Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Engineering live cell surfaces with polyphenol-functionalized nanoarchitectures
Yunxiang
He
 a,
Qinling
Liu
a,
Qinling
Liu
 ab,
Yuanmeng
He
a,
Siqi
Deng
a and
Junling
Guo
ab,
Yuanmeng
He
a,
Siqi
Deng
a and
Junling
Guo
 *acd
*acd
aBMI Center for Biomass Materials and Nanointerfaces, National Engineering Laboratory for Clean Technology of Leather Manufacture, Ministry of Education Key Laboratory of Leather Chemistry and Engineering, College of Biomass Science and Engineering, Sichuan University, Chengdu, Sichuan 610065, China. E-mail: junling.guo@scu.edu.cn; junling.guo@ubc.ca
bTea Refining and Innovation Key Laboratory of Sichuan Province, College of Horticulture, Sichuan Agricultural University, Chengdu, Sichuan 611130, China
cBioproducts Institute, Department of Chemical and Biological Engineering, The University of British Columbia, Vancouver, BC V6T 1Z4, Canada
dState Key Laboratory of Polymer Materials Engineering, Sichuan University, Chengdu, Sichuan 610065, China
First published on 11th February 2025
Abstract
Cell surface functionalization has emerged as a powerful strategy for modulating cellular behavior and expanding cellular capabilities beyond their intrinsic biological limits. Natural phenolic molecules present as ‘green’ and versatile building blocks for constructing cell-based biomanufacturing and biotherapeutic platforms. Due to the abundant catechol or galloyl groups, phenolic molecules can dynamically and reversibly bind to versatile substrates via multiple molecular interactions. A range of self-assembled cytoadhesive polyphenol-functionalized nanoarchitectures (cytoPNAs) can be formed via metal coordination or macromolecular self-assembly that can rapidly attach to cell surfaces in a cell-agnostic manner. Additionally, the cytoPNAs attached on the cell surface can also provide active sites for the conjunction of bioactive payloads, further expanding the structural repertoire and properties of engineered cells. This Perspective introduces the wide potential of cytoPNA-mediated cell engineering in three key applications: (1) creating inorganic–organic biohybrids as cell factories for efficient production of high-value chemicals, (2) constructing engineered cells for cell-based therapies with enhanced targeting specificity and nano–bio interactions, and (3) encapsulating microbes as biotherapeutics for the treatment of gastrointestinal tract-related diseases. Collectively, the rapid, versatile, and modular nature of cytoPNAs presents a promising platform for next-generation cell engineering and beyond.
1 Introduction
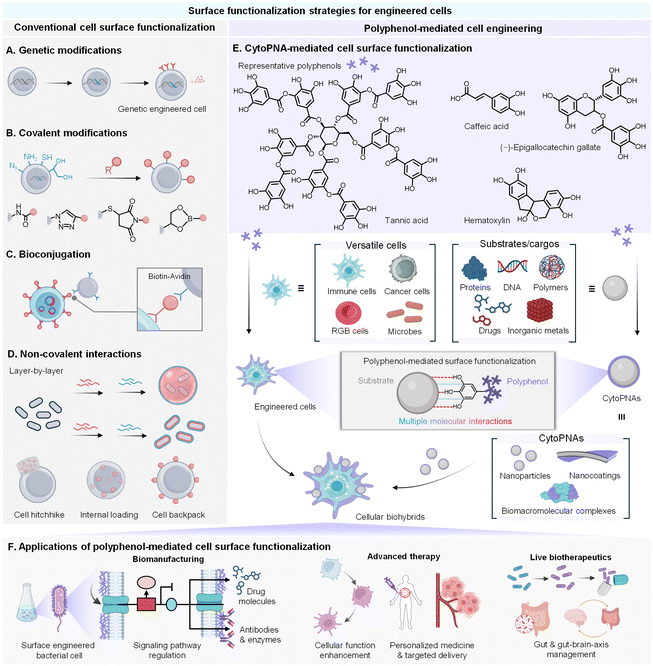
Synthetic biology and cell surface functionalization are two prevalent strategies of cell engineering that have significantly enhanced our understanding on the underlying mechanisms governing various biological behaviors in cells, enabling control over cellular interactions and new functionalities that go beyond natural biological limits.1,2 Cell surface functionalization emerges as a rapid and versatile tool to integrate material systems (such as inorganic particles, organic compounds, and biological macromolecules) with living cells. For example, the introduction of cyto-attached nanosystems can shield the cells from external environmental stresses such as toxins, immune responses, or harsh conditions, while maintaining their viability and functionality. Moreover, cell surface functionalization also allows precise control over cell–environment interactions, introduces new functionalities, and enhances biocompatibility, thereby broadening the potential applications of cells in biomanufacturing and cell-based therapies.3–5Several approaches have been developed for cell surface functionalization, ranging from genetic methods and chemical synthesis techniques to non-covalent interactions (Fig. 1A–D).6,7 Genetic methods involve engineering cells to express specific surface proteins, enabling the attachment of functional groups or the self-assembly of protective layers.8 Chemical synthesis techniques achieve stable covalent attachments between the reactive functional groups to the synthetic molecules or polymers on the cell surface.9 While effective, these approaches face potential concerns regarding intricate and expensive genetic modifications, limited flexibility and adaptability of functionalization, and compromises in long-term cell viability. In contrast, non-covalent methods, especially those based on supramolecular interactions, offer significant advantages due to their reversible, biocompatible, and modular nature.10 These methods use weak interactions like hydrogen bonding and π–π stacking to modify the cell surface, providing a non-invasive and adaptable platform for adding functional motifs dynamically.11 More importantly, the single-cell encapsulation can be tailored for multifunctionality while maintaining cell integrity.
Natural polyphenols, containing rich catechol or galloyl groups in their molecular structures, exhibit capabilities to bind with various substrates via multiple molecular interactions.12–14 These unique structural and chemical features of polyphenols facilitate the formation of a range of polyphenol-functionalized nanoarchitectures (PNAs), including nanocomplexes,15 nanoparticles,16,17 and nanocoatings,18,19 driven by metal coordination or macromolecular self-assembly. Importantly, the catechol or galloyl groups on the surface of PNAs also endow the functional substrates with cytoadhesion capability to attach to a different type of cells (referred to as cytoadhesive PNAs, cytoPNAs), offering a cell-independent, modular cell surface functionalization.20 Our group, along with others, demonstrated that cytoPNAs can be used to construct cell-based biohybrids integrated with various functional cargoes such as inorganic nanoparticles, therapeutic agents, enzymes, or imaging probes for the applications of biomanufacturing and cell-based therapies (Fig. 1E and F).21–23 The dynamic and modular nature makes cytoPNAs highly advantageous for applications requiring adaptable and reversible modifications, positioning them as a promising strategy for next-generation cell engineering.
This Perspective aims to introduce the rapidly developing research and applications of cytoPNAs in cell surface functionalization, showcasing its transformative potential in cell-based biomanufacturing and biotherapeutics. The modular and rational design of cytoPNAs opens new avenues for enhancing cellular functions, creating biohybrids with tailored properties, and ultimately, revolutionizing the next-generation biosynthetic production and personalized medicine.
2 Molecular interactions in cytoPNAs and engineered cells
Polyphenol-based materials can directly interact with cells and functional cargoes to construct engineered cells and cytoPNAs, respectively, through covalent and diverse non-covalent binding mechanisms, serving as a platform for constructing functional biointerfaces.24 Understanding these interactions is crucial for optimizing the functionality and stability of cytoPNAs and derived cellular biohybrids.2.1 Covalent interactions
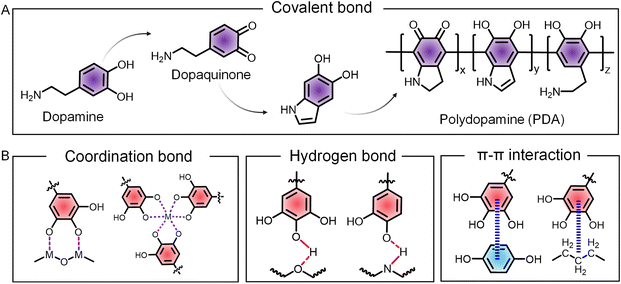
Engineered cells with dopamine and its derivatives are pioneer examples of covalent decoration of phenolic molecules to form nanocoatings on cells.25 The catecholamine of dopamine undergoes spontaneous oxidation in alkaline conditions to form dopaquinone, followed by further oxidation and polymerization to yield a complex network of polydopamine (PDA) containing covalently linked phenolic units attached on the surface of both cells and functional cargoes, enabling the construction of engineered cells and cytoPNAs (Fig. 2A). In addition, the PDA-induced cytoPNAs form a strong adhesive layer on cell surfaces, providing a stable interface for further functionalization. The catechol groups in PDA can react with nucleophilic groups such as amines and mercaptans present on membrane proteins, forming covalent bonds and enhancing the stability and longevity of the engineered cell surface. For instance, therapeutic agents, imaging probes, or other bioactive molecules can be covalently linked to the PDA coating through reactions such as Michael addition or Schiff base reactions. PDA-induced cytoPNAs can assist the precise delivery of these cargoes to specific cellular targets,26 enhance the efficacy and specificity of cell-based therapies,27 and also expand potential applications in catalysis and sensing.282.2 Non-covalent interactions
Non-covalent interactions, such as coordination, hydrogen bonding, hydrophobic forces, and π–π stacking, are the predominant methods for the cytoPNAs and engineered cells (Fig. 2B).29 Coordination with metal ions is a characteristic reaction of polyphenols, where the deprotonated phenolic groups donate the electrons to metal ion centers to form the coordination bonds. The coordination bond dominates the structure and properties of cytoPNAs consisting of polyphenols and metal ions. Hydrogen bonding is formed between the phenolic groups in polyphenols with various hydroxyl and carboxyl groups on cell or functional cargo surfaces, which is characterized by bridging the oxygen atom of the phenolic group and a highly electronegative atom (such as oxygen or nitrogen) on the cell or cargo surface via a hydrogen atom. Hydrophobic interactions and π–π interactions are significant in forming cytoPNAs and are raised by the hydrophobic aromatic rings. The tendency of hydrophobic groups to aggregate in an aqueous environment and the overlap of π-electrons significantly enhance the attachment of phenolic molecules on the cell and cargo surface.Typically, tannic acid (TA) and ferric ions (Fe3+) can form a stable metal–phenolic network on a variety of substrate surfaces from metal oxide rods, polymeric nanowires, and nanosheets to biological cells.30 These substrates with different sizes, shapes, and compositions are transformed into cytoPNAs by the coordination-induced surface functionalization and are further constructed into complex superstructures through metal ion-mediated intermolecular interactions and particle interlocking. Particularly, the non-covalent strategies are effective for cellular biohybrids with cytoPNAs and impart stimuli responsiveness such as pH or temperature by utilizing the dynamic properties of the coordination bonds.12,31 These phenolic modifications can be tailored for functional applications, such as the immobilization of growth factors like vascular endothelial growth factor to enhance endothelial cell survival and tissue regeneration.
Non-covalent interactions offer reversibility and adaptability, which are generally considered less invasive and promising preservation for cell viability and functions. On the other hand, the dynamic nature of non-covalent interactions suggests a less stable form compared with that of covalent bonding, especially in the high temperatures of some biomanufacturing processes or the complicated and fluctuated physical conditions. Attention must be paid to designing cytoPNAs, ensuring that the interactions are strong enough to maintain functionality while still allowing for reversibility and adaptability. Hence, covalent modifications are still prevalently employed in many practices due to their high stability and permanence, which are essential for long-term applications and in environments where reversibility is not required.
Collectively, the dynamic and tunable nature of phenolic-based materials provides a versatile toolkit for engineering cell–material interactions. The integration of phenolic materials onto cell and functional cargo surfaces enhances cell survival, modulates cellular behavior, and introduces new functionalities. The ability to construct cytoPNAs and biohybrids with polyphenols opens the door to a wide range of applications, from biomanufacturing to personalized medicine and cell-based therapies.
3 Inorganic–organic biohybrids for biosynthesis
Cell engineering aims to effectively regulate and control cell behaviors to augment the properties or achieve new functions, where engineered cells as ‘cell factories’ for designed chemical production stand as a representative practice. The underlying mechanism is to improve the efficiency of energy utilization.Photosynthesis is the most efficient natural process for using light and covert into chemical energy, driving the chemical conversion of CO2 into organic matter and splitting water to produce oxygen.32 To artificially harness this potential, light-harvesting semiconductors and metabolically engineered microbes are paired to enable the biohybrids to efficiently carry out the complex biosynthetic pathways for aimed chemicals. The modified anaerobic bacterium Moorella thermoacetica can absorb cadmium ion (Cd2+) from the solution by the surface transport protein, together with the dispersed cysteine, producing the insoluble cadmium sulfide (CdS) nanoparticles deposited on the bacterial surface. CdS can absorb light and pass the excited photoelectrons to the bacteria to produce acetic acid from CO2.33 The production of acetic acid can be optimized with high selectivity and yields by turning the composition of inorganic–organic biohybrids. Organic semiconductor–bacteria biohybrid systems have also been reported to efficiently increase the production of acetic acid. Perylene diimide derivatives with cationic electron-transporting (n-type) and hydrophobic hole-transporting (p-type) structures were constructed to form p–n heterojunction and coated to the bacterial surface, affording higher hole/electron separation efficiency and ensuring the efficient electron transfer to bacteria for promoting the biosynthesis (Fig. 3A).34 A more complicated system has been established by integrating Au nanoparticle enzymes into membrane-free organelles in non-photosynthetic bacteria, which greatly improves solar energy conversion and hydrogen production (Fig. 3B).35 All these biohybrids demonstrate the regulatory capabilities of functional cargoes to cellular behavior when integrated as a biohybrid via cell surface functionalization.
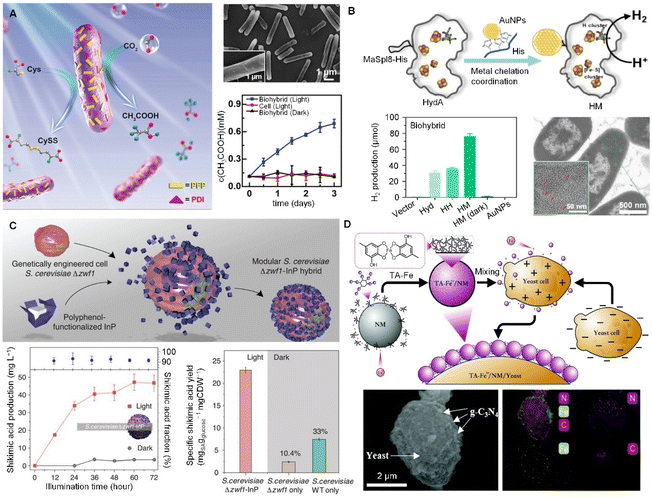 | ||
| Fig. 3 Cell surface functionalization aimed at regulating cellular pathways for the production of high-value chemicals. (A) Organic semiconductor–bacteria biohybrid system for the production of acetic acid, where the semiconductor was constructed on the bacterial surface, affording higher hole/electron separation efficiency and ensuring the efficient electron transfer to bacteria for promoting biosynthesis.34 Copyright 2020, Wiley-VCH GmbH. (B) Assembly of gold nanoparticles (AuNPs) with hydrogenases in membrane-free organelles and a comparison of hydrogen production in different systems.35 Copyright 2024, Elsevier. (C) Assembly of Saccharomyces cerevisiae–InP biohybrids and their effect on the production of shikimic acids.36 Copyright 2018, AAAS. (D) TA-modified yeast biohybrids regulate the balanced levels of NADH and NAD within the yeast.37 Copyright 2021, Royal Society of Chemistry. | ||
The cytoPNAs have been applied to a series of microbial cells to construct cellular biohybrids for producing high-value chemicals from simple inputs as a cell factory. A representative example would be the cytoPNAs composed of TA-functionalized indium phosphide (InP) nanoparticles to construct cellular biohybrids for the carbon- and energy-efficient production of shikimic acid (Fig. 3C).36 The TA-InP cytoPNAs adhered to the surface of the engineered yeast Saccharomyces cerevisiae to facilitate the transfer of the electrons from InP to the NADP+ to produce NADPH, supporting the continuous biosynthesis of shikimic acid. Similar results have been observed with the biohybrids composed of bismuth (Bi)- and graphitic carbon nitride (g-C3N4)-containing cytoPNAs on yeast cells, where the NADH (important intermediate in the electron pathway in cellular energy conversion and metabolism) content is found to increase significantly (Fig. 3D).37,38
Conventional strategies in biomanufacturing often rely solely on genetically engineered biological systems. For example, genetically engineered microbes are generally highly efficient in terms of selectivity and specificity but can be limited by their metabolic capacity and sensitivity to environmental conditions.5,10 Inorganic–organic biohybrids combine the strengths of both systems and can enhance overall efficiency and versatility. The combination generally produces synergistic effects that result in higher selectivity and productivity compared to using the biological system alone.36,37 However, there are certain limitations to consider. The fabrication and maintenance of these hybrid systems can be complex and costly, and the stability and reproducibility of the hybrid interface under various conditions remain a challenge. Additionally, the scalability of these systems for industrial applications may require further optimization. The integration of inorganic material-based cytoPNAs with biological systems demonstrates the remarkable potential for the efficient production of high-value chemicals and the conversion of solar energy into chemical energy.
4 Engineered cells for advanced cell-based therapy
Adoptive cell therapy (ACT) is an emerging and highly promising approach in cancer immunotherapy, where immune cells (typically T cells) are engineered to target and eliminate diseased cells. Conventionally, the T cells are genetically engineered by introducing transgenes, such as chimeric antigen receptors (CARs) or tumor-specific T cell receptors (TCRs), to enhance their ability to recognize and attack cancer cells. Significant successes have been achieved with the FDA-approved tisagenlecleucel and axicabtagene ciloleucel, which are two products that have revolutionized the treatment of hematologic malignancies.39,40 Material-based engineering involves the use of biomaterials to modify or enhance the immune cell functions via surface modification, encapsulation, and the delivery of bioactive agents and provides a less invasive and dynamic strategy for realizing the survival increase, targeting specificity, and rapid activation of immune cells.41 Diverse functional motifs such as biorthogonal chemical groups,42 synthetic polymers,43 and biomacromolecules44 have been employed to modify cell surface via electrostatic interactions, hydrophobic insertion, and bioconjugation reactions, holding potential in therapies.Due to the strong binding capabilities of catechol and galloyl groups, polyphenols exhibit unique advantages in cell surface functionalization and the creation of cellular biohybrids by employing cytoPNAs through reversible and tunable non-covalent interactions.30,36 ‘Cellnex’ technology presents a typical technology and has been extensively investigated for cellular biohybrid fabrication via polyphenol-based surface functionalization. Typically, polyphenol-functionalized nanocomplexes are first attached to the surface of targeted cells and provide multifunctional adsorption sites for further cytoPNAs to bind. The simplicity and modularity of ‘Cellnex’ enable the functionalization of red blood cells with a diverse array of biomolecules, encompassing functional proteins, DNA, mRNA, and viral vectors, all while preserving the intrinsic biological characteristics of the cells (Fig. 4A).45
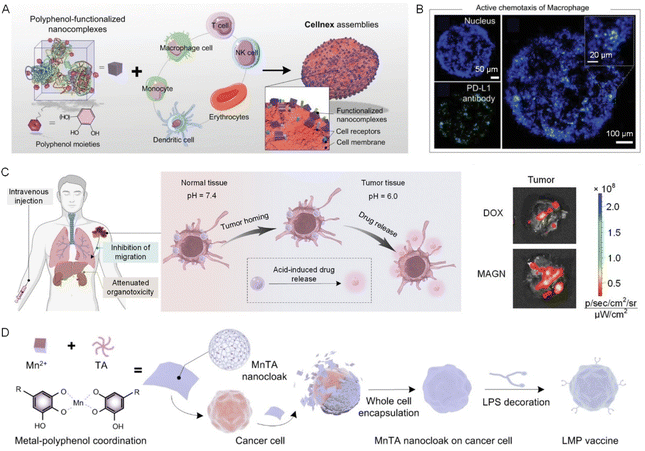 | ||
| Fig. 4 Engineered cells via polyphenol-based or cytoPNA-based cell surface functionalization for advanced cell-based therapy. (A) Modular assembly of ‘Cellnex’ by assembling polyphenol-functionalized bioactive nanocomplexes on cells. (B) Therapeutic antibodies (green) can be delivered precisely by the macrophages to the tumor spheroid and nucleus (blue).45 Copyright 2020, Wiley-VCH GmbH. (C) Schematic illustration on the macrophage-based biohybrids to load chemotherapeutic drugs with responsive release properties for antitumor applications. The biohybrid system demonstrated improved targeted delivery efficiency.46 Copyright 2023, Wiley-VCH GmbH. (D) A schematic illustration of the preparation of a novel whole-cell cancer vaccine (referred to as LMP vaccine) developed by cloaking living cancer cells with lipopolysaccharide (LPS)-modified manganese-phenolic networks (MnTA nanocloaks).49 Copyright 2023, Wiley-VCH GmbH. | ||
The cytoPNA-mediated cell engineering performs as a less invasive strategy and the biohybrids can harness the functionals of both encapsulated cells and attached functional cargoes. For example, cytoPNA-assisted conjugation of PD-L1 monoclonal antibodies to the surfaces of macrophages facilitates the targeted accumulation of antibody-based therapeutics at tumor sites, thereby promoting immune response (Fig. 4B). The chemotactic mobility and effective tumor-homing capabilities of macrophages can be preserved in a cytoPNAs composed of polyphenol-functionalized polymeric gel on the surfaces of tumor-homing macrophages. Upon exposure to the acidic conditions characteristic of the tumor microenvironment, the disassembly of the metal–phenolic interactions facilitates the controlled release of therapeutics from the nanomatrix at the targeted site, realizing the precision of delivery (Fig. 4C).46 CytoPNAs have also been reported to control the cellular behavior of immune cells, such as maintaining the anti-inflammatory phenotype of regulatory T cells when attached on the surface, further guiding macrophage polarization, enhancing stromal cell osteogenic differentiation, and promoting hard tissue reparation.47
Other than therapeutic applications, cytoPNAs can enable convenient and rapid cell labeling via a non-transmembrane approach. This technique harnesses the adhesive properties of catechol groups to non-invasively coat the surfaces of cells with fluorescent markers, thereby offering a novel strategy for in vivo cell tracing and imaging.48 Leakage and loss prevention of the functional components in cells are significant for vaccine technologies. Intracellular materials from cancer cells can serve as novel cancer antigens, stimulating the immune system to develop a memory effect against such tumors, thereby inducing immune suppression. However, a significant challenge lies in minimizing the transmembrane diffusion of small molecules, such as RNA, into the extracellular environment. By constructing a manganese ion (Mn2+)-TA artificial cell membrane on the surface of cancer cells, we effectively reduce the diffusion of intracellular materials into the bloodstream, enabling precise delivery of immune cells and offering a new strategy for tumor immunotherapy (Fig. 4D).49
Although modifying the cell surface with cytoPNA can enrich cell function, ‘Cellnex’ has some challenges to consider in terms of cell surface functionalization. One of the primary challenges is the potential impact on cell viability and function. Although polyphenols provide a versatile platform for functionalizing cell surfaces, the encapsulation process can sometimes disrupt the cell membrane, leading to reduced cell viability or altered cellular behavior.41,43 Additionally, the stability of the polyphenol coatings under various physiological conditions needs further investigation. While these coatings are designed to be biocompatible, they may degrade or detach from the cell surface over time, affecting the longevity and effectiveness of the therapy.42,45 Furthermore, the complexity of the cellular microenvironment, including interactions with other cells and the extracellular matrix, can influence the performance of the encapsulated cells, making it essential to thoroughly understand and optimize these interactions for successful therapeutic outcomes.47 In general, the application of cytoPNAs in cell-based therapies offers a versatile and non-invasive platform for enhancing cellular function and therapeutic precision. The modularity and biocompatibility of phenolic chemistries hold significant promise for the next generation of cell therapies, from cancer treatment to vaccine development.
5 CytoPNAs on microbes for enhanced biomedical performance
Utilizing cytoPNAs for biohybrids has emerged as a versatile and powerful approach for developing advanced microbial-based therapies, where bacteria have drawn great attention while fungi, viruses, and algae are also employed. This strategy facilitates the targeted delivery, viability, and stability of microbia, and the customized/personalized therapeutic strategies, providing a robust foundation for applications in bioimaging, diagnostics, and treatments on diseases such as inflammatory bowel disease (IBD), cancer, and other immune-related disorders.5.1 Microbial protection and enhanced targeted delivery
The efficacy of microbial-based therapies often faces significant obstacles such as low cell viability and off-target delivery, due to the encountered hostile environments of the gastrointestinal tract.50 CytoPNAs offer a robust solution to these challenges, either by encapsulating the bacterial cells for protection or utilizing the strong binding effect of catechol and galloyl groups with the endothelium cells in the gastrointestinal tract to assist bacterial colonization.Metal–phenolic nanocomplexes have shown effective in enhancing the viability and stability of anaerobic bacteria (Bacteroides thetaiotaomicron) by providing robust protection against oxygen exposure and processing stressors.51 TA and Fe3+ ions can assemble to form a nanoarmor on the bacterial cell surface, protecting the bacteria from the antibiotics in the gastrointestinal tract (Fig. 5A and B). This nanoarmor effectively shields both Gram-positive and Gram-negative bacteria from various antibiotics and promotes their colonization.52 In addition, combining the metal–phenolic coordination and layer-by-layer assembly with mucin and carboxymethylated β-glucan has significantly enhanced the colonization and targeted delivery of live bacterial therapeutics, particularly in the treatment of colitis (Fig. 5C and D).53,54 This versatility has also been extended to carrying diverse therapeutic cargoes, including small drug molecules and macromolecular proteins, enabling multimodal therapy across various biointerfaces such as the gastrointestinal tract, skin, and mucosae (Fig. 5E and F).55
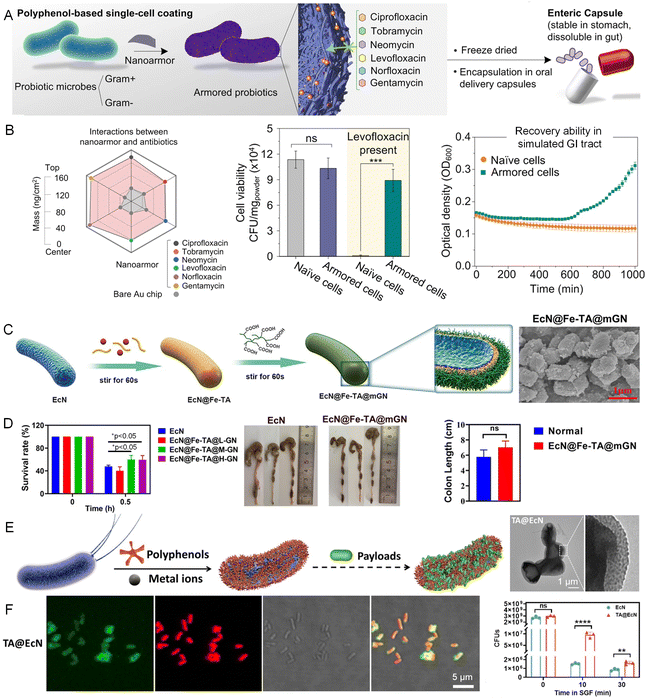 | ||
| Fig. 5 CytoPNAs on the cell surface to provide protection and enhance colonization of microbes in the gastrointestinal tract. (A) The polyphenol-based nanoarmor facilitates rapid, biocompatible bacterial cell surface functionalization, offering broad-spectrum protection against antibiotic treatments. (B) The interactions of polyphenol-based nanoarmor and various antibiotics. Polyphenol-based nanoarmor can improve the viability of bacteria in simulated gastrointestinal (GI) tract.52 Copyright 2022, Springer Nature. (C) Engineered bacterial cells by cytoPNAs via metal–phenolic coordination and synthetic polymers via layer-by-layer techniques. (D) Survival rate of bacteria with different loading amounts of functional cargoes on the cell surface, and the corresponding lengths of colons harvested from mice.54 Copyright 2023, American Chemical Society. (E) CytoPNAs induced bacterial cell surface functionalization with payloads. (F) Fluorescence characterization indicates the successful preparation of the nanocoatings on the bacterial cell surface. The survival rate of engineered bacteria being exposed to drugs.55 Copyright 2023, Elsevier. Statistical analysis was performed using one-way analysis of variance (ANOVA) analysis and Student's t-test. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 represented different statistical significances. ns stands for not significant. | ||
Polyphenols have also been pivotal in directly forming nanocoatings that advance the viability and targeting capability of biohybrids. For instance, a double-layer coating of TA and enteric L100 has been employed to optimize the selective release and retention of the bacteria (Escherichia coli Nissle 1917) within the gastrointestinal tract, enhancing its therapeutic potential against conditions like colitis.56 Curcumin-loaded liposomes can also be attached to the microbial cell surface to further boost bacterial survival and adhesion.57 Moreover, hydrogel microspheres designed for targeted delivery provide a sophisticated platform for the co-delivery of probiotics and auxiliary molecules (indole-3-propionic acid, IPA), demonstrating their efficacy in modulating flora in the gastrointestinal tract and reducing intestinal inflammation.58 These strategies underscore the vital role of cytoPNAs in advancing microbial-based biohybrids, offering a refined approach for microbial protection and targeted delivery.
5.2 Tailored therapeutic actions
In microbial-based therapy, apart from focusing on microbial activity and targeted delivery, it is also significant to employ the appropriate therapeutic cargoes to construct cellular biohybrids for the correct therapeutic actions on specific diseases. Ideally, the microbial-based therapeutic biohybrids are expected to accurately reach the lesion and effectively intervene in the disease pathological process.Polyphenol-based surface functionalization offers a versatile platform for imparting bacterial biohybrids with a diverse range of therapeutic cargoes, including small molecule drugs, antibodies, enzymes, and gene therapies.56,59 By combining the inherent antioxidant and anti-inflammatory properties of polyphenols, these bacterial-based biohybrids can be precisely tuned to respond to specific stimuli in diseased tissues, enhancing the therapeutic potential (Fig. 6A and B).57 For instance, a nanostructured polyphenol-based platform (polymerizable aromatic dithiol-TA with sodium alginate) has been developed to enhance the bacteria resistance to oxidative and inflammatory stress, thereby improving the colonization and therapeutic efficacy in IBD treatment (Fig. 6C–E).60 This strategy allows the bacterial-based biohybrids with therapeutic actions of stimuli responsiveness for drug release at desired lesions.
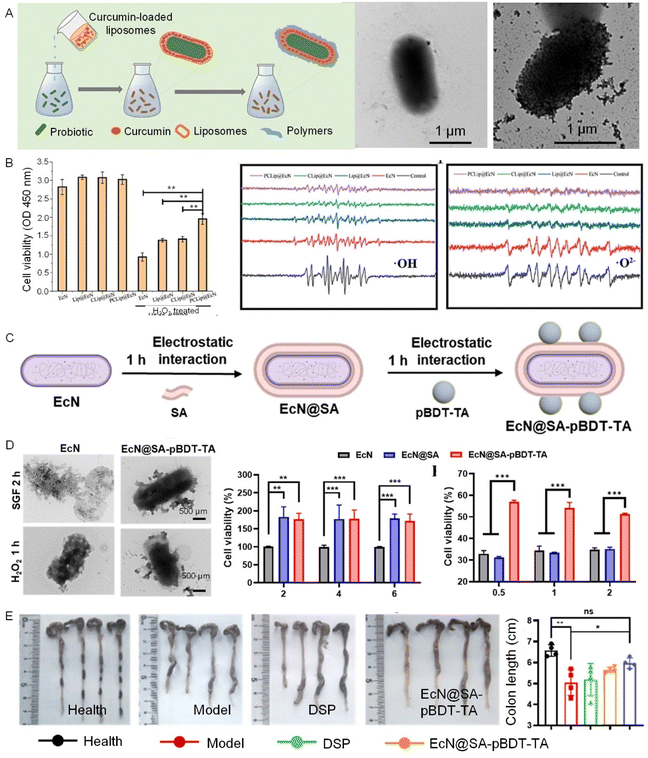 | ||
| Fig. 6 Tailored therapeutic actions enabled by bacterial cell surface functionalization with cytoPNAs. (A) Schematic illustration of the sequential process of preparation of bacterial-based biohybrids. (B) Cell viability under H2O2 treatment and scavenging effects of different treatments on hydroxyl (·OH) and superoxide (·O2−) radicals toward surface-modified bacteria.57 Copyright 2023, Elsevier. (C) Schematic illustration of the bacterial cell functionalization with a sequential process of polyphenol-based molecular interactions and layer-by-layer techniques. (D) The survival rate of bacteria under the treatment of active radical species. (E) Schematic diagram of colitis model mice and administration methods.60 Copyright 2024, American Chemical Society. Statistical analysis was performed using one-way analysis of variance (ANOVA) analysis and student's t-test. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 represented different statistical significances. ns stands for not significant. | ||
Due to the edibility and biosafety, cytoPNA-containing biohybrids are primarily designed for gastrointestinal diseases, while preserving the potential for cancer treatment. The intratumoral microbiota plays a critical role in cancer progression and therapy, influencing tumor development and the immune response through mechanisms such as chronic inflammation and signaling pathway activation.61 Engineered bacteria, modified to deliver therapeutic agents directly to tumors, have shown promise in modulating the tumor immune microenvironment.62,63 Although cytoPNAs have yet to be widely applied in tumor therapy, the inherent properties of polyphenols, including their ability to inhibit tumor cell growth, prevent metastasis, and activate the immune response, indicate strong potential for future cancer treatments by tailoring therapeutic actions.15,46,49,64 Utilizing cytoPNAs to modify bacteria could enhance their ability to regulate tumor microenvironments, offering a novel approach to targeted cancer therapy by combining microbial colonization capabilities.
5.3 Bioimaging and diagnostics
CytoPNAs also hold wide potential applications in bioimaging and diagnostics. By integrating metal-based photosensitizers, magnetic nanoparticles, and carbon-based nanomaterials, polyphenol-based biohybrids can be engineered to enhance imaging capabilities.65 This integration allows for real-time visualization of bacterial biohybrids within the body, which is particularly valuable for tracking the efficacy of therapies, such as monitoring probiotic colonization in gastrointestinal diseases or visualizing tumor-targeting bacteria in cancer treatments. Additionally, the stability and biocompatibility of polyphenol-based coatings could enhance diagnostic accuracy and sensitivity, enabling earlier and more precise disease detection, as well as facilitating advancements in non-invasive monitoring and personalized medicine.Although cytoPNAs of metal–phenolic nanocomplexes have been applied to a variety of bacterial species, expanding the range of applications in microbial protection and targeted delivery,66 several pitfalls are worth considering. The potential toxicity of the metal ions used in the cytoPNAs would be the first concern. While metal ions like Fe3+ and zinc ions (Zn2+) are generally biocompatible, other metal ions may exhibit cytotoxic effects, especially at high concentrations.65 Additionally, the choice of polyphenols must be tailored to the specific application, as different polyphenols can influence the stability and functionality of the prepared cytoPNAs.52,60 Furthermore, the stability of the coatings under physiological conditions and their impact on bacterial metabolic activity need to be thoroughly evaluated.51,56 Addressing these challenges will be essential for the successful development and clinical translation of cytoPNA–bacterial biohybrid for biomedical applications.
6 Conclusion and outlook
Natural polyphenols possess rich catechol or galloyl groups in the molecular structures, which can effectively impart novel functionalities or regulate surface properties of a wide range of substrates, including inorganic particles, organic polymers, and biomacromolecules. This polyphenol-mediated surface functionalization strategy has enabled a series of nanoarchitectures termed cytoPNAs, where the surface-exposed phenolic groups impart cell-adhesion properties. Both the polyphenols and cytoPNAs can be employed in cell surface functionalization in a cell-agnostic manner. The rapid formation and modular composition of cytoPNA-mediated cell engineering offer a powerful tool to overcome challenges encountered in biomanufacturing and cell-based therapies. In this Perspective, we present a discussion on the advances in cell surface modification using cytoPNAs. We discuss the molecular interactions that underpin the formation of cytoPNAs and their integration with engineered cells. The non-covalent interactions realize the capability and versatility of cytoPNA-mediated cell engineering in regulating cell survival and behaviors with new functionalities. The use of cytoPNAs to create inorganic–organic biohybrids can promote the production of high-value chemicals from cells, harnessing both the increased energy utilization and the regulation of the biosynthetic pathway. CytoPNAs can also advance cell- and microbial-based therapies with tailored therapeutic actions. We highlight the non-invasive surface functionalization for the enhancement of therapeutic precision, exhibiting the promise of cytoPNAs in therapies and vaccine development. In general, these versatile, dynamic, and biocompatible strategies open up new windows for next-generation cell engineering and beyond.Despite the versatility and advantages of polyphenols and cytoPNAs in bio-manufacturing and cell-based therapy, they have limited applications due to ongoing challenges that need to be addressed before further exploration. From the technical perspective, preserving essential cell functions such as adhesion, proliferation, signaling, and immune interactions is crucial for cell surface modification. The non-covalent interactions among catechol or galloyl groups in cytoPNAs with cells are generally mild, reducing the risk of disrupting membrane proteins and receptors critical for cell functions. CytoPNAs of TA–Fe nanocomplexes attached to the bacterial cell surface are engineered sufficiently thin and porous, allowing for the free diffusion of small molecules and the unhindered operation of transmembrane receptors.52,67 The thickness and distribution densities of cytoPNAs on the cell surface are key parameters to ensure the surface modification does not impede the cellular process. In addition, the nanostructure of cytoPNAs also determines the success of cell surface modification, which can be turned via the parameters of compositions, molar ratios, pH, and ionic strength. CytoPNAs with high stability by fine-tuning the parameters play an essential role in mitigating the risk of vascular occlusion due to the potential aggregation. Ongoing research into the in vivo behavior of these nanocomplexes is crucial to ensure their safety in clinical applications. Moreover, the tunable nature of cytoPNAs allows for the incorporation of specific ligands or peptides that can facilitate or mimic natural adhesion processes, thereby supporting the formation of functional immunological synapses.68 Apart from the widely studied pH variations, additional mild and diverse stimuli such as temperature, enzyme activity, or specific molecular triggers can be integrated into the cytoPNAs to control cargo release.69 From the regulatory perspective, challenges lie in the urgent need for comprehensive validation of safety, efficacy, and reproducibility for clinical applications. The long-term biosafety profile and the in vivo metabolism of cytoPNAs are also critical concerns that needed for further assessments in clinical applications. Scalable and standardized fabrication processes for cytoPNAs are essential and require interdisciplinary collaboration.
Several directions can be proposed for further development of cytoPNA-mediated cell-engineering:
- Advanced theragnostic technology: rational design cytoPNAs with responsiveness to light, sound, and magnetic fields at physiological conditions to combine the inherent targeted delivery with photo- and sono-based therapy and diagnosis.
- Enhanced targeted delivery for plants: cytoPNAs for targeted drug delivery can be expanded from biomedicine to agriculture, which can promote the efficacy of pesticides and fertilizers to facilitate crop production and alleviate the current environmental pollution caused by indiscriminate usage.
- Artificial intelligence-assisted cell engineering platforms: metal–phenolic chemistry has plenty of combinations due to the variations of both polyphenols and metal ions. Machine learning algorithms can be trained to predict the properties of metal–phenolic complexes, accelerate the discovery process, and optimize drug release and cellular uptake profiles.
- Optimized fabrication for clinical translation: polyphenol is generally considered safe as a food additive and adjuvant in pharmaceutics. Certain metal ions and most biomacromolecules are also clinically approved. Clinical translation of cytoPNAs would accelerate the development of novel therapies. Chemically demonstration of the uniformity of the assemblies in composition, size, and morphology from different batches would be the primary goal to reach.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
Conceptualization: J. Guo and Y. He; writing – original draft: Y. He; writing – review & editing: Y. He, Q. Liu, Y. He and S. Deng; validation: Y. He and J. Guo.Conflicts of interest
The authors have no conflicts to disclose.Acknowledgements
The authors thank Yue Wu and Yajing Zhang for the discussion. We acknowledge financial support from the National Key R&D Program of China (2022YFA0912800), National Excellent Young Scientists Fund (00308054A1045), National Natural Science Foundation of China (22178233, 22408241), Talents Program of Sichuan Province, Double First-Class University Plan of Sichuan University, State Key Laboratory of Polymer Materials Engineering (sklpme 2020–03−01), Tianfu Emei Program of Sichuan Province (2022-EC02-00073-CG), Postdoctoral special funding of Sichuan Province (TB2022063), Ministry of Education Key Laboratory of Leather Chemistry and Engineering, National Engineering Research Center of Clean Technology in Leather Industry.References
- J. Park, B. Andrade, Y. Seo, M.-J. Kim, S. C. Zimmerman and H. Kong, Engineering the surface of therapeutic “living” cells, Chem. Rev., 2018, 118, 1664–1690 CrossRef CAS PubMed.
- K. Adebowale, R. Liao, V. C. Suja, N. Kapate, A. Lu, Y. S. Gao and S. Mitragotri, Materials for cell surface engineering, Adv. Mater., 2024, 36, 2210059 Search PubMed.
- W. Youn, J. Y. Kim, J. Park, N. Kim, H. Choi, H. Cho and I. S. Choi, Single-cell nanoencapsulation: from passive to active shells, Adv. Mater., 2020, 32, 1907001 Search PubMed.
- K. Liang, J. J. Richardson, J. Cui, F. Caruso, C. J. Doonan and P. Falcaro, Metal–organic framework coatings as cytoprotective exoskeletons for living cells, Adv. Mater., 2016, 28, 7910–7914 Search PubMed.
- T.-C. Tang, B. An, Y. Huang, S. Vasikaran, Y. Wang, X. Jiang, T. K. Lu and C. Zhong, Materials design by synthetic biology, Nat. Rev. Mater., 2020, 6, 332–350 Search PubMed.
- J. Almeida-Pinto, M. R. Lagarto, P. Lavrador, J. F. Mano and V. M. Gaspar, Cell surface engineering tools for programming living assemblies, Adv. Sci., 2023, 10, 2304040 CrossRef CAS PubMed.
- H. Yang, L. Yao, Y. Wang, G. Chen and H. Chen, Advancing cell surface modification in mammalian cells with synthetic molecules, Chem. Sci., 2023, 14, 13325–13345 RSC.
- G. J. Knott and J. A. Doudna, CRISPR-Cas guides the future of genetic engineering, Science, 2018, 361, 866–869 CrossRef CAS PubMed.
- Z. Zhou, K. Maxeiner, D. Y. W. Ng and T. Weil, Polymer chemistry in living cells, Acc. Chem. Res., 2022, 55, 2998–3009 CrossRef CAS PubMed.
- B. J. Kim, H. Cho, J. H. Park, J. F. Mano and I. S. Choi, Strategic advances in formation of cell-in-shell structures: from syntheses to applications, Adv. Mater., 2018, 30, 1706063 CrossRef PubMed.
- O. Hasturk and D. L. Kaplan, Cell armor for protection against environmental stress: advances, challenges and applications in micro- and nanoencapsulation of mammalian cells, Acta Biomater., 2019, 95, 3–31 CrossRef CAS PubMed.
- H. Ejima, J. J. Richardson, K. Liang, J. P. Best, M. P. van Koeverden, G. K. Such, J. Cui and F. Caruso, One-step assembly of coordination complexes for versatile film and particle engineering, Science, 2013, 341, 154–157 Search PubMed.
- J. Guo, Y. Ping, H. Ejima, K. Alt, M. Meissner, J. J. Richardson, Y. Yan, K. Peter, D. von Elverfeldt, C. E. Hagemeyer and F. Caruso, Engineering multifunctional capsules through the assembly of metal–phenolic networks, Angew. Chem., Int. Ed., 2014, 53, 5546–5551 CrossRef CAS PubMed.
- S. Quideau, D. Deffieux, C. Douat-Casassus and L. Pouysegu, Plant polyphenols: chemical properties, biological activities, and synthesis, Angew. Chem., Int. Ed., 2011, 50, 586–621 CrossRef CAS PubMed.
- K. Li, G. Xiao, J. J. Richardson, B. L. Tardy, H. Ejima, W. Huang, J. Guo, X. Liao and B. Shi, Targeted therapy against metastatic melanoma based on self-assembled metal–phenolic nanocomplexes comprised of green tea catechin, Adv. Sci., 2019, 6, 1801688 CrossRef PubMed.
- Y. Han, Z. Lin, J. Zhou, G. Yun, R. Guo, J. J. Richardson and F. Caruso, Polyphenol-mediated assembly of proteins for engineering functional materials, Angew. Chem., Int. Ed., 2020, 59, 15618–15625 CrossRef CAS PubMed.
- X. Jin, Q. Wang, J. Pan, J. Wang, Y. He, J. Shang, M. Chen, X. He, Y. Zhang, B. Wang, Y. Wang, G. Gong and J. Guo, A biologically stable, self-catalytic DNAzyme machine encapsulated by metal–phenolic nanoshells for multiple microRNA imaging, Chin. Chem. Lett., 2023, 34, 108200 CrossRef CAS.
- W. Luo, G. Xiao, F. Tian, J. J. Richardson, Y. Wang, J. Zhou, J. Guo, X. Liao and B. Shi, Engineering robust metal–phenolic network membranes for uranium extraction from seawater, Energy Environ. Sci., 2019, 12, 607–614 RSC.
- Y. Wang, Y. He, Q. Wang, X. Wang, B. L. Tardy, J. J. Richardson, O. J. Rojas and J. Guo, Microporous membranes for ultrafast and energy-efficient removal of antibiotics through polyphenol-mediated nanointerfaces, Matter, 2023, 6, 260–273 CrossRef CAS.
- Z. Jia, M. Wen, Y. Cheng and Y. Zheng, Strategic advances in spatiotemporal control of bioinspired phenolic chemistries in materials science, Adv. Funct. Mater., 2021, 31, 2008821 CrossRef CAS.
- W. Jeong, E. Kim, J. Jeong, H. Bisht, H. Kang and D. Hong, Development of stimulus-responsive degradable film via codeposition of dopamine and cystamine, Chem.–Asian J., 2020, 15, 2622–2626 CrossRef CAS PubMed.
- Y. Ju, H. T. Liao, J. J. Richardson, J. L. Guo and F. Caruso, Nanostructured particles assembled from natural building blocks for advanced therapies, Chem. Soc. Rev., 2022, 51, 4287–4336 RSC.
- W. Chen, Y. Cai, Q. Fu, B. Chen, J. Guo and J. J. Chou, Unidirectional presentation of membrane proteins in nanoparticle-supported liposomes, Angew. Chem., Int. Ed., 2019, 58, 9866–9870 CrossRef CAS PubMed.
- J. J. Richardson, J. W. Maina, H. Ejima, M. Hu, J. Guo, M. Y. Choy, S. T. Gunawan, L. Lybaert, C. E. Hagemeyer, B. G. De Geest and F. Caruso, Versatile loading of diverse cargo into functional polymer capsules, Adv. Sci., 2015, 2, 1400007 CrossRef PubMed.
- Y. Liu, K. Ai and L. Lu, Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields, Chem. Rev., 2014, 114, 5057–5115 CrossRef CAS PubMed.
- L. Dong, M. Liu, M. Fang, Q. Lu, X. Li, Y. Ma and T. Zhao, Nucleation-inhibited emulsion interfacial assembled polydopamine microvesicles as artificial antigen-presenting cells, Small, 2024, 20, e2400714 CrossRef PubMed.
- S. Pan, W. Zhong, Y. Lan, S. Yu, L. Yang, F. Yang, J. Li, X. Gao and J. Song, Pathology-guided cell membrane-coated polydopamine nanoparticles for efficient multisynergistic treatment of periodontitis, Adv. Funct. Mater., 2024, 34, 2312253 CrossRef CAS.
- Z. Wang, Y. Zou, Y. Li and Y. Cheng, Metal-containing polydopamine nanomaterials: catalysis, energy, and theranostics, Small, 2020, 16, 1907042 CrossRef CAS PubMed.
- J. Zhou, Z. Lin, Y. Ju, M. A. Rahim, J. J. Richardson and F. Caruso, Polyphenol-mediated assembly for particle engineering, Acc. Chem. Res., 2020, 53, 1269–1278 CrossRef CAS PubMed.
- J. Guo, B. L. Tardy, A. J. Christofferson, Y. Dai, J. J. Richardson, W. Zhu, M. Hu, Y. Ju, J. Cui, R. R. Dagastine, I. Yarovsky and F. Caruso, Modular assembly of superstructures from polyphenol-functionalized building blocks, Nat. Nanotechnol., 2016, 11, 1105–1111 Search PubMed.
- B. J. Kim, J. K. Lee and I. S. Choi, Iron gall ink revisited: hierarchical formation of Fe(III)–tannic acid coacervate particles in microdroplets for protein condensation, Chem. Commun., 2019, 55, 2142–2145 RSC.
- D. K. Dogutan and D. G. Nocera, Artificial photosynthesis at efficiencies greatly exceeding that of natural photosynthesis, Acc. Chem. Res., 2019, 52, 3143–3148 CrossRef CAS PubMed.
- K. K. Sakimoto, A. B. Wong and P. Yang, Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production, Science, 2016, 351, 74–77 CrossRef CAS PubMed.
- P. Gai, W. Yu, H. Zhao, R. Qi, F. Li, L. Liu, F. Lv and S. Wang, Solar-powered organic semiconductor–bacteria biohybrids for CO2 reduction into acetic acid, Angew. Chem., Int. Ed., 2020, 59, 7224–7229 CrossRef CAS PubMed.
- H. Li, X. Yu, Y. Wu, C. Li, Z. Xu, W. Liu, S. Chen, H. Sun, Y. Ge and Z. Qi, Membraneless organelles assembled by AuNPs-enzyme integration in non-photosynthetic bacteria: achieving high specificity and selectivity for solar hydrogen production, Chem. Eng. J., 2024, 492, 152207 CrossRef CAS.
- J. Guo, M. Suástegui, K. K. Sakimoto, V. M. Moody, G. Xiao, D. G. Nocera and N. S. Joshi, Light-driven fine chemical production in yeast biohybrids, Science, 2018, 362, 813–816 CrossRef CAS PubMed.
- X. Ren, P. Yin, J. Liang, X. Liu, W. Zhan, J. Niu, T. Si, T. Yu, D. Wu and B. Wang, Insight into the tannic acid-based modular-assembly strategy based on inorganic–biological hybrid systems: a material suitability, loading effect, and biocompatibility study, Mater. Chem. Front., 2021, 5, 3867–3876 RSC.
- T. Yu, Y. J. Zhou, M. Huang, Q. Liu, R. Pereira, F. David and J. Nielsen, Reprogramming yeast metabolism from alcoholic fermentation to lipogenesis, Cell, 2018, 174, 1549–1558 CrossRef CAS PubMed.
- R. Awasthi, H. J. Maier, J. Zhang and S. Lim, Kymriah® (tisagenlecleucel) – an overview of the clinical development journey of the first approved CAR-T therapy, Hum. Vaccines Immunother., 2023, 19, 2210046 CrossRef PubMed.
- F. L. Locke, D. B. Miklos, C. A. Jacobson, M.-A. Perales, M.-J. Kersten, O. O. Oluwole, A. Ghobadi, A. P. Rapoport, J. McGuirk, J. M. Pagel, J. Muñoz, U. Farooq, T. van Meerten, P. M. Reagan, A. Sureda, I. W. Flinn, P. Vandenberghe, K. W. Song, M. Dickinson, M. C. Minnema, P. A. Riedell, L. A. Leslie, S. Chaganti, Y. Yang, S. Filosto, J. Shah, M. Schupp, C. To, P. Cheng, L. I. Gordon and J. R. Westin, Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma, N. Engl. J. Med., 2022, 386, 640–654 Search PubMed.
- P. Lavrador, V. M. Gaspar and J. F. Mano, Engineering mammalian living materials towards clinically relevant therapeutics, EBioMedicine, 2021, 74, 103717 Search PubMed.
- M. Hao, L. Zhu, S. Hou, S. Chen, X. Li, K. Li, N. Zhu, S. Chen, L. Xue, C. Ju and C. Zhang, Sensitizing tumors to immune checkpoint blockage via sting agonists delivered by tumor-penetrating neutrophil cytopharmaceuticals, ACS Nano, 2023, 17, 1663–1680 CrossRef CAS PubMed.
- W. Youn, E. H. Ko, M. H. Kim, M. Park, D. Hong, G. A. Seisenbaeva, V. G. Kessler and I. S. Choi, Cytoprotective encapsulation of individual jurkat t cells within durable TiO2 shells for T-cell therapy, Angew. Chem., Int. Ed., 2017, 56, 10702–10706 CrossRef CAS PubMed.
- S. Shi, J. Chen, X. Wang, M. Xiao, A. R. Chandrasekaran, L. Li, C. Yi and H. Pei, Biointerface engineering with nucleic acid materials for biosensing applications, Adv. Funct. Mater., 2022, 32, 2201069 CrossRef CAS.
- Z. Zhao, D. C. Pan, Q. M. Qi, J. Kim, N. Kapate, T. Sun, C. W. Shields, L. L. W. Wang, D. Wu, C. J. Kwon, W. He, J. Guo and S. Mitragotri, Engineering of living cells with polyphenol-functionalized biologically active nanocomplexes, Adv. Mater., 2020, 32, 2003492 CrossRef CAS PubMed.
- X. Liao, G. Gong, M. Dai, Z. Xiang, J. Pan, X. He, J. Shang, A. M. Blocki, Z. Zhao, C. W. Shields and J. Guo, Systemic tumor suppression via macrophage-driven automated homing of metal–phenolic-gated nanosponges for metastatic melanoma, Adv. Sci., 2023, 10, 2207488 CrossRef CAS PubMed.
- B. Zhang, G. Gong, Y. He, J. Liu, B. Wang, Y. Li, J. Fang, Z. Zhao and J. Guo, Regulatory T cells engineered with polyphenol-functionalized immunosuppressant nanocomplexes for rebuilding periodontal hard tissue under inflammation-challenged microenvironment, Biomaterials, 2025, 315, 122961 Search PubMed.
- Y. Zhang, J. Wang, Y. He, J. Pan, X. Jin, J. Shang, G. Gong, J. J. Richardson, I. Manners and J. Guo, Customizable supraparticles constructed from catechol-terminated molecular building blocks with controllable intermolecular interactions, Angew. Chem., Int. Ed., 2023, 62, e202303463 CrossRef CAS PubMed.
- X. He, G. Gong, M. Chen, H. Zhang, Y. Zhang, J. J. Richardson, W. Y. Chan, Y. He and J. Guo, Metal–phenolic nanocloaks on cancer cells potentiate sting pathway activation for synergistic cancer immunotherapy, Angew. Chem., Int. Ed., 2024, 63, e202314501 Search PubMed.
- J. Liu, Y. X. Wang, W. J. Heelan, Y. Chen, Z. T. Li and Q. Y. Hu, Mucoadhesive probiotic backpacks with ROS nanoscavengers enhance the bacteriotherapy for inflammatory bowel diseases, Sci. Adv., 2022, 8, eabp8798 Search PubMed.
- G. Fan, P. Wasuwanich, M. R. Rodriguez-Otero and A. L. Furst, Protection of anaerobic microbes from processing stressors using metal–phenolic networks, J. Am. Chem. Soc., 2021, 144, 2438–2443 CrossRef PubMed.
- J. Z. Pan, G. D. Gong, Q. Wang, J. J. Shang, Y. X. He, C. Catania, D. Birnbaum, Y. Li, Z. J. Jia, Y. Y. Zhang, N. S. Joshi and J. L. Guo, A single-cell nanocoating of probiotics for enhanced amelioration of antibiotic-associated diarrhea, Nat. Commun., 2022, 13, 2117 CrossRef CAS PubMed.
- X. Yang, J. Yang, Z. Ye, G. Zhang, W. Nie, H. Cheng, M. Peng, K. Zhang, J. Liu, Z. Zhang and J. Shi, Physiologically inspired mucin coated Escherichia coli Nissle 1917 enhances biotherapy by regulating the pathological microenvironment to improve intestinal colonization, ACS Nano, 2022, 16, 4041–4058 Search PubMed.
- A. Q. Xie, H. H. Ji, Z. Y. Liu, Y. Q. Wan, X. C. Zhang, H. H. Xiong, S. P. Nie and H. Wan, Modified prebiotic-based “shield” armed probiotics with enhanced resistance of gastrointestinal stresses and prolonged intestinal retention for synergistic alleviation of colitis, ACS Nano, 2023, 17, 14775–14791 CrossRef CAS PubMed.
- H. Luo, F. Wu, X. Wang, S. Lin, M. Zhang, Z. Cao and J. Liu, Encoding bacterial colonization and therapeutic modality by wrapping with an adhesive drug-loadable nanocoating, Mater. Today, 2023, 62, 98–110 Search PubMed.
- J. Liu, W. Li, Y. Wang, Y. Ding, A. Lee and Q. Hu, Biomaterials coating for on-demand bacteria delivery: selective release, adhesion, and detachment, Nano Today, 2021, 41, 101291 Search PubMed.
- M. Han, S. Yang, J. Song and Z. Gao, Layer-by-layer coated probiotics with chitosan and liposomes demonstrate improved stability and antioxidant properties in vitro, Int. J. Biol. Macromol., 2024, 258, 128826 CrossRef CAS PubMed.
- X. Yang, W. Nie, C. Wang, Z. Fang and L. Shang, Microfluidic-based multifunctional microspheres for enhanced oral co-delivery of probiotics and postbiotics, Biomaterials, 2024, 308, 122564 Search PubMed.
- M. Chen, L. Xia, C. Wu, Z. Wang, L. Ding, Y. Xie, W. Feng and Y. Chen, Microbe-material hybrids for therapeutic applications, Chem. Soc. Rev., 2024, 53, 8306–8378 Search PubMed.
- Q. Hu, J. Li, T. Wang, X. Xu, Y. Duan and Y. Jin, Polyphenolic nanoparticle-modified probiotics for microenvironment remodeling and targeted therapy of inflammatory bowel disease, ACS Nano, 2024, 18, 12917–12932 CAS.
- D. Jia, Q. Wang, Y. Qi, Y. Jiang, J. He, Y. Lin, Y. Sun, J. Xu, W. Chen, L. Fan, R. Yan, W. Zhang, G. Ren, C. Xu, Q. Ge, L. Wang, W. Liu, F. Xu, P. Wu, Y. Wang, S. Chen and L. Wang, Microbial metabolite enhances immunotherapy efficacy by modulating T cell stemness in pan-cancer, Cell, 2024, 187, 1651–1665 CrossRef CAS PubMed.
- F. Cao, L. Jin, C. Zhang, Y. Gao, Z. Qian, H. Wen, S. Yang, Z. Ye, L. Hong, H. Yang, Z. Tong, L. Cheng, Y. Ding, W. Wang, G. Yu, Z. Mao and X. Chen, Engineering clinically relevant probiotics with switchable “nano-promoter” and “nano-effector” for precision tumor therapy, Adv. Mater., 2024, 36, e2304257 CrossRef PubMed.
- Y. Fan, J. Ye, Y. Kang, G. Niu, J. Shi, X. Yuan, R. Li, J. Han and X. J. S. A. Ji, Biomimetic piezoelectric nanomaterial-modified oral microrobots for targeted catalytic and immunotherapy of colorectal cancer, Sci. Adv., 2024, 10, eadm9561 CrossRef CAS PubMed.
- X. L. He, H. F. Zhu, J. J. Shang, M. F. Li, Y. Y. Zhang, S. C. Zhou, G. D. Gong, Y. X. He, A. Blocki and J. L. Guo, Intratumoral synthesis of transformable metal–phenolic nanoaggregates with enhanced tumor penetration and retention for photothermal immunotherapy, Theranostics, 2022, 12, 6258–6272 CrossRef CAS PubMed.
- W. Lin, Y. Liu, J. Wang, Z. Zhao, K. Lu, H. Meng, R. Luoliu, X. He, J. Shen, Z. W. Mao and W. Xia, Engineered bacteria labeled with iridium(III) photosensitizers for enhanced photodynamic immunotherapy of solid tumors, Angew. Chem., Int. Ed., 2023, 62, e202310158 Search PubMed.
- G. Fan, J. Cottet, M. R. Rodriguez-Otero, P. Wasuwanich and A. L. Furst, Metal–phenolic networks as versatile coating materials for biomedical applications, ACS Appl. Bio Mater., 2022, 5, 4687–4695 Search PubMed.
- W. Chen, Z. Yang, X. Fu, L. Du, Y. Tian, J. Wang, W. Cai, P. Guo and C. Wu, Synthesis of a removable cytoprotective exoskeleton by tea polyphenol complexes for living cell encapsulation, ACS Biomater. Sci. Eng., 2021, 7, 764–771 CrossRef CAS PubMed.
- J. Li, Q. Xia, H. Guo, Z. Fu, Y. Liu, S. Lin and J. Liu, Decorating bacteria with triple immune nanoactivators generates tumor-resident living immunotherapeutics, Angew. Chem., Int. Ed., 2022, 61, e202202409 CrossRef CAS PubMed.
- H. Ejima, J. J. Richardson and F. Caruso, Metal–phenolic networks as a versatile platform to engineer nanomaterials and biointerfaces, Nano Today, 2017, 12, 136–148 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |