Open Access Article
Open Access ArticleSynthesis of organic molecules via spray-drying†
Gerard
Pena
 ab,
Jorge
Albalad
ab,
Jorge
Albalad
 ab,
Daniel
Maspoch
ab,
Daniel
Maspoch
 *abc and
Inhar
Imaz
*abc and
Inhar
Imaz
 *ab
*ab
aCatalan Institute of Nanoscience and Nanotechnology (ICN2), CSIC and The Barcelona Institute of Science and Technology, Campus UAB, Bellaterra, 08193 Barcelona, Spain. E-mail: inhar.imaz@icn2.cat; daniel.maspoch@icn2.cat
bDepartament de Química, Facultat de Ciències, Universitat Autònoma de Barcelona (UAB), Cerdanyola del Vallès 08913, Barcelona, Spain
cICREA, Pg. Lluís Companys 23, Barcelona 08010, Spain
First published on 26th February 2025
Abstract
Confining chemical reactions within microdroplets has attracted significant attention from chemists due to the accelerated reaction rates resulting from the drastically smaller reaction volumes than in standard solutions. Herein we report that, beyond its widespread use for producing dry-powder formulations for industries (e.g. pharmaceuticals and food) via the atomization of microdroplets followed by drying in a hot gas stream, spray-drying can also be employed in organic synthesis. Specifically, we used spray-drying to run three model reactions: a Schiff-base condensation, a Claisen–Schmidt reaction, and acylation of amines, for synthesizing small organic molecules. Our results showcase that, compared to traditional methods, spray-drying can reduce reaction times without compromising (high) yields, paving the way for its use as a scalable method for industrial-scale organic synthesis.
Introduction
Synthetic organic chemistry is a perpetual wellspring of discovery and synthesis of small molecules, macromolecules, and polymers, all of which are subject to further exploration and application across myriad uses. Progress in this field is linked to development of novel reactions, synthesis of new compounds, as well as method and fabrication technology development for known syntheses.Since the 2010s, a few studies have demonstrated the enormous potential of using aerosols as microscale reactors for conducting organic reactions. Initially, aerosols were primarily important within the context of environmental chemistry, as many atmospheric reactions occur within microdroplets,1 such as the atmospheric oxidation of SO2, which leads to acid rain.2 Soon after, several studies revealed that charged aqueous aerosols could spontaneously generate nucleosides and peptides from their constituents (e.g. natural amino acids, monosaccharides, or nitrogenous bases),3 even though these reactions are typically slow in bulk and require a constant energy supply. More recently, important advances on the use of aerosol-based technologies in synthetic organic chemistry have been reported by Cooks et al. and Zare et al., who have demonstrated its utility to run and accelerate4–6 several reactions, such as epoxy ring-opening,7 amination of benzylic sp3 carbon atoms,8 and aza-Michael additions,9 as well as to enable unusual transformations10 by employing electrospray ionization coupled to mass spectrometry (ESI-MS). Using this technique, multiple groups have expanded the scope of organic reactions that can be performed using aerosols.11–16 Additionally, other techniques, such as the microdroplet/thin film method, have also proven effective for aerosol-based organic reactions, enabling processes like two-phase oxidations,17 phosphorylations,18 and more.19–22 Most recently, Zare's and Cooks' groups have also begun the first efforts to scale up these aerosol technologies (on the order of a few grams per hour),23,24 utilizing either a heated ultrasonic-nebulization device (Zare et al.) or a custom-built atomic sprayer apparatus with a solvent-recirculation system (Cooks et al.). Remarkably, these set-ups have been used to test various organic reactions, including Claisen–Schmidt condensations, Schiff-base formation, Katritzky salt reactions, and Suzuki couplings.
Building on these pioneering efforts, a next step is to make aerosol technologies more universally accessible for synthetic organic chemistry, both in academia and industry. This means developing and utilizing systems that are readily available in laboratories and industry, user-friendly, scalable, and ideally, commercially viable. A promising technology that meets these criteria is spray-drying,25 which is already widely applied in various industrial processes, especially for drying and encapsulation.26,27 Recently, spray-drying has also begun to be used for synthesizing inorganic materials and related composites in remarkably short synthetic times.28 For instance, our group pioneered its use for porous metal–organic frameworks (MOFs)29 and related composites.30–32 Nonetheless, an important question remains: can spray-drying facilitate the synthesis of small organic compounds? Such an approach could shorten reaction times and enable continuous organic reactions with the potential for solvent-recycling. In fact, as early as 2017, in our attempts to covalently post-synthesize MOFs by spray-drying, we observed such potential in control Schiff-base condensation reactions of small aldehydes and amines.33 Herein, we describe the value of spray-drying in synthetic organic chemistry.
Results and discussion
In our spray-drying process, all chemistry begins with the atomization of a solution of reagents, into a spray of microdroplets, which is facilitated by a two-fluid nozzle (Fig. 1). This involves simultaneous injection of the solution, at a specified feed rate, and nitrogen gas, at another specified flow rate. In our reactions, the flow rate was maintained at 357 L h−1 and the feed rate, at 3.0 mL min−1. Consequently, each precursor droplet comes into contact with — and is suspended by — a gas stream heated to a designated temperature (the inlet temperature), thereby initiating the evaporation of the solvent. This in turn leads to the formation of a dried micro-structured powder, which is then directed through a cyclone, separated from the gas stream, and finally, collected inside a vessel. In this study, after each spray-drying synthesis, the powder is collected from the vessel and characterized using 1H NMR to determine its purity. At this stage, the reaction was deemed complete for products with purities equal to or above 95%, while products with purities below 95% underwent further purification using standard work-up processes.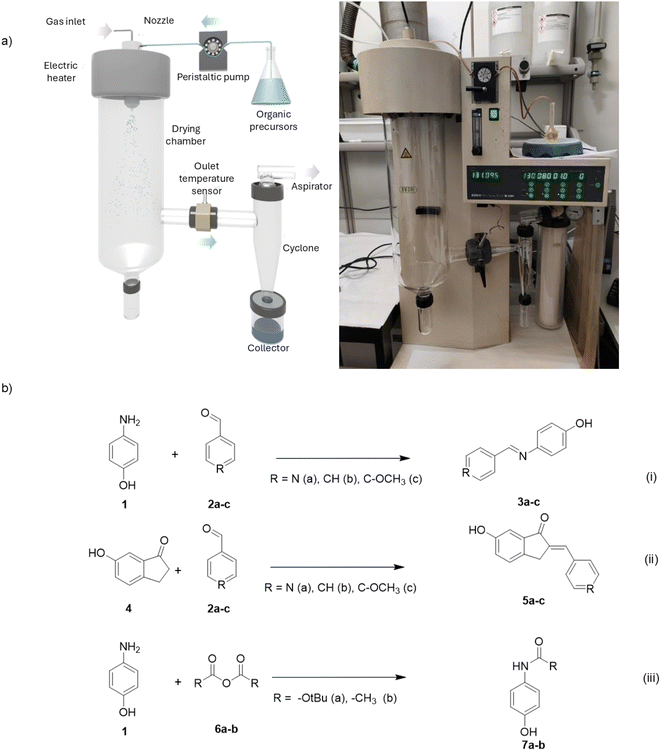 | ||
| Fig. 1 (a) Schematic (left) and photo (right) of the lab-scale spray-dryer used for the organic reactions. (b) The reactions run with spray-drying. | ||
To confirm our previously reported observations, we first extended the use of spray-drying to conduct Schiff-base condensations. We examined the formation of (E)-4-((pyridin-4-ylmethylene)amino)phenol (3a) using 4-aminophenol (1) and 4-pyridinecarboxaldehyde (2a) as reagents (Fig. 1b(i)). The synthesis began with the dissolution of both reagents (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) in 25 mL of ethanol. The resultant solution was then spray-dried at an inlet temperature of 130 °C for 8.33 min, using a Mini Spray Dryer (Buchi, model B-290). The inlet temperature was selected to ensure evaporation of both the ethanol and the water generated during the reaction, as a way to facilitate the formation of imines according to Le Chatelier's principle. The reaction afforded a powder structured in the form of microspheres that was analyzed by 1H NMR without any further purification, which confirmed the formation of 3a (Fig. S1–S5†) at a purity of 98% (0.917 g, yield: 90%; Table 1). Remarkably, comparing these results to the reported values for batch synthesis, the reaction time was 88% shorter, yet the yield was comparable (yield: 93%, reaction time: 240 min).34
1 molar ratio) in 25 mL of ethanol. The resultant solution was then spray-dried at an inlet temperature of 130 °C for 8.33 min, using a Mini Spray Dryer (Buchi, model B-290). The inlet temperature was selected to ensure evaporation of both the ethanol and the water generated during the reaction, as a way to facilitate the formation of imines according to Le Chatelier's principle. The reaction afforded a powder structured in the form of microspheres that was analyzed by 1H NMR without any further purification, which confirmed the formation of 3a (Fig. S1–S5†) at a purity of 98% (0.917 g, yield: 90%; Table 1). Remarkably, comparing these results to the reported values for batch synthesis, the reaction time was 88% shorter, yet the yield was comparable (yield: 93%, reaction time: 240 min).34
| Reagent A | Reagent B | Product | Reagent ratio (A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B) B) |
Inlet temperature (°C) | Puritya (%) | Yield (%) |
|---|---|---|---|---|---|---|
| a After collecting the product from the spray-drier. b Measured after acidifying the crude and isolating the solid. c After purification. | ||||||
| 1 | 2a | 3a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 98 | 90 |
| 1 | 2b | 3b | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
130 | 97 | 85 |
| 1 | 2c | 3c | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
130 | 97 | 80 |
| 4 | 2a | 5a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7 1.7 |
80 | 96b | 79b |
| 4 | 2b | 5b | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 95b | 67b |
| 4 | 2c | 5c | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
80 | 97b | 93b |
| 1 | 6a | 7a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
100 | 91 | 81c |
| 1 | 6b | 7b | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
80 | 97 | 83 |
Next, to study how the spray-dryer circumvents the effects of neutral or electron-donor substituents at the electrophile moiety, we expanded our chemistry to two other Schiff-base condensations: that of 1 and benzaldehyde (2b) to produce (E)-4-(N-benzylidene)aminophenol (3b); and that of 1 and 4-methoxybenzaldehyde (2c) to give (E)-4-[N-(4-methoxybenzylidene)amino]phenol (3c). Under stoichiometric synthetic conditions, we collected both imines (3b and 3c) from the spray-drier with purities of 90% and 44%, respectively, and then purified each one by liquid/liquid extraction to get 3b and 3c in yields of 62% and 16%, respectively. Although these yields were low compared to those reported for the corresponding batch syntheses (91% for 3b,35 and 97% for 3c36), we were able to increase the purity and yield for each one by tuning the molar ratio of the reagents. Thus, we reproduced the spray-drying protocols using molars of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 (1
1.3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2b), which afforded 3b in 85% yield, and of 1
2b), which afforded 3b in 85% yield, and of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (1
3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2c), which afforded 3c in 80% yield (Tables 1, S1 and S2, and Fig. S6–S19†). Crucially, use of excess aldehyde in each case favored purity of the corresponding crude product (97%), thus obviating the need for any tedious and solvent-consuming additional purification.
2c), which afforded 3c in 80% yield (Tables 1, S1 and S2, and Fig. S6–S19†). Crucially, use of excess aldehyde in each case favored purity of the corresponding crude product (97%), thus obviating the need for any tedious and solvent-consuming additional purification.
Having demonstrated the efficacy of spray-drying in Schiff-base condensations, we next applied it to a second reaction: the Claisen–Schmidt reaction, an aldol condensation variant that involves a ketone and a non-enolizable aldehyde and is widely used in medicinal chemistry (Fig. 1b(ii)). Moreover, the Claisen–Schmidt reaction has been widely used as a model reaction in many microdroplet chemistry studies.23,24,37–42 As in Schiff-based condensations, in Claisen–Schmidt condensations spray-drying can facilitate formation of the desired products by forcing evaporation of the water generated during the reaction. We tested this by separately reacting 6-hydroxy-1-indanone (4) with each of the same three aldehydes that we had previously used (2a–c). The condensations were run in methanol in the presence of two equivalents of KOH as base, using similar spray-drying conditions as in the Schiff-base reactions, except that the inlet temperature was lowered to 80 °C to avoid formation of by-products.43 After spray-drying, the collected solids were treated with aqueous HCl because of their ionic character, and re-collected by filtration. All three isolated condensation adducts 5a–c had purities below 95% (Tables S3–S5†). After purification, the products were obtained in yields of 55% (5a), 41% (5b) and 78% (5c). However, similarly to the Schiff-based condensation reactions, increasing the molar ratios to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7 (4
1.7 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a), 1
2a), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (4
2 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2b) and 1
2b) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 (4
1.3 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2c) enabled spray-drying synthesis of 5a–c without the need for any further purification (Fig. S20–S39†). Thus, 5a was obtained at 96% purity (79% yield); 5b, at 95% purity (67% yield); and 5c, at 97% purity (93% yield) (Table 1). These values are comparable or superior to those obtained from batch procedures,44–46 while offering much shorter reaction times and requiring far less of the (catalytic) base. Moreover, compared to the initial works by Cooks and co-workers,38–40 we were able to eliminate the need for high voltage while maintaining high yields. Additionally, our final observations suggest that electron-rich aldehydes facilitate the formation of relatively pure unsaturated products when Claisen–Schmidt reactions are performed via spray-drying.
2c) enabled spray-drying synthesis of 5a–c without the need for any further purification (Fig. S20–S39†). Thus, 5a was obtained at 96% purity (79% yield); 5b, at 95% purity (67% yield); and 5c, at 97% purity (93% yield) (Table 1). These values are comparable or superior to those obtained from batch procedures,44–46 while offering much shorter reaction times and requiring far less of the (catalytic) base. Moreover, compared to the initial works by Cooks and co-workers,38–40 we were able to eliminate the need for high voltage while maintaining high yields. Additionally, our final observations suggest that electron-rich aldehydes facilitate the formation of relatively pure unsaturated products when Claisen–Schmidt reactions are performed via spray-drying.
Having validated spray-drying in both condensations, we then extended its scope to include acylation of a primary amine — namely, N-Boc protection of 4-aminophenol (Fig. 1b(iii)). Accordingly, an equimolar mixture of amine 1 and Boc2O (6a) in methanol, containing 1.5 equivalents of TEA, was spray-dried for form carbamate 7a.47 These conditions proved successful, affording the protected product at a purity of 88%, which, after purification, gave 7a in 66% yield (Table S6, and Fig. S40–S44†). Consistent with the previous reactions, spraying 1 with excess Boc2O (1.3 mol eq.) streamlined the process, giving the protected carbamate at 91% purity, and in 81% yield after purification (Table 1).
As acylation of amines to obtain amides is among the most frequent reactions in medicinal chemistry, we assessed our spray-drying approach in the synthesis of N-acetyl-para-aminophenol (the analgesic known as paracetamol or acetaminophen) (8a). It is considered an Essential Medicine by the World Health Organization48 and is widely used as a synthetic intermediate.49 Thus, a solution containing 4-aminophenol and acetic anhydride (molar ratio: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
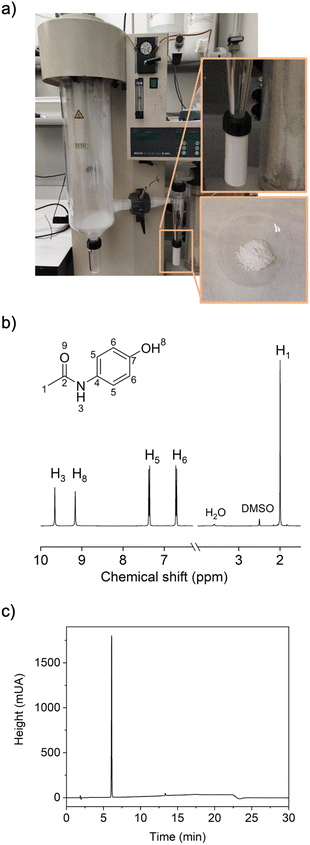
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3) in 25 mL of THF was spray-dried at an inlet temperature of 80 °C for 8.33 min. Next, a solid was collected from the spray-drier, and then analyzed by 1H NMR (Fig. S45 and S46†), HPLC (Fig. S49†) and X-ray powder diffraction (XRPD, Fig. S50†), which together confirmed the direct formation of crystalline paracetamol (in its monoclinic form)50 at 97% purity and in 83% yield. Importantly, spray-drying not only enables continuous synthesis of paracetamol at shorter-than-standard reaction times, it also does so at high purity, without the need for any work-up, because the by-product, acetic acid, is volatile and therefore, evaporates off with the solvent. Another interesting observation is that our spray-drying reaction provides chemoselective N-acylation of 4-aminophenol: 1H NMR analysis did not reveal the possible O-acylated derivative.
1.3) in 25 mL of THF was spray-dried at an inlet temperature of 80 °C for 8.33 min. Next, a solid was collected from the spray-drier, and then analyzed by 1H NMR (Fig. S45 and S46†), HPLC (Fig. S49†) and X-ray powder diffraction (XRPD, Fig. S50†), which together confirmed the direct formation of crystalline paracetamol (in its monoclinic form)50 at 97% purity and in 83% yield. Importantly, spray-drying not only enables continuous synthesis of paracetamol at shorter-than-standard reaction times, it also does so at high purity, without the need for any work-up, because the by-product, acetic acid, is volatile and therefore, evaporates off with the solvent. Another interesting observation is that our spray-drying reaction provides chemoselective N-acylation of 4-aminophenol: 1H NMR analysis did not reveal the possible O-acylated derivative.
Finally, having synthesized paracetamol as a powder in a single, continuous step, without the need for isolation from the solvent, motivated us to explore the scalability of this process (×10 relative to the first reaction) using our lab-scale spray-drier (Fig. 2). To achieve this, we reproduced the above-mentioned spray-drying synthesis, increasing the amount of reagents with a precursor solution of 250 mL of THF containing 4-aminophenol (5.45 g) and acetic anhydride (6.63 g). Remarkably, after spray-drying the solution for 83.3 minutes, we were able to directly collect paracetamol as a white powder (6.60 g) at the same purity (98%) and in the same yield (85%) as in the milligram-scale synthesis, as confirmed by 1H NMR and HPLC (Fig. 2).
Conclusions
In summary, we have shown that spray-drying can be an interesting method for the continuous synthesis of small organic compounds. We have demonstrated its utility in three different reactions (Schiff-base condensations, Claisen–Schmidt reactions, and acylation of amines), to synthesize a total of eight such compounds. In these reactions, spray-drying allows the fast synthesis of these molecules with high purities and yields. Moreover, in some cases, spray-drying bypasses the need of purification protocols, further simplifying the production of these molecules. We believe that, as spray-drying is a widely available technique in industry allowing the processability of liters of solutions within minutes, the results shown herein will contribute to easily scale-up aerosol technologies to produce small organic molecules in a continuous and fast way.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
G. P.: methodology, investigation, and writing-original draft. J. A.: validation and investigation. D. M.: funding acquisition, conceptualization, supervision and writing-review and editing. I. I.: funding acquisition, conceptualization, supervision and writing-review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has received funding from the Catalan AGAUR (project 2021 SGR 00458). It was also funded by the CERCA Programme/Generalitat de Catalunya. ICN2 is supported by the Severo Ochoa Centres of Excellence programme, Grant CEX2021-001214-S, funded by MCIN/AEI/10.13039.501100011033. G. P. acknowledges funding from the Spanish Research Agency (PID2021-123265NB-I00).Notes and references
- W. G. Tsui, J. L. Woo and V. F. McNeill, Atmosphere, 2019, 10, 666 CAS.
- H. M. Hung and M. R. Hoffmann, Environ. Sci. Technol., 2015, 49, 13768–13776 Search PubMed.
- Y. Ju, H. Zhang, W. Wang, Q. Liu, K. Yu, G. Kan, L. Liu and J. Jiang, J. Phys. Chem. Lett., 2022, 13, 567–573 Search PubMed.
- L. Qiu, Z. Wei, H. Nie and R. G. Cooks, Chempluschem, 2021, 86, 1362–1365 Search PubMed.
- X. Yan, R. M. Bain and R. G. Cooks, Angew. Chem., Int. Ed., 2016, 55, 12960–12972 CAS.
- M. Girod, E. Moyano, D. I. Campbell and R. G. Cooks, Chem. Sci., 2011, 2, 501–510 CAS.
- Y. H. Lai, S. Sathyamoorthi, R. M. Bain and R. N. Zare, J. Am. Soc. Mass Spectrom., 2018, 29, 1036–1043 CAS.
- Y. Meng, E. Gnanamani and R. N. Zare, J. Am. Chem. Soc., 2022, 144, 19709–19713 CAS.
- J. Ghosh, J. Mendoza and R. G. Cooks, Angew. Chem., Int. Ed., 2022, 61, e202214090 CrossRef CAS.
- S. Banerjee, H. Prakash and S. Mazumdar, J. Am. Soc. Mass Spectrom., 2011, 22, 1707–1717 CrossRef CAS PubMed.
- Z. Song, C. Liang, K. Gong, S. Zhao, X. Yuan, X. Zhang and J. Xie, J. Am. Chem. Soc., 2023, 145, 26003–26008 CrossRef CAS.
- C. Gong, D. Li, X. Li, D. Zhang, D. Xing, L. Zhao, X. Yuan and X. Zhang, J. Am. Chem. Soc., 2022, 144, 3510–3516 CrossRef CAS PubMed.
- P. Basuri, J. S. Kumar, S. Das and T. Pradeep, ACS Sustainable Chem. Eng., 2022, 10, 8577–8587 CrossRef CAS.
- P. Basuri, S. Mukhopadhyay, K. S. S. V. P. Reddy, K. Unni, B. K. Spoorthi, J. Shantha Kumar, S. S. R. K. C. Yamijala and T. Pradeep, Angew. Chem., Int. Ed., 2024, 63, e202403229 CrossRef CAS PubMed.
- C. Salvitti, G. de Petris, A. Troiani, M. Managò, A. Di Noi, A. Ricci and F. Pepi, J. Am. Soc. Mass Spectrom., 2023, 34, 2748–2754 CrossRef CAS PubMed.
- C. Salvitti, G. de Petris, A. Troiani, M. Managò, C. Villani, A. Ciogli, A. Sorato, A. Ricci and F. Pepi, J. Am. Soc. Mass Spectrom., 2022, 33, 565–572 CAS.
- W. Zhang, H. Cheng and J. Liu, ACS Sustainable Chem. Eng., 2018, 6, 8125–8129 CAS.
- B. Zheng, L. Xue, C. Dai, J. Liu and H. Cheng, J. Org. Chem., 2022, 87, 5287–5295 CAS.
- W. Zhang, S. Yang, Q. Lin, H. Cheng and J. Liu, J. Org. Chem., 2019, 84, 851–859 CAS.
- B. Zheng, X. Jin, J. Liu and H. Cheng, ACS Sustainable Chem. Eng., 2021, 9, 4383–4390 Search PubMed.
- X. Jin, Y. Wu, J. Sun, J. Liu and H. Cheng, Analysis Sensing, 2023, 3, e202300031 CAS.
- H. M. Brown, J. E. Estevez, J. C. Bottaro, B. G. Harvey and P. W. Fedick, React. Chem. Eng., 2023, 8, 1576–1582 Search PubMed.
- C. Liu, J. Li, H. Chen and R. N. Zare, Chem. Sci., 2019, 10, 9367–9373 RSC.
- H. Nie, Z. Wei, L. Qiu, X. Chen, D. T. Holden and R. G. Cooks, Chem. Sci., 2020, 11, 2356–2361 CAS.
- S. R. Percy, US Pat. US125406A, 1872 Search PubMed.
- J. Broadhead, S. K. Edmond Rouan and C. T. Rhodes, Drug Dev. Ind. Pharm., 2008, 18, 1169–1206 CrossRef.
- S. S. Santos, L. M. Rodrigues, S. C. Costa and G. S. Madrona, Food Packag. Shelf Life, 2019, 20, 100177 Search PubMed.
- S. Wintzheimer, L. Luthardt, K. L. A. Cao, I. Imaz, D. Maspoch, T. Ogi, A. Bück, D. P. Debecker, M. Faustini and K. Mandel, Adv. Mater., 2023, 35, 2306648 Search PubMed.
- A. Carne-Sanchez, I. Imaz, M. Cano-Sarabia and D. Maspoch, Nat. Chem., 2013, 5, 203–211 CrossRef CAS.
- G. Boix, X. Han, I. Imaz and D. Maspoch, ACS Appl. Mater. Interfaces, 2021, 13, 17835–17843 CrossRef CAS.
- G. Boix, J. Troyano, L. Garzon-Tovar, C. Camur, N. Bermejo, A. Yazdi, J. Piella, N. G. Bastus, V. F. Puntes, I. Imaz and D. Maspoch, ACS Appl. Mater. Interfaces, 2020, 12, 10554–10562 Search PubMed.
- J. Troyano, C. Çamur, L. Garzón-Tovar, A. Carné-Sánchez, I. Imaz and D. Maspoch, Acc. Chem. Res., 2020, 53, 1206–1217 CrossRef CAS PubMed.
- L. Garzon-Tovar, S. Rodriguez-Hermida, I. Imaz and D. Maspoch, J. Am. Chem. Soc., 2017, 139, 897–903 CrossRef CAS PubMed.
- H. Ö. Demir, I. Kaya and M. Saçak, Russ. Chem. Bull. Int. Ed., 2006, 55, 1852–1855 Search PubMed.
- J. Xia, E. B. Stephens, M. G. Mason, J. W. Miley, L. J. Starks and E. K. Stephenson, US Pat. US6667392B2, 2003 Search PubMed.
- Z. Tang, C. Wu, T. Wang, K. Lao, Y. Wang, L. Liu, M. Muyaba, P. Xu, C. He, G. Luo, Z. Qian, S. Niu, L. Wang, Y. Wang, H. Xiao, Q. You and H. Xiang, Eur. J. Med. Chem., 2016, 118, 328–339 CAS.
- V. Calvino, M. Picallo, A. J. López-Peinado, R. M. Martín-Aranda and C. J. Durán-Valle, Appl. Surf. Sci., 2006, 252, 6071–6074 Search PubMed.
- T. Müller, A. Badu-Tawiah and R. G. Cooks, Angew. Chem., Int. Ed., 2012, 51, 11832–11835 CrossRef PubMed.
- Y. Li, X. Yan and R. G. Cooks, Angew. Chem., Int. Ed., 2016, 55, 3433–3437 Search PubMed.
- R. M. Bain, C. J. Pulliam, X. Yan, K. F. Moore, T. Müller and R. G. Cooks, J. Chem. Educ., 2014, 91, 1985–1989 CAS.
- R. M. Bain, C. J. Pulliam, F. Thery and R. G. Cooks, Angew. Chem., Int. Ed., 2016, 55, 10478–10482 Search PubMed.
- Z. Wei, M. Wleklinski, C. Ferreira and R. G. Cooks, Angew. Chem., Int. Ed., 2017, 56, 9386–9390 CAS.
- B. Lantaño, J. M. Aguirre, E. V. Drago, M. Bollini, D. J. de la Faba and J. D. Mufato, Synth. Commun., 2017, 47, 2202–2214 Search PubMed.
- T. M. Kadayat, S. Banskota, G. Bist, P. Gurung, T. B. T. Magar, A. Shrestha, J.-A. Kim and E.-S. Lee, Bioorg. Med. Chem. Lett., 2018, 28, 2436–2441 CAS.
- H. Wu, H. Zhao, T. Lu, B. Xie, C. Niu and A. H. Aisa, Med. Chem., 2023, 19, 686–703 Search PubMed.
- P.-C. Huo, X.-Q. Guan, P. Liu, Y.-Q. Song, M.-R. Sun, R.-J. He, L.-W. Zou, L.-J. Xue, J.-H. Shi, N. Zhang, Z.-G. Liu and G.-B. Ge, Eur. J. Med. Chem., 2021, 209, 112856 CAS.
- A. De Dios, T. Li, L. M. Martin-Cabrejas, M. A. Pobanz, C. Shih, Y. Wang and B. Zhong, Eur Pat. EP1609789A1, 2005 Search PubMed.
- W. H. Organization, World Health Organization Model List of Essential Medicines, World Health Organization, 2023 Search PubMed.
- G. A. Dziwornu, D. Coertzen, M. Leshabane, C. M. Korkor, C. K. Cloete, M. Njoroge, L. Gibhard, N. Lawrence, J. Reader, M. van der Watt, S. Wittlin, L.-M. Birkholtz and K. Chibale, J. Med. Chem., 2021, 64, 5198–5215 CAS.
- D. Y. Naumov, M. A. Vasilchenko and J. A. K. Howard, Acta Crystallogr., Sect. C:Cryst. Struct. Commun., 1998, 54, 653–655 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc00126a |
| This journal is © The Royal Society of Chemistry 2025 |