Open Access Article
Open Access Articleα-N-phthalimido-oxy isobutyrate-mediated deoxygenative arylation: total synthesis of alanenses A and B†
Young Eum Hyun a,
Jeonguk Kweon
a,
Jeonguk Kweon ba,
Thi Hieu Linh Phan
ba,
Thi Hieu Linh Phan a,
Dongwook Kim
a,
Dongwook Kim ba and
Sunkyu Han
ba and
Sunkyu Han *ab
*ab
aDepartment of Chemistry, Korea Advanced Institute of Science and Technology (KAIST), Daejeon 34141, Republic of Korea. E-mail: sunkyu.han@kaist.ac.kr
bCenter for Catalytic Hydrocarbon Functionalizations, Institute for Basic Science (IBS), Daejeon 34141, Republic of Korea
First published on 25th April 2025
Abstract
Inspired by a biosynthetic hypothesis of alanense A, we developed two distinct methods for the deoxygenative arylation of α-N-phthalimido-oxy isobutyrate (NPIB), derived from hydroxyl groups adjacent to or conjugated with a carbonyl moiety. One approach utilizes photoredox catalysis to achieve a radical-mediated arylation reaction. The other approach involves an acid-mediated arylation method that proceeds through a cationic intermediate. The acid-mediated approach was successfully applied to the total syntheses of alanenses A, B, and O7′-methyllacinilene E.
Introduction
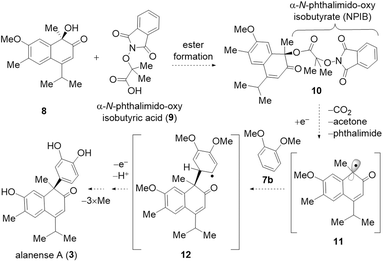
Two-phase biosynthesis of terpene natural products, encompassing a cyclization phase followed by an oxidation phase, is a well-established process.1,2 Cadinane sesquiterpenoids conform to this paradigm. The cadinane framework, biosynthesized via the cyclization of farnesyl pyrophosphate, undergoes biosynthetic oxidations, resulting in remarkable structural diversity.3,4 Lacinilene C (1), isolated from the cotton plant Gossypium hirsutum L., is an example of a highly oxidized cadinane sesquiterpenoid.5 Notably, in 2018, lacinilene E (2), the benzyl substituted derivative of lacinilene C (1), was isolated.6 More recently, alanenses A (3) and B (4), C1- and C2-arylated cadinane sesquiterpenoids, were isolated as racemates from the leaves of Alangium chinense (Scheme 1A).7 Importantly, alanenses A and B inhibit spontaneous calcium channel oscillations (SCOs)8 at low micromolar concentrations. Initial structure–activity relationship studies revealed that the aromatic group at C1- and C2-positions of alanense natural products is essential for the observed inhibitory activity toward SCOs.7We hypothesized that alanense A (3) and lacinilene C (1) share a common biosynthetic precursor, namely 2,7-dihydroxycadalene (5). Previous studies have shown that 2,7-dihydroxycadalene undergoes air oxidation to form lacinilene C (1).9 We proposed that this oxidation involves the reaction of a radical intermediate 6 with triplet oxygen. For the biosynthesis of alanense A (3), we speculated that the same radical intermediate (6) might instead react with catechol (7a). While the biosynthetic pathways of alanense A (3) and lacinilene C (1) diverge from the radical intermediate 6, we questioned whether it might be possible to chemically revert lacinilene C (1) back to alanense A (3). Inspired by recent advancements in deoxygenative functionalization via radical intermediates,10,11 we envisioned accessing alanense A (3) through a deoxygenative arylation12–17 of lacinilene C (1) or its derivative in a “contra-biosynthetic” manner (Scheme 1B),18 intending to use the redox-active ester as a radical precursor.19
We recently reported a radical-mediated deoxygenative transformation of tertiary alcohol derivatives to nitriles.20 The key to success was the development of α-N-phthalimido-oxy isobutyrate (NPIB) as a novel redox-active handle for alcohols.20 We envisioned utilizing the NPIB moiety for the deoxygenative arylation of the lacinilene C derivative as described in Scheme 2. Specifically, we planned to install the NPIB group to the tertiary alcohol moiety of lacinilene C7-methyl ether (8). Single-electron transfer (SET) to the NPIB derivative 10 would result in α-carbonyl radical intermediate 11 upon release of phthalimide, carbon dioxide, and acetone (Scheme 2). Radical intermediate 11 was designed to react with catechol derivative 7b to yield C–C coupled intermediate 12. SET (oxidation) and deprotonation of radical intermediate 12, followed by subsequent demethylations of the resulting intermediate, would afford alanense A (3).
Results and discussion
Our initial investigations focused on the deoxygenative arylation of NPIB derivative 13a using [Ir(dtbbpy)(ppy)2]PF6 as a photoredox catalyst and methanol as a hydrogen bonding-mediated activator of the NPIB moiety.21 To our delight, when NPIB derivative 13a was allowed to react with 1,2,4-trimethoxybenzene (7c) in the presence of [Ir(dtbbpy)(ppy)2]PF6 (1 mol%) under 427 nm Kessil lamp irradiation in a methanol/acetonitrile (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5, v/v) mixture, arylated product 14a was isolated in 71% yield (Scheme 3A, entry 1). Notably, when the reaction was conducted without methanol, the product yield was significantly reduced (28%), highlighting the importance of hydrogen bonding-mediated activation of the NPIB moiety (Scheme 3A, entry 2).21 On the same token, when the reaction was conducted in dimethylformamide (DMF), a strong hydrogen bonding acceptor, product 14a was obtained in 32% yield consistent with diminished hydrogen bonding activation of the NPIB moiety (Scheme 3A, entry 3). In the absence of light, the reaction was not operative (Scheme 3A, entry 4). Markedly, the addition of BHT to the reaction mixture completely shut down the arylated product formation and resulted in 15 (81% yield) and 16 (40% yield based on the equivalence of NPIB derivative 13a), consistent with the intermediacy of the radical intermediate (Scheme 3A, entry 5).
2.5, v/v) mixture, arylated product 14a was isolated in 71% yield (Scheme 3A, entry 1). Notably, when the reaction was conducted without methanol, the product yield was significantly reduced (28%), highlighting the importance of hydrogen bonding-mediated activation of the NPIB moiety (Scheme 3A, entry 2).21 On the same token, when the reaction was conducted in dimethylformamide (DMF), a strong hydrogen bonding acceptor, product 14a was obtained in 32% yield consistent with diminished hydrogen bonding activation of the NPIB moiety (Scheme 3A, entry 3). In the absence of light, the reaction was not operative (Scheme 3A, entry 4). Markedly, the addition of BHT to the reaction mixture completely shut down the arylated product formation and resulted in 15 (81% yield) and 16 (40% yield based on the equivalence of NPIB derivative 13a), consistent with the intermediacy of the radical intermediate (Scheme 3A, entry 5).
Based on our experimental and DFT-calculation results as well as related previous studies,20,21 we proposed the catalytic cycle depicted in Scheme 3B. Photoexcited [IrIII]* (E1/2 (IV/III*) = −0.96 V vs. SCE) species would engage in hydrogen-bond assisted SET with 13a to produce radical anion A which undergoes highly facile fragmentation (ΔG‡ = 4.4 kcal mol−1) to deliver electrophilic radical species B. The formation of the radical intermediate B was calculated to be a thermodynamically favorable process (−86.4 kcal mol−1), and the subsequent radical addition to C is also smooth (ΔG‡ = 27.1 kcal mol−1). The photoredox cycle is then completed by SET between D and the oxidizing [IrIV] species leading to the formation of a cation, which undergoes aromatization to furnish 14a (for details, see Fig. S2 and S3†).
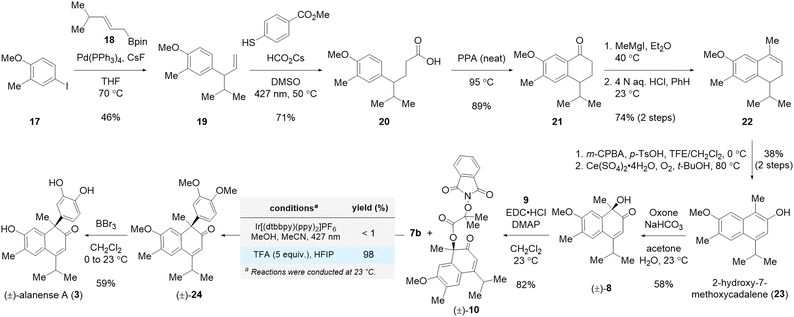
After developing the NPIB-mediated photocatalytic deoxygenative arylation, we turned our attention to its application in the total synthesis of alanense natural products.22,23 Our synthesis commenced with a regioselective reverse-prenylation of commercially available aryl iodide 17 using prenylboronic ester 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 in the presence of Pd(PPh3)4 and CsF25 to afford coupled product 19 in 46% yield (Scheme 4). With terminal alkene 19 in hand, we then employed Wickens' radical hydrocarboxylation to produce homologated carboxylic acid 20 in 71% yield.26 Subsequently, carboxylic acid 20 was transformed into cadinane framework 22 based on McCormick's protocol.27 Epoxidation of compound 22 followed by an oxidative epoxide opening reaction afforded natural 2-hydroxy-7-methoxycadalene (23) in 38% yield over two steps.28 Oxone-mediated dearomatization of naphthol derivative 23 yielded α-hydroxyketone 8 in 58% yield.29 Esterification of the tertiary alcohol group in compound 8 with α-N-phthalimido-oxy isobutyric acid (9) was achieved in 82% yield in the presence of EDC·HCl and DMAP. With NPIB derivative 10 in hand, the stage was set for the deoxygenative arylation reaction. However, an intractable mixture of products was observed when NPIB derivative 10 and 1,2-dimethoxybenzene (7b) were subjected to the aforementioned standard photoredox catalytic conditions (Scheme 4).
24 in the presence of Pd(PPh3)4 and CsF25 to afford coupled product 19 in 46% yield (Scheme 4). With terminal alkene 19 in hand, we then employed Wickens' radical hydrocarboxylation to produce homologated carboxylic acid 20 in 71% yield.26 Subsequently, carboxylic acid 20 was transformed into cadinane framework 22 based on McCormick's protocol.27 Epoxidation of compound 22 followed by an oxidative epoxide opening reaction afforded natural 2-hydroxy-7-methoxycadalene (23) in 38% yield over two steps.28 Oxone-mediated dearomatization of naphthol derivative 23 yielded α-hydroxyketone 8 in 58% yield.29 Esterification of the tertiary alcohol group in compound 8 with α-N-phthalimido-oxy isobutyric acid (9) was achieved in 82% yield in the presence of EDC·HCl and DMAP. With NPIB derivative 10 in hand, the stage was set for the deoxygenative arylation reaction. However, an intractable mixture of products was observed when NPIB derivative 10 and 1,2-dimethoxybenzene (7b) were subjected to the aforementioned standard photoredox catalytic conditions (Scheme 4).
We speculated that the increased electron density of the α-keto radical intermediate and the decreased electron density of the aromatic coupling partner caused a polarity mismatch during the key C–C bond formation. In fact, DFT calculations revealed that the activation barrier for the C–C coupling between radical intermediate B (Scheme 3B) and 1,2,4-trimethoxybenzene is 27.1 kcal mol−1, while the activation energy for the coupling between radical 11 and 1,2-dimethoxybenzene is 32.4 kcal mol−1 (for details, see Fig. S4†). Extensive experimentations were conducted with a model system to remedy the observed lack of desired reactivity under our previously optimized photocatalytic reaction conditions. To our delight, we discovered that NPIB derivative 10 and 1,2-dimethoxybenzene (7b) could be coupled in the presence of 5 equiv. of TFA in hexafluoroisopropanol (HFIP) to yield product 24 in 98% yield (vide infra). It is noteworthy that the carbocationic moiety is efficiently formed at the α-position of the ketone from the NPIB group under the newly discovered optimized reaction conditions. Finally, treatment of trimethylated compound 24 with 10 equiv. of BBr3 afforded the synthetic sample of alanense A (3) in 59% yield.
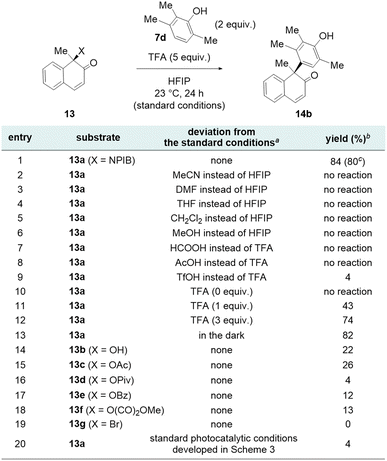
The optimization studies of the key NPIB-mediated Friedel–Crafts-type arylation are shown in Scheme 5. Treatment of NPIB derivative 13a and 2,3,6-trimethylphenol (7d, 2 equiv.) with TFA (5 equiv.) in HFIP solution produced deoxygenative arylated product 14b in 80% isolation yield along with α-N-phthalimido-oxy isobutyric acid 9 (Scheme 5, entry 1). The use of HFIP as a solvent was critical as the reaction did not proceed when acetonitrile, DMF, tetrahydrofuran, methylene chloride, or methanol were employed as a solvent (Scheme 5, entries 2–6).30,31 TFA was the optimal acid for the reaction since other Brønsted acids such as formic acid or acetic acid resulted in full recovery of NPIB derivative 13a (Scheme 5, entries 7 and 8). When stronger triflic acid was employed, NPIB derivative 13a underwent undesired decomposition leading to only 4% of coupled product 14b (Scheme 5, entry 9). In the absence of TFA, the reaction did not proceed at all (Scheme 5, entry 10). The use of 1 or 3 equiv. of TFA resulted in lower product yields due to slower conversion (Scheme 5, entries 11 and 12). The reaction proceeded in the dark, indicating that the mechanism does not involve a photoinduced radical pathway (Scheme 5, entry 13).
Next, the reactivity of the NPIB group was compared with that of other leaving groups. When hydroxyl derivative 13b was employed as a substrate under the optimized reaction conditions, the desired arylated product 14b was obtained in 22% yield (Scheme 5, entry 14).32,33 Other carboxylate-based leaving groups, such as acetate (13c), pivalate (13d), benzoate (13e), and methyl oxalate (13f) exhibited lower yields compared to the NPIB group (Scheme 5, entries 15–18), indicating the superior leaving group ability of NPIB (for details, see Fig. S6†). Even though the product yields were lower, the formation of the coupled product 14b with these leaving groups suggests the involvement of a carbocation intermediate. Subjection of bromide derivative 13g under the optimized reaction conditions did not yield the desired coupled product (Scheme 5, entry 19) and only reduced product 15 and 4-bromo-2,3,6-trimethylphenol were observed. Under the standard photocatalytic deoxygenative arylation conditions, a 4% yield of the coupled product was observed. The lower O–H bond dissociation energy of the phenol moiety facilitated a hydrogen atom transfer to the radical intermediate, leading to the formation of 15, which was obtained in 81% yield, along with 28% of the oxidative dimer of the phenol derivative 7d (Scheme 5, entry 20).
With the reaction conditions for acid-mediated deoxygenative arylation in hand, we hypothesized that the reaction may proceed via protonation-mediated NPIB dissociation followed by the Friedel–Crafts-type arylation process. Indeed, the formation of α-N-phthalimido-oxy isobutyric acid 9 was observed experimentally, supporting this mechanistic proposal. To further evaluate its plausibility, we conducted DFT calculations (Fig. 1). As expected, subsequent processes including protonation of 13a to form [13a-H], intramolecular proton transfer providing [13a-H]′, and dissociation of 9 to furnish carbocationic intermediate B′ were found to be kinetically accessible at room temperature (ΔG‡ = 21.5 kcal mol−1) although they are slight endergonic processes (ΔG = 7.3 kcal mol−1). Consequently, the Friedel–Crafts-type reaction with B′ and electron rich aryl substrate C′ to provide C–C coupled intermediate D′ also turned out to be thermodynamically and kinetically plausible (ΔG = −5.3 kcal mol−1 and ΔG‡ = 2.5 kcal mol−1). As a final step, highly exergonic deprotonation of D′ can provide the observed corresponding deoxygenative arylation product 14b (ΔG = −40.2 kcal mol−1).
With the two standard conditions established for NPIB-mediated deoxygenative arylations, the substrate scope was first investigated under photocatalytic conditions (Fig. 2, conditions A). Nitrogen-containing arenes participated in the reaction, affording arylated products (14c and 14d). Notably, acid- and/or base-sensitive functional groups on the arene, such as Boc (14c), MOM (14e), Bn (14f), and silyl (14g and 14h), were compatible with the reaction conditions. The substrate with the NPIB moiety juxtaposed between two carbonyl groups produced the arylated product 14ah in 41% yield. However, arylated products were obtained only from electron-rich 1,2,4-tri-substituted arenes.
 | ||
| Fig. 2 Substrate scope of the NPIB-mediated arylation reaction. All reactions were carried out on a 0.1 mmol scale and at 0.25 M. aTFA (20 equiv.) was used. | ||
Next, the substrate scope of aryl coupling partners was investigated under acidic conditions (Fig. 2, conditions B). Triflate (14i), acetate (14j), iodide (14l), bromide (14m and 14n), chloride (14o), and fluoride (14p and 14q) groups were compatible with our newly discovered reaction conditions. It is noteworthy that the presence of inductively electron-withdrawing halides did not hamper the reaction outcomes. The carboxylic acid, ketone and terminal olefin groups were compatible under the standard reaction conditions (14r–14t). The substrate with a primary hydroxyl group could be employed in our coupling reaction conditions. In this case, the hydroxyl group underwent trifluoroacetylation (14u). The phenolic moiety, which was problematic under the previously described photocatalytic reaction conditions, was compatible under the optimized TFA + HFIP reaction conditions (14v–14x). Bicyclic (14y–14aa) and tricyclic (14ab) aromatic systems yielded the coupled products in good yields. Notably, when anisole was employed as a coupling partner, ortho- and para-regioisomers were obtained in 22% (14ae) and 54% (14af) isolation yields, respectively. It is noteworthy that for all other cases delineated here, products were obtained as a single regioisomer. Unlike under photocatalytic conditions, no arylated products were obtained from nitrogen-containing arenes (14c and 14d) or those bearing Boc (14c), MOM (14e), or Bn (14f) groups. Additionally, silyl groups (14g and 14h) were not well tolerated.
We subsequently surveyed the scope of NPIB derivatives under acidic conditions. Structurally diverse benzylic and tertiary NPIB derivatives yielded coupling products with 1,2,4-trimethoxybenzene in good yields. The presence of an ester (14al and 14am) or an amide moiety (14an) at the α-position of NPIB did not hinder the reaction. As long as the ketone group is conjugated to the carbon attached to the NPIB group either via an aromatic ring (14ao and 14ap) or an olefin (14aq), the arylated products were formed in moderate to good yield. Notably, secondary NPIB derivatives were successfully transformed into arylated products under the standard reaction conditions (14ap and 14aq). Interestingly, when the NPIB derivative of 2-phenylpropan-2-ol was subjected to the standard reaction conditions, homodimerization product 25 was formed in 82% yield. It is reasoned that the benzylic carbocationic intermediate is trapped with in situ generated α-methylstyrene. When an analogous NPIB derivative with an ortho-fluoride moiety at the phenyl group was employed, the desired arylated product (14ar) was obtained in 61% yield. Unlike under photocatalytic conditions, the formation of a carbocation at the 1,3-diketone moiety proved challenging, preventing the formation of the desired product (14ah).
We next embarked on the total synthesis of alanense B (4). α-Methylation of common ketone precursor 21 and subsequent α-hydroxylation of the resulting methylated product via a protocol reported by the Schoenebeck group34 afforded alcohol 26 in 53% yield over two steps (Scheme 6). Oxidation of compound 26 with DDQ produced conjugated alcohol 27 in 57% yield. Treatment of alcohol 27 with NPIB acid 9 in the presence of EDC·HCl and DMAP forged NPIB derivative 28 as a single regioisomer. However, NPIB derivative 28 was partially isomerized to 29 during silica gel column chromatography to yield 28 and 29 in 59% and 10% yield, respectively. Interestingly, treatment of 28 with 1,2-dimethoxybenzene (7b) and 5 equiv. of TFA in HFIP solution yielded desired arylated product 30 (19%) along with [3+2] cycloaddition product 31 (17%), likely formed via cationic intermediate 32. When 28 was allowed to react with 20 equiv. of TFA in HFIP, only cycloaddition product 31 was obtained in 43% yield. The structure of [3+2] cycloaddition product 31 was unambiguously confirmed by a single crystal X-ray diffraction analysis. It is notable that the bicyclo[3.2.1]octadienone framework in 31 constitutes the backbone of various natural products including naphthocyclinone.35 The subjection of the NPIB regioisomer 29 to these reaction conditions revealed analogous reaction outcomes.
To circumvent the formation of the [3+2] cycloaddition byproduct, phenol (7x) was selected as the coupling partner. We reasoned that the undesired intramolecular Friedel–Crafts reaction-based cyclization would not be feasible because the meta position of the phenol group is deactivated by an inductively electron-withdrawing oxygen atom. This strategic design was possible owing to the broad substrate scope of the newly developed TFA/HFIP-based arylation reaction. In the event, when NPIB derivative 28 (or 29) was allowed to react with phenol in the presence of TFA (5 equiv.) in HFIP, the desired α-arylated product 33 was exclusively formed in 66% yield (63% yield from 29). The exclusive arylation at the α-position of the ketone group is noteworthy. Based on DFT calculations, nucleophilic phenol addition at the α-position is kinetically favored (ΔG‡ = 14.5 kcal mol−1) over addition at the benzylic position (ΔG‡ = 17.2 kcal mol−1), presumably due to the significant steric hindrance posed by the benzylic isopropyl substituent (for details, see Fig. S7†).
For the endgame of the synthesis, phenol derivative 33 was oxidized to the ortho-quinone derivative in the presence of IBX at −25 °C. Subsequent one-pot addition of aqueous sodium dithionite solution to the reaction mixture resulted in catechol derivative 34 in 71% yield. The use of chloroform/methanol co-solvent was critical for the efficient transformation.36 The final demethylation of the methoxy group in 34 was achieved by treating it with ethanethiol and sodium hydride in DMF to produce the first synthetic sample of alanense B (4) in 48% yield.
Finally, we envisioned applying the newly developed NPIB-based deoxygenative transformation into the synthesis of lacinilene E (2) (Scheme 7). To this end, 2-hydroxy-7-methoxycadalene (23) was allowed to react with NPIB derivative 35a in the presence of TFA in HFIP. However, only the decomposition of NPIB derivative 35a was observed under these reaction conditions. We speculated that the electron-rich NPIB derivative 35a acts as a dominant nucleophile over 2-hydroxy-7-methoxycadalene (23). To temper the nucleophilic reactivity of the NPIB derivative, we designed fluorinated NPIB derivatives 35b and 35c. To our delight, when 23 was allowed to react with fluorinated NPIB derivatives 35b and 35c in the presence of TFA in HFIP, coupled products 36 and 37 were formed in 50% and 32% yield, respectively. NiCl2-catalyzed hydrodefluorination using superhydride as a reductant37 afforded the defluorinated allylic alcohol product from compound 37. Subsequent DMP-mediated oxidation of the resulting alcohol resulted in dimethylated lacinilene E derivative 38 (67% yield over two steps). Markedly, the defluorinated product of compound 36 was not observed under the various conditions tested. Demethylation at the O7′ position of compound 38 was extremely challenging. 2,7-dihydroxycadalene (5) was obtained as a major product under various demethylation conditions used. We reasoned that the demethylation at O7′ induced the C–C bond cleavage via the formation of an ortho-quinone methide. In fact, when compound 38 was reacted with boron tribromide as a demethylating agent, a trace amount of lacinilene E (2) was obtained along with 2,7-dihydroxycadalene as a major product (for details, see Fig. S1†). Subjection of compound 38 to ethanethiol and sodium hydride in DMF delivered the methylated congener O7′-methyllacinilene E (39) in 61% yield along with 2,7-dihydroxycadalene (5) in 23% yield.
Conclusions
In conclusion, we have completed the total synthesis of alanenses A and B. To achieve the biosynthetically inspired38,39 arylation, we designed deoxygenative arylation reactions of α-hydroxycarbonyl derivatives. By utilizing α-N-phthalimido-oxy isobutyrate (NPIB) as a redox-active handle for hydroxyl groups, we have established a photoredox-catalyzed deoxygenative arylation reaction that proceeds via radical intermediates. Although challenges arose when applying this photocatalytic approach to the synthesis of alanense A, we overcame these obstacles by developing an alternative method using TFA/HFIP, which enabled the arylation of the NPIB derivative en route to the target natural products. Notably, our work demonstrated that the combination of the NPIB group with TFA and HFIP effectively promotes Friedel–Crafts reactions, even in the challenging context of generating carbocations adjacent (or conjugated) to electron-withdrawing groups such as carbonyl moieties. This methodology enabled streamlined total syntheses of alanenses A, B, and O7′-methyllacinilene E. Ongoing studies aim to further explore and expand the versatile reactivity of the NPIB group, with findings to be presented in future reports.Data availability
The experimental procedures and additional data can be found in the ESI.† Crystallographic data for the structure reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers 2403465 (14u), 2403466 (26), 2403461 (31), and 2403468 (33). Copies of the data can be obtained free of charge from the CCDC via https://www.ccdc.cam.ac.uk/structures/.Author contributions
Y. E. H. and S. H. conceived the study and obtained funding. S. H. supervised the project. Y. E. H. played a key role in the experimentation. J. K. carried out computational studies. T. H. L. P. investigated the substrate scope. D. K. performed the single crystal X-ray diffraction analysis. Y. E. H., J. K. and S. H. wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2019R1A6A1A10073887). This work was also supported by the National Research Foundation of Korea (NRF-2021R1A2C2011203), KAIST Grand Challenge 30 project, and KAIST Cross-Generation Collaborative Lab Project.References
- D. W. Christianson, Chem. Rev., 2017, 117, 11570–11648 CrossRef CAS PubMed.
- I. Pateraki, A. M. Heskes and B. Hamberger, Adv. Biochem. Eng./Biotechnol., 2015, 148, 107–139 CrossRef CAS PubMed.
- X.-Y. Chen, Y. Chen, P. Heinstein and V. J. Davisson, Arch. Biochem. Biophys., 1995, 324, 255–266 CrossRef CAS PubMed.
- C. A. Citron, J. Gleitzmann, G. Laurenzano, R. Pukall and J. S. Dickschat, Chembiochem, 2012, 13, 202–214 CrossRef CAS PubMed.
- R. D. Stipanovic, P. J. Wakelyn and A. A. Bell, Phytochemistry, 1975, 14, 1041–1043 CrossRef CAS.
- Y.-G. Xie, S.-l. Zhu, Y.-y. Huang, Y.-g. Guo, G.-j. Wu, I. Muhammad, S.-k. Yan, H.-z. Jin and W.-d. Zhang, Phytochem. Lett., 2018, 28, 64–68 CrossRef CAS.
- C.-L. Zhang, J. Liu, C.-C. Xi, Y.-G. Cao, J. He, S.-C. Li, F. Zhang, C. B. Naman and Z.-Y. Cao, J. Nat. Prod., 2022, 85, 599–606 CrossRef CAS PubMed.
- X.-s. Wang and E. I. Gruenstein, Brain Res., 1997, 767, 239–249 Search PubMed.
- R. D. Stipanovic, G. A. Greenblatt, R. C. Beier and A. A. Bell, Phytochemistry, 1981, 20, 729–730 CrossRef CAS.
- T. Mandal, S. Mallick, M. Islam and S. De Sarkar, ACS Catal., 2024, 14, 13451–13496 CrossRef CAS.
- A. Cook and S. G. Newman, Chem. Rev., 2024, 124, 6078–6144 CrossRef CAS PubMed.
- Z. Dong and D. W. C. MacMillan, Nature, 2021, 598, 451–456 Search PubMed.
- X. Zhang and D. W. C. MacMillan, J. Am. Chem. Soc., 2016, 138, 13862–13865 CrossRef CAS PubMed.
- L. Reginald Mills, J. J. Monteith, G. dos Passos Gomes, A. Aspuru-Guzik and S. A. L. Rousseaux, J. Am. Chem. Soc., 2020, 142, 13246–13254 CrossRef PubMed.
- W. Xu, C. Fan, X. Hu and T. Xu, Angew. Chem., Int. Ed., 2024, 63, e202401575 CrossRef CAS PubMed.
- S. K. Jana, R. Bhattacharya, P. Dey, S. Chakraborty and B. Maji, ACS Catal., 2024, 14, 14172–14182 CrossRef CAS.
- E. C. Gentry, L. J. Rono, M. E. Hale, R. Matsuura and R. R. Knowles, J. Am. Chem. Soc., 2018, 140, 3394–3402 CrossRef CAS PubMed.
- M. A. Hardy, J. Hayward Cooke, Z. Feng, K. Noda, I. Kerschgens, L. A. Massey, D. J. Tantillo and R. Sarpong, Angew. Chem., Int. Ed., 2024, 63, e202317348 CrossRef CAS PubMed.
- Z. Huang and J.-P. Lumb, Nat. Chem., 2021, 13, 24–32 CrossRef CAS PubMed.
- S. Lee, G. Kang and S. Han, Org. Lett., 2024, 26, 5640–5645 CrossRef CAS PubMed.
- A. Tlahuext-Aca, R. A. Garza-Sanchez and F. Glorius, Angew. Chem., Int. Ed., 2017, 56, 3708–3711 CrossRef CAS PubMed.
- K. Makino, R. Fukuda, S. Sueki and M. Anada, J. Org. Chem., 2024, 89, 2050–2054 CrossRef CAS PubMed.
- M. Kadarauch, T. A. Moss and R. J. Phipps, J. Am. Chem. Soc., 2024, 146, 34970–34978 CrossRef CAS PubMed.
- Y. T. Boni, J. Vaitla and H. M. L. Davies, Org. Lett., 2023, 25, 5–10 CrossRef CAS PubMed.
- S. Kotha, M. Behera and V. Shah, Synlett, 2005, 12, 1877–1880 CrossRef.
- S. N. Alektiar, J. Han, Y. Dang, C. Z. Rubel and Z. K. Wickens, J. Am. Chem. Soc., 2023, 145, 10991–10997 CrossRef CAS PubMed.
- J. P. McCormick, T. Shinmyozu, J. Paul Pachlatko, T. R. Schafer, J. W. Gardner and R. D. Stipanovic, J. Org. Chem., 1984, 49, 34–40 CrossRef CAS.
- Y. Zhang, Y. Liao, X. Liu, X. Xu, L. Lin and X. Feng, Chem. Sci., 2017, 8, 6645–6649 RSC.
- M. J. Cabrera-Afonso, M. Carmen Carreño and A. Urbano, Adv. Synth. Catal., 2019, 361, 4468–4473 CrossRef CAS.
- I. Colomer, A. E. R. Chamberlain, M. B. Haughey and T. J. Donohoe, Nat. Rev. Chem., 2017, 1, 0088 CrossRef CAS.
- K. Morimoto, K. Sakamoto, Y. Ohnishi, T. Miyamoto, M. Ito, T. Dohi and Y. Kita, Chem.–Eur. J., 2013, 19, 8726–8731 CrossRef CAS PubMed.
- A. Kumar, T. V. Singh, S. P. Thomas and P. Venugopalan, Eur. J. Org Chem., 2020, 2020, 2530–2536 Search PubMed.
- L. Chen and J. Zhou, Chem.–Asian J., 2012, 7, 2510–2515 CrossRef CAS PubMed.
- A. S.-K. Tsang, A. Kapat and F. Schoenebeck, J. Am. Chem. Soc., 2016, 138, 518–526 CrossRef CAS PubMed.
- Y. Ando, T. Hoshino, N. Tanaka, M. M. Maturi, Y. Nakazawa, T. Fukazawa, K. Ohmori and K. Suzuki, Angew. Chem., Int. Ed., 2025, 64, e202415108 CrossRef CAS PubMed.
- A. Pezzella, L. Lista, A. Napolitano and M. d'Ischia, Tetrahedron Lett., 2005, 46, 3541–3544 CrossRef CAS.
- J. Wu and S. Cao, ChemCatChem, 2011, 3, 1582–1586 CrossRef CAS.
- G. Kang and S. Han, Bull. Korean Chem. Soc., 2024, 45, 876–879 CrossRef CAS.
- R. C. Godfrey, H. E. Jones, N. J. Green and A. L. Lawrence, Chem. Sci., 2022, 13, 1313–1322 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details including characterization data and NMR spectra. CCDC 2403465, 2403466, 2403461 and 2403468. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc00341e |
| This journal is © The Royal Society of Chemistry 2025 |