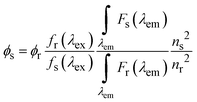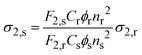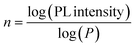Open Access Article
Open Access ArticleTwo-photon brightness of NIR-emitting, atomically precise DNA-stabilized silver nanoclusters†
Agata
Hajda
 a,
Rweetuparna
Guha
a,
Rweetuparna
Guha
 b,
Stacy Marla
Copp
b,
Stacy Marla
Copp
 bcde and
Joanna
Olesiak-Bańska
bcde and
Joanna
Olesiak-Bańska
 *a
*a
aInstitute of Advanced Materials, Wroclaw University of Science and Technology, Wrocław, Poland. E-mail: joanna.olesiak-banska@pwr.edu.pl
bDepartment of Materials Science and Engineering, University of California, Irvine, CA 92697, USA
cDepartment of Chemistry, University of California, Irvine, CA 92697, USA
dDepartment of Physics and Astronomy, University of California, Irvine, CA 92697, USA
eDepartment of Chemical and Biomolecular Engineering, University of California, Irvine, CA 92697, USA
First published on 16th December 2024
Abstract
Near-infrared (NIR) emitters with high two-photon absorption (2PA) cross-sections are of interest to enable in vivo imaging in the tissue transparency windows. This study explores the potential of DNA-stabilized silver nanoclusters (AgN-DNAs) as water-soluble two-photon absorbers. We investigate 2PA of four different atomically precise AgN-DNA species with far-red to NIR emission and varying nanocluster and ligand compositions. 2PA cross-sections, σ2, were determined by two-photon excited luminescence (2PEL) technique for a wide wavelength range from 810 to 1400 nm. The AgN-DNAs exhibited reversed strength of corresponding transitions in the two-photon regime, as compared to one-photon, which further demonstrates the complex photophysics of these emitters. Maximal 2PA cross-section value (∼582 GM) was observed for (DNA)3[Ag21]15+, which is stabilized by 3 DNA oligomers. (DNA)2[Ag16Cl2]8+ presented distinct 2PA behavior from the AgN-DNAs without chlorido ligands, with a high 2PA of 176 GM at 1050 nm. Our findings support the potential of AgN-DNAs as NIR-to-NIR two-photon probes that are both excited and emit in the NIR. Their high σ2 and fluorescence quantum yield values result in superior two-photon brightness on the order of ∼102 GM, significantly higher than water-soluble organic fluorophores.
Introduction
DNA-stabilized silver nanoclusters (AgN-DNAs) are an emerging class of biocompatible fluorescent nanomaterials with sequence-tuned optical properties.1–3 These ultrasmall emitters have unique and diverse photoluminescence properties that span visible and near-infrared (NIR) wavelengths. The optical properties of AgN-DNAs depend strongly on the size, shape, and effective valence electron count of the nanocluster core, which are governed by the nucleobase sequence of the DNA oligomer template.4–6 Thus, DNA oligomer selection enables tunable design of AgN-DNA properties,7,8 making AgN-DNAs promising and versatile emitters to meet the needs of bioimaging.Recent tandem efforts to harness machine learning-guided experiments and to prepare and investigate atomically precise AgN-DNA species have accelerated the discovery of AgN-DNAs.9–11 Many recently discovered AgN-DNAs emit at NIR-I and NIR-II wavelengths (700–1000 nm and 1000–1300 nm, respectively)12–15 with high fluorescence quantum yields16 (FQY, ϕ) compared to other NIR emitters, such as organic dyes. Water soluble NIR emitting probes are highly desirable for optical imaging of biological samples because scattering, absorption, and autofluorescence of tissues are significantly reduced at NIR wavelengths compared to visible wavelengths.17,18 The remarkably high FQY and inherent water solubility of NIR AgN-DNAs make these promising emitters for overcoming the issue of low FQY of NIR-emitting organic fluorophores, which limit in vivo fluorescence imaging.
In contrast to their one-photon (1P) properties, the nonlinear optical (NLO) properties of AgN-DNAs have been far less explored. Two-photon absorption (2PA) is a third order NLO phenomenon that occurs upon simultaneous absorption of two photons, each with half the excitation energy of the chromophore, thereby exciting an electron from the ground state to the excited state. This excitation process requires high-intensity pulsed laser irradiation and is induced at long wavelengths, which are more favorable for deeper penetration into biological samples. 2PA can be followed by two-photon excited fluorescence (2PEF), which enables two-photon fluorescence microscopy (2PFM). 2PFM at NIR-I or NIR-II wavelengths19 presents multiple advantages compared to the standard 1P fluorescence microscopy, such as deeper sample penetration, reduced phototoxicity and photobleaching, 3D imaging within tissues, selective excitation of femtoliter-scale volumes, and visualization over long time periods (longitudinal imaging).20–25
Atomically precise silver or gold nanoclusters (Ag/Au NCs) were reported as strong two-photon absorbers.26–30 2PA cross-section (σ2, expressed in Goeppert-Mayer units, GM) for Ag/Au NCs varies from ∼102 GM to ∼105 GM and is dictated by nanocluster properties, such as the number of metal atoms, protecting ligands, and doping with other metal atoms.26–28 However, Ag/Au NCs generally display low ϕ and low two-photon brightness (defined as σ2,b = ϕ × σ2), limiting their relevance in 2PFM. Early studies of 2PA of AgN-DNAs suggested the promise of these emitters for 2PFM, but these studies preceded the robust development of purification strategies to produce AgN-DNA emitters with defined chemical compositions.31,32 While the 2PA of atomically precise AgN-DNAs has not been explored in detail, application of this effect is now emerging.33
To advance in vivo fluorescence imaging, it is a major goal to develop water-soluble, 2PEF probes that both excite and emit at NIR wavelengths with high FQY and high two-photon brightness (σ2,b above 50 GM).34,35 In this study, we investigate 2PA of four different atomically precise AgN-DNAs with far-red to NIR emission, which were recently investigated by Guha, et al.5 We find that 2PA spectra of the four AgN-DNAs, measured from 810 nm to 1400 nm, show significant differences between 1P and two-photon (2P) response. We then present a detailed analysis of NLO properties (specifically two-photon absorption) of the NIR-emissive AgN-DNA with chlorido ligands and known X-ray crystal structure, (DNA)2[Ag16Cl2]8+. Finally, we compare the 2P brightness of AgN-DNAs to organic fluorophores and commercially used NIR markers, demonstrating their utility for applications in NIR-to-NIR 2P bioimaging.
Results and discussion
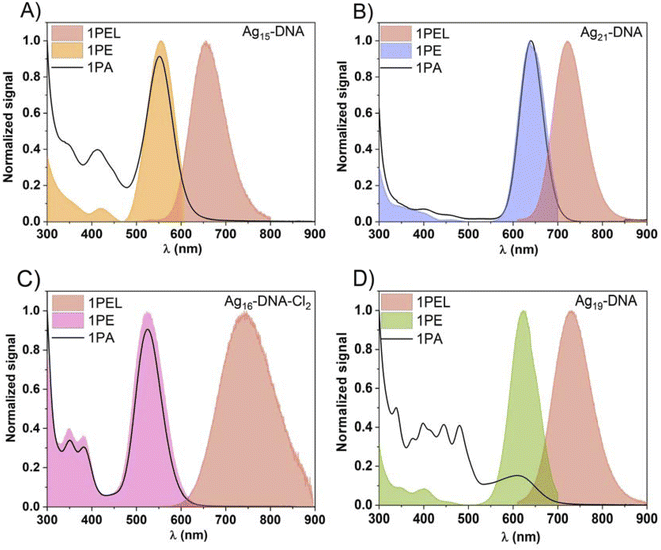
To investigate the NLO properties of far red and NIR emitting AgN-DNAs, we selected four AgN-DNAs with different molecular compositions, including total number of Ag atoms, effective valence electron count (N0), and the numbers of DNA oligomer ligands (ns) and chlorido ligands (Table 1). Emitters were selected from a recent study by Guha, et al., which reported the molecular compositions and one-photon absorbance (1PA) and emission spectra of four distinct structural/compositional groups of HPLC-purified far-red to NIR emitting AgN-DNAs.5 One emitter from each group was selected to study the breadth of possible 2PE properties of far red and NIR emitting AgN-DNAs. To ensure agreement with past studies,5 molecular compositions were confirmed by negative-ion mode electrospray ionization mass spectrometry (see Experimental methods and ESI†), and 1P optical properties of the AgN-DNAs (absorption and emission) were measured to ensure consistency with prior reports. For the first time, we present one-photon excitation (1PE) spectra of these clusters (Fig. 1). 1PA and 1PEL maxima, FQY, and Stokes shifts are reported in Table 1, and Fig. 1 presents 1P properties of the four AgN-DNAs.| Name | Molecular formula | DNA sequence (5′ to 3′) | n s and no. of Cl− ligands | N 0 | 1PA (nm) | 1PEL (nm) | FQY, ϕ [%] | Stokes shift (eV) |
|---|---|---|---|---|---|---|---|---|
| Ag15-DNA | (DNA)2[Ag15]9+ | ACCAATGACC | 2 and 0 | 6 | 550 | 652 | 11 | 0.35 |
| Ag21-DNA | (DNA)3[Ag21]15+ | CCCGGAGAAG | 3 and 0 | 6 | 640 | 721 | 73 (ref. 16) | 0.22 |
| Ag16-DNA-Cl2 | (DNA)2[Ag16Cl2]8+ | CACCTAGCGA | 2 and 2 | 6 | 525 | 744 | 26 (ref. 15) | 0.70 |
| Ag19-DNA | (DNA)2[Ag19]11+ | GCGCAAGATG | 2 and 0 | 8 | 480, 615 | 730 | 16 | 0.31 |
Fig. 1 shows that the most prominent AgN-DNA excitation peaks are Gaussian-shaped bands located at the longest wavelength transition in 1PA, indicating that these emitters follow Kasha's rule. We observe no difference in shape or intensity of peaks in 1PA and one-photon excitation (1PE) spectra for Ag21-DNA (Fig. 1b) and Ag16-DNA-Cl2 (Fig. 1c). For Ag15-DNA, the peak bands in 1PA and 1PE occur at the same wavelength, but the ratio of the longest-wavelength band and the band located between 400 and 450 nm is significantly higher in 1PE than in 1PA (Fig. 1a). The most significant deviations between 1PA and 1PE are observed for Ag19-DNA (Fig. 1d). This emitter's 1PE spectrum contains four distinct peaks (347 nm, 401 nm, 450 nm, and 620 nm), while far more peaks are observed in the 1PA spectrum, as previously reported.5 This suggests that absorption at certain wavelengths does not result in radiative recombination and fluorescence emission for Ag19-DNA. Ag15 DNA, Ag21-DNA, Ag19-DNA exhibit Stokes shifts between 0.2 and 0.3 eV whereas, Ag16-DNA-Cl2 displays a Stokes shift of 0.7 eV (Table 1).15 Comparison with FQY shows that energy gap law does not hold for these nanoclusters. Ag21-DNA displays a high 73% FQY at room temperature in aqueous solution,16 which is unprecedented for NIR emitters, however it displays the lowest Stokes shift.
Ag16-DNA-Cl2 has molecular composition (DNA)2[Ag16Cl2]8+; i.e. the species is stabilized by two copies of the ssDNA template and two additional chlorido ligands, which significantly contribute to nanocluster stability and optical properties.9Ag16-DNA-Cl2 is the only AgN-DNA in this study with a reported high-resolution X-ray crystal structure (and one of only two AgN-DNA species with high-resolution structural information about both nanocluster core and ligand shell reported to date).36 This emitter has 26% FQY and a high Stokes shift of 0.7 eV, implicating a large energy difference between the Franck–Condon excited state and the S1–S0 transition (fluorescence). The emitter has been well-studied by the Vosch group.15,37–40 Theoretical analysis of its 1PA properties identified silver → silver, silver → base and base → silver electronic transitions.41
Finally, Ag19-DNA has molecular composition (DNA)2[Ag19]11+ and contains 8 effective valence electrons (N0 = 8), unlike the N0 = 6 of the rest of the AgN-DNAs studied here. Because N0 = 8 is a magic number for spherical superatoms,42 and due to its distinctly different absorption and circular dichroism spectra, Ag19-DNA has been hypothesized to possess spheroidal nanocluster geometry43 rather than the rod-shaped structures of N0 = 6 AgN-DNAs.44
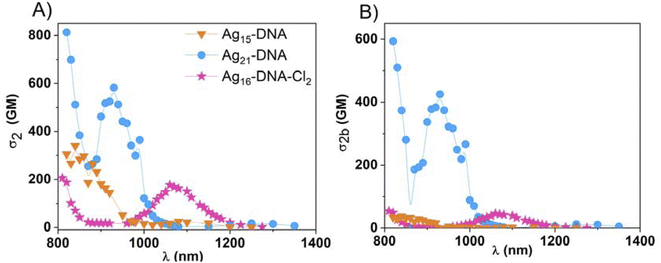
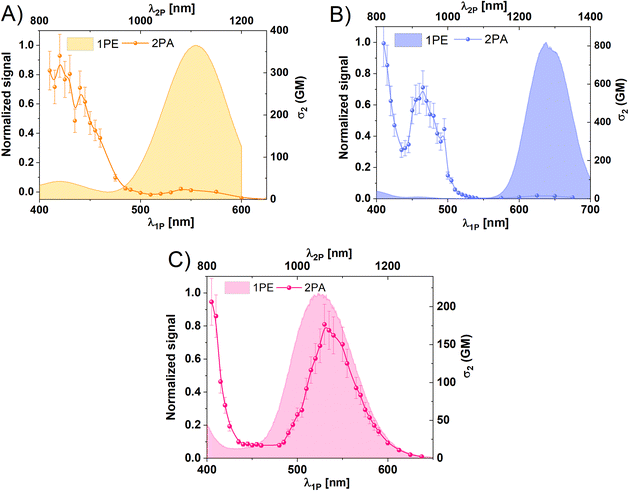
After characterization of one-photon properties, we measured the NLO properties of the AgN-DNA species. The 2PA cross-sections, σ2, of AgN-DNAs and reference samples (Styryl 9M or LDS-698) were evaluated by two-photon excited luminescence (2PEL) technique using femtosecond pulsed laser excitation in a wide range of wavelengths (see Experimental section).45 As a luminescence-based technique, 2PEL provides information about 2PA cross-section values only for wavelengths at which 2PE leads to luminescence.26 In systems for which absorption perfectly overlaps with excitation, 2PA spectrum determined with 2PEL can be directly compared with 1PA. However, in systems for which absorption differs from excitation, the measured 2PA spectrum should be compared with the 1PE spectrum. Because excitation and absorption spectra differ for Ag15-DNA and Ag19-DNA (Fig. 1), we compare 2PA cross-sections to 1PE spectrum. Wavelength ranges were selected such that emission wavelengths do not interfere with the excitation wavelength range. Moreover, the 2P nature of the observed processes was determined based on the quadratic dependence of photoluminescence intensity (PL intensity) on the incident laser power, P, which indicates a two-photon process in AgN-DNAs (the slope of log(PL intensity) vs. log(P) plots equal ∼2.0, Fig. S7†).46 The values of ∼1.9 support a 2P process (small deviations from 2.0 were also seen for reference organic dyes with known 2P behaviour). In case of lower values, contributions from upconversion may be present, as is observed for Ag21-DNA for excitation <850 nm (Fig. S7b†). Therefore, it should be noted that σ2 values for Ag21DNA at 810 and 820 nm may have contributions from 1P processes. Ag19DNA seems to have significant one-photon process contribution at wavelengths below 980 nm (Fig. S7†), nevertheless transitions involving higher energy states are the most prominent under fs laser excitation. Due to this fact, we present results for Ag19DNA in the ESI,† and the measured 2PA spectra for Ag19DNA presented in Fig. S8† need to be considered as qualitative. Taking into account, that there is no other experimental or theoretical data for this nanocluster, its response under fs laser illumination needs extended studies, out of the scope of this manuscript. Ag19-DNA will be excluded from further comparisons with the other nanoclusters. We ensure lack of photobleaching by using low laser power ∼10 mW and a short 5 s exposure time, with samples exposed to laser irradiation only during signal collection.
Fig. 2 compares the σ2 values of AgN-DNAs to their 1PE spectra. AgN-DNAs exhibit maximum σ2 values of several hundred GM at <950 nm, which corresponds to one-photon transitions at <475 nm. At longer wavelengths, two different types of σ2 behavior are observed. Ag15-DNA and Ag21-DNA exhibit significantly lower σ2 above ca. 1000 nm, in the range of the most prominent S1 → S0 transitions in the 1P regime. In contrast, the 2PA spectrum of Ag16-DNA-Cl2 is distinctly different, with similar σ2 values at 800 nm and 1050 nm (Fig. 2c).
 | ||
| Fig. 2 Comparison between one photon excitation (1PE, filled bands) and two photon absorption (2PA, points and lines) of (A) Ag15-DNA, (B) Ag21-DNA, (C) Ag16-DNA-Cl2. | ||
1PE and 2PA spectra can be compared using the ratio of intensity of higher energy to lower energy transitions in 1PE and 2PA spectra. For Ag15-DNA, the ratio between 1PE transitions at 420/550 nm is ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. In contrast, the 2PA ratio at 840/1100 nm is 17
20. In contrast, the 2PA ratio at 840/1100 nm is 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Thus, this emitter displays a reversal of the intensities of 2P transitions compared to 1P transitions.
1. Thus, this emitter displays a reversal of the intensities of 2P transitions compared to 1P transitions.
For Ag21-DNA, σ2 is largest for 820 nm and 940 nm excitation, which overlap with low-intensity peaks in the 1PE spectrum (Fig. 2b). The 2PA is nearly zero between 1000 and 1150 nm, similar to 1PE in the 500–550 nm range. However, as mentioned before, the dominant 1PE peak at 640 nm is not the most prominent in 2PA (Fig. 2b). A closer look at 1PE between 400–500 nm (Fig. S2†) shows that high σ2 bands correlate with very weak 1P S0 → Sn transitions (where n > 1). The ratio of 1P transition intensity at 410/460/635 nm is ∼3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67, while for 2PA (at double wavelength) the ratio is 50
67, while for 2PA (at double wavelength) the ratio is 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
For Ag16-DNA-Cl2, the locations of 2PA transitions are well-aligned with 1PE transitions (Fig. 2c). Surprisingly, however, the 2PA band at longer wavelengths has one order of magnitude higher σ2 value than the other three AgN-DNA species. This Ag16-DNA-Cl2 is discussed in more detail later.
A trend of opposite strength of 1PE and 2PA bands is observed in Ag15-DNA, Ag21-DNA and Ag16-DNA-Cl2. Past studies of unpurified AgN-DNAs by Patel, et al.32 reported a similar phenomenon where two-photon excitation (2PE) was more prominent at shorter wavelengths. Thus, such opposite strength of 1PA and 2PE bands could be a more general observation for this type of nanostructures could be a more general observation for this type of nanostructures. 1P and 2P transitions may follow distinct selection rules and resonant enhancements of selected transitions in 2PA, thus giving rise to the possibility of such phenomena. Generally, in molecules with more complex energy landscapes like porphyrins47 or fluorescent proteins,48 resonance effects with various electronic or vibrational states contribute to the enhancement of certain 2P transitions. In fluorescent proteins, 2PA corresponding to S0 → S1 transition is often less prominent than for transitions at shorter wavelengths, which gain intensity due to the resonant enhancement effect related to the proximity of virtual state energy (at 1/2 transition energy) and real Sn state.49 Such a phenomenon may exist in AgN-DNAs.
Ag16-DNA-Cl2 has one order of magnitude higher σ2 at longer wavelengths than the other AgN-DNAs. Ag16-DNA-Cl2 is distinct for containing chlorido ligands. As one of the most electronegative atoms, chlorine influences electron density, electric transition dipole moment and increases the inter- and intra-molecular charge-transfer effects. Indeed, computational modelling41 shows that chloridos modulate electron density and optical properties of Ag16-DNA-Cl2, stabilizing the electronic structure by reducing the electron density in the metal core and thereby leading to shifts in the nanocluster energy levels, affecting both the HOMO (highest occupied molecular orbital) and LUMO (lowest unoccupied molecular orbital) energies. Calculations also revealed that the experimentally observed absorbance peak at 525 nm consists only of a HOMO-to-LUMO molecular transition (S0–S1*) located at the silver core of Ag16-DNA-Cl2,41 while higher energy transitions also contain contributions from silver–base and base–silver transitions, making these transitions more complex. Ag16-DNA-Cl2 presents also complex emission, with nanosecond and microsecond components. In previous reports, excited state dynamics was probed by transient absorption (TA) spectroscopy for Ag16-DNA-Cl2, revealing that upon excitation, electrons are transferred to the Franck–Condon state and then rapidly depopulate to vibrationally hot levels of the fluorescent state within 100 fs.51 Hot vibrational levels thermalize on a timescale of 6.2 ps. Radiative relaxation (emission) to the ground state occurs with a lifetime of 4.7 ns. In a fraction of cases, the transition to a microsecond-lived state is observed with a decay time of ca. 70 μs. TA spectra showed a positive signal between 1000–1200 nm, which blue-shifts over several ns.51 Recently, the simulated TA spectra by Malola, et al. presented a broad peak between 1100–1400 nm related to S1 and T1 transitions with absorption maxima at 1244 nm and 1181 nm, respectively.50 Because this range of wavelengths overlaps with the 2PA band of Ag16-DNA-Cl2, a possible contribution of transitions in a long-lived T1 state in measurements at NIR wavelengths were considered. Therefore, we investigated the optical properties of Ag16-DNA-Cl2 in more detail. Standard 2PEL measurements involve the use of fs lasers with 80 MHz repetition rate, corresponding to a 12.5 ns window between consecutive pulses. Because the long-lived state in Ag16-DNA-Cl2 has 70 μs lifetime, we also performed 2PEL measurements using a laser system with a 1 kHz repetition rate (1 ms window between pulses) Fig. S6† compares 2PA spectra obtained at 1 kHz and 80 MHz laser repetition rates, which show no significant changes in σ2 value or maximum wavelength. We do observe a slight alteration in the shape of the 2PA band at 1000–1150 nm, with higher values of σ2 at 980–1060 nm.
Finally, 2P brightness (σ2,b = ϕ × σ2) of each AgN-DNA species was calculated and compared with commercially available probes to evaluate the potential of AgN-DNAs for 2PFM.52–54Fig. 3a and b present σ2 and σ2,b of the AgN-DNA species, respectively, over a range of 810–1400 nm, which spans the NIR-I and NIR-II biological tissue transparency windows. Most notably, Ag21-DNA has both high σ2 and exceptionally high FQY compared to commonly used NIR-emitting fluorophores, resulting in the highest value of 2P brightness in the NIR-I window (ca. 582 GM at 930 nm, Fig. 3b). Ag16-DNA-Cl2 exhibits high σ2,b in the NIR-II window. Moreover, the combined use of Ag15-DNA with other emitters could enable two-color, 2P imaging,55,56 as the emission of this species is blue-shifted by 50 to 100 nm from the other three AgN-DNAs, which would enable simultaneous 2PE of two distinct probes using a single laser line.
There is a major need for water-soluble NIR-emitting probes with 2PE > 1000 nm, which currently are scarce because most available probes are insoluble in water due to the presence of highly hydrophobic units. Table 2 compares the NLO properties of the four AgN-DNA species with commercially available water-soluble dyes and fluorescent proteins used in 2PFM. σ2 and 2P brightness values of AgN-DNAs are comparable or significantly greater than the commercial fluorescent probes. The ability of AgN-DNAs to combine water solubility with NIR-I emission, high 2P brightness, low toxicity, and functionalization via click-chemistry for applications such as targeted staining of certain cell lines57 positions AgN-DNA as promising emitters to address the shortage of probes that are suitable for 2PFM.
| Probe | σ 2 [GM] | σ 2,b [GM] | Emission [nm] | Excitation window |
|---|---|---|---|---|
| mCherry58 | 25 | 5.5 | 610 | NIR-II |
| tdTomato58 | 108 | 60 | 581 | NIR-II |
| Alexa Fluor (ref. 21) 647 | 133 | 44 | 671 | NIR-II |
| Cy5 (ref. 21) | 143 | 40 | 670 | NIR-II |
| Cy7 (ref. 21) | 200 | 60 | 779 | NIR-II |
| Alexa Fluor (ref. 21) 680 | 203 | 73 | 704 | NIR-II |
| ICG59 | 210 | 6.3 | 813 | NIR-II |
| Cy5.5 (ref. 21) | 286 | 60 | 695 | NIR-II |
| Ag21-DNA | 582 | 425 | >700 | NIR-I |
| 17 | 12 | NIR-II | ||
| Ag16-DNA-Cl2 | 211 | 54 | >700 | NIR-I |
| 176 | 45 | NIR-II | ||
| Ag15-DNA | 340 | 37 | 650 | NIR-II |
| 25 | 3 | NIR-I |
Conclusions
We investigated the 2P properties of four atomically precise AgN-DNAs with far-red to NIR-I emission. These four species present distinct ligand compositions and valence electron counts, enabling us to probe the diversity of optical properties that may vary with nanocluster composition and structure. AgN-DNA species were measured with the same optical setup and the use of HPLC purification and ESI-MS allowed us to accurately estimate nanocluster concentration, an essential property for accurate σ2 calculation. Thus, our results provide quantitative information to advance understanding of structure–optical properties relationship of these nanoclusters. For Ag15-DNA and Ag16-DNA-Cl2, the same transitions take part in 1PA and radiative relaxation (1PA and 1PE spectra overlap). In contrast, some excited states are not involved in radiative relaxation for Ag21-DNA and Ag19-DNA, which results in differences in 1PA and 1PE spectra, particularly in case of Ag19-DNA. All nanocluster species present high 2PA cross-sections. For Ag15-DNA and Ag21-DNA, maximum σ2 values are obtained at the low-wavelength transitions, contrary to 1PA. In case of Ag16-DNA-Cl2, strong 2PA is also present at NIR-II wavelengths. For Ag19-DNA, one-photon process contributes to excitation under fs laser illumination, and further studies on this cluster are needed to fully understand its nonlinear optical properties. At present, the relationship between nanocluster structure and two-photon properties still remains poorly understood, and additional AgN-DNA crystal structures are needed to enable theoretical studies that investigate how chemical composition and structure relate to observed 2P properties. However, our findings confirm that AgN-DNAs are a new class of promising markers for NIR imaging and 2PFM, presenting high values of two-photon brightness together with other properties beneficial for bioimaging, such as NIR emission, large Stokes shifts, and water solubility. Our findings further identify the optimal excitation wavelengths to achieve a strong 2P fluorescence signal from these emitters. A deeper understanding of their photophysics and NLO properties could enable rational design of these unique emitters for advanced two-photon deep tissue bioimaging applications.Methods
Synthesis and purification of AgN-DNAs
An aqueous solution of single stranded DNA oligomer and AgNO3 in 10 mM ammonium acetate (pH 7) was prepared and incubated at room temperature for 15 minutes, followed by reduction by a freshly prepared aqueous solution of sodium borohydride (more details in ESI and Table S1†). The solution was stored in the dark at 4 °C for several days to allow the fluorescent AgN-DNA to form. Each AgN-DNA was then purified by high-performance liquid chromatography (HPLC) to obtain atomically precise AgN-DNAs. The molecular compositions of HPLC-purified AgN-DNAs were determined using negative-ion mode electrospray ionization mass spectrometry (ESI-MS). The ESI-MS methods and the mass spectra of the AgN-DNAs studied are shown in Fig. S1–S4.† The concentrations of HPLC-purified AgN-DNAs were estimated using the sample's absorption at 260 nm, the known molar extinction coefficient of the ssDNA oligomer template, and the number of DNA oligomers per nanocluster (Table 1). Detailed synthesis procedures (including concentrations of DNA oligomer and AgNO3, and storage conditions), HPLC chromatograms, and mass spectra of AgN-DNAs are reported in ref. 5.One-photon measurements
All measurements were carried out in 10 mM ammonium acetate solution (pH 7). Absorption spectra were recorded using a Jasco V-670 spectrophotometer. Excitation and fluorescence spectra using an FS5 Spectrofluorometer (Edinburgh Instruments) equipped with a Xenon lamp.FQY measurements
First, the FQY of the LDS-698 in CHCl3 was determined using an integrating sphere (FS5 Spectrofluorometer) and by the comparative method. The standard in the comparative method was DCM (4-(dicyanomethylene)-2-methyl-6-(4-dimethylaminostyryl)-4H-pyran) in EtOH with FQY = 43.5%.60 Finally, the FQY (ϕ) was determined using the following formula:| fs(λex) = 1 − 10Ax(λex) |
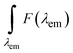 corresponds to the fluorescence integral of the sample or reference, and fi(λex) refers to the corresponding absorption factor at the excitation wavelength. The results obtained using the comparative method and the integrating sphere were almost identical, within the standard deviation margin for the integrating sphere (2%). FQY of LDS-698 in CHCl3 was 14% for the absolute method and 15.5% for the comparative method. Hence, LDS-698 was used as a standard to determine the quantum yield for Ag15-DNA and Ag19-DNA. For Ag21-DNA and Ag16-DNA-Cl2, FQY values were taken from previous reports.15,16
corresponds to the fluorescence integral of the sample or reference, and fi(λex) refers to the corresponding absorption factor at the excitation wavelength. The results obtained using the comparative method and the integrating sphere were almost identical, within the standard deviation margin for the integrating sphere (2%). FQY of LDS-698 in CHCl3 was 14% for the absolute method and 15.5% for the comparative method. Hence, LDS-698 was used as a standard to determine the quantum yield for Ag15-DNA and Ag19-DNA. For Ag21-DNA and Ag16-DNA-Cl2, FQY values were taken from previous reports.15,16
NLO measurements
Two-photon excited photoluminescence (2PEL) was measured using a custom-built multiphoton setup. The excitation source was femtosecond mode-locked Ti:Sapphire laser (∼100 fs, 80 MHz, Chameleon, Coherent Inc. with a wavelength range 680 to 1080 nm), combined with an optical parametric oscillator Chameleon OPO (Coherent Inc., applied in 1100–1600 nm range). The signal was collected, and emission spectra were measured with a spectrograph – Shamrock 303i (Andor) with an iDUS camera (Andor). Depending on excitation wavelength ranges, optical filters were also used: FELH0950 – Ø 25.0 mm Longpass Filter (Thorlabs), FELH0800 – Ø 25.0 mm Longpass Filter (Thorlabs). The power of the laser irradiating the sample was measured and calibrated for each wavelength. Laser power was kept at ∼10 mW for most measured wavelengths, to ensure quality signal of detectors and no photobleaching. Samples were irradiated only at the time of signal collection to avoid photobleaching. The sample and reference dye were always measured at the same excitation power. 2PA cross-sections were calculated with the following equation.45,61where, r and s denote reference and sample, respectively. ϕr and ϕs is the fluorescence quantum yield. F2,s and F2,r is the integrated two-photon fluorescence intensity at a particular excitation wavelength, n is the refractive index of the solvent. Cs and Cr is the concentration of the sample and reference, respectively. Styryl 9M and LDS-698 in CHCl3 were used as a reference. FQY of Styryl was taken from literature.27 FQY of LDS-698 in CHCl3 was measured for this work and is described in the FQY measurements section. 2PA cross-sections of Styryl 9M and LDS-698 were obtained from previously reported literature.45,61 Laser system employed in additional measurements of Ag16-DNA-Cl2 was a Coherent Ti: Sapphire Astrella regenerative amplifier combined with an OPA TOPAS Prime (Coherent) optical parametric amplifier providing tunable ∼60 fs pulses at 1 kHz.
Two-photon brightness (σ2,b) was calculated using the equation:
| σ2,b = σ2 × ϕ |
Power dependence of luminescence intensity
To confirm the two-photon nature of the observed fluorescence excited by laser pulses, we measured fluorescence intensity vs. incident laser excitation power and determined the power exponent, n. 2PEL was collected by photon-counting avalanche photodiode (IDQ id100) or spectrograph – Shamrock 303i (Andor) with an iDUS camera (Andor). To avoid photobleaching, we recorded three separate spectra at each power or recorded for 30 s on an avalanche photodiode to detect any change in intensity. The power exponent was calculated using the equation:where PL intensity is a 2P excited photoluminescence intensity and P is the average incident laser power.
Data availability
The data that support the findings of this study are available within the article and ESI.†Author contributions
A. H. conducted optical experiments and analysed the data. R. G. prepared and characterized atomically precise nanocluster solutions. S. M. C. and J. O. B. supervised the research, analyzed the data and acquired the funding. All authors took part in writing and editing the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Sonata Bis project from National Science Centre (2019/34/E/ST5/00276). R. G. and S. M. C. acknowledge support from the Hellman Faculty Fellowship and grant 2024-337801 from the Chan Zuckerberg Initiative DAF, an advised fund of the Silicon Valley Community Foundation. The authors acknowledge Anna Gonzàlez-Rosell for preparing one of the nanoclusters used in this study.References
- J. T. Petty, J. Zheng, N. V. Hud and R. M. Dickson, DNA-Templated Ag Nanocluster Formation, J. Am. Chem. Soc., 2004, 126(16), 5207–5212 CrossRef CAS PubMed.
- Y. Chen, M. L. Phipps, J. H. Werner, S. Chakraborty and J. S. Martinez, DNA Templated Metal Nanoclusters: From Emergent Properties to Unique Applications, Acc. Chem. Res., 2018, 51(11), 2756–2763 CrossRef CAS PubMed.
- A. Gonzàlez-Rosell, C. Cerretani, P. Mastracco, T. Vosch and S. M. Copp, Structure and luminescence of DNA-templated silver clusters, Nanoscale Adv., 2021, 3(5), 1230–1260 RSC.
- S. M. Copp, D. Schultz, S. Swasey, J. Pavlovich, M. Debord, A. Chiu, K. Olsson and E. Gwinn, Magic Numbers in DNA-Stabilized Fluorescent Silver Clusters Lead to Magic Colors, J. Phys. Chem. Lett., 2014, 5(6), 959–963 CrossRef CAS PubMed.
- R. Guha, A. Gonzàlez-Rosell, M. Rafik, N. Arevalos, B. B. Katz and S. M. Copp, Electron count and ligand composition influence the optical and chiroptical signatures of far-red and NIR-emissive DNA-stabilized silver nanoclusters, Chem. Sci., 2023, 14(41), 11340–11350 RSC.
- A. Gonzàlez-Rosell and S. M. Copp, An Atom-Precise Understanding of DNA-Stabilized SilverNanoclusters, Acc. Chem. Res., 2024, 57, 2117–2129 CrossRef PubMed.
- A. V. Pinheiro, D. Han, W. M. Shih and H. Yan, Challenges and opportunities for structural DNA nanotechnology, Nat. Nanotechnol., 2011, 6(12), 763–772 CrossRef CAS PubMed.
- S. M. Copp and A. Gonzàlez-Rosell, Large-scale investigation of the effects of nucleobase sequence on fluorescence excitation and Stokes shifts of DNA-stabilized silver clusters, Nanoscale, 2021, 13, 4602–4613 RSC.
- A. Gonzàlez-Rosell, S. Malola, R. Guha, N. R. Arevalos, M. F. Matus, M. E. Goulet, E. Haapaniemi, B. B. Katz, T. Vosch, J. Kondo, H. Häkkinen and S. M. Copp, Chloride Ligands on DNA-Stabilized Silver Nanoclusters, J. Am. Chem. Soc., 2023, 145(19), 10721–10729 CrossRef PubMed.
- R. Guha and S. M. Copp, 12 Nucleic Acid-Templated Metal Nanoclusters, in Modern Avenues in Metal-Nucleic Acid Chemistry, ed. Müller, J. and Lippert, B., CRC Press, 2023, pp. 291–342 Search PubMed.
- P. Mastracco and S. M. Copp, Beyond nature's base pairs: machine learning-enabled design of DNA-stabilized silver nanoclusters, Chem. Commun., 2023, 59, 10360–10375 RSC.
- M. B. Liisberg, Z. Shakeri Kardar, S. M. Copp, C. Cerretani and T. Vosch, Single-Molecule Detection of DNA-Stabilized Silver Nanoclusters Emitting at the NIR I/II Border, J. Phys. Chem. Lett., 2021, 12(4), 1150–1154 CrossRef CAS PubMed.
- J. T. Petty, C. Fan, S. P. Story, B. Sengupta, M. Sartin, J.-C. Hsiang, J. W. Perry and R. M. Dickson, Optically Enhanced, Near-IR, Silver Cluster Emission Altered by Single Base Changes in the DNA Template, J. Phys. Chem. B, 2011, 115(24), 7996–8003 CrossRef CAS PubMed.
- J. T. Petty, C. Fan, S. P. Story, B. Sengupta, A. St. John Iyer, Z. Prudowsky and R. M. Dickson, DNA Encapsulation of 10 Silver Atoms Producing a Bright, Modulatable, Near-Infrared-Emitting Cluster, J. Phys. Chem. Lett., 2010, 1(17), 2524–2529 CrossRef CAS PubMed.
- S. A. Bogh, M. R. Carro-Temboury, C. Cerretani, S. M. Swasey, S. M. Copp, E. G. Gwinn and T. Vosch, Unusually large Stokes shift for a near-infrared emitting DNA-stabilized silver nanocluster, Methods Appl. Fluoresc., 2018, 6(2), 024004 CrossRef PubMed.
- V. A. Neacşu, C. Cerretani, M. B. Liisberg, S. M. Swasey, E. G. Gwinn, S. M. Copp and T. Vosch, Unusually large fluorescence quantum yield for a near-infrared emitting DNA-stabilized silver nanocluster, Chem. Commun., 2020, 56(47), 6384–6387 RSC.
- R. Weissleder, A clearer vision for in vivo imaging, Nat. Biotechnol., 2001, 19(4), 316–317 CrossRef CAS PubMed.
- J. V. Frangioni, In vivo near-infrared fluorescence imaging, Curr. Opin. Chem. Biol., 2003, 7(5), 626–634 CrossRef CAS PubMed.
- A. M. Smith, M. C. Mancini and S. Nie, Second window for in vivo imaging, Nat. Nanotechnol., 2009, 4(11), 710–711 CrossRef CAS PubMed.
- S. E. Crowe and G. C. R. Ellis-Davies, Longitudinal in vivo two-photon fluorescence imaging, J. Comp. Neurol., 2014, 522(8), 1708–1727 CrossRef PubMed.
- D. Kobat, M. E. Durst, N. Nishimura, A. W. Wong, C. B. Schaffer and C. Xu, Deep tissue multiphoton microscopy using longer wavelength excitation, Opt. Express, 2009, 17(16), 13354–13364 CrossRef PubMed.
- D. Kobat, N. Horton and C. Xu, In vivo two-photon microscopy to 1.6-mm depth in mouse cortex, J. Biomed. Opt., 2011, 16(10), 106014 Search PubMed.
- K. W. Dunn and P. A. Young, Principles of Multiphoton Microscopy, Nephron Exp. Nephrol., 2006, 103(2), e33–e40 CrossRef PubMed.
- C. Xu, W. Zipfel, J. B. Shear, R. M. Williams and W. W. Webb, Multiphoton fluorescence excitation: new spectral windows for biological nonlinear microscopy, Proc. Natl. Acad. Sci. U.S.A., 1996, 93(20), 10763–10768 CrossRef CAS PubMed.
- W. R. Zipfel, R. M. Williams and W. W. Webb, Nonlinear magic: multiphoton microscopy in the biosciences, Nat. Biotechnol., 2003, 21(11), 1369–1377 CrossRef CAS PubMed.
- J. Olesiak-Banska, M. Waszkielewicz, P. Obstarczyk and M. Samoc, Two-photon absorption and photoluminescence of colloidal gold nanoparticles and nanoclusters, Chem. Soc. Rev., 2019, 48(15), 4087–4117 RSC.
- A. Pniakowska, K. Kumaranchira Ramankutty, P. Obstarczyk, M. Perić Bakulić, Ž. Sanader Maršić, V. Bonačić-Koutecký, T. Bürgi and J. Olesiak-Bańska, Gold-Doping Effect on Two-Photon Absorption and Luminescence of Atomically Precise Silver Ligated Nanoclusters, Angew. Chem., Int. Ed., 2022, 61(43), e202209645 CrossRef CAS PubMed.
- V. Bonačić-Koutecký and R. Antoine, Enhanced two-photon absorption of ligated silver and gold nanoclusters: theoretical and experimental assessments, Nanoscale, 2019, 11(26), 12436–12448 RSC.
- J. Olesiak-Banska, M. Waszkielewicz, K. Matczyszyn and M. Samoc, A closer look at two-photon absorption, absorption saturation and nonlinear refraction in gold nanoclusters, RSC Adv., 2016, 6(101), 98748–98752 RSC.
- A. S. Reyna, I. Russier-Antoine, F. Bertorelle, E. Benichou, P. Dugourd, R. Antoine, P.-F. Brevet and C. B. de Araújo, Nonlinear Refraction and Absorption of Ag29 Nanoclusters: Evidence for Two-Photon Absorption Saturation, J. Phys. Chem. C, 2018, 122(32), 18682–18689 CrossRef CAS.
- S. H. Yau, N. Abeyasinghe, M. Orr, L. Upton, O. Varnavski, J. H. Werner, H.-C. Yeh, J. Sharma, A. P. Shreve, J. S. Martinez and T. Goodson Iii, Bright two-photon emission and ultra-fast relaxation dynamics in a DNA-templated nanocluster investigated by ultra-fast spectroscopy, Nanoscale, 2012, 4(14), 4247–4254 RSC.
- S. A. Patel, C. I. Richards, J.-C. Hsiang and R. M. Dickson, Water-Soluble Ag Nanoclusters Exhibit Strong Two-Photon-Induced Fluorescence, J. Am. Chem. Soc., 2008, 130(35), 11602–11603 CrossRef CAS PubMed.
- X. Wang, M. B. Liisberg, G. L. Nolt, X. Fu, C. Cerretani, L. Li, L. A. Johnson, T. Vosch and C. I. Richards, DNA-AgNC Loaded Liposomes for Measuring Cerebral Blood Flow Using Two-Photon Fluorescence Correlation Spectroscopy, ACS Nano, 2023, 17(13), 12862–12874 CrossRef CAS PubMed.
- H. J. Chun, E. S. Kim and B. R. Cho, Scope and limitation of label-free multiphoton microscopy and probe-labeled two-photon microscopy for the endomicroscopic diagnosis, Scanning, 2014, 36(4), 462–464 CrossRef CAS PubMed.
- H. M. Kim and B. R. Cho, Small-Molecule Two-Photon Probes for Bioimaging Applications, Chem. Rev., 2015, 115(11), 5014–5055 CrossRef CAS PubMed.
- C. Cerretani, H. Kanazawa, T. Vosch and J. Kondo, Crystal structure of a NIR-Emitting DNA-Stabilized Ag16 Nanocluster, Angew. Chem., Int. Ed., 2019, 58(48), 17153–17157 CrossRef CAS PubMed.
- C. Cerretani, M. B. Liisberg, V. Rück, J. Kondo and T. Vosch, The effect of inosine on the spectroscopic properties and crystal structure of a NIR-emitting DNA-stabilized silver nanocluster, Nanoscale Adv., 2022, 4(15), 3212–3217 RSC.
- C. Cerretani, G. Palm-Henriksen, M. B. Liisberg and T. Vosch, The effect of deuterium on the photophysical properties of DNA-stabilized silver nanoclusters, Chem. Sci., 2021, 12(48), 16100–16105 RSC.
- V. Rück, C. Cerretani and T. Vosch, How Inert is Single-Stranded DNA Towards DNA-Stabilized Silver Nanoclusters? A Case Study, ChemPhotoChem, 2024, e202400014 CrossRef.
- C. Cerretani, J. Kondo and T. Vosch, Removal of the A10 adenosine in a DNA-stabilized Ag16 nanocluster, RSC Adv., 2020, 10(40), 23854–23860 RSC.
- S. Malola, M. F. Matus and H. Häkkinen, Theoretical Analysis of the Electronic Structure and Optical Properties of DNA-Stabilized Silver Cluster Ag16Cl2 in Aqueous Solvent, J. Phys. Chem. C, 2023, 127(33), 16553–16559 CrossRef CAS.
- H. Häkkinen, Atomic and electronic structure of gold clusters: understanding flakes, cages and superatoms from simple concepts, Chem. Soc. Rev., 2008, 37(9), 1847–1859 RSC.
- A. Gonzàlez-Rosell, R. Guha, C. Cerretani, V. Rück, M. B. Liisberg, B. B. Katz, T. Vosch and S. M. Copp, DNA Stabilizes Eight-Electron Superatom Silver Nanoclusters with Broadband Downconversion and Microsecond-Lived Luminescence, J. Phys. Chem. Lett., 2022, 13(35), 8305–8311 CrossRef PubMed.
- S. M. Copp, D. Schultz, S. M. Swasey, A. Faris and E. G. Gwinn, Cluster Plasmonics: Dielectric and Shape Effects on DNA-Stabilized Silver Clusters, Nano Lett., 2016, 16(6), 3594–3599 CrossRef CAS PubMed.
- N. S. Makarov, M. Drobizhev and A. Rebane, Two-photon absorption standards in the 550–1600 nm excitation wavelength range, Opt. Express, 2008, 16(6), 4029–4047 CrossRef CAS PubMed.
- C. Li, Nonlinear Optics. Principles and Applications. 1 edn, Springer Singapore, 2016, vol. XVII, p. 386 Search PubMed.
- M. Drobizhev, F. Meng, A. Rebane, Y. Stepanenko, E. Nickel and C. W. Spangler, Strong Two-Photon Absorption in New Asymmetrically Substituted Porphyrins: Interference between Charge-Transfer and Intermediate-Resonance Pathways, J. Phys. Chem. B, 2006, 110(20), 9802–9814 CrossRef CAS PubMed.
- M. Drobizhev, N. S. Makarov, T. Hughes and A. Rebane, Resonance Enhancement of Two-Photon Absorption in Fluorescent Proteins, J. Phys. Chem. B, 2007, 111(50), 14051–14054 CrossRef CAS PubMed.
- M. Drobizhev, N. S. Makarov, S. E. Tillo, T. E. Hughes and A. Rebane, Two-photon absorption properties of fluorescent proteins, Nat. Methods, 2011, 8(5), 393–399 CrossRef CAS PubMed.
- S. Malola and H. Häkkinen, On transient absorption and dual emission of the atomically precise, DNA-stabilized silver nanocluster Ag16Cl2, Chem. Commun., 2024, 60(24), 3315–3318 RSC.
- J. Chen, A. Kumar, C. Cerretani, T. Vosch, D. Zigmantas and E. Thyrhaug, Excited-State Dynamics in a DNA-Stabilized Ag16 Cluster with Near-Infrared Emission, J. Phys. Chem. Lett., 2023, 14(17), 4078–4083 CrossRef CAS PubMed.
- S. Wang, B. Li and F. Zhang, Molecular Fluorophores for Deep-Tissue Bioimaging, ACS Cent. Sci., 2020, 6(8), 1302–1316 CrossRef CAS PubMed.
- S. Pascal, S. David, C. Andraud and O. Maury, Near-infrared dyes for two-photon absorption in the short-wavelength infrared: strategies towards optical power limiting, Chem. Soc. Rev., 2021, 50(11), 6613–6658 RSC.
- P. A. Shaw, E. Forsyth, F. Haseeb, S. Yang, M. Bradley and M. Klausen, Two-Photon Absorption: An Open Door to the NIR-II Biological Window?, Front. Chem., 2022, 10, 921354 CrossRef CAS PubMed.
- H. Kawano, T. Kogure, Y. Abe, H. Mizuno and A. Miyawaki, Two-photon dual-color imaging using fluorescent proteins, Nat. Methods, 2008, 5(5), 373–374 CrossRef CAS PubMed.
- S. E. Tillo, T. E. Hughes, N. S. Makarov, A. Rebane and M. Drobizhev, A new approach to dual-color two-photon microscopy with fluorescent proteins, BMC Biotechnol., 2010, 10(1), 6 CrossRef PubMed.
- V. Rück, N. K. Mishra, K. K. Sørensen, M. B. Liisberg, A. B. Sloth, C. Cerretani, C. B. Mollerup, A. Kjaer, C. Lou, K. J. Jensen and T. Vosch, Bioconjugation of a Near-Infrared DNA-Stabilized Silver Nanocluster to Peptides and Human Insulin by Copper-Free Click Chemistry, J. Am. Chem. Soc., 2023, 145(30), 16771–16777 CrossRef PubMed.
- M. Drobizhev, S. Tillo, N. S. Makarov, T. E. Hughes and A. Rebane, Absolute Two-Photon Absorption Spectra and Two-Photon Brightness of Orange and Red Fluorescent Proteins, J. Phys. Chem. B, 2009, 113(4), 855–859 CrossRef CAS PubMed.
- M. Y. Berezin, C. Zhan, H. Lee, C. Joo, W. J. Akers, S. Yazdanfar and S. Achilefu, Two-Photon Optical Properties of Near-Infrared Dyes at 1.55 μm Excitation, J. Phys. Chem. B, 2011, 115(39), 11530–11535 CrossRef CAS PubMed.
- K. Rurack and M. Spieles, Fluorescence Quantum Yields of a Series of Red and Near-Infrared Dyes Emitting at 600–1000 nm, Anal. Chem., 2011, 83(4), 1232–1242 CrossRef CAS PubMed.
- N. S. Makarov, J. Campo, J. M. Hales and J. W. Perry, Rapid, broadband two-photon-excited fluorescence spectroscopy and its application to red-emitting secondary reference compounds, Opt. Mater. Express, 2011, 1(4), 551–563 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc05853d |
| This journal is © The Royal Society of Chemistry 2025 |