Nickel foam supported biochar doped Ni–Mo bimetallic oxide for supercapacitor application†
Zhongxin
Jin‡
 *ab,
Kaijia
Hu‡
a,
Feng
Lin
a,
Siqi
Liu
a,
Ruining
Gu
a,
Wei
Zhang
a,
Siyu
Liu
a,
Caiying
Li
a,
Hongyang
Liao
a,
Xinping
Cai
a,
Haijun
Pang
*ab,
Kaijia
Hu‡
a,
Feng
Lin
a,
Siqi
Liu
a,
Ruining
Gu
a,
Wei
Zhang
a,
Siyu
Liu
a,
Caiying
Li
a,
Hongyang
Liao
a,
Xinping
Cai
a,
Haijun
Pang
 b,
Chunjing
Zhang
b,
Chunjing
Zhang
 *cd and
Huiyuan
Ma
*b
*cd and
Huiyuan
Ma
*b
aKey Laboratory of Oilfield Applied Chemistry and Technology, College of Chemical Engineering, Daqing Normal University, Daqing 163712, P. R. China. E-mail: jzx1128@126.com; Fax: +86 0451 86392716; Tel: +86 0451 86392716
bSchool of Materials Science and Chemical Engineering, Harbin University of Science and Technology, Harbin 150040, P. R. China. E-mail: mahy017@163.com; Fax: +86 0451 86392716; Tel: +86 0451 86392716
cCollege of Pharmaceutical Sciences, Heilongjiang University of Chinese Medicine, Harbin 150040, P. R. China. E-mail: zhangcj922@163.com; Fax: +86 0451 86392716; Tel: +86 0451 86392716
dKey Laboratory of Optic-electric Sensing and Analytical Chemistry for Life Science, MOE, P. R. China
First published on 31st October 2024
Abstract
As novel energy storage devices that have garnered significant attention, supercapacitors offer merits including long cycle life, high power density, ease of fabrication, and rapid charge/discharge rates. The core component of supercapacitors is an electrode material. Carbon materials are the most widely used in supercapacitors. However, their intrinsic charge storage mechanism results in relatively low capacitance performance, which falls short of the requirements for high-performance electrode materials. In this study, rice husks were converted into biochar. The porous biochar produced exhibits characteristics such as a well-developed porous structure, high specific surface area, tunable architecture, and low cost. Polyoxometalates exhibit excellent redox properties and high stability, offering advantages such as acting as electron reservoirs or electron sponges. C-MoO3-NiO2/NF was synthesized on nickel foam (NF) by using polyoxometalate (NH4)4[Ni(II)Mo6O24H6]·5H2O as a precursor, doping with rice husk biochar and utilizing KOH for porosity development. The supercapacitor test results indicate that the C-MoO3-NiO2/NF electrode material exhibits a charge–discharge time reaching 374.4 s and a specific capacitance of 180.77 F g−1 at a current density of 1 A g−1 in 6 mol L−1 KOH solution. After 1000 cycles of charge–discharge testing, the capacitance retention rate was still 75%. This indicates that the electrode material is an excellent supercapacitor material, laying a foundation for the development of novel supercapacitor materials.
1. Introduction
As modern industrial society progresses rapidly, the demand for energy has increased significantly. However, the limitations of traditional fossil fuels, along with the variable availability of wind, solar, and tidal energy due to geographic, climatic, and economic factors, present a significant challenge for the modern world.1–3 Therefore, the exploitation of novel energy reserve devices is an important benchmark for technological advancement in the new era. Furthermore, research on new energy and the development of novel energy storage devices represent a significant exploration in China's energy progression and an inevitable choice under the dual carbon goals. Supercapacitors, as a novel type of energy storage device,4–7 exhibit excellent cyclic stability, reversibility and high power density. Moreover, they combine the characteristics of traditional capacitors and secondary batteries, making them one of the most promising green energy storage devices.8–10Developing an environmentally friendly and highly efficient electrode material is currently at the core of research in the field of supercapacitors. These outstanding advantages have led to the widespread application of supercapacitors in renewable energy systems, hybrid electric vehicles, and portable electronic products.11–14 One of the key aspects of supercapacitor research is the development of electrode materials with good conductivity and stability, which mainly include carbon-based materials,15–17 metal (hydr)oxides18–21 and conductive polymer materials.22–24
As a major agricultural country, China generates a substantial amount of plant waste annually from its agricultural and pastoral activities. If plant waste was not properly managed, this can have a profound impact on economic development and the environment. Consequently, the rational development and utilization of biomass waste are of significant importance in achieving the dual carbon goals. Biochar is a low-dimensional carbon material characterized by its unique porous structure,25–27 which possesses developed porous structures, high temperature resistance, good electrical conductivity, large specific surface area, light weight and corrosion resistance.28,29 Compared to low-dimensional carbon materials like carbon nanotubes, graphene, and mesoporous carbon, biochar is abundant, environmentally friendly, and cost-effective. Moreover, it offers advantages such as renewability, thus becoming the most favourite precursor for porous carbon materials.30–32 Most biomass can't be directly used as electrode materials for supercapacitors; specific methods are required to convert it into biochar. These processes enhance its porosity and specific surface area, and tailor its surface chemical properties and morphology.33 At high temperatures, biomass is gradually converted into biochar through thermochemical decomposition during the pyrolysis process.34
Polyoxometalates (POMs) are a class of high-nuclearity metal oxo compounds formed by covalent bonding of early transition metals such as vanadium, niobium, tantalum, molybdenum, and tungsten with oxygen.35–39 They exhibit excellent redox properties, diversity, and stability, offering characteristics of multiple metal elements.40 They also possess a rich structural variety, high surface negative charge density, and unique redox and acid–base properties. Additionally, they feature modifiable structures and sizes, commonly utilized in critical areas such as catalysis, host–guest chemistry, and energy conversion.41,42 POMs are also often combined with other materials to exhibit synergistic effects in the field of electrode materials for supercapacitors. In 2020, Song reported a method, incorporating PMo12 into CNT@MoS2 materials resulting in the synthesis of the CNT@MoS2/PDDA/PMo12 composite material, which displayed a specific capacitance of 110 F g−1 and a power density of 152 W kg−1, as well as an energy density of 15.27 W h kg−1. Its capacitance retention rate remained as high as 89% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.43 Different types of polyoxometalates were commonly used as functional materials in chemistry including Keggin, Dawson, Lindquist, and Anderson structures.44,45 In the Anderson (XM6O24n−) structure, the group [XM6O24H6] consists of a plane symmetric D3d configuration formed by six Mo6 octahedra surrounding an XO6 polyhedron.46 The central X ion in the cluster is modifiable, providing an ideal model for studying the structure–function relationships of metal-induced isomorphic complexes.47 The nanocluster (NH4)4[Ni(II)Mo6O24H6]·5H2O features Ni(II) as the heteroatom, incorporated at the centre of the octahedron. (NH4)4[Ni(II)Mo6O24H6]·5H2O (NiMo6) stands out due to its ability to provide a dual metal source of nickel and molybdenum.45,48 Studies have shown that metal oxide doping of biochar can modify the superficial characteristics of biochar, and enlarge the pore volume and specific surface area, thereby enhancing its catalytic activity.42 For instance, Wan49 reported a core–shell structure composite material consisting of lignocellulosic biochar and manganese dioxide nanosheets in 2016. At a current density of 0.05 A g−1, it exhibited a specific capacitance of 101 F g−1, and the capacitance retention rate was 85% through 10
000 cycles.43 Different types of polyoxometalates were commonly used as functional materials in chemistry including Keggin, Dawson, Lindquist, and Anderson structures.44,45 In the Anderson (XM6O24n−) structure, the group [XM6O24H6] consists of a plane symmetric D3d configuration formed by six Mo6 octahedra surrounding an XO6 polyhedron.46 The central X ion in the cluster is modifiable, providing an ideal model for studying the structure–function relationships of metal-induced isomorphic complexes.47 The nanocluster (NH4)4[Ni(II)Mo6O24H6]·5H2O features Ni(II) as the heteroatom, incorporated at the centre of the octahedron. (NH4)4[Ni(II)Mo6O24H6]·5H2O (NiMo6) stands out due to its ability to provide a dual metal source of nickel and molybdenum.45,48 Studies have shown that metal oxide doping of biochar can modify the superficial characteristics of biochar, and enlarge the pore volume and specific surface area, thereby enhancing its catalytic activity.42 For instance, Wan49 reported a core–shell structure composite material consisting of lignocellulosic biochar and manganese dioxide nanosheets in 2016. At a current density of 0.05 A g−1, it exhibited a specific capacitance of 101 F g−1, and the capacitance retention rate was 85% through 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, demonstrating good cyclic stability. M. Genovese and K. Lian utilized polyoxometalate clusters PMo12 to modify pine cone biochar further to use as an electrode material in supercapacitors. This improved the structural characteristics of the pine cone biochar, and significantly enhanced its electrochemical performance.50
000 cycles, demonstrating good cyclic stability. M. Genovese and K. Lian utilized polyoxometalate clusters PMo12 to modify pine cone biochar further to use as an electrode material in supercapacitors. This improved the structural characteristics of the pine cone biochar, and significantly enhanced its electrochemical performance.50
This work synthesized biochar based on rice husk, after alkaline activation and combining with polyoxometalates. The porous structure and high surface area of biochar provide an excellent platform for depositing metal oxides derived from POMs.51 This deposition not only immobilizes the POMs but also modifies the biochar surface, potentially enhancing its catalytic activity. POMs are known for their redox properties and ability to facilitate electron transfer reactions.52 When combined with biochar, which can also act as an electron conductor, the system may exhibit improved catalytic performance in redox-driven processes. The C-MoO3-NiO2/NF electrode material was fabricated by calcination in a tubular furnace. For the first time, polyoxometalate (NH4)4[Ni(II)Mo6O24H6]·5H2O was doped with biochar made from rice husks to enhance its performance. Supercapacitor tests show that the C-MoO3-NiO2/NF electrode material exhibits a charge–discharge duration reaching 374.4 s and a specific capacitance of 180.77 F g−1 at a current density of 1 A g−1. The capacitance retention was still 75% after 1000 charge–discharge cycles. In conclusion, this work makes full use of the synergistic effect between biochar and the polyoxometalate to obtain a better superelectric effect.
2. Experimental
2.1. Preparation of porous biochar at different carbon-to-potassium hydroxide ratios
2.0000 g of the prepared biochar was accurately weighed (Fig. S1†) and mixed with 2.0000 g of KOH (denoted as C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1
KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; C for biochar). The mixture was dissolved in 20.00 mL of deionized water and stirred for 2 h. The activated carbon and KOH are thoroughly mixed, then the mixture was placed in an oven at 60 °C for 24 h.53 The mixture mentioned above was calcined in a tube furnace with 500 °C for 2 h at a heating rate of 5 min °C−1 in a N2 environment (Fig. S2†). The mixture was cooled to room temperature. The resulting sample was washed multiple times with a large amount of deionized water and 0.5 mol L−1 HCl solution until the pH is neutral. The final product was dried at 60 °C for 12 h to obtain gray powdered porous biochar. To investigate the optimal ratio of C
1; C for biochar). The mixture was dissolved in 20.00 mL of deionized water and stirred for 2 h. The activated carbon and KOH are thoroughly mixed, then the mixture was placed in an oven at 60 °C for 24 h.53 The mixture mentioned above was calcined in a tube furnace with 500 °C for 2 h at a heating rate of 5 min °C−1 in a N2 environment (Fig. S2†). The mixture was cooled to room temperature. The resulting sample was washed multiple times with a large amount of deionized water and 0.5 mol L−1 HCl solution until the pH is neutral. The final product was dried at 60 °C for 12 h to obtain gray powdered porous biochar. To investigate the optimal ratio of C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH mixtures, three sets of control experiments were conducted as shown in Table S1.† The mass of KOH was altered, and the rest of the experimental process was the same as that described above. Porous biochar was obtained by mixing 2.0000 g of biochar with 1.0000 g (denoted as C
KOH mixtures, three sets of control experiments were conducted as shown in Table S1.† The mass of KOH was altered, and the rest of the experimental process was the same as that described above. Porous biochar was obtained by mixing 2.0000 g of biochar with 1.0000 g (denoted as C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 2
KOH = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and 4.0000 g (denoted as C
1) and 4.0000 g (denoted as C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1
KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) of KOH, respectively; the charge–discharge performance for the electrode material is the best when C
2) of KOH, respectively; the charge–discharge performance for the electrode material is the best when C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1
KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
2.2. Preparation of electrode materials at different carbon-to-alkali ratios
Nickel foam (NF) was cut into 1 × 1.5 cm2 pieces and ultrasonically treated with acetone, ethanol, and distilled water for 30 minutes, respectively. Then, the nickel foam was rinsed several times with distilled water. The nickel foam was dried at 60 °C for 24 h.540.0080 g of porous biochar with different carbon-to-alkali ratios was weighed and mixed uniformly with 0.0009 g of acetylene black. The mixture was placed in an agate mortar, and after grinding, 0.00047 g of polytetrafluoroethylene (PTFE) was added. Finally, 5 mL of anhydrous ethanol was added and ultrasonicated for 1 h, and then placed in a bake oven at 60 °C to dry until it reaches an ink-like consistency. A micropipette was used to apply 60 μL of the suspension onto a 1.5 cm × 1.0 cm piece of nickel foam, and it was placed in an oven at 60 °C to dry for 12 h. Electrode materials with different carbon-to-alkali ratios were obtained according to the above method.
2.3. Preparation of C-MoO3-NiO2/NF electrodes with different material ratios
0.2000 g of C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1
KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture was accurately weighed, and mixed with 0.2000 g, 0.4000 g, and 0.6000 g of polyoxometalate (NH4)4[Ni(II)Mo6O24H6]·5H2O (abbreviated as NiMo6), respectively (denoted as C-MoO3-NiO2/NF-1, C-MoO3-NiO2/NF-2, and C-MoO3-NiO2/NF-3). The synthesis method and IR spectra of NiMo6 are listed in the ESI.† The mixture was dissolved in 20.00 mL of deionized water and stirred for two hours. Then the samples were placed at 60 °C for 12 hours.53 The prepared samples were transferred into an alumina crucible boat, and the boat was placed in a tubular furnace. The mixture was heated to 500 °C for 2 h at a rate of 5 °C min−1 under a nitrogen gas atmosphere. Then the tubular furnace was cooled naturally to room temperature. Finally, the C-MoO3-NiO2 electrode material was obtained. Other samples with different ratios were prepared using the same method.
1 mixture was accurately weighed, and mixed with 0.2000 g, 0.4000 g, and 0.6000 g of polyoxometalate (NH4)4[Ni(II)Mo6O24H6]·5H2O (abbreviated as NiMo6), respectively (denoted as C-MoO3-NiO2/NF-1, C-MoO3-NiO2/NF-2, and C-MoO3-NiO2/NF-3). The synthesis method and IR spectra of NiMo6 are listed in the ESI.† The mixture was dissolved in 20.00 mL of deionized water and stirred for two hours. Then the samples were placed at 60 °C for 12 hours.53 The prepared samples were transferred into an alumina crucible boat, and the boat was placed in a tubular furnace. The mixture was heated to 500 °C for 2 h at a rate of 5 °C min−1 under a nitrogen gas atmosphere. Then the tubular furnace was cooled naturally to room temperature. Finally, the C-MoO3-NiO2 electrode material was obtained. Other samples with different ratios were prepared using the same method.
Then, the working electrodes C-MoO3-NiO2/NF were prepared using the same procedure as described in section 2.2. The mixture of biochar and alkali previously pre-prepared (0.008 g) and acetylene black (0.0009 g) were weighed, respectively. They were placed in an agate mortar and fully ground. The ground powder, PTFE (0.00047 g) and 5 mL alcohol were placed in a small beaker with ultrasonication for 2 h, and finally the above mixture was dried and coated on the nickel foam weighed earlier (Fig. S3†). Table S2† presents three sets of experiments with different mass ratios of the C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH mixture and polyoxometalate (NH4)4[Ni(II)Mo6O24H6]·5H2O (NiMo6), and with the increasing content of NiMo6, the constant current charge–discharge time of the samples first increases and then decreases. It can be concluded that the optimal mass ratio of the carbon–alkali mixture to NiMo6 is 1
KOH mixture and polyoxometalate (NH4)4[Ni(II)Mo6O24H6]·5H2O (NiMo6), and with the increasing content of NiMo6, the constant current charge–discharge time of the samples first increases and then decreases. It can be concluded that the optimal mass ratio of the carbon–alkali mixture to NiMo6 is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (C-MoO3-NiO2/NF-2).
2 (C-MoO3-NiO2/NF-2).
Table S3† presents comparative experiments on the carbon–alkali mixture with a ratio of C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1
KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, C-MoO3-NiO2/NF-2 electrode material and C
1, C-MoO3-NiO2/NF-2 electrode material and C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NiMo6 (only C mixed with NiMo6). The C-MoO3-NiO2/NF-2 electrode material presents the best supercapacitor performance. The corresponding weight gain of the material is shown in Table S8.†
NiMo6 (only C mixed with NiMo6). The C-MoO3-NiO2/NF-2 electrode material presents the best supercapacitor performance. The corresponding weight gain of the material is shown in Table S8.†
2.4. Characterization
The phase structure of the C-MoO3-NiO2/NF electrode samples with different material ratios was analyzed using an X' Pert powder X-ray diffractometer. The operating conditions were as follows: a tube voltage of 40 kV, a tube current of 40 mA and Cu Kα radiation. The morphology, microstructure, and elemental distribution of the electrode materials were observed using an EM-30AX+ scanning electron microscope. The chemical composition and element state of the C-MoO3-NiO2/NF electrode materials can be obtained by XPS (Thermo Scientific K-Alpha XPS spectrometer).2.5. Electrochemical measurements
The electrochemical capabilities of the electrodes were evaluated by using a CHI 660D electrochemical workstation from Shanghai Chenhua Instrument in China. The test was performed in 6 mol L−1 KOH electrolyte solution with a standard three-electrode configuration, the nickel foam-supported electrode material as the working electrode, a platinum sheet as the counter electrode and Hg/HgO as the reference electrode. The scan rate of the cyclic voltammetry (CV) curves was 10–100 mV s−1, and the current density for galvanostatic charge–discharge (GCD) was measured as 1–10 A g−1; electrochemical impedance spectroscopy (EIS) was performed in the frequency from 0.01 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Hz. Based on the three-electrode system, the specific capacitance formula using the GCD technique is as follows:55
000 Hz. Based on the three-electrode system, the specific capacitance formula using the GCD technique is as follows:55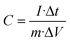 | (1) |
3. Results and discussion
Powder X-ray diffraction (XRD) is an important approach for characterizing the crystal structure and chemical composition of samples. To determine the phase composition of the products, XRD for the samples was performed. Fig. 1(a–c) show the XRD patterns of MoO3 (PDF#76-1003); the characteristic peaks at 2θ = 27.324°, 12.768°, and 25.698° correspond to the (021), (020), and (040) crystal planes, respectively. The intensity of these characteristic peaks is notably high. The characteristic peaks located at 2θ = 18.557°, 37.118°, 44.705°, and 58.661° correspond to the (003), (101), (104), and (107) crystal planes of NiO2 (PDF#85-1977), respectively. The characteristic peaks located at 2θ = 26.309°, 46.501°, and 49.170° correspond to the (111), (010), and (110) crystal planes of C (PDF#75-0444), respectively. Fig. 1(a–c) show the XRD patterns of C-MoO3-NiO2/NF-2, C-MoO3-NiO2/NF-1, C-MoO3-NiO2/NF-3, respectively.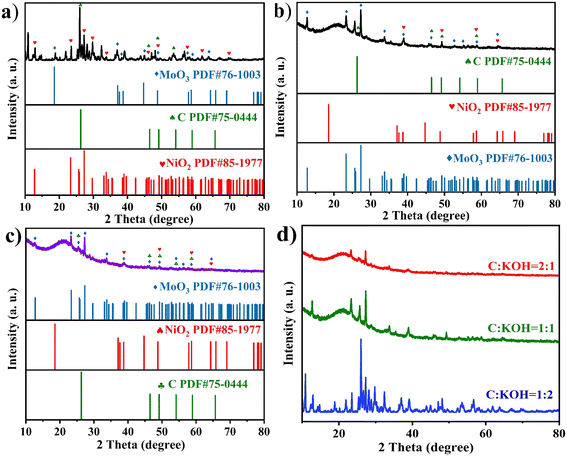 | ||
Fig. 1 XRD patterns of (a)–(c) C-MoO3-NiO2/NF-2, C-MoO3-NiO2/NF-1, C-MoO3-NiO2/NF-3 electrode materials, respectively. (d) The three ratios of C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH electrode materials. KOH electrode materials. | ||
Fig. 1(d) shows the XRD patterns of the three ratios with C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH, indicating that both the electrode materials when C
KOH, indicating that both the electrode materials when C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 2
KOH = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and C
1 and C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1
KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 exhibit a broad hump at around 21°, which is characteristic of carbon peaks, representing typical weakly graphitized carbon materials.56 The carbon peak of the electrode material when C
1 exhibit a broad hump at around 21°, which is characteristic of carbon peaks, representing typical weakly graphitized carbon materials.56 The carbon peak of the electrode material when C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1
KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 is not prominent because the incomplete treatment during the pH adjustment after mixing and activating the activated carbon with KOH resulted in the presence of K salts. Additionally, as the content of KOH increases, the peak shifts to the right.
2 is not prominent because the incomplete treatment during the pH adjustment after mixing and activating the activated carbon with KOH resulted in the presence of K salts. Additionally, as the content of KOH increases, the peak shifts to the right.
To observe the morphological characteristics of the C-MoO3-NiO2/NF electrode material, SEM was carried out. Fig. 2(a–c) show the SEM images of the C-MoO3-NiO2/NF electrode material at different magnifications, from which we can clearly observe that the material presents a porous structure. Fig. 2(d) provides the mapping images of elements including Mo, O, C, N and Ni. Fig. 2(e–i) show the distribution of Mo, O, C, N and Ni elements in the C-MoO3-NiO2/NF electrode material, respectively. These elements were distributed uniformly. This finding indicates that porous carbon and NiMo6 have been successfully combined to form the C-MoO3-NiO2/NF electrode material.
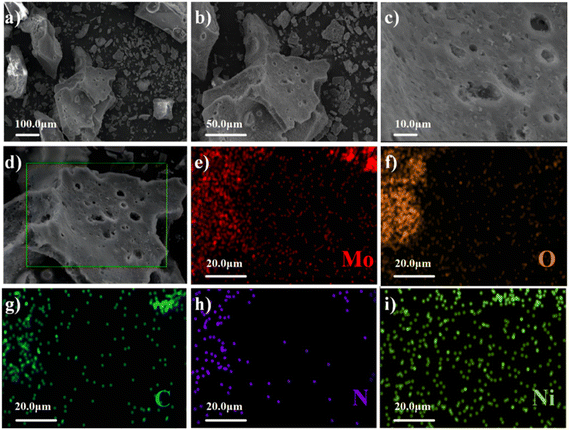 | ||
| Fig. 2 SEM images of the C-MoO3-NiO2/NF electrode material. (a)–(c) SEM images at different magnifications. (d)–(i) Elemental mappings of the material. | ||
Nitrogen adsorption–desorption isotherm test and the BET analysis were performed to determine the specific surface area of C-MoO3-NiO2/NF (Fig. 3). It can be clearly observed from Fig. 3(a) that the adsorption curve of the nitrogen adsorption–desorption isotherm of C-MoO3-NiO2/NF belongs to a typical type IV isotherm and the BET surface area is estimated to be 23.82 m2 g−1 by the Brunauer–Emmett–Teller (BET) method, and the total pore volume is 0.13 cm3 g−1. The pore size of the material is mainly distributed in 3–6 nm as shown in Fig. 3(b);57,58 the aperture distribution chart, derived via the Barrett–Joyner–Halenda (BJH) method, highlights the predominance of mesoporous pores within the C-MoO3-NiO2/NF composites. This feature facilitates the accessibility of a greater number of active sites and boosts the penetration of electrolytes, which is advantageous to electrochemical performance for supercapacitor application.59,60
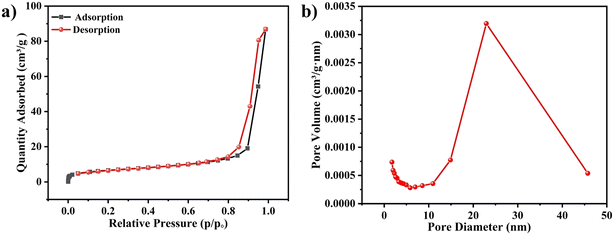 | ||
| Fig. 3 (a) and (b) Nitrogen adsorption–desorption isotherm and pore size distributions of C-MoO3-NiO2/NF. | ||
Fig. 4(a) shows the XPS survey spectrum of the C-MoO3-NiO2/NF electrode material; the elements including Ni, Mo, O and N can be observed. The valence states and chemical environments of the elements of the C-MoO3-NiO2/NF electrode material can be elucidated by high-resolution XPS. In the high-resolution C 1s spectrum in Fig. 4(b), three distinct peaks can be observed, including 285.85 eV, 284.40 eV, and 288.32 eV, which are assigned to C–N/C–O bonds, C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, and C
C bonds, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively.61–64 The double peaks at 862.34 eV and 880.25 eV match the characteristic satellite peaks of Ni 2p3/2 and Ni 2p1/2. The peaks at binding energies of 855.90 eV and 873.97 eV are attributed to the peak positions of Ni3+ 2p3/2 and Ni3+ 2p1/2, respectively.65–68 The peaks observed at 854.90 eV and 872.83 eV correspond to Ni2+ 2p3/2 and Ni2+ 2p1/2, respectively,69 reflecting Ni2+ and Ni3+ coexisting in the electrode material.
O bonds, respectively.61–64 The double peaks at 862.34 eV and 880.25 eV match the characteristic satellite peaks of Ni 2p3/2 and Ni 2p1/2. The peaks at binding energies of 855.90 eV and 873.97 eV are attributed to the peak positions of Ni3+ 2p3/2 and Ni3+ 2p1/2, respectively.65–68 The peaks observed at 854.90 eV and 872.83 eV correspond to Ni2+ 2p3/2 and Ni2+ 2p1/2, respectively,69 reflecting Ni2+ and Ni3+ coexisting in the electrode material.
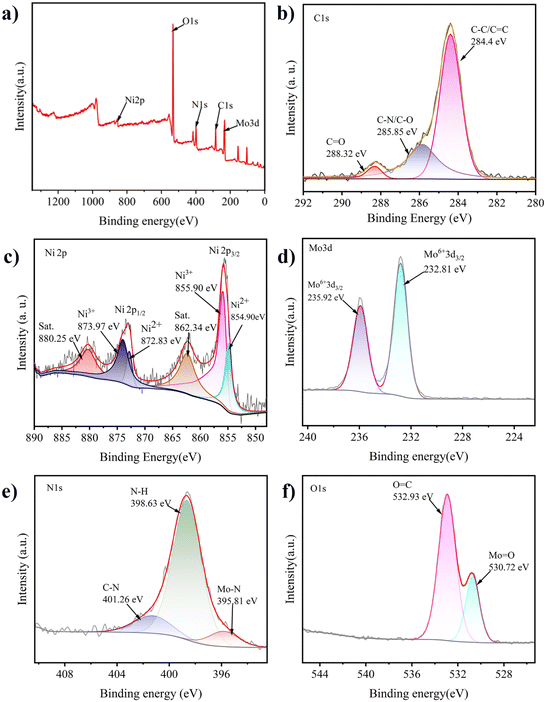 | ||
| Fig. 4 High-resolution XPS spectra of the C-MoO3-NiO2/NF composite electrode material. (a) Full-scan spectrum; (b) C 1s; (c) Ni 2p; (d) Mo 3d; (e) N 1s; (f) O 1s. | ||
From the high-resolution Mo 3d spectrum in Fig. 4(d), the peaks at binding energies of 232.81 eV and 235.92 eV indicate the presence of Mo6+.70 From the high-resolution N 1s spectrum in Fig. 4(e), three significant characteristic peaks can be observed at 395.81 eV, 398.76 eV, and 402.01 eV. The peak at 395.81 eV represents the Mo–N bond, which may result from the nitridation reaction between the nitrogen gas introduced during the activation process and a small amount of molybdenum in the material. The peak at 398.76 eV corresponds to the N–H bond, which may be due to the NH3 gas produced during the high-temperature reaction process. The peak at 402.01 eV is attributed to the C–N bond, indicating that a small amount of nitrogen was doped into the activated carbon during the activation process.71 From the high-resolution O 1s spectrum in Fig. 4(f), characteristic peaks can be observed at binding energies of 530.720 eV and 532.93 eV. The peak at 530.720 eV represents the Mo–O bond in MoO3, while the peak at 532.93 eV is attributed to the C–O bond.
3.1. Analysis of supercapacitor performance of electrode materials with different carbon–alkali ratios
As shown in Fig. 5(a)–(c), the cyclic voltammetry (CV) curves of the C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1
KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, C
1, C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 2
KOH = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and C
1, and C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1
KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 electrode materials at varying scan rates (10–100 mV s−1) were obtained. It can be observed that the curve shapes are irregular and exhibit redox peaks, which are consistent with the characteristics of pseudocapacitance, indicating that the electrode material exhibits pseudocapacitance performance. The innermost to the outermost curves represent the scan rates increasing from 10 mV s−1 to 100 mV s−1, respectively. As the scan rate increases, the CV curves remain basically unchanged, indicating that the electrode material exhibits good redox capacitive performance and excellent rate capability.72
2 electrode materials at varying scan rates (10–100 mV s−1) were obtained. It can be observed that the curve shapes are irregular and exhibit redox peaks, which are consistent with the characteristics of pseudocapacitance, indicating that the electrode material exhibits pseudocapacitance performance. The innermost to the outermost curves represent the scan rates increasing from 10 mV s−1 to 100 mV s−1, respectively. As the scan rate increases, the CV curves remain basically unchanged, indicating that the electrode material exhibits good redox capacitive performance and excellent rate capability.72
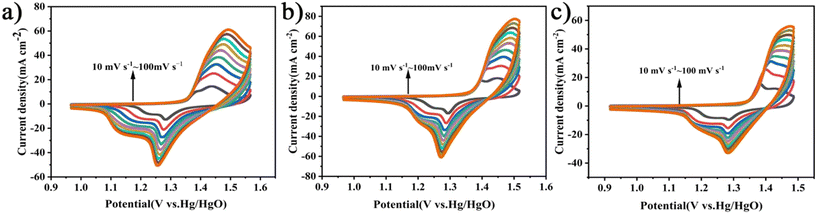 | ||
Fig. 5 (a)–(c) Cyclic voltammetry curves of electrode materials when C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1 KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, C 1, C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1 KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and C 2, and C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 2 KOH = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. 1, respectively. | ||
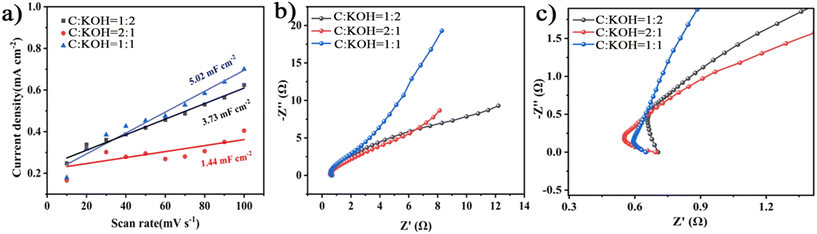
As shown in Fig. 6(a) and (b), the electrochemically active surface areas and electrochemical impedance at different carbon–alkali ratios (C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH) are presented. The active surface area was compared based on the values obtained from the CV curves. It can be seen that the active surface areas for the C
KOH) are presented. The active surface area was compared based on the values obtained from the CV curves. It can be seen that the active surface areas for the C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1
KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, C
1, C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 2
KOH = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and C
1, and C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1
KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 electrode materials are 5.02 mF cm−2, 1.44 mF cm−2, and 3.73 mF cm−2, respectively. As shown in Fig. 6(c), the C
2 electrode materials are 5.02 mF cm−2, 1.44 mF cm−2, and 3.73 mF cm−2, respectively. As shown in Fig. 6(c), the C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1
KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 electrode material has the smallest semicircle diameter in the high-frequency region and the highest slope in the low-frequency region, indicating its good conductivity and ion diffusion characteristics.73 Based on the above, the C
1 electrode material has the smallest semicircle diameter in the high-frequency region and the highest slope in the low-frequency region, indicating its good conductivity and ion diffusion characteristics.73 Based on the above, the C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1
KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 electrode material has the largest electrochemically active area and lowest resistance, which is consistent with the simulated impedance values, as shown in Table S5.†
1 electrode material has the largest electrochemically active area and lowest resistance, which is consistent with the simulated impedance values, as shown in Table S5.†
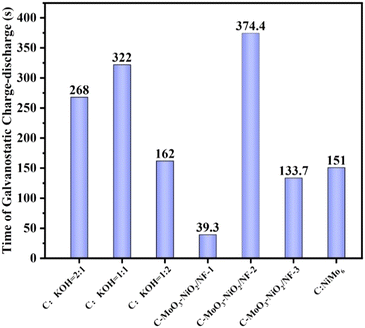
As shown in Fig. 7(a)–(c), the GCD tests for C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1
KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, C
1, C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 2
KOH = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and C
1, and C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1
KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 exhibit irregular and nonlinear shapes, confirming that the materials are pseudocapacitive. The charge–discharge time for C
2 exhibit irregular and nonlinear shapes, confirming that the materials are pseudocapacitive. The charge–discharge time for C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1
KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is the longest, reaching 322 s. At a current density of 1 A g−1, the specific capacitance reaches up to 225 F g−1. Subsequently, cycling tests were conducted for 1000 cycles. As shown in Fig. 7(d), the capacitance retention rates are 66% for C
1 is the longest, reaching 322 s. At a current density of 1 A g−1, the specific capacitance reaches up to 225 F g−1. Subsequently, cycling tests were conducted for 1000 cycles. As shown in Fig. 7(d), the capacitance retention rates are 66% for C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1
KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 43% for C
1, 43% for C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 2
KOH = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 41% for C
1, and 41% for C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1
KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Combining these results with previous tests, it can be concluded that the C
2. Combining these results with previous tests, it can be concluded that the C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1
KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 electrode material exhibits the best performance.
1 electrode material exhibits the best performance.
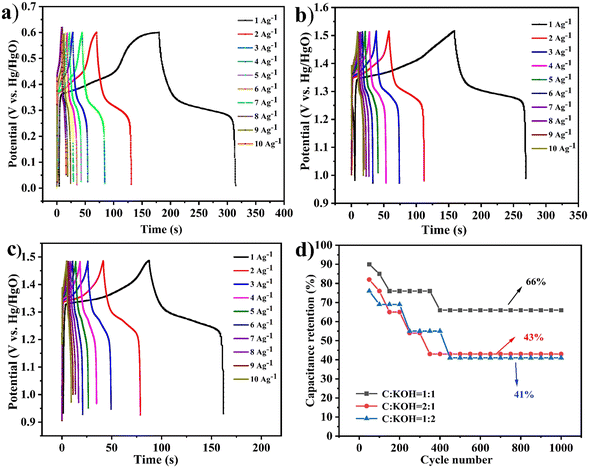 | ||
Fig. 7 The different ratios of the C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH (1 KOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 1 1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) electrode materials. (a)–(c) GCD plots. (d) Cycling test. 2) electrode materials. (a)–(c) GCD plots. (d) Cycling test. | ||
3.2. Analysis of the supercapacitor performance of C-MoO3-NiO2/NF electrode materials
In the study of carbon–alkali ratios (C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH), it was found that C
KOH), it was found that C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1
KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 exhibited the best performance. Therefore, the performance of C
1 exhibited the best performance. Therefore, the performance of C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1
KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 combined with polyoxometalate NiMo6 (C-MoO3-NiO2/NF) and the performance of polyoxometalate NiMo6 combined with biochar without mixing with KOH (C
1 combined with polyoxometalate NiMo6 (C-MoO3-NiO2/NF) and the performance of polyoxometalate NiMo6 combined with biochar without mixing with KOH (C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NiMo6) were investigated.
NiMo6) were investigated.
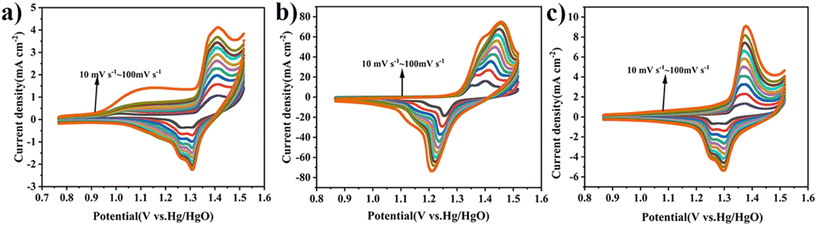
As shown in Fig. 8(a)–(c), the CV scan rate plots for C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1
KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, C-MoO3-NiO2/NF, and C
1, C-MoO3-NiO2/NF, and C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NiMo6 exhibit distinct redox peaks, which are consistent with the characteristics of pseudocapacitance.
NiMo6 exhibit distinct redox peaks, which are consistent with the characteristics of pseudocapacitance.
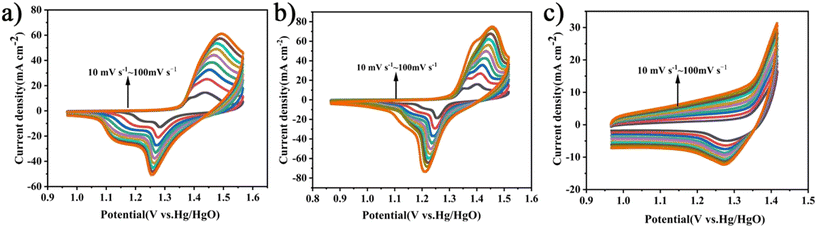 | ||
Fig. 8 Cyclic voltammetry curves of the electrode materials. (a) C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1 KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. (b) C-MoO3-NiO2/NF. (c) C 1. (b) C-MoO3-NiO2/NF. (c) C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NiMo6. NiMo6. | ||
Fig. 9(a) shows the electrochemically active surface areas of C-MoO3-NiO2/NF, C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NiMo6, and C
NiMo6, and C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1
KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which are 68.17 mF cm−2, 7.35 mF cm−2, and 5.02 mF cm−2, respectively. In Fig. 9(b), it can be seen that the C-MoO3-NiO2/NF electrode material has the smallest semicircle radius in the high-frequency region and the highest slope in the low-frequency region, which are consistent with the simulated impedance values as shown in Tables S4 and S6.† Therefore, the C-MoO3-NiO2/NF electrode material has the largest active surface area and lowest resistance.
1, which are 68.17 mF cm−2, 7.35 mF cm−2, and 5.02 mF cm−2, respectively. In Fig. 9(b), it can be seen that the C-MoO3-NiO2/NF electrode material has the smallest semicircle radius in the high-frequency region and the highest slope in the low-frequency region, which are consistent with the simulated impedance values as shown in Tables S4 and S6.† Therefore, the C-MoO3-NiO2/NF electrode material has the largest active surface area and lowest resistance.
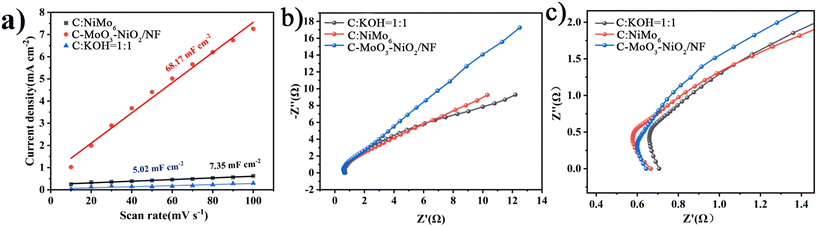 | ||
| Fig. 9 Different electrode materials. (a) Electrochemically active surface areas. (b) Electrochemical impedance spectra. (c) Magnified view of the electrochemical impedance spectra. | ||
As shown in Fig. 10(a)–(c), the GCD tests for the three different electrode materials indicate that the charge–discharge time for C-MoO3-NiO2/NF at 1 A g−1 reaches up to 374.4 seconds with a specific capacitance of 180.77 F g−1. The charge–discharge time for C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1
KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is 322 seconds, while C
1 is 322 seconds, while C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NiMo6 has a charge–discharge time of only 151 seconds. Fig. 10(d) shows that the C-MoO3-NiO2/NF and C
NiMo6 has a charge–discharge time of only 151 seconds. Fig. 10(d) shows that the C-MoO3-NiO2/NF and C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH materials exhibit good capacitance retention rates of 75% and 66%, while C
KOH materials exhibit good capacitance retention rates of 75% and 66%, while C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NiMo6 has a capacitance retention rate of only 23.1%. Combining these results, it can be concluded that the C-MoO3-NiO2/NF electrode material demonstrates superior performance.
NiMo6 has a capacitance retention rate of only 23.1%. Combining these results, it can be concluded that the C-MoO3-NiO2/NF electrode material demonstrates superior performance.
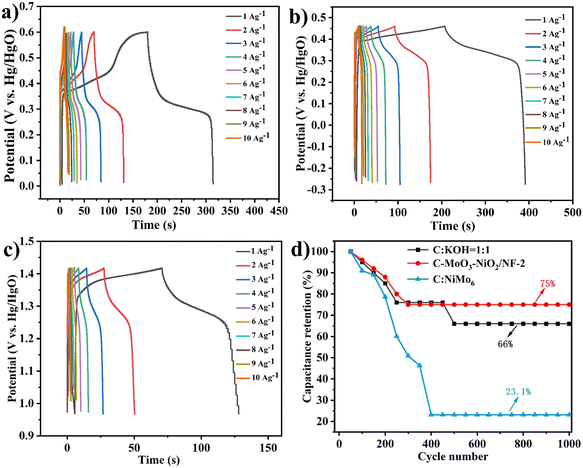 | ||
Fig. 10 (a)–(c) GCD curves of C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH = 1 KOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, C-MoO3-NiO2/NF, and C 1, C-MoO3-NiO2/NF, and C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NiMo6, respectively. (d) Cycling stability test of electrode materials. NiMo6, respectively. (d) Cycling stability test of electrode materials. | ||
3.3. Analysis of the supercapacitor performance of C-MoO3-NiO2/NF electrode materials with different material ratios
Currently, C-MoO3-NiO2/NF exhibits the best performance. To explore the optimal ratio for the supercapacitor performance of electrode materials combined with polyoxometalates, the mass ratios of polyoxometalates were varied to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (denoted as C-MoO3-NiO2/NF-1), 1
1 (denoted as C-MoO3-NiO2/NF-1), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (denoted as C-MoO3-NiO2/NF-2) and 1
2 (denoted as C-MoO3-NiO2/NF-2) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (denoted as C-MoO3-NiO2/NF-3).
3 (denoted as C-MoO3-NiO2/NF-3).
Fig. 11(a)–(c) show the CV scan rate plots for C-MoO3-NiO2/NF-1, C-MoO3-NiO2/NF-2, and C-MoO3-NiO2/NF-3. Fig. 12(a) shows the electrochemically active surface areas with 6.35 mF cm−2 for C-MoO3-NiO2/NF-1, 68.17 mF cm−2 for C-MoO3-NiO2/NF-2, and 9.88 mF cm−2 for C-MoO3-NiO2/NF-3. Fig. 12(b) shows the electrochemical impedance of C-MoO3-NiO2/NF, indicating that C-MoO3-NiO2/NF-2 has the smallest semicircle radius and the highest slope, and therefore the lowest resistance; this is consistent with the simulated impedance values, as shown in Table S7.†
 | ||
| Fig. 11 Cyclic voltammetry curves of the electrode materials. (a) C-MoO3-NiO2/NF-1. (b) C-MoO3-NiO2/NF-2. (c) C-MoO3-NiO2/NF-3. | ||
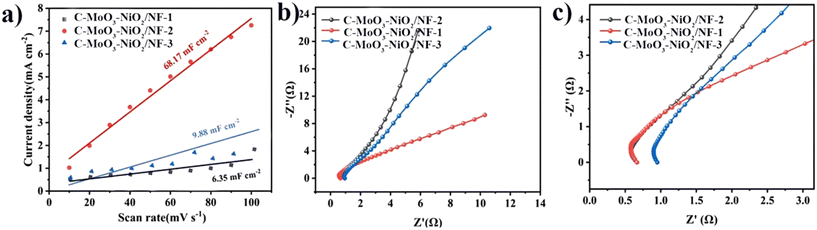 | ||
| Fig. 12 The different ratios of C-MoO3-NiO2/NF. (a) Electrochemically active surface areas. (b) Electrochemical impedance spectra. (c) Magnified view of the electrochemical impedance spectra. | ||
As shown in Fig. 14(a)–(c), the GCD plots for the C-MoO3-NiO2/NF electrode materials are presented. At 1 A g−1, the total charge–discharge time for C-MoO3-NiO2/NF-2 is 374.4 s, while C-MoO3-NiO2/NF-1 is 40 s and C-MoO3-NiO2/NF-3 is 133.7 s, preliminarily indicating that C-MoO3-NiO2/NF-2 has the best performance. Fig. 14(d) shows the cyclic stability test, with capacitance retention of 59% for C-MoO3-NiO2/NF-1, 75% for C-MoO3-NiO2/NF-2, and 68.4% for C-MoO3-NiO2/NF-3. Combining these results with previous tests, it can be concluded that the C-MoO3-NiO2/NF-2 electrode material exhibits the best performance (Fig. 13). Furthermore, a comparison has been made with those reported in previous studies49,74–85 as shown in Fig. 15 and Table S9,† which further proved that the C-MoO3-NiO2/NF-2 electrode material has excellent performance for supercapacitor application.
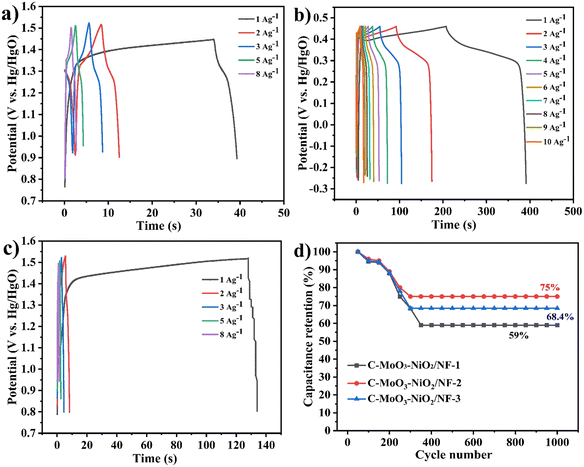 | ||
| Fig. 14 (a)–(c) GCD curves of C-MoO3-NiO2/NF-1, C-MoO3-NiO2/NF-2, and C-MoO3-NiO2/NF-3 electrodes, respectively. (d) Cycling stability. | ||
4. Conclusions
Using polyoxometalate (NH4)4[Ni(II)Mo6O24H6]·5H2O as a precursor, rice husk biomass was carbonized and combined with potassium hydroxide for porosity creation. After combining with the polyoxometalate, the sample was coated onto nickel foam to prepare the C-MoO3-NiO2/NF electrode material. Additionally, by varying the carbon–alkali ratios, when the amount of KOH added is small, the porosity of the biomass-activated carbon is incomplete, resulting in smaller pores. Conversely, when too much alkali is added, the pores in the activated carbon tend to collapse, and the porosity of the biomass-activated carbon is optimized with a carbon–alkali ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. It was found that at the same carbon–alkali ratio, combining with polyoxometalate NiMo6 enhances the supercapacitor performance. The optimal supercapacitor performance of the C-MoO3-NiO2/NF-2 electrode material exhibits a charge–discharge time reaching 374.4 s and a specific capacitance of 180.77 F g−1 with a current density of 1 A g−1, and the capacitance retention rate was 75% after 1000 charge–discharge cycles. This indicates that the electrode material prepared with the porous biomass-activated carbon with the polyoxometalate has an excellent superelectric value.
1. It was found that at the same carbon–alkali ratio, combining with polyoxometalate NiMo6 enhances the supercapacitor performance. The optimal supercapacitor performance of the C-MoO3-NiO2/NF-2 electrode material exhibits a charge–discharge time reaching 374.4 s and a specific capacitance of 180.77 F g−1 with a current density of 1 A g−1, and the capacitance retention rate was 75% after 1000 charge–discharge cycles. This indicates that the electrode material prepared with the porous biomass-activated carbon with the polyoxometalate has an excellent superelectric value.
Data availability
The authors confirm that most of the data supporting the findings of this study are available within the article and/or its ESI.†Author contributions
Zhongxin Jin and Kaijia Hu: conceptualization, methodology, validation, formal analysis, investigation, and writing – original draft. Feng Lin, Siqi Liu and Ruining Gu: conceptualization, resources, writing – review & editing, and supervision. Wei Zhang and Siyu Liu: revising the manuscript. Caiying Li: formal analysis and investigation. Xinping Cai and Hongyang Liao: formal analysis and investigation. Chunjing Zhang: visualization and investigation. Haijun Pang and Huiyuan Ma: formal analysis, investigation and supervision.Conflicts of interest
We declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This study was funded by the Innovation and Entrepreneurship Training Program for College Students at Daqing Normal University (S202410235051). This work was also financially supported by the Open Research Fund Program of Key Laboratory of Optic-electric Sensing and Analytical Chemistry for Life Science, MOE (M2022-4). We also thank the Heilongjiang Province Basic Scientific Research Expenses (2023KYYWF0022), the Project of Daqing Science and Technology Bureau (zd-2024-68), and the Daqing Normal University school fund (14ZR18).References
- L. Yang, Z. Zhang, C. N. Zhang and X. L. Wang, Rare Met., 2023, 43, 236–246 CrossRef.
- J. J. Xin, H. J. Pang, C. J. Gómez-García, Z. X. Jin, Y. Wang, C. M. Au, H. Y. Ma, X. M. Wang, G. X. Yang and W. Y. Yu, J. Colloid Interface Sci., 2024, 659, 312–319 CrossRef CAS.
- S. H. Fu, S. U. Khan, R. R. Yang, H. J. Pang, C. M. Au, H. Y. Ma, X. M. Wang, G. X. Yang, W. L. Sun and W. Y. Yu, J. Colloid Interface Sci., 2024, 666, 496–504 CrossRef CAS PubMed.
- X. J. Wei, X. Q. Jiang, J. S. Wei and S. Y. Gao, Chem. Mater., 2016, 28, 445–458 CrossRef CAS.
- N. Zhao, J. Chen, D. S. Yu, C. J. Tang, J. W. Zhao and T. M. Zhao, Mater. Today Commun., 2024, 40, 109538 CrossRef CAS.
- Y. L. Hu, Y. Y. Wang, J. W. Zhao and L. J. Chen, Coord. Chem. Rev., 2024, 506, 215724 CrossRef CAS.
- T. T. Deng, H. S. Li, S. Ding, F. Chen, J. B. Fu and J. W. Zhao, Nanomaterials, 2024, 14, 973 CrossRef CAS.
- B. S. Chikkatti, A. M. Sajjan, N. R. Banapurmath, J. K. Bhutto, R. Verma and T. M. Y. Khan, Polymers, 2023, 15, 4587 CrossRef CAS PubMed.
- S. Tanwar, N. Singh and A. L. Sharma, Energy Storage, 2022, 45, 103797 CrossRef.
- H. Li, L. Cao, F. Wang, F. Wang, G. Duan, W. Xu, C. Mei, G. Zhang, K. Liu, M. Yang and S. Jiang, Front. Chem., 2020, 8, 89 CrossRef CAS.
- Y. J. Li, W. Ou-yang, X. T. Xu, M. Wang, S. J. Hou, T. Lu, Y. F. Yao and L. K. Pan, Electrochim. Acta, 2018, 271, 591–598 CrossRef CAS.
- Z. Bi, Q. Kong, Y. Cao, S. Sun, F. Su, X. Wei, X. Li, A. Ahmad, L. Xie and C. Chen, J. Mater. Chem. A, 2019, 7, 16028–16045 RSC.
- Y. Zhang, P. Yu, M. Zheng, Y. Xiao, H. Hu, Y. Liang, Y. Liu and H. Dong, New J. Chem., 2021, 45, 5712–5719 RSC.
- L. L. Xing, X. Chen, Z. Tan, M. Chi, W. Xie, J. Huang, Y. Liang, M. Zheng, H. Hu, H. Dong, Y. Liu and Y. Xiao, ACS Sustainable Chem. Eng., 2019, 7, 6601–6610 CrossRef CAS.
- C. Guo and C. M. Li, Energy Environ. Sci., 2011, 4, 4504–4507 RSC.
- C. Kim and K. Yang, Appl. Phys. Lett., 2003, 83, 1216–1218 CrossRef CAS.
- K. Wang, Y. Wang and Y. Wang, J. Phys. Chem. C, 2009, 113, 1093–1097 CrossRef CAS.
- P. Karandikar, D. B. Talange, U. P. Mhaskar and R. Bansal, Energy, 2012, 40, 131–138 CrossRef CAS.
- Z. Huang, Z. Zhang, X. Qi, X. Ren, G. Xu, P. Ren, X. Wan and H. Zhang Sun, Nanoscale, 2016, 8, 13273–13279 RSC.
- S. Vijayakumar, A. K. Ponnalagi, S. Nagamuthu and G. Muralidharan, Electrochim. Acta, 2013, 106, 500–505 CrossRef CAS.
- X. Zhou, D. Cao, J. Huang, K. Ye, S. Yang, T. Liu, X. Liu, J. Yin and G. Wang, J. Electroanal. Chem., 2014, 720, 115–120 CrossRef.
- D. D. Potphode, S. P. Mishra, P. Sivaraman and M. Patri, Electrochim. Acta, 2017, 230, 29–38 CrossRef CAS.
- S. Patra and N. Munichandraiah, J. Appl. Polym. Sci., 2007, 106, 1160–1171 CrossRef CAS.
- T. Liu, L. Finn, M. Yu, H. Wang, T. Zhai, X. Lu, Y. Tong and Y. Li, Nano Lett., 2014, 14, 2522–2527 CrossRef CAS PubMed.
- J. W. Li, W. l. Yao, F. C. Zhang, X. F. Rao, Q. Zhang, S. W. Zhong, H. W. Cheng and Z. Q. Yan, Electrochim. Acta, 2021, 32, 138582 CrossRef.
- B. Liu, Y. J. Liu, H. B. Chen, M. Yang and H. M. Li, J. Power Sources, 2017, 341, 309–317 CrossRef CAS.
- X. Deng, B. Zhao, L. Zhu and Z. P. Shao, Carbon, 2015, 93, 48–58 CrossRef CAS.
- L. Guo, K. Wan, B. Liu, Y. Wang and G. Wei, Nanotechnology, 2021, 32, 442001 CrossRef CAS PubMed.
- J. L. Goldfarb, G. Dou, M. Salari and M. W. Grinstaff, ACS Sustainable Chem. Eng., 2017, 5, 3046–3054 CrossRef CAS.
- J. H. Wang, X. Zhang, Z. Li, Y. Q. Ma and L. Ma, J. Power Sources, 2020, 451, 227794 CrossRef CAS.
- L. Ren and B. W. Zhang, Exploration, 2022, 2, 20210182 CrossRef CAS PubMed.
- R. J. Shi, S. L. Jiao, Q. Q. Yue, G. Q. Gu, K. Zhang and Y. Zhao, Exploration, 2022, 2, 20220066 CrossRef CAS.
- Y. A. Kumar, G. Koyyada, T. Ramachandran, J. H. Kim, S. Sajid, M. Moniruzzaman, S. Alzahmi and I. M. Obaidat, Nanomaterials, 2023, 13, 1049 CrossRef PubMed.
- T. Y. A. Fahmy, Y. Fahmy, F. Mobarak, M. El-Sakhawy and R. E. Abou-Zeid, Environ. Dev. Sustain., 2020, 22, 17–23 CrossRef.
- A. A. Vannathan, T. Kella, D. Shee and S. S. Mal, Ionics, 2023, 29, 4227–4241 CrossRef CAS.
- H. Guo, H. L. Ren, J. Y. Tian, J. X. Xu, Y. R. Hao, L. P. Peng, Y. S. Liu and W. Yang, J. Alloys Compd., 2024, 1000, 175107 CrossRef CAS.
- B. R. Biradar, N. Thathron, P. P. Das and S. S. Mal, J. Electroanal. Chem., 2024, 960, 118192 CrossRef CAS.
- Y. Y. Yan, X. Cao, C. J. Wang, J. J. Liu, L. Y. Fu, Y. Yang, T. Wang, Y. Lu, W. F. Liu, X. G. Liu, R. Y. Wang, J. D. Zhou and M. L. Wang, Nano Today, 2024, 59, 102476 CrossRef CAS.
- H. T. Cui, T. Liu, M. L. Yang, A. Tian, J. Ying and X. L. Wang, J. Mater. Chem. C, 2024, 39, 15797–16240 Search PubMed.
- H. Guo, Y. Chen, N. Wu, L. P. Peng, F. Yang, Z. L. Pan, B. Q. Liu, H. Zhang, C. L. Li and W. Yang, J. Alloys Compd., 2022, 921, 165730 CrossRef CAS.
- M. S. Petronek, B. G. Allen, G. Luthe and J. M. Stolwijk, Int. J. Mol. Sci., 2022, 23, 8263 CrossRef CAS PubMed.
- Y. Deng, Y. Zhao, K. Peng and L. Yu, ACS Appl. Mater. Interfaces, 2022, 14, 49909–49918 CrossRef CAS.
- P. E. P. Win, J. X. Wang, X. Y. Jia, B. Qi, W. Chen, L. He and Y. F. Song, J. Alloys Compd., 2020, 844, 156194 CrossRef CAS.
- S. S. Wang and G. Y. Yang, Recent advances in polyoxometalate-catalyzed reactions, Chem. Rev., 2015, 115, 4893–4962 CrossRef CAS PubMed.
- R. Sivakumar, J. Thomas and M. Yoon, J. Photochem. Photobiol., C, 2012, 13, 277–298 CrossRef CAS.
- M. J. Muñoz-Batista, G. R. Bertolini, C. I. Cabello, R. Luque, E. Rodríguez-Castellón, A. Kubacka and M. Fernández-García, Appl. Catal., B, 2018, 238, 381–392 CrossRef.
- R. Ma, N. F. Liu, T. T. Lin, T. B. Zhao, S. L. Huang and G. Y. Yang, J. Mater. Chem. A, 2020, 8, 8548–8553 RSC.
- Y. Yang, Q. Y. Wu, Y. H. Guo, C. W. Hu and E. B. Wang, J. Mol. Catal. A: Chem., 2005, 225, 203–212 CrossRef CAS.
- C. Wan, Y. Jiao and J. Li, RSC Adv., 2016, 6, 64811–64817 RSC.
- M. Genovese and K. Lian, J. Mater. Chem. A, 2017, 5, 3939–3947 RSC.
- X. X. Yang, K. Li, J. Q. Lv, X. Y. Chen, H. Y. Zang, H. Q. Tan, Y. H. Wang and Y. G. Li, ChemElectroChem, 2018, 21, 3279–3286 CrossRef.
- J. Alcañiz-Monge, G. Trautwein, S. Parres-Esclapez and J. A. Maciá-Agulló, Microporous Mesoporous Mater., 2008, 115, 440–446 CrossRef.
- C. Yuan, L. Yang, L. Hou, L. Shen, X. Zhang and X. W. D. Lou, Energy Environ. Sci., 2012, 5, 7883–7887 RSC.
- Z. L. Li, J. Ren, C. M. Yang, Y. X. He, Y. Liang, J. L. Liu, G. I. N. Waterhouse, J. H. Li and D. Qian, J. Alloys Compd., 2021, 889, 161661 CrossRef.
- Z. Zhao, J. P. Sun, X. Li, Z. S. Zhang and X. C. Meng, Appl. Catal., B, 2024, 340, 123277 CrossRef CAS.
- C. R. Zhao, W. K. Wang and Z. B. Yu, J. Mater. Chem., 2010, 20, 976–980 RSC.
- B. Sun, S. Wang and M. Zhang, Polymers, 2023, 15, 4538 CrossRef CAS PubMed.
- H. Xu, Y. H. Bao, S. S. Zuo, P. D. Chen, Y. Q. Zhu, X. Q. Kong and Y. Chen, J. Electrochem. Soc., 2022, 169, 010514 CrossRef CAS.
- K. Liu, X. W. Chi, Y. Guo, K. Q. Hu, L. Mei, J. P. Yu and W. Q. Shi, New J. Chem., 2022, 46, 14711–14723 RSC.
- L. H. Zhu, F. Yang, X. Lin, D. Zhang, X. X. Duan, J. Y. Shi and Z. Sun, Process Saf. Environ. Prot., 2023, 172, 425–436 CrossRef CAS.
- S. J. Martínez, A. Lavacchi, E. Berreti, L. Capozzoli, C. Evangelisti, A. Arranz, J. L. Rodríguez and E. Pastor, Inorg. Chim. Acta, 2024, 566, 122008 CrossRef.
- M. Zheng, K. Shi, Y. X. Zhao, T. Zhang, F. X. Liu, J. P. Liu, Y. N. Sun, Y. F. Zhang and H. Wang, Int. J. Hydrogen Energy, 2024, 72, 1077–1090 CrossRef CAS.
- Y. Liu, C. L. Yue, F. Y. Sun, W. J. Bao, L. L. Chen, Z. Zeb, C. Z. Wang, S. Y. Ma, C. Zhang, D. F. Sun, Y. Pan, Y. C. Huang, Y. K. Lu and Y. G. Wei, Chem. Eng. J., 2023, 454, 140105 CrossRef CAS.
- P. Wang, H. P. Wang, N. Li, J. F. Sun and B. Hong, J. Colloid Interface Sci., 2024, 658, 497–505 CrossRef CAS PubMed.
- Q. L. Kang, M. Y. Li, J. W. Shi, Q. Y. Lu and F. Gao, ACS Appl. Mater. Interfaces, 2020, 12, 19447–19456 CrossRef CAS PubMed.
- S. C. Lee, S. D. Liu, P. A. Shinde, K. Y. Chung and S. C. Jun, Electrochim. Acta, 2020, 353, 136578 CrossRef CAS.
- X. Ren, Y. H. Zhou, Y. Y. Du, Y. C. Jiang, Y. J. Chen, J. F. Wan and F. W. Ma, Appl. Surf. Sci., 2020, 514, 145951 CrossRef CAS.
- X. Y. He, R. M. Li, J. Y. Liu, Q. Liu, R. R. Chen, D. L. Song and J. Wang, Chem. Eng. J., 2018, 334, 1573–1583 CrossRef CAS.
- Q. Wang, H. Y. Zhao, F. M. Li, W. Y. She, X. M. Wang, L. Xu and H. Jiao, J. Mater. Chem. A, 2019, 7, 7636–7643 RSC.
- X. K. Hu, Y. T. Qian, Z. T. Song, J. R. Huang, R. Cao and J. Q. Xiao, Chem. Mater., 2008, 20, 1527–1533 CrossRef CAS.
- I. Matanovic, K. Artyushkova, M. B. Strand, M. J. Dzara, S. Pylypenko and P. Atanassov, J. Phys. Chem. C, 2016, 120, 29225–29232 CrossRef CAS.
- X. J. He, P. H. Ling, M. X. Yu, X. T. Wang, X. Y. Zhang and M. D. Zheng, Electrochim. Acta, 2013, 105, 635–641 CrossRef CAS.
- C. Liu, K. Wang, Y. Du, Y. Shan, P. Duan and N. Ramzan, Polymers, 2023, 15, 4478 CrossRef CAS PubMed.
- Y. Ding, T. Wang, D. Dong and Y. S. Zhang, Front. Energy Res., 2020, 7, 159 CrossRef.
- L. Kouchachvili, N. Maffei and E. Entchev, J. Porous Mater., 2015, 22, 979–988 CrossRef CAS.
- Y. Gao, R. X. Sun, A. M. Li and G. Z. Ji, J. Electroanal. Chem., 2021, 882, 114986 CrossRef CAS.
- M. Gao, W. K. Wang, Y. M. Zheng, Q. B. Zhao and H. Q. Yu, Chem. Eng. J., 2020, 402, 126171 CrossRef CAS.
- Z. X. Sun and W. Thielemans, J. Energy Chem., 2023, 76, 165–174 CrossRef CAS.
- L. Y. Niu, C. Shen, L. J. Yan, J. H. Zhang, Y. Lin, Y. Y. Gong, C. Li, C. Q. Sun and S. Q. Xu, J. Colloid Interface Sci., 2019, 547, 92–101 CrossRef CAS PubMed.
- S. Dong, Z. Y. Xie, Y. Z. Fang, K. Zhu, Y. Y. Gao, G. L. Wang, J. Yan, K. Cheng, K. Ye and D. X. Cao, ChemistrySelect, 2019, 2711–2715 CrossRef CAS.
- F. Ma, S. L. Ding, H. J. Ren and Y. H. Liu, RSC Adv., 2019, 9, 2474 RSC.
- X. J. Liang, Y. Q. Chen, Z. C. Jiao, M. Demir, M. Du and J. J. Han, J. Energy Storage, 2024, 88, 11634 Search PubMed.
- J. H. He, Y. Zhou, S. B. Wu, L. Jin, J. Cao, M. Demir and P. P. Ma, Inorg. Chem., 2024, 29, 13755–13765 CrossRef PubMed.
- G. F. Liu, P. P. Ma, Y. Qiao, R. H. Xu, D. M. Huang, R. Y. Hu, L. Y. Liu, G. H. Jiang and M. Demir, J. Energy Storage, 2022, 52, 104942 CrossRef.
- Z. C. Jiao, Y. Q. Chen, M. Demir, M. Du, M. M. Gu, C. Wang, X. X. Zhang, Y. F. Deng, Z. J. Wang, T. Wang and W. Zhong, J. Energy Storage, 2022, 52, 104929 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4re00471j |
| ‡ These authors share co-first-authorship. |
| This journal is © The Royal Society of Chemistry 2025 |