 Open Access Article
Open Access ArticleOpportunities and challenges of lead-free metal halide perovskites for luminescence
Run
Tan
b,
Zhenyu
Liu
c,
Zhigang
Zang
 *ab and
Shuangyi
Zhao
*b
*ab and
Shuangyi
Zhao
*b
aSchool of Information Science and Engineering, Yanshan University, Qinhuangdao 066004, P. R. China. E-mail: zangzg@cqu.edu.cn
bKey Laboratory of Optoelectronic Technology and Systems (Ministry of Education), Chongqing University, Chongqing 400044, China. E-mail: shyzhao@cqu.edu.cn
cCDGM Glass Company Limited, Chengdu, Sichuan, China
First published on 25th October 2024
Abstract
Metal halide perovskites (MHPs) have been developed rapidly for application in light-emitting diodes (LEDs), lasers, solar cells, photodetectors and other fields in recent years due to their excellent photoelectronic properties, and they have attracted the attention of many researchers. Perovskite LEDs (PeLEDs) show great promise for next-generation lighting and display technologies, and the external quantum efficiency (EQE) values of polycrystalline thin-film PeLEDs exceed 20%, which is undoubtedly a big breakthrough in lighting and display fields. However, the toxicity and instabilities of lead-based MHPs remain major obstacles limiting their further commercial applications. The exploration and development of lead-free MHPs (LFMHPs) are regarded as the most facile strategies to solve these problems. Compared with lead-based perovskites, LFMHPs exhibit better stabilities and broadband emission. With continuous development of LFMHPs, their photoluminescence quantum yields (PLQYs) have reached 99%, facilitating their use as ideal emitters. In this review, the structures and features of LFMHPs are analyzed, and the preparation methods of LFMHPs with various structures and configurations are discussed. Then, the mechanisms and strategies for improving the emission performance of white LEDs based on LFMHPs are demonstrated. Finally, their challenges in commercial production and perspectives are prospected.
Introduction
In the field of solid-state lighting technology, white-light-emitting diodes (WLEDs) have been identified as ideal light sources to replace incandescent bulbs due to their long lifetime, low energy consumption, wide spectrum, and controllability of color temperature.1 Light-emitting diodes (LEDs) were first demonstrated from the development of gallium nitride (GaN)-based semiconductors in the early 1990s.2 As one of the 2014 Nobel Prize winners, Nakamura created a representative high-brightness blue GaN-LED with an external quantum efficiency of 7.20% in 1993, which shows good prospects for application in WLEDs. Therefore, WLEDs have gradually been recognized as excellent candidates in cost-effective and high-efficiency solid-state lighting.1–10As a new type of semiconductor material, metal halide perovskites (MHPs) possess many advantages,11 such as high photoluminescence quantum yields, tunable bandgaps,12 high carrier mobility,13 solution processibility,14 and low preparation cost.15 All of these superior photoelectronic properties give MHPs great potential to perform well in LEDs.11,13,14,16–23 MHPs are materials with the formula ABX3 with a crystal structure similar to that of the mineral perovskite CaTiO3, where A is a monovalent cation, such as CH3NH3+ (MA+), CH(NH2)+ (FA+), and Cs+; B is a bivalent metal cation, such as Pb2+, Sn2+, Cu2+, (Ag+ and Bi3+ are double perovskites); and X represents the halide anions, including Cl−, Br−, and I−. By changing the halogen and cation composition,24 the structures and chemical bonds of MHPs can be regulated, resulting in changes to their photoelectronic properties. With the further study of perovskites, novel A2BX4, A3BX6 and A3B2X5 metal halide perovskites are now being researched in addition to the classic ABX3 metal halides. Since Tan et al. fabricated the first LEDs based on solution-processed organometallic halide perovskites in 2014,25 perovskite-based LEDs have been developed. In just a few years, internal quantum efficiencies (IQEs) and external quantum efficiencies (EQEs) of perovskite LEDs (PeLEDs) increased from 3.4% and 0.76% in the near-infrared region and 0.4% and 0.1% in the green-light region to more than 20% in the near-infrared26,27 and green-light28 regions, respectively. Due to the excellent photoelectronic performance of MHPs and the rapid development of PeLEDs, certain progress has been made in the material preparation and device fabrication of WLEDs. For example, warm and cold WLEDs were achieved by incorporating 1% and 10% Sb3+ doped Cs2ZrCl6 on a 310 nm UV-LED chip, respectively.29 However, the inherent toxicity of lead, the complex preparation process, and the low operational lifetime of PeLEDs, as well as their instability under high temperature, light, humidity and other conditions,16,17,30 limits the application of MHPs in solid-state lighting.31,32
To solve these problems, lead-free metal halide perovskites (LFMHPs), in which Pb2+ ions are completely replaced by bivalent cations, such as Sn2+, Ge2+, and Zn2+,33 have been studied and applied in photoelectronic applications. Additionally, ions with different valence states can also replace Pb2+ ions, such as trivalent Sb3+,34 Eu3+, Tb3+,35 and monovalent Cu+,36 and this occurs via heterovalent substitution. At the same time, replacing Pb2+ in a perovskite with monovalent, trivalent and quadrivalent cations, as well as vacancies, can not only improve perovskite stability, but also lead to enhancement of their photoelectronic properties. For LFMHPs, their band gap values can be adjusted according to the different metal components, so that more stable electroluminescence in LEDs can be obtained. The outstanding performance of LFMHPs has attracted the attention of many researchers, and up to now, there has been a lot of progress in WLEDs based on LFMHPs. Sb3+-doped Cs2InCl5·H2O:Sb3+ exhibits broadband yellow light emission, and its PLQY is as high as 95.5%.34 Cs2AgInCl6 prepared by Li et al. exhibited an enhanced PLQY of 87.2%, assisted by sodium (Na+) alloying and bismuth (Bi3+) doping, and was used in WLEDs to achieve efficient white-light emission with International Commission on Illumination (CIE) color coordinates of (0.38, 0.44)37 and a color render index (CRI) of 87.8, showing remarkable operational stability under ambient conditions. In this review, the structures and characteristics of LFMHPs are firstly analysed, and preparation methods of LFMHPs with different structures and configurations are discussed. Furthermore, mechanisms, key parameters and strategies for improving the luminous efficiencies of WLEDs based on LFMHPs are described. Finally, their challenges and prospects in commercial applications are discussed (Fig. 1).
 | ||
| Fig. 1 Summary of the review, which includes synthesis and structures of LFMHPs, as well as mechanisms and applications of WLEDs based on LFMHPs. | ||
Structures, dimension and optical properties of LFMHPs
As is well-known, perovskite configurations can be divided into zero-dimensional (0D), one-dimensional (1D), two-dimensional (2D) and three-dimensional (3D) according to their atomic arrangement order. In addition, perovskites can exist in various structures, such as nanocrystals (NCs), powders, thin films, and single crystals. Different structures can affect the photoelectronic performance of LFMHPs and PeLEDs.38 Similar to traditional 3D Pb-based perovskites, 3D LFMHPs possess a 3D configuration, where corner-shared [BX6]4− octahedra are the basic units.39 In a [BX6]4− octahedron, a metal cation is located in the center of the octahedron surrounded by monovalent cations (e.g. Cs+, Rb+, and CH3NH3+). For example, Sn(II)-based ASnX3 perovskites (X = Cl−, Br−, I−) show a similar three-dimensional configuration, as shown in Fig. 2a.40 By introducing larger cations such as butylammonium (BA) and phenethylamine (PEA), the 3D octahedral framework can be isolated into octahedral layers with shared angles, resulting in reduction of dimension from 3D to 2D. 2D perovskites can be viewed as a plate or layer cut along a crystallographic direction. By cutting 3D perovskites along the (001) plane, Ruddlesden–Popper-type (R2An−1MnX3n+1, R = 1+ cation) or Dion–Jacobson-type (RAn−1MnX3n+1, R = 2+ cation) perovskites can be obtained.46,47 For two typical perovskites with layered structures, A-site ions are regarded as organic ligands. The dimension of LFMHPs can be further reduced to 1D with side- and angular-shared chains (formula A3BX6),48 and unconnected (isolated) octahedral 0D structures (formula A3B2X6).49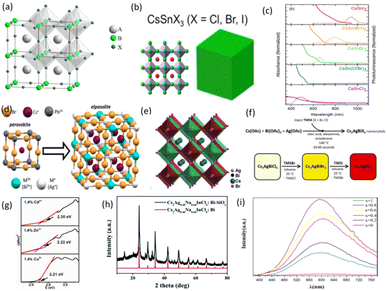 | ||
| Fig. 2 (a) ABX3 perovskite structure. BX6 corner-sharing octahedra are in evidence.40 Copyright 2008 American Chemical Society. (b) Structure of CsSnX3.41 Copyright 2016 American Chemical Society. (c) Absorption and steady-state PL of NCs containing pure and mixed halogens.41 Copyright 2016 American Chemical Society. (d) Structure of double perovskites with M+ and M3+ ions replacing Pb2+ ions.42 Copyright 2018 American Chemical Society. (e) Crystal structure of cubic Cs2AgBiBr6.43 Copyright 2018 American Chemical Society. (f) Synthesis diagram of Cs2AgBiX6 NCs.42 Copyright 2018 American Chemical Society. (g) Tauc plots for 1.4% Cd2+, 1.4% Zn2+, and 1.4% Cu2+-doped Cs2AgInCl6, respectively.44 Copyright 2020 American Chemical Society. (h) XRD of Cs2Ag0.40Na0.60InCl6:Bi–SiO2.45 Copyright 2021 Royal Society of Chemistry. (i) Emission spectra of Cs2Ag1−xNaxInCl6:Bi (x = 0, 0.2, 0.4, 0.6, 0.8, 1).14 Copyright 2021 Electrochemical Society. | ||
3D LFMHPs
In 2014, Felix Deschler et al.25 prepared methylammonium iodide (CH3NH3I) by a solvent evaporation method, and then dissolved CH3NH3I and lead(II) chloride (PbCl2) in anhydrous N,N′-dimethylformamide at a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. CH3NH3PbI3−xClx thin films were prepared by spin-coating from a precursor solution. The photoluminescence (PL) observed from CH3NH3PbI3−xClx thin films are mainly attributed to recombination of free charges (electron and hole) within the films. At room temperature, free charges are formed under 1 ps photoexcitation and exhibit a long lifetime, resulting in very efficient PL with a high PLQY of 70%. This reveals the potential of 3D perovskite semiconductors in emission applications due to their facile processing and high luminous efficiency. Additionally, Protesescu et al. have opened a new avenue for the development of MHPs by designing highly efficient perovskite colloidal quantum dots (QDs).50 They synthesized CsPbX3 QDs using a simple preparation method and the emission of the prepared perovskites was in the visible region of 410–700 nm with PLQYs up to 90%, indicating tunable bandgap values of MHPs. It can be seen that photoelectronic performance of QDs is better than that of thin films, and their enhanced PLQYs and stabilities enable them to be applied in PeLEDs.
1 molar ratio. CH3NH3PbI3−xClx thin films were prepared by spin-coating from a precursor solution. The photoluminescence (PL) observed from CH3NH3PbI3−xClx thin films are mainly attributed to recombination of free charges (electron and hole) within the films. At room temperature, free charges are formed under 1 ps photoexcitation and exhibit a long lifetime, resulting in very efficient PL with a high PLQY of 70%. This reveals the potential of 3D perovskite semiconductors in emission applications due to their facile processing and high luminous efficiency. Additionally, Protesescu et al. have opened a new avenue for the development of MHPs by designing highly efficient perovskite colloidal quantum dots (QDs).50 They synthesized CsPbX3 QDs using a simple preparation method and the emission of the prepared perovskites was in the visible region of 410–700 nm with PLQYs up to 90%, indicating tunable bandgap values of MHPs. It can be seen that photoelectronic performance of QDs is better than that of thin films, and their enhanced PLQYs and stabilities enable them to be applied in PeLEDs.
Subsequently, Jellicoe et al. reported nontoxic LFMHP CsSnX3 nanocrystals (NCs), which were synthesized by a hot-injection method.41 As demonstrated in Fig. 2b, the prepared CsSnX3 NCs possess a compact cubic structure. With the change of halogen in CsSnX3 NCs, their optical spectra were found to extend from the visible-light range to the near-infrared (NIR) region (Fig. 2c). Owing to similar atomic sizes and orbital structures of Sn2+ ions with those of Pb2+ ions, Sn-based LFMHPs exhibit ideal 3D perovskite structures, but the measured PLQYs of CsSnX3 are below 1%, which results from the existence of high-density defects. It has been found that the defect formation energy in Sn-based perovskites is as low as 250 meV, which leads to defect densities of up to ∼1019 cm−3. Due to excellent stabilities and photoelectronic properties of three-dimensional lead-free double perovskites, they have attracted the attention of a large number of researchers. The density functional theory (DFT) results show that their stabilities can be evaluated with proper t (tolerance factor) and μ (octahedral factor) values (0.813 < t < 1.107, 0.337 < μ < 0.895),51 indicating 3D structures and good photoelectronic performance of lead-free double perovskites. For example, Cs2AgBiBr6 and Cs2AgInCl6 were characterized and found to possess 3D structures, in which Ag+ and Bi3+ (In3+) together replace a pair of adjacent B2+ ions and then form two octahedrons with halogens, as demonstrated in Fig. 2d. In 2018, Zhou et al. prepared Cs2AgBiBr6 double perovskite NCs by the hot-injection method, and their structure is shown in Fig. 2e. Cs2AgBiBr6 NCs possess good photoelectronic properties and stabilities under high temperature and humidity. However, it has been reported that most double perovskites exhibit indirect band structures, resulting in weak PL performance.43 In addition, a series of Cs2AgBiX6 NCs composed of different halogens were prepared by an ion exchange method. Sidney E. et al. prepared Cs2AgBiX6 (X = Cl, Br) NCs by the hot-injection method. The synthesis and conversion diagram of Cs2AgBiX6 NCs in Fig. 2f further demonstrates that Cs2AgBiI6 can be synthesized by an anion exchange reaction. The product maintains the symmetry of cubic Fm3m with the structure of CsAgBiBr6.42 During the ion exchange process, the spectral characteristics of larger-size NCs illustrate their broadband emission with low-energy shifts, as well as an adjusted emission range in the 350–600 nm region. Furthermore, Yang et al. measured the carrier dynamics of double perovskite nanocrystals using time-resolved PL and femtosecond (fs) transient absorption (TA) spectroscopies, and found that the double perovskite NCs show an obvious sub-band-gap capture process, which originates from surface defects. To solve this problem, they used the surfactant oleic acid (OA) as ligands to passivate the defects, increasing the PLQY by 100 times. Even so, the PLQY is only 6.7%.52 Li et al. proposed the Al3+ doping strategy to change an indirect band gap of Cs2AgBiX6 into a direct band gap, thus enhancing its PL performance. As a result, its PLQY was improved to 17.2% and white LEDs with a CRI of 81 were fabricated by integrating Cs2AgBiX6 with K3SbCl6 on 365 nm UV chips.53
Among reported lead-free double perovskites, Cs2AgInCl6 has the characteristics of a direct band gap. Locardi et al. synthesized intrinsic double perovskite Cs2AgInCl6 NCs by using a colloidal method. They are stable in air and exhibit broadband white-light emission. The PL emission intensity of Mn-doped Cs2AgInCl6 NCs increases as the Mn doping concentration increases from 0.5% to 1.5%, and the highest PLQY achieved was 16 ± 4%.54 In order to better understand the mechanism of the doping strategy, Liao et al. doped Cu2+, Zn2+ and Cd2+ ions into Cs2AgInCl6 NCs, and the subsequent band gap values were 2.21 eV, 2.22 eV and 2.30 eV, respectively. The influence of different dopants on direct gaps shows that metal dopants enable the band gaps in Cs2AgInCl6 to be changed (Fig. 2g).44
In 2021, Li et al. synthesized Bi3+-doped Cs2AgxNa1−xInCl6 QDs by the hot-injection method. Based on the broadband spectrum originating from self-trapped excitons in Bi3+-doped Cs2AgxNa1−xInCl6, they obtained excellent white light emission. The best PLQY of Cs2AgxNa1−xInCl6 was achieved by doping a small amount of Bi3+ ions. The PLQY increased to 57.3%, and the emission peak at 600 nm was obvious. The Na-alloy and Bi-doped Cs2AgxNa1−xInCl6 QDs possess the same cubic structure as Cs2AgInCl6. The stability of the QDs was further improved by preparing Cs2AgxNa1−xInCl6:Bi–SiO2 thin films using perhydropolysilazane as the coating precursor, which was confirmed from Fig. 2h. XRD patterns of the Cs2Ag0.40Na0.60InCl6:Bi–SiO2 thin films exhibited dominant peaks of SiO2 and Cs2Ag0.40Na0.60InCl6 QDs. Compared with Cs2Ag0.40Na0.60InCl6:Bi QDs at room temperature, they exhibited nearly all the main diffraction peaks, therefore the temperature change did not cause a phase transition of the QDs, and it could be concluded that SiO2 plays a protective role and thus prevented the phase transition of the QDs. It is known that coating passivation can inhibit surface-related non-radiative processes and PL intensity is improved after sufficient coating. In addition, after heat treatment at 200 °C, the PL intensity of the film based on coating QDs is enhanced, and its PLQY is higher than that of uncoated QDs.45 Subsequently, Wang et al. studied the phase structures, morphologies and luminescence properties of Cs2Ag1−xNaxInCl6:Bi with different Na contents. Cs2Ag1−xNaxInCl6:Bi has a wide emission peak at 600 nm, as shown in Fig. 2i. When x = 0.8, its PLQY reaches 86.91%, which has been identified as the best value among reported double perovskites.14
2D LFMHPs
Recently, researchers have used long-chain alkylammonium ions to separate 3D octahedra, with adjacent layers stacked on top of each other by van der Waals forces between organic chains. As shown in Fig. 3a, long hydrophobic chains provide 2D perovskites with better moisture resistance and thermal stability than 3D perovskites. Wang et al. reported emissive 2D (OCTAm) 2SnX4 (OCTAm = octylammonium cation) LFMHPs with a near-unity PLQY and ultrahigh stability in acidic aqueous solutions.62 Hou et al. reported that different carbon chains can affect the photoelectronic effect of 2D tin-based perovskites. They reported the synthesis of 2D LFMHPs by selecting different long-chain amines (RNH2), as shown in Fig. 3b. (C8H17NH2)2SnBr4 (PLQY = 54%), (C12H25NH2)2SnBr4 and (C18H37NH2)2SnBr4 (PLQY = 2%) emit bright yellow light (∼615 nm), and (OAm)2SnBr4 (PLQY = 60%) emits excellent orange light (∼628 nm). This also proves that organic carbon chains have an effect on the properties, in which the flexibility of the carbon chains influences the arrangement of the carbon chains and the distortion of the octahedral layers. As a result, changes in binding energy, which are attributed to the arrangement of the carbon chains and distortion of the [SnBr6]4− octahedra, affect the band gap of tin-based LFMHPs.56 Hou et al. found that the energy level of a Mn 4T1 orbital was close to that of the self-induced exciton, which could effectively reduce the energy loss during the energy transfer process. Therefore, they doped 2D Sn-based perovskites with Mn ions through a solid grinding method. The structure of the prepared (C8H17NH2)2Sn1−xMnxBr4 is shown in Fig. 3c. Its emitted light is orange and red, and originates due to the emission of self-trapped excitons (STEs) from the host crystal and the effective emission of the d–d orbital transition (4T1–6A1) in Mn ions. However, the prepared LFMHPs exhibit PL as a single broad band due to the competition between STEs and the doped Mn d–d transition emission.57 In order to explore the broadband emission of STEs from a 2D lead-free perovskite, Li et al. synthesized Sn-based perovskites of R2+xSnI4+x, which has broadband emission with the highest PLQY reaching 99%. Its stability and good photoelectronic performance make it suitable for potential application in solid-state lighting.63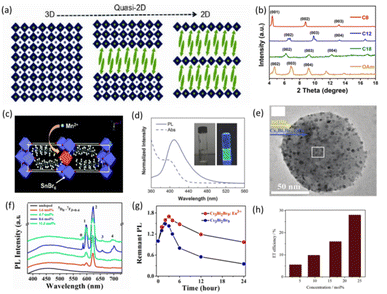 | ||
| Fig. 3 (a) Perovskite lattices with different dimensions (n = ∞, 3D structure; n = 1, pure 2D structure; and n = defined integer, quasi-2D structure).55 Copyright 2014 Wiley-VCH. (b) XRD patterns for tin-based perovskites.56 Copyright 2019 American Chemical Society. (c) Schematic representation of 2D single-layered (C8H17NH2)2Sn1−xMnxBr4 (x = 0.25).57 Copyright 2020 Royal Society of Chemistry. (d) Absorption and PL spectra of Cs3Bi2Br9 QD synthesis. Insets: typical optical images of the QD solution in ambient light and under 325 nm UV lamp illumination.58 Copyright 2017 Wiley-VCH. (e) TEM images of the water-induced Cs3Bi2Br9/BiOBr nanocomposites.59 Copyright 2020 Royal Society of Chemistry. (f) Emission spectra of undoped and Eu3+ ions-doped Cs3Bi2Br9 QDs with different doping concentrations.60 Copyright 2019 American Chemical Society. (g) PL intensity of undoped and Eu3+ ions-doped Cs3Bi2Br9 PeQDs as a function of time in water.60 Copyright 2019 American Chemical Society. (h) Energy transfer efficiency from PQDs to Sm3+versus Sm3+ doping concentrations.61 Copyright 2020 Chinese Society of Rare Earths. | ||
In addition, other A3B2X9 (B = Bi, Sb) LFMHPs exhibit a 2D layered structure. Leng et al. synthesized 2D Cs3Bi2Br9 QDs with blue light emission using a modified ligand-assisted recrystallization method. Absorption and PL spectra of Cs3Bi2Br9 QDs are shown in Fig. 3d. In the PL spectrum, an emission peak is observed at 410 nm with a FWHM of 48 nm and a PLQY of ∼19.4%. Compared with single crystals, the PL peak of QDs shows a blue shift of 60 nm, indicating a strong quantum confinement effect. In addition, colloidal Cs3Bi2Br9 quantum dots exhibit good stability when exposed to water due to the formation of self-passivation layers of BiOBr.58 Based on the water stability of Cs3Bi2Br9 QDs, Ma et al. proposed a strategy to improve the PLQY of Cs3Bi2X9 QDs by using water-induced nanocomposites. The QDs are encapsulated in a BiOBr matrix. By optimizing the water treatment in Cs3Bi2Br9 QDs, as shown in Fig. 3e, Cs3Bi2Br9 QDs were encapsulated into the BiOBr matrix, which led to an enhancement of their PLQY by approximately 130% (from 20.2% to 46.4%) and improved color purity.59 The low PLQY of Cs3Bi2Br9 QDs can be attributed to the intrinsic surface defects, and the lattice defects of Cs3Bi2Br9 can be reduced by the doping strategy, allowing doping ions to occupy defect states. Ding et al. doped Eu3+ ions into Cs3Bi2Br9 QDs, which demonstrated an increased PLQY of 42.4% and multicolor emission using an improved ligand-assisted reprecipitation method. As shown in Fig. 3f, the ratio of PL intensity between Eu3+ ion emission and exciton emission increases with increasing Eu3+ doping concentration, which implies a higher effective energy transfer (ET) from Cs3Bi2Br9 QDs host to the Eu3+ ion level. However, with the further increase of Eu3+ doping concentration, a self-quenching process occurs among the Eu3+ ions, resulting in the reduction of PL intensity of the Eu3+ ions. As shown in Fig. 3g, Eu3+-doped Cs3Bi2Br9 QDs exhibit better stability in water than undoped Cs3Bi2Br QDs.60 Zhu et al. also doped Sm3+ ions into Cs3Bi2Br9 QDs using an improved ligand-assisted recrystallization method. The PLQY of Cs3Bi2Br9 QDs increased from 10.9% to 20.8%, and the water resistance stability also significantly improved. As shown in Fig. 3h, the efficiency of ET increases with the increase of Sm3+ dopant concentration, indicating that doping is an effective method to improve the PLQYs of LFMHPs.61
1D LFMHPs
BX6 octahedra of 1D perovskites are linked in chains (corner-, side-, and face-sharing) and surrounded by organic cations. In 2017, Zhou et al. prepared the 1D-structure of C4N2H14SnBr4 from a hydrothermal reaction of equal amounts of SnBr2 and C4H14N2Br2 in a mixture of 48% w/w hydrobromic acid (HBr) and 50% w/w hypochromic acid (H3PO2) aqueous solution, where H3PO2 can prevent the oxidation of Sn2+ ions. As shown in Fig. 4a, 1D C4N2H14SnBr4 has a similar structure to C4N2H14PbBr4, where the metal halide chains of [SnBr4]2− are surrounded by C4N2H142+ cations. However, unlike the intense white light emission observed in 1D lead bromide perovskite (C4N2H14PbBr4), 1D C4N2H14SnBr6 does not emit white light because the size of Sn is smaller than Pb, leading to structural distortion and suppressed recombination of excitons in 1D C4N2H14SnBr6.64 In terms of Mn-based LFMHPs, because of the 3d5 structure in Mn ions, the luminescence changes of Mn vary greatly under different crystal field environments. Mn ions enable green light emission in weak fields, and orange to red light emission in strong fields, which also has a positive effect on the luminescence efficiency in low-dimensional perovskites.67 Xiao et al. prepared 1D CsMnCl3(H2O)2 single crystals using a typical crystallization method, and mixed isomolar MnCl2(H2O)4 and CsCl in concentrated hydrochloric acid at 80 °C. After cooling and crystallization, pink single crystals of CsMnCl3(H2O)2 were obtained, which emit red light under ultraviolet (UV) excitation. The 1D crystal structure of CsMnCl3(H2O)2 is shown in Fig. 4c. Each Mn2+ ion is coordinated with four Cl− ions and two O2− ions to form a twisted [MnCl4(H2O)2]2− octahedron, which is connected by a common vertex to form a zigzag chain. In addition, each Cs+ ion is surrounded by eight nearest Cl− ions, thus connecting the octahedra. They measured temperature-dependent PL spectra from 20 to 300 °C, as shown in Fig. 4b. The PL intensity initial decreased to 100 °C and slightly increased at 120 °C, then decreased again. This was attributed to the loss of crystal water. Compared with CsMnCl3(H2O)2, the PLQY of CsMnCl3 increases from 13% to 36%, which is due to the strong coupling of excitons with tensile vibration of OH− groups, which eliminates nonradioactive recombination and quenching fluorescence.65 Pandurangan et al. adopted simple ultra-sonication and the modified ligand-assisted reprecipitation method to prepare 1D lead-free Mn halide NCs in methanol instead of DMF, so that the synthesized Mn-based perovskites are highly stable.68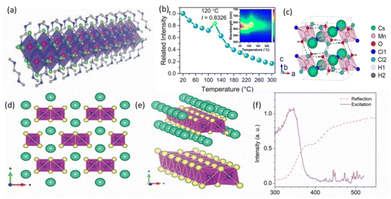 | ||
| Fig. 4 (a) Views of a 1D Sn bromide wire wrapped by organic cations.64 Copyright 2017 Wiley-VCH. (b) The variation in the integrated emission intensity (λex = 416 nm) of CsMnCl3(H2O)2vs. the heating temperature (20–300 °C). Inset in the upper right shows the temperature-dependent PL spectra of CsMnCl3(H2O)2.65 Copyright 2020 Wiley-VCH. (c) Crystal structure of CsMnCl3(H2O)2, hydrogen bonds (O–H) depicted as black dashed lines.65 Copyright 2020 Wiley-VCH. (d) Structure of 1D CsCu2I3 SC (green: Cs atom, yellow: I atom, dark blue: Cu atom, purple octahedron: Cu–I octahedron).66 Copyright 2019 Wiley-VCH. (e) Cu–I 1D chain surrounded by Cs atoms and the independent Cu–I chain that is likea “nanowire”.66 Copyright 2019 Wiley-VCH. (f) Ultraviolet excitation spectrum collected at 568 nm and reflection spectrum.66 Copyright 2019 Wiley-VCH. | ||
A new type of Cu+-based LFMHP has attracted the extensive attention of researchers. Taking CsCu2I3 as an example, it possesses a typical 1D structure on the molecular level.69 Huang et al. successfully prepared 1D CsCu2I3 SCs by dropping methanol into the mixture of saturated solutions (dimethyl sulfoxide (DMSO) and dimethyl formamide (DMF) at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and dissolved a certain proportion of CsI and CuI) through the antisolvent impregnation method. The 1D crystal structure of CsCu2I3 with isolated [Cu2I3]− chains in the second-dimension direction is shown in Fig. 4d and e. The emission spectra at room temperature are shown in Fig. 4f. The strong absorption peak is located at 328 nm, the optical band gap is measured as 3.78 eV, and a Stokes shift of 1.51 eV is found, indicating recombination of self-trapped excitons.66 Shi et al. dissolved different proportions of CsI and CuI in γ-butyrolactone through a rapid antisolvent crystallization process with isopropanol. After stirring the solution at 80 °C for 2 hours, the mixture was cooled naturally to room temperature. Then the supernatant of the mixture was quickly injected into isopropyl alcohol (10 mL) and stirred vigorously in the air at room temperature, producing white Cs3Cu2I5 and CsCu2I3 precipitates immediately. Under UV excitation, blue and yellow light was emitted, respectively. The PL spectra cover almost the entire visible range of 370–750 nm. By evaluating the absorption spectra of the two products, the optical band gap of 0D Cs3Cu2I5 is 3.78 eV, and that of 1D CsCu2I3 is smaller than 4.11 eV. Therefore, larger Stokes shifts indicate that Cu-based perovskites with a 1D structure have greater lattice distortion. The two compounds can be converted into each other by a mechanochemical reaction. Thus, standard white light emission can be obtained from the mixture of these two compounds in appropriate proportions, showing good optical properties.70
4, and dissolved a certain proportion of CsI and CuI) through the antisolvent impregnation method. The 1D crystal structure of CsCu2I3 with isolated [Cu2I3]− chains in the second-dimension direction is shown in Fig. 4d and e. The emission spectra at room temperature are shown in Fig. 4f. The strong absorption peak is located at 328 nm, the optical band gap is measured as 3.78 eV, and a Stokes shift of 1.51 eV is found, indicating recombination of self-trapped excitons.66 Shi et al. dissolved different proportions of CsI and CuI in γ-butyrolactone through a rapid antisolvent crystallization process with isopropanol. After stirring the solution at 80 °C for 2 hours, the mixture was cooled naturally to room temperature. Then the supernatant of the mixture was quickly injected into isopropyl alcohol (10 mL) and stirred vigorously in the air at room temperature, producing white Cs3Cu2I5 and CsCu2I3 precipitates immediately. Under UV excitation, blue and yellow light was emitted, respectively. The PL spectra cover almost the entire visible range of 370–750 nm. By evaluating the absorption spectra of the two products, the optical band gap of 0D Cs3Cu2I5 is 3.78 eV, and that of 1D CsCu2I3 is smaller than 4.11 eV. Therefore, larger Stokes shifts indicate that Cu-based perovskites with a 1D structure have greater lattice distortion. The two compounds can be converted into each other by a mechanochemical reaction. Thus, standard white light emission can be obtained from the mixture of these two compounds in appropriate proportions, showing good optical properties.70
0D LFMHPs
In 0D LFMHPs, the structures of the metal halide polyhedra are independent and not connected to the surrounding cations. As a result, a flat band structure is formed, which effectively inhibits photoexcitons, leading to the emission of strong excitons and significantly promoting radiation recombination.74 Under excitation, independent 0D structures cause lattice deformation to produce self-trapped exciton (STE) emission, thus achieving efficient luminescence. In recent years, a large number of lead-free perovskite compounds with 0D structures have been explored and synthesized, and their highly tunable emission and remarkable PLQYs have attracted the attention of many researchers.75 In 2018, Kovalenko et al. used a simple solid-state heating approach to heat a mixture of CsBr and SnBr2 at 350 °C for 60 hours. In this process, Cs4SnX6 NCs were ground in a glove box and prepared by annealing. As shown in Fig. 5a, the crystallization of Cs4SnX6 belongs to a trigonal crystal system, forming an R3c space group, where Cs+ ions occupy two different crystal positions and separate [SnBr6]4−octahedra.4 Compared with the CsSnBr3 perovskite, Cs4SnBr6 shows excellent stability in air.41 In addition, they prepared Cs4−xAxSn(Br1−yIy)6 (A = Rb, K; x ≤ 1, y ≤ 1), which exhibits 500–620 nm broadband emission. As shown in Fig. 5b, both broadband emission and long radiation lifetime indicated the formation of STEs. Zhang et al. reported a simple room-temperature antisolvent synthesis of lead-free green-light-emitting Cs4SnBr6 (∼524 nm) and cyan-green-light-emitting Cs3KSnBr6 (∼500 nm) with 0D structures. By evaluating the PL, PLE and absorption spectra of Cs4SnBr6 and Cs3KSnBr6 (Fig. 5c and d), large FWHM values greater than 100 nm were measured, which is desirable, indicating the existence of a strong phonon–electron coupling effect.30 In addition, Stokes shifts were found to decrease from 204 nm (Cs4SnBr6) to 180 nm (Cs3KSnBr6). It may be attributed to changes in the Jahn–Teller distortion in the Sn–Br octahedra, where the elongation of axial Sn–Br bonds and the contraction of the equatorial Sn–Br bonds are included.4 However, the air stability of Sn-based 0D LFMHPs was not greatly improved, which may be due to oxidation of Sn2+ ions in the air. Thus, it is urgent to explore 0D LFMHPs with high-valence lead-free metal ions. 0D [Na(DMSO)2]3SbBr6 NCs were synthesized by Zang et al. using the antisolvent strategy under standard atmospheric conditions, in which toluene acted as the antisolvent. During the preparation process, the precursor solution was obtained by dissolving NaBr and SbBr3 into DMSO. Isolated [SbBr6]4− octahedra are surrounded by [Na(DMSO)2]3, and the prepared NCs exhibit bright broadband yellow-light emission under UV light excitation.71 The photoluminescence of [Na(DMSO)2]3SbBr6 is regarded as originating from STEs, and its colloidal solution, powders and single crystals exhibit high PLQYs of 60%, 60%, and 56%, respectively, as shown in Fig. 5e.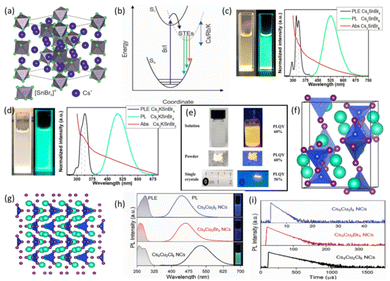 | ||
| Fig. 5 (a) The crystal structure of Cs4SnBr6 viewed along the (111) axis with [SnBr6]4− octahedra (gray with green bromine atoms) separated by Cs+ cations (blue).4 Copyright 2018 Wiley-VCH. (b) Configurational coordinate diagram illustrating the origin of STE PL in Cs4−xAxSn(Br1−yIy)6.4 Copyright 2018 Wiley-VCH. PL, PLE, and Abs of (c) Cs4SnBr6 and (d) Cs3KSnBr6 perovskites. Photographs of colloidal NCs captured under daylight and 302 nm UV light, respectively.30 Copyright 2020 American Chemical Society. (e) Photographs of [Na(DMSO)2]3SbBr6 solution, powder, and single crystals with/without UV light.71 Copyright 2022 Wiley-VCH. (f) and (g) Crystal structure of Cs3Cu2I5.72 Copyright 2018 Wiley-VCH. (h) PLE and PL spectra of colloidal Cs3Cu2X5 NC solutions in hexane. Right insets show photographs of Cs3Cu2X5 NC solutions under UV lamp excitation with λ = 254 nm.73 Copyright 2019 Wiley-VCH. (i) Time-resolved PL decay spectra of Cs3Cu2X5 NC solutions.73 Copyright 2019 Wiley-VCH. | ||
Cs3Cu2X5 (X = Cl, Br, I), with a formula of A3B2X5, is one of the representative 0D LFMHPs in which there are two polyhedra (triangular planes [CuI3]2− and [CuI4]3−), as shown in Fig. 5f and g. The unique 0D structure results in the presence of strong local electrons.51 In 2018, Hosono et al. synthesized a Cs3Cu2I5 single crystal with a size of about 5 mm using a solvent-resistant steam saturation method. Strong blue PL was observed at 445 nm and the PLQY reached 90%. A single-crystal Cs3Cu2I5 thin film was successfully prepared that possesses bright blue PL with a high PLQY of 62% at room temperature, and the PLQY was almost unchanged after being stored for two months, while the PLQY in the QD film suffered from PLQY degradation, which was attributed to the aggregation of QDs.72 Wu et al. utilized a vacuum dual-source thermal deposition strategy to prepare Cs3Cu2I5 thin films, which were directly deposited on a hot quartz substrate with a CsI/CuI molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Annealing treatment was applied to the films in the deposition process, thus their crystals began to grow when precursors were attached to the substrate, avoiding the incomplete reaction of precursors and optimizing the morphologies of the Cs3Cu2I5 thin films.76 In order to explore the tunable broadband emission of Cs3Cu2X5 NCs, Quan et al. synthesized Cs3Cu2X5 using the hot-injection method. As shown in Fig. 5h, the PLE and PL spectra of Cs3Cu2X5 (X = I, Br, and Cl) NCs show clear exciton characteristics, and the emission peaks of the Cs3Cu2X5 NCs change from 445 to 527 nm, which can be attributed to STE emission. Fig. 5i shows the transient PL spectra of Cs3Cu2I5 NCs with a different halogen. The long lifetime of Cs3Cu2X5 NCs is 1.56 μs (X = I), 14.12 μs (X = Br), and 135.97 μs (X = Cl). In addition, Cs3Cu2I5 NCs exhibit excellent PL stability over 45 days, while PL quenching is found in Cs3Cu2Br5 and Cs3Cu2Cl5 due to oxidation.73 During the preparation of Cs3Cu2X5 NCs, the purity of the products was difficult to enhance. To solve this problem, Zang et al. prepared Cs3Cu2Cl3 and Cs3Cu2Cl5 NCs emitting bright blue and green light, respectively, by controlling the reaction temperature (70 °C and 120 °C) during the hot-injection process. The PLQY of Cs3Cu2I5 was up to 87.2% and the emission spectrum was broadband. The results show that the reaction temperature can determine the final composition of Cu-based LFMHPs.77
2. Annealing treatment was applied to the films in the deposition process, thus their crystals began to grow when precursors were attached to the substrate, avoiding the incomplete reaction of precursors and optimizing the morphologies of the Cs3Cu2I5 thin films.76 In order to explore the tunable broadband emission of Cs3Cu2X5 NCs, Quan et al. synthesized Cs3Cu2X5 using the hot-injection method. As shown in Fig. 5h, the PLE and PL spectra of Cs3Cu2X5 (X = I, Br, and Cl) NCs show clear exciton characteristics, and the emission peaks of the Cs3Cu2X5 NCs change from 445 to 527 nm, which can be attributed to STE emission. Fig. 5i shows the transient PL spectra of Cs3Cu2I5 NCs with a different halogen. The long lifetime of Cs3Cu2X5 NCs is 1.56 μs (X = I), 14.12 μs (X = Br), and 135.97 μs (X = Cl). In addition, Cs3Cu2I5 NCs exhibit excellent PL stability over 45 days, while PL quenching is found in Cs3Cu2Br5 and Cs3Cu2Cl5 due to oxidation.73 During the preparation of Cs3Cu2X5 NCs, the purity of the products was difficult to enhance. To solve this problem, Zang et al. prepared Cs3Cu2Cl3 and Cs3Cu2Cl5 NCs emitting bright blue and green light, respectively, by controlling the reaction temperature (70 °C and 120 °C) during the hot-injection process. The PLQY of Cs3Cu2I5 was up to 87.2% and the emission spectrum was broadband. The results show that the reaction temperature can determine the final composition of Cu-based LFMHPs.77
The preparation methods of LFMHP materials
It is known that LFMHPs can be divided into 0D, 1D, 2D and 3D structures according to their atomic arrangement order, and they can also be divided into films, single crystals, powders and NCs according to their morphologies and composition.78 Owing to the diverse crystal configuration and low formation energy of LFMHPs, various preparation methods, such as the antisolvent reprecipitation method,71 the hot-injection method,79 and the solvent thermal method,80 have been proposed and developed to prepare LFMHPs. Different preparation methods have an impact on the characteristics and structures of the LFMHPs, as well as affect their optoelectronic performance.81–89 As a result, the preparation methods can determine the manufacture and performance of perovskite LEDs (PeLEDs).Antisolvent-induced reprecipitation method
During the antisolvent reprecipitation method, the solubility of the solvent is reduced due to the introduction of antisolvent-induced reprecipitation into the solution. In general, according to the polarity and electrolytic capacity of the solvent, an organic solvent can be divided into good solvents (high solubility of precursors in given solvents) and weak solvents (low solubility of precursors in given solvents). Among them, good solvents, including dimethyl sulfoxide (DMSO), N,N-dimethylformamide (DMF), gamma-hydroxybutyrolactone (GBL) and dichloromethane (DCM), generally have a high polarity, which can dissolve highly ionic precursors. The polarity of weak solvents is weak or even non-polar, thus it is difficult for them dissolve some highly ionic precursors. Examples of weak solvents include toluene, chlorobenzene, ether, methanol and n-octane.18 As shown in Fig. 6a, during the antisolvent-assisted crystallization process, mixed precursors can be dissolved efficiently in a good solvent, and it is well known that a mixture of a weak solvent and a good solvent is completely immiscible. A weak solvent enables the content of a good solvent in a precursor solution to be continuously consumed during the diffusion process, resulting in a decrease of solubility and the achievement of saturation states. As a result, precipitate of LFMHP single crystals can be obtained from solution.90 The decreased rate of solubility determines the precipitation rate of single crystals. This method is relatively simple and facile, and there are many kinds of solvent and antisolvent.52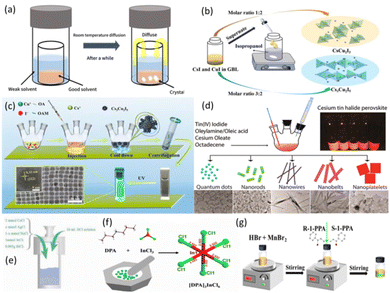 | ||
| Fig. 6 (a) Diagram of antisolvent-assisted crystallization.90 Copyright 2020 Elsevier Ltd. (b) Schematic illustration of the synthetic process for cesium copper iodide with different stoichiometric ratios.70 Copyright 2012 Royal Society of Chemistry. (c) Schematic diagram for the colloidal synthesis of blue-light-emitting Cs3Cu2I5 NCs.79 Copyright 2020 American Chemical Society. (d) Schematic diagram of controlled synthesis procedure of perovskite Cs2SnI6 NCs (left) and photo of prepared Cs2SnI6 sample under UV light (right).91 Copyright 2016 American Chemical Society. (e) Diagram of preparation of perovskites by a hydrothermal method.14 Copyright 2021 Electrochemical Society. (f) Mechanochemical grinding solid state preparation method, and [InCl6]3− unit structure of [DPA]3InCl6.92 Copyright 2021 Chinese Chemical Society. (g) The experimental operation step diagram of preparation of (R/S-1-PPA)2MnBr4.93 Copyright 2022 Elsevier B.V. | ||
As shown in Fig. 6b, Fang et al. synthesized a CsI–CuI solution using the antisolvent reprecipitation method,70 and the resulting CsCu2I3 single crystals were filtered and washed thoroughly with isopropyl alcohol. Finally, CsCu2I3 single crystals exhibited strong yellow-light emission with a FWHM of ∼120 nm under 305 nm UV excitation. However, when the molar ratio of CsI to CuI is changed to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the resulting precipitate is regarded as Cs3Cu2I5, which shows emission of blue light with a FWHM of ∼82 nm under UV excitation. When the molar ratio is 1
2, the resulting precipitate is regarded as Cs3Cu2I5, which shows emission of blue light with a FWHM of ∼82 nm under UV excitation. When the molar ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, there are both CsCu2I3 and CsCu2I5 in the precipitate. The luminescence spectra of Cs3Cu2I5 and CsCu2I3 show strong and broadband emission, which almost covers the whole visible spectrum range of 370–750 nm.
1, there are both CsCu2I3 and CsCu2I5 in the precipitate. The luminescence spectra of Cs3Cu2I5 and CsCu2I3 show strong and broadband emission, which almost covers the whole visible spectrum range of 370–750 nm.
Another common strategy for preparing LFMHPs is the co-precipitation crystallization method, which is commonly used for growing single crystals. In 2021, Chen et al. reported the synthesis of Cs2ZrCl6 single crystals with the assistance of concentrated hydrochloric acid via a simple co-precipitation method, where CsCl, ZrOCl·8H2O, SbCl3 and 37% hydrochloric acid (HCl) were the raw materials. Cs2Zr1−xCl6:xSb3+ (x = 1, 5, 10, 20%) single crystals were obtained from different molar ratios of SbCl3 and ZrOCl·8H2O, and they showed different PL performance. It was found that intrinsic Cs2ZrCl6 shows blue-light STE emission centered at 445 nm under 260 nm excitation, and the FWHM is 135 nm. Cs2ZrCl6 doped with 10% Sb3+ shows the strongest broadband blue-light emission peaks at 495 nm and red-light emission peaks at 622 nm with a PLQY of about 78%.29 Sb3+ doping was proved to facilitate the strong electron–phonon coupling in the soft crystal structure, leading to the formation of STEs.
Although the antisolvent reprecipitation method is an effective method for preparing MHPs, the antisolvent-assisted crystallization method shows a high selectivity for organic solvents with low solubility. In addition, during the synthesis process, the fast diffusion rate of a weak solvent may cause fast crystallinity, resulting in the production of metal-halide powders instead of single crystals. In comparison, the slow diffusion rate of a solvent has been found to affect the crystal quality of LFMHP single crystals.58 Furthermore, the single-crystal growth period is slow in this process, and the crystallization process is uncontrollable, so the antisolvent-assisted crystallization method is not suitable for fabricating large-size single crystals.30,70,94
Hot-injection method
Hot injection is a rapid preparation method, in which a precursor solution is injected into a hot solution containing ligands at a high temperature (usually above 120 °C).95 Due to its fast injection speed, when the reaction solution is saturated, fast nucleation and growth occur immediately, facilitating dispersal of the final product in solution. This synthesis method can be used to synthesize small, monodisperse and high-crystallinity NCs. The sizes, distribution, and shapes of the NCs depend on the ratios of surfactants to precursors, as well as the concentration of precursor, injection temperature, and reaction time.79 As shown in Fig. 6c, Wang et al. synthesized Cs3Cu2I5 NCs using a modified hot-injection method. The synthesis process can be described simply as dissolving copper iodide precursors into cesium oleate, and cooling the mixture after a short reaction time. Cu+ ions combine with Cs+ and I− ions to form Cs3Cu2I5 NCs stabilized by oleic acid (OA) and octylamine (OAM) ligands. Under excitation of UV light, the colloidal solution of NCs shows bright blue-light emission at 445 nm with a FWHM of 63 nm and a PLQY of 87%, as well as negligible PL degradation of Cs3Cu2I5.91 Cesium oleate and tin iodide(IV) act as precursors in the solution containing OA and OAm. Even after exposure of the cesium oleate Cs3Cu2I5 to an air environment for 35 days, its stability against water and oxygen was maintained.79Wang et al. synthesized well-structured lead-free and stable Cs2SnI6 NCs with different morphologies by the simple hot-injection method, as shown in Fig. 6d.91 White products were observed at first, which then turned dark-brown. The crude solution was then purified with a mixture of toluene and hexane (1/1 v/v) under ambient conditions. Finally, the NCs were dried in a bottle. When adjusting the reaction temperature and time, as well as the ratios of precursors, spherical QDs, nanorods, nanowires and nanosheets of the synthesized Cs2SnI6 are obtained.
The hot-injection method is regarded as a common method in the preparation of LFMHPs. According to previous studies about LFMHPs, the hot-injection method is usually used for the synthesis of NCs96 and QDs,70 because this method can obtain more evenly distributed products. However, there is often solvent residue on the surface of the products, which has an impact on the performance of the prepared products.94 Therefore, improving the solubility of the solvent and exploring new good solvents are required to optimize the hot-injection method.
Solvent thermal method
A hydrothermal method refers to a chemical reaction in a sealed pressure vessel with water as the solvent under high temperature and pressure conditions. According to different reaction types, it can be divided into hydrothermal oxidation, reduction, precipitation, and crystallization. Among them, the hydrothermal crystallization method is the most commonly used, and its main principle is dissolution and recrystallization. Firstly, reactants are dissolved in a hydrothermal medium in a certain proportion, facilitating ionization in solution.64 Then, the strong convection of the autoclaves enables transport of these ions to the low-temperature area, forming a saturated solution and crystallization of the precipitate. In terms of the solubility of various LFMHPs, the solvothermal method is beneficial for the preparation of LFMHPs with different structures.97 Wang et al. prepared Bi-doped Cs2Ag1−xNaxInCl6 using the hydrothermal method,14 in which a mixture of InCl3, CsCl, AgCl, and a small amount of BiCl3 were dissolved in hydrochloric acid solution, as shown in Fig. 6e. The solution was sealed in PTFE bottles and heated in an oven to 180 °C for 12 hours, and then cooled to 50 °C. It was found that the highest PLQY of 86.91% in Bi-doped Cs2Ag1−xNaxInCl6 was obtained when x = 0.8, and its PL spectrum was in the range of 400–780 nm. Jiang et al. also prepared Cs2NaBiCl6 (ref. 98) single crystals by the hydrothermal method, and studied the relationship between the optical response and phase transition of Cs2NaBiCl6 under high pressure. Li et al. used the solvothermal method to synthesize all-inorganic lead-free perovskite Cs2Zr1−xTexCl6 (x = 0–0.3) microcrystals (MCs).80 The prepared pure Cs2ZrCl6 MCs showed blue-light emission at 460 nm, while Cs2Zr1−xTexCl6 perovskite MCs doped with Fe4+ showed bright yellow-light emission with a PLQY up to 79.46% at room temperature.The solvothermal method can not only be used with different solvents to obtain products with different structures, but also exhibits the advantages of simple processability, low-energy consumption, and controllable particle shapes.99 However, the product yields of LFMHPs prepared from this method are low and their purities are not high enough.100
Other methods
In addition to the methods described above, there are many other ways to prepare LFMHPs. Zhu et al. used a modified ligand-assisted recrystallization method61 to dope Sm3+ ions into Cs3Bi2Br9 QDs, which then exhibited effective white-light emission. Two new crystalline hybrid indium chloride materials [DAPEDA]InCl6·Cl·H2O and [DPA]3InCl6 (DAPEDA = C8H22N4, DPA = C6H15N) were prepared by Sun et al. using a facile wet-chemistry method, as shown in Fig. 6f,92 and they exhibited strong broadband green-light emission with PLQY values of 40% and 34%, respectively. Zang et al. also used a solid mechanical grinding method to prepare 0D hybrid copper halide (TMA)3Cu2Br5−xClx powders, which possess strong broadband emission and a PLQY up to 75%, as well as good stability to heat and air.7 Wang et al. successfully prepared a pair of lead-free manganese halide enantiomers (R/S-1-PPA)2MnBr4 (R/S-1-PPA = R/S-1-phenylpropane-1-amine) using a solution method, as shown in Fig. 6g.93 However, its PLQY dropped sharply when the film was prepared.101 Therefore, the preparation technology of LFMHPs needs to be further improved.WLEDs based on LFMHPs
In solid-state lighting, the white light of ideal light sources should be continuous and stable, and the chromaticity coordinate of ideal white light is (0.33, 0.33). Researchers often use a correlated color temperature (CCT), color rendering index (CRI), energy conversion efficiency (ECE), and lifetime to evaluate the luminous quality of white light. Various CCT values of light sources can cause different feelings to humans. CCT of warm white light is about 2700 K, neutral white light is about 4000 K, and when the CCT is above 5000 K, it is known as cold white light.105 The CRI ranges from 0 to 100, and white light with a higher CRI enables the true color of objects to be illustrated.106 ECE(η) represents the energy conversion efficiency in lumens/watts and can be expressed by the formula: η = Φv/P1, where Φv is the total luminous flux (the energy radiated per unit of time from the source at the visible wavelength) and P1 is the total lamp power input or radiant power emission.107 The lifetime is defined as the time it takes for the brightness to decline to a specific percentage of its initial value.108 Directly mixing lead-based perovskites does not lead to emission of white light due to an anion exchange reaction. Therefore, by mixing LFMHPs with other emitting materials, a reasonable device structure can be designed and fabricated to emit white light.109 The white light produced by LFMHPs can be divided into two forms: (1) multiple LED chips or emitters of different colors are connected, and perovskite electroluminescence (EL) enables ideal white light through voltage and current excitation; (2) the LFMHPs can be activated by blue or UV chips to emit high-performance white light through photoluminescence (PL). In this section, a brief description on the development of WLEDs based on LFMHPs from these two aspects is given.Electroluminescent (EL) WLEDs based on LFMHPs
The main components of electroluminescent devices are luminescent layers; electron transport layers (ETLs), and hole transport layers (HTLs), as well as upper and lower electrodes. The two kinds of carrier recombination processes of electroluminescent devices are as follows: the first one is that electrons and holes are injected into the luminescent layer under a driving voltage, forming excitons to emit photons through radiation recombination. The second type is Förster resonance energy transfer, in which electrons or holes are not confined in the luminescent material layers, but in hole transport layers or electron transport layers, and then transferred to the luminescent layers through Förster resonance. Composite emission of photons then occurs in the luminescent layers. These two carrier recombination processes can exist simultaneously and have no influence on each other. Key indicators to evaluate an electroluminescent LED are the external quantum efficiency, current efficiency and opening voltage.In 2018, Luo et al. reported a lead-free double perovskite Cs2(Ag0.60Na0.40)InCl6 film that showed a high PLQY of 86%. Due to the existence of STEs, one-component warm white-light emission can be realized in Cs2(Ag0.60Na0.40)InCl6. Fig. 7a shows the structure of the fabricated EL device, in which carrier injection and transport can be regulated in carrier transporting layers.16 Under driving voltages, the EL device exhibits broadband emission, demonstrating a peak current efficiency of 0.11 cd A−1 (Fig. 7b).16 To improve the quality of white light based on lead-free double perovskites, Chen et al. doped Tb3+ ions into the Cs2Na0.4Ag0.6In0.97Bi0.03Cl6 double perovskite (TDP) thin film via a solution method and the Tb3+ dopants were proven to enhance the restructuring of STEs in the TDP. In addition, TPD shows effective injection and carrier recombination abilities. Therefore, a WLED with TDP/host and green carbon dots (G-CDs) as emission layers was fabricated, and its structure is shown in Fig. 7c. It was found that the WLED exhibited excellent white-light emission performance, including a color coordinate of (0.328, 0.329), a CRI as high as 87.7, a CCT value of 5702 K, an EQE of 0.695%, and a high brightness of 3163 cd m−2.19 In addition, Liu's team reported a one-component warm WLED based on an ultra-wideband MA2CuCl3 emitter with a visible light range of 400 to 800 nm, and the prepared electroluminescent WLED is shown in Fig. 7d.102 The valence band position of the hole injection layer (HIL) was reduced by incorporation of 20% 4,4′-bis(carbazol-9-yl) biphenyl (CBP) into PVK (poly(9-vinylcarbazole)), resulting in significant white-light emission at a bias voltage of 7 to 13 V. Fig. 7e gives the current–volt–brightness (I–V–L) characteristics of the MA2CuCl3 LED, and the inset shows a photograph of the LED driven at a 10 V bias. The required on-voltage of the LED is about 6 V, the maximum brightness is 54 cd m−2, and the maximum EQE is 0.035% (Fig. 7f).
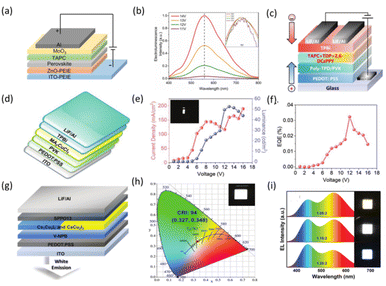 | ||
| Fig. 7 (a) The electroluminescent device structure.16 Copyright 2018 Springer Nature Limited. (b) Electroluminescence spectra at an applied voltage of 11 V, 12 V, 13 V and 14 V, respectively. The inset is the normalized spectra.16 Copyright 2018 Springer Nature. (c) Schematic illustration of white light-emitting diode (WLED) device structure.19 Copyright 2023 Wiley-VCH. (d) Device structure of MA2CuCl3 thin-film-based LEDs.102 Copyright 2022 Wiley-VCH. (e) I–V–L characteristics of MA2CuCl3 LED device.102 Inset: photograph of the LED driven by a 10 V bias. Copyright 2022 Wiley-VCH. (f) EQE versus voltage of these devices.102 Copyright 2022 Wiley-VCH. (g) Schematic structure of WLEDs.103 Copyright 2020 Royal Society of Chemistry. (h) CIE chromaticity diagram (inset: photograph of the white device).103 Copyright 2020 Royal Society of Chemistry. (i) EL spectra of the devices fabricated with different CsI/CuI molar ratios. The insets of (i) show a series of white-light photographs of the devices from cold-white light to warm-white light.104 Copyright 2020 Wiley-VCH. | ||
However, the CRI values of reported single-component white emitters are generally small and not suitable for efficient white-lighting performance.51 Therefore, multi-component Cu-based perovskite WLEDs with STE characteristics have attracted the interest of researchers. Zhu et al. easily realized a controllable and reversible phase transition between Cs3Cu2I5 and CsCu2I3via solvent treatment, and achieved white-light emission. The electroluminescent device structure and CIE chromaticity diagram are shown in Fig. 7g and h, respectively. The on-voltage is only 2.9 V at 1 cd m−2, and the maximum current efficiency is 0.11 cd A−1. The EQE is 0.053%.103 At the same time, Shan et al. prepared a CsCu2I3@CsCu2I5 composite using different ratios of CsI and CuI, and the composite showed ideal white-light emission with cold and warm white-light tuning as shown in Fig. 7i. They produced electroluminescent WLEDs with a multi-layer structure of ITO/PEDOT:PSS/poly-TPD/PVK/CsCu2I3@Cs3Cu2I5/TPBi/LiF/Al, and the device schematic diagram is shown in Fig. 8a. They experimented with WLEDs by creating three devices with a Csl/Cul molar ratio of 1.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1.15
2, 1.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 1.25
2, and 1.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, respectively. As shown in Fig. 8b, the current density–voltage–brightness curves of the three devices showed that, above the initial voltage, the current density and brightness of the devices increased sharply, yielding a maximum luminance of 145 cd m−2 at 8.4 V for the WLED with the Csl/Cul molar ratio of 1.15
2, respectively. As shown in Fig. 8b, the current density–voltage–brightness curves of the three devices showed that, above the initial voltage, the current density and brightness of the devices increased sharply, yielding a maximum luminance of 145 cd m−2 at 8.4 V for the WLED with the Csl/Cul molar ratio of 1.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and an EQE value of 0.15%. It is worth mentioning that the CRI value obtained was as high as 91.6.104 However, the electronic properties of cesium copper iodides are disadvantageous for LEDs because of the large effective mass of the carriers,113 and poor charge transport due to the large band gap.114 They found that the photoelectronic properties of cesium copper iodides could be significantly enhanced by simply chemically adsorbing ether groups to the metal-halide surfaces as electron donors. Based on this strategy, Wang et al. fabricated WLEDs based on a prepared 0D Cs3Cu2I5 and 1D CsCu2I3 mixture, as shown in Fig. 8c.110 WLEDs exhibit broadband EL spectra with a CIE coordinate of (0.44, 0.53), and display luminance up to 1570 cd m−2 at a low voltage of 5.4 V. Perovskite quantum dots have been widely used in WLEDs because of their high quantum yields, adjustable band gap and simple preparation.111 In addition to white-light emitting perovskites as the emission layer of the device, perovskites that emit non-white light such as blue, red or green light can also be used as light-emitting layers. Zhang et al. prepared an electroluminescent WLED based on lead-free double perovskite Cs2AgIn0.9Bi0.1Cl6 quantum dots with red and purple dual-color emission. They chose PVK as the hole transport layer, and purple radiation of PVK effectively compensated for purple radiation of quantum dots, and luminous performance remained unchanged. The device structure is shown in Fig. 8d. The current density–voltage (J–V) and light–emitting voltage (L–V) curves of QD WLEDs are shown in Fig. 8e. The on-voltage (at 1 cd m−2) is 10 V, and the brightness value is 34.7 cd m−2 (15 V). As shown in Fig. 8f, the maximum current efficiency and EQE are 0.058 cd A−1 and 0.064%, respectively. Finally, they chose triphenylphosphine oxide (TPPO) to fill tiny gaps among the quantum dots, and increased the maximum brightness and EQE to 158 cd m−2 and 0.08%, respectively.111 Su et al. combined a successively stacked pure-red-light-emitting lead-free perovskite with a sky-blue-light-emitting organic p–i–n heterojunction to achieve coordinated white-light emission. The device structure is shown in Fig. 8g, where an ultra-thin undoped organic phosphorescent intermediate layer (FIrpic) is sandwiched between a p-type hole transport layer (p-HTL) (TAPC) and an n-type electron transport layer (n-ETL) (BOCzPh) to form a sky-blue-light glowing organic p–i–n heterojunction unit. They investigated different thicknesses of perovskite layers at 50, 40 and 30 nm in devices designated as W2, W4 and W5, respectively. It can be seen from Fig. 8h that the brightness gradually increases as the thickness of the perovskite layer decreases. As shown in Fig. 8i, although W5's maximum brightness climbed to 1162 cd m−2, its EQE peak decreased to 5.54%. By adjusting the thickness, the peak EQE of the final device is 7.35%, the brightness is 746 cd m−2, the CIE coordinate is (0.424, 0.363), and the CCT value is 2868 K.112 Similarly, other perovskites with high luminous efficiency and stable red-light emission can also be applied; for example, the red LED made by Lee's team can also be used to prepare white LEDs with this strategy.115 It is noteworthy that lead-free perovskites with other emitters enable electroluminescent emission using this strategy. Wang et al. utilized the efficient blue-light emission of Cs3Cu2I5 to realize white-light emission.79
2 and an EQE value of 0.15%. It is worth mentioning that the CRI value obtained was as high as 91.6.104 However, the electronic properties of cesium copper iodides are disadvantageous for LEDs because of the large effective mass of the carriers,113 and poor charge transport due to the large band gap.114 They found that the photoelectronic properties of cesium copper iodides could be significantly enhanced by simply chemically adsorbing ether groups to the metal-halide surfaces as electron donors. Based on this strategy, Wang et al. fabricated WLEDs based on a prepared 0D Cs3Cu2I5 and 1D CsCu2I3 mixture, as shown in Fig. 8c.110 WLEDs exhibit broadband EL spectra with a CIE coordinate of (0.44, 0.53), and display luminance up to 1570 cd m−2 at a low voltage of 5.4 V. Perovskite quantum dots have been widely used in WLEDs because of their high quantum yields, adjustable band gap and simple preparation.111 In addition to white-light emitting perovskites as the emission layer of the device, perovskites that emit non-white light such as blue, red or green light can also be used as light-emitting layers. Zhang et al. prepared an electroluminescent WLED based on lead-free double perovskite Cs2AgIn0.9Bi0.1Cl6 quantum dots with red and purple dual-color emission. They chose PVK as the hole transport layer, and purple radiation of PVK effectively compensated for purple radiation of quantum dots, and luminous performance remained unchanged. The device structure is shown in Fig. 8d. The current density–voltage (J–V) and light–emitting voltage (L–V) curves of QD WLEDs are shown in Fig. 8e. The on-voltage (at 1 cd m−2) is 10 V, and the brightness value is 34.7 cd m−2 (15 V). As shown in Fig. 8f, the maximum current efficiency and EQE are 0.058 cd A−1 and 0.064%, respectively. Finally, they chose triphenylphosphine oxide (TPPO) to fill tiny gaps among the quantum dots, and increased the maximum brightness and EQE to 158 cd m−2 and 0.08%, respectively.111 Su et al. combined a successively stacked pure-red-light-emitting lead-free perovskite with a sky-blue-light-emitting organic p–i–n heterojunction to achieve coordinated white-light emission. The device structure is shown in Fig. 8g, where an ultra-thin undoped organic phosphorescent intermediate layer (FIrpic) is sandwiched between a p-type hole transport layer (p-HTL) (TAPC) and an n-type electron transport layer (n-ETL) (BOCzPh) to form a sky-blue-light glowing organic p–i–n heterojunction unit. They investigated different thicknesses of perovskite layers at 50, 40 and 30 nm in devices designated as W2, W4 and W5, respectively. It can be seen from Fig. 8h that the brightness gradually increases as the thickness of the perovskite layer decreases. As shown in Fig. 8i, although W5's maximum brightness climbed to 1162 cd m−2, its EQE peak decreased to 5.54%. By adjusting the thickness, the peak EQE of the final device is 7.35%, the brightness is 746 cd m−2, the CIE coordinate is (0.424, 0.363), and the CCT value is 2868 K.112 Similarly, other perovskites with high luminous efficiency and stable red-light emission can also be applied; for example, the red LED made by Lee's team can also be used to prepare white LEDs with this strategy.115 It is noteworthy that lead-free perovskites with other emitters enable electroluminescent emission using this strategy. Wang et al. utilized the efficient blue-light emission of Cs3Cu2I5 to realize white-light emission.79
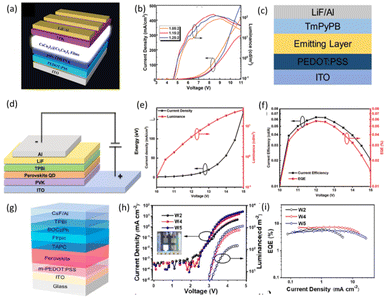 | ||
| Fig. 8 (a) Schematic structure of the electrically driven WLEDs based on CsCu2I3@Cs3Cu2I5 composite films.104 Copyright 2020 Wiley-VCH. (b) Current density–voltage–luminance curves of WLEDs with different CsI/CuI molar ratios.104 Copyright 2020 Wiley-VCH. (c) Schematic diagram of the WLED structure.110 Copyright 2021 Springer Nature. (d) Structure diagram of Cs2AgIn0.9Bi0.1Cl6 QDs-based electroluminescence WLEDs.111 (e) Dependence of the current density and luminance on the driving voltage.111 (f) Dependence of the current efficiency and EQE on the driving voltage.111 Copyright 2021 Wiley-VCH. (g) Schematic diagram of the device structure of the perovskite/organic hybrid white LEDs.112 (h) J–V–L (inset: photograph of working device W4) and (i) EQE–J characteristics of devices W2, W4, and W5.112 Copyright 2022 American Chemical Society. | ||
However, up to now, electroluminescent WLEDs based on lead-free environment-friendly metal halides are still at the initial stage, and the performance of the devices obtained is far less than that of WLEDs based on lead-based perovskites.117–119 Therefore, it is also necessary to improve the efficiencies of the electroluminescent WLEDs based on LFMHP materials to meet the requirements of various applications.
Photoluminescent (PL) WLEDs based on LFMHPs
It has been reported that a simple mixture of yellow and blue light facilitates white-light emission. In 2017, Ma et al. reported a series of lead-free organic-methane halides (C4N2H14X)4SnX6 (X = Br, I) and (C9NH20)2SbX5 (X = Cl) with 0D structures. The yellow-light-emitting (C4N2H14Br)4SnBr6 is mixed with commercial blue-light-emitting phosphors (BaMgAl10O17:Eu2+) in different ratios. The emission spectra for the mixtures with different ratios under UV irradiation are shown in Fig. 9a. The variety of CCT values implies that the white light can be adjusted from “cold” to “warm” (Fig. 9b). When the ratio of two emitters is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the CIE coordinates of the obtained white-light emission are (0.35, 0.39), and its CCT and CRI values are 4946 K and 70, respectively. Under different operating currents, the color stability of this white-light LED is very good, as shown in Fig. 9c.116 However, the low PLQYs of intrinsic LFMHPs still hinder their practical application in optoelectronic devices. Xia et al. doped Sb3+ ions into 0D-structure Cs2InCl5·H2O single crystals to achieve broadband yellow-light emission with a PLQY of up to 95.5%, as shown in Fig. 9d. They then prepared WLEDs with Cs2InCl5·H2O:Sb3+ and the blue-light-emitting BaMgAl10O17:Eu2+ phosphor in epoxy resin. Fig. 9e shows the PL spectrum of the WLED device at 20 mA current. As shown in Fig. 9f, the CIE coordinate of the fabricated WLED is (0.3576, 0.3547), corresponding to a CRI of 86, and a CCT value of 4556 K.34 However, due to the lack of red and orange emissions, WLEDs with yellow phosphors combined with blue chips usually emit cold white light. Therefore, Li et al. doped Mn2+ into yellow-light-emitting (C8H17NH2)2SnBr4 to enable orange-red-light emission, and combined prepared phosphors with commercial blue phosphors to manufacture WLEDs. As shown in Fig. 9g, h and i, the color coordinates of the WLEDs based on doped metal halides are located in the warmer color region, and they exhibited an increased CRI of 88.12 and a CCT of 3542 K.57
1, the CIE coordinates of the obtained white-light emission are (0.35, 0.39), and its CCT and CRI values are 4946 K and 70, respectively. Under different operating currents, the color stability of this white-light LED is very good, as shown in Fig. 9c.116 However, the low PLQYs of intrinsic LFMHPs still hinder their practical application in optoelectronic devices. Xia et al. doped Sb3+ ions into 0D-structure Cs2InCl5·H2O single crystals to achieve broadband yellow-light emission with a PLQY of up to 95.5%, as shown in Fig. 9d. They then prepared WLEDs with Cs2InCl5·H2O:Sb3+ and the blue-light-emitting BaMgAl10O17:Eu2+ phosphor in epoxy resin. Fig. 9e shows the PL spectrum of the WLED device at 20 mA current. As shown in Fig. 9f, the CIE coordinate of the fabricated WLED is (0.3576, 0.3547), corresponding to a CRI of 86, and a CCT value of 4556 K.34 However, due to the lack of red and orange emissions, WLEDs with yellow phosphors combined with blue chips usually emit cold white light. Therefore, Li et al. doped Mn2+ into yellow-light-emitting (C8H17NH2)2SnBr4 to enable orange-red-light emission, and combined prepared phosphors with commercial blue phosphors to manufacture WLEDs. As shown in Fig. 9g, h and i, the color coordinates of the WLEDs based on doped metal halides are located in the warmer color region, and they exhibited an increased CRI of 88.12 and a CCT of 3542 K.57
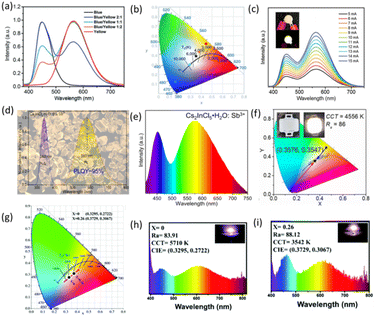 | ||
| Fig. 9 (a) Emission spectra of UV-pumped LEDs with different blending ratios of blue and yellow phosphors.116 Copyright 2017 Royal Society of Chemistry. (b) CIE coordinates and CCTs for UV-pumped LEDs plotted on the CIE 1931 chromaticity chart.116 Copyright 2017 Royal Society of Chemistry. (c) Emission spectra of a white LED at different driving currents; insets show the operating device.116 Copyright 2017 Royal Society of Chemistry. (d) PLE (with monitoring wavelength at 580 nm) and PL (with excitation wavelength at 340 nm) spectra of Cs2InCl5·H2O:5% Sb3+.34 Copyright 2020 American Chemical Society. (e) PL spectra of the UV-pumped WLED based on Cs2InCl5·H2O:Sb3+ at 20 mA drive current.34 Copyright 2020 American Chemical Society. (f) CIE coordinates and CCTs for the UV pumped LEDs plotted on the CIE1931 chromaticity chart.34 Copyright 2020 American Chemical Society. (g) Color coordinates and (h) image and emission spectrum of a white LED fabricated by (C8H17NH2)2Sn1−xMnxBr4 (x = 0) phosphors combined with BAM commercial blue powder driven by a UV chip (λem = 365 nm).57 Copyright 2022 Royal Society of Chemistry. (i) Image and emission spectrum of a white LED fabricated by (C8H17NH2)2Sn1−xMnxBr4 (x = 0.26) phosphors combined with BAM commercial blue powder driven by a UV chip (λem = 365 nm).57 Copyright 2022 Royal Society of Chemistry. | ||
Additionally, luminescent WLEDs can be fabricated with the assistance of mixed red, green and blue emitters. High-efficiency blue-light-emitting lead-free perovskite phosphors are firstly used to make WLEDs. Jing et al. synthesized [H2AMPd]ZnBr4·H2O with strong blue-light emission and mixed it with commercial (Sr, Ca)AlSiN3:Eu as a red phosphor to prepare WLEDs, which displayed bright white light, as shown in Fig. 10a and b, with a CCT of 5273 K, CIE chrominance coordinates of (0.33, 0.33), and a CRI of 94.5.120 Hu et al. doped Bi3+ into Cs2ZrCl6 to make it emit blue light and they found that Cs2ZrCl6:Bi3+ has a strong water-resistant core–shell structure and durable luminescence characteristics. After two hours of immersion in water, the luminescence intensity even increased to 115.94% of the initial level. Mixed with green and red commercial phosphors on UV chips, as shown in Fig. 10c, the WLEDs demonstrated CIE coordinates of (0.37, 0.35) and a CCT of 4179 K.121
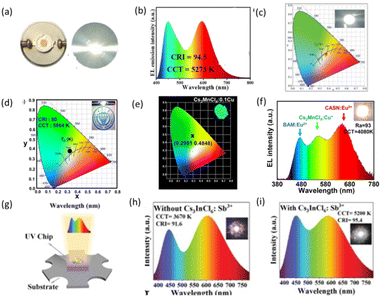 | ||
| Fig. 10 (a) Photograph of obtained white LED.120 Copyright 2023 Elsevier Ltd. (b) EL emission spectrum under 120 mA drive current.120 Copyright 2023 Elsevier Ltd. (c) CIE color coordinates of the WLED device is denoted by the red star. The inset is a photo of the WLED device in operation.121 Copyright 2020 WILEY-VCH. (d) A CIE chromaticity diagram of the WLEDs, with the inset showing a photograph of a WLED. Copyright Elsevier B.V. (e) Chromaticity coordinates (1931) of Cs2MnCl4:0.1Cu+.122 (f) Electroluminescence spectrum of the WLED fabricated using a 310 nm chip and BaMgAl10O17:Eu2+, CaAlSiN3:Eu2+ and Cs2MnCl4:0.1Cu+ phosphors (the inset shows the photograph of the as-packaged LED lighting).122 Copyright 2023 Royal Society of Chemistry. (g) Schematic configuration of the WLED device fabricated from a 317 nm chip encapsulated with a mixture of phosphors consisting of Cs2NaInCl6:Sb3+, Cs3InCl6:Sb3+, and Cs2InCl5(H2O):Sb3+.123 (h) Electroluminescence spectrum of a WLED based on Cs2NaInCl6:Sb3+ and Cs2InCl5(H2O):Sb3+ pumped by 317 nm chip.123 (i) EL spectrum of a WLED based on Cs2NaInCl6:Sb3+, Cs2InCl5(H2O):Sb3+, and Cs3InCl6:Sb3+ pumped by a 317 nm chip. The inset shows the corresponding EL photograph of the WLEDs.123 Copyright 2023 Wiley-VCH GmbH. | ||
In addition, lead-free perovskites with green-light luminescence have also been developed. Zang et al. mixed green (TBA)2MnBr4 single crystals with commercial red CaAlSiN3:Eu2+ phosphors (630 nm) and blue InGaN chips (450 nm) to fabricate WLEDs. As shown in Fig. 10d, their CCT and CRI values were 5864 K and 80, respectively.84 In order to further enhance the green luminescence, Chen et al. doped Cu+ into Cs2MnCl4, whose CIE coordinates are displayed in Fig. 10e, and prepared WLEDs with a coating of blue BAM:Eu2+ and red CASN:Eu2+ phosphors. As shown in Fig. 10f, the prepared WLEDs have a high CRI of 93 and a low CCT of 4080 K.122 More interestingly, Jiang et al. doped Sb3+ ions into the In-based lead-free halides of Cs2InCl5(H2O):Sb3+: yellow, Cs3InCl6:Sb3+: green, and Cs2NaInCl6:Sb3+: blue. Three-color phosphors were mixed to fabricate WLEDs, as shown in Fig. 10g. It was seen from a comparison of Fig. 10h and i that the WLEDs with Cs3InCl6:Sb3+ have a higher CRI of 95.4.123 This has greatly promoted the development of lead-free halide emitters, providing new possibilities for manufacture of high-color rendering WLEDs.
Furthermore, the mergence of WLEDs based on single-component LFMHPs can solve some issues of multi-component metal-halide WLEDs.127–133 In 2021, He Shao et al. prepared lead-free 0D K3SbCl6 perovskites with a blue-light emission center at 440 nm and a PLQY of 22.3%.74 By introducing Mn2+ ions into 0D K3SbCl6 perovskites, the white light was realized by controlling the doping concentration of Mn2+ ions. As shown in Fig. 11a, the energy transition from the 4T1–6A1 levels to the Mn2+ states is proved, which is due to the deformation of the lattices with the increase of Mn2+ ion concentration.134 In Mn2+-K3SbCl6 NCs, STEs in the perovskites together with the intrinsic transition of the dopant Mn2+ ions results in white-light emission. When the doping concentration of Mn2+ ions is 3.7%, 4.2% and 5.8%, the white-light luminescence CIE coordinates of the Mn2+-K3SbCl6 NCs were (0.29, 0.29), (0.34, 0.31) and (0.33, 0.30), respectively. In addition, as shown in Fig. 11b, they found that upon further increasing the doping concentration of Mn2+ ions, the relative emission intensity of the STEs for the intrinsic transition of Mn2+ ions continuously decreased due to the concentration quenching of dopants.74 The intrinsic lead-free perovskites were found to exhibit extra spectral ranges induced by incorporation of dopants, and single-component white-light emission can also be realized. Xie et al. synthesized (C8NH12)6InBr9·H2O, which showed dual-band emission including strong cyan-light emission and a weak red-light emission tail (Fig. 11c).124 The doping with Sb3+ ions greatly enhanced the low-energy ultra-broadband red-light emission tail, optimized the band gap structure, and promoted red-light STE emission (Fig. 11d), resulting in single-component warm white-light emission with a CIE coordinates of (0.400, 0.361), a CCT of 3347 K, and a CRI of 84 at the Sb concentration of 0.1%.48 Although a 35% PLQY was obtained when the Sb3+ concentration was 5% (Fig. 11e), it cannot emit white light efficiently, indicating that the doping concentration may affect the efficiency of white-light emission. Pan et al. doped Sm3+ ions into 2D CsBi2Br9 QDs, which not only enhanced the PLQY from 10.9% to 20.8% (Fig. 11f), but also led to emission of tunable warm white light. The optimized white-light emission possesses CIE coordinates of (0.296, 0.289) and a CCT of 8967 K, as well as better water resistance stability.61 Wang et al. grew 0D white-light-emitting (C4H16N3)InBr6 (C4H13N3(DETA) = diethylenetriamine) LFMHPs through a simple mechanochemical method. The blue-light emission band at 400 nm and the yellow-light emission band at 550 nm are coupled together to produce cold-white-light emission with a PLQY of 1.4%. This phenomenon was caused by the radiative recombination of free excitons and STEs. The CCT and CRI of (DETA)InBr6 are 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 059 K and 91, respectively. Its CIE coordinates are (0.26, 0.26), corresponding to cold-white-light emission (Fig. 11g).125 In order to achieve highly tunable emission, they doped with Sb3+ ions to regulate the photoluminescence. After Sb3+ doping, the band-edge absorption was significantly enhanced in the range of 350–430 nm. Moreover, the doping amount of Sb3+ had a great influence on the PLQYs, and a maximum PLQY of 24.12% was achieved in (DETA)InBr6:1.5% Sb3+ (Fig. 11h). It can be seen that with the increase of Sb3+ concentration, the increase of luminescent centers as the energy receptors are transferred from the exciton band led to an enhancement of photoluminescence intensity. Li et al.97 reported 0D (TTA)2SbCl5 (TTA = tetraethyl ammonium) with dual-band emission under high-energy photon excitation (Fig. 11i and 12a). It can be seen that under 300 nm excitation, dual-band emission and an additional blue-light emission band in (TTA)2SbCl5 enabled single-component white-light emission with a PLQY of 68%, which was much higher than that of other low-dimensional hybrid LFMHPs.97 WLEDs based on (TTA)2SbCl5 exhibited a CCT of 2360 K and a CRI of 84. In addition, their excellent water resistance and thermal stability was proved, as shown in Fig. 12b.126 Double perovskites with broadband white-light emission, high absorption coefficients and fast carrier recombination rates are also of interest to researchers.136 Cs2NaInCl6 is a representative cubic double perovskite, which is similar to classic lead halide perovskites, as shown in Fig. 12c. However, due to a forbidden transition in Cs2NaInCl6, its PLQY is very low. To enhance its optoelectronic properties, Liu et al. synthesized millimeter-scale Cs2NaInCl6 single crystals and co-doped Sb–Mn into them. The co-doped Cs2NaInCl6 was found to emit warm white light with an enhanced PLQY of 84%, as shown in Fig. 12d.135 However, blue light with a wavelength below 450 nm is harmful to human bodies and is not suitable for long-term indoor lighting. To solve this problem, Chen et al. doped Sb3+ ions into Cs2NaInCl6−xBrx double perovskites and optimized the halogen ratios. With the increase of Br/Cl ratios, the emission spectra continuously red-shifted from 445 nm (blue light) to 480 nm (cyan light), indicating that they could be employed in WLEDs.137
059 K and 91, respectively. Its CIE coordinates are (0.26, 0.26), corresponding to cold-white-light emission (Fig. 11g).125 In order to achieve highly tunable emission, they doped with Sb3+ ions to regulate the photoluminescence. After Sb3+ doping, the band-edge absorption was significantly enhanced in the range of 350–430 nm. Moreover, the doping amount of Sb3+ had a great influence on the PLQYs, and a maximum PLQY of 24.12% was achieved in (DETA)InBr6:1.5% Sb3+ (Fig. 11h). It can be seen that with the increase of Sb3+ concentration, the increase of luminescent centers as the energy receptors are transferred from the exciton band led to an enhancement of photoluminescence intensity. Li et al.97 reported 0D (TTA)2SbCl5 (TTA = tetraethyl ammonium) with dual-band emission under high-energy photon excitation (Fig. 11i and 12a). It can be seen that under 300 nm excitation, dual-band emission and an additional blue-light emission band in (TTA)2SbCl5 enabled single-component white-light emission with a PLQY of 68%, which was much higher than that of other low-dimensional hybrid LFMHPs.97 WLEDs based on (TTA)2SbCl5 exhibited a CCT of 2360 K and a CRI of 84. In addition, their excellent water resistance and thermal stability was proved, as shown in Fig. 12b.126 Double perovskites with broadband white-light emission, high absorption coefficients and fast carrier recombination rates are also of interest to researchers.136 Cs2NaInCl6 is a representative cubic double perovskite, which is similar to classic lead halide perovskites, as shown in Fig. 12c. However, due to a forbidden transition in Cs2NaInCl6, its PLQY is very low. To enhance its optoelectronic properties, Liu et al. synthesized millimeter-scale Cs2NaInCl6 single crystals and co-doped Sb–Mn into them. The co-doped Cs2NaInCl6 was found to emit warm white light with an enhanced PLQY of 84%, as shown in Fig. 12d.135 However, blue light with a wavelength below 450 nm is harmful to human bodies and is not suitable for long-term indoor lighting. To solve this problem, Chen et al. doped Sb3+ ions into Cs2NaInCl6−xBrx double perovskites and optimized the halogen ratios. With the increase of Br/Cl ratios, the emission spectra continuously red-shifted from 445 nm (blue light) to 480 nm (cyan light), indicating that they could be employed in WLEDs.137
 | ||
| Fig. 11 (a) The proposed PL mechanism for the Mn2+-K3SbCl6 NCs.74 Copyright 2020 Elsevier B.V. (b) PL emission spectra for Mn2+-K3SbCl6 NCs with different doping concentrations under excitation of 365 nm.74 Copyright 2020 Elsevier B.V. (c) Absorption, PLE, and PL spectra of (C8NH12)6InBr9·H2O.124 Copyright 2020 American Chemical Society. (d) Absorption, PLE, and PL spectra of (C8NH12)6InBr9·H2O:5%Sb sample.124 Copyright 2020 American Chemical Society. (e) PLQE variation vs. different Sb doping contents upon 365 nm excitation.124 Copyright 2020 American Chemical Society. (f) PLQYs of exciton emission versus Sm3+ doping concentrations in Cs3Bi2Br9 PQDs.61 Copyright 2020 Chinese Society of Rare Earths. (g) PLE and PL spectra of (DETA)InBr6 (λex = 353 nm) and (DETA)Br3.125 Copyright 2023 American Chemical Society. (h) PLQYs of (DETA)InBr6:1.5%Sb3+.125 Copyright 2023 American Chemical Society. (i) PLE and PL spectra of (TTA)2SbCl5.126 Copyright 2019 American Chemical Society. | ||
 | ||
| Fig. 12 (a) Photographs of (TTA)2SbCl5 powder under ambient light (top) and 365 nm UV light (bottom).126 Copyright 2019 American Chemical Society. (b) The PL spectra of (TTA)2SbCl5 in the range of 250–375 K.126 Copyright 2019 American Chemical Society. (c) Crystal structure of Cs2NaInCl6.135 (d) Normalized PL spectra of Cs2NaInCl6 NCs with different doping ratios.135 (e) Optical absorption (solid lines) and PL (dashed lines) spectra of intrinsic Cs2AgInCl6 and Cs2Ag0.60Na0.40InCl6.16 Copyright 2018 Springer Nature. (f) Photoluminescence stability of Cs2Ag0.60Na0.40InCl6 against continuous heating at 150 °C on a hotplate, measured after cooling to room temperature.16 Copyright 2018 Springer Nature. | ||
Furthermore, Tang et al. prepared Cs2(Ag0.60Na0.40)InCl6 by alloying Na+ ions into Cs2AgInCl6, and then observed white-light emission by doping with 0.04% Bi ions. After Na alloying, an obvious exciton absorption peak appeared near 365 nm. Fig. 12e shows that the alloyed perovskites exhibited 1000 times higher emission intensity than intrinsic Cs2AgInCl6, and the highest PLQY of (86 ± 5) % was obtained when the content of Na was about 40%. The powders were directly coated onto commercial UV-LEDs to fabricate WLEDs, which showed CIE coordinates of (0.396, 0.448) and a CCT of 4054 K. When the WLEDs were operated in air for more than 1000 hours at 5000 cd m−2, their luminous performance hardly changed, as shown in Fig. 12f, showing their good operating stability.16 For a PL WLED, stability is crucial, and Table 1 summarizes stability details of reported WLEDs against light, air and water, implying the effect of emitters on the stability of WLEDs.
| Materials | Color | CIE (x, y) | CCT (K) | CRI | WLED stability | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Light | Air | Water | ||||||
| a T n : required time for luminescence intensity to decrease to n% of the initial values in WLEDs. | ||||||||
| (C4N2H14Br)4SnBr6 | Yellow | (0.35, 0.39) | 4946 | 70 | — | T 98 = 6 h | — | 116 |
| [Na(DMSO)2]3SbBr6 | Yellow | (0.36, 0.28) | 3603 | 85 | T 98 = 120 h@440 nm | T 57 = 130 h | — | 71 |
| OTA2+xSnI4+x | Yellow | (0.45, 0.38) | 3300 | 92 | T 98 = 25 min@380 nm | — | — | 63 |
| CsMnBr3 | Red | (0.33, 0.36) | 5342 | 94 | — | — | T 95 = 90 days | 138 |
| [H2AMPd]ZnBr4·H2O | Blue | (0.33, 0.33) | 5273 | 94.5 | — | T 99 = 8 h | — | 120 |
| Cs2ZrCl6:Bi3+ | Blue | (0.37, 0.35) | 4486 | 81.9 | T 99 = 24 h@350 nm | — | T 99 = 2 h | 121 |
| [DAPEDA]InCl6·Cl·H2O:Sb3+ | Green | (0.33, 0.34) | 5669 | 93.2 | T 90 = 240 min@450 nm | — | — | 92 |
| [DPA]3InCl6:Sb3+ | Green | (0.34, 0.35) | 5864 | 80 | — | T 50 = 240 h | — | 84 |
| Cs2MnCl4:Eu2+ | White | (0.33, 0.27) | — | 90 | — | T 90 = 20 h | — | 21 |
| K3SbCl6:Mn2+ | White | (0.29, 0.28) | 8173 | >80 | T 50 = 12 h@365 nm | — | — | 74 |
| K3SbCl6:Mn2+ | White | (0.32, 0.30) | 5068 | >80 | — | — | — | 74 |
| K3SbCl6:Mn2+ | White | (0.35, 0.30) | 4779 | >80 | — | — | — | 74 |
| Cs3Cu2Cl5@CsCu2Cl3 | White | (0.34, 0.34) | 5285 | 94 | — | T 64 = 60 h | — | 77 |
| Cs3Cu2Cl5@CsCu2Cl3 | White | (0.36, 0.37) | 4516 | 89 | — | T 98 = 120 h | — | 139 |
| Cs2Ag0.6Na0.4InCl6:30% Bi | White | — | 6585 | — | — | T 95 = 10 h | T 90 = 10 h | 140 |
| Cs2Ag0.6Na0.4InCl6:0.05% Bi | White | (0.40, 0.45) | 4054 | — | — | T 92 = 1000 h | — | 16 |
| Cs2Ag0.4Na0.6In0.9Bi0.1Cl:Ho3+/Yb3+ | White | (0.34, 0.36) | — | — | — | T 89 = 5 h | — | 141 |
Challenges and perspectives
In recent years, LFMHPs have been widely utilized in solar cells, light-emitting diodes, lasers and photodetectors due to their excellent optoelectronic characteristics and properties. Importantly, the attractive luminescence performance of LFMHPs enables them to be used as emitting sources of WLEDs, facilitating potential applications in display and lighting. Therefore, researchers have paid more attention to the structures and emission mechanisms of LFMHPs, as well as optimization of WLED performance. In this work, we have reviewed structures of LFMHPs with different dimensions and common preparation methods. We focused on the principles of photoluminescent and electroluminescent WLEDs based on LFMHPs, and summarized and analyzed their performance. However, WLEDs based on LFMHPs face several problems and challenges, which may impede their further enhancement of performance and limit their potential applications. The current challenges and perspectives of WLEDs based on LFMHPs are summarized and highlighted as follows:Light-emitting devices based on LFMHPs have been the subject of numerous studies and improvements in the performance optimization of environmentally-friendly WLEDs has been shown. LFMHPs possess high fluorescence efficiencies, narrow luminescence peaks, and adjustable emission spectra covering the entire visible range, but there are still challenges with regard to their high-efficiency photoluminescence and electroluminescence operation. For photoexcited WLEDs, their luminous efficiencies often depend on the output power of the excitation chips and the PLQYs of the LFMHPs. However, the luminous efficiencies of the photoluminescent WLEDs are far below those of commercial sources. In addition, LFMHPs exhibit strong absorption in the ultraviolet and deep ultraviolet regions, however, short-wavelength chips (below 365 nm) used as a common excitation source for WLEDs have lower output power than blue chips, resulting in a large loss of efficiency. Therefore, enhancement of PLQYs in LFMHPs under UV excitation enables the efficiencies of WLEDs based on LFMHPs to be increased. By optimizing and minimizing the lattice deformation energy and self-trapping energy of LFMHPs, the structure of the LFMHPs can be tuned and their defects suppressed, thereby improving their PLQYs. According to previous reports, ion doping is considered to be an effective strategy to regulate the band structures of LFMHPs and improve their luminescence performance. In addition, compared with multi-component WLEDs, fabrication of single-component WLEDs simplifies the preparation processes and can decrease the deterioration of luminous efficiencies caused by the mismatch of LFMHPs.
Apart from high luminous efficiencies, excellent stabilities of WLEDs are also required. It has been reported that the operating lifetime of an LFMHP-based photoluminescent WLED is below 1000 hours, which cannot meet the requirements of >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours for commercial lighting applications. During long-term operation of a WLED, heat is generated, and with the increase of temperature, this may lead to disintegration of the LFMHPs, which deteriorates the performance of the WLED. In addition, some LFMHPs are found to decompose when exposed to water, so it is very important to explore and optimize LFMHPs with excellent heat and water resistance. Therefore, further research on the degradation mechanism of LFMHPs is needed, and various modification and encapsulation technologies should continue to be explored. Researchers have proposed doping and coating strategies to protect LFMHPs from heat and water, thereby improving the working performance of WLEDs. Additionally, good surface ligands can be introduced onto LFMHP NCs to reduce their sensitivity to heat and water. In summary, research of WLEDs based on LFMHPs has made remarkable progress in materials development, device design and performance improvement, but there are still many challenges, such as improving electroluminescence efficiencies and stabilities, as well as exploring materials synthesis and luminescence mechanisms. These studies are important for promoting the development of next-generation display and lighting technologies.
000 hours for commercial lighting applications. During long-term operation of a WLED, heat is generated, and with the increase of temperature, this may lead to disintegration of the LFMHPs, which deteriorates the performance of the WLED. In addition, some LFMHPs are found to decompose when exposed to water, so it is very important to explore and optimize LFMHPs with excellent heat and water resistance. Therefore, further research on the degradation mechanism of LFMHPs is needed, and various modification and encapsulation technologies should continue to be explored. Researchers have proposed doping and coating strategies to protect LFMHPs from heat and water, thereby improving the working performance of WLEDs. Additionally, good surface ligands can be introduced onto LFMHP NCs to reduce their sensitivity to heat and water. In summary, research of WLEDs based on LFMHPs has made remarkable progress in materials development, device design and performance improvement, but there are still many challenges, such as improving electroluminescence efficiencies and stabilities, as well as exploring materials synthesis and luminescence mechanisms. These studies are important for promoting the development of next-generation display and lighting technologies.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
Z. Z. and S. Z. directed this review. R. T. performed the literature search and organized the content. All authors collaborated on the data analysis and participated in writing the paper.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is funded by National Natural Science Foundation of China (No. 11974063); Young Elite Scientists Sponsorship Program by CAST (No. 2022QNR001).Notes and references
- E. F. Schubert and J. K. Kim, Science, 2005, 308, 1274–1278 CrossRef PubMed.
- H. Amano, M. Kito, K. Hiramatsu and I. Akasaki, Jpn. J. Appl. Phys., 1989, 28, L2112–L2114 CrossRef.
- S. Nakamura, T. Mukai and M. Senoh, Appl. Phys. Lett., 1994, 64, 1687–1689 CrossRef.
- B. M. Benin, D. N. Dirin, V. Morad, M. Worle, S. Yakunin, G. Raino, O. Nazarenko, M. Fischer, I. Infante and M. V. Kovalenko, Angew. Chem., Int. Ed., 2018, 57, 11329–11333 CrossRef PubMed.
- S. Zhao, J. Zhao, S. M. H. Qaid, D. Liang, K. An, W. Cai, Q. Qian and Z. Zang, Appl. Phys. Rev., 2024, 11, 011408 Search PubMed.
- S. Zhao, S. Jiang, W. Cai, R. Li, Q. Mo, B. Wang and Z. Zang, Cell Rep. Phys. Sci., 2021, 2, 100585 CrossRef.
- D. Liang, Z. Sun, S. Lu, J. Zhao, Y. Zhou, K. An and Z. Zang, Inorg. Chem., 2023, 62, 7296–7303 CrossRef.
- S. Wang, X. Han, T. Kou, Y. Zhou, Y. Liang, Z. Wu, J. Huang, T. Chang, C. Peng, Q. Wei and B. Zou, J. Mater. Chem. C, 2021, 9, 4895–4902 RSC.
- R. Fan, S. Fang, C. Liang, Z. Liang and H. Zhong, Photonics Res., 2021, 9, 694–700 CrossRef.
- A. Yan, K. Li, Y. Zhou, Y. Ye, X. Zhao and C. Liu, J. Alloys Compd., 2020, 822, 153528 CrossRef.
- H. Cho, Y. H. Kim, C. Wolf, H. D. Lee and T. W. Lee, Adv. Mater., 2018, 30, e1704587 CrossRef PubMed.
- C. Zhao, Y. Yu, J. Dai, Y. Zu and Z. Wu, Chin. Sci. Bull., 2021, 66, 2139–2150 CrossRef.
- F. Liu, C. Ding, Y. Zhang, T. Kamisaka, Q. Zhao, J. M. Luther, T. Toyoda, S. Hayase, T. Minemoto, K. Yoshino, B. Zhang, S. Dai, J. Jiang, S. Tao and Q. Shen, Chem. Mater., 2019, 31, 798–807 CrossRef CAS.
- X. Wang, X. Hong, B. Yang, Y. Chen and J. Zou, ECS J. Solid State Sci. Technol., 2021, 10, 065011 CrossRef CAS.
- X. Zhang, Z. Jin, J. Zhang, D. Bai, H. Bian, K. Wang, J. Sun, Q. Wang and S. F. Liu, ACS Appl. Mater. Interfaces, 2018, 10, 7145–7154 CrossRef CAS.
- J. Luo, X. Wang, S. Li, J. Liu, Y. Guo, G. Niu, L. Yao, Y. Fu, L. Gao, Q. Dong, C. Zhao, M. Leng, F. Ma, W. Liang, L. Wang, S. Jin, J. Han, L. Zhang, J. Etheridge, J. Wang, Y. Yan, E. H. Sargent and J. Tang, Nature, 2018, 563, 541–545 CrossRef CAS.
- R. Wang, H. Xiang, J. Chen, Y. Li, Y. Zhou, W. C. H. Choy, Z. Fan and H. Zeng, ACS Energy Lett., 2022, 7, 2173–2188 CrossRef CAS.
- S. Zhao, W. Cai, H. Wang, Z. Zang and J. Chen, Small Methods, 2021, 5, e2001308 CrossRef PubMed.
- S. Jin, H. Yuan, T. Pang, M. Zhang, J. Li, Y. Zheng, T. Wu, R. Zhang, Z. Wang and D. Chen, Adv. Mater., 2024, 36, e2308487 CrossRef.
- Q. Mo, Y. Shi, W. Cai, S. Zhao, Y. Ying and Z. Zang, J. Phys. D: Appl. Phys., 2022, 55, 333003 CrossRef CAS.
- J. Hou, J. Wu, Y. Fang, Y. Wei, L. Dong, G. Zhang, Y. Liu, H. Chen and G. Li, ACS Appl. Mater. Interfaces, 2024, 16, 30176–30184 CrossRef CAS.
- D. Liang, L. Tan, S. Lu, Z. Sun, H. Wang, W. Cai and Z. Zang, ACS Appl. Mater. Interfaces, 2023, 15, 24622–24628 CrossRef CAS.
- D. Liang, H. Xiao, W. Cai, S. Lu, S. Zhao, Z. Zang and L. Xie, Adv. Opt. Mater., 2023, 11, 2202997 CrossRef CAS.
- G. E. Eperon, S. D. Stranks, C. Menelaou, M. B. Johnston, L. M. Herz and H. J. Snaith, Energy Environ. Sci., 2014, 7, 982–988 RSC.
- Z. K. Tan, R. S. Moghaddam, M. L. Lai, P. Docampo, R. Higler, F. Deschler, M. Price, A. Sadhanala, L. M. Pazos, D. Credgington, F. Hanusch, T. Bein, H. J. Snaith and R. H. Friend, Nat. Nanotechnol., 2014, 9, 687–692 CrossRef CAS PubMed.
- Y. Hassan, J. H. Park, M. L. Crawford, A. Sadhanala, J. Lee, J. C. Sadighian, E. Mosconi, R. Shivanna, E. Radicchi, M. Jeong, C. Yang, H. Choi, S. H. Park, M. H. Song, F. De Angelis, C. Y. Wong, R. H. Friend, B. R. Lee and H. J. Snaith, Nature, 2021, 591, 72–77 CrossRef CAS.
- B. Zhao, S. Bai, V. Kim, R. Lamboll, R. Shivanna, F. Auras, J. M. Richter, L. Yang, L. Dai, M. Alsari, X.-J. She, L. Liang, J. Zhang, S. Lilliu, P. Gao, H. J. Snaith, J. Wang, N. C. Greenham, R. H. Friend and D. Di, Nat. Photonics, 2018, 12, 783–789 CrossRef CAS.
- K. Lin, J. Xing, L. N. Quan, F. P. G. de Arquer, X. Gong, J. Lu, L. Xie, W. Zhao, D. Zhang, C. Yan, W. Li, X. Liu, Y. Lu, J. Kirman, E. H. Sargent, Q. Xiong and Z. Wei, Nature, 2018, 562, 245–248 CrossRef PubMed.
- C. Chen, J. Xiang, Y. Chen, M. Jin, J. Zheng, N. Zhang and C. Guo, Ceram. Int., 2022, 48, 1851–1856 CrossRef.
- X. Zhang, H. Wang, S. Wang, Y. Hu, X. Liu, Z. Shi, V. L. Colvin, S. Wang, W. W. Yu and Y. Zhang, Inorg. Chem., 2020, 59, 533–538 CrossRef.
- G. Yang, S. Bai, X. Li, H. Liang, C. Li, J. Sun, Y. Wang, J. Huang, G. Pan and Y. Zhu, ACS Appl. Mater. Interfaces, 2023, 15, 24629–24637 CrossRef.
- J. Mao, D. Venugopal, Y. Zhang, P. Zhu and G. Wang, Chem. Eng. J., 2023, 470, 144160 CrossRef.
- X. Hao, H. Liu, W. Ding, F. Zhang, X. Li and S. Wang, J. Phys. Chem. Lett., 2022, 13, 4688–4694 CrossRef PubMed.
- Y. Jing, Y. Liu, X. Jiang, M. S. Molokeev, Z. Lin and Z. Xia, Chem. Mater., 2020, 32, 5327–5334 CrossRef.
- E. Cui, X. Yuan, L. Tang, L. Yang, X. Yang, X. Liao, J. Tang, Y. Zhao, W. Sun, K. Liu, Y. Liu and J. Liu, Appl. Surf. Sci., 2023, 609, 155472 CrossRef.
- M. Lyu, J. H. Yun, P. Chen, M. Hao and L. Wang, Adv. Energy Mater., 2017, 7, 1602512 CrossRef.
- S. Li, Z. Shi, F. Zhang, L. Wang, Z. Ma, D. Wu, D. Yang, X. Chen, Y. Tian, Y. Zhang, C. Shan and X. Li, ACS Appl. Mater. Interfaces, 2020, 12, 46330–46339 CrossRef.
- P. Han, C. Luo, S. Yang, Y. Yang, W. Deng and K. Han, Angew. Chem., Int. Ed., 2020, 59, 12709–12713 CrossRef PubMed.
- H. Peng, B. Zou, Y. Guo, Y. Xiao, R. Zhi, X. Fan, M. Zou and J. Wang, J. Mater. Chem. C, 2020, 8, 6488–6495 RSC.
- I. Borriello, G. Cantele and D. Ninno, Phys. Rev. B, 2008, 77, 235214 CrossRef.
- T. C. Jellicoe, J. M. Richter, H. F. J. Glass, M. Tabachnyk, R. Brady, S. E. Dutton, A. Rao, R. H. Friend, D. Credgington, N. C. Greenham and M. L. Böhm, J. Am. Chem. Soc., 2016, 138, 2941–2944 CrossRef.
- S. E. Creutz, E. N. Crites, M. C. De Siena and D. R. Gamelin, Nano Lett., 2018, 18, 1118–1123 CrossRef.
- L. Zhou, Y. F. Xu, B. X. Chen, D. B. Kuang and C. Y. Su, Small, 2018, 14, e1703762 CrossRef.
- Q. Liao, J. Chen, L. Zhou, T. Wei, L. Zhang, D. Chen, F. Huang, Q. Pang and J. Z. Zhang, J. Phys. Chem. Lett., 2020, 11, 8392–8398 CrossRef PubMed.
- Z. Li, F. Sun, H. Song, H. Zhou, Y. Zhou, Z. Yuan, P. Guo, G. Zhou, Q. Zhuang and X. Yu, Dalton Trans., 2021, 50, 9804–9811 RSC.
- M. D. Smith, B. A. Connor and H. I. Karunadasa, Chem. Rev., 2019, 119, 3104–3139 CrossRef.
- S. Ma, M. Cai, T. Cheng, X. Ding, X. Shi, A. Alsaedi, T. Hayat, Y. Ding, Z. a. Tan and S. Dai, Sci. China Mater., 2018, 61, 1257–1277 CrossRef.
- L. Mao, P. Guo, M. Kepenekian, I. Hadar, C. Katan, J. Even, R. D. Schaller, C. C. Stoumpos and M. G. Kanatzidis, J. Am. Chem. Soc., 2018, 140, 13078–13088 CrossRef PubMed.
- M. I. Saidaminov, O. F. Mohammed and O. M. Bakr, ACS Energy Lett., 2017, 2, 889–896 CrossRef.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nano Lett., 2015, 15, 3692–3696 CrossRef.
- Y. Wang, F. Zhang, J. Ma, Z. Ma, X. Chen, D. Wu, X. Li, Z. Shi and C. Shan, EcoMat, 2022, 4, e12160 CrossRef.
- B. Yang, J. Chen, S. Yang, F. Hong, L. Sun, P. Han, T. Pullerits, W. Deng and K. Han, Angew. Chem., Int. Ed., 2018, 57, 5359–5363 CrossRef.
- L. Li, H. Shao, X. Wu, W. Chen, J. Zhu, B. Dong, L. Xu, W. Xu, J. Hu, M. Zhou, Y. Ji, H. Song and X. Bai, Mater. Res. Bull., 2022, 147, 111645 CrossRef.
- F. Locardi, M. Cirignano, D. Baranov, Z. Dang, M. Prato, F. Drago, M. Ferretti, V. Pinchetti, M. Fanciulli, S. Brovelli, L. De Trizio and L. Manna, J. Am. Chem. Soc., 2018, 140, 12989–12995 CrossRef.
- K. G. Lim, H. B. Kim, J. Jeong, H. Kim, J. Y. Kim and T. W. Lee, Adv. Mater., 2014, 26, 6461–6466 CrossRef PubMed.
- L. Hou, Y. Zhu, J. Zhu and C. Li, J. Phys. Chem. C, 2019, 123, 31279–31285 CrossRef CAS.
- L. Hou, Y. Zhu, J. Zhu, Y. Gong and C. Li, J. Mater. Chem. C, 2020, 8, 8502–8506 RSC.
- M. Leng, Y. Yang, K. Zeng, Z. Chen, Z. Tan, S. Li, J. Li, B. Xu, D. Li, M. P. Hautzinger, Y. Fu, T. Zhai, L. Xu, G. Niu, S. Jin and J. Tang, Adv. Funct. Mater., 2018, 28, 1704446 CrossRef.
- Z. Z. Ma, Z. F. Shi, L. T. Wang, F. Zhang, D. Wu, D. W. Yang, X. Chen, Y. Zhang, C. X. Shan and X. J. Li, Nanoscale, 2020, 12, 3637–3645 RSC.
- N. Ding, D. Zhou, G. Pan, W. Xu, X. Chen, D. Li, X. Zhang, J. Zhu, Y. Ji and H. Song, ACS Sustainable Chem. Eng., 2019, 7, 8397–8404 CrossRef.
- Y. Zhu, J. Zhu, H. Song, J. Huang, Z. Lu and G. Pan, J. Rare Earths, 2021, 39, 374–379 CrossRef.
- A. Wang, Y. Guo, Z. Zhou, X. Niu, Y. Wang, F. Muhammad, H. Li, T. Zhang, J. Wang, S. Nie and Z. Deng, Chem. Sci., 2019, 10, 4573–4579 RSC.
- Z. Li, Z. Deng, A. Johnston, J. Luo, H. Chen, Y. Dong, R. Sabatini and E. H. Sargent, Adv. Funct. Mater., 2022, 32, 2111346 CrossRef.
- C. Zhou, Y. Tian, M. Wang, A. Rose, T. Besara, N. K. Doyle, Z. Yuan, J. C. Wang, R. Clark, Y. Hu, T. Siegrist, S. Lin and B. Ma, Angew. Chem., Int. Ed., 2017, 56, 9018–9022 CrossRef PubMed.
- H. Xiao, P. Dang, X. Yun, G. Li, Y. Wei, Y. Wei, X. Xiao, Y. Zhao, M. S. Molokeev, Z. Cheng and J. Lin, Angew. Chem., Int. Ed., 2021, 60, 3699–3707 CrossRef PubMed.
- R. Lin, Q. Guo, Q. Zhu, Y. Zhu, W. Zheng and F. Huang, Adv. Mater., 2019, 31, e1905079 CrossRef PubMed.
- B. Su, M. S. Molokeev and Z. Xia, J. Phys. Chem. Lett., 2020, 11, 2510–2517 CrossRef PubMed.
- A. M. Anthony, M. K. Pandian, P. Pandurangan and M. Bhagavathiachari, ACS Appl. Mater. Interfaces, 2022, 14, 29735–29743 CrossRef PubMed.
- S. Zhao, C. Chen, W. Cai, R. Li, H. Li, S. Jiang, M. Liu and Z. Zang, Adv. Opt. Mater., 2021, 9, 2100307 CrossRef CAS.
- S. Fang, Y. Wang, H. Li, F. Fang, K. Jiang, Z. Liu, H. Li and Y. Shi, J. Mater. Chem. C, 2020, 8, 4895–4901 RSC.
- Q. Mo, J. Yu, C. Chen, W. Cai, S. Zhao, H. Li and Z. Zang, Laser Photonics Rev., 2022, 16, 2100600 CrossRef CAS.
- T. Jun, K. Sim, S. Iimura, M. Sasase, H. Kamioka, J. Kim and H. Hosono, Adv. Mater., 2018, 30, e1804547 CrossRef PubMed.
- Z. Luo, Q. Li, L. Zhang, X. Wu, L. Tan, C. Zou, Y. Liu and Z. Quan, Small, 2020, 16, e1905226 CrossRef PubMed.
- H. Shao, X. Wu, J. Zhu, W. Xu, L. Xu, B. Dong, J. Hu, B. Dong, X. Bai, H. Cui and H. Song, Chem. Eng. J., 2021, 413, 127415 CrossRef CAS.
- S. Liu, X. Fang, B. Lu and D. Yan, Nat. Commun., 2020, 11, 4649 CrossRef CAS PubMed.
- X. Liu, Y. Yu, F. Yuan, C. Zhao, H. Dong, B. Jiao and Z. Wu, ACS Appl. Mater. Interfaces, 2020, 12, 52967–52975 CrossRef CAS PubMed.
- S. Zhao, Q. Mo, W. Cai, H. Wang and Z. Zang, Photonics Res., 2021, 9, 187–192 CrossRef.
- Q. Fan, G. V. Biesold-McGee, J. Ma, Q. Xu, S. Pan, J. Peng and Z. Lin, Angew. Chem., Int. Ed., 2020, 59, 1030–1046 CrossRef CAS.
- L. Wang, Z. Shi, Z. Ma, D. Yang, F. Zhang, X. Ji, M. Wang, X. Chen, G. Na, S. Chen, D. Wu, Y. Zhang, X. Li, L. Zhang and C. Shan, Nano Lett., 2020, 20, 3568–3576 CrossRef CAS PubMed.
- Z. Li, Z. Rao, Q. Li, L. Zhou, X. Zhao and X. Gong, Adv. Opt. Mater., 2021, 9, 2100804 CrossRef CAS.
- P. Zhu, S. Thapa, H. Zhu, S. Wheat, Y. Yue and D. Venugopal, J. Alloys Compd., 2023, 960, 170836 CrossRef CAS.
- B. Wang, X. Ouyang, X. He, Z. Deng, Y. Zhou and P. Li, Adv. Opt. Mater., 2023, 11, 2300388 CrossRef CAS.
- W. Ma, Q. Qian, S. M. H. Qaid, S. Zhao, D. Liang, W. Cai and Z. Zang, Nano Lett., 2023, 23, 8932–8939 CrossRef PubMed.
- W. Ma, D. Liang, Q. Qian, Q. Mo, S. Zhao, W. Cai, J. Chen and Z. Zang, eScience, 2023, 3, 100089 CrossRef.
- Q. Chen, H. Fu, J. Jiang, Z. Fang, H. Zhang, W. Yang, W. Liu and J. Zheng, J. Lumin., 2023, 258, 119783 CrossRef.
- M. Zhu, X. Du, G. Niu, W. Liu, W. Pan, J. Pang, W. Wang, C. Chen, Y. Xu and J. Tang, Fundam. Res., 2022, 2, 108–113 CrossRef.
- D. Yan, S. Zhao, Y. Zhang, H. Wang and Z. Zang, Opto-Electron. Adv., 2022, 5, 200075 Search PubMed.
- Y. Zhou, J. Chen, O. M. Bakr and O. F. Mohammed, ACS Energy Lett., 2021, 6, 739–768 CrossRef.
- B. Wang, D. Liang, S. M. H. Qaid, W. Cai, X. Yang, Z. Xu, R. Li, H. Xiao and Z. Zang, Appl. Phys. Lett., 2024, 124, 243302 CrossRef.
- S. Bhaumik, S. Ray and S. K. Batabyal, Mater. Today Chem., 2020, 18, 100363 CrossRef.
- A. Wang, X. Yan, M. Zhang, S. Sun, M. Yang, W. Shen, X. Pan, P. Wang and Z. Deng, Chem. Mater., 2016, 28, 8132–8140 CrossRef.
- C. Sun, J.-P. Zang, Y.-Q. Liu, Q.-Q. Zhong, X.-X. Xing, J.-P. Li, C.-Y. Yue and X.-W. Lei, CCS Chem., 2022, 4, 3106–3121 CrossRef.
- B. Wang, C. Wang, Y. Chu, H. Zhang, M. Sun, H. Wang, S. Wang and G. Zhao, J. Alloys Compd., 2022, 910, 164892 CrossRef.
- M. Zhang, Z. Wang, B. Zhou, X. Jia, Q. Ma, N. Yuan, X. Zheng, J. Ding and W. H. Zhang, Sol. RRL, 2018, 2, 1700213 CrossRef.
- S. A. Kulkarni, S. G. Mhaisalkar, N. Mathews and P. P. Boix, Small Methods, 2019, 3, 1800231 CrossRef.
- H. Li, L. Tian, Z. Shi, Y. Li, C. Li, J. Feng and H. Zhang, J. Mater. Chem. C, 2022, 10, 10609–10615 RSC.
- A. Yangui, R. Roccanova, T. M. McWhorter, Y. Wu, M.-H. Du and B. Saparov, Chem. Mater., 2019, 31, 2983–2991 CrossRef CAS.
- J. Jiang, G. Niu, L. Sui, X. Wang, Y. Zhang, L. Che, G. Wu, K. Yuan and X. Yang, J. Phys. Chem. Lett., 2021, 12, 7285–7292 CrossRef CAS PubMed.
- S. Si, X. Guo, W. Gan, X. Zhang, B. Lan, M. R. Li and J. Wang, J. Lumin., 2022, 251, 119212 CrossRef CAS.
- Z. Tan, M. Hu, G. Niu, Q. Hu, J. Li, M. Leng, L. Gao and J. Tang, Sci. Bull., 2019, 64, 904–909 CrossRef CAS PubMed.
- J. Lu, C. Yan, W. Feng, X. Guan, K. Lin and Z. Wei, EcoMat, 2021, 3, e12082 CrossRef CAS.
- X. Meng, S. Ji, Q. Wang, X. Wang, T. Bai, R. Zhang, B. Yang, Y. Li, Z. Shao, J. Jiang, K. L. Han and F. Liu, Adv. Sci., 2022, 9, e2203596 CrossRef.
- S. Liu, Y. Yue, X. Zhang, C. Wang, G. Yang and D. Zhu, J. Mater. Chem. C, 2020, 8, 8374–8379 RSC.
- Z. Ma, Z. Shi, D. Yang, Y. Li, F. Zhang, L. Wang, X. Chen, D. Wu, Y. Tian, Y. Zhang, L. Zhang, X. Li and C. Shan, Adv. Mater., 2021, 33, e2001367 CrossRef.
- B. Chiou, H. Lee, Y. Jang, Z. Yang, Y. Wang, M. Sarma, H. Su and K. Wong, Org. Electron., 2017, 48, 248–253 CrossRef CAS.
- S. Ye, F. Xiao, Y. X. Pan, Y. Y. Ma and Q. Y. Zhang, Mater. Sci. Eng., R, 2010, 71, 1–34 CrossRef.
- N. Narendran, J. D. Bullough, N. Maliyagoda and A. Bierman, J. Illum. Eng. Soc., 2001, 30, 57–67 CrossRef.
- N. Narendran and Y. Gu, J. Disp. Technol., 2005, 1, 167–171 CrossRef.
- G. Nedelcu, L. Protesescu, S. Yakunin, M. I. Bodnarchuk, M. J. Grotevent and M. V. Kovalenko, Nano Lett., 2015, 15, 5635–5640 CrossRef.
- H. Chen, L. Zhu, C. Xue, P. Liu, X. Du, K. Wen, H. Zhang, L. Xu, C. Xiang, C. Lin, M. Qin, J. Zhang, T. Jiang, C. Yi, L. Cheng, C. Zhang, P. Yang, M. Niu, W. Xu, J. Lai, Y. Cao, J. Chang, H. Tian, Y. Jin, X. Lu, L. Jiang, N. Wang, W. Huang and J. Wang, Nat. Commun., 2021, 12, 1421 CrossRef.
- Y. Zhang, Z. Zhang, W. Yu, Y. He, Z. Chen, L. Xiao, J. J. Shi, X. Guo, S. Wang and B. Qu, Adv. Sci., 2022, 9, e2102895 CrossRef.
- D. Liu, X. Liu, Y. Gan, Z. Liu, G. Sun, C. Shen, X. Peng, W. Qiu, D. Li, Z. Zhou, Z. Li, H. L. Yip and S. J. Su, ACS Energy Lett., 2022, 7, 523–532 CrossRef.
- T. Jun, T. Handa, K. Sim, S. Iimura, M. Sasase, J. Kim, Y. Kanemitsu and H. Hosono, APL Mater., 2019, 7, 111113 CrossRef.
- Z. Ma, Z. Shi, C. Qin, M. Cui, D. Yang, X. Wang, L. Wang, X. Ji, X. Chen, J. Sun, D. Wu, Y. Zhang, X. J. Li, L. Zhang and C. Shan, ACS Nano, 2020, 14, 4475–4486 CrossRef PubMed.
- H. C. Jung-Min Heo, S.-C. Lee, M.-H. Park, J. S. Kim, H. Kim, J. Park, Y.-H. Kim, H. J. Yun, E. Yoon, D.-H. Kim, S. Ahn, S.-J. Kwon, C.-Y. Park and T.-W. Lee, ACS Energy Lett., 2022, 7, 2807–2815 CrossRef.
- C. Zhou, H. Lin, Y. Tian, Z. Yuan, R. Clark, B. Chen, L. J. van de Burgt, J. C. Wang, Y. Zhou, K. Hanson, Q. J. Meisner, J. Neu, T. Besara, T. Siegrist, E. Lambers, P. Djurovich and B. Ma, Chem. Sci., 2018, 9, 586–593 RSC.
- W. Liang, L. Wang, Y. Li, F. Zhang, X. Chen, D. Wu, Y. Tian, X. Li, C. Shan and Z. Shi, Mater. Today Phys., 2021, 18, 100398 CrossRef.
- J. Chen, J. Wang, X. Xu, J. Li, J. Song, S. Lan, S. Liu, B. Cai, B. Han, J. T. Precht, D. Ginger and H. Zeng, Nat. Photonics, 2021, 15, 238–244 CrossRef.
- C. Y. Chang, A. N. Solodukhin, S. Y. Liao, K. P. O. Mahesh, C. L. Hsu, S. A. Ponomarenko, Y. N. Luponosov and Y. h. Chao, J. Mater. Chem. C, 2019, 7, 8634–8642 RSC.
- D. Y. Li, Y. H. Liu, Q. Wang, X. W. Lei, C. Y. Yue and Z. H. Jing, Mater. Today Chem., 2023, 31, e101604 CrossRef.
- G. Xiong, L. Yuan, Y. Jin, H. Wu, Z. Li, B. Qu, G. Ju, L. Chen, S. Yang and Y. Hu, Adv. Opt. Mater., 2020, 8, e2000779 CrossRef.
- J. Hou, J. Wu, Z. Qin, Y. Fang, L. Shen, X. Qiao, L. Dong, G. Zhang, Y. Liu, G. Zhao and H. Chen, J. Mater. Chem. C, 2023, 11, 2137–2143 RSC.
- J. Li, Y. Sheng, G. Tong, H. Zhu, X. Tao, C. Wu, Y. Chang, Z. Tang, J. Yang, S. Zhang and Y. Jiang, Adv. Opt. Mater., 2023, 11, e2300100 CrossRef.
- Z. Li, G. Song, Y. Li, L. Wang, T. Zhou, Z. Lin and R. J. Xie, J. Phys. Chem. Lett., 2020, 11, 10164–10172 CrossRef CAS.
- H. Wang, C. Wang, Y. Wang, M. Sun and G. Zhao, J. Phys. Chem. C, 2023, 127, 11616–11622 CrossRef CAS.
- Z. Li, Y. Li, P. Liang, T. Zhou, L. Wang and R. J. Xie, Chem. Mater., 2019, 31, 9363–9371 CrossRef CAS.
- Y. Sheng, P. Chen, Y. Gao, Y. He, J. Li, Muhammad, X. Xie, C. Cheng, J. Yang, Y. Chang, G. Tong and Y. Jiang, ACS Appl. Mater. Interfaces, 2024, 16, 19175–19183 CrossRef CAS.
- Muhammad, Z. Liu, J. Li, Y. Sheng, Y. Chang, P. Chen and Y. Jiang, J. Mater. Chem. C, 2024, 12, 6606–6614 RSC.
- S. Li, Y. Zhao, L. Du, Y. He, R. Wang, Y. Guo, C. Wang, T. Tian, L. Wang and H. Liu, Chem. Eng. J., 2024, 482, 148966 CrossRef CAS.
- H. Xu, J. Liu, Q. Hu, J. Yu, Q. Han and W. Wu, Adv. Opt. Mater., 2024, 12, 2302288 CrossRef.
- Z. Hu, X. Wang, K. Nie, R. Zhou, W. Dai, X. Duan, X. Zhang, H. Wang, L. Wang, L. Mei, Y. Liu and X. Ma, Chem. Eng. J., 2024, 482, 148829 CrossRef.
- H. Mai, X. Wen, X. Li, N. S. L. Dissanayake, X. Sun, Y. Lu, T. C. Le, S. P. Russo, D. Chen, D. A. Winkler and R. A. Caruso, Mater. Today, 2024, 74, 12–21 CrossRef.
- D. Liang, X. Liu, B. Luo, Q. Qian, W. Cai, S. Zhao, J. Chen and Z. Zang, EcoMat, 2023, 5, e12296 CrossRef.
- A. K. Guria, S. K. Dutta, S. D. Adhikari and N. Pradhan, ACS Energy Lett., 2017, 2, 1014–1021 CrossRef.
- X. Liu, X. Xu, B. Li, L. Yang, Q. Li, H. Jiang and D. Xu, Small, 2020, 16, e2002547 CrossRef PubMed.
- X. Deng, S. Cheng, X. Chen, M. Wang, X. Li, G. Li, D. Zhu, M. Jia, X. Li and Z. Shi, J. Lumin., 2024, 269, 120525 CrossRef.
- F. Chen, X. Wang, X. Zhang and C. Zhang, Opt. Mater., 2023, 139, 113750 CrossRef.
- G. Xu, C. Wang, Y. Li, W. Meng, G. Luo, M. Peng, B. Xu and Z. Deng, Chem. Sci., 2023, 14, 5309–5315 RSC.
- W. Meng, C. Wang, Y. Li, G. Hu, S. Sui, G. Xu, M. Peng and Z. Deng, Chem. - Eur. J., 2023, 29, e202202675 Search PubMed.
- W. Zheng, R. Sun, Y. Liu, X. Wang, N. Liu, Y. Ji, L. Wang, H. Liu and Y. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 6404–6410 CrossRef PubMed.
- H. Xu, J. Yu, Q. Hu, Q. Han and W. Wu, Mater. Today Chem., 2023, 30, 101580 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |
