Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dynamic selection in metallo-organic cube CdII8L4 conformations induced by perfluorooctanoate encapsulation†
Yu-Qing
Li‡
a,
He
Zhao‡
a,
Ermeng
Han
a,
Zhiyuan
Jiang
*c,
Qixia
Bai
b,
Yu-Ming
Guan
b,
Zhe
Zhang
 b,
Tun
Wu
*b and
Pingshan
Wang
b,
Tun
Wu
*b and
Pingshan
Wang
 *ab
*ab
aHunan Key Laboratory of Micro & Nano Materials Interface Science, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China. E-mail: chemwps@csu.edu.cn
bInstitute of Environmental Research at Greater Bay Area, Key Laboratory for Water Quality and Conservation of the Pearl River Delta, Ministry of Education, Guangzhou University, Guangzhou 510006, China. E-mail: chemwt@gzhu.edu.cn
cDepartment of Chemistry, The University of Hong Kong, Hong Kong SAR 999077. E-mail: chemjzy@hku.hk
First published on 22nd November 2024
Abstract
Metallo-organic cages possess flexibility comparable to that of biological receptors and can alter their conformations to better accommodate guest species due to the dynamic reversibility of the coordination bond. Induced fit is widely accepted involving conformation change of the host, while few definitive examples are related to conformation selection. Herein, we report the generation of metallo-organic cube CdII8L4 with two coexisting conformations, which have been fully confirmed by NMR, ESI-MS and single-crystal X-ray diffraction analysis. The specific guest perfluorooctanoate PFOA selectively binds to the active conformer C2h-1 to form the PFOA⊂C2h-1 complex. Furthermore, conformer D2-2 isomerizes to conformer C2h-1 in the presence of PFOA, for maximizing the guest binding affinity. This study provides an effective working paradigm for conformation selection, facilitating the understanding of the fundamental mechanism of molecular recognition.
Introduction
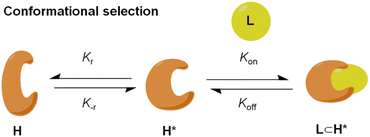
Molecular recognition, described as the binding between a substrate molecule and a protein-host, plays an essential role in various biochemical and physiological functions in an organism.1,2 The “conformational selection” hypothesis postulates that there are a series of discrete conformers for receptors in equilibrium (H and H*), in which the substrate molecule selectively interacts with the active one to form a host–guest complex, subsequently shifting the equilibrium distribution of receptor conformers (Fig. 1).3,4 It greatly facilitates the comprehensive understanding of molecular recognition in favour of the structure-based drug design, enzymatic catalysis and allosteric regulation of cell signaling.5–8 Metallo-organic cages (MOCs),9–18 constructed by the coordination between organic ligands and metal ions, serve as an effective model to simulate the molecular recognition of bio-receptors since their characteristic vacant cavities enclose central guest species, enabling various functional applications including chemical separation,19–21 sensing,22–24 mimic catalysis25–27 and luminescent materials.28–30Moreover, the dynamic reversibility of the dative bond within these three-dimensional (3D) metallo-organic structures provides them with recombination ability in response to external stimuli, specifically metallo-organic cages altering their conformation to fit target guests.31–40 This guest-induced structural change of metallo-organic cages generally involves an “induced fit” mechanism, in which a guest binds to the receptor in an inactive state, and then undergoes a structural rearrangement into an active model for an optimum fit.41,42 However, the molecular recognition of metallo-organic cages based on conformation selection is relatively rare and presents a great challenge. An ideal model for conformational selection should meet the criteria that there are two or more pre-existing conformations in equilibrium and that all of them can be detected.43,44 In general, the conformers that coexist possess similar energies, presenting great obstacles for their interconversion. Meanwhile, the complete differentiation of different conformers is still highly challenging due to their structural nuances.45
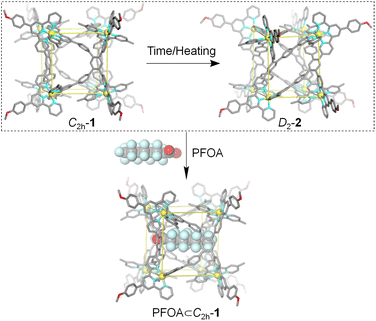
Herein, we report the generation of metallo-organic cages CdII8L4 in good accordance with the conformation selection mechanism. The self-assembly between tetratopic ligand L and CdII ions afforded metallo-organic cages CdII8L4 with the coexistence of two conformers. Conformer 1 is a cuboid metallo-organic cage in which four ligands L cap the equatorial faces with C2h symmetry. Differing only in the location orientation of terpyridine units, two parallel ligands L together occupy the equatorial faces of conformer 2, displaying an unprecedented helical cubic structure with D2 symmetry. Conformer C2h-1 converts to conformer D2-2 at a quite slow rate and an elevated temperature can facilitate this process. Owing to the different cavity volume (1: 1263 Å3, 2: 867 Å3) and shape, the inclusion of guest perfluorooctanoate PFOA, classified as a persistent organic pollutant (POP), can only be achieved by conformer C2h-1 based on the shape and size complementarity. Furthermore, the addition of PFOA into conformer D2-2 can shift the equilibrium distribution to conformer C2h-1 (Scheme 1).
 | ||
| Scheme 1 Conformational change of metallo-organic cages CdII8L4 in response to temperature/time and guest PFOA based on the conformational selection mechanism. | ||
Results and discussion
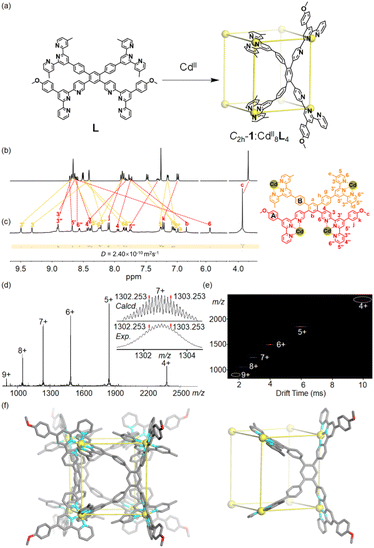
The tetratopic ligand L contains two kinds of terpyridine units: one connects with the 5-position of the lateral pyridine (part A) and the other is substituted on the para-position of benzene attached to the central pyridine (part B) (Fig. 2). It was facilely synthesized through successive Suzuki–Miyaura reactions from commercially available 1,5-dibromo-2,4-diiodobenzene (Scheme S1 and Fig. S1–S13†). Part A and part B possess inwardly converging and outwardly extending conformations, respectively, showing geometric complementarity. So, hetero-connection of parts A and B (tpyA-CdII-tpyB) is more favourable than homo-connections (tpyA-CdII-tpyA and tpyB-CdII-tpyB) in terms of geometric conformation. Self-assembled supramolecules are normally favoured thermodynamically over oligomeric or polymeric systems because they are synthesized under thermodynamic conditions, facilitating the formation of finite structures at the expense of increased angle strain. In addition, entropy favours closed structures with a minimum number of components rather than polymeric structures, which involve a far larger number of components.46,47 So, self-assembly was conducted under thermodynamic conditions: direct reaction of ligand L and two equivalents of cadmium nitrate tetrahydrate Cd(NO3)2·4H2O at 65 °C in a mixed solvent (CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 3
MeOH = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) for 12 h. After cooling to room temperature, excess lithium bis(trifluoromethanesulphonyl)imide (LiNTf2) was added to give a precipitate, which was collected by filtration and further washed with D.I. water and MeOH (Fig. 2a and Scheme S2†). The product conformer 1 was quantitatively obtained as a pale white solid after being dried in vacuo. The 1H NMR spectrum of 1 showed a significant upfield shift of H6,6′′ (part B in ligand L) and H6 (part A in ligand L), attributed to the characteristic electron shielding effect caused by the pseudo-octahedral bis(terpyridine) complex.48 The presence of two singlets of H3′,5′ and only one singlet of H-OMe attributed to two kinds of terpyridine moieties strongly demonstrated the equivalent environments of each terpyridine unit, indicating the generation of a highly symmetric species (Fig. 2b, f and S14–S16†). Diffusion-ordered 1H NMR spectroscopy (DOSY) analysis confirmed the presence of single discrete species in solution with extracted diffusion coefficient D values of 2.4 × 10−10 m2 s−1 (Fig. 2c and S17†). In addition, electrospray ionization mass spectrometry (ESI-MS) was performed to provide composition information of conformer 1. It displays a series of multicharged ions from [CdII8L4 + 5NTf2− + 2NO3−]9+ to [CdII8L4 + 10NTf2− + 2NO3−]4+, demonstrating the exclusive formation of CdII8L4 type assemblies. It's noted that the experimental isotopic patterns for each charge state agreed well with the calculated distributions (Fig. 2d and S18†). 2D travelling wave ion migration mass spectrometry (TWIM-MS) exhibited a narrow drift time distribution of each charge state for conformer C2h-1, ruling out other isomers and conformers (Fig. 2e). The results of NMR, MS and computational simulation strongly indicate a metallo-organic cube with C2h symmetry of conformer 1. It's noted that self-assembly between ligand L and ZnII gives an isostructural metallo-organic cage [ZnII8L4] (Fig. S43 and S44†).
4) for 12 h. After cooling to room temperature, excess lithium bis(trifluoromethanesulphonyl)imide (LiNTf2) was added to give a precipitate, which was collected by filtration and further washed with D.I. water and MeOH (Fig. 2a and Scheme S2†). The product conformer 1 was quantitatively obtained as a pale white solid after being dried in vacuo. The 1H NMR spectrum of 1 showed a significant upfield shift of H6,6′′ (part B in ligand L) and H6 (part A in ligand L), attributed to the characteristic electron shielding effect caused by the pseudo-octahedral bis(terpyridine) complex.48 The presence of two singlets of H3′,5′ and only one singlet of H-OMe attributed to two kinds of terpyridine moieties strongly demonstrated the equivalent environments of each terpyridine unit, indicating the generation of a highly symmetric species (Fig. 2b, f and S14–S16†). Diffusion-ordered 1H NMR spectroscopy (DOSY) analysis confirmed the presence of single discrete species in solution with extracted diffusion coefficient D values of 2.4 × 10−10 m2 s−1 (Fig. 2c and S17†). In addition, electrospray ionization mass spectrometry (ESI-MS) was performed to provide composition information of conformer 1. It displays a series of multicharged ions from [CdII8L4 + 5NTf2− + 2NO3−]9+ to [CdII8L4 + 10NTf2− + 2NO3−]4+, demonstrating the exclusive formation of CdII8L4 type assemblies. It's noted that the experimental isotopic patterns for each charge state agreed well with the calculated distributions (Fig. 2d and S18†). 2D travelling wave ion migration mass spectrometry (TWIM-MS) exhibited a narrow drift time distribution of each charge state for conformer C2h-1, ruling out other isomers and conformers (Fig. 2e). The results of NMR, MS and computational simulation strongly indicate a metallo-organic cube with C2h symmetry of conformer 1. It's noted that self-assembly between ligand L and ZnII gives an isostructural metallo-organic cage [ZnII8L4] (Fig. S43 and S44†).
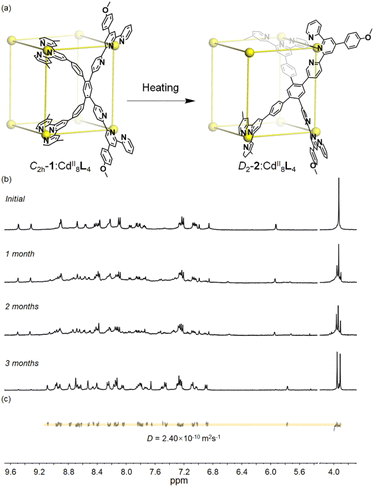
As time went by, an unexpected growth in the number of proton resonances was observed in the 1H NMR spectra of C2h-1. Three months later, a brand-new and highly complicated 1H NMR spectrum can be detected, showing four sets of terpyridine signals in the aromatic region along with two singlets derived from methoxy (Fig. 3b and S19–S21†). Its 1H DOSY NMR spectrum confirms that all signals have the same diffusion coefficient D of 2.4 × 10−10 m2 s−1 which is similar to that of conformer C2h-1 (Fig. 3c and S22†). The identical CdII8L4 composition of metallo-organic cage 2 was further verified by ESI-MS coupled with TWIM-MS (Fig. S23 and S24†). Therefore, it can be concluded that the product self-assembled from ligand L and CdII ion has two conformers (Fig. 3a) with different symmetries and thus different peak patterns in the 1H NMR spectra. Conformational conversion from conformer C2h-1 to conformer 2 is slow on the NMR timescale, enabling their direct differentiation in 1H NMR spectra. In order to accelerate conformational conversion, the NMR tube of C2h-1 was heated at 65 °C, achieving a complete transformation to conformer 2 after four weeks. It's noted that conformer C2h-1 cannot be recovered even by freezing treatment of conformer 2 (208 K, 1 month). The above results illustrate that conformer 2 is thermodynamically preferred compared to metastable and kinetic conformer C2h-1.
 | ||
| Fig. 3 (a) Conformational conversion from conformer C2h-1 to conformer 2. (b) Time-resolved 1H NMR spectra, and (c) DOSY NMR spectra of metallo-organic cage conformer 2 (500 MHz, 298 K, CD3CN-d3). | ||
Subsequently, by slow diffusion of isopropyl ether over a month or three days' diffusion of toluene into an acetonitrile solution of the product assembled from ligand L and CdII ions, single crystals suitable for X-ray diffraction (SC-XRD) were achieved (Table S1†). There are two problems in obtaining high-quality crystals: (1) during the crystallization process, gel is always generated and (2) due to the presence of large voids and highly disordered solvents/anions, the reflections in the high θ angle are too weak to obtain completeness and good data/parameter ratios. Fortunately, the satisfactory refinement results are sufficient for the cage structure determination. It revealed a helical cuboid structure in which the eight CdII ions occupy the vertices. The neighbouring CdII⋯CdII distances were measured to be 11.4–13.5 Å corresponding to the different edges of the cube (Fig. S35†). Along the equatorial plane of the cubic structure, three terpyridine arms (two tpy-A, one tpy-B) in ligand L bridge the metal centers and the last one (tpy-B) extends to the opposite plane and coordinates to the metal center on the body diagonal. In this manner, two ligands L together form a face of the helical cube which is totally different from the ligand face-capped cuboid metallo-organic cages.49,50 The solid-state structure of the helical cube shows multiple extended orientations of terpyridine units which is consistent with the increased number of proton resonances in the 1H NMR spectra of conformer D2-2 rather than highly-symmetric conformer C2h-1 with only two kinds of terpyridine units. There are two C2 axes that are perpendicular to each other and no symmetry plane can be observed, demonstrating the D2 symmetry of helical cube 2 (Fig. 4). Despite many attempts, only single crystals attributed to D2-2 can be detected, probably because it's easier to crystallize in contrast to C2h-1.
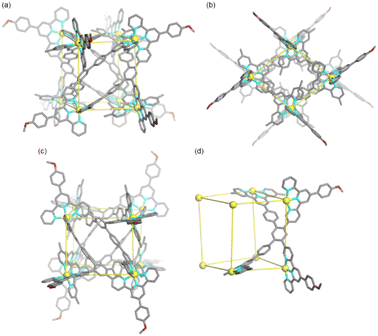 | ||
| Fig. 4 (a–c) Three views of the single crystal X-ray structure of metallo-organic cage D2-2. (d) The geometrical illustration of metallo-organic cage D2-2. | ||
As we fully confirmed the conformational conversion from C2h-1 to D2-2 and their exact structures, calculations using Forcite of Materials Studio were performed to investigate the energy difference between these conformers. Conformer D2-2 is 28.19 kcal mol−1 more stable than conformer C2h-1 (Fig. S39†), rationalizing the thermodynamically controlled conversion. The cavities of the two conformers (C2h-1 and D2-2) were well-enclosed by ligand L, and the methyl groups of part B further blocked the pores of the two apical vacant planes (Fig. 4b and S36†). Calculated using VOIDOO,51 the internal cavity volumes of two conformers were different, at 1263 Å3 and 867 Å3 for C2h-1 and D2-2, respectively (Fig. S37 and S38†).
The two conformers C2h-1 and D2-2 may possess drastically different binding affinities toward the same guest due to their different cavity size and shape. So, it's an ideal model for conformational selection, in which a guest will only be encapsulated by one conformation and will not bind to the other at all. Moreover, when accommodating a specific guest, the original equilibrium between the conformers will be broken, leading to the exclusive generation of one kind of host–guest complex owing to the dynamic reversible nature of dative bonds. After screening (Fig. S25–S28†), perfluorooctanoate (PFOA) was found to be a suitable guest. PFOA accumulates in water resources and poses serious environmental and health threats due to its non-biodegradable nature and long environmental persistence time.52–54 Effective recognition of PFOA by artificial hosts may bring about the sensing or even adsorption materials for PFOA pollutant.55–57 The addition of excess PFOA into the solution of conformer D2-2 resulted in a reduction in the number of terpyridine units from 4 to 2, along with the appearance of only one singlet assigned to methoxy for part A of ligand L, strongly indicating the main structure of conformer D2-2's conversion to conformer C2h-1. And, compared to the 1H NMR spectra of conformer C2h-1, proton resonance of H3 on part B of ligand L displayed a moderate downfield shift (from 9.31 ppm to 9.51 ppm) and the other signals shifted to the upfield. In addition, 19F NMR signals of the fluorine atoms on C2h-1 derived from NTf2− showed obvious upfield shift with the addition of PFOA, along with the upfield shift and broadening of 19F signals on guest PFOA, which is consistent with previous reports.58,59 The obvious changes in 1H and 19F NMR spectra were indicative of a molecule of PFOA encapsulated within host C2h-1 (Fig. 5b and S29–S31†). In addition, ESI-MS results exhibited a series of multicharged ions from [CdII8L4 + PFOA + 7NTf2− + KNO3]8+ to [CdII8L4 + PFOA + 9NTf2− + KNO3]6+, indicating the presence of host–guest complex PFOA⊂C2h-1 (Fig. S32†). That is, as the cavity of conformer D2-2 could not provide a suitable environment for PFOA, it transformed into conformer C2h-1 with the aim to accommodate the guest. Thus, the binding mechanism can be unambiguously assigned to conformational selection (Fig. 5a).
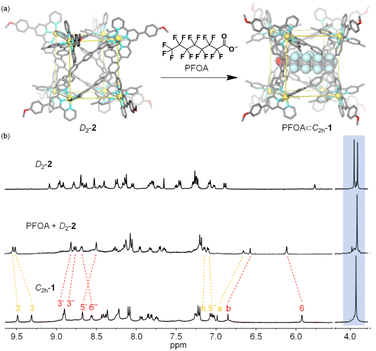 | ||
| Fig. 5 (a) PFOA-induced conformational selection from D2-2 to PFOA⊂C2h-1. (b) 1H NMR spectra of (top) D2-2, (middle) D2-2 + PFOA, (bottom) C2h-1 (500 MHz, CD3CN-d3, 298 K). | ||
To further understand the mechanism of guest-induced conformational selection, theoretical calculations were conducted by using Forcite of Materials Studio (molecular-level interactions of the host–guest complex are shown in the ESI†). The calculated results are in good agreement with the experimental phenomenon, that is, the binding energy of host–guest complex PFOA⊂C2h-1 is −31.5 kcal mol−1, which is more than that of PFOA⊂D2-2 (ΔE = −7.9 kcal mol−1) (Fig. S40–S42†). It's noted that the addition of PFOA into the solution of C2h-1 afforded the same result as PFOA⊂C2h-1. In the 1H and 19F NMR titration experiments performed by continuously adding PFOA into C2h-1, no free host C2h-1 was detected, suggesting PFOA's fast exchange binding model on the NMR timescale (Fig. S29†). The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest stoichiometry was confirmed by the Job plot method (Fig. S33†).60 The host–guest binding constant (Ka) was estimated by 1H NMR titration and calculated to be 690 ± 20 M−1 based on Bindfit (Fig. S34†).61
1 host–guest stoichiometry was confirmed by the Job plot method (Fig. S33†).60 The host–guest binding constant (Ka) was estimated by 1H NMR titration and calculated to be 690 ± 20 M−1 based on Bindfit (Fig. S34†).61
Conclusions
We have presented a metallo-organic cube CdII8L4 with two discrete conformations based on the different location orientations of tetratopic ligand L. NMR, ESI-MS and SC-XRD techniques clearly supported the coexistence of two conformers. Time-resolved 1H NMR spectra confirmed conformer C2h-1's extremely slow conversion to conformer D2-2. Due to the different cavity volume and shape of the two conformers, a specific guest PFOA was selectively encapsulated by C2h-1. Moreover, in the presence of PFOA, the conformational equilibrium between C2h-1 and D2-2 can be shifted to conformer C2h-1 with the aim to maximize the binding affinity. This host–guest behaviour strictly follows the conformation selection model, which can serve as a standard paradigm to study the deep mechanism of molecular recognition. In addition, the metallo-organic cube provides a suitable host, possessing the potential as a sorbent material and phase transfer or extraction system for the PFOA pollutant. Further studies will concentrate on improving the binding affinity through reasonable modifications of metallo-organic cubic cages.Data availability
Crystallographic data for D2-2 have been deposited at the CCDC database under CCDC number 2359196† and can be obtained from The Cambridge Crystallographic Data Centre viahttps://www.ccdc.cam.ac.uk/data_request/cif. Further analytical data are reported in the ESI† to this article. Data are available upon request from the authors.Author contributions
T. Wu and J. Z. conceived the study. Y.-Q. Li and Z. H. performed the synthesis. E. Han and Q. Bai performed characterization of the materials. Y.-M. Guan and Z. Zhang assisted in structural characterization (X-ray, NMR, and MS analyses). T. Wu wrote the original draft. P. Wang reviewed and edited the paper.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Dr Yi Huang (https://www.castest.net. No. Jin Tel: 400 096 8959) for the crystal structure determination and analysis in the work. This research was supported by the National Natural Science Foundation of China (22101061 and 22471047 to Z. Z. and 22401054 to T. W.), GuangDong Basic and Applied Basic Research Foundation (2023A1515110911 to T. W.), Guangzhou Basic and Applied Basic Research of City and University (Institute) Joint Funding Project (2023A03J0624 to P. W. and 2023A03J0023 to Z. Z.), and the Natural Science Foundation of Guangdong Province-Youth Enhancement Programme (2024A1515030235 to Z. Z.). The authors extend their gratitude to Theoretical and Computational Chemistry Team from Shiyanjia Lab (https://www.shiyanjia.com) for providing invaluable assistance.Notes and references
- R. Baron and J. A. McCammon, Molecular Recognition and Ligand Association, Annu. Rev. Phys. Chem., 2013, 64, 151–175 CrossRef CAS PubMed.
- E. Persch, O. Dumele and F. Diederich, Molecular recognition in chemical and biological systems, Angew. Chem., Int. Ed., 2015, 54, 3290–3327 CrossRef CAS.
- J. Monod, J. Wyman and J.-P. Changeux, On the nature of allosteric transitions: A plausible model, J. Mol. Biol., 1965, 12, 88–118 CrossRef CAS.
- D. D. Boehr, R. Nussinov and P. E. Wright, The role of dynamic conformational ensembles in biomolecular recognition, Nat. Chem. Biol., 2009, 5, 789–796 CrossRef CAS.
- H. Steuber, M. Zentgraf, C. L. Motta, S. Sartini, A. Heine and G. Klebe, Evidence for a Novel Binding Site Conformer of Aldose Reductase in Ligand-Bound State, J. Mol. Biol., 2007, 369, 186–197 CrossRef CAS PubMed.
- D. Ajami, L. Liu and J. Rebek Jr, Soft templates in encapsulation complexes, Chem. Soc. Rev., 2015, 44, 490–499 RSC.
- D. M. Kaphan, M. D. Levin, R. G. Bergman, K. N. Raymond and F. D. Toste, A supramolecular microenvironment strategy for transition metal catalysis, Science, 2015, 350, 1235–1238 CrossRef CAS.
- Z. Lu, T. K. Ronson, A. W. Heard, S. Feldmann, N. Vanthuyne, A. Martinez and J. R. Nitschke, Enantioselective fullerene functionalization through stereochemical information transfer from a self-assembled cage, Nat. Chem., 2023, 15, 405–412 CrossRef CAS.
- D. Fujita, Y. Ueda, S. Sato, N. Mizuno, T. Kumasaka and M. Fujita, Self-assembly of tetravalent Goldberg polyhedra from 144 small components, Nature, 2016, 540, 563–566 CrossRef CAS PubMed.
- M. Yoshizawa and L. Catti, Bent Anthracene Dimers as Versatile Building Blocks for Supramolecular Capsules, Acc. Chem. Res., 2019, 52, 2392–2404 CrossRef CAS.
- L.-J. Wang, X. Li, S. Bai, Y.-Y. Wang and Y.-F. Han, Self-Assembly, Structural Transformation, and Guest-Binding Properties of Supramolecular Assemblies with Triangular Metal–Metal Bonded Units, J. Am. Chem. Soc., 2020, 142, 2524–2531 CrossRef CAS.
- T. Wu, Z. Jiang, Q. Bai, Y. Li, S. Mao, H. Yu, L. Wojtas, Z. Tang, M. Chen, Z. Zhang, T.-Z. Xie, M. Wang, X. Li and P. W, Supramolecular triangular orthobicupola: Self-assembly of a giant Johnson solid J27, Chem, 2021, 7, 2429–2441 CAS.
- S. Sudan, F. Fadaei-Tirani, R. Scopelliti, K. E. Ebbert, G. H. Clever and K. Severin, LiBF4-Induced Rearrangement and Desymmetrization of a Palladium-Ligand Assembly, Angew. Chem., Int. Ed., 2022, 61, e202201823 CrossRef CAS.
- B. Chen, J. J. Holstein, A. Platzek, L. Schneider, K. Wu and G. H. Clever, Cooperativity of steric bulk and H-bonding in coordination sphere engineering: heteroleptic PdII cages and bowls by design, Chem. Sci., 2022, 13, 1829–1834 RSC.
- Q. Bai, Y.-M. Guan, T. Wu, Y. Liu, Z. Zhai, Q. Long, Z. Jiang, P. Su, T.-Z. Xie, P. Wang and Z. Zhang, Anion-Regulated Hierarchical Self-Assembly and Chiral Induction of Metallo-Tetrahedra, Angew. Chem., Int. Ed., 2023, 62, e202309027 CrossRef CAS.
- A. C. Pearcy, L. S. Lisboa, D. Preston, N. B. Page, T. Lawrence, L. J. Wright, C. G. Hartinger and J. D. Crowley, Exploiting reduced-symmetry ligands with pyridyl and imidazole donors to construct a second-generation stimuli-responsive heterobimetallic [PdPtL4]4+ cage, Chem. Sci., 2023, 14, 8615–8623 RSC.
- K. Wu, T. K. Ronson, P. Su, Z. Chen, L. Goh, A. W. Heard, X. Li, F. Klautzsch, C. A. Schalley, M. Vinković and J. R. Nitschke, Systematic construction of progressively larger capsules from a fivefold linking pyrrole-based subcomponent, Nat. Synth., 2023, 2, 789–797 CrossRef CAS.
- Y.-H. Huang, Y.-L. Lu, J. Ruan, S.-P. Zheng, X.-D. Zhang, C.-H. Liu, Y.-H. Qin, Z.-M. Cao, Z. Jiao, H.-S. Xu and C.-Y. Su, Dynamic Metallosupramolecular Cages Containing 12 Adaptable Pockets for High-Order Guest Binding Beyond Biomimicry, J. Am. Chem. Soc., 2023, 145, 23361–23371 CrossRef CAS.
- D. Zhang, T. K. Ronson, Y.-Q. Zou and J. R. Nitschke, Metal–organic cages for molecular separations, Nat. Rev. Chem, 2021, 5, 168–182 CrossRef CAS PubMed.
- D. Chakraborty, R. Saha, J. K. Clegg and P. S. Mukherjee, Selective separation of planar and non-planar hydrocarbons using an aqueous Pd6 interlocked cage, Chem. Sci., 2022, 13, 11764–11771 RSC.
- D. Prajapati, J. K. Clegg and P. S. Mukherjee, Formation of a low-symmetry Pd8 molecular barrel employing a hetero donor tetradentate ligand, and its use in the binding and extraction of C70, Chem. Sci., 2024, 15, 12502–12510 RSC.
- P. Howlader, B. Mondal, P. C. Purba, E. Zangrando and P. S. Mukherjee, Self-Assembled Pd(II) Barrels as Containers for Transient Merocyanine Form and Reverse Thermochromism of Spiropyran, J. Am. Chem. Soc., 2018, 140, 7952–7960 CrossRef CAS PubMed.
- A. J. Plajer, E. G. Percástegui, M. Santella, F. J. Rizzuto, Q. Gan, B. W. Laursen and J. R. Nitschke, Fluorometric Recognition of Nucleotides within a Water-Soluble Tetrahedral Capsule, Angew. Chem., Int. Ed., 2019, 58, 4200–4204 CrossRef CAS PubMed.
- M. Canton, A. B. Grommet, L. Pesce, J. Gemen, S. Li, Y. Diskin-Posner, A. Credi, G. M. Pavan, J. Andréasson and R. Klajn, Improving Fatigue Resistance of Dihydropyrene by Encapsulation within a Coordination Cage, J. Am. Chem. Soc., 2020, 142, 14557–14565 CrossRef CAS.
- E. Ubasart, O. Borodin, C. Fuertes-Espinosa, Y. Xu, C. García-Simón, L. Gómez, J. Juanhuix, F. Gándara, I. Imaz, D. Maspoch, M. von Delius and X. Ribas, A three-shell supramolecular complex enables the symmetry-mismatched chemo- and regioselective bis-functionalization of C60, Nat. Chem., 2021, 13, 420–427 CrossRef CAS PubMed.
- W.-L. Jiang, B. Huang, X.-L. Zhao, X. Shi and H.-B. Yang, Strong halide anion binding within the cavity of a conformation-adaptive phenazine-based Pd2L4 cage, Chem, 2023, 9, 2655–2668 CAS.
- T. K. Piskorz, V. Martí-Centelles, R. L. Spicer, F. Duarte and P. J. Lusby, Picking the lock of coordination cage catalysis, Chem. Sci., 2023, 14, 11300–11331 RSC.
- J. Gemen, M. J. Białek, M. Kazes, L. J. W. Shimon, M. Feller, S. N. Semenov, Y. Diskin-Posner, D. Oron and R. Klajn, Ternary host-guest complexes with rapid exchange kinetics and photoswitchable fluorescence, Chem, 2022, 8, 2362–2379 CAS.
- I. Heckelmann, Z. Lu, J. C. A. Prentice, F. Auras, T. K. Ronson, R. H. Friend, J. R. Nitschke and S. Feldmann, Supramolecular Self-Assembly as a Tool to Preserve the Electronic Purity of Perylene Diimide Chromophores, Angew. Chem., Int. Ed., 2023, 62, e202216729 CrossRef CAS PubMed.
- Z. Zhang, Q. Bai, Z. Zhai, Q. Long, E. Han, H. Zhao, C.-W. Zhou, H. Lin, W. Zhang, G.-H. Ning, T.-Z. Xie, P. Wang and T. Wu, Multiple-stimuli fluorescent responsive metallo-organic helicated cage arising from monomer and excimer emission, Nat. Commun., 2024, 15, 7261 CrossRef.
- W. Wang, Y.-X. Wang and H.-B. Yang, Supramolecular transformations within discrete coordination-driven supramolecular architectures, Chem. Soc. Rev., 2016, 45, 2656–2693 RSC.
- W. Liu and J. F. Stoddart, Emergent behavior in nanoconfined molecular containers, Chem, 2021, 7, 919–947 CAS.
- D.-N. Yan, L.-X. Cai, P.-M. Cheng, S.-J. Hu, L.-P. Zhou and Q.-F. Sun, Photooxidase Mimicking with Adaptive Coordination Molecular Capsules, J. Am. Chem. Soc., 2021, 143, 16087–16094 CrossRef CAS PubMed.
- X.-Q. Guo, L.-P. Zhou, S.-J. Hu, L.-X. Cai, P.-M. Cheng and Q.-F. Sun, Hexameric Lanthanide–Organic Capsules with Tertiary Structure and Emergent Functions, J. Am. Chem. Soc., 2021, 143, 6202–6210 CrossRef CAS PubMed.
- Y.-H. Huang, Y.-L. Lu, X.-D. Zhang, C.-H. Liu, J. Ruan, Y.-H. Qin, Z.-M. Cao, J. Jiang, H.-S. Xu and C.-Y. Su, Dynamic Stereochemistry of M8Pd6 Supramolecular Cages Based on Metal-Center Lability for Differential Chiral Induction, Resolution, and Recognition, Angew. Chem., Int. Ed., 2023, 62, e202315053 Search PubMed.
- D.-N. Yan, L.-X. Cai, S.-J. Hu, Y.-F. Zhou, L.-P. Zhou and Q.-F. Sun, An Organo-Palladium Host Built from a Dynamic Macrocyclic Ligand: Adaptive Self-Assembly, Induced-Fit Guest Binding, and Catalysis, Angew. Chem., Int. Ed., 2022, 61, e202209879 CrossRef CAS.
- E. Benchimol, B.-N. T. Nguyen, T. K. Ronson and J. R. Nitschke, Transformation networks of metal–organic cages controlled by chemical stimuli, Chem. Soc. Rev., 2022, 51, 5101–5135 RSC.
- H.-Y. Lin, Y.-T. Wang, X. Shi, H.-B. Yang and L. Xu, Switchable metallacycles and metallacages, Chem. Soc. Rev., 2023, 52, 1129–1154 RSC.
- Y. Domoto, M. Abe, G. R. Genov, Z. Yu and M. Fujita, Interconversion of Highly Entangled Polyhedra into Concave Polyhedra by Nitrate-Induced Ternary Coordination, Angew. Chem., Int. Ed., 2023, 62, e202303714 CrossRef CAS.
- N. M. A. Speakman, A. W. Heard and J. R. Nitschke, A CuI6L4 Cage Dynamically Reconfigures to Form Suit[4]anes and Selectively Bind Fluorinated Steroids, J. Am. Chem. Soc., 2024, 146, 10234–10239 CrossRef CAS.
- E. Fischer and J. D. Dermatol, Einfluss der Configuration auf die Wirkung der Enzyme, Ges, 1894, 27, 2985–2993 CrossRef CAS.
- D. E. Koshland Jr, Application of a Theory of Enzyme Specificity to Protein Synthesis, Proc. Natl. Acad. Sci. U.S.A., 1958, 44, 98–104 CrossRef.
- C. M. Hong, D. M. Kaphan, R. G. Bergman, K. N. Raymond and F. D. Toste, Conformational Selection as the Mechanism of Guest Binding in a Flexible Supramolecular Host, J. Am. Chem. Soc., 2017, 139, 8013–8021 CrossRef CAS.
- L.-P. Yang, L. Zhang, M. Quan, J. S. Ward, Y.-L. Ma, H. Zhou, K. Rissanen and W. Jiang, A supramolecular system that strictly follows the binding mechanism of conformational selection, Nat. Commun., 2020, 11, 2740 CrossRef CAS PubMed.
- T. K. Ronson, J. P. Carpenter and J. R. Nitschke, Dynamic optimization of guest binding in a library of diastereomeric heteroleptic coordination cages, Chem, 2022, 8, 557–568 CAS.
- R. Chakrabarty, P. S. Mukherjee and P. J. Stang, Supramolecular Coordination: Self-Assembly of Finite Two- and Three-Dimensional Ensembles, Chem. Rev., 2015, 115, 7001–7045 CrossRef.
- T. R. Cook and P. J. Stang, Recent Developments in the Preparation and Chemistry of Metallacycles and Metallacages via Coordination, Chem. Rev., 2015, 115, 7001–7045 CrossRef CAS.
- S. Chakraborty and G. R. Newkome, Terpyridine-based metallosupramolecular constructs: tailored monomers to precise 2D-motifs and 3D-metallocages, Chem. Soc. Rev., 2018, 47, 3991–4016 RSC.
- N. Fujita, K. Biradha, M. Fujita, S. Sakamoto and K. Yamaguchi, A Porphyrin Prism: Structural Switching Triggered by Guest Inclusion, Angew. Chem., Int. Ed., 2001, 40, 1718–1721 CrossRef CAS PubMed.
- G. Cecot, M. Marmier, S. Geremia, R. De Zorzi, A. V. Vologzhanina, P. Pattison, E. Solari, F. F. Tirani, R. Scopelliti and K. Severin, The Intricate Structural Chemistry of MII2nLn-Type Assemblies, J. Am. Chem. Soc., 2017, 139, 8371–8381 CrossRef CAS.
- G. J. Kleywegt and T. A. Jones, Detection, delineation, measurement and display of cavities in macromolecular structures, Acta Crystallogr., Sect. D:Biol. Crystallogr., 1994, 50, 178–185 CrossRef CAS PubMed.
- M. M. Renfrew, Drinking Water Health Advisory: Pesticides, J. Chem. Educ., 1990, 67, A55 Search PubMed.
- W. Ji, L. Xiao, Y. Ling, C. Ching, M. Matsumoto, R. P. Bisbey, D. E. Helbling and W. R. Dichtel, Removal of GenX and Perfluorinated Alkyl Substances from Water by Amine-Functionalized Covalent Organic Frameworks, J. Am. Chem. Soc., 2018, 140, 12677–12681 CrossRef CAS PubMed.
- M. Van den Bergh, A. Krajnc, S. Voorspoels, S. R. Tavares, S. Mullens, I. Beurroies, G. Maurin, G. Mali and D. E. De Vos, Highly Selective Removal of Perfluorinated Contaminants by Adsorption on All-Silica Zeolite Beta, Angew. Chem., Int. Ed., 2020, 59, 14086–14090 CrossRef CAS PubMed.
- M. J. Klemes, Y. Ling, C. Ching, C. Wu, L. Xiao, D. E. Helbling and W. R. Dichtel, Reduction of a Tetrafluoroterephthalonitrile-β-Cyclodextrin Polymer to Remove Anionic Micropollutants and Perfluorinated Alkyl Substances from Water, Angew. Chem., Int. Ed., 2019, 58, 12049–12053 CrossRef CAS PubMed.
- Z. Chen, Y.-L. Lu, L. Wang, J. Xu, J. Zhang, X. Xu, P. Cheng, S. Yang and W. Shi, Efficient Recognition and Removal of Persistent Organic Pollutants by a Bifunctional Molecular Material, J. Am. Chem. Soc., 2023, 145, 260–267 CrossRef CAS.
- Y. He, D. Luo, V. M. Lynch, M. Ahmed, J. L. Sessler and X. Chi, Porous adaptive luminescent metallacage for the detection and removal of perfluoroalkyl carboxylic acids, Chem, 2023, 9, 93–101 CAS.
- C. Dalvit and A. Vulpetti, Ligand-Based Fluorine NMR Screening: Principles and Applications in Drug Discovery Projects, J. Med. Chem., 2019, 62, 2218–2244 Search PubMed.
- Y. Yang, Y. Du, T. K. Ronson and J. R. Nitschke, Steric Control over Interligand Dihedrals and Splay Leads to the Formation of FeII6L6 and FeII8L8 Antiprisms, CCS Chem., 2024, 6, 2411–2419 CrossRef CAS.
- Z. Zhang, L. Ma, F. Fang, Y. Hou, C. Lu, C. Mu, Y. Zhang, H. Liu, K. Gao, M. Wang, Z. Zhang, X. Li and M. Zhang, Porphyrin-Based Multicomponent Metallacage: Host–Guest Complexation toward Photooxidation-Triggered Reversible Encapsulation and Release, JACS Au, 2022, 2, 1479–1487 CrossRef CAS.
- C.-W. Zhou, X.-Z. Wang, M. Xie, R.-Q. Xia, D. Luo, Z.-X. Lian, G.-H. Ning, W. Lu, X.-P. Zhou and D. Li, A Self-Assembled Capsule for Propylene/Propane Separation, Angew. Chem., Int. Ed., 2023, 62, e202315020 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2359196. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc07105k |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |