Open Access Article
Open Access ArticleNickel-catalyzed diastereoselective hydroboration of acrylates with a vinylborane reagent†
Guanwen
Hu
,
Peiqi
Zhang
 ,
Xinmou
Wang
,
Chunteng
Wan
,
Yiyi
Fu
,
Wa Hung
Leung
,
Xinmou
Wang
,
Chunteng
Wan
,
Yiyi
Fu
,
Wa Hung
Leung
 ,
Zhenyang
Lin
,
Zhenyang
Lin
 * and
Yangjian
Quan
* and
Yangjian
Quan
 *
*
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong SAR, China. E-mail: chzlin@ust.hk; chyjquan@ust.hk
First published on 13th June 2025
Abstract
Organoboron compounds exhibit unique properties and valuable applications in organic synthesis, catalysis, materials science, and drug discovery, driving researchers to improve their structural and functional diversity. Despite significant advancements, challenges remain in accessing complex organoboron compounds, particularly in constructing boron-stereogenic skeletons. Here we report a nickel catalyzed diastereoselective hydroboration of olefins using a newly developed vinylborane reagent, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–BH2L (where L is a dative donor). This protocol enables the efficient synthesis of versatile vinylborane derivatives featuring a boron-stereogenic center. Mechanistic studies, including the isolation and structural characterization of a key B–H–Ni bonded intermediate, control experiments, and DFT calculations reveal a σ-coordination enabled B(sp3)–H activation. The σ-coordination induces an oxidative metalation, simultaneously forming B–Ni, C–Ni and C–H bonds. The C
CH–BH2L (where L is a dative donor). This protocol enables the efficient synthesis of versatile vinylborane derivatives featuring a boron-stereogenic center. Mechanistic studies, including the isolation and structural characterization of a key B–H–Ni bonded intermediate, control experiments, and DFT calculations reveal a σ-coordination enabled B(sp3)–H activation. The σ-coordination induces an oxidative metalation, simultaneously forming B–Ni, C–Ni and C–H bonds. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C unit in the vinylborane reagent plays a pivotal role in facilitating the otherwise challenging oxidative metalation of the B(sp3)–H bond. This unique B(sp3)–H metalation mode may have broader implications for achieving other forms of selective B–H functionalization.
C unit in the vinylborane reagent plays a pivotal role in facilitating the otherwise challenging oxidative metalation of the B(sp3)–H bond. This unique B(sp3)–H metalation mode may have broader implications for achieving other forms of selective B–H functionalization.
Introduction
Organoboron compounds are finding increasingly valuable applications in synthetic chemistry, materials science, and drug discovery. Notably, due to the unique interactions between borane motifs and bioactive centers, five boron-based drugs have been approved by the FDA, and more candidates are under clinical trials (Fig. 1a).1–3 Furthermore, boron-containing units such as azaborines4–6 and carboranes7,8 serve as benzene analogues to enhance or alter the bioactivity of the lead drug molecules. Given these advancements, it remains a compelling goal to enhance the structural and functional complexity of organoboron molecules. Despite significant progress, challenges persist in the synthesis of complex organoboranes, particularly in constructing boron-stereogenic skeletons.9,10 To address these challenges and expand the chemical space of organoboranes, design and development of a versatile reagent would be an ideal solution.In view of the inherent versatility of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–B units, vinylboron reagents containing both active centers have been developed and proven to be powerful synthons for organoborane synthesis and organic transformations (Fig. 1b).11–19 These reagents typically undergo three main reaction modes, including (1) C
C and C–B units, vinylboron reagents containing both active centers have been developed and proven to be powerful synthons for organoborane synthesis and organic transformations (Fig. 1b).11–19 These reagents typically undergo three main reaction modes, including (1) C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond modifications,20–30 (2) C–B bond transformations,13,31–34 and (3) 1,2-migrations of “boron-ate” complexes.35–39 However, functionalization at the boron center assisted by the C
C double bond modifications,20–30 (2) C–B bond transformations,13,31–34 and (3) 1,2-migrations of “boron-ate” complexes.35–39 However, functionalization at the boron center assisted by the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C motif in vinylboron reagents remains unexplored, despite its potential to access boron-stereogenic compounds. In this connection, we have designed a bench-stable vinylborane reagent, CH2
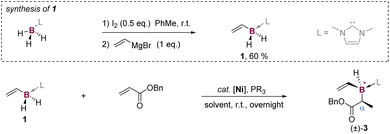
C motif in vinylboron reagents remains unexplored, despite its potential to access boron-stereogenic compounds. In this connection, we have designed a bench-stable vinylborane reagent, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–BH2L (1, L is a dative donor). This reagent is hypothesized to undergo B(sp3)–H functionalization to generate a broad range of vinylborane derivatives distinguished by a chiral-at-boron center (Fig. 1b).9,10,40–44 For realizing effective B(sp3)–H modification, the activation strategy should be able to differentiate the B(sp3)–H site from other active centers in 1 and allow for precise selectivity control.
CH–BH2L (1, L is a dative donor). This reagent is hypothesized to undergo B(sp3)–H functionalization to generate a broad range of vinylborane derivatives distinguished by a chiral-at-boron center (Fig. 1b).9,10,40–44 For realizing effective B(sp3)–H modification, the activation strategy should be able to differentiate the B(sp3)–H site from other active centers in 1 and allow for precise selectivity control.
A promising approach for selective B–H derivatization involves transition metal catalysis,45–52 which has been widely applied in borylative transformations53–65 but remains relatively underdeveloped in B(sp3)–H activation.66–68 Pioneering studies by Weller,69 Chuzel and Parrain,43 and Shi70 demonstrated noble metal catalysts (e.g., Rh, Au) for B(sp3)–H functionalization, but successful examples remain scarce. This difficulty arises because B(sp3)–H bonds are electron-rich, weakly polarized, and lack an empty p-orbital on boron, making their oxidative addition on transition metals challenging compared to B(sp2)–H bonds. Interestingly, several studies document σ-coordination of B(sp3)–H bonds to transition metal centers, which weakens the B(sp3)–H bond and may facilitate its activation.71–75 Building on this premise, we have developed a nickel-catalyzed diastereoselective hydroboration of olefins using the vinylborane reagent (Fig. 1c). The successful isolation and structural characterization of a key B–H–Ni bonded intermediate, along with control experiments and DFT calculations, reveal a unique σ-coordination enabled oxidative metalation that simultaneously generates B–Ni, C–Ni and C–H bonds while cleaving the B–H bond and olefin π bond. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C unit in the vinylborane reagent plays a crucial role in facilitating the key oxidative metalation of the B(sp3)–H bond.
C unit in the vinylborane reagent plays a crucial role in facilitating the key oxidative metalation of the B(sp3)–H bond.
Results and discussion
In the presence of NiBr2(PPh3)2 as a catalyst, reaction of the vinylborane reagent 1 with benzyl acrylate in dioxane at room temperature overnight gave the coupling product 3 in 80% yield with a diastereomeric ratio (dr) of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (entry 1, Table 1). Using NiCl2 as the catalyst without a phosphine ligand provided a comparable yield of 75%, but with a significantly reduced dr of 1.1
1 (entry 1, Table 1). Using NiCl2 as the catalyst without a phosphine ligand provided a comparable yield of 75%, but with a significantly reduced dr of 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (entry 2, Table 1). We then screened a series of ligands to improve the dr of product 3 (Table 1 and S1b in the ESI†). Mono-phosphine ligands, P(p-F–Ph)3 and P(p-Cl–Ph)3, proved optimal, leading to excellent drs of > 20
1 (entry 2, Table 1). We then screened a series of ligands to improve the dr of product 3 (Table 1 and S1b in the ESI†). Mono-phosphine ligands, P(p-F–Ph)3 and P(p-Cl–Ph)3, proved optimal, leading to excellent drs of > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The most electron-deficient P(C6F5)3, electron-rich PCy3, or bidentate phosphine ligands offered low drs ranging from 1
1. The most electron-deficient P(C6F5)3, electron-rich PCy3, or bidentate phosphine ligands offered low drs ranging from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2
1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (entries 7 and 9, Tables 1 and S1b in the ESI†). Using less polar toluene as the solvent increased the yield to 97% without compromising the dr (entry 12, Table 1). The employment of Ni(COD)2 instead of NiCl2 resulted in a decreased yield of 84% with a reduced dr of 16
1 (entries 7 and 9, Tables 1 and S1b in the ESI†). Using less polar toluene as the solvent increased the yield to 97% without compromising the dr (entry 12, Table 1). The employment of Ni(COD)2 instead of NiCl2 resulted in a decreased yield of 84% with a reduced dr of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (entry 13, Table 1). Reducing the loadings of NiCl2 and P(p-F–Ph)3 to 5 mol% and 12.5 mol%, respectively, did not affect the reaction efficiency (entry 14, Table 1) and was therefore chosen as the optimal reaction condition. Replacing the vinylborane reagent 1 with NHC–BH3 or NHC–BH2Ph failed to give the target product (Section 4.4 in the ESI†), highlighting the important role of the “C
1 (entry 13, Table 1). Reducing the loadings of NiCl2 and P(p-F–Ph)3 to 5 mol% and 12.5 mol%, respectively, did not affect the reaction efficiency (entry 14, Table 1) and was therefore chosen as the optimal reaction condition. Replacing the vinylborane reagent 1 with NHC–BH3 or NHC–BH2Ph failed to give the target product (Section 4.4 in the ESI†), highlighting the important role of the “C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C” motif in 1 in facilitating B–H metalation/activation. In contrast, the previously developed boryl radical strategy proved to give β-borylation products with poor diastereoselectivity (Fig. S14†).66,76–80
C” motif in 1 in facilitating B–H metalation/activation. In contrast, the previously developed boryl radical strategy proved to give β-borylation products with poor diastereoselectivity (Fig. S14†).66,76–80
| Entry | [Ni] (10 mol%) | PR3 (25 mol%) | Solvent | Yieldb (%) | d r |
|---|---|---|---|---|---|
| a Reactions were conducted at 0.05 mmol scale in 0.5 mL of solvent in a closed flask. b Yields and drs were determined by 1H NMR with 1,3,5-trimethoxybenzene as an internal standard. c 5 mol% NiCl2 and 12.5 mol% P(p-F–Ph)3 were used, isolated yield in parentheses. | |||||
| 1 | NiBr2(PPh3)2 | — | Dioxane | 80 | 8/1 |
| 2 | NiCl2 | — | Dioxane | 75 | 1.1/1 |
| 3 | NiCl2 | PPh3 | Dioxane | 88 | 19/1 |
| 4 | NiCl2 | P(p-F–Ph)3 | Dioxane | 92 | >20/1 |
| 5 | NiCl2 | P(p-Cl–Ph)3 | Dioxane | 88 | >20/1 |
| 6 | NiCl2 | P(p-OMe–Ph)3 | Dioxane | 89 | 18/1 |
| 7 | NiCl2 | P(C6F5)3 | Dioxane | 72 | 1.1/1 |
| 8 | NiCl2 | P(p-Me–Ph)3 | Dioxane | 91 | 12/1 |
| 9 | NiCl2 | PCy3 | Dioxane | 42 | 2/1 |
| 10 | NiCl2 | P(p-F–Ph)3 | MeCN | 37 | 11/1 |
| 11 | NiCl2 | P(p-F–Ph)3 | DMA | 87 | 14/1 |
| 12 | NiCl2 | P(p-F–Ph)3 | Toluene | >95 | >20/1 |
| 13 | Ni(COD)2 | P(p-F–Ph)3 | Toluene | 84 | 16/1 |
| 14 | NiCl 2 | P(p-F–Ph) 3 | Toluene | >95 (97) | >20/1 |
To gain insights into the reaction mechanism, control experiments were conducted. Treatment of 1, Ni(COD)2, and P(p-F–Ph)3 in THF at room temperature for 6 h produced a B–H–Ni bonded complex INT1 in 60% isolated yield (Fig. 2a). The 1H NMR spectrum of INT1 exhibited a significantly up-field peak at −0.88 ppm (Fig. 2c). Its assignment to one BH was supported by the subsequent 11B–1H HSQC analysis, with another BH being detected at 0.66 ppm (Fig. 2c). The B(sp3)–H coordination mode was further verified by the single-crystal structure of INT1 (Fig. 2a). Upon hydrogen addition through modeling, the bond length of the B(sp3)–H σ-coordinated to the Ni center was measured as 1.29 Å, longer than the 1.13 Å length measured for the free B(sp3)–H bond. Moreover, the distance between the B and Ni centers was found to be 2.28 Å, suggesting a potential interaction. A combination of the NMR and X-ray diffraction findings indicated (1) the σ-coordination of one B(sp3)–H in the vinylborane reagent to the Ni center and (2) the significant activation of this B(sp3)–H bond by Ni. Such a bonding and activation mode differs from the deprotonation or C–H metalation reaction observed in forming transition metal-allylic complexes,81,82 probably due to the distinct electronic nature of B(sp3)–H (hydridic) versus C–H (protonic). Compound INT1 reacted with ethyl (E)-2-hexenoate under the standard conditions to deliver 41 in 21% yield with a dr of over 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 2b). It was also proved effective as a catalyst in the hydroboration reaction (Fig. 2b). These results support the role of INT1 as a key intermediate involved in the reaction pathway.
1 (Fig. 2b). It was also proved effective as a catalyst in the hydroboration reaction (Fig. 2b). These results support the role of INT1 as a key intermediate involved in the reaction pathway.
DFT calculations were carried out to gain insight into the reaction mechanism. Fig. 2g shows the energy profile calculated. Starting from INT1 (using its single crystal structure for geometry optimization), a ligand substitution of benzyl acrylate for P(p-F–Ph)3 leads to the formation of INT2, from which the crucial oxidative metalation occurs, establishing B–Ni, C–Ni, and C–H bonds in INT3 concurrently, while cleaving a B–H bond and benzyl acrylate π bond. The energy barrier for this metalation step is 19.9 kcal mol−1, being the highest in the calculated pathway (Fig. 2g). Subsequently, INT3 undergoes reductive elimination, followed by product release to deliver the final product and regenerate INT1. Diastereoselectivity control is closely associated with the rate-determining step. Therefore, optimization was conducted for the four diastereomers of TS1, originating from three chiral B, Ni, and C (bonded with the ester group) centers (Fig. S15 in the ESI†). Among them, the diastereomer with the least steric repulsion between the olefin substrate and the phosphine ligand was most favored (Fig. 2g), leading to product 3 with a configuration identical to experimental observations (see single crystal structures of 4 and 21 in Fig. 3).
The deuterated vinylborane reagent 1-D was prepared and reacted with 2-furanone to yield 49-D, wherein one hydrogen of the β-CH2, with respect to boron, was deuterated at a rate of 67% (Fig. 2d). The addition of 1.5 equiv. of D2O into the reaction mixture did not cause any B–H or C–H deuteration (Fig. 2d). Competitive and parallel experiments using 1 and 1-D displayed significant kinetic isotope effect (KIE) with kH/kD ratios of 2.5 and 2.4, respectively (Fig. 2e). These experimental findings suggested that the B(sp3)–H cleavage might be involved in the rate-determining step, consistent with the computational results. Based on the above experiments and DFT calculations, a plausible reaction pathway is proposed in Fig. 2f.
The reaction mode of B(sp3)–H metalation is very similar to the documented ligand-to-ligand hydrogen transfer (LLHT) mechanism observed in C(sp2)–H nickelation.83,84 The difference lies in the bond strength between Ni–B(sp3) and Ni–C(sp2). The former one is believed to be relatively weak due to the lack of π-interaction between metal and the boron unit.85 Therefore, its formation via LLHT is involved in the rate-determining step.
We then explored the scope of various types of olefins. Terminal vinyl esters containing a diverse array of functional groups, including alkyl, trifluoromethyl, alkenyl, alkynyl, cyano, methoxyl, ether, halide, silyl, carbonyl, amide, ester, bpin, and pyridinyl groups, reacted with the vinylborane reagent smoothly (3–29, Fig. 3). Most reactions exhibited excellent diastereomeric ratios of >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Notably, carbonyl and pyridinyl substituents, which can coordinate to nickel, were well tolerated (21 and 29). The hydroboration occurred regioselectively at the relatively electron-poor C
1. Notably, carbonyl and pyridinyl substituents, which can coordinate to nickel, were well tolerated (21 and 29). The hydroboration occurred regioselectively at the relatively electron-poor C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C site, when the substrates involved multiple unsaturated functionalities (10–14). The ferrocene-containing substrate worked well (30), while the cyclobutylamino substituent proved compatible (31). A series of aryl acrylates were evaluated, giving 32–38 in moderate to excellent isolated yields, yet with relatively low diastereomeric ratios of around 10
C site, when the substrates involved multiple unsaturated functionalities (10–14). The ferrocene-containing substrate worked well (30), while the cyclobutylamino substituent proved compatible (31). A series of aryl acrylates were evaluated, giving 32–38 in moderate to excellent isolated yields, yet with relatively low diastereomeric ratios of around 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Alkyl thioacrylate was also an effective coupling partner, delivering product 39.
1. Alkyl thioacrylate was also an effective coupling partner, delivering product 39.
A series of internal olefins were subsequently evaluated as substrates. Various functional groups including piperidine, tetrahydropyrane, nerolin, and phthalimide were well tolerated (42–45). The substrate featuring a cyclopropylethene motif underwent this hydroboration effectively to afford 46, where the cyclopropyl ring remained intact, negating the involvement of a radical pathway. In addition to 1,2-disubstituted olefins, the 1,1′-disubstituted one also worked well (50), constructing a quaternary C–B(sp3) bond. Tri-substituted olefins afforded the corresponding products (51, 53–55) effectively, albeit with compromised diastereomeric ratios. However, the tetra-substituted olefin failed to react with the vinylborane reagent 1 (Section 4.4 in the ESI†). These results imply the influence of steric factors on the reaction efficiency and diastereoselectivity. Less bulky cyclic olefins were compatible, yielding 49 and 52 with diastereomeric ratios of >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
This protocol also enabled the straightforward introduction of a versatile vinylborane motif into a broad array of bioactive molecules, including derivatives of commercially available medicines (Fig. 4a): diclofenac (61, anti-inflammatory), probenecid (62, for treating gout and hyperuricemia), febuxostat (63, for treating gout), telmisartan (66, for treating high blood pressure, heart failure, and diabetic nephropathy), ciprofibrate (68, a hypolipidemic agent), and indometacin (65 and 67, anti-inflammatory). Additionally, cholesterol (64), estrone (60), Boc-Ser-OMe (59, an amino acid), diacetone-D-glucose (58, a pharmaceutical intermediate), menthol (57, anti-irritation), isoborneol (56, antiviral), and butenolide (69, a marine drug) derivatives reacted well with the vinylborane reagent, achieving good to excellent diastereomeric ratios. These findings highlight the remarkable compatibility and promising potential of this nickel-catalytic protocol.
This nickel-catalyzed hydroboration was conveniently scaled up to give 3 and 50 in 97% and 72% yields, respectively (Fig. 4b). The introduced vinylborane motif is anticipated to be versatile for further molecular modifications, alike the previously reported vinylboron fragments. Treatment of 3 with in situ generated [Fe]–H species86,87 delivered a new α-boryl(sp3) radical, which effectively attacked olefins or diazo compounds to give 72–74 and 70–71, respectively. The ester groups in 3 and 50 were conveniently reduced to give 75 and 76. Attempts to construct a four-membered oxaboracycle from 76via Brønsted acid catalyzed B–H/O–H dehydrogenative coupling failed. However, an unexpected product 78 was isolated and characterized (see Fig. S3 in the ESI†). A plausible pathway including a sequence of borenium-induced alkenyl metathesis, nucleophilic 1,2-boryl shift, and carbocation hydridation was proposed accordingly (see Fig. 4b and S4 in the ESI†). Reaction of 50 with the diazo compound upon visible light irradiation yielded 77 bearing a bicyclo[3.1.0] ring,62 rather than the B–H insertion species.58
Conclusions
In summary, we have designed a new vinylborane reagent that permits the facile modification at the boron center via B(sp3)–H bond functionalization. The developed nickel catalytic protocol demonstrates advantages of remarkable diastereoselectivity control, good compatibility, and mild operation conditions. The isolation and structural characterization of a B–H–Ni bonded intermediate, along with DFT calculations, underscore the important bidentate coordination of the vinylborane reagent to the nickel center. This coordination induces a unique oxidative metalation, distinct from the metalation modes observed for allylic C–H bonds. The potential of this vinylborane reagent in synthetic chemistry awaits further research efforts.Data availability
Crystallographic data for INT1, 4, 21 have been deposited at the CCDC under 2381595, 2381592, 2381593 and can be obtained from https://www.ccdc.cam.ac.uk/. Detailed synthetic procedures, characterization of boranes, and mechanistic investigations supporting this article have been uploaded as ESI.†Author contributions
G. H., X. W., and Y. Q. conceived and designed the experiments. G. H., P. Z., X. W., C. W., and Y. F. performed experiments. C. W. and W. H. L. performed crystallization and structural resolution. P. Z. and Z. L. performed DFT calculations. G. H., P. Z., Z. L., and Y. Q. wrote the manuscript. Z. L. and Y. Q. directed the research.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the start-up funding (Project No. R9804 to Y. Q.) from the Hong Kong University of Science and Technology (HKUST), Early Career Scheme from the Research Grants Council of Hong Kong (Project Number: 26307123 to Y. Q.), NSFC Young Scholar Fund (Project Number: NSFC23SC02 to Y. Q.), and the General Research Fund from the Research Grants Council of Hong Kong (HKUST 16302222 to Z. L.). Dr Herman Ho-Yung SUNG and Prof. Dr Ian Williams are acknowledged for X-ray crystallography. Dr Yu CAO, Prof. Dr Wan CHAN and Ms. Wing-Laam LUK are acknowledged for HRMS test.Notes and references
- R. J. Grams, W. L. Santos, I. R. Scorei, A. Abad-García, C. A. Rosenblum, A. Bita, H. Cerecetto, C. Viñas and M. A. Soriano-Ursúa, The Rise of Boron-Containing Compounds: Advancements in Synthesis, Medicinal Chemistry, and Emerging Pharmacology, Chem. Rev., 2024, 124, 2441–2511 CrossRef CAS PubMed.
- S. X. Tian, H.-B. Li and J. Yang, Monoanion BH4− Can Stabilize Zwitterionic Glycine with Dihydrogen Bonds, ChemPhysChem, 2009, 10, 1435–1437 CrossRef CAS PubMed.
- A. Prester, M. Perbandt, M. Galchenkova, D. Oberthuer, N. Werner, A. Henkel, J. Maracke, O. Yefanov, J. Hakanpää, G. Pompidor, J. Meyer, H. Chapman, M. Aepfelbacher, W. Hinrichs, H. Rohde and C. Betzel, Time-resolved crystallography of boric acid binding to the active site serine of the β-lactamase CTX-M-14 and subsequent 1,2-diol esterification, Commun. Chem., 2024, 7, 152 CrossRef CAS PubMed.
- Z. X. Giustra and S.-Y. Liu, The State of the Art in Azaborine Chemistry: New Synthetic Methods and Applications, J. Am. Chem. Soc., 2018, 140, 1184–1194 CrossRef CAS PubMed.
- A. Bhattacharjee, G. H. M. Davies, B. Saeednia, S. R. Wisniewski and G. A. Molander, Selectivity in the Elaboration of Bicyclic Borazarenes, Adv. Synth. Catal., 2021, 363, 2256–2273 CrossRef CAS PubMed.
- J. J. Blackner, O. M. Schneider, W. O. Wong and D. G. Hall, Removing Neighboring Ring Influence in Monocyclic B–OH Diazaborines: Properties and Reactivity as Phenolic Bioisosteres with Dynamic Hydroxy Exchange, J. Am. Chem. Soc., 2024, 146, 19499–19508 CrossRef CAS PubMed.
- P. Stockmann, M. Gozzi, R. Kuhnert, M. B. Sárosi and E. Hey-Hawkins, New keys for old locks: carborane-containing drugs as platforms for mechanism-based therapies, Chem. Soc. Rev., 2019, 48, 3497–3512 RSC.
- A. Marfavi, P. Kavianpour and L. M. Rendina, Carboranes in drug discovery, chemical biology and molecular imaging, Nat. Rev. Chem., 2022, 6, 486–504 CrossRef PubMed.
- A. Abdou-Mohamed, C. Aupic, C. Fournet, J.-L. Parrain, G. Chouraqui and O. Chuzel, Stereoselective formation of boron-stereogenic organoboron derivatives, Chem. Soc. Rev., 2023, 52, 4381–4391 RSC.
- X. Li, G. Zhang and Q. Song, Recent advances in the construction of tetracoordinate boron compounds, Chem. Commun., 2023, 59, 3812–3820 RSC.
- Y.-L. Feng, B.-W. Zhang, Y. Xu, S. Jin, D. Mazzarella and Z.-Y. Cao, The reactivity of alkenyl boron reagents in catalytic reactions: recent advances and perspectives, Org. Chem. Front., 2024, 11, 7249–7277 RSC.
- J. Carreras, A. Caballero and P. J. Perez, Alkenyl Boronates: Synthesis and Applications, Chem Asian J., 2019, 14, 329–343 CrossRef CAS PubMed.
- G. A. Molander and N. Ellis, Organotrifluoroborates: protected boronic acids that expand the versatility of the Suzuki coupling reaction, Acc. Chem. Res., 2007, 40, 275–286 CrossRef CAS PubMed.
- T. Nishikawa, Radical polymerization of alkenyl boronates and C–B bond transformation: polymer synthesis through side-chain replacement for overcoming synthetic limitations, Polym. J., 2024, 56, 873–886 CrossRef CAS.
- B. E. Uno, E. P. Gillis and M. D. Burke, Vinyl MIDA boronate: a readily accessible and highly versatile building block for small molecule synthesis, Tetrahedron, 2009, 65, 3130–3138 CrossRef CAS.
- C. Jiang and D. W. Stephan, Hydrophosphination of vinyl-boranes with phosphinoamines, Dalton Trans., 2013, 42, 3318–3325 RSC.
- V. Fasano, J. Cid, R. J. Procter, E. Ross and M. J. Ingleson, Selective Boryl-Anion Migration in a Vinyl sp(2) -sp(3) Diborane Induced by Soft Borane Lewis Acids, Angew. Chem., Int. Ed., 2018, 57, 13293–13297 CrossRef CAS PubMed.
- M. Shimoi, T. Watanabe, K. Maeda, D. P. Curran and T. Taniguchi, Radical trans-Hydroboration of Alkynes with N-Heterocyclic Carbene Boranes, Angew. Chem., Int. Ed., 2018, 57, 9485–9490 CrossRef CAS PubMed.
- A. Marotta, C. E. Adams and J. J. Molloy, The Impact of Boron Hybridisation on Photocatalytic Processes, Angew. Chem., Int. Ed., 2022, 61, e202207067 CrossRef CAS PubMed.
- K. Sasaki and T. Hayashi, Rhodium-catalyzed asymmetric conjugate addition of arylboroxines to borylalkenes: asymmetric synthesis of beta-arylalkylboranes, Angew. Chem., Int. Ed., 2010, 49, 8145–8147 CrossRef CAS PubMed.
- J. Carreras, A. Caballero and P. J. Perez, Enantio- and Diastereoselective Cyclopropanation of 1-Alkenylboronates: Synthesis of 1-Boryl-2,3-Disubstituted Cyclopropanes, Angew. Chem., Int. Ed., 2018, 57, 2334–2338 CrossRef CAS PubMed.
- A. Noble, R. S. Mega, D. Pflasterer, E. L. Myers and V. K. Aggarwal, Visible-Light-Mediated Decarboxylative Radical Additions to Vinyl Boronic Esters: Rapid Access to gamma-Amino Boronic Esters, Angew. Chem., Int. Ed., 2018, 57, 2155–2159 CrossRef CAS PubMed.
- J. Levi Knippel, A. Z. Ni, A. W. Schuppe and S. L. Buchwald, A General Strategy for the Asymmetric Preparation of alpha-Stereogenic Allyl Silanes, Germanes, and Boronate Esters via Dual Copper Hydride- and Palladium-Catalysis, Angew. Chem., Int. Ed., 2022, 61, e202212630 CrossRef CAS PubMed.
- J. C. Lee, R. McDonald and D. G. Hall, Enantioselective preparation and chemoselective cross-coupling of 1,1-diboron compounds, Nat. Chem., 2011, 3, 894–899 CrossRef CAS PubMed.
- S. Bera, C. Fan and X. Hu, Enantio- and diastereoselective construction of vicinal C(sp3) centres via nickel-catalysed hydroalkylation of alkenes, Nat. Catal., 2022, 5, 1180–1187 CrossRef CAS.
- L. Liu, M. C. Aguilera, W. Lee, C. R. Youshaw, M. L. Neidig and O. Gutierrez, General method for iron-catalyzed multicomponent radical cascades-cross-couplings, Science, 2021, 374, 432–439 CrossRef CAS PubMed.
- M. L. Lepage, S. Lai, N. Peressin, R. Hadjerci, B. O. Patrick and D. M. Perrin, Direct Access to MIDA Acylboronates through Mild Oxidation of MIDA Vinylboronates, Angew. Chem., Int. Ed., 2017, 56, 15257–15261 CrossRef CAS PubMed.
- Y. F. Zeng, W. W. Ji, W. X. Lv, Y. Chen, D. H. Tan, Q. Li and H. Wang, Stereoselective Direct Chlorination of Alkenyl MIDA Boronates: Divergent Synthesis of E and Z alpha-Chloroalkenyl Boronates, Angew. Chem., Int. Ed., 2017, 56, 14707–14711 CrossRef CAS PubMed.
- J. J. Molloy, M. Schäfer, M. Wienhold, T. Morack, C. G. Daniliuc and R. Gilmour, Boron-enabled geometric isomerization of alkenes via selective energy-transfer catalysis, Science, 2020, 369, 302–306 CrossRef CAS PubMed.
- N. Hanania, N. Eghbarieh and A. Masarwa, PolyBorylated Alkenes as Energy-Transfer Reactive Groups: Access to Multi-Borylated Cyclobutanes Combined with Hydrogen Atom Transfer Event, Angew. Chem., Int. Ed., 2024, 63, e202405898 CrossRef CAS PubMed.
- S. Liao, A. Porta, X. Cheng, X. Ma, G. Zanoni and L. Zhang, Bifunctional Ligand Enables Efficient Gold-Catalyzed Hydroalkenylation of Propargylic Alcohol, Angew. Chem., Int. Ed., 2018, 57, 8250–8254 CrossRef CAS PubMed.
- A. J. Lennox and G. C. Lloyd-Jones, Selection of boron reagents for Suzuki-Miyaura coupling, Chem. Soc. Rev., 2014, 43, 412–443 RSC.
- H. Kim and D. W. MacMillan, Enantioselective organo-SOMO catalysis: the alpha-vinylation of aldehydes, J. Am. Chem. Soc., 2008, 130, 398–399 CrossRef CAS PubMed.
- Z. He and A. K. Yudin, Amphoteric alpha-boryl aldehydes, J. Am. Chem. Soc., 2011, 133, 13770–13773 CrossRef CAS PubMed.
- S. Namirembe and J. P. Morken, Reactions of organoboron compounds enabled by catalyst-promoted metalate shifts, Chem. Soc. Rev., 2019, 48, 3464–3474 RSC.
- M. Silvi, C. Sandford and V. K. Aggarwal, Merging Photoredox with 1,2-Metallate Rearrangements: The Photochemical Alkylation of Vinyl Boronate Complexes, J. Am. Chem. Soc., 2017, 139, 5736–5739 CrossRef CAS PubMed.
- L. Zhang, G. J. Lovinger, E. K. Edelstein, A. A. Szymaniak, M. P. Chierchia and J. P. Morken, Catalytic conjunctive cross-coupling enabled by metal-induced metallate rearrangement, Science, 2016, 351, 70–74 CrossRef CAS PubMed.
- M. Kischkewitz, K. Okamoto, C. Mück-Lichtenfeld and A. Studer, Radical-polar crossover reactions of vinylboron ate complexes, Science, 2017, 355, 936–938 CrossRef CAS PubMed.
- H. Wang, C. Jing, A. Noble and V. K. Aggarwal, Stereospecific 1,2-Migrations of Boronate Complexes Induced by Electrophiles, Angew. Chem., Int. Ed., 2020, 59, 16859–16872 CrossRef CAS PubMed.
- C. Aupic, A. Abdou Mohamed, C. Figliola, P. Nava, B. Tuccio, G. Chouraqui, J.-L. Parrain and O. Chuzel, Highly diastereoselective preparation of chiral NHC-boranes stereogenic at the boron atom, Chem. Sci., 2019, 10, 6524–6530 RSC.
- B. Zu, Y. Guo and C. He, Catalytic EnantioselectivConstruction of Chiroptical Boron-Stereogenic Compounds, J. Am. Chem. Soc., 2021, 143, 16302–16310 CrossRef CAS PubMed.
- G. Zhang, Z. Zhang, M. Hou, X. Cai, K. Yang, P. Yu and Q. Song, Construction of boron-stereogenic compounds via enantioselective Cu-catalyzed desymmetric B–H bond insertion reaction, Nat. Commun., 2022, 13, 2624 CrossRef CAS PubMed.
- M. Toure, O. Chuzel and J.-L. Parrain, Asymmetric Rhodium-Directed anti-Markovnikov Regioselective Boracyclopentannulation, J. Am. Chem. Soc., 2012, 134, 17892–17895 CrossRef CAS PubMed.
- N. M. Ankudinov, N. V. Alexeev, E. S. Podyacheva, D. A. Chusov, K. A. Lyssenko and D. S. Perekalin, Catalytic insertion of nitrenes into B–H bonds, Chem. Sci., 2025, 16, 6298–6306 RSC.
- D. A. Evans and G. C. Fu, Amide-directed, iridium-catalyzed hydroboration of olefins: documentation of regio- and stereochemical control in cyclic and acyclic systems, J. Am. Chem. Soc., 1991, 113, 4042–4043 CrossRef CAS.
- J. F. Hartwig, K. S. Cook, M. Hapke, C. D. Incarvito, Y. Fan, C. E. Webster and M. B. Hall, Rhodium Boryl Complexes in the Catalytic, Terminal Functionalization of Alkanes, J. Am. Chem. Soc., 2005, 127, 2538–2552 CrossRef CAS PubMed.
- W. J. Jang, S. M. Song, J. H. Moon, J. Y. Lee and J. YunCopper-Catalyzed, Enantioselective Hydroboration of Unactivated 1,1-Disubstituted Alkenes, J. Am. Chem. Soc., 2017, 139, 13660–13663 CrossRef CAS PubMed.
- W. Su, J. Zhu, Y. Chen, X. Zhang, W. Qiu, K. Yang, P. Yu and Q. Song, Copper-catalysed asymmetric hydroboration of alkenes with 1,2-benzazaborines to access chiral naphthalene isosteres, Nat. Chem., 2024, 16, 1312–1319 CrossRef CAS PubMed.
- S. J. Geier, C. M. Vogels, J. A. Melanson and S. A. Westcott, The transition metal-catalysed hydroboration reaction, Chem. Soc. Rev., 2022, 51, 8877–8922 RSC.
- Y. Quan and Z. Xie, Controlled functionalization of o-carborane via transition metal catalyzed B–H activation, Chem. Soc. Rev., 2019, 48, 3660–3673 RSC.
- L. Chen and S. J. S. Xu, Ligand-Enabled Regio-and/or Stereoselective Hydroboration of Alkenes, Synlett, 2023, 34, 2103–2109 CrossRef CAS.
- R. Bisht, C. Haldar, M. M. M. Hassan, M. E. Hoque, J. Chaturvedi and B. Chattopadhyay, Metal-catalysed C–H bond activation and borylation, Chem. Soc. Rev., 2022, 51, 5042–5100 RSC.
- Q.-Q. Cheng, S.-F. Zhu, Y.-Z. Zhang, X.-L. Xie and Q.-L. Zhou, Copper-Catalyzed B–H Bond Insertion Reaction: A Highly Efficient and Enantioselective C–B Bond-Forming Reaction with Amine–Borane and Phosphine–Borane Adducts, J. Am. Chem. Soc., 2013, 135, 14094–14097 CrossRef CAS PubMed.
- X. Li and D. P. Curran, Insertion of Reactive Rhodium Carbenes into Boron–Hydrogen Bonds of Stable N-Heterocyclic Carbene Boranes, J. Am. Chem. Soc., 2013, 135, 12076–12081 CrossRef CAS PubMed.
- A. Yamamoto and M. Suginome, Nickel-Catalyzed trans-Alkynylboration of Alkynes via Activation of a Boron−Chlorine Bond, J. Am. Chem. Soc., 2005, 127, 15706–15707 CrossRef CAS PubMed.
- T. Furukawa, M. Tobisu and N. Chatani, Nickel-catalyzed borylation of arenes and indoles via C–H bond cleavage, Chem. Commun., 2015, 51, 6508–6511 RSC.
- C. F. Meng, B. B. Zhang, Q. Liu, K. Q. Chen, Z. X. Wang and X. Y. Chen, Achieving Nickel-Catalyzed Reductive C(sp(2))-B Coupling of Bromoboranes via Reversing the Activation Sequence, J. Am. Chem. Soc., 2024, 146, 7210–7215 CrossRef CAS PubMed.
- L. Tendera, F. Fantuzzi, T. B. Marder and U. Radius, Nickel boryl complexes and nickel-catalyzed alkyne borylation, Chem. Sci., 2023, 14, 2215–2228 RSC.
- C.-L. Ji, H. Chen, Q. Gao, J. Han, W. Li and J. Xie, Dinuclear gold-catalyzed divergent dechlorinative radical borylation of gem-dichloroalkanes, Nat. Commun., 2024, 15, 3721 CrossRef CAS PubMed.
- H. Lyu, I. Kevlishvili, X. Yu, P. Liu and G. Dong, Boron insertion into alkyl ether bonds via zinc/nickel tandem catalysis, Science, 2021, 372, 175–182 CrossRef CAS PubMed.
- X. Yang, C. Yuan and S. Ge, Ligand-enabled stereodivergence in nickel-catalyzed regioselective hydroboration of internal allenes, Chem, 2023, 9, 198–215 CAS.
- Z.-L. Chen, C. Empel, K. Wang, P.-P. Wu, B.-G. Cai, L. Li, R. M. Koenigs and J. Xuan, Enabling Cyclopropanation Reactions of Imidazole Heterocycles via Chemoselective Photochemical Carbene Transfer Reactions of NHC-Boranes, Org. Lett., 2022, 24, 2232–2237 CrossRef CAS PubMed.
- W. Srimontree, L. Guo and M. Rueping, Hydride Transfer Enables the Nickel-Catalyzed ipso-Borylation and Silylation of Aldehydes, Chem. Eur. J., 2020, 26, 423–427 CrossRef CAS PubMed.
- J. Hu, M. Ferger, Z. Shi and T. B. Marder, Recent advances in asymmetric borylation by transition metal catalysis, Chem. Soc. Rev., 2021, 50, 13129–13188 RSC.
- S. K. Bose, L. Mao, L. Kuehn, U. Radius, J. Nekvinda, W. L. Santos, S. A. Westcott, P. G. Steel and T. B. Marder, First-Row d-Block Element-Catalyzed Carbon-Boron Bond Formation and Related Processes, Chem. Rev., 2021, 121, 13238–13341 CrossRef CAS PubMed.
- T. Y. Peng, F. L. Zhang and Y. F. Wang, Lewis Base-Boryl Radicals Enabled Borylation Reactions and Selective Activation of Carbon-Heteroatom Bonds, Acc. Chem. Res., 2023, 56, 169–186 CrossRef CAS PubMed.
- X. Wang, P. Zhang, Z. Yang, W. Sun, H. Lyu, Z. Lin and Y. Quan, Synthesis of strained, air-stable boracycles via boron-carbon-centred diradicals, Nat. Chem., 2025, 17, 663–671 CrossRef CAS PubMed.
- Z. Zhu, W. C. Chan, B. Gao, G. Hu, P. Zhang, Y. Fu, K. S. Ly, Z. Lin and Y. Quan, Borenium-Catalyzed “Boron Walking” for Remote Site-Selective Hydroboration, J. Am. Chem. Soc., 2025, 147, 880–888 CrossRef PubMed.
- H. C. Johnson, C. L. McMullin, S. D. Pike, S. A. Macgregor and A. S. Weller, Dehydrogenative Boron Homocoupling of an Amine-Borane, Angew. Chem., Int. Ed., 2013, 52, 9776–9780 CrossRef CAS PubMed.
- Q. Wang, S. E. Motika, N. G. Akhmedov, J. L. Petersen and X. Shi, Synthesis of Cyclic Amine Boranes through Triazole-Gold(I)-Catalyzed Alkyne Hydroboration, Angew. Chem., Int. Ed., 2014, 53, 5418–5422 CrossRef CAS PubMed.
- K. B. Kolpin and D. J. H. Emslie, η3-Vinylborane Complexes of Platinum and Nickel: Borataallyl- and Alkyl/Borataalkene-Like Coordination Modes, Angew. Chem., Int. Ed., 2010, 49, 2716–2719 CrossRef CAS PubMed.
- D. J. H. Emslie, B. E. Cowie and K. B. Kolpin, Acyclic boron-containing π-ligand complexes: η2- and η3-coordination modes, Dalton Trans., 2012, 41, 1101–1117 RSC.
- S. Gomosta, K. Saha, U. Kaur, K. Pathak, T. Roisnel, A. K. Phukan and S. Ghosh, Hydroboration of Alkynes: η4-Alkene–Borane versus η4-E-Boratabutadiene, Inorg. Chem., 2019, 58, 9992–9997 CrossRef CAS PubMed.
- Y. Kawano, T. Yasue and M. Shimoi, BH Bond Activation of Trimethylphosphineborane by Transition Metal Complexes: Synthesis of Metal Complexes Bearing Nonsubstituted Boryl−Trimethylphosphine, Cp*M(CO)3(BH2·PMe3) (M = Mo, W), J. Am. Chem. Soc., 1999, 121, 11744–11750 CrossRef CAS.
- K. Saha, D. K. Roy, R. D. Dewhurst, S. Ghosh and H. Braunschweig, Recent Advances in the Synthesis and Reactivity of Transition Metal σ-Borane/Borate Complexes, Acc. Chem. Res., 2021, 54, 1260–1273 CrossRef CAS PubMed.
- H. Tao and H. Lyu, Functionalization of Boranes through Thiol/Oxygen Catalysis, Chin. J. Chem., 2024, 42, 2804–2810 CrossRef CAS.
- C. Zhu, J. Dong, X. Liu, L. Gao, Y. Zhao, J. Xie, S. Li and C. Zhu, Photoredox-Controlled beta-Regioselective Radical Hydroboration of Activated Alkenes with NHC-Boranes, Angew. Chem., Int. Ed., 2020, 59, 12817–12821 CrossRef CAS PubMed.
- P. J. Xia, D. Song, Z. P. Ye, Y. Z. Hu, J. A. Xiao, H. Y. Xiang, X. Q. Chen and H. Yang, Photoinduced Single-Electron Transfer as an Enabling Principle in the Radical Borylation of Alkenes with NHC-Borane, Angew. Chem., Int. Ed., 2020, 59, 6706–6710 CrossRef CAS PubMed.
- F. X. Li, X. Wang, J. Lin, X. Lou, J. Ouyang, G. Hu and Y. Quan, Selective multifunctionalization of N-heterocyclic carbene boranes via the intermediacy of boron-centered radicals, Chem. Sci., 2023, 14, 6341–6347 RSC.
- G. Li, G. Huang, R. Sun, D. P. Curran and W. Dai, Regioselective Radical Borylation of alpha,beta-Unsaturated Esters and Related Compounds by Visible Light Irradiation with an Organic Photocatalyst, Org. Lett., 2021, 23, 4353–4357 CrossRef CAS PubMed.
- G. Liu and Y. Wu, in C-H Activation, ed. J.-Q. Yu and Z. Shi, Springer Berlin Heidelberg, Berlin, Heidelberg, 2010, pp. 195–209, DOI:10.1007/128_2009_16.
- P.-S. Wang and L.-Z. Gong, Palladium-Catalyzed Asymmetric Allylic C–H Functionalization: Mechanism, Stereo- and Regioselectivities, and Synthetic Applications, Acc. Chem. Res., 2020, 53, 2841–2854 CrossRef CAS PubMed.
- J. S. Bair, Y. Schramm, A. G. Sergeev, E. Clot, O. Eisenstein and J. F. Hartwig, Linear-selective hydroarylation of unactivated terminal and internal olefins with trifluoromethyl-substituted arenes, J. Am. Chem. Soc., 2014, 136, 13098–13101 CrossRef CAS PubMed.
- S. Tang, O. Eisenstein, Y. Nakao and S. Sakaki, Aromatic C–H σ-Bond Activation by Ni0, Pd0, and Pt0 Alkene Complexes: Concerted Oxidative Addition to Metal vs. Ligand-to-Ligand H Transfer Mechanism, Organometallics, 2017, 36, 2761–2771 CrossRef CAS.
- Z. Ye, C. Y. Kwok, S. L. Lam, L. Wu and H. Lyu, Copper-Catalyzed C-B(sp(3)) Bond Formation through the Intermediacy of Cu-B(sp(3)) Complex, J. Am. Chem. Soc., 2025, 147, 14915–14923 CrossRef CAS PubMed.
- J. C. Lo, J. Gui, Y. Yabe, C. M. Pan and P. S. Baran, Functionalized olefin cross-coupling to construct carbon-carbon bonds, Nature, 2014, 516, 343–348 CrossRef CAS PubMed.
- J. Zheng, J. Qi and S. Cui, Fe-Catalyzed Olefin Hydroamination with Diazo Compounds for Hydrazone Synthesis, Org. Lett., 2016, 18, 128–131 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2381595, 2381592 and 2381593. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc02695d |
| This journal is © The Royal Society of Chemistry 2025 |