Developing self-reflection in students: a case study in chemistry education†
Maila Pentucci a,
Andrea Mascitti
a,
Andrea Mascitti b,
Nicola d’Alessandro
b,
Nicola d’Alessandro b,
Lucia Tonucci
b,
Lucia Tonucci *c and
Francesca Coccia
*c and
Francesca Coccia c
c
aDepartment of Humanities, Art and Social Science, University “G. d’Annunzio” of Chieti-Pescara, Via dei Vestini 31, Chieti, Italy
bDepartment of Engineering and Geology, University “G. d’Annunzio” of Chieti-Pescara, Viale Pindaro 42, Pescara, Italy
cDepartment of Socio-Economic, Managerial and Statistical Studies, University “G. d’Annunzio” of Chieti-Pescara, Viale Pindaro 42, Pescara, Italy. E-mail: lucia.tonucci@unich.it
First published on 12th May 2025
Abstract
This study investigates innovative reflective practices in chemistry class through a combination of practical laboratory experiments and theoretical lectures in a high school setting. Conducted in three chemistry classes, the research engaged 33 students in acid–base titration experiments using a conductometer, combined with reflective and metacognitive activities. The aim was to assess students' self-reflection and their ability to integrate new knowledge with existing concepts. A flipped learning approach was utilized, where students first performed the titration experiment and then received theoretical lecture. Reflective tools, such as the 3-2-1 Bridge Thinking Routine and the One Minute Paper, facilitated metacognitive engagement and self-reflection on their own learning processes. Results indicated that while students effectively grasped essential conductometry concepts through practical engagement, their understanding was primarily content-focused. The reflective questions posed by students highlighted their interest in both theoretical and operational aspects, suggesting a need for a balanced approach that integrates cognitive, practical, and emotional dimensions in chemistry education.
Introduction
Conductometry is a traditional electrochemical analytical method to obtain electrolytes concentration by measuring conductivity of an electric current in the solution. Several applications, across different fields, utilize this analytical technique; it is widely used in industrial settings for quality control purposes, to monitor the concentration of ions in solutions (Richardson et al., 2023), such as in the production of chemicals, pharmaceutical, food and beverages (Adley and Ryan, 2015; Volmer et al., 2017; Alhazmi et al., 2020); in the water treatment processes; in soil science and agriculture to assess soil fertility, salinity levels and nutrient concentrations (Burton et al., 2020; Keshavarzi et al., 2020; Kulmatov et al., 2020); in biomedical research and diagnostics, particularly in the analysis of biological fluids, like blood and urine (Mierzejewska et al., 2007; Upadhyay and Verma, 2015).The ions concentration can be obtained by measuring the change in electrical conductivity during a titration (Edimeh, 2011). Conductometric analysis works effectively for following neutralization, substitution, redox and precipitation reactions; in the case of neutralization analysis, you can plot a titration curve from the added volume of the titration solution (Veq) and the measured conductivity. Then, for the acid–base titrations, the curve shows a V-shape, where the minimum value is the equivalence point (peq).
Among the typical laboratory/demonstration activities for high school students, the acid–base titrations are quite common; students learn to use indicators or pH-meters to monitor the reaction, detect the peq, and then calculate the unknown concentration of an analyte. Furthermore, they can apply and consolidate their knowledge about general chemistry, physics, physico-chemistry.
The theoretical nature and the requirement for a strong understanding of fundamental chemistry/physics concepts make conductometry a challenging topic for high school students (Nyachwaya, 2016). Conductometry experiments, which involve measuring the electrical conductivity in solutions, can be difficult to deeply interpret for high school students (Donkersloot, 1991): understanding how and why ions in solution affect the conductivity and how the conductivity relates to concentration require a solid foundation in general chemistry (Preoteasa and Ionescu-Tirgoviste, 2015). Moreover, the conductometric calculations frequently involve mathematical tools as well as stoichiometry and molarity calculations (Lubert and Kalcher, 2010). The topic requires interpreting abstract representations, such as conductivity curves: the students may find them challenging to visualize and to interpret (Nyachwaya, 2016). However, laboratory experiments are crucial for students to visualize and to experience the principles of conductometry firsthand, providing opportunities to explore and manipulate this equipment and collect and analyze data. Before introducing conductometry, the teacher should ensure that students have a solid knowledge of general chemistry/physics concepts, akin the nature of matter, ions in solutions, stoichiometry, electricity, acid–base, at both macroscopic and microscopic levels. Learning concepts while, at the same time, engaging in the chemistry laboratory is essential: we can consider it as a combination of hands-on and mind-on activities (Hofstein and Hugerat, 2021; Seery et al., 2023).
Recent research on science education emphasizes the role of the laboratory in terms of reflection, knowledge reconstruction and metacognition. Science is also a practice; therefore, students and teachers should be involved in scientific practices in the classroom (Lehrer and Schauble, 2006). This “practical turn” points up that students should be engaged in the work of constructing and evaluating knowledge (Ford and Forman, 2006) and that it is through reflective participation in scientific practices that students develop usable forms of both, the epistemology underlying the scientific enterprise and explanatory scientific ideas (Lehrer and Schauble, 2006; Duschl, 2008). Thus, the emphasis on scientific practices, as learning goals and as a pedagogical approach, is designed to focus teachers’ attention on students’ construction and application of knowledge, rather than on the achievement of discrete scientific and epistemic ideas. In fact, science education needs to shift from relying on students' knowledge of scientific and epistemic ideas to using these understandings as tools for making sense of the world. This perspective moves students to go beyond the mechanical execution of scientific actions or processes and to engage, instead, in knowledge construction work (Berland et al., 2016). Metacognition, in Flavell's original definition, means “thinking about thinking” (Flavell, 1979): it is an internal psychological process necessary for effective learning and problem solving. Extensive and existing literature reviews on metacognition, to which we refer, (Dimmitt and McCormick, 2012; Avargil et al., 2018), say that it is a fuzzy (Perry et al., 2019) and widely applicable concept. It is possible to conclude that metacognition can be successfully taught from primary school to graduate level (Veenman and Beishuizen, 2004; Veenman and Spaans, 2005; van der Stel and Veenman, 2010). To maximize its impact, metacognition should be incorporated into the curriculum: the metacognitive element must be made explicit and clearly visible to students (Hattie, 2008). A valuable tool for fostering metacognition is self-reflective questioning (Malthouse et al., 2015). When students learn to question their own learning processes, they gain two significant benefits. Epistemologically, they engage in discovery-based learning (Laurillard, 2012), leading to deeper knowledge retention. When this knowledge is reconstructed, instead of passively assimilated, it gains greater meaning and becomes an integral part of their core identity. From a pedagogical perspective, students develop awareness of their learning approaches and of the often-implicit mechanisms they employ, enabling them to assess both their strengths and areas for improvement (Bain et al., 2002).
This paper presents the results of a research project focused on innovative reflective practices in chemistry education applied to conductometry topic, carried out at high school level through a laboratory experiment about acid–base titration and, later, a theoretical lecture. The primary objective of these inquiries was to observe the development of students' self-reflection and how they align and integrate newly acquired knowledge with established concepts (van Velzen, 2015; Chang, 2019).
From a didactic standpoint, adopting this approach means emphasizing the development of self-feedback skills (Van der Kleij, 2024) and metacognitive abilities. In a learning process where students are required to infer and reconstruct theoretical assumptions directly from practice, it is crucial for them to reflect not only on what they are learning but, even more importantly, on how they are learning.
The structured approach, incorporating validated tools, like Thinking Routine, called 3-2-1 Bridge, and the One Minute Paper tool (OMP), provided insights into students' self-reflection processes and their ability to apply metacognitive strategies effectively (Angelo and Cross, 1993; Laici, 2021).
Thinking Routines are structured sets of questions or a brief sequence of steps, used to scaffold and support student thinking. They are designed to deepen students’ thinking and to help turning that thinking “visible”. Thinking Routines aid in revealing students' thinking to the teacher and, also, help students themselves to recognize and identify particular “thinking moves”, making those moves more accessible and useful to them even in other settings.
Especially, 3-2-1 Bridge enables the introduction and exploration of ideas and the generation of new possibilities or making analogies, comparing naive thinking on a topic with structured thinking upstream of a training or teaching intervention (Stewart and Dake, 2019). The OMP may be defined as a noticeably short (taking one minute to complete) in-class writing activity in response to teacher questions, which prompts students to reflect on the day's lesson and provides the instructor with useful feedback. The idea behind this strategy was originally created by Charles Schwartz, a physics professor at the University of California, Berkeley, (Davis et al., 1983), but it was later made popular by Angelo and Cross (Angelo and Cross, 1993). It was aimed at giving teachers anonymous feedback on what students are learning in class.
These tools were aggregated into an integrated learning ecosystem (Walcutt and Schatz, 2019; Pentucci and Laici, 2020), designed by hybridizing known strategies, teaching methods and tools in order to realize a more targeted, context-situated use, aligned to the aims of the proposed learning pathway.
The aim of our experimentation was to observe the learning postures of students in order to understand their approach to savoir savant (Chevallard, 1991). According to Durand and Poizat, learning is a transformative process that involves two key steps: (1) appropriation. It indicates the set of transformations by which the activity absorbs newly significant elements and their properties (Theureau, 2011). It consists of a triple integration with respect to the contextual situation (in-situation), with respect to the body (in-corporation or embodiment), with respect to the reference culture (in-culturation); (2) individuation, which means reconstructing and rethinking oneself using these new elements (Durand and Poizat, 2022).
The research questions, that guided the analysis and interpretation of the data, were as follows:
RQ1 [chem]: In the autonomous processing of knowledge of chemistry, do students produce conceptions or misconceptions?
RQ2 [ped]: How do students' perceptions of their own teaching–learning processes change before and after practice?
RQ3 [ped]: What self-reflective questions do students ask themselves about their learning?
The tag “chem” identifies the question concerning the chemistry subjects and the tag “ped” the pedagogical aspects.
To our knowledge, the use of this structured approach (flipped lesson, Thinking Routines, OMP, chemistry laboratory) was not reported in literature, almost in chemistry class at high school level. Understanding how these transformations occur within the contextual situations of specific disciplines is of interest both for teachers, who can enhance their teaching processes by supporting and encouraging transformation, and for students, who can gain the necessary self-awareness about the learning mechanisms to mobilize when faced with new concepts and experiences.
Methodology
Context
This study was conducted in three small classes in the fourth year of a State high school institute in Pescara (High School IIS “A. Volta”, Italy); the students (age 17–18 years), of the Chemistry curriculum, were 33. Their natural language is Italian. The Institutional steering board and all students approved this research.The students have learned the concepts of electricity, conductivity, acid–base titrations and solutions concentration before this laboratory experience; they were able to calculate molarity, pH, and they have already experienced acid–base titrations by indicators and pH-meter.
The teachers gave only procedural information introducing the experiment as an acid–base titration to students, as well as all the safety information required to carry out the experiment, in accordance with the regulations in force in the school system. The instructors remembered the importance of the Veq and introduced the conductometer as an instrument to measure the ions in solution by mS values. The students worked in groups of 2–3; each group had its own experimental setup.
From the perspective of teaching methodology, the teachers used a flipped learning approach (Rahman et al., 2020). Rather than starting with the theoretical aspects, they engaged students in an experiment, designed to autonomously construct knowledge, through inferential and deductive reasoning, supported by the tools described above: the Thinking Routines and OMP. In terms of transversal skills, the goal was to develop the ability to engage in metacognitive reflection on foundational knowledge. This is a crucial competence for mastering key concepts and applying them in future studies and professional settings.
Students, according to a perspective close to learning by doing (Dewey, 1938), are invited to deduce and construct knowledge from the experiment, which becomes a space for reflection and re-elaboration on knowledge and not only for action and proof of knowledge. In this approach, known as reasoned scientific investigation, the student is encouraged to develop their own hypothesis and, subsequently, design experimental setups or logical reasoning to test them (Bot et al., 2005).
Conductometric experiment
The conductometer was a XS Cond 50 VioLab, Giorgio Bormac S.r.L., Carpi (Italy), with accuracy ±2%, equipped with XS Sensor Cella 2301 T VioLab, an epoxy cell containing a platinum electrode. Before use, it was calibrated with standard solutions to obtain accurate and reliable measurements.In this acid–base conductometric titration, a strong acid solution (HCl) was titrated with a standardized solution of a strong base (NaOH, 0.1 M). Here's how the experience typically worked: first, the students observed and prepared the titration apparatus, which consisted of a burette to deliver the standardized NaOH solution, a flask containing the unknown concentration solution of HCl, and a conductivity cell connected to the conductometer. The students measured the initial conductivity of the HCl solution; then, they slowly added the NaOH solution under continuous stirring. During the neutralization reaction, the solution conductivity diminished as H+ ions were neutralized by OH−. The peq was reached when the stoichiometrically equivalent amounts of base were added and the conductivity underwent a sharp change reaching a minimum value, due to the complete neutralization. Plotting measured conductivity versus added volume of NaOH solution resulted in a typical conductivity curve. So, the students, by the Veq required to reach the peq, calculated the concentration of the analyte solution (HCl) using stoichiometric principles; repeating the titration another time, they verified the accuracy of the titration results.
Data collection tools
To observe how students' approach reshapes and questions their own learning processes, thereby activating their reflective abilities, validated tools were used to support the development of awareness and self-analysis of learning mechanisms. The first tool employed was the 3-2-1 Bridge (traditional scheme in Fig. 1), developed by Harvard University as part of the work for “Project Zero” (https://pz.harvard.edu/thinking-routines). It was employed to encourage metacognitive processes. This routine prompts the students to articulate their initial thoughts, questions, and visions, and later, to connect them to new insights after instruction (Laici, 2021). Specifically, students were asked, by 3-2-1-Bridge forms, to reflect, before and after the conductometry experiment and lesson, and to list three ideas, two questions, and a metaphor, stimulated by the learning process (real form used in this study, Fig. S1, ESI†). Responding in the pre-operative phase, the students’ expectations and pre-conceptions become explicit. Responding in the post-operative stage (and after the theoretical lecture), students can express the revised or reconstructed beliefs, the hypotheses for further study, and the new conceptions.To systematize the acquired knowledge, the teachers provided a theoretical lesson, focused on learning through acquisition processes: “it is this sense of enabling students to build on the work of others that is fundamental to formal education and the progressive development of ideas” (Laurillard, 2012, p. 144). In this way, students can juxtapose their practical insights with formal knowledge through an inductive process.
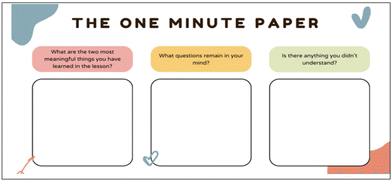
The second tool employed (after the laboratory experience) was the OMP, a tool for active learning (Stead, 2005), developed by Angelo and Cross (Angelo and Cross, 1993); it was used to prompt immediate reflection on learning mechanisms. This structured reflection, consisting of three simple questions, helps students evaluate their learning content and process, as well as clarify remaining questions or uncertainties (traditional scheme in Fig. 2 and real form used in this study in Fig. S2, ESI†). Questions are about: (1) What were the two most meaningful things you have learned in the lesson? (2) What do questions remain in your mind? (3) Is there anything you didn’t understand?
According to Nicol and Macfarlane-Dick (Nicol and MacFarlane-Dick, 2006), this tool supports deep metacognitive and self-regulatory processes. The research has highlighted the effectiveness of question-based strategies within Flipped Lesson frameworks, as utilized in this context. Through posing questions, students can integrate new information with prior knowledge, construct their own perspectives, and develop their self-reflection abilities (Lin et al., 2021).
According to Cheng et al., a lot of “researchers have indicated that using systematic prompts to reflect on the learning status can guide students to further understand the essence of questions and to realize their insufficiency in learning performance during the process of completing a learning task, which can then improve their learning results. Among various guidance strategies, systematic questioning to facilitate in-depth thinking and reflection in learning has been recommended by numerous researchers” (Cheng et al., 2024, p. 7082).
In this study, all tools were translated from English to Italian by the authors and checked by an English-Italian mother-tongue (researcher in natural sciences) before being submitted to the students. Data, paper-based, were collected in the classrooms/laboratories during the lessons time by their teachers. The students were informed about the purpose of this research and asked to answer honestly, while being reminded that the documents were anonymous and not subjected to the evaluation from the school. All the original data (in Italian) are shown in Fig. S3 and S4 (ESI†). The filled forms were received by the authors in-person directly from the school's teachers. The high school teachers provided the authors with 6 topics that they considered essential to appropriately interpret this laboratory activity: (1) relationship between conductivity and ions concentration; (2) titration analysis; (3) ions movement; (4) shape, and (5) use of the chart; (6) use and function of cell and conductometer. The filled forms were analyzed in Italian from the authors and then, translated in English for this article. The translation was carefully checked by the English-Italian mother-tongue.
Data analysis
Beginning with the initial focus of investigation, i.e. the testing of self-interrogation tools and Thinking Routines to promote students' reflection on their learning processes and metacognitive strategies, the research process entailed an in-depth analysis of qualitative and inductive practices. Due to the limited number of students (33) who participated in the study, a qualitative approach was chosen. With this method, meaningful insights can be drawn, even from small datasets, allowing for comprehensive and context-specific exploration.From a methodological perspective, the students' written responses, collected through the OMP and the 3-2-1 Bridge, were divided into two distinct corpora and analyzed recursively using various approaches, depicted in Table 1.
| Type of analysis | Mode | Tools |
|---|---|---|
| Reflexive thematic analysis | Manual post hoc tagging of students' statements | OMP: section 1 – concepts |
| 3-2-1 Bridge: section Ideas/Thoughts pre- and post-activity | ||
| Sentiment analysis | Classification of students' statements as negative, positive, or neutral | 3-2-1 Bridge: section Ideas/Thoughts pre- and post-activity |
| Post hoc classification | Classification of self-reflective questions based on Altet's Model (2003) | OMP: section 3: questions and reflections |
Thematic analysis
We conducted a semantic analysis of both corpora using the Reflexive Thematic Analysis Model, developed by Braun and Clarke. This interpretive method, firmly rooted in the qualitative paradigm, is widely applied in educational research contexts (Braun and Clarke, 2019). Within this analytical framework, the researcher's subjectivity is not viewed as problematic but is instead recognized and valued as an integral component of the analytical process (Campbell et al., 2021).After normalizing the dataset (that is, taking the action to ensure that the data are not redundant or unstructured, and therefore much more difficult to manage and use because they are more difficult to organize), the researchers proceeded with coding, by carefully adhering to the participants' words and perspectives, allowing explicit meanings to guide the process, in line with an inductive approach. As a result, the coding framework was not predetermined but, instead, emerged organically from the data. Following the methodological guidelines proposed by Braun and Clarke (Braun and Clarke, 2006, 2019), the analysis process was carried out in six stages: (1) familiarization with the data; (2) generation of initial codes for the units of analysis; (3) identification of recurring and significant themes across the dataset; (4) revision of themes; (5) definition of final themes; (6) production of a comprehensive final report.
The researchers continuously refined and redefined codes and categories through constant comparative analysis of the data, returning to the empirical dimension when necessary to address information and knowledge gaps (Salvini, 2015).
Sentiment analysis
To capture the polarizations in perceptions, we applied techniques from Sentiment Analysis to the data collected through the 3-2-1 Bridge. Sentiment Analysis is a natural language processing technique designed to identify and categorize the sentiments expressed in a text. Using this analysis, it is possible to determine if an opinion or comment is positive, negative, or neutral.Given the limited sample size in this case, we conducted the analysis manually, assigning polarity to the analyzed texts. This approach involved carefully reading the content, interpreting it, and classifying each sentence or document as positive, negative, or neutral, based on predefined criteria. For example, a statement expressing enthusiasm or curiosity about the experiment was classified as positive, an expression of difficulty or concern as negative, and an objective or unemotional comment as neutral (Mite-Baidal et al., 2018; Zhou and Ye, 2023).
In this case the sentiment analysis was conducted manually by a single researcher trained in qualitative analysis. Given the limited sample size and the qualitative nature of the study, a single coder approach was deemed appropriate to ensure consistent and context-sensitive classification. Special attention was paid to the contextual and emotional nuances of the texts to accurately capture sentiment beyond surface-level expressions. Examples of ambiguous cases and how sentiment was interpreted are provided in Table S1 (ESI†).
Manual analysis has the advantage of capturing context, sarcasm, and linguistic nuances that automated algorithms might overlook. This method is particularly effective in contexts where it is essential to precisely identify and interpret the emotions expressed in the texts.
Post hoc analysis: classification of questions
Furthermore, a posteriori, the self-reflective questions expressed by the OMP were classified, using Marguerite Altet's model. This model classifies the dimensions of cognitive operations that are activated during information processing, which occur during learning processes. This classification allows the underlying learning mechanisms to emerge from the questions themselves (Altet, 2003). It is divided into six dimensions that will be explained in detail in the Results and Discussion section: (1) collection operations, (2) transformation operations, (3) memorization operations, (4) inference operations, (5) production operations, (6) metacognition operations.As already indicated, the research questions were:
RQ1 [chem]: In the autonomous processing of knowledge of chemistry, do students produce conceptions or misconceptions?
RQ2 [ped]: How do students' perceptions of their own teaching–learning processes change before and after practice?
RQ3 [ped]: What self-reflective questions do students ask themselves about their learning?
Ethical considerations
The school was contacted by the authors in October 2023, during the initial organization of the scholastic year. The school's teachers of Chemistry were informed about the goal of this research and the procedure that would be utilized; they discussed with their Principal and, when approved, with the students.The Chemistry Committee and Institutional steering board of the school approved this project's methodologies. Students were asked to anonymously participate in the project and their consent was obtained and collected from the teachers. Prior to participants providing consent, they were given information on the study, particularly that they could withdraw their consent at any time, and that they can contact the authors with any questions or concerns.
Results and discussion
Disciplinary perspective: answering RQ1
In Table S2 (ESI†), the students’ answers to the first question in OMP (During the experiment execution, what are the three main concepts that you could grasp?, Fig. 2 and Fig. S2, S3, ESI†), resumed by tags, were reported. We collected 93 tags from 33 students, which were analyzed identifying 6 misconceptions. Two students showed difficulties in interpreting the graph, confusing, respectively, the V-shape of this experiment with a bell shape or with a curve having two peq; one outlined that NaOH solution was in burette because of its chemical and reactive nature but it is characteristic of the titrations instead (representative excerpts in Table S3, ESI†). Other mistakes were born from previous knowledge, without understanding the differences with this technique (they confused conductometry with volumetric analyses) or were real errors, like thinking to follow the trend of pH rather than the changes of the conductivity. The identified errors and misconceptions, akin acid–base neutralization or graphs, are like those reported in literature (Smith et al., 2010; Nyachwaya, 2016). We considered 6 topics essential to appropriately interpret this laboratory activity, according to the high school teachers: (1) relationship between conductivity and ions concentration; (2) titration analysis; (3) ions movement; (4) shape, and (5) use of the chart; (6) use and function of cell and conductometer. These arguments were correctly present in 24 answers (25%): 12 were related to conductivity/concentration relationship; 6 to the conductometer; 3 to the ions movement; 2 to the graph and 1 to the titration.Among the other tags, 16 talked about the observed values of conductivity and its changes, 6 named peq, 5 the curves and the graph and 4 the potentiometry; 5 outlined the link between the ions and the conductivity; 6 viewed the method and the precision/characteristic of the analysis.
Answers to the third question in OMP (What is unclear or did you not understand and would you like to be explained?, Fig. S2 and S3, ESI†) revealed the students interest in theory concepts: What are the theoretical bases?; What is the real correlation between conductivity and concentration?; Why does the conductivity increase after the peq and why the slopes (before and after the peq) were different?; I’d like to deep the conductivity and its use; How does the temperature influence the conductivity?; What are the differences between the measure units related to the electricity (e.g., Siemens, Volt, Ohm)?. Many responses were strictly focused on the construction of the chart, the table, the calculations or on the operational activities, akin the instrument calibration, the different cell K, the operation of the conductometer. Some students wondered why you utilize this titration and not a volumetric one or other. Even misconceptions were present: Why was the conductivity value at peq different to zero even if the acid ions concentration was equal to the base one?; Why did I put NaOH (and not HCl) in burette?; How did work function change?.
Discussing OMP data, the students demonstrated a partial alignment with the teachers’ target concepts, as only 25% of responses referred to the six core topics, meaning that the experiment alone was significative but not satisfactory for the comprehension of the subject.
Due to the theoretical aspect not being yet explained, many students provided answers linked to the experience features, like the visual (e.g., chart) or practical ones (e.g., conductometer, calibration) or to prior knowledge (e.g., pH, potentiometry). Most of the answers were not about general chemistry but about the use of conductometry analysis in a future workplace, and for their school reports.
Their curiosity suggests that these students are engaged and eager to deepen their understanding. However, the theoretical aspects were not fully understood during the experiment. Furthermore, they used the previous knowledge to depict this experience and its understanding.
Students’ perceptions: answering RQ2
To address RQ2, we analyzed the section of the 3-2-1 Bridge tool, titled “3 Thoughts/Ideas”, where students expressed their thoughts, ideas, questions, and understandings about the topic of the experiment (Fig. 1 and Fig. S1, S4, ESI†), connected to their new thinking after they executed the experiment and the lesson on conductometry.We tagged all the answers given before and after; then the tags assigned to the answers related to preconceptions were compared with those related to conceptions and realigned in terms of linguistic identity and semantic coherence (Table 2, columns Before and After).
| Tag | Before | After | Sentiment |
|---|---|---|---|
| CONTENT | 25 | 49 | = |
| BRIDGE | 14 | 7 | = |
| AIM/PURPOSE | 11 | 4 | = |
| DIFFICULT | 8 | 1 | − |
| INTERDISCIPLINARITY | 7 | 2 | = |
| TOOL | 6 | 6 | = |
| FUNCTION | 3 | 1 | = |
| METHODOLOGY | 3 | 0 | = |
| INTERESTING | 2 | 1 | + |
| COMFORTABLE | 1 | 0 | + |
| HARD TO ANTICIPATE | 1 | 0 | − |
| EASY | 1 | 7 | + |
| UNDERSTANDING | 0 | 5 | + |
| CONFIRMATION OF THE CONTENTS | 0 | 1 | + |
| PROCEDURE | 0 | 1 | = |
In Table 2, the complete situation is shown: the tag CONTENT was prevalent in both moments of the question, before and after (N = 25 before and N = 49 after); it was followed by the tag BRIDGE (N = 14 before and N = 7 after). To analyze the difference in answers between before and after, in addition to the two previously mentioned tags, we highlighted the tags EASY and UNDERSTANDING, which were present respectively N = 7 and N = 5 times in the final question and nearly absent in the initial one. In contrast, the tags DIFFICULT and INTERDISCIPLINARY were in the opposite way.
This overview suggests that when students were asked to reflect on a school activity, their ideas and thoughts were oriented towards the content, i.e. the purely notional and epistemological aspects of the activity were prevalent. Students, before starting the lesson, for example, wrote: (1) it is related to Ohm's law; or, very specifically, (2) in my opinion, conductivity is based on a charged solution that attracts or repels ions outside. The focus on content increased after the experience, with 45 occurrences. This suggests that students tend to emphasize the factual content of disciplinary constructs in their learning process. During self-reflection, they focused predominantly on the main notions and in-depth content of what they had learnt, rather than on the methods of their learning. Probably, this attitude is linked to a widespread habit in high school, particularly in STEM disciplines, where students are required to acquire the content, focusing on the cognitive ability and considering the metacognitive one, called “learning to learn”, less relevant.
About this kind of habitus, international research suggests that a major curriculum revision and a revolution in approaches to teaching and learning through the teaching of cognitive and metacognitive skills are needed (Chapman and Aspin, 1997; Cornford, 2004; Sunkel, 2019). As Cornford states, “the vast majority of teachers at all levels of education have good intentions to promote effective learning by their students, but they have never been taught how to incorporate the teaching of cognitive and metacognitive skills into their sessions. In short, along with changes in curriculum, a revolution in approaches to teacher education may well be necessary” (Cornford, 2004, p. 13).
In terms of metacognitive skills, the tag BRIDGE refers to a typical strategy that involves recalling familiar situations and concepts in order to grasp new concepts, by utilizing similarities and differences between case (Rossi and Pentucci, 2021). In fact, according to Hennessey, metacognition is also “awareness of one's own thinking, awareness of the content of one's conceptions, an active monitoring of one's cognitive processes, an attempt to regulate one's cognitive processes in relationship to further learning, and an application of a set of heuristics as an effective device for helping people organize their methods of attack on problems in general” (Hennessey, 1999, p. 3). In the Before stage, students' sentences recalling familiar situations by, for example, I think it looks like … or It has connections with … were numerous. This tag is complemented by the AIM/PURPOSE tag (N = 11 in Before stage) which indicates the ideas students had for using the knowledge they had learned, in terms of remobilizing or anticipating the meaning to be given to the knowledge in question. This may indicate that students prepared themselves to learning through anticipation and connection strategies; they tried to implement bridging analogies and anchoring intuitions in constructing their preconceptions, as already pointed out by Clement for physics education (Clement, 1993).
In the After phase, tags of this kind are halved: the student, focused on the experiment and the content, lost sight of the connections with what he/she already knew or had already dealt with in previous school activities. This is an important wake-up call for teachers, who in addition to knowledge should take care of the skills of putting it into perspective, contextualizing and systematizing it.
The dynamics of the EASY and DIFFICULT tags provide interesting information. Indeed, it is possible to hypothesize that there was a positive change in the emotional state of the students' expectations regarding their understanding and ability to engage in the proposed topic: before the activity, the students expected a difficult activity (N = 8) but after the activity, the perception reversed. Only one student pointed out the difficulty of the topic, while the tag EASY (N = 7) emerged, which was previously only identified in one response. For example, students said: The practical experiment was easy for me even though I thought I would have difficulties.
We are convinced, in accordance with the international authors we cite below, that, in education, the interaction between cognition and emotion is crucial in the activation or inhibition of learning mechanisms. In fact, according to Fishman and Dede, every teaching/learning process contributes to the simultaneous development of cultural, intrapersonal and interpersonal dimensions of competence, in a holistic perspective, in which the idea that learning is only linked to the cognitive domain and consists in the shaping of mere content is definitely outdated (Fishman and Dede, 2016).
Student success is multidimensional, extending beyond the outcomes of acquiring notions to include identity development, as well as cognitive and social maturity (Renner et al., 2016).
Therefore, we found it interesting to apply the principles of Sentiment Analysis (Sentiment column, Table 2) through a basic approach, with the aim of capturing students' initial emotional feedback and obtaining information on their attitudes towards learning (Mite-Baidal et al., 2018). Given the limited amount of data, a reduced sentiment analysis was applied, manually classifying the tags as positive, negative and neutral (three-level polarity scale; Hattie, 2008). Although neutral emotional perceptions and ideas predominated, Table 3 shows a shift from negative to positive mood between before and after the experiment. The neutral tags were equally presented (69 Before and 70 After).
| Negative | Positive | |
|---|---|---|
| 9 | 4 | Before |
| 1 | 14 | After |
The initial confusion and apprehension associated with using a new and sensitive instrument, like the conductometer, without any theoretical teacher's explanation before laboratory experience, led some students to a negative perception. The work procedure, required from the use of the conductometer, can be daunting for students who are accustomed to more robust tools, such as pH-meter or litmus paper, susceptible to fewer experimental variations; in fact, the sensitivity of the conductometer requires precise handling and an understanding of factors that can affect conductivity measurements, such as temperature, concentration of ions, and presence of interfering substances.
Effect of self-questioning on learning processes: answering RQ3
To answer question RQ3, we considered the students' answers to the second section of the OMP (What reflections or questions did you have during the work?, Fig. S2, ESI†).Malthouse suggests that questions become reflective when emergent ideas are related to the existing senses of knowledge, self and the world, and as new understanding emerges (Malthouse et al., 2015). These questions possess the ability to recursively return to the past in terms of introspection and restructuring, to focus on the present by describing experiential contexts and bringing them to light, and to project into the future, stimulating new reflections and generating knowledge.
Considering this, the self-questioning of students was studied as a verbalized trace of their cognitive processes, captured when they were elaborating and processing information and disciplinary concepts experienced during the practice. According to the literature, the self-questioning is highly effective in the learning of scientific disciplines, as it promotes systemic and metacognitive approaches to knowledge and develops independent thinking (Bsharh Al-Swelmyeen et al., 2020; Daniel and Williams, 2021). The questions that students explicitly stated in the OMP were classified using Marguerite Altet's model, model that allows us to place our findings, albeit limited in number, in the landscape of research related to self-reflection questions that stimulate metacognitive postures (as argued even by Van Helvert et al.) who have connected reflection and metacognition (Van Helvert et al., 2016). The Altet's model makes it possible to reveal, from the questions themselves, the following cognitive operations, that are activated during information processing and that allow the underlying learning mechanisms to emerge (Altet, 2003).
1. Collection operations: these involve mechanisms of attention, decoding, identification, and all mechanisms that allow understanding and situating information.
2. Transformation operations: these concern discrimination, comparison, categorization, establishing relationships, and anticipation through the structuring of action schemes.
3. Memorization operations: these allow the construction of a bridge with previously stored preliminary knowledge and the retrieval and selection of information.
4. Inference operations: these pertain to the overall notion of reasoning.
5. Production operations: these relate to the construction of new knowledge or problem-solving.
6. Metacognitive operations: these involve the awareness of applied strategies.
In Table 4, the number of questions posed by students for each expected cognitive operation is shown.
| Collect | Transform | Memorize | Infer | Produce | Reflect |
|---|---|---|---|---|---|
| 20 | 13 | 5 | 4 | 5 | 5 |
| Informative aspect of learning | Formative aspect of learning | ||||
The analysis of self-reflection questions in the OMP confirms the content-focused and assimilative approach to knowledge that students displayed. Indeed, the questions they posed, classified according to Altet and summarized in Table 4 (representative examples in Table S4, ESI†), are predominantly linked to an informative and reproductive dimension of learning (Altet, 2003). Students primarily engaged in mechanisms of reception, reproduction, imitation, memorization, assimilation, and integration. Questions related to the formative and generative aspects of learning were less frequent. Only 5 students engaged in a process that can be described as metacognitive reflection, and their questions, rather than focusing on mobilizing the competencies learned, centered on the potential reuse of acquired knowledge in the workplace, reflecting a more instrumental than functional idea of mobilization for generating new learning.
To summarize, the interpretation of the collected data allows us to answer the research questions as depicted in Table 5.
| Research question | Results | Analysis |
|---|---|---|
| In the autonomous processing of knowledge of chemistry, do students produce conceptions or misconceptions? | The experience features, such as visual or practical aspects, or prior knowledge, were addressed by many students. Most of the answers were focused on the use of conductometry analysis in their future careers and for their school reports. | Thematic analysis of the first question of OMP and thematization into macrocategories |
| How did students' perceptions of their own teaching–learning processes change before and after practice? | Students were oriented towards mere content rather than learning modes and mechanisms. | Thematic analysis of the section “Ideas/Thoughts” of 3-2-1 Bridge |
| Students used bridging strategies to anticipate the knowledge brought into play. | ||
| Students tended to perceive the new topic as difficult. | Sentiment Analysis | |
| What self-reflective questions did students ask themselves about their learning? | Students mainly asked themselves questions related to the informational aspects of learning. Formative and generative aspects remained in the background. | Classification of self-reflective questions collected through Section 2 of the OMP |
Limitations of this research
The research has inherent limitations due to the small sample size and the lack of a control group that participated in the same activity without the use of the same path and tools. Given the limited number of students, these results are not generalizable and remain specific to the reference context. Nevertheless, our goal is not to generalize or to build models, but to describe a specific context that may provide valuable insights into students' approaches to learning scientific disciplines. Nonetheless, they offer valuable insights for implementing both the reversed lesson approach and the use of reflective and metacognitive tools in chemistry education. Such approaches aim to enhance students’ awareness not only of what they study but also of how they study.Implications for research on STEM teaching practices
Bringing attention to the metacognitive mechanisms activated through self-reflection questions may be useful for extending and disseminating research and training on teaching practices related to STEM disciplines to broader samples. Specifically, it appears beneficial to work with teachers not only on what students need to learn, but also on how learning processes are developed.Educational research, for example, reflects on the relationship between awareness of one's own cognitive mechanisms and time (Murphy et al., 2021; Thompson and Markovits, 2025).
Specifically, the strategic use of past information may be involved in focusing on important future information, and the metacognitive processes, that enable this prioritization of memory, may be related to more general problem-solving abilities that involve identifying crucial features of information to guide cognition in a broader context. It is therefore important to examine whether the self-reflection questions, that students spontaneously ask themselves, are intended to revisit or integrate past activities in order to assess their current preparedness and awareness, or whether they aim to explore how to apply their knowledge in the future. Teachers can develop structured and routine self-reflection practices by addressing this question (Bain et al., 2002; Altet, 2003; Malthouse et al., 2015). Such practices could guide students in referring to distinct points in their learning journey, aiding them in retrieving useful information to establish connections with past experiences, reinforcing and confirming their current skills, and fostering reflection and anticipation of new concepts to be explored in the future. Analyzing how students question knowledge is a valuable tool for rethinking learning design. In particular, studies on designing for the unexpected, as well as reflections by pedagogues—particularly Italian scholars—on the ontological unexpected in education (Rossi and Pentucci, 2021; Pentucci et al., 2023; Capolla, 2024; Capolla et al., 2024), can benefit from the feedback generated by students' self-reflection regarding the learning mechanisms involved. This feedback can support the development of co-design frameworks of a multidimensional nature, attentive not only to the cognitive and informational aspects of science teaching but also to metacognitive dimensions and the consolidation of students' awareness of how they learn.
Conclusions
In terms of chemistry education, the proposed conductometry experiment could provide a practical context for students to apply and engage with their theoretical knowledge. The students should demonstrate an ability to connect their prior knowledge in chemistry, such as titration method, acid–base, neutralization reaction, with new concepts from the conductometry experiment (e.g., conductometer, ions movement in aqueous solution).The dominance of cognitive operations that focus on assimilation and content suggests a notion view of scientific knowledge. This case study shows that the educational intervention reinforced these notions, as students prioritized content over the mobilization of skills, particularly after the lesson. Meanwhile, the use of laboratorial practice is key for developing a deeper understanding of chemical concepts but also for providing valuable skills to the students for their future workplace.
Building on existing literature, this study reinforces the view that self-questioning is a highly effective strategy in the learning of scientific disciplines. By fostering both systemic and metacognitive approaches to knowledge, self-questioning not only enhances students' ability to structure and integrate scientific concepts but also cultivates independent and critical thinking skills. Encouraging students to actively engage in self-reflection and inquiry thus emerges as a crucial pedagogical approach, enabling them to develop a deeper understanding of scientific reasoning while strengthening their autonomy as learners. These findings highlight that incorporating structured self-questioning practices in science education is crucial to encourage more reflective and adaptive learning processes.
Author contributions
M. P.: methodology, conceptualization, writing – review & editing; A. M.: writing – original draft; N. d’A.: conceptualization; L. T.: formal analysis, validation, writing – review & editing; F. C.: supervision, investigation, writing – original draft.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank High School IIS “A. Volta” in Pescara, Italy, for the collaboration and, in particular, prof. Graziella D’Ambrosio, prof. Primiano D’Ambrosio and prof. Sabrina Trimigno, for the participation to the research project and for the discussions about this topic. The research project was partially supported by the FSE-REACTEU, PON Ricerca e Innovazione 2014-2020 DM 1062/2021, Cod: MUR 53-G-14753.Notes and references
- Adley C. C. and Ryan M. P., (2015), Conductometric biosensors for high throughput screening of pathogens in food, in Bhunia A. K., Kim M. S. and Taitt C. R. (ed.), High Throughput Screening for Food Safety Assessment, Woodhead Publishing, pp. 315–326 DOI:10.1016/B978-0-85709-801-6.00014-9.
- Alhazmi H. A., Ali Bokar Nasib A., Musleh Y. A., Hijri K. Q., Rehman Z. ur, Khuwaja G. et al., (2020), Application of drug–metal ion interaction principle in conductometric determination of imatinib, sorafenib, gefitinib and bosutinib, Open Chem., 18(1), 798–807 DOI:10.1515/chem-2020-0123.
- Altet M., (2003), La ricerca sulle pratiche d’insegnamento in Francia, La scuola.
- Angelo T. A. and Cross K. P., (1993), Classroom Assessment Techniques: A Handbook for College Teachers, 2nd edn, Jossey-Bass.
- Avargil S., Lavi R. and Dori Yehudit Judy, (2018), Students’ Metacognition and Metacognitive Strategies in Science Education, in Dori Y. J., Mevarech Z. R. and Baker D. R. (eds), Cognition, Metacognition, and Culture in STEM Education. Innovations in Science Education and Technology, Springer, pp. 33–64 DOI:10.1007/978-3-319-66659-4_3.
- Bain J. D., Ballantyne R., Mills C. and Lester N. C., (2002), Reflecting on practice: Student teachers’ perspective, Post Pressed.
- Berland L. K., Schwarz C. V., Krist C., Kenyon L., Lo A. S. and Reiser B. J., (2016), Epistemologies in practice: making scientific practices meaningful for students, J. Res. Sci. Teach., 53(7), 1082–1112 DOI:10.1002/tea.21257.
- Bot L., Gossiaux P.-B., Rauch C.-P. and Tabiou S., (2005), ‘Learning by doing’: a teaching method for active learning in scientific graduate education, Eur. J. Eng. Educ., 30(1), 105–119 DOI:10.1080/03043790512331313868.
- Braun V. and Clarke V., (2006), Using thematic analysis in psychology, Qual. Res. Psychol., 3(2), 77–101 DOI:10.1191/1478088706qp063oa.
- Braun V. and Clarke V., (2019), Reflecting on reflexive thematic analysis, Qual. Res. Sport. Exerc. Heal., 11(4), 589–597 DOI:10.1080/2159676X.2019.1628806.
- Bsharh Al-Swelmyeen M., Abed Sakarneh M. and Pernec Al zaben G., (2020), The Effectiveness of Self-Questioning Strategy in Developing Independent Thinking in teaching Physics, Cypriot J. Educ. Sci., 15(3), 502–510 DOI:10.18844/cjes.v%vi%i.4918.
- Burton L., Jayachandran K. and Bhansali S., (2020), Review—The “Real-Time” Revolution for In situ Soil Nutrient Sensing, J. Electrochem. Soc., 167(3), 037569 DOI:10.1149/1945-7111/ab6f5d.
- Campbell K., Orr E., Durepos P., Nguyen L., Li L. and Whitmore C. et al., (2021), Reflexive Thematic Analysis for Applied Qualitative Health Research, Qual. Rep., 26(6), 2011–2028 DOI:10.46743/2160-3715/2021.5010.
- Capolla L. M., (2024), Shaping Educational Strategies: A Literature Review on Uncertainty and the Unexpected, Educ. Sci., 14(3) 309 DOI:10.3390/educsci14030309.
- Capolla L. M., Giannandrea L., Gratani F., Pentucci M. and Rossi P. G., (2024), Rethinking and formalizing initial teacher training on learning design for and in uncertainty, Front. Educ., 9, 1–6 DOI:10.3389/feduc.2024.1268936.
- Chang B., (2019), Reflection in Learning, Online Learn., 23(1), 95–110 DOI:10.24059/olj.v23i1.1447.
- Chapman J. and Aspin D., (1997), Schools as Centres of Lifelong Learning for All, in Hatton M. J. (ed.), Lifelong Learning: Policies, Practices, and Programs, pp. 155–166.
- Cheng S.-C., Hwang G.-J. and Chen C.-H., (2024), Fostering students’ scientific literacy by reflective questioning: an identification, summarization, self-reflective questioning, and application (ISSA)-based flipped learning approach, Educ. Inf. Technol., 29(6), 7081–7104 DOI:10.1007/s10639-023-12121-9.
- Chevallard Y., (1991), La transposition didactique, du savoir savant au avoir enseigné, augmentée edn, La Pensée Sauvage.
- Clement J., (1993), Using bridging analogies and anchoring intuitions to deal with students’ preconceptions in physics, J. Res. Sci. Teach., 30(10), 1241–1257 DOI:10.1002/tea.3660301007.
- Cornford I. R., (2004), Cognitive and Metacognitive Strategies as a Basis for Effective Lifelong Learning: How Far Have We Progressed? AARE Annual Conference, Australian Association for Research in Education, pp. 1–15.
- Daniel J. and Williams K. J., (2021), Self-Questioning Strategy for Struggling Readers: A Synthesis, Remedial Spec. Educ., 42(4), 248–261 DOI:10.1177/0741932519880338.
- Davis B. G., Wood L. and Wilson R. C., (1983), ABCs of Teaching with Excellence: A Berkeley Compendium of Suggestions for Teaching with Excellence, Berkeley: University of California.
- Dewey J., (1938), Experience and Education, Kappa Delta Pi.
- Dimmitt C. and McCormick Christine B., (2012), Metacognition in education, in Harris K. R., Graham S., Urdan T., McCormick C. B., Sinatra G. M. and Sweller J. (ed.), APA educational psychology handbook, Theories, constructs, and critical issues, American Psychological Association, vol. 1, pp. 157–187 DOI:10.1037/13273-007.
- Donkersloot M. C. A., (1991), Teaching conductometry: another perspective, J. Chem. Educ., 68(2), 136 DOI:10.1021/ed068p136.
- Durand M. and Poizat G., (2022), Enazione, attività umana e ambienti di formazione, in Rivoltella P. C. and Rossi P. G. (ed.), L’agire didattico. Manuale per l’insegnante, La scuola, pp. 217–232.
- Duschl R., (2008), Science Education in Three-Part Harmony: Balancing Conceptual, Epistemic, and Social Learning Goals, Rev. Res. Educ., 32(1), 268–291 DOI:10.3102/0091732X07309371.
- Edimeh K., (2011), Conductometry Titrations, Appl. Conduct. Electrogravimetry Coulom., 68–71.
- Fishman B. and Dede C., (2016), Teaching and Technology: New Tools for New Times, Handbook of Research on Teaching, American Educational Research Association, pp. 1269–1334 DOI:10.3102/978-0-935302-48-6_21.
- Flavell J. H., (1979), Metacognition and cognitive monitoring: a new area of cognitive–developmental inquiry, Am. Psychol., 34(10), 906–911 DOI:10.1037/0003-066X.34.10.906.
- Ford M. J. and Forman E. A., (2006), Chapter 1: Redefining Disciplinary Learning in Classroom Contexts, Rev. Res. Educ., 30(1), 1–32 DOI:10.3102/0091732X030001001.
- Hattie J., (2008), Visible Learning, Routledge DOI:10.4324/9780203887332.
- Hennessey G. M., (1999), Probing the dimensions of metacognition: implications for conceptual change teaching–learning, Proceedings of the Annual Meeting of the National Association for Research in Science Teaching, NARST, pp. 1–33.
- Hofstein A. and Hugerat M., (2021), The Chemistry Laboratory: From Theory to Practice, Teaching and Learning in the School Chemistry Laboratory, The Royal Society of Chemistry, pp. 46–90 10.1039/9781839164712-00046.
- Keshavarzi A., Tuffour H. O., Bagherzadeh A., Tattrah L. P., Kumar V., Gholizadeh A. and Rodrigo-Comino J., (2020), Using fuzzy-AHP and parametric technique to assess soil fertility status in Northeast of Iran, J. Mt. Sci., 17(4), 931–948 DOI:10.1007/s11629-019-5666-6.
- Kulmatov R., Mirzaev J., Abuduwaili J. and Karimov B., (2020), Challenges for the sustainable use of water and land resources under a changing climate and increasing salinization in the Jizzakh irrigation zone of Uzbekistan, J. Arid Land, 12(1), 90–103 DOI:10.1007/s40333-020-0092-8.
- Laici C., (2021), Il feedback come pratica trasformativa nella didattica universitaria, FrancoAngeli.
- Laurillard D., (2012), Teaching as a Design Science Building Pedagogical Patterns for Learning and Technology, Routledge.
- Lehrer R. and Schauble L., (2006), Scientific thinking and science literacy: supporting development in learning in contexts, in Eisenberg N., Damon W. and Lerner R. M. (ed.), andbook of child psychology: Social, emotional, and personality development, 6th edn, John Wiley & Sons, Inc., pp. 153–196.
- Lin H. C., Hwang G. J., Chang S. C. and Hsu Y. D., (2021), Facilitating critical thinking in decision making-based professional training: an online interactive peer-review approach in a flipped learning context, Comput. Educ., 173, 104266 DOI:10.1016/j.compedu.2021.104266.
- Lubert K. H. and Kalcher K., (2010), History of electroanalytical methods, Electroanalysis, 22(17–18), 1937–1946 DOI:10.1002/elan.201000087.
- Malthouse R., Watts M. and Roffey-Barentsen J., (2015), Reflective Questions, Self-Questioning and Managing Professionally Situated Practice, Res. Educ., 94(1), 71–87 DOI:10.7227/RIE.0024.
- Mierzejewska D., Marciniak-Darmochwa K., Kostyra H. and Rudnicka B., (2007), Application of the conductometric method for differentiation of proteins and peptides, Polish J. Food Nutr. Sci., 57(1), 77–82.
- Mite-Baidal K., Delgado-Vera C., Solís-Avilés E., Espinoza A. H., Ortiz-Zambrano J. and Varela-Tapia E., (2018), Sentiment Analysis in Education Domain: A Systematic Literature Review, in Valencia-García, R., Alcaraz-Mármol, G., Del Cioppo-Morstadt, J., Vera-Lucio, N. and Bucaram-Leverone M. (ed.), Communications in Computer and Information Science, Springer, pp. 285–297 DOI:10.1007/978-3-030-00940-3_21.
- Murphy D. H., Agadzhanyan K., Whatley M. C. and Castel A. D., (2021), Metacognition and fluid intelligence in value-directed remembering. Metacognition Learn., 16(3), 685–709 DOI:10.1007/s11409-021-09265-9.
- Nicol D. and MacFarlane-Dick D., (2006), Formative assessment and selfregulated learning: a model and seven principles of good feedback practice, Stud. High. Educ., 31(2), 199–218 DOI:10.1080/03075070600572090.
- Nyachwaya J. M., (2016), General chemistry students’ conceptual understanding and language fluency: acid–base neutralization and conductometry, Chem. Educ. Res. Pract., 17(3), 509–522 10.1039/C6RP00015K.
- Pentucci M. and Laici C., (2020), An integrated blended learning ecosystem for the development of the design skills of teachers-to-be, Proceedings of ICERI2020 Conference, IATED, pp. 2145–2154 DOI:10.21125/iceri.2020.0516.
- Pentucci M., Rossi P. G. and Capolla L., (2023), Designing regulation in action to manage the unforeseen in teaching–learning contexts, EDULEARN23 Proceedings, IATED, pp. 1151–1158 DOI:10.21125/edulearn.2023.0393.
- Perry J., Lundie D. and Golder G., (2019), Metacognition in schools: what does the literature suggest about the effectiveness of teaching metacognition in schools? Educ. Rev., 71(4), 483–500 DOI:10.1080/00131911.2018.1441127.
- Preoteasa E. A. and Ionescu-Tirgoviste C., (2015), Ionic structure in mineral waters. a conductometry study, Proc. Rom. Acad., Ser. B, 17(3), 201–213.
- Project Zero's Thinking Routine Toolbox, https://pz.harvard.edu/thinking-routines.
- Rahman S. F. A., Yunus M. M. and Hashim H., (2020), The Uniqueness of Flipped Learning Approach, Int. J. Educ. Pract., 8(3), 394–404 DOI:10.18488/journal.61.2020.83.394.404.
- Renner P., O’Dea B., Sheehan J., Tebbutt J. and Davis K., (2016), The importance of cognitive, intrapersonal, and interpersonal attributes to student success: an exploration of University students’ and staff views, J. Aust. New Zeal. Student Serv. Assoc., 2016(48), 14–23.
- Richardson E. T., Hansen A. M., Kraus T. E. C., Downing B. D., Forsberg D. and Stillian J. et al., (2023), A novel boat-based field application of a high-frequency conductometric ammonium analyzer to characterize spatial variation in aquatic ecosystems, Limnol. Oceanogr. Methods, 21(12), 761–774 DOI:10.1002/lom3.10579.
- Rossi P. G. and Pentucci M., (2021), Progettazione come azione simulata, FrancoAngeli.
- Salvini A., (2015), Percorsi di analisi dei dati qualitativi, UTET Università.
- Seery M. K., Agustian H. Y., Christiansen F. V., Gammelgaard B. and Malm R. H., (2023), 10 Guiding principles for learning in the laboratory, Chem. Educ. Res. Pract., 383–402 10.1039/d3rp00245d.
- Smith K. C., Edionwe E. and Michel B., (2010), Conductimetric Titrations: A Predict–Observe–Explain Activity for General Chemistry, J. Chem. Educ., 87(11), 1217–1221 DOI:10.1021/ed100538q.
- Stead D. R., (2005), A review of the one-minute paper, Act. Learn. High. Educ., 6(2), 118–131 DOI:10.1177/1469787405054237.
- Stewart J. J. and Dake G. R., (2019), Activating Students’ Prior Knowledge Using a Bridge Activity as an Initial Interactive Discussion in a Flipped Organic Chemistry Course, J. Chem. Educ., 96(11), 2426–2431 DOI:10.1021/acs.jchemed.9b00370.
- Sunkel D. B., (2019), Developing Metacognitive Competence for Governing Self-Influence on Learning To Improve Collaborative STEM Learning, Doctorate Thesis, University of Washington.
- Theureau J., (2011), Appropriation, Incorporation & In-culturation, Journée Ergo-Idf, 1–31.
- Thompson V. A. and Markovits H., (2025), Fast reasoning and metacognition, Psychon. Bull. Rev. DOI:10.3758/s13423-025-02662-0.
- Upadhyay L. S. B. and Verma N., (2015), Alkaline phosphatase inhibition based conductometric biosensor for phosphate estimation in biological fluids, Biosens. Bioelectron., 68, 611–616 DOI:10.1016/j.bios.2015.01.064.
- Van der Kleij F. M., (2024), Supporting students to generate self-feedback: critical reflections on the special issue, Stud. Educ. Eval., 81, 101348 DOI:10.1016/j.stueduc.2024.101348.
- van der Stel M. and Veenman M. V. J., (2010), Development of metacognitive skillfulness: a longitudinal study, Learn. Individ. Differ., 20(3), 220–224 DOI:10.1016/j.lindif.2009.11.005.
- Van Helvert J., Petukhova V., Stevens C., de Weerd H., Börner D., Van Rosmalen P. et al., (2016), Observing, Coaching and Reflecting: Metalogue – A Multi-modal Tutoring System with Metacognitive Abilities, EAI Endorsed Trans. Futur. Intell. Educ. Environ., 2(6), 151525 DOI:10.4108/eai.27-6-2016.151525.
- van Velzen J. H., (2015), Are students intentionally using self-reflection to improve how they learn? Conceptualising self-induced self-reflective thinking, Reflective Pract., 16(4), 522–533 DOI:10.1080/14623943.2015.1064378.
- Veenman M. V. J. and Beishuizen J. J., (2004), Intellectual and metacognitive skills of novices while studying texts under conditions of text difficulty and time constraint, Learn. Instr., 14(6), 621–640 DOI:10.1016/j.learninstruc.2004.09.004.
- Veenman M. V. J. and Spaans M. A., (2005), Relation between intellectual and metacognitive skills: age and task differences, Learn. Individ. Differ., 15(2), 159–176 DOI:10.1016/j.lindif.2004.12.001.
- Volmer D. A., Curbani L., Parker T. A., Garcia J., Schultz L. D. and Borges E. M., (2017), Determination of Titratable Acidity in Wine Using Potentiometric, Conductometric, and Photometric Methods, J. Chem. Educ., 94(9), 1296–1302 DOI:10.1021/acs.jchemed.6b00891.
- Walcutt J. J. and Schatz S., (2019), Modernizing Learning: Building the Future Learning Ecosystem, Government Publishing Office.
- Zhou J. and Ye J., (2023), Sentiment analysis in education research: a review of journal publications, Interact. Learn. Environ., 31(3), 1252–1264 DOI:10.1080/10494820.2020.1826985.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4rp00368c |
| This journal is © The Royal Society of Chemistry 2025 |