Open Access Article
Open Access ArticlePeriodic law-guided design of highly stable O3-type layered oxide cathodes for practical sodium-ion batteries†
Yuan-Bo
Wu
ab,
Hai-Yan
Hu
*abc,
Jia-Yang
Li
c,
Hang-Hang
Dong
b,
Yan-Fang
Zhu
*ab,
Shuang-Qiang
Chen
ab,
Na-Na
Wang
c,
Jia-Zhao
Wang
 b and
Yao
Xiao
b and
Yao
Xiao
 *abd
*abd
aCollege of Chemistry and Materials Engineering, Wenzhou University, Wenzhou, 325035, P. R. China. E-mail: hyh927@uowmail.edu.au; yanfangzhu@wzu.edu.cn; xiaoyao@wzu.edu.cn
bWenzhou Key Laboratory of Sodium-Ion Batteries, Wenzhou University Technology Innovation Institute for Carbon Neutralization, Wenzhou, 325035, P. R. China
cInstitute for Superconducting and Electronic Materials, Australian Institute for Innovative Materials, University of Wollongong Innovation Campus, North Wollongong, NSW 2522, Australia
dKey Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), Nankai University, Tianjin, 300071, P. R. China
First published on 15th January 2025
Abstract
O3-type NaNi0.5Mn0.5O2 cathode material exhibits significant potential for sodium-ion batteries (SIBs) owing to its high theoretical capacity and ample sodium reservoir. Nonetheless, its practical implementation encounters considerable obstacles, such as impaired structural integrity, sensitivity to moisture, inadequate high-temperature stability, and being unstable under high-voltage conditions. This study investigates the co-substitution of Cu, Mg, and Ti, guided by principles of the periodic law, to enhance the material's stability under varying conditions. The substituent elements were selected based on their atomic properties and introduced into specific sites within the structure: Cu and Mg were substituted at Ni sites, while Ti replaced Mn sites. These modifications strengthened the crystal lattice, mitigating phase transitions, and improved electrochemical performance. The O3-type NaNi0.4Cu0.05Mg0.05Mn0.3Ti0.2O2 material exhibited remarkable moisture stability, maintaining 85% of its capacity after 1000 cycles at 5C in 2.0–4.0 V. It also exhibited reversible phase transitions at voltages up to 4.3 V, with no oxygen release observed even when charged to 4.5 V. Furthermore, it exhibited remarkable high-temperature stability in half-cell testing and excellent cycling performance in full-cell evaluations. These results are very helpful for designing high-performance SIB cathodes that can withstand a variety of operating circumstances and ensuring structural stability.
Introduction
Sodium-ion batteries (SIBs) have emerged as a plausible and affordable alternative to lithium-ion batteries.1–3 Their appeal lies in the plentiful and evenly distributed nature of sodium resources relative to lithium, which bodes well for large-scale, low-cost energy storage.4,5 In addition, SIBs have greater safety thanks to the more stable electrochemical behaviours of sodium, reducing risks such as thermal runaway.6 It is for these reasons that SIBs are particularly fascinating in areas where reliable and secure energy is required, emphasizing both cost-effectiveness and material abundance.7 Nonetheless, amidst these promising aspects, the development of efficient SIBs in turn depends largely on the breakthroughs in high-performance cathode materials. This is the key to determining energy density, life span, and safety like yet a viable, less expensive and lower-risk candidate for today's batteries.8,9Currently, O3-type NaNi0.5Mn0.5O2 (NaNM) is acknowledged as a promising candidate for SIB cathodes due to its impressive capacity and cycle stability, along with its relatively simple synthesis process.10 These attributes make it a strong contender for Na+ storage applications.11 However, NaNM suffers from a number of limitations that place obstacles in the way of practical application. These include the following: (a) structural instability: NaNM undergoes multiple phase transitions  , which cause severe distortion of the crystal structure during Na+ extraction and insertion.12 This leads to problems such as grain cracking, voltage hysteresis and reduction in capacity durability.13 Repetition of these charge–discharge cycles aggravates these problems, and high-voltage cycling further accelerates the inevitable phase changes and lattice distortions that damage the long-term stability of the material.14,15 (b) Moisture sensitivity: NaNM is highly sensitive to moisture, which results in surface degradation.16 The reaction of moisture with NaNM leads to production of unwanted by-products, such as NaOH and Na2CO3, on the particle surfaces.17 These increase interfacial resistance, even further lowering the electrical performance of the material, reducing the chances that it will work well in real-world settings.18 (c) Poor high-temperature performance: NaNM's performance deteriorates at elevated temperatures due to its sensitivity to thermal fluctuations, which affect the stability of the crystal lattice. Prolonged exposure at high temperatures speeds up phase transitions and introduces distortions to the lattice, which in turn affect the material's structural integrity.19 After that, the material's durability and performance at high temperature fall off as well. While NaNM holds significant potential as a SIB cathode material, addressing these challenges through further material optimization is crucial for its practical application in commercial energy storage solutions.20
, which cause severe distortion of the crystal structure during Na+ extraction and insertion.12 This leads to problems such as grain cracking, voltage hysteresis and reduction in capacity durability.13 Repetition of these charge–discharge cycles aggravates these problems, and high-voltage cycling further accelerates the inevitable phase changes and lattice distortions that damage the long-term stability of the material.14,15 (b) Moisture sensitivity: NaNM is highly sensitive to moisture, which results in surface degradation.16 The reaction of moisture with NaNM leads to production of unwanted by-products, such as NaOH and Na2CO3, on the particle surfaces.17 These increase interfacial resistance, even further lowering the electrical performance of the material, reducing the chances that it will work well in real-world settings.18 (c) Poor high-temperature performance: NaNM's performance deteriorates at elevated temperatures due to its sensitivity to thermal fluctuations, which affect the stability of the crystal lattice. Prolonged exposure at high temperatures speeds up phase transitions and introduces distortions to the lattice, which in turn affect the material's structural integrity.19 After that, the material's durability and performance at high temperature fall off as well. While NaNM holds significant potential as a SIB cathode material, addressing these challenges through further material optimization is crucial for its practical application in commercial energy storage solutions.20
In response to the shortcomings of NaNM, several strategies have been suggested, including those involving surface coatings, ion substitution, and modifications to the structure of crystals.21–23 Among these strategies, ion substitution has shown promising potential for improving material stability. Therefore, this work examines the strategic co-substitution within the O3-type NaNM structure of Cu, Mg, and Ti guided by principles periodic law in order to get materials that are more stable. Co-substitution in practice reinforces the material's structure and helps to suppress phase transitions, particularly under high-charge conditions. The choice of Cu, Mg, and Ti as substitution elements was based on a detailed analysis of their atomic orbital configurations, electron arrangement, ionic radii and charge state.24 These properties were carefully considered to optimize their effects on the host structure and to determine the appropriate substitution sites within the material. Optimization of these properties allows the co-substitution strategy to address both the structural and electrochemical challenges implicit in NaNM. Specifically, substituting Cu and Mg for Ni enhances the material by suppressing irreversible phase transitions and improving structural stability. Substituting Mn with Ti will enhance the stability by increasing high-voltage stability and stabilizing lattice oxygen. These synergistic effects together give the material moisture stability, high voltage stability, structural stability, and high-temperature stability. The O3-NaNi0.4Cu0.05Mg0.05Mn0.3Ti0.2O2 (NaNCMMT) material demonstrates excellent moisture stability, achieving 85% capacity retention after 1000 cycles at 5C in 2–4 V, with completely reversible phase transitions during cycling. Even under extended voltage conditions up to 4.3 V, the phase transitions remain reversible. Notably, there is no oxygen release observed even when it is charged to 4.5 V. The cathode also exhibits strong high-temperature stability, demonstrating favorable capacity retention. In full-cell evaluations, it shows outstanding cycling stability and reversibility, even without pre-sodiation. This study provides scientific insights into tuning the structure and electrochemical balance of sodium-layered oxides to support the systematic design of sodium-ion cathodes with enhanced performance under diverse practical conditions.
Results and discussion
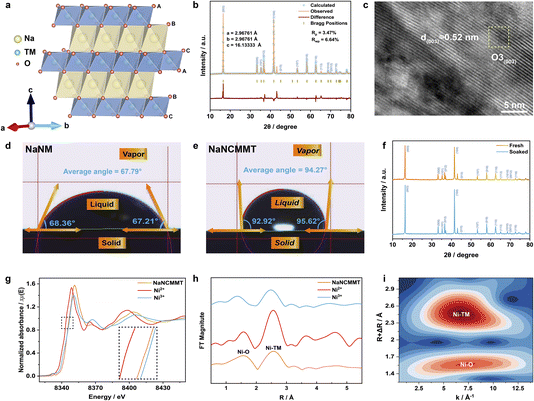
To enhance the electrochemical performance of O3-type layered NaNM cathodes, the strategic selection of substituent elements is essential.25,26 The principle that structure dictates properties guided the choice of Cu, Mg, and Ti, leveraging their periodic characteristics.27,28 The elemental properties, such as electronic configurations, atomic radii, and predominant oxidation states, display periodic trends due to the structured variation in electron arrangements as nuclear charge increases.29–31 This periodicity stems from the fundamental arrangement of electrons outside the atomic nucleus, influencing how atoms and their resulting ions interact with surrounding environments.32,33 As shown in Fig. 1a, Cu and Mg were selected for substitutions at the Ni sites based on similarities in electron configuration and ionic radius. Both Cu2+ and Mg2+ share an ionic radius of approximately 72 pm and exhibit a consistent +2 charge state, which helps maintain structural stability within the NaNM framework. Additionally, Ti was chosen to occupy Mn sites due to comparable ionic properties, supporting uniform distribution without substantial lattice distortion.34 The similar electron cloud densities and shapes of these substituent elements—such as spherically symmetric s-orbitals or p-orbitals—were also considered (Fig. 1b). These orbitals affect how the electrons contribute to structural stability and ionic conductivity.35 From a microscale perspective, electrons move rapidly within confined atomic spaces (diameters around 10−10 m) with unpredictable trajectories, characterized as electron clouds. This understanding emphasizes the significance of electron probability density in assessing substituent elements' behavior. For instance, spherical s-orbitals distribute electron density evenly, while p- and d-orbitals create specific, directional bonding effects. The nuanced orbital interactions of Cu, Mg, and Ti ensure tailored modifications to NaNM's electronic structure and ion diffusion pathways. This methodical selection and placement of substituent elements enable substantial advancements in material performance, aligning with the desired electrochemical enhancements.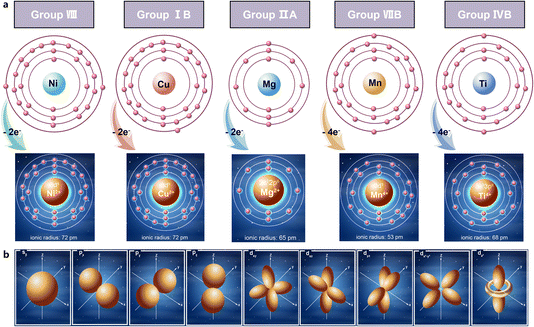 | ||
| Fig. 1 (a) The atomic orbital configurations and outer electron arrangements of Ni, Cu, Mg, Mn, Ti. (b) Schematic of the distribution of s, p, d atomic orbital types. | ||
The crystal structure of NaNCMMT was analyzed using powder X-ray diffraction (XRD) with Rietveld refinement, as illustrated in Fig. 2a and b.36 The XRD results confirmed that the material exhibits a hexagonal O3 phase with an R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group, indicating the successful synthesis of the O3-type layered structure.37 The detailed structural parameters, such as lattice constants (a = 2.96761 Å, b = 2.96761 Å, c = 16.13333 Å), and the low values of Rp and Rwp, signify a strong correlation between the experimental and calculated patterns, validating the accuracy of the crystallographic data.38 The morphology of NaNCMMT was further examined using scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The SEM image (Fig. S1, ESI†) revealed that NaNCMMT exhibits chunk-like morphology. High-resolution TEM (HR-TEM) images (Fig. 2c; and S2, ESI†) provided detailed information about the crystalline facets, showing interplanar lattice spacings of 0.52 nm, which correspond to the (003) planes of the O3 phase.39 The HR-TEM analysis corroborates the XRD findings, confirming the well-defined O3 structure of NaNCMMT. Besides, inductively coupled plasma mass spectrometry (ICP-MS) analysis of the sample, as shown in Table S1, ESI† confirms the successful synthesis of the NaNCMMT cathode material. The wettability of NaNCMMT was assessed by analyzing the three-phase interaction among gas, solid, and liquid. As shown in Fig. S3a, ESI†, the interfacial tensions between solid–vapor (γSV), solid–liquid (γSL), and liquid–vapor (γLV) were considered, and Young's equation, γLV
m space group, indicating the successful synthesis of the O3-type layered structure.37 The detailed structural parameters, such as lattice constants (a = 2.96761 Å, b = 2.96761 Å, c = 16.13333 Å), and the low values of Rp and Rwp, signify a strong correlation between the experimental and calculated patterns, validating the accuracy of the crystallographic data.38 The morphology of NaNCMMT was further examined using scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The SEM image (Fig. S1, ESI†) revealed that NaNCMMT exhibits chunk-like morphology. High-resolution TEM (HR-TEM) images (Fig. 2c; and S2, ESI†) provided detailed information about the crystalline facets, showing interplanar lattice spacings of 0.52 nm, which correspond to the (003) planes of the O3 phase.39 The HR-TEM analysis corroborates the XRD findings, confirming the well-defined O3 structure of NaNCMMT. Besides, inductively coupled plasma mass spectrometry (ICP-MS) analysis of the sample, as shown in Table S1, ESI† confirms the successful synthesis of the NaNCMMT cathode material. The wettability of NaNCMMT was assessed by analyzing the three-phase interaction among gas, solid, and liquid. As shown in Fig. S3a, ESI†, the interfacial tensions between solid–vapor (γSV), solid–liquid (γSL), and liquid–vapor (γLV) were considered, and Young's equation, γLV![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos(¢) = γSV − γSL, was applied to determine the contact angle.40 The measured wetting angle for NaNCMMT was 94.27°, indicating hydrophobic behavior, while NaNM exhibited a wetting angle of 67.79°, reflecting hydrophilic properties (Fig. 2d, e; and Tables S2, S3, ESI†).41 The hydrophobic nature of NaNCMMT suggests that its surface repels water, thereby reducing interaction with moisture and preventing penetration. The material's stability was further confirmed through soaking experiments. The consistent XRD patterns before and after soaking (Fig. 2f) demonstrate that NaNCMMT maintains its structural integrity, with robust metal–oxygen bonds that resist hydrolysis and moisture-induced degradation. Furthermore, the infrared spectroscopy (IR) analysis of the material post-soaking only exhibited weak CO32− peaks, indicating minimal surface reaction and strong chemical stability in aqueous conditions (Fig. S3b, ESI†).42,43 These findings suggest that the material's intrinsic structural stability, combined with its hydrophobic surface properties, effectively prevents oxidation and ensures durability in moist environments. To explore the influence of Cu, Mg, and Ti co-substitution on the valence states of elements within NaNCMMT, X-ray photoelectron spectroscopy (XPS) was employed, as presented in Fig. S4, ESI†. The weak satellite peaks observed in Fig. S4c, ESI† suggest the possible presence of both divalent and trivalent nickel.44 This conclusion is consistent with the X-ray absorption near-edge structure (XANES) spectra at the Ni K-edge (Fig. 2g), which provide complementary evidence for the divalent and trivalent states of Ni after co-substitution.45 Further structural insights were gained from Fourier transform of extended X-ray absorption fine structure (FT-EXAFS) and wavelet transform EXAFS (WT-EXAFS). These analyses clearly visualized the Ni–O and Ni-transition metal (TM) bonds, providing a deeper understanding of the local coordination environment and the effects of co-substitution on the Ni sites (Fig. 2h and i).
cos(¢) = γSV − γSL, was applied to determine the contact angle.40 The measured wetting angle for NaNCMMT was 94.27°, indicating hydrophobic behavior, while NaNM exhibited a wetting angle of 67.79°, reflecting hydrophilic properties (Fig. 2d, e; and Tables S2, S3, ESI†).41 The hydrophobic nature of NaNCMMT suggests that its surface repels water, thereby reducing interaction with moisture and preventing penetration. The material's stability was further confirmed through soaking experiments. The consistent XRD patterns before and after soaking (Fig. 2f) demonstrate that NaNCMMT maintains its structural integrity, with robust metal–oxygen bonds that resist hydrolysis and moisture-induced degradation. Furthermore, the infrared spectroscopy (IR) analysis of the material post-soaking only exhibited weak CO32− peaks, indicating minimal surface reaction and strong chemical stability in aqueous conditions (Fig. S3b, ESI†).42,43 These findings suggest that the material's intrinsic structural stability, combined with its hydrophobic surface properties, effectively prevents oxidation and ensures durability in moist environments. To explore the influence of Cu, Mg, and Ti co-substitution on the valence states of elements within NaNCMMT, X-ray photoelectron spectroscopy (XPS) was employed, as presented in Fig. S4, ESI†. The weak satellite peaks observed in Fig. S4c, ESI† suggest the possible presence of both divalent and trivalent nickel.44 This conclusion is consistent with the X-ray absorption near-edge structure (XANES) spectra at the Ni K-edge (Fig. 2g), which provide complementary evidence for the divalent and trivalent states of Ni after co-substitution.45 Further structural insights were gained from Fourier transform of extended X-ray absorption fine structure (FT-EXAFS) and wavelet transform EXAFS (WT-EXAFS). These analyses clearly visualized the Ni–O and Ni-transition metal (TM) bonds, providing a deeper understanding of the local coordination environment and the effects of co-substitution on the Ni sites (Fig. 2h and i).
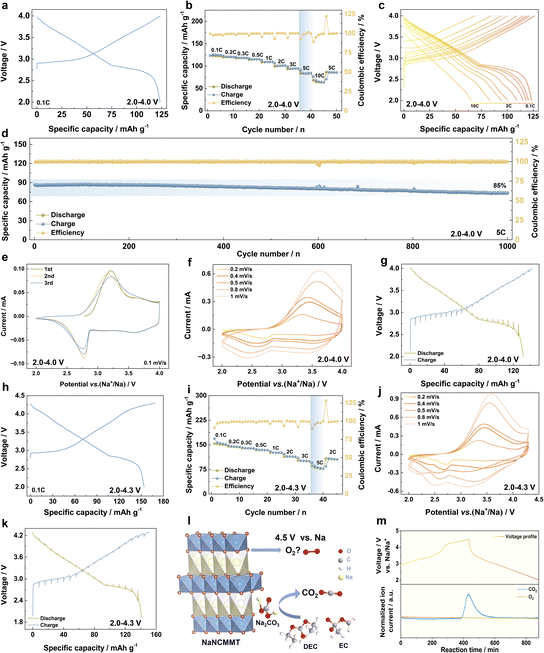
The Na+ storage performance of the NaNCMMT cathode was systematically assessed using coin-type half-cells with a Na metal anode.46,47 At a voltage window of 2.0–4.0 V, the NaNCMMT cathode demonstrated a reversible capacity of 123.43 mA h g−1 at 0.1C (12 mA g−1, Fig. 3a). Furthermore, it exhibited excellent rate performance, retaining 68.3% of its initial capacity at 5C compared to 0.1C (Fig. 3b and c). The cathode's impressive cycling stability was evident with 85% capacity retention after 1000 cycles at 5C (Fig. 3d), and consistent retention rates of 93.4% and 98.1% after 300 cycles at 1C and 100 cycles at 2C, respectively (Fig. S5, ESI†). Cyclic voltammetry (CV) at 0.1 mV s−1 revealed peaks that aligned well with the galvanostatic charge–discharge profiles, suggesting the absence of complex phase transitions and highlighting stable electrochemical characteristics (Fig. 3e).48,49 The study of Na+ transport kinetics using CV scans at different rates highlighted a primarily capacitive sodium storage mechanism, as indicated by a calculated b-value of 0.85 (Fig. 3f; S6a and b, ESI†). The galvanostatic intermittent titration technique (GITT) analysis supported this finding, with minimal ohmic (13.7 mV) and voltage polarization (77.9 mV) observed, signifying efficient ionic transport (Fig. 3g; S6c and d, ESI†).50 To further understand the material's performance across a wider voltage range, tests were extended to 2.0–4.3 V.51 In this range, the NaNCMMT cathode achieved a higher reversible capacity of 153.9 mA h g−1 at 0.1C (16 mA g−1, Fig. 3h). Even under high-rate conditions, the capacity retention was steady, maintaining 66.3% at 5C (Fig. 3i; S7a, ESI†) and 64.4% after 100 cycles at 2C (Fig. S7b, ESI†). Consistent with previous observations, CV curves at 0.1 mV s−1 aligned with the charge–discharge profiles (Fig. S7c, ESI†), and the Na+ kinetics analysis yielded a b-value of 0.93, further confirming the dominant capacitive mechanism (Fig. 3j; S8a and b, ESI†). GITT analysis showed low ohmic (26.2 mV) and voltage polarization (85.8 mV, Fig. 3k; S8c and d, ESI†), emphasizing stable electrochemical performance. The extended voltage window testing provided crucial insights into the high-voltage performance of the layered oxide cathode.52 As illustrated in the schematic (Fig. 3l), charging up to 4.5 V induces oxygen release from the structure due to intensified overlap and electronic interactions between the O 2p orbitals and TM 3d orbitals at elevated oxidation states. This interaction redistributes electron density away from the oxygen atoms, weakening the TM–O bonds, destabilizing the lattice, and ultimately facilitating lattice oxygen release.53 This reflects the intrinsic instability of layered oxides at elevated potentials, where extensive Na extraction and overlapping electronic states promote oxygen oxidation.54 Additionally, the observed CO2 release predominantly originated from electrolyte decomposition rather than from the cathode material itself.55 Differential electrochemical mass spectrometry (DEMS) characterized the gas evolution, showing negligible O2 outgassing but significant CO2 emission (Fig. 3m). The electrochemical performance of the NaNCMMT cathode is shown in Fig. S9 (ESI†). Even when charged to 4.5 V, the cathode retains 62.4% of its capacity at 2C. Additionally, at 0.2C, the material maintains 75.3% of its capacity after 100 cycles, demonstrating excellent stability. Post-cycling SEM images reveal that the electrode morphology remains consistent with that of the pristine state, without any visible cracks (Fig. S10a–c, ESI†). Elemental distribution analysis using energy dispersive spectroscopy (EDS) confirms the uniform dispersion of Na, Ni, Cu, Mg, Mn, Ti, and O within the material (Fig. S10d, ESI†). These findings highlight the robustness of lattice oxygen in the NaNCMMT cathode under high-voltage conditions, providing valuable insights into its electrochemical stability and long-term durability.56
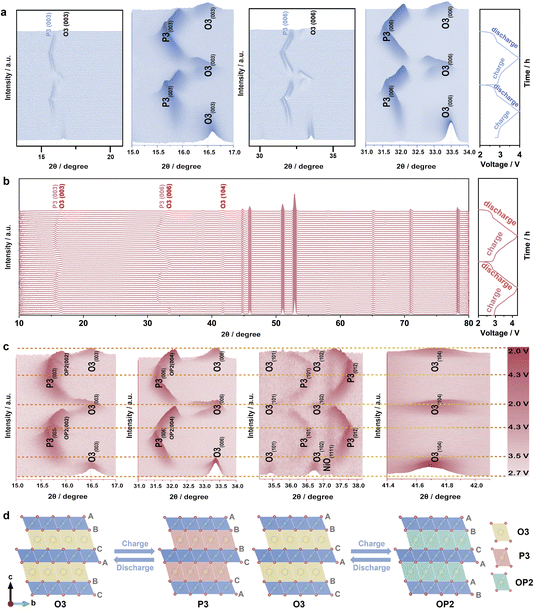
In situ XRD analyses were employed to investigate the crystal structure evolution of the NaNCMMT electrode during cycling in the voltage window of 2.0–4.0 V (Fig. 4a).57–60 The results revealed a distinct phase-change process, which was captured through 3D contour graphs and intensity contour maps (Fig. S11a and S12a, ESI†). As Na+ ions were extracted from the electrode, the (003) peak of the P3 phase appeared, signaling the transition from the O3 phase to the P3 phase.61 Concurrently, a clear reduction in the (104) peaks associated with the O3 phase was observed, indicating a phase transition from O3 to P3. During Na+ re-intercalation, the diffraction peaks reversed their trends, aligning well with the observed charge–discharge profiles. Notably, the second cycle mirrored the phase behavior of the initial one, affirming the reversibility of the O3 → P3 phase transition.62,63 The voltage window was extended to 2.0–4.3 V to examine structural stability under more extreme conditions (Fig. 4b, c; and S11b, S12b, ESI†). Remarkably, the phase transition behavior observed in the 2.0–4.3 V range differed from that in the 2.0–4.0 V range due to the emergence of the OP2 phase. This phase, characterized by distinctive diffraction peaks associated with a mixed O–P stacking structure, became evident as Na+ extraction progressed further. The extended voltage window triggered this additional transition, underscoring the electrode's capacity to accommodate significant structural changes. This reliable behavior suggests that Cu, Mg, and Ti co-substitution induces a fully reversible phase transition (O3 → P3 → OP2), as depicted more clearly in the crystal structure change diagram during cycling in 2.0–4.3 V (Fig. 4d). The incorporation of these co-substituent elements effectively stabilizes the crystal structure, enhancing the electrode's electrochemical performance by mitigating phase instability and reducing irreversible transitions.
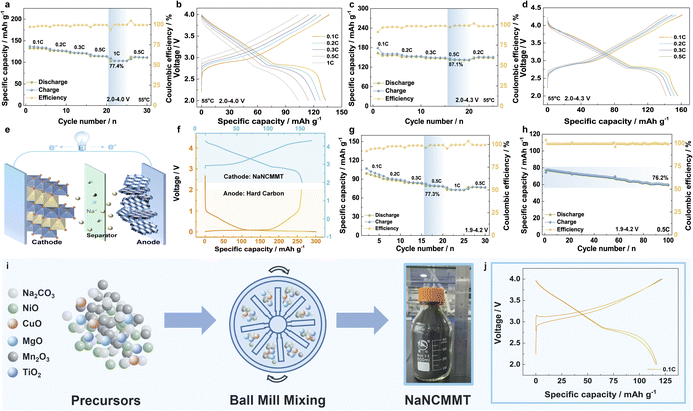
High-temperature testing is crucial for evaluating the practical performance and stability of the NaNCMMT cathode.64,65 The electrochemical properties of the sodium half-cell were assessed at 55 °C, a temperature known to induce structural changes in layered oxide cathodes, such as lattice destabilization and accelerated phase transitions, which can significantly affect the electrochemical stability. The analysis focused on two voltage ranges, 2.0–4.0 V and 2.0–4.3 V, providing a comprehensive assessment of the material's performance under high-temperature conditions. These conditions were selected to explore the material's behavior under realistic, thermally elevated scenarios, providing insights into its potential for high-temperature applications.66 The rate performance data (Fig. 5a and b) revealed that, at 2.0–4.0 V, the NaNCMMT electrode retained 77.4% of its capacity when cycled at 1C compared to 0.1C. The NaNCMMT cathode exhibited a high reversible capacity of 132.8 mA h g−1 at 0.1C, which was notably greater than the capacity measured at room temperature. This enhancement in capacity under elevated temperature conditions is attributed to improved electrochemical reaction kinetics at the electrode–electrolyte interface.67 Specifically, the increase in temperature accelerates charge and discharge processes, facilitating faster ion transport and more efficient electrochemical activity. Despite these gains, the cycling stability remained satisfactory, with a 73.9% retention rate after 100 cycles at 0.5C (Fig. S13a and b, ESI†). Expanding the voltage window to 2.0–4.3 V further demonstrated the material's steady performance. At 0.5C, the NaNCMMT electrode displayed an impressive 87.1% capacity retention compared to that at 0.1C, with cycle retention reaching 74.8% after 50 cycles at 0.2C (Fig. 5c and d; S13c, ESI†). The high-temperature testing confirmed that the Cu, Mg, and Ti co-doped NaNCMMT cathode maintains excellent electrochemical performance even under extreme conditions possibly due to the suppression of the Jahn–Teller effect by Ti substitution, thereby mitigating Mn dissolution. To investigate the cathode's practical application potential, a full cell was constructed pairing NaNCMMT as the cathode with hard carbon (HC) as the anode.68Fig. 5e illustrates schematically the operation of the full battery, and Fig. 5f presents the independent voltage distributions of the HC anode and NaNCMMT cathode in half-cells. Without pre-sodiation of the anode, the cell exhibited a specific capacity of 77.91 mA h g−1 within the 1.9–3.9 V range at 0.1C, maintaining 80.1% of its capacity at 0.5C compared to that at 0.1C (Fig. S14a and b, ESI†). Even after 100 cycles at 0.5C, the capacity retention was 77.6% (Fig. S14c, ESI†). Extending the voltage window to 1.9–4.2 V improved capacity to 102.72 mA h g−1, with a retention rate of 77.3% at 0.5C compared to that at 0.1C and 76.2% after 100 cycles (Fig. 5g and h; S14d, ESI†). To further validate the practicality of the NaNCMMT cathode, the production process for industrial-grade NaNCMMT was examined (Fig. 5i; S15, ESI†).69–71 Key characterizations reveal that the industrial-grade NaNCMMT exhibits a particle size distribution centered around 6.75 μm (Fig. S16a, ESI†), and shows uniform elemental distribution of Na, Ni, Cu, Mg, Mn, Ti, and O as confirmed by EDS mapping (Fig. S16b, ESI†). Although the electrochemical performance of the industrial-grade NaNCMMT is slightly reduced compared to lab-scale samples, it retains significant application potential. Performance could be further improved through optimizations like electrolyte adjustments or surface modifications (Fig. 5j; and S17, ESI†).
Conclusions
In summary, the Cu–Mg–Ti co-substitution strategy, guided by the principles of the periodic law, significantly improves the stability and electrochemical performance of O3-NaNi0.5Mn0.5O2 cathodes, addressing key challenges such as structural instability, moisture sensitivity, and high-temperature degradation. The optimized material, O3-NaNi0.4Cu0.05Mg0.05Mn0.3Ti0.2O2, benefits from the synergistic effects of substituting Cu and Mg for Ni and Ti for Mn. Substituting Ni sites with Cu and Mg helps suppress irreversible phase transitions and enhance structural stability. Meanwhile, Mn site replacement with Ti not only enhances the material's high-voltage stability but also stabilizes the lattice oxygen (Scheme 1). These improvements in structure contribute to the material's excellent electrochemical performance: after 1000 cycles at 5C within the 2–4 V window, it retains 85% of its capacity, with fully reversible phase transitions. Additionally, the material is also highly resistant to moisture, maintaining a stable structure even in humid conditions. Moreover, it exhibits outstanding high-voltage stability. During charge–discharge cycling at a terminal voltage of 4.5 V, no oxygen release was observed, nor were any cracks detected in its morphology. Furthermore, the cathode exhibits excellent high-temperature stability in a half-cell and shows promising performance in a full cell, even without pre-sodiation. These findings illustrate the effectiveness of a periodic law-guided co-substitution approach in improving both stability and performance for sodium-ion cathodes, thereby offering valuable insights on how to design materials that will satisfy the demanding conditions typically experienced by systems for practical energy storage.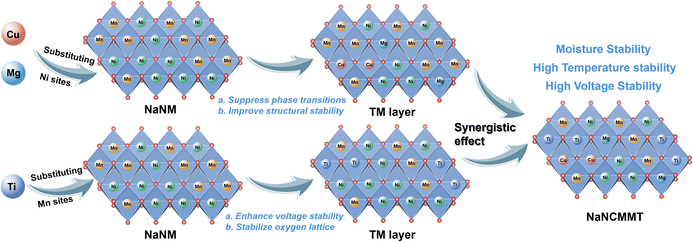 | ||
| Scheme 1 Synergistic effects of Cu/Mg substitution for Ni and Ti substitution for Mn in NaNCMMT cathode. | ||
Data availability
Essential data are fully provided in the main text and ESI.†Author contributions
Yuan-Bo Wu: conceptualization, data curation, writing-original draft. Hai-Yan Hu: conceptualization, methodology, data analysis, manuscript revision. Jia-Yang Li: software, data curation. Hang-Hang Dong: methodology, data curation. Yan-Fang Zhu: project administration. Shuang-Qiang Chen: technical support. Na-Na Wang, Jia-Zhao Wang: resources. Yao Xiao: supervision, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Key R&D Program of China (2024YFA1211900), the National Natural Science Foundation of China (52402301, 52472240, 52202284), the Natural Science Foundation of Zhejiang Province (LQ23E020002), the Wenzhou Key Scientific and Technological Innovation Research Project (ZG2023053), the China Scholarship Council (202106370062), the Doctoral Innovation Foundation of Wenzhou University (3162023001001), and the Australia Research Council (DP240102926, FT240100596).References
- C. Vaalma, D. Buchholz, M. Weil and S. Passerini, Nat. Rev. Mater., 2018, 3, 18013 CrossRef.
- J. Wang, Y. F. Zhu, Y. Su, J. X. Guo, S. Chen, H. K. Liu, S. X. Dou, S. L. Chou and Y. Xiao, Chem. Soc. Rev., 2024, 53, 4230–4301 RSC.
- D. Eum, B. Kim, J.-H. Song, H. Park, H.-Y. Jang, S. J. Kim, S.-P. Cho, M. H. Lee, J. H. Heo, J. Park, Y. Ko, S. K. Park, J. Kim, K. Oh, D.-H. Kim, S. J. Kang and K. Kang, Nat. Mater., 2022, 21, 664–672 CrossRef CAS PubMed.
- Y. Su, B. Johannessen, S. Zhang, Z. Chen, Q. Gu, G. Li, H. Yan, J. Y. Li, H. Y. Hu, Y. F. Zhu, S. Xu, H. Liu, S. Dou and Y. Xiao, Adv. Mater., 2023, 35, e2305149 CrossRef PubMed.
- H. Fu, Y.-P. Wang, G. Fan, S. Guo, X. Xie, X. Cao, B. Lu, M. Long, J. Zhou and S. Liang, Chem. Sci., 2022, 13, 726–736 RSC.
- X. Wang, Q. Zhang, C. Zhao, H. Li, B. Zhang, G. Zeng, Y. Tang, Z. Huang, I. Hwang, H. Zhang, S. Zhou, Y. Qiu, Y. Xiao, J. Cabana, C.-J. Sun, K. Amine, Y. Sun, Q. Wang, G.-L. Xu, L. Gu, Y. Qiao and S.-G. Sun, Nat. Energy, 2024, 9, 184–196 CrossRef CAS.
- Y. Tang, Q. Zhang, W. Zuo, S. Zhou, G. Zeng, B. Zhang, H. Zhang, Z. Huang, L. Zheng, J. Xu, W. Yin, Y. Qiu, Y. Xiao, Q. Zhang, T. Zhao, H.-G. Liao, I. Hwang, C.-J. Sun, K. Amine, Q. Wang, Y. Sun, G.-L. Xu, L. Gu, Y. Qiao and S.-G. Sun, Nat. Sustain., 2024, 7, 348–359 CrossRef.
- J. Y. Li, H. Y. Hu, L. F. Zhou, H. W. Li, Y. J. Lei, W. H. Lai, Y. M. Fan, Y. F. Zhu, G. Peleckis, S. Q. Chen, W. K. Pang, J. Peng, J. Z. Wang, S. X. Dou, S. L. Chou and Y. Xiao, Adv. Funct. Mater., 2023, 33, 2213215 CrossRef CAS.
- Y. J. Guo, R. X. Jin, M. Fan, W. P. Wang, S. Xin, L. J. Wan and Y. G. Guo, Chem. Soc. Rev., 2024, 53, 7828–7874 RSC.
- G. T. Park, N. Y. Park, H. H. Ryu, H. H. Sun, J. Y. Hwang and Y. K. Sun, Chem. Soc. Rev., 2024 10.1039/d3cs01110k.
- Q. Wang, D. Zhou, C. Zhao, J. Wang, H. Guo, L. Wang, Z. Yao, D. Wong, G. Schuck, X. Bai, J. Lu and M. Wagemaker, Nat. Sustain., 2024, 7, 338–347 CrossRef.
- H. Y. Hu, H. Wang, Y. F. Zhu, J. Y. Li, Y. Liu, J. Wang, H. X. Liu, X. B. Jia, H. Li, Y. Su, Y. Gao, S. Chen, X. Wu, S. X. Dou, S. Chou and Y. Xiao, ACS Nano, 2023, 17, 15871–15882 CrossRef CAS PubMed.
- Y. Yu, Q. Mao, D. Wong, R. Gao, L. Zheng, W. Yang, J. Yang, N. Zhang, Z. Li, C. Schulz and X. Liu, J. Am. Chem. Soc., 2024, 146, 22220–22235 CrossRef CAS PubMed.
- D. Yang, X. W. Gao, G. Gao, Q. Lai, T. Ren, Q. Gu, Z. Liu and W. B. Luo, Carbon Energy, 2024, 6, e574 CrossRef CAS.
- L. Qiu, M. Zhang, Y. Song, Z. Wu, Y. F. Zhu, J. Zhang, D. Wang, H. Y. Hu, H. W. Li, H. R. Liu, X. B. Jia, J. Peng, S. Chen, Z. Yang, Y. Xiao and X. Guo, Carbon Energy, 2022, 5, e298 CrossRef.
- S. Gao, Z. Zhu, H. Fang, K. Feng, J. Zhong, M. Hou, Y. Guo, F. Li, W. Zhang, Z. Ma and F. Li, Adv. Mater., 2024, 36, e2311523 CrossRef PubMed.
- Y.-F. Liu, H.-Y. Hu, J.-Y. Li, H. Wang, Y. Zhao, J. Wang, Y.-B. Wu, Y.-J. Li, G.-Y. Zhang, Q.-Q. Sun, Y.-F. Zhu, R.-R. Tang, X.-W. Wu, J.-Z. Wang, S.-X. Dou, S.-L. Chou and Y. Xiao, Sci. China:Chem., 2024, 67, 4242–4250 CrossRef CAS.
- J. Li, H. Hu, J. Wang and Y. Xiao, Carbon Neutralization, 2022, 1, 96–116 CrossRef.
- Z. Li, H. Yi, H. Ren, J. Fang, Y. Du, W. Zhao, H. Chen, Q. Zhao and F. Pan, Adv. Funct. Mater., 2023, 33, 2307913 CrossRef CAS.
- Y. Xiao, H. R. Wang, H. Y. Hu, Y. F. Zhu, S. Li, J. Y. Li, X. W. Wu and S. L. Chou, Adv. Mater., 2022, 34, e2202695 CrossRef PubMed.
- Z. Xu, K. Song, X. Chang, L. Li, W. Zhang, Y. Xue, J. Zhang, D. Lin, Z. Liu, Q. Wang, Y. Yu and C. Yang, Carbon Neutralization, 2024, 3, 832–856 CrossRef.
- S. He, X. Shen, M. Han, Y. Liao, L. Xu, N. Yang, Y. Guo, B. Li, J. Shen, C. Zha, Y. Li, M. Wang, L. Wang, Y. Su and F. Wu, ACS Nano, 2024, 18, 11375–11388 CrossRef CAS PubMed.
- Y. F. Liu, K. Han, D. N. Peng, L. Y. Kong, Y. Su, H. W. Li, H. Y. Hu, J. Y. Li, H. R. Wang, Z. Q. Fu, Q. Ma, Y. F. Zhu, R. R. Tang, S. L. Chou, Y. Xiao and X. W. Wu, InfoMat, 2023, 5, e12422 CrossRef CAS.
- H. Dong, H. Liu, Y. J. Guo, Y. H. Feng, X. Zhu, S. W. Xu, F. Sui, L. Yu, M. Liu, J. Z. Guo, Y. X. Yin, B. Xiao, X. L. Wu, Y. G. Guo and P. F. Wang, J. Am. Chem. Soc., 2024, 146, 22335–22347 CrossRef CAS PubMed.
- X.-Y. Zhang, H.-Y. Hu, X.-Y. Liu, J. Wang, Y.-F. Liu, Y.-F. Zhu, L.-Y. Kong, Z.-C. Jian, S.-L. Chou and Y. Xiao, Nano Energy, 2024, 128, 109905 CrossRef CAS.
- C. Zhao, Q. Wang, Z. Yao, J. Wang, B. Sanchez-Lengeling, F. Ding, X. Qi, Y. Lu, X. Bai, B. Li, H. Li, A. Aspuru-Guzik, X. Huang, C. Delmas, M. Wagemaker, L. Chen and Y.-S. Hu, Science, 2020, 370, 708 CrossRef CAS PubMed.
- P. F. Wang, H. R. Yao, X. Y. Liu, J. N. Zhang, L. Gu, X. Q. Yu, Y. X. Yin and Y. G. Guo, Adv. Mater., 2017, 29, 1700210 CrossRef PubMed.
- H. R. Yao, P. F. Wang, Y. Gong, J. Zhang, X. Yu, L. Gu, C. OuYang, Y. X. Yin, E. Hu, X. Q. Yang, E. Stavitski, Y. G. Guo and L. J. Wan, J. Am. Chem. Soc., 2017, 139, 8440–8443 CrossRef CAS PubMed.
- D. A. Edelman, D. Eum and W. C. Chueh, Nat. Sustain., 2024, 7, 234–235 CrossRef.
- Y. Huang, Q. Zhang, X. G. Sun, K. Liu, W. Sun, M. Zhi, Y. Guo, S. Zheng and S. Dai, Angew. Chem., Int. Ed., 2024, 136, e202406277 CrossRef.
- H. Wang, K. Lai, F. Guo, B. Long, X. Zeng, Z. Fu, X. Wu, Y. Xiao, S. Dou and J. Dai, Carbon Neutralization, 2022, 1, 59–67 CrossRef.
- L.-Y. Kong, J.-Y. Li, H.-X. Liu, Y.-F. Zhu, J. Wang, Y. Liu, X.-Y. Zhang, H.-Y. Hu, H. Dong, Z.-C. Jian, C. Cheng, S. Chen, L. Zhang, J.-Z. Wang, S. Chou and Y. Xiao, J. Am. Chem. Soc., 2024, 146, 32317–32332 CrossRef CAS PubMed.
- S. Huang, Y. Sun, T. Yuan, H. Che, Q. Zheng, Y. Zhang, P. Li, J. Qiu, Y. Pang, J. Yang, Z. F. Ma and S. Zheng, Carbon Neutralization, 2024, 3, 584–596 CrossRef CAS.
- T. Zhang, M. Ren, Y. Huang, F. Li, W. Hua, S. Indris and F. Li, Angew. Chem., Int. Ed., 2024, 63, e202316949 CrossRef CAS PubMed.
- X. B. Jia, J. Wang, Y. F. Liu, Y. F. Zhu, J. Y. Li, Y. J. Li, S. L. Chou and Y. Xiao, Adv. Mater., 2024, 36, e2307938 CrossRef PubMed.
- K. Momma and F. Izumi, J. Appl. Crystallogr., 2011, 44, 1272–1276 CrossRef CAS.
- T. Yuan, S. Li, Y. Sun, J. H. Wang, A. J. Chen, Q. Zheng, Y. Zhang, L. Chen, G. Nam, H. Che, J. Yang, S. Zheng, Z. F. Ma and M. Liu, ACS Nano, 2022, 16, 18058–18070 CrossRef CAS PubMed.
- H. Y. Hu, J. Y. Li, Y. F. Liu, Y. F. Zhu, H. W. Li, X. B. Jia, Z. C. Jian, H. X. Liu, L. Y. Kong, Z. Q. Li, H. H. Dong, M. K. Zhang, L. Qiu, J. Q. Wang, S. Q. Chen, X. W. Wu, X. D. Guo and Y. Xiao, Chem. Sci., 2024, 15, 5192–5200 RSC.
- L. Yu, X. He, B. Peng, F. Wang, N. Ahmad, Y. Shen, X. Ma, Z. Tao, J. Liang, Z. Jiang, Z. Diao, B. He, Y. Xie, B. Qing, C. Wang, Y. Wang and G. Zhang, Adv. Funct. Mater., 2024, 34, 2406771 CrossRef CAS.
- P. Masset and R. A. Guidotti, J. Power Sources, 2007, 164, 397–414 CrossRef CAS.
- Z.-C. Jian, Y.-F. Liu, Y.-F. Zhu, J.-Y. Li, H.-Y. Hu, J. Wang, L.-Y. Kong, X.-B. Jia, H.-X. Liu, J.-X. Guo, M.-Y. Li, Y.-S. Xu, J.-F. Mao, S.-L. Zhang, Y. Su, S.-X. Dou, S.-L. Chou and Y. Xiao, Nano Energy, 2024, 125, 109528 CrossRef CAS.
- S. Jia, S. Kumakura and E. McCalla, Energy Environ. Sci., 2024, 17, 4343–4389 RSC.
- S. Guo, Q. Li, P. Liu, M. Chen and H. Zhou, Nat. Commun., 2017, 8, 135 CrossRef PubMed.
- A. P. L. Oleksandr Bondarchuk, A. Kvasha, T. Thieu, E. Ayerbe and I. Urdampilleta, Appl. Surf. Sci., 2021, 535, 147699 CrossRef.
- Y. G. Zou, H. Mao, X. H. Meng, Y. H. Du, H. Sheng, X. Yu, J. L. Shi and Y. G. Guo, Angew. Chem., Int. Ed., 2021, 60, 26535–26539 CrossRef CAS PubMed.
- C. Jiang, Y. Wang, Y. Xin, X. Ding, S. Liu, Y. Pang, B. Chen, Y. Wang, L. Liu, F. Wu and H. Gao, Carbon Neutralization, 2024, 3, 233–244 CrossRef CAS.
- J. Y. Li, H. Y. Hu, H. W. Li, Y. F. Liu, Y. Su, X. B. Jia, L. F. Zhao, Y. M. Fan, Q. F. Gu, H. Zhang, W. K. Pang, Y. F. Zhu, J. Z. Wang, S. X. Dou, S. L. Chou and Y. Xiao, ACS Nano, 2024, 18, 12945–12956 CrossRef CAS PubMed.
- X. Z. Wang, Y. Zuo, Y. Qin, X. Zhu, S. W. Xu, Y. J. Guo, T. Yan, L. Zhang, Z. Gao, L. Yu, M. Liu, Y. X. Yin, Y. Cheng, P. F. Wang and Y. G. Guo, Adv. Mater., 2024, 36, e2312300 CrossRef PubMed.
- Y. F. Liu, H. Y. Hu, Y. F. Zhu, D. N. Peng, J. Y. Li, Y. J. Li, Y. Su, R. R. Tang, S. L. Chou and Y. Xiao, Chem. Commun., 2024, 60, 6496–6499 RSC.
- Y. Xiao, Y. F. Liu, H. W. Li, J. Y. Li, J. Q. Wang, H. Y. Hu, Y. Su, Z. C. Jian, H. R. Yao, S. Q. Chen, X. X. Zeng, X. W. Wu, J. Z. Wang, Y. F. Zhu, S. X. Dou and S. L. Chou, InfoMat, 2023, 5, e12475 CrossRef CAS.
- Y. Wang, J. Jin, X. Zhao, Q. Shen, X. Qu, L. Jiao and Y. Liu, Angew. Chem., Int. Ed., 2024, 63, e202409152 CrossRef CAS PubMed.
- Z. Chen, Y. Deng, J. Kong, W. Fu, C. Liu, T. Jin and L. Jiao, Adv. Mater., 2024, 36, e2402008 CrossRef PubMed.
- T. Cui, L. Liu, Y. Xiang, C. Sheng, X. Li and Y. Fu, J. Am. Chem. Soc., 2024, 146, 13924–13933 CrossRef CAS PubMed.
- Y. J. Guo, P. F. Wang, Y. B. Niu, X. D. Zhang, Q. Li, X. Yu, M. Fan, W. P. Chen, Y. Yu, X. Liu, Q. Meng, S. Xin, Y. X. Yin and Y. G. Guo, Nat. Commun., 2021, 12, 5267 CrossRef CAS PubMed.
- S. Kim, H. S. Kim, B. Kim, Y. J. Kim, J. W. Jung and W. H. Ryu, Adv. Energy Mater., 2023, 13, 2301983 CrossRef CAS.
- Y.-F. Zhu, Y. Xiao, S.-X. Dou, Y.-M. Kang and S.-L. Chou, eScience, 2021, 1, 13–27 CrossRef.
- H. Y. Hu, Y. F. Zhu, Y. Xiao, S. Li, J. Y. Li, Z. Q. Hao, J. H. Zhao and S. L. Chou, Adv. Energy Mater., 2022, 12, 2201511 CrossRef CAS.
- W. Zhang, Y. Wu, Y. Dai, Z. Xu, L. He, Z. Li, S. Li, R. Chen, X. Gao, W. Zong, F. Guo, J. Zhu, H. Dong, J. Li, C. Ye, S. Li, F. Wu, Z. Zhang, G. He, Y. Lai and I. Parkin, Chem. Sci., 2023, 14, 8662–8671 RSC.
- L.-Y. Kong, H.-X. Liu, Y.-F. Zhu, J.-Y. Li, Y. Su, H.-W. Li, H.-Y. Hu, Y.-F. Liu, M.-J. Yang, Z.-C. Jian, X.-B. Jia, S.-L. Chou and Y. Xiao, Sci. China:Chem., 2023, 67, 191–213 CrossRef.
- M. Ren, S. Zhao, S. Gao, T. Zhang, M. Hou, W. Zhang, K. Feng, J. Zhong, W. Hua, S. Indris, K. Zhang, J. Chen and F. Li, J. Am. Chem. Soc., 2023, 145, 224–233 CrossRef CAS PubMed.
- Q. Wang, G. Yu, B. Luo, W. Ji, Z. Liu, M. Li, Y. Nong, Y. Tian, X. Wang, J. Zhang, C. L. Chen, C. K. Chang, Z. Sang, Z. Zhao, R. Zhao and J. Liang, ACS Nano, 2024, 18, 18622–18634 CrossRef CAS PubMed.
- Y. Xiao, T. Wang, Y. F. Zhu, H. Y. Hu, S. J. Tan, S. Li, P. F. Wang, W. Zhang, Y. B. Niu, E. H. Wang, Y. J. Guo, X. Yang, L. Liu, Y. M. Liu, H. Li, X. D. Guo, Y. X. Yin and Y. G. Guo, Research, 2020, 2020, 1469301 CAS.
- C. Zheng, S. He, J. Gan, Z. Wu, L. She, Y. Gao, Y. Yang, J. Lou, Z. Ju and H. Pan, Carbon Energy, 2024, e605, DOI:10.1002/cey2.605.
- Y. Zhu, W. Li, L. Zhang, W. Fang, Q. Ruan, J. Li, F. Zhang, H. Zhang, T. Quan and S. Zhang, Energy Environ. Sci., 2023, 16, 2825–2855 RSC.
- F. Zhang, B. He, Y. Xin, T. Zhu, Y. Zhang, S. Wang, W. Li, Y. Yang and H. Tian, Chem. Rev., 2024, 124, 4778–4821 CrossRef CAS PubMed.
- Z. Cui and A. Manthiram, Angew. Chem., Int. Ed., 2023, 62, e202307243 CrossRef CAS PubMed.
- H. Liu, L. Kong, H. Wang, J. Li, J. Wang, Y. Zhu, H. Li, Z. Jian, X. Jia, Y. Su, S. Zhang, J. Mao, S. Chen, Y. Liu, S. Chou and Y. Xiao, Adv. Mater., 2024, 36, e2407994 CrossRef PubMed.
- H.-Y. Hu, Y. Xiao, W. Ling, Y.-B. Wu, P. Wang, S.-J. Tan, Y.-S. Xu, Y.-J. Guo, W.-P. Chen, R.-R. Tang, X.-X. Zeng, Y.-X. Yin and X.-W. Wu, Energy Technol., 2020, 9, 2000730 CrossRef.
- Y. Liao, L. Yuan, Y. Han, C. Liang, Z. Li, Z. Li, W. Luo, D. Wang and Y. Huang, Adv. Mater., 2024, 36, e2312287 CrossRef PubMed.
- Z.-C. Jian, J.-X. Guo, Y.-F. Liu, Y.-F. Zhu, J. Wang and Y. Xiao, Chem. Sci., 2024, 15, 19698–19728 RSC.
- B. Zhu, W. Zhang, Z. Jiang, J. Chen, Z. Li, J. Zheng, N. Wen, R. Chen, H. Yang, W. Zong, Y. Dai, C. Ye, Q. Zhang, T. Qiu, Y. Lai, J. Lia and Z. Zhang, Chem. Sci., 2024, 15, 14104–14121 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc08351b |
| This journal is © The Royal Society of Chemistry 2025 |