a rapid and effective rate, and that the ZCG10 electrode had retain its high capacitance at high current density.
 | ||
| Fig. 14 GCD at different current densities of (a) ZG, (b) ZCG2, (c) ZCG4, (d) ZCG6, (e) ZCG8 and (f) ZCG10. | ||
The cyclic stability of ZCG electrodes was measured at different current densities. After 1000 cycles, coulombic efficiency and capacitance retention of ZCG10 electrode was 89% and 93% Fig. 15. The GCD measurements are in Table 3.
 | ||
| Fig. 15 Cyclic performance of (a) ZG, (b) ZCG2, (c) ZCG4, (d) ZCG6, (e) ZCG8 and (f) ZCG10 after 1000 cycles. | ||
Fig. 16 illustrates the relationship between frequency and phase angle using a Bode plot, whereby the knee frequency is seen at a phase angle of 45° (ref. 70) which is 790.9, 510.5, 524.6, 510.5, 518.3, and 503.5 s−1. The phase angle and response time mentioned in Table 4.
 | ||
| Fig. 16 Graph between frequency and phase angle of, (a) ZG, (b) ZCG2, (c) ZCG4, (d) ZCG6, (e) ZCG8 and (f) ZCG10 before and after GCD. | ||
| Samples | Phase angle (degree) | Knee frequency (Hz) | Response time constant (s) |
|---|---|---|---|
| ZG | 78 | 790.9 | 0.0002 |
| ZCG2 | 76 | 510.5 | 0.00031 |
| ZCG4 | 76 | 524.6 | 0.00031 |
| ZCG6 | 76 | 510.5 | 0.00031 |
| ZCG8 | 75 | 518.3 | 0.00031 |
| ZCG10 | 77 | 503.5 | 0.00031 |
The Nyquist plot of ZCG electrodes were plotted (Fig. 17) represent the depressed semicircles with inclined lines were displayed in the high-frequency and low-frequency region, respectively (Fig. 17). Curiously, all of the electrodes had comparable EIS having negligible semicircle region, which is known as charge transfer resistance (Rct), which could be indicates a low faradaic rate of charge–discharge.71
 | ||
| Fig. 17 Nyquist plot of (a) ZG, (b) ZCG2, (c) ZCG4, (d) ZCG6, (e) ZCG8 and (f) ZCG10 after 1000 cycles before and after GCD. | ||
Fig. 18 shows the EIS study of ZCG10 nanocomposite. Bode plot illustrating the frequency response of the phase angle and impedance of the ZCG10 nanocomposite (Fig. 18a). (Fig. 18b) illustrates the relationship between frequency and phase angle using a Bode plot, whereby the knee frequency is seen at a phase angle of 45° (ref. 70) which is 503.5 s−1.
Fig. 18c shows the Nyquist plots of the ZCG10 nanocomposite electrode before and after the GCD cyclic stability. In both irradiated and dark cases, Nyquist plots exhibit a straight slope line at low frequencies and a prominent semicircle at high frequencies. The charge-transfer resistance at the electrode interface is represented by the diameters of the semicircles, while diffusion process of reactive species is represented by the straight sloping line. The model fitting was done by the Gamry Echem Analyst (inset in Fig. 18c). The term “Rs” refers to the resistance of the electrolyte, which includes the resistances related to contact and charge transfer at counter electrode/electrolyte interface.72 Rct refers to the resistance produced during electron transport. The fitting results showed the calculated values of Re and Rct for the electrodes. The ZCG10 electrode exhibits respective Re and Rct which are 60.2 Ω and 7 kΩ. The constant phase element (CPE) is a term used to describe the double layer capacitance. The Warburg impedance (Zw) represents the diffusion of reactive material at electrode surface and is depicted as a straight line in Nyquist plots.65,73
Additionally, ZCG electrodes exhibited high specific capacitance which is calculated by Creal (F) over frequency (Hz) (Fig. 18d). The response time of supercapacitors are calculated by Cimag (F) over frequency (Hz) (Fig. 18e). Fig. 18d in the electrolyte shows a higher specific capacitance before and after GCD which is 24 and 77 μF. Fig. 18e shows that the response time of the supercapacitors which is 0.17 s calculated from frequency and Cimag graph in the KOH electrolyte solution which ascribed to the charge transfer resistance.71 Table 5 represent the comparative study of ZCG10 electrode with previous published literature.
| Sr# | Material | Synthesis method | Electrolyte | Scan rate/current density | Specific capacitance | Potential window (V) | Cyclic stability | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | ZnS/graphene | Hydrothermal | 2 M KOH | 5 mV s−1 | 315.1 F g−1 | −0.2 to 0.3 | 63.2% after 1000 cycles | 74 |
| 2 | Cu-doped ZnS | Single-step glycol-assisted process | 3 M KOH | 1 A g−1 | 468 F g−1 | 0.0 to 0.5 | 90.5% after 5000 cycles | 16 |
| 3 | ZnS:Cu | Solvothermal | 3 M KOH | 1 A g−1 | 545 F g−1 | 0 to 0.4 | 87.4% after 5000 cycles | 75 |
| Cu doped ZnS/G | Coprecipitation | 1 M KOH | 100 A g−1 | 743 F g−1 | 0 to 0.6 | 89% after 1000 cycles | This work |
ZCG10 symmetric coin cell
To evaluate the practical application of ZCG10 electrodes, a coin cell was fabricated utilizing 1 M KOH aqueous electrolyte. Fig. 19 displays the CV curves of coin cell obtained at various potential windows. The results demonstrate that the greatest operational potential window achievable is 1 V. The CVs of the supercapacitor obtained using varying scan rates ranging from 10 to 50 mV s−1 (Fig. 19a). The CV curves exhibiting symmetric and rectangular shape with little lean, indicate typical capacitive characteristics, similar to the results obtained from three-electrode experiment. The specific capacitance of ZCG10 coin cell is 72 F g−1 calculated using the eqn (9). | ||
| Fig. 19 (a) CV curve of ZCG10 coin cell (b) Nyquist plot of ZCG10 coin cell (inset model fitting) (c) GCD curves of ZCG10 coin cell (d) cyclic performance o ZCG10 coin cell after 1000 cycles. | ||
Fig. 19b depicts the Nyquist plot of ZCG10 coin cell.
The GCD curves of ZCG10 coin cell in Fig. 19c. The fabricated cell exhibited a specific capacitance of 130.8 F g−1, an energy density of 18.06 W h kg−1 and a power density of 2400 W kg−1 when operating at a current density of 0.12 A g−1 calculated by eqn (10)–(12).76
The cycle stability of the ZCG10 coin cell after 1000 cycles was evaluated at a current density of 0.1 A g−1 (Fig. 19d). The capacitance retention dropped to about 75% after 200 cycles, but the tendency of the capacitance retention drops gradually in subsequent cycles. Finally, capacitance retention of the cell remained at 55% after 1000 cycles. The coulombic efficiency is about 99% at 0.1 A g−1 after 1000 cycles.58 Table 6 represents the electrochemical measurements of ZCG10 coin cell. Table 6 represents the electrochemical measurements of ZCG10 coin cell. Table 7 represent the comparative study of ZCG10 coin cell with previous published literature.
| Current density (A g−1) | Specific capacitance (F g−1) | Energy density (W h kg−1) | Power density (W kg−1) | Capacitive retention (%) | Columbic efficiency (%) |
|---|---|---|---|---|---|
| 1.2 | 130.8 | 18.06 | 2400 | 55 after 1000 cycles | 99 after 1000 cycles |
| Material | Electrolyte | Current density | Specific capacitance | Potential window (V) | Energy density (W h kg−1) | Power density (W kg−1) | Ref. |
|---|---|---|---|---|---|---|---|
| rGO/ZnO/PDAN | PVA/KOH | 0.6 A g−1 | 40 F g−1 | 0 to 1.2 | 12.66 | 933 | 77 |
| ZnS/g-C3N4 | 6 M KOH | 0.5 A g−1 | 92.8 F g−1 | 0 to 0.9 | 10.4 | 187.3 | 78 |
| ZnS/NiO | 1 M KOH | 0.8 A g−1 | 44.43 F g−1 | 0 to 1 | 5.52 | 1600 | 63 |
| Cu doped ZnS/G | 1 M KOH | 1.2 A g−1 | 130.78 F g−1 | 0 to 1 | 18 | 2400 | This work |
Conclusions
In this work, a Cu doped ZnS (ZCS) was synthesized via chemical coprecipitation method and graphene (G) is synthesized via electrochemical exfoliation method. After that ZCS/G (ZCG) nanocomposite prepared via ultrasonication method. Using appropriate analytical characterization techniques, the successful formation of ZCS, G and ZCG nanocomposite electrode was confirmed. XRD analysis confirmed the cubic phase of pure ZnS and ZCS. FE-SEM observation demonstrated that ZnS, ZCS and ZCG nanocomposite contains nanomaterials. The ZnS/NiO electrode exhibited remarkable electrochemical performance as an electrode material. The ZCG10 electrode exhibited an ultrahigh specific capacitance of 2295.04 F g−1 at a relatively low scan rate of 5 mV s−1 in a 1 M KOH solution from CV and 743 F g−1 at 100 A g−1 from GCD. After 1000 cycles, the ZCG10 nanomaterial demonstrated an enhanced and praiseworthy stability performance of 89%. Furthermore, the ZCG10 device exhibited a specific capacitance, energy density, power density of 130.8 F g−1, 18 W h kg−1, 2400 W kg−1 at current density of 1.2 A g−1 in a 1 M KOH solution from GCD. After 1000 cycles, the ZCG10 nanomaterial demonstrated stability performance of 55%. This ZCG nanocomposite is a potential candidate for high performance of supercapacitor devices and hybrid material design is an effective strategy for developing high-performance electrode materials.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank Mr Muzzamil (Experimental High Energy Physics (EHEP) Department, NCP Islamabad) for providing technical support.Notes and references
- B. E. Conway, Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications, Springer Science & Business Media, 2013 Search PubMed.
- F. Blaabjerg and D. M. Ionel, Renewable energy devices and systems–state-of-the-art technology, research and development, challenges and future trends, Electr. Power Compon. Syst., 2015, 43(12), 1319–1328 CrossRef.
- I. Staffell, et al., The role of hydrogen and fuel cells in the global energy system, Energy Environ. Sci., 2019, 12(2), 463–491 RSC.
- M. R. Thalji, et al., Cobalt-doped tungsten suboxides for supercapacitor applications, Chem. Eng. J., 2023, 473, 145341 CrossRef CAS.
- Z. Han, et al., Introduction to supercapacitors, Nanoscale Adv., 2023, 5(16), 4015–4017 RSC.
- S. Mohammadi and S. M. Mousavi-Khoshdel, Preparation of a Cu-Doped Graphene Oxide–Glutamine Nanocomposite for Supercapacitor Electrode Applications: An Experimental and Theoretical Study, ACS Appl. Electron. Mater., 2024, 6(6), 4108–4119 CrossRef CAS.
- W. Chu, et al., Trifunctional of phosphorus-doped NiCo2O4 nanowire materials for asymmetric supercapacitor, oxygen evolution reaction, and hydrogen evolution reaction, ACS Appl. Mater. Interfaces, 2019, 12(2), 2763–2772 CrossRef PubMed.
- J. Rehman, et al., Engineering of transition metal sulfide nanostructures as efficient electrodes for high-performance supercapacitors, ACS Appl. Energy Mater., 2022, 5(6), 6481–6498 CrossRef CAS.
- J. Theerthagiri, et al., Recent progress and emerging challenges of transition metal sulfides based composite electrodes for electrochemical supercapacitive energy storage, Ceram. Int., 2020, 46(10), 14317–14345 CrossRef CAS.
- W. Liu, et al., Ternary transition metal sulfides embedded in graphene nanosheets as both the anode and cathode for high-performance asymmetric supercapacitors, Chem. Mater., 2018, 30(3), 1055–1068 CrossRef CAS.
- S. Khalid, et al., Transition metal sulfides for supercapacitors, in Emerging Nanotechnologies for Renewable Energy, Elsevier, 2021, pp. 407–445 Search PubMed.
- A. M. Golsheikh, Graphene-based Metal/metal Sulphide Nanocomposites: Fabrication, Characterization and its Applications, University of Malaya, Malaysia, 2014 Search PubMed.
- M. Bhushan, et al., Graphene-doped ZnS nanoparticles synthesized via hydrothermal route for enhanced electrocatalytic performance, Int. J. Appl. Ceram. Technol., 2021, 18(5), 1510–1526 CrossRef CAS.
- X. Xu, et al., A graphene P–N junction induced by single-gate control of dielectric structures, J. Mater. Chem. C, 2019, 7(29), 8796–8802 RSC.
- C. S. Kamal, et al., Effect of structure, size and copper doping on the luminescence properties of ZnS, Mater. Res. Bull., 2016, 81, 127–133 CrossRef CAS.
- I. Hussain, et al., Glycol-assisted Cu-doped ZnS polyhedron-like structure as binder-free novel electrode materials, J. Saudi Chem. Soc., 2022, 26(4), 101510 CrossRef CAS.
- X. Zheng, et al., Rational design of a dual-gradient zincophilic–conductive interphase for dendrite-free zinc batteries, J. Mater. Chem. A, 2024, 12(25), 15352–15360 RSC.
- Z. Deng, et al., High-quality manganese-doped zinc sulfide quantum rods with tunable dual-color and multiphoton emissions, J. Am. Chem. Soc., 2011, 133(14), 5389–5396 CrossRef CAS PubMed.
- S. C. Erwin, et al., Doping semiconductor nanocrystals, Nature, 2005, 436(7047), 91–94 CrossRef CAS PubMed.
- Y. Yang, et al., Radial-position-controlled doping in CdS/ZnS core/shell nanocrystals, J. Am. Chem. Soc., 2006, 128(38), 12428–12429 CrossRef CAS PubMed.
- M. Sajjad, et al., Structural and optical properties of pure and copper doped zinc oxide nanoparticles, Results Phys., 2018, 9, 1301–1309 CrossRef.
- P. M. Pandian and A. Pandurangan, Copper nanoparticles anchored onto boron-doped graphene nanosheets for use as a high performance asymmetric solid-state supercapacitor, RSC Adv., 2019, 9(6), 3443–3461 RSC.
- A. Al Mahmud, et al., Copper-doped strontium metal-organic framework: Dual-function active material for supercapacitor and oxygen evolution reaction, Electrochim. Acta, 2024, 503, 144857 CrossRef.
- A. Kuzmin, et al., Examining the effect of Cu and Mn dopants on the structure of zinc blende ZnS nanopowders, Materials, 2023, 16(17), 5825 CrossRef CAS PubMed.
- Z. Zhang, et al., Construction of Cu-doped Ni–Co-based electrodes for high-performance supercapacitor applications, ACS Appl. Energy Mater., 2022, 5(6), 6642–6653 CrossRef CAS.
- A. Asfaram, et al., Simultaneous ultrasound-assisted ternary adsorption of dyes onto copper-doped zinc sulfide nanoparticles loaded on activated carbon: optimization by response surface methodology, Spectrochim. Acta, Part A, 2015, 145, 203–212 CrossRef CAS PubMed.
- C. Lee, et al., Measurement of the elastic properties and intrinsic strength of monolayer graphene, science, 2008, 321(5887), 385–388 CrossRef CAS PubMed.
- X. Xu, et al., Interface characteristics of graphene/ZnS hybrid-dimensional heterostructures, Opt. Express, 2022, 30(23), 42605–42613 CrossRef CAS PubMed.
- M. Guo, et al., Rational fabrication of nickel vanadium sulfide encapsulated on graphene as an advanced electrode for high-performance supercapacitors, Molecules, 2024, 29(15), 3642 CrossRef CAS PubMed.
- A. Dumbrava, et al., The influence of Triton X-100 surfactant on the morphology and properties of zinc sulfide nanoparticles for applications in azo dyes degradation, Mater. Chem. Phys., 2017, 193, 316–328 CrossRef CAS.
- M. H. Ali, et al., Effect of Triton X-100 surfactant on structural, morphological, and optical properties of ZnS thin films deposited by spin coating method, J. Sol-Gel Sci. Technol., 2024, 110(2), 560–566 CrossRef CAS.
- C.-Y. Su, et al., High-quality thin graphene films from fast electrochemical exfoliation, ACS Nano, 2011, 5(3), 2332–2339 CrossRef CAS PubMed.
- M. P. Dojčinović, I. Stojković Simatović and M. V. Nikolić, Supercapacitor electrodes: is nickel foam the right substrate for active materials?, Materials, 2024, 17(6), 1292 CrossRef PubMed.
- B. S. Wardhana, et al., Highly Nanoporous Nickel Foam as Current Collectors in 3D All-Solid-State Microsupercapacitors, ACS Omega, 2024, 9(35), 37355–37364 CrossRef CAS PubMed.
- Y. Zhang, et al., Self-template synthesis of ZnS/Ni3S2 as advanced electrode material for hybrid supercapacitors, Electrochim. Acta, 2019, 328, 135065 CrossRef CAS.
- K. D. Dahm and D. J. Dahm, Principles of diffuse reflectance spectroscopy, Handbook of Near-Infrared Analysis, 2021, pp. 27–43 Search PubMed.
- A. Kole and P. Kumbhakar, Effect of manganese doping on the photoluminescence characteristics of chemically synthesized zinc sulfide nanoparticles, Appl. Nanosci., 2012, 2, 15–23 CrossRef CAS.
- M. Z. Iqbal, M. M. Faisal and S. R. Ali, Integration of supercapacitors and batteries towards high-performance hybrid energy storage devices, Int. J. Energy Res., 2021, 45(2), 1449–1479 CrossRef CAS.
- I. F. Gul, et al., Fe/Co doped ZIF derived nitrogen doped nanoporous carbon as electrode material for supercapacitors, J. Ind. Eng. Chem., 2022, 116, 595–605 CrossRef CAS.
- M. R. Islam, et al., Hydrothermal synthesis of an MoS 2/MnO 2 nanocomposite: a unique 3D-nanoflower/1D-nanorod structure for high-performance energy storage applications, Mater. Adv., 2024, 5(12), 5307–5321 RSC.
- L.-Q. Mai, et al., Synergistic interaction between redox-active electrolyte and binder-free functionalized carbon for ultrahigh supercapacitor performance, Nat. Commun., 2013, 4(1), 2923 CrossRef PubMed.
- V. Sunil, et al., Characterization of supercapacitive charge storage device using electrochemical impedance spectroscopy, Mater. Today: Proc., 2021, 46, 1588–1594 CAS.
- N. O. Laschuk, E. B. Easton and O. V. Zenkina, Reducing the resistance for the use of electrochemical impedance spectroscopy analysis in materials chemistry, RSC Adv., 2021, 11(45), 27925–27936 RSC.
- M. S. Kolathodi, M. Palei and T. S. Natarajan, Electrospun NiO nanofibers as cathode materials for high performance asymmetric supercapacitors, J. Mater. Chem. A, 2015, 3(14), 7513–7522 RSC.
- P. Pegallapati, et al., Synthesis of Cu-doped ZnS nano-powder by chemical co-precipitation process, Mater. Today: Proc., 2023 DOI:10.1016/j.matpr.2023.02.419.
- S. Sagadevan, et al., One pot synthesis of hybrid ZnS–Graphene nanocomposite with enhanced photocatalytic activities using hydrothermal approach, J. Mater. Sci.: Mater. Electron., 2018, 29, 9099–9107 CrossRef CAS.
- M. Sathishkumar, M. Saroja and M. Venkatachalam, Influence of (Cu, Al) doping concentration on the structural, optical and antimicrobial activity of ZnS thin films prepared by Sol-Gel dip coating techniques, Optik, 2019, 182, 774–785 CrossRef CAS.
- M. Taoufiq, et al., DFT theoretical and experimental investigations of the effect of Cu doping within SILAR deposited ZnS, Opt. Mater., 2024, 147, 114607 CrossRef CAS.
- D. Ayodhya and G. Veerabhadram, Facile fabrication, characterization and efficient photocatalytic activity of surfactant free ZnS, CdS and CuS nanoparticles, J. Sci.:Adv. Mater. Devices, 2019, 4(3), 381–391 Search PubMed.
- N. Dengo, et al., In-depth study of ZnS nanoparticle surface properties with a combined experimental and theoretical approach, J. Phys. Chem. C, 2020, 124(14), 7777–7789 CrossRef CAS.
- M. B. Mohamed, Z. K. Heiba and N. Imam, Optical and thermogravimetric analysis of Zn1-xCuxS/PVA nanocomposite films, J. Mol. Struct., 2018, 1163, 442–448 CrossRef CAS.
- X. Chen, et al., Visible-light-driven photocatalytic activities of monodisperse ZnS-coated reduced graphene oxide nanocomposites, Mater. Chem. Phys., 2019, 227, 368–374 CrossRef CAS.
- Y. Feng, et al., A ZnS nanocrystal/reduced graphene oxide composite anode with enhanced electrochemical performances for lithium-ion batteries, Phys. Chem. Chem. Phys., 2016, 18(44), 30630–30642 RSC.
- H. Bayoka, et al., Evidencing the synergistic effects of carbonization temperature, surface composition and structural properties on the catalytic activity of biochar/bimetallic composite, J. Anal. Appl. Pyrolysis, 2023, 173, 106069 CrossRef CAS.
- G.-J. Lee, et al., Sonochemical synthesis of hollow copper doped zinc sulfide nanostructures: optical and catalytic properties for visible light assisted photosplitting of water, Ind. Eng. Chem. Res., 2014, 53(21), 8766–8772 CrossRef CAS.
- M. Shuai, et al., Room-temperature ferromagnetism in Cu+ implanted ZnO nanowires, J. Phys. D: Appl. Phys., 2008, 41(13), 135010 CrossRef.
- H. Hassan, et al., Enhanced the performance of zinc strontium sulfide-based supercapattery device with the polyaniline doped activated carbon, J. Solid State Electrochem., 2023, 27(1), 125–137 CrossRef CAS.
- W. Liu, et al., Synergistic adsorption-photocatalytic degradation effect and norfloxacin mechanism of ZnO/ZnS@ BC under UV-light irradiation, Sci. Rep., 2020, 10(1), 11903 CrossRef CAS PubMed.
- S. Kannan, N. Subiramaniyam and M. Sathishkumar, A novel green synthesis approach for improved photocatalytic activity and antibacterial properties of zinc sulfide nanoparticles using plant extract of Acalypha indica and Tridax procumbens, J. Mater. Sci.: Mater. Electron., 2020, 31(12), 9846–9859 CrossRef CAS.
- X. Yang, et al., Tailoring ion-accessible pores of robust nitrogen heteroatomic carbon nanoparticles for high-capacity and long-life Zn-ion storage, J. Energy Storage, 2024, 104, 114509 CrossRef.
- J. Yu and J. Ran, Facile preparation and enhanced photocatalytic H 2-production activity of Cu (OH) 2 cluster modified TiO 2, Energy Environ. Sci., 2011, 4(4), 1364–1371 RSC.
- R. Ramachandran, et al., Solvothermal synthesis of Zinc sulfide decorated Graphene (ZnS/G) nanocomposites for novel Supercapacitor electrodes, Electrochim. Acta, 2015, 178, 647–657 CrossRef CAS.
- S. Saleem, et al., Electrochemical investigation of ZnS/NiO nanocomposite based symmetric supercapattery, ChemistrySelect, 2024, 9(25), e202400809 CrossRef CAS.
- M. Khairy, et al., Studies on characterization, magnetic and electrochemical properties of nano-size pure and mixed ternary transition metal ferrites prepared by the auto-combustion method, J. Mater. Res., 2020, 35(20), 2652–2663 CrossRef CAS.
- M. S. Javed, et al., Design and fabrication of highly porous 2D bimetallic sulfide ZnS/FeS composite nanosheets as an advanced negative electrode material for supercapacitors, Energy Fuels, 2021, 35(18), 15185–15191 CrossRef CAS.
- S. Chen, et al., Ultrasensitive cracking-assisted strain sensors based on silver nanowires/graphene hybrid particles, ACS Appl. Mater. Interfaces, 2016, 8(38), 25563–25570 CrossRef CAS PubMed.
- J.-l. Hong, et al., Temperature-dependent pseudocapacitive behaviors of polyaniline-based all-solid-state fiber supercapacitors, Electrochem. Commun., 2023, 148, 107456 CrossRef CAS.
- S. S. Delekta, et al., Fully inkjet printed ultrathin microsupercapacitors based on graphene electrodes and a nano-graphene oxide electrolyte, Nanoscale, 2019, 11(21), 10172–10177 RSC.
- S. Lv, et al., Eco-friendly wood-based solid-state flexible supercapacitors from wood transverse section slice and reduced graphene oxide, Electron. Mater. Lett., 2015, 11, 633–642 CrossRef CAS.
- D. Kumar, et al., A 1 V supercapacitor device with nanostructured graphene oxide/polyaniline composite materials, Bull. Mater. Sci., 2015, 38, 1507–1517 CrossRef CAS.
- Y. Tian, et al., Understanding MXene-based “symmetric” supercapacitors and redox electrolyte energy storage, ACS Appl. Energy Mater., 2020, 3(5), 5006–5014 CrossRef CAS.
- H. Wang, et al., Nitrogen-doped graphene nanosheets with excellent lithium storage properties, J. Mater. Chem., 2011, 21(14), 5430–5434 RSC.
- W. Zhao, et al., Enhanced photocatalytic activity for H2 evolution under irradiation of UV–Vis light by Au-modified nitrogen-doped TiO2, PLoS One, 2014, 9(8), e103671 CrossRef PubMed.
- R. Balu, S. Sagadevan and A. Dakshanamoorthy, A cost effective, facile hydrothermal approach of zinc sulfide decorated on graphene nanocomposite for supercapacitor applications, J. Nanosci. Nanotechnol., 2019, 19(11), 6987–6994 CrossRef CAS PubMed.
- I. Hussain, et al., Binder-free cupric-ion containing zinc sulfide nanoplates-like structure for flexible energy storage devices, Chemosphere, 2023, 314, 137660 CrossRef CAS PubMed.
- M. Wu, et al., Nitrogen-doped porous carbon composite with three-dimensional conducting network for high rate supercapacitors, J. Alloys Compd., 2020, 844, 156217 CrossRef CAS.
- C. Saravanan, et al., Pseudocapacitive electrode performance of zinc oxide decorated reduced graphene oxide/poly (1, 8-diaminonaphthalene) composite, J. Energy Storage, 2024, 76, 109792 CrossRef.
- B. Wei, et al., One-step synthesis of graphitic-C3N4/ZnS composites for enhanced supercapacitor performance, J. Energy Chem., 2018, 27(2), 472–477 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra01710f |
| This journal is © The Royal Society of Chemistry 2025 |