Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Metallic sealants increase flux and change selectivity in supported molten-salt membranes
Liam A.
McNeil
,
Guannan
Chen
,
Wenting
Hu
 ,
Evangelos I.
Papaioannou
,
Evangelos I.
Papaioannou
 ,
Ian S.
Metcalfe
,
Ian S.
Metcalfe
 and
Greg A.
Mutch
and
Greg A.
Mutch
 *
*
Materials, Concepts, and Reaction Engineering (MatCoRE) Group, School of Engineering, Newcastle University, Newcastle upon Tyne, NE1 7RU, UK. E-mail: greg.mutch@newcastle.ac.uk
First published on 3rd December 2024
Abstract
Metallic sealants are widely used with high-temperature membranes. Here we show that their use in supported molten-salt membranes results in order-of-magnitude differences in CO2 flux and introduces O2 co-permeation. The ‘short-circuiting’ effect they introduce has important implications for the design of future experiments, and the interpretation of past work.
A key but often overlooked component of membrane modules and reactors is the sealant used to ensure that the fluid streams on the two sides of a membrane do not mix. However, to achieve this, the sealant must be in contact with both streams. Thus, the role of the sealant on flux and selectivity through a membrane should be considered. In high-temperature ceramic membranes, metallic sealants are frequently employed in the hot zone. Their use can, in principle, introduce poorly defined, transmembrane electronic conductivity.
Supported molten-salt membranes comprise a porous solid support, typically a metal oxide or metal, with molten salts infiltrated into the pore space via capillarity.1 Current literature suggests that they selectively permeate CO2 when they comprise molten carbonates supported in an oxygen-ion conductor viareaction 1, and that they co-permeate CO2 and O2 when an electronic conductor is employed viareaction 2.
| CO2(g) + O2−(s) ⇌ CO32−(l) | (1) |
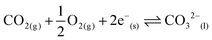 | (2) |
There are, however, contradictory results in the literature, which suggests there is unappreciated mechanistic nuance. The wide variety of support geometries and materials, salt compositions, and sealants used may be responsible for some of the contradictions as they introduce the potential for the superposition of different mechanisms in a single membrane (e.g., reaction 2 introduced via the use of a metallic sealant to a membrane otherwise expected to follow reaction 1).
The lack of consensus around the CO2 permeation mechanism in arguably the simplest class of supported molten-carbonate membrane, i.e., molten carbonates supported in a nominally inert material (e.g., Al2O3), best highlights this issue. A variety of oxide-, hydroxide-, bicarbonate-, and carbonate-like species are posited as being stable in molten carbonates,2–6 which would in theory allow CO2 transport via a mechanism similar to reaction 1 in the molten phase alone. There have also been suggestions that electronic conductivity in molten carbonates (rather than in the support as in reaction 2) due to the formation of dissolved metal/cation pairs is responsible.7 Finally, the transport of neutral, dissolved CO2 (a solution–diffusion mechanism) is also possible, although comparing the physical and chemical (reactive) solubilities of CO2 in molten carbonates suggests such a mechanism likely does not contribute significantly.1,8
Despite inert supports providing lower CO2 fluxes than oxide ion- and electron-conducting supports, they provide very useful permeation and mechanistic data as they simplify the membrane by restricting permeation to the molten-carbonate salt alone.9–13 Moreover, with their low intrinsic CO2 fluxes, modifications to the membrane leading to increased fluxes can be easier to measure. For example, in our previous work, the growth of an electronically-conductive, transmembrane Ag structure within an Al2O3 support increased CO2 and O2 flux compared to the Al2O3 support alone.12 This was achieved by doping the molten-carbonate salt with Ag. Selectivity was also influenced, whereby O2 co-permeation (with CO2) occurred only in the membrane with Ag. Work on cermet-supported, molten-salt membranes has also shown that the incorporation of Ag into the membrane support provides high CO2 fluxes.14,15
Our previous work on Ag doping encouraged us to consider the potential impact of metallic sealants, as these are frequently employed with supported molten-salt membranes due to the high operating temperatures of these membranes (∼400–1000 °C). This has included Ag,14–31 and Au sealants,32,33 despite suggestions (but no experimental proof) that they might introduce poorly-defined, transmembrane electronic conductivity.34,35 An alternative approach is to avoid sealing in the hot zone, employing a cold-zone seal instead. However, at the lab-scale this has rarely been employed, as it can involve preparing larger and more complex dense membrane supports, followed by the introduction of porosity using e.g., micro-scale subtractive manufacturing.12,13,36 Overall, this results in a much more expensive membrane and one which may not be suitable for real applications. However, as the sealant remains in the cold zone, the quality of the seal can be greatly improved which is important for interpreting permeation data and deriving mechanism.
Here we show that the use of metallic sealants with supported molten-salt membranes results in changes in flux and selectivity from feed-gas mixtures of importance for carbon dioxide separation. The use of a cold-zone-sealed Al2O3 support restricted permeation to the molten carbonate alone and demonstrated that molten carbonates selectively permeate only CO2 from both CO2/N2 and CO2/O2/N2 feed-gas mixtures. The use of both Au and Ag sealants in the hot zone with Al2O3 supports resulted in CO2 and O2 co-permeation from a CO2/O2/N2 feed-gas mixture, indicating a clear and significant impact on selectivity due to the transmembrane electronic conductivity introduced by the sealant. Furthermore, the metallic sealants increased CO2 flux significantly (∼1 × 10−2 ml min−1 cm−2 with Au, and ∼16 × 10−2 ml min−1 cm−2 with Ag). If supported molten-salt membranes are to progress towards scale-up, robust permeation and mechanistic data will be required; our results clearly demonstrate that consideration must be given to the sealants used.
Experimental
Membrane fabrication
Two membrane support geometries were employed: pressed pellets with a random porous architecture which required a hot-zone seal (Au or Ag), and tubular membranes with laser-drilled pores which required a cold-zone seal (O-ring and vacuum grease). These are hereafter referred to by the nature of the sealant, i.e., as hot- or cold-zone-sealed supports (before carbonate infiltration) and hot- or cold-zone-sealed membranes (following carbonate infiltration).To produce the hot-zone-sealed supports, Al2O3 powder (Alpha Aesar, ACS, >99.5%) was mixed with a 10 wt% PVA binder in a 1 ml binder![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 g Al2O3 ratio. 1 g of this mixture was uniaxially pressed at 3 tonnes to form ∼1.75 mm thick, ∼20 mm diameter pellets using a hydraulic press. Pellets were sintered at 1200 °C for 5 h at a ramp rate of 2 °C min−1, before being sealed to Al2O3 tubes with two open ends (∼200 mm length, 12 mm outer diameter, 9 mm inner diameter) using commercial hot-zone sealants (Au and Ag pastes from Fuel Cell Materials). The metallic pastes were applied to the rim of the Al2O3 tube, before the tubes were pressed on to the pellets from above. Further metallic paste was applied around the external circumference of the Al2O3 tube, and the entire assembly was held in place with a clamp for ∼1 h at room temperature for initial adhesion. Metallic sealants were set at 850 °C in air, employing a heating rate of 1 °C min−1. After holding the support and sealant at 850 °C for 1 h, they were cooled at a rate of 1 °C min−1 to room temperature.
1.5 g Al2O3 ratio. 1 g of this mixture was uniaxially pressed at 3 tonnes to form ∼1.75 mm thick, ∼20 mm diameter pellets using a hydraulic press. Pellets were sintered at 1200 °C for 5 h at a ramp rate of 2 °C min−1, before being sealed to Al2O3 tubes with two open ends (∼200 mm length, 12 mm outer diameter, 9 mm inner diameter) using commercial hot-zone sealants (Au and Ag pastes from Fuel Cell Materials). The metallic pastes were applied to the rim of the Al2O3 tube, before the tubes were pressed on to the pellets from above. Further metallic paste was applied around the external circumference of the Al2O3 tube, and the entire assembly was held in place with a clamp for ∼1 h at room temperature for initial adhesion. Metallic sealants were set at 850 °C in air, employing a heating rate of 1 °C min−1. After holding the support and sealant at 850 °C for 1 h, they were cooled at a rate of 1 °C min−1 to room temperature.
For the cold-zone-sealed supports, Al2O3 tubes (∼200 mm length, 20 mm outer diameter, 15 mm inner diameter) with one closed end of thickness ∼500 μm were laser drilled to form ∼2000 parallel pores within the central ∼15 mm diameter of the closed end. These laser-drilled tubes have been described in detail previously.12,13,36 They do not require a hot-zone sealant.
To prepare the carbonate phase of the membranes, individual lithium, sodium, and potassium carbonate powders (Alpha Aesar, ACS, >99.5%) were dried at ∼300 °C for ∼24 h in air, before being mixed in a ∼43.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31.5
31.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 (Li
25 (Li![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na
Na![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K) mol% ratio.
K) mol% ratio.
Membrane reactor
To seal both the hot- and cold-zone-sealed supports to the membrane reactor, a rubber O-ring and high-quality vacuum greases were applied to the open end of the Al2O3 tubes (noting that the hot-zone-sealed supports have the additional metallic sealant between the pressed pellet and Al2O3 tube). The open end of the tubes was situated in the base of the membrane reactor. The membrane reactor has been described in detail previously.12,13,36Infiltration of supports was achieved by pressing the eutectic carbonate mixture into pellets which were placed on the surface of the membrane supports (∼20 and 15 mm diameter pellets of mass ∼0.6 and 0.06 g were employed for the hot- and cold-zone-sealed supports respectively, i.e., they were of the same diameter as the porous areas of the supports and of a mass required to occupy the volume of the pores). The supports with carbonate pellets were enclosed within a quartz tube and heated to 450 °C at 1 °C min−1 under a flow of 50 mol% CO2 in N2 supplied to both feed and permeate sides. They were held at 450 °C for ∼1 h to permit infiltration of the molten carbonate (Tm ≈ 400 °C), before the reactor was heated to the experimental temperature (650 °C) at 1 °C min−1. At the experimental temperature the permeate-side gas was switched to Ar and the feed gas was either 50 mol% CO2 in N2 or 50 mol% CO2, 25 mol% O2 in N2. Detection of N2 at the permeate-side outlet above any pre-existing background level indicated a leaking membrane, at which point the experiment was abandoned. Similarly, CO2 or O2 permeation was assumed when signals exceeded their background level in Ar. All flow rates were 50 ml min−1, controlled by Brooks Smart II mass flow controllers, and measured at NTP.
CO2 and O2 flux measurement
During permeation, the permeate-side outlet gas composition was monitored using a quadrupole mass spectrometer (Hiden Analytical QGA). The mass spectrometer was calibrated using Ar (background), and ∼0.04 and 1 mol% CO2 mixtures. Mole fractions of permeated gases were converted to volumetric flux, Ji (ml min−1 cm−2) using eqn (1),| Ji = yi × Q × 1/A | (1) |
Membrane characterisation
Scanning electron microscopy (SEM), performed using a Tescan Vega 3LMU instrument, was used to analyse a membrane following gas permeation measurements. Digital images were collected using a digital camera and digital microscope.Results and discussion
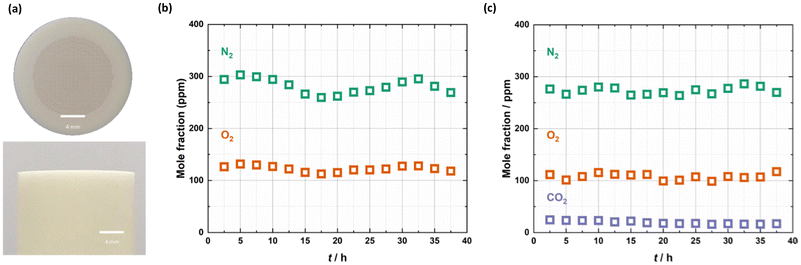
Fig. 1 demonstrates that the cold-zone-sealed support and membrane possessed an exceptionally high-quality seal. First, a cold-zone-sealed support without laser-drilled pores was supplied with a 50 ml min−1 Ar sweep gas to determine the background mole fractions of N2 and O2 present due to e.g., air leaks into the membrane reactor, mass spectrometer, and tubing/fittings used to connect the apparatus, and due to impurities in the Ar. N2 was present at ∼275 ppm and O2 at ∼125 ppm in the outlet of the sweep gas, suggesting that there were minimal leaks into the apparatus (Fig. 1b). Based on the ∼2000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (mole fraction in the air
1 (mole fraction in the air![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mole fraction in the reactor) ratio for N2 and O2, this would suggest that the mole fraction of CO2 in the reactor as a result of air leaks would be on the order of 0.1 ppm.
mole fraction in the reactor) ratio for N2 and O2, this would suggest that the mole fraction of CO2 in the reactor as a result of air leaks would be on the order of 0.1 ppm.
Subsequently, the cold-zone-sealed membrane was supplied with a 50 mol% CO2, 25 mol% O2 in N2 feed gas and an Ar sweep gas at 650 °C. The lack of any significant change in the level of N2 indicated that despite the support having been laser drilled and infiltrated with molten carbonates, there were no measurable transmembrane leaks (N2 is not expected to permeate a molten-carbonate membrane) (Fig. 1c). The presence of CO2 at ∼20 ppm (two orders of magnitude higher mole fraction than that expected due to air leaks into the reactor) in the permeate-side outlet is due to selective permeation through the molten-carbonate salt (discussed below). We note that if the N2 and O2 background in Fig. 1b was subtracted from the permeation experiment in Fig. 1c, their mole fractions would become ∼0 ppm. This background subtraction methodology was applied in all experiments reported hereafter. Clearly, there is no measurable O2 flux, however, using the CO2 mole fraction from Fig. 1c, a low CO2 flux (<0.1 × 10−3 ml min−1 cm−2) can be calculated. Together, these results suggest that the molten-carbonate phase does not possess sufficient electronic conductivity to contribute significantly to CO2 permeation via a reaction like reaction 2, contrary to some previous proposals.7
As discussed above, we suspected that metallic sealants may introduce transmembrane electronic conductivity. Indeed, when hot-zone-sealed membranes were exposed to the same conditions (feed gases, sweep gas, and temperature) used with the cold-zone-sealed membrane, the results were quite different (Fig. 2). With a 50 mol% CO2, 25 mol% O2 in N2 feed gas ((1) and (3) in Fig. 2b), CO2 and O2 co-permeated the membrane sealed with Au, with a CO2 flux of up to ∼1 × 10−2 ml min−1 cm−2. We suggest that the introduction of transmembrane electronic conductivity to the membrane facilitated the co-permeation of CO2 and O2viareaction 2 by ‘short-circuiting’ the membrane (Fig. 2c).34,35 Moreover, the CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 flux ratio in (1) and (3) of Fig. 2 is ∼2.5
O2 flux ratio in (1) and (3) of Fig. 2 is ∼2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, but if the CO2 flux in (2) (note that the O2 flux is zero), where a 50 mol% CO2 in N2 feed gas was employed are subtracted, then the ratio is ∼2
1, but if the CO2 flux in (2) (note that the O2 flux is zero), where a 50 mol% CO2 in N2 feed gas was employed are subtracted, then the ratio is ∼2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, consistent with reaction 2. This suggests that in (1) and (3), there is a superposition of reaction 2 (on top of the reaction responsible for permeation in (2)) due to the presence of O2 and transmembrane electronic conductivity via the Au sealant. Overall, Fig. 2 clearly demonstrates that the Au sealant ‘switches on’ selectivity for O2, and that when reaction 2 can occur due to the presence of O2 in the feed gas, CO2 flux is increased by ∼400% (comparing (1) and (3) to (2) in Fig. 2).
1, consistent with reaction 2. This suggests that in (1) and (3), there is a superposition of reaction 2 (on top of the reaction responsible for permeation in (2)) due to the presence of O2 and transmembrane electronic conductivity via the Au sealant. Overall, Fig. 2 clearly demonstrates that the Au sealant ‘switches on’ selectivity for O2, and that when reaction 2 can occur due to the presence of O2 in the feed gas, CO2 flux is increased by ∼400% (comparing (1) and (3) to (2) in Fig. 2).
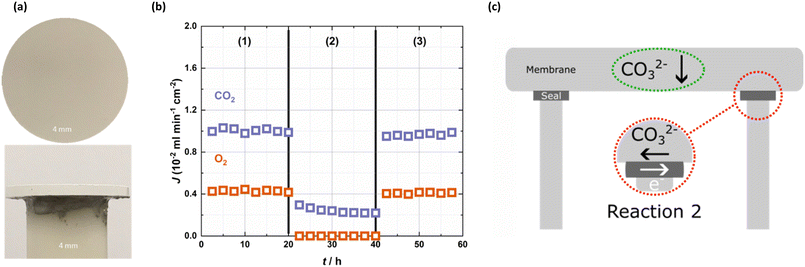 | ||
| Fig. 2 Short-circuiting due to the Au sealant. (a) Images of a hot-zone-sealed membrane. (b) CO2 and O2 fluxes as a function of feed gas at 650 °C in the Au, hot-zone-sealed membrane. An Ar sweep gas was used across all sections, with feed gas varying: (1) 50% CO2, 25% O2 in N2, (2) 50% CO2 in N2 and (3) 50% CO2, 25% O2 in N2. (c) Schematic of the short-circuiting effect due to metallic sealants, where reaction 2 is introduced via the metallic sealant. | ||
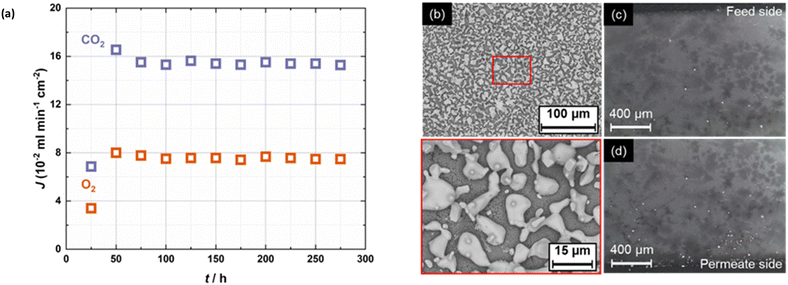
The impact of metallic sealants was further evidenced by using the Ag sealant in place of the Au sealant (Fig. 3). In this case, again O2 co-permeation was observed with a 50 mol% CO2, 25 mol% O2 in N2 feed gas, but this time with significant migration of the Ag sealant across the surface of, and into the bulk, of the membrane (Fig. 3b–d). Although not shown here, migration of Au was not observed (this, and the mechanism of Ag migration is discussed in our previous work).12 Notably, the use of the Ag sealant resulted in the highest CO2 fluxes in this work (∼16 × 10−2 ml min−1 cm−2, an order of magnitude higher than with the Au sealant under identical conditions). The flux remained at this level for >200 h. Also, we note the CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 flux ratio of ∼2
O2 flux ratio of ∼2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in line with reaction 2. In this case, with such high CO2 and O2 fluxes, the small contribution of any other permeation mechanism is concealed.
1, in line with reaction 2. In this case, with such high CO2 and O2 fluxes, the small contribution of any other permeation mechanism is concealed.
The very significant impact that the Ag sealant had on flux and selectivity is particularly concerning due to the aforementioned widespread use of Ag sealants in the supported molten-salt membrane literature.14–31Table 1 shows that a variety of Ag sealant forms (pastes, gaskets etc.) have been employed at the laboratory scale. This variety likely means that the effects we discuss (changes in flux and selectivity) will differ quantitatively between studies. Moreover, the sealant quantity and sealing area in laboratory-scale studies is relatively large when compared to membrane geometries used in industrial applications. Thus, one might expect quantitative differences here also. However, it is very important to note that this could result in performance (e.g., flux) at the laboratory scale that cannot be realised upon scale up.
Finally, we note that whilst we have studied the impact of metallic seals, graphite seals (which have an electronic conductivity approximately one order of magnitude lower than metals) are also used with supported molten-salt membranes, and therefore may unintentionally introduce contributions to permeation viareaction 2 also.6,17,37–42 It is possible that oxide-ion conducting sealants may introduce reaction 1 contributions similarly.
Conclusions
The influence that sealants have on flux and selectivity in membranes at the lab-scale should be carefully investigated as efforts towards the scale-up of membrane modules and reactors may otherwise encounter unforeseen difficulties. Here we have shown that metallic hot-zone sealants ‘switch on’ O2 selectivity in supported molten-salt membranes. This conclusion was supported by comparison to a cold-zone-sealed membrane, which showed no measurable O2 permeation under the same conditions. Furthermore, the metallic sealants significantly increased CO2 flux, with an order-of-magnitude difference observed between Au and Ag sealants. Going forwards, mechanistic interpretation, and comparisons of the performance between different membranes should consider the significant impact that sealant choice can have.Data availability
Data for this article are available at data.ncl at https://doi.org/10.25405/data.ncl.27045505.Author contributions
Funding acquisition (EIP, ISM, GAM); investigation (LM, GC, WH); supervision (WH, EIP, ISM, GAM); validation (WH); visualization (GAM); writing – original draft (GAM); writing – review & editing (WH, EIP, ISM, GAM).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Engineering & Physical Sciences Research Council [grant numbers EP/P009050/1, EP/P007767/1, EP/M01486X/1, EP/V047078/1, EP/W03395X/1, EP/Y034961/1]. The research leading to these results has received funding from the European Research Council under the European Union's Seventh Framework Programme (FP/2007-2013)/ERC grant agreement no. 320725. Support from the UK Catalysis Hub funded by grant reference EP/R027129/1 is gratefully acknowledged. ISM acknowledges funding from the Royal Academy of Engineering through a Chair in Emerging Technologies Award entitled “Engineering Chemical Reactor Technologies for a Low-Carbon Energy Future” (Grant CiET1819/2/57). GAM was supported by the Royal Academy of Engineering under the Research Fellowship scheme.Notes and references
- G. A. Mutch, L. Qu, G. Triantafyllou, W. Xing, M. L. Fontaine and I. S. Metcalfe, J. Mater. Chem. A, 2019, 7, 12951–12973 RSC.
- W. R. Carper, P. G. Wahlbeck and T. R. Griffiths, J. Phys. Chem. B, 2012, 116, 5559–5567 CrossRef CAS.
- W. Xing, Z. Li, T. Peters, M. L. Fontaine, M. McCann, A. Evans, T. Norby and R. Bredesen, Sep. Purif. Technol., 2019, 212, 723–727 CrossRef CAS.
- M. Cassir, G. Moutiers and J. Devynck, J. Electrochem. Soc., 1993, 140, 3114 CrossRef CAS.
- W. Xing, T. Peters, M. L. Fontaine, A. Evans, P. P. Henriksen, T. Norby and R. Bredesen, J. Membr. Sci., 2015, 482, 115–119 CrossRef CAS.
- M. R. Cerón, L. S. Lai, A. Amiri, M. Monte, S. Katta, J. C. Kelly, M. A. Worsley, M. D. Merrill, S. Kim and P. G. Campbell, J. Membr. Sci., 2018, 567, 191–198 CrossRef.
- H. Näfe, ECS J. Solid State Sci. Technol., 2014, 3, N23–N29 CrossRef.
- P. Claes, D. Moyaux and D. Peeters, Eur. J. Inorg. Chem., 1999, 3, 583–588 CrossRef.
- J. L. Wade, C. Lee, A. C. West and K. S. Lackner, J. Membr. Sci., 2011, 369, 20–29 CrossRef CAS.
- M. Kazakli, G. A. Mutch, L. Qu, G. Triantafyllou and I. S. Metcalfe, J. Membr. Sci., 2020, 600, 117855 CrossRef CAS.
- M. Kazakli, G. A. Mutch, G. Triantafyllou, A. G. Gil, T. Li, B. Wang, J. J. Bailey, D. J. L. Brett, P. R. Shearing, K. Li and I. Metcalfe, J. Membr. Sci., 2021, 617, 118640 CrossRef CAS.
- L. McNeil, G. A. Mutch, F. Iacoviello, J. Bailey, G. Triantafyllou, D. Neagu, T. Miller, E. I. Papaioannou, W. Hu, D. Brett, P. Shearing and I. S. Metcalfe, Energy Environ. Sci., 2020, 13, 1766–1775 RSC.
- I. S. Metcalfe, G. A. Mutch, E. I. Papaioannou, S. Tsochataridou, D. Neagu, D. J. L. Brett, F. Iacoviello, T. S. Miller, P. R. Shearing and P. A. Hunt, Nat. Energy, 2024, 9, 1074–1083 CAS.
- C. G. Mendoza-Serrato, R. López-Juárez, A. Reyes-Montero, J. A. Romero-Serrano, C. Gómez-Yáñez, J. A. Fabián-Anguiano and J. Ortiz-Landeros, Chem. Eng. Sci., 2022, 255, 117673 CrossRef CAS.
- J. A. Fabián-Anguiano, M. J. Ramírez-Moreno, H. Balmori-Ramírez, J. A. Romero-Serrano, I. C. Romero-Ibarra, X. Ma and J. Ortiz-Landeros, J. Membr. Sci., 2021, 637, 119607 CrossRef.
- T. T. Norton and Y. S. Lin, Solid State Ionics, 2014, 263, 172–179 CrossRef CAS.
- T. T. Norton, B. Lu and Y. S. Lin, J. Membr. Sci., 2014, 467, 244–252 CrossRef CAS.
- J. Tong, L. Zhang, M. Han and K. Huang, J. Membr. Sci., 2015, 477, 1–6 CrossRef CAS.
- L. Zhang, Y. Gong, K. S. Brinkman, T. Wei, S. Wang and K. Huang, J. Membr. Sci., 2014, 455, 162–167 CrossRef CAS.
- P. Zhang, J. Tong and K. Huang, J. Mater. Chem. A, 2017, 5, 12769–12773 RSC.
- J. Fang, J. Tong and K. Huang, J. Membr. Sci., 2016, 505, 225–230 CrossRef CAS.
- J. Tong, F. Si, L. Zhang and J. Fang, Chem. Commun., 2015, 51, 2936–2938 RSC.
- P. Zhang, J. Tong, Y. Jee and K. Huang, Chem. Commun., 2016, 52, 9817–9820 RSC.
- P. Zhang, J. Tong and K. Huang, ACS Sustainable Chem. Eng., 2018, 6, 14162–14169 CrossRef CAS.
- J. A. Fabian-Anguiano, R. Ortega-Lugo, M. J. Ramirez-Moreno, B. Zeifert, C. Gomez-Yanez and J. Ortiz-Landeros, Int. J. Appl. Ceram. Technol., 2021, 18, 1307–1320 CrossRef CAS.
- Z. Xu, Q. Zheng, S. Wang, Z. Zhang, Z. Liu, G. Zhang and W. Jin, J. Membr. Sci., 2021, 635, 119506 CrossRef CAS.
- S. Wang, J. Tong, L. Cui, P. Zhang and F. Zhou, J. Membr. Sci., 2022, 647, 120278 CrossRef CAS.
- L. Zhang, J. Tong, Y. Gong, M. Han, S. Wang and K. Huang, J. Membr. Sci., 2014, 468, 373–379 CAS.
- L. Zhang, Y. Gong, J. Yaggie, S. Wang, K. Romito and K. Huang, J. Membr. Sci., 2014, 453, 36–41 CAS.
- Z. Tong, X. Qiao, L. Hou, J. Tong and P. Zhang, ACS Sustainable Chem. Eng., 2024, 12, 8139–8147 CAS.
- A. Gili, B. Bischoff, U. Simon, F. Schmidt, D. Kober, O. Görke, M. F. Bekheet and A. Gurlo, Membranes, 2019, 9, 108 CrossRef CAS PubMed.
- G. Zhang, E. I. Papaioannou and I. S. Metcalfe, Energy Environ. Sci., 2015, 8, 1220 RSC.
- L. Grima, G. A. Mutch, P. B. Oliete, W. Bucheli, R. I. Merino, E. I. Papaioannou, J. J. Bailey, M. D. Kok, D. J. L. Brett, P. R. Shearing, I. S. Metcalfe and M. L. Sanjuán, J. Membr. Sci., 2021, 630, 119057 CrossRef CAS.
- M. Starykevich, A. Jamale, K. A. Yasakau and F. M. B. Marques, J. Membr. Sci., 2022, 659, 120806 CrossRef CAS.
- A. Jamale, M. Starykevich and F. M. B. Marques, J. Membr. Sci., 2022, 662, 120968 CrossRef CAS.
- S. Tsochataridou, G. A. Mutch, D. Neagu, E. I. Papaioannou, M. L. Sanjuán, B. Ray, R. I. Merino, V. M. Orera and I. S. Metcalfe, ACS Appl. Mater. Interfaces, 2020, 12, 16436–16441 CrossRef CAS.
- L. Yang, S. Ricote, S. T. B. Lundin and J. D. Way, Ind. Eng. Chem. Res., 2020, 59, 13706–13715 CrossRef CAS.
- B. Lu and Y. S. Lin, Ind. Eng. Chem. Res., 2014, 53, 13459–13466 CrossRef CAS.
- B. Lu and Y. S. Lin, J. Membr. Sci., 2013, 444, 402–411 CrossRef CAS.
- Y. Li, Z. Rui, C. Xia, M. Anderson and Y. S. Lin, Catal. Today, 2009, 148, 303–309 CrossRef CAS.
- O. Ovalle-Encinia and J. Y. S. Lin, J. Membr. Sci., 2022, 646, 120249 CrossRef CAS.
- O. Ovalle-Encinia and J. Y. S. Lin, Chem. Eng. J., 2022, 448, 137652 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |