Open Access Article
Open Access ArticleA strategy of chiral cation coordination to achieve a large luminescence dissymmetry factor in 1D hybrid manganese halides†
Fei Wang abc,
Xingjun Li
abc,
Xingjun Li *abc,
Tianqi Chenabc,
Liqing Wangac,
Chenliang Liac,
Wei Zhangac,
Wen Yuanabc,
Shan Luabc,
Lina Li
*abc,
Tianqi Chenabc,
Liqing Wangac,
Chenliang Liac,
Wei Zhangac,
Wen Yuanabc,
Shan Luabc,
Lina Li abc and
Xueyuan Chen
abc and
Xueyuan Chen abc
abc
aState Key Laboratory of Structural Chemistry, Fujian Key Laboratory of Nanomaterials, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, China. E-mail: lixj@fjirsm.ac.cn
bCollege of Chemistry and Materials Science, Fujian Normal University, Fuzhou, Fujian 350117, China
cFujian College, University of Chinese Academy of Sciences, Fuzhou, Fujian 350116, China
First published on 14th May 2025
Abstract
Chiral organic–inorganic metal halides (OIMHs) have emerged as a new class of promising circularly polarized luminescence (CPL) materials owing to their structural tunability and fascinating optoelectronic properties. However, the development of high-performance chiral hybrid OIMHs remains a critical challenge, largely attributed to the absence of effective strategies for modulating chiroptical activity. Herein, we present enantiomeric hybrid manganese bromides, denoted as R/S-DACAMnBr3, featuring a one-dimensional chain structure alternately coordinated by organic cations via edge-sharing MnOBr5 octahedra, which establishes a robust chiral transfer pathway from organic cations to inorganic emissive centers. This structural design synergizes with the high intrinsic emission efficiency of Mn2+ centers to achieve intense orange CPL at 626 nm, yielding a remarkable luminescence dissymmetry factor (glum) of 0.292 for S-DACAMnBr3, which surpasses most reported chiral OIHMs by 1–3 orders of magnitude. Remarkably, a positive magneto-chiroptical effect under a 1.6 T magnetic field amplifies the glum value to 0.321 at room temperature, demonstrating the first example of magnetic-field-enhanced CPL in lead-free OIMHs. The practical viability is further evidenced by S-DACAMnBr3-based circularly polarized light-emitting diodes exhibiting a strong CPL signal at 620 nm with a glum of 6.4 × 10−3, alongside single-crystal photodetectors achieving a switching ratio of 7.72. These findings contribute valuable insights for amplifying the chiroptical activity of hybrid OIMHs via a strategy of chiral cation coordination, which may pave the way for the development of effective CPL materials toward diverse applications in the future.
Introduction
Circularly polarized luminescence (CPL) materials hold immense potential for advancing optoelectronic technologies,1 such as circularly polarized light-emitting diodes (CP-LEDs),2–6 three-dimensional (3D) displays, CPL switches, CPL detectors,7,8 and spintronic devices.9–11 Conventional CPL systems such as organic small molecules, polymers, and inorganic nanomaterials often face a fundamental trade-off between achieving high luminescence efficiency and large luminescence dissymmetry factors (glum).12–15 While liquid crystalline composites demonstrate strong chiroptical activity, their practical deployment is hindered by complex synthetic protocols.16 In contrast, chiral organic–inorganic metal halides (OIMHs) have emerged as a promising alternative, offering structural versatility, superior optical properties, and scalable fabrication.17–20 Among these, chiral manganese-based halides stand out due to their exceptional photochemical stability, high photoluminescence quantum yields (PLQY), and low toxicity,21–24 rendering them as versatile platforms for developing high-performance CPL materials with additional functionalities such as ferroelectricity and magneto-chiroptical coupling.25–27Despite these advantages, reconciling intense emission with large glum values poses a considerable challenge in chiral OIMHs.28 Recent strategies to amplify chiroptical activity have focused on modulating inorganic framework distortions via weak non-covalent interactions (e.g., hydrogen bonding or halogen–halogen contacts).29–32 For example, Quan et al. proved that solvent-induced lattice distortions in zero-dimensional (0D) [MnBr4]2− tetrahedra can elevate glum from 10−4 to 10−3.33 Similarly, halogenation of chiral cations in layered lead iodides enhanced the rotatory strength of the electronic transition, yielding a glum of 3.1 × 10−3.34 However, such approaches suffer from inefficient chirality transfer from organic ligands to inorganic emitters due to the weak and long-range nature of these interactions. Recent breakthroughs in coordination-driven structural design have addressed this limitation by leveraging stronger covalent coordination bonds. Notably, Mao et al. achieved a record glum of 0.328 in chiral hybrid manganese bromides through direct Mn–O coordination, surpassing hydrogen-bonded analogues by 40 fold.35 Despite this progress, critical gaps persist, including the limited library of chiral amines capable of forming such coordination architecture, unclear mechanisms of chiral signal amplification, and a lack of practical demonstrations in optoelectronic devices.
In this work, we report enantiomeric hybrid manganese bromides, denoted as R/S-DACAMnBr3, featuring a one-dimensional (1D) chain structure constructed from edge-sharing MnOBr5 octahedra alternately coordinated by chiral (1R, 2R)/(1S, 2S)-2-aminocyclohexanol (DACA) cations. This unique architecture establishes a robust chiral transfer pathway from organic cations to Mn2+ emitters, synergizing with the intrinsic high PLQY of Mn2+ to achieve intense orange CPL at 626 nm with an exceptional glum of 0.292, which surpasses most chiral OIMHs by 1–3 orders of magnitude. Furthermore, positive magneto-chiroptical effect under a 1.6 T magnetic field enhances the glum to 0.321 at room temperature (RT). The practical viability of the obtained chiral OIMHs is illustrated through R/S-DACAMnBr3-based CP-LEDs and single-crystal photodetectors. This work not only provides an effective strategy for amplifying the chiroptical activity of OIMHs through coordination-driven chirality transfer but also advances the development of versatile CPL materials for next-generation optoelectronics.
Results and discussion
Structural characteristics
Single crystals of the enantiomeric hybrid manganese bromides, R/S-DACAMnBr3, were synthesized via a solution reaction of Mn(CH3COO)2 with chiral (1R, 2R)/(1S, 2S)-2-aminocyclohexanol (DACA) in hydrobromic acid. Single-crystal X-ray diffraction analysis confirmed that both compounds crystallize in a non-centrosymmetric orthorhombic system with the chiral space group P212121, showing identical unit cell parameters (Table S1†). Fig. 1a presents the molecular stacking pattern of the unit cell for R-DACAMnBr3, and the asymmetric unit of R-DACAMnBr3 comprises one MnBr3 subunit coordinated to a DACA cation. Notably, the structure adopted a 1D chain motif along the a-axis, formed by edge-sharing MnOBr5 octahedra alternately coordinated by chiral DACA ligands (Fig. 1b). This alternating coordination mode, previously observed in axial staggered systems,36 contrasted with conventional chiral OIMHs where organic–inorganic interactions are dominated by weak hydrogen bonding. Here, the Mn2+ center was directly bonded to the hydroxyl group of DACA and five bromide ions, forming a distorted MnOBr5 octahedron, which is a structural hallmark critical for efficient chirality transfer. Recent work demonstrated that the lattice distortion induced by the chiral molecules was likely to be the dominant factor for the chirality.8 The octahedral distortion was quantified by significant variations in bond lengths and angles (Tables S2–S5†). For R-DACAMnBr3, Mn–O/Br bond lengths ranged from 2.218 Å to 2.711 Å, while intraoctahedral angles spanned from 84.15° to 176.33°. Similar distortions were observed in S-DACAMnBr3 (2.223–2.709 Å, 83.99–176.25°), confirming the structural equivalence of the enantiomers. The pronounced distortion arose from the asymmetric coordination environment imposed by the chiral DACA ligand, which disrupted the octahedral symmetry and induced a helical twist in the 1D chain. This structural asymmetry was further stabilized by multiple hydrogen bonds (O–H⋯Br, N–H⋯Br, and C–H⋯Br; Fig. S1†), ensuring tight integration between organic and inorganic components. The chirality of R/S-DACAMnBr3 was unequivocally demonstrated by their non-superimposable mirror-image geometries (Fig. 1c and S2†). The alternating coordination architecture established a short-range chiral transfer pathway from the chiral DACA ligand to the Mn2+ emitter, circumventing the limitations of long-range non-covalent interactions in conventional systems. This direct coupling mechanism, combined with the high intrinsic luminescence characteristics of Mn2+, is anticipated to synergistically enhance CPL efficiency. The 1D chains showed Mn2+–Mn2+ distances from 3.900 Å to 15.769 Å (Fig. S3†), reflecting the periodic arrangement of octahedra along the chain. This structural periodicity, coupled with the chiral distortion, may facilitate the amplification of chiroptical activity in R/S-DACAMnBr3.X-ray photoelectron spectroscopy (XPS) further confirmed the presence of C, O, N, Br, and Mn elements in R-DACAMnBr3 crystals, with the Mn 2p3/2 and 2p1/2 peaks centered at 641.2 eV and 653.5 eV, respectively, consistent with the +2 oxidation state of Mn2+ ion (Fig. S4†). Powder X-ray diffraction (PXRD) patterns of both enantiomers exhibited excellent agreement with simulations derived from single-crystal data (Fig. 1d), confirming their high phase purity and long-range crystallographic order. Fourier-transform infrared (FT-IR) spectroscopy provided additional evidence of the organic–inorganic integration. The disappearance of –OH stretching vibration and a redshift of the –NH2 stretching vibration indicated coordination and hydrogen bonding interactions between the DACA ligands and the Mn2+ centers (Fig. 1e).
Photoluminescence properties
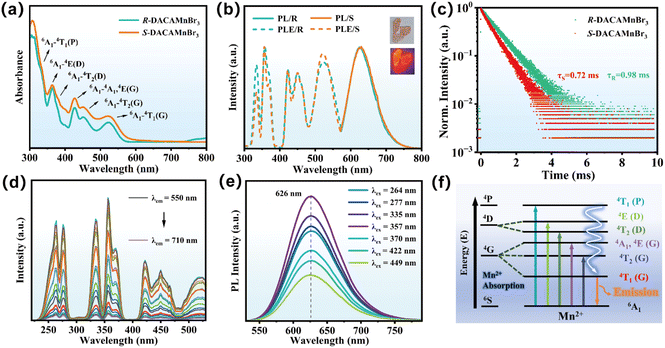
The ultraviolet-visible (UV-Vis) absorption spectra of R/S-DACAMnBr3 crystals revealed a strong absorption band in the ultraviolet (UV) region at 300 nm. This absorption can be attributed to the absorption of the chiral DACA ligand, as illustrated in Fig. 2a and S5.† Additionally, several absorption peaks were identified within the wavelength range of 310 nm to 600 nm. These peaks correspond to the electronic transitions of Mn2+ from the ground state 6A1(S) to the excited states 4T1(P), 4E(D), 4T2(D) 4A1/4E(G), 4T2(G), and 4T1(G), respectively, as reported in ref. 37 As depicted in the insets of Fig. 2b, under ambient light conditions, R-DACAMnBr3 crystals demonstrated a light pink and transparent appearance. When irradiated with 365 nm UV light, they displayed bright orange PL emission. Due to the similar chemical components and structural characteristics, both enantiomeric crystals showed a broad orange emission band upon irradiation with 357 nm UV light. This emission band extended from 550 nm to 800 nm, peaking around 626 nm with a full-width at half-maximum (FWHM) of 266 meV (85 nm), and it exhibited a large Stokes shift of 105 nm. A large Stokes shift is beneficial as it helps to mitigate self-absorption, thereby enhancing the emission efficiency. Upon 357 nm excitation, the average PLQY was determined to be 32.2% for R-DACAMnBr3, and 18.7% for S-DACAMnBr3 (Fig. S6†). The PLE spectra of the crystals displayed a high degree of overlap with the absorption bands of both DACA ligands and Mn2+ ions, which indicates an efficient energy transfer process from the chiral organic ligands to the Mn2+ centers. Time-resolved PL decay curves for both enantiomers fit well to a single-exponential model, yielding average PL lifetimes of 0.98 ms (R) and 0.72 ms (S) at 626 nm under ambient excitation conditions (Fig. 2c and S7†). Based on the PL lifetimes and PLQYs, we calculated the non-radiative rate constants (knr) of R/S-DACAMnBr3 crystals at RT (Table S6†). The R-DACAMnBr3 crystals manifested a smaller knr value than S-DACAMnBr3 (6.92 × 102 s−1 vs. 1.13 × 103 s−1), demonstrating a reduced non-radiative decay pathway in R-DACAMnBr3 crystals, which endowed them with an enhanced PL emission.To gain deep insights into the origin of the broadband orange emission in R/S-DACAMnBr3, wavelength-dependent PLE and PL emission spectra were systematically analyzed. As depicted in Fig. 2d and e for R-DACAMnBr3, the PL emission maximum at 626 nm and PLE peaks remain invariant across varying wavelengths, confirming the absence of multiple emissive centers. Furthermore, the integrated emission intensity displayed a linear relationship with incident excitation power density, ruling out defect-mediated recombination mechanisms (Fig. S8†).38 This result combining with the PLE and excitation wavelength-dependent PL spectra unambiguously attributed the broadband emissions of R/S-DACAMnBr3 crystals to a single Mn2+ luminescent center in octahedra from the same excited state, featuring a distinct d–d (4T1(G)–6A1) transition.39 The invariance of spectral features under varying excitation conditions underscored the stability of the Mn2+ coordination environment. Fig. 2f illustrates the proposed energy-level diagram for Mn2+ in R/S-DACAMnBr3, comparing the electronic states of free Mn2+ ions with those in the distorted MnOBr5 octahedral field.40 The crystal field splitting induced by the asymmetric coordination lowers the energy of the 4T1(G) excited state, resulting in the observed orange emission at 626 nm.
To elucidate the excited state dynamics, we conducted temperature-dependent steady-state PL spectroscopic analysis on R-DACAMnBr3. Under 357 nm excitation, photophysical properties such as the PL peak position, PL intensity, and FWHM were monitored over a temperature range of 80–300 K. The pseudo-color PL emission spectra showed pronounced temperature-dependent behavior. As temperature decreased from 300 K to 80 K, the excitation and emission intensities of R-DACAMnBr3 crystals exhibited a substantial enhancement (Fig. 3a and S9†), which can be attributed to reduced electron–phonon coupling and suppression of non-radiative recombination of Mn2+ at cryogenic temperatures.41,42 Meanwhile, a distinct redshift was observed in the Mn2+ emission band, shifting from 626 nm at 300 K to 640 nm at 80 K. This phenomenon can be ascribed to lattice contraction-induced enhancement of d–d splitting in Mn2+, which consequently reduced the energy gap for the 4T1 → 6A1 transition. By quantitatively analyzing the relationship between PL intensity and reciprocal temperature through Arrhenius plotting (Fig. 3b), the thermal activation energy was determined by using the following equation:43
 | (1) |
Further in-depth analysis of the emission FWHM as a function of temperature was carried out to quantify the electron–phonon coupling interactions (Fig. 3c), which is evaluated by using eqn (2):40
 | (2) |
Subsequently, the stability of R-DACAMnBr3 crystals was evaluated. The XRD pattern, PL spectra and PL lifetime of R-DACAMnBr3 crystals stored in a desiccator for 3 months remained essentially unchanged, confirming the good stability of the crystals in dry air (humidity: <30%) (Fig. S11†). Thermogravimetric analysis (TGA) demonstrated that both enantiomers possessed excellent thermal stability, with high decomposition temperature of approximately 270 °C (Fig. S12†). As depicted in Fig. 3f, R-DACAMnBr3 crystals were capable of maintaining 88% and 77% of their room-temperature PL intensity when heated to 90 °C and 140 °C, respectively. This clearly indicated their remarkable resistance to thermal quenching, which arose from the effective suppression of non-radiative decay pathways.46 Moreover, when subjected to continuous UV irradiation at 365 nm for 200 min at temperatures of 25 °C, 90 °C, and 150 °C, R-DACAMnBr3 crystals retained more than 95% of their original PL intensity at the corresponding temperature (Fig. S13†). These findings convincingly proved their outstanding thermal stability and photostability.
Chiroptical properties
The chiroptical properties of R/S-DACAMnBr3 were investigated to elucidate the interplay between their alternating coordination structure and CPL activity. Circular dichroism (CD) spectra revealed mirror-image signals for the enantiomers in the 250–600 nm range (Fig. 4a), with peak positions aligning closely with the Mn2+ d–d transition bands observed in UV-vis absorption spectra. Notably, this contrasts with the CD response of the free chiral DACA ligand, which showed mirror symmetry only in the 200–250 nm UV region (Fig. S14†). The pronounced CD activity in the UV-vis range (300–600 nm) directly implicated the inorganic MnOBr5 octahedra as chiral centers, confirming successful chirality transfer from the organic ligand to the Mn2+ emitter via the alternating coordination architecture. The excited-state chiral activity of R/S-DACAMnBr3 was probed through CPL spectroscopy. Under a bias of 0.8–0.9 V, the R- and S-enantiomers exhibited distinct orange CPL signals at 550–800 nm, corresponding to right-handed (negative) and left-handed (positive) circularly polarized emission, respectively (Fig. 4b). The glum factor, quantifying the degree of CPL asymmetry, is calculated using:47
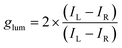 | (3) |
To the best of our knowledge, the glum values of R/S-DACAMnBr3 ranked among the highest reported chiral Mn-based halides (Fig. 4d and Table S8†). This remarkable chiroptical response can be attributed to the unique structural features of these materials. Firstly, rather than relying on the conventional weak hydrogen-bonding forces, the chiral organic ligand is directly coordinated into the inorganic octahedral structure via the oxygen atom. This coordination occurs with a shorter interaction distance and stronger interaction force, which is crucial for the chiroptical properties of hybrid OIMHs. Secondly, due to the distinct coordination interaction forces of oxygen and bromine with Mn2+, along with the significant difference in their ionic radii, the octahedron is induced to undergo substantial lattice distortion. This distortion plays a key role in influencing the chiroptical properties. Thirdly, within the octahedral geometry, chiral organic ligands exhibit directionally staggered coordination, leading to the formation of a slightly helical inorganic octahedral 1D chain. These engineered structural features enabled optimized chirality transfer from organic ligands to the inorganic lattice, while coupling with the high emission efficiency of Mn2+ to synergistically enhance the generation of a strong CPL signal.
To investigate the influence of structural anisotropy of R/S-DACAMnBr3 crystals on their CPL properties, we systematically evaluated the CPL performance of R/S-DACAMnBr3 by rotating the single-crystal sample through 360° at 45° increments.48,49 As shown in Fig. 4e, the handedness of CPL remained unaltered across all rotation angles, with some fluctuations in glum from −0.246 to −0.298 (Fig. S15 and Table S9†). The slight variation of glum values may be attributed to the different thickness and surface morphology of the sample during rotation. Furthermore, CPL measurements were conducted on polycrystalline powder samples of R/S-DACAMnBr3. The polycrystalline forms displayed pronounced weakening CPL signals, with glum values reduced by an order of magnitude (−5.2 × 10−2 and 5.7 × 10−2 for R- and S-polycrystals, respectively; Fig. S16†). Such a significant reduction in chiroptical activity stemmed from the loss of long-range crystallographic order and anisotropic alignment in the polycrystalline state, consistent with prior observations in lead-free chiral OIMHs.35 These controlled experiments conclusively verified that the robust CPL activity in R/S-DACAMnBr3 arose from genuine circularly polarized emission governed by the intrinsic chirality of the alternating coordination structure, rather than optical artifacts. The retention of high glum values in single crystals underscored the critical role of crystal orientation and ordered molecular arrangement within the crystal lattice for achieving high performance CPL materials.
To explore the interplay between chiroptical activity and magnetism, we investigated the magneto-chiroptical coupling effects in R/S-DACAMnBr3 under an external magnetic field. Hitherto, studies on magnetic-field-modulated CPL in chiral manganese-based halides remain exceptionally rare.50,51 At room temperature, the CPL responses of both enantiomers were measured under a 1.6 T magnetic field applied parallel (N → S) or antiparallel (S → N) to the excitation light propagation direction (Fig. 4f). Strikingly, the CPL intensity of R/S-DACAMnBr3 was amplified by the magnetic field, independent of its orientation. The glum increases by 12.4% (from −0.282 to −0.317) and 9.93% (from 0.292 to 0.321) for the R- and S-enantiomers, respectively (Fig. S17 and Table S10†). Furthermore, magnetic circular dichroism (MCD) and electron paramagnetic resonance (EPR) were employed to investigate the positive magneto-chiroptical activity of R/S-DACAMnBr3 crystals (Fig. S18 and S19†). The MCD spectra of R/S-DACAMnBr3 clearly revealed that the intensities of CD signals noticeably increased when an external magnetic field (1.6 T) was applied, illustrating the enhancement in light absorption of the crystals. The EPR spectrum of R-DACAMnBr3 crystals exhibited a single broad peak with a large line width (ΔH = 45.1 mT) instead of six fine-line spectra, indicating that there were strong dipole–dipole interactions between Mn2+ ions in R-DACAMnBr3. Therefore, the MCD and EPR results verified that the positive magneto-chiroptical properties were due to the enhanced light absorption and dipole–dipole interactions of R/S-DACAMnBr3 upon application of an external magnetic field.52,53 To our knowledge, the positive magneto-chiroptical properties of R/S-DACAMnBr3 are unprecedented in chiral lead-free OIMHs, which may unlock the potential of coordination-engineered chiral OIMHs as versatile platforms for magneto-chiroptical applications.54
Optoelectronic applications
Inspired by the excellent CPL performance, optical and thermal stability, and photophysical properties of R/S-DACAMnBr3, we fabricated CP-LEDs by integrating R-DACAMnBr3 with UV-curing adhesive onto commercial 365 nm UV chips (Fig. 5a). The CP-LEDs emitted a bright and visible orange light with the Commission Internationale de l'Eclairage (CIE) coordinate of (0.62, 0.37) (Fig. 5b and S20†). Systematic characterization of the CP-LEDs under varying operating currents (40–200 mA) revealed excellent color stability and a linear relationship between the emission intensity and driving currents of the crystals (Fig. 5c and S20†). To assess the chiral optical performance, we analyzed the CPL activity of the R/S-DACAMnBr3-based CP-LEDs by connecting a DC power supply with the device (3.3 V, 0.6 W) (Fig. S21†). We observed that the R/S-DACAMnBr3-based CP-LEDs show mirror-symmetric CPL signals centered arround 620 nm with glum values of −5.7 × 10−3 and 6.4 × 10−3, respectively (Fig. 5d and S22†). The almost unchanged emission intensities of the fabricated CP-LEDs under different cyclic driving currents varying from 30 to 150 mA demonstrated their excellent cycling stability (Fig. 5e). Furthermore, the CP-LEDs retained 80.1% of their original intensity after 3 h of continuous power-on at a constant current of 70 mA (Fig. 5f), indicating their good operational stability. The above findings emphasized the potential of R/S-DACAMnBr3 as a high-performance, single-component CPL source for advanced optoelectronic applications.Bulk single crystals with narrow bandgaps, high carrier mobility, and steady-state photocurrent are excellent materials for fabricating efficient optoelectronic devices.55–58 Over recent years, hybrid Mn-based halides have demonstrated significant potential in X-ray scintillators, spintronics, and LEDs, while their application in photodetection remains largely unexplored. On this basis, we integrated UV photodetectors using large-sized R-DACAMnBr3 single crystals with silver electrodes (Figs. S23 and S24a†). Under 266 nm laser excitation with biases from −5 V to 5 V, the device exhibited pronounced photoresponse, as evidenced by the current–voltage (I–V) curves measured under both illuminated and dark conditions. The photodetector illustrated an on/off ratio of 7.72 with symmetric I–V characteristics (Fig. S24b†). We also investigated the linear dynamic range and detectivity of the R-DACAMnBr3-based photodetector (Fig. S24c and d†), which manifested the photocurrent density associated with the light power intensity, showing linear dependence with a linear dynamic range (LDR) of 30.82 dB and a specific detectivity of 6.19 × 108 Jones. Furthermore, the fabricated photodetector exhibited good reproducibility over multiple switching cycles (Fig. S24e†). Overall, exploring its potential in photodetectors is conducive to broadening the application scope of chiral manganese-based halides in optoelectronics.
Conclusions
In summary, we have successfully designed and synthesized a unique enantiomeric chiral hybrid manganese bromide, R/S-DACAMnBr3, featuring an infinite 1D chain structure constructed from edge-sharing MnOBr5 octahedra alternately coordinated by chiral DACA ligands. This distinct alternating coordination architecture established a direct and efficient chiral transfer pathway from organic ligands to Mn2+ emission centers, synergizing with the intrinsic high PLQY of Mn2+ to realize intense orange CPL at 626 nm. The resulting glum reaches 0.292 for S-DACAMnBr3, surpassing most reported chiral OIMHs by 1–3 orders of magnitude. Remarkably, positive magneto-chiroptical effect under a 1.6 T magnetic field further amplified the glum value to 0.321 at RT, marking the first demonstration of magnetic-field-enhanced CPL in lead-free OIMHs and highlighting their potential for spintronic applications. The exceptional stability of R/S-DACAMnBr3 has been evidenced by its resistance to thermal quenching (77% emission intensity retention at 140 °C) and UV irradiation (more than 95% intensity retention after 200 minutes at λex = 365 nm), suggesting its robustness for practical optoelectronic deployment. S-DACAMnBr3-based CP-LEDs displayed stable orange emission with a glum value up to 6.4 × 10−3, while single-crystal photodetectors possessed a switching ratio of 7.72. These findings not only advance the rational design of high-performance CPL materials but also open new avenues for developing chiral optoelectronic systems with tailored functionalities toward next-generation 3D displays, spin-based optoelectronics, and magneto-chiroptical devices.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
X. Li and X. Chen conceived the project and revised the manuscript. F. Wang performed the research and wrote the manuscript. T. Chen and L. Wang helped with the CD and CPL spectra measurements and analyses. C. Li, W. Zhang, and W. Yuan helped with the PL spectra measurements. S. Lu and L. Li helped with the design of the experiments and data analyses. All authors contributed to the general discussion and analysis of the manuscript.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22135008, 22175179, U22A20398, 12174392), and the Self-deployment Project Research Program of Haixi Institutes, Chinese Academy of Sciences (No. CXZX-2022-GH10, CXZX-2022-GS01).Notes and references
- K.-H. Jin, Y. Zhang, K.-J. Li, M.-E. Sun, X.-Y. Dong, Q.-L. Wang and S.-Q. Zang, Angew. Chem., Int. Ed., 2022, 61, e202205317 CrossRef CAS.
- F. Zinna, M. Pasini, F. Galeotti, C. Botta, L. Di Bari and U. Giovanella, Adv. Funct. Mater., 2017, 27, 1603719 CrossRef.
- F. Zinna, U. Giovanella and L. Di Bari, Adv. Mater., 2015, 27, 1791–1795 CrossRef CAS PubMed.
- L. Wan, J. Wade, X. Shi, S. Xu, M. J. Fuchter and A. J. Campbell, ACS Appl. Mater., 2020, 12, 39471–39478 CrossRef CAS.
- D.-W. Zhang, M. Li and C.-F. Chen, Chem. Soc. Rev., 2020, 49, 1331–1343 RSC.
- J. Han, S. Guo, H. Lu, S. Liu, Q. Zhao and W. Huang, Adv. Opt. Mater., 2018, 6, 1800538 CrossRef.
- J. Wang, C. Fang, J. Ma, S. Wang, L. Jin, W. Li and D. Li, ACS Nano, 2019, 13, 9473–9481 CrossRef CAS.
- J. Ma, C. Fang, C. Chen, L. Jin, J. Wang, S. Wang, J. Tang and D. Li, ACS Nano, 2019, 13, 3659–3665 CrossRef CAS.
- Y. Zhang, S. Yu, B. Han, Y. Zhou, X. Zhang, X. Gao and Z. Tang, Matter, 2022, 5, 837–875 CrossRef CAS.
- C. Zhang, S. Li, X.-Y. Dong and S.-Q. Zang, Aggregate, 2021, 2, e48 CrossRef CAS.
- J. Hu, X. Wen, D. Yang, Y. Chen, Z. Liu and D. Li, Nano Lett., 2024, 24, 1001–1008 CrossRef CAS.
- Y. Guo, Y. Xue, C. Geng and C. Li, Mater. Today Commun., 2022, 33, 104524 CrossRef CAS.
- J.-Y. Wang, Y. Si, X.-M. Luo, Z.-Y. Wang, X.-Y. Dong, P. Luo, C. Zhang, C. Duan and S.-Q. Zang, Adv. Sci., 2023, 10, 2207660 CrossRef CAS PubMed.
- M. Deng, N. F. M. Mukthar, N. D. Schley and G. Ung, Angew. Chem., Int. Ed., 2020, 59, 1228–1231 CrossRef CAS PubMed.
- C.-M. Shi, H. Lu, J.-Y. Wang, G. Long, L.-J. Xu and Z.-N. Chen, Nat. Commun., 2025, 16, 1505 CrossRef CAS.
- Y. Wu, M. Li, Z.-g. Zheng, Z.-Q. Yu and W.-H. Zhu, J. Am. Chem. Soc., 2023, 145, 12951–12966 CrossRef CAS PubMed.
- X.-H. Zhao, X. Hu, M.-E. Sun, X.-M. Luo, C. Zhang, G.-S. Chen, X.-Y. Dong and S.-Q. Zang, J. Mater. Chem. C, 2022, 10, 3440–3446 RSC.
- S. Feng, Y. Ma, S. Wang, S. Gao, Q. Huang, H. Zhen, D. Yan, Q. Ling and Z. Lin, Angew. Chem., Int. Ed., 2022, 61, e202116511 CrossRef CAS.
- X.-Y. Luo and M. Pan, Coord. Chem. Rev., 2022, 468, 214640 CrossRef CAS.
- W. Zhang, W. Zheng, L. Li, P. Huang, J. Xu, W. Zhang, Z. Shao and X. Chen, Adv. Mater., 2024, 36, 2408777 CrossRef CAS PubMed.
- Z. Zhou, H. Meng, F. Li, T. Jiang, Y. Yang, S. Liu and Q. Zhao, Inorg. Chem., 2023, 62, 5729–5736 CrossRef CAS.
- Y. Qin, P. She, X. Huang, W. Huang and Q. Zhao, Coord. Chem. Rev., 2020, 416, 213331 CrossRef CAS.
- D. Liang, H. Xiao, W. Cai, S. Lu, S. Zhao, Z. Zang and L. Xie, Adv. Opt. Mater., 2023, 11, 2202997 CrossRef CAS.
- Z.-L. He, J.-H. Wei, J.-B. Luo, Z.-Z. Zhang and D.-B. Kuang, J. Mater. Chem. C, 2023, 11, 1251–1257 RSC.
- K. Taniguchi, P.-J. Huang, S. Kimura and H. Miyasaka, Dalton Trans., 2022, 51, 17030–17034 RSC.
- Y. Zhang, W.-Q. Liao, D.-W. Fu, H.-Y. Ye, C.-M. Liu, Z.-N. Chen and R.-G. Xiong, Adv. Mater., 2015, 27, 3942–3946 CrossRef CAS PubMed.
- A. Sen, D. Swain, T. N. G. Row and A. Sundaresan, J. Mater. Chem. C, 2019, 7, 4838–4845 RSC.
- Y. Nagata and T. Mori, Front. Chem., 2020, 8, 448 CrossRef CAS.
- Z. Song, X. Liu, C. Yang, Q. Wu, X. Guo, G. Liu, Y. Wei, L. Meng and Y. Dang, Adv. Opt. Mater., 2024, 12, 2301272 CrossRef CAS.
- J. Son, G. Jang, S. Ma, H. Lee, C. U. Lee, S. Yang, J. Lee, S. Moon, W. Jeong, J. H. Park, J. Kim, D. H. Kim, J.-S. Park and J. Moon, Adv. Funct. Mater., 2025, 35, 2413041 CrossRef CAS.
- Y. Liu, Y. Wei, Z. Luo, B. Xu, M. He, P. Hong, C. Li and Z. Quan, Chem. Sci., 2024, 15, 15480–15488 RSC.
- Y. Liu, Z. Luo, Y. Wei, C. Li, Y. Chen, X. He, X. Chang and Z. Quan, Angew. Chem., Int. Ed., 2023, 62, e202306821 Search PubMed.
- C. Li, Y. Wei, Y. Li, Z. Luo, Y. Liu, M. He, Y. Zhang, X. He, X. Chang and Z. Quan, Small, 2024, 20, 2400338 CrossRef CAS.
- J.-T. Lin, D.-G. Chen, L.-S. Yang, T.-C. Lin, Y.-H. Liu, Y.-C. Chao, P.-T. Chou and C.-W. Chiu, Angew. Chem., Int. Ed., 2021, 60, 21434–21440 CrossRef CAS PubMed.
- J. Chen, S. Zhang, X. Pan, R. Li, S. Ye, A. K. Cheetham and L. Mao, Angew. Chem., Int. Ed., 2022, 61, e202205906 CrossRef CAS.
- D. P. Panda, D. Swain, R. Raghunathan and A. Sundaresan, Chem. Mater., 2024, 36, 5698–5708 CrossRef CAS.
- S. Zhang, Y. Zhao, J. Zhou, H. Ming, C.-H. Wang, X. Jing, S. Ye and Q. Zhang, Chem. Eng. J., 2021, 421, 129886 CrossRef CAS.
- X. Cheng, X. Chang, Y. Lin, L. Lv, L. Cong, Y. Jia, J. Yin, J. Li and B.-B. Cui, Small, 2024, 20, 2307216 Search PubMed.
- H. Xiao, P. Dang, X. Yun, G. Li, Y. Wei, X. Xiao, Y. Zhao, M. S. Molokeev, Z. Cheng and J. Lin, Angew. Chem., Int. Ed., 2021, 60, 3699–3707 CrossRef CAS PubMed.
- X. Liu, J. Yang, W. Chen, F. Yang, Y. Chen, X. Liang, S. Pan and W. Xiang, Nano Res., 2023, 16, 5894–5899 Search PubMed.
- W. Zhang, J. Wei, Z. Gong, P. Huang, J. Xu, R. Li, S. Yu, X. Cheng, W. Zheng and X. Chen, Adv. Sci., 2020, 7, 2002210 CrossRef CAS.
- C. Li, D. Tu, Y. Liu, L. Wang, M. Yang, Z. Xie, S. Yu, J. Xu and X. Chen, J. Lumin., 2024, 269, 120513 Search PubMed.
- T. Chang, Y. Dai, Q. Wei, X. Xu, S. Cao, B. Zou, Q. Zhang and R. Zeng, ACS Appl. Mater., 2023, 15, 5487–5494 CrossRef CAS.
- Y. Wang, S. Zhang, H. Hou, Y. Yin, B. Zou and R. Zeng, J. Lumin., 2024, 273, 120674 CrossRef CAS.
- G. Blasse and B. C. Grabmaier, Luminescent Materials, ed. G. Blasse and B. C. Grabmaier, Springer Berlin Heidelberg, Berlin, Heidelberg, 1994, pp. 33–70 Search PubMed.
- W. Zhang, P. Sui, W. Zheng, L. Li, S. Wang, P. Huang, W. Zhang, Q. Zhang, Y. Yu and X. Chen, Angew. Chem., Int. Ed., 2023, 62, e202309230 CrossRef CAS PubMed.
- G. Longhi, E. Castiglioni, J. Koshoubu, G. Mazzeo and S. Abbate, Chirality, 2016, 28, 696–707 CrossRef CAS PubMed.
- C. Yang, S. Xiao, H. Xiao, L. Xu and Z. Chen, ACS Nano, 2023, 17, 7830–7836 Search PubMed.
- D.-H. Kong, Y. Wu, C.-M. Shi, H. Zeng, L.-J. Xu and Z.-N. Chen, Chem. Sci., 2024, 15, 16698–16704 Search PubMed.
- H. Lu, F. Qi, H. Wang, T. He, B. Sun, X. Gao, A. H. Comstock, S. Gull, Y. Zhang, T. Qiao, T. Shao, Y.-X. Zheng, D. Sun, Y. Chen, H.-L. Zhang, Z. Tang and G. Long, Angew. Chem., Int. Ed., 2024, 64, e202415363 CrossRef.
- M. P. P. Davydova, L. Meng, M. I. I. Rakhmanova, Z. Jia, A. S. S. Berezin, I. Y. Bagryanskaya, Q. Lin, H. Meng and A. V. V. Artem'ev, Adv. Mater., 2023, 35, 2303611 CrossRef CAS PubMed.
- S. Sutariya, M. Bsatee, O. Gololobova, D. Diaz-Diestra, B. Thapa, B. R. Weiner, G. Morell, W. M. Jadwisienczak and J. Beltran-Huarac, ACS Omega, 2021, 6, 7598–7604 Search PubMed.
- I. Sarkar, M. K. Sanyal, S. Takeyama, S. Kar, H. Hirayama, H. Mino, F. Komori and S. Biswas, Phys. Rev. B:Condens. Matter Mater. Phys., 2009, 79, 054410 CrossRef.
- Z. Shang, T. Liu, Q. Yang, S. Cui, K. Xu, Y. Zhang, J. Deng, T. Zhai and X. Wang, Small, 2022, 18, 2203015 Search PubMed.
- V.-H. Vuong, S. V. N. Pammi, S. Ippili, V. Jella, T. N. Thi, K. S. Pasupuleti, M.-D. Kim, M. J. Jeong, J.-R. Jeong, H. S. Chang and S. G. Yoon, Chem. Eng. J., 2023, 458, 141473 CrossRef CAS.
- G. Zhou, Z. Liu, M. S. Molokeev, Z. Xiao, Z. Xia and X.-M. Zhang, J. Mater. Chem. C, 2021, 9, 2047–2053 RSC.
- Y. Wu, W. Fan, Z. Gao, Z. Tang, L. Lei, X. Sun, Y. Li, H.-L. Cai and X. Wu, Nano Energy, 2020, 77, 105170 Search PubMed.
- H. Wang, Z. Li, D. Li, P. Chen, L. Pi, X. Zhou and T. Zhai, Adv. Funct. Mater., 2021, 31, 2103106 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, spectral data and additional data. CCDC 2378510 and 2378511. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc01615k |
| This journal is © The Royal Society of Chemistry 2025 |