Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Valorization systems based on electrocatalytic nitrate/nitrite conversion for energy supply and valuable product synthesis
Yi
Feng
,
Jin-Tao
Ren
,
Ming-Lei
Sun
and
Zhong-Yong
Yuan
 *
*
School of Materials Science and Engineering, Smart Sensing Interdisciplinary Science Center, Nankai University, Tianjin 300350, China. E-mail: zyyuan@nankai.edu.cn
First published on 29th November 2024
Abstract
The excessive accumulation of nitrate/nitrite (NOx−) in surface and groundwater has severely disrupted the global nitrogen cycle and jeopardized public health. The electrochemical conversion of NOx− to ammonia (NH3) not only holds promise for ecofriendly NOx− removal, but also provides a green alternative to the energy-intensive Haber–Bosch process for NH3 production. Recently, in addition to the electrocatalyst design explosion in this field, many innovative valorization systems based on NOx−-to-NH3 conversion have been developed for generating energy and expanding the range of value-added products. Collective knowledge of advanced conversion systems is indispensable for restoring the global nitrogen cycle and promoting a N-based economy. Herein, a timely and comprehensive review is provided on the important progress of valorization systems based on NOx− conversion, including waste treatment systems, novel electrolytic systems, and energy conversion and storage systems. Some mechanism explorations, device designs, key electrode developments and feasibility analyses are involved to gain deeper understanding of various systems and facilitate implementing these cleaning systems in industry. Finally, challenges and future prospects are outlined in the NOx− conversion field with an aim to promote large-scale electrocatalytic system development and prosperous N-based electrochemistry.
1. Introduction
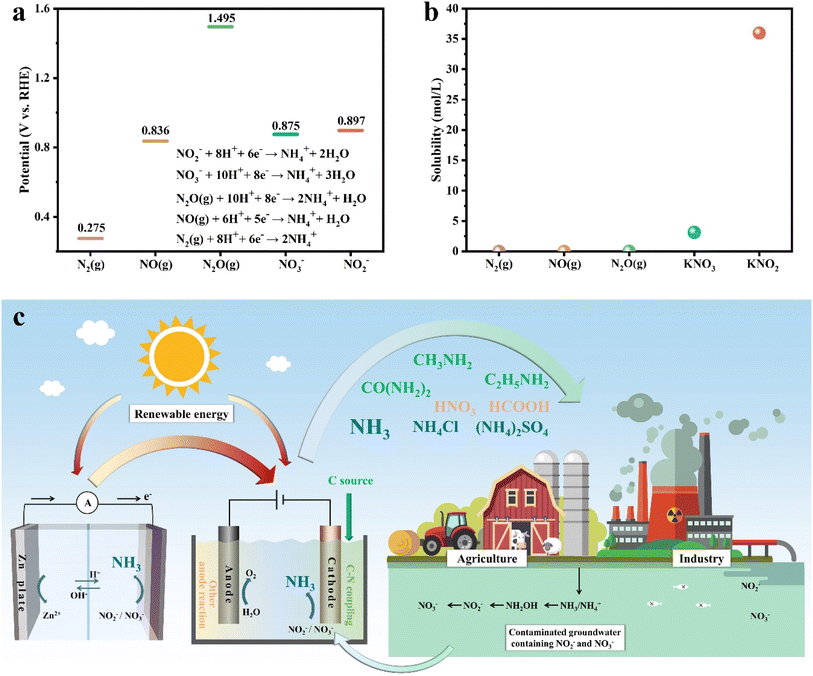
Nitrogen is an indispensable element in the biological processes of organisms, and the global nitrogen cycle plays a crucial role in material exchange within the biosphere.1–5 The severe disruption of the global nitrogen cycle is caused by the abusive use of synthetic N-containing fertilizers and chemicals,6–8 causing excessive accumulation of N-species such as nitrate/nitrite (NOx−) in surface and groundwater, which may lead to eutrophic water bodies, devastated aquatic ecosystems and jeopardized public health.9 For restoring the global nitrogen cycle and alleviating the pollutant effect, an electrochemistry-assisted strategy has surged as a promising approach for the degradation of NOx− in wastewater,10 in contrast with the most widely employed conventional biotechnology method requiring an adequate organic carbon source,11 which stands at the midpoint of pursuing sustainable NOx− removal and carbon neutrality.12–14 Compared with the direct conversion to harmless and low-value N2,15 more and more efforts are being devoted to electrocatalytic conversion of NOx− to NH3 due to easier N–H bond formation and more valuable products.NH3 is a versatile chemical raw material accounting for 5% of the chemical market value and has also been acknowledged as an intriguing carbon-free energy carrier containing 17.5 wt% hydrogen (H2).16 However, industrial synthesis of NH3 heavily relies on the Haber–Bosch process which consumes a considerable amount of energy of 5.5 EJ per year and emits about 3.0 t of CO2 per metric ton of NH3 produced.17 Thus, further advancements are necessary to achieve lower temperature ammonia synthesis with a low or zero carbon footprint. So far, several attractive routes for electrocatalytic NH3 synthesis under ambient conditions have been proposed, which can be achieved by electrochemical reduction of N-containing species such as nitrogen (N2)18 and nitrogen oxides (NO, N2O).19 However, the electrochemical reduction efficiencies of N2, NO and N2O are limited by the vigorous competition from the H2 evolution reaction (HER) due to their ultralow solubility in water (Fig. 1a and b). In contrast, electrocatalytic reduction reactions of NOx− are much easier to mitigate against the HER competition due to their higher solubility and more positive potentials.20
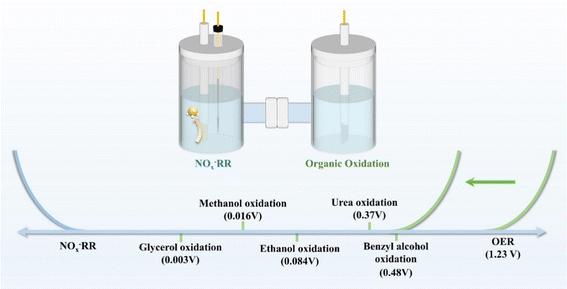
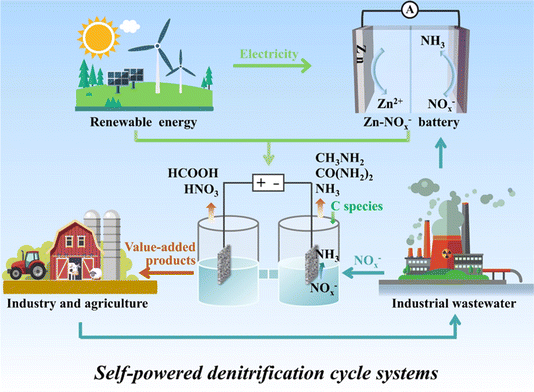
The period since 2020 has witnessed an explosive growth in the literature devoted to designing advanced electrocatalysts with high faradaic efficiency (FE) and NH3 yield rate, reflecting a comprehensive and intensive exploration of diverse electrode materials.21–23 In recent years, a variety of valorization systems (Fig. 1c) based on electrochemical NOx−-to-NH3 conversion have been developed,24–27 including sewage treatment systems for NOx− removal and systems for producing NH3-based chemicals, energy storage systems including metal–NOx− batteries and N2H4–NOx− batteries for energy supply and storing intermittent renewable energies, and novel electrolytic systems for production of multiple value-added chemicals. The design of novel electrolytic systems can include the modification and substitution of anode and cathode reactions. According to the cathodic reaction, integrated and tandem reactions based on NOx− reduction and C species conversion can yield high-value-added chemicals such as urea28 and methylamine,29 which are generally synthesized through energy- and emission-intensive processes. More intriguingly, the anodic reaction is commonly the oxygen evolution reaction (OER) in an electrolyzer for electrocatalytic NO3−/NO2− reduction to NH3, which possesses sluggish kinetics and produces low-value O2 ($25 per ton).30 Numerous reactions including oxidation of small organic molecules could then be employed to replace the anodic OER for reducing overall energy consumption and obtaining other high-value-added products.31
In view of the significance of the collective knowledge on advanced conversion systems for prosperous N-based chemistry and the scarcity of systematic reviews towards NOx− reduction applications, a timely and comprehensive review is provided on the recent fundamental insights and achievements of valorization systems based on NOx− conversion, including waste treatment systems, novel electrolytic systems, and energy conversion and storage systems. In this review, the basic knowledge of NOx− reduction is firstly provided including reaction mechanisms, reaction devices and design principles of catalysts. Then, the obtained technological innovations and existing challenges are elaborated on with regards to the applications of NOx− reduction by summarizing mechanism explorations, key electrode developments, and feasibility analyses. Finally, challenges and future prospects are outlined in the NOx− conversion field with an aim to promote large-scale electrocatalytic system development and prosperous N-based electrochemistry.
2. Fundamentals of electrocatalytic NO3−/NO2− reduction
2.1 Reaction mechanisms
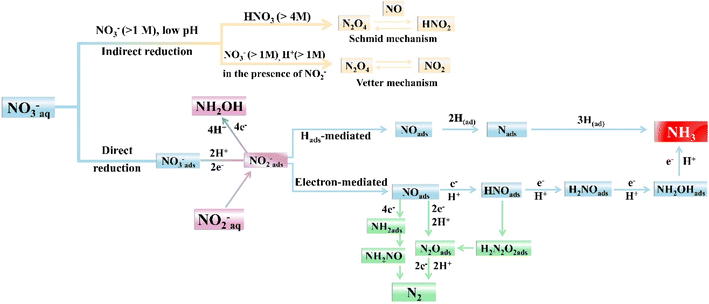
Recognizing and mastering the mechanisms of the electrocatalytic conversions of NO3− and NO2− is a prerequisite for the development of self-powered denitrification systems. Electrochemical reduction of NO3− is a complex process with multielectron reactions,32 which involves various nitrogen-containing intermediates and products ranging from −3 to +5 valence states. N2 and NH3/NH4+ are widely recognized as the most thermodynamically stable products,33 but the final result can be altered due to several causes, such as cathode materials and the pH value of the solution. The indirect autocatalytic reduction mechanism and the direct electrocatalytic reduction mechanism comprise the currently accepted mechanism of NO3− electroreduction.34 As depicted in Fig. 2, the indirect autocatalytic reaction is considered as the reduction process without NO3− involved in the electron transfer, only existing under the conditions of nitrate concentration exceeding 1 M and low pH value. However, more efforts are put into the mechanism of NO3− electroreduction occurring at concentrations lower than 1 M (direct reaction).35 The active adsorbed hydrogen atom (Hads)-mediated route and the electron-mediated pathway are pursued simultaneously in the direct mechanism (Fig. 2), which leads to the complexity of the electrocatalytic NO3− reduction.The electroreduction of NO3− is initiated by the adsorption of NO3− ions onto the cathodic electrodes. The adsorbed NO3− is transformed into NO2− by a tripartite electrochemical–chemical–electrochemical process, which is recognized as the dominant rate-controlling step.36 Later, the nitric oxide (NOads) intermediate is derived by NO2− conversion. As depicted in Fig. 2, NOads can be reduced to NH3 as the ultimate product and occupy a dominant position in the N2 formation pathway.
In addition, the reduction process of NO3− can be mediated by Hads. NO2ads−, NO3−, and NOads can be reduced by Hads.15 The predominant final product in this Hads-mediated process is NH3, which is caused by the fact that the formation of N–N bonds mediated by Hads is kinetically more challenging than the formation of N–H bonds. The specific Hads-mediated pathways are described by reactions (1)–(7).
| H2O + e− → Hads + OH− | (1) |
| NO3− + 2Hads → NO2ads− + H2O | (2) |
| NO2ads− + Hads → NOads + OH− | (3) |
| NOads + 2Hads → Nads + H2O | (4) |
| Nads + Hads → NHads | (5) |
| NHads + Hads → NH2ads | (6) |
| NH2ads + Hads → NH3ads | (7) |
NOads is also essential for the formation of N2. As illustrated in eqn (8)–(11), unstable HN2O2 can be formed by NOads, sequentially forming N2Oads and N2 through electron transfer. The generation of N2 can also be obtained by the rapid decomposition of NH2NO. It is known from eqn (12)–(14) that the stable NH2ads can be formed through the NOads reduction process, which can react with NOads to form NH2NO.
| NOads + NOads + e− + H+ → HN2O2 | (8) |
| HN2O2ads + e− + H+ → N2Oads + H2O | (9) |
| N2O + e− → N2O− | (10) |
| N2O− + e− + 2H+ → N2 + H2O | (11) |
| NOads + 3H2O + 4e− → NH2ads + 4OH− | (12) |
| NOads + NH2ads → NH2NOads | (13) |
| NH2NOads → N2 + H2O | (14) |
The electrocatalytic NO2− reduction to NH3 is roughly identical to the reduction process following the conversion of NO3− to NO2−. In fact, NO2− reduction is easier than NO3−reduction due to less charge transfer involved. In the NO3− reduction process on many catalysts, the rate-limiting step is always the conversion of NO3 to NO2ads−.37 The in situ Fourier transform infrared spectroscopy in most studies is employed to explore the formation of hydroxylamine (NH2OH) during the NO2−/NO3− reduction process, which is a vital feedstock for caprolactam synthesis and pesticide production.38 However, in most cases, NH2OH is inclined to be converted to NH4+.
2.2 Design principles of NOx− reduction electrocatalysts
It is evident from the above that the selectivity and energy efficiency of NOx− reduction can be influenced by the pH of the electrolyte and the reactor designs. In general, the required potential of NOx− reduction in the alkaline environment is much lower than that in neutral and acidic environments.39–41 Alkaline conditions are more conducive to inhibiting the competing HER compared with acidic conditions providing more active hydrogen species. In neutral or alkaline environments, the required protons for NOx− reduction are obtained from dissociation of water molecules. Diffusion of water molecules in neutral solutions is the rate-determining step of proton formation, while the rate-determining step in alkaline environments is the transport of adsorbed *OH. Intriguingly, the *OH produced facilitates the generation of NH4OH due to the attraction of nitrogenous species, which accelerates NOx− reduction and reduces the competition of the HER. Thus, the design of NOx− reduction catalysts for alkaline conditions is discussed in the majority of efforts available currently, since NOx− reduction under alkaline conditions is easier to conduct with decent selectivity. In view of the neutral or acidic conditions of most wastewater, the associated design of catalysts under neutral and acidic conditions will also be discussed in the wastewater treatment section. In this section, the design principles of NOx− reduction catalysts under alkaline conditions will be primarily discussed.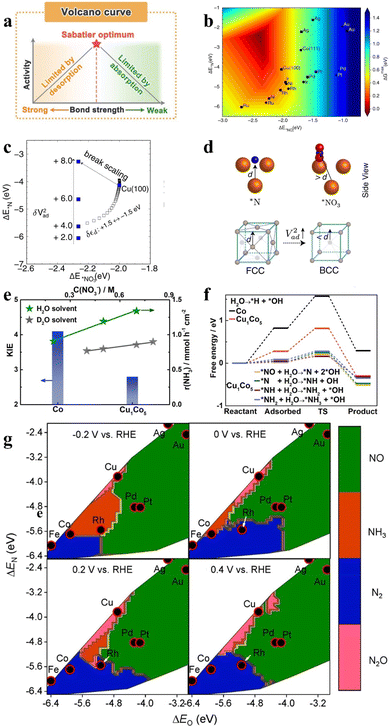 | ||
| Fig. 3 (a) Volcanic curves of activity based on Sabatier's principle. Reproduced with permission.47 Copyright 2022, Elsevier. (b) The activity volcano plot of various metal elements for the conversion of NO3− to NH3, (c) adsorption energies of *NO3 and *N on Cu (100) under different Vad2. (d) The models of the (100) facet and (111) facet. Reproduced with permission.48 Copyright 2022, Springer Nature. (e) Reaction kinetics and H/D kinetic isotope effect of the NO3−RR with Cu1Co5 and (f) Gibbs free energies on Co and Cu1Co5. Reproduced with permission.49 Copyright 2023, American Chemical Society. (g) Theoretical selectivity maps to various nitrogen-containing products from electrocatalytic NO3− reduction on the basis of ΔEO and ΔEN under different applied voltages. Reproduced with permission.33 Copyright 2019, American Chemical Society. | ||
Apart from the excellent energy conversion efficiency, high selectivity is also required in catalysts. The activity and selectivity of transition metals are exhibited in Fig. 3g for NO3− reduction, which is predicted by exploring the adsorption energies of O and N atoms under different potentials.33 It is revealed that catalysts with moderate ΔEO and ΔEN are inclined to exhibit more remarkable NH3 selectivity at more negative potentials. The modulation of ΔEO and ΔEN can be achieved by adjusting the d-band center of the catalyst. For example, Cu50Ni50 was obtained by introducing Ni into Cu, which exhibited six-fold higher NO3− reduction activity than pure Cu at the same potential.54 After the introduction of Ni, the d-band center of Cu50Ni50 was shifted by 0.28 eV, compared to the d-band center position of pure Cu (−2.84 eV). The regulation of the d-band center has been confirmed to contribute to modulating the adsorption energies of intermediates including *NO3−, *NO2, and *NH2, leading to the enhanced performance of Cu50Ni50 for NO3− reduction.
Moreover, reducing competition from other side reactions is also worthy of attention for NO3− reduction in aqueous systems,55 including (1) the coupling reactions between Nads and Nads for blocking NH3 production and causing the generation of N2, N2H4, and N2O, (2) the competition for active hydrogen species with the HER only involving two-electron transfer. Single-atom metal-based catalysts offer new insights into reducing the direct coupling of Nads in adjacent active sites due to the lack of adjacent sites,56 which is promising for inhibiting the generation of by-products including N2 and N2O and enhancing NH3 selectivity. In addition, the advantages of low metal loading and high metal utilization ratio in single-atom metal-based catalysts endow single-atom metal-based catalysts with the potential to be extremely cost-effective catalysts. Direct solution-phase synthesis (Fig. 4a) is employed for obtaining Cu/CuAu core/shell nanocrystals with tunable single-atom alloy layers.57 The synthetic Cu/CuAu nanocrystals reach a decent FE of 85.5% in NO3− reduction at −0.5 V vs. RHE with high densities of single atoms. The weakened anchoring of Nads (Fig. 4b) is conducive to the decent performance of Cu/CuAu nanocrystals, which is caused by strong repulsion from the gold ligand in subsurface or single-atom gold in the surface.
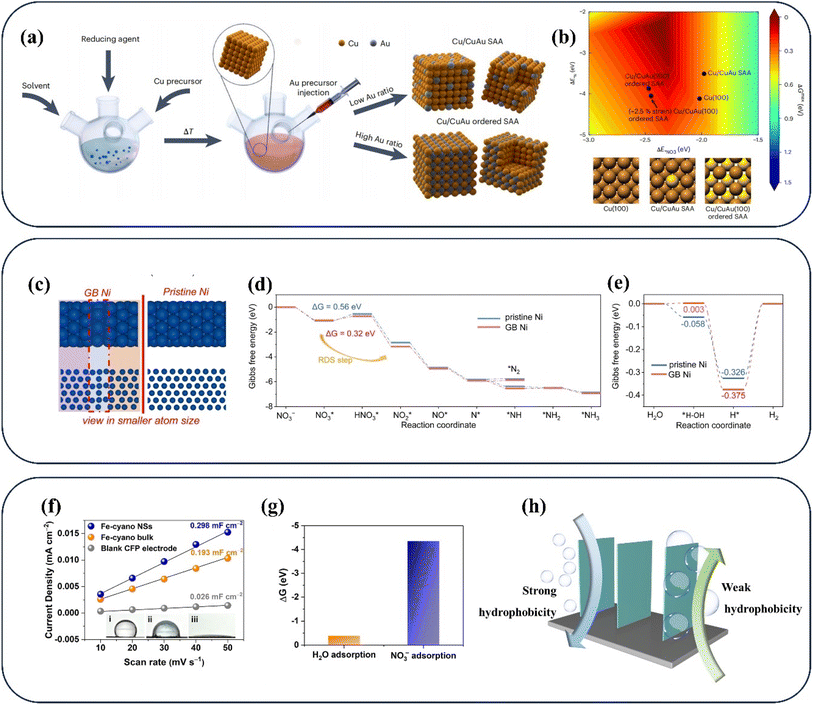 | ||
| Fig. 4 (a) Schematic diagram of synthesizing Cu/CuAu core/shell nanocrystals, (b) the binding energies of *NO3 and *N in related catalysts. Reproduced with permission.57 Copyright 2023, Springer Nature. (c) The structure model, (d) Gibbs free energy diagrams for NO3− reduction (e) for the HER of pristine Ni and GB Ni. Reproduced with permission.58 Copyright 2023, Royal Society of Chemistry. (f) Contact angle measurement and (g) adsorption energy on Fe-cyano NSs. Reproduced with permission.59 Copyright 2022, American Chemical Society. (h) The states of bubbles on different aerophobic surfaces. | ||
Furthermore, the NOx− reduction process can be hampered by the existence of the HER, which is due to the competition for active hydrogen species60 and the blockage of active sites by the H2 generated.61 The relatively inert metals including Cu during the water splitting process have gained much attention in numerous studies,62–64 with a view to eliminating competition with the HER in NOx− reduction. However, considering that the NOx− reduction process relies heavily on the active hydrogen produced by H2O splitting, the blind inhibition of active hydrogen generation can hinder the subsequent hydrogenation process of the NOx reduction process. In the common design strategy, the hydrogenation process of N species is promoted by lowering the energy barrier of *N formation when active hydrogen is abundant. Numerous efforts have employed diverse strategies such as doping and defects to lower the energy barrier of Nads formation,65,66 thus promoting the subsequent hydrogenation processes. Ni nanoparticles with grain boundary defects have been developed for opening up an entirely new strategy of utilizing water splitting and preventing H2 formation.58 Abundant active hydrogen species can be generated in the catalytic process due to the decent HER activity of Ni. Besides, the formation of H2 is difficult on the surface owing to the strong retention capacity of active hydrogen species in grain boundary (GB) regions (Fig. 4c). With the presence of grain boundary regions, the active hydrogen species are transferred to the neighbouring adsorbed intermediates for accelerating NOx− reduction. The Gibbs free energy diagrams of GB Ni for NO3− reduction and the HER are exhibited in Fig. 4d and e. The excellent selectivity of GB Ni for NH3 is confirmed by the lower formation energy of the N–H bond than that of N2 in Fig. 4d. The stronger retention capacity of GB Ni for active hydrogen species is evidenced by the higher energy barrier for obtaining H2 (Fig. 4e). In addition, the energy barrier for the conversion of *NO3 to *NO2 in the GB region is lower than that of forming H2, confirming that this strategy facilitates the inhibition of the HER, where the active hydrogen species tends to reduce *NO3 rather than form H2. The above strategy employing two sites has opened up a new avenue for achieving sufficient active hydrogen species and excellent NH3 selectivity. In this strategy, the production of protons is promoted in one site acting as a proton warehouse, while the active hydrogen species are stored temporarily at the other site to facilitate subsequent hydrogenation processes.
It is seen above that a series of hurdles are present in NOx− reduction catalysts including inferior selectivity and unpromising energy conversion efficiency. In order to achieve a sustainable and competitive electrochemical ammonia production route, the following points could be considered for constructing outstanding NOx− reduction electrocatalysts: (1) the enhanced adsorption of NOx− can promote the reduction reaction, but accompanied with the dilemma of difficult product desorption. The key to solving the problem for significantly improving performance lies in breaking the scaling relationship between the adsorption energies of intermediates and reactants; (2) the competition with the HER is difficult to escape for NOx− reduction under aqueous conditions. Boosting NH3 selectivity and reducing H2 formation can be achieved by employing appropriate strategies utilizing water splitting for achieving sufficient supply of active hydrogen species and hindering direct coupling of active hydrogen species; (3) the stability of intermediates including *NO2ads or *NOHads should not be ignored for more efficient NH3 production, which can decrease other side reactions generating N2 or NO; (4) the hydrophilic and aerophobic properties on the catalyst surface are also deserving of emphasis. The strongly hydrophilic and aerophobic surface of catalysts is more conducive to the adsorption of reactants and circumventing blocked active sites.
2.3 Reactor design
The current reactor designs under different scenarios are summarized in Fig. 5. The most common reaction devices available in the current laboratory are shown in Fig. 5a and b, which can be divided into single-chamber cells and dual-chamber cells (H-type cells). The difference between the single-chamber reactor and dual-chamber reactor is caused by the ion exchange membrane and electrode spacing. The single-chamber reactor possesses the advantages of smaller internal resistance, more simplified design and lower cost than the double-chamber reactor due to the absence of an ion exchange membrane and smaller electrode spacing.76 However, it is difficult to avoid that the dissolved metal ions generated by the anode may also be deposited on the catalyst surface in a single-chamber reactor, thus reducing the FE of ammonia production.77 Therefore, two chambers separated by ion exchange membranes are commonly employed in experimental studies to form a dual-chamber reactor. In spite of the higher ohmic resistance and larger energy consumption, the double chamber reactor can substantially decrease the occurrence of side reactions and enhance the FE of ammonia production. Notably, the dual-chamber reactors employed in a majority of the studies are typically operated under constant voltage. The dual-chamber reactor may not be more advantageous than a single-chamber reactor when the “multi-potential steps” are employed. For example, the in situ reconstruction of the Cu surface during NO3− reduction was achieved by pulsed electrolysis of the Cu electrode.78 Both the anodic pulsed oxidation reaction of Cu and the cathodic pulsed nitric acid reduction reaction occur in a single compartment, with the possibility of reoxidation of the reduction intermediates during the anodic pulse. In the process of electrolysis, the Cu oxidation by the anodic pulse and NO3− reduction by the cathodic pulse occurred in the same chamber, where the re-oxidation of reducing intermediates may occur during the anodic pulse. In this process, the selection of single-chamber reactors is caused by the unavoidable intermediate crossover in dual-chamber cells.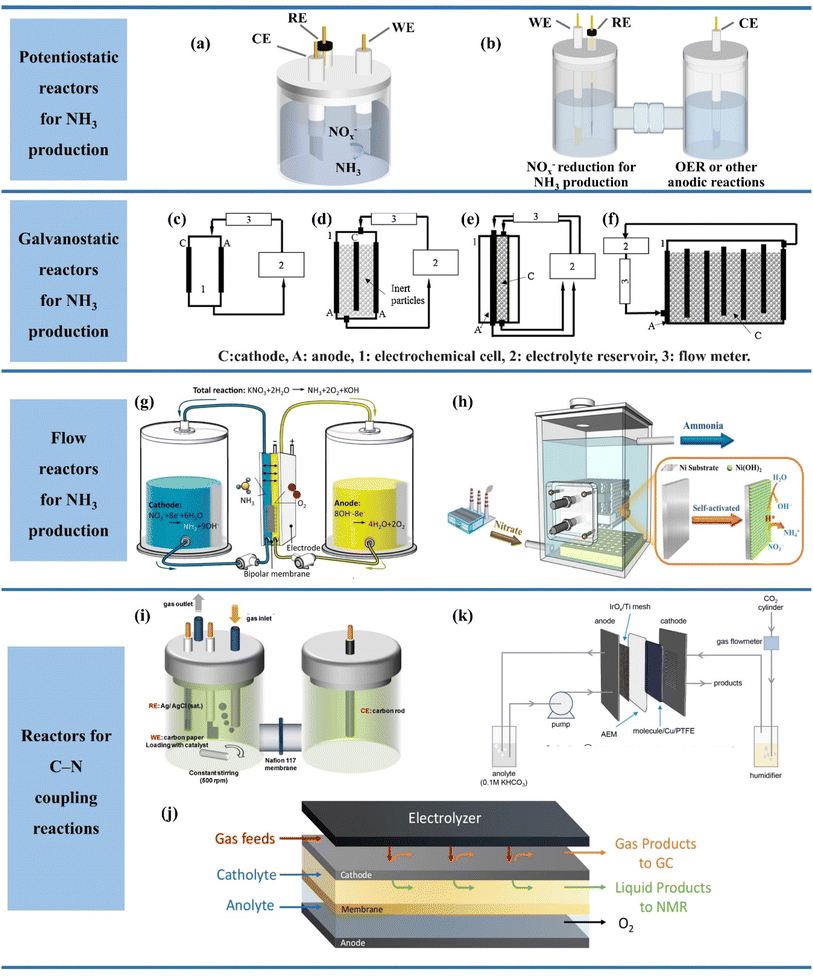 | ||
| Fig. 5 (a) Single-chamber and (b) dual-chamber reactors, (c) plate electrode cell, (d) fluidizing bed of the inert particle cell, (e) packed bed cathode cell, (f) vertical moving particle bed cell. Reproduced with permission.74 Copyright 2018, Elsevier. (g) Continuous NH3 electrosynthesis with a bipolar membrane reactor and (h) electrochemical reactor in a pilot scale used in electrocatalytic reduction of NO3−/NO2−. (i) H-cells, (j) membrane-based flow reactors and (k) membrane electrode assembly electrolyzers for C–N coupling reactions. Reproduced with permission.75 Copyright 2023, Elsevier. | ||
Nevertheless, the reactor systems employed in experimental studies are still restricted for industrial applications. The three-electrode system (potentiostatic electrolysis) often employed in experimental studies tends to result in slower nitrate reduction owing to higher activation energy in comparison to galvanostatic electrolysis.74 In addition, the struggle of the three-electrode system for large-scale application in wastewater treatment is also attributed to the complex power supply conditions.79 Consequently, the galvanostatic system without the involvement of reference electrodes for control is more promising for industrial applications, but it is worth noting that the required constant applied voltage should be evaluated in advance when using galvanostatic electrolysis to suppress side reactions and maximize economic efficiency.
The designs of the reactors for galvanostatic electrolysis are displayed in Fig. 5c–f, which are more favourable for industrial applications due to the requirement of only two electrodes in the electrolyser containing the cathode and anode. The designed reactors are composed of three components, including the electrochemical cell, electrolyte reservoir and flow meter. The simplest reactor (Fig. 5c) is shown with one anode and one cathode in the electrochemical cell, which contributes to the decreased solution resistivity and reduced operating cost due to the small spacing between the pole plate electrodes. However, the mass transfer process is not desirable enough in Fig. 5c. In order to accelerate the mass transfer, the reactor (Fig. 5d) is designed with various turbulence promotors or fluidized bed inert particles in the inter-electrode space. In addition, NOx− reduction can be facilitated due to the production of reduction promoter H2 when hydrogenated catalysts are employed as fluidized particles. Moreover, the further optimization of reactors is achieved in Fig. 5e and f with enhanced active area and faster mass transfer, which is caused by the packed bed cathode cells.
The reactor volume in the laboratory can hardly exceed 500 mL, which makes it extremely difficult to meet the industrial demands and achieve treating large quantities of wastewater.11 In order to achieve uniform NOx− reduction, the device is operated in continuous flow state. In continuous flow reactors, more challenges are imposed on electrode design, hydraulic flow state and ion-exchange membrane. The inferior hydraulic flow state can be enhanced by employing the strategies of improving reactor configuration including spiral tube reactors. Obviously, more attention is being paid to electrode design80 and the improvement of the ion-exchange membrane.81
In terms of electrode design, reasonable electrode space and sufficient active sites are required to achieve accelerated mass transfer and decent reduction efficiency. Considering the technical difficulties and increased internal resistance of electrodes associated with directly enlarging the electrodes, the formation of an electrode module by multiple electrode sheets provides a reasonable solution in Fig. 5h.82 The electrode module assembly is placed in a continuous flow reactor, which can overcome the drawback of insufficient reaction sites on the electrode surface and alleviate the limited treatment capacity of intermittent reactors.
In addition, ion-exchange membranes are also essential for electrochemical NH3 synthesis, being required to isolate the asymmetric electrolytes on two compartments and inhibit the re-oxidation of NH3 diffused to the anode. Compared with the severe ion crossover in the unipolar ion-exchange membrane, a bipolar membrane (BM) has been proposed with a mortise–tenon joint interlayer (Fig. 5g), which is composed of an anion exchange layer and a cation exchange layer.83 In this modified bipolar membrane, ion selectivity is formed by electrostatic repulsion of the bipolar membrane. The total dissociation rate and the stability of the bipolar membrane are improved due to the increased hydrolysis dislocation sites. The electrolytic ammonia production device assembled with this membrane lays the foundation for the achievement of continuous and stable electrochemical ammonia synthesis at high current density exceeding 1000 mA cm−2.
As for the electrochemical C–N coupling reactions based on NOx− reduction, some differences will exist in the design of the reaction equipment due to the injection of gases involved in some C–N coupling reactions. Three types of electrochemical reactors are illustrated in Fig. 5i–k demonstrating potential for application in electrochemical C–N coupling reactions, which consist of an H-type cell, membrane-based flow reactor and membrane electrode assembly (MEA) electrolyser.75 The H-type cell is the most commonly employed electrochemical reactor for C–N coupling reactions in laboratories currently due to its low cost and easy installation.84 The most obvious difference between the H-type cell for C–N coupling reactions and that for NOx− reduction is the increase of gas inlet and outlet ports. The gaseous reactants including CO2 in the H-type cell are dissolved in aqueous solution and then diffuse to the interface between the cathode electrolyte and the working electrode. The concentration of gaseous reactants on the cathode surface is lowest due to the distance required for diffusion to the cathode in this process. In addition, the H-type cell is also still restricted for further industrial application due to its inferior mass transfer and high electrical resistance.
Membrane-based flow reactors (Fig. 5j) employing gas diffusion electrodes (GDEs) as cathodes hold promise for overcoming the drawbacks of the H-electrolyser, which possesses a continuous flow of electrolyte in both anode and cathode compartments.85 Due to the presence of GDEs, the gaseous reactants can enter the cathode directly instead of diffusing from the cathode electrolyte. Consequently, the presence of GDEs ensures sufficient supply of gaseous reactants near the catalyst surface to allow the reaction to proceed at high current density. However, the membrane-based flow reactor is also hampered by poor stability, which is mainly attributed to hindered transport of gaseous reactants caused by electrolyte penetration in GDEs during the electrolysis process.
The emerging MEA electrolyzer provides novel insights into circumventing the poor stability of the membrane-based flow cell, which eliminates the cathode fluid based on the membrane-based flow cell and employs dampened reactant streams as feeding gases (Fig. 5k).86 The elimination of the cathode electrolyte facilitates enhanced stability and energy efficiency of the electrochemical system, which is caused by reducing the ohmic resistance and bypassing the poor stability of GDEs due to the penetration of the electrolyte. In addition, compared to products dissolved in the electrolyte of the membrane-based flow cell, products obtained by the MEA electrolyser remain in the gaseous phase, which is easy to collect by condensation for dramatically reducing the cost of separating the product. In spite of the unexplored application of the MEA electrolyser in C–N coupling reactions currently, it has provided a promising approach to achieve ultrastable C–N coupling reactions at high current density.
3. Wastewater treatment
Enormous efforts have been made towards the removal of NO3−/NO2− in underground water for restoring the global nitrogen cycle. Public health is directly threatened by NOx− ions which impair oxygen transport and trigger the “blue baby syndrome”. More importantly, the destroyed ozone layer and worsened global warming can be caused by N2O generated through bacterial denitrification in nature.87 The electrochemical NOx− reduction has gained much attention as a promising denitrification alternative, which possesses the advantages of mild operating conditions, no deleterious residues and small installation footprint. Although the NOx− reduction to NH3 pathway has demonstrated more benefits than NOx− reduction to N2 owing to the easier formation of the N–H bond and higher application value of NH3, it is worth noting that employing the NOx−—NH3 approach for treating wastewater has also been doubted due to the following two reasons: (1) the uncompetitive economic benefits due to insufficient NOx− concentration;88 (2) the difficult extraction of dissolved NH3 causing worse environmental implications.89 Thus, the treatment of wastewater containing nitrate/nitrite is recommended in many efforts to convert low-concentration NOx− ions into N2 or recycle high-concentration NOx− ions to other N-containing fertilizers.90–92 For achieving the optimal balance between efficiency and economy in large-scale wastewater treatment employing the NOx−—NH3 approach, it is necessary to delve into electrocatalyst development, actual wastewater composition, reaction system design and product separation.3.1 Factors influencing electrochemical NOx− reduction
In view of the complex composition of wastewater, several studies have explored the influences of ion concentration, other existing inorganic ions, and pH on NOx− reduction in wastewater to facilitate more efficient utilization of wastewater streams for ammonia production.93–95 The concentrations and species of ions in some common wastewater sources are illustrated in Table 1. Indeed, NO2− reduction to NH3 is thermodynamically and kinetically facile, making it promising for NH3 synthesis. Despite the mass of NO2− in wastewaters is far less than that of NO3− (Table 1), NO3− ions in wastewater are always transformed to NO2− by micro-organisms. With respect to the effects of other ions, it is obvious that NO3− in sufficiently large concentrations is far more competitive than other ions, and thus the reaction rate is hardly affected.100 However, such wastewater is not especially common and therefore concentrated NO3−-containing wastewater offers greater potential for ammonia production. The effects of pH and other inorganic ions on the reaction rate of the nitrate reduction process should not be neglected. The NOx− reduction reaction can be conducted over a broad range of pH; however, the performance and selectivity of NOx− reduction differ dramatically at different pH conditions. In addition, the drastic changes in the pH of the electrode interface and solution probably cause altered reaction mechanisms during the reduction process with proton depletion or ammonium hydroxide production.101 Thus, buffer solutions with high concentrations are employed to mitigate drastic changes of pH in the majority of studies on electrochemical NOx− removal. The dominant HER under acidic conditions can diminish the selectivity and FE of NOx− reduction. Despite the fact that the selectivity of NOx− reduction can be enhanced under alkaline conditions, the reduction kinetics can be boosted with the presence of indirect catalytic processes in acidic environment. It has been confirmed that NO2− is the dominant product for NO3− reduction in an alkaline environment, and the generation of NH3 can be accelerated with the increase of proton concentration.11| Type of wastewater | pH | Main composition | NO3− concentration | NO2− concentration | Ref. |
|---|---|---|---|---|---|
| Textile wastewater | Neutral | NO3−, Cl− | 7.4 mM | — | 96 |
| Industrial wastewater | Alkalescent | NO3−, NH4+, Cl− | 41.6 mM | — | 97 |
| Polluted ground water | Unknown | NO3−, NO2−, NH4+ | 0.88–1.26 mM | 0.22–1.27 mM | 98 |
| Low-level nuclear wastewater | Alkaline | NO3−, NO2−, SO42−, CO32−, Cl−, F−, SiO32−, CrO42− | 1.95 M | 0.55 M | 99 |
For inorganic ions, the impacts on nitrate reactions are diverse, including positive and negative effects. It is found that alkali metal cations follow the order of Li+ < Na+ < K+ < Cs+ to enhance the rate of NO3− reduction.11 The cations weaken the repulsive force between the negative ions and the cathode and facilitate the reduction of NO3− on the cathode since the cations modify the bimolecular structure of the cathode and form transient neutral ion pairs.102 As for multivalent cations, the presence of NH4+, Ca2+ and La3+ can achieve higher rates than alkali metal cations, but some cations such as Ca2+ and Mg2+ can be adsorbed on the cathode surface to form precipitates, resulting in poisoning of the cathode active sites and reduced reaction rates.103 Similarly, the reduction reaction of NO3− is also affected by anions. The negative effect of anions on the reaction rate is caused by the competition of anions for adsorption sites. The anions are ranked in the order of I− > Br− > Cl− > F− to reduce the rate of nitrate reduction.104
3.2 Reactors for NOx− removal and product collection
The continuous flow state is usually adopted in the operation of industrialized reactors. In spite of the preliminary discussion of continuous flow reactors in Section 2.2, the feasibility of reactors is of significant importance for enhancing the scale and commercial value of wastewater treatment.105 Regretfully, the feasibility of continuous flow reactors has rarely been explored for removing NOx− ions in current studies. Makover et al. have explored the feasibility of Cu-dimensionally stable anode (DSA) electrodes in a continuous flow reactor (Fig. 6a) for treating sewage after Donnan dialysis.106 The anode (DSA) employed is Ti covered with RuO2/IrO2. In Donnan dialysis, the NOx− ions in sewage will transfer to the receiver compartment from the feed compartment due to electroneutrality induction, accompanied with the transfer of high concentration Cl− and SO42− ions into the receiver driven by a concentration gradient. The excellent results employing Cu-DSA electrodes are obtained in high salinity NOx− contaminated solution generated by Donnan dialysis. Optimal NO3− removal can be reached at a low current density of 10 mA cm−2 and short residence time of 90 min, reaching 63% in high salinity Na2SO4 and 44% in high salinity NaCl. The promotion effect of high salinity SO42− ions and the inhibition effect of high salinity Cl− ions on nitrate removal have also been confirmed.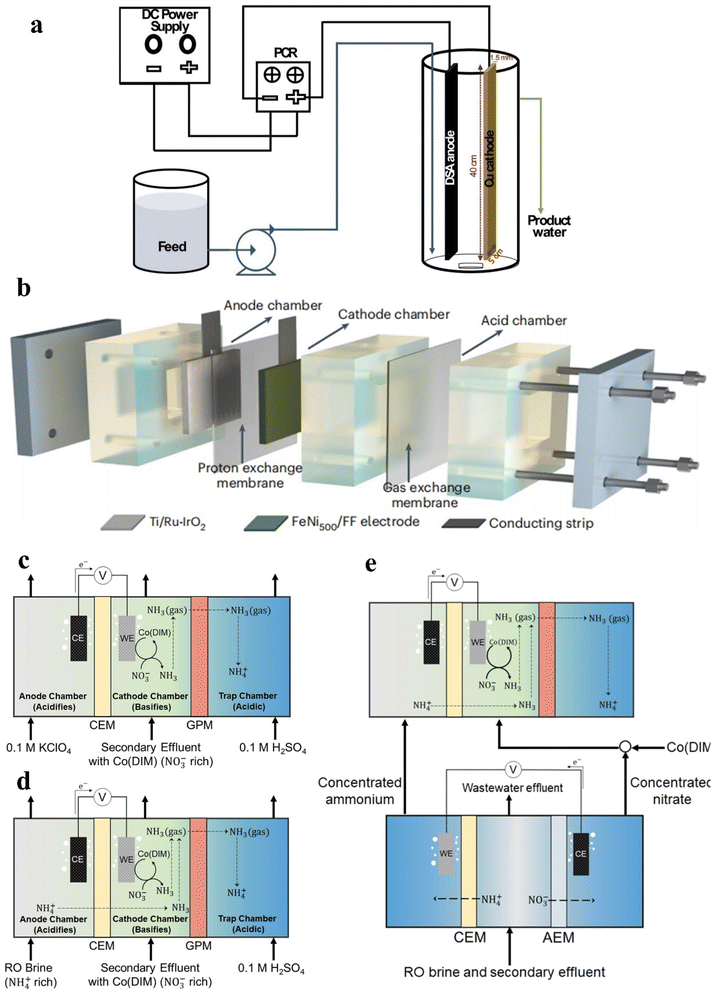 | ||
| Fig. 6 (a) Electrochemical continuous flow system. Reproduced with permission.106 Copyright 2020, Elsevier. (b) Schematic of the three-chamber membrane distillation reactor. Reproduced with permission.5 Copyright 2023, Springer Nature. (c) The ECS reactor, (d) ECS reactor with NH4+-rich RO brine and (e) ECS reactor with ED. Reproduced with permission.107 Copyright 2023, American Chemical Society. | ||
In the current studies of separating products, converting NH3 to (NH4)2SO4 by coupling acid adsorption is a reasonable route. Fig. 6b exhibits a three-chamber membrane distillation reactor by coupling electrocatalysis with acid absorption.5 The electrodes involved in Fig. 6b are generally self-supported electrodes with active sites that fail to be fully utilized, compared with the homogeneous molecular catalyst achieving precise atomic coordination between reactants and catalytic active sites. Thus, a series of electrochemical stripping (ECS) reactors are developed using homogeneous molecular catalyst Co(DIM) for degrading contaminants and extracting products in large-volume and nitrate-rich wastewaters (typically <4 mM).107 The ECS recirculating batch process is shown in Fig. 6c. A cation exchange membrane (CEM) is used to avoid NOx− diffusion from the cathode chamber to the anode compartment. During the reduction process, the increased pH of the catholyte is expected to exceed the pKa of NH3 (9.25), causing the majority of products to be in the form of volatile NH3. Thus, a hydrophobic breathable membrane is utilized for dispersing volatile NH3 from the cathode chamber. The operation for 42 h in this reactor witnessed 70.5% NOx− removal, which makes the treated water meet the drinking water limit. Moreover, the NH3 selectivity remains over 98.5% throughout this period which confirms that the Co(DIM)-mediated NOx− reduction is rarely influenced by wastewater constituents.
In order to reduce electrical energy consumption and enhance the ammonia recovery rate, two additional process configurations are explored in Fig. 6d and e. In a parallel feed configuration (Fig. 6d), the KClO4 solution is replaced by NH4+-rich reverse osmosis (RO) brine, enhancing the NH3 recovery rate by 17 times and halving electrical energy consumption. An electrodialysis (ED) cell is added in Fig. 6e for concentrating NO3− and NH4+ to provide a greater driving force for reaction and separation, which effectively enhances the rates of NO3− removal and NH3 recovery by 10 and 95 times, respectively. Moreover, Co(DIM) is added in the concentrated NO3− solution instead of wastewater in this ED-concentrated parallel configuration for preventing catalysts from separating from the wastewater.
3.3 Catalyst design for large-scale wastewater treatment
For industrial treatment of NOx−-containing wastewater, it is necessary to take into account the concentrated electrolyte, the pH of electrolytes and the electrode amplification for improving the economic viability of electrosynthesis.107–110 Currently, the NOx− concentration is typically lower than 1 M in a majority of efforts. Fig. 7a shows the nonmonotonic change of NH3 yield on Cu2O from 0.01 to 3 M NO3−. In the dilute regime (0.01 to 0.1 M), the production rate increases with nitrate concentrations due to promoted mass transfer and reaction kinetics. However, a decline is experienced in the range from 0.1 to 3 M, which is caused by the excessive NO3− adsorption for reducing water molecule adsorption on surface sites. To solve the dilemma of mismatched reaction kinetics between the HER and NO3− reduction, Ru is introduced in Cu2O for more efficient NO3− reduction.111 The water density profiles from the Cu2O and Ru/Cu2O surface are exhibited in Fig. 7b and c through molecular dynamics simulations. The higher H2O density in the Ru/Cu2O model is more conducive to promoting the collision between NO3− ions and H2O molecules for more efficient hydrogen transfer, which endows Ru/Cu2O with 89% NH3 FE under 9.9 A for 3 M NO3− reduction.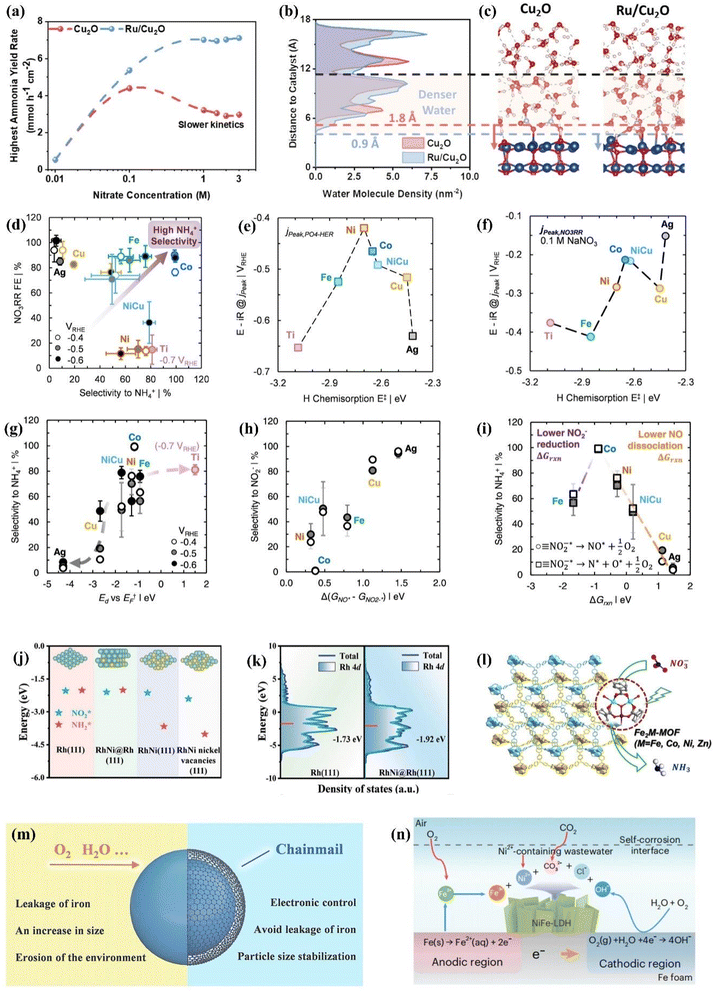 | ||
Fig. 7 (a) Optimized NH3 yield rate of various catalysts in an electrolyte containing 1 M KOH and different NO3− concentrations and (b) densities of water molecules, and (c) representative molecular dynamics simulation snapshots. Reproduced with permission.111 Copyright 2024, American Chemical Society. (d) NO3− reduction FE and selectivity on different metals. Relationships between the H chemisorption energy and potential in mass-transfer limited (e) phosphate-mediated HER and (f) NO3− reduction in 0.1 M NaNO3. (g) Relationship between selectivity and Edvs. EF. Relationship between reaction free energies (ΔGrxn) for converting *NO2− to *NO and selectivity to (h) NO2− and (i) NH4+. Reproduced with permission.112 Copyright 2022, American Chemical Society. (j) Adsorption energy of  and and  and (k) d-band centers of different catalysts. Reproduced with permission.113 Copyright 2024, Wiley-VCH. (l) 3D framework of Fe2M-MOF. Reproduced with permission.114 Copyright 2023, Wiley-VCH. (m) The role of graphene nano-chainmail. Reproduced with permission.115 Copyright 2023, Wiley-VCH. (n) The specific reactions of the self-corrosion process. Reproduced with permission.5 Copyright 2023, Springer Nature. and (k) d-band centers of different catalysts. Reproduced with permission.113 Copyright 2024, Wiley-VCH. (l) 3D framework of Fe2M-MOF. Reproduced with permission.114 Copyright 2023, Wiley-VCH. (m) The role of graphene nano-chainmail. Reproduced with permission.115 Copyright 2023, Wiley-VCH. (n) The specific reactions of the self-corrosion process. Reproduced with permission.5 Copyright 2023, Springer Nature. | ||
As for the amplified synthesis of electrodes, the electrode pieces are often present individually on a laboratory scale, so if they were to be scaled up directly, not only would there be modification, but also the internal resistance would increase. The direct amplification of electrodes faces the challenges of technical limitation for processing and sharp increases in resistance; the stacked electrode module mentioned in Fig. 5h provides a decent solution for avoiding the limitations induced by direct electrode amplification. Despite the extremely wide range of pH values in sewage collected, the majority of sewage is in neutral and acid environments. The catalysts discussed in Section 2 are designed for alkaline conditions; the HER competition will be more intense in neutral and acidic media than that in alkaline media.
As for the catalysts in a neutral environment, a series of transition metals and alloys have been explored including Ti, Fe, Co, Ni, Cu, Ag and Ni0.68Cu0.32, employing 0.1 M NaxH3−xPO4 solution to simulate the neutral environment.112 The promising catalysts have been screened under neutral conditions with exploration of the associated thermodynamic and kinetic parameters of catalysts. As demonstrated in Fig. 7d, metallic Co exhibits promising NO3− reduction FE and selectivity for NH3 formation. Co with moderate H chemisorption energy (Fig. 7e and f) shows smaller cathode mass transfer limiting the nitrate reduction potential compared with transition metals binding H strongly such as Fe and Ti, indicating that strong H chemisorption energy leads to sluggish proton-coupled electron transfer and hydrogenation kinetics in NO3− reduction. Further explorations of the origin of the excellent Co activity are demonstrated in Fig. 7g–i by probing the d-band center energy (Ed) and calculated reaction free energies of NO2− reduction to NO and further dissociation. The theoretical calculations have confirmed that more negative ΔGNOads can be achieved when Ed approaches the Fermi level (EF), which is caused by the increasingly unoccupied antibonding molecular orbital formed between NOads and the catalyst surface. However, as illustrated in Fig. 7g, Co exhibits superior selectivity beyond 95% over a broad range of potentials, which significantly exceeds the selectivity of metals (Ni, Fe) with similar Edvs. EF. The calculated reaction free energies of NO2− reduction to NO and further dissociation (Fig. 7h and i) are considered for better exploring the origin of the extraordinary NH3 selectivity of Co. As described in Fig. 7h, the selectivity for NO2− decreases roughly with the reduced difference between ΔG*NO and ΔG*NO2−. In spite of the lower NO2− reduction energy barrier in Ni compared to Co, Ni exhibits inferior NH3 selectivity due to weaker decomposition for subsequently produced NO. In contrast, the lower NH3 selectivity of Fe possessing more favourable NO dissociation is caused by adverse NO2− reduction activity. The volcanic trend of selectivity is described in Fig. 7i based on NO2− reduction activity and NO dissociation energy of metals explored. An ideal state of catalysts with promising NH3 selectivity can be represented by Co with sufficiently strong tendency for NO binding and dissociation and adequate activity for NO2− reduction.
The design of excellent catalysts can be conducted by approaching a series of parameters on Co including hydrogen affinity and Edvs. EF. For example, in spite of high hydrogen binding energy of the NiFe alloy for unfavorable NO3− reduction, the NiFe alloy possesses Edvs. EF and work function similar to Co, which cause better NO3− reduction activity and selectivity than the mono-component metals (Ni, Fe). On the flip side, the performance of Co can also be further improved including the possible improvement of the energy conversion efficiency. Enhancing NO3− affinity by pairing of metal oxides and electrolyte modulation are both effective approaches for boosting the NO3− reduction activity of Co.
As for the catalysts under acidic conditions, there are major challenges including (1) stronger HER competition and (2) drastically reduced stability caused by the dissolution of metal catalysts in strong acidic environments.110,111 However, several advantages are also presented in acidic environments, which can avoid the subsequent extraction process and spillage loss of aqueous NH3 owing to the direct generation of nitrogen fertilizers such as (NH4)2SO4 and NH4Cl under acidic conditions.112 In order to resist acid-induced corrosion, Rh has recently been employed to construct an electrocatalyst and is one of the few metals that can withstand aqua regia. The RhNi@Rh bimetallenes are synthesized for weakening the adsorption of  and
and  to resist adsorption-induced Rh dissolution (Fig. 7j), which exhibit a declined d-band center (Fig. 7k) due to the compressive stress induced from the inside out by the RhNi alloy core. With the modified adsorption behavior of Rh, the catalyst demonstrates exceptional stability over an extended 400 h test in acidic environments.
to resist adsorption-induced Rh dissolution (Fig. 7j), which exhibit a declined d-band center (Fig. 7k) due to the compressive stress induced from the inside out by the RhNi alloy core. With the modified adsorption behavior of Rh, the catalyst demonstrates exceptional stability over an extended 400 h test in acidic environments.
The immobilisation also provides a plausible strategy for enhancing the stability of transition metal catalysts capable of effectively inhibiting the HER. Fe2Co-MOF (Fig. 7l) is obtained by assembling Fe2Co clusters and H4TPBD ligands,115 which bypasses the decreased catalytic efficiencies due to saturated metal centres in the majority of MOFs. Fe2Co-MOF exhibits superior electrocatalytic stability up to 75 h at −1.1 V vs. RHE in pH = 1 electrolyte, accompanied by NH3 yield approaching 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 653.5 μg h−1 mgsite−1 and FE of 90.55%. The decent activity of Fe2Co-MOF derives from the suppressed HER and high turnover adsorption of NO3− due to the unsaturated metal sites induced by trinuclear clusters. In addition, the catalytic efficiency is further enhanced in acidic environments filled with protons, since the transfer of electrons and reactants can be promoted by the redox-active dinitrogen ligand. The remarkable stability of Fe2Co-MOF can be primarily ascribed to the highly connected structure with robust coordinative bonds formed by the combination of high-valence Fe3+ and carboxylate ligands.
653.5 μg h−1 mgsite−1 and FE of 90.55%. The decent activity of Fe2Co-MOF derives from the suppressed HER and high turnover adsorption of NO3− due to the unsaturated metal sites induced by trinuclear clusters. In addition, the catalytic efficiency is further enhanced in acidic environments filled with protons, since the transfer of electrons and reactants can be promoted by the redox-active dinitrogen ligand. The remarkable stability of Fe2Co-MOF can be primarily ascribed to the highly connected structure with robust coordinative bonds formed by the combination of high-valence Fe3+ and carboxylate ligands.
In addition to the approach mentioned above, armoured catalysts and self-corrosion reconstruction strategies also deserve to be utilized for enhancing long-term stabilities in the acid environment. Fig. 7m depicts the merits of armoured catalysts for NO3− conversion to N2 protected by ultrathin graphene nanolayers, which could provide more insights for enhancing the stabilities of NOx− reduction catalysts.115 Moreover, novel insights on expanding electrocatalyst and wastewater treatment can be provided by the economical self-corrosion approach (Fig. 7n) utilizing heavy metal ions in wastewater (Ni2+, Co2+ and Zn2+) for inducing the Fe surface to generate LDH nanosheets.5 This corrosion strategy is conducive to generating the active phase and avoiding conventional corrosion passivation. Furthermore, the contact between active sites and NOx− is facilitated in the corroded interface with an enlarged and turbulent region.
4. Production of multiple value-added chemicals
The comfortable survival of human beings in contemporary society is ensured by the mass production of organic N-containing compounds. Currently, more than half of the NH3 produced globally is consumed in organic N-containing compounds produced industrially by thermo-catalyzed reactions under harsh conditions (150–500 °C, 20–250 bar), causing disruptions in global carbon and nitrogen cycles due to the excess emissions of CO2 and nitrogen oxides (NOx).116 Recently, with the creation of the carbon-neutral vision and the boom of CO2 reduction and NOx− reduction, electrocatalytic C–N coupling reactions based on NOx− reduction have proposed new horizons for achieving the synthesis of high-value organic N-containing compounds, which employ useless or harmful wastes including C-containing species (CO2, CO) and N-containing species (NO2−, NO3−).117 A brighter future is emerging for electrocatalytic synthesis than traditional thermo-catalytic synthesis due to the sustainability and more favourable on-site/on-demand production owing to the features of decentralisation and modularity.118More intriguingly, the hidden surprises of NOx− reduction extend far beyond the synthesis of high-value organic N-containing compounds. The H-type electrolyser commonly employed in the laboratory offers an uninterrupted environment for NOx− reduction and also provides an opportunity for the anodic reaction to be thoroughly explored.119 The anodic oxygen evolution reaction (OER) commonly coupled with the NOx− reduction reaction has motivated researchers to seek alternative anodic reactions including small organic molecule oxidation reaction with low energy consumption and appealing products,120,121 which is caused by high energy barriers and difficult collection of products in the OER. Therefore, current research advances and potential challenges will be discussed in this section on the C–N coupling reactions and alternative anodic reactions based on NOx− reduction.
4.1 C–N coupling reactions
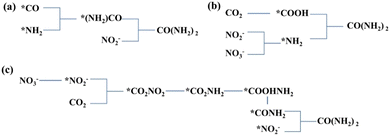
The C–N coupling reactions based on NOx− reduction can be categorized into coupling and cascade reactions, and the difference between tandem reaction and integrated reaction is illustrated in Fig. 8a. The integrated reaction is achieved by coupling key intermediates to build preset chemical bonds and generate advanced products, while tandem electrocatalytic reactions employ the in situ desorbed products.122 The integrated reaction based on CO2 and NO3− reduction is commonly used for the synthesis of urea. Indeed, the NO2− reduction process has also been confirmed to be promoted by CO2 on copper catalysts,123 which can reach a drastically enhanced NH3 FE of approximately 100% within a wide range of potentials. The significantly enhanced NO2− reduction performance is due to COads generated by CO2 reduction, which accelerates the deoxygenation and subsequent hydrogenation of intermediates.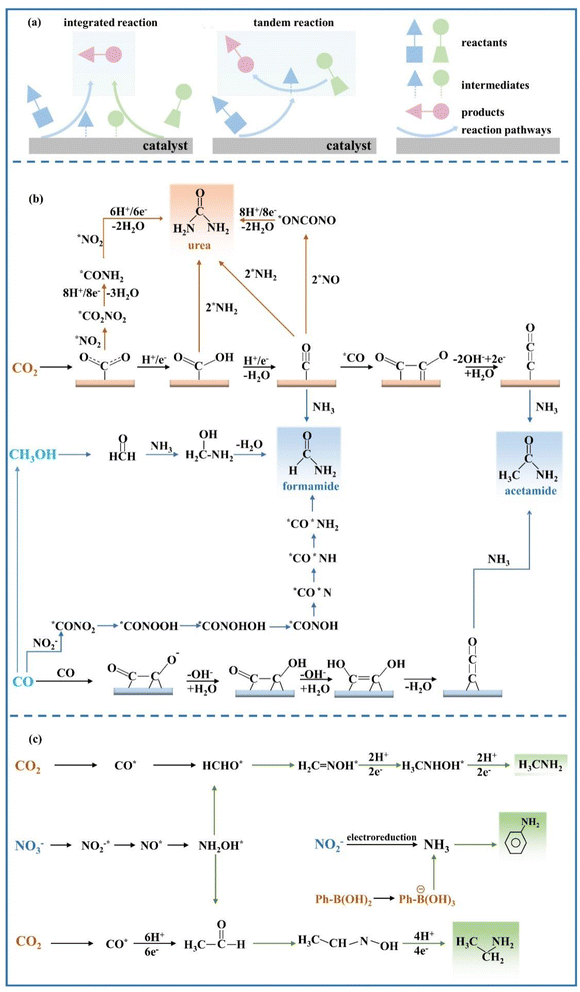 | ||
Fig. 8 (a) Schematic diagrams of tandem reaction and integrated reaction. Reaction pathways of (b) C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–N bond formation and (c) C–N bond formation. O)–N bond formation and (c) C–N bond formation. | ||
The C–N coupling reaction demonstrates promising prospects for the synthesis of high value N-containing chemicals; however, a number of challenges are faced such as sluggish kinetics and low selectivity due to stubborn bonding structures of the reactants and competition of CO2/NO2−/NO3− reduction and the HER.75 Numerous factors can exert an influence on electrocatalytic C–N coupling, including reactants, electrocatalysts and the reactors. The reactors have already been discussed in Fig. 5. The research status and the existing challenges of the C–N coupling reaction will be discussed in the part including the related reaction mechanisms and design principles of catalysts, hopefully providing a comprehensive understanding of C–N coupling reactions based on NOx− reduction.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) O)–N bonds.
Amides are a group of compounds with a characteristic C(
O)–N bonds.
Amides are a group of compounds with a characteristic C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–N unit, which can be constituted by CO and NHy intermediates. Amides available currently based on CO/CO2 reduction and NOx− reduction include urea, formamide, and acetamide. The possible reaction pathways of forming C(
O)–N unit, which can be constituted by CO and NHy intermediates. Amides available currently based on CO/CO2 reduction and NOx− reduction include urea, formamide, and acetamide. The possible reaction pathways of forming C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–N bonds are demonstrated in Fig. 8b employing various C-containing species.
O)–N bonds are demonstrated in Fig. 8b employing various C-containing species.
4.1.1.1 Urea. Urea accounts for approximately 70% of nitrogen-containing manure in the world, contributing to the sustainable development of human society.124 However, it is primarily obtained through the combination of NH3 and CO2 under harsh conditions, which is adverse to alleviating fossil energy consumption and decreasing CO2 emissions.125 The synthesis of urea from CO2 and NO3−/NO2− has recently been considered as a mild alternative route, but the current production efficiency is struggling to reach the standard for industrial applications.126 Thus, emphasis will be placed here on the formation mechanism of urea and the related studies on catalysts, in order to promote the industrial application process of electrocatalytic urea synthesis.
An in-depth understanding of the reaction mechanism of electrocatalytic urea synthesis is beneficial for improving the efficiency of the reaction. Numerous studies have been reported on the reaction mechanisms of electrocatalytic urea synthesis (Fig. 9), with controversies mainly existing in the key intermediates of C–N coupling. In this section, three representative mechanisms are introduced. It was proposed the formation of *(NH2)CO intermediates from *CO and *NH2 as a key step in C–N coupling through comparative experiments for the co-reduction of CO2 + NH3 and CO + NO2−.126 Meng et al. proposed that urea was generated by the coupling of *NH2 and *COOH intermediates in NO2−-integrated CO2 reduction,127 which was inferred from the disappearance of the signal peaks of *COOH in in situ diffuse reflectance infrared Fourier transform spectroscopy during the coexistence of CO2 and NO2−. Meanwhile, Yu et al. proposed that the intermediates in NO3−-integrated CO2 reduction are *NO2− and *CO2 instead of *CO and *NH2.128 The early coupling of *NO2− and *CO2 forms *CO2NO2, and subsequently, the *CO2NO2 intermediate undergoes several electron and proton transfer steps to generate *CO2NH2. The later protonation of the *CO2NH2 intermediate to *COOHNH2 is considered as the potential determining step (PDS) in the urea electrosynthesis process.
Despite the various controversies surrounding the current mechanisms, it is definitely evident that the adsorption configuration of CO2 can have a significant impact on the activity and selectivity of the urea synthesis reaction. The adsorption configurations of intermediates for the reaction can be modulated by the charged state of the catalyst surface with changes in catalyst compositions. Cu–In catalysts possessing different charge states were developed to explore the influences of different CO2 adsorption configurations on the activity and selectivity of the electrocatalytic synthesis of urea.129 The urea yield on a negatively charged Cu97In3–C catalyst with a C-bound surface was approximately thirteen times that of the positively charged Cu30In70–C catalyst possessing an O-bound surface. It was confirmed that the subsequent C–N coupling process was facilitated by the C-bound configuration (*COOH) on the catalyst surface, while the O-bond configuration (*OCHO) was a terminal blocking further non-electrochemical steps and causing inferior performance of urea formation.
With the further studies on the mechanisms of urea electrosynthesis, the focus on urea electrosynthesis has shifted to the pursuit of high FE catalysts.130–132 In contrast, the focus on other C–N coupling reaction organics has remained on exploring more synthetic pathways and expanding the variety of obtained N-containing organics, while less emphasis has been placed on the development of catalysts and Cu-based catalysts available commercially have usually been chosen directly. A diverse variety of catalysts have currently been developed for urea production including bimetallic catalysts, metal oxide catalysts, and monoatomic catalysts. Obviously, decent electrocatalysts with enhanced conductivity and abundant active sites are favourable to promote the simultaneous reduction of CO2 and NO3−/NO2−. Bimetallic electrocatalysts have exhibited promising performance in urea electrochemical synthesis, which is caused by the fact that the binding energy of the reaction intermediates can be controlled by modifying the electronic structure and composition of electrocatalysts.133–135 For example, Te-doped Pd nanocrystals significantly promoted the reaction between *CO and *NH2 in the reaction process of CO2 and NO2− and inhibited the formation of N2 through NO2− reduction owing to the synergistic effect between Te and Pd.136 The synergistic effect of bimetallic electrocatalysts was also demonstrated for specific morphological and structural regulation. Self-supported core–shell Cu@Zn nanowires, obtained by a simple electroreduction process, reached a higher urea yield rate of 7.29 μmol cm−2 h−1 and a corresponding faradaic efficiency of 9.28% compared to Zn (0.77 μmol cm−2 h−1, 1.00%) and Cu (0 μmol cm−2 h−1, 0.00%).135 Theoretical calculations revealed that the catalytic performance of urea electrosynthesis was enhanced by electron transfer from the Zn shell to the Cu core due to the reduction in the critical coupling energy barriers of the *CO and *NH2 intermediates. In comparison to metal catalysts, metal oxide catalysts were prone to introduce oxygen vacancies that acted as catalytic centres with rich electron densities.137 Oxygen vacancy-rich anatase TiO2 (Cu–TiO2) nanotubes could be easily obtained by low-valence Cu doping,138 and the high-density oxygen vacancies facilitated the selectivity of NOx to *NH2 and exposure of bi-Ti3+ active sites.
For single-atom catalysts anchoring isolated atoms on carriers by ligands, they have aroused growing interest due to the optimal atom utilization, the explicit catalytic active sites and the absence of aggregated metal atoms.139 Leverett et al. prepared Cu–N–C single-atom catalysts for electrochemical urea synthesis and studied the effect of Cu coordination on the electrochemical reduction reactions of CO2 (CO2RR) and NO3− (NO3RR).140 The experiments combined with theoretical calculations indicate that the Cu–N4 site exhibited higher activity for the CO2RR, while the Cu–N4−x–Cx site demonstrated a higher NH4+ yield rate in the NO3RR. The catalyst on the Cu–N4 site exhibited the best urea synthesis performance with an FE of 28% and a yield of 4.3 nmol s−1 cm−2 at −0.9 V versus RHE. In contrast to isolated single-atom catalysts, bonded diatomic catalysts thermodynamically and kinetically strengthen pivotal C–N coupling due to the presence of effective sites for coordinated adsorption and coactivation of carbon and nitrogen sources. The bonded diatomic Fe–Ni catalyst demonstrated excellent performance, reaching a high urea yield of 20.2 mmol h−1 g−1 with FE of 17.8%,141 which was up to an order of magnitude higher than those of single-atom and isolated diatomic electrocatalysts. Such excellent performance is mainly due to two factors: (1) the simultaneous introduction of Fe and Ni sites overcomes the restriction of unilateral selective adsorption and activation of carbon or nitrogen reactants. (2) The bridge sites of Fe–Ni pairs boost the C–N coupling process thermodynamically and kinetically, and the bridged configuration inhibits the HER effectively.
The metal-based catalysts mentioned above have exhibited promising performance; nonetheless, metal-based catalysts are still hampered by high cost,142 destabilization under adverse operating conditions143 and vulnerability to small molecule toxicity.144 Carbon-based metal-free electrocatalysts have shown brilliant application prospects in the electrocatalytic synthesis of urea due to abundant sources, competitive cost and superior stability. The doping of heteroatoms such as N, B and F has been widely employed for optimizing the electrocatalytic properties of carbon materials for urea synthesis due to new surface charge distribution induced by doping. The HER activity can be suppressed in carbon materials doped with F, facilitating enhanced urea synthesis activity. The high urea yield rate of carbon nanotubes with a fluorine-rich surface (F-CNT) could reach up to 6.36 mmol gcat−1 h−1, which has been confirmed to be caused by more favorable *COOH generation and *NH2 formation processes on the F-CNT.143 The abundant nitrogen-containing active intermediates are also more conducive for urea synthesis. The remarkable urea yield rate of 610.6 mg h−1 gcat−1 was exhibited in porous N-doped carbon obtained by pyrolysis of the coordination polymer, which even exceeded those of some noble metal-based catalysts.145
The synthesis of urea by simultaneous electrochemical reduction of CO2 and NO2−/NO3− has gained growing attention, especially when the coupled CO2RR is of high significance for achieving carbon neutrality. However, the synthesis mechanisms are full of arguments. In regard to studies on catalysts, numerous experiments in the design of high-performance catalysts have confirmed that it is imperative to attach importance to the coactivation and reaction of reactants, as well as the construction of efficient sites conducive to C–N coupling by optimizing the adsorption of intermediate components. Nevertheless, the Faraday efficiencies of current catalysts for urea synthesis are generally lower than 70%,146 which are far from the actual requirements. Furthermore, the precise regulation of the interfacial microenvironment should not be ignored for comprehensively improving the electrocatalytic performance, since efficient and stable three-phase interfaces are required to supply reactants and accelerate mass transfer.147 Three optimization strategies can be employed for controlling the microenvironment, including adjusting the hydrophobicity of electrocatalysts, improving proton supply in the electrolyte and regulating experimental conditions in the electrolyzer.
4.1.1.2 Organic amides. Amides including formamide and acetamide are of significant commercial value, and are widely employed in polymer manufacture and biological compounds. In view of the energy crisis and environmental pollution aggravated by the current industrial synthesis of amides, electrosynthesis routes have been developed employing CO2 and NH3. The combination of NH3 in the liquid phase and CO2 in the gas phase has been proven to successfully synthesize formamides and acetamides over non-homogeneous Cu catalysts,148 the detailed mechanisms are shown in Fig. 8b. However, the FEs obtained are considerably low, not even exceeding 1% at the highest. In addition, the electrolysis process will be disrupted by the undesired carbonate formation at the electrode–electrolyte interface, owing to the inevitable reaction of OH− ions with CO2 under alkaline conditions.
In order to solve such dilemma, CO reduction is a promising strategy to replace direct CO2 reduction, which can yield CO from electrochemical CO2 reduction under non-alkaline conditions. The higher selectivity for acetic acid in CO reduction has been demonstrated compared to CO2 reduction on the Cu catalyst surface, implying an easy reaction between the ketene intermediates in the CO reduction process with the nucleophilic agent NH3. The FE of generating acetamide can reach 40% at −0.68 V vs. RHE on the Cu nanoparticle catalyst, when the molar ratio of CO to NH3 is up to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.149 The synthesis mechanism and competitive reactions for acetamide formation are shown in Fig. 8b. Under the conditions of high pH and less negative potential, CO reduction is more biased towards the generation of the C
1.149 The synthesis mechanism and competitive reactions for acetamide formation are shown in Fig. 8b. Under the conditions of high pH and less negative potential, CO reduction is more biased towards the generation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O intermediate compared with the generation of the C
O intermediate compared with the generation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) COH intermediate for producing ethylene and ethanol. Although the formation of ethylene and ethanol is inhibited at high NH3 concentration, the formation of acetamide is also faced with competition for forming acetate due to OH− on the surface of the catalyst.
COH intermediate for producing ethylene and ethanol. Although the formation of ethylene and ethanol is inhibited at high NH3 concentration, the formation of acetamide is also faced with competition for forming acetate due to OH− on the surface of the catalyst.
The N-containing nucleophilic reagent in the above C–N coupling pathways directly employs NH3. With the flourishing of studies on NO2− reduction to NH3 due to the low dissociation energy of the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond (204 kJ mol−1), a credible route is provided for green NH3 production with renewable electricity. Electrocatalytic coupling of NO2− with CO has been confirmed to be an alternative avenue to achieve formamide synthesis. The reaction pathway is shown in Fig. 8b for electrocatalytic coupling of NO2− with CO obtained by theoretical calculation, and the key challenge for reaching decent formamide selectivity is the construction of highly active and stable catalysts for enhancing CO and NO2− activity reduction and promoting C–N coupling. Ru atoms dispersed on Cu nanoclusters (Ru–Cu) have been developed to achieve decent formamide FE of 45.65% with a yield of 2483.77 μg h−1 mgcat−1 at −0.5 V vs. RHE.150 The design of dual active sites in Ru–Cu catalysts could achieve synergistic catalysis for C and N activation, which can significantly improve the C–N coupling efficiency compared to monometallic catalysts. The adsorption and subsequent hydrogenation process of NO2− could be promoted by Ru atoms, while the dissociation adsorption of CO could be accelerated by adjacent Cu sites. Therefore, the decent activity and selectivity for formamide formation can be reached by the synergistic catalysis in Ru–Cu catalysts.
O bond (204 kJ mol−1), a credible route is provided for green NH3 production with renewable electricity. Electrocatalytic coupling of NO2− with CO has been confirmed to be an alternative avenue to achieve formamide synthesis. The reaction pathway is shown in Fig. 8b for electrocatalytic coupling of NO2− with CO obtained by theoretical calculation, and the key challenge for reaching decent formamide selectivity is the construction of highly active and stable catalysts for enhancing CO and NO2− activity reduction and promoting C–N coupling. Ru atoms dispersed on Cu nanoclusters (Ru–Cu) have been developed to achieve decent formamide FE of 45.65% with a yield of 2483.77 μg h−1 mgcat−1 at −0.5 V vs. RHE.150 The design of dual active sites in Ru–Cu catalysts could achieve synergistic catalysis for C and N activation, which can significantly improve the C–N coupling efficiency compared to monometallic catalysts. The adsorption and subsequent hydrogenation process of NO2− could be promoted by Ru atoms, while the dissociation adsorption of CO could be accelerated by adjacent Cu sites. Therefore, the decent activity and selectivity for formamide formation can be reached by the synergistic catalysis in Ru–Cu catalysts.
The electrocatalytic C–N coupling system developed currently is generally employed under aqueous conditions, and the further enhancement of C–N coupling efficiency is limited by severe HER competition due to the low-soluble CO in the aqueous solution. The extremely water-soluble methanol can be obtained from CO conversion, which can be an attractive alternative C-containing species for C–N coupling reactions. The combination of methanol and NH3 has provided an interesting process to produce formamide under ambient conditions, which utilizes the nucleophilic attack of NH3 on a formaldehyde-like intermediate from methanol electrooxidation. The most likely reaction pathway is illustrated in Fig. 8c. The C–N bond formation in formamide is caused by a nucleophilic attack process, where the positively charged C in *CH2O is attacked by the electronegative N atom in NH3. The conversion of methanol and NH3 to formamide can reach a selectivity of 74.26% and FE of 40.39% on PtO2 at 100 mA cm−2.151 The decent formamide production efficiency was due to the moderate affinity of the reaction intermediate on PtO2. However, the large-scale production of formamides based on methanol and NH3 is faced with the limitation of poor mass transfer at high current density exceeding 100 mA cm−2. Compared with the easy dissolution of precious metal Pt under large oxidation current density, a boron-doped diamond (BDD) electrode is highly promising for large-scale formamide electrosynthesis due to the outstanding stability on the most corrosive electrolytes with a wide potential window.152 The BDD electrode exhibits high durability after a continuous 20-cycle test on a laboratory scale, which also shows decent FE of 33.5% and output of 36.9 g h−1 at 264 A in the pilot plant test.
The combination of methanol and ammonia has provided a favorable idea for the electrochemical synthesis of formamide, and this strategy has been proven to be extensible for the synthesis of other organic N compounds. More intriguingly, acetamide and propenamide can be obtained by lengthening the C chain of C-containing species, and formyl methylamine can be obtained by replacing the N source (CH3NH2).
Methylamine is the simplest alkylamine and is employed as the major commercial chemical intermediate in pesticide production, solvent fabrication and water treatment.154 For industrial production, methylamine is currently obtained from methanol (CH3OH) and NH3 under high-temperature high-pressure conditions. CoPc-NH2/CNT was developed as a working electrode for driving the co-reduction process of CO2 and NO3− to methylamine involving the transfer of 14 electrons and 15 protons.29 The total FE of the co-reduction process reaches 13%, with no performance degradation after at least 16 hours of uninterrupted operation. More significantly, the intermediates (NH2OH and HCHO) involved in the key C–N coupling step are confirmed; thus, eight consecutive reaction steps regarding the formation of methylamine are proposed. As shown in Fig. 10a, formaldoxime is generated by spontaneous condensation through NH2OH derived from NO3− with HCHO obtained from CO2, and then formaldoxime is reduced to form methylamine.
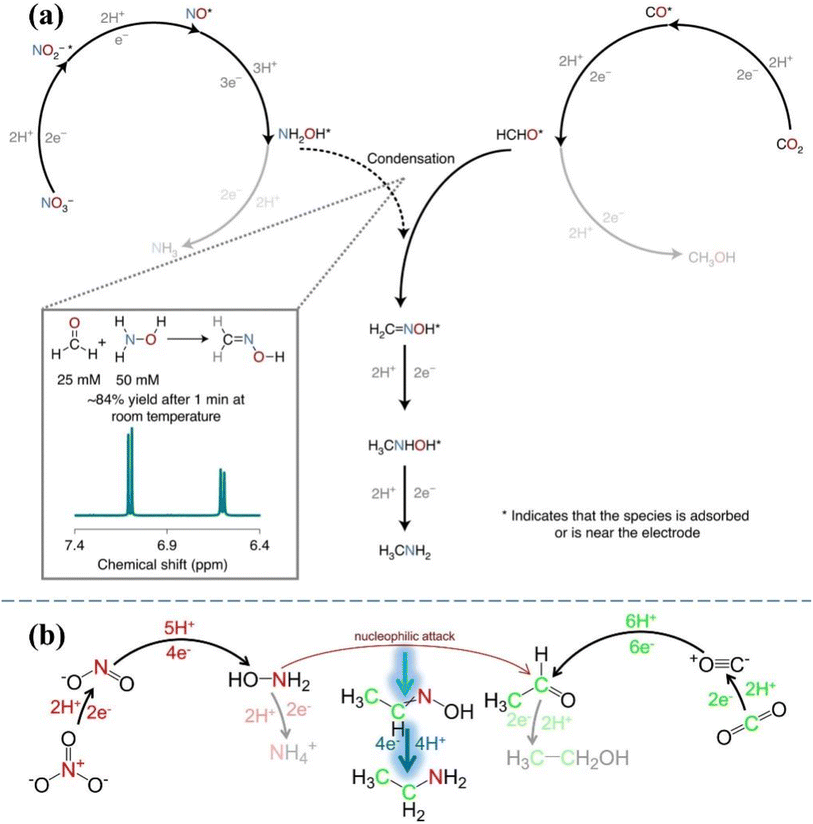 | ||
| Fig. 10 (a) The proposed reaction pathways to form methylamine. Reproduced with permission.29 Copyright 2021, Springer Nature. (b) Reaction pathways for obtaining ethylamine. Reproduced with permission.155 Copyright 2022, Elsevier. | ||
The synthesis of ethylamine is obviously more challenging than that of methylamine, which is caused by the process of transferring 20 electrons and 21 protons in total. The cascade electrocatalytic synthesis of ethylamine from CO2 and NO3− is achieved by the catalysis of oxide-derived Cu nanoparticles.153 In addition, the related synthesis mechanism is proposed. The mechanism of ethylamine synthesis displayed in Fig. 10b is similar to that of methylamine, and the critical C–N coupling step is the condensation of hydroxylamine (NH2OH) with aldehyde (CH3CHO) to form acetaldoxime. However, the FE of ethylamine production by this process is as low as 0.3%. The poor yield of ethylamine is primarily attributed to the following factors: (1) it is adversely affected by the competition between the rapid side reactions of NH2OH to NH4+ and CH3CHO to CH3CH2OH. (2) The reduction rate of acetaldoxime is significantly slower than that of the CO2RR, NH3RR and HER. (3) The selectivity of the CO2 to CH3CHO reduction pathway on Cu-based catalysts is inferior.
While significant breakthroughs have been made in the synthesis of methylamine and ethylamine, it is hard to implement them immediately in industry. Apparently, the electrocatalytic synthesis of propylamines or alkylamines with more C atoms will also be more arduous. The route for generating arylamine has recently been explored by coupling arylboronic acid with NH3 produced from NO2− reduction. Intriguingly, the synthesis of arylamine was carried out at pulsed potentials,156 which was owing to the consideration that metal electrodes were easily reduced to zero-valent metals during NO2− reduction and deactivated due to excessive oxidation in the C–N coupling process. Therefore, low-coordinated Cu nano-coral was employed under pulse potential for efficient NO2− reduction and C–N coupling, which experienced an alternating transition from the zero-valent state to the divalent state.
The environmental pollutant CO could be a promising C source substituting CO2, which facilitates environmental restoration and averts the disruption of the electrolysis process due to undesirable carbonate formation from the inevitable reaction of OH− with CO2 at the electrode–electrolyte interface. The possible C and N sources and the corresponding theoretical products are depicted in Table 2, presumably contributing to the broadening of the organonitrogen product scope to obtain more high-value products.
| C source | N source | Organonitrogen compound |
|---|---|---|
| CO | NH3 | Acetamide |
| CO | CH3NH2 | N-Methylacetamide |
| CO | C2H5NH2 | N-Ethylacetamide |
| CO | (CH3)2NH | N,N-Dimethylacetamide |
4.2 Valuable anode reactions coupled with NOx− reduction
The traditional NOx− reduction reaction is usually coupled with the OER, which consumes up to 90% of the input energy and produces low-value O2.158 The OER with high energy consumption results in twice the production cost of the electrocatalytic NH3 production compared to the conventional Haber–Bosch route for producing NH3 with the same quality.2 Currently, appeals to develop alternative oxidation reactions are progressively increasing for enhancing energy utilization efficiency and obtaining value-added chemicals. The development of anodic oxidation reactions employing inexpensive or hazardous reactants has received considerable attention. Electrooxidation of N2 to HNO3 is a promising alternative reaction, which can both generate high-value HNO3 and provide reactants for NOx− reduction.119 In spite of the inferior FE of only 1.23% for N2 oxidation on Pt foil,159 this strategy employing anodic N2 oxidation has provided a promising route for obtaining HNO3 and NH3 at distributed sources.In addition, the immense interest in electrooxidation reactions of biomass and related derivatives has been driven not only by lower theoretical potentials than those of the OER (Fig. 11) but also by the sustainable nature for accelerating the creation of a carbon-neutral society.160 Biomass and its derivatives have been considered as renewable carbon-neutral resources including 5-hydroxymethylfurfural, glycerol and benzyl alcohol with abundant proton content.161–165 Commercial Ni foam was usually employed as an anode in earlier studies for initially verifying the feasibility of replacing the anodic reaction. With the deeper cognition on electrocatalytic reconfiguration, excellent bifunctional catalysts can be obtained by adjusting the reconfiguration direction of catalysts in different reaction processes.166 Decent bifunctional NiCu based catalysts have been developed for NO3− reduction and glycerol oxidation reactions, which can be reconstructed under different operating conditions.167 Under the cathodic reduction environment, the materials were transformed into amorphous Ni(OH)2 coupled Cu nanoparticles. The NO3− reduction performance of the material was enhanced by the synergistic effect of Cu and Ni(OH)2. Meanwhile, composites including NiOOH and CuO with rich Cu vacancies were obtained by the reconstruction of NiCu based catalysts during the glycerol oxidation process. The glycerol oxidation process was promoted by the increased exposure of active NiOOH species due to the leaching of Cu in Cu vacancy-rich CuO. Compared to the traditional electrolyzer coupled with the OER, the electrolyzer coupled with the GOR exhibited an incredible overpotential reduction of 285 mV at a current density of 100 mA cm−2.
Recently, some efforts have been focussed on further improving the economic efficiency of electrochemical systems coupled with NO3− reduction and glycerol oxidation. A CO2 capture strategy has been explored for upgrading products,168 which can convert anodic formate to potassium diformate and cathodic NH3 to NH4HCO3. This strategy is conducive to product separation holding promising prospects for industrial applications.
The strategy of replacing the OER with biomass electrooxidation has provided an innovative insight for reducing electricity consumption and enhancing product value. However, biomass electrooxidation also faces the challenges of higher cost and higher requirements for membrane, which typically drives current density below 200 mA cm−2 under potential exceeding 1.23 V.169–171 The undesirable overpotential in the biomass electrooxidation process may be attributed to the high energy required for destroying C–H and O–H bonds in biomass.172,173 Compared to the C–H and O–H bonds in alcohols or aldehydes, more easily dissociated hydrogen atoms are manifested in the more reactive enol structure.174 Consequently, substances including ascorbic acid with a highly active enol structure can be considered as an anode additive to accelerate NH3 production in future efforts.
5. Energy conversion and storage systems
Metal–NO2−/NO3− batteries have received growing attention as a paradigm of simultaneous NH3 production and energy output.175–178 The anodes currently employed in metal–NO2−/NO3− batteries are dominated by Zn anodes, which are especially appealing in the treatment of industrial wastes containing NO2−/NO3− due to their low cost, easy recycling and high stability in alkaline solutions.179 The working mechanism, performance, hurdles and opportunities of Zn–NO2−/NO3− batteries are primarily discussed in this section.5.1 Metal–NO3− batteries
In 2021, the viability of galvanic metal–NO3− batteries (Fig. 12a) was evidenced by Zhi's group for the first time, shedding renewed light on the field of sustainable NH3 generation and zinc-based batteries.21 A Pd-doped TiO2 nanoarray was employed as the cathode in this experiment, which exhibited impressive NO3− reduction activity due to attenuated intermediate adsorption (Fig. 12b) with introducing Pd. The electrochemical reactions of the discharge process in this Zn–NO3− cell are as follows:| Cathode reaction: NO3− + 7H2O + 8e− → NH4OH + 9OH− |
| Anode reaction: 4Zn + 8OH− → 4ZnO + 4H2O + 8e− |
| Overall reaction: 4Zn + NO3− + 3H2O → 4ZnO + NH4OH + OH− |
 | ||
| Fig. 12 (a) Schematic representation of the Zn–NO3− battery, (b) the calculated intermediate adsorption energies on Pd/TiO2. Reproduced with permission.21 Copyright 2021, Royal Society of Chemistry. (c) Schematic diagram of a rechargeable Zn–NO3− battery. Catalyst screening studies of (d) the NO3RR and (e) OER. (f) Photovoltaic driven Zn–NO3− battery. (g) Galvanostatic discharge–charge cycling curves of a Zn–NO3− battery assembled from DM-Co. Reproduced with permission.180 Copyright 2022, Wiley-VCH. (h) Schematic diagram of an alkaline–acidic hybrid Zn–NO3− battery. (i) Pathways for active hydrogen production in the NO3−RR process under different conditions. (j) Environmental sulfur recovery powered by a hybrid Zn–NO3− battery. Reproduced with permission.181 Copyright 2023, Springer Nature. (k) Schematic diagram of a Zn–NO2− battery. (l) The energy barriers of C/Co3O4 and Co3O4 for the rate-determining step in the NO2RR. (m) NO2RR performance of C/Co3O4 at different potentials. Reproduced with permission.23 Copyright 2022, Royal Society of Chemistry. | ||
However, the rechargeability of Zn–NO3− batteries has not been sufficiently investigated in the Zn–NO3− battery with Pd-doped TiO2 as the cathode. Lin et al. have developed rechargeable Zn–NO3− batteries (Fig. 12c), inspired by soybean which could exploit nitrogen and generate oxygen simultaneously. Bifunctional DM-Co catalysts were designed for the NO3RR and OER after theoretical pre-screening (Fig. 12d and e), which reveals the great potential of Co-based catalysts.180 The aqueous rechargeable Zn–NO3− battery constructed with DM-Co as the cathode achieves a high power density of over 25 mW cm−2, which is much higher than that of the Zn–NO3− battery with a Pd-doped TiO2 cathode (0.87 mW cm−2). In addition, the successive discharge–charge cycle curves illustrated in Fig. 12g reveal the excellent robustness of the rechargeable Zn–NO3− battery, presenting 76 cycles at a low potential of 2.1 V. The discharge process in this rechargeable Zn–NO3− cell is analogous to that of the Zn–NO3− cell with a Pd-doped TiO2 cathode.
The following reactions take place when the aqueous Zn–NO3− battery is charged:
| Cathode reaction: 4OH− → 2H2O + O2 + 4e− |
| Anode reaction: Zn(OH)24− → Zn2+ + 4OH− |
| Zn2+ → Zn − 2e− |
| Overall reaction: 2Zn(OH)24− → 2Zn + 2H2O + 4OH− + O2 |
The total battery reaction of the rechargeable Zn–NO3− cell can be described as follows:
| NO3− + 3H2O → NH4+ + 2OH− + 2O2 |
It is worth mentioning that the Zn–NO3− battery system driven by a photovoltaic cell (Fig. 12f) has also been attempted. When the Zn–NO3− battery is charged, the electrical energy is stored in the chemical bonds of the Zn anode, which is converted from the solar energy absorbed by the photovoltaic cell. The optimal NO3RR FE of 95% and solar-to-NH3 efficiency of 19.5% are obtained in the Zn–NO3− cell system driven by a photovoltaic cell. The cells described above are operated in alkaline environments; however, corrosion issues of equipment and limitations on the type of battery components are unavoidable under extreme pH conditions.182 A Zn–NO3− cell with a Co2AlO4 cathode has also recently been constructed in a neutral environment,40 which is also beneficial for simulating the actual textile wastewater environment. The introduction of Al ions improves CO3O4 with poor ammonia production performance in a neutral environment, which achieved optimal adsorption of NO3− on Co sites by reducing the electron cloud density on the Co surface. The power density of 3.43 mW cm−2 offered by the Zn–NO3− battery was higher than that of the Zn–NO3− battery reported for the first time. However, the output voltages are limited in current Zn–NO3− batteries equipped with NO3− reduction under neutral/alkaline conditions, which indicates more challenges on the conditions of the cathodic part severely affecting the power density and NH3 yield of the related battery. Recently, an alkaline–acidic hybrid Zn–NO3− battery (Fig. 12h) is developed to exhibit higher output power density, due to enhanced NO3− conversion rate and more energy-efficient NH3 generation with abundant protons provided in an acidic environment (Fig. 12i). FePc/TiO2 is developed as a stable and active electrocatalyst for energy-efficient acid NO3− reduction with an impressive NH3 yield rate of 17.4 mg h−1 cm−2 and NH3 FE of 90.6%.181 The developed alkaline–acid hybrid Zn–NO3− battery based on the FePc/TiO2 cathode shows high open-circuit voltage up to 1.99 V with high power density of 91.4 mW cm−2, which can be applied for efficient environmental sulfur recovery by driving the electrolyzer composed of cathodic HER and anodic sulfur oxidation reaction with the current density of 35.6 mA cm−2 (Fig. 12j).
Some progress has been made in terms of rechargeability studies of Zn–NO3− batteries and the design of cathodes under different conditions. However, the performance of the cells requires further enhancement and the electrolyte environment in the cells should be brought closer to that of real wastewater. Notably, the potential value of the NO3RR under basic conditions is 0.6 V vs. the RHE, which is higher than the 0.4 V of the oxygen reduction reaction (ORR) O2 + 2H2O + 4e− → 4OH−.183 Consequently, NO3−-based batteries potentially produce a higher voltage output than metal–air batteries. The performances of Zn–NO3− batteries and Zn–air batteries as reported in current literature are presented in Table 3. It is clear that a significant discrepancy can be found between the performance of the Zn–NO3− batteries and the Zn–air batteries; reducing the discrepancy can be achieved by seeking decent-efficiency and high-selectivity catalysts and developing more Zn–NO3− batteries with an abundant electrolyte environment.
| Batteries | Catalyst | OCV (V) | FE (%) | NH3 yield (mg h−1 cm−2) | Power density (mW cm−2) | Ref. |
|---|---|---|---|---|---|---|
| Zn–NO3− | Pd/TiO2 | 0.81 | 81.3 | 0.54 | 0.87 | 21 |
| Zn–NO3− | ZnCo2O4 | 0.6 | 98.33 | 1.55 | 4.62 | 22 |
| Zn–NO3− | Fe/Ni2P | 1.22 | 85 | 4.17 | 3.25 | 176 |
| Zn–NO3− | Co2AlO4 | 1.862 | 92.6 | 0.75 | 3.43 | 40 |
| Zn–NO3− | NiCo2O4/CC | 1.30 | 96.1 | 0.82 | 3.94 | 175 |
| Zn–NO3− | CeO2−x@NC | 1.45 | 96.09 | 2.46 | 3.44 | 184 |
| Zn–NO3− | DM-Co | 0.62 | 91 | 2.04 | 25 | 180 |
| Zn–NO3− | CuNi NPs/CF | 0.94 | 97.03 | 94.57 | 70.7 | 185 |
| Zn–NO3− | FePc/TiO2 | 1.99 | 88.2 | 12.3 | FePc/TiO2 | 181 |
| Zn–NO2− | TiO2−x | 0.6 | 91.1 | 12.230 | 2.38 | 177 |
| Zn–NO2− | C/Co3O4 | 1.589 | 95.1 | 0.802 | 6.03 | 23 |
| Hydrazine-nitrate | Bimetallic RuCo | — | — | 6.64 | 12 | 178 |
| Zn–N2 | CoPi/NPCS | ∼1.4 | 16.35 | 0.0147 | 0.49 | 186 |
| Zn–N2 | OV-Ti2O3 | — | 19.29 | 0.03724 | 1.02 | 187 |
| Zn–N2 | VN@NSC-900 | ∼0.55 | — | 0.000172 | 0.01642 | 18 |
| Zn–N2 | CoPi/HSNPC | ∼1 | 24.42 | — | 0.31 | 188 |
| Zn–N2 | NbS2 | 0.5 | 10.12 | 0.03758 | 0.31 | 189 |
| Zn–N2 | Fe1.0HTNs | — | — | 0.00014 | 0.028 | 190 |
| Zn–NO | MoS2 | 2.03 | 85.0 | 0.4118 | 1.04 | 191 |
| Zn–NO | CoS1−x | 1.83 | 53.62 | 1.49 | 2.06 | 192 |
| Zn–NO | Bi@C | 2.08 | 93 | 0.36 | 2.35 | 193 |
| Zn–O2 | S–FeCo3P/NPSG | — | — | — | 38 | 194 |
| Zn–O2 | Fe@Co-NMC | — | — | — | 98.7 | 195 |
| Zn–O2 | AP-CONPs/NF | 1.37 | — | — | 89.1 | 196 |
5.2 Metal–NO2− batteries
The cathode conditions of Zn–NO2− batteries (Fig. 12k) developed currently are an alkaline environment and neutral environment,177 and the cathode reaction in the alkaline environment is identical to that in the neutral environment. Carbon-doped Co3O4 nanotubes have been developed as cathodic catalysts under neutral conditions for the assembly of novel Zn–NO2− batteries.23 During the NO2− reduction process, the energy barrier of *N hydrogenation in C/Co3O4 (Fig. 12l) is significantly reduced due to accelerated charge transfer caused by the C dopant inducing a local electric field. Carbon-doped Co3O4 possessed decent catalytic activity (Fig. 12m) for NO2− reduction, achieving a high FE approaching 100% for ammonia production within a wide range (−0.1 V to −0.6 V vs. RHE). The assembled Zn–NO2− battery demonstrated a power density of 6.03 mW cm−2 and a FE of 95.1% for NH3 production.The electrochemical reactions in the Zn–NO2− battery are presented as follows:
| Cathode reaction: NO2− + 6H2O + 6e− → NH4OH + 7OH− |
| Anode reaction: 3Zn + 6OH− → 3ZnO + 3H2O + 6e− |
| Overall reaction: 3Zn + NO2− + 3H2O → 3ZnO + NH4OH + OH− |
Some advances have been made in Zn–NO2− batteries with cathodes under neutral and alkaline conditions. Indeed, little effort has been devoted to catalysts in acidic environments, while the theoretical voltage (2.146 V) of the Zn–NO2− cell in the acidic environment is higher than those in alkaline (1.089 V) and neutral environments (1.589 V). Furthermore, a bottleneck currently exists in the development of rechargeable Zn–NO2− batteries, which may be due to the challenge in avoiding the OER coinciding with the conversion of nitrite to nitrate.
5.3 Hurdles and opportunities on batteries
The comparative performances of the currently developed Zn–NO2− batteries, Zn–NO3− batteries and Zn–gas batteries are exhibited in Table 3, thus providing a better understanding of the development status of Zn–NO2− batteries and Zn–NO3− batteries. It is evident that Zn–NOx− batteries are much more promising than Zn–N2 for simultaneous NH3 generation and electricity output. However, the insufficiently desirable state of current Zn–NOx− batteries must also be confirmed. Strategies to improve the performance of Zn–NOx− batteries cannot be limited in designing more advanced cathode catalysts with reduced HER competition.197 As shown in Table 3, despite facing fierce competition from the HER, the FEs for Zn–NOx− batteries range from 81.3% to 98.3%, which suggests that the HER at most leads to 1.7–18.7% loss in FE. In view of the higher theoretical voltage of the Zn–NOx− cell and more abundant proton supply in an acidic environment, it is essential to develop a Zn–NOx− cell with acidic cathodic NOx− reduction and enhance the corrosion resistance of the corresponding electrodes for effectively increasing the open circuit voltage and power density.Moreover, pure metallic Zn electrodes are widely employed in rechargeable battery systems, including zinc–air, silver–zinc and zinc–nickel batteries;198–200 however, many adverse side reactions exist between Zn electrodes and the electrolyte during the reaction process, such as electrode passivation, zinc dendrite growth and electrode distortion.201–203 In particular, the growth of zinc dendrites is caused by the inhomogeneous deposition of Zn during the charging process,204 which can cause drastic degradation of the coulomb efficiencies and capacities of the batteries. More seriously, the two electrodes of the battery will come into contact when the dendrites pierce the membrane of the battery, leading to internal short-circuiting and termination of the battery. Various approaches have been proposed to combat zinc dendrites, including electrolyte optimization,205 electrode surface modification206 and electrode structure design;207 nevertheless, comprehensive criteria for evaluating the state of metal anodes in aqueous metal–NO2−/NO3− batteries are currently lacking. Thus, the behavior of metal anodes in aqueous metal–NO2−/NO3− batteries could be considered as a priority for future studies.
Apart from the influence of Zn, the robustness of metal–NO2−/NO3− batteries is also affected by the consumption of NO2−/NO3−. To overcome this issue, a continuously stirring flow system can be introduced with steady NO2−/NO3− concentration and stable current density. Future efforts can be attempted in the design and construction of highly efficient and selective electrocatalysts applied in a flow metal–NO2−/NO3− battery system.
In addition to enhancing the metal–NO2−/NO3− battery performance by optimizing electrode catalysts and metal anodes, some attempts have recently been made to replace the Zn oxidation reaction with other oxidation reactions including hydrazine (N2H4) oxidation. Theoretically, a battery can be composed of anodic N2H4 oxidation to N2 and cathodic NO3− reduction to NH3. In this battery, a high theoretical discharge voltage can be generated up to 1.04 V (NO3− + 2N2H4 → NH3 + 2N2 + 2H2O + OH−),208,209 accompanied by sewage purification and NH3 production. Currently, in view of the importance of electrolyte renewal, a novel N2H4–NO3− flow battery has been developed, which employs RuCo precatalysts as electrodes for accelerating both the N2H4 oxidation and NO3− reduction.178 The RuCo precatalysts have been confirmed to be reconstructed into Ru/Co(OH)2 heterostructures during the electrocatalysis (Fig. 13a). The positive shift of 0.2 eV could be observed in the Ru 3p XPS peaks of the precatalyst after the test (Fig. 13b), suggesting the formation of electron-deficient Ru sites and strong interfacial interactions between Ru and Co(OH)2. The battery performances influenced by wastewater concentration are depicted in Fig. 13c. The discharge power density is gradually enhanced from 2.8 to 16.8 mW cm−2 with the increase of concentration from 1 to 1000 mM. Fig. 13d illustrates the stability test of the N2H4–NO3− flow battery in 0.1 M wastewater, which was operated continuously for 20 hours at 100 mA cm−2 and maintains the NH4 production rate of roughly 0.38 mmol h−1 cm−2. In addition to the remarkable stability of Ru/Co(OH)2 heterostructures, the lack of significant voltage degradation during the test may also be caused by the promotion of electrolyte renewal and recovery in the flow state. In addition, the promising potential of the N2H4–NO3− flow battery has been demonstrated on electricity supply. As indicated in Fig. 13e, a low-voltage-driven anion exchange membrane hydrazine electrolyzer can be spontaneously driven by two tandem N2H4–NO3− flow batteries, achieving a H2 production rate of 0.35 mmol h−1 cm−2, at output current density reaching 18.76 mA cm−2.
 | ||
| Fig. 13 (a) XRD pattern and (b) Ru 3p spectra of RuCo catalysts before and after testing. (c) Power density curves and (d) stability test of the N2H4–NO3− flow battery. (e) Digital photograph of the tandem N2H4–NO3− flow battery and hydrogen production electrolyzer. Reproduced with permission.178 Copyright 2023, Wiley-VCH. | ||
6. Summary and outlook
The electrocatalytic reduction of NOx− to ammonia has drawn renewed interest for the restoration of the nitrogen cycle and the promotion of ammonia-based economies. This paper reviews current studies of three valorization systems based on electrocatalytic NOx− conversion for energy supply and multifarious value-added chemicals synthesis, including waste treatment systems, novel electrolytic systems, and energy conversion and storage systems. In spite of the remarkable advances made in each application, some challenges and opportunities still exist.6.1 Establishing uniform comparison standards and real experimental conditions
Uniform criteria are lacking in the field of NOx− reduction including benchmark materials, test conditions and ammonia production units, which can potentially misguide readers and peers in judging and comparing catalyst performance. In order to achieve comparison under similar conditions, a majority of efforts focus on creating an identical test environment by establishing analogous test conditions including the electrolyte pH, NO3−/NO2− concentration, and electrochemical test parameters such as applied potential and stability test duration. However, the pH, NOx− ion concentration and operating voltage in the electrolytic environment hardly remain constant for practical application situations. Most catalysts currently developed are primarily targeted at specific pH and NOx− ion concentration, ignoring the challenges of environments with variable pH, NOx− ion concentration and operating voltage. Therefore, electrodes are required to be developed which generally exhibit remarkable FE and energy exchange efficiency over an extensive range of pH, NOx− ion concentration and electrode potentials. In addition, the effects of wastewater constituents on reaction activity and electrode fouling still need be elucidated. For example, transition metal-based catalysts may be inactivated by complexing with interfering SCN− ions in wastewater. Therefore, the effects of ionic strength, cation type, and competing anions should be thoroughly studied.6.2 Deepening the mechanism research and improving the theoretical model
Although it is commonly recognized that the NO3−/NO2− reduction catalysts in alkaline environments are more inclined to exhibit higher FE and energy conversion efficiencies compared to those in other environments, the exploration of mechanisms in different environments is unclear. Currently, apart from the knowledge of excess OH− ions inhibiting the HER, more intrinsic mechanisms have not been probed accompanied by the absence of reliable experimental evidence.Moreover, in the majority of microkinetic models currently developed for screening efficient NOx− reduction catalysts, the microkinetic models are often simplified by assuming that Nads is directly coupled to Hads. However, this theoretical model only embodies the competition from the HER, but neglects the side reaction generating N2 and NO. A comprehensive competitive kinetic model that can adequately display the competition of NOx− reduction reactions, the HER and other side reactions generating N-containing species is currently lacking.
6.3 Optimizing product separation strategies
The current efforts on NOx− reduction to NH3 are mainly focused on enhancing the economic efficiency and reducing the energy consumption of NH3, but the discussions on the subsequent NH3 separation are severely lacking. In electrochemical denitrification systems, the pH is constantly increased due to the consumption of H+ in the NOx− reduction process. The majority of NH3 produced at the electrolysis interface exists in the gaseous form. In addition to the traditional techniques separating NH3 including the air stripping method and extraction-condensation method, acid trapping and CO2 capture strategies have also been demonstrated to achieve NH3 separation, but the availability of pure NH3 using such strategies is facing safety risks and cost challenges. More reasonable separation strategies need to be developed with competitive cost.6.4 Constructing self-powered denitrification systems
The current electrocatalytic reduction of NOx− to NH3 still relies on external electric power, posing a critical obstacle to practical applications in mobile devices. Therefore, a hypothetical integrated system called a “self-powered denitrification cycle system” is proposed, where NOx− reduction is powered by metal–NOx− batteries storing intermittent renewable energies such as wind and solar energy. This hypothetical system (Fig. 14) can be coupled with the global nitrogen cycle, where high-value-added products based on NOx− reduction re-enter the nitrogen cycle to provide abundant NOx− ions. The concept of self-powered denitrification cycle system not only opens up new insights for restoring the disturbed nitrogen cycle and boosting the nitrogen-based economy, but also provides a referable model for NOx− reduction in a realistic scenario.The key to the successful construction of self-powered denitrification systems lies in the development of the ammonia economy. The flourishing of the ammonia economy is linked not only to advances in catalytic technology but also to the acceptance of ammonia as an energy source by the public and the promotion of ammonia by government policies.
The chemical conversion of other N species could be introduced into the self-powered denitrification cycle system based on NOx− reduction, which could further promote the restoration of the global N cycle and the prosperity of the N-based economy. N2, NO and NO2 could be converted to NOx−, providing a more stable approach for obtaining NOx− and high-value product HNO3. Ammonia can be oxidized in various cells to provide hydrogen as a power source for denitrification systems.
6.5 From C–N coupling to artificial life synthesis
In order to enhance the efficiency and economic benefit of aqueous C–N coupling reactions and to broaden the range of N-containing organic products, it is crucial to promote the accumulation and generation of key N- and C-containing intermediates. A deeper knowledge of electrocatalytic reduction of individual organic molecules is required to genuinely facilitate the development of C–N coupling reactions.More intriguingly, amino acid molecules have been confirmed to be formed by CO2 and NOx−. This corresponds to the first stage towards the origin of life, the evolution of inorganic molecules into organic substances in the primitive atmosphere and oceans, accompanied by energies from cosmic rays, lightning and volcanic explosion. Large accumulation of amino acid molecules could form primitive biomolecule proteins in the early oceans, and proteins could promote the process of dehydration and condensation of nucleic acids to form deoxyribonucleic acid (DNA). Subsequently, biomolecules evolved into multimolecular systems through condensation and polymerization. Finally, the organic polymolecular system evolves into primitive life, completing the intricate and lengthy process of gradual evolution from simple inorganic molecules into primitive life forms with self-replicating functions. The success of synthetic amino acids is of great importance for exploring the origin of life; moreover, with the reduction of electricity price and the development of alternative energy sources, artificial life synthesis and accelerated evolution of life is on the horizon using CO2, N-containing substances and H2O.
Data availability
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.Author contributions
Yi Feng: conceptualization, writing – original draft, writing – review & editing; Jin-Tao Ren: writing – review & editing; Ming-Lei Sun: writing – review & editing; Zhong-Yong Yuan: conceptualization, writing – review & editing, supervision.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22179065, 22105108).References
- X. Fu, J. B. Pedersen, Y. Zhou, M. Saccoccio, S. Li, R. Sažinas, K. Li, S. Z. Andersen, A. Xu, N. H. Deissler, J. B. V. Mygind, C. Wei, J. Kibsgaard, P. C. K. Vesborg, J. K. Nørskov and I. Chorkendorff, Science, 2023, 379, 707 CrossRef PubMed
.
- P. H. van Langevelde, I. Katsounaros and M. T. M. Koper, Joule, 2021, 5, 290 CrossRef
.
- H. Wang, J. Ren, M. Sun, W. Tian, Y. Feng and Z. Yuan, Adv. Energy Mater., 2024, 14, 2302515 CrossRef
.
- H. Luo, S. Li, Z. Wu, M. Jiang, M. Kuang, Y. Liu, W. Luo, D. Zhang and J. Yang, Adv. Funct. Mater., 2024, 34, 2403838 CrossRef
.
- K. Wang, R. Mao, R. Liu, J. Zhang, H. Zhao, W. Ran and X. Zhao, Nat. Mater., 2023, 1, 1068 Search PubMed
.
- H. Xu, Y. Ma, J. Chen, W. X. Zhang and J. Yang, Chem. Soc. Rev., 2022, 51, 2710 RSC
.
- M. Sun, H. Wang, Y. Feng, J. Ren, L. Wang and Z. Yuan, Chem. Soc. Rev., 2024 10.1039/d4cs00517a
.
- Z. Wu, M. Karamad, X. Yong, Q. Huang, D. A. Cullen, P. Zhu, C. Xia, Q. Xiao, M. Shakouri, F. Chen, J. Y. T. Kim, Y. Xia, K. Heck, Y. Hu, M. S. Wong, Q. Li, I. Gates, S. Siahrostami and H. Wang, Nat. Commun., 2021, 12, 2870 CrossRef PubMed
.
- D. R. Macfarlane, P. V. Cherepanov, J. Choi, B. H. R. Suryanto, R. Y. Hodgetts, J. M. Bakker, F. M. Ferrero Vallana and A. N. Simonov, Joule, 2020, 4, 1186 CrossRef
.
- Y. Pang and J. Wang, Sci. Total Environ., 2021, 794, 148699 CrossRef PubMed
.
- X. Zou, J. Xie, C. Wang, G. Jiang, K. Tang and C. Chen, Chin. Chem. Lett., 2022, 34, 107908 CrossRef
.
- J. Li, G. Zhan, J. Yang, F. Quan, C. Mao, Y. Liu, B. Wang, F. Lei, L. Li, A. W. M. Chan, L. Xu, Y. Shi, Y. Du, W. Hao, P. K. Wong, J. Wang, S. Dou, L. Zhang and J. C. Yu, J. Am. Chem. Soc., 2020, 142, 7036 CrossRef PubMed
.
- X. Fan, X. He, X. Ji, L. Zhang, J. Li, L. Hu, X. Li, S. Sun, D. Zheng, Y. Luo, Y. Wang, L. Xie, Q. Liu, B. Ying and X. Sun, Inorg. Chem. Front., 2023, 10, 1431–1435 RSC
.
- P. Gao, Z. Xue, S. Zhang, D. Xu, G. Zhai, Q. Li, J. Chen and X. Li, Angew. Chem., Int. Ed., 2021, 60, 20711 CrossRef CAS PubMed
.
- R. Li, W. Ma, Y. Liu, L. Zhang and Z. Zhou, J. Mater. Chem. A, 2023, 11, 18626–18645 RSC
.
- J. Lim, C. A. Fernández, S. W. Lee and M. C. Hatzell, ACS Energy Lett., 2021, 6, 3676 CrossRef CAS
.
- C. Wang, Y. Zhang, H. Luo, H. Zhang, W. Li, W. X. Zhang and J. Yang, Small Methods, 2022, 6, 2200790 CrossRef CAS PubMed
.
- X. Lv, Y. Liu, Y. Wang, X. Liu and Z. Yuan, Appl. Catal., B, 2021, 280, 119434 CrossRef CAS
.
- J. Shi, C. Wang, R. Yang, F. Chen, N. Meng, Y. Yu and B. Zhang, Sci. China:Chem., 2021, 64, 1493 CrossRef CAS
.
- X. Lv, X. Liu, Y. Suo, Y. Liu and Z. Yuan, ACS Nano, 2021, 15, 12109 CrossRef PubMed
.
- X. Zhang, A. Chen, L. Chen and Z. Zhou, Adv. Energy Mater., 2022, 12, 2003841 CrossRef
.
- Z. Li, J. Liang, Q. Liu, L. Xie, L. Zhang, Y. Ren, L. Yue, N. Li, B. Tang, A. A. Alshehri, M. S. Hamdy, Y. Luo, Q. Kong and X. Sun, Mater. Today Phys., 2022, 23, 100619 CrossRef
.
- R. Zhang, S. Zhang, Y. Guo, C. Li, J. Liu, Z. Huang, Y. Zhao, Y. Li and C. Zhi, Energy Environ. Sci., 2022, 15, 3024 RSC
.
- W. Tian, Y. Ying, J. Ren and Z. Yuan, J. Mater. Chem. A, 2023, 11, 8024 RSC
.
- J. Ren, L. Chen, L. Wang, X. Song, Q. Kong and Z. Yuan, J. Mater. Chem. A, 2023, 11, 2899 RSC
.
- J. Qu, Z. Wang, W. Gan, R. Xiao, X. Yao, Z. Khanam, L. Ouyang, H. Wang, H. Yang, S. Zhang and M. Balogun, Small, 2024, 20, 2304541 CrossRef PubMed
.
- R. Yang, X. Zheng, M. Qin, B. Lin, X. Shi and Y. Wang, Adv. Sci., 2022, 9, 2201594 CrossRef PubMed
.
- S. Liu, S. Yin, Z. Wang, Y. Xu, X. Li, L. Wang and H. Wang, Cell Rep. Phys. Sci., 2022, 3, 100869 CrossRef
.
- Y. Wu, Z. Jiang, Z. Lin, Y. Liang and H. Wang, Nat. Sustain., 2021, 4, 725 CrossRef
.
- T. Ren, Z. Yu, H. Yu, K. Deng, Z. Wang, X. Li, H. Wang, L. Wang and Y. Xu, ACS Nano, 2023, 17, 12422 CrossRef PubMed
.
- L. Xiao, W. Dai, S. Mou, X. Wang, Q. Cheng and F. Dong, Energy Environ. Sci., 2023, 16, 2696 RSC
.
- X. Zhang, Y. Wang, C. Liu, Y. Yu, S. Lu and B. Zhang, Chem. Eng. J., 2021, 403, 126269 CrossRef
.
- J. Liu, D. Richards, N. Singh and B. R. Goldsmith, ACS Catal., 2019, 9, 7052 CrossRef
.
- Y. Wang, C. Wang, M. Li, Y. Yu and B. Zhang, Chem. Soc. Rev., 2021, 50, 6720 RSC
.
- J. Theerthagiri, J. Park, H. T. Das, N. Rahamathulla, E. S. F. Cardoso, A. P. Murthy, G. Maia, D. V. N. Vo and M. Y. Choi, Environ. Chem. Lett., 2022, 20, 2929 CrossRef
.
- R. Jia, Y. Wang, C. Wang, Y. Ling, Y. Yu and B. Zhang, ACS Catal., 2020, 10, 3533 CrossRef
.
- J. Long, S. Chen, Y. Zhang, C. Guo, X. Fu, D. Deng and J. Xiao, Angew. Chem., Int. Ed., 2020, 59, 9711 CrossRef PubMed
.
- X. Lan, C. Cheng, C. Guo, M. Guo, T. Li, Y. Wu, Y. Yu and B. Zhang, Sci. China:Chem., 2023, 66, 1758 CrossRef
.
- C. Wang, Y. Zhang, H. Luo, H. Zhang, W. Li, W. Zhang and J. Yang, Small Methods, 2022, 6, 2200790 CrossRef
.
- Z. Deng, J. Liang, Q. Liu, C. Ma, L. Xie, L. Yue, Y. Ren, T. Li, Y. Luo, N. Li, B. Tang, A. Ali Alshehri, I. Shakir, P. O. Agboola, S. Yan, B. Zheng, J. Du, Q. Kong and X. Sun, Chem. Eng. J., 2022, 435, 135104 CrossRef
.
- L. Xie, Q. Liu, S. Sun, L. Hu, L. Zhang, D. Zhao, Q. Liu, J. Chen, J. Li, L. Ouyang, A. A. Alshehri, M. S. Hamdy, Q. Kong and X. Sun, ACS Appl. Mater. Interfaces, 2022, 14, 33242 CrossRef
.
- W. Chen, X. Yang, Z. Chen, Z. Ou, J. Hu, Y. Xu, Y. Li, X. Ren, S. Ye, J. Qiu, J. Liu and Q. Zhang, Adv. Funct. Mater., 2023, 33, 2300512 CrossRef
.
- H. Jiang, G. Chen, O. Savateev, J. Xue, L. Ding, Z. Liang, M. Antonietti and H. Wang, Angew. Chem., Int. Ed., 2023, 62, 202218717 CrossRef
.
- A. Khorshidi, J. Violet, J. Hashemi and A. A. Peterson, Nat. Catal., 2018, 1, 263 CrossRef
.
- J. Pérez-Ramírez and N. López, Nat. Catal., 2019, 2, 971 CrossRef
.
- M. Zhang, K. Zhang, X. Ai, X. Liang, Q. Zhang, H. Chen and X. Zou, Chin. J. Catal., 2022, 43, 2987 CrossRef
.
- Z. Zhao, S. Liu, S. Zha, D. Cheng, F. Studt, G. Henkelman and J. Gong, Nat. Rev. Mater., 2019, 4, 792 CrossRef
.
- Q. Gao, H. S. Pillai, Y. Huang, S. Liu, Q. Mu, X. Han, Z. Yan, H. Zhou, Q. He, H. Xin and H. Zhu, Nat. Commun., 2022, 13, 2338 CrossRef
.
- Y. Zhou, R. Duan, H. Li, M. Zhao, C. Ding and C. Li, ACS Catal., 2023, 13, 10846 CrossRef
.
- S. Ma and Z. Liu, ACS Catal., 2020, 10, 13213 CrossRef CAS
.
- L. Chen, X. Zhang, A. Chen, S. Yao, X. Hu and Z. Zhou, Chin. J. Catal., 2022, 43, 11 CrossRef
.
- W. Ma, S. Xie, T. Liu, Q. Fan, J. Ye, F. Sun, Z. Jiang, Q. Zhang, J. Cheng and Y. Wang, Nat. Catal., 2020, 3, 478 CrossRef
.
- K. Fan, W. Xie, J. Li, Y. Sun, P. Xu, Y. Tang, Z. Li and M. Shao, Nat. Commun., 2022, 13, 7958 CrossRef PubMed
.
- Y. Wang, A. Xu, Z. Wang, L. Huang, J. Li, F. Li, J. Wicks, M. Luo, D. Nam, C. Tan, Y. Ding, J. Wu, Y. Lum, C. Dinh, D. Sinton, G. Zheng and E. H. Sargent, J. Am. Chem. Soc., 2020, 142, 5702 CrossRef PubMed
.
- H. Xu, Y. Ma, J. Chen, W. Zhang and J. Yang, Chem. Soc. Rev., 2022, 51, 2710 RSC
.
- J. Liu, H. Xiao and J. Li, J. Am. Chem. Soc., 2020, 142, 3375 CrossRef
.
- Q. Gao, B. Yao, H. S. Pillai, W. Zang, X. Han, Y. Liu, S. Yu, Z. Yan, B. Min, S. Zhang, H. Zhou, L. Ma, H. Xin, Q. He and H. Zhu, Nat. Synth., 2023, 2, 624 CrossRef
.
- J. Zhou, M. Wen, R. Huang, Q. Wu, Y. Luo, Y. Tian, G. Wei and Y. Fu, Energy Environ. Sci., 2023, 16, 2611 RSC
.
- Z. Fang, Z. Jin, S. Tang, P. Li, P. Wu and G. Yu, ACS Nano, 2022, 16, 1072–1081 CrossRef PubMed
.
- J. Yu, Y. Qin, X. Wang, H. Zheng, K. Gao, H. Yang, L. Xie, Q. Hu and C. He, Nano Energy, 2022, 103, 107705 CrossRef
.
- A. Angulo, P. van der Linde, H. Gardeniers, M. Modestino and D. Fernández Rivas, Joule, 2020, 4, 555 CrossRef
.
- S. Zhang, J. Wu, M. Zheng, X. Jin, Z. Shen, Z. Li, Y. Wang, Q. Wang, X. Wang, H. Wei, J. Zhang, P. Wang, S. Zhang, L. Yu, L. Dong, Q. Zhu, H. Zhang and J. Lu, Nat. Commun., 2023, 14, 3634 CrossRef
.
- L. Wang, L. Zhang, W. Ma, H. Wan, X. Zhang, X. Zhang, S. Jiang, J. Y. Zheng and Z. Zhou, Adv. Funct. Mater., 2022, 32, 2203342 CrossRef CAS
.
- Y. Xue, Q. Yu, Q. Ma, Y. Chen, C. Zhang, W. Teng, J. Fan and W. Zhang, Environ. Sci. Technol., 2022, 56, 14797 CrossRef PubMed
.
- Y. Zhang, X. Chen, W. Wang, L. Yin and J. C. Crittenden, Appl. Catal., B, 2022, 310, 121346 CrossRef
.
- H. Liu, X. Lang, C. Zhu, J. Timoshenko, M. Rüscher, L. Bai, N. Guijarro, H. Yin, Y. Peng, J. Li, Z. Liu, W. Wang, B. R. Cuenya and J. Luo, Angew. Chem., Int. Ed., 2022, 61, 202202556 CrossRef
.
- J. Wang, C. Liang, X. Ma, P. Liu, W. Pan, H. Zhu, Z. Guo, Y. Sui, H. Liu, L. Liu and C. Yang, Adv. Mater., 2023, 45, 2307925 Search PubMed
.
- H. Liu, X. Li, L. Chen, X. Zhu, P. Dong, M. O. L. Chee, M. Ye, Y. Guo and J. Shen, Adv. Funct. Mater., 2022, 32, 2107308 CrossRef
.
- Y. Xu, H. Liang, R. Li, Z. Zhang, C. Qin, D. Xu, H. Fan, B. Hou, J. Wang, X. Gu and M. Ding, Angew. Chem., Int. Ed., 2023, 62, 202306786 CrossRef
.
- Y. Feng, J. Ren, Y. Song, W. Tian, H. Wang, L. Wang, M. Sun and Z. Yuan, CCS Chem., 2024 DOI:10.31635/ccschem.024.202404299
.
- C. Weng, X. Lv, J. Ren, T. Ma and Z. Yuan, Electrochem. Energy Rev., 2022, 5, 19 CrossRef
.
- W. Xu, Z. Lu, X. Sun, L. Jiang and X. Duan, Acc. Chem. Res., 2018, 51, 1590 CrossRef
.
- G. Liu, W. S. Y. Wong, M. Kraft, J. W. Ager, D. Vollmer and R. Xu, Chem. Soc. Rev., 2021, 50, 10674 RSC
.
- S. Garcia-Segura, M. Lanzarini-Lopes, K. Hristovski and P. Westerhoff, Appl. Catal., B, 2018, 236, 546 CrossRef
.
- Y. Zhong, H. Xiong, J. Low, R. Long and Y. Xiong, eScience, 2023, 3, 100086 CrossRef
.
- J. M. Mcenaney, S. J. Blair, A. C. Nielander, J. A. Schwalbe, D. M. Koshy, M. Cargnello and T. F. Jaramillo, ACS Sustain. Chem. Eng., 2020, 8, 2672 CrossRef CAS
.
- I. Katsounaros, Curr. Opin. Electrochem., 2021, 28, 100721 CrossRef CAS
.
- Y. Bu, C. Wang, W. Zhang, X. Yang, J. Ding and G. Gao, Angew. Chem., Int. Ed., 2023, 62, 202217337 Search PubMed
.
- J. Gao, B. Jiang, C. Ni, Y. Qi, Y. Zhang, N. Oturan and M. A. Oturan, Appl. Catal., B, 2019, 254, 391 CrossRef CAS
.
- J. Wang, Y. Wang, C. Cai, Y. Liu, D. Wu, M. Wang, M. Li, X. Wei, M. Shao and M. Gu, Nano Lett., 2023, 23, 1897 CrossRef CAS PubMed
.
- C. Zhong, B. Liu, J. Ding, X. Liu, Y. Zhong, Y. Li, C. Sun, X. Han, Y. Deng, N. Zhao and W. Hu, Nat. Energy, 2020, 5, 440 CrossRef CAS
.
- Z. Wenxiao, Z. Liuyi, Y. Zhang, L. Zichao, L. Zhenchao, Z. Yifan, X. Haolin, D. Zhi, W. Chaohai and F. Chunhua, Environ. Sci. Technol., 2021, 55, 13231 Search PubMed
.
- Z. Xu, L. Wan, Y. Liao, M. Pang, Q. Xu, P. Wang and B. Wang, Nat. Commun., 2023, 14, 1619 CrossRef CAS
.
- C. Chen, X. Zhu, X. Wen, Y. Zhou, L. Zhou, H. Li, L. Tao, Q. Li, S. Du, T. Liu, D. Yan, C. Xie, Y. Zou, Y. Wang, R. Chen, J. Huo, Y. Li, J. Cheng, H. Su, X. Zhao, W. Cheng, Q. Liu, H. Lin, J. Luo, J. Chen, M. Dong, K. Cheng, C. Li and S. Wang, Nat. Chem., 2020, 12, 717 CrossRef CAS PubMed
.
- J. Lv, M. Jouny, W. Luc, W. Zhu, J. Zhu and F. Jiao, Adv. Mater., 2018, 30, 1803111 CrossRef
.
- F. Li, A. Thevenon, A. Rosas-Hernández, Z. Wang, Y. Li, C. M. Gabardo, A. Ozden, C. T. Dinh, J. Li, Y. Wang, J. P. Edwards, Y. Xu, C. Mccallum, L. Tao, Z. Liang, M. Luo, X. Wang, H. Li, C. P. O Brien, C. Tan, D. Nam, R. Quintero-Bermudez, T. Zhuang, Y. C. Li, Z. Han, R. D. Britt, D. Sinton, T. Agapie, J. C. Peters and E. H. Sargent, Nature, 2020, 577, 509 CrossRef CAS PubMed
.
- Y. Zeng, C. Priest, G. Wang and G. Wu, Small Methods, 2020, 4, 2000672 CrossRef CAS
.
- Z. Wu, Y. Song, Y. Liu, W. Luo, W. Li and J. Yang, Chem Catal., 2023, 3, 100786 CrossRef CAS
.
- Y. Liu, M. Chen, X. Zhao, H. Zhang, Y. Zhao and Y. Zhou, Chem. Eng. J., 2023, 475, 146176 CrossRef CAS
.
- H. Xu, J. Chen, Z. Zhang, C. Hung, J. Yang and W. Li, Adv. Mater., 2023, 35, 2207522 CrossRef CAS
.
- J. Sun, H. Yang, W. Gao, T. Cao and G. Zhao, Angew. Chem., Int. Ed., 2022, 61, 202211373 CrossRef
.
- H. Huang, K. Peramaiah and K. Huang, Energy Environ. Sci., 2024, 17, 2682–2685 RSC
.
- C. Fu, S. Shu, L. Hu, Z. Liu, Z. Yin, X. Lv, S. Zhang and G. Jiang, Chem. Eng. J., 2022, 435, 134969 CrossRef CAS
.
- X. Wang, M. Zhu, G. Zeng, X. Liu, C. Fang and C. Li, Nanoscale, 2020, 12, 9385 RSC
.
- F. Ni, Y. Ma, J. Chen, W. Luo and J. Yang, Chin. Chem. Lett., 2021, 32, 2073 CrossRef CAS
.
- L. Su, K. Li, H. Zhang, M. Fan, D. Ying, T. Sun, Y. Wang and J. Jia, Water Res., 2017, 120, 1 CrossRef
.
- R. Chauhan and V. C. Srivastava, Chem. Eng. J., 2020, 386, 122065 CrossRef
.
- T. T. P. Nguyen, B. K. D. Do, N. N. Bui, M. A. Pham and T. V. Nguyen, ECS Trans., 2013, 53, 41 CrossRef
.
- I. Katsounaros, M. Dortsiou and G. Kyriacou, J. Hazard. Mater., 2009, 171, 323 CrossRef
.
- G. Chen, Y. Yuan, H. Jiang, S. Ren, L. Ding, L. Ma, T. Wu, J. Lu and H. Wang, Nat. Energy, 2020, 5, 605 CrossRef
.
- T. F. Beltrame, D. Carvalho, L. Marder, M. A. Ulla, F. A. Marchesini and A. M. Bernardes, J. Environ. Chem. Eng., 2020, 8, 104120 CrossRef
.
- Y. Zeng, C. Priest, G. Wang and G. Wu, Small Methods, 2020, 4, 2000672 CrossRef
.
- G. Jiang, M. Peng, L. Hu, J. Ouyang, X. Lv, Z. Yang, X. Liang, Y. Liu and H. Liu, Chem. Eng. J., 2022, 435, 134853 CrossRef
.
- I. Katsounaros and G. Kyriacou, Electrochim. Acta, 2007, 52, 6412 CrossRef
.
- S. Meng, Y. Ling, M. Yang, X. Zhao, A. I. Osman, A. A. H. Al-Muhtaseb, D. W. Rooney and P. Yap, J. Environ. Chem. Eng., 2023, 11, 109418 CrossRef
.
- J. Makover, D. Hasson, R. Semiat and H. Shemer, J. Environ. Chem. Eng., 2020, 8, 103727 CrossRef
.
- M. J. Liu, D. M. Miller and W. A. Tarpeh, Environ. Sci. Technol. Lett., 2023, 10, 458 CrossRef CAS
.
- S. L. Foster, S. I. P. Bakovic, R. D. Duda, S. Maheshwari, R. D. Milton, S. D. Minteer, M. J. Janik, J. N. Renner and L. F. Greenlee, Nat. Catal., 2018, 1, 490 CrossRef
.
- B. H. Ko, B. Hasa, H. Shin, Y. Zhao and F. Jiao, J. Am. Chem. Soc., 2022, 144, 1258 CrossRef CAS
.
- X. Deng, Y. Yang, L. Wang, X. Fu and J. Luo, Adv. Sci., 2021, 8, 2004523 CrossRef CAS
.
- Q. Hu, K. Yang, O. Peng, M. Li, L. Ma, S. Huang, Y. Du, Z. Xu, Q. Wang, Z. Chen, M. Yang and K. P. Loh, J. Am. Chem. Soc., 2024, 146, 668 CrossRef CAS
.
- O. Q. Carvalho, R. Marks, H. K. K. Nguyen, M. E. Vitale-Sullivan, S. C. Martinez, L. árnadóttir and K. A. Stoerzinger, J. Am. Chem. Soc., 2022, 144, 14809 CrossRef CAS PubMed
.
- W. Zhong, Q. Hong, X. Ai, C. Zhang, F. Li, X. Li and Y. Chen, Adv. Mater., 2024, 46, 2314351 CrossRef PubMed
.
- Y. Lv, S. Ke, Y. Gu, B. Tian, L. Tang, P. Ran, Y. Zhao, J. Ma, J. Zuo and M. Ding, Angew. Chem., Int. Ed., 2023, 62, 202305246 CrossRef
.
- H. Zhang, C. Wang, H. Luo, J. Chen, M. Kuang and J. Yang, Angew. Chem., Int. Ed., 2023, 62, e202217071 CrossRef PubMed
.
- Y. Feng, L. Chen and Z. Yuan, Inorg. Chem. Front., 2023, 10, 5225 RSC
.
- K. Yu, H. Wang, W. Yu, S. Li, X. Zhang and Z. Bian, Appl. Catal., B, 2024, 341, 123292 CrossRef
.
- C. Tang, Y. Zheng, M. Jaroniec and S. Qiao, Angew. Chem., Int. Ed., 2021, 60, 19572 CrossRef PubMed
.
- J. Liang, Z. Li, L. Zhang, X. He, Y. Luo, D. Zheng, Y. Wang, T. Li, H. Yan, B. Ying, S. Sun, Q. Liu, M. S. Hamdy, B. Tang and X. Sun, Chem, 2023, 9, 1768 Search PubMed
.
- S. Ye, Z. Chen, G. Zhang, W. Chen, C. Peng, X. Yang, L. Zheng, Y. Li, X. Ren, H. Cao, D. Xue, J. Qiu, Q. Zhang and J. Liu, Energy Environ. Sci., 2022, 15, 760 RSC
.
- C. Wang, W. Zhou, Z. Sun, Y. Wang, B. Zhang and Y. Yu, J. Mater. Chem. A, 2021, 9, 239 RSC
.
- W. R. Leow, Y. Lum, A. Ozden, Y. Wang, D. Nam, B. Chen, J. Wicks, T. Zhuang, F. Li, D. Sinton and E. H. Sargent, Science, 2020, 368, 1228 CrossRef
.
- Y. Zhang, Y. Wang, L. Han, S. Wang, T. Cui, Y. Yan, M. Xu, H. Duan, Y. Kuang and X. Sun, Angew. Chem., Int. Ed., 2022, 61, 202213711 Search PubMed
.
- Z. Mei, Y. Zhou, W. Lv, S. Tong, X. Yang, L. Chen and N. Zhang, ACS Sustain. Chem. Eng., 2022, 10, 12477 CrossRef
.
- J. Leverett, T. Tran Phu, J. A. Yuwono, P. Kumar, C. Kim, Q. Zhai, C. Han, J. Qu, J. Cairney, A. N. Simonov, R. K. Hocking, L. Dai, R. Daiyan and R. Amal, Adv. Energy Mater., 2022, 12, 2201500 CrossRef
.
- Z. Tao, C. L. Rooney, Y. Liang and H. Wang, J. Am. Chem. Soc., 2021, 143, 19630 CrossRef
.
- N. Meng, Y. Huang, Y. Liu, Y. Yu and B. Zhang, Cell Rep. Phys. Sci., 2021, 2, 100378 CrossRef
.
- C. Lv, L. Zhong, H. Liu, Z. Fang, C. Yan, M. Chen, Y. Kong, C. Lee, D. Liu, S. Li, J. Liu, L. Song, G. Chen, Q. Yan and G. Yu, Nat. Sustain., 2021, 4, 868 CrossRef
.
- Y. Liu, X. Tu, X. Wei, D. Wang, X. Zhang, W. Chen, C. Chen and S. Wang, Angew. Chem., Int. Ed., 2023, 62, 202300387 CrossRef
.
- S. Liu, M. Wang, Q. Cheng, Y. He, J. Ni, J. Liu, C. Yan and T. Qian, ACS Nano, 2022, 16, 17911 CrossRef
.
- Y. Luo, K. Xie, P. Ou, C. Lavallais, T. Peng, Z. Chen, Z. Zhang, N. Wang, X. Li, I. Grigioni, B. Liu, D. Sinton, J. B. Dunn and E. H. Sargent, Nat. Catal., 2023, 6, 939–948 CrossRef
.
- X. Huang, Y. Li, S. Xie, Q. Zhao, B. Zhang, Z. Zhang, H. Sheng and J. Zhao, Angew. Chem., Int. Ed., 2024, 63, 202403980 CrossRef PubMed
.
- Y. Feng, L. Chen and Z. Yuan, J. Ind. Eng. Chem., 2023, 120, 27 CrossRef
.
- A. V. Munde, B. B. Mulik, R. P. Dighole and B. R. Sathe, ACS Appl. Energy Mater., 2021, 4, 13172 CrossRef
.
- N. Meng, X. Ma, C. Wang, Y. Wang, R. Yang, J. Shao, Y. Huang, Y. Xu, B. Zhang and Y. Yu, ACS Nano, 2022, 16, 9095 CrossRef PubMed
.
- Y. Feng, H. Yang, Y. Zhang, X. Huang, L. Li, T. Cheng and Q. Shao, Nano Lett., 2020, 20, 8282 CrossRef PubMed
.
- X. Lv, Y. Liu, R. Hao, W. Tian and Z. Yuan, ACS Appl. Mater. Interfaces, 2020, 12, 17502 CrossRef PubMed
.
- N. Cao, Y. Quan, A. Guan, C. Yang, Y. Ji, L. Zhang and G. Zheng, J. Colloid Interface Sci., 2020, 577, 109 CrossRef PubMed
.
- Q. Zhang and J. Guan, Nano Res., 2022, 15, 38 CrossRef
.
- J. Leverett, T. Tran-Phu, J. A. Yuwono, P. Kumar, C. Kim, Q. Zhai, C. Han, J. Qu, J. Cairney, A. N. Simonov, R. K. Hocking, L. Dai, R. Daiyan and R. Amal, Adv. Energy Mater., 2022, 12, 2201500 CrossRef
.
- X. Zhang, X. Zhu, S. Bo, C. Chen, M. Qiu, X. Wei, N. He, C. Xie, W. Chen, J. Zheng, P. Chen, S. P. Jiang, Y. Li, Q. Liu and S. Wang, Nat. Commun., 2022, 13, 5337 CrossRef CAS PubMed
.
- L. Chen and Z. Yuan, J. Ind. Eng. Chem., 2022, 108, 1 CrossRef CAS
.
- X. Liu, P. V. Kumar, Q. Chen, L. Zhao, F. Ye, X. Ma, D. Liu, X. Chen, L. Dai and C. Hu, Appl. Catal., B, 2022, 316, 121618 CrossRef CAS
.
- C. Hu, Y. Lin, J. W. Connell, H. Cheng, Y. Gogotsi, M. Titirici and L. Dai, Adv. Mater., 2019, 31, 1806128 CrossRef
.
- C. Chen, S. Li, X. Zhu, S. Bo, K. Cheng, N. He, M. Qiu, C. Xie, D. Song, Y. Liu, W. Chen, Y. Li, Q. Liu, C. Li and S. Wang, Carbon Energy, 2023, 5, 345 CrossRef
.
- Y. Huan, Y. Jiang, L. Li, Y. He, Q. Cheng, Y. Cao, M. Wang, C. Yan and T. Qian, ACS Mater. Lett., 2023, 5, 3347 CrossRef CAS
.
- J. Liang, Z. Cai, Z. Li, Y. Yao, Y. Luo, S. Sun, D. Zheng, Q. Liu, X. Sun and B. Tang, Nat. Commun., 2024, 15, 2950 CrossRef CAS
.
- J. Li and N. Kornienko, Chem. Sci., 2022, 13, 3957 RSC
.
- M. Jouny, J. Lv, T. Cheng, B. H. Ko, J. Zhu, W. A. Goddard and F. Jiao, Nat. Chem., 2019, 11, 846 CrossRef CAS
.
- J. Lan, Z. Wei, Y. Lu, D. Chen, S. Zhao, T. Chan and Y. Tan, Nat. Commun., 2023, 14, 2870 CrossRef
.
- N. Meng, J. Shao, H. Li, Y. Wang, X. Fu, C. Liu, Y. Yu and B. Zhang, Nat. Commun., 2022, 13, 5452 CrossRef CAS PubMed
.
- J. Shao, N. Meng, Y. Wang, B. Zhang, K. Yang, C. Liu, Y. Yu and B. Zhang, Angew. Chem., Int. Ed., 2022, 61, 202213009 CrossRef PubMed
.
- Z. Tao, Y. Wu, Z. Wu, B. Shang, C. Rooney and H. Wang, J. Energy Chem., 2022, 65, 367 CrossRef CAS
.
- F. Rieck Genannt Best, A. Mundstock, H. Richter, P. A. Kißling, K. D. J. Hindricks, A. Huang, P. Behrens and J. Caro, Microporous Mesoporous Mater., 2022, 337, 111920 CrossRef CAS
.
- Z. Tao, Y. Wu, Z. Wu, B. Shang, C. Rooney and H. Wang, J. Energy Chem., 2022, 65, 367 CrossRef CAS
.
- M. He, Y. Wu, R. Li, Y. Wang, C. Liu and B. Zhang, Nat. Commun., 2023, 14, 5088 CrossRef CAS PubMed
.
- J. Wu, L. Xu, Z. Kong, K. Gu, Y. Lu, X. Wu, Y. Zou and S. Wang, Angew. Chem., Int. Ed., 2023, e202311196 CAS
.
- H. Yadegari, A. Ozden, T. Alkayyali, V. Soni, A. Thevenon, A. Rosas-Hernández, T. Agapie, J. C. Peters, E. H. Sargent and D. Sinton, ACS Energy Lett., 2021, 6, 3538 CrossRef
.
- Y. Wang, Y. Yu, R. Jia, C. Zhang and B. Zhang, Natl. Sci. Rev., 2019, 6, 730 CrossRef
.
- H. Wang, L. Wang, J. Ren, W. Tian, M. Sun and Z. Yuan, Nano-Micro Lett., 2023, 15, 155 CrossRef
.
- J. Wu, L. Xu, Z. Kong, K. Gu, Y. Lu, X. Wu, Y. Zou and S. Wang, Angew. Chem., Int. Ed., 2023, e202311196 Search PubMed
.
- D. Li, Z. Li, R. Zou, G. Shi, Y. Huang, W. Yang, W. Yang, C. Liu and X. Peng, Appl. Catal., B, 2022, 307, 121170 CrossRef
.
- J. Wu, J. Chen, T. Yu, Z. Zhai, Y. Zhu, X. Wu and S. Yin, ACS Catal., 2023, 13, 13257–13266 CrossRef
.
- R. Zhong, P. Wu, Q. Wang, X. Zhang, L. Du, Y. Liu, H. Yang, M. Gu, Z. C. Zhang, L. Huang and S. Ye, Green Chem., 2023, 25, 4674 RSC
.
- C. Huang, Y. Huang, C. Liu, Y. Yu and B. Zhang, Angew. Chem., Int. Ed., 2019, 58, 12014 CrossRef PubMed
.
- Y. Wu, C. Liu, C. Wang, Y. Yu, Y. Shi and B. Zhang, Nat. Commun., 2021, 12, 3881 CrossRef PubMed
.
- S. Li, P. Ma, C. Gao, L. Liu, X. Wang, M. Shakouri, R. Chernikov, K. Wang, D. Liu, R. Ma and J. Wang, Energy Environ. Sci., 2022, 15, 3004 RSC
.
- J. Li, H. Li, K. Fan, J. Y. Lee, W. Xie and M. Shao, Chem Catal., 2023, 3, 100638 CrossRef
.
- Z. Li, Y. Yan, S. Xu, H. Zhou, M. Xu, L. Ma, M. Shao, X. Kong, B. Wang and L. Zheng, Nat. Commun., 2022, 13, 147 CrossRef CAS PubMed
.
- Z. Yin, Y. Zheng, H. Wang, J. Li, Q. Zhu, Y. Wang, N. Ma, G. Hu, B. He, A. Knop-Gericke, R. Schlögl and D. Ma, ACS Nano, 2017, 11, 12365 CrossRef CAS PubMed
.
- H. Huang, C. Yu, X. Han, H. Huang, Q. Wei, W. Guo, Z. Wang and J. Qiu, Energy Environ. Sci., 2021, 14, 6055 RSC
.
- M. Axelsson, C. F. Marchiori, P. Huang, C. M. Araujo and H. Tian, J. Am. Chem. Soc., 2021, 143, 21229 CrossRef CAS
.
- H. Zhou, Z. Li, S. Xu, L. Lu, M. Xu, K. Ji, R. Ge, Y. Yan, L. Ma, X. Kong, L. Zheng and H. Duan, Angew. Chem., Int. Ed., 2021, 60, 8976 CrossRef CAS PubMed
.
- Z. Chen, J. Dong, J. Wu, Q. Shao, N. Luo, M. Xu, Y. Sun, Y. Tang, J. Peng and H. Cheng, Nat. Commun., 2023, 14, 4210 CrossRef CAS PubMed
.
- Q. Liu, L. Xie, J. Liang, Y. Ren, Y. Wang, L. Zhang, L. Yue, T. Li, Y. Luo, N. Li, B. Tang, Y. Liu, S. Gao, A. A. Alshehri, I. Shakir, P. O. Agboola, Q. Kong, Q. Wang, D. Ma and X. Sun, Small, 2022, 18, 2106961 CrossRef CAS PubMed
.
- R. Zhang, Y. Guo, S. Zhang, D. Chen, Y. Zhao, Z. Huang, L. Ma, P. Li, Q. Yang, G. Liang and C. Zhi, Adv. Energy Mater., 2022, 12, 2103872 CrossRef CAS
.
- Z. Ren, Q. Chen, X. An, Q. Liu, L. Xie, J. Zhang, W. Yao, M. S. Hamdy, Q. Kong and X. Sun, Inorg. Chem., 2022, 61, 12895 CrossRef CAS
.
- W. Zhu, X. Zhang, F. Yao, R. Huang, Y. Chen, C. Chen, J. Fei, Y. Chen, Z. Wang and H. Liang, Angew. Chem., Int. Ed., 2023, 62, e202300390 CrossRef CAS
.
- F. Zhou and C. Sun, Small, 2022, 18, 2200436 CrossRef CAS
.
- W. Lin, E. Zhou, J. Xie, J. Lin and Y. Wang, Adv. Funct. Mater., 2022, 32, 2209464 CrossRef CAS
.
- R. Zhang, C. Li, H. Cui, Y. Wang, S. Zhang, P. Li, Y. Hou, Y. Guo, G. Liang, Z. Huang, C. Peng and C. Zhi, Nat. Commun., 2023, 14, 8036 CrossRef CAS
.
- X. Lv, J. Ren, Y. Wang, Y. Liu and Z. Yuan, ACS Sustain. Chem. Eng., 2019, 7, 8993 CrossRef CAS
.
- H. Wang, C. Weng and Z. Yuan, J. Energy Chem., 2021, 56, 470 CrossRef CAS
.
- Z. Li, Z. Deng, L. Ouyang, X. Fan, L. Zhang, S. Sun, Q. Liu, A. A. Alshehri, Y. Luo, Q. Kong and X. Sun, Nano Res., 2022, 15, 8914 CrossRef CAS
.
- W. Yu, J. Yu, M. Huang, Y. Wang, Y. Wang, J. Li, H. Liu and W. Zhou, Energy Environ. Sci., 2023, 16, 2991 RSC
.
- J. Ren, L. Chen, H. Wang and Z. Yuan, ACS Appl. Mater. Interfaces, 2021, 13, 12106 CrossRef CAS
.
- H. Chen, Z. Xu, S. Sun, Y. Luo, Q. Liu, M. S. Hamdy, Z. Feng, X. Sun and Y. Wang, Inorg. Chem. Front., 2022, 9, 4608 RSC
.
- J. Ren, L. Chen, Y. Liu and Z. Yuan, J. Mater. Chem. A, 2021, 9, 11370 RSC
.
- H. Wang, J. Si, T. Zhang, Y. Li, B. Yang, Z. Li, J. Chen, Z. Wen, C. Yuan, L. Lei and Y. Hou, Appl. Catal., B, 2020, 270, 118892 CrossRef
.
- X. Lv, X. Liu, L. Gao, Y. Liu and Z. Yuan, J. Mater. Chem. A, 2021, 9, 4026 RSC
.
- L. Zhang, J. Liang, Y. Wang, T. Mou, Y. Lin, L. Yue, T. Li, Q. Liu, Y. Luo, N. Li, B. Tang, Y. Liu, S. Gao, A. A. Alshehri, X. Guo, D. Ma and X. Sun, Angew. Chem., Int. Ed., 2021, 60, 25263 CrossRef PubMed
.
- L. Zhang, Q. Zhou, J. Liang, L. Yue, T. Li, Y. Luo, Q. Liu, N. Li, B. Tang, F. Gong, X. Guo and X. Sun, Inorg. Chem., 2022, 61, 8096 CrossRef PubMed
.
- Q. Liu, Y. Lin, L. Yue, J. Liang, L. Zhang, T. Li, Y. Luo, M. Liu, J. You, A. A. Alshehri, Q. Kong and X. Sun, Nano Res., 2022, 15, 5032 CrossRef
.
- J. Ren, Y. Ying, Y. Liu, W. Li and Z. Yuan, J. Energy Chem., 2022, 71, 619 CrossRef
.
- Y. Zhang, Y. Li, K. Shi, Z. Zhu, X. Li, H. Xu and J. Gao, J. Alloys Compd., 2022, 925, 166680 CrossRef
.
- X. Lv, Y. Liu, W. Tian, L. Gao and Z. Yuan, J. Energy Chem., 2020, 50, 324 CrossRef
.
- J. Islam, M. Shareef, H. M. Zabed, X. Qi, F. I. Chowdhury, J. Das, J. Uddin, Y. V. Kaneti, M. U. Khandaker, M. H. Ullah and M. K. Masud, Energy Storage Mater., 2023, 54, 98 CrossRef
.
- Z. Zhao, B. Liu, Y. Shen, T. Wu, X. Zang, Y. Zhao, C. Zhong, F. Ma and W. Hu, J. Power Sources, 2021, 510, 230393 CrossRef
.
- S. Schismenos, M. Chalaris and G. Stevens, Saf. Sci., 2021, 140, 105290 CrossRef
.
- W. Tian, J. Ren, X. Lv and Z. Yuan, Chem. Eng. J., 2022, 431, 133210 CrossRef
.
- W. Tian, J. Ren and Z. Yuan, Appl. Catal., B, 2022, 317, 121764 CrossRef
.
- Y. Zuo, K. Wang, P. Pei, M. Wei, X. Liu, Y. Xiao and P. Zhang, Mater. Today Energy, 2021, 20, 100692 CrossRef
.
- W. Lu, C. Xie, H. Zhang and X. Li, ChemSusChem, 2018, 11, 3996 CrossRef
.
- Z. Xu, Q. Fan, Y. Li, J. Wang and P. D. Lund, Renewable Sustainable Energy Rev., 2020, 127, 109838 CrossRef
.
- X. Guo, Z. Zhang, J. Li, N. Luo, G. Chai, T. S. Miller, F. Lai, P. Shearing, D. J. L. Brett, D. Han, Z. Weng, G. He and I. P. Parkin, ACS Energy Lett., 2021, 6, 395 CrossRef
.
- Y. Kim and K. Ryu, Appl. Surf. Sci., 2019, 480, 912 CrossRef
.
- Q. Li, Y. Chen, H. Wang, H. Yu, W. Wei, X. Ji, B. Qu and L. Chen, Sustainable Energy Fuels, 2022, 6, 3501 RSC
.
- N. Singh and B. R. Goldsmith, ACS Catal., 2020, 10, 3365 CrossRef
.
- Y. Feng, Q. Shi, J. Lin, E. Chai, X. Zhang, Z. Liu, L. Jiao and Y. Wang, Adv. Mater., 2022, 34, 2207747 CrossRef
.
| This journal is © The Royal Society of Chemistry 2025 |