 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Unexpected structural isomers of AlFe2O4+ and AlCo2O4+: vibrational spectroscopy and ion mobility combined with quantum chemistry†
Winni Schwedland‡
 a,
Tatiana C. Penna‡
a,
Tatiana C. Penna‡ cd,
Henning Windeck
cd,
Henning Windeck a,
Fabian Müller
a,
Fabian Müller acd,
Stephen Leach
acd,
Stephen Leach a,
Joachim Sauer
a,
Joachim Sauer *a,
Xavier R. Advincula
*a,
Xavier R. Advincula b,
Fabian Berger
b,
Fabian Berger *ab,
Nanako Ishidae,
Keijiro Ohshimo
*ab,
Nanako Ishidae,
Keijiro Ohshimo e,
Fuminori Misaizu
e,
Fuminori Misaizu *e,
Ya-Ke Li
*e,
Ya-Ke Li cd,
Arghya Chakraborty
cd,
Arghya Chakraborty cd,
Francine Horn
cd,
Francine Horn cd and
Knut R. Asmis
cd and
Knut R. Asmis *c
*c
aInstitut für Chemie, Humboldt-Universität zu Berlin, Unter den Linden 6, 10099 Berlin, Germany. E-mail: js@chemie.hu-berlin.de
bYusuf Hamied Department of Chemistry, University of Cambridge, Lensfield Rd, Cambridge CB2 1EW, UK. E-mail: fb593@cam.ac.uk
cWilhelm-Ostwald-Institut für Physikalische und Theoretische Chemie, Universität Leipzig, Linnéstraße 2, 04103 Leipzig, Germany. E-mail: knut.asmis@uni-leipzig.de
dFritz-Haber-Institut der Max-Planck-Gesellschaft, Faradayweg, 4-6, 14195 Berlin, Germany
eGraduate School of Science, Tohoku University, 6-3 Aoba, Aramaki, Aoba-ku, Sendai 980-8578, Japan. E-mail: misaizu@tohoku.ac.jp
First published on 19th May 2025
Abstract
The structure and reactivity of the mixed metal oxide clusters Al2MO4+ and AlM2O4+ (M = Fe, Co), formally obtained by transition metal ion substitution from Al3O4+, are studied using infrared photodissociation (IRPD) spectroscopy, ion-mobility mass-spectrometry (IM-MS) and quantum chemistry. We use density functional theory (DFT) in combination with global structure optimization to identify low energy structures and to connect them to the IRPD and IM-MS data. Insights into anharmonic and temperature effects are obtained from machine learning-based molecular dynamics simulations. While all metal ions are equal in the cone-shaped structure of M3O4+, the mixed metal oxide clusters attain different, more stable structures, in which the metal ions are either in different oxidation states (Al2MO4+) or have different coordination numbers (AlM2O4+). The present results illustrate that different DFT functionals may accurately describe local minimum structures, but reliable relative energies of isomers with differently coordinated transition metal ions require multi-reference wavefunction calculations.
1 Introduction
Corundum (α-Al2O3) and hematite (α-Fe2O3) belong to Earth's most ubiquitous metal oxides. Together, they play important roles in geochemical processes, as industrial materials, and in catalysis.1–3 For example, Al3+-substituted hematite nanoparticles represent an important active component in soils, affecting the sequestration and bioavailability of contaminants. However, how the Al-substitution-induced morphological changes affect the adsorption behavior of hematite for contaminants remains poorly understood.4Both materials, corundum and hematite, share the same rhombohedral crystal structure, in which a metal ion M3+ binds to six O2− ions to form an octahedron. The isomorphous substitution of Fe3+ by Al3+ in hematite is well documented and there is much interest in understanding how the presence of the smaller, less electronegative Al3+ ions affect the material properties.1,5 Strain is induced upon Fe3+-substitution as a consequence of the smaller lattice parameters for α-Fe2O3 compared to α-Al2O3. Hence, substitution is limited to about 8%, leading to the well-known miscibility gap of the corundum-hematite system.6 Intermediate orthorhombic AlFeO3 can be prepared at temperatures above 1300 °C but is unstable at 298 °C (with respect to the pure phases).7 Recently, morphology variations of hematite crystals by Al-substitution have been studied by electron microscopy and motivated subsequent investigations using density functional theory (DFT).1,8 Still, the impact of Al-substitution on the structural and electronic properties of hematite remains ill-characterized, and tools that allow predicting such properties reliably are in demand.
Metal oxide clusters isolated in the gas phase represent well-defined model systems that can be studied using the highly sensitive and selective tool kit of action spectroscopy.9–11 Cryogenic ion vibrational spectroscopy in combination with electronic structure calculations yields important structural information and allows to analyze structure–reactivity correlations.12–14 Additional information about the presence of different structural isomers can be obtained from ion-mobility measurements.15,16
The simplest positively charged cluster models for pure alumina and pure hematite are Al3O4+ and Fe3O4+, respectively. They are isoelectronic with the solid materials, with all metal and O ions in their bulk oxidation states of +3 and −2, respectively. Both Al3O4+,17 and Fe3O4+,18 have “cone”-like structures with a central trivalent oxygen ion (Scheme 1).
Here, we examine the substitution of a transition metal (M = Fe, Co) by an Al ion in M3O4+, yielding AlM2O4+, and the substitution of Al by a transition metal ion in Al3O4+, yielding Al2MO4+. We are particularly interested in the question whether the substitution is isomorphous and, if not, which factors drive the structural changes and how do such changes affect the reactivity towards methane. It is known that substitution of Al by Fe in Al3O4+ is not isomorphic and that a change of the oxidation states from Fe+III/O−II to Fe+II/O˙−I is accompanied by the change to a planar bicyclic structure (Scheme 2).19 The terminal oxygen radical site explains why Al2FeO4+ abstracts hydrogen from methane,19 whereas Al3O4+ is unreactive towards methane.
Specifically, we use infrared photodissociation (IRPD) spectroscopy to measure vibrational spectra and employ ion-mobility mass-spectrometry (IM-MS) measurements to distinguish between differently shaped isomers. Additionally, we use DFT in combination with global structure optimization to identify low energy structures and to connect them to the experimental IRPD spectra and IM-MS data. The reactivity of the clusters towards methane is measured in an ion trap and compared to a DFT-based reaction energy profile.
2 Experimental results
2.1. Mass spectrometry
The metal oxide cations are produced by laser vaporization of a single mixed-metal rod (see the Methods section for details). The ablation of metal ions of two different elements yields more congested mass spectra compared to those metal oxide cations which contain only one metal type20 and, consequently, more stringent settings are required to ensure that only the cation of interest is probed. First, unit mass resolution was used for measuring the IRPD spectra. Second, the possibility of forming multiple isobaric species needs to be considered. While no obvious overlap is apparent for Al56Fe216O4+ (m/z 203), the only naturally occurring Co isotope (59Co) is isobaric with 27Al16O2. Therefore, the latter experiments were performed on Al2Co18O4+ (m/z 185) and AlCo218O4+ (m/z 217) using molecular 18O to avoid ion signal overlap with Al316O6+(m/z 177), Al2Co16O6+ (m/z 209) and Al316O8+ (m/z 209). The mass spectra obtained in this way are shown in Fig. S1.†2.2. Ion-trap reactivity
We performed ion-trap reactivity measurements towards methane under multiple collision conditions to test for the presence of reactive sites (see Fig. S2†). These measurements show that the doubly substituted cations AlFe2O4+ and AlCo2O4+ are not reactive towards methane, similar to Al3O4+.21 In contrast, the singly substituted cations Al2FeO4+ and Al2CoO4+ readily react with methane, with H atom abstraction efficiencies of 54% and 75%, respectively. Previously, the corresponding analogs Al2MO4+ (M = Zn, Ni) have been found to abstract an H atom from methane under formation of a methyl radical.21,222.3. Vibrational action spectroscopy
To obtain structural information on the mixed metal oxide cations we measured vibrational action spectra through IRPD spectroscopy of the corresponding He-tagged species.23 This method provides vibrational spectra in the linear absorption regime, typically a prerequisite for the unambiguous structure assignment based on a comparison to calculated infrared (IR) frequencies and intensities.14Fig. 1 compares the IRPD spectra of the metal oxide cations Al3O4+ (top panel) and Fe3O4+ (bottom panel), which exhibit similar structures of C3v symmetry,17,18,24–26 as well as the spectra of the mixed metal oxide cations Al3−nMnO4+ (M = Fe, Co; n = 1, 2) in the spectral range from 500 to 1200 cm−1. Band positions and assignments are summarized in Table 1. While Fe3O4+ and Al3O4+ share the same structure type, their IRPD spectra are shifted and have a different appearance. This is attributed to a combined effect of the higher atomic mass of the metal ions and the weaker bonds in Fe3O4+ (see Fig. S4†).
 | ||
| Fig. 1 IRPD spectra (from top to bottom) of Al3O4+(He3),17 Al2FeO4+(He1,2),19 Al2Co18O4+(He1,2), AlFe2O4+(He1,2), AlCo218O4+(He1,2), and Fe3O4+(He).24 See Table 1 for band positions and assignments. Characteristic spectral regions are separated by the broken lines. | ||
| n | Experiment | Calculated | Experiment | Calculated | Assignment | ||
|---|---|---|---|---|---|---|---|
| a The values for Al3O4+, Al2FeO4+ and Fe3O4+ are from ref. 17, 19 and 24, respectively. Experimental relative intensities are determined from the ratios of the integrated peak areas. | |||||||
| 0 | Al | ||||||
| a1 | 871 (100) | 846 (100) | νas(O–Al–O) | ||||
| a2 | 750 (30) | 730 (27) | νs(O–Al–O) | ||||
| a3 | 722 (79) | 697 (41) | νs(O–Al–O) | ||||
| a4 | 700 (36) | 692 (60) | νs(O(–Al)3) | ||||
| a5 | 678 (25) | ||||||
| a6 | 602 (23) | 576 (12) | νs(O(–Al)3) | ||||
| a7 | 543 (40) | 532 (7) | νas(O(–Al)3) | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| 1 | Fe | Co | |||||
| b1 | 1004 (100) | 986 (80) |  |
976 (72) | 953 (62) | νas(O–Al–O) | |
| b2 | 953 (100) | 936 (100) |  |
926 (100) | 913 (100) | νs(Al–Ot) | |
| b3 | 778 (56) | 763 (76) |  |
740 (82) | 725 (65) | νap(M–O–Al) | |
| b4 | 735 (34) | 724 (18) |  |
703 (31) | 687 (19) | νs(O–Al–O) | |
| b5 | 708 (30) | 690 (19) | 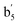 |
664 (33) | 650 (12) | νs(O–Al–O) | |
| b6 | 602 (32) | 599 (25) |  |
583 (19) | 569 (21) | νas(O–M–O) | |
| b7 | 541 (27) | 539 (32) |  |
531 (23) | 528 (27) | δ(M–O–Al) | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| 2 | Fe2 | Co2 | |||||
| c1 | 1091 (28) | 1097 (100) |  |
1082 (47) | 1067 (100) | νap(Al–O–M) | |
| c2 | 893 (3) |  |
839 (1) | ν(M–Ot) | |||
| c3 | 803 (75) | 760 (9) |  |
798 (57) | 714 (8) | νs(O–M–O) | |
| c4 | 727 (100) | 689 (11) |  |
681 (100) | 650 (9) | νs(O–Al–O) | |
| 687 (11) | 646 (6) | νas(O–M–O) | |||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| 3 | Fe3 | ||||||
 |
669 (100) | 678 (95) | νas(O–M–O) | ||||
 |
609 (91) | 641 (100) | νs(O–M–O) | ||||
 |
542 (39) | 527 (55) | νs(O(–M)3) | ||||
There are two main observations: (i) the spectra of the mixed metal oxide cations for the same n but different transition metals are very similar, suggesting identical structural motifs with the remaining differences being due to slightly different masses and bond strengths. (ii) The IRPD spectra for different n are so different that substantial structural changes are very likely upon Al/M (or M/Al) substitution. In other words, there are no indications for isomorphous substitution, neither for substitution of Al by M (n = 0 → 1), nor for substitution of M by Al (n = 3 → 2).
The IRPD spectra for n = 1 shown in Fig. 1 have seven bands (Fe: b1 to b7; Co: b′1 to b′7), with similar relative positions and relative intensities for Fe and Co (see Table 1). The IRPD bands in the Al2Co18O4+ spectrum are slightly red-shifted (up to 25 cm−1) with respect to the corresponding IRPD bands of Al2FeO4+, which can be explained by the higher mass of the O isotope in the Co-containing ions. As reported previously, the replacement of one Al by either an Fe ion19 or a Ni ion21 leads to a change from the “cone”-like structure with a central trivalent O ion (C3v symmetry) to a planar bicyclic frame (Cs symmetry) with a terminal Al–O˙−I radical site. The Al2MO4+ spectra suggest that a similar structural change occurs for the substitution with a Co ion.
In analogy to Al2MO4+, the IRPD spectra of the two AlM2O4+ cations are very similar to each other, exhibiting three characteristic absorption features (Fe: c1 to c4; Co: c′1 to c′4). The bands in the Co-substituted spectrum are again slightly red-shifted due to the presence of 18O. The AlM2O4+ IRPD spectra differ from those of all other compositions by a characteristic band (Fe: c1; Co: c′1) above 1050 cm−1. Moreover, they exhibit no bands between 900 and 1050 cm−1 or between 500 and 650 cm−1, which are the spectral regions where prominent features are observed for Al3O4+, Al2MO4+, and M3O4+.
2.4. Ion mobility measurements
Additional structural information is obtained from IM-MS measurements,15,16 which allow discerning structural isomers based on shape differences. These measurements reveal a bimodal feature in the arrival time distribution of AlFe2O4+, with an intensity ratio of 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and a single peak in the arrival time distribution of AlCo2O4+ (see Fig. 2). From the peak position of the bimodal distribution in Fig. 2, the AlFe2O4+ collision cross sections (CCSs) were determined to be 67.0 ± 0.9 and 70.6 ± 0.9 Å2, and 71.4 ± 0.8 Å2 for AlCo2O4+ (error bars were determined from eight independent measurements). We also studied monosubstituted Al2FeO4+ for which a single peak is observed in the arrival time distribution corresponding to a CCS of 68.4 ± 1.1 Å2 (see Fig. S3†).
1, and a single peak in the arrival time distribution of AlCo2O4+ (see Fig. 2). From the peak position of the bimodal distribution in Fig. 2, the AlFe2O4+ collision cross sections (CCSs) were determined to be 67.0 ± 0.9 and 70.6 ± 0.9 Å2, and 71.4 ± 0.8 Å2 for AlCo2O4+ (error bars were determined from eight independent measurements). We also studied monosubstituted Al2FeO4+ for which a single peak is observed in the arrival time distribution corresponding to a CCS of 68.4 ± 1.1 Å2 (see Fig. S3†).
3 Computational results
3.1. Isomer structures and harmonic IR spectra
The energetically most stable structures of Al2CoO4+, AlFe2O4+, and AlCo2O4+ were identified using a genetic algorithm27,28 with DFT for the respective high spin states. The most stable isomers were locally reoptimized with the TPSSh29 exchange-correlation functional and the def2-TZVPP30,31 basis set. The same method was used to calculate IR spectra (wavenumbers and intensities) using the (double) harmonic approximation. Analogous to Al2FeO4+, the oxidation states of the Al2CoO4+ isomers Cs-1 and Cs-2 are Co+III/O−II and Co+II/O˙−I, respectively. The oxidation states for all isomers of the composition AlM2O4+ shown in Fig. 3 are M+III/O−II, except for C2v and C1-2 which also contain an O radical with M+II/O˙−I. | ||
| Fig. 3 IRPD spectra of Al2FeO4+(He1,2),19 Al2Co18O4+(He1,2), AlFe2O4+(He1,2), and AlCo218O4+(He1,2) as well as the corresponding harmonic IR spectra of the bare cations as calculated with TPSSh/def2-TZVPP for their high spin states. For each composition, different isomers are considered, and the respective point groups are reported. Relative energies (including zero-point vibrational energies) are given in kJ mol−1. Color code: O – red, Al – gray, Fe – green, and Co – blue. | ||
Fig. 3 compares the IRPD spectra of the He-tagged clusters Al2FeO4+,19 Al2CoO4+, AlFe2O4+, and AlCo2O4+ to calculated harmonic IR spectra of different isomers of the bare cations. He-tagging, which is experimentally necessary to measure IRPD spectra in the linear absorption regime, does not significantly affect the calculated IR spectra (see Fig. S9†) and is therefore neglected in the further discussion.19
For these isomers, none of the calculated harmonic IR spectra agrees as well with the corresponding IRPD spectra as it is the case for the Cs-2 isomer of the Al2MO4+ cations, see Fig. 3. The predicted spectra of C1-2 and Cs-2, the doubly substituted analogs of Cs-2 for Al2MO4+, do not exhibit any resemblance to the experimental spectra. The D2d and Cs-1 isomers, which TPSSh predicts to be the lowest energy structures, do not provide good agreement between predicted and experimental vibrational spectra either.
The IRPD spectra of AlM2O4+ show a characteristic band just above 1050 cm−1 (Fe: c1; Co: c′1 in Table 1). It falls into the range in which the O–O stretching vibrations of superoxide (O2−I) moieties can be observed.12 However, such a unit is unlikely to be present in these systems as this would imply an oxidation state of M+I for one of the transition metal ions, which is considered unfavorable for Fe and Co.
| Al(M+III)2(O−II)4+ ⇔ Al(M+I)(M+II)(O2–I)(O−II)2+ | (1) |
Indeed, the most stable isomer with a superoxide moiety (Cs-3) is 271 and 165 kJ mol−1 less stable than the TPSSh global minimum (D2d) for AlFe2O4+ and AlCo2O4+, respectively, see Fig. S10.† This is outside the expected TPSSh uncertainty for transition metal containing gas phase clusters of about 100 kJ mol−1.19 A superoxide-containing isomer is therefore discarded as a candidate for the experimentally observed structure. More importantly, for this isomer the overall agreement between predicted and experimental spectra is poor (see Fig. S10†).
The “key”-like C1-1 isomer is the only one without a superoxide unit which can explain the observed band above 1050 cm−1. It is assigned to an antiphase Al–O–M stretching vibration (Table 1, c1/c′1). In addition, for C1-1 two bands are predicted in the region of 600 to 850 cm−1 which are also experimentally observed (Table 1, c3/c′3 and c4/c′4). For a complete list of all vibrations of C1-1, see Table S6.† Therefore, the best agreement between the predicted and experimental vibrational spectra is obtained for the “key”-like isomer C1-1. Even though the experimental c3/c′3 and c4/c′4 bands exhibit substantially larger relative band intensities with respect to c1 than the predicted ones, there is no doubt that, if at all, it is the C1-1 isomer that gives rise to the observed IRPD spectrum.
3.2. IR spectra of AlFe2O4+ and AlCo2O4+ based on molecular dynamics simulations
To see if the experimental and predicted intensity ratios for the bands c1/c3 (c′1/c′3) and c1/c4 (c′1/c′4) for C1-1 can be improved by accounting for anharmonicity and finite temperature effects, we performed molecular dynamics (MD) simulations and determined the IR spectra by Fourier transformation of the dipole moments. To reach simulation times that are long enough to yield converged IR spectra with sufficient resolution, we employed machine learning interatomic potentials (MLIPs) derived from training data obtained from short DFT MD simulations (ab initio MD, AIMD). We have chosen this approach because directly performing MD simulations with forces and dipoles evaluated with hybrid exchange-correlation functionals such as TPSSh is computationally not feasible, see the Computational details section for further information.Fig. 4 shows the IRPD spectra of C1-1 (“key”-like isomer) compared to the anharmonic IR spectra obtained from MD simulations and the harmonic spectra. Taking anharmonicities into account, the band positions remain similar, but the relative band intensities change substantially. The bands below 800 cm−1 gain in intensity relative to the band above 1050 cm−1, improving the agreement with the experimental IRPD spectra. We attribute the observed intensity differences between the harmonic and anharmonic IR spectra of C1-1 to the large amplitude motion of the O–M+III–O−II unit, which results in a wide range of bending angles present in the MD simulations (see Section 5.4 of the ESI†). In contrast, the MD-based IR spectra of D2d and Cs-1 remain similar to the harmonic IR spectra in terms of band positions and relative band intensities (see Fig. S17†).
The IR spectra derived from the MD simulations improve the intensity ratios of c1/c3 (c′1/c′3) and c1/c4 (c′1/c′4) compared to the harmonic IR spectra (see Table S8†). Nevertheless, the intensity ratios are not the same as in the experiment and the c1 band of C1-1 in AlCo2O4+ remains the most intense. However, the improvement of intensity ratios provides additional support for the assignment of the observed IRPD spectra of AlFe2O4+ and AlCo2O4+ to the “key”-like C1-1 isomer.
3.3 Relative stability of isomers: dependence on the exchange-correlation functional
Multireference configuration interaction calculations with Davidson corrections (MRCI-D) confirm that Cs-2 is indeed the global minimum structure, which is 56 and 100 kJ mol−1 more stable than Cs-1 for Al2FeO4+ (ref. 19) and Al2CoO4+, respectively, see Tables S2 and S3.† In contrast, the computationally much more affordable multi-configuration pair density functional theory (MC-PDFT),32,33 which uses the density from MC calculations in a translated PBE functional (tPBE),34 fails to give the right stability ordering for Al2CoO4+, whereas it gave the correct result for Al2FeO4+.19
So far, we have assumed that both transition metal ions are in a high spin state and ferromagnetically coupled. The transition metal and O ions of D2d, Cs-1, and C1-1 are all in a +III and –II oxidation state, respectively (see Fig. S7†). When lower spin states at the transition metal ions or antiferromagnetic coupling are considered, the stability order does not change and D2d remains the global minimum structure with TPSSh (see Tables S4 and S5†). Furthermore, the agreement of the predicted IR spectra of C1-1 with the experimental IRPD spectra does not improve when considering lower spin states (see Fig. S12†).
In summary, none of the tested DFT functionals is able to correctly predict the relative stability of different AlFe2O4+ and AlCo2O4+ isomers.
3.4. Collision cross sections
Since the “key”-like C1-1 isomer is structurally so different from the compact “spiro” D2d found to be most stable with TPSSh and also from other low-energy structures such as Cs-1, we provide additional evidence for the presence of the C1-1 structure from ion mobility measurements (see Section 2.4).15,16 Table 4 compares the experimental and theoretical CCSs of each isomer of the AlM2O4+ cations. The “key”-like C1-1 structures (71.4 and 72.5 Å2 for M = Fe and Co, respectively) are unequivocally assigned as the experimentally observed structures. The deviations between the experimental and theoretical CCSs and arrival times are within the experimental uncertainty limits. The second, less intense peak in the arrival time distribution for AlFe2O4+ (Fig. 2, 67.0 ± 0.9 Å2) is assigned to the planar bicyclic structure-type Cs-2 or C1-2 structure. The CCS deviation between prediction and experiment (−2.5 ± 0.9 Å2) is larger, but this assignment is supported by the results for the mono-substituted Al2FeO4+ cation. Consistent with the original assignment based on the IRPD and MRCI data,16 the best agreement between experiment and theory for the CCS is obtained for the planar bicyclic structure Cs-2. For Al2FeO4+, the deviation is also −2.5 ± 1.1 Å2. The intensity ratio between the 70.6 and 67.0 Å2 peaks for AlFe2O4+, assigned to the C1-1 (“key”) and Cs-2 (“ladder”) isomers, is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3.
0.3.
| Al2FeO4+ | AlFe2O4+ | AlCo2O4+ | |||||
|---|---|---|---|---|---|---|---|
| Isomer | CCS | Type | Isomer | CCS | t | CCS | t |
| Spiro | D2d | 63.6 | 289 | 67.4 | 308 | ||
| Cs-1 | 61.8 | Cone | Cs-1 | 59.4 | 275 | 64.3 | 297 |
| C2v | 63.7 | 290 | 67.9 | 310 | |||
| Key | C1-1 | 71.4 | 316 | 72.5 | 326 | ||
| Cs-2 | 65.9 | Ladder | C1-2 | 64.5 | 292 | 68.6 | 312 |
| Ladder | Cs-2 | 64.8 | 293 | 68.5 | 312 | ||
| Exp | 68.4 ± 1.1 | Exp | 70.6 ± 0.9 | 312 ± 3 | 71.4 ± 0.8 | 322 ± 3 | |
| (Cs-2) | (−2.5 ± 1.1) | (C1-1) | (0.8 ± 0.9) | (4 ± 3) | (1.1 ± 0.9) | (4 ± 3) | |
| Exp | 67.0 ± 0.9 | 298 ± 3 | |||||
| (C1-2) | (−2.5 ± 0.9) | (−6 ± 3) | |||||
| (Cs-2) | (−2.2 ± 0.9) | (−5 ± 3) | |||||
3.5. Reactivity calculations
Whereas Al3O4+ exhibits a “cone”-like C3v structure,17 substitution with an Fe3+ ion results in a structural rearrangement, forming a planar bicyclic “ladder”-like Cs structure for Al2FeO4+ (Cs-2 in Fig. 3) with a highly reactive terminal Al–O˙−I oxygen radical site.22,35 This is accompanied by the change from the Fe+III/O−II to the Fe+II/O˙−I valence state which is also observed for Co+III in Al2CoO4+ (see Fig. 3) and for Ni+III in Al2NiO4+(see ref. 21). The experiments conducted in this work confirm that Al2FeO4+ and Al2CoO4+ readily react with methane (see Fig. S2†).In agreement with the experimental observation that AlFe2O4+ and AlCo2O4+ exhibit no signs of reactivity towards methane (see Fig. S2†) our TPSSh calculations for the “key”-like C1-1 isomer yield positive apparent energy barriers ΔE0 of 44 and 29 kJ mol−1, respectively, for the C–H bond activation (see Fig. S18†). Methane initially attaches to the two-fold coordinated transition metal ion in the four-membered ring with binding energies of −75 and −86 kJ mol−1 for AlFe2O4+ and AlCo2O4+, respectively. Given that all transition metal and O ions in C1-1 are in a +III and –II oxidation state, respectively, H atom abstraction proceeds via proton coupled electron transfer, M+III(dn)/O2− + H˙ → M+II(dn+1)/OH−, rather than via H atom transfer as found for the planar bicyclic Cs-2 structure of Al2FeO4+, M+II(dn)/O˙− + H• → M+II(dn)/OH−. However, due to the positive apparent barrier, methane would dissociate again from the cluster before any type of C–H bond activation could occur.
4 Discussion and conclusions
All three metal oxide clusters, Al3O4+,17 Fe3O4+,26 and presumably Co3O4+ (ESI, Section S3†) share the same “cone”-like C3v structure which has a threefold coordinated O ion on top of a six-membered (MO)3 ring. Fig. 5 summarizes the structural changes caused by substitution of an Al ion for a transition metal (M = Fe, Co) in M3O4+ yielding AlM2O4+, and by substitution of a transition metal ion for Al in Al3O4+ yielding Al2MO4+. For both substitutions, the observed IR spectra (Fig. 1) indicate a significant structural change compared to the respective parent cluster, but no structural differences between Fe- and Co-containing clusters of the same composition. | ||
| Fig. 5 Schematic representation of the change in structure and valence upon composition change from Al3O4+ to Al2MO4+ and M3O4+ to AlM2O4+ with M = Fe, Co. | ||
Substitution of M = Fe19 and Co (this work) for Al in Al3O4+ changes the “cone”-like C3v structure into a planar bicyclic structure (Cs-2 in Fig. 3) which is accompanied by a change of the valence state from M+III/O−II to M+II/O˙−I. The unpaired electron at the terminal oxygen site explains the observed hydrogen abstraction from methane. In contrast, the Al substitution in M3O4+ (M = Fe, Co) changes the “cone”-like C3v structure of M3O4+ to a different, “key”-like C1 structure of AlM2O4+ (C1-1 in Fig. 3) which does not show reactivity towards methane. It consists of a four-membered ring connected via a central three-fold coordinated Al3+ ion to a nearly linear O−II–M+III–O−II unit.
Comparison of the IRPD spectra and IM-MS data together with the computational predictions for the “key”-like C1-1 structure leaves no doubt that this is the experimentally observed AlM2O4+ isomer. It is the only isomer which explains the observed characteristic band above 1050 cm−1. But, according to the DFT calculations, C1-1 is not the global minimum energy structure. For AlFe2O4+ and AlCo2O4+, it is 96 to 101 and 37 to 50 kJ mol−1, respectively, less stable than the respective global minimum structures. Such failures of DFT with transition metal compounds are not unexpected. Although TPSSh does not yield C1-1 as the global minimum structure, it still provides good agreement with the CCSs and IRPD band positions. This illustrates that the different functionals may accurately describe the local minimum structures and the shape of the PES around them, but not the relative energies of isomers with differently coordinated transition metal ions.
Reliable results for transition metal oxides require multi-reference wavefunction calculations. Whereas for the Al2MO4+ ions MRCI-D calculations could be completed, see ref. 19 and Section 3.3, for AlM2O4+, the presence of two transition metal ions would require larger active spaces for which such calculations cannot be converged with the currently available methods. However, we may learn something about the differences between TPSSh and MRCI-D results from available calculations on related, even smaller gas phase systems. FeO2− is a subunit in both the “spiro” D2d isomer (most stable with TPSSh) and the “key”-like C1-1 isomer (best match with experiment) of AlM2O4+. Previously, Müller found that FeO2− ions prefer a bent structure according to TPSSh, but a linear one according to MRCI.36 The “spiro” D2d structure has two bend FeO2− subunits connected by the central Al ion. If one of them opens and becomes linear the “key”-like C1-1 isomer is obtained. With MRCI this transition would be connected with an energy gain making the C1-1 structure more stable than the “spiro” D2d structure. This consideration could be made more quantitative with calculations using the localized active space (LAS) approach, which defines active spaces separately on connected sub-units.37
In conclusion, isovalent metal substitution in metal oxides induces structural changes that are much larger for gas phase clusters than for the corresponding bulk oxides. Starting from cone-shaped M3O4+ structures in which all metal ions are equal (the same M+III oxidation state and the same three-fold coordination with oxygen ions) and in the absence of periodic constraints, the substituted clusters (M = Fe, Co) attain more stable structures of different type in which the metal ions are in different oxidation states (M+II in Al2MO4+, “ladder”) or have different coordination numbers (two-fold coordination in AlM2O4+, “key”-structure).
5 Experimental methods
5.1. Infrared photodissociation spectroscopy
The infrared photodissociation (IRPD) experiments were performed employing a cryogenic ion trap tandem mass spectrometer38,39 using the widely tunable, intense IR radiation from the Fritz-Haber-Institute Free-Electron-Laser (FHI FEL).40 The gas phase ions were generated in a pulsed laser vaporization source by focusing a frequency-doubled Nd:YAG laser (50 Hz, 10–15 mJ) onto a rotating mixed metal rod (Al/TM of 70/30 and 18/82 at% for the mono and doubly substituted clusters, respectively). The resulting plasma was quenched with a gas pulse of 0.5% O2 seeded in He. Throughout all experiments, the Oxygen-18 isotope was used with the Co/Al target to reduce the number of isobars formed by avoiding clusters containing Al16O2 moieties, which are isobaric with Co (59 u). Cluster ions were formed during expansion through a clustering channel downstream from the rod and passed through a 4 mm diameter skimmer. The beam of ions was then collimated and thermalized close to room temperature in a He-gas filled radio frequency (RF) ion guide, mass-selected using a quadrupole mass-filter, and focused into a cryogenically cooled RF ring-electron ion-trap. The trap was continuously filled with He-gas at a trap temperature of 11 to 15 K or with a reactant gas/buffer gas mix of 0.01% CH4 in He. Many collisions of the trapped ions with the gas particles provided gentle cooling of the internal degrees of freedom close to the ambient temperature. Under these conditions, the He-tagged species are formed by three-body collisions.23 All ions are extracted from the ion trap and focused both temporally and spatially into the center of the extraction region of an orthogonally mounted reflectron time-of-flight (TOF) tandem mass spectrometer. Here, the ions are irradiated with a single counterpropagating IR laser macropulse (duration: 10 µs) produced by the FHI FEL (430–1200 cm−1, 5 Hz), with a bandwidth of ∼0.5% fwhm and pulse energy of 0.7–2.5 mJ. Parent as well as photofragment ion yields are monitored simultaneously as a function of the irradiation wavenumber. IRPD scans are recorded by averaging over 100 TOF mass spectra per wavenumber step (2 cm−1). Typically, at least three scans are summed to obtain the final IRPD spectrum. The photodissociation cross section σIRPD is determined as described previously.14,415.2. Ion mobility-mass spectrometry
Ion mobility-mass spectrometry was performed using a home-built vacuum apparatus composed of a cluster ion source, an ion drift tube, and a time-of-flight mass spectrometer. Details of experiments were already reported elsewhere.16 Fe/Al alloy oxide cluster cations were generated by a combination of laser vaporization of a Fe/Al alloy rod and supersonic expansion of 5% O2/He mixture gas (stagnation pressure = 0.3 MPa). For formation of Co/Al alloy oxide cluster cations, we used double-rod type laser vaporization source where Co and Al rods were vaporized by two YAG lasers independently to reduce the number of isobars possible. In the present experiment, the power of laser for vaporization of a Co rod (5.7 mJ per pulse) was slightly higher than that of an Al rod (3.6 mJ per pulse). With this condition, we confirmed that the contribution of CoAl2O6+ can be negligible in the arrival time distribution of Co2AlO4+. The generated ions were injected into the drift tube with a kinetic energy of 50 eV by a pulsed electric field at a given time (t = t0). The drift tube was 107 mm long and was filled with He buffer gas with a pressure of 1.00 Torr at 100 K. The drift electric field in the tube was E = 11.2 V cm−1. The E/N value was 11.5 Td (N is the number density of the buffer gas, 1 Td = 10−17 V cm2). After running through the drift tube, the ions were reaccelerated to ∼1.8 keV by another pulsed electric fields in an acceleration region of the time-of-flight mass spectrometer at a given time later from the first pulse: t = t0 + Δt. The ions were introduced to the mass spectrometer and detected by a dual microchannel plate.The delay time between the two pulses, Δt, was defined as “arrival time”. The drift velocity of the ions in the drift tube, vd, was calculated numerically to satisfy the measured arrival time. We also obtained the time that an ion spends in the drift tube, td, from the measured arrival time. It is known that vd is proportional to E, and the proportional constant (K) is called as ion mobility. The mobility K depends on the number density of the buffer gas N. To compare the mobility under different experimental conditions, the reduced mobility K0 is defined as K0 = K·(N/N0), where N0 is Loschmidt's number. From the Mason–Schamp equation, the reduced mobility K0 in the drift tube was given as
 | (2) |
Theoretical collision cross sections (CCScalc) were calculated by using the trajectory method in the MOBCAL program. We used the parameters of Lennard-Jones potentials in the trajectory calculations. These parameters were determined to reproduce the experimental collision cross sections (CCSexp) of cobalt, iron, and aluminum oxide cluster ions.
5.3. Computational methods
All multireference calculations were performed using the correlation consistent polarized core valence triple-ζ (cc-pwCVTZ)61–64 basis sets. CASSCF, CASPT2, NEVPT2, and MRCI calculations were performed with the Molpro program package V2015.1,65 and multiconfiguration pair-density functional theory (MC-PDFT)32,33 calculations with the translated PBE functional (tPBE) using the openMOLCAS program V18.0.66–68
We build on the MACE architecture,69 as it allows for fast and highly data-efficient training with high-order equivariant message passing and has been successfully used to generate IR spectra in other contexts.70–72 To represent the PES and dipole moment surface (DMS), we train MLIPs to both (i) energies and forces, and (ii) dipole moments. For each isomer, independent training data sets are generated. For this, DFT MD simulations were run in a canonical NVT ensemble using the Nose–Hoover thermostat at a simulation temperature of 600 K and a time step of 0.5 fs for a total simulation time of 16 ps with the PBE0 (ref. 44) exchange correlation functional and the def2-TZVPP30,31 basis set. A total of 1600 structures were randomly selected from four independent DFT MD runs, the structures were shifted to the coordinate system origin, aligned by minimizing their RMSD, and the energy, gradient and dipole moment were recalculated with the same computational method. To accurately reproduce the PES, we used MACE models with two layers, a spherical expansion up to lmax = 3, a cutoff distance of 5 Å, and 128 equivariant messages. To reproduce the DMS, we used MACE models with 32 channels, a spherical expansion up to lmax = 2, and a cutoff distance of 5 Å.
The MLIPs reproduce the underlying DFT data accurately (see Fig. S13†). The MDs are run by the Atomic Simulation Environment (ASE)73 using the MLIPs trained to reproduce the PESs. We conducted the MLIP MD simulations at a simulation temperature of 100 K and simulation time of 250 ps for D2d and Cs-1 as well as 500 ps for C1-1 with a 0.5 fs time step using a Langevin thermostat with a friction coefficient of 5 ps−1. The longer simulation time for C1-1 was chosen to provide fully converged MD-based IR spectra (Fig. S16†). We then evaluated the dipole moments for the structures of the MD trajectories using the MLIPs trained to reproduce the DMSs. MD based IR spectra were subsequently generated by Fourier-transforming the dipole moments.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its ESI.†Author contributions
W. Schwedland: conceptualization, data curation, formal analysis, investigation, methodology, project administration, validation, visualization, writing – original draft, writing – review & editing. T. Penna: conceptualization, data curation, formal analysis, investigation, project administration, validation, visualization, writing – review & editing. H. Windeck: data curation, formal analysis, investigation, writing – original draft, writing – review & editing. F. Müller: data curation, investigation, methodology, supervision. S. Leach: data curation, investigation. J. Sauer: conceptualization, funding acquisition, project administration, resources, supervision, writing – original draft, writing – review & editing. X. R. Advincula: data curation, investigation, software. F. Berger: conceptualization, formal analysis, investigation, methodology, project administration, supervision, writing – original draft, writing – review & editing. N. Ishida: data curation, formal analysis, investigation, validation. K. Ohshimo: data curation, formal analysis, investigation, writing – original draft, writing – review & editing. F. Misaizu: conceptualization, funding acquisition, project administration, resources, supervision, writing – original draft, writing – review & editing. Y. Li: conceptualization, project administration, data curation, formal analysis, investigation, validation A. Chakraborty: conceptualization, project administration, data curation, formal analysis, investigation, validation F. Horn: data curation, formal analysis, investigation, validation. K. R. Asmis: conceptualization, funding acquisition, project administration, resources, supervision, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is funded by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), project 430942176. H. W. is member of the International Max Planck Research School for Elementary Processes in Physical Chemistry and holds a Kekulé Fellowship of the Fonds der Chemischen Industrie. X. R. A. acknowledges support from the European Union under the “n-AQUA” European Research Council project (Grant No. 101071937). F. B. acknowledges support from the Alexander von Humboldt Foundation through a Feodor Lynen Research Fellowship, from the Isaac Newton Trust through an Early Career Fellowship, and from Churchill College, Cambridge, through a Postdoctoral By-Fellowship. X. R. A. and F. B. additionally acknowledge access to CSD3 obtained through a University of Cambridge EPSRC Core Equipment Award (EP/X034712/1). Y.-K. L. thanks the Alexander von Humboldt Foundation for a postdoctoral research fellowship. This work also used the Cirrus UK National Tier-2 HPC Service at EPCC, funded by the University of Edinburgh and the EPSRC (EP/P020267/1).References
- W. Li, X. Liang, P. An, X. Feng, W. Tan, G. Qiu, H. Yin and F. Liu, Mechanisms on the morphology variation of hematite crystals by Al substitution: The modification of Fe and O reticular densities, Sci. Rep., 2016, 6, 35960, DOI:10.1038/srep35960.
- M. Zhu and I. E. Wachs, Iron-Based Catalysts for the High-Temperature Water–Gas Shift (HT-WGS) Reaction: A Review, ACS Catal., 2016, 6, 722–732, DOI:10.1021/acscatal.5b02594.
- A. P. Amrute, Z. Łodziana, H. Schreyer, C. Weidenthaler and F. Schüth, High-surface-area corundum by mechanochemically induced phase transformation of boehmite, Science, 2019, 366, 485–489, DOI:10.1126/science.aaw9377.
- Y. Liang, Y. Xiang, Z. Wei, M. Avena, J. Xiong, J. Hou, M. Wang and W. Tan, Complexation mechanism of Pb(2+) on Al-substituted hematite: A modeling study and theoretical calculation, Environ. Res., 2024, 252, 118935, DOI:10.1016/j.envres.2024.118935.
- A. Zoppi, C. Lofrumento, E. M. Castellucci and P. Sciau, Al-for-Fe substitution in hematite: the effect of low Al concentrations in the Raman spectrum of Fe2O3, J. Raman Spectrosc., 2008, 39, 40–46, DOI:10.1002/jrs.1811.
- A. Feenstra, S. SÄMann and B. Wunder, An Experimental Study of Fe–Al Solubility in the System Corundum–Hematite up to 40 kbar and 1300°C, J. Petrol., 2005, 46, 1881–1892, DOI:10.1093/petrology/egi038.
- A. Feenstra, S. SÄMann and B. Wunder, An Experimental Study of Fe–Al Solubility in the System Corundum–Hematite up to 40 kbar and 1300°C, J. Petrol., 2005, 46, 1881–1892, DOI:10.1093/petrology/egi038.
- W. G. Guimarães, G. F. de Lima and H. A. Duarte, Exploring the effect of isomorphically Al-substituted on the thermodynamical and structural properties of hematite and goethite – A DFT investigation, Hydrometallurgy, 2023, 221, 106125, DOI:10.1016/j.hydromet.2023.106125.
- E. Andris, R. Navrátil, J. Jašík, T. Terencio, M. Srnec, M. Costas and J. Roithová, Chasing the Evasive Fe
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O Stretch and the Spin State of the Iron(IV)–Oxo Complexes by Photodissociation Spectroscopy, J. Am. Chem. Soc., 2017, 139, 2757–2765, DOI:10.1021/jacs.6b12291.
O Stretch and the Spin State of the Iron(IV)–Oxo Complexes by Photodissociation Spectroscopy, J. Am. Chem. Soc., 2017, 139, 2757–2765, DOI:10.1021/jacs.6b12291. - J. Sauer and H.-J. Freund, Models in Catalysis, Catal. Lett., 2015, 145, 109–125, DOI:10.1007/s10562-014-1387-1.
- D. K. Böhme and H. Schwarz, Gas-Phase Catalysis by Atomic and Cluster Metal Ions: The Ultimate Single-Site Catalysts, Angew. Chem., Int. Ed., 2005, 44, 2336–2354, DOI:10.1002/anie.200461698.
- K. R. Asmis and J. Sauer, Mass-selective vibrational spectroscopy of vanadium oxide cluster ions, Mass Spectrom. Rev., 2007, 26, 542–562, DOI:10.1002/mas.20136.
- H. Schwarz and K. R. Asmis, Identification of Active Sites and Structural Characterization of Reactive Ionic Intermediates by Cryogenic Ion Trap Vibrational Spectroscopy, Chem.–Eur. J., 2019, 25, 2112–2126, DOI:10.1002/chem.201805836.
- N. Heine and K. R. Asmis, Cryogenic ion trap vibrational spectroscopy of hydrogen-bonded clusters relevant to atmospheric chemistry, Int. Rev. Phys. Chem., 2015, 34, 1–34, DOI:10.1080/0144235X.2014.979659.
- F. Lanucara, S. W. Holman, C. J. Gray and C. E. Eyers, The power of ion mobility-mass spectrometry for structural characterization and the study of conformational dynamics, Nat. Chem., 2014, 6, 281–294, DOI:10.1038/nchem.1889.
- Y. Nakajima, M. A. Latif, T. Nagata, K. Ohshimo and F. Misaizu, Size-Dependent Geometrical Structures of Platinum Oxide Cluster Cations Studied by Ion Mobility–Mass Spectrometry, J. Phys. Chem. A, 2023, 127, 3570–3576, DOI:10.1021/acs.jpca.2c09017.
- G. Santambrogio, E. Janssens, S. Li, T. Siebert, G. Meijer, K. R. Asmis, J. Döbler, M. Sierka and J. Sauer, Identification of Conical Structures in Small Aluminum Oxide Clusters: Infrared Spectroscopy of (Al2O3)1−4(AlO)+, J. Am. Chem. Soc., 2008, 130, 15143–15149, DOI:10.1021/ja805216e.
- R. Logemann, G. A. de Wijs, M. I. Katsnelson and A. Kirilyuk, Geometric, electronic, and magnetic structure of FexOy+ clusters, Phys. Rev. B, 2015, 92, 144427, DOI:10.1103/PhysRevB.92.144427.
- F. Müller, J. B. Stückrath, F. A. Bischoff, L. Gagliardi, J. Sauer, S. Debnath, M. Jorewitz and K. R. Asmis, Valence and Structure Isomerism of Al2FeO4+: Synergy of Spectroscopy and Quantum Chemistry, J. Am. Chem. Soc., 2020, 142, 18050–18059, DOI:10.1021/jacs.0c07158.
- G. Santambrogio, Infrared Spectroscopy of Gas Phase Cluster Ions, Doctoral thesis, Freie Universität Berlin, 2007.
- Y.-K. Li, F. Müller, W. Schöllkopf, K. R. Asmis and J. Sauer, Gas-Phase Mechanism of O.−/Ni2+-Mediated Methane Conversion to Formaldehyde, Angew. Chem., Int. Ed., 2022, 61, e202202297, DOI:10.1002/anie.202202297.
- S. Zhou, L. Yue, M. Schlangen and H. Schwarz, Electronic Origin of the Competitive Mechanisms in the Thermal Activation of Methane by the Heteronuclear Cluster Oxide [Al2ZnO4]+, Angew. Chem., Int. Ed., 2017, 56, 14297–14300, DOI:10.1002/anie.201704979.
- M. Brümmer, C. Kaposta, G. Santambrogio and K. R. Asmis, Formation and photodepletion of cluster ion–messenger atom complexes in a cold ion trap: Infrared spectroscopy of VO+, VO2+, and VO3+, J. Chem. Phys., 2003, 119, 12700–12703, DOI:10.1063/1.1634254.
- M. R. Fagiani, Cryogenetic ion vibrational spectroscopy of gas-phase clusters, Doctoral thesis, Freie Universität Berlin, 2017.
- R. Logemann, G. A. de Wijs, M. I. Katsnelson and A. Kirilyuk, Geometric, electronic, and magnetic structure of FeO+ clusters, Phys. Rev. B, 2015, 92, 144427, DOI:10.1103/PhysRevB.92.144427.
- A. Kirilyuk, A. Fielicke, K. Demyk, G. von Helden, G. Meijer and T. Rasing, Ferrimagnetic cagelike Fe4O6 cluster: Structure determination from infrared dissociation spectroscopy, Phys. Rev. B, 2010, 82, 020405, DOI:10.1103/PhysRevB.82.020405.
- M. Sierka, J. Döbler, J. Sauer, G. Santambrogio, M. Brümmer, L. Wöste, E. Janssens, G. Meijer and K. R. Asmis, Unexpected Structures of Aluminum Oxide Clusters in the Gas Phase, Angew. Chem., Int. Ed., 2007, 46, 3372–3375, DOI:10.1002/anie.200604823.
- D. M. Deaven and K. M. Ho, Molecular Geometry Optimization with a Genetic Algorithm, Phys. Rev. Lett., 1995, 75, 288–291, DOI:10.1103/PhysRevLett.75.288.
- V. N. Staroverov, G. E. Scuseria, J. Tao and J. P. Perdew, Comparative assessment of a new nonempirical density functional: Molecules and hydrogen-bonded complexes, J. Chem. Phys., 2003, 119, 12129–12137, DOI:10.1063/1.1626543.
- F. Weigend and R. Ahlrichs, Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305, 10.1039/B508541A.
- F. Weigend, Accurate Coulomb-fitting basis sets for H to Rn, Phys. Chem. Chem. Phys., 2006, 8, 1057–1065, 10.1039/B515623H.
- G. Li Manni, R. K. Carlson, S. Luo, D. Ma, J. Olsen, D. G. Truhlar and L. Gagliardi, Multiconfiguration Pair-Density Functional Theory, J. Chem. Theory Comput., 2014, 10, 3669–3680, DOI:10.1021/ct500483t.
- L. Gagliardi, D. G. Truhlar, G. Li Manni, R. K. Carlson, C. E. Hoyer and J. L. Bao, Multiconfiguration Pair-Density Functional Theory: A New Way To Treat Strongly Correlated Systems, Acc. Chem. Res., 2017, 50, 66–73, DOI:10.1021/acs.accounts.6b00471.
- R. Pandharkar, M. R. Hermes, D. G. Truhlar and L. Gagliardi, A New Mixing of Nonlocal Exchange and Nonlocal Correlation with Multiconfiguration Pair-Density Functional Theory, J. Phys. Chem. Lett., 2020, 11, 10158–10163, DOI:10.1021/acs.jpclett.0c02956.
- C. A. Gaggioli, J. Sauer and L. Gagliardi, Hydrogen Atom or Proton Coupled Electron Transfer? C–H Bond Activation by Transition-Metal Oxides, J. Am. Chem. Soc., 2019, 141, 14603–14611, DOI:10.1021/jacs.9b04006.
- F. Müller, Theoretical Investigation and Structural Assignment of Small Metal Oxide Clusters, Humboldt-Universität zu Berlin, 2021 Search PubMed.
- V. Agarawal, D. S. King, M. R. Hermes and L. Gagliardi, Automatic State Interaction with Large Localized Active Spaces for Multimetallic Systems, J. Chem. Theory Comput., 2024, 20, 4654–4662, DOI:10.1021/acs.jctc.4c00376.
- D. J. Goebbert, T. Wende, R. Bergmann, G. Meijer and K. R. Asmis, Messenger-Tagging Electrosprayed Ions: Vibrational Spectroscopy of Suberate Dianions, J. Phys. Chem. A, 2009, 113, 5874–5880, DOI:10.1021/jp809390x.
- D. J. Goebbert, G. Meijer and K. R. Asmis, 10 K Ring Electrode Trap—Tandem Mass Spectrometer for Infrared Spectroscopy of Mass Selected Ions, AIP Conf. Proc., 2009, 1104, 22–29, DOI:10.1063/1.3115605.
- S. Wieland, G. Sandy, J. Heinz, P. Alexander, H. Gert von, P. B. Hans and M. M. T. Alan, in The new IR and THz FEL facility at the Fritz Haber Institute in Berlin, Proc. SPIE, 2015, p 95121L Search PubMed.
- N. Heine and K. R. Asmis, Cryogenic Ion Trap Vibrational Spectroscopy of Hydrogen-Bonded Clusters Relevant to Atmospheric Chemistry, Int. Rev. Phys. Chem., 2016, 35, 507, DOI:10.1080/0144235X.2016.1203533.
- J. P. Perdew, Density-functional approximation for the correlation energy of the inhomogeneous electron gas, Phys. Rev. B, 1986, 33, 8822–8824, DOI:10.1103/PhysRevB.33.8822.
- A. D. Becke, Density-functional exchange-energy approximation with correct asymptotic behavior, Phys. Rev. A, 1988, 38, 3098–3100, DOI:10.1103/PhysRevA.38.3098.
- C. Adamo and V. Barone, Toward reliable density functional methods without adjustable parameters: The PBE0 model, J. Chem. Phys., 1999, 110, 6158–6170, DOI:10.1063/1.478522.
- A. D. Becke, Density‐functional thermochemistry. III. The role of exact exchange, J. Chem. Phys., 1993, 98, 5648–5652, DOI:10.1063/1.464913.
- A. Schäfer, H. Horn and R. Ahlrichs, Fully optimized contracted Gaussian basis sets for atoms Li to Kr, J. Chem. Phys., 1992, 97, 2571–2577, DOI:10.1063/1.463096.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77, 3865–3868, DOI:10.1103/PhysRevLett.77.3865.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1997, 78, 1396, DOI:10.1103/PhysRevLett.78.1396.
- TURBOMOLE V7.2 2017, a development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989-2007,TURBOMOLE GmbH, since 2007, available from http://www.turbomole.com.
- R. Ahlrichs, M. Bär, M. Häser, H. Horn and C. Kölmel, Electronic structure calculations on workstation computers: The program system turbomole, Chem. Phys. Lett., 1989, 162, 165–169, DOI:10.1016/0009-2614(89)85118-8.
- O. Treutler and R. Ahlrichs, Efficient molecular numerical integration schemes, J. Chem. Phys., 1995, 102, 346–354, DOI:10.1063/1.469408.
- K. Eichkorn, F. Weigend, O. Treutler and R. Ahlrichs, Auxiliary basis sets for main row atoms and transition metals and their use to approximate Coulomb potentials, Theor. Chem. Acc., 1997, 97, 119–124, DOI:10.1007/s002140050244.
- P. J. Knowles and H.-J. Werner, An efficient method for the evaluation of coupling coefficients in configuration interaction calculations, Chem. Phys. Lett., 1988, 145, 514–522, DOI:10.1016/0009-2614(88)87412-8.
- H. J. Werner and P. J. Knowles, An efficient internally contracted multiconfiguration–reference configuration interaction method, J. Chem. Phys., 1988, 89, 5803–5814, DOI:10.1063/1.455556.
- S. R. Langhoff and E. R. Davidson, Configuration interaction calculations on the nitrogen molecule, Int. J. Quantum Chem., 1974, 8, 61–72, DOI:10.1002/qua.560080106.
- J. A. Pople, R. Seeger and R. Krishnan, Variational configuration interaction methods and comparison with perturbation theory, Int. J. Quantum Chem., 1977, 12, 149–163, DOI:10.1002/qua.560120820.
- H.-J. Werner, Third-order multireference perturbation theory The CASPT3 method, Mol. Phys., 1996, 89, 645–661, DOI:10.1080/002689796173967.
- C. Angeli, M. Pastore and R. Cimiraglia, New perspectives in multireference perturbation theory: the n-electron valence state approach, Theor. Chem. Acc., 2007, 117, 743–754, DOI:10.1007/s00214-006-0207-0.
- C. Angeli, R. Cimiraglia and J.-P. Malrieu, n-electron valence state perturbation theory: A spinless formulation and an efficient implementation of the strongly contracted and of the partially contracted variants, J. Chem. Phys., 2002, 117, 9138–9153, DOI:10.1063/1.1515317.
- C. Angeli, R. Cimiraglia, S. Evangelisti, T. Leininger and J. P. Malrieu, Introduction of n-electron valence states for multireference perturbation theory, J. Chem. Phys., 2001, 114, 10252–10264, DOI:10.1063/1.1361246.
- T. H. Dunning Jr, Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen, J. Chem. Phys., 1989, 90, 1007–1023, DOI:10.1063/1.456153.
- K. A. Peterson and T. H. Dunning Jr, Accurate correlation consistent basis sets for molecular core–valence correlation effects: The second row atoms Al–Ar, and the first row atoms B–Ne revisited, J. Chem. Phys., 2002, 117, 10548–10560, DOI:10.1063/1.1520138.
- D. E. Woon and T. H. Dunning Jr, Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon, J. Chem. Phys., 1993, 98, 1358–1371, DOI:10.1063/1.464303.
- N. B. Balabanov and K. A. Peterson, Systematically convergent basis sets for transition metals. I. All-electron correlation consistent basis sets for the 3d elements Sc–Zn, J. Chem. Phys., 2005, 123, 064107, DOI:10.1063/1.1998907.
- H.-J. K. P. J. Werner, G. Knizia, F. R. Manby and M. Schütz, MOLPRO package of ab initio programs, 2015, vol. 1, http://www.molpro.net.
- F. Aquilante, J. Autschbach, R. K. Carlson, L. F. Chibotaru, M. G. Delcey, L. De Vico, I. Fdez Galván, N. Ferré, L. M. Frutos, L. Gagliardi, M. Garavelli, A. Giussani, C. E. Hoyer, G. Li Manni, H. Lischka, D. Ma, P. Å. Malmqvist, T. Müller, A. Nenov, M. Olivucci, T. B. Pedersen, D. Peng, F. Plasser, B. Pritchard, M. Reiher, I. Rivalta, I. Schapiro, J. Segarra-Martí, M. Stenrup, D. G. Truhlar, L. Ungur, A. Valentini, S. Vancoillie, V. Veryazov, V. P. Vysotskiy, O. Weingart, F. Zapata and R. Lindh, Molcas 8: New capabilities for multiconfigurational quantum chemical calculations across the periodic table, J. Comput. Chem., 2016, 37, 506–541, DOI:10.1002/jcc.24221.
- J. Olsen, B. O. Roos, P. Jo/rgensen and H. J. r. A. Jensen, Determinant based configuration interaction algorithms for complete and restricted configuration interaction spaces, J. Chem. Phys., 1988, 89, 2185–2192, DOI:10.1063/1.455063.
- P. A. Malmqvist, A. Rendell and B. O. Roos, The restricted active space self-consistent-field method, implemented with a split graph unitary group approach, J. Chem. Phys., 1990, 94, 5477–5482, DOI:10.1021/j100377a011.
- I. Batatia, D. P. Kovacs, G. Simm, C. Ortner and G. Csanyi, MACE: Higher Order Equivariant Message Passing Neural Networks for Fast and Accurate Force Fields, ed. Koyejo S., Mohamed, S., Agarwal, A., Belgrave, D., Cho, K. and Oh, A., 2022, vol. 35, pp 11423–11436 Search PubMed.
- M. Gastegger, J. Behler and P. Marquetand, Machine learning molecular dynamics for the simulation of infrared spectra, Chem. Sci., 2017, 8, 6924–6935, 10.1039/c7sc02267k.
- R. Beckmann, F. Brieuc, C. Schran and D. Marx, Infrared Spectra at Coupled Cluster Accuracy from Neural Network Representations, J. Chem. Theory Comput., 2022, 18, 5492–5501, DOI:10.1021/acs.jctc.2c00511.
- V. Kapil, D. P. Kovács, G. Csányi and A. Michaelides, First-principles spectroscopy of aqueous interfaces using machine-learned electronic and quantum nuclear effects, Faraday Discuss., 2024, 249, 50–68, 10.1039/D3FD00113J.
- A. Hjorth Larsen, J. Jørgen Mortensen, J. Blomqvist, I. E. Castelli, R. Christensen, M. Dułak, J. Friis, M. N. Groves, B. Hammer, C. Hargus, E. D. Hermes, P. C. Jennings, P. Bjerre Jensen, J. Kermode, J. R. Kitchin, E. Leonhard Kolsbjerg, J. Kubal, K. Kaasbjerg, S. Lysgaard, J. Bergmann Maronsson, T. Maxson, T. Olsen, L. Pastewka, A. Peterson, C. Rostgaard, J. Schiøtz, O. Schütt, M. Strange, K. S. Thygesen, T. Vegge, L. Vilhelmsen, M. Walter, Z. Zeng and K. W. Jacobsen, The atomic simulation environment—a Python library for working with atoms, J. Condens. Matter Phys., 2017, 29, 273002, DOI:10.1088/1361-648X/aa680e.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc02681d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |




