Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Annulated carbocyclic gallylene and bis-gallylene with two-coordinated Ga(I) atoms†
Arne
Merschel
 ,
Shkelqim
Heda
,
Yury V.
Vishnevskiy
,
Shkelqim
Heda
,
Yury V.
Vishnevskiy
 ,
Beate
Neumann
,
Hans-Georg
Stammler
and
Rajendra S.
Ghadwal
,
Beate
Neumann
,
Hans-Georg
Stammler
and
Rajendra S.
Ghadwal
 *
*
Molecular Inorganic Chemistry and Catalysis, Inorganic and Structural Chemistry, Center for Molecular Materials, Faculty of Chemistry, Universität Bielefeld, Universitätsstrasse 25, D-33615, Bielefeld, Germany. E-mail: rghadwal@uni-bielefeld.de; Web: http://www.ghadwalgroup.de
First published on 14th November 2024
Abstract
The first carbocyclic gallylene [(ADC)2Ga(GaI2)] and bis-gallylene [(ADC)Ga]2 (ADC = PhC{N(Dipp)C}2; Dipp = 2,6-iPr2C6H3) featuring a central C4Ga2 ring annulated between two 1,3-imidazole rings are prepared by KC8 reductions of [(ADC)GaI2]2. Treatment of [(ADC)Ga]2 with Fe2(CO)9 affords complex [(ADC)GaFe(CO)4]2 in which each Ga(I) atom serves as a two-electron donor. [(ADC)Ga]2 activates white phosphorus (P4) and the Csp2–F bond of aryl fluorides (ArF) to yield compounds [(ADC)Ga(P4)]2 and cis-/trans-[(ADC)GaF(Ar)]2, respectively. [(ADC)Ga]2 undergoes oxidation with (Me2S)AuCl to give [(ADC)GaCl2]2, while with PhN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NPh it forms [1 + 4]-cycloaddition product [(ADC)GaN(Ph)N
NPh it forms [1 + 4]-cycloaddition product [(ADC)GaN(Ph)N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C6H5]2 by the dearomatization of one of the phenyl rings.
C6H5]2 by the dearomatization of one of the phenyl rings.
Introduction
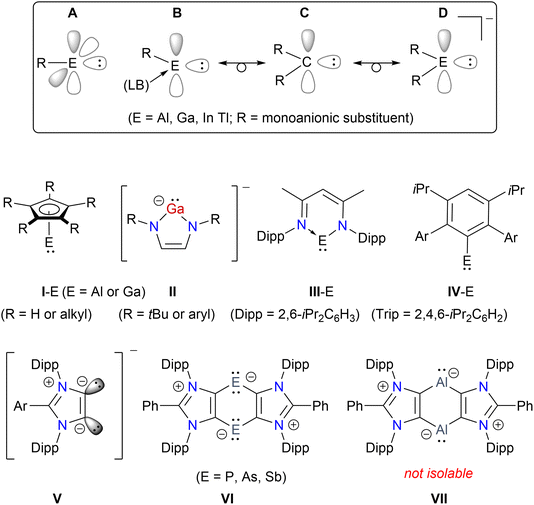
The isolation of first crystalline N-heterocyclic carbene (NHC) by Arduengo1 prompted the search for stable carbene analogues of heavier Group 13 and 14 elements, i.e. metallylenes.2 Like carbenes, metallylenes are in general highly reactive species. The first Al(I) and Ga(I) compounds were reported as tetrameric species (I-E)4 (ref. 3) and [GaC(SiMe3)3]4 (ref. 4) in the solid state (Fig. 1). In 1999, Schmidbaur reported the first anionic Ga(I) compound II (R = tBu).5 Subsequently, serval other anionic as well as neutral compounds with a two-coordinated Ga(I) or Al(I) atom were reported.2,6 In 2000, Roesky and Power independently reported the first aluminylene7 and gallylene8 compounds (III-E), respectively, based on a bulky β-diketiminate ligand. Over the past years, III-E have been extensively investigated to access a variety of Al/Ga-compounds with intriguing structures and properties.6a The fascinating chemistry of these species prompted further interests in the isolation of new thermally stable Group 13 metallylenes.9 Among mono-coordinated metallylenes, Power et al. reported the first Ga(I) species Ar(Me3Si)NGa (Ar = 2,6-Mes2C6H3; Mes = 2,4,6-Me3C6H2) in 2006 (ref. 10) and the first Al(I) compound IV-E in 2020.11 Subsequently, the research groups of Liu,12 Hinz,13 and Tan14 reported monocoordinated Group 13 metallylene compounds using a bulky carbozole ligand. Very recently, Kretschmer and colleagues isolated a mono-coordinated Ga(I) compound.15 Like singlet carbenes, Group 13 metallylenes are promising ligands in organometallic chemistry.12,16 In general, most of the known examples of neutral as well as anionic metallylene species9b,17 are based on chelating N-donor ligands.2,6 The use of singlet carbenes for the stabilization of borylene species has been shown,18 however, related heavier metallylenes remained rather scarce.19 Like other main-group homonuclear heavier alkenes (i.e. for example the dimers of metallylenes),20 digallenes may also be regarded as dimers of gallylenes.10,21 Among three coordinated Ga(I) compounds, Lewis base-stabilized neutral22 as well as dicationic23 digallene compounds are known. We have shown the suitability of 1,3-imidazole-based anionic dicarbenes (ADCs) V in accessing a variety of low-valent main-group heterocycles.24 Compounds VI featuring formally P(I),25 As(I)26 or Sb(I)27 atoms are accessible as crystalline solids. Attempts to isolate the aluminylene species VII were unfortunately unsuccessful as it underwent C–H bond activation to yield an annulated Al(III) compound in 76% yield.28 Herein, we report the first carbocyclic gallylene as well as bis-gallylene compounds (Schemes 1 and 2) as crystalline solids and showcase the reactivity of the bis-gallylene towards transition metal, white phosphorus, organofluorine, and azobenzene substrates.Results and discussion
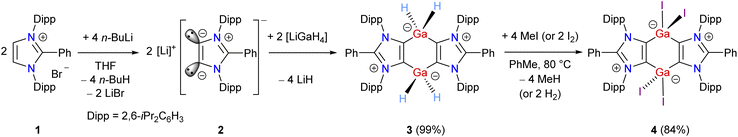
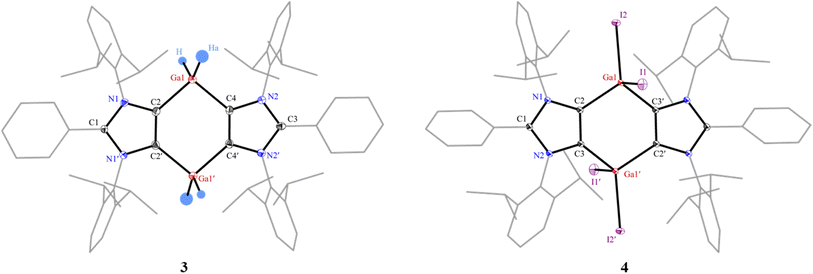
The starting Ga(III) hydride [(ADC)GaH2]2 (3) (ADC = PhC{N(Dipp)C}2, Dipp = 2,6-iPr2C6H3) was prepared by reacting freshly prepared LiGaH4 and Li(ADC) (2)29 as a colorless crystalline solid in 99% yield (Scheme 1). Compound 1 was synthesized by the direct C2-arylation of the corresponding NHC under nickel catalysis.30 Compound 3 is stable under an inert gas (N2 or Ar) atmosphere but slowly decomposes when exposed to air. The 1H and 13C{1H} NMR spectra of 3 exhibit well-resolved signals, which are fully consistent with the related aluminium species [(ADC)AlH2].31 The 1H NMR spectrum of 3 shows a broad signal at 4.16 ppm for the GaH2 moieties that is comparable with those of NHC-stabilized Ga(III) hydrides.32 The FT-IR spectrum of 3 displays two characteristic bands at 1800 and 1830 cm−1 for the Ga–H stretching vibrations.32,33 Treatment of 3 with methyl iodide (or iodine) at 80 °C affords the Ga(III) iodide 4 as a white solid. Compound 4 is insoluble in benzene and toluene but sparingly dissolves in chloroform. The 1H and 13C{1H} NMR spectra of 4 show broad signals for the isopropyl groups, which is in line with an easily polarizable nature of iodides.28 The 13C{1H} NMR signal for the gallium bound carbon atoms of 4 (164.0 ppm) is slightly downfield shifted compared to that of 3 (158.3 ppm).The solid-state molecular structures of 3 and 4 (Fig. 2) show the expected atom connectivity.34 The four-fold coordinated Ga(III) atoms in the C4Ga2 ring of 3 and 4 have a distorted tetrahedral coordination geometry. The C2–Ga1 (2.016(3) Å) and C4–Ga1 (2.015(3) Å) bond lengths of 3 are slightly smaller than those of NHC–Ga(III) hydrides (2.071(5) Å).32 This may be attributed to the stronger σ-donor strength of mesoionic carbenes (iMICs) than NHCs.35 The gallium bound hydrogen atoms of 3 were refined isotropically. The C2–Ga1–H (108.1(2)°) and C2–Ga1–Ha (113.7(2)°) bond angles of 3 are distinct. Like the Ga–H/Ha bond lengths in 3 (1.492(3)/1.574(3) Å), the Ga1–I1 (2.6117(1) Å) and Ga1–I2 (2.5230(1) Å) bond lengths of 4 are dissimilar (see below the NBO (Natural Bond Orbital) analysis). The I1 atom of 4 is situated out of the C4Ga2-ring plane, resulting the C3–C2–Ga1–I1 torsion angle of 104.1(1)°. The C2, Ga1, C3′, and I2 atoms are positioned in a semi-trigonal plane with the C3–C2–Ga1–I1 torsion angle of 168.1(1)°.
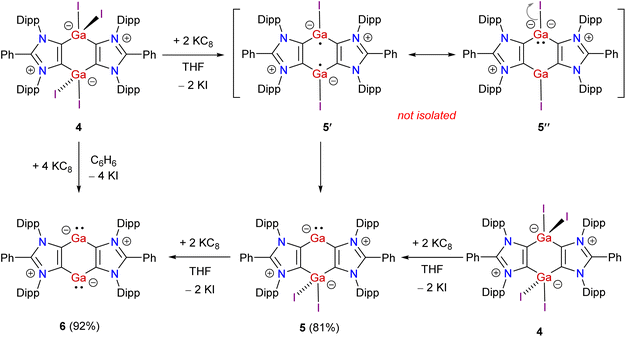
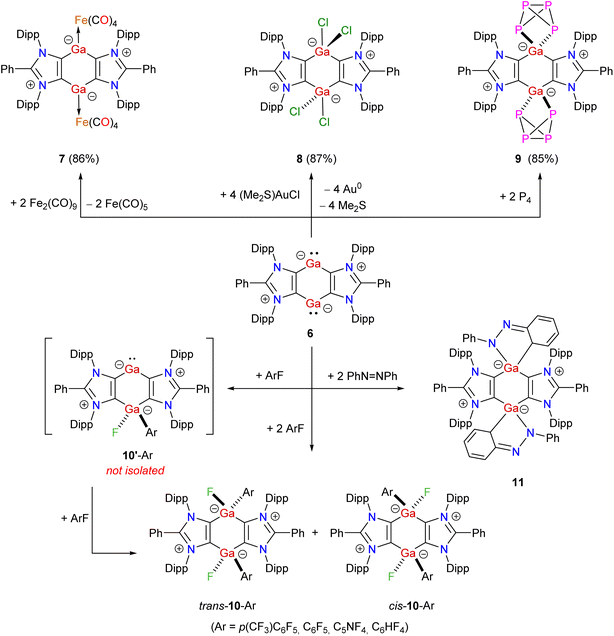
Having the desired Ga(III) compound 4 in hand, we prompted to perform its reduction. Treatment of 4 with 2 equivalents of KC8 in THF affords the mixed-valent Ga(I/III) compound 5 as a brown solid in 81% yield (Scheme 2). The exact mechanism of the formation of 5 is currently not known. Direct reduction of one of the Ga(III) atoms of 4 by two equivalents of KC8 to give 5 is likely. The reduction of 4 to give the putative Ga(II) species 5′, which may also be viewed as a zwitterionic species 5′′, cannot be ruled out. Finally, the disproportionation of 5′ (i.e. formally an intramolecular iodide ion transfer in 5′′) into 5 is calculated to be thermodynamically favored by 17.6 kcal mol−1 (see below). Reaction of 4 with 4 equivalents of KC8 in benzene leads to the clean formation of bis-gallylene 6 as a red-brown solid in 92% yield. 6 can also be prepared by reacting 5 with KC8. 5 and 6 are crystalline solids, soluble in common organic solvents (THF, toluene, benzene), and stable under an inert atmosphere (of N2 or Ar) for several weeks. The 1H NMR spectrum of mixed-valent Ga(I/III) compound 5 exhibits four doublets and two septets for the isopropyl groups, suggesting unsymmetrical coordination settings at the Ga atoms. In contrast, the 1H NMR spectrum of bis-gallylene 6 shows two doublets and one septet for the isopropyl groups, which are consistent with the higher symmetry of 6 than 5. A similar 1H NMR signal pattern is also observed for isostructural compounds featuring Group 14 (ref. 36) or 15 (ref. 25–27) elements in a formally +1 oxidation state. The 13C{1H} NMR spectrum of 5 shows two resonances at 163.6 (CGaI2) and 176.4 (CGa) ppm for the C4Ga2 moiety. The 13C{1H} NMR spectrum of 6 exhibits one signal at 173.6 ppm for the C4Ga2 unit.
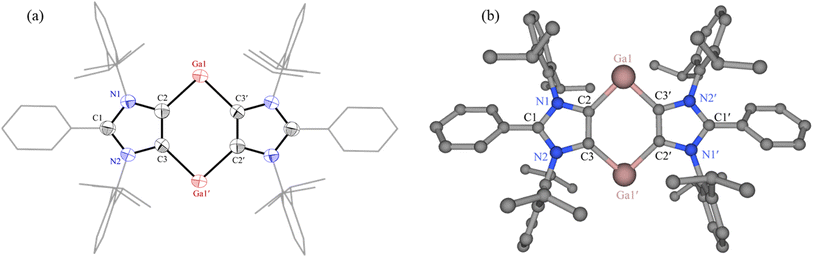
The solid-state molecular structure of 6 features a planar C4Ga2 core embedded between two 1,3-imidazole moieties (Fig. 3a). Compound 6 with two-coordinated Ga(I) atoms may be regarded as a base-stabilized bis-gallylene. Due to the phase transition below 220 K, the reflection data for 6 were collected at 220 K, giving rise to large thermal displacement ellipsoids. The C2–Ga1 (2.092(4) Å) and C3′–Ga1 (2.107(4) Å) bond lengths of 6 are marginally larger than those of Ga(III) compounds 3 (2.016(3) Å) and 4 (1.993(1) Å). The C2–Ga1–C3′ bond angle of 6 (92.6(1)°) is smaller than those of 3 (101.2(1)°) and 4 (107.4(1)°).
To obtain a further insight into the electronic structures of 5 and 6, we performed quantum chemical calculations at the r2SCAN-3c level of theory. The optimized molecular structure of 6 is in good agreement with that of sc-XRD structure (Fig. 3). Calculations reveal closed-shell singlet (CS) ground state for 5 (Fig. S60†) and 6. The triplet (T) solution for the putative intermediate 5′ (Fig. S61†) is 0.26 kcal mol−1 more stable than the CS solution. The conversion 5′ → 5 is calculated to be thermodynamically favored by 17.6 kcal mol−1. The NBO charges and WBIs (Wiberg Bond Indices) calculated at the PBE0/def2-TZVPP level of theory for 4, 5, and 6 (Table S7†) are consistent with their solid-state structures. With the WBIs of 0.45–0.54 and NBO charges of ca. −0.32e on C atoms, the Ga–C bonds of 4, 5, and 6 are essentially polar covalent bonds that are polarized towards the C atoms. The NBO charge(s) on the Ga atom(s) of 4 (1.02), 5 (1.02/0.52), and 6 (0.48) is consistent with its formal (I/III) oxidation state.
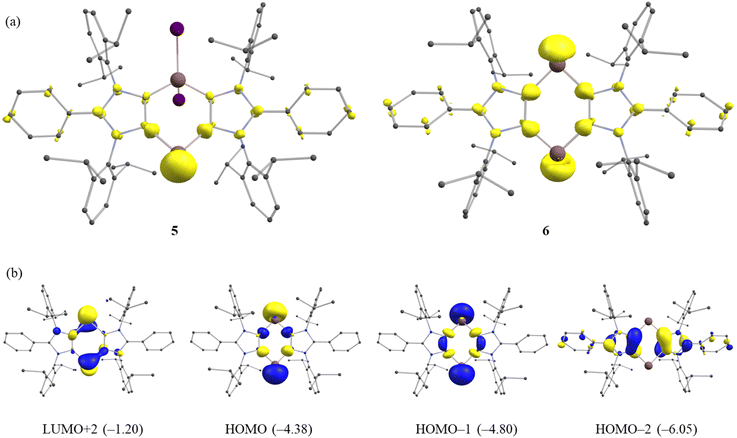
We also performed FOD (fractional occupation number weighed density) calculations37 at the PBE0/def2-TZVPP level of theory to analyze the electron correlation in 5 and 6. The FOD analyses provide reliable information on the localization of ‘hot’ (strongly correlated and chemical active) electrons of the molecule.37 The FOD calculations (PBE0, T = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 K) reveal a moderate level of electron correlation in 5 (NFOD = 2.50 e) and 6 (NFOD = 2.85 e) (Fig. 4a). The NFOD of 6 is smaller than that of the transient bis-aluminylene VII (NFOD = 3.10 e).28
000 K) reveal a moderate level of electron correlation in 5 (NFOD = 2.50 e) and 6 (NFOD = 2.85 e) (Fig. 4a). The NFOD of 6 is smaller than that of the transient bis-aluminylene VII (NFOD = 3.10 e).28
The HOMO and HOMO−1 of 6 (Fig. 4b) are essentially the electron lone-pairs on the Ga(I) atoms. The LUMO (Fig. S64†) of 6 is based on the ligand, while the LUMO+2 is mainly located on the Ga atoms. The HOMO–LUMO energy gap (ΔEH–L) for 6 amounts to 2.83 eV, which is larger than that of the corresponding fleeting Al(I) species VII (1.94 eV).28 In line with this, the HOMO (Fig. S63†) of mono-gallylene 5 is the lone-pair on Ga(I) atom, while the LUMO+2 is located mainly at the Ga(I) atom. The HOMO of 5 (−4.84 eV) is low-lying than that of 6 (−4.38 eV), suggesting a higher Lewis basicity of the latter. A rather smaller ΔEH–L for 6 (2.83 eV) than 5 (3.18 eV) also implies a greater reactivity of 6. The UV-Vis spectrum of 5 and 6 each exhibits a main absorption band (λmax) at 300 and 312 nm, respectively (Fig. S48 and S49†). This may be assigned to HOMO−1 → LUMO (5) and HOMO−2 → LUMO (6) transition.
Treatment of 6 with Fe2(CO)9 yields the dinuclear Fe(0) complex 7 (Scheme 3). Compound 6 readily oxidizes with (Me2S)AuCl to yield Ga(III) compound 8 as a colorless solid. Reaction of 6 with white phosphorus affords compound 9 as a brown solid. Prompted by the use of low-valent main-group compounds in the activation of C–F bonds,38 we performed reactions of 6 with different aryl fluorides (ArF) at room temperature that gave Ga(III) compounds cis-/trans-10-Ar (Ar = p-(CF3)C6F4, C6F5, C5NF4, C6HF4) as off-white solids. The formation of cis-/trans-10-Ar isomers is likely due to the stepwise addition of Ar–F to 6via mixed-valent Ga(I)/Ga(III) species 10′-Ar. Treatment of 6 with azobenzene at room temperature leads to the [1 + 4]-cycloaddition product 11 in which one of the phenyl rings of azobenzene has dearomatized. The 1H and 13C{1H} NMR spectra of 7–11 exhibit expected resonances for the ADC moieties. The 13C{1H} NMR spectrum of 7 features a signal at 216.6 ppm for the carbonyl carbon atoms of the Fe(CO)4 fragments.39 The 1H NMR spectrum of 8 compares well with that of the related aluminium chloride.28 The 31P{1H} NMR spectrum of 9 shows two triplets at 152.3 and −298.7 ppm (1JP–P = 157 Hz), which are consistent with those of a related isostructural P4-activation product.40 The 19F{1H} NMR spectra of cis-/trans-10-Ar reveal expected signals for the aryl fluoride moieties as well as a signal for the GaF group in the −205.6 to −207.8 ppm region.38
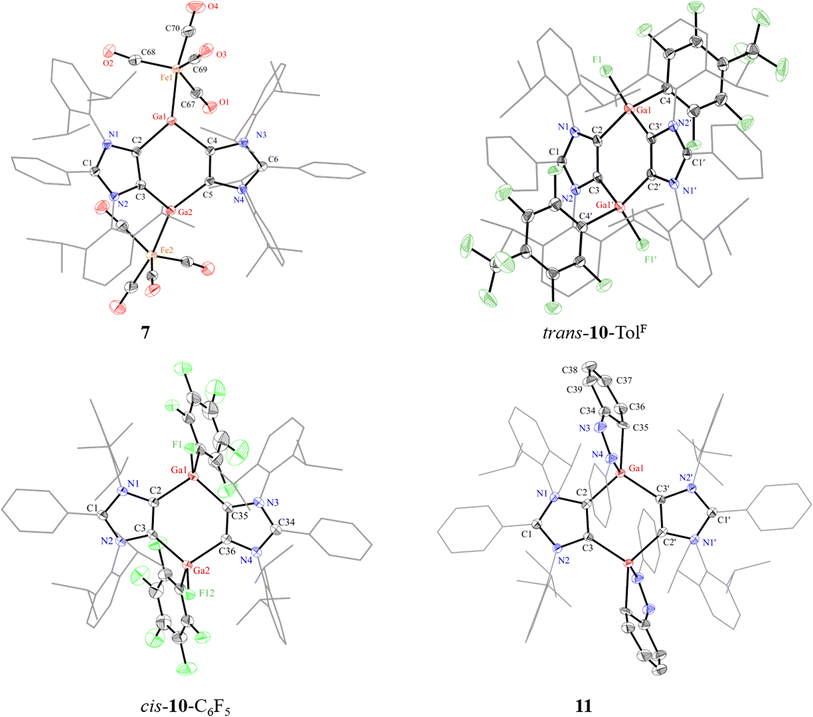
The solid-state molecular structures of 7 (Fig. 5), 8 (Fig. S54†), trans-10-TolF, cis-10-C6F5 (Fig. 5), trans-10-C5NF4 (Fig. S57†), and 11 (Fig. 5) show the expected atom connectivity.41 The structure of 7 revealed a minor impurity (∼3%) with two iodine atoms instead of one Fe(CO)4 group. This is likely due to the presence of a trace of 5 with 6 used to prepare 7, which was however not observed in the NMR spectroscopic analysis of the same sample. The sum of the angles at each of three-coordinated Ga atoms of 7 amounts to 359° that is consistent with a trigonal planar coordination environment. The Ga1–Fe1 (2.327(1) Å) and Ga2–Fe2 (2.320(1) Å) bond lengths of 7 are larger than that of [(Cp*Ga)Fe(CO)4] (2.273(1)Å).39 The four-coordinated Ga atoms of 8, 9, cis-/trans-10-Ar, and 11 show a distorted tetrahedral coordination environment. As expected for an oxidized product, the C2–Ga1 (1.997(3) Å) and C3′–Ga1 (1.997(3) Å) bond lengths of trans-10-TolF are smaller than that of 6 (Ga1–C2: 2.092(4) Å), while the Ga1–F1 (1.795(2) Å) bond lengths of trans-10-TolF agree well with other Ga(III) fluorides.38 A similar trend was shown by cis-10-C6F5 and trans-10-C5NF4. In 11, the N3–N4 (1.394(3) Å) and C34–C35 (1.472(4) Å) bond lengths are consistent with a single bond,42 while N3–C34 (1.294(4) Å) bond length corresponds to a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond.43 These features show the dearomatization of one of the phenyl groups of azobenzene in 11.
N double bond.43 These features show the dearomatization of one of the phenyl groups of azobenzene in 11.
Conclusions
In conclusion, the first carbocyclic gallylene 5 and bis-gallylene 6 have been reported as crystalline solids. The formation of the mixed-valent Ga(I/III) compound 5 may likely a result of the disproportionation of the transient Ga(II) species 5′. In addition to the sc-XRD structures of 4 and 6, electronic structures of 4, 5, 5′, and 6 have been investigated by quantum chemical calculations. Preliminary reactivity studies of 6 have been presented with Fe2(CO)9 (coordination chemistry), Au(I) chloride (oxidation), white phosphorus (small molecule activation), aryl fluoride (C–F activation), and azobenzene (dearomative cycloaddition) to afford compounds 7, 8, 9, cis-/trans-10-Ar, and 11, respectively. Further reactivity studies and use of 5 and 6 as ligands as well as substrates for new gallium compounds, in particular aromatic systems and radicals, are expected to add new facets in low-valent gallium chemistry.Data availability
Experimental details, the plots of NMR, FT-IR, and UV-Vis spectra as well as the detail of X-ray crystallography and theoretical studies of the reported compounds are given in the ESI.† The assigned CCDC identification numbers and the compounds in the study are as follows: CCDC 2334945 (3), 2334948 (4), 2334949 (6), 2334950 (7), 2334951 (trans-10-TolF), 2334952 (8), 2379059 (cis-10-C6F5), 2379060 (trans-10-C5NF4), and 2334953 (11).Author contributions
AM and SH: experimental investigation, data collection and analysis, writing. YVV: calculations, data collection and analysis, writing. BN and HGS: sc-XRD, data collection and analysis, writing. RSG: conceptualization, investigation, data analysis, writing, editing, supervision. All authors approved the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We are grateful to the Deutsche Forschungsgemeinschaft (DFG) for support [GH 129/16-1 (Project No. 549520861), GH 129/9-1 (Project No. 466111525), GH 129/12-1 (Project No. 514566227); VI 713/3-1 (Project No. 243500032)]. The authors thank Professor Norbert W. Mitzel for his constant encouragement. Arne Merschel thanks Mr Johannes Witte for assistance in starting materials preparation. The HPC facilities at the Universität zu Köln are acknowledged for computing time and programs. Dedicated to Professor Hubert Schmidbaur on the occasion of his 90th birthday.References
- A. J. Arduengo, R. L. Harlow and M. Kline, J. Am. Chem. Soc., 1991, 113, 361–363 CrossRef CAS.
- (a) M. He, C. Hu, R. Wei, X. F. Wang and L. L. Liu, Chem. Soc. Rev., 2024, 53, 3896–3951 RSC; (b) M. Asay, C. Jones and M. Driess, Chem. Rev., 2011, 111, 354–396 CrossRef CAS PubMed.
- (a) C. Dohmeier, D. Loos and H. Schnöckel, Angew. Chem., Int. Ed., 1996, 35, 129–149 CrossRef CAS; (b) C. Dohmeier, C. Robl, M. Tacke and H. Schnöckel, Angew. Chem., Int. Ed., 1991, 30, 564–565 CrossRef; (c) D. Loos, E. Baum, A. Ecker, H. Schnöckel and A. J. Downs, Angew. Chem., Int. Ed., 1997, 36, 860–862 CrossRef CAS.
- W. Uhl, W. Hiller, M. Layh and W. Schwarz, Angew. Chem., Int. Ed., 1992, 31, 1364–1366 CrossRef.
- E. S. Schmidt, A. Jockisch and H. Schmidbaur, J. Am. Chem. Soc., 1999, 121, 9758–9759 CrossRef CAS.
- (a) M. Zhong, S. Sinhababu and H. W. Roesky, Dalton Trans., 2020, 49, 1351–1364 RSC; (b) M. P. Coles and M. J. Evans, Chem. Commun., 2023, 59, 503–519 RSC; (c) X. Zhang, Y. Mei and L. L. Liu, Chem.–Eur. J., 2022, 28, e202202102 CrossRef CAS; (d) Y. S. Liu, J. Li, X. L. Ma, Z. Yang and H. W. Roesky, Coord. Chem. Rev., 2018, 374, 387–415 CrossRef CAS; (e) S. Nagendran and H. W. Roesky, Organometallics, 2008, 27, 457–492 CrossRef CAS.
- C. Cui, H. W. Roesky, H. G. Schmidt, M. Noltemeyer, H. Hao and F. Cimpoesu, Angew. Chem., Int. Ed., 2000, 39, 4274–4276 CrossRef CAS.
- N. J. Hardman, B. E. Eichler and P. P. Power, Chem. Commun., 2000, 1991–1992 RSC.
- (a) N. Saito, J. Takaya and N. Iwasawa, Angew. Chem., Int. Ed., 2019, 58, 9998–10002 CrossRef CAS PubMed; (b) T. Kodama, N. Mukai and M. Tobisu, Inorg. Chem., 2023, 62, 6554–6559 CrossRef CAS PubMed.
- R. J. Wright, M. Brynda, J. C. Fettinger, A. R. Betzer and P. P. Power, J. Am. Chem. Soc., 2006, 128, 12498–12509 CrossRef CAS PubMed.
- J. D. Queen, A. Lehmann, J. C. Fettinger, H. M. Tuononen and P. P. Power, J. Am. Chem. Soc., 2020, 142, 20554–20559 CrossRef CAS PubMed.
- X. Zhang and L. L. Liu, Angew. Chem., Int. Ed., 2021, 60, 27062–27069 CrossRef CAS PubMed.
- A. Hinz and M. P. Muller, Chem. Commun., 2021, 57, 12532–12535 RSC.
- (a) Q. Yu, L. Zhang, Y. He, J. Pan, H. Li, G.-q. Bian, X. Chen and G. Tan, Chem. Commun., 2021, 57, 9268–9271 RSC; (b) H. Li, Y. He, C. Liu and G. Tan, Dalton Trans., 2021, 50, 12674–12680 RSC.
- S. H. F. Schreiner, T. Rüffer and R. Kretschmer, Nat. Synth., 2024 DOI:10.1038/s44160-44024-00639-w.
- (a) G. Feng, K. L. Chan, Z. Lin and M. Yamashita, J. Am. Chem. Soc., 2024, 146, 7204–7209 CrossRef CAS; (b) S. G. Minasian, J. L. Krinsky, J. D. Rinehart, R. Copping, T. Tyliszczak, M. Janousch, D. K. Shuh and J. Arnold, J. Am. Chem. Soc., 2009, 131, 13767–13783 CrossRef CAS; (c) M. Wiecko and P. W. Roesky, Organometallics, 2007, 26, 4846–4848 CrossRef CAS; (d) C. Jones, A. Stasch and W. D. Woodul, Chem. Commun., 2009, 113–115 RSC; (e) D. Dange, C. P. Sindlinger, S. Aldridge and C. Jones, Chem. Commun., 2017, 53, 149–152 RSC; (f) R. Y. Kong and M. R. Crimmin, Dalton Trans., 2021, 50, 7810–7817 RSC; (g) P. Dabringhaus and I. Krossing, Chem. Sci., 2022, 13, 12078–12086 RSC; (h) J. T. Boronski, L. R. Thomas-Hargreaves, M. A. Ellwanger, A. E. Crumpton, J. Hicks, D. F. Bekiş, S. Aldridge and M. R. Buchner, J. Am. Chem. Soc., 2023, 145, 4408–4413 CrossRef CAS; (i) V. A. Dodonov, V. G. Sokolov, E. V. Baranov, A. A. Skatova, W. Xu, Y. Zhao, X.-J. Yang and I. L. Fedushkin, Inorg. Chem., 2022, 61, 14962–14972 CrossRef CAS.
- (a) J. Kretsch, A. Kreyenschmidt, T. Schillmöller, C. Sindlinger, R. Herbst-Irmer and D. Stalke, Inorg. Chem., 2021, 60, 7389–7398 CrossRef CAS; (b) B. Wang, W. Chen, J. Yang, L. Lu, J. Liu, L. Shen and D. Wu, Dalton Trans., 2023, 52, 12454–12460 RSC; (c) D. Dange, S. L. Choong, C. Schenk, A. Stasch and C. Jones, Dalton Trans., 2012, 41, 9304–9315 RSC.
- (a) M. Soleilhavoup and G. Bertrand, Angew. Chem., Int. Ed., 2017, 56, 10282–10292 CrossRef CAS; (b) F. Dahcheh, D. Martin, D. W. Stephan and G. Bertrand, Angew. Chem., Int. Ed., 2014, 53, 13159–13163 CrossRef CAS; (c) R. Kinjo, B. Donnadieu, M. A. Celik, G. Frenking and G. Bertrand, Science, 2011, 333, 610–613 CrossRef CAS; (d) C. Pranckevicius, J. O. C. Jimenéz-Halla, M. Kirsch, I. Krummenacher and H. Braunschweig, J. Am. Chem. Soc., 2018, 140, 10524–10529 CrossRef CAS; (e) Y.-J. Lin, W.-C. Liu, Y.-H. Liu, G.-H. Lee, S.-Y. Chien and C.-W. Chiu, Nat. Commun., 2022, 13, 7051 CrossRef CAS; (f) A. D. Ledet and T. W. Hudnall, Dalton Trans., 2016, 45, 9820–9826 RSC; (g) M.-A. Légaré, M. Rang, G. Bélanger-Chabot, J. I. Schweizer, I. Krummenacher, R. Bertermann, M. Arrowsmith, M. C. Holthausen and H. Braunschweig, Science, 2019, 363, 1329–1332 CrossRef; (h) M.-A. Légaré, G. Bélanger-Chabot, R. D. Dewhurst, E. Welz, I. Krummenacher, B. Engels and H. Braunschweig, Science, 2018, 359, 896–900 CrossRef.
- X. Zhang and L. L. Liu, Angew. Chem., Int. Ed., 2022, 61, e202116658 CrossRef CAS PubMed.
- (a) P. P. Power, Organometallics, 2020, 39, 4127–4138 CrossRef CAS; (b) R. C. Fischer and P. P. Power, Chem. Rev., 2010, 110, 3877–3923 CrossRef CAS PubMed; (c) P. P. Power, A. Moezzi, D. C. Pestana, M. A. Petrie, S. C. Shoner and K. M. Waggoner, Pure Appl. Chem., 1991, 63, 859–866 CrossRef CAS.
- (a) M. L. McCrea-Hendrick, C. A. Caputo, C. J. Roberts, J. C. Fettinger, H. M. Tuononen and P. P. Power, Organometallics, 2016, 35, 579–586 CrossRef CAS; (b) Z. Zhu, R. C. Fischer, B. D. Ellis, E. Rivard, W. A. Merrill, M. M. Olmstead, P. P. Power, J. D. Guo, S. Nagase and L. Pu, Chem.–Eur. J., 2009, 15, 5263–5272 CrossRef CAS; (c) N. J. Hardman, R. J. Wright, A. D. Phillips and P. P. Power, Angew. Chem., Int. Ed., 2002, 41, 2842–2844 CrossRef CAS.
- Z. Feng, Y. Fang, H. Ruan, Y. Zhao, G. Tan and X. Wang, Angew. Chem., Int. Ed., 2020, 59, 6769–6774 CrossRef CAS PubMed.
- (a) P. Dabringhaus, H. Scherer and I. Krossing, Nat. Synth., 2024, 3, 732–743 CrossRef CAS; (b) A. Barthélemy, H. Scherer, H. Weller and I. Krossing, Chem. Commun., 2023, 59, 1353–1356 RSC; (c) A. Barthélemy, H. Scherer, M. Daub, A. Bugnet and I. Krossing, Angew. Chem., Int. Ed., 2023, 62, e202311648 CrossRef.
- R. S. Ghadwal, Angew. Chem., Int. Ed., 2023, 62, e202304665 CrossRef CAS.
- D. Rottschäfer, B. Neumann, H.-G. Stammler, T. Sergeieva, D. M. Andrada and R. S. Ghadwal, Chem.–Eur. J., 2021, 27, 3055–3064 CrossRef PubMed.
- D. Rottschäfer, T. Glodde, B. Neumann, H.-G. Stammler, D. M. Andrada and R. S. Ghadwal, Angew. Chem., Int. Ed., 2021, 60, 15849–15853 CrossRef.
- H. Steffenfauseweh, D. Rottschäfer, Y. V. Vishnevskiy, B. Neumann, H.-G. Stammler, D. W. Szczepanik and R. S. Ghadwal, Angew. Chem., Int. Ed., 2023, 62, e202216003 CrossRef CAS PubMed.
- A. Merschel, Y. Vishnevskiy, B. Neumann, G. Stammler and R. Ghadwal, Chem.–Eur. J., 2024, 30, e202400293 CrossRef CAS PubMed.
- D. Rottschäfer, F. Ebeler, T. Strothmann, B. Neumann, H.-G. Stammler, A. Mix and R. S. Ghadwal, Chem.–Eur. J., 2018, 24, 3716–3720 CrossRef PubMed.
- (a) D. Rottschäfer, B. Neumann, H.-G. Stammler, M. v. Gastel, D. M. Andrada and R. S. Ghadwal, Angew. Chem., Int. Ed., 2018, 57, 4765–4768 CrossRef PubMed; (b) N. K. T. Ho, B. Neumann, H.-G. Stammler, V. H. Menezes da Silva, D. G. Watanabe, A. A. C. Braga and R. S. Ghadwal, Dalton Trans., 2017, 46, 12027–12031 RSC.
- A. Merschel, Y. V. Vishnevskiy, B. Neumann, H.-G. Stammler and R. S. Ghadwal, Chem.–Eur. J., 2023, 29, e202301037 CrossRef CAS PubMed.
- (a) M. D. Francis, D. E. Hibbs, M. B. Hursthouse, C. Jones and N. A. Smithies, J. Chem. Soc., Dalton Trans., 1998, 3249–3254 RSC; (b) A. Hock, L. Werner, C. Luz and U. Radius, Dalton Trans., 2020, 49, 11108–11119 RSC.
- X. Wang and L. Andrews, J. Phys. Chem. A, 2003, 107, 11371–11379 CrossRef CAS.
- See the data availability section.
- (a) A. Merschel, Y. V. Vishnevskiy, B. Neumann, H. G. Stammler and R. S. Ghadwal, Angew. Chem., Int. Ed., 2024, 63, e202318525 CrossRef CAS PubMed; (b) A. Merschel, T. Glodde, B. Neumann, H.-G. Stammler and R. S. Ghadwal, Angew. Chem., Int. Ed., 2021, 60, 2969–2973 CrossRef CAS; (c) A. Merschel, D. Rottschäfer, B. Neumann, H.-G. Stammler and R. S. Ghadwal, Organometallics, 2020, 39, 1719–1729 CrossRef CAS.
- (a) M. K. Sharma, F. Ebeler, T. Glodde, B. Neumann, H.-G. Stammler and R. S. Ghadwal, J. Am. Chem. Soc., 2021, 143, 121–125 CrossRef CAS PubMed; (b) M. K. Sharma, D. Rottschäfer, T. Glodde, B. Neumann, H. G. Stammler and R. S. Ghadwal, Angew. Chem., Int. Ed., 2021, 60, 6414–6418 CrossRef CAS; (c) F. Ebeler, Y. V. Vishnevskiy, B. Neumann, H. G. Stammler, D. W. Szczepanik and R. S. Ghadwal, J. Am. Chem. Soc., 2024, 146, 30584–30595 CrossRef CAS.
- (a) C. A. Bauer, A. Hansen and S. Grimme, Chem.–Eur. J., 2017, 23, 6150–6164 CrossRef CAS PubMed; (b) S. Grimme and A. Hansen, Angew. Chem., Int. Ed., 2015, 54, 12308–12313 CrossRef CAS.
- (a) O. Kysliak, H. Görls and R. Kretschmer, J. Am. Chem. Soc., 2021, 143, 142–148 CrossRef CAS; (b) M. R. Crimmin, M. J. Butler and A. J. White, Chem. Commun., 2015, 51, 15994–15996 RSC.
- P. Jutzi, B. Neumann, G. Reumann and H.-G. Stammler, Organometallics, 1998, 17, 1305–1314 CrossRef CAS.
- (a) G. Prabusankar, A. Doddi, C. Gemel, M. Winter and R. A. Fischer, Inorg. Chem., 2010, 49, 7976–7980 CrossRef CAS; (b) M. Scheer, G. Balázs and A. Seitz, Chem. Rev., 2010, 110, 4236–4256 CrossRef CAS.
- For 9, the proposed structural motif was ascertained by preliminary X-ray diffraction outcomes, unfortunately poor diffracting nature of crystals resulted in an unpublishable data set.
- P. Pyykkö and M. Atsumi, Chem.–Eur. J., 2009, 15, 186–197 CrossRef.
- P. Pyykkö and M. Atsumi, Chem.–Eur. J., 2009, 15, 12770–12779 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2334945, 2334948–2334953, 2379059 and 2379060. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc06782g |
| This journal is © The Royal Society of Chemistry 2025 |