 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
In situ transmission electron microscopy characterization and manipulation of the morphology, composition and phase evolution of nanomaterials under microenvironmental conditions
Na
Li†
ab,
Xinyang
Li†
a,
Tian
Wang†
a,
Bo
Wen
a,
Zicheng
Yin
a,
Jie
Feng
a,
Shengchun
Yang
 *b,
Yawei
Yang
*b,
Yawei
Yang
 *c,
Guorui
Yang
*c,
Guorui
Yang
 a and
Shujiang
Ding
a and
Shujiang
Ding
 *a
*a
aSchool of Chemistry, Engineering Research Center of Energy Storage Materials and Devices of Ministry of Education, National Innovation Platform (Center) for Industry-Education Integration of Energy Storage Technology, Xi'an Jiaotong University, No. 28, West Xianning Road, Xi'an 710049, P. R. China. E-mail: dingsj@mail.xjtu.edu.cn
bMOE Key Laboratory for Non-equilibrium Synthesis and Modulation of Condensed Matter, Key Laboratory of Shaanxi for Advanced Materials and Mesoscopic Physics, State Key Laboratory for Mechanical Behavior of Materials, School of Physics, Xi'an Jiaotong University, No. 28 West Xianning Road, Xi'an 710049, China. E-mail: ysch1209@mail.xjtu.edu.cn
cElectronic Materials Research Laboratory, Key Laboratory of the Ministry of Education, International Center for Dielectric Research, Shaanxi Engineering Research Center of Advanced Energy Materials and Devices, School of Electronic Science and Engineering, Xi'an Jiaotong University, Xi'an 710049, PR China. E-mail: ywyang@xjtu.edu.cn
First published on 7th May 2025
Abstract
Nanomaterials possess a broad range of applications in areas such as catalysis, energy, and biomedicine because of their unique properties. However, from the perspective of materials synthesis, there are numerous challenges in the controllable preparation of nanomaterials. These include the control of their size, morphology, crystal structure, and surface properties, which are essential for their performance in specific applications. The fundamental cause of these issues is the limitation in the real-time observation of the growth process of nanomaterials. In situ transmission electron microscopy (TEM), on the other hand, overcomes the limitations of traditional in situ testing techniques. It enables the real-time observation and analysis of the dynamic structural evolution during the growth of nanomaterials at the atomic scale. This contributes to a profound understanding of the nucleation and growth mechanisms of nanomaterials and facilitates the controlled preparation of nanomaterials. This review centers on the utilization of in situ TEM to observe and study the complex dynamic processes of zero-, one-, and two-dimensional nanomaterial growth and evolution in different environments (liquid, gas, and solid phases) at the atomic scale. This is of great significance for the design and preparation of nanomaterials with specific properties. The proposed future development of in situ TEM, in combination with advanced data analysis and integration with other techniques, holds great potential for the further advancement of nanotechnology and its applications.
1 Introduction
Nanomaterials, defined by their size typically ranging from 1 to 100 nanometers, have ignited a revolution in the field of materials science due to their unique properties and broad spectrum of applications. These materials exhibit distinctive characteristics that are not observed in their bulk counterparts, primarily due to their high surface-to-volume ratios, quantum confinement effects, and the ability to manipulate their structures at the atomic level. Nanomaterials have extremely extensive application prospects in the domains of catalysis,1,2 energy,3 and biomedicine.4,5 Despite their immense potential, the development and application of nanomaterials also present challenges. These include issues related to their synthesis, stability, toxicity, and environmental impact. The controlled fabrication of nanomaterials, or the precise control over their size, shape, crystal structure, and surface properties, is a complex and challenging process that is critical for tailoring their behaviors and optimizing their performance in various applications.6–8 The quest for precision in crafting nanomaterials with tailored properties is hindered by a complex interplay of factors that govern their formation, growth, and resultant characteristics. One of the primary obstacles in the controlled synthesis of nanomaterials is the deep understanding and manipulation of nucleation and growth mechanisms at the atomic scale.9,10 As demonstrated in the accompanying documents, the journey from monomers to stable nanocrystals is fraught with complexity. Classical and non-classical nucleation theories attempt to explain the aggregation of intermediates into crystalline structures, although the reality of atomic migration dynamics, interfacial evolution, and structural transformation during synthesis often deviates from these theoretical predictions.11,12 The uniformity and scalability of nanomaterial synthesis are also significant challenges. The high surface-to-volume ratio of nanomaterials, which is a source of their unique properties, also introduces variability in their synthesis. Techniques such as wet chemical synthesis and solid-state reactions often yield nanomaterials with a broad size distribution and morphological diversity, which can compromise their performance in specific applications. Moreover, the influence of environmental factors during synthesis, such as temperature, pressure, and the presence of surfactants or solvents, adds another layer of complexity. These factors can significantly affect the crystallinity, phase, and surface properties of nanomaterials, leading to a lack of reproducibility in their synthesis. The stability of nanomaterials during synthesis and under various conditions is a critical concern. Phase transformations and structural changes under different stimuli, such as thermal, mechanical, or chemical influences, can alter the intended properties of nanomaterials. For instance, the thermal stability of magnetic nanoparticles13 is crucial for their application in high-temperature environments, and any phase change could render them ineffective.14,15 In conclusion, the controlled synthesis of nanomaterials is a multifaceted challenge that requires a profound understanding of atomic-scale processes, mastery over environmental influences, ensuring stability and safety, and the development of scalable and environmentally friendly methods. Overcoming these challenges is crucial for the advancement of nanotechnology and the realization of its vast potential in diverse fields of application.In the quest to unravel the intricate processes underlying the formation and evolution of nanomaterials, in situ transmission electron microscopy (TEM) has emerged as a transformative tool, providing a platform for real-time observation and manipulation of nanostructures with atomic accuracy.16 The journey of in situ TEM began with the desire to transcend the limitations of traditional ex situ characterization techniques, which often fell short in capturing the dynamic nature of material synthesis and phase transformations. In situ TEM has evolved to fill this void, offering a suite of capabilities that allow researchers to peer into the heart of nanomaterial formation processes. With its ability to operate under a variety of conditions, including high temperatures, pressures, and in the presence of various chemical environments,17in situ TEM has become an indispensable ally in the quest to understand and control material properties at the fundamental level.18,19 The importance of in situ TEM in nanomaterial preparation cannot be overstated. It has facilitated the visualization of nucleation events, the tracking of growth pathways, and the exploration of structural dynamics in real time.20 This has been particularly crucial in advancing our understanding of phenomena such as Ostwald ripening, phase separation, and defect evolution, which are pivotal in determining the final properties of nanomaterials.21–23 Furthermore, in situ TEM has been instrumental in elucidating the mechanisms of size-dependent phase transformations and the role of surface energy in stabilizing metastable phases. One of the key strengths of in situ TEM lies in its multimodal approach, which integrates imaging with spectroscopic techniques such as energy dispersive X-ray spectroscopy (EDS) and electron energy loss spectroscopy (EELS). This synergy allows for comprehensive characterization of nanomaterials, capturing not only their morphology but also their chemical composition and electronic structure. The implementation of aberration-corrected lenses and the development of advanced imaging modalities, such as high-angle annular dark field (HAADF), scanning TEM (STEM) and electron tomography, have further enhanced the spatial resolution and analytical prowess of in situ TEM. The impact of in situ TEM extends beyond academic research. It has played a pivotal role in the development of new materials for energy storage, catalysis, electronics, and medicine. For instance, in the realm of catalysis, in situ TEM has been used to study the active sites of nanoparticles under reaction conditions, providing insights into their catalytic mechanisms and enabling the design of more efficient catalysts.24,25 Similarly, in the field of electronics, the technique has been harnessed to investigate the conduction properties of nanomaterials and their response to electrical stimuli, which is vital for the development of nanoscale devices.26,27 As we stand on the precipice of new discoveries, the future of in situ TEM holds promise for even greater advancements. The integration of machine learning algorithms and artificial intelligence is set to enhance data analysis and automate the identification of complex structural transformations. Moreover, the ongoing miniaturization of TEM components and the development of more sensitive detectors will further push the limits of spatial and temporal resolution, enabling the capture of fleeting events and transient states in nanomaterial systems. The development of in situ TEM has been a monumental achievement in the field of nanoscience, providing a powerful means to study and control the synthesis of nanomaterials. Its ability to offer insights into the atomic-scale processes that govern material properties has not only enriched our fundamental understanding but also paved the way for innovative applications across diverse industries.28 As we continue to push the boundaries of this technology, the potential for transformative breakthroughs in material design and engineering remains limitless.
In this review, we focused on the application of in situ TEM in analyzing the morphology, composition, and phase evolution of nanomaterials under microenvironmental conditions (Fig. 1). It highlights the challenges in controlling the synthesis of nanomaterials, such as nucleation and growth mechanisms, environmental influences, and stability issues. In situ TEM is presented as a transformative tool that allows real-time observation and manipulation of nanostructures with atomic precision, overcoming the limitations of traditional ex situ techniques. The review categorizes in situ TEM methodologies into several types, including heating chips, gas-phase cells, and liquid cells, each serving specific roles in nanomaterial synthesis. It discusses the insights gained from in situ TEM in understanding the nucleation and growth of nanocrystals, the formation of 0D, 1D, and 2D nanomaterials, and the effects of electron-beam interactions. The article also addresses the challenges associated with in situ TEM, such as replicating realistic synthesis conditions, achieving high temporal and spatial resolution, managing electron beam interactions, and integrating with other analytical techniques. Despite these challenges, the review emphasizes the significant contributions of in situ TEM to advancing our understanding of nanomaterial synthesis and its potential for future breakthroughs in material design and engineering.
 | ||
| Fig. 1 Schematic illustration of key topics in this review. Reproduced with permission.29–32 Copyright 2022, Tsinghua University Press. Copyright 2008, American Chemical Society. Copyright 2020, American Chemical Society. Copyright 2019, American Chemical Society. | ||
2 Classifications of in situ TEM for nanomaterials synthesis
In situ TEM methodologies possess the capability to monitor the developmental stages of a particular system through the establishment and activation of its external conditions. Integral to in situ TEM are the application of external triggers and the ability to perform real-time monitoring. The former is facilitated by a range of specialized TEM holders, while the latter benefits from the implementation of advanced, rapid recording systems, which are not the focus of this review. To date, the exploration of TEM holders for nanomaterial synthesis has led to the identification of five types: the in situ heating chip, the electrochemical liquid cell,33–35 the graphene liquid cell,36–38 the gas-phase cell,39,40 and the environmental TEM,41,42 each playing a significant role in this field (Fig. 2). | ||
| Fig. 2 Schematics of TEM and in situ chips and cells fabricated by MEMS. Reproduced with permission.43–46 Copyright 2013, Elsevier. Copyright 2024, Elsevier. Copyright 2016, American Chemical Society. Copyright 2019, American Chemical Society. | ||
2.1 In situ TEM thermal engineering—heating chip
The “heating chip” is a specialized tool utilized in the field of materials science, particularly for the in situ study of nanomaterials. It allows for the precise control and application of heat to samples within a TEM, enabling researchers to observe the dynamic evolution of the material structure and chemistry under elevated temperatures. Classifications of heating chips typically focus on the type of heating element and the method of temperature control.47–49 For instance, there are furnace-type heating holders, which use a resistive heating element to heat the entire sample, and microelectromechanical system (MEMS)-based heating holders, which offer localized heating through a nanopatterned metal element.50 MEMS-based heating chips are often pre-calibrated for temperature control but may require verification under specific experimental conditions due to probable electron-beam-induced heating effects.In the synthesis of nanomaterials, heating chips play a crucial role by facilitating the study of thermally induced transformations. They allow researchers to monitor processes such as phase transitions, crystal growth, and structural changes in real time. The localized heating provided by MEMS-based chips is particularly beneficial for studying nanostructured and focused ion beam samples, as it minimizes thermal drift and enables rapid temperature stabilization. Moreover, heating chips can be integrated with other in situ TEM techniques, such as gas or liquid environments, to simulate realistic conditions for the material under study. This integration provides a comprehensive understanding of the material's behavior under various stimuli, which is vital for the rational design and optimization of nanomaterials for energy-related applications. Heating chips are indispensable for in situ TEM studies, providing a controlled thermal environment to observe and analyze the structural and chemical evolution of nanomaterials, thereby contributing significantly to the advancement of materials science and technology.51
2.2 In situ TEM gas environment study—gas cell and environmental TEM
In situ TEM for gas environments is primarily classified into two types: environmental TEM (ETEM)41 and windowed holders.18 ETEMs feature advanced pumping systems that maintain a high vacuum in the microscope column while allowing gas to be introduced around the sample, thus simulating realistic reaction conditions. They have progressed from early models with limited resolution to modern versions capable of atomic-scale imaging. Windowed holders (gas cell), in contrast, are designed to contain both the material and gas within a sealed reactor that can be heated, enabling studies under controlled gas and temperature conditions. These holders are compatible with conventional TEM instruments and allow for experiments under high pressures, making them versatile for various catalytic studies.52 In the context of nanomaterial synthesis, in situ TEM plays a vital role. It has been used to monitor the transformation of metal particles into single atoms, a process that significantly enhances catalytic efficiency. For example, the evolution of subnanometric metal species within specific spatial confinements has been studied under different gas atmospheres, revealing the dynamics of sintering and redispersion. Additionally, in situ TEM has been instrumental in visualizing the diffusion of atoms within matrices, leading to the formation of individual atoms and clusters, which is essential for the development of highly dispersed catalysts.2.3 In situ TEM liquid environment study—electrochemical liquid cell and graphene liquid cell
Liquid cell technology has revolutionized the field of nanomaterial synthesis by enabling in situ observations under TEM. These cells are designed to contain liquid samples within a high-vacuum TEM environment, allowing researchers to directly visualize dynamic processes such as nanoparticle growth, transformation, and motion at the nanoscale. There are primarily two types of liquid cells: microfabricated silicon cells33,53 and graphene liquid cells.38 Microfabricated silicon cells are made from silicon wafers and feature electron-beam-transparent windows, typically composed of thin Si3N4 membranes, which allow for high-resolution imaging. These cells can be static or flow-type, with the latter enabling controlled introduction of reactants for reactions requiring precise temporal and spatial control. Graphene liquid cells, on the other hand, use graphene sheets as the window material, offering even higher spatial resolution due to the thinness and electron transparency of graphene.54In the context of nanomaterial synthesis, liquid cells have been instrumental in studying various phenomena. They have been used to observe the nucleation and growth of nanoparticles, including metal nanoparticles, through processes such as electrochemical reduction.55 The real-time imaging capabilities of liquid cells have shed light on the mechanisms of nanoparticle formation, revealing insights into the role of prenucleation intermediates and the dynamics of aggregative growth.56 Furthermore, liquid cells have facilitated the investigation of nanoparticle transformations, such as galvanic replacement reactions and etching processes, which are crucial for synthesizing nanoparticles with complex structures and desired properties. The ability to control the chemical environment within liquid cells has also been vital for studying the effects of various factors on nanoparticle synthesis, such as the concentration of precursors, the presence of stabilizing agents, and the influence of the electron beam itself.17 By manipulating these parameters, researchers can gain a deeper understanding of the underlying chemistry and optimize the synthesis of nanomaterials for various applications. Liquid cell technology has expanded the horizons of nanomaterial research by providing a platform for in situ TEM studies, offering unprecedented insights into the formation, transformation, and behavior of nanoparticles in liquid media. This technology has become an indispensable tool for scientists in the fields of materials science, chemistry, and nanotechnology.
3 Nucleation and growth of nanocrystals
3.1 Nucleation of nanocrystals
Over the past few years, there has been a consistent rise in the utilization of functional nanocrystals across different sectors, with a notable increase in energy, catalysis, and biomedicine applications.1,3 This surge is primarily attributed to the distinctive structural characteristics of nanocrystals, such as their extensive specific surface area, one of a kind surface, and electron configurations, which hold promise for these applications. Nonetheless, crafting nanoparticles with the desired structures and attributes poses considerable difficulties. To advance further, a variety of theories regarding the nucleation and development of nanocrystals have been put forward, encompassing both classical and non-classical nucleation concepts, as well as growth mechanisms like ripening, clustering, and merging.12 Despite these theoretical advancements, the underlying atomic movements, regulatory elements, and propelling forces of these phenomena are not yet fully understood.21 In this context, the use of in situ TEM is emerging as an indispensable instrument for scientific inquiry, enhancing our comprehension of the mechanisms behind nanocrystal development. The insights gained from these studies are instrumental in the deliberate alteration of the structure and form of nanocrystals, ultimately guiding the production of high-caliber nanocrystals tailored for diverse applications.Classical nucleation theory is a fundamental concept in understanding the formation of nanocrystals.57 The classical LaMer model (Fig. 3A)58 is an extension of classical nucleation theory. It describes the process by which a new thermodynamic phase forms through the aggregation of monomer units. This theory outlines the process by which nanoparticles nucleate and grow in a solution phase. Here is a summary of the key points related to classical nucleation theory:
 | ||
| Fig. 3 Nucleation of nanocrystals. (A) LaMer model describing nucleation and growth of nanocrystals as a function of reaction time and concentration of precursor atoms. Reproduced with permission.58 Copyright 1950, American Chemical Society. (B) Classical nucleation model showing the free energy diagram for nucleation. Reproduced with permission.59 Copyright 2009, American Chemical Society. (C) TEM images showing the formation of NiO nanocrystals in SiNx liquid cells. Reproduced with permission.29 Copyright 2022, Tsinghua University Press. | ||
Classical nucleation theory provides a framework for understanding and controlling the initial stages of nanoparticle formation, which is crucial for the synthesis of colloidal nanoparticles with tailored properties (Fig. 3C).29
3.2 Growth of 0D nanomaterials
The realm of zero-dimensional (0D) nanomaterials has witnessed remarkable advancements in both applications and fabrication techniques, marking a significant evolution in nanotechnology.61 0D nanomaterials, with their distinct properties arising from quantum confinement effects and large surface-to-volume ratios, have catalyzed a surge of interest across various fields, which encompass energy conversion,62,63 catalysis,64,65 photonics,66 and biology.67,68 For instance, in the energy sector, these nanomaterials have been utilized in solar cells69–71 and batteries,72–74 capitalizing on their size-dependent electronic properties. In catalysis,75 0D nanomaterials have demonstrated enhanced activity and selectivity for various chemical reactions, attributed to their well-defined surface structures and compositions. The photonics industry has also benefited from the unique optical properties of 0D nanomaterials, such as in light-emitting diodes and photodetectors.76,77 Moreover, in the biomedical field,78 their use in drug delivery systems and imaging agents has shown great promise. The synthesis of 0D nanomaterials has been refined through various approaches, ensuring control over size, shape, and composition–crucial parameters that dictate their properties and performance. Wet chemical methods, including sol–gel, precipitation, and hydrothermal synthesis, have been refined to produce monodisperse 0D nanomaterials with high yields.15,79,80 Advanced physical techniques such as sputtering and laser ablation have also been employed to synthesize nanomaterials with unique properties.Furthermore, the integration of in situ TEM allows researchers to directly visualize the dynamic processes of nanomaterial synthesis, including nucleation, growth, and phase transformations, which are crucial for understanding the underlying mechanisms and optimizing the material properties.56,81,82 The ability to apply various external stimuli, such as mechanical, thermal, electrical, and chemical influences, within the TEM environment provides a unique platform for the precise control and tuning of the nanomaterial properties. Advancements in in situ TEM techniques have led to the development of new experimental setups that enable the study of 0D nanomaterials under more realistic and complex conditions. These include the use of nanomanipulation for mechanical testing, MEMS for precise temperature control, and environmental cells for gas and liquid exposure.23,52,53,83 Furthermore, the integration of spectroscopic tools like EELS and EDS within the TEM has enhanced the chemical and electronic characterization capabilities. Looking forward, the direction of research in in situ TEM for 0D nanomaterials is expected to focus on achieving higher spatial and temporal resolutions, improving the stability and controllability of external stimuli, and expanding the range of accessible materials and conditions. The development of novel in situ holders and the integration of additional physical fields, such as magnetic and optical stimuli, will further broaden the applicability of in situ TEM in the synthesis and study of 0D nanomaterials. Moreover, the combination of in situ TEM with computational modeling and simulation will provide deeper insights into atomic-scale processes and enable the prediction and design of new materials with tailored properties.
Meanwhile, studies on the thermal stability and growth dynamics of the structures of novel functional nanoparticles (bimetallic Janus nanostructures (JNs)32,104–106 and high-entropy oxides (HEOs)97) have extended our understanding of the evolutionary behavior of nanoparticles at elevated temperatures and provided important scientific insights into the control of the nanoparticle structure and morphology. These findings not only deepen our understanding of the behavior of nanoparticles at high temperatures, but also provide an important scientific basis for the design of materials with specific properties, especially for applications in catalysts, energy storage, and conversion. JNs have garnered significant attention due to their distinctive interfacial properties that can be finely tuned for a myriad of applications. The size of these nanoparticles plays a pivotal role in dictating the structural and orientational characteristics of their heterointerfaces, which in turn significantly influences their performance in practical applications. Sun et al. utilized in situ annealing HRTEM and revealed a novel sub-10 nm heterostructure with a unique interface, which offered fresh insights into the role of particle size in interfacial evolution during thermal annealing (Fig. 4A).32 The study underscores the importance of understanding the atomic motion mechanism that governs the formation of different heterointerfaces, influenced by particle size. The findings are instrumental in the development of nanoelectronic devices and catalytic systems where precise control over the heterointerfaces is essential for optimizing performance. In the realm of materials science, HEOs have emerged as a novel class of multicomponent solid-solution materials, showcasing immense potential for applications in catalysis, energy storage, and thermal barrier coatings. The ability to fine-tune their composition and crystal structures offers a vast landscape for enhancing material properties. However, unraveling the atomic-scale mechanisms of nucleation and growth of HEOs has been a formidable challenge, impeding the rational design of their structure and function. Gao et al. leveraged atomic resolution in situ STEM to visualize the entire formation process of a high-entropy fluorite oxide (HEFO) from a polymeric precursor, as schematically shown in Fig. 4B.97 The findings of this study are pivotal, providing critical insights into the rational synthesis of HEOs with controlled grain sizes and morphologies, which in turn are essential for tailoring the material's properties. The research demonstrates that the random and uniform distribution of elements in the designed polymeric precursor is fundamental to the low-temperature oxidation and nucleation process. Furthermore, the study elucidates that the formation of HEFO entails slow grain growth through atom diffusion at temperatures below 900 °C and a subsequent liquid-phase-assisted grain growth process at elevated temperatures. This work not only advances the scientific understanding of HEOs but also opens new avenues for the development of advanced materials with targeted properties for specific technological applications.
 | ||
| Fig. 4 Phase transition of 0D nanomaterials in solid-state interactions. (A) Schematic representations of the reasonable atomic motion in sub-10 nm CuAg nanoparticles during annealing to form a Cu (100)/Ag (100) heterointerface, and STEM-HAADF images of CuAg nanoparticles before and after annealing under vacuum at 500 °C for 10 min. The corresponding high-magnification STEM-HAADF images and EDS elemental maps. Reproduced with permission.32 Copyright 2019, American Chemical Society. (B) Schematic representations of the HEFO from an amorphous precursor and characterization of the structures and compositions of high-entropy fluorite oxides at different temperatures. Reproduced with permission.97 Copyright 2022, American Chemical Society. | ||
As an important class of nanomaterials, twinned noble metal nanoparticles show a wide range of applications and significant technological advantages in many fields, such as catalysis, optics, electronics, and biomedicine, due to their excellent physicochemical properties and tunable surface plasmon resonance.127–130 The growth process of such noble metal nanoparticles in liquids is mostly kinetically controlled, with a large deviation from the equilibrium state defined by thermodynamics. When the temperature increases, various changes in the arrangement of atoms occur, which affect the geometrical morphology of the nanocrystals, the spatial distribution of the elements, the internal structure and the phase structure. Ma et al. used an in situ LCTEM technique to study in depth multi-twinned gold nanoparticles, especially decahedra and icosahedra, which have a wide range of applications and significant technological advantages (Fig. 5A).131 The growth of multi-twinned nanoparticles mainly proceeds through two paths: path A involves core-based laminar growth, starting from rounded multi-twinned seeds and growing in a laminar manner (Fig. 5B and C), and path B involves continuous twinning and tetrahedral (tetrahedra) growth (Fig. 5D). There were differences in the internal strain relaxation mechanisms and growth kinetics of the two pathways: in path A, multi-twinned nanoparticles grew by opening and closing of re-entrant grooves at twin boundaries, which was not found in path B. The researchers discussed the preferred pathway (path A) and the preferred pathway (path B) further. The researchers further discussed the relationship between the preferred path (A or B) and the initial seed yield as well as the size- and morphology-dependent multi-twinned nanoparticle formation, revealing the mechanism of formation and evolution of multi-twinned structures. Zhu et al. conducted an in situ study of the growth mechanism of citrate-stabilized gold nanoparticles by oriented attachment (Fig. 5E).132 It was observed that the process of oriented attachment between pairs of nanoparticles in the liquid phase was tightly controlled by the adsorbed ligand layer, and when two gold nanoparticles approached a certain distance, their ligand layers started to overlap, resulting in the rotation of the pairs of particles shifting from a random mode to an oriented mode until their <111> crystalline surfaces were completely aligned, followed by a rapid contact. The thickness and interaction energy of the ligand layer in this process are the key factors determining the oriented attachment behavior of nanoparticles. For the core–shell growth mechanism, Tan et al. thoroughly explored the epitaxial growth process of silver (Ag) on gold nanocubes (Au nanocubes) in solution (Fig. 5F).124 It was found that the formation of the Au–Ag core–shell structure proceeds through two mechanisms: the first mechanism is fusion, where silver nanoparticles are adsorbed onto Au nanocubes (Fig. 5G), and the second mechanism is monolithic attachment, where silver atoms are epitaxially deposited on Au nanocubes (Fig. 5H). Both paths end up with the same Au–Ag core–shell nanostructure. Further analysis reveals that the growth of silver shells is controlled kinetically and thermodynamically. When the surface diffusion rate is faster than the atomic deposition rate, the reaction is driven by thermodynamics, and the silver atoms diffuse and migrate to the sites with the lowest surface free energy, the growth of the shell layer will proceed along the <100> and <110> directions. However, when the atomic deposition rate is higher than the surface diffusion rate, the reaction is controlled by kinetics, with preferential attachment of silver atoms to certain crystalline surfaces, and the shell layer growth pattern may shift to promote the formation of more complex shapes, such as concave cubes or octopods; the discovery of these phenomena is of great significance for the understanding and optimization of the synthesis process of core–shell nanostructures.
 | ||
| Fig. 5 Nucleation, growth and structural transformation of 0D nanomaterials in the liquid phase. (A) Schematic of different formation routes of decahedral and icosahedral multiply twinned nanoparticles. Time-sequenced TEM images of nucleation-based layer-by-layer growth of decahedral and icosahedral Au NPs from the rounded multiply twinned seeds viewed along (B) [011] and (C) [111] orientations, respectively. (D) Time-sequenced TEM images of successive twinning growth of an icosahedral Au MTP from an initial tetrahedral seed viewed along [111] orientation. Reproduced with permission.131 Copyright 2020, American Chemical Society. (E) Schematic illumination of the whole oriented attachment process and imaging of oriented attachment at the atomic level. Reproduced with permission.132 Copyright 2018, Springer Nature. (F) Schematic illustrations showing the shape evolution of a cubic seed under thermodynamic control for two conditions: absence or presence of capping agents. Time-lapse TEM images of Au nanocubes interacting with the Ag precursor solution inside a flow cell via (G) the monomer attachment process and (H) monomer attachment and nuclei coalescence. Reproduced with permission.124 Copyright 2016, American Chemical Society. | ||
 | ||
| Fig. 6 Structural and phase transformation of 0D nanomaterials in gas–solid interaction. (A) Pt–NiO1−x interfacial structure formation of a Pt3Ni nanoparticle after O2 annealing and in situ TEM of the spreading process. Reproduced with permission.145 Copyright 2020, American Chemical Society. (B) The growing and spreading process of Pt islands on Ru branched nanoparticles to create single-Pt-atom-on-Ru catalysts, and the HRTEM images and corresponding cartoons of the island as it transforms during in situ heating under a flow of H2. Scale bars, 2.5 nm. Reproduced with permission.146 Copyright 2022, Springer Nature. | ||
3.3 Growth of 1D nanomaterials
1D nanomaterials, with their distinct structural and functional attributes, have emerged as a cornerstone in the realm of nanotechnology.149 The defining features of 1D nanomaterials include their ultra-high aspect ratios, tunable cross-sectional dimensions ranging from 1 to 100 nanometers, and variable lengths that can span from hundreds of nanometers to millimeters. These attributes endow 1D nanomaterials with a suite of unique properties that are leveraged across a myriad of applications. The high surface-to-volume ratio of 1D nanomaterials facilitates exceptional interaction with their environment, making them ideal candidates for applications in sensing, catalysis, and energy storage. Their quantum confinement effects, stemming from their small cross-sectional dimensions, lead to size-dependent electronic and optical properties, which are pivotal for the development of advanced photonic and electronic devices. Moreover, the crystalline nature of 1D nanomaterials ensures high purity and fewer defects, contributing to their superior performance in various applications. The tunable bandgap and efficient charge transport properties of semiconductor nanowires have been particularly instrumental in the advancement of integrated photonics, energy conversion, and storage technologies.150,151 The ability to precisely control the nucleation and growth of these nanomaterials is crucial for tailoring their structures and properties to meet specific application requirements. In this context, in situ TEM has emerged as a powerful tool for providing real-time, atomic-scale insights into the formation and evolution of 1D nanomaterials.In situ TEM allows researchers to directly observe the dynamics of nanocrystal nucleation, growth, and structural transformations under controlled conditions, which is essential for understanding the fundamental mechanisms governing their formation. This technique has been particularly instrumental in studying the effects of various reaction parameters, such as temperature, atmosphere, and substrate interactions, on the nucleation and growth behaviors of 1D nanomaterials. By employing in situ TEM, researchers have been able to reveal the atomic migration dynamics, interfacial evolution, and phase transformations during the synthesis of nanowires, nanotubes, and other 1D structures. Advancements in in situ TEM have also led to the development of new synthetic strategies, such as catalyst-assisted growth mechanisms, including vapor–liquid–solid (VLS)152–154 and vapor–solid–solid (VSS)155–157 pathways, which have been extensively studied for the controlled fabrication of 1D nanomaterials. These in situ studies have provided valuable insights into the role of catalysts, the influence of gas environments, and the mass transfer processes during nanowire growth, enabling the optimization of synthesis conditions for the production of high-quality 1D nanomaterials with desired properties. Moreover, in situ TEM has been pivotal in exploring the growth of 1D nanomaterials under various conditions, including the effects of strain, defects, and surface/interface engineering. The technique has also been used to investigate the dynamics of light elemental atoms in metal nanocrystals during catalytic reactions, which is crucial for understanding their catalytic performance and potential applications. Looking forward, the continued development of in situ TEM techniques, such as the integration of more complex growth environments and advanced characterization methods, holds great promise for gaining a deeper comprehension of the nucleation and growth processes of 1D nanomaterials.
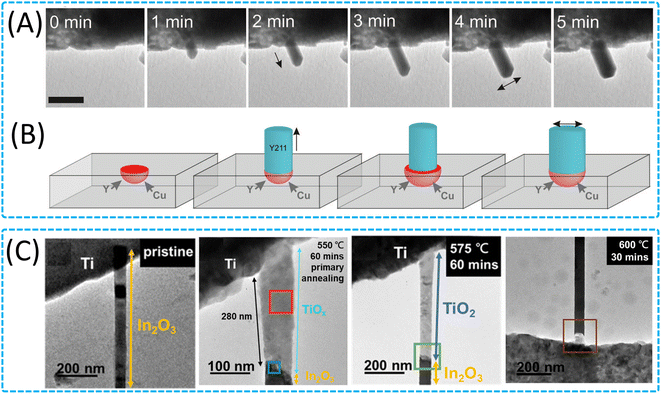 | ||
| Fig. 7 Growth of 1D nanomaterials through solid-state interactions. (A) TEM images and (B) schematic of a nanowire spontaneously broadening as a result of microcrucible creep and expansion on continuing heating at 500 °C. Reproduced with permission.165 Copyright 2014, AAAS. (C) Characterization of the pristine In2O3 nanowire and different temperature annealed In2O3/TiOx heterostructure nanowire. Reproduced with permission.166 Copyright 2022, Elsevier. | ||
 | ||
| Fig. 8 Growth of 1D nanomaterials in the liquid phase. (A) Sequential color TEM images showing the evolution from the initial nucleation and growth in the molecular precursor solution to a later stage of nanowire formation by shape-directed nanoparticle attachment. Reproduced with permission.176 Copyright 2012, AAAS. (B) Schematic depiction of Ge nanowire growth and frame grabs with a Ga nanodroplet immersed in an aqueous solution. Reproduced with permission.55 Copyright 2020, American Chemical Society. | ||
 | ||
| Fig. 9 Catalyst-assisted 1D nanomaterial growth through gas–solid interactions. (A) Nucleation and growth process of a single-walled carbon nanotubes from a nanoparticle catalyst on a substrate. Reproduced with permission.30 Copyright 2008, American Chemical Society. (B) Schematic illustration of the catalyst evolution and growth process of single-walled carbon nanotubes as revealed by in situ ETEM. Reproduced with permission.186 Copyright 2022, American Chemical Society. (C) The phase evolution of cobalt catalyst nanoparticles during the incubation, nucleation, and growth stages of carbon nanotubes under near-atmospheric pressure using an in situ close-cell ETEM and phase structure of an active cobalt catalyst nanoparticle during carbon nanotube growth. Reproduced with permission.31 Copyright 2020, American Chemical Society. | ||
3.4 Growth of 2D nanomaterials
2D nanomaterials are characterized by their ultrathin profiles and exhibit distinctive properties due to quantum confinement effects, which differentiate them from their bulk forms.192–194 These materials demonstrate remarkable potential in various high-tech applications, including next-generation electronics, optoelectronics, magnetism, spintronics, catalysis, and energy storage due to their unique geometric structures and extraordinary properties.195–197 The functionality of 2D nanomaterials is strongly related to their atomic structure, morphology, and the presence of defects and interfaces, which can be precisely engineered. Defects, which are common in both natural and synthesized crystals, can act as active sites, introducing localized electronic states and significantly enhancing the properties of the 2D materials. Interfaces between 2D materials and other components, as well as heterostructures created by stacking different 2D materials, can exhibit unique electronic and optoelectronic properties.198In situ TEM has emerged as a cutting-edge technique for studying the growth mechanisms of 2D nanomaterials at the atomic scale.199,200 It provides real-time monitoring capabilities under various stimuli, including electron irradiation, thermal excitation, and voltage bias, offering unprecedented insights into nucleation, growth, and phase transformations.19,201 Advanced techniques such as aberration-corrected STEM and EELS have further enhanced the understanding of the structure–property relationships in 2D materials by enabling detailed examinations of their atomic structures, chemical compositions, and electronic properties.46,202 The current state of research leveraging in situ TEM showcases the ability to identify various polymorphs, defects, and interfaces in 2D nanomaterials. It also highlights the capacity for atom-by-atom chemical analysis and the study of excitons and phonons, which are crucial for understanding the properties of the 2D nanomaterials.
Researchers have utilized in situ TEM with an in situ heating holder to delve into the growth mechanisms of various 2D nanomaterials (WS2,206 MoS2,49,51,203,204,207–209 V2O5 (ref. 50), etc.) at high temperatures during solid-state reactions. The study reveals precise control over the vertical and horizontal growth of different 2D materials through the thermolysis of solid precursors, as well as multiple growth stages and various growth modes formed on different substrates. Gavhane et al. achieved control over the vertical and horizontal growth of WS2 by altering the thickness of the precursor (Fig. 10A–C),206 and the study found that on different metal-deposited heating chips, two layers of WS2 formed interference patterns by rotating at various angles relative to each other, providing a new perspective for understanding the growth dynamics of WS2. Additionally, Kondekar et al. discovered that a low concentration of Ni can significantly alter the crystallization and growth process of MoS2, leading to an increase in MoS2 crystal size, which may be due to changes in the migration rate of grain boundaries during the growth process (Fig. 10D).203 In contrast, a higher concentration of Ni inhibits the formation of MoS2, instead forming Ni and nickel sulfides. These findings indicate that the addition of other metal elements during synthesis plays a crucial role in the evolution of 2D nanomaterials. Regarding 2D V2O5 nanomaterials, Gavhane et al. utilized an in situ heating holder to observe in real time the formation process of two-dimensional V2O5 nanostructures, including the growth of orthogonal V2O5 2D nanosheets and 1D nanobelts.50 The study also revealed the phase transition process of V2O5 to VO2 and optimized the temperature range required for the growth of V2O5 nanostructures. These studies provide in-depth insights into the growth dynamics of WS2, MoS2, and V2O5 and offer effective pathways for the preparation of 2D nanomaterials. Furthermore, Kotakoski et al. employed in situ STEM combined with a deep learning framework to explore the dynamic process of MoS2 restructuring from 2D to 3D configurations and its growth on graphene.209 These studies not only provide an in-depth atomic-level understanding of the growth and structure of 2D nanomaterials but also demonstrate the potential of deep learning technology in 2D material research, offering new avenues for exploring novel structures and properties. Concurrently, the study investigated the dynamic behavior and structural changes of nanocrystalline graphene under high-temperature conditions, as well as the structural evolution of vertically aligned 2D MoS2 layers, providing key insights into the structural stability of general van der Waals 2D crystals and offering valuable technical guidance for material design and optimization. Overall, the results from in situ heating studies on pure solid-state reactions are crucial for guiding the synthesis, manufacturing, and customization of functional characteristics of 2D nanomaterials, providing new understanding for the controlled synthesis of large-area 2D nanomaterials, and holding the potential for achieving atomic-level precise control and growth of 2D nanomaterials during solid-state reactions.
 | ||
| Fig. 10 Structure evolution and growth of 2D nanomaterials through solid-state interactions. (A) Schematic illustration of the evolution of polycrystalline WS2 through thermolysis of an ammonium tetra-thiotungstate precursor, showing the growth of vertical layers and horizontal structures with heating in thick and thin precursor areas, respectively. (B) Growth of vertically aligned layers of WS2 at different temperatures. (C) Horizontal growth of WS2 layers at different temperatures.206 Copyright 2022, Wiley. (D) Illustration summarizing the formation of MoS2 crystals from the pure ammonium tetrathiomolybdate precursor and in situ TEM images showing the evolution of the ammonium tetrathiomolybdate precursor in the presence of a 5 nm Ni film during heating to different temperatures.203 Copyright 2019, American Chemical Society. | ||
 | ||
| Fig. 11 Growth and shape transformation of 2D nanomaterials in the liquid phase. (A) Schematic showing the formation of a 2D nanosheet from a molecular precursor solution with the pathway of 3D nanoparticle growth and subsequent 3D-to-2D transformations. Sequential images show a few cobalt nickel oxide nanoparticles transforming into a 2D nanosheet and the formation of cobalt nickel oxide nanosheets through the growth of 3D nanoparticles and 3D-to-2D transformations. Reproduced with permission.215 Copyright 2019, Springer Nature. (B) Schematic illustration of an LPTEM cell for observing diverse shape transformations of Au nanocrystals. Reproduced with permission.226 Copyright 2023, American Chemical Society. | ||
The structure–property relationship of noble metal nanocrystals is crucial for their applications in various fields such as catalysis and sensing. Noble metal nanosheets exhibit unique behaviours during shape transformation due to their high surface-to-volume ratio and dynamic surface reactivity, which include adsorption, desorption, and diffusion of surface atoms, processes that are critical for the overall shape change. Therefore, researchers have delved into the growth kinetics and formation mechanisms of gold nanosheets,212,213,227 silver nanosheets,228 and palladium dendritic nanosheets.211 Alloyeau et al. found that the growth of colloidal nanoparticles is affected by a combination of kinetic and thermodynamic effects and that by controlling the electron dosage, it is possible to control the growth rate directly, thus quantifying the influence of kinetic effects on planar nanoparticle formation.227 Park et al. revealed that at lower electron doses, the growth of gold nanosheets is driven by thermodynamics, and the formation and shape of nanosheets are directly related to the formation of twinned surfaces during growth.212 Jin et al. also found that the growth rate of Au nanocrystals can be precisely controlled by adjusting the solution chemistry, in particular pH and chloride ion concentration, which is important for the design and synthesis of nanostructures with specific shapes and structures.213 In addition, Choi et al.226 explored the shape change mechanism of Au nanosheets (Fig. 11B–E) and found that the diffusion of nanocrystal surface atoms is the main determinant of the final structure in the shape change and that this rapid diffusion of surface atoms leads to a truncated morphology transition of unstable crystal surfaces, thus minimising the surface energy. Liquid in situ experiments revealed that oxidative etching of gold nanoprisms and subsequent structural remodelling of the crystal faces were induced by changing the chemical potential in the reaction solution and that diffusion of surface atoms on the exposed crystal faces led to the development of unstable {220} crystal faces into stable {111} crystal faces, resulting in truncated morphologies with minimal surface energy. This finding not only provides a new perspective for understanding the formation mechanism of nanocrystals of various shapes, but also has important implications for the controlled synthesis of colloidal nanocrystals. Meanwhile, for silver nanosheets, E studies revealed a dissolution-re-growth mechanism from triangular to hexagonal shapes, providing a potential pathway for the synthesis of Ag HNPs with controllable shapes and sizes. These findings not only deepen the understanding of the microscopic formation process of nanosheets, but also elucidate the origin of the observed reversible shape changes, providing new insights into the rational design of controllable nanocrystal shapes in the future, as well as key prerequisites for the understanding of the growth mechanism of nanomaterials and the control of shape-dependent properties.
 | ||
| Fig. 12 Growth of 2D nanomaterials through gas–solid interactions. (A) ETEM experimental setup and typical observations of in situ graphene growth. (B) Nucleation and growth of graphene from an amorphous C layer. (C) Time-resolved HRTEM images showing the lateral epitaxial growth of graphene on the Cu edge. Reproduced with permission.230 Copyright 2020, American Chemical Society. | ||
4 Electron-beam induced synthesis for nanomaterials
Electron beams play a pivotal role in the synthesis of nanostructures, offering a versatile and precise method for manipulating materials at the nanoscale.233–236 The high energy of electron beams allows them to induce a range of physical and chemical changes in materials, such as knock-on displacement,237 sputtering,238 and radiolysis,239 which are essential for nanostructure synthesis. These interactions can lead to the formation of nanoparticles,233,240 nanowires,241 nanosheets,234,242,243 and more complex geometries, all through in situ processes within TEM. One significant advantage of using electron beams for nanostructure synthesis is the ability to control the process with atomic precision, which can directly affect the final properties of the nanostructures. TEM provides real-time temporal resolution, enabling researchers to observe the microstructural evolution as nanostructures are synthesized. On the other hand, electron-beam-induced synthesis can be conducted without specialized specimen holders or peripheral equipment, making it a simpler and rapidly growing approach. It discusses various protocols for synthesizing different dimensional nanostructures, including 0D nanoparticles,235 1D nanowires/nanotubes,244 2D films,238 and other exotic geometries like nano-trees or nano-dendrites.245There has been a lot of research focus on the investigation of nanomaterials and their transformations under electron beam irradiation, utilizing advanced TEM techniques. These studies explore the structural changes,234,243 decomposition,239,246 and phase transitions247–250 processes of nanomaterials with different morphologies such as 0D, 1D and 2D materials. Zhu et al. investigated the effects of electron-beam irradiation on the structural transformation of silicon (Si) and zinc oxide (ZnO) nanowires. They revealed that electron-beam irradiation can induce a crystal-to-amorphous transition in Si nanowires and surface reconstruction in ZnO nanowires (Fig. 13A).244 These transformations demonstrate the potential for localized modification of one-dimensional nanomaterials using electron beams. Kim et al. observed the rapid decomposition of Bi2S3 under electron beam irradiation in water (Fig. 13B),246 providing insights into the stability and potential applications of such photocatalysts in addressing energy and environmental issues. Meanwhile, more and more researchers are focusing on the transformative effects of electron beam irradiation on nanomaterials, specifically on transition metal dichalcogenides (Fig. 13C)46 and cesium lead halide perovskites (Fig. 13D).249 Mendes et al.46 and Manna et al.249 employed TEM to investigate the structural and compositional changes induced by electron irradiation. They explored how electron beams can stimulate desorption of atoms, induce phase transformations, and trigger the nucleation and growth of nanoparticles within these materials. The research underscored the potential of electron beam manipulation for material property tuning and the development of nanodevices, while also highlighting the challenges associated with controlling the electron irradiation-induced processes for stable material synthesis and applications in optoelectronics and photovoltaics.
 | ||
| Fig. 13 Electron-beam induced synthesis of nanomaterials: electron-beam-induced (A) in situ structural transformation in 1D nanomaterials. Reproduced with permission.244 Copyright 2015, Science China Press. (B) Decomposition of Bi2S3 nanorods in water. Reproduced with permission.246 Copyright 2021, IOP. Phase transition of (C) 2D transition metal dichalcogenides. Reproduced with permission.46 Copyright 2019, American Chemical Society. (D) Colloidal cesium lead halide perovskite nanocrystals. Reproduced with permission.249 Copyright 2017, American Chemical Society. | ||
Furthermore, the electron-beam specimen interactions are crucial for understanding the physical background behind the growth mechanisms. The section also discusses the challenges and limitations of using electron beams, such as specimen charging and the risk of inducing uncontrollable structural transformations. The electron-beam-induced synthesis of nanostructures is a powerful technique that leverages the precise interactions between electron beams and materials to create nanostructures with tailored properties.248 This method is particularly valuable for research and development in nanotechnology, offering a platform for innovation and the potential for large-scale industrial applications once the challenges are addressed.249
5 Challenges and conclusion
In situ TEM has proven to be an invaluable tool for investigating the synthesis of nanomaterials at the atomic scale, offering unprecedented insights into nucleation, growth, and structural transformations. However, despite its substantial contributions to the field, there are several challenges associated with in situ TEM that need to be addressed to fully harness its potential in nanomaterial synthesis.5.1 Integration with other techniques
Although in situ TEM can provide structural information, real-time chemical characterization at the atomic scale during synthesis is still limited. Firstly, combining in situ TEM with advanced spectroscopic methods for real-time chemical analysis is an area that requires further development. Therefore, to gain a comprehensive understanding of nanomaterial synthesis, in situ TEM needs to be integrated with other characterization techniques such as spectroscopy, diffraction, and tomography. Secondly, although in situ TEM excels at providing vivid, real-time images of nanomaterials' structural and chemical transformations within their chemical milieus, a complete grasp of their synthetic intricacies demands a multifaceted investigational approach. To counter this, an array of innovative in situ characterization techniques has swiftly come to the forefront, including but not limited to in situ Raman spectroscopy,251–253in situ infrared spectroscopy,254–256in situ X-ray diffraction (XRD),257–259in situ nuclear magnetic resonance (NMR),260–263in situ X-ray photoelectron spectroscopy (XPS),264–266 and in situ X-ray absorption fine structure (XAFS).267,268 These advanced techniques have filled the gaps left by conventional approaches. Moreover, the harmonization of these cutting-edge in situ methods with established characterization tools is forging new avenues for the invention of novel analytical methodologies. This integrated approach is set to facilitate a more rounded appreciation of nanomaterial synthesis mechanisms, thereby enhancing our ability to design and fabricate materials with tailored properties for specific applications (Fig. 14).269 | ||
| Fig. 14 The most important in situ characterization techniques with their spatial resolution scales and the corresponding detection targets in the synthesis of nanomaterials. | ||
5.2 Complex reaction environments
Replicating realistic synthesis conditions within the high-vacuum environment of a TEM remains a challenge. The need to integrate multiple external stimuli, such as liquid, gas, heat, and light, into a single in situ TEM experiment is crucial for mimicking real-world synthesis processes. On the one hand, it is difficult to maintain precise control of the gas and liquid environments within the TEM, including pressure and composition. The spatial and temporal resolution can be compromised due to gas scattering and the need for high-pressure compatibility. On the other hand, accurate temperature measurement at the nanoscale is challenging, particularly when considering the heat effect of the electron beam. This can affect the actual phase transformation temperature and dynamics.5.3 Data acquisition and analysis
The acquisition of high-quality, high-resolution data in a timely manner is essential for understanding complex nanomaterial synthesis processes. However, the current limitations in data acquisition systems, such as frame rates and image quality, can hinder the detailed analysis of dynamic processes. In addition, understanding the role of interfaces and compositional changes during nanomaterial synthesis is critical. However, current in situ TEM techniques may struggle to provide detailed information on the chemical state, valence, and distribution of elements, particularly light elements, which are often involved in catalytic processes. Hence, the interpretation of in situ TEM observations can be complex, particularly when distinguishing between different growth mechanisms or understanding the influence of various reaction parameters. Developing a comprehensive understanding that links observations to underlying mechanisms is an ongoing challenge.5.4 Temporal and spatial resolution
Capturing the dynamics of nanomaterial synthesis requires high temporal resolution to follow fast processes and high spatial resolution to observe atomic-scale changes. Current in situ TEM techniques may not always provide the necessary resolution to capture all relevant details, particularly for very fast or small-scale phenomena.5.5 Electron beam interaction
The electron beam used in TEM can interact with the sample, causing effects such as heating, knock-on damage, or charging. These interactions can alter the sample's structure and chemistry, potentially leading to observations that do not accurately represent the undisturbed synthesis process.5.6 Sample preparation and stability
Preparing samples that are representative of actual synthesis conditions and maintaining their stability under electron beams are non-trivial tasks. The need for specialized holders and the potential for sample contamination or damage during preparation and observation add layers of complexity. Meanwhile, achieving stable loading of nanomaterials within the TEM and maintaining the sample under test conditions without drift is a significant challenge, especially for quantitative nanomechanical tests that require precision.As mentioned above, the in situ TEM method is essential for obtaining high-resolution data on nanocrystal growth in relation to space, time, and energy. We anticipate the development of more intricate in situ cultivation settings and a variety of experimental approaches within TEM, including hydrothermal and CVD techniques. Furthermore, the integration of cutting-edge characterization methodologies and advanced data analytics, such as high-throughput experimentation and artificial intelligence algorithms, is anticipated. This synergy will facilitate a more profound comprehension of the underlying nucleation and growth mechanisms of nanocrystals, enabling the meticulous design and crafting of nanocrystals tailored with specific structural and functional attributes. By leveraging these advanced techniques, researchers will gain the ability to elucidate the intricate dynamics of nanocrystal formation with unprecedented clarity, leading to advancements in the precise engineering of materials with customized properties for a wide array of applications. These advances hold immense promise for a wide range of applications in nanomaterials.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
All the authors contributed to the literature search, writing, and editing of this review.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22105153, 52433002 and 52273081), the Fundamental Research Funds for the Central Universities (xzy012024040), the Young Talent Support Plan of Xi'an Jiaotong University, and the Young Talent Fund of Xi'an Science and Technology Association (no. 959202313002).References
- Z. Y. Zhou, N. Tian, J. T. Li, I. Broadwell and S. G. Sun, Nanomaterials of high surface energy with exceptional properties in catalysis and energy storage, Chem. Soc. Rev., 2011, 40, 4167–4185 RSC.
- X. Qiu, Y. Zhang, Y. Zhu, C. Long, L. Su, S. Liu and Z. Tang, Applications of nanomaterials in asymmetric photocatalysis: recent progress, challenges, and opportunities, Adv. Mater., 2021, 33, 2001731 CrossRef CAS.
- E. Pomerantseva, F. Bonaccorso, X. Feng, Y. Cui and Y. Gogotsi, Energy storage: The future enabled by nanomaterials, Science, 2019, 366, eaan8285 CrossRef CAS.
- X. Yang, M. Yang, B. Pang, M. Vara and Y. Xia, Gold nanomaterials at work in biomedicine, Chem. Rev., 2015, 115, 10410–10488 CrossRef CAS PubMed.
- X. Wang, X. Zhong, J. Li, Z. Liu and L. Cheng, Inorganic nanomaterials with rapid clearance for biomedical applications, Chem. Soc. Rev., 2021, 50, 8669–8742 RSC.
- Z. Fan and H. Zhang, Crystal phase-controlled synthesis, properties and applications of noble metal nanomaterials, Chem. Soc. Rev., 2016, 45, 63–82 RSC.
- N. Baig, I. Kammakakam and W. Falath, Nanomaterials: a review of synthesis methods, properties, recent progress, and challenges, Mater. Adv., 2021, 2, 1821–1871 RSC.
- N. Abid, A. M. Khan, S. Shujait, K. Chaudhary, M. Ikram, M. Imran, J. Haider, M. Khan, Q. Khan and M. Maqbool, Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review, Adv. Colloid Interface Sci., 2022, 300, 102597 CrossRef CAS PubMed.
- N. T. K. Thanh, N. Maclean and S. Mahiddine, Mechanisms of nucleation and growth of nanoparticles in solution, Chem. Rev., 2014, 114, 7610–7630 CrossRef CAS.
- J. Lee, J. Yang, S. G. Kwon and T. Hyeon, Nonclassical nucleation and growth of inorganic nanoparticles, Nat. Rev. Mater., 2016, 1, 1–16 Search PubMed.
- C. Jia, A. Xiao, J. Zhao, P. Wang, X. Fang, H. Zhang and B. Guan, A new perspective on crystal nucleation: a classical view on non-classical nucleation, Cryst. Growth Des., 2024, 24, 601–612 CrossRef CAS.
- Y. S. Jun, Y. Zhu, Y. Wang, D. Ghim, X. Wu, D. Kim and H. Jung, Classical and nonclassical nucleation and growth mechanisms for nanoparticle formation, Annu. Rev. Phys. Chem., 2022, 73, 453–477 CrossRef CAS PubMed.
- M. J. Ansari, M. M. Kadhim, B. A. Hussein, H. A. Lafta and E. Kianfar, Synthesis and stability of magnetic nanoparticles, J. Bionanosci., 2022, 12, 627–638 CrossRef.
- B. Rezaei, P. Yari, S. M. Sanders, H. Wang, V. K. Chugh, S. Liang, S. Mostufa, K. Xu, J. P. Wang, J. Gómez-Pastora and K. Wu, Magnetic nanoparticles: a review on synthesis, characterization, functionalization, and biomedical applications, Small, 2024, 20, 2304848 CrossRef CAS PubMed.
- Z. Ma, J. Mohapatra, K. Wei, J. P. Liu and S. Sun, Magnetic nanoparticles: synthesis, anisotropy, and applications, Chem. Rev., 2023, 123, 3904–3943 CrossRef CAS PubMed.
- Y. Han, L. Wang, K. Cao, J. Zhou, Y. Zhu, Y. Hou and Y. Lu, In situ TEM characterization and modulation for phase engineering of nanomaterials, Chem. Rev., 2023, 123, 14119–14184 Search PubMed.
- J. Wu, H. Shan, W. Chen, X. Gu, P. Tao, C. Song, W. Shang and T. Deng, In situ environmental TEM in imaging gas and liquid phase chemical reactions for materials research, Adv. Mater., 2016, 28, 9686–9712 CrossRef CAS.
- R. R. Unocic and E. A. Stach, Gas-phase electron microscopy for materials research, MRS Bull., 2023, 48, 828–832 CrossRef.
- C. Luo, C. Wang, X. Wu, J. Zhang and J. Chu, In situ transmission electron microscopy characterization and manipulation of two-dimensional layered materials beyond graphene, Small, 2017, 13, 1604259 Search PubMed.
- F. M. Alcorn, P. K. Jain and R. M. Van Der Veen, Time-resolved transmission electron microscopy for nanoscale chemical dynamics, Nat. Rev. Chem., 2023, 7, 256–272 Search PubMed.
- H. Ye, Z. Zhang and R. Wang, Nucleation and growth of nanocrystals investigated by in situ transmission electron microscopy, Small, 2023, 19, 2303872 CrossRef CAS PubMed.
- Z. Xu and Z. Ou, Direct imaging of the kinetic crystallization pathway: simulation and liquid-phase transmission electron microscopy observations, Materials, 2023, 16, 2026 CrossRef CAS.
- Q. Chen, J. M. Yuk, M. R. Hauwiller, J. Park, K. S. Dae, J. S. Kim and A. P. Alivisatos, Nucleation, growth, and superlattice formation of nanocrystals observed in liquid cell transmission electron microscopy, MRS Bull., 2020, 45, 713–726 CrossRef.
- H. Y. Chao, K. Venkatraman, S. Moniri, Y. Jiang, X. Tang, S. Dai, W. Gao, J. Miao and M. Chi, In situ and emerging transmission electron microscopy for catalysis research, Chem. Rev., 2023, 123, 8347–8394 CrossRef CAS PubMed.
- B. He, Y. Zhang, X. Liu and L. Chen, In situ transmission electron microscope techniques for heterogeneous catalysis, ChemCatChem, 2020, 12, 1853–1872 CrossRef CAS.
- J. Xie, J. Li, W. Mai and G. Hong, A decade of advanced rechargeable batteries development guided by in situ transmission electron microscopy, Nano Energy, 2021, 83, 105780 CrossRef CAS.
- Y. Li, H. Xu, Q. Ning, S. Li, J. Wang, J. Wang, Z. Hu, J. Tian, X. Li, Y. Han and Y. Zhu, Visualizing structure, growth, and dynamics of Li dendrite in batteries: from atomic to device scales, Adv. Funct. Mater., 2024, 34, 2401361 CrossRef CAS.
- H. Zhao, Y. Zhu, H. Ye, Y. He, H. Li, Y. Sun, F. Yang and R. Wang, Atomic-scale structure dynamics of nanocrystals revealed by in situ and environmental transmission electron microscopy, Adv. Mater., 2023, 35, 2206911 CrossRef CAS PubMed.
- J. Zhang, M. Li, Z. Kang, B. Xiao, H. Lin, J. Lu, H. Liu, X. Zhang, D. L. Peng and Q. Zhang, Atomic mechanisms of hexagonal close-packed Ni nanocrystallization revealed by in situ liquid cell transmission electron microscopy, Nano Res., 2022, 15, 6772–6778 CrossRef CAS.
- H. Yoshida, S. Takeda, T. Uchiyama, H. Kohno and Y. Homma, Atomic-scale in situ observation of carbon nanotube growth from solid state iron carbide nanoparticles, Nano Lett., 2008, 8, 2082–2086 CrossRef CAS PubMed.
- Y. Wang, L. Qiu, L. Zhang, D. M. Tang, R. Ma, Y. Wang, B. Zhang, F. Ding, C. Liu and H. M. Cheng, Precise identification of the active phase of cobalt catalyst for carbon nanotube growth by in situ transmission electron microscopy, ACS Nano, 2020, 14, 16823–16831 CrossRef CAS.
- L. Tang, W. Wu, L. He, K. Yu, T. Xu, Q. Zhang, L. Zhang and L. Sun, Novel interface in CuAg nanostructure induced by size effect, J. Phys. Chem. Lett., 2019, 10, 1973–1980 CrossRef CAS.
- Z. Zeng, X. Zhang, K. Bustillo, K. Niu, C. Gammer, J. Xu and H. Zheng, In situ study of lithiation and delithiation of MoS2 nanosheets using electrochemical liquid cell transmission electron microscopy, Nano Lett., 2015, 15, 5214–5220 CrossRef CAS PubMed.
- R. Yang, L. Mei, Y. Fan, Q. Zhang, H. G. Liao, J. Yang, J. Li and Z. Zeng, Fabrication of liquid cell for in situ transmission electron microscopy of electrochemical processes, Nat. Protoc., 2023, 18, 555–578 CrossRef CAS PubMed.
- N. Hodnik, G. Dehm and K. J. J. Mayrhofer, Importance and challenges of electrochemical in situ liquid cell electron microscopy for energy conversion research, Acc. Chem. Res., 2016, 49, 2015–2022 CrossRef CAS.
- J. Park, K. Koo, N. Noh, J. H. Chang, J. Y. Cheong, K. S. Dae, J. S. Park, S. Ji, I. D. Kim and J. M. Yuk, Graphene liquid cell electron microscopy: progress, applications, and perspectives, ACS Nano, 2021, 15, 288–308 CrossRef CAS.
- D. J. Kelly, M. Zhou, N. Clark, M. J. Hamer, E. A. Lewis, A. M. Rakowski, S. J. Haigh and R. V. Gorbachev, Nanometer resolution elemental mapping in graphene-based TEM liquid cells, Nano Lett., 2018, 18, 1168–1174 CrossRef CAS PubMed.
- J. M. Yuk, H. K. Seo, J. W. Choi and J. Y. Lee, Anisotropic lithiation onset in silicon nanoparticle anode revealed by in situ graphene liquid cell electron microscopy, ACS Nano, 2014, 8, 7478–7485 CrossRef CAS.
- F. Ye, M. Xu, S. Dai, P. Tieu, X. Ren and X. Pan, In situ TEM studies of catalysts using windowed gas cells, Catalysts, 2020, 10, 779 CrossRef CAS.
- F. Wu and N. Yao, Advances in windowed gas cells for in situ TEM studies, Nano Energy, 2015, 13, 735–756 CrossRef CAS.
- L. Zhang, T. Yang, C. Du, Q. Liu, Y. Tang, J. Zhao, B. Wang, T. Chen, Y. Sun, P. Jia, H. Li, L. Geng, J. Chen, H. Ye, Z. Wang, Y. Li, H. Sun, X. Li, Q. Dai, Y. Tang, Q. Peng, T. Shen, S. Zhang, T. Zhu and J. Huang, Lithium whisker growth and stress generation in an in situ atomic force microscope-environmental transmission electron microscope set-up, Nat. Nanotechnol., 2020, 15, 94–98 CrossRef CAS PubMed.
- J. R. Jinschek, Advances in the environmental transmission electron microscope (ETEM) for nanoscale in situ studies of gas-solid interactions, Chem. Commun., 2014, 50, 2696–2706 RSC.
- D. Su, F. Wang, C. Ma and N. Jiang, Engineering nano-composite Li4Ti5O12 anodes via scanning electron-probe fabrication, Nano Energy, 2013, 2, 343–350 CrossRef CAS.
- M. Xu, T. Deng, C. Li, H. Zhao, J. Wang, Y. Liu, J. Wang, G. Feng, N. Li, S. Ding and K. Xi, Oxygen deficient Eu2O3-synchronizes the shielding and catalytic conversion of polysulfides toward high-performance lithium sulfur batteries, Chin. Chem. Lett., 2024, 110372 CrossRef.
- N. Li, Y. Zhang, H. Zhao, Z. Liu, X. Zhang and Y. Du, Synthesis of high-quality α-MnSe nanostructures with superior lithium storage properties, Inorg. Chem., 2016, 55, 2765–2770 CrossRef CAS.
- R. G. Mendes, J. Pang, A. Bachmatiuk, H. Q. Ta, L. Zhao, T. Gemming, L. Fu, Z. Liu and M. H. Rümmeli, Electron-driven in situ transmission electron microscopy of 2D transition metal dichalcogenides and their 2D heterostructures, ACS Nano, 2019, 13, 978–995 CAS.
- R. Podor, V. Trillaud, G. I. N. Bouala, N. Dacheux, C. Ricolleau and N. Clavier, A multiscale in situ high temperature high resolution transmission electron microscopy study of ThO2 sintering, Nanoscale, 2021, 13, 7362–7374 Search PubMed.
- A. Chauvin, L. Molina-Luna, J. Ding, C. H. Choi, P. Y. Tessier and A. A. El Mel, Study of the coarsening of nanoporous gold nanowires by in situ scanning transmission electron microscopy during annealing, Phys. Status Solidi RRL, 2019, 13, 1900376 CrossRef CAS.
- Y. Lee, J. Lee, H. Chung, J. Kim and Z. Lee, In situ scanning transmission electron microscopy study of MoS2 formation on graphene with a deep-learning framework, ACS Omega, 2021, 6, 21623–21630 CrossRef CAS PubMed.
- D. S. Gavhane, A. D. Sontakke and M. A. van Huis, Thermolysis-driven growth of vanadium oxide nanostructures revealed by in situ transmission electron microscopy: implications for battery applications, ACS Appl. Nano Mater., 2023, 6, 7280–7289 CrossRef CAS.
- L. Fei, S. Lei, W. B. Zhang, W. Lu, Z. Lin, C. H. Lam, Y. Chai and Y. Wang, Direct TEM observations of growth mechanisms of two-dimensional MoS2 flakes, Nat. Commun., 2016, 7, 12206 CrossRef CAS PubMed.
- M. Xiao, H. Sun, Y. Meng and F. Zhu, Advances of in situ transmission electron microscopy research on gas phase catalyst particles, Catal. Sci. Technol., 2024, 14, 2040–2063 RSC.
- Y. Chen, K. Yin, T. Xu, H. Guo and L. Sun, Characterization of nanomaterials using in situ liquid-cell transmission electron microscopy: a review, ACS Appl. Nano Mater., 2023, 6, 22545–22567 CrossRef CAS.
- S. Pu, C. Gong and A. W. Robertson, Liquid cell transmission electron microscopy and its applications, R. Soc. Open Sci., 2020, 7, 191204 CrossRef CAS PubMed.
- Q. Cheek, E. Fahrenkrug, S. Hlynchuk, D. H. Alsem, N. J. Salmon and S. Maldonado, In situ transmission electron microscopy measurements of Ge nanowire synthesis with liquid metal nanodroplets in water, ACS Nano, 2020, 14, 2869–2879 CrossRef CAS PubMed.
- I. A. Moreno-Hernandez, M. F. Crook, V. Jamali and A. P. Alivisatos, Recent advances in the study of colloidal nanocrystals enabled by in situ liquid-phase transmission electron microscopy, MRS Bull., 2022, 47, 305–313 CrossRef.
- Y. Sun, Controlled synthesis of colloidal silver nanoparticles in organic solutions: empirical rules for nucleation engineering, Chem. Soc. Rev., 2013, 42, 2497–2511 RSC.
- V. K. LaMer and R. H. Dinegar, Theory, production and mechanism of formation of monodispersed hydrosols, J. Am. Chem. Soc., 1950, 72, 4847–4854 CrossRef CAS.
- D. Erdemir, A. Y. Lee and A. S. Myerson, Nucleation of crystals from solution: classical and two-step models, Acc. Chem. Res., 2009, 42, 621–629 CrossRef CAS PubMed.
- S. Jeon, T. Heo, S. Y. Hwang, J. Ciston, K. C. Bustillo, B. W. Reed, J. Ham, S. Kang, S. Kim, J. Lim, K. Lim, J. S. Kim, M. H. Kang, R. S. Bloom, S. Hong, K. Kim, A. Zettl, W. Y. Kim, P. Ercius, J. Park and W. C. Lee, Reversible disorder-order transitions in atomic crystal nucleation, Science, 2021, 371, 498–503 CrossRef CAS PubMed.
- K. A. Altammar, A review on nanoparticles: characteristics, synthesis, applications, and challenges, Front. Microbiol., 2023, 14, 1155622 CrossRef.
- J. Park, T. Kwon, J. Kim, H. Jin, H. Y. Kim, B. Kim, S. H. Joo and K. Lee, Hollow nanoparticles as emerging electrocatalysts for renewable energy conversion reactions, Chem. Soc. Rev., 2018, 47, 8173–8202 RSC.
- A. Fereydooni, C. Yue and Y. Chao, A brief overview of silicon nanoparticles as anode material: a transition from lithium-ion to sodium-ion batteries, Small, 2024, 20, 2307275 CrossRef CAS PubMed.
- R. Saha, B. Mondal and P. S. Mukherjee, Molecular cavity for catalysis and formation of metal nanoparticles for use in catalysis, Chem. Rev., 2022, 122, 12244–12307 CrossRef CAS PubMed.
- D. Astruc, Introduction: nanoparticles in catalysis, Chem. Rev., 2020, 120, 461–463 CrossRef CAS PubMed.
- M. S. Rider, Á. Buendía, D. R. Abujetas, P. A. Huidobro, J. A. Sánchez-Gil and V. Giannini, Advances and prospects in topological nanoparticle photonics, ACS Photonics, 2022, 9, 1483–1499 CrossRef CAS.
- E. C. Wang and A. Z. Wang, Nanoparticles and their applications in cell and molecular biology, Integr. Biol., 2014, 6, 9–26 CrossRef CAS.
- W. J. Stark, Nanoparticles in biological systems, Angew. Chem., Int. Ed., 2011, 50, 1242–1258 CrossRef CAS.
- M. Notarianni, K. Vernon, A. Chou, M. Aljada, J. Liu and N. Motta, Plasmonic effect of gold nanoparticles in organic solar cells, Sol. Energy, 2014, 106, 23–37 CrossRef CAS.
- D. Garcia Romero, L. Di Mario, F. Yan, C. M. Ibarra-Barreno, S. Mutalik, L. Protesescu, P. Rudolf and M. A. Loi, Understanding the surface chemistry of SnO2 nanoparticles for high performance and stable organic solar cells, Adv. Funct. Mater., 2024, 34, 2307958 CrossRef CAS.
- G. Zhang, Q. Chen, Z. Zhang, Z. Gao, C. Xiao, Y. Wei and W. Li, NiOx nanoparticles hole-transporting layer regulated by ionic radius-controlled doping and reductive agent for organic solar cells with efficiency of 19.18%, Adv. Mater., 2024, 36, 2310630 CrossRef CAS.
- H. Wu, W. Yan, Y. Xing, L. Li, J. Liu, L. Li, P. Huang, C. Lai, C. Wang, W. Chen and S. Chou, Tailoring the interfacial electric field using silicon nanoparticles for stable zinc-ion batteries, Adv. Funct. Mater., 2024, 34, 2213882 CrossRef CAS.
- Y. Liu, F. Bettels, Z. Lin, Z. Li, Y. Shao, F. Ding, S. Liu and L. Zhang, Recent advances in transition-metal-based catalytic material for room-temperature sodium-sulfur batteries, Adv. Funct. Mater., 2024, 34, 2302626 CrossRef CAS.
- M. Khan, S. Yan, M. Ali, F. Mahmood, Y. Zheng, G. Li, J. Liu, X. Song and Y. Wang, Innovative solutions for high-performance silicon anodes in lithium-ion batteries: overcoming challenges and real-world applications, Nano-Micro Lett., 2024, 16, 179 CrossRef CAS.
- M. Cai, C. Li, X. An, B. Zhong, Y. Zhou, K. Feng, S. Wang, C. Zhang, M. Xiao, Z. Wu, J. He, C. Wu, J. Shen, Z. Zhu, K. Feng, J. Zhong and L. He, Supra-photothermal CO2 methanation over greenhouse-like plasmonic superstructures of ultrasmall cobalt nanoparticles, Adv. Mater., 2024, 36, 2308859 CrossRef CAS.
- I. Venditti, Gold nanoparticles in photonic crystals applications: a review, Materials, 2017, 10, 97 CrossRef.
- T. An, X. Jiang, F. Gao, C. Schäfer, J. Qiu, N. Shi, X. Song, M. Zhang, C. E. Finlayson, X. Zheng, X. Li, F. Tian, B. Zhu, T. Sui, X. Han, J. J. Baumberg, T. Fan and Q. Zhao, Strain to shine: stretching-induced three-dimensional symmetries in nanoparticle-assembled photonic crystals, Nat. Commun., 2024, 15, 5215 CrossRef CAS PubMed.
- Y. Hang, A. Wang and N. Wu, Plasmonic silver and gold nanoparticles: shape and structure-modulated plasmonic functionality for point-of-caring sensing, bio-imaging and medical therapy, Chem. Soc. Rev., 2024, 53, 2932–2971 RSC.
- R. Ghosh Chaudhuri and S. Paria, Core/shell nanoparticles: classes, properties, synthesis mechanisms, characterization, and applications, Chem. Rev., 2012, 112, 2373–2433 CrossRef CAS PubMed.
- S. Anu Mary Ealia and M. P. Saravanakumar, A review on the classification, characterisation, synthesis of nanoparticles and their application, IOP Conf. Ser.: Mater. Sci. Eng., 2017, 263, 032019 Search PubMed.
- J. Kim, S. Kang, F. Cheng, Y. Wang, X. Ye and J. Park, Recent advances in liquid phase transmission electron microscopy of nanoparticle growth and self-assembly, MRS Bull., 2024, 49, 365–376 CrossRef.
- B. H. Kim, J. Yang, D. Lee, B. K. Choi, T. Hyeon and J. Park, Liquid-phase transmission electron microscopy for studying colloidal inorganic nanoparticles, Adv. Mater., 2018, 30, 1703316 CrossRef.
- A. Chen, T. U. Dissanayake, J. Sun and T. J. Woehl, Unraveling chemical processes during nanoparticle synthesis with liquid phase electron microscopy and correlative techniques, Chem. Commun., 2023, 59, 12830–12846 RSC.
- H. J. Han, G. R. Lee, Y. Xie, H. Jang, D. J. Hynek, E. N. Cho, Y. J. Kim, Y. S. Jung and J. J. Cha, Unconventional grain growth suppression in oxygen-rich metal oxide nanoribbons, Sci. Adv., 2021, 7, eabh2012 CrossRef CAS PubMed.
- W. Gao, P. Tieu, C. Addiego, Y. Ma, J. Wu and X. Pan, Probing the dynamics of nanoparticle formation from a precursor at atomic resolution, Sci. Adv., 2019, 5, eaau9590 CrossRef PubMed.
- T. M. Diallo, M. R. Aziziyan, R. Arvinte, J. C. Harmand, G. Patriarche, C. Renard, S. Fafard, R. Arès and A. Boucherif, In situ transmission electron microscopy observation of germanium growth on freestanding graphene: unfolding mechanism of 3D crystal growth during van der waals epitaxy, Small, 2022, 18, 2101890 CrossRef CAS PubMed.
- L. Fei, S. M. Ng, W. Lu, M. Xu, L. Shu, W. B. Zhang, Z. Yong, T. Sun, C. H. Lam, C. W. Leung, C. L. Mak and Y. Wang, Atomic-scale mechanism on nucleation and growth of Mo2C nanoparticles revealed by in situ transmission electron microscopy, Nano Lett., 2016, 16, 7875–7881 CrossRef CAS.
- V. Sudheeshkumar, C. Soong, S. Dogel and R. W. J. Scott, Probing the thermal stability of (3-mercaptopropyl)-trimethoxysilane-protected Au25 clusters by in situ transmission electron microscopy, Small, 2021, 17, 2004539 CrossRef CAS.
- T. R. Henninen, M. Bon, F. Wang, D. Passerone and R. Erni, The structure of sub-nm platinum clusters at elevated temperatures, Angew. Chem., Int. Ed., 2020, 59, 839–845 CrossRef CAS.
- H. M. Ashberry, X. Zhan and S. E. Skrabalak, Identification of nanoscale processes associated with the disorder-to-order transformation of carbon-supported alloy nanoparticles, ACS Mater. Au, 2022, 2, 143–153 CrossRef CAS.
- Y. Zhou, X. Deng, H. Xing, H. Zhao, Y. Liu, L. Guo, J. Feng, W. Feng, Y. Zong, X. Zhu, X. Li, Y. Peng and X. Zheng, Dynamically observing the formation of MOFs-driven Co/N-doped carbon nanocomposites by in situ transmission electron microscope and their application as high-efficient microwave absorbent, Nano Res., 2022, 15, 6819–6830 CrossRef CAS.
- M. M. Rusu, A. Vulpoi, I. Maurin, L. C. Cotet, L. C. Pop, C. I. Fort, M. Baia, L. Baia and I. Florea, Thermal evolution of C-Fe-Bi nanocomposite system: from nanoparticle formation to heterogeneous graphitization stage, Microsc. Microanal., 2022, 28, 317–329 CrossRef CAS.
- R. Bergamaschini, B. A. Rosen, F. Montalenti and J. Colin, Motion of crystalline inclusions by interface diffusion in the proximity of free surfaces, J. Nanopart. Res., 2019, 21, 271 CrossRef.
- F. Li, Y. Zong, Y. Ma, M. Wang, W. Shang, P. Tao, C. Song, T. Deng, H. Zhu and J. Wu, Atomistic imaging of competition between surface diffusion and phase transition during the intermetallic formation of faceted particles, ACS Nano, 2021, 15, 5284–5293 CrossRef CAS PubMed.
- Y. Shimada, Y. Ikeda, K. Yoshida, M. Sato, J. Chen, Y. Du, K. Inoue, R. Maaß, Y. Nagai and T. J. Konno, In situ thermal annealing transmission electron microscopy of irradiation induced Fe nanoparticle precipitation in Fe-Si alloy, J. Appl. Phys., 2022, 131, 164902 CrossRef CAS.
- M. Z. Hussain, M. Bahri, W. R. Heinz, Q. Jia, O. Ersen, T. Kratky, R. A. Fischer, Y. Zhu and Y. Xia, An in situ investigation of the thermal decomposition of metal-organic framework NH2-MIL-125 (Ti), Microporous Mesoporous Mater., 2021, 316, 110957 CrossRef CAS.
- L. Su, X. Chen, L. Xu, T. Eldred, J. Smith, C. DellaRova, H. Wang and W. Gao, Visualizing the formation of high-entropy fluorite oxides from an amorphous precursor at atomic resolution, ACS Nano, 2022, 16, 21397–21406 CrossRef CAS.
- M. Gocyla, S. Kuehl, M. Shviro, H. Heyen, S. Selve, R. E. Dunin-Borkowski, M. Heggen and P. Strasser, Shape stability of octahedral PtNi nanocatalysts for electrochemical oxygen reduction reaction studied by in situ transmission electron microscopy, ACS Nano, 2018, 12, 5306–5311 CrossRef CAS PubMed.
- Z. Lyu, R. Chen, M. Mavrikakis and Y. Xia, Physical transformations of noble-metal nanocrystals upon thermal activation, Acc. Chem. Res., 2021, 54, 1–10 CrossRef CAS PubMed.
- M. Zhao, Z. Chen, Z. Lyu, Z. D. Hood, M. Xie, M. Vara, M. Chi and Y. Xia, Ru octahedral nanocrystals with a face-centered cubic structure, {111} facets, thermal stability up to 400 °C, and enhanced catalytic activity, J. Am. Chem. Soc., 2019, 141, 7028–7036 CrossRef CAS.
- M. Gatalo, F. Ruiz-Zepeda, N. Hodnik, G. Dražić, M. Bele and M. Gaberšček, Insights into thermal annealing of highly-active PtCu3/C oxygen reduction reaction electrocatalyst: an in situ heating transmission electron microscopy study, Nano Energy, 2019, 63, 103892 CrossRef CAS.
- X. Li, Y. He, S. Cheng, B. Li, Y. Zeng, Z. Xie, Q. Meng, L. Ma, K. Kisslinger, X. Tong, S. Hwang, S. Yao, C. Li, Z. Qiao, C. Shan, Y. Zhu, J. Xie, G. Wang, G. Wu and D. Su, Atomic structure evolution of Pt-Co binary catalysts: single metal sites versus intermetallic nanocrystals, Adv. Mater., 2021, 33, 2106371 CrossRef CAS.
- X. Li, S. Cheng, Y. He, L. Qian, D. Zakharov, G. Wu, C. Shan, L. Zhang and D. Su, Revealing the dynamics of the alloying and segregation of Pt-Co nanoparticles via in situ environmental transmission electron microscopy, Nano Res., 2023, 16, 3055–3062 CrossRef CAS.
- L. Tang, W. Wu, L. He, T. Xu, H. Dong, L. Zhang, L. Shi and L. Sun, In situ observation of the solid solution-induced sublimation of CuAg Janus nanoparticles, J. Alloys Compd., 2021, 877, 160168 CrossRef CAS.
- L. Tang, W. Wu, L. He, K. Yu, T. Xu, Q. Zhang, L. Zhang and L. Sun, Novel interface in CuAg nanostructure induced by size effect, J. Phys. Chem. Lett., 2019, 10, 1973–1980 CrossRef CAS.
- M. A. Asoro, D. Kovar and P. J. Ferreira, In situ transmission electron microscopy observations of sublimation in silver nanoparticles, ACS Nano, 2013, 7, 7844–7852 CrossRef CAS PubMed.
- N. D. Loh, S. Sen, M. Bosman, S. F. Tan, J. Zhong, C. A. Nijhuis, P. Král, P. Matsudaira and U. Mirsaidov, Multistep nucleation of nanocrystals in aqueous solution, Nat. Chem., 2017, 9, 77–82 CrossRef CAS.
- W. Dachraoui and R. Erni, Nonclassical nucleation and growth of Pd nanocrystals from aqueous solution studied by in situ liquid transmission electron microscopy, Chem. Mater., 2023, 35, 1201–1208 CrossRef CAS.
- T. J. Woehl, Metal nanocrystal formation during liquid phase transmission electron microscopy: thermodynamics and kinetics of precursor conversion, nucleation, and growth, Chem. Mater., 2020, 32, 7569–7581 CrossRef CAS.
- B. Mei, A. J. Moreno and K. S. Schweizer, Unified understanding of the structure, thermodynamics, and diffusion of single-chain nanoparticle fluids, ACS Nano, 2024, 18, 15529–15544 CrossRef CAS.
- M. Borowska, K. Jankowski, M. Trzaskowski, W. Wojtasiak, P. Korpas, S. Kozłowski and D. Gryglewski, Nucleation and growth of zinc-templated mesoporous selenium nanoparticles and potential non-thermal effects during their microwave-assisted synthesis, Sci. Rep., 2024, 14, 31444 CrossRef CAS.
- Y. Sun, X. Zhang, R. Huang, D. Yang, J. Kim, J. Chen, E. H. Ang, M. Li, L. Li and X. Song, Revealing microscopic dynamics: in situ liquid-phase TEM for live observations of soft materials and quantitative analysis via deep learning, Nanoscale, 2024, 16, 2945–2954 RSC.
- J. H. Nam, G. Nayak, S. Exarhos, C. M. Mueller, D. Xu, G. C. Schatz and P. J. Bruggeman, Mechanisms of controlled stabilizer-free synthesis of gold nanoparticles in liquid aerosol containing plasma, Chem. Sci., 2024, 15, 11643–11656 RSC.
- R. G. Finke, M. A. Watzky and C. B. Whitehead, Response to “Particle size is a primary determinant for sigmoidal kinetics of nanoparticle formation: a ‘disproof’ of the finke-watzky (F-W) nanoparticle nucleation and growth mechanism.”, Chem. Mater., 2020, 32, 3657–3672 CrossRef CAS.
- Y. S. Pestovsky and T. Srichana, Formation of aggregate-free gold nanoparticles in the cyclodextrin-tetrachloroaurate system follows finke-watzky kinetics, Nanomaterials, 2022, 12, 583 CrossRef CAS PubMed.
- D. Keller, T. R. Henninen and R. Erni, Atomic mechanisms of gold nanoparticle growth in ionic liquids studied by in situ scanning transmission electron microscopy, Nanoscale, 2020, 12, 22511–22517 RSC.
- S. Y. Hou, C. Y. Huang, S. B. Tsai, J. Y. Chen and W. W. Wu, In situ atomic-scale TEM observation of Ag nanoparticle-mediated coalescence in liquids, Appl. Surf. Sci., 2021, 546, 149057 CrossRef CAS.
- Z. Aabdin, J. Lu, X. Zhu, U. Anand, N. D. Loh, H. Su and U. Mirsaidov, Bonding pathways of gold nanocrystals in solution, Nano Lett., 2014, 14, 6639–6643 CrossRef CAS.
- T. Su, Z. L. Wang and Z. Wang, In situ observations of shell growth and oxidative etching behaviors of Pd nanoparticles in solutions by liquid cell transmission electron microscopy, Small, 2019, 15, 1900050 CrossRef.
- W. Gao, A. O. Elnabawy, Z. D. Hood, Y. Shi, X. Wang, L. T. Roling, X. Pan, M. Mavrikakis, Y. Xia and M. Chi, Atomistic insights into the nucleation and growth of platinum on palladium nanocrystals, Nat. Commun., 2021, 12, 3215 CrossRef CAS PubMed.
- J. Wu, W. Gao, J. Wen, D. J. Miller, P. Lu, J. M. Zuo and H. Yang, Growth of Au on Pt icosahedral nanoparticles revealed by low-dose in situ TEM, Nano Lett., 2015, 15, 2711–2715 CrossRef CAS.
- W. Wei, T. Bai, R. Fu, L. Sun, W. Wang, M. Dong, L. Chen, Z. Guo and F. Xu, Unravelling the shell growth pathways of Au-Ag core-shell nanoparticles by in situ liquid cell transmission electron microscopy, Nanoscale, 2021, 13, 3136–3143 RSC.
- S. B. Tsai, J. Y. Chen, C. Y. Huang, S. Y. Hou and W. W. Wu, Observing growth and crystallization of Au@ZnO core-shell nanoparticles by in situ liquid cell transmission electron microscopy: implications for photocatalysis and gas-sensing applications, ACS Appl. Nano Mater., 2021, 4, 612–620 CrossRef CAS.
- S. F. Tan, S. W. Chee, G. Lin, M. Bosman, M. Lin, U. Mirsaidov and C. A. Nijhuis, Real-time imaging of the formation of Au-Ag core-shell nanoparticles, J. Am. Chem. Soc., 2016, 138, 5190–5193 CrossRef CAS.
- W. I. Liang, X. Zhang, Y. Zan, M. Pan, C. Czarnik, K. Bustillo, J. Xu, Y. H. Chu and H. Zheng, In situ study of Fe3Pt-Fe2O3 core-shell nanoparticle formation, J. Am. Chem. Soc., 2015, 137, 14850–14853 CrossRef CAS PubMed.
- N. Ahmad, M. Bon, D. Passerone and R. Erni, Template-assisted in situ synthesis of Ag@Au bimetallic nanostructures employing liquid-phase transmission electron microscopy, ACS Nano, 2019, 13, 13333–13342 CrossRef CAS PubMed.
- M. Song, D. Zhang, D. Leng, J. Lee, Z. Yang, J. Chen, D. Li, L. Wang, G. Zhou, R. Yang and K. Zhou, In situ atomic observations of aggregation growth and evolution of penta-twinned gold nanocrystals, Nat. Commun., 2024, 15, 9217 CrossRef CAS.
- R. Mendoza-Cruz, J. P. Palomares-Báez, S. M. López-López, J. M. Montejano-Carrizales, J. L. Rodríguez López, M. José Yacamán and L. Bazán-Díaz, Experimental high-resolution observation of the truncated double-icosahedron structure: a stable twinned shell in alloyed Au-Ag core@shell nanoparticles, Nano Lett., 2024, 24, 4072–4081 CrossRef CAS PubMed.
- H. Kim, T. Y. Yoo, M. S. Bootharaju, J. H. Kim, D. Y. Chung and T. Hyeon, Noble metal-based multimetallic nanoparticles for electrocatalytic applications, Adv. Sci., 2022, 9, 2104054 CrossRef CAS PubMed.
- X. Geng, S. Li, J. Heo, Y. Peng, W. Hu, Y. Liu, J. Huang, Y. Ren, D. Li, L. Zhang and L. Luo, Grain-boundary-rich noble metal nanoparticle assemblies: synthesis, characterization, and reactivity, Adv. Funct. Mater., 2022, 32, 2204169 CrossRef CAS.
- X. Ma, F. Lin, X. Chen and C. Jin, Unveiling growth pathways of multiply twinned gold nanoparticles by in situ liquid cell transmission electron microscopy, ACS Nano, 2020, 14, 9594–9604 CrossRef CAS PubMed.
- C. Zhu, S. Liang, E. Song, Y. Zhou, W. Wang, F. Shan, Y. Shi, C. Hao, K. Yin, T. Zhang, J. Liu, H. Zheng and L. Sun, In situ liquid cell transmission electron microscopy investigation on oriented attachment of gold nanoparticles, Nat. Commun., 2018, 9, 421 CrossRef PubMed.
- Z. Q. Zhang, Y. C. Pei, M. J. Xiao, G. Hu, Z. P. Huang, T. Song, Q. Wang, W. Y. Huang, Y. Peng and H. L. Zhang, In situ observation of the crystal structure transition of Pt-Sn intermetallic nanoparticles during deactivation and regeneration, Chem. Commun., 2021, 57, 5454–5457 RSC.
- M. Kamp, A. Tymoczko, U. Schürmann, J. Jakobi, C. Rehbock, K. Rätzke, S. Barcikowski and L. Kienle, Temperature-dependent ultrastructure transformation of Au-Fe nanoparticles investigated by in situ scanning transmission electron microscopy, Cryst. Growth Des., 2018, 18, 5434–5440 CrossRef CAS.
- T. Vystavel, G. Palasantzas, S. A. Koch, J. Th and M. De Hosson, Nanosized iron clusters investigated with in situ transmission electron microscopy, Appl. Phys. Lett., 2003, 82, 197–199 CrossRef CAS.
- K. Y. Jang, S. J. Ahn, J. H. Kwon, K. M. Nam and Y. H. Kim, Novel route from a wurtzite to a rock-salt structure in CoO nanocrystals: in situ transmission electron microscopy study, J. Phys. Chem. C, 2019, 123, 10689–10694 CrossRef CAS.
- B. Jin, F. Zhang, G. Wu, T. Yuan, Q. Wang, H. Zhou, Y. Zhao, G. Zhang and X. Hong, Structural evolution induced by Au atom diffusion in Ag2S, Chem. Commun., 2019, 55, 13176–13178 RSC.
- A. Govender, E. J. Olivier, S. J. Haigh, D. Kelly, M. Smith, H. van Rensburg, R. P. Forbes and E. van Steen, Performance of a NiFe2O4@Co core-shell fischer-tropsch catalyst: effect of low temperature reduction, ACS Omega, 2020, 5, 32975–32983 CrossRef CAS.
- W. Xia, Y. Yang, Q. Meng, Z. Deng, M. Gong, J. Wang, D. Wang, Y. Zhu, L. Sun, F. Xu, J. Li and H. L. Xin, Bimetallic nanoparticle oxidation in three dimensions by chemically sensitive electron tomography and in situ transmission electron microscopy, ACS Nano, 2018, 12, 7866–7874 CrossRef CAS.
- X. Shen, S. Dai, S. Zhang, Z. Lu, C. Zhang, G. W. Graham, Y. Lei, X. Pan and Z. Peng, Oxidation-induced atom diffusion and surface restructuring in faceted ternary Pt-Cu-Ni nanoparticles, Chem. Mater., 2019, 31, 1720–1728 CrossRef CAS.
- C. Ma, Y. Yun, T. Zhang, H. Suo, L. Yan, X. Shen, Y. Li and Y. Yang, Insight into the structural evolution of the cobalt oxides nanoparticles upon reduction process: an in situ transmission electron microscopy study, ChemCatChem, 2021, 13, 4350–4354 CrossRef CAS.
- A. P. LaGrow, D. C. Lloyd, P. L. Gai and E. D. Boyes, In situ scanning transmission electron microscopy of Ni nanoparticle redispersion via the reduction of hollow NiO, Chem. Mater., 2018, 30, 197–203 CrossRef CAS.
- T. Wang, W. Lu, X. Xu, J. Qiu and S. F. Yu, Study of crystallization and coalescence of nanocrystals in amorphous glass at high temperature, Inorg. Chem., 2019, 58, 9500–9504 CrossRef CAS.
- D. Wang, Z. Zhao, B. Shi, J. X. Wang and J. F. Chen, Real-time imaging and quantitative evolution for pyrolysis of carbon dots-encapsulated metal-organic frameworks at the nanoscale by in situ environmental transmission electron microscopy, ACS Appl. Mater. Interfaces, 2023, 15, 35358–35365 CrossRef CAS PubMed.
- T. S. Kim, J. Kim, H. C. Song, D. Kim, B. Jeong, J. Lee, J. W. Shin, R. Ryoo and J. Y. Park, Catalytic synergy on PtNi bimetal catalysts driven by interfacial intermediate structures, ACS Catal., 2020, 10, 10459–10467 CrossRef CAS.
- A. R. Poerwoprajitno, L. Gloag, J. Watt, S. Cheong, X. Tan, H. Lei, H. A. Tahini, A. Henson, B. Subhash, N. M. Bedford, B. K. Miller, P. B. O'Mara, T. M. Benedetti, D. L. Huber, W. Zhang, S. C. Smith, J. J. Gooding, W. Schuhmann and R. D. Tilley, A single-Pt-atom-on-Ru-nanoparticle electrocatalyst for CO-resilient methanol oxidation, Nat. Catal., 2022, 5, 231–237 CrossRef CAS.
- B. Song, Y. Yang, T. T. Yang, K. He, X. Hu, Y. Yuan, V. P. Dravid, M. R. Zachariah, W. A. Saidi, Y. Liu and R. Shahbazian-Yassar, Revealing high-temperature reduction dynamics of high-entropy alloy nanoparticles via in situ transmission electron microscopy, Nano Lett., 2021, 21, 1742–1748 CrossRef CAS.
- B. Song, Y. Yang, M. Rabbani, T. T. Yang, K. He, X. Hu, Y. Yuan, P. Ghildiyal, V. P. Dravid, M. R. Zachariah, W. A. Saidi, Y. Liu and R. Shahbazian-Yassar, In situ oxidation studies of high-entropy alloy nanoparticles, ACS Nano, 2020, 14, 15131–15143 CrossRef CAS.
- E. Garnett, L. Mai and P. Yang, Introduction: 1D nanomaterials/nanowires, Chem. Rev., 2019, 119, 8955–8957 CrossRef CAS.
- I. Cho, J. Ko, D. D. O. Henriquez, D. Yang and I. Park, Recent advances in 1D nanostructure assembly and direct integration methods for device applications, Small Methods, 2024, 8, 2400474 CrossRef.
- Q. Wei, F. Xiong, S. Tan, L. Huang, E. H. Lan, B. Dunn and L. Mai, Porous one-dimensional nanomaterials: design, fabrication and applications in electrochemical energy storage, Adv. Mater., 2017, 29, 1602300 CrossRef PubMed.
- T. Pham, K. Reidy, J. D. Thomsen, B. Wang, N. Deshmukh, M. A. Filler and F. M. Ross, Salt-assisted vapor-liquid-solid growth of 1D van der waals materials, Adv. Mater., 2024, 36, 2309360 CrossRef CAS.
- P. Sutter and E. Sutter, Tunable 1D van der waals nanostructures by vapor-liquid-solid growth, Acc. Chem. Res., 2023, 56, 3235–3245 CrossRef CAS.
- H. Wang, J. T. Wang, Z. X. Cao, W. J. Zhang, C. S. Lee, S. T. Lee and X. H. Zhang, A surface curvature oscillation model for vapour-liquid-solid growth of periodic one-dimensional nanostructures, Nat. Commun., 2015, 6, 6412 CrossRef CAS.
- Q. Sun, D. Pan, M. Li, J. Zhao, P. Chen, W. Lu and J. Zou, In situ TEM observation of the vapor-solid-solid growth of <001> InAs nanowires, Nanoscale, 2020, 12, 11711–11717 RSC.
- T. Hu, Y. Cao, S. M. Franzén, D. Jacobsson, M. S. Seifner, M. E. Messing and K. A. Dick, In situ manipulation of growth mechanisms in the vapor-solid-solid growth of GaP nanowires, Adv. Mater. Interfaces, 2024, 2400805 CrossRef.
- E. Bellet-Amalric, F. Panciera, G. Patriarche, L. Travers, M. den Hertog, J. C. Harmand, F. Glas and J. Cibert, Regulated dynamics with two monolayer steps in vapor-solid-solid growth of nanowires, ACS Nano, 2022, 16, 4397–4407 CrossRef CAS.
- S. Song, K. Y. Kim, S. H. Lee, K. K. Kim, K. Lee, W. Lee, H. Jeon and S. H. Ko, Recent advances in 1D nanomaterial-based bioelectronics for healthcare applications, Adv. NanoBiomed Res., 2022, 2, 2100111 CrossRef CAS.
- T. Jin, Q. Han, Y. Wang and L. Jiao, 1D nanomaterials: design, synthesis, and applications in sodium-ion batteries, Small, 2018, 14, 1703086 CrossRef.
- G. W. Liu, Y. Zhang, M. P. Thomas, A. Ullah, M. Pharr, B. S. Guiton and S. Banerjee, Negative thermal expansion HfV2O7 nanostructures for alleviation of thermal stress in nanocomposite coatings, ACS Appl. Mater. Interfaces, 2021, 13, 44723–44732 CrossRef CAS.
- X. Li, S. Cheng, S. Deng, X. Wei, J. Zhu and Q. Chen, Direct observation of the layer-by-layer growth of ZnO nanopillar by in situ high resolution transmission electron microscopy, Sci. Rep., 2017, 7, 40911 CrossRef CAS.
- J. H. Kim, J. G. Kim, J. Song, T. S. Bae, K. H. Kim, Y. S. Lee, Y. Pang, K. H. Oh and H.-S. Chung, Investigation of the growth and in situ heating transmission electron microscopy analysis of Ag2S-catalyzed ZnS nanowires, Appl. Surf. Sci., 2018, 436, 556–561 CrossRef CAS.
- Z. Fan, J. L. Maurice, I. Florea, W. Chen, L. Yu, S. Guilet, E. Cambril, X. Lafosse, L. Couraud, S. Bouchoule and P. Roca i Cabarrocas, In situ observation of droplet nanofluidics for yielding low-dimensional nanomaterials, Appl. Surf. Sci., 2022, 573, 151510 CrossRef CAS.
- S. Choi, J. Lee, M. Pin, J. H. Kwon, I. Kim, M. S. Yeom, C. S. Kim, H. S. Lee, S. J. Ahn, S. H. Yi and Y. H. Kim, Anisotropic atomistic evolution during the sublimation of polar InAs nanowires, Nanoscale, 2019, 11, 6685–6692 RSC.
- R. Boston, Z. Schnepp, Y. Nemoto, Y. Sakka and S. R. Hall, In situ TEM observation of a microcrucible mechanism of nanowire growth, Science, 2014, 344, 623–626 CrossRef CAS.
- J. H. Chang, Y. T. Tseng, A. Y. Ho, H. Y. Lo, C. Y. Huang, S. C. Tsai, T. H. Yu, Y. L. Wu, H. K. Yen, P. H. Yeh, K. C. Lu and W. W. Wu, In situ TEM investigation of indium oxide/titanium oxide nanowire heterostructures growth through solid state reactions, Mater. Charact., 2022, 187, 111832 CrossRef CAS.
- K. M. Vailonis, K. Gnanasekaran, X. B. Powers, N. C. Gianneschi and D. M. Jenkins, Elucidating the growth of metal-organic nanotubes combining isoreticular synthesis with liquid-cell transmission electron microscopy, J. Am. Chem. Soc., 2019, 141, 10177–10182 CrossRef CAS.
- K. He, A. Nie, Y. Yuan, S. M. Ghodsi, B. Song, E. Firlar, J. Lu, Y. Lu, T. Shokuhfar, C. M. Megaridis and R. Shahbazian-Yassar, In situ transmission electron microscopy explores a new nanoscale pathway for direct gypsum formation in aqueous solution, ACS Appl. Nano Mater., 2018, 1, 5430–5440 CrossRef CAS.
- K. Gnanasekaran, K. M. Vailonis, D. M. Jenkins and N. C. Gianneschi, In situ monitoring of the seeding and growth of silver metal-organic nanotubes by liquid-cell transmission electron microscopy, ACS Nano, 2020, 14, 8735–8743 CrossRef CAS PubMed.
- É. Ngo, W. Wang, P. Bulkin, I. Florea, M. Foldyna, P. Roca i Cabarrocas and J. L. Maurice, Liquid-assisted vapor-solid-solid silicon nanowire growth mechanism revealed by in situ TEM when using Cu-Sn bimetallic catalysts, J. Phys. Chem. C, 2021, 125, 19773–19779 CrossRef.
- K. Gnanasekaran, J. Korpanty, O. Berger, N. Hampu, M. Halperin-Sternfeld, D. Cohen-Gerassi, L. Adler-Abramovich and N. C. Gianneschi, Dipeptide nanostructure assembly and dynamics via in situ liquid-phase electron microscopy, ACS Nano, 2021, 15, 16542–16551 CrossRef CAS.
- X. Ye, M. R. Jones, L. B. Frechette, Q. Chen, A. S. Powers, P. Ercius, G. Dunn, G. M. Rotskoff, S. C. Nguyen, V. P. Adiga, A. Zettl, E. Rabani, P. L. Geissler and A. P. Alivisatos, Single-particle mapping of nonequilibrium nanocrystal transformations, Science, 2016, 354, 874–877 CrossRef CAS PubMed.
- K. Aliyah, J. Lyu, C. Goldmann, T. Bizien, C. Hamon, D. Alloyeau and D. Constantin, real-time in situ observations reveal a double role for ascorbic acid in the anisotropic growth of silver on gold, J. Phys. Chem. Lett., 2020, 11, 2830–2837 CrossRef CAS.
- M. Dong, W. Wang, W. Wei, X. Hu, M. Qin, Q. Zhang, L. Sun and F. Xu, Understanding the ensemble of growth behaviors of sub-10-nm silver nanorods using in situ liquid cell transmission electron microscopy, J. Phys. Chem. C, 2019, 123, 21257–21264 CrossRef CAS.
- M. S. A. Asghar, B. J. Inkson and G. Möbus, In situ formation of 1D nanostructures from ceria nanoparticle dispersions by liquid cell TEM irradiation, J. Mater. Sci., 2020, 55, 2815–2825 CrossRef CAS.
- H. G. Liao, L. Cui, S. Whitelam and H. Zheng, Real-time imaging of Pt3Fe nanorod growth in solution, Science, 2012, 336, 1011–1014 CrossRef CAS.
- K. Y. Niu, M. Liu, K. A. Persson, Y. Han and H. Zheng, Strain-mediated interfacial dynamics during Au-PbS core-shell nanostructure formation, ACS Nano, 2016, 10, 6235–6240 CrossRef CAS.
- M. Xin, Z. Chang, H. Luo, R. Zeng, G. Zhang and Q. Lei, An electrical super-insulator prototype of 1D gas-solid Al2O3 nanocell, Nano Energy, 2017, 39, 95–100 CrossRef CAS.
- Y. Ma, W. Gao, H. Shan, W. Chen, W. Shang, P. Tao, C. Song, C. Addiego, T. Deng, X. Pan and J. Wu, Platinum-based nanowires as active catalysts toward oxygen reduction reaction: in situ observation of surface-diffusion-assisted, solid-state oriented attachment, Adv. Mater., 2017, 29, 1703460 CrossRef.
- L. C. Campos, M. Tonezzer, A. S. Ferlauto, V. Grillo, R. Magalhães-Paniago, S. Oliveira, L. O. Ladeira and R. G. Lacerda, Vapor-solid-solid growth mechanism driven by epitaxial match between solid AuZn alloy catalyst particles and ZnO nanowires at low temperatures, Adv. Mater., 2008, 20, 1499–1504 CrossRef CAS.
- F. Panciera, Z. Baraissov, G. Patriarche, V. G. Dubrovskii, F. Glas, L. Travers, U. Mirsaidov and J. C. Harmand, Phase selection in self-catalyzed GaAs nanowires, Nano Lett., 2020, 20, 1669–1675 CrossRef CAS.
- M. Marnauza, R. Sjökvist, S. Lehmann and K. A. Dick, Diameter control of GaSb nanowires revealed by in situ environmental transmission electron microscopy, J. Phys. Chem. Lett., 2023, 14, 7404–7410 CrossRef CAS PubMed.
- B. M. Hudak, Y. J. Chang, L. Yu, G. Li, D. N. Edwards, M. E. Park and B. S. Guiton, Understanding nanomaterial synthesis with in situ transmission electron microscopy, Microsc. Microanal., 2015, 21, 1507–1508 CrossRef.
- W. Xin, I. M. D. Rosa, Y. Cao, X. Yin, H. Yu, P. Ye, L. Carlson and J. M. Yang, Ultrasonication-assisted synthesis of high aspect ratio gold nanowires on a graphene template and investigation of their growth mechanism, Chem. Commun., 2018, 54, 4124–4127 RSC.
- Y. C. Chou, F. Panciera, M. C. Reuter, E. A. Stach and F. M. Ross, Nanowire growth kinetics in aberration corrected environmental transmission electron microscopy, Chem. Commun., 2016, 52, 5686–5689 RSC.
- X. Zhao, S. Sun, F. Yang and Y. Li, Atomic-scale evidence of catalyst evolution for the structure controlled growth of single-walled carbon nanotubes, Acc. Chem. Res., 2022, 55, 3334–3344 CrossRef CAS PubMed.
- L. Zhang, M. He, T. W. Hansen, J. Kling, H. Jiang, E. I. Kauppinen, A. Loiseau and J. B. Wagner, Growth termination and multiple nucleation of single-wall carbon nanotubes evidenced by in situ transmission electron microscopy, ACS Nano, 2017, 11, 4483–4493 CrossRef CAS.
- R. Ma, L. Qiu, L. Zhang, D. M. Tang, Y. Wang, B. Zhang, F. Ding, C. Liu and H. M. Cheng, Nucleation of single-wall carbon nanotubes from faceted Pt catalyst particles revealed by in situ transmission electron microscopy, ACS Nano, 2022, 16, 16574–16583 CrossRef CAS.
- X. Huang, R. Farra, R. Schlögl and M. G. Willinger, Growth and termination dynamics of multiwalled carbon nanotubes at near Ambient pressure: an in situ transmission electron microscopy study, Nano Lett., 2019, 19, 5380–5387 CrossRef CAS.
- H. Fan, L. Qiu, A. Fedorov, M. G. Willinger, F. Ding and X. Huang, Dynamic state and active structure of Ni-Co catalyst in carbon nanofiber growth revealed by in situ transmission electron microscopy, ACS Nano, 2021, 15, 17895–17906 CrossRef CAS PubMed.
- M. Choe, H. Chung, W. Kim, Y. Jang, Z. Wang and Z. Lee, In situ transmission electron microscopy of temperature-dependent carbon nanofiber and carbon nanotube growth from ethanol vapor, Carbon, 2024, 219, 118843 CrossRef CAS.
- H. Yoshida, S. Takeda, T. Uchiyama, H. Kohno and Y. Homma, Atomic-scale in situ observation of carbon nanotube growth from solid state iron carbide nanoparticles, Nano Lett., 2008, 8, 2082–2086 CrossRef CAS PubMed.
- H. Zhang, H. M. Cheng and P. Ye, 2D nanomaterials: beyond graphene and transition metal dichalcogenides, Chem. Soc. Rev., 2018, 47, 6009–6012 RSC.
- H. Zhang, Ultrathin two-dimensional nanomaterials, ACS Nano, 2015, 9, 9451–9469 CrossRef CAS PubMed.
- Y. Zhu, L. Peng, Z. Fang, C. Yan, X. Zhang and G. Yu, Structural engineering of 2D nanomaterials for energy storage and catalysis, Adv. Mater., 2018, 30, 1706347 CrossRef.
- Y. Wu, Y. Wu, Y. Sun, W. Zhao and L. Wang, 2D nanomaterials reinforced organic coatings for marine corrosion protection: state of the art, challenges, and future prospectives, Adv. Mater., 2024, 36, 2312460 CrossRef CAS.
- Y. Chao, Y. Han, Z. Chen, D. Chu, Q. Xu, G. Wallace and C. Wang, Multiscale structural design of 2D nanomaterials-based flexible electrodes for wearable energy storage applications, Adv. Sci., 2024, 11, 2305558 CrossRef CAS.
- C. Tan, X. Cao, X. J. Wu, Q. He, J. Yang, X. Zhang, J. Chen, W. Zhao, S. Han, G. H. Nam, M. Sindoro and H. Zhang, Recent advances in ultrathin two-dimensional nanomaterials, Chem. Rev., 2017, 117, 6225–6331 CrossRef CAS PubMed.
- X. Li, M. Sun, C. Shan, Q. Chen and X. Wei, Mechanical properties of 2D materials studied by in situ microscopy techniques, Adv. Mater. Interfaces, 2018, 5, 1701246 CrossRef.
- W. Sohn, M. Kim and H. W. Jang, Atomic-scale insights into the 2D materials from aberration-corrected scanning transmission electron microscopy: progress and future, Small Sci., 2024, 4, 2300073 CrossRef CAS PubMed.
- R. Luo, M. Gao, C. Wang, J. Zhu, R. Guzman and W. Zhou, Probing functional structures, defects, and interfaces of 2D transition metal dichalcogenides by electron microscopy, Adv. Funct. Mater., 2024, 34, 2307625 CrossRef CAS.
- Y. Zhang, Z. Zhang, Y. Cheng, F. Cheng, L. Wang, N. Liu, L. Li, J. Su and Y. Gao, In situ TEM observation of controlled growth of two-dimensional WS2 with vertically aligned layers and high-temperature stability, Nano Energy, 2020, 67, 104221 CrossRef CAS.
- N. Kondekar, M. G. Boebinger, M. Tian, M. H. Kirmani and M. T. McDowell, The effect of nickel on MoS2 growth revealed with in situ transmission electron microscopy, ACS Nano, 2019, 13, 7117–7126 CrossRef CAS.
- X. Sang, X. Li, A. A. Puretzky, D. B. Geohegan, K. Xiao and R. R. Unocic, Atomic insight into thermolysis-driven growth of 2D MoS2, Adv. Funct. Mater., 2019, 29, 1902149 CrossRef CAS.
- C. N. S. Kumar, M. Konrad, V. S. K. Chakravadhanula, S. Dehm, D. Wang, W. Wenzel, R. Krupke and C. Kübel, Nanocrystalline graphene at high temperatures: insight into nanoscale processes, Nanoscale Adv., 2019, 1, 2485–2494 RSC.
- D. S. Gavhane, A. D. Sontakke and M. A. van Huis, Selective vertical and horizontal growth of 2D WS2 revealed by in situ thermolysis using transmission electron microscopy, Adv. Funct. Mater., 2022, 32, 2106450 CrossRef CAS.
- M. Wang, J. H. Kim, S. S. Han, M. Je, J. Gil, C. Noh, T. J. Ko, K. S. Lee, D. I. Son, T. S. Bae, H. I. Ryu, K. H. Oh, Y. Jung, H. Choi, H. S. Chung and Y. Jung, Structural evolutions of vertically aligned two-dimensional MoS2 layers revealed by in situ heating transmission electron microscopy, J. Phys. Chem. C, 2019, 123, 27843–27853 CrossRef CAS.
- B. C. Bayer, R. Kaindl, M. Reza Ahmadpour Monazam, T. Susi, J. Kotakoski, T. Gupta, D. Eder, W. Waldhauser and J. C. Meyer, Atomic-scale in situ observations of crystallization and restructuring processes in two-dimensional MoS2 films, ACS Nano, 2018, 12, 8758–8769 CrossRef CAS PubMed.
- H. Inani, D. H. Shin, J. Madsen, H. Jeong, M. H. Kwon, N. McEvoy, T. Susi, C. Mangler, S. W. Lee, K. Mustonen and J. Kotakoski, Step-by-step atomic insights into structural reordering from 2D to 3D MoS2, Adv. Funct. Mater., 2021, 31, 2008395 CrossRef CAS.
- J. Y. Zhang, L. P. Xiao and M. Lu, In situ liquid-phase transmission electron microscopy for two-dimensional energy materials, Sci. China: Chem., 2025, 68, 414–429 CrossRef CAS.
- G. Zhu, Y. Jiang, F. Lin, H. Zhang, C. Jin, J. Yuan, D. Yang and Z. Zhang, In situ study of the growth of two-dimensional palladium dendritic nanostructures using liquid-cell electron microscopy, Chem. Commun., 2014, 50, 9447–9450 RSC.
- J. H. Park, N. M. Schneider, J. M. Grogan, M. C. Reuter, H. H. Bau, S. Kodambaka and F. M. Ross, Control of electron beam-induced Au nanocrystal growth kinetics through solution chemistry, Nano Lett., 2015, 15, 5314–5320 CrossRef CAS PubMed.
- B. Jin, H. Wang, M. L. Sushko, C. Jin and R. Tang, The formation and shape transformation mechanism of a triangular Au nanoplate revealed by liquid-cell TEM, Nanoscale, 2020, 12, 19592–19596 RSC.
- J. Zhang, Y. Jiang, Q. Fan, M. Qu, N. He, J. Deng, Y. Sun, J. Cheng, H. G. Liao and S. G. Sun, Atomic scale tracking of single layer oxide formation: self-peeling and phase transition in solution, Small Methods, 2021, 5, 2001234 CrossRef CAS PubMed.
- J. Yang, Z. Zeng, J. Kang, S. Betzler, C. Czarnik, X. Zhang, C. Ophus, C. Yu, K. Bustillo, M. Pan, J. Qiu, L. W. Wang and H. Zheng, Formation of two-dimensional transition metal oxide nanosheets with nanoparticles as intermediates, Nat. Mater., 2019, 18, 970–976 CrossRef CAS.
- K. S. Vasu, E. Prestat, J. Abraham, J. Dix, R. J. Kashtiban, J. Beheshtian, J. Sloan, P. Carbone, M. Neek-Amal, S. J. Haigh, A. K. Geim and R. R. Nair, Van der waals pressure and its effect on trapped interlayer molecules, Nat. Commun., 2016, 7, 12168 CrossRef CAS.
- Q. Zhang, X. Peng, Y. Nie, Q. Zheng, J. Shangguan, C. Zhu, K. C. Bustillo, P. Ercius, L. Wang, D. T. Limmer and H. Zheng, Defect-mediated ripening of core-shell nanostructures, Nat. Commun., 2022, 13, 2211 CrossRef CAS PubMed.
- J. Zhang, G. Li, H. G. Liao and S. G. Sun, Tracking the atomic pathways of Pt3Ni-Ni(OH)2 core-shell structures at the gas-liquid interface by in situ liquid cell TEM, Sci. China Chem., 2020, 63, 513–518 CrossRef CAS.
- J. Zhang, Atomic-scale imaging of the growth and transformation of Pt3Ni-NiO nanoparticles, New J. Chem., 2021, 45, 2217–2220 RSC.
- L. Xiao, G. Wang, X. Huang, S. Zhou, R. Zhou, Y. Jiang, S. Liu, G. Li, H. Zheng, S. G. Sun and H. G. Liao, Efficient CO2 reduction MOFs derivatives transformation mechanism revealed by in situ liquid phase TEM, Appl. Catal. B Environ., 2022, 307, 121164 CrossRef CAS.
- W. Wang, H. Yan, U. Anand and U. Mirsaidov, Visualizing the conversion of metal-organic framework nanoparticles into hollow layered double hydroxide nanocages, J. Am. Chem. Soc., 2021, 143, 1854–1862 CrossRef CAS PubMed.
- X. Peng, P. M. Pelz, Q. Zhang, P. Chen, L. Cao, Y. Zhang, H. G. Liao, H. Zheng, C. Wang, S. G. Sun and M. C. Scott, Observation of formation and local structures of metal-organic layers via complementary electron microscopy techniques, Nat. Commun., 2022, 13, 5197 CrossRef CAS.
- N. A. Jose, J. J. Varghese, S. H. Mushrif, H. C. Zeng and A. A. Lapkin, Assembly of two-dimensional metal organic framework superstructures via solvent-mediated oriented attachment, J. Phys. Chem. C, 2021, 125, 22837–22847 CrossRef CAS.
- Z. Ou, Z. Wang, B. Luo, E. Luijten and Q. Chen, Kinetic pathways of crystallization at the nanoscale, Nat. Mater., 2020, 19, 450–455 CrossRef CAS.
- W. Wang, S. W. Chee, H. Yan, I. Erofeev and U. Mirsaidov, Growth dynamics of vertical and lateral layered double hydroxide nanosheets during electrodeposition, Nano Lett., 2021, 21, 5977–5983 CrossRef CAS PubMed.
- B. K. Choi, J. Kim, Z. Luo, J. Kim, J. H. Kim, T. Hyeon, S. Mehraeen, S. Park and J. Park, Shape transformation mechanism of gold nanoplates, ACS Nano, 2023, 17, 2007–2018 CrossRef CAS PubMed.
- D. Alloyeau, W. Dachraoui, Y. Javed, H. Belkahla, G. Wang, H. Lecoq, S. Ammar, O. Ersen, A. Wisnet, F. Gazeau and C. Ricolleau, Unravelling kinetic and thermodynamic effects on the growth of gold nanoplates by liquid transmission electron microscopy, Nano Lett., 2015, 15, 2574–2581 CrossRef CAS PubMed.
- M. Dong, R. Fu, H. Min, Q. Zhang, H. Dong, Y. Pan, L. Sun, W. Wei, M. Qin, Z. Zhu and F. Xu, In situ liquid cell transmission electron microscopy investigation on the dissolution-regrowth mechanism dominating the shape evolution of silver nanoplates, Cryst. Growth Des., 2021, 21, 1314–1322 CrossRef CAS.
- J. Yu, X. Y. Li, J. Miao, W. Yuan, S. Zhou, B. Zhu, Y. Gao, H. Yang, Z. Zhang and Y. Wang, Atomic mechanism in layer-by-layer growth via surface reconstruction, Nano Lett., 2019, 19, 4205–4210 CrossRef CAS.
- Y. Liu, L. Xu, L. Zhang, Z. Dong, S. Wang and L. Luo, Direct visualization of atomic-scale graphene growth on Cu through environmental transmission electron microscopy, ACS Appl. Mater. Interfaces, 2020, 12, 52201–52207 CrossRef CAS PubMed.
- J. Kling, T. W. Hansen and J. B. Wagner, Quantifying the growth of individual graphene layers by in situ environmental transmission electron microscopy, Carbon, 2016, 99, 261–266 CrossRef CAS.
- K. Yu, W. Zhao, X. Wu, J. Zhuang, X. Hu, Q. Zhang, J. Sun, T. Xu, Y. Chai, F. Ding and L. Sun, In situ atomic-scale observation of monolayer graphene growth from SiC, Nano Res., 2018, 11, 2809–2820 CrossRef CAS.
- N. Kurtyka, B. van Devener, B. W. Chung and L. W. I. McDonald, In situ liquid cell transmission electron microscopy study of studtite particle formation and growth via electron beam radiolysis, ACS Omega, 2023, 8, 48336–48343 CrossRef CAS PubMed.
- K. L. Tai, C. W. Huang, R. F. Cai, G. M. Huang, Y. T. Tseng, J. Chen and W. W. Wu, Atomic-scale fabrication of in-plane heterojunctions of few-layer MoS2 via in situ scanning transmission electron microscopy, Small, 2020, 16, 1905516 CrossRef CAS PubMed.
- Y. Fei, T. Tong, J. Bao and Y. H. Hu, In situ observation of electron-beam-induced NaH decomposition in graphene nanoreactors by transmission electron microscopy, J. Phys. Chem. Lett., 2023, 14, 1–8 CrossRef CAS PubMed.
- T. Clark, C. A. Taylor, C. M. Barr and K. Hattar, Sample preparation and experimental design for in situ multi-beam transmission electron microscopy irradiation experiments, J. Vis. Exp., 2022, e61293 CAS.
- H. Ma, D. Kim, S. I. Park, B. K. Choi, G. Park, H. Baek, H. Lee, H. Kim, J. S. Yu, W. C. Lee, J. Park and J. Yang, Direct observation of off-stoichiometry-induced phase transformation of 2D CdSe quantum nanosheets, Adv. Sci., 2023, 10, 2205690 CrossRef CAS.
- K. K. Neelisetty, X. Mu, S. Gutsch, A. Vahl, A. Molinari, F. Von Seggern, M. Hansen, T. Scherer, M. Zacharias, L. Kienle, V. K. Chakravadhanula and C. Kübel, Electron beam effects on oxide thin films—structure and electrical property correlations, Microsc. Microanal., 2019, 25, 592–600 CrossRef CAS.
- O. Dyck, A. R. Lupini, P. D. Rack, J. Fowlkes and S. Jesse, Controlling hydrocarbon transport and electron beam induced deposition on single layer graphene: Toward atomic scale synthesis in the scanning transmission electron microscope, Nano Sel., 2022, 3, 643–654 CrossRef CAS.
- S. J. Kim, K. S. Dae, J. Y. Park, J. Y. Lee and J. M. Yuk, Hollow Ag2S nanosphere formation via electron beam-assisted oxidative etching of Ag nanoparticles, Chem. Commun., 2017, 53, 11122–11125 RSC.
- Y. Ding, K. C. Pradel and Z. L. Wang, In situ transmission electron microscopy observation of ZnO polar and non-polar surfaces structure evolution under electron beam irradiation, J. Appl. Phys., 2016, 119, 015305 CrossRef.
- Y. T. Tseng, L. S. Lu, F. C. Shen, C. H. Wang, H. Y. Sung, W. H. Chang and W. W. Wu, In situ atomic-scale observation of monolayer MoS2 devices under high-voltage biasing via transmission electron microscopy, Small, 2022, 18, 2106411 CrossRef CAS.
- Y. T. Tseng, K. L. Tai, C. W. Huang, C. Y. Huang and W. W. Wu, Atomic-scale localized thinning and reconstruction of two-dimensional WS2 layers through in situ transmission electron microscopy/scanning transmission electron microscopy, J. Phys. Chem. C, 2020, 124, 14935–14940 CrossRef CAS.
- S. Dai, M. He and J. Zhu, E-beam-induced in situ structural transformation in one-dimensional nanomaterials, Sci. Bull., 2015, 60, 71–75 CrossRef CAS.
- M. Owusu-Mensah, J. Cooper, A. L. Morales, K. Yano, S. D. Taylor, D. K. Schreiber, B. P. Uberuaga and D. Kaoumi, Surprisingly high irradiation-induced defect mobility in Fe3O4 as revealed through in situ transmission electron microscopy, Mater. Charact., 2022, 187, 111863 CrossRef CAS.
- S. Y. Kim, J. H. Kim, T. Jeong, K. B. Kim, H. J. Kim, K. M. Nam, S. J. Ahn, J. H. Kwon and Y. H. Kim, Accelerated decomposition of Bi2S3 nanorods in water under an electron beam: a liquid phase transmission electron microscopy study, Nanotechnology, 2021, 32, 195702 CrossRef CAS PubMed.
- H. Zhang, W. Wang, T. Xu, F. Xu and L. Sun, Phase transformation at controlled locations in nanowires by in situ electron irradiation, Nano Res., 2020, 13, 1912–1919 CrossRef CAS.
- X. Huang, T. Jones, H. Fan and M. G. Willinger, Atomic-scale observation of irradiation-induced surface oxidation by in situ transmission electron microscopy, Adv. Mater. Interfaces, 2016, 3, 1600751 CrossRef.
- Z. Dang, J. Shamsi, F. Palazon, M. Imran, Q. A. Akkerman, S. Park, G. Bertoni, M. Prato, R. Brescia and L. Manna, In situ transmission electron microscopy study of electron beam-induced transformations in colloidal cesium lead halide perovskite nanocrystals, ACS Nano, 2017, 11, 2124–2132 CrossRef CAS.
- I. G. Gonzalez-Martinez, A. Bachmatiuk, V. Bezugly, J. Kunstmann, T. Gemming, Z. Liu, G. Cuniberti and M. H. Rümmeli, Electron-beam induced synthesis of nanostructures: a review, Nanoscale, 2016, 8, 11340–11362 RSC.
- X. Guo, H. Xu, Y. Tang, Z. Yang, F. Dou, W. Li, Q. Li and H. Pang, Confining iodine into metal-organic framework derived metal-nitrogen-carbon for long-life aqueous zinc-iodine batteries, Adv. Mater., 2024, 36, 2408317 CrossRef CAS.
- C. Guo, Y. Cao, Y. Gao, C. Zhi, Y. X. Wang, Y. Luo, X. J. Yang and X. Luo, Cobalt single-atom electrocatalysts enhanced by hydrogen-bonded organic frameworks for long-lasting zinc-iodine batteries, Adv. Funct. Mater., 2024, 34, 2314189 CrossRef CAS.
- E. M. You, Y. Gu, J. Yi, D. Y. Wu, J. F. Li and Z. Q. Tian, Unraveling the multilayer solid-electrolyte interphase in lithium batteries through depth-sensitive plasmon-enhanced Raman spectroscopy: a theoretical and experimental study, Electrochim. Acta, 2024, 498, 144689 CrossRef CAS.
- J. Wang, W. Tang, Z. Zhu, Y. Lin, L. Zhao, H. Chen, X. Qi, X. Niu, R. Wu and J. S. Chen, Stabilizing lattice oxygen of Bi2O3 by interstitial insertion of indium for efficient formic acid electrosynthesis, Angew. Chem., Int. Ed., 2025, e202423658 CAS.
- A. Duan, S. Luo, Y. Tang, Y. Feng, M. Li, B. Zhang and W. Sun, In situ monitoring of dynamic adsorption-induced interfacial buffering toward highly stable zinc metal batteries, Adv. Energy Mater., 2025, 2404693 CrossRef.
- É. A. Santos, M. C. Policano, M. J. Pinzón, I. Galantini, V. A. Gonçalves, F. C. B. Maia, L. J. A. Macedo, G. Doubek, R. G. Freitas and H. Zanin, Operando FTIR study on water additive in lithium-sulfur batteries to mitigate shuttle effect, J. Energy Chem., 2024, 98, 702–713 CrossRef.
- X. Liu, M. Zhang, X. Wang, Y. Peng, Y. Liu, S. Ullah, Z. Duan, W. Gao, B. Song, M. Wei, J. He, Z. Li and Y. Wu, Evidence of quasi-Na metallic clusters in sodium ion batteries through in situ X-Ray diffraction, Adv. Mater., 2025, 37, 2410673 CrossRef CAS.
- X. Li, W. Hao, H. Wang, T. Li, D. Trikkaliotis, X. Zhou, D. Hou, K. Chang, A. M. Hashem, Y. Liu, Z. Yang, S. Cao, G. Hwang, G. Z. Kyzas, S. Yang, C. B. Mullins, C. M. Julien and L. Zhu, Lithium storage mechanisms and electrochemical behavior of a molybdenum disulfide nanoparticle anode, Energy Environ. Mater., 2024, e12855 CrossRef.
- L. Li, A. Huang, H. Jiang, Y. Li, X. Pan, T. Y. Chen, H. Y. Chen and S. Peng, Encapsulation of Sn sub-nanoclusters in multichannel carbon matrix for high-performance potassium-ion batteries, Angew. Chem., Int. Ed., 2024, 63, e202412077 CrossRef CAS.
- K. Romanenko, N. Avdievich and E. Foy, A universal plug-and-play approach to in situ multinuclear magnetic resonance analysis of electrochemical phenomena in commercial battery cells, J. Am. Chem. Soc., 2024, 146, 29407–29416 CrossRef CAS.
- M. Gabrijelčič, B. Tratnik, G. Kapun, E. Tchernychova, N. Z. Logar, A. Krajnc, R. Dominko and A. Vizintin, Probing sodium structures and dynamics in hard carbon for Na-ion batteries using 23Na operando solid-state NMR spectroscopy, J. Mater. Chem. A, 2025, 13, 1042–1056 RSC.
- I. E. Gunathilaka, S. A. Ferdousi, F. Chen, M. Armand, A. A. H. Padua, P. C. Howlett, M. Forsyth and L. A. O'Dell, Studying the growth and morphology of metal microstructures in sodium metal batteries with ionic liquid electrolytes by operando 23Na NMR spectroscopy, Nano Energy, 2025, 133, 110479 CrossRef CAS.
- Y. Kwon, A. Svirinovsky-Arbeli, J. C. Hestenes, P. J. Buitrago Botero, K. R. M. Corpus, P. Lepucki, O. Pecher and L. E. Marbella, Elucidating the role of cathode identity: voltage-dependent reversibility of anode-free batteries, Chem, 2024, 10, 3159–3183 CAS.
- D. J. Yun, S. Kim, S. Heo, H. Choi, J. Baik, J. Chung, S. Park, D. Yu, J. Lee, S. Kang, C. Jung and D. S. Ko, Understanding the role of metal Interlayers in all-solid-electrolyte batteries using operando X-Ray photoelectron spectroscopy, Adv. Energy Mater., 2024, 14, 2470140 CrossRef.
- R. Lee, T. S. Nunney, M. Isaacs, R. G. Palgrave and A. Dey, Monitoring the behavior of Na ions and solid electrolyte interphase formation at an aluminum/ionic liquid electrode/electrolyte interface via operando electrochemical X-ray photoelectron spectroscopy, ACS Appl. Mater. Interfaces, 2024, 16, 35675–35685 CrossRef CAS.
- F. G. Capone, J. Sottmann, V. Meunier, L. P. Ramírez, A. Grimaud, A. Iadecola, M. Scardamaglia, J. P. Rueff and R. Dedryvère, Operando observation of the dynamic SEI formation on a carbonaceous electrode by near-ambient pressure XPS, Energy Environ. Sci., 2024, 17, 1509–1519 RSC.
- A. Krishnan, D. C. Lee, I. Slagle, S. Ahsan, S. Mitra, E. Read and F. M. Alamgir, Monitoring redox processes in lithium-ion batteries by laboratory-scale operando X-ray emission spectroscopy, ACS Appl. Mater. Interfaces, 2024, 16, 16096–16105 CrossRef CAS.
- W. Huang, M. Cao, H. Mao, L. An, Z. Chen, W. Xu, X. Li and H. Wei, Temperature-tunable operando nondestructive detection of electronic and geometrical structures in battery electrodes, Anal. Chem., 2024, 96, 1178–1184 CrossRef CAS.
- Z. Li, H. Yang, W. Cheng and L. Tian, Recent progress of in situ/operando characterization techniques for electrocatalytic energy conversion reaction, Chin. Chem. Lett., 2024, 35, 109237 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |




