Open Access Article
Open Access ArticleTailored engineering of primary catalytic sites and secondary coordination spheres in metalloenzyme-mimetic MOF catalysts for boosting efficient CO2 conversion†
Jiawei
Li
 *a,
Fan
Yang
a,
Benling
Yu
a,
Zhongke
Dai
a,
Shiyuan
Wei
a,
Ying
Wu
*bc,
Liuqing
He
d,
Fa
Zhou
a,
Jianhan
Huang
*a,
Fan
Yang
a,
Benling
Yu
a,
Zhongke
Dai
a,
Shiyuan
Wei
a,
Ying
Wu
*bc,
Liuqing
He
d,
Fa
Zhou
a,
Jianhan
Huang
 *a and
You-Nian
Liu
*a and
You-Nian
Liu
 a
a
aCollege of Chemistry and Chemical Engineering, Hunan Provincial Key Laboratory of Micro and Nano Material Interface Science, Central South University, Changsha 410083, Hunan, P. R. China. E-mail: lijiawei@csu.edu.cn; jianhanhuang@csu.edu.cn
bGuangDong Engineering Technology Research Center of Modern Fine Chemical Engineering, School of Chemical Engineering and Light Industry, Guangdong University of Technology, Guangzhou 510006, P. R. China
cSchool of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, Guangdong 510641, P. R. China. E-mail: yingwu@gdut.edu.cn
dThe Second Xiangya Hospital of Central South University, Changsha 410000, Hunan, P. R. China
First published on 8th April 2025
Abstract
The fabrication of metalloenzyme-mimetic artificial catalyst is a promising approach to achieve maximum catalytic efficiency, but the rational integration of sophisticatedly optimized primary catalytic sites (PCS) and secondary coordination spheres (SCS) for specific transformation poses a grand challenge. Here in this work, we report the tailored engineering of Cu PCS and perfluoroalkyl SCS onto a zirconium-based framework [UiO-67-(BPY-Cu)-Fx (x = 3, 5, 7, 11)] [BPY = 2,2′-bipyridine-5,5′-dicarboxylate] that can be utilized in the highly efficient carboxylic cyclization reaction between propargylamines and flue gas CO2. The perfluoroalkyl groups act as tunable SCS that can facilely adjust the surface electronegativity, hydrophobicity, as well as the CO2 affinity and water vapor-resistance by simply varying the chain length. Meanwhile, the synergy between the Cu PCS and perfluoroalkyl SCS significantly facilitated the cyclization step by stabilizing the critical transition state, leading to the fast cyclization to the oxazolidinone ring. Owing to these features, UiO-67-(BPY-Cu)-F7 exhibited remarkable metalloenzyme-mimetic catalytic behavior by greatly facilitating the binding of propargylamines and CO2, promoting the stabilization of the critical transition state to cyclization, and boosting the releasing of oxazolidinones, which have been systematically investigated by the combination of substrate adsorption tests, in situ Fourier transform infrared spectra, grand canonical Monte Carlo simulations, density functional theory calculations, etc. Consequently, UiO-67-(BPY-Cu)-F7 showed outstanding catalytic performance in the carboxylic cyclization of propargylamines and flue gas CO2 under ambient conditions, exhibiting 64 times higher turnover frequency (TOF) than that of homogeneous or other MOF catalysts, and exhibiting the highest TOF under similar conditions. The present work not only provides an alternative strategy for the construction of advanced carboxylic cyclization systems, but also paves a new direction in the development of CO2 conversion with exceptional activity through the tailored engineering of PCS and SCS in metalloenzyme-mimetic artificial catalysts.
Introduction
As the primary greenhouse gas contributing to climate change, the escalating levels of carbon dioxide (CO2) emission present formidable environment challenges on a global scale. In this context, capturing and converting CO2 into high-value products have emerged as a pivotal frontier in the realm of sustainable chemistry.1 The carboxylic cyclization of propargylamine and CO2 serves as a facile pathway for the preparation of 2-oxazolidinones,2 a N-containing heterocyclic compound with widespread applications in antibacterial drugs, monoamine oxidase inhibitors, etc., which have a global market of billions of dollars annually.3 From the perspectives of economy and ecology, the utilization of low-cost catalysts such as non-noble-metals and CO2 directly from flue gas under ambient conditions is highly favorable, but the inherent inertness of both the non-noble-metal and low-concentration CO2 in flue gas led to largely compromised efficiency.4 This paradox between economy, ecology and efficiency unambiguously underlines the most critical challenge in advancing towards a more practical CO2 conversion paradigm.Metalloenzymes, as the most powerful catalysts towards specific biochemical transformation, offer unique insights from nature to the fabrication of advanced CO2-converting systems.5 The exceptional activity of metalloenzymatic catalysis arises from the sophisticatedly optimized primary catalytic sites (PCS) and secondary coordination spheres (SCS) within a metalloenzyme, which supports highly cooperative substrate binding, confined catalysis, and product releasing processes (Scheme 1a).6 Generally, the PCS are composed of non-noble-metals and an inner coordination sphere to drive the biochemical catalysis, while the SCS play an essential role in facilitating substrate binding, stabilizing the transition state and conformational structure, and promoting the release of products.7 Therefore, the key fundamental principle to fabricate a highly efficient metalloenzyme-mimetic artificial catalyst is the construction of catalytic pockets with judiciously engineered PCS and SCS, where the entire structure can proceed in a concerted manner to emulate the essential features of a metalloenzyme. In this context, metal–organic-frameworks (MOFs) as a highly porous, versatile and tunable platform should be an ideal selection by rationally incorporating essential metal active sites and an appropriate surrounding microenvironment.8
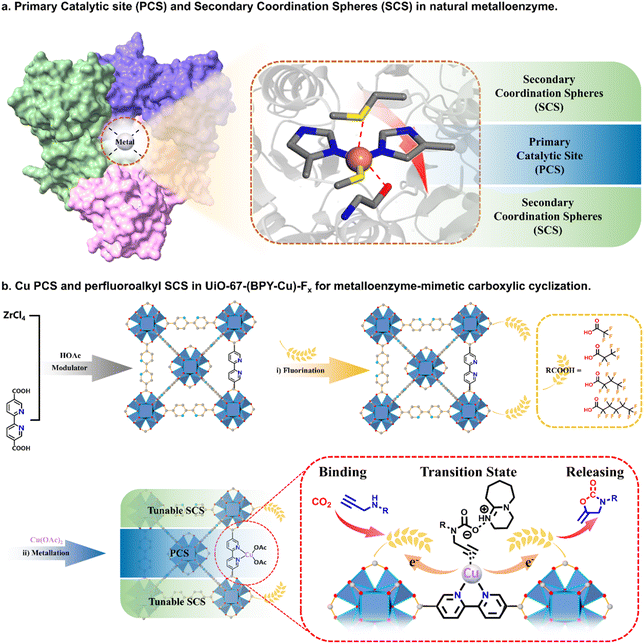 | ||
| Scheme 1 (a) PCS and SCS in a natural metalloenzyme. (b) Synthetic scheme of [UiO-67-(BPY-Cu)-Fx (x = 3, 5, 7, 11)]. | ||
Here we attempted the tailored engineering of PCS and SCS in MOF catalysts for the development of a metalloenzyme-mimetic carboxylic cyclization process by a systematic evaluation of the molecular properties. Firstly, for the substrate propargylamine, whose skeleton consists of both hydrophobic alkynyl and hydrophilic –NH groups, an appropriate surface hydrophobicity of SCS is necessary for the favorable binding of propargylamine. For the other substrate flue gas CO2, which is always accompanied by dilute CO2 and water vapor,9 excellent CO2-philicity, as well as superior H2O-resistance properties are indispensable for maintaining long-term activity and stability. Secondly, the charge distributions of the product oxazolidinone skeletons were investigated by density functional theory (DFT) calculations. By using N-methyl propargylic amine (1a) and the corresponding 2-oxazolidinone (2a) as the basic model substances, the simulation results showed that 2a is overall more electronegative than 1a, suggesting that prominent electronegative SCS may be conducive to the efficient release of oxazolidinone. Additionally, the metal catalyst has been proved to promote the cyclization to oxazolidinone through stepwise charge transfer processes.4b Thus, the rational regulation of the electronic state of PCS through the synergy with SCS might be reasonable for improving its intrinsic activity.
Bearing these in mind, we report the fabrication of metalloenzyme-mimetic noble-metal-free MOF catalysts [UiO-67-(BPY-Cu)-Fx (x = 3, 5, 7, 11)] for the highly efficient carboxylic cyclization reaction between propargylamine and flue gas CO2 (Scheme 1b). Through the tailored engineering of Cu as the PCS and perfluoroalkyl as tunable SCS onto the framework, UiO-67-(BPY-Cu)-F7 exhibited remarkable metalloenzyme-mimetic catalytic behavior by greatly facilitating the binding of propargylamines and CO2, boosting the confined catalytic reaction, and promoting the release of oxazolidinones, which leads to a 64 times higher turnover frequency (TOF) than that of homogeneous or other MOF catalysts, and exhibiting the highest TOF in a non-noble-metal-catalyzed carboxylic cyclization reaction under ambient conditions.
Results and discussion
Synthesis and characterization of MOF catalysts
UiO-67-BPY was prepared according to the reported method.10 As illustrated in Scheme 1b, UiO-67-BPY was initially synthesized by the combination of ZrCl4 and BPY in DMF at 120 °C for 24 hours, utilizing HOAc as the modulator. The resulting UiO-67-BPY exhibited a highly crystalline structure, as evidenced by the powder X-ray diffraction (PXRD) pattern, which matched well with the simulated one (Fig. 1a). The N2 adsorption–desorption isotherms at 77 K revealed a reversible type I microporous adsorption isotherm according to the IUPAC classification, with a calculated BET surface area of 2369 m2 g−1 (Fig. 1b). Scanning electron microscopy (SEM) images depicted UiO-67-BPY as regular and uniform octahedral morphology, with a diameter of 1 μm (Fig. S1†). It is noteworthy that the treatment of excess modulator in the synthesis of UiO series MOFs usually leads to a residual modulator coordinated in the Zr-clusters, which could be further exchanged by other functional groups.11 To identify the quantity of the residual HOAc in UiO-67-BPY, 1H NMR analysis of the digested UiO-67-BPY was first conducted, indicating a BPY/HOAc ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.38 (Fig. 1c). Thus, the molecular formula of the defective UiO-67-BPY can be derived as Zr6O4(OH)4(BPY)5.04(OAc)1.91. To further verify the molecular formula of the defective UiO-67-BPY, thermogravimetry analysis was conducted. As shown in Fig. 1d, the first weight loss (4.95%) from 30 to 100 °C corresponds to the removal of adsorbed solvents in the MOF. The second weight loss (62.77%) from 100–800 °C corresponds to decomposition of UiO-67-BPY to 6ZrO2 (739.34 g mol−1), consistent with a weight loss of 63.39% based on the conversion of Zr6O4(OH)4(BPY)5.04(OAc)1.91 to 6ZrO2.
0.38 (Fig. 1c). Thus, the molecular formula of the defective UiO-67-BPY can be derived as Zr6O4(OH)4(BPY)5.04(OAc)1.91. To further verify the molecular formula of the defective UiO-67-BPY, thermogravimetry analysis was conducted. As shown in Fig. 1d, the first weight loss (4.95%) from 30 to 100 °C corresponds to the removal of adsorbed solvents in the MOF. The second weight loss (62.77%) from 100–800 °C corresponds to decomposition of UiO-67-BPY to 6ZrO2 (739.34 g mol−1), consistent with a weight loss of 63.39% based on the conversion of Zr6O4(OH)4(BPY)5.04(OAc)1.91 to 6ZrO2.
After determining the exact quantity of the defects, we attempted the direct binding of perfluoroalkyl carboxylic acids with the unsaturated Zr6-clusters. The UiO-67-BPY crystals were immersed in the solutions of perfluoroalkyl carboxylic acids with varying chain lengths (CnF2n+1COOR, n = 1, 2, 3, 5) in DMF at room temperature for 24 hours, resulting in the formation of UiO-67-BPY-Fx (x = 3, 5, 7, 11). The successful fluorination was first confirmed using the 19F NMR spectra of the acid-digested samples of UiO-67-BPY-Fx, which clearly showed the characteristic peaks of different perfluoroalkyl chains (Fig. 1e). Meanwhile, 1H NMR analysis of the digested UiO-67-BPY-Fx showed that the peaks corresponding to HOAc almost disappeared (Fig. 1f), indicating the complete substitution of the regulator HOAc by the perfluoroalkyl groups. Moreover, the water contact angle tests manifested a notable increase with the elongation of the length of perfluoroalkyl chains, increasing from 75° for UiO-67-BPY to 140° for UiO-67-BPY-F11 (Fig. S2†). These results demonstrated the successful transformation of the surface microenvironment from hydrophilic to hydrophobic by the incorporation of perfluoroalkyl chains.
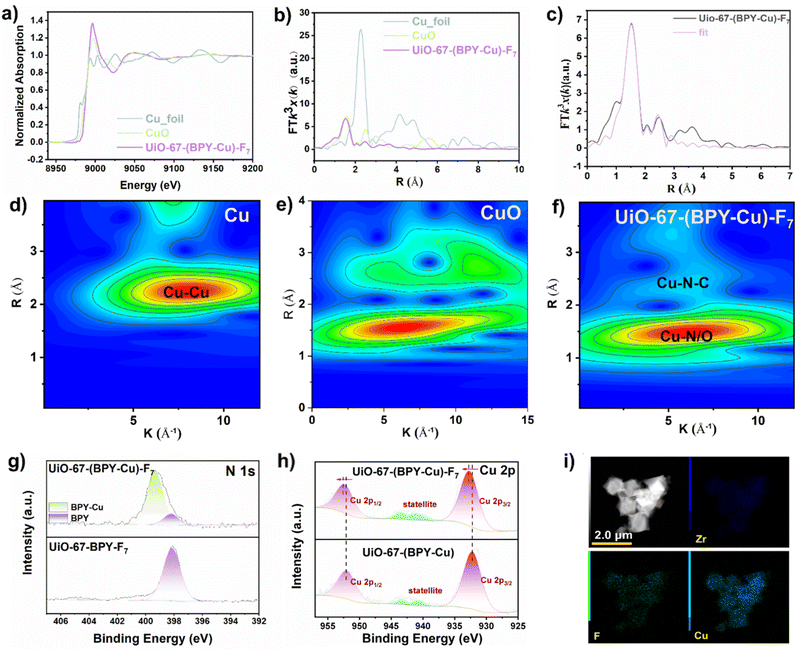
Finally, the post-synthetic metalation of UiO-67-BPY-Fx with Cu(OAc)2·H2O was carried out in acetonitrile at room temperature for 24 hours to afford UiO-67-(BPY-Cu)-Fx (x = 3, 5, 7, 11). The integrity of the MOF structures after the metalation was confirmed using the PXRD patterns (Fig. 1a). The N2 adsorption results at 77 K indicated a decrease in the adsorption amounts and SBET of UiO-67-(BPY-Cu)-Fx, as a result of the pore space occupation by perfluoroalkyl chains and metal species (Fig. 1b). The TEM and elemental mapping images displayed a uniform distribution of Cu over the block crystals (Fig. 2i). Inductively coupled plasma mass spectrometry (ICP-MS) indicated that the Cu content in the UiO-67-(BPY- Cu)-Fx was about 10.5 wt%. To ascertain the valence state and coordination modes of the Cu species in the metallized MOFs, X-ray photoelectron spectroscopy (XPS) analyses were conducted. As shown in Fig. 2g, the binding energy (BE) of the N 1s peak at 398.2 eV can be ascribed to the N atoms in the free BPY ligand, while the BE of the N atom at 399.2 eV is attributed to the BPY-Cu species for UiO-67-(BPY-Cu)-F7,12 verifying the successful coordination of Cu(OAc)2 to the framework. In addition, the characteristic peaks of BEs of Cu 2p3/2 at 934 eV and Cu 2p1/2 at 945 eV, as well as the satellite peaks, both suggest that the oxidation state of Cu is +2 in UiO-67-(BPY-Cu)-Fx (Fig. 2h).13 It is noteworthy that the peaks of BEs of Cu in UiO-67-(BPY-Cu) shifted to lower positions than that in UiO-67-(BPY-Cu)-F7, suggesting the electron-withdrawing effect of perfluoroalkyl groups on Cu centers. Moreover, we calculated the charge density difference of Cu in UiO-67-(BPY-Cu) and UiO-67-(BPY-Cu)-F7, which indicated that after the decoration of perfluoroalkyl, the Cu centers mainly lose electrons, and the positive electric field is more significant in UiO-67-(BPY-Cu)-F7 (Fig. S4†). The above results verified the apparent interactions between the Cu PCS and perfluoroalkyl SCS in UiO-67-(BPY-Cu)-F7.
To further determine the coordination environments and oxidation states of Cu in UiO-67-(BPY-Cu)-F7, X-ray absorption fine structure (XAFS) was carried out. X-ray absorption near-edge structure (XANES) spectroscopy of different standard samples confirmed the CuII oxidation state in UiO-67-(BPY-Cu)-F7 (Fig. 2a). Fitting of the extended X-ray absorption fine structure (EXAFS) data for UiO-67-(BPY-Cu)-F7 at the Cu K-edge manifests that the coordination number of Cu is about 4, suggesting the coordination to two N atoms from BPY and two O atoms from two OAc- groups with the average Cu–N/O bond length of 1.98 Å (Fig. 2b, Table S1†). The wavelet-transform EXAFS analysis further supported that the Cu coordination bond length parameters in UiO-67-(BPY-Cu)-F7 (k = 5.4 Å, r = 1.5 Å) were comparable to those observed in CuO (k = 5.5 Å, r = 1.6 Å) rather than Cu foil (k = 7.8 Å, r = 2.3 Å), demonstrating the presence of Cu–N/O bonds in these structures (Fig. 2d–f). The above results clearly confirmed the successful construction of UiO-67-(BPY-Cu)-Fx.
Catalytic performances in the cyclization reaction
With the well-prepared UiO-67-(BPY-Cu)-Fx in hand, we started to examine their catalytic performances in the carboxylic cyclization of propargylamine and CO2. Initially, the reaction between 1a and CO2 in the presence of Cu(OAc)2 (5 mol%) at 25 °C gave the corresponding product with a yield of only 17% (Table 1, entry 2). Similarly, when 1,8-diazabicyclo [5,4,0] undecene-7 (DBU) (25 mol%) was used alone, the yield of the product was 22% (Table 1, entry 1). By using the mixture of Cu(OAc)2 (5 mol%) and DBU (25 mol%) as the co-catalyst, the product yield elevated to 52% (Table 1, entry 3). Upon employing UiO-67-(BPY-Cu) (5 mol% Cu) as the catalyst, a further increase of the yield to 67% can be observed (Table 1, entry 4). Impressively, the introduction of perfluoroalkyl groups on the framework gave greatly increased catalytic efficiency. For example, incorporating a short perfluoroalkyl (F3) group can give the product with a yield of 66% with largely reduced Cu loading (0.5 mol%) (Table 1, entry 5). When the length of the perfluoroalkyl chain was extended from F5 to F7, an optimal yield of 99% and a remarkably elevated TOF value of 49.5 can be achieved (Table 1, entries 6 and 7), which is 19 and 15 times higher than that of the homogeneous counterpart and the unfluorinated MOF, respectively, and is among the highest TOF in non-noble-metal-catalyzed carboxylic cyclization reactions under similar conditions (Table S2†). Further extending the perfluoroalkyl chain to F11 led to a slight decline in the activity, possibly due to the pore size limitation and mass transfer hindrance (Table 1, entry 8). These findings showed the prominent impact of the perfluoroalkyl decoration on the catalytic performance.| Entry | Catalyst | CO2 | Yieldb (%) | TONc | TOFd (h−1) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (1 mmol), CH3CN (2 ml), DBU (0.25 mmol), catalyst (0.5 mol% Cu), 25 °C, 4 h (pure CO2) or 9 h (15% CO2). b Yields are determined by NMR with trimethylbenzene as the internal standard. c TON: moles of product/catalyst. d TOF: TON per t. e 5 mol% Cu and without DBU. f 5 mol% Cu. g Catalyst was exposed to humid conditions (RH: 70%) for 12 h before the reaction. h N2: 80.5%, CO2: 15%, O2: 4%, SO2: 0.5%. i Fifth catalytic cycle. | |||||
| 1 | — | Pure | 22% | — | — |
| 2e | Cu(OAc)2 | Pure | 17% | 3.4 | 0.85 |
| 3f | Cu(OAc)2 | Pure | 52% | 10.4 | 2.6 |
| 4f | UiO-67-(BPY-Cu) | Pure | 67% | 13.4 | 3.35 |
| 5 | UiO-67-(BPY-Cu)-F3 | Pure | 66% | 132 | 33 |
| 6 | UiO-67-(BPY-Cu)-F5 | Pure | 88% | 176 | 44 |
| 7 | UiO-67-(BPY-Cu)-F7 | Pure | 99% | 198 | 49.5 |
| 8 | UiO-67-(BPY-Cu)-F11 | Pure | 93% | 186 | 46.5 |
| 9f | UiO-67-(BPY-Cu) | CO2 (15%) | 35% | 7 | 0.78 |
| 10 | UiO-67-(BPY-Cu)-F7 | CO2 (15%) | 91% | 182 | 20.22 |
| 11f | UiO-67-(BPY-Cu) | CO2 (15%)g | 15% | 3 | 0.33 |
| 12 | UiO-67-(BPY-Cu)-F7 | CO2 (15%)g | 95% | 190 | 21.11 |
| 13 | UiO-67-(BPY-Cu)-F7 | Flue gash | 91% | 182 | 20.22 |
| 14i | UiO-67-(BPY-Cu)-F7 | Flue gash | 90% | 180 | 20 |
This discrepancy in the activity becomes more pronounced when diluted CO2 (CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2 = 15
N2 = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85) was utilized in the catalytic reaction. When UiO-67-(BPY-Cu) (5 mol% Cu) was employed as the catalyst, an obvious reduced yield of 35% was observed in the diluted CO2 atmosphere (Table 1, entry 9). In stark contrast, UiO-67-(BPY-Cu)-F7 (0.5 mol% Cu) still exhibited outstanding catalytic performance under the same reaction conditions, emphasizing its proficiency in the adsorption and conversion of dilute CO2 (Table 1, entry 10). In order to further assess the catalyst's performance under conditions mimicking a real industrial setting, we first conducted the catalytic reaction with diluted CO2 in a humid environment. This choice stems from the fact that industrial flue gas typically contains considerable amount of water vapor. Impressively, the results turned out that UiO-67-(BPY-Cu) can only give a yield of 15% (Table 1, entry 11), while the existence of water vapor exerts no influence on the reactivity of UiO-67-(BPY-Cu)-F7 under identical reaction conditions (Table 1, entry 12), exhibiting a significant 64 times higher TOF value. This dominant contrast in activity can be attributed to their distinct difference in the hydrothermal stability. As illustrated in Fig. 3a, the water vapor adsorption amount of UiO-67-(BPY-Cu)-F7 significantly decreased compared to its unfluorinated counterpart, highlighting its superior resistance towards water vapor. Furthermore, PXRD measurement revealed that UiO-67-(BPY-Cu)-F7 still retained its framework integrity after exposure to water vapor treatment, while the diffraction peak of UiO-67-(BPY-Cu) totally disappeared, indicating the decomposition of the framework (Fig. S6†). Moreover, by utilizing a more complex flue gas (N2: 80.5%, CO2: 15%, O2: 4%, SO2: 0.5%), UiO-67-(BPY-Cu)-F7 still exhibited a high yield of 91% in the carboxylic cyclization, and could be reused for five times without apparent loss of activity, highlighting its potential application under practical flue gas conditions (Table 1, entries 13 and 14). The combination of PXRD, N2 adsorption and XPS characterization studies of UiO-67-(BPY-Cu)-F7 after the catalysis was conducted, showing that the crystallinity, porosity and the Cu active sites are well retained after the carboxylic cyclization test (Fig. S7–S9†). These findings underlined the pivotal role played by perfluoroalkyl decoration in imparting superior stability to the MOF catalyst, thereby preserving its structural integrity and catalytic efficacy under the challenging conditions of practical flue gas environment. These results are particularly crucial in the context of evaluating the catalyst's adaptability and efficiency in real-world scenarios.
85) was utilized in the catalytic reaction. When UiO-67-(BPY-Cu) (5 mol% Cu) was employed as the catalyst, an obvious reduced yield of 35% was observed in the diluted CO2 atmosphere (Table 1, entry 9). In stark contrast, UiO-67-(BPY-Cu)-F7 (0.5 mol% Cu) still exhibited outstanding catalytic performance under the same reaction conditions, emphasizing its proficiency in the adsorption and conversion of dilute CO2 (Table 1, entry 10). In order to further assess the catalyst's performance under conditions mimicking a real industrial setting, we first conducted the catalytic reaction with diluted CO2 in a humid environment. This choice stems from the fact that industrial flue gas typically contains considerable amount of water vapor. Impressively, the results turned out that UiO-67-(BPY-Cu) can only give a yield of 15% (Table 1, entry 11), while the existence of water vapor exerts no influence on the reactivity of UiO-67-(BPY-Cu)-F7 under identical reaction conditions (Table 1, entry 12), exhibiting a significant 64 times higher TOF value. This dominant contrast in activity can be attributed to their distinct difference in the hydrothermal stability. As illustrated in Fig. 3a, the water vapor adsorption amount of UiO-67-(BPY-Cu)-F7 significantly decreased compared to its unfluorinated counterpart, highlighting its superior resistance towards water vapor. Furthermore, PXRD measurement revealed that UiO-67-(BPY-Cu)-F7 still retained its framework integrity after exposure to water vapor treatment, while the diffraction peak of UiO-67-(BPY-Cu) totally disappeared, indicating the decomposition of the framework (Fig. S6†). Moreover, by utilizing a more complex flue gas (N2: 80.5%, CO2: 15%, O2: 4%, SO2: 0.5%), UiO-67-(BPY-Cu)-F7 still exhibited a high yield of 91% in the carboxylic cyclization, and could be reused for five times without apparent loss of activity, highlighting its potential application under practical flue gas conditions (Table 1, entries 13 and 14). The combination of PXRD, N2 adsorption and XPS characterization studies of UiO-67-(BPY-Cu)-F7 after the catalysis was conducted, showing that the crystallinity, porosity and the Cu active sites are well retained after the carboxylic cyclization test (Fig. S7–S9†). These findings underlined the pivotal role played by perfluoroalkyl decoration in imparting superior stability to the MOF catalyst, thereby preserving its structural integrity and catalytic efficacy under the challenging conditions of practical flue gas environment. These results are particularly crucial in the context of evaluating the catalyst's adaptability and efficiency in real-world scenarios.
Under the optimized reaction conditions, we then evaluated the catalytic activity of the catalyst towards different substrates, and the outcomes are summarized in Table S3.† In the presence of UiO-67-(BPY-Cu)-F7 and a pure CO2 atmosphere, diverse propargylamine compounds with different substituents can lead to the corresponding 2-oxazolidinone products with excellent activity. For example, different propargylamines bearing aliphatic groups 2a–c (-CH3, n-Bu, and n-Hex) can be well tolerated in this catalytic system. Meanwhile, benzyl propargylamines bearing both electron-donating (OMe, Me) and electron-withdrawing (F, Cl, and CF3) groups 2d–i can convert to the corresponding oxazolidinones smoothly. It is noteworthy that these reactions also proceeded efficiently when using low-concentration CO2 under humid conditions, with no significant loss of activity. These results highlighted the wide applicability of UiO-67-(BPY-Cu)-F7 in the carboxylic cyclization reaction between propargylamine and flue gas CO2.
Investigation of the metalloenzyme-mimetic catalytic mechanism
In pursuit of unraveling the metalloenzyme-mimetic catalytic process of UiO-67-(BPY-Cu)-Fx in the carboxylic cyclization reaction, we conducted an in-depth mechanism investigation combining several experiments and theoretical calculations. Initially, the substrate binding processes of different catalytic systems were evaluated. For propargylamine, its affinity performance on UiO-67-(BPY-Cu)-Fx was first examined by contact angle experiments using 1a as the model substance. The results showed that 1a exhibited excellent affinity towards all these perfluoroalkyl-decorated MOF catalysts with contact angles ranging from 10–16° (Fig. S10†). Then, we further investigated the adsorption kinetics of propargylamine to distinguish the binding behaviors of the different catalysts. Among the various MOFs tested, UiO-67-(BPY-Cu)-F7 exhibited discernibly superior enrichment and adsorption behaviors of 1a, probably owing to its most appropriate surface hydrophobicity and electronegativity (Fig. 3b and 4a). For the other substrate CO2, the adsorption and desorption isotherms were measured at 0 °C (∼273 K) and 25 °C (∼298 K) for all obtained MOFs (Fig. 3c and S11†). A comparison of the isotherms between UiO-67-BPY and UiO-67-(BPY-Cu)-Fx indicated increased CO2 uptake with the inclusion of the perfluoroalkyl chains. This phenomenon is attributed to the interaction of C–F dipoles with the quadrupole of CO2, rendering preferential positioning of CO2 molecules around the perfluoroalkyl groups within the framework, as verified by DFT calculations (Fig. 4b). The isosteric heat of adsorption (Qst) ranged from 13.9 to 32.6 kJ mol−1 for these MOFs, lower than that of chemical adsorption (41.7–84.0 kJ mol−1) (Fig. S12†). The above results clearly suggested the remarkable effect of the SCS on the efficient binding of both propargylamine and CO2.Next, in situ FT-IR spectra were recorded for UiO-67-(BPY-Cu) and UiO-67-(BPY-Cu)-F7 to study their desorption behavior of 2-oxazolidinones (2a), which helps to shed light on the product releasing process. As shown in Fig. 3d, an obvious characteristic stretching vibration band of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond at 1768 cm−1 and the asymmetric and symmetric stretching vibration bands of the C–O bond at around 1247 and 1050 cm−1 assignable to 2a were similarly observed for both UiO-67-(BPY-Cu) and UiO-67-(BPY-Cu)-F7 after the adsorption of 2a. However, after further purging the samples with N2, the characteristic peaks of 2a at 1768, 1247 and 1050 cm−1 almost vanished in UiO-67-(BPY-Cu)-F7, while the adsorption of 2a was still apparently detectable in UiO-67-(BPY-Cu). These results implied the obvious weaker adsorption interaction between 2a and UiO-67-(BPY-Cu)-F7, which can be ascribed to the repulsive effect by the electronegative pore structure of the MOF and the skeleton of 2a. Indeed, our grand canonical Monte Carlo simulation results indicated that UiO-67-(BPY-Cu)-F7 preferentially interacts with the reactant 1a and CO2 rather than the product 2a, where 1a and CO2 molecules stabilize closer together due to the attraction effects of F7 chains, with the C (CO2)–F and H (1a)–F distance of 3.12 Å and 5.17 Å, respectively, compared to the H (2a)–F distance of 5.84–7.25 Å (Fig. 4b). This competitive adsorption behavior may be attributed to the different electrostatic nature of 1a and 2a. As illustrated in Fig. 4a, compared to the negative charges of oxygen atoms (−0.41 to −0.26 e) in 2a, the relatively positive charges of H atoms (0.06–0.21 e) in 1a allow the molecule to be thermodynamically more favorable towards F atoms with negative charges (about −0.25 e). This feature was significantly beneficial to the desorption of 2a from the surface of UiO-67-(BPY-Cu)-F7, thereby promoting the product unleashing process.
O bond at 1768 cm−1 and the asymmetric and symmetric stretching vibration bands of the C–O bond at around 1247 and 1050 cm−1 assignable to 2a were similarly observed for both UiO-67-(BPY-Cu) and UiO-67-(BPY-Cu)-F7 after the adsorption of 2a. However, after further purging the samples with N2, the characteristic peaks of 2a at 1768, 1247 and 1050 cm−1 almost vanished in UiO-67-(BPY-Cu)-F7, while the adsorption of 2a was still apparently detectable in UiO-67-(BPY-Cu). These results implied the obvious weaker adsorption interaction between 2a and UiO-67-(BPY-Cu)-F7, which can be ascribed to the repulsive effect by the electronegative pore structure of the MOF and the skeleton of 2a. Indeed, our grand canonical Monte Carlo simulation results indicated that UiO-67-(BPY-Cu)-F7 preferentially interacts with the reactant 1a and CO2 rather than the product 2a, where 1a and CO2 molecules stabilize closer together due to the attraction effects of F7 chains, with the C (CO2)–F and H (1a)–F distance of 3.12 Å and 5.17 Å, respectively, compared to the H (2a)–F distance of 5.84–7.25 Å (Fig. 4b). This competitive adsorption behavior may be attributed to the different electrostatic nature of 1a and 2a. As illustrated in Fig. 4a, compared to the negative charges of oxygen atoms (−0.41 to −0.26 e) in 2a, the relatively positive charges of H atoms (0.06–0.21 e) in 1a allow the molecule to be thermodynamically more favorable towards F atoms with negative charges (about −0.25 e). This feature was significantly beneficial to the desorption of 2a from the surface of UiO-67-(BPY-Cu)-F7, thereby promoting the product unleashing process.
Subsequently, we elucidated the confined catalytic process by DFT calculations. We utilized the BPY-Cu(OAc)2 moiety and the adjacent Zr6 cluster in both UiO-67-(BPY-Cu)-F7 (Cat_CuAc_F) and UiO-67-(BPY-Cu) (Cat_CuAc) as the simulation models to clarify the synergy of the Cu PCS and the perfluoroalkyl SCS. Owing to the favorable substrate binding properties of UiO-67-(BPY-Cu)-F7, propargylamine 1a and CO2 were preferentially adsorbed and accumulated on the catalyst's surface. Then, the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C in 1a was activated through the coordination to Cu. Subsequently, –NH group in 1a undergoes electrophilic attack by CO2 with the assistance of DBU to produce the critical transition state (TS2). Impressively, DFT calculations indicated that the energy barrier of the TS2 step for Cat_CuAc_F is significantly lower compared to that of Cat_CuAc (−2.98 vs. −1.24 kcal mol−1). This apparent discrepancy results from the much more electron-deficient Cu site in Cat_CuAc_F due to the intense electron-withdrawing effect of the perfluoroalkyl group, which facilitates the electron transfer from the alkynyl group to Cu and thus promotes the nucleophilic attack of the negatively charged oxygen atom on the alkynyl. Eventually, cyclization from the TS2 complex to TS3 occurred, and the formed intermediate undergoes H transfer to regenerate the base, and give the final product 2a. Note that the product 2a can be rapidly released from the catalyst's surface and start another catalytic cycle.
C in 1a was activated through the coordination to Cu. Subsequently, –NH group in 1a undergoes electrophilic attack by CO2 with the assistance of DBU to produce the critical transition state (TS2). Impressively, DFT calculations indicated that the energy barrier of the TS2 step for Cat_CuAc_F is significantly lower compared to that of Cat_CuAc (−2.98 vs. −1.24 kcal mol−1). This apparent discrepancy results from the much more electron-deficient Cu site in Cat_CuAc_F due to the intense electron-withdrawing effect of the perfluoroalkyl group, which facilitates the electron transfer from the alkynyl group to Cu and thus promotes the nucleophilic attack of the negatively charged oxygen atom on the alkynyl. Eventually, cyclization from the TS2 complex to TS3 occurred, and the formed intermediate undergoes H transfer to regenerate the base, and give the final product 2a. Note that the product 2a can be rapidly released from the catalyst's surface and start another catalytic cycle.
After determining the substrate binding, confined catalytic reaction and product releasing processes of UiO-67-(BPY-Cu)-F7, we further investigated its rate constant to evaluate the reaction kinetics. We first monitored the catalytic yields of UiO-67-(BPY-Cu) and UiO-67-(BPY-Cu)-F7 in the reaction between 1a and diluted CO2 over its reaction time. The results showed that UiO-67-(BPY-Cu)-F7 gave apparently overwhelming activity compared to its unfluorinated counterpart (Fig. 3e). Specifically, UiO-67-(BPY-Cu)-F7 exhibited an obviously higher catalytic efficiency from the initiation of the reaction, and quickly reached 91%, while the catalytic rate of UiO-67-(BPY-Cu) was notably slow, ultimately achieving a yield of only 35%. In order to quantitatively evaluate the reactivities of UiO-67-(BPY-Cu) and UiO-67-(BPY-Cu)-F7, their apparent reaction rate constants were calculated. As is evident from Fig. 3f, UiO-67-(BPY-Cu)-F7 (k = 106.84 ml h−1 mmolCu−1) results in 55 times higher rate than the unfluorinated UiO-67-(BPY-Cu) (k = 1.96 ml h−1 mmolCu−1), implying that the synergy between the Cu PCS and perfluoroalkyl SCS facilitates greatly enhanced reaction kinetics in the carboxylic cyclization reaction. All the above results unambiguously highlighted the metalloenzyme-mimetic catalytic process of UiO-67-(BPY-Cu)-F7 in the carboxylic cyclization reaction.
Conclusions
In summary, we have reported the tailored engineering of metalloenzyme-mimetic non-noble-metal MOF catalysts by introducing the single-site Cu as the PCS and perfluoroalkyl groups as tunable SCS. The combination of substrate adsorption tests, in situ Fourier transform infrared spectra, GCMC simulations and DFT calculations highlighted the remarkable metalloenzyme-mimetic catalytic behavior of UiO-67-(BPY-Cu)-F7 in the carboxylic cyclization reaction by greatly promoting the binding of propargylamines and flue gas CO2, stabilizing the transition state to cyclization, and facilitating the oxazolidinone release. These metalloenzyme-mimetic features of UiO-67-(BPY-Cu)-Fx led to overwhelming catalytic performance in the carboxylic cyclization reaction, exhibiting the highest TOF in non-noble-metal-catalyzed carboxylic cyclization reactions under ambient conditions. This work proposes an alternative strategy for the construction of advanced carboxylic cyclization catalytic systems for the facile preparation of 2-oxazolidinones. It also offers a promising new insight into the development of highly efficient CO2 conversion through the tailored engineering of PCS and SCS in metalloenzyme-mimetic artificial catalysts.Data availability
The data that support the findings of this study are available in the ESI† of this article.Author contributions
Jiawei Li and Fan Yang contributed the central idea, analyzed most of the data, and wrote the initial draft of the paper. Jianhan Huang acquired of the financial support for the project leading to this publication. The remaining authors contributed to refining the ideas, carrying out additional analyses and finalizing this paper.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully thank the National Natural Science Foundation of China (No. 22308389 and No. 22478442), the Guangdong Basic and Applied Basic Research Foundation (2023A1515030274), the Science and Technology Projects in Guangzhou (2023A04J1378), and the Fundamental Research Funds for the Central Universities of Central South University (2023ZZTS0321) for the financial support.Notes and references
- (a) P. D. Luna, C. Hahn, D. Higgins, S. A. Jaffer, T. F. Jaramillo and E. H. Sargent, Science, 2019, 364, eaav3506 CrossRef PubMed; (b) C. A. Trickett, A. Helal, B. A. Al-Maythalony, Z. H. Yamani, K. E. Cordova and O. M. Yaghi, Nat. Rev. Mater., 2017, 2, 17045 CrossRef CAS; (c) M. Ding, R. W. Flaig, H.-L. Jiang and O. M. Yaghi, Chem. Soc. Rev., 2019, 48, 2783–2828 RSC; (d) G. A. Olah, G. K. S. Prakash and A. Goeppert, J. Am. Chem. Soc., 2011, 133, 12881–12898 CrossRef CAS PubMed.
- (a) X. Yang, L. Xu, Y. Zhu, S. Zhang, G. Jia and J. Du, J. CO2 Util., 2023, 74, 102531 CrossRef CAS; (b) A. Sattar, V. Esmail, S. Marjan, H. Akram and B. Ahmedreza, J. CO2 Util., 2017, 19, 120–129 CrossRef.
- (a) J. S. Karen and R. B. Michael, Ann. N. Y. Acad. Sci., 2011, 1241, 48–70 CrossRef PubMed; (b) D. J. Diekema and R. N. Jones, Lancet, 2001, 358, 1975–1982 CrossRef CAS PubMed; (c) Q. Zhao, L. Xin, Y. Liu, C. Liang, J. Li, Y. Jian, H. Li, Z. Shi, H. Liu and W. Cao, J. Med. Chem., 2021, 64, 10557–10580 CrossRef CAS PubMed.
- (a) H.-N. Qin, M.-W. He, J. Wang, H.-Y. Li, Z.-Y. Wang, S.-Q. Zang and C. W. M. Thomas, J. Am. Chem. Soc., 2024, 146, 3545–3552 CrossRef CAS PubMed; (b) W. Wang, T. Wang, S. Chen, Y. Lv, L. Salmon, B. Espuche, S. Moya, O. Morozova, Y. Yun, D. D. Silvio, N. Daro, M. Berlande, P. Hapiot, J.-L. Pozzo, H. Yu, J.-R. Hamon and D. Astruc, Angew. Chem., Int. Ed., 2024, e202407430 CAS; (c) X. Chen, J.-Y. Song, J. Zheng, Y.-M. Wang, J. Luo, P. Weng, B.-C. Cai, X.-C. Lin, G.-H. Ning and D. Li, J. Am. Chem. Soc., 2024, 146, 19271–19278 CrossRef CAS PubMed; (d) Z.-W. Zheng, J.-J. Zhou, H. Liu, X.-Y. Zhang, J. Zhao, D.-S. Zheng, K. Huang and D.-B. Qin, Inorg. Chem., 2024, 63, 16878–16887 CrossRef CAS PubMed; (e) J. Qiu, X. Qi, K. Zhu, Y. Zhao, H. Wang, Z. Li, H. Wang, Y. Zhao and J. Wang, Green Chem., 2024, 26, 6172–6179 RSC; (f) Z. Zhang, K. Shen, Q. Zhang, C. Duan and X. Jing, Dalton Trans., 2024, 53, 10060–10064 RSC; (g) Q. Cao, W. Sun, Z. Xiao, X. Zhou, L. Lu, H. Hou, Y. Chen and L. Wang, Small, 2024, 20, 2404202 CrossRef CAS PubMed; (h) V. Parihar, G. Singh, N. Duhan, S. Kumar, T. J. D. Kumar and C. M. Nagaraja, ChemSusChem, 2024, e202401497 Search PubMed; (i) A.-L. Gu, Y.-X. Zhang, Z.-L. Wu, H.-Y. Cui, T.-D. Hu and B. Zhao, Angew. Chem., Int. Ed., 2024, e202114817 Search PubMed; (j) C.-S. Cao, S.-M. Xia, Z.-J. Song, H. Xu, Y. Shi, L.-N. He, P. Cheng and B. Zhao, Angew. Chem., Int. Ed., 2020, 59, 8586–8593 CrossRef CAS PubMed.
- (a) M. Can, F. A. Armstrong and S. W. Ragsdale, Chem. Rev., 2014, 114, 4149–4174 CrossRef CAS PubMed; (b) V. Waser, M. Mukherjee, R. Tachibana, N. V. Igareta and T. R. Ward, J. Am. Chem. Soc., 2023, 145, 14823–14830 CrossRef CAS PubMed.
- (a) F. Schwizer, Y. Okamoto, T. Heinisch, Y. Gu, M. M. Pellizzoni, V. Lebrun, R. Reuter, V. Köhler, J. C. Lewis and T. R. Ward, Chem. Rev., 2018, 118, 142–231 CrossRef CAS PubMed; (b) E. N. Mirts, A. Bhagi-Damodaran and Y. Lu, Acc. Chem. Res., 2019, 52, 935–944 CrossRef CAS PubMed.
- (a) M. Zhao, H.-B. Wang, L.-N. Ji and Z.-W. Mao, Chem. Soc. Rev., 2013, 42, 8360–8375 RSC; (b) S. T. Stripp, B. R. Duffus, V. Fourmond, C. Léger, S. Leimkühler, S. Hirota, Y. Hu, A. Jasniewski, H. Ogata and M. W. Ribbe, Chem. Rev., 2022, 122, 11900–11973 CrossRef CAS PubMed.
- (a) H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Nature, 1999, 402, 276–279 CrossRef CAS; (b) H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed; (c) W. Xu, Y. Wu, W. Gu, D. Du, Y. Lin and C. Zhu, Chem. Soc. Rev., 2024, 53, 137–162 RSC; (d) K.-Y. Wang, J. Zhang, Y.-C. Hsu, H. Lin, Z. Han, J. Pang, Z. Yang, R.-R. Liang, W. Shi and H.-C. Zhou, Chem. Soc. Rev., 2023, 123, 5347–5420 CrossRef CAS PubMed; (e) J. Liang, M. Y. B. Zulkifli, J. Yong, Z. Du, Z. Ao, A. Rawal, J. A. Scott, J. R. Harmer, J. Wang and K. Liang, J. Am. Chem. Soc., 2022, 144, 17865–17875 CrossRef CAS PubMed; (f) J. Wu, Z. Wang, X. Jin, S. Zhang, T. Li, Y. Zhang, H. Xing, Y. Yu, H. Zhang, X. Gao and H. Wei, Adv. Mater., 2021, 33, 2005024 CrossRef CAS PubMed; (g) S. Rojas-Buzo, P. Concepción, J. L. Olloqui-Sariego, M. Moliner and A. Corma, ACS Appl. Mater. Interfaces, 2021, 13, 31021–31030 CrossRef CAS PubMed; (h) J. R. Bour, A. M. Wright, X. He and M. Dincă, Chem. Sci., 2020, 11, 1728–1737 RSC; (i) J. Chen, H. Gao, Z. Li, Y. Li and Q. Yuan, Chin. Chem. Lett., 2020, 31, 1398–1401 CrossRef CAS; (j) Q. Sun, B. Aguila and S. Ma, Chem, 2018, 4, 2736–2738 CrossRef CAS.
- (a) G. T. Rochelle, Science, 2009, 325, 1652–1654 CrossRef CAS PubMed; (b) T. M. McDonald, W. R. Lee, J. A. Mason, B. M. Wiers, C. S. Hong and J. R. Long, J. Am. Chem. Soc., 2012, 134, 7056–7065 CrossRef CAS PubMed; (c) H. Lyu, O. I.-F. Chen, N. Hanikel, M. I. Hossain, R. W. Flaig, X. Pei, A. Amin, M. D. Doherty, R. K. Impastato, T. G. Glover, D. R. Moore and O. M. Yaghi, J. Am. Chem. Soc., 2022, 144, 2387–2396 CrossRef CAS PubMed.
- J. Li, L. He, Q. Liu, Y. Ren and H. Jiang, Nat. Commun., 2022, 13, 928 CrossRef CAS PubMed.
- (a) X. Feng, J. Hajek, H. S. Jena, G. Wang, S. K. P. Veerapandian, R. Morent, N. De Geyter, K. Leyssens, A. E. J. Hoffman and V. Meynen, et al , J. Am. Chem. Soc., 2020, 142, 3174–3183 CrossRef CAS PubMed; (b) G. Fu, P. Wu, J. Yang, S. Zhang, L. Wang, M. Xu and X. Huai, Inorg. Chem., 2022, 61, 17943–17950 CrossRef CAS PubMed.
- (a) F. Yang, J. Wang, Y. Wang, B. Yu, Y. Cao, J. Li, L. Wu, J. Huang and Y.-N. Liu, Angew. Chem., Int. Ed., 2024, e202318115 CAS; (b) J. Li, J. Liao, Y. Ren, C. Liu, C. Yue, J. Lu and H. Jiang, Angew. Chem., Int. Ed., 2019, 58, 17148–17152 CrossRef CAS PubMed.
- M. C. Biesinger, L. W. M. Lau, A. R. Gerson and R. S. C. Smart, Appl. Surf. Sci., 2010, 257, 887–898 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc01004g |
| This journal is © The Royal Society of Chemistry 2025 |