Open Access Article
Open Access ArticleMetallacycle-cored luminescent ionic liquid crystals with trigonal symmetry†
Long
Chen
ac,
Yu
Cao
 *a,
Haohui
Huo
a,
Shuai
Lu
b,
Yali
Hou
a,
Tianyi
Tan
a,
Xiaopeng
Li
*a,
Haohui
Huo
a,
Shuai
Lu
b,
Yali
Hou
a,
Tianyi
Tan
a,
Xiaopeng
Li
 b,
Feng
Liu
b,
Feng
Liu
 a and
Mingming
Zhang
a and
Mingming
Zhang
 *a
*a
aShaanxi International Research Center for Soft Matter, State Key Laboratory for Mechanical Behavior of Materials, Xi'an Jiaotong University, Xi'an 710049, P. R. China. E-mail: yu.cao@xjtu.edu.cn; mingming.zhang@xjtu.edu.cn
bCollege of Chemistry and Environmental Engineering, Shenzhen University, Shenzhen 518055, P. R. China
cKey Laboratory of Catalytic Materials and Technology of Shaanxi Province, Kaili Catalyst & New Materials Co., Ltd, Xi'an 710201, P. R. China
First published on 17th February 2025
Abstract
Herein, we report the preparation of a series of metallacycle-cored liquid crystals with hexagonal and trigonal symmetries based on the self-assembly of tri(ethyl glycol) (TEG)-functionalized diplatinum(II) ligands and alkyl chain-appendant tetraphenylethylene (TPE) derivatives. Interestingly, with the increase of the density of the TEG units in the metallacycles, the phase separation between TEG and alkyl chains reduces the symmetry of the columnar phase from hexagonal p6mm to trigonal p3m1, which significantly enhances the aggregation of TPE units and thus increases the emission of the system, resulting in fluorescence quantum yield as high as 47.4% in the mesogenic phase. Moreover, the positive charges of the metallacycles endow these liquid crystals with good ionic conductivity at room temperature, making them potential candidates for optoelectronics.
Introduction
Macrocycles play a vital role in the development of supramolecular chemistry owing to their molecular recognition and complexation properties.1–5 Furthermore, the self-assembly of macrocycles can generate favorable channels via molecular stacking, leading to the design of functional materials for the transportation of ions and molecules.6–8 In particular, the formation of mesogenic phases endows the materials with good orientation and ordered nanostructures, making them useful materials for absorption, separation, sensing and transportation.8–15 Therefore, various covalent macrocycles have been used as the cores to prepare macrocycle-cored liquid crystals to deliver different functions.16–19 However, this strategy generally requires tedious chemical synthesis to decorate flexible chains onto the macrocycles to provide them sufficient mobility for suitable molecular stacking and liquid crystallinity.20 In order to solve this problem, macrocycles formed by non-covalent interactions are developed,21 with the aim to simplify the synthesis with their liquid crystalline properties well-retained.Metal-coordination interactions have been widely applied for the construction of supramolecular coordination complexes (SCCs)22–28 with certain stability owing to their good directionality and moderate bond strength.29–32 These SCCs, including metallacycles and metallacages, possess well-defined shapes, sizes and geometries, making them an ideal platform for further constructing ordered supramolecular assemblies with increased complexity via hierarchical self-assembly. Therefore, various interesting supramolecular structures, including supramolecular polymers, networks and gels, and liquid crystals have been prepared through SCC-based hierarchical self-assembly. Recently, we have reported a type of emissive rhomboidal metallacycle-cored liquid crystals, which exhibits a columnar phase and shows potential for the preparation of optoelectronic materials.33 However, these liquid crystals only show moderate emission, and their conductivity has not been explored. For liquid crystal displays (LCDs), the mesogenic materials should be both highly emissive and sufficiently conductive to offer the device certain brightness, efficiency as well as electroluminescence.34–37 Therefore, liquid crystals with high quantum yields and ion-conductivities are urgently needed for both devices and fundamental research.
Herein, we report four hexagonal metallacycles with varied densities of tri(ethylene glycol) (TEG) units (Scheme 1) which show both emission and ionic conductivity in the mesogenic phase. All these metallacycles show a hexagonal columnar mesophase (Colhex) over a wide temperature range. In the film state, associated with the reduction of symmetry from p6mm to p3m1, the quantum yield of the metallacycle reaches 47.4% at room temperature, much higher than that of previously reported rhomboidal metallacycle-cored liquid crystals and many other luminescent liquid crystals.33,38 More interestingly, the ionic nanochannels formed by the stacking of these hexagonal metallacycles endow the metallacycles with good ionic conductivity (1.1 × 10−6 S cm−1 at 30 °C) in the liquid crystalline film at room temperature. This work reveals that hollow hexagonal-shaped metallacycles can form stable mesophases driven by metal coordination and explores a type of luminescent ionic liquid crystals that may be employed as one-dimensional ion-conductive materials in energy-related devices, optoelectronics and sensing.
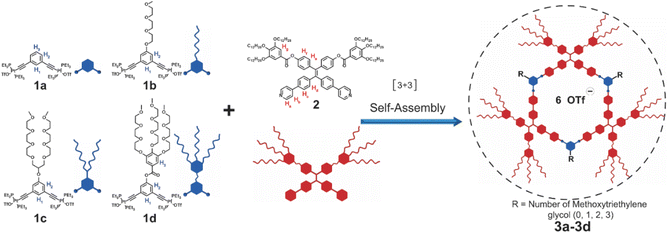 | ||
| Scheme 1 Cartoon representations of the formation of hexagonal metallacycles 3a–dvia metal-coordination-driven self-assembly. | ||
Results and discussion
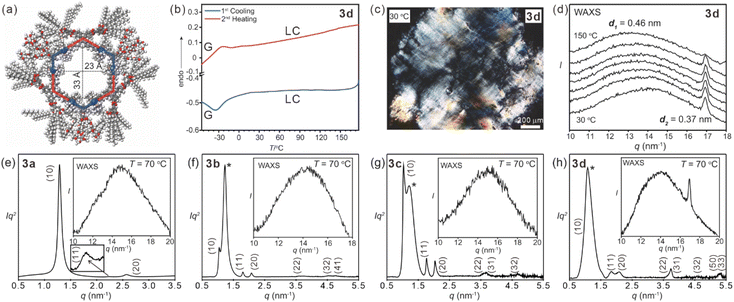
Based on the self-assembly of 120° platinum (II) ligands 1a–d and a dipyridyl tetraphenylethylene (TPE) derivative 2, hexagonal metallacycles 3a–d were prepared in good yields (Scheme 1). Metallacycles 3a–d were characterized by multiple NMR (31P{1H}, 1H, and 13C) analysis and electrospray ionization time-of-flight mass spectrometry (ESI-TOF-MS) (ESI Fig. S1–S60†). All these observations agreed well with previously reported results33 and suggested the successful preparation of hexagonal metallacycles 3a–d.The liquid crystalline properties of metallacycles 3a–d were investigated by differential scanning calorimetry (DSC), polarized optical microscopy (POM), and small/wide angle X-ray scattering (SAXS/WAXS). Taking 3d as an example (Fig. 1a), no obvious exothermic/endothermic peaks were observed above room temperature from the DSC curves (Fig. 1b and S61 ESI†). Clear birefringent textures were found for all the metallacycles upon heating (Fig. 1c and S62–S65 ESI†). Due to the same phase type and close melting points of TEG chains and dodecane, the DSC curves and POM textures of 3a–d were similar to each other. WAXS temperature scans showed obvious diffused peaks at ca. 0.45 nm for all the metallacycles (Fig. 1d and S66a–d, ESI†). Particularly, an extra sharp peak at 0.37 nm was seen for 3d until 130 °C (Fig. 1d), corresponding to the π–π stacking of the adjacent metallacycles, suggesting the increased packing order with the increase of the density of TEG units. All the results suggested the formation of thermotropic liquid crystals above room temperature for all the metallacycles.
In order to decipher the liquid crystalline phase, SAXS measurements were performed for metallacycles 3a–d (Fig. 1e–h, S66e–l and Tables S1–S4 ESI†). For 3a, the scattering pattern indicated a typical hexagonal lattice with 2D symmetry as the columnar phase (ratio of d-spacings: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/31/2
1/31/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
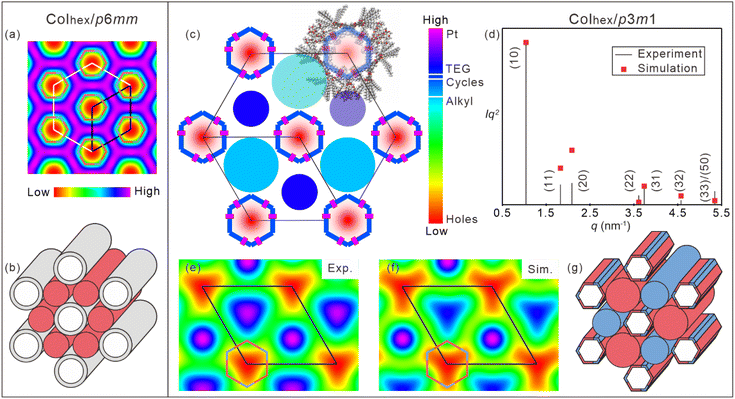
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2) (Fig. 1e). The lattice parameter ahex of 3a was 5.61 nm. The reconstructed electron density (ED) map (Fig. 2a) suggested that metallacycle 3a self-organized into columns (Fig. 2b) owing to the steric effect and nano-segregation caused by the peripheral alkyl chains. From the hollow center (red circular region) to the metallacycles (green region) and then to the peripheral alkyl chains (purple region), the electron density increased gradually, which was consistent with the stacking of 3a in the columnar phase. Owing to the free-rotating metallacycles and adjacent holes, the spatial and temporal average ED of metallacycles was lower than that of peripheral alkyl chains. Interestingly, for metallacycles 3b–d, an extra broad peak adjacent to (10) was observed (Fig. 1f–h), indicating the local clusters of TEG units, which indicates the columnar phase of the p3m1 plane group (for more details, see Section S7 and Fig. S67†).33 Correspondingly, the lattice parameter of metallacycles 3b–d swelled to ca. 6.96 nm. The phase separation would inevitably induce the symmetry breaking and reduction of symmetry from hexagonal p6mm for 3a to trigonal p3m1 for 3b–d. The rarely found p3m1 phase was only observed in the bent-core bola-polyphile liquid crystal due to steric effect.39 Due to its non-centrosymmetric property, the phase combination becomes arbitrary which hinders the reconstruction of the ED map by a simple trial and error. Thus, a theoretical packing model with actual lattice parameters, molecular size and electron density was constructed to estimate the phase angle combination (Fig. 2c and ESI Section 7†). The Fourier transform of the model, based on positions and shape factor of circular and rectangular components in the proposed model, gives analytical scattering intensities that fit well with experimental results with phase angle combination (Fig. 2d and ESI Table S5†). An alternating ED distribution was seen at the corners of metallacycles 3b–d based on both experimental and simulated results (Fig. 2e–f and S68 ESI†), suggesting that the rotation of the metallacycles was restricted by the aggregation of peripheral TEG and alkyl chains (Fig. 2g), which would benefit the emission of these metallacycles.
1/2) (Fig. 1e). The lattice parameter ahex of 3a was 5.61 nm. The reconstructed electron density (ED) map (Fig. 2a) suggested that metallacycle 3a self-organized into columns (Fig. 2b) owing to the steric effect and nano-segregation caused by the peripheral alkyl chains. From the hollow center (red circular region) to the metallacycles (green region) and then to the peripheral alkyl chains (purple region), the electron density increased gradually, which was consistent with the stacking of 3a in the columnar phase. Owing to the free-rotating metallacycles and adjacent holes, the spatial and temporal average ED of metallacycles was lower than that of peripheral alkyl chains. Interestingly, for metallacycles 3b–d, an extra broad peak adjacent to (10) was observed (Fig. 1f–h), indicating the local clusters of TEG units, which indicates the columnar phase of the p3m1 plane group (for more details, see Section S7 and Fig. S67†).33 Correspondingly, the lattice parameter of metallacycles 3b–d swelled to ca. 6.96 nm. The phase separation would inevitably induce the symmetry breaking and reduction of symmetry from hexagonal p6mm for 3a to trigonal p3m1 for 3b–d. The rarely found p3m1 phase was only observed in the bent-core bola-polyphile liquid crystal due to steric effect.39 Due to its non-centrosymmetric property, the phase combination becomes arbitrary which hinders the reconstruction of the ED map by a simple trial and error. Thus, a theoretical packing model with actual lattice parameters, molecular size and electron density was constructed to estimate the phase angle combination (Fig. 2c and ESI Section 7†). The Fourier transform of the model, based on positions and shape factor of circular and rectangular components in the proposed model, gives analytical scattering intensities that fit well with experimental results with phase angle combination (Fig. 2d and ESI Table S5†). An alternating ED distribution was seen at the corners of metallacycles 3b–d based on both experimental and simulated results (Fig. 2e–f and S68 ESI†), suggesting that the rotation of the metallacycles was restricted by the aggregation of peripheral TEG and alkyl chains (Fig. 2g), which would benefit the emission of these metallacycles.
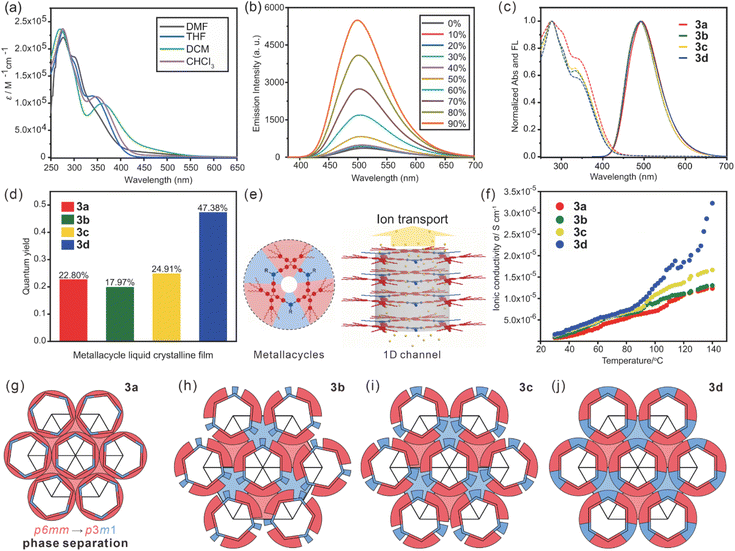
The UV-vis absorption and emission spectra of metallacycles 3a–d in dichloromethane were collected (Fig. 3a and ESI Fig. S69a–h†). All these metallacycles exhibited three absorption bands centered at ca. 266 nm, 299 nm and 357 nm and a broad emission band centered at 510 nm, which was attributed to the TPE units.33 Upon the gradual addition of hexane into the dichloromethane solution of metallacycles 3a–d, their emission increased remarkably (Fig. 3b and ESI Fig. S69i–l†), suggesting that the aggregation-induced emission (AIE)40 feature of TPE was well retained. Thin films of metallacycles 3a–d were further prepared to study their absorption and emission in the liquid crystalline state at room temperature (Fig. 3c). Three absorption bands centered at 276 nm, 304 and 339 nm were found owing to n–σ*, n–π* and π–π* transitions, respectively. These metallacycles exhibited a single emission band with the maximum emission centered at 493 nm. The quantum yields (ΦF) were measured to be 22.8% for 3a, 18.0% for 3b, 24.9% for 3c, and 47.4% for 3d (Fig. 3d), which were among the highest values of luminescent liquid crystals at room temperature (Fig. S70a†).38
Apart from the luminescence from aggregation, the columnar phase provided intrinsic 1D channels (Fig. 3e). Combining with the positive-charged nature of these metallacycles and the ionic conductivity of TEG units,41 the ionic conductivities of 3a–d along the columnar axis were measured by electrochemical impedance spectroscopy. As shown in Fig. 3f, the ionic conductivities of metallacycles 3a–d increased along with temperature due to the enhanced mobility. At 30 °C, the ionic conductivity was 5.5 × 10−7 S cm−1 for 3a, 6.9 × 10−7 S cm−1 for 3b, 9.4 × 10−7 S cm−1 for 3c and 1.1 × 10−6 S cm−1 for 3d. With the increase of the density of TEG units, the ionic mobility of the metallacycles in the mesophase was enhanced, giving better ionic conductivity comparable with other ionic liquid crystals at room temperature (Fig. S70b†).42 Integrating with their highly emissive nature and ease of alignment, these metallacycles could be applied in the construction of optoelectrical devices such as LCDs.
Both liquid crystalline phase and properties of these metallacycles were closely related to the phase separation and symmetry breaking. As indicated by ED maps (Fig. 2a and e), metallacycles 3a–d were packed in the columnar phase with a central hollow of ca. 2.1 nm, close to their geometrically optimized molecular model (Fig. 1a). Such a similarity suggested a minor local shift in the crystallographic plane, generating well-organized channels along the columnar axis capable of transporting ions. In order to evaluate the influence of the in-plane steric effect, molecular packing models based on the actual molecular shape and peripheral chain distribution were proposed (Fig. 3g–j). Alkyl chains in red uniformly filled the space between columns of 3a, providing liquid crystallinity and conventional p6mm group.43 Alkyl chains of 3b–d were rearranged with blue TPE units as intervals due to phase separation, forming the p3m1 phase. Obviously, the volume of TPE units was essential for the metallacycle packing. Theoretically, 54.1% volume ratio between TEG and alkyl chains (ESI Fig. S71†) was needed to properly fill the space between neighboring columns. The volume ratio was 11.9% for 3b, leading to an inevitable local rotation and the lowest ΦF of 17.8%. Increasing the volume of TEG units gradually restricted the rotation of the metallacycles and thus enhanced the emission of metallacycles 3b–d. 3d provided a more desirable volume ratio as large as 42.6%, giving a strong phase separation and good space filling to regulate the stacking of metallacycles and eventually boost the ΦF to 47.4%.
Conclusions
In summary, we have successfully constructed a series of hexagonal metallacycles that formed a thermotropic hexagonal columnar mesophase at room temperature. The phase separation inside these liquid crystals further restricts the molecular motion of these metallacycles, providing them with good emission in the film state. Moreover, the ordered packing of these metallacycles in the mesophase offers an organized one-dimensional channel, giving them good ionic conductivity. This study provides a simple yet effective strategy for the construction of highly emissive and ionic conductive liquid crystals via metal-coordination-driven self-assembly, which will open a new avenue for advanced supramolecular materials towards optoelectrical applications.44,45Experimental methods
All reagents and deuterated solvents were purchased as analytical grade and used without further purification. Column chromatography was performed using 300–400 mesh silica gel. Nuclear magnetic resonance spectra were recorded with a Bruker Avance 400 MHz or 600 MHz spectrometer. 1H and 13C NMR chemical shifts are reported relative to residual solvent signals, and 31P{1H} NMR chemical shifts are referenced to an external unlocked sample of 85% H3PO4 (δ 0.0). Mass spectra were recorded on a Micromass Quattro II triple-quadrupole mass spectrometer using electrospray ionization with a MassLynx operating system. The UV-vis experiments were conducted on a Lambda 950 absorption spectrophotometer. The fluorescence experiments were conducted on a Hitachi F-7000 fluorescence spectrophotometer. Phase textures of all compounds were fully characterized by polarizing optical microscopy (Olympus BX51-P) in conjunction with a heating stage (Linkam LTS420E) and controller (T95-HS). Optical investigations were carried out under equilibrium conditions between two glass slides that were used without further treatment. Transition enthalpies were determined as obtained from differential scanning calorimetry (DSC) which were recorded on a TA DSC250 (heating and cooling rate: 10 K min−1, peak temperatures). SAXS was conducted in a BL16B1 of SSRF and processed using Nika and Irena macros from the Igor platform.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
L. C.: data curation, investigation, validation, writing – original draft. Y. C.: formal analysis, funding acquisition, investigation, methodology, visualization, writing – review & editing. H. H.: investigation, writing – review & editing. S. L.: investigation, writing – review & editing. Y. H.: investigation, writing – review & editing. T. T.: investigation, writing – review & editing. X. L.: project administration, writing – review & editing. F. L.: project administration, supervision, writing – review & editing. M. Z.: conceptualization, funding acquisition, project administration, resources, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22171219, 22222112 and 12204369), China Postdoctoral Science Foundation (2022M712551, 2023T160505), Innovation Talent Promotion Plan of Shaanxi Province for Science and Technology Innovation Team (2023-CX-TD-51), and Science and Technology Agency of Shaanxi Province (2023-YBGY-459, 2024JC-YBQN-0116). The authors thank beamlines BL16B1 at Shanghai Synchrotron Radiation Facility (SSRF) for providing the beam time. We also thank Dr Gang Chang and Dan He at the Instrument Analysis Center of Xi'an Jiaotong University for NMR and fluorescence measurements.References
- Z. Liu, S. K. M. Nalluri and J. F. Stoddart, Chem. Soc. Rev., 2017, 46, 2459–2478 RSC.
- D. Prochowicz, A. Kornowicz and J. Lewiński, Chem. Rev., 2017, 117, 13461–13501 CrossRef CAS PubMed.
- R. Kumar, A. Sharma, H. Singh, P. Suating, H. S. Kim, K. Sunwoo, I. Shim, B. C. Gibb and J. S. Kim, Chem. Rev., 2019, 119, 9657–9721 Search PubMed.
- S. J. Barrow, S. Kasera, M. J. Rowland, J. Del Barrio and O. A. Scherman, Chem. Rev., 2015, 115, 12320–12406 CrossRef CAS PubMed.
- T. Ogoshi, T. Yamagishi and Y. Nakamoto, Chem. Rev., 2016, 116, 7937–8002 CrossRef CAS PubMed.
- Q. Wang, Y. Zhong, D. P. Miller, X. Lu, Q. Tang, Z. L. Lu, E. Zurek, R. Liu and B. Gong, J. Am. Chem. Soc., 2020, 142, 2915–2924 CrossRef CAS PubMed.
- M. Stepien, B. Donnio and J. L. Sessler, Angew. Chem., Int. Ed., 2007, 46, 1431–1435 CrossRef CAS PubMed.
- Y. Luo, N. Marets and T. Kato, Chem. Sci., 2018, 9, 608–616 RSC.
- M. Fritzsche, A. Bohle, D. Dudenko, U. Baumeister, D. Sebastiani, G. Richardt, H. W. Spiess, M. R. Hansen and S. Hoger, Angew. Chem., Int. Ed., 2011, 50, 3030–3089 CrossRef CAS PubMed.
- M. Amorin, A. Perez, J. Barbera, H. L. Ozores, J. L. Serrano, J. R. Granja and T. Sierra, Chem. Commun., 2014, 50, 688–690 Search PubMed.
- K. Guy, P. Ehni, S. Paofai, R. Forschner, C. Roiland, M. Amela-Cortes, S. Cordier, S. Laschat and Y. Molard, Angew. Chem., Int. Ed., 2018, 57, 11692–11696 CrossRef CAS PubMed.
- P. L. Champagne, D. Ester, D. Polan, V. E. Williams, V. Thangadurai and C. C. Ling, J. Am. Chem. Soc., 2019, 141, 9217–9224 CrossRef CAS PubMed.
- Y. X. Hu, X. Hao, L. Xu, X. Xie, B. Xiong, Z. Hu, H. Sun, G. Q. Yin, X. Li, H. Peng and H. B. Yang, J. Am. Chem. Soc., 2020, 142, 6285–6294 Search PubMed.
- Y. X. Hu, X. Hao, D. Wang, Z. C. Zhang, H. Sun, X. D. Xu, X. Xie, X. Shi, H. Peng, H. B. Yang and L. Xu, Angew. Chem., Int. Ed., 2024, 63, e202315061 CrossRef CAS PubMed.
- T. Kato, J. Uchida, T. Ichikawa and T. Sakamoto, Angew. Chem., Int. Ed., 2018, 57, 4355–4371 Search PubMed.
- Z. Yu, X. M. Chen, Z. Y. Liu, M. Wang, S. Huang and H. Yang, Chem. Commun., 2021, 57, 911–914 RSC.
- K. Sato, Y. Itoh and T. Aida, J. Am. Chem. Soc., 2011, 133, 13767–13769 CrossRef CAS PubMed.
- S. Kawano, Y. Ishida and K. Tanaka, J. Am. Chem. Soc., 2015, 137, 2295–2302 CrossRef CAS PubMed.
- Y. Li, H. Su, X. Feng, K. Yue, Z. Wang, Z. Lin, X. Zhu, Q. Fu, Z. Zhang, S. Z. D. Cheng and W. B. Zhang, Polym. Chem., 2015, 6, 827–837 Search PubMed.
- W. Zhang and J. S. Moore, Angew. Chem., Int. Ed., 2006, 45, 4416–4439 CrossRef CAS PubMed.
- H. Y. Lin, Y. T. Wang, X. Shi, H. B. Yang and L. Xu, Chem. Soc. Rev., 2023, 52, 1129–1154 RSC.
- Z. Zhang, Z. Zhao, Y. Hou, H. Wang, X. Li, G. He and M. Zhang, Angew. Chem., Int. Ed., 2019, 58, 8862–8866 CrossRef CAS PubMed.
- Z. Zhang, Z. Zhao, L. Wu, S. Lu, S. Ling, G. Li, L. Xu, L. Ma, Y. Hou, X. Wang, X. Li, G. He, K. Wang, B. Zou and M. Zhang, J. Am. Chem. Soc., 2020, 142, 2592–2600 CrossRef CAS PubMed.
- Y. Hou, Z. Zhang, S. Lu, J. Yuan, Q. Zhu, W. P. Chen, S. Ling, X. Li, Y.-Z. Zheng, K. Zhu and M. Zhang, J. Am. Chem. Soc., 2020, 142, 18763–18768 CrossRef CAS PubMed.
- C. Mu, Z. Zhang, Y. Hou, H. Liu, L. Ma, X. Li, S. Ling, G. He and M. Zhang, Angew. Chem., Int. Ed., 2021, 133, 12401–12405 CrossRef.
- Y. Hou, Z. Zhang, L. Ma, R. Shi, S. Ling, X. Li, G. He and M. Zhang, CCS Chem., 2022, 4, 2604–2611 CrossRef CAS.
- C. Mu, L. Zhang, G. Li, Y. Hou, H. Liu, Z. Zhang, R. Zhang, T. Gao, Y. Qian, C. Guo, G. He and M. Zhang, Angew. Chem., Int. Ed., 2023, 135, e202311137 CrossRef.
- K. Gao, Y. Cheng, Z. Zhang, X. Huo, C. Guo, W. Fu, J. Xu, G. L. Hou, X. Shang and M. Zhang, Angew. Chem., Int. Ed., 2024, 136, e202319488 CrossRef.
- B. H. Northrop, Y. R. Zheng, C. Ki-Whan and P. J. Stang, Acc. Chem. Res., 2009, 42, 1554–1563 CrossRef CAS PubMed.
- T. R. Cook, Y.-R. Zheng and P. J. Stang, Chem. Rev., 2013, 113, 734–777 CrossRef CAS PubMed.
- T. R. Cook and P. J. Stang, Chem. Rev., 2015, 115, 7001–7045 CrossRef CAS PubMed.
- Z. L. Gong, Z. Q. Li and Y. W. Zhong, Aggregate, 2022, 3, e177 CrossRef CAS.
- L. Chen, C. Chen, Y. Sun, S. Lu, H. Huo, T. Tan, A. Li, X. Li, G. Ungar, F. Liu and M. Zhang, Angew. Chem., Int. Ed., 2020, 132, 10143–10150 CrossRef PubMed.
- Y. F. Wang, J. W. Shi, J. H. Chen, W. G. Zhu and E. Baranoff, J. Mater. Chem. C, 2015, 3, 7993–8005 RSC.
- D. Zhao, H. He, X. Gu, L. Guo, K. S. Wong, J. W. Y. Lam and B. Z. Tang, Adv. Opt. Mater., 2016, 4, 534–539 CrossRef CAS.
- J. Han, S. Guo, H. Lu, S. Liu, Q. Zhao and W. Huang, Adv. Opt. Mater., 2018, 6, 1800538 CrossRef.
- Y. Wu, L. H. You, Z.-Q. Yu, J.-H. Wang, Z. Meng, Y. Liu, X.-S. Li, K. Fu, X.-K. Ren and B. Z. Tang, ACS Mater. Lett., 2020, 2, 505–510 CrossRef CAS.
- C. Cuerva, M. Cane and C. Lodeiro, Chem. Rev., 2021, 121, 12107–13010 CrossRef PubMed.
- B. Glettner, F. Liu, X. B. Zeng, M. Prehm, U. Baumeister, G. Ungar and C. Tschierske, Angew. Chem., Int. Ed., 2008, 47, 6080–6083 CrossRef CAS PubMed.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed.
- Z. Su, J. Huang, W. Shan, X. Y. Yan, R. Zhang, T. Liu, Y. Liu, Q. Y. Guo, F. Bian, X. Miao, M. Huang and S. Z. D. Cheng, CCS Chem., 2021, 3, 1434–1444 CrossRef CAS.
- S. Dou, S. Zhang, R. J. Klein, J. Runt and R. H. Colby, Chem. Mater., 2006, 18, 4288–4295 CrossRef CAS.
- K. Goossens, K. Lava, C. R. Bielawski and K. Binnemans, Chem. Rev., 2016, 116, 4643–4807 CrossRef CAS PubMed.
- B. Soberats, M. Yoshio, T. Ichikawa, H. Ohno and T. Kato, J. Mater. Chem. A, 2015, 3, 11232–11238 RSC.
- B. Soberats, M. Yoshio, T. Ichikawa, X. B. Zeng, H. Ohno, G. Ungar and T. Kato, J. Am. Chem. Soc., 2015, 137, 13212–13215 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc07318e |
| This journal is © The Royal Society of Chemistry 2025 |