Open Access Article
Open Access ArticleHow primary school students use their disciplinary drawings to navigate between everyday and scientific discourses of water
Bodil Sundberg *a,
Johanna Anderssonb,
Sofie Areljungc,
Carina Hermanssond and
Marianne Skooge
*a,
Johanna Anderssonb,
Sofie Areljungc,
Carina Hermanssond and
Marianne Skooge
aDepartment of Biology and Environmental Science, Linnaeus University, Sweden. E-mail: Bodil.sundberg@lnu.se
bDepartment of Behavioural Sciences and Learning, Linköping University, Sweden. E-mail: Johanna.andersson@liu
cDepartment of Educational Science, Umeå university, Sweden. E-mail: Sofie.areljung@umu.se
dDepartment of Educational Work, University of Borås, Sweden. E-mail: Carina.hermansson@hb.se
eSchool of Humanities, Education and Social Sciences, Örebro University, Sweden. E-mail: Marianne.skoog@oru.se
First published on 5th March 2025
Abstract
In this study, we investigate how young students can make use of their own disciplinary drawings to support transitions between everyday and scientific discourses of water. The empirical data consists of video-recorded stimulated recall interviews with six student pairs (age 8 years), conducted six months after they had been introduced to a water theme that included disciplinary drawing techniques. During the interviews, we provided students with their drawings as recall material. To stimulate a stalled discussion further or to support a new line of thought, we also asked supporting questions and provided the students with plastic models of water molecules, and a bottle of water. To trace their reasoning over time during the interview, the empirical material was used to construct semantic profiles for all student pairs underpinned by Legitimation Code Theory (LCT). Our findings show that most students used their drawings to bridge everyday experiences and scientific explanations of phenomena involving water. The plastic models and the water bottle however had varying effects, sometimes leading to adding a scientific discourse, and sometimes leading to off-topic reasoning. The students generally needed adult guidance to use their own drawings for navigating between everyday and scientific reasoning. However, our findings also show that some students were able to independently use their drawings to move between everyday and scientific discourse, in a way that suggests a gradual deepening of their understanding of the chemical properties of water. Based on these findings, we advocate for emergent disciplinary drawing, in combination with guided discussions, as an age-appropriate method for supporting primary students to navigate between everyday and scientific discourses in chemistry class. This approach could ensure that the educational value of students' creative efforts when drawing extends beyond the moment of creation, to also foster a richer language that can open for new ways of understanding and making sense of the world.
Introduction
Chemistry is commonly viewed as both challenging and devoid of meaning by students across academic levels. One reason that students struggle to master chemistry is because of its inherently abstract nature. In our daily lives, we encounter chemical processes and properties of matter through our senses at a macroscopic level. However, the scientific explanations for why substances react in a specific way or have specific properties, are found at the submicroscopic level of atoms and molecules. Consequently, an essential aspect of learning chemistry is to develop the ability to move back and forth between observable everyday experiences at the macroscopic level, and scientific explanations grounded in invisible processes at the submicroscopic level.The transitions between everyday experience and scientific explanations have proved to be problematic for students at all educational levels (Johnstone, 1982; Taber, 2013). One proposed solution to this problem is to introduce students to conceptual resources early, concomitant with supporting their reasoning about these resources (Fleer, 2009; Åkerblom et al., 2019). Another suggested solution is to introduce students early to the particle model, that is, to the concept of matter at both macroscopic and submicroscopic levels. Several researchers support an early introduction to the particle model, highlighting that students in their early school years are able to grasp, and enjoy learning about, the particle nature of matter (Samarapungavan et al., 2017; Adbo and Vidal Carulla, 2019; Haeusler and Donovan, 2020; Berg and Hultén, 2024). However, these researchers underscore that an early introduction needs to be carefully structured to correspond with appropriate primary teaching approaches. In this study, we draw on research that suggests that incorporating drawing into science education supports students to organise their thoughts, communicate knowledge and provides them with shared reference points for reasoning about scientific concepts (Ainsworth et al., 2011; Tytler et al., 2020). The overarching aim of the study is to contribute knowledge about the pedagogical potential of using children's emergent disciplinary drawings as an age-appropriate method to support transitions between everyday and scientific discourses about water.
We draw on empirical data obtained during a teaching sequence in grade two (students aged 8 years) where the teacher concurrently presented basic information about water as a chemical substance while guiding students to represent the content using chemistry-specific semiotic resources. The data comprises video recordings of stimulated recall interviews with student pairs conducted six months after the teaching sequence. During the interviews, the students’ individual drawings were introduced as recall material. Supporting questions, a bottle of water, and plastic models of water molecules were also introduced to stimulate a stalled discussion further or to support a new line of thought. To analyse the students' reasoning we use the semantic dimension of Legitimation Code Theory (LCT) (Maton, 2014). This sociological theory supplies us with two analytical concepts, semantic gravity (context dependence) and semantic density (complexity), that enable us to identify and describe how students navigate back and forth between every day and disciplinary discourse when reasoning about water.
To meet the aim of the article, we seek to respond to the following research question: In what ways, if any, do the students’ disciplinary drawings support them in navigating back and forth between everyday and scientific discourse?
Background
Students face challenges in chemistry learning due to difficulties in connecting personal experiences of matter at the macroscopic level with explanations described at the submicroscopic level. Additionally, they struggle with the literacy demands of learning new scientific concepts that have specific disciplinary meanings related to matter (Johnstone, 1982; Taber, 2013; Blackie, 2014). In the following we present research addressing these challenges within chemistry education, focusing on early years learning.An early introduction of the invisible levels of chemistry and scientific terms
The submicroscopic and atomic understanding of matter that ‘makes chemistry make sense’ is neither intuitive nor obvious to students (e.g. Musengimana et al., 2021). In most countries, including Sweden where this study was conducted, the chemistry curriculum at the primary level emphasises macroscopic experiences of phenomena without providing corresponding mechanistic explanations on submicroscopic levels (Haeusler and Donovan, 2020; Samarapungavan et al., 2017; Berg and Hultén, 2024). Consequently, students typically do not learn about the particle nature of matter until late primary or early secondary school. This one-sided focus on the macroscopic perspective of matter leaves young students unaware of the fundamental concept underpinning chemistry. An increasing number of researchers argue that primary science curricula thereby fail young children in two ways. Firstly, by not preparing them to understand chemistry education later in the educational system. Secondly, by missing out on supporting them to develop ‘a taste for science’ by omitting the key scientific principle that corresponds to the question why? at an early age when children often express interest in such questions (Anderhag et al., 2016; Haeusler and Donovan, 2020).The reason for only focusing on the macroscopic level has been ascribed to Piagetian views on what is appropriate in early childhood teaching (Haeusler and Donovan, 2020). Several researchers in science education now challenge this view, suggesting an early introduction of the particle nature of matter. Empirical studies from the last two decades demonstrate how students at an early age can handle and understand simple particle models. For instance, Berg and Hultén (2024) illustrate how primary students (9–10-year-olds) can engage in mechanistic reasoning and navigate across different representational levels. Samarapungavan et al. (2017) describe how second-grade students, involved in model-based inquiry lessons, could use simple particle models to describe and explain various material phenomena. The findings of Haeusler and Donovan (2020) suggest that 9-year-old students not only engaged with and learned aspects of atomic theory. Introducing atomic theory also seemed to foster further interest in science and provided the students with a welcome intellectual challenge. Additionally, Adbo and Vidal Carulla's (2019) study at the preschool level showed that even three-year-olds could deduce notions of particulate matter by themselves.
It is however crucial to emphasise that proponents of introducing the particle nature of matter early underscore the necessity of incorporating the submicroscopic perspective into carefully structured teaching approaches. These are suggested to specifically focus on establishing connections to students' everyday experiences with matter at a macroscopic level, and align with primary teaching approaches such as model-based inquiry (Samarapungavan et al., 2017; Berg and Hultén, 2024), integration of brief episodes of direct instruction, group work, hands-on activities, and simple experiments (Haeusler and Donovan, 2020), play-based activities (Adbo and Vidal Carulla, 2019) and the importance of introducing children to conceptual resources and the meaning of these (Fleer, 2009; Åkerblom, et al., 2019). Building on these suggestions, we aim to contribute new insights into the pedagogical potential of using student-generated drawings to support children's use of new conceptual resources and the meaning of these resources when reasoning about water.
The continuum of everyday and academic discourses
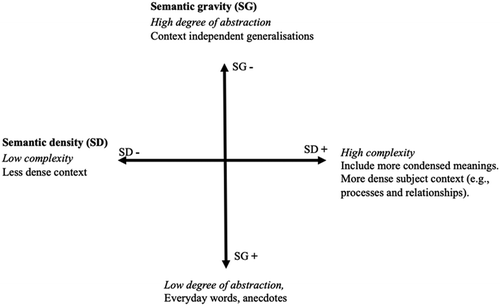
In many discussions about students' encounters with discourses within school subjects, a common distinction is drawn between informal, everyday language and decontextualised academic and scientific discourse. This distinction was highlighted by Vygotsky (1986) and has since been a fundamental topic in educational research. In line with the Vygotskian perspective several researchers in early years science have emphasised the importance of introducing children to conceptual resources and to invite them to reflect on the meaning of these. For example, Fleer (2009) describes how introducing children to new concepts in a playful way invites them to a broader membership of the problem-solving practices of basic science. Åkerblom et al. (2019), who interviewed 6-year-old children before and after a playfully enacted introduction of the concepts water, molecule and chemistry conclude, based on the children's reasoning before and after the playful intervention, that even young children are able to develop their use of scientific concepts to give a more generalised and abstract description of an experience. Their findings exemplify how most of the children approached a scientific understanding of the concepts. Moreover, the possibility to reason about their understanding of the concepts made some of the children identify their own learning gap on a meta level.Over time, the distinction between informal, everyday language and decontextualised academic and scientific discourse has been further developed from various perspectives, including systemic functional linguistics (SFL) (Halliday and Martin, 1993), and Bernstein's (1999) distinction between horizontal (everyday) and vertical (academic) discourse. These frameworks have helped highlight the literacy demands placed on students as they are introduced to new school subjects. However, the emphasis on distinguishing between everyday languages and disciplinary school languages has sometimes led to a perceived dichotomy between these languages, rather than viewing them as parts of a continuum. This perceived dichotomy might create the notion that there are two distinct linguistic domains from which educators must choose. To address this issue, Maton (2014) developed the semantic dimension of the Legitimation Code Theory (LCT) framework and a model that enables visualisations of how informal everyday discourses and decontextualized scientific discourses are continuous and complementary in educational practices. This model is known as the semantic plane. The semantic plane is grounded in Bernstein's framework on codes and knowledge structures and is constructed by combining two concepts: semantic gravity (SG) and semantic density (SD) (Fig. 1). Semantic gravity refers to the degree of context dependence, or abstraction of a concept or idea. Thus, the strength or weakness of semantic gravity is related to how concrete or abstract a concept is (Maton, 2013). A concept with stronger semantic gravity is termed (SG+) and is, for example, something that can be observed. Semantic density (SD) refers to the degree of disciplinary meanings condensed within symbols, terms, concepts, phrases, expressions, and gestures.
 | ||
| Fig. 1 The semantic plane with four principal quadrants adapted after Maton (2020). | ||
In the semantic plane, semantic gravity and semantic density are combined to produce a four-quadrant model of semantic codes. For example, an advanced conversation that is characterised by a high degree of abstraction (SG−) as well as high degree of complexity (SD+) corresponds to the semantic code (SG−, SD+) and the upper right quadrant of the plane. Even though the semantic plane is a typical four quadrant model, it should not be interpreted as comprising four separate boxes that describe four qualitatively different types of communications. Rather, the plane is meant to visualise how communication within educational practices is underpinned by a dynamic interplay of various combinations of degrees of abstraction and complexity. Moreover, semantic planes are always constructed with reference to the context where the communication is taking place (Maton, 2014). Thus, the criteria for a conversation to belong in the top right quadrant (i.e. a conversation on advanced or expert level) is expected to differ between a primary and secondary chemistry class.
In recent years, the semantic dimension of the LCT framework has been increasingly used in empirical educational research to explore discourses across various educational contexts (Maton, 2014, 2020; Nygård Larsson 2018; Dankenbring et al., 2024). In science education, it has proven particularly effective in analysing whether, and how, teaching oscillates between unpacking and repacking dense concepts and ideas to help secondary, or university students connect disciplinary and everyday language (Blackie, 2014; Hipkiss and Windsor, 2023). We find the use of LCT as particularly relevant to this study, as the framework is grounded in the view that understanding disciplinary language is essential for anyone seeking to ‘break into’ a new disciplinary culture (Maton, 2014). This notion aligns with the primary students in our study, who are in the process of navigating and understanding the spoken and visual scientific language of chemistry.
Drawing to learn and learning to draw in chemistry
Chemists have at all times used detailed drawings, symbols, and models to describe the invisible and abstract aspects of matter (Kozma et al., 2000). In chemistry teaching, it is common for teachers to use these drawings, symbols, and models to support their student's learning (Harrison and Treagust, 2002). These semiotic resources can serve as a means for meaning-making about chemical processes and how the macroscopic and submicroscopic levels are integrated. However, they also constitute a new symbolic and information-dense visual language for the students to master. One way to support students' mastery of this disciplinary visual language is to make them create their own representation. A growing number of studies have suggested that letting students create representations supports not only their science learning and interest but also their representational skills and science repertoire. For example, drawing can help students sort their thoughts out, communicate, recall disciplinary content, and come up with new thoughts and ideas (Ainsworth et al., 2011; Prain and Tytler, 2012; Caiman and Jakobson, 2019). Students may also use drawings to communicate and develop their understanding in interaction with others (Park et al., 2021; Areljung et al., 2024), to practice careful observation (Monteira and Jiménez-Aleixandre, 2016; Skoog et al., 2025), to think creatively and solve problems (Tytler et al., 2020) and to become visually literate in science (García Fernández and Ruiz-Gallardo, 2017).In primary school, students often draw in connection to science class. However, our findings from primary science classrooms show that the teachers rarely help the students focus on the scientific content or develop disciplinary drawing skills (Areljung et al., 2021). In addition, there is a lack of empirically based research to inform teachers on how they can support their students to develop disciplinary drawing skills. Most research on drawing in primary science classes has focused on the drawings per se and the cognitive abilities of individual students (Danish and Saleh, 2014). The few studies that recognize the teacher's role in drawing activities indicate that teachers’ ways of interacting with students matter to how they draw in science class. These studies have focused on the teacher's introduction to the drawing activity (Wilson and Bradbury, 2016; Areljung, Skoog and Sundberg, 2022), feedback on students’ finished drawings (Danish and Saleh, 2015), and methodological perspectives on using young students’ drawings as a starting point for their meaning-making (Andersson, 2019).
Taken together, a growing body of research supports the early introduction of the particle nature of matter in primary school education. At the same time, the role of drawing in primary science classrooms is gaining attention as a potential tool for enhancing science learning. In line with this, our study examines how primary students use their disciplinary drawings to navigate between everyday and scientific language when reasoning about water at both macroscopic and submicroscopic levels. This will provide empirical data to contribute to the development of these research domains. To emphasise that disciplinary drawings that students make at the primary level are ‘early attempts of using science-specific forms of visual language, different from those used in other school subjects or when drawing for fun’ we will use the term emergent disciplinary drawings as proposed by Areljung et al. (2022 p. 924).
Theoretical and analytical perspectives
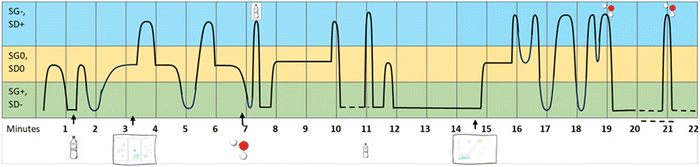
In this study, we wanted to investigate how primary students used their emergent disciplinary drawings to navigate between everyday and scientific discourse when reasoning about water. Therefore, we required an analytical tool that allowed us to explore their shifts between abstraction and complexity over time. To achieve this we used semantic profiling, an analytical tool based on the LCT framework (Maton, 2013, 2014). Semantic profiles are dual-axis charts that describe how discourses within educational practices vary over time (see Fig. 2). Depending on the context and the research questions, these profiles can either visualise variations in semantic gravity (SG) or semantic density (SD), or combine both dimensions into semantic codes (e.g., SG+/SD− and SG−/SG+) which move inversely within the profile. | ||
| Fig. 2 Semantic profile types. (a) Flatline (b) escalator and (c) semantic wave. Inspired by Maton (2020). | ||
In our study we have constructed semantic profiles where the two dimensions are merged to capture how the students navigate between everyday and more abstract and complex knowledge. Merging the two dimensions can help simplify the analysis when the goal, as in our case, is to understand general movement or trends in how students navigate between everyday and more complex and abstract language (Maton, 2013; Nygård Larsson, 2018). Merging the dimensions is generally not advised, referring to the risks of losing valuable analytical precision that the separate dimensions provide. In our study however, we focus on primary science education where the science content is less abstract and complex and more accessible compared to higher-level disciplines. As a result, the distinction between semantic gravity (concrete vs. abstract) and semantic density (simple vs. complex) is less pronounced. In such cases, merging the semantic dimensions can still provide meaningful insights without losing too much precision. In addition, merging the dimensions may have practical benefits for designing learning interventions or exploring pedagogical strategies that address both the complexity and abstraction of content simultaneously. In primary education, focusing on both dimensions together may help identify general supports or teaching strategies that target both the movement between concrete and abstract ideas and the increasing complexity of the content.
Fig. 2 displays three schematic types of semantic profiles based on semantic codes, inspired by Maton (2020). A high level on the Y-axis corresponds to a high level of abstraction and complexity (SG−, SD+), whereas a low level corresponds to a low level of abstraction and complexity (SG+, SD−). The first type, the semantic flat line, visualises a discourse that remains at a constant everyday level over time. The second type, the semantic escalator, visualizes discourses that begin at an everyday level, then introduce abstractions and dense concepts that are not unpacked (upward escalator). The third profile type, the semantic wave, describes a discourse that continuously oscillates between abstract, complex language and everyday language. For clarity, the middle points (SD0, SG0) are omitted in the figures.
According to the underpinnings of LCT, semantic waves are indicative of teaching that supports cumulative knowledge-building, as they suggest that students are supported to move back and forth between meanings constructed from previous everyday experiences and new, abstract and complex ideas. In contrast, discourses described as semantic flatlines or escalators indicate educational practices where the students lack support to make connections between everyday and scientific abstractions and ideas. For example, a lesson that initially is held at an informal level with occasional introductions of out-of-context or unexplained scientific terminology, will produce a semantic escalator type profile. Semantic escalators can also describe discourses where students' discussions are based on firsthand everyday experiences, with occasional additions of unexplained scientific, dense concepts that they sense might fit into the context (cf. buzzwords). This may, according to the framework, may lead to fragmented knowledge and ‘semantic gaps’.
Method
The empirical data of this study is a subsample of a larger project (Drawing to learn and learning to draw in primary school science) that aimed to generate knowledge about the pedagogical potentials and limitations of drawing in science education. The project was based on formative interventions (Penuel, 2014) where teams consisting of primary school teachers, researchers, and art educators collaborated to generate a pedagogical vocabulary and practical repertoire for teaching disciplinary drawing in science class. During team meetings, the teachers and researchers discussed possible ways of supporting the students’ disciplinary drawing in relation to the curriculum core content that the teachers intended to cover. The teachers then made concrete lesson plans in ways they found suitable for their context. The subsample analysed in this study consists of video-recorded stimulated recall interviews (DeWitt and Osborne, 2009) with six student pairs, conducted six months after such science lessons, where both basic content knowledge about water and disciplinary drawing techniques were introduced.We selected 12 students, 6 from each of two classes aiming to follow two criteria: (a) an even distribution between girls and boys, and (b) the content and design of their drawings of water should together represent the diversity displayed by all drawings. On the day of the interview, the teachers made some adjustments to our original selection to ensure that the pairs would feel comfortable with each other and to adjust to the fact that some of the students were not present. Still, recall interviews were held with six pairs that represented an equal number of boys and girls.
Context, data collection and analysis
The study was performed at a primary school situated in a multicultural area of a middle-sized city in central Sweden. In Sweden, early primary school includes grades 1–3 for students between 7–9 years. The science curriculum for grades 1–3 specifies certain goals grouped into central areas to cover.The content areas of water and matter are described within the central area “Materials and substances in our environment”. At the time of the study this theme included (National Agency for Education, 2018, pp. 189–190):
• Properties of materials and how materials and objects can be categorised based on such properties as appearance, magnetism, conductivity, and whether they float or sink in water.
• Man's use and development of different materials during the course of history. The different materials used to manufacture daily objects and how they can be recycled.
• Various forms of water: solids, liquids and gases. Transition between the forms: evaporation, boiling, condensation, melting and solidification.
• Basic properties of air and how they can be observed.
• Simple solutions and mixtures and how these can be divided into different constituents, such as through evaporation and filtering.
The curriculum also states that students should be encouraged to observe, measure, and systematically discuss natural phenomena, emphasising the importance of acknowledging students’ interests and developing their ability to ask questions and describe and discuss scientific concepts. By the end of grade 3, students are expected to be able to document their scientific investigations using various forms of expression and use their documentation to participate in discussions and conversations.
The drawings
The drawings used as recall material were made by the students as an assignment after the initial lesson about water. In line with the formative intervention method the teachers and researchers had discussed possible ways of introducing water on both macroscopic and submicroscopic level, and how to support the students’ disciplinary drawing in relation to this content. The teachers then made concrete lesson plans that would be appropriate for their students. The introductory lesson covered factual knowledge about water on earth, common symbols in scientific visualisations of water (Fig. 3) and included an educational film culminating in a memorable “H2O-song”. The students were then asked to “draw water in solid, liquid and gaseous states”. They were asked to “use symbols” and reminded “do not forget the water molecules and how they align to each other in the three states”. | ||
| Fig. 3 Collage of symbols and concepts used in PowerPoint presentation by the teachers when introducing the water theme (Translated from Swedish). | ||
A paper template with three squares, one for each phase, was provided, and the students were instructed to use symbols and specific colours to represent water and molecular arrangements. After the initial lesson, the water theme continued for a couple of weeks with simple experiments connected to water and extra opportunities for the students to finish their drawings. Due to pandemic restrictions, no observations could be made of the introductory lesson when the drawings were conducted. However, we participated in the lesson planning, and the teachers shared the final plans along with the PowerPoint used in class (excerpt in Fig. 3). When the students had completed their drawings, copies were made for our research.
Most students produced three sets of drawings illustrating the phases: (a) on a macroscopic level (b) on a submicroscopic (molecular) level and (c) a combination of (a) and (b) (Fig. 4). Six students also (d) depicted the water cycle simultaneously on macro and submicroscopic level. While most drawings adhered to the semiotic resources provided in the instruction, such as symbols, colour, and composition, some included extra elements such as stick figures, contextual details (e.g. boats, fishes), and representations of personal or imaginary experiences of water.
 | ||
| Fig. 4 A selection of student drawings showing water on (a) macroscopic level, (b) submicro (molecular) level, (c) a combination of (a) and (b), and (d) integrated into the water cycle. | ||
Recall interviews
The same two researchers attended each of the six interviews, and the same semi-structured guide was followed during all interviews. This guide served to ask questions that encouraged the students to explain their thoughts when creating their drawings and about water as a subject or substance. We began the interviews with an open question about what the students could recall from the water theme: You worked on the theme of water this past spring. You talked about it and made drawings. What do you remember from last spring when you worked on the theme of water? If the students needed further encouragement for responding to this question, we asked follow up questions about their recollections of any experiments or showed a bottle of water as a prompt. When the students seemed to run out of recollections to share from the water theme, we brought forward their drawings of water, focusing on the drawings where both macro and submicroscopic levels were displayed (variant c in Fig. 4). We then asked the children to describe their drawings. To guide the conversation, we asked supporting questions such as: What do you think was important to convey in your image? What did you want to express by drawing an arrow/using that color/drawing a molecule? How do you think the different parts of your drawing are connected? For example, how does the snowman relate to the group of molecules?Depending on how the discussions progressed, we introduced two plastic molecule models or a bottle of water (again) as ‘tools for thinking’ (Schoultz et al., 2001, p. 103) and asked more guiding questions. This approach aimed to either stimulate a stalled discussion further or support a new line of thought introduced by the students. When the students were grasping for the right words, we supported them with Swedish vocabulary or scientific terms such as experiment, molecule, gas and steam. The student pairs needed support to varying extent, but all were provided with artifacts, supporting questions and help with the vocabulary during the interview. Each interview lasted 10–20 minutes. We ended the interviews when we had discussed both students' drawings, and both students had had opportunities to give rich descriptions of their thoughts about the content of their drawings, alternatively if the students showed signs of getting tired. In one case (student pair 1), one of the students was clearly uncomfortable with the situation so we ended the interview at an early stage. Each interview was concluded with the final question: Is there anything important that you've learned about water that we haven't talked about?
All six interviews were video recorded to capture students’ verbal and non-verbal interactions with each other, the researchers, and the recall material. Notes were also taken.
When all transcripts were coded for semantic gravity and semantic density, we constructed semantic profiles to visualise how the students' reasoning moved between everyday and scientific discourse during the interviews.
Additionally, most of the students in our study had only recently been introduced to the spoken and visual scientific language of chemistry. Another limitation in the design of our study is therefore that our findings are likely influenced by how we, as interviewers, interacted with the students to encourage them to articulate their views in more detail. Aware of this, we ensured that the students had space to express their own recollections and understandings before we supported them with guiding questions and specific terms. We also used the same semi-structured interview guide for all participants to ensure consistency. Nevertheless, we suggest that these limitations be addressed in future research building on our findings.
Findings
The overarching aim of this study was to contribute to the understanding of the pedagogical potential of using students’ emergent disciplinary drawings to support transitions between everyday and scientific discourses about water. Our findings show that most students used their drawings to transition between everyday and scientific discourses. However, it also became clear that many of them required challenge and support from a knowledgeable adult when reasoning about the scientific content of their drawings.Through semantic profiling, we identified two examples in which student pairs used their drawings, along with supportive questions from the researchers, to make a temporary upward shift to a scientific discourse (the supported upshift escalator profile); two examples where they used their drawings and supportive questions to oscillate between everyday and scientific discourse (the supported semantic wave); and one example where the students independently used their drawings to oscillate between everyday and scientific discourse, gradually building new knowledge (the student-generated semantic wave).
In the following section, we present three cases that illustrate how the student pairs used their drawings and other types of support to navigate between everyday and scientific language when reasoning about the properties of water. To capture the richness of the students' reasoning in more detail, each case is presented together with excerpts from the interview transcripts.
Case one. Supported upshift escalator: using disciplinary-specific concepts without connections or explanations
The interviews of two student pairs could be described by upshift escalator profiles. Fig. 5 illustrates the semantic profile produced by one of the pairs: Charlie and Drew. | ||
| Fig. 5 Example of an upshift escalator profile, indicating where different recall material was provided during the interview. | ||
In this pair, Charlie is driving the discussion. Drew uses affirmative nods and facial expressions and occasionally contributes verbally with a word or two throughout the interview. When asked about their recollection of the water theme, Charlie immediately exclaims “H2O!”. Upon further inquiry regarding the meaning of H2O, neither student however recalls what it is or might be, and both clarify their overall limited recollection of the water theme. Hence, the specific scientific concept of H2O is used as a single utterance (SG0, SD0). This is visualised in Fig. 5 by the initial escalator peak which represents how a dense concept is introduced but never unpacked.
When queried further about the water theme, Charlie and Drew recollect having made experiments where they had mixed water, salt, and sand but they have vague recollections of the experiments’ objectives and outcomes (30 seconds into the interview):
Drew: We mixed water, and…(frowns)
Charlie: yeees, water and salt and we put it on a shelf and waited for it to, what was it…
Drew: Be taken away?
The water bottle is introduced but doesn't support their thinking. It is not until they are presented with their drawings (2.58 minutes into the interview) that they recall and expand further upon context-independent ideas and specific scientific terms (SG−, SD+) connected to water:
Researcher: you also drew this [drawing] depicting the various appearances water can take, let's see… (puts the drawings in front of the students). Do you recognize your drawings?
Both Drew and Charlie look at their drawing and smile, Drew then covers her eyes with her hands, Charlie nods.
Researcher: do you remember what you were thinking when you made them? What did you want to describe?
Charlie: (looks down at her drawing) I wanted to describe ehh, solid, and a cup and ice (Drew looks at Charlie's drawing)
Researcher: yes exactly, that… that's in the first square there? (Fig. 4, pair 2)
Charlie: aha (affirmatively, points to the first square)
Researcher: ah, that's what water looks like when it's solid?
(Charlie pushes the drawing towards the researcher, then brings the hands under the table)
Researcher: and, what is this then? (points to the next square)
Charlie: liquid
Researcher: and these things then? (points to the molecules depicting molecular arrangement in liquid phase)
Charlie: them, what's their name… they are also H2O but they are not together, they are kind of spread out
It appears that initially, Charlie feels somewhat uneasy in the situation, requiring additional support from the researchers to articulate the content of her drawings. However, she subsequently uses her drawing to describe the scientific term H2O in a denser meaning when she uses it as a symbol embedded in a scientific explanation to describe the molecular arrangement underlying the phases. This shift in discourse is illustrated in the profile as a temporary upshift, moving from everyday discourse (SG+, SD−) toward a more abstract and condensed language (SG−, SD+). Despite the researcher's gentle prompting and assistance, Charlie does not relate this disciplinary description back to an everyday example. Consequently, the upward shift lacks a subsequent downwards connection, resulting in a fragmented upward wave, or ‘upward escalator’ (2 min 58 s – 4 min 05 s).
The interview continues with small talk for a while, during which the students comment on their pictures, mostly noting their inability to recall more details. The term H2O resurfaces (6 min, 15 s) in response to questions about symbols on the drawings. However, the students do so without further elaborations on the complex and context-independent ideas behind these concepts or connections with everyday examples, resulting in another upward escalator. Attempting to prompt the students to unpack the concept further, the plastic model is introduced (6 min, 52 s). One student points out discrepancies between the colours of the atoms in her drawing and those in the plastic model. However, none of the students draw connections between the plastic model and the term H2O previously used in the interview. As the students start to display signs of waning interest, the interview is concluded.
In summary, this profile is characterised by a couple of upward escalators that lack subsequent downward connections, resulting in a fragmented upward escalator pattern. Neither the researchers’ support nor student interaction or other recall material contributes to further elaborations by the students.
Case two. Supported semantic wave: drawings and adult guidance create oscillations between discourses
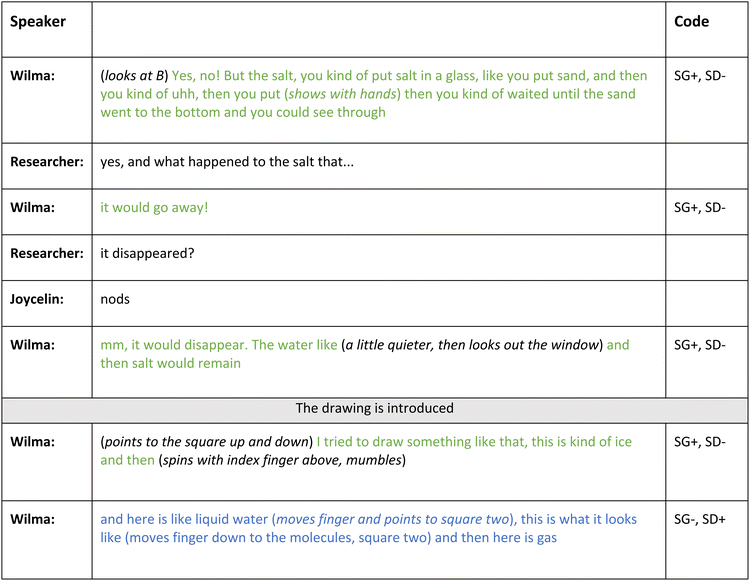
Two student pairs’ conversations could be visualised by supported semantic wave profiles. In Fig. 6 the semantic profile of the interview with Wilma and Jocelyn is described. | ||
| Fig. 6 Example of a supported semantic wave profile, indicating where different recall material was provided. | ||
Before the drawings are presented, Wilma and Jocelyn both have hazy recollections of the water theme. Wilma initiates the discussion by introducing the term “gaseous phase” just one minute into the interview but does not provide further details (upward escalator, SG0, SD0). The students spend the next two minutes struggling together to recall an experiment conducted during the water theme. Their conversation is characterised by everyday language:
Wilma: we did some experiments
Researcher: what did you do then?
Jocelyn: water, that we put salt in and then I think there was something else too (looks into the camera)
Wilma: we put sand and… (looks at Jocelyn for support)
Jocelyn: sand in a glass, and sand…
Researcher: in different glasses?
Jocelyn: and then we placed it in the front (points to the window) because it…
Wilma: (interrupts) we would put sand
Jocelyn: – went away
Here one of the researchers questions that the water just “went away”, which triggers Wilma to be more specific.
Wilma: Nooo, it evaporated!
However, she does not further elaborate on the meaning of this scientific concept, which is illustrated with an upward escalator (2 min, 50 s).
When the drawings (Fig. 4, student pair 5) are introduced (3 min, 30 s) the students first need some time to familiarise themselves with them. After some supporting questions from one of the researchers, Wilma uses her drawing by pointing towards the different symbols that represent the details she is describing:
Wilma: I tried to draw that sort of thing, solid, this is kind of ice and then (spins with index finger above the ice cubes, mumbles). And here is, like liquid water (moves finger and points to square two), this is what it looks like (spins her finger over the molecules), and then here is gas and here I drew the (mumbles) I wrote…
Researcher: yes right, you've made some equal signs like this here (points to the bottom of square one)
Wilma: yes! It shows that this (points to the ice cube and snowflake in the first square) inside is like this (points to the arrangements of molecules, first square), and this (points to the running water in square two) inside is like this (points to the molecules, square two). I think that's what I learned (looks up at the ceiling)
Wilma and Jocelyn then take turns describing the phases of water, using scientific terminology, and referencing their drawings by pointing to details (4 min, 10 s–4 min, 55 s). However, they refrain from elaborating further to unpack the scientific meanings and eventually drift off to other topics (7 minutes). To sustain the conversation, the researchers offer encouraging questions and supportive comments, resulting in consecutive upward shifts since no unpacking occurs. A notable change in the semantic pattern however follows the introduction of new drawings, depicting the water cycle (9 min, 50 s). Both Wilma and Joycelyn, now change their reasoning into a pattern of unpacking and repacking concepts aided by their drawings and the researchers' guidance:
Jocelyn: when the sun goes down into the water, the water evaporates, it rises to the vapor. And then it goes up to the cloud, then it rains
After a while, the students appear to lose interest in their drawings and the plastic molecules are introduced. Both students reach forward to grasp one each. One of the researchers comments that these are models of water molecules and points out the different atoms that constitute the molecule. Wilma holds the molecule with both hands, one hydrogen atom in each, and exclaims:
H2! Whoa, whoa whoa! These two are H (squeezes the hydrogen atoms) and this (grabs the oxygen atom with her right hand) is O! H2 look! H, H it's two… two, two, and one O, H2O! (14 min, 25 s)
This expression of a potentially new insight into the water molecule structure (SG−, SD+) however, swiftly transitions into imaginative thinking (14 min, 38 s) veering into off-topic reasoning generating an upward escalator. Similarly, the initial impression that the water bottle (16 min, 15 s) leads to new insights is short-lived. Instead, discussions about prehistoric animals and old things in general take over:
Researcher: so this water could have been in many places (holds the water bottle)
Wilma: We have been drinking the same water since the Stone Age
Researcher: yes, it's certainly exciting to think like that
Jocelyn is silent but looks at the water bottle that the researcher is wiggling back and forth
Wilma: it's the same as dinosaurs…
Researcher: mm
Wilma: the dinosaurs did not exist in Sweden//
Jocelyn: I once found a really old coin
In summary, this profile features upward escalators and sections of semantic waves. Researchers’ support is as crucial as the drawings for students’ recollections of the scientific content, and the generation of a semantic wave. Student interaction is infrequent, and primarily focused on their own drawings. Other recall material triggers new perspectives but doesn't contribute to semantic waves.
Case three. Student-generated semantic wave: gradually building knowledge with support from drawings and peers
The third case differs from the others in that the students, Benjamin and Felix, do not need support from the researchers to make connections between everyday and scientific descriptions of water (Fig. 7).Benjamin and Felix initiate a semantic wave even before the drawings are introduced. After a brief introduction and with some encouraging questions from the researchers, the students collaboratively introduce scientific terms related to water, drawing connections to their personal experiences of observing transformations between different forms of water (first and second wave, SG0, SD0):
Benjamin: first, we were taught about water. About ice and then gas, and then, things like… (looks at Felix, smiles) like… No, I don't know (laughs)!
Researcher: like ordinary water? That kind of water? (shows the bottle, 1 min, 39 s)
Benjamin: yes, that kind of water
Felix: yes, that kind of water, made into ice
Benjamin: yes, that kind of water, if you put it in the freezer, then water, it becomes ice, it becomes ice
Researcher: yes
Felix: and then we did another one (inaudible, but points with hand to forehead, Benjamin looks at Felix)
Benjamin: and then it heats up, it melts//
Benjamin: and then gas comes out! And you kind of heat it on the stove, then gas comes out.
When the first drawings are shown (Fig. 4, pair 6), the students' reasoning continues to oscillate between everyday and disciplinary discourses, where they describe water based on everyday experiences and as a chemical substance. However, there is an upward shift on the semantic scale, as the everyday terms water, and ice are replaced with liquid and solid states. Additionally, a submicroscopic perspective is introduced to describe the molecular arrangements in the different states (SG−, SD+) (3 min, 10 seconds):
Researcher: there's your [drawing] huh? (to Benjamin)
Benjamin: yes, that's it (smiles and points to the left square, in Fig. 4c), first they sit together (shows by crossing the arms close to the body), and then they spread out a little
Researcher: aha
Benjamin: and then, these here (slaps hands down on the third square in Fig. 4c, that shows the gas phase), so when they are see… they go out like this (slaps outwards with hands) nobody wants to be with each other (puts hands down on lap, Felix looks on Benjamin's drawing and appears to be listening, then looks down at his drawing)
Researcher: so then they are like further apart?
Benjamin: aha
During the whole interview, both students are active. They interact with each other and the drawings to explain their thoughts about water. Both body language and verbal language are used to communicate, and they do not need supporting questions from the researchers to prompt the conversation further, thus driving a semantic wave on their own. They, however, interact with the researchers when searching for terms they have forgotten. For example (6 min, 47 s):
Benjamin: and P [the teacher] had things like that, things like this (pointing to the molecules on Felix's drawing)
Felix: yees
Researcher: Molecules?
Felix: yes
Benjamin: yes, Molecules! (smiles)
To support this reasoning further, the plastic models are introduced which inspires the students into a new line of thought, the concept of scientific models (6 min, 56 s).
Benjamin: but this, it's kind of like this (places one plastic model on top of one of his illustrations of a molecule), the red one and then the white one (holds the molecule up in the air again and shows by holding on to the different atomic types with his hands) are up here (looks up at the researchers).
Now Felix also puts his molecule on top of one molecule in his drawing
Researcher: did P show them [the plastic models] when you were working on this?
Benjamin: yes, we saw
Felix: (waves his plastic molecule) They are so small that you can't see them!
Researcher: well, exactly, so then you make a model of them
Felix: mm
One example of how the students drive the conversation, and the oscillation between everyday and scientific language is how this remark leads Felix to ponder further and ask:
Felix: are these everywhere?
Researcher: do you mean water molecules?
Felix: yes
Researcher: aha (picks up the water bottle)
Felix: is it in there now? (Benjamin also looks at the bottle)
Researcher: yes, that's what water consists of
Felix smiles big, turns his whole body away for a moment, and looks back, clearly fascinated by the new information.
This realisation appears to be a turning point for the students, resulting in another instance of leveraging their discussion on the semantic scale (10 min, 59 s). From here on they use their drawings, the water bottle, and the plastic molecule as prompts to support a playful conversation that oscillates back and forth between everyday experiences and scientific explanations, on submicroscopic and macroscopic levels. This lively exchange, which continues the semantic wave pattern, continues until 12 minutes into the interview when the discussion remains on an everyday level for a while as they delve into how both ice and ice cream are made in a freezer. The drawings of the water cycle are introduced (14 min, 40 s), and this sparks a new semantic wave where the students contemplate the phase transitions occurring in various parts of the cycle.
In summary, the students initiate a semantic wave even before the first drawings are introduced and continue to shift between everyday and disciplinary discourse throughout the interview. The semantic wave is leveraged each time the students encounter a new type of recall material and new terms suggested by the researchers are seamlessly integrated into their discussion.
Discussion and implications
Our study draws on recent suggestions to introduce the particle model, and related conceptual resources, early in chemistry education, as well as on concerns that such an early introduction must be appropriate for primary teaching practices (Haeusler and Donovan, 2020; Samarapungavan et al., 2017; Adbo and Vidal Carulla, 2019; Berg and Hultén, 2024). Recognising these concerns, we used semantic profiling to explore if and how young students’ emergent disciplinary drawings may support them to transit between everyday and scientific language when reasoning about water at macroscopic and submicroscopic level.Our findings suggest that introducing emergent disciplinary drawing in primary chemistry education can serve as an age-appropriate method for helping young students use new conceptual resources when making meaning of water ‘as a phenomenon on both macroscopic and submicroscopic levels’ (Berg and Hultén, 2024). In their emergent disciplinary drawing, the students represented water using specific semiotic resources that chemists have used for centuries (e.g. words, images, and symbols), intertwined with decorations as well as depictions of personal everyday experiences or imaginative worlds. Although these drawings did not strictly align with disciplinary standards in chemistry, they supported the students to recall the scientific content of the water theme, even six months later. Before they were provided with their drawings, most of the students did not recall the scientific content of the water theme and few of them used scientific language when describing their recollections. However, when provided with their drawings most of them could, with support from researchers’ questions, navigate between both everyday and scientific language and macroscopic and submicroscopic levels. Moreover, our findings align with previous research showing that young students not only grasp but also enjoy learning about the particle nature of matter (Samarapungavan et al., 2017; Adbo and Vidal Carulla, 2019; Haeusler and Donovan, 2020; Berg and Hultén, 2024).
We propose that the pedagogical potential of these emergent disciplinary drawings lies in their deviation from strict disciplinary standards within the field of chemistry. The students’ drawings effectively convey the particle nature of matter, which is a fundamental aspect of understanding chemistry. Furthermore, the drawings facilitated a student's perspective, in the sense that they enabled students to relate to their everyday or imagined experiences with water. Building on the underpinnings of LCT theory, we suggest that these emergent disciplinary drawings helped the students to bridge hierarchical and horizontal knowledge structures (Bernstein, 1999), thus serving as a tool to connect their everyday perspectives with the disciplinary view of matter.
Our findings reveal significant variations in the level of support the students required to ‘make waves’ which are indicative of cumulative learning. Although all student pairs received similar support from the researchers during all parts of the interview (before and after the drawings were introduced), this support was not enough for every student. For instance, Charlie's and Drew's discussions (Case One) did not generate semantic waves, whereas Wilma and Jocelyn (Case Two) generated waves with the researchers' help and Benjamin and Felix (Case Three) generated waves on their own. This variation in terms of need for adult support likely reflects a broader trend across science classrooms. A similar pattern was observed by Åkerblom et al. (2019) in their study of children's reasoning about water before and after a playful learning intervention with eleven 6-year-old children. Initially, all children discussed water using everyday concepts grounded in their rich practical experiences. However, two of the children also interlaced their everyday descriptions with scientific terms using “language in an expansive way that transcends the local context of the lesson and the interview, respectively” (p. 892). These findings underscore the importance of guided discussions following disciplinary activities to help all students to “make waves”, to avoid ‘semantic gaps’ that can lead to fragmented learning. To address the varying levels of scaffolding needed by students, we recommend that primary teachers collaborate with students to make semantic waves in the classroom, by unpacking and repacking dense disciplinary meanings together (Maton, 2013). Such an approach might be particularly crucial in chemistry education, where unpacking and repacking disciplinary meanings related to matter is a significant challenge for students (Johnstone, 1982; Taber, 2013; Blackie, 2014). In line with Hipkiss and Windsor (2023) and Blackie (2014), we propose that teachers use semantic profiling to provide opportunities for cumulative learning in the science classroom.
While previous studies have focused on higher educational levels, we present this article as a first step towards a guide for primary teachers aiming to create semantic waves that support cumulative learning in primary chemistry. Here, the significant variations in the level of support that the students required addresses an aspect that needs to be considered both in future implications and research building on our findings.
Finally, we want to draw attention to how students made use of the molecule model and the water bottle during the interviews. Whereas the drawings supported all students to use a more scientific discourse, the molecule model had a variable effect on students’ reasoning. In some cases, the model helped the students develop their scientific reasoning. In other cases, the plastic molecule model became a toy or triggered anthropomorphic reasoning. Interestingly, the same student could exhibit both approaches to the model. For example, in an excerpt from Case Two, Joycelin first examines the different parts of the plastic molecule from a scientific perspective, then imagines it as a little person with two legs, which she plays with. Joycelin's example is particularly interesting in the light of research on anthropomorphic reasoning in chemistry education. Although mainly portrayed as a problem in previous research, anthropomorphic reasoning has lately been recognised as a potential resource for fostering students’ understanding in chemistry (Dorion, 2011; Manneh et al., 2018). Åkerblom et al. (2019) highlight the inherent tension between creating educational experiences that are true to scientific explanations and those that invite young students to learn in a playful and age-appropriate way that include anthropomorphic narratives. In their study, an actor's impersonation of a water molecule resulted in both joy and confusion among the children. In our study, the instances where the model inspired students to create anthropomorphic narratives did not at first sight, indicate a deeper understanding of the molecular structure of water. However, one could argue that these anthropomorphic narratives were part of a playful approach to chemistry that could enhance young students' positive feelings towards the subject. We concur with Åkerblom et al. (2019) that fostering a young students' feeling of joy and inclusion might be an equally important goal that should be interwoven with concept learning.
We note that many students in our study appeared to form a particularly positive connection with the term H2O. As previously described, the plastic H2O model often prompted anthropomorphic narratives or play. However, beyond this, most students seemed to have intrinsic appreciation for the term H2O itself. We documented several examples where students spontaneously called out the term without further explanations, typically accompanied by big smiles or laughter. Such spontaneous exclamation is displayed by the upwards escalator in Fig. 5. According to the LCT framework, upwards escalators indicate educational practices that overly focus on out of context disciplinary terms, which can potentially lead to fragmented knowledge and ‘semantic gaps’. An alternative interpretation could be that these spontaneous exclamations are signs of emergent meaning-making and that the students are experimenting with a science-specific term that differs from the everyday language. A child first learns that there is something that, for example, is called “H2O”. The next step is to connect this term to concrete examples, followed by the ability to define it in more abstract manners (Vygotsky, 1986). The 8-year-old students in our study are in a stage of discovering new terms that connect to their previous experiences of water. Possibly, they are eager to explore these terms. Following the Vygotskyan interpretation of children's learning of scientific concepts, the spontaneous exclamations of “H2O!” by Charlie in Case one, as well as “Gaseous phase!” by Wilma in Case Two, can be interpreted as examples of their initial step to appropriate a new concept, rather than signs of fragmented learning. Similarly, Åkerblom et al. (2019) found instances in their transcripts where children used scientific terms like “oxygen” and “steam” although they only had a vague understanding of their meanings. Hence, spontaneous use of scientific terms, even when seemingly out of context, are not necessarily signs of students’ mindless use of “buzzwords” and fragmented learning. Instead, these spontaneous and joyful exclamations of scientific concepts can be viewed as valuable resources for them to, with help from their teacher, develop a richer language that can open for new ways of understanding and making sense of the world. As Ludwig Wittgenstein aptly stated (1922): “The limits of my language means the limits of my world”. By expanding their scientific vocabulary, students are not just learning new terms that are required by educational curricula – they are broadening the horizons of their understanding, allowing them to explore and articulate the complexities of the world around them.
Concluding remarks
The final destiny of completed drawings in primary classrooms is often on the classroom walls or tucked away in students’ desks or drawers. Our findings suggest that the educational value of students' disciplinary drawings extends beyond the moment of creation. When revisiting their drawings, most students used them to expand their science-specific terminology and make personal, affective connections to a scientific content when reasoning about water at both macroscopic and submicroscopic levels. We recognise that our study design could be further developed to provide a more comprehensive account and offer stronger recommendations for classroom practice. Additional research is needed to explore how emergent disciplinary drawing, in combination with other supportive strategies, can enhance science learning in primary schools. We hope that our study offers valuable insights to guide future research in advancing this area of work.Author contributions
All authors contributed to the design of this study. Data collection was performed by authors 1 and 5. The LCT-analysis was designed and performed by author 1, who also drafted the manuscript with support from all authors. Findings and implications were discussed and agreed upon by all authors. All authors read and approved the final manuscript.Data availability
All relevant data generated or analyzed during this study are included in the published article. However, the raw empirical data, consisting of video-recorded interviews with underaged children, cannot be shared due to ethical considerations and the need to protect the privacy and confidentiality of the participants.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was financed by the Swedish Research Council, grant 2019-03429. We would also like to thank the teachers and their students who took part in the study.References
- Adbo K. and Vidal Carulla C., (2019), Designing play-based learning chemistry activities in the preschool environment, Chem. Educ. Res. Pract., 20(3), 542–553.
- Ainsworth S., Prain V. and Tytler R., (2011), Drawing to learn in science, Science, 333(6046), 1096–1097.
- Åkerblom A., Součková D. and Pramling N., (2019), Preschool children's conceptions of water, molecule, and chemistry before and after participating in a playfully dramatized early childhood education activity, Cult. Stud. Sci. Educ., 14, 879–895.
- Anderhag P., Wickman P.-O., Bergqvist K., Jakobson B., Hamza K. M. and Säljö R., (2016), Why do secondary school students lose their interest in science? Or does it never emerge? A possible and overlooked explanation, Sci. Educ., 100(5), 791–813.
- Andersson J., (2019), Barns teckningar som utgångspunkt i det naturvetenskapliga samtalet [Children's Drawings as Starting Point for Dialogues in Science], Doctoral Dissertation, Linköping University Electronic Press, vol. 106.
- Areljung S., Andersson J., Hermansson C., Skoog M. and Sundberg B., (2024), Co-drawing to Learn in Science, Res. Sci. Educ. DOI:10.1007/s11165-024-10217-x.
- Areljung S., Due K., Ottander C., Skoog M. and Sundberg B., (2021), Why and how teachers make use of drawing activities in early childhood science education, Int. J. Sci. Educ., 43(13), 2127–2147.
- Areljung S., Skoog M. and Sundberg B., (2022), Teaching for emergent disciplinary drawing in science? Comparing teachers’ and children's ways of representing science content in early childhood classrooms, Res. Sci. Educ., 52, 909–926.
- Berg A. and Hultén M., (2024), Children's emergent mechanistic reasoning in chemistry: a case study about early primary students’ reasoning about the phenomenon of thermal expansion of air, Chem. Educ. Res. Pract., 25(1), 92–114.
- Bernstein B., (1999), Vertical and Horizontal Discourse: an essay, Br. J. Soc. Educ., 20(2), 157–173.
- Blackie M. A. L., (2014) Creating semantic waves: using Legitimation Code Theory as a tool to aid the teaching of chemistry, Chem. Educ. Res. Pract., 15, 462–469.
- Caiman C. and Jakobson B., (2019), The Role of Art Practice in Elementary School Science, Sci. Educ., 28(1–2), 153–175.
- Dankenbring C. A., Guzey S. S. and Bryan L. A., (2024), Legitimation Code Theory as an analytical framework for integrated STEM curriculum and its enactment, Res. Sci. Educ., 54, 49–64.
- Danish J. A. and Saleh A., (2014), Examining how activity shapes students’ interactions while creating representations in early elementary science, Int. J. Sci. Educ., 36(14), 2314–2334.
- Danish J. A. and Saleh A., (2015), The impact of classroom context upon 1st and 2nd grade students’ critical criteria for science representations, Instruct. Sci., 43, 665–682.
- DeWitt J. and Osborne J., (2009), Recollections of Exhibits: stimulated-recall interviews with primary school children about science centre visits, Int. J. Sci. Educ., 32(10), 1365–1388.
- Dorion K., (2011), A learner's tactic: How secondary students’ anthropomorphic language may support learning of abstract science concepts, Electron. J. Sci. Educ., 12(2), 1–22.
- Fleer M., (2009), Supporting scientific conceptual consciousness or learning in ‘a roundabout way’ in play-based contexts, Int. J. Sci. Educ., 31(8), 1069–1089.
- García Fernández B. and Ruiz-Gallardo J. R., (2017), Visual literacy in primary science: Exploring anatomy cross-section production skills, J. Sci. Educ. Technol., 26, 161–174.
- Haeusler C. and Donovan J., (2020), Challenging the Science Curriculum Paradigm: Teaching Primary Children Atomic-Molecular Theory, Res. Sci. Educ., 50(1), 23–52.
- Halliday M. A. K. and Martin J. R., (1993), Writing science, Literacy and discursive power, Pittsburgh: University of Pittsburgh Press.
- Harrison A. G. and Treagust D. F., (2002), The Particulate Nature of Matter: Challenges in Understanding the Submicroscopic World, in J. K. Gilbert, O. De Jong, R. Justi, D. F. Treagust, & J. H. Van Driel (ed.), Chemical Education: Towards Research-based Practice, Science & Technology Education Library, Springer, vol. 17, pp. 189–212.
- Hipkiss A. M. and Windsor S., (2023), Surfing semantic waves: Using semantic profiling to focus on knowledge in practicum lessons, Action Teach. Educ., 45(1), 68–85.
- Johnstone A. H., (1982), Macro- and microchemistry, School Sci. Rev., 64, 377–379.
- Kozma R., Chin E., Russell J. and Marx N., (2000), The role of representations and tools in the chemistry laboratory and their implications for chemistry learning, J. Learn. Sci., 9(2), 105–143.
- Manneh I. L., Hamza K. M., Rundgren C.-J. and Eriksson L., (2018), The Role of Anthropomorphisms in Students’ Reasoning about Chemical Structure and Bonding, Asia-Pac. Forum Sci. Learn. Teach., 19(2), 1–16.
- Maton K., (2013), Making semantic waves: a key to cumulative knowledge-building. Linguist. Educ., 24(1), 8–22.
- Maton K., (2014), Knowledge and Knowers: Towards a Realist Sociology of Education, Knowledge and Knowers: Towards a Realist Sociology of Education, Routledge.
- Maton K., (2020), Semantic waves: context, complexity and academic discourse, in J. R. Martin, K. Maton and Y. J. Doran (ed.) Accessing academic discourse. Systemic Functional Linguistics and Legitimation Code Theory, Routledge.
- Monteira S. F. and Jiménez-Aleixandre M. P., (2016), The practice of using evidence in kindergarten: the role of purposeful observation, J. Res. Sci. Teach., 53(8), 1232–1258.
- Musengimana J., Kampire E. and Ntawiha, P., (2021), Factors Affecting Secondary Schools Students’ Attitudes toward Learning Chemistry: A Review of Literature, Eurasia J. Math., Sci. Technol. Educ., 17(1), 1–12.
- National Agency for Education, (2018), Curriculum for the compulsory school, preschool class and school-age educare 2011 (revised 2018), Stockholm: Skolverket.
- Nygård Larsson P., (2018), “We’re talking about mobility:” discourse strategies for promoting disciplinary knowledge and language in educational contexts, Linguist. Educ., 48, 61–75.
- Park J., Tang K.-S. and Chang J., (2021), Plan-Draw-Evaluate (PDE) pattern in students' collaborative drawing: interaction between visual and verbal modes of representation, Sci. Educ., 105(5), 1013–1045.
- Penuel W. R., (2014), Emerging Forms of Formative Intervention Research in Education, Mind, Culture, Activity, 21(2), 97–117.
- Prain V. and Tytler R., (2012), Learning through constructing representations in science: A framework of representational construction affordances, Int. J. Sci. Educ., 34(17), 2751–2773.
- Samarapungavan A., Bryan L. and Wills J., (2017), Second graders’ emerging particle models of matter in the context of learning through model-based inquiry, J. Res. Sci. Teach., 54(8), 988–1023.
- Schoultz J., Säljö R. and Wyndhamn J., (2001), Heavenly Talk: Discourse, Artifacts, and Children's Understanding of Elementary Astronomy, Human Dev., 44(2/3), 103–118.
- Sigsgaard Meidell A.-V., Heinrich S., Pagaard D. M. and Olsen P. S., (2023), Semantiske bølger i natur/teknologi-undervisningen: – sprogligt arbejde med fagligt fokus, Matematik-Og Naturfagsdidaktik, 23(3), 26–43.
- Skoog M., Areljung S., Andersson J., Hermansson C. and Sundberg B., (2025), Sharpening the gaze, sharpening the pencil: Supporting observational drawing in primary education, J. Biol. Educ. DOI:10.1080/00219266.2025.2452188.
- Swedish Research Council, (2017), Good Research Practice. VR1710, Stockholm: Swedish Research Council.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14(2), 156–168.
- Tytler R., Prain V., Aranda G., Ferguson J. and Gorur R., (2020), Drawing to reason and learn in science, J. Res. Sci. Teach., 57(2), 209–231.
- Vygotsky L., (1986), Thought and language, Cambridge: MIT Press.
- Wilson R. E. and Bradbury L. U., (2016), The pedagogical potential of drawing and writing in a primary science multimodal unit, Int. J. Sci. Educ., 38(17), 2621–2641.
- Wittgenstein L., (1922), Tractatus logico-philosophicus (C. K. Ogden, Trans.), Kegan Paul, Trench, Trubner & Co. (Original work published in 1921).
| This journal is © The Royal Society of Chemistry 2025 |