Open Access Article
Open Access ArticleSynthesis of polysubstituted cyclobutanes through a photoredox strain-release/[3,3]-rearrangement cascade†
Fangqing Zhang‡
ab,
Chun Xu‡bc,
Zichun Zhangb,
Zhuang Yangd,
Tao Peng a,
Wen Shao
a,
Wen Shao *c,
Xiaoming Feng
*c,
Xiaoming Feng *be and
Yangbin Liu
*be and
Yangbin Liu *ab
*ab
aState Key Laboratory of Chemical Oncogenomics, Guangdong Provincial Key Laboratory of Chemical Genomics, Peking University Shenzhen Graduate School, Shenzhen, Guangdong 518055, China
bInstitute of Chemical Biology, Shenzhen Bay Laboratory, Shenzhen, 518055, China. E-mail: liuyb@szbl.ac.cn
cKey Laboratory for Green Organic Synthesis and Application of Hunan Province, Key Laboratory of Environmentally Friendly Chemistry and Application of Ministry of Education, College of Chemistry, Xiangtan University, Xiangtan 411105, China. E-mail: shaowen@xtu.edu.cn
dLaboratory of Natural and Targeted Small Molecule Drugs, State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University and Collaborative Innovation Center of Biotherapy, Chengdu 610041, China
eKey Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064, China. E-mail: xmfeng@scu.edu.cn
First published on 14th May 2025
Abstract
Small saturated carbocycles, such as cyclobutanes, with elevated three-dimensionality and rich Csp3 centers are privileged scaffolds in naturally occurring molecules and drug discovery. It remains highly desirable and challenging to develop modular and straightforward strategies to craft densely substituted cyclobutanes. Herein, a photoredox-catalyzed radical strain-release/[3,3]-rearrangement cascade (SRRC) strategy for efficient synthesis of polysubstituted cyclobutanes is disclosed. This protocol operates with readily available α-silylamines as radical precursors, and strained bicyclo[1.1.0]butanes (BCBs) and cyclobutenes as radical acceptors, to access an array of structurally diverse 1,1,3- and 1,1,2-trisubstituted cyclobutanes containing a unique non-natural amino acid scaffold. Mechanistic studies reveal the pivotal reactivity of the silylketene acetal intermediate and the origin of diastereoselectivity. The power and utility of this method are illustrated with diverse transformations and preliminary anticancer assessment.
Introduction
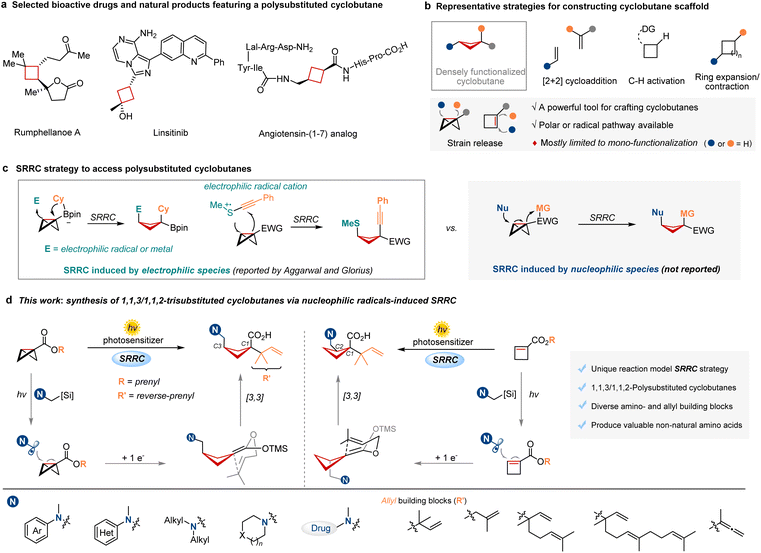
Polysubstituted cyclobutanes are not only prevalent in natural products, but also serve as privileged motifs in drug molecules (Fig. 1a).1 Compared to the common planar systems, cyclobutane cores possess a more rigid three-dimensionality and an increased content of Csp3 centers, thus exhibiting a greater structural modularity and enhanced pharmacologically relevant properties.2 As such, angiotensin (1–7) analogs containing a non-natural amino acid [cis-3-(aminomethyl)cyclobutanecarboxylic acid (ACCA)] are used as effective therapeutic compositions to treat or prevent a broader range of diseases.1e Consequently, development of efficient and modular strategies to construct polysubstituted cyclobutanes remains a fundamentally important research topic and has attracted substantial attention from synthetic chemists.Among various approaches to prepare cyclobutanes (Fig. 1b), such as [2 + 2] cycloaddition,3 C–H functionalization4 and ring contraction/expansion,5 strain-release-driven syntheses have been popularized and emerged as a divergent strategy to build complex cyclobutanes. Particularly, bicyclo[1.1.0]butanes (BCBs) and cyclobutenes are two privileged building blocks in efficient access to cyclobutane scaffolds under mild conditions due to their relatively high ring-strain energies.6–13 However, developing straightforward and potent strategies for the simultaneous installation of two functional groups into these strained rings to rapidly assemble polysubstituted cyclobutanes still poses significant challenges.14,15
The strain-release/rearrangement cascade (SRRC) strategy provides an effective approach for achieving regioselective difunctionalization of BCBs, thereby affording more densely polysubstituted cyclobutanes (Fig. 1c). For example, Aggarwal and coworkers reported the addition of electron-deficient species (aryl palladium, allyl iridium, electrophilic radicals) to the strained C–C σ-bond, triggering a 1,2-boronate rearrangement to produce 1,1,3-trisubstituted cyclobutanes.16 Glorius and coworkers described the addition of sulfur radical cations to BCBs and subsequent alkynyl rearrangement by C–S σ-bond scission, leading to the thio-alkynyl difunctionalization of BCBs.17 Although such advances have been made in the SRRC strategy, they are still limited to electrophilic species as initiators. Additionally, BCBs as highly strained molecules can undergo thermally driven ring-opening reactions with nucleophiles,4e,8,18 and can also couple with single-electron radical nucleophiles for the ring-opening processes.9b–d,19 This reactivity was exemplified by the pioneering works of Cintrat and Jui,9c,19a who independently reported the addition of nucleophilic α-amino radicals to BCBs, yielding hydroalkylated cyclobutanes in moderate to good yields. Nevertheless, the combination of nucleophilic strain-release and rearrangement cascade transformations in BCBs to achieve difunctionalization and produce polysubstituted cyclobutanes remains unexplored.
Recently, our group has disclosed a photocatalytic radical dearomatization of indoles and [3,3]-rearrangement cascade reaction to produce valuable prenylated indolines.20 Inspired by this precedent, we envisioned that the nucleophilic radicals (for instance, α-aminoalkyl radicals) could add to the central C–C σ-bond of BCBs driven by strain-release force. Subsequent single-electron reduction could generate a ketene acetal intermediate and trigger an intramolecular Claisen-type [3,3]-rearrangement, thus enabling regioselective synthesis of 1,1,3-trisubstituted cyclobutanes (Fig. 1d, left). Should this approach prove successful, it would conveniently incorporate two privileged motifs, amino and allyl, into a saturated carbocycle to produce novel non-natural amino acids containing the polysubstituted cyclobutane skeleton. Moreover, the complementary 1,1,2-trisubstituted cyclobutanes could be easily accessed by trapping with cyclobutene partners, followed by a similar procedure (Fig. 1d, right). A major challenge of this cascade process would be the competition between [3,3]-rearrangement and protonation of the active ketene acetal intermediate. Herein, we report a nucleophilic radical-induced SRRC reaction of BCBs and cyclobutenes to forge 1,1,3- and 1,1,2-trisubstituted cyclobutane products, respectively. This protocol covers a range of readily available amines with high functional group tolerance and a variety of allyl fragments, such as geranyl, farnesyl and allenyl. The synthetic utility of the resulting products is also demonstrated by further transformations to form versatile building blocks.
Results and discussion
Reaction development
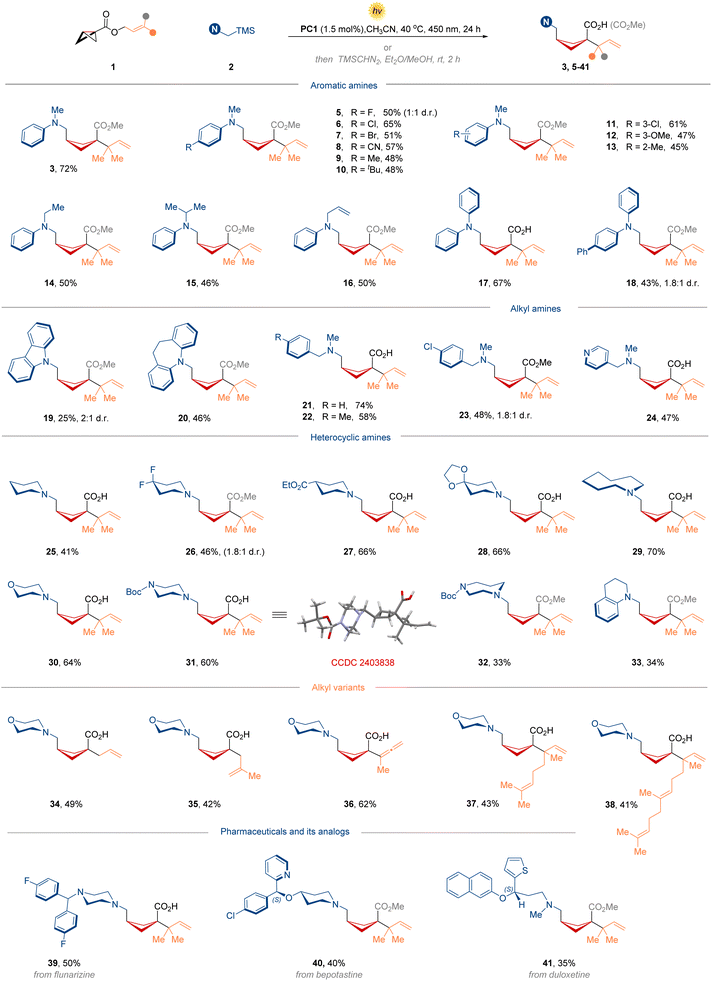
Given that prenyl and reverse-prenyl motifs are widely found in bioactive natural products and medicinal compounds,21 our design plan began with a prenyl bicyclo[1.1.0]butane-1-carboxylate 1 as the radical acceptor, potentially producing reverse-prenyl-functionalized cyclobutanecarboxylic acid after the desired radical SRRC process. For radical donors, tertiary amines containing a trimethylsilyl (TMS) functionality are suitable precursors (2) in photoredox reactions:22,23 (1) they have relatively low oxidation potentials to readily generate highly nucleophilic α-aminoalkyl radicals; (2) the fragment TMS can combine with the ketene acetal species and promote the subsequent [3,3]-rearrangement; (3) organic amines have a rich structural diversity in FDA approved drugs, thus making the amine-functionalized cyclobutanes even more valuable in drug discovery. Preliminary optimization experiments highlighted PC1 as the optimal photocatalyst with a moderate oxidation potential, providing the amino- and reverse-prenyl 1,3-difunctionalized cyclobutane product 3 in 30% yield (Fig. 2). Notably, such congested vicinal quaternary centers in product 3 were constructed smoothly due to the inherent intramolecular advantages of [3,3]-sigmatropic rearrangement. Further investigation of other photocatalysts with a higher or lower oxidation potential led to an unproductive reaction (PC2-4). During the selection of the ideal PC, we found that amine 2 was almost fully consumed and the majority of BCB 1 was left. It is assumed that the desired radical strain-release addition of BCB is slower than the decomposition rate of the α-aminoalkyl radical. Thus, we changed the ratio between 1 and 2 to achieve a higher yield (from 30% to 50%) as the amount of BCB increased (2.0 equiv.). Examination of different solvents identified CH3CN as the optimal solvent for this transformation. Delightedly, fine-tuning of the reaction parameters revealed that a higher concentration was the most beneficial to improve reactivity (86% yield at 0.8 M). Additional control experiments confirmed that both the photocatalyst and visible light were essential for the desired product.Substrate scope for the synthesis of 1,1,3-trisubstituted cyclobutanes
With the optimized conditions in hand, we next evaluated the substrate scope of this photocatalytic SRRC reaction of BCBs to afford 1,1,3-trisubstituted cyclobutanes (Fig. 3). Various substituted N,N-dimethyl anilines (5–13) were well tolerated to give the corresponding cyclobutane products in moderate to good yields. A series of functional groups including electron-withdrawing and electron-donating groups on the aromatic ring, such as halogens, cyano and methoxy groups, were well tolerated. Altering the substituent on the nitrogen atom, ethyl (14), isopropyl (15), allyl (16), and phenyl (17 and 18) groups were also compatible with this protocol. Under the same conditions, carbazole (19) and 5H-dibenzo[b,f]azepine (20) were incorporated into the cyclobutane scaffold, albeit in slightly lower yields. Additionally, alkyl amines as substrates were shown to perform similarly, delivering the corresponding cyclobutylated amino acid products in good yields (21–24). Given the frequency and importance of nitrogen-containing saturated heterocycles in drug development, we sought to evaluate the tolerance of various N-heterocyclic functionalities. As such, piperidines bearing functional groups, 1H-azepine, morpholine, piperazine, 1,4-diazepane, and tetrahydroquinoline readily participated in the radical addition/rearrangement cascade transformation, providing the desired products smoothly (25–33). The structure and relative configuration were unambiguously confirmed by X-ray crystallographic analysis of 31. Next, we turned our attention to BCB acceptors. By varying diverse allylic esters as substrates, this protocol was successfully extended to achieve allyl segment diversification beyond the reverse-prenyl group, such as unsubstituted allyl (34), 2-methyl substituted allyl (35), valuable allenyl group (36, from propargylic esters), geranyl (37), and even more complex farnesyl (38). Further generality for the developed method was demonstrated in more systems with biologically relevant scaffolds. Remarkably, such core amino-fragments in flunarizine (39), bepotastine (40) and serotonin reuptake inhibitor duloxetine (41) underwent this transformation smoothly, affording the corresponding cyclobutylated amino acid products in moderate yields.Substrate scope for the synthesis of 1,1,2-trisubstituted cyclobutanes
Having established the above process for accessing C3-aminoalkyl-type cyclobutanes via the radical addition to the central C–C σ-bond of BCBs, we wondered if the complementary site-isomers, C2-aminoalkyl-functionalized cyclobutanes, would be obtained through the radical addition to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of cyclobutenes, followed by a [3,3]-rearrangement reaction. Pleasingly, this protocol was compatible to access 1,2-difunctionalization of cyclobutenes with minor adjustments in the reaction setup (Fig. 4). Aniline and N-heterocyclic amine derivatives could be well tolerated, such as piperidine, morpholine, piperazine, and complex bioactive motifs (42–47). It is worth mentioning that aminoalkyl and reverse-prenyl were incorporated into cyclobutanes in an anti-selective manner, providing 1,1,2-trisubstituted cyclobutane products in excellent diastereoselectivities (20
C bond of cyclobutenes, followed by a [3,3]-rearrangement reaction. Pleasingly, this protocol was compatible to access 1,2-difunctionalization of cyclobutenes with minor adjustments in the reaction setup (Fig. 4). Aniline and N-heterocyclic amine derivatives could be well tolerated, such as piperidine, morpholine, piperazine, and complex bioactive motifs (42–47). It is worth mentioning that aminoalkyl and reverse-prenyl were incorporated into cyclobutanes in an anti-selective manner, providing 1,1,2-trisubstituted cyclobutane products in excellent diastereoselectivities (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 d.r. for most cases). Moreover, different cyclobutene derivatives bearing diverse allylic esters were also reactive, providing the corresponding allenyl, simple allyl, or 2-methyl-allyl products in good yields (48–53).
1 d.r. for most cases). Moreover, different cyclobutene derivatives bearing diverse allylic esters were also reactive, providing the corresponding allenyl, simple allyl, or 2-methyl-allyl products in good yields (48–53).
Mechanistic investigation
As shown in Fig. 5a, possible mechanistic details on the photoredox radical SRRC process are proposed. Firstly, single-electron transfer (SET) between the excited Ir(III) photocatalyst PC1 and α-silylaniline 2 should generate radical cation I that undergoes fragmentation to form an α-aminoalkyl radical II and a trimethylsilylium ion stabilized by acetonitrile solvent. Then, the strain-release addition of this highly nucleophilic species II to the BCB (1) would produce α-ester radical III. The subsequent reduction by the Ir(II) catalyst would give enolate IV, which could combine with a trimethylsilylium ion to form the key silylketene acetal intermediate V. Then, the Claisen-type [3,3]-rearrangement of V would furnish the 1,1,3-trisubstituted cyclobutane product 3. Similarly, the synthesis of 1,1,2-trisubstituted cyclobutanes could be achieved through a radical addition to cyclobutene followed by the corresponding rearrangement pathway.To gain insights into the above mechanism, a series of control experiments and spectroscopy studies were conducted. First, a light on/off experiment showed that continuous irradiation was essential for product formation (Fig. 5b). UV/vis absorption experiments confirmed the primary absorption of the photocatalyst at the irradiated wavelength (for details, see the ESI†). Stern−Volmer luminescence quenching revealed that the excited state of PC1 was significantly quenched by the α-silylaniline (Fig. 5c). Then, such a radical addition/rearrangement process was completely inhibited in the presence of 2,2,6,6-tetramethylpiperidinyloxy (TEMPO) and the corresponding TEMPO-aminoalkyl adduct was confirmed by HRMS (Fig. 5d, for details, see the ESI†), suggesting the involvement of aminoalkyl radical species in product formation. Moreover, when the reaction was carried out with the addition of external water, the aminoalkylation-protonation products 54 and 55 were detected exclusively, while the [3,3]-rearrangement process was completely interrupted, which supports the generation of α-ester anion species IV (Fig. 5e). Interestingly, we also compared the reactivity difference between the BCB and cyclobutene by an intermolecular competition experiment. The cyclobutene 4 was proved to be more reactive than BCB 1, affording the corresponding rearrangement product 42 in 63% yield (Fig. 5f).
To further evaluate the reaction's energy profile and rationalize the observed reaction outcome, we performed density functional theory (DFT) calculations. As shown in Fig. 6a, the relative energies for morpholine radical (INT) addition to BCB 1 and cyclobutene 4 have been computed. It is found that the BCB as the radical acceptor with 20.4 kcal mol−1 free energy of activation is energetically much higher than the cyclobutene scenario with a modest activation barrier (12.8 kcal mol−1, Fig. 6a). This result is in good accordance with the competition experiment observation that radical addition to the BCB is less kinetically favored than to the cyclobutene. To elucidate the observed reactivity difference, we conducted a comparative analysis of strain energies between BCBs and cyclobutenes. It is found that BCBs display a remarkable ring strain energy (64 kcal mol−1), which is much higher than that of cyclobutenes (28.7 kcal mol−1).6b As such, the strain energy is not the key factor leading to the enhanced reactivity of cyclobutenes. Further geometric analysis demonstrates distinct structural reorganization pathways: the BCB-to-cyclobutane conversion requires substantial geometric distortion, while the cyclobutene-to-cyclobutane transformation proceeds with minimal conformational changes (as evidenced by bond angle analysis). These structural insights suggest that the reduced geometric perturbation during cyclobutene activation may contribute to the observed preference for radical addition to cyclobutenes over BCBs.
 | ||
| Fig. 6 Insights from DFT calculations. (a) aminoalkyl radical addition to BCB and cyclobutene. (b) relative energies for [3,3]-rearrangement of the BCB-TMS intermediate. (c) relative energies for [3,3]-rearrangement of the (E)-cyclobutene-TMS intermediate. Calculations were performed at the B3LYP/6-31G(d,p)/SMD (acetonitrile) level of theory. More details on the [3,3]-rearrangement of cyclobutene-radical intermediate (Z)-INT-TMS-B are provided in ESI Fig. S7.† | ||
To gain insight into the origin of diastereoselectivity, possible [3,3]-rearrangement transition states of the silylketene acetal intermediate were also investigated. The computational analysis of BCB-radical intermediate INT-TMS-A suggests that it can undergo two transition states TS-A1 and TS-A2 with very close Gibbs free-energy (ΔG = 18.0 kcal mol−1 vs. 18.2 kcal mol−1), thus resulting in a similar ratio of anti-INT-C1 and syn-INT-C2 rearrangement products (Fig. 6b). These calculations explain why the SRRC process of BCBs tends to give the product in a low diastereomeric ratio (1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 d.r., Fig. 3). Comparatively, the computational analysis of [3,3]-rearrangement involving the 2-aminoalkyl substituted cyclobutane was also conducted (Fig. 6c). By taking (E)-INT-TMS-B as an example, the transition state TS-B1 that leads to the formation of the observed diastereomer anti-INT-D1 is more kinetically favorable than the other transition state TS-B1 by at least 3.9 kcal mol−1, which generates the diastereomer syn-INT-D2. This substantial energy difference suggests that the prenyl group prefers to approach the four-membered ring from the opposite direction of the morpholine substituent. The scenario of the (Z)-INT-TMS-B intermediate is also considered (see the SI for details).
1 d.r., Fig. 3). Comparatively, the computational analysis of [3,3]-rearrangement involving the 2-aminoalkyl substituted cyclobutane was also conducted (Fig. 6c). By taking (E)-INT-TMS-B as an example, the transition state TS-B1 that leads to the formation of the observed diastereomer anti-INT-D1 is more kinetically favorable than the other transition state TS-B1 by at least 3.9 kcal mol−1, which generates the diastereomer syn-INT-D2. This substantial energy difference suggests that the prenyl group prefers to approach the four-membered ring from the opposite direction of the morpholine substituent. The scenario of the (Z)-INT-TMS-B intermediate is also considered (see the SI for details).
Furthermore, Hirshfeld partition of molecular density (IGMH) analysis was performed to investigate the non-covalent interactions in both transition states TS-B1 and TS-B2. Notably, TS-B2 suffers from the obvious steric repulsion due to the stronger CH···HC and CH⋯N interactions between the terminal methyl on the ally moiety and the morpholine fragment. Thus, the corresponding diastereomer bearing the reverse-prenyl and the morpholine in a syn-manner is unfavorable. These results are consistent with the experimental findings in Fig. 4, wherein the SRRC process of cyclobutene often affords an enhanced diastereoselectivity (for most cases 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 d.r).
1 d.r).
Synthetic utility and biological activity evaluation
We next performed divergent synthetic manipulations of cyclobutane products to further reveal the potential utility of the enclosed functionalization protocol by taking advantage of the functionalities, including carboxylic acid, terminal olefin and amino groups (Fig. 7a). Structurally appealing tripeptides 56 and 57 containing 1,3- and 1,2-cyclobutylated amino acids were readily achieved under the classical condensation conditions. The carboxylic acid group was also amenable to deliver primary alcohol 58 by LiAlH4 reduction and to engage in Schmidt rearrangement, giving rise to the cyclobutylated amine 59. Iodine-mediated lactonization led to more complex cyclobutylated spirolactone 60 in 50% yield. Interestingly, when targeting the preparation of an acyl chloride intermediate through the oxalyl chloride and DMF procedure, we obtained the unexpected yet attractive cyclobutane ring-fused lactam scaffold 61 in a single step. Lastly, the hydrogenation of the reverse-prenyl to a bulky tert-amyl group was furnished with a Pd/C catalyst (62). A sequential hydroboration-oxidation of the terminal olefin could formally introduce the isopentyl alcohol motif to cyclobutane (63).Given that cyclobutanes are privileged in pharmaceuticals, we decided to evaluate the anticancer activity of selected products by detecting the in vitro cytotoxicity against the human diffuse large B-cell lymphoma (TMD-8) cell line (Fig. 7b). Pleasingly, a series of cyclobutane products showcased preliminary inhibitory activity at 10 μM. Among these candidates, cyclobutylated amino acid 31 bearing a piperazine motif displayed superior cytotoxicity to inhibit diverse cancer cell lines, including TMD-8, mouse pancreatic ductal adenocarcinoma (KPC) and human colorectal adenocarcinoma (HCT-116), with IC50 values ranging from 5.7 to 8.2 μM. This indicates such cyclobutane compounds have potential applications in medicinal chemistry.
Conclusions
In summary, we have established a photoredox-mediated radical strain-release/[3,3]-rearrangement cascade strategy to achieve polysubstituted cyclobutanes, with the utility of bicyclo[1.1.0]butanes and cyclobutenes as radical acceptors and readily available tertiary amines as radical donors. An array of structurally diverse 1,1,3- and 1,1,2-trisubstituted cyclobutanes containing a non-natural amino acid scaffold were smoothly accessed in moderate to good yields. This protocol features mild reaction conditions, good functional group tolerance, and an extremely wide range of amine precursors and allyl fragments. Mechanistic investigations reveal that the pivotal reactivity of the silylketene acetal intermediate is generated by the strain-release radical addition and subsequent single electron reduction. The origin of the anti-selective formation of densely substituted cyclobutanes via the steric repulsion effect in the transition states of [3,3]-rearrangement is explained by computational calculations. The synthetic applications and anticancer activity assessment further substantiate the potential of this photocatalytic SRRC protocol. We anticipate that the development of other types of transformations based on the SRRC strategy will expand the synthetic chemical space of saturated carbocycles.Data availability
The data that support the findings of this study are available in the ESI† of this article.Author contributions
F. Q. Z. performed the experiments and prepared the ESI.† C. X. investigated several substrates and performed some experiments. Z. C. Z. performed the DFT calculations. Z. Y. performed the biological experiments. T. P. helped with the Stern–Volmer luminescence quenching experiment. W. S. supported the project. X. M. F. supported the project. Y. B. L. designed and supervised the project, and wrote the manuscript. All the authors contributed to the manuscript revisions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We appreciate the National Natural Science Foundation of China (22471174), Shenzhen Bay Laboratory (S201100003 and S211101001-4), Shenzhen Bay Qihang Fellow Program (QH23001), Guangdong Pearl River Talent Program (2021QN020268) and the Natural Science Foundation of Guangdong Province (2024A1515012381) for generous financial support.Notes and references
- (a) H.-M. Chung, Y.-H. Chen, M.-R. Lin, J.-H. Su, W.-H. Wang and P.-J. Sung, Tetrahedron Lett., 2010, 51, 6025–6027 CrossRef CAS; (b) V. M. Dembitsky, Phytomedicine, 2014, 21, 1559–1581 CrossRef CAS PubMed; (c) M. D. Tricklebank, Drugs, 2000, 3, 228–231 CAS; (d) M. R. Van Der Kolk, M. A. C. H. Janssen, F. P. J. T. Rutjes and D. Blanco-Ania, ChemMedChem, 2022, 17, e202200020 CrossRef CAS PubMed; (e) P. Gallagher, A. Tallant, D. Yohannes and K. A. Gruber, PCT Int. Patent Appl. US2016/066181, 2017.
- (a) F. Lovering, J. Bikker and C. Humblet, J. Med. Chem., 2009, 52, 6752–6756 CrossRef CAS PubMed; (b) F. Lovering, MedChemComm, 2013, 4, 515–519 RSC.
- For selected examples of construction of cyclobutanes by [2 + 2] cycloaddition, see: (a) S. Poplata, A. Tröster, Y.-Q. Zou and T. Bach, Chem. Rev., 2016, 116, 9748–9815 Search PubMed; (b) F. Strieth-Kalthoff, M. J. James, M. Teders, L. Pitzer and F. Glorius, Chem. Soc. Rev., 2018, 47, 7190–7202 Search PubMed; (c) M. R. Fructos and A. Prieto, Tetrahedron, 2016, 72, 355–369 CrossRef CAS; (d) S. Das, C. Zhu, D. Demirbas, E. Bill, C. K. De and B. List, Science, 2023, 379, 494–499 CrossRef CAS PubMed; (e) Y. Liu, D. Ni and M. K. Brown, J. Am. Chem. Soc., 2022, 144, 18790–18796 CrossRef CAS PubMed; (f) Z. Liu, C. Zhou, T. Lei, X.-L. Nan, B. Chen, C.-H. Tung and L.-Z. Wu, CCS Chem., 2020, 2, 582–588 CrossRef CAS; (g) Z. C. Girvin, L. F. Cotter, H. Yoon, S. J. Chapman, J. M. Mayer, T. P. Yoon and S. J. Miller, J. Am. Chem. Soc., 2022, 144, 20109–20117 CrossRef CAS PubMed; (h) F. Pecho, Y. Sempere, J. Gramüller, F. M. Hörmann, R. M. Gschwind and T. Bach, J. Am. Chem. Soc., 2021, 143, 9350–9354 CrossRef CAS PubMed.
- (a) K.-J. Xiao, D. W. Lin, M. Miura, R.-Y. Zhu, W. Gong, M. Wasa and J.-Q. Yu, J. Am. Chem. Soc., 2014, 136, 8138–8142 CrossRef CAS PubMed; (b) W. R. Gutekunst and P. S. Baran, J. Am. Chem. Soc., 2011, 133, 19076–19079 CrossRef CAS PubMed; (c) L. M. Chapman, J. C. Beck, L. Wu and S. E. Reisman, J. Am. Chem. Soc., 2016, 138, 9803–9806 CrossRef CAS PubMed; (d) F. Frébault and N. Maulide, Angew. Chem., Int. Ed., 2012, 51, 2815–2817 CrossRef PubMed; (e) R. A. Panish, S. R. Chintala and J. M. Fox, Angew. Chem., Int. Ed., 2016, 55, 4983–4987 CrossRef CAS PubMed.
- (a) C. Liu, R. Chen, Y. Shen, Z. Liang, Y. Hua and Y. Zhang, Angew. Chem., Int. Ed., 2017, 56, 8187–8190 CrossRef CAS PubMed; (b) R. Meier and D. Trauner, Angew. Chem., Int. Ed., 2016, 55, 11251–11255 CrossRef CAS PubMed; (c) C. Hui, L. Brieger, C. Strohmann and A. P. Antonchick, J. Am. Chem. Soc., 2021, 143, 18864–18870 CrossRef CAS PubMed; (d) F. Kleinbeck and F. D. Toste, J. Am. Chem. Soc., 2009, 131, 9178–9179 CrossRef CAS PubMed; (e) X.-Z. Shu, M. Zhang, Y. He, H. Frei and F. D. Toste, J. Am. Chem. Soc., 2014, 136, 5844–5847 CrossRef CAS PubMed.
- (a) K. B. Wiberg, G. M. Lampman, R. P. Ciula, D. S. Connor, P. Schertler and J. Lavanish, Tetrahedron, 1965, 21, 2749–2769 CrossRef CAS; (b) J. Qi, C. Wang, G. Wang, P. O'Neill, S. Reddy Dubbaka, H. Ting Ang, X. Chen and J. Wu, Angew. Chem., Int. Ed., 2025, 64, e202413723 CrossRef CAS PubMed.
- For selected reviews on the functionalization of BCBs and cyclobutenes: (a) J. Chen, Q. Zhou, H. Fang and P. Lu, Chin. J. Chem., 2022, 40, 1346–1358 CrossRef CAS; (b) X. Zhan, H.-X. He, Q. Peng and J.-J. Feng, Synthesis, 2024, 56, 3829–3848 CrossRef CAS; (c) M. Golfmann and J. C. L. Walker, Commun. Chem., 2023, 6, 9 CrossRef CAS PubMed; (d) Y. Xiao, L. Tang, X.-C. Yang, N.-Y. Wang, J. Zhang, W.-P. Deng and J.-J. Feng, CCS Chem., 2025 DOI:10.31635/ccschem.025.202505825.
- R. Panish, S. R. Chintala, D. T. Boruta, Y. Fang, M. T. Taylor and J. M. Fox, J. Am. Chem. Soc., 2013, 135, 9283–9286 CrossRef CAS PubMed.
- (a) R. Gianatassio, J. M. Lopchuk, J. Wang, C.-M. Pan, L. R. Malins, L. Prieto, T. A. Brandt, M. R. Collins, G. M. Gallego, N. W. Sach, J. E. Spangler, H. Zhu, J. Zhu and P. S. Baran, Science, 2016, 351, 241–246 CrossRef CAS PubMed; (b) X. Wu, W. Hao, K.-Y. Ye, B. Jiang, G. Pombar, Z. Song and S. Lin, J. Am. Chem. Soc., 2018, 140, 14836–14843 CrossRef CAS PubMed; (c) G. Ernouf, E. Chirkin, L. Rhyman, P. Ramasami and J.-C. Cintrat, Angew. Chem., Int. Ed., 2020, 59, 2618–2622 CrossRef CAS PubMed; (d) X. Yu, M. Lübbesmeyer and A. Studer, Angew. Chem., Int. Ed., 2021, 60, 675–679 CrossRef CAS PubMed; (e) M. Ociepa, A. J. Wierzba, J. Turkowska and D. Gryko, J. Am. Chem. Soc., 2020, 142, 5355–5361 CrossRef CAS PubMed; (f) L. Tang, Q.-N. Huang, F. Wu, Y. Xiao, J.-L. Zhou, T.-T. Xu, W.-B. Wu, S. Qu and J.-J. Feng, Chem. Sci., 2023, 14, 9696–9703 RSC; (g) G. Chen, D. Tian, X. Wang and H.-J. Zhang, ACS Catal., 2024, 14, 14928–14936 CrossRef CAS; (h) A. Guin, S. Bhattacharjee, M. S. Harariya and A. T. Biju, Chem. Sci., 2023, 14, 6585–6591 RSC.
- (a) Y.-J. Chen, T.-J. Hu, C.-G. Feng and G.-Q. Lin, Chem. Commun., 2015, 51, 8773–8776 RSC; (b) M. Guisán-Ceinos, A. Parra, V. Martín-Heras and M. Tortosa, Angew. Chem., Int. Ed., 2016, 55, 6969–6972 CrossRef PubMed; (c) S. Feng, H. Hao, P. Liu and S. L. Buchwald, ACS Catal., 2020, 10, 282–291 CrossRef CAS PubMed; (d) F. W. Goetzke, A. M. L. Hell, L. van Dijk and S. P. Fletcher, Nat. Chem., 2021, 13, 880–886 CrossRef CAS PubMed.
- For selected examples of racemic peripheral cyclization of BCBs, see: (a) R. Kleinmans, T. Pinkert, S. Dutta, T. O. Paulisch, H. Keum, C. G. Daniliuc and F. Glorius, Nature, 2022, 605, 477–482 CrossRef CAS PubMed; (b) K. Dhake, K. J. Woelk, J. Becica, A. Un, S. E. Jenny and D. C. Leitch, Angew. Chem., Int. Ed., 2022, 61, e202204719 CrossRef CAS PubMed; (c) Y. Liang, R. Kleinmans, C. G. Daniliuc and F. Glorius, J. Am. Chem. Soc., 2022, 144, 20207–20213 CrossRef CAS PubMed; (d) R. Guo, Y.-C. Chang, L. Herter, C. Salome, S. E. Braley, T. C. Fessard and M. K. Brown, J. Am. Chem. Soc., 2022, 144, 7988–7994 CrossRef CAS PubMed; (e) Y. Zheng, W. Huang, R. K. Dhungana, A. Granados, S. Keess, M. Makvandi and G. A. Molander, J. Am. Chem. Soc., 2022, 144, 23685–23690 CrossRef CAS PubMed; (f) Y. Liang, F. Paulus, C. G. Daniliuc and F. Glorius, Angew. Chem., Int. Ed., 2023, 62, e202305043 CrossRef CAS PubMed; (g) D. Ni, S. Hu, X. Tan, Y. Yu, Z. Li and L. Deng, Angew. Chem., Int. Ed., 2023, 62, e202308606 CrossRef CAS PubMed; (h) R. Kleinmans, S. Dutta, K. Ozols, H. Shao, F. Schäfer, R. E. Thielemann, H. T. Chan, C. G. Daniliuc, K. N. Houk and F. Glorius, J. Am. Chem. Soc., 2023, 145, 12324–12332 CrossRef CAS PubMed; (i) S. Agasti, F. Beltran, E. Pye, N. Kaltsoyannis, G. E. M. Crisenza and D. J. Procter, Nat. Chem., 2023, 15, 535–541 CrossRef CAS PubMed; (j) Y. Liu, S. Lin, Y. Li, J.-H. Xue, Q. Li and H. Wang, ACS Catal., 2023, 13, 5096–5103 CrossRef CAS; (k) N. Radhoff, C. G. Daniliuc and A. Studer, Angew. Chem., Int. Ed., 2023, 62, e202304771 CrossRef CAS PubMed; (l) J.-J. Wang, L. Tang, Y. Xiao, W.-B. Wu, G. Wang and J.-J. Feng, Angew. Chem., Int. Ed., 2024, 63, e202405222 CrossRef CAS PubMed; (m) J. L. Tyler, F. Schäfer, H. Shao, C. Stein, A. Wong, C. G. Daniliuc, K. N. Houk and F. Glorius, J. Am. Chem. Soc., 2024, 146, 16237–16247 CrossRef CAS PubMed; (n) S. Dutta, Y.-L. Lu, J. E. Erchinger, H. Shao, E. Studer, F. Schäfer, H. Wang, D. Rana, C. G. Daniliuc, K. N. Houk and F. Glorius, J. Am. Chem. Soc., 2024, 146, 5232–5241 CrossRef CAS PubMed; (o) J.-L. Zhou, Y. Xiao, L. He, X.-Y. Gao, X.-C. Yang, W.-B. Wu, G. Wang, J. Zhang and J.-J. Feng, J. Am. Chem. Soc., 2024, 146, 19621–19628 CrossRef CAS PubMed; (p) S. Hu, Y. Pan, D. Ni and L. Deng, Nat. Commun., 2024, 15, 6128 CrossRef CAS; (q) S. Dutta, D. Lee, K. Ozols, C. G. Daniliuc, R. Shintani and F. Glorius, J. Am. Chem. Soc., 2024, 146, 2789–2797 CrossRef CAS PubMed; (r) Y. Liu, Z. Wu, J.-R. Shan, H. Yan, E.-J. Hao and L. Shi, Nat. Commun., 2024, 15, 4374 CrossRef CAS PubMed; (s) L. Tang, Y. Xiao, F. Wu, J.-L. Zhou, T.-T. Xu and J.-J. Feng, Angew. Chem., Int. Ed., 2023, 62, e202310066 CrossRef CAS PubMed; (t) Y. Xiao, F. Wu, L. Tang, X. Zhang, M. Wei, G. Wang and J.-J. Feng, Angew. Chem., Int. Ed., 2024, 63, e202408578 CrossRef CAS; (u) M. Xu, Z. Wang, Z. Sun, Y. Ouyang, Z. Ding, T. Yu, L. Xu and P. Li, Angew. Chem., Int. Ed., 2022, 61, e202214507 CrossRef CAS PubMed; (v) T. Yu, J. Yang, Z. Wang, Z. Ding, M. Xu, J. Wen, L. Xu and P. Li, J. Am. Chem. Soc., 2023, 145, 4304–4310 CrossRef CAS PubMed; (w) D. Sarkar, S. Deswal, R. Chandra Das and A. T. Biju, Chem. Sci., 2024, 15, 16243–16249 RSC; (x) Y. Liu, S. Lin, Z. Ding, Y. Li, Y.-J. Tang, J.-H. Xue, Q. Li, P. Li and H. Wang, Chem, 2024, 10, 3699–3708 CrossRef CAS; (y) S. Nicolai and J. Waser, Chem. Sci., 2024, 15, 10823–10829 RSC.
- (a) J. Zhang, J.-Y. Su, H. Zheng, H. Li and W.-P. Deng, ACS Catal., 2024, 14, 17837–17849 Search PubMed; (b) K. Zhang, Z. Gao, Y. Xia, P. Li, P. Gao, X.-H. Duan and L.-N. Guo, Chem. Sci., 2025, 16, 1411–1416 Search PubMed; (c) H. Liao, J. Dong, X. Zhou, Q. Jiang, Z. Lv, F. Lei and D. Xue, Chem. Sci., 2025, 16, 4654–4660 RSC.
- For selected examples of asymmetric peripheral cyclization of BCBs, see: (a) M. de Robichon, T. Kratz, F. Beyer, J. Zuber, C. Merten and T. Bach, J. Am. Chem. Soc., 2023, 145, 24466–24470 CAS; (b) J. Jeong, S. Cao, H.-J. Kang, H. Yoon, J. Lee, S. Shin, D. Kim and S. Hong, J. Am. Chem. Soc., 2024, 146, 27830–27842 Search PubMed; (c) Q. Fu, S. Cao, J. Wang, X. Lv, H. Wang, X. Zhao and Z. Jiang, J. Am. Chem. Soc., 2024, 146, 8372–8380 CrossRef CAS PubMed; (d) F. Wu, W.-B. Wu, Y. Xiao, Z. Li, L. Tang, H.-X. He, X.-C. Yang, J.-J. Wang, Y. Cai, T.-T. Xu, J.-H. Tao, G. Wang and J.-J. Feng, Angew. Chem., Int. Ed., 2024, 63, e202406548 CrossRef CAS PubMed; (e) W.-B. Wu, B. Xu, X.-C. Yang, F. Wu, H.-X. He, X. Zhang and J.-J. Feng, Nat. Commun., 2024, 15, 8005 CrossRef CAS PubMed; (f) X. Wang, R. Gao and X. Li, J. Am. Chem. Soc., 2024, 146, 21069–21077 CrossRef CAS PubMed; (g) X.-G. Zhang, Z.-Y. Zhou, J.-X. Li, J.-J. Chen and Q.-L. Zhou, J. Am. Chem. Soc., 2024, 146, 27274–27281 CrossRef CAS PubMed; (h) Y.-J. Li, Z.-L. Wu, Q.-S. Gu, T. Fan, M.-H. Duan, L. Wu, Y.-T. Wang, J.-P. Wu, F.-L. Fu, F. Sang, A.-T. Peng, Y. Jiang, X.-Y. Liu and J.-S. Lin, J. Am. Chem. Soc., 2024, 146, 34427–34441 CrossRef CAS PubMed; (i) E. F. Plachinski, R. Z. Qian, R. Villanueva, D. L. Poole, T. Rosenthal and T. P. Yoon, J. Am. Chem. Soc., 2024, 146, 31400–31404 Search PubMed; (j) X.-C. Yang, F. Wu, W.-B. Wu, X. Zhang and J.-J. Feng, Chem. Sci., 2024, 15, 19488–19495 Search PubMed.
- For selected examples of the difunctionalization of BCBs: (a) J. Majhi, R. K. Dhungana, Á. Rentería-Gómez, M. Sharique, L. Li, W. Dong, O. Gutierrez and G. A. Molander, J. Am. Chem. Soc., 2022, 144, 15871–15878 CrossRef CAS PubMed; (b) K. Das, A. Pedada, T. Singha and D. P. Hari, Chem. Sci., 2024, 15, 3182–3191 RSC.
- For selected examples of the difunctionalization of cyclobutenes: (a) A. K. Simlandy, M.-Y. Lyu and M. K. Brown, ACS Catal., 2021, 11, 12815–12820 CrossRef CAS PubMed; (b) L. Nóvoa, L. Trulli, A. Parra and M. Tortosa, Angew. Chem., Int. Ed., 2021, 60, 11763–11768 Search PubMed; (c) Z. Wang, J. Zhu, M. Wang and P. Lu, J. Am. Chem. Soc., 2024, 146, 12691–12701 CrossRef CAS PubMed.
- (a) M. Silvi and V. K. Aggarwal, J. Am. Chem. Soc., 2019, 141, 9511–9515 Search PubMed; (b) A. Fawcett, T. Biberger and V. K. Aggarwal, Nat. Chem., 2019, 11, 117–122 Search PubMed; (c) S. H. Bennett, A. Fawcett, E. H. Denton, T. Biberger, V. Fasano, N. Winter and V. K. Aggarwal, J. Am. Chem. Soc., 2020, 142, 16766–16775 CrossRef CAS PubMed; (d) H.-C. Shen, M. V. Popescu, Z.-S. Wang, L. de Lescure, A. Noble, R. S. Paton and V. K. Aggarwal, J. Am. Chem. Soc., 2023, 145, 16508–16516 CrossRef CAS PubMed; (e) L. Guo, A. Noble and V. K. Aggarwal, Angew. Chem., Int. Ed., 2021, 60, 212–216 CrossRef CAS PubMed.
- H. Wang, J. E. Erchinger, M. Lenz, S. Dutta, C. G. Daniliuc and F. Glorius, J. Am. Chem. Soc., 2023, 145, 23771–23780 Search PubMed.
- (a) Y. Gaoni, Tetrahedron Lett., 1982, 23, 5215–5218 CrossRef CAS; (b) S. Hoz, C. Azran and A. Sella, J. Am. Chem. Soc., 1996, 118, 5456–5461 CrossRef CAS; (c) Y. Gaoni, Tetrahedron Lett., 1982, 23, 5219–5220 Search PubMed; (d) Y. Gaoni, Tetrahedron Lett., 1988, 29, 1591–1594 Search PubMed; (e) Y. Gaoni, A. Tomazic and E. Potgieter, J. Org. Chem., 1985, 50, 2943–2947 Search PubMed; (f) Y. Gaoni, Tetrahedron, 1989, 45, 2819–2840 CrossRef CAS; (g) J. M. Lopchuk, K. Fjelbye, Y. Kawamata, L. R. Malins, C.-M. Pan, R. Gianatassio, J. Wang, L. Prieto, J. Bradow, T. A. Brandt, M. R. Collins, J. Elleraas, J. Ewanicki, W. Farrell, O. O. Fadeyi, G. M. Gallego, J. J. Mousseau, R. Oliver, N. W. Sach, J. K. Smith, J. E. Spangler, H. Zhu, J. Zhu and P. S. Baran, J. Am. Chem. Soc., 2017, 139, 3209–3226 CrossRef CAS PubMed; (h) J. A. Milligan, C. A. Busacca, C. H. Senanayake and P. Wipf, Org. Lett., 2016, 18, 4300–4303 CrossRef CAS PubMed; (i) P. Zhang, R. Zhuang, X. Wang, H. Liu, J. Li, X. Su, X. Chen and X. Zhang, Bioconjugate Chem., 2018, 29, 467–472 CrossRef CAS PubMed; (j) B. D. Schwartz, M. Y. Zhang, R. H. Attard, M. G. Gardiner and L. R. Malins, Chem. Eur. J., 2020, 26, 2808–2812 CrossRef CAS PubMed; (k) K. Tokunaga, M. Sato, K. Kuwata, C. Miura, H. Fuchida, N. Matsunaga, S. Koyanagi, S. Ohdo, N. Shindo and A. Ojida, J. Am. Chem. Soc., 2020, 142, 18522–18531 CrossRef CAS PubMed; (l) A. Kaur, W. Lin, V. Dovhalyuk, L. Driutti, M. L. Di Martino, M. Vujasinovic, J. M. Löhr, M. E. Sellin and D. Globisch, Chem. Sci., 2023, 14, 5291–5301 RSC.
- (a) C. J. Pratt, R. A. Aycock, M. D. King and N. T. Jui, Synlett, 2020, 31, 51–54 CrossRef CAS PubMed; (b) H. Gao, L. Guo, C. Shi, Y. Zhu, C. Yang and W. Xia, Adv. Synth. Catal., 2022, 364, 2140–2145 CrossRef CAS; (c) P.-F. Chen, D.-S. Li, W.-T. Ou, F. Xue and H.-P. Deng, Org. Lett., 2023, 25, 6184–6188 CrossRef CAS PubMed; (d) W. Huang, Y. Zheng, S. Keess and G. A. Molander, J. Am. Chem. Soc., 2023, 145, 5363–5369 CrossRef CAS PubMed; (e) Y. Xiao, T.-T. Xu, J.-L. Zhou, F. Wu, L. Tang, R.-Y. Liu, W.-B. Wu and J.-J. Feng, Chem. Sci., 2023, 14, 13060–13066 RSC.
- X. X. Chang, F. Q. Zhang, S. B. Zhu, Z. Yang, X. M. Feng and Y. B. Liu, Nat. Commun., 2023, 14, 3876 CrossRef CAS PubMed.
- (a) H.-j. Wang, J. B. Gloer, D. T. Wicklow and P. F. Dowd, J. Nat. Prod., 1998, 61, 804–807 CrossRef CAS PubMed; (b) S.-M. Li, Nat. Prod. Rep., 2010, 27, 57–78 RSC; (c) L. Gu, F.-J. Sun, C.-P. Li, L.-T. Cui, M.-H. Yang and L.-Y. Kong, Phytochem. Lett., 2021, 42, 77–81 CrossRef CAS; (d) Y. Liu, Y. Li, M. Chen, Y. Liu, J. Liang, Y. Zhang and Z.-J. Qian, Int. Immunopharmacol., 2022, 109, 108931 CrossRef CAS PubMed.
- For selected reviews on α-silylamines as radical precursors: (a) K. Nakajima, Y. Miyake and Y. Nishibayashi, Acc. Chem. Res., 2016, 49, 1946–1956 CrossRef CAS PubMed; (b) A. L. Gant Kanegusuku and J. L. Roizen, Angew. Chem., Int. Ed., 2021, 60, 21116–21149 CrossRef CAS PubMed.
- For selected examples of α-silylamines as radical precursors: (a) L. Ruiz Espelt, I. S. McPherson, E. M. Wiensch and T. P. Yoon, J. Am. Chem. Soc., 2015, 137, 2452–2455 Search PubMed; (b) R. Kleinmans, L. E. Will, J. L. Schwarz and F. Glorius, Chem. Sci., 2021, 12, 2816–2822 RSC; (c) S. Zheng, W. Wang and W. Yuan, J. Am. Chem. Soc., 2022, 144, 17776–17782 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2403838 and 2403839. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc01431j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |