Open Access Article
Open Access ArticleA thermally and photoresponsive luminescent single-molecule magnet based on dysprosium–anthracene: effect of temperature on anthracene photocycloaddition†
Xiu-Fang
Ma‡
 a,
Xin-Lan
Hou‡
b,
Ye-Hui
Qin
a,
Qian
Teng
a,
Song-Song
Bao
a,
Xin-Lan
Hou‡
b,
Ye-Hui
Qin
a,
Qian
Teng
a,
Song-Song
Bao
 a,
Yu-Xi
Tian
a,
Yu-Xi
Tian
 *b and
Li-Min
Zheng
*b and
Li-Min
Zheng
 *a
*a
aState Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing 210023, China. E-mail: lmzheng@nju.edu.cn
bState Key Laboratory of Analytical Chemistry for Life Science, Key Laboratory of Mesoscopic Chemistry of MOE, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210023, China. E-mail: tyx@nju.edu.cn
First published on 22nd April 2025
Abstract
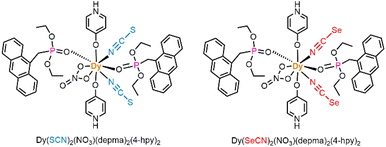
Stimuli-responsive lanthanide-based single-molecule magnets (Ln-SMMs) are attractive for their potential in information storage, sensors and molecular devices. However, challenges remain in synergistically tuning the magnetic and optical properties of Ln-SMMs at the same temperature. Herein, we report a mononuclear dysprosium complex containing anthracene units, namely [Dy(SeCN)2(NO3)(depma)2(4-hpy)2] (1DySeCN) (depma = 9-diethylphosphonomethylanthracene, 4-hpy = 4-hydroxypyridine). It shows a thermally induced phase transition attributed to an order–disorder transition of the axial 4-hpy ligand, and a thermochromism due to a slight slipping of the anthracene pairs. Compound 1DySeCN can undergo [4 + 4] photocycloaddition reaction in a single-crystal-to-single-crystal (SC–SC) transformation manner at room temperature to form the 1D coordination polymer [Dy(SeCN)2(NO3)(depma2)(4-hpy)2]n (2DySeCN), accompanied by a luminescence switch from yellow to blue-white. Meanwhile, significant changes in SMM properties are observed with the reduction of energy barrier from 334 K to 144 K and the narrowing of the butterfly-like hysteresis loop. We further investigated the effect of temperature on the photodimerization reaction of anthracene in 1DySeCN, and found that the compound can still undergo efficient photodimerization at temperatures as low as 200 K. At this temperature, the magnetic susceptibility (χMT) value of the dilute compound 1DySeCN@Y also changed significantly before and after light irradiation. This study provides the first example of lanthanide–anthracene compounds synergistically modulating the magnetic and luminescent properties at the same temperature.
Introduction
Molecular materials showing light-responsive magnetic and luminescent properties have received considerable attention in recent years owing to their potential applications in molecular spintronics, sensors, and devices.1–4 Lanthanide-based single-molecule magnets (Ln-SMMs) are appealing because they not only exhibit characteristic photoluminescence (PL) associated with the f–f/d–f electronic transitions, but also the strong magnetic anisotropy arising from spin–orbit coupling.5–11 However, although the magnetic properties of Ln-SMMs are sensitive to external stimuli,12–15 their luminescent properties are not easily modulated. So far, reports on the synergistic modulation of the magnetic and luminescent properties of Ln-SMMs are still rare,16 which have been realized by external stimuli such as solvent exchange or removal,17,18 mechanical force,19 and light irradiation.20–26 The latter is particularly desired because light can be manipulated quickly and easily. Recent work has shown that the incorporation of photoactive components into Ln-SMMs can achieve changes in SMM and optical properties such as photochromism20–22 and photo-switchable luminescence.23–26 For example, Wang and co-workers found that photogenerated radicals from organic guest molecules induced a switch in the SMM behavior of a Dy chain compound as well as a decrease in luminescence intensity.23 We found that lanthanide–anthracene compounds can undergo reversible [4 + 4] photocycloaddition reaction, accompanied by dramatic changes in magnetic and luminescent properties.26–31 Notably, the temperature at which the photocycloaddition occurred for Dy–anthracene complexes did not generally coincide with the temperature at which the magnetic changes were observed, the former usually at room temperature and the latter at very low temperatures. This raises the question about what is the minimum temperature at which the photocycloaddition reaction of lanthanide–anthracene complexes can efficiently take place? Can the modulation of their magnetic and luminescent properties be achieved by photo-stimulation at the same temperature?To address these questions, we herein report a new Dy–anthracene complex, [Dy(SeCN)2(NO3)(depma)2(4-hpy)2] (1DySeCN, 4-hpy = 4-hydroxypyridine, depma = 9-diethylphosphonomethylanthracene, Scheme 1), and its photophysical, photochemical and magnetic properties at and below room temperature. 1DySeCN was designed and synthesized because of its structural similarity to the previously reported compound [Dy(SCN)2(NO3)(depma)2(4-hpy)2] (DySCN, Scheme 1)31 except for the replacement of SCN− with SeCN−. We envisioned that the incorporation of SeCN− would lead to a weakening of the Dy–N bond at the equatorial position, which in turn would enhance the magnetic anisotropy. Indeed, 1DySeCN shows SMM behaviour at zero dc field with an effective energy barrier (Ueff) of 334 K, which is higher than that of DySCN (Ueff = 277 K). The dilute sample 1DySeCN@Y displays an open butterfly-shaped magnetic hysteresis below the blocking temperature (TB = 4.2 K) with a coercivity (Hc) of 1200 Oe at 2.0 K. To the best of our knowledge, the Ueff, TB and Hc values are the highest among the known photoresponsive luminescent Ln-SMMs. In addition, 1DySeCN undergoes photocycloaddition reaction in a single-crystal-to-single-crystal (SC–SC) manner at room temperature to form a 1D chain compound [Dy(SeCN)2(NO3)(depma2)(4-hpy)2]n (2DySeCN, depma2 is the photo-dimerized depma), accompanied by remarkable changes in photoluminescent (PL) and magnetic properties. Interestingly, the photocycloaddition reaction of 1DySeCN can occur at temperatures down to 140 K. The in situ PL measurements on a single crystal of 1DySeCN revealed a significant photo-induced change in luminescence when the temperature was as low as 200 K. Impressively, the dilute sample 1DySeCN@Y showed photo-triggered luminescence and magnetic changes at 200 K. This work opens up the possibility of synergistically modulating the magnetic and luminescent properties of Ln-SMMs by photocycloaddition at the same temperature.
Results and discussion
Crystal structure of 1DySeCN and its reversible thermally induced SC–SC structural transformation
Compound 1DySeCN was obtained by the reaction of Dy(NO3)3·6H2O, KSeCN, 4-hpy, and depma in CH3CN (Scheme S1†). The purity was confirmed by the PXRD measurement (Fig. S1†). Single crystal structural analysis of 1DySeCN at 293 K (named 1DySeCN-293) reveals that it crystallizes in the monoclinic system, space group P21/m (Tables 1 and S1†). The asymmetric unit contains 0.5 DyIII ion, one SeCN−, 0.5 NO3−, one depma and two half 4-hpy ligands. The DyIII ion is eight-coordinated, surrounded by two N atoms from two SeCN−, and six O atoms from one NO3−, two depma, and two 4-hpy ligands (Fig. 1a and Table S2†). The SHAPE 2.1 calculation suggests that the geometry of {DyN2O6} is close to the Snub diphenoid J84 (D2d) (CShM = 1.802) (Table S3†).32 However, if we consider the chelating NO3− as occupying one coordination site, the geometry of DyIII can be viewed as having a pseudo-D5h symmetry with the two axial positions filled with O atoms from the zwitterionic 4-hpy ligands [Dy1–Oax: 2.208(9), 2.231(8) Å; ∠O7–Dy1–O8: 163.2(4)°; Dy1–O/Neq: 2.313(9)–2.576(8) Å]. The neighbouring molecules are stacked through face-to-face π–π interacting anthracene rings to form a one-dimensional (1D) supramolecular chain (Fig. 1b). The centre-to-centre (dcc) and plane-to-plane (dpp) distances of the two anthracene rings are 3.759 Å and 3.428 Å, respectively, which satisfy the Schmidt's rule for photocycloaddition reaction.33 The chains are further connected by multiple hydrogen bonds of N–H⋯O/Se and C–H⋯O, thus providing a flexible supramolecular network that may tolerate structural changes induced by photocycloaddition reaction (Fig. S2 and Table S4†). In addition, we note that the axial Dy–Oax bond lengths in 1DySeCN-293 (2.208/2.231 Å) are slightly shortened compared with those in DySCN (2.210/2.239 Å), while the Dy1–Neq bond lengths (2.462 Å vs. 2.456 Å in DySCN) and the shortest Dy⋯Dy distance are slightly elongated (9.754 Å vs. 9.54 Å in DySCN),31 suggesting that the magnetic anisotropy could be further enhanced in 1DySeCN.| Complex | 1DySeCN-293 | 1DySeCN-193 | 1DySeCN-130 | 2DySeCN-293 | 2DySeCN-193 |
|---|---|---|---|---|---|
| Temperature | 293 K | 193 K | 130 K | 293 K | 193 K |
| Crystal system | Monoclinic | Triclinic | Triclinic | Monoclinic | Monoclinic |
| Space group | P21/m |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P21/m | P21/m |
| a (Å) | 9.7539(12) | 9.6729(5) | 9.7055(12) | 9.585(9) | 9.5847(12) |
| b (Å) | 25.787(3) | 11.1667(6) | 11.1138(13) | 25.97(2) | 25.809(3) |
| c (Å) | 11.2182(14) | 25.6156(16) | 25.416(3) | 11.340(12) | 11.2751(13) |
| α (0) | 90 | 90.315(2) | 90.469(3) | 90 | 90 |
| β (0) | 111.587(4) | 92.411(2) | 92.518(4) | 112.620(19) | 112.946(3) |
| γ (0) | 90 | 112.001(2) | 112.134(3) | 90 | 90 |
| V (Å3) | 2623.8(5) | 2562.4(3) | 2536.1(5) | 2606(4) | 2568.4(6) |
| Dy–O (axial)/Å | 2.208/2.231 | 2.228/2.240 | 2.227/2.243 | 2.250/2.222 | 2.219/2.225 |
| Dy–O (equatorial)/Å | 2.313–2.576 | 2.317–2.563 | 2.310–2.557 | 2.307–2.568 | 2.326–2.546 |
| Dy–N/Å | 2.463 | 2.464/2.468 | 2.462/2.474 | 2.456 | 2.459 |
| O–Dy–O (axial)/° | 163.2 | 162.0 | 162.2 | 163.2 | 162.4 |
| Dy⋯Dy/Å | 9.754 | 9.673 | 9.705 | 9.584 | 9.585 |
| Slip angle θ/° | 21.91 | 22.04/23.03 | 22.44/24.49 | — | — |
| d cc/Å | 3.759 | 3.835/3.713 | 3.846/3.685 | — | — |
| d pp/Å | 3.428 | 3.433/3.439 | 3.418/3.442 | — | — |
| d C2–C9A/Å | 3.753 | 3.832/3.707 | 3.843/3.664 | 1.658 | 1.645 |
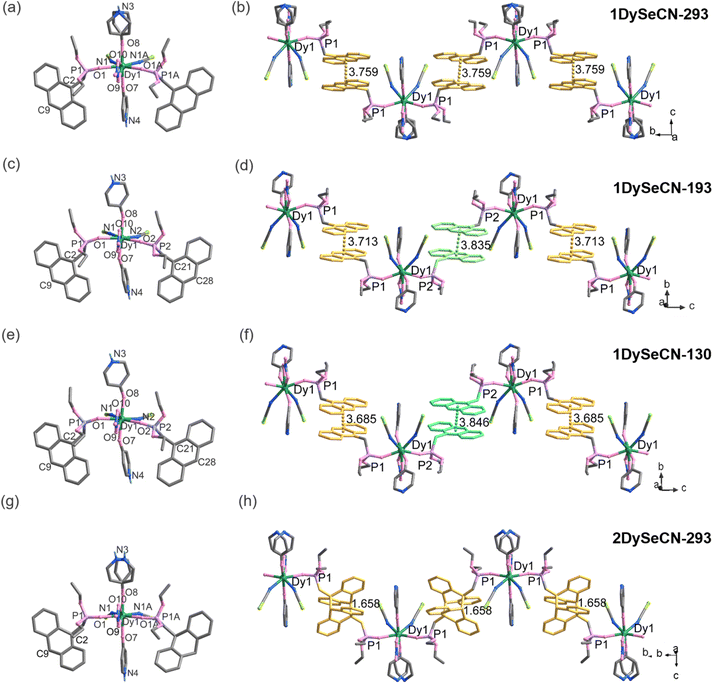 | ||
| Fig. 1 The molecule structures (a, c, e and g) and 1D chain (b, d, f and h) of 1DySeCN-293, 1DySeCN-193, 1DySeCN-130 and 2DySeCN-293. | ||
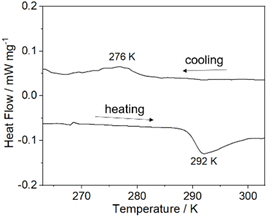
Since one of the two 4-hpy ligands in 1DySeCN is disordered at 293 K, we measured the differential scanning calorimetry (DSC) curve to examine whether an order–disorder phase transition may occur. As shown in Fig. 2 and S3,† the DSC curve of 1DySeCN shows an exothermic peak at 276 K upon cooling and an endothermic peak at 292 K upon warming (ΔT = 16 K). Based on the endothermic peak, we can calculate the enthalpy change (ΔH) to be 1.29 kJ mol−1, and the entropy change (ΔS) to be 4.42 J mol−1 K−1. According to the Boltzmann equation ΔS = R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(N), where R is the gas constant and N represents the number of disordered states, the value N was estimated to be 1.69, suggesting that the phase transition originates from the order–disorder transition of the axial 4-hpy ligand.34 The thermo-induced phase transition of 1DySeCN was also confirmed by variable temperature PXRD measurements (Fig. S4†). Notably, an order–disorder phase transition was not observed for the related compound DySCN down to 193 K, though one of the two axial 4-hpy ligands was also disordered in the latter.31
ln(N), where R is the gas constant and N represents the number of disordered states, the value N was estimated to be 1.69, suggesting that the phase transition originates from the order–disorder transition of the axial 4-hpy ligand.34 The thermo-induced phase transition of 1DySeCN was also confirmed by variable temperature PXRD measurements (Fig. S4†). Notably, an order–disorder phase transition was not observed for the related compound DySCN down to 193 K, though one of the two axial 4-hpy ligands was also disordered in the latter.31
To investigate whether the phase transition affects the local geometry of the DyIII ion and the stacking of the anthracene groups, we determined the single-crystal structure of 1DySeCN at 193 K and named it 1DySeCN-193. Interestingly, 1DySeCN-193 crystallizes in a triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group, which is different from that measured at 293 K (Tables 1 and S1†). The asymmetric unit consists of one DyIII ion, two SeCN−, one NO3−, two depma and two 4-hpy ligands (Fig. 1c). Apparently, the phase transition upon cooling is accompanied by a shift from disorder to order in the axial 4-hpy (Fig. S5†). In addition, we also observe slight changes in bond lengths and angles. Compared with those in 1DySeCN-293, the Dy–Oax bond lengths in 1DySeCN-193 are slightly elongated while the Oax–Dy–Oax angles and Dy–Oeq bond lengths are slightly reduced (Tables 1 and S2†). The most significant structural change is probably the stacking of the anthracene units. In 1DySeCN-293, there is only one type of depma ligand in the structure, and the neighbouring anthracene rings are face-to-face π–π interacted with a dcc distance of 3.759 Å, forming an equally spaced supramolecular chain (Fig. 1b). While in 1DySeCN-193, there are two types of depma ligands (P1-An, P2-An) in the structure, each of them is face-to-face π–π interacted with its equivalent and the dcc distances are 3.713 Å for P1-An⋯P1-An and 3.835 Å for P2-An⋯P2-An, respectively, hence forming an alternatively spaced supramolecular chain (Fig. 1d).
space group, which is different from that measured at 293 K (Tables 1 and S1†). The asymmetric unit consists of one DyIII ion, two SeCN−, one NO3−, two depma and two 4-hpy ligands (Fig. 1c). Apparently, the phase transition upon cooling is accompanied by a shift from disorder to order in the axial 4-hpy (Fig. S5†). In addition, we also observe slight changes in bond lengths and angles. Compared with those in 1DySeCN-293, the Dy–Oax bond lengths in 1DySeCN-193 are slightly elongated while the Oax–Dy–Oax angles and Dy–Oeq bond lengths are slightly reduced (Tables 1 and S2†). The most significant structural change is probably the stacking of the anthracene units. In 1DySeCN-293, there is only one type of depma ligand in the structure, and the neighbouring anthracene rings are face-to-face π–π interacted with a dcc distance of 3.759 Å, forming an equally spaced supramolecular chain (Fig. 1b). While in 1DySeCN-193, there are two types of depma ligands (P1-An, P2-An) in the structure, each of them is face-to-face π–π interacted with its equivalent and the dcc distances are 3.713 Å for P1-An⋯P1-An and 3.835 Å for P2-An⋯P2-An, respectively, hence forming an alternatively spaced supramolecular chain (Fig. 1d).
When the measured temperature was further lowered to 130 K, the obtained 1DySeCN-130 crystallizes in the same triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group, but the cell volume decreases from 2562.4(3) Å3 at 193 K to 2536.1(5) Å3 at 130 K (Tables 1 and S1†). Again, the mononuclear molecules are stacked forming an alternatively arranged supramolecular chain (Fig. 1e and f). Compared to 1DySeCN-193, the dcc and dC2–C9A spacings of the P1-An⋯P1-An pair in 1DySeCN-130 increase slightly by 0.011 Å and 0.011 Å, while those of the P2-An⋯P2-An pair decrease by 0.028 Å and 0.043 Å, respectively (Table 1).
space group, but the cell volume decreases from 2562.4(3) Å3 at 193 K to 2536.1(5) Å3 at 130 K (Tables 1 and S1†). Again, the mononuclear molecules are stacked forming an alternatively arranged supramolecular chain (Fig. 1e and f). Compared to 1DySeCN-193, the dcc and dC2–C9A spacings of the P1-An⋯P1-An pair in 1DySeCN-130 increase slightly by 0.011 Å and 0.011 Å, while those of the P2-An⋯P2-An pair decrease by 0.028 Å and 0.043 Å, respectively (Table 1).
Clearly, lowering temperature leads to a more pronounced differentiation of the two pairs of anthracene rings and a greater deviation of each pair from the face-to-face stacking pattern. The extent to which the anthracene rings deviate from strict face-to-face stacking can be expressed in terms of the slipping angle (θ), i.e., the angle between the centroid–centroid line and the vertical line. The slipping angles of the anthracene rings are 21.910 for 1DySeCN-293, 22.040/23.030 for 1DySeCN-193, and 22.440/24.490 for 1DySeCN-130 (Fig. S6†). Indeed, the slipping angle of the anthracene pairs increases with decreasing temperature. This deviation of anthracene stacking in 1DySeCN may affect its photophysical properties.
Photophysical properties and photoinduced SC–SC structural transformation of 1DySeCN at room temperature
We first measured the solid-state UV-visible diffuse reflectance spectrum of 1DySeCN at room temperature. As shown in Fig. 3a, a broad absorption band is observed in the range of 250–450 nm, attributed to the π → π* transition of the depma ligand. In addition, there appears weak absorption peaks at 747 and 756 nm, arising from the f–f transition of the DyIII from 6H15/2 to 6F3/2.Fig. 3b shows the PL spectra of the bulk sample of 1DySeCN as a function of irradiation time, measured on an Edinburgh FLS 980 instrument. The irradiation of the sample at 395 nm (100 mW cm−2) was performed outside the spectrometer prior to PL measurement at 365 nm. Clearly, before UV light irradiation, 1DySeCN exhibits a broad and strong emission band peaking at 550 nm (λex = 365 nm) with an average lifetime (τ) of 25.35 ns, attributed to the excimer emission of the face-to-face stacked anthracene pair (Table S5†). The quantum yield is 6.22%. After exposure to the 395 nm light, the intensity of the excimer emission decreases continuously with increasing irradiation time. At the same time, new peaks appeared at 424, 446, and 575 nm. The average lifetimes of the peaks at 422 and 446 nm are 2.3 and 4.2 ns, respectively, which are much shorter than that of the excimer emission at 550 nm and can be attributed to the π ← π* transition of the dianthracene moiety.35,36 The shoulder peak at 575 nm correspond to the f–f transition of the DyIII ion from 4F9/2 to 6H13/2, with phosphorescent lifetime of 7.01 μs (Table S5†). Clearly, photodimerization of the anthracene pairs in 1DySeSCN occurs after UV illumination, and this enhances the energy of the 3T state leading to the sensitization of the f–f transition of the dysprosium ion.
Single crystal structural analysis can provide structural information after photoinduced phase transition. By irradiating single crystals of 1DySeCN under 395 nm UV light (100 mW cm−2) at room temperature for 30 min, we obtained [DyIII(SeCN)2(NO3)(depma2)(4-hpy)2]n (2DySeCN). Although the resulting crystals were slightly cracked, they were still suitable for single crystal structure determination. Compound 2DySeCN measured at 293 K (named as 2DySeCN-293) crystallizes in the monoclinic system, space group P21/m. It has a 1D chain structure in which the equivalent DyIII ions are connected by the photo-dimerized depma2 ligands. The central C2–C9A distance in depma2 is 1.658 Å, which is remarkably shortened compared to that in 1DySeCN-293 (3.735 Å). The photodimerization of anthracene pairs also causes changes in the coordination environment of the dysprosium ion (Tables 1 and S2†). The axial Dy–O bond lengths are slightly shortened [2.208(9)/2.231(8) Å vs. 2.250(10)/2.222(11) Å in 1DySeCN-293], and the O7–Dy1–O8 angle becomes slightly smaller [162.4(6)° vs. 163.2(4)° in 1DySeCN-293]. Meanwhile, the equatorial Dy–O/N bond lengths are either elongated or shortened [2.307(7)–2.568(10) Å vs. 2.313(6)–2.576(8) Å in 1DySeCN-293]. The shortest Dy⋯Dy distance (9.584 Å) is smaller than that in 1DySeCN-293 (9.754 Å). Variations in these structural parameters explain the changes in the PL profiles of 1DySeCN-293 upon UV irradiation. Also, they will lead to changes in the magnetic properties (shown below).
Notably, the SC–SC structural transformation from 1DySeCN to 2DySeCN is reversible by annealing 2DySeCN at 105 °C. The process can be repeated at least five times with retaining the single crystallinity (Table S6 and Fig. S7†). It is worth mentioning that although one axial 4-hpy in 2DySeCN-293 is disordered, we did not see an order–disorder phase transition down to 150 K, as confirmed by the single crystal structural analysis at 193 K (Table S2 and Fig. S8†) as well as the DSC analysis in the temperature range 150–303 K (Fig. S3b†).
To ensure the completeness of the photocycloaddition reaction of the bulk sample for physical measurements, we irradiated the crystals of 1DySeCN under 395 nm UV light for 12 hours at room temperature. The purity of the resulting 2DySeCN was confirmed by its PXRD pattern which agrees well with that simulated from the single crystal data (Fig. S1†). In addition, the 1H NMR spectrum of 2DySeCN digested in DMSO-d6 solution was also measured. By integrating the area of the two sets of signals (anthracene and dianthracene), we calculated the yield of the photochemical reaction to be 95.2% (Fig. S9–S11†). The DSC curve of 2DySeCN shows an exothermic peak at 105 °C (ΔH = −51.27 kJ mol−1), indicating the occurrence of de-dimerization of the dianthracene ligand in 2DySeCN (Fig. S12†). Indeed, by annealing 2DySeCN at 105 °C for 10 min, it can be transformed back to the pristine 1DySeCN (named as 1DySeCN-re). This reversed structural transformation was supported by the PXRD, IR, UV-vis, and PL spectra measurements (Fig. 3a, S1, S13 and S14†).
Effect of temperature on the photophysical and photochemical properties of 1DySeCN
To investigate the temperature effect on the photoluminescent properties of 1DySeCN, we selected a single crystal and investigated the temperature dependent PL spectra down to 77 K on a Horiba-JY HR Evolution instrument. As shown in Fig. 4a, 1DySeCN displays a broad and nearly symmetric emission band at 550 nm in the temperature range of 300–180 K, originating from the excimer emission of the π–π stacked anthracene pairs. The emission band is gradually blue-shifted and becomes more asymmetric at temperatures lower than 160 K. When the temperature is lowered to 100 K, we observe a significant blueshift of the excimer emission from 550 nm to 510 nm. If the temperature is further lowered to 77 K, the position of the excimer emission remains unchanged. At the same time, the luminescence colour changes from bright yellow at 150–300 K to cyan-green at 77–100 K (Fig. 4b), which is a feature of thermochromism. Apparently, this thermochromic property is related to the structural changes of the compound at low temperatures. As mentioned earlier, 1DySeCN-193 and 1DySeCN-130 contain two types of anthracene pairs with slightly different dcc distances and slipping angles, which may account for the blue-shift of the excimer emission (Fig. 1b–f). The fluorescence lifetime of 1DySeCN at 77 K was 32.17 ns, which is an enhancement of 6.82 ns over that at room temperature (Table S5†), attributed to the weakening of non-radiative processes at low temperatures.37The effect of temperature is more pronounced for the photochemical reaction of 1DySeCN. In general, lowering the temperature will result in a decrease in the number of molecules in the activated state, thus reducing the rate of reaction. In order to determine the lowest temperature at which the photocycloaddition reaction can occur in a reasonable amount of time, we placed 1DySeCN crystals on a hot pot and irradiated them in situ with 395 nm UV light for 2 h at different temperatures (140–200 K). The resulting samples were subjected to 1H NMR, IR and PXRD measurements (Fig. 5, S15 and S16†). As shown in Fig. 5, we calculated the yields of photochemical reactions of 1DySeCN in the temperature range of 200–140 K to be 40.8%, 21.6%, 9.5% and 6.8%, respectively. In addition, at temperatures as low as 180 K, the weak vibration peak (686 cm−1) characteristic of the dimerized anthracycline of 1DySeCN can still be observed after UV illumination (Fig. S15†). Moreover, the PXRD pattern of 1DySeCN after UV irradiation at 180 K also shows peaks corresponding to 2DySeCN (Fig. S16†). When the temperature further decreases to 160 and 140 K, these weak characteristic peaks almost disappeared in the IR and PXRD profiles.
 | ||
| Fig. 5 The 1H NMR spectra in the range of 9.0–3.0 ppm of 1DySeCN irradiated in situ with 395 nm UV light for 2 h at different temperatures (140–200 K) in DMSO-d6. | ||
To examine the changes in luminescence after UV light irradiation, we selected a single crystal of 1DySeCN and studied the PL spectra after irradiation for different times at low temperatures (77–275 K) using a home-built fluorescence microscope. Due to the limited light sources available for this instrument, we used a 375 nm laser for UV irradiation and excitation with power of 9570 W cm−2. As shown in Fig. 6, the PL spectrum of the 1DySeCN single crystal at 300 K is identical to that of the bulk sample, showing a broad excimer emission peaking at 550 nm. Upon 375 nm light irradiation, photocycloaddition reactions occurred which can be monitored by its PL spectra. The intensity of the excimer emission decreased with increasing irradiation time, while the intensity of the dianthracene emission at 422 and 446 nm increased. In addition, shoulder peaks corresponding to the f–f transition of the DyIII ion appeared at 575 nm. A similar change in the PL profile was observed when the photoreaction temperature was lowered to 200–275 K. Notably, when the temperature was further reduced, the emission intensity of dianthracene and the DyIII ion became very weak at 175 K and invisible below 150 K. The results imply that at temperatures as low as 175–200 K, 1DySeCN can undergo an efficient photocycloaddition reaction concomitant with significant changes in luminescence.
Similar experiments were conducted for crystals of the dilute sample [Dy0.05Y0.95(SeCN)2(NO3)(depma)2(4-hpy)2] (1DySeCN@Y) at low temperatures (77–300 K). Upon UV light irradiation, we observed a significant reduction of the excimer emission intensity (Fig. S17†). Although the PL spectra did not show distinct characteristic emission peaks of dianthracene, the 1H NMR characterization showed the yields of photochemical reactions of 1DySeCN@Y to be 33.3% at 200 K, 25.4% at 180 K, 15.7% at 160 K and 8.3% at 140 K, respectively (Fig. S18†). Notably, the yield may be affected by the amount of sample placed on the hot stage and how it is placed.
We also tried to analyse the structure of the resulting crystal (named as 2DySeSCN-200) after irradiating 1DySeCNin situ with 395 nm UV light for 30 min at 200 K. Unfortunately, the atoms in 2DySeSCN-200 were severely disordered, so we were unable to solve the structure. However, the fact that the atoms are heavily disordered indirectly supports the occurrence of photodimerization of anthracene pairs in 1DySeCN at 200 K.
Photo-switchable magnetic properties of 1DySeCN
Fig. S19† shows the dc magnetic susceptibilities of 1DySeCN and 2DySeCN measured under an applied dc field of 1 kOe in the temperature range of 2–300 K. The χMT values at 300 K are 14.10 and 14.01 cm3 K mol−1 for 1DySeCN and 2DySeCN, respectively, consistent with the theoretical value of 14.17 cm3 K mol−1 for an isolated DyIII (6H15/2, S = 5/2, L = 5, gJ = 4/3). In both cases, χMT decreases gradually with decreasing temperature until 5 K, ascribed to the depopulation of Stark sublevels. Below 5 K, the χMT declines sharply, which may be due to the blocking of the magnetic moment.38 The unsaturated magnetization (M) values at 2 K and 70 kOe (5.19 Nβ for 1DySeCN, 5.68 Nβ for 2DySeCN) as well as the non-superposition of the M vs. H/T plots at different temperatures suggested the presence of magnetic anisotropy and/or low-lying excited states (Fig. S20†). Interestingly, both compounds exhibit butterfly-shaped hysteresis loops (Fig. S21†), but the loop for 2DySeCN is significantly narrowed compared to that of 1DySeCN (Fig. S21b†). The closing of the hysteresis loops at zero dc field indicates that strong quantum tunnelling of magnetization (QTM) is operative in both cases,39 possibly due to the weak magnetic interactions between the dysprosium ions.To inhibit the QTM effect, we prepared the diluted sample [Dy0.05Y0.95(SeCN)2(NO3)(depma)2(4-hpy)2] (1DySeCN@Y), and its photodimerization product [Dy0.05Y0.95(SeCN)2(NO3)(depma2) (4-hpy)2] (2DySeCN@Y). 1DySeCN@Y also exhibited reversible photochemical reactions upon UV irradiation, as confirmed by PXRD, IR, UV-vis diffuse reflectance and emission spectra as well as dc magnetic susceptibility measurements (Fig. S22† and 7a). The magnetization values are again not saturated for 1DySeCN@Y and 2DySeCN@Y at 2 K and 70 kOe (Fig. S23†). Remarkably, 1DySeCN@Y shows open butterfly-shaped hysteresis loops below 4 K characteristic of SMM behaviour (Fig. 7b and S24a†), indicating that QTM is effectively suppressed below this temperature (TB).40 The remnant and coercivity values at 2 K are 0.56 Nβ and 1200 Oe, respectively. By contrast, 2DySeCN@Y displays open hysteresis loops below 3 K with smaller remnant (0.24 Nβ) and coercivity (200 Oe) values at 2 K (Fig. 7b and S24b†). The zero-field-cooling (ZFC) and field-cooling (FC) χMvs. T curves show clear divergences at 4.2 K for 1DySeCN@Y and 3.4 K for 2DySeCN@Y, indicating that the blocking temperatures (TB) are 4.2 K and 3.4 K for the two compounds, respectively (Fig. 7c and d). Notably, the remnant, coercivity and TB values of 1DySeCN@Y are all the highest among the known photo-responsive luminescent Ln-SMMs (Table S7†).
To further understand the magnetic dynamics, we performed the alternating current (ac) susceptibility measurements of 1DySeCN and 2DySeCN under zero dc field. Both show temperature and frequency dependences of the in-phase (χ′) and out-of-phase (χ′′) susceptibility components typical for SMMs (Fig. S25 and S26†). The relaxation times (τ) were extracted using the generalized Debye model,41 and both exhibit a broad distribution of relaxation coefficients, e.g. α = 0.08–0.31 (2–22 K) for 1DySeCN and α = 0.15–0.32 (2–14 K) for 2DySeCN (Tables S8 and S9†). The ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) τ vs. T−1 curves can be fitted using eqn (1), which considers the QTM, Raman, and Orbach processes.
τ vs. T−1 curves can be fitted using eqn (1), which considers the QTM, Raman, and Orbach processes.
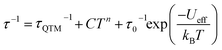 | (1) |
 | (2) |
The diluted samples 1DySeCN@Y and 2DySeCN@Y also show slow magnetic relaxation at zero dc field (Fig. 7e, f, S27, S28, Tables S10 and S11†). The ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) τ–T−1 curves for 1DySeCN@Y and 2DySeCN@Y were fitted with eqn (2) containing Orbach and Raman processes. The resulting parameters are Ueff = 347(11) K, τ0 = 5.9(3) × 10−12 s, C = 7(1) × 10−6 K−6.23 s−1, and n = 6.2(1) for 1DySeCN@Y, and Ueff = 207(12) K, τ0 = 1.4(3) × 10−9 s, C = 1.2(4) × 10−3 K−4.56 s−1, and n = 4.6(2) for 2DySeCN@Y, respectively. Apparently, the energy barrier of the diluted sample is larger than that of the pristine samples, attributed to the suppression of the QTM effect.
τ–T−1 curves for 1DySeCN@Y and 2DySeCN@Y were fitted with eqn (2) containing Orbach and Raman processes. The resulting parameters are Ueff = 347(11) K, τ0 = 5.9(3) × 10−12 s, C = 7(1) × 10−6 K−6.23 s−1, and n = 6.2(1) for 1DySeCN@Y, and Ueff = 207(12) K, τ0 = 1.4(3) × 10−9 s, C = 1.2(4) × 10−3 K−4.56 s−1, and n = 4.6(2) for 2DySeCN@Y, respectively. Apparently, the energy barrier of the diluted sample is larger than that of the pristine samples, attributed to the suppression of the QTM effect.
The above results demonstrate that the photocycloaddition reaction of 1DySeCN and its dilute sample 1DySeCN@Y will cause remarkable changes in their SMM behavior, i.e., the reduction of effective energy barrier and the narrowing of the hysteresis loop. However, the temperature at which the SMM behaviour was observed (TB ≤ 4.2 K) differed significantly from the temperature at which the photocycloaddition reaction was observed (≥140 K). An interesting finding is that the χMT values of 1DySeCN@Y and its photodimerization product 2DySeCN@Y are quite different at about 175–200 K (Fig. 7a). This result, combined with the fact that 1DySeCN@Y can undergo photocycloaddition reaction at 175–200 K and produce notable luminescence changes (Fig. S16†), makes it possible to synergistically modulate the magnetic and luminescent properties of Ln-SMMs at the same temperature via a reversible in situ photocycloaddition reaction. Similar work has not yet been seen in the literature.
Conclusions
We report a dysprosium–anthracene single-molecule magnet, namely [Dy(SeCN)2(NO3)(depma)2(4-hpy)2] (1DySeCN). The significance of this work is three-fold. First, 1DySeCN and its dilute sample (1DySeCN@Y) show the highest effective energy barrier for magnetization reversal and blocking temperature among the known photoresponsive luminescent Ln-SMMs. Second, 1DySeCN is capable of undergoing photoinduced SC–SC structural transformation accompanied by remarkable changes in luminescence and SMM behaviour. Third, the temperature at which the anthracene groups in 1DySeCN and 1DySeCN@Y undergo efficient photodimerization can be as low as 175–200 K, along with notable changes in magnetic and luminescent properties. This work may shed light on the development of photoresponsive luminescent Ln-SMMs for practical applications.Data availability
Crystallographic data for complexes 1DySeCN (1DySeCN-293, 1DySeCN-193, 1DySeCN-130) and 2DySeCN (2DySeCN-293, 2DySeCN-193) have been deposited at the https://www.ccdc.cam.ac.uk/structures/ with the deposition numbers CCDC 2421525–2421527, 2424230 and 2424231. The data supporting this article have been included as part of the ESI.†Author contributions
X.-F. M. conducted most of the experiments and wrote the draft of the manuscript. X.-L. H. and Y.-X. T. performed single crystal PL measurements at low temperature and the related data processing. Y.-H. Q. assisted in the NMR measurements of the photo-dimerized products. Q. T. assisted in performing the single-crystal X-ray diffraction data collection. S.-S. B. assisted in single crystal structural analysis. L.-M. Z. conceived the project and revised the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (22273037, 21731003).Notes and references
- E. Coronado, Molecular magnetism: from chemical design to spin control in molecules, materials and devices, Nat. Rev. Mater., 2020, 5, 87–104 CrossRef.
- J. J. Zakrzewski, M. Liberka, J. Wang, S. Chorazy and S.-i. Ohkoshi, Chem. Rev., 2024, 124, 5930–6050 CrossRef CAS PubMed.
- N. Deorukhkar, C. Egger, L. Guénée, C. Besnard and C. Piguet, J. Am. Chem. Soc., 2024, 146, 308–318 Search PubMed.
- P. Sadhukhan, S.-Q. Wu, S. Kanegawa, S.-Q. Su, X. Zhang, T. Nakanishi, J. I. Long, K. Gao, R. Shimada, H. Okajima, A. Sakamoto, J. G. Chiappella, M. S. Huzan, T. Kroll, D. Sokaras, M. L. Baker and O. Sato, Nat. Commun., 2023, 14, 3394 CrossRef CAS.
- R. Marin, G. Brunet and M. Murugesu, Angew. Chem., Int. Ed., 2021, 60, 1728–1746 CrossRef CAS.
- K. Bernot, C. Daiguebonne, G. Calvez, Y. Suffren and O. Guillou, Acc. Chem. Res., 2021, 54, 427–440 CrossRef CAS PubMed.
- J. Long, Y. Guari, R. A. S. Ferreira, L. D. Carlos and J. Larionova, Coord. Chem. Rev., 2018, 363, 57–70 CrossRef CAS.
- Y.-C. Chen and M.-L. Tong, Chem. Sci., 2022, 13, 8716–8726 RSC.
- V. Vieru, S. Gómez-Coca, E. Ruiz and L. F. Chibotaru, Angew. Chem., Int. Ed., 2024, 63, e202303146 CrossRef CAS PubMed.
- K. Dhbaibi, M. Grasser, H. Douib, V. Dorcet, O. Cador, N. Vanthuyne, F. Riobé, O. Maury, S. Guy, A. Bensalah-Ledoux, B. Baguenard, G. L. J. A. Rikken, C. Train, B. Le Guennic, M. Atzori, F. Pointillart and J. Crassous, Angew. Chem., Int. Ed., 2023, 62, e202215558 CrossRef CAS.
- H. Huang, R. Sun, X.-F. Wu, Y. Liu, J.-Z. Zhan, B.-W. Wang and S. Gao, Dalton Trans., 2023, 52, 7646–7651 Search PubMed.
- O. Cador, B. Le Guennic and F. Pointillart, Inorg. Chem. Front., 2019, 6, 3398–3417 RSC.
- Z. Zhu, X.-L. Li, S. Liu and J. Tang, Inorg. Chem. Front., 2020, 7, 3315–3326 RSC.
- X. Gou, Y. Wu, M. Wang, N. Liu, W. Lan, Y.-Q. Zhang, W. Shi and P. Cheng, Dalton Trans., 2024, 53, 148–152 RSC.
- J. Li, M. Kong, L. Yin, J. Zhang, F. Yu, Z.-W. Ouyang, Z. Wang, Y.-Q. Zhang and Y. Song, Inorg. Chem., 2019, 58, 14440–14448 CrossRef CAS.
- R. Jankowski, M. Wyczesany and S. Chorazy, Chem. Commun., 2023, 59, 5961–5986 RSC.
- L. Münzfeld, M. Dahlen, A. Hauser, N. Mahieu, S. K. Kuppusamy, J. Moutet, M. Tricoire, R. Köppe, L. La Droitte, O. Cador, B. Le Guennic, G. Nocton, E. Moreno-Pineda, M. Ruben and P. W. Roesky, Angew. Chem., Int. Ed., 2023, 62, e202218107 CrossRef PubMed.
- N. Monni, J. J. Baldoví, V. García-López, M. Oggianu, E. Cadoni, F. Quochi, M. Clemente-León, M. L. Mercuri and E. Coronado, Chem. Sci., 2022, 13, 7419–7428 Search PubMed.
- M.-J. Liu, Z.-Y. Fu, R. Sun, J. Yuan, C.-M. Liu, B. Zou, B.-W. Wang and H.-Z. Kou, ACS Appl. Electron. Mater., 2021, 3, 1368–1374 CrossRef CAS.
- (a) M. Hojorat, H. Al Sabea, L. Norel, K. Bernot, T. Roisnel, F. Gendron, B. L. Guennic, E. Trzop, E. Collet, J. R. Long and S. Rigaut, J. Am. Chem. Soc., 2020, 142, 931–936 CrossRef CAS PubMed; (b) K. Rogacz, M. Magott, S. Baś, M. Foltyn, M. Rams and D. Pinkowicz, RSC Adv., 2024, 14, 14515–14522 RSC.
- Y.-J. Ma, J.-X. Hu, S.-D. Han, J. Pan, J.-H. Li and G.-M. Wang, J. Am. Chem. Soc., 2020, 142, 2682–2689 CrossRef CAS.
- H. Kong, J.-Y. Wang, J.-C. Liu, L. Zhang, P.-Y. Liao, Y.-Q. Qi, Z. Liu, S.-G. Wu and M.-L. Tong, Angew. Chem., Int. Ed., 2025, 64, e202422557 CrossRef CAS.
- Q. Zhang, S.-D. Han, Q. Li, J.-X. Hu and G.-M. Wang, Sci. China Mater., 2022, 65, 788–794 CrossRef CAS.
- P.-Y. Liao, Y. Liu, Z.-Y. Ruan, H.-L. Wang, C.-G. Shi, W. Deng, S.-G. Wu, J.-H. Jia and M.-L. Tong, Inorg. Chem., 2023, 62, 1075–1085 CrossRef CAS PubMed.
- L. Wu, X.-D. Huang, W. Li, X. Cao, W.-H. Fang, L.-M. Zheng, M. Dolg and X. Chen, JACS Au, 2024, 4, 3606–3618 CrossRef CAS.
- X.-D. Huang, Y. Xu, K. Fan, S.-S. Bao, M. Kurmoo and L.-M. Zheng, Angew. Chem., Int. Ed., 2018, 57, 8577–8581 CrossRef CAS PubMed.
- X.-F. Ma, P.-F. Mi, S.-S. Bao and L.-M. Zheng, Chin. J. Inorg. Chem., 2024, 40, 270–280 CAS.
- X.-D. Huang, G.-H. Wen, S.-S. Bao, J.-G. Jia and L.-M. Zheng, Chem. Sci., 2021, 12, 929–937 RSC.
- X.-D. Huang, X.-F. Ma, T. Shang, Y.-Q. Zhang and L.-M. Zheng, Inorg. Chem., 2023, 62, 1864–1874 CrossRef CAS PubMed.
- X.-F. Ma, X.-D. Huang and L.-M. Zheng, Cryst. Growth Des., 2023, 23, 1095–1103 CrossRef CAS.
- X.-D. Huang, X.-F. Ma and L.-M. Zheng, Angew. Chem., Int. Ed., 2023, 62, e202300088 CrossRef CAS.
- D. Casanova, M. Llunell, P. Alemany and S. Alvarez, Chem.–Eur. J., 2005, 11, 1479–1494 CrossRef CAS PubMed.
- G. M. J. Schmidt, Pure Appl. Chem., 1971, 27, 647–678 CrossRef CAS.
- D.-S. Sun, Y.-Z. Zhang, J.-X. Gao, X.-N. Hua, X.-G. Chen, G.-Q. Mei and W.-Q. Liao, CrystEngComm, 2019, 21, 2669–2674 RSC.
- X.-D. Huang, J.-G. Jia, M. Kurmoo, S.-S. Bao and L.-M. Zheng, Dalton Trans., 2019, 48, 13769–13779 RSC.
- X.-F. Ma, X.-D. Huang, G.-H. Wen, S.-S. Bao, Y.-Q. Zhang and L.-M. Zheng, Dalton Trans., 2022, 51, 12026–12030 RSC.
- D.-S. Sun, Y.-Z. Zhang, J.-X. Gao, X.-N. Hua, X.-G. Chen, G.-Q. Mei and W.-Q. Liao, CrystEngComm, 2019, 21, 2669–2674 RSC.
- Y.-S. Meng, L. Xu, J. Xiong, Q. Yuan, T. Liu, B.-W. Wang and S. Gao, Angew. Chem., Int. Ed., 2018, 57, 4673–4676 CrossRef CAS.
- J. Wang, Q.-W. Li, S.-G. Wu, Y.-C. Chen, R.-C. Wan, G.-Z. Huang, Y. Liu, J.-L. Liu, D. Reta, M. J. Giansiracusa, Z.-X. Wang, N. F. Chilton and M.-L. Tong, Angew. Chem., Int. Ed., 2021, 60, 5299–5306 CrossRef CAS.
- F. Habib, P.-H. Lin, J. Long, I. Korobkov, W. Wernsdorfer and M. Murugesu, J. Am. Chem. Soc., 2011, 133, 8830–8833 CrossRef CAS PubMed.
- K. S. Cole and R. H. Cole, J. Chem. Phys., 1941, 9, 341–351 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, crystallographic details, and full characterization (SC-XRD, PXRD, IR, UV-vis and photoluminescence spectra, thermal analysis, photographs) of all described compounds. CCDC 2421525–2421527, 2424230 and 2424231. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc01302j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |