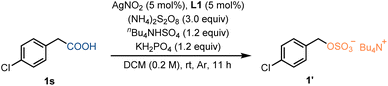Open Access Article
Open Access ArticleDecarboxylative sulfation by persulfates†
Zhen Xiaab,
Ting Denga,
Chunlan Song a and
Jiakun Li
a and
Jiakun Li *a
*a
aState Key Laboratory of Chemo and Biosensing, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, China. E-mail: jkli@hnu.edu.cn
bHunan Provincial Engineering Technology Research Center for Polyvinyl Alcohol Based New Functional Materials, College of Chemistry and Materials Engineering, Huaihua University, Huaihua 418000, China
First published on 29th May 2025
Abstract
Direct decarboxylative sulfation via C–O bond formation is an unexplored disconnection strategy for the synthesis of organosulfates, with the potential to overcome the significant limitations of O-sulfonation, which is restricted to hydroxyl-containing compounds. Reported here is a radical process for direct decarboxylative sulfation by persulfates. In this reaction, persulfates serve a dual role: acting as a versatile oxidant to generate carbon-centered radicals in decarboxylation, and providing an O–O source to facilitate the synthesis of organosulfates via C–OSO3− bond formation. This method enables the replacement of diverse carboxylic acids with ionizable organosulfate groups, which could be potential isosteres to improve molecules’ metabolic profiles.
Introduction
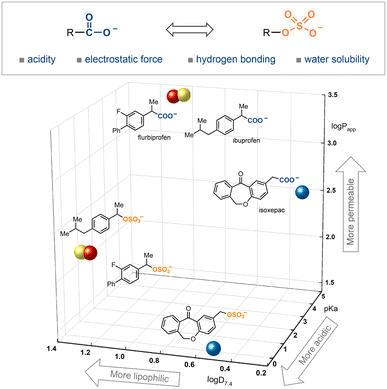
Carboxylic acids are prevalent motifs in natural products and pharmacologically active molecules. The acidity of this functional group, along with its ability to form strong electrostatic interactions and hydrogen bonds, makes it highly versatile.1 Consequently, the carboxylic acid group often plays a crucial role in drug–target interactions. Additionally, these characteristics suggest that carboxylic acid groups can impart relatively high water solubility, a critical attribute for a drug-like molecule. On occasion, the presence of this functional group in a drug or a drug candidate may result in adverse effects, such as metabolic instability, potential idiosyncratic toxicities and limited permeability across biological membranes.2 To mitigate one or more of these issues, medicinal chemists often resort to isosteric replacement or ester-prodrug strategies.3Organosulfates (–OSO3−), possessing similar physicochemical properties to carboxylic acids, may serve as potential isosteres (Scheme 1). They are also prevalent in various biological compounds, ranging from exogenous metabolites to post-translationally modified endogenous bioactive molecules.4 The incorporation of polar sulfate groups into target molecules can dramatically alter their solubility, acidity, electrostatic forces, and hydrogen bonding interactions. This modification has been implicated in the regulation of various biological and disease processes, including cell-signaling, molecular recognition, neurobiology, inflammation and cancer metastasis.5 The intrinsically anionic character of sulfates enhances their excretion properties and reduces potential toxicity.6 Considering the unique physicochemical properties between organosulfates and carboxylic acids, it is highly desirable to develop a method for the replacement of carboxylic acids with organosulfates to create improved drug derivatives, particularly in terms of pKa values, lipophilicity (i.e., log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4), and permeability coefficients (Papp).7
D7.4), and permeability coefficients (Papp).7
In contrast to the successful development of various decarboxylative functionalizations,8,9 the synthesis of organosulfates from carboxylic acids has not been reported. The primary challenge in this approach is the formation of the C–OSO3− bond. The weak nucleophilicity of the sulfate anion (SO42−) restricts C–OSO3− bond formation via a nucleophilic substitution pathway.10 Furthermore, the electron-withdrawing nature of the sulfate anion contributes substantially to the ionic character of the metal–sulfate bond, which increases the energy barrier for C–OSO3− reductive elimination in transition-metal-catalyzed cross-coupling reactions.11 A radical approach was alternatively employed to construct challenging C–O bonds.12 As a persistent radical trapping reagent, tetramethylpiperidine-1-oxyl (TEMPO˙) can form C–O bonds with carbon-centered radicals.13 This stable aminoxyl radical was recently investigated in frustrated Lewis pairs (FLPs) and applied to regioselective C–H oxygenation by Lin and co-workers.14 In 2020, Gooßen and co-workers reported an electrodecarboxylative etherification through a radical C–O coupling strategy; the success of this reaction is attributed to the facile oxidation of 1-hydroxybenzotriazole (HOBt) to form an O-centered radical (˙OBt).15
In addition, the formation of C–O bonds through the reaction of a carbon radical with an oxygenating reagent would be a more versatile synthetic method, as it allows for the use of a wide range of radical precursors.16 Malonoyl peroxide was utilized for aromatic C–H oxygenation by Tomkinson et al.,17 while bis(methanesulfonyl) peroxide has been reported by Ritter for late-stage C–O bond formation in arenes and benzylic C–H compounds.18 In 2024, our group reported benzylic C–H sulfation with persulfates, a classic oxidant that has been rarely employed for sulfate-transfer functions.19,20 This radical C–O bond formation exhibits distinct reactivity compared to previous O-sulfonation methods, which are a common way to access organosulfates via an O–S bond, and therein limited to hydroxyl substrates (Scheme 2A).21
In this study, our objective was to establish an efficient protocol for decarboxylative sulfation via a radical C–OSO3− bond, using persulfates as both oxidants and sulfating reagents (Scheme 2B). Persulfates initially act as the oxidant to generate carbon-centered radicals from decarboxylation, and then supply an O–O source to afford the organosulfates via C–OSO3− bond formation. Notably, the oxidative properties of persulfates and their roles as sulfate sources can be modulated by their positively charged counterions.19
Results and discussion
We initiated our investigation into the decarboxylative sulfation of 4-chlorophenylacetic acid (1s) with AgNO2 as the catalyst, 4,7-diphenyl-1,1-phenanthroline (L1) as the ligand, KH2PO4 as the base, and (NH4)2S2O8 serving as both the oxidant and sulfating agent. The desired sulfate 1 was obtained in a 69% isolated yield with nBu4NHSO4 as an additive (Table 1, entry 1). Substitution of the phenanthroline ligand with another variant still led to the desired product, albeit in a reduced yield (Table 1, entry 2). Use of bipyridine type ligands had a deleterious effect (Table 1, entries 3 and 4). The choice of ligand influenced the oxidation potential of the in situ conversion of the silver(I) carboxylate complex to a silver(II) carboxylate complex, which is crucial for the subsequent decarboxylation.22 Changing the reaction solvent from DCM to 1,2-dichloroethane resulted in a slight decrease in yield (Table 1, entry 5). In contrast, polar solvents led to either slower or no reaction (Table 1, entries 6–8). Persulfate salts K2S2O8 and Na2S2O8 were less productive (Table 1, entries 9 and 10). Moreover, when the additive nBu4NHSO4 was replaced with other ammonium salts in the reaction, a noticeable decrease in yield was observed (Table 1, entries 11 and 12). Control experiments confirmed the requirement of a silver catalyst, ligand, and ammonium salt additive in this decarboxylative sulfation protocol (Table 1, entries 13–16).| Entry | Variation from standard conditions | Yield of 1′ |
|---|---|---|
| a Standard reaction conditions: substrate (0.2 mmol), (NH4)2S2O8 (3.0 equiv.), AgNO2 (5 mol%), L1 = 4,7-diphenyl-1,1-phenanthroline (5 mol%), KH2PO4 (1.2 equiv.), nBu4NHSO4 (1.2 equiv.), DCM (0.2 M), Ar, rt, 11 h. Yields were determined by integration of the 1H NMR spectrum using dibromomethane as an internal standard.b Isolated yield. | ||
| 1 | None | 75% (69%)b |
| 2 | L2 as ligand | 49% |
| 3 | L3 as ligand | Trace |
| 4 | L4 as ligand | 6% |
| 5 | DCE as solvent | 64% |
| 6 | MeCN as solvent | 9% |
| 7 | 1,4-Dioxane as solvent | Trace |
| 8 | DMSO as solvent | Trace |
| 9 | K2S2O8 instead of (NH4)2S2O8 | 59% |
| 10 | Na2S2O8 instead of (NH4)2S2O8 | 47% |
| 11 | Et4NHSO4 instead of nBu4NHSO4 | 16% |
| 12 | nBu4NOAc instead of nBu4NHSO4 | 26% |
| 13 | Without AgNO2 | Trace |
| 14 | Without ligand | Trace |
| 15 | Without nBu4NHSO4 | Trace |
| 16 | (nBu4N)2S2O8 instead of (NH4)2S2O4, nBu4NHSO4 | 28% |
 |
||
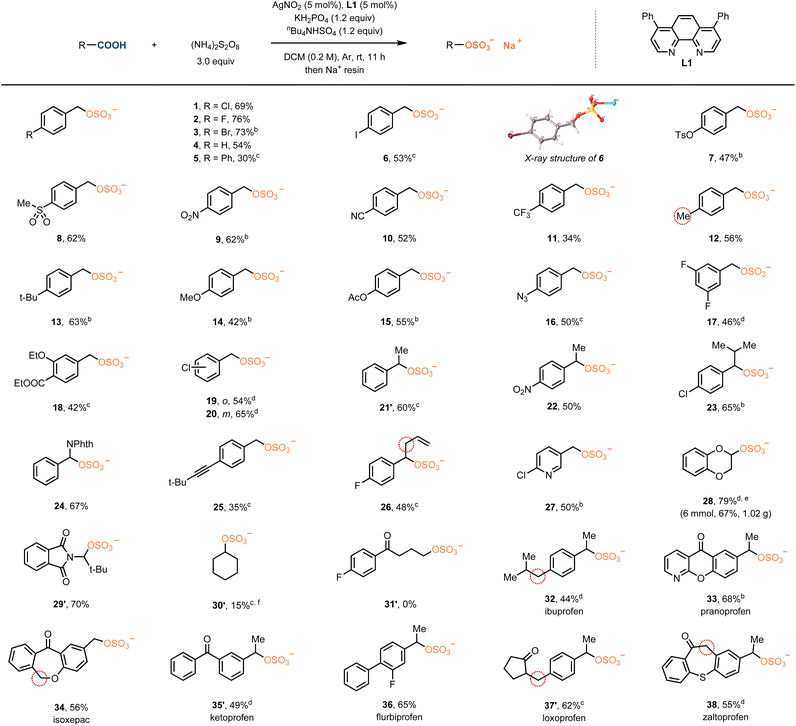
The optimized reaction conditions were then used to explore the substrate scope. As shown in Table 2, a diverse range of primary and secondary carboxylic acids, spanning from electron-withdrawing to electron-donating substituents on the arenes, were well-tolerated to give organosulfates in moderate to good isolated yields. Compatible functional groups include halide (2–3), tosyl (7), sulfonyl (8), nitro (9), nitrile (10), trifluoromethyl (11), alkoxyl (14), azide (16), ester (15) and aromatic heterocycle (27) groups. Oxidatively labile functional groups, such as alkyne (25) and alkene (26), are viable with this method. Substrates with two substituents (17, 18), or substitution at different positions on the aromatic ring (1, 19, 20) also performed smoothly. The sulfation occurred selectively at the carboxylic acid site in the presence of benzylic (12, 32, 34, 37′, 38) and allylic (26) C–H bonds, both of which can easily undergo hydrogen atom abstraction (HAA) and form corresponding sulfate products. Tertiary carboxylic acids failed due to their inherent lability and sluggish reactivity.23 In addition, this method is currently restricted to arylacetic acids that form a stabilized radical. To our delight, the substrate scope can be extended to carboxylic acid fragments adjacent to oxygen (28) and nitrogen (29′) atoms. A non-activated aliphatic acid could afford the sulfated product (30′) in 15% yield, though broader substrate generality remains constrained (31′). The identity of the target sulfate group was unambiguously confirmed by the X-ray crystal structure of 6. The generality and mild reaction conditions of this method make it suitable for late-stage sulfation of drugs, including ibuprofen (32), pranoprofen (33), isoxepac (34), ketoprofen (35′), flurbiprofen (36), loxoprofen (37′) and zaltoprofen (38). Meanwhile, the synthetic robustness was further demonstrated on a gram scale to give the product 28 without obviously detrimental effect on reaction efficiency.
| a Carboxylic acid (0.2 mmol), (NH4)2S2O8 (3.0 equiv.), AgNO2 (5 mol%), L1 = 4,7-diphenyl-1,1-phenanthroline (5 mol%), KH2PO4 (1.2 equiv.), nBu4NHSO4 (1.2 equiv.), DCM (0.2 M), Ar, rt, 11 h, isolated yield.b K2HPO4 as the base.c Et3N (1.5 equiv.) instead of KH2PO4, nBu4NHSO4.d K2CO3 as the base.e H2O (5.0 equiv.) was added.f 3,8-Dibromo-1,10-phenanthroline instead of L1. 29: Na as cation; 29′: nBu4N as cation. (′) indicates the countercation is nBu4N for other compounds as well. |
|---|
 |
To gain insight into the mechanism of the decarboxylative sulfation, a few experiments were conducted. The addition of the radical scavenger TEMPO to the standard reaction conditions effectively inhibited the sulfation process, resulting in a low conversion of 21s (Scheme 3A, top). Meanwhile, the reaction of cyclopropylacetic acid 39s afforded the ring-opened product 39 in a 39% yield (Scheme 3A, bottom). These results suggest the involvement of a benzyl radical intermediate in the silver-catalyzed decarboxylative sulfation. Subsequently, a Hammett analysis of the relative rate of decarboxylative sulfation across a series of para-substituted arylacetic acids was performed. The measured Hammett slope of −1.2 is consistent with ρ values indicative of benzyl radical formation as the rate-determining step in most processes (Scheme 3B).24
Aside from the radical pathway of C–OSO3− bond formation outlined in our initial mechanistic hypothesis (Scheme 2B), a nucleophilic attack by SO42− toward a benzylic carbocation—which could be generated from further oxidation of a benzylic radical—is an alternative process to form the C–OSO3− bond. To validate this, substrate 40s, containing a pendent sulfate group, was subjected to the standard conditions, leading to the exclusive formation of bisulfated product 40′, with no detectable cyclized product 40a (Scheme 3C, top). Furthermore, the bisulfated product 40′ was not generated from nucleophilic substitution of SO42− on sulfate diester 40a (Scheme 3C, bottom). These findings indicate that the decarboxylation sulfation proceeds via a radical pathway rather than a nucleophilic attack process. Notably, treatment of 4s with a stoichiometric silver(II)–sulfate complex did not yield any sulfate product,25 suggesting that high-valent Ag(II)SO4 may not engage in C–OSO3− bond formation; moreover, the Ag(III) sulfate complex was not detected in the reaction mixture (see Fig. S5†).26
Based on the above results and pertinent literature,22,27 a mechanism is proposed (Scheme 2B). Initially, a silver(I)–carboxylate complex was formed in the presence of the base and catalyst system. The Ag(I) complex was then oxidized to Ag(II) by either persulfate or a sulfate radical. The resulting silver(II) complex underwent a ligand-to-metal charge transfer (LMCT) with the carboxylate to produce a benzylic radical followed by decarboxylation. Finally, the benzylic radical participated in a bimolecular homolytic substitution (SH2) reaction with persulfate,19a leading to the desired sulfated product via C–O bond formation.
Conclusions
In summary, we have developed a method for decarboxylative sulfation by persulfates. Persulfates serve as both versatile oxidant and sulfating agents to facilitate the challenging C–OSO3− bond formation. The mild reaction conditions and operational simplicity of this method allow for a broad substrate scope and high functional-group tolerance. The improved synthetic utility is demonstrated by the late-stage modification of drugs, as a potential isosteric replacement for carboxylic acids to modify their metabolic profiles.Data availability
Detailed synthetic procedures and complete characterization data for all new compounds can be found in the ESI.†Author contributions
J. L. conceived the idea, directed the project, and wrote the paper. Z. X. and T. D. performed the experiments. Z. X. and C. S. analyzed the results and participated in the preparation of the manuscript.Conflicts of interest
A patent application (No. 202310019868.5, China), dealing with the use of persulfates for sulfation, has been filed, and J. L. may benefit from royalty payments.Acknowledgements
This work was supported by the Natural Science Foundation of Hunan Province (2025JJ40012). We thank Dr Dingyan Wang (Lingang Laboratory) for computational support, Zeting Zhang and Prof. Hong Yi (Wuhan University) for the acquisition and interpretation of EPR spectra, and the Analytical Instrumentation Center of Hunan University for mass spectrometry analysis.Notes and references
- (a) G.-I. Badea and G. L. Radu, Carboxylic Acids – Key Role in Life Sciences, IntechOpen, 2018 CrossRef; (b) A. S. Kalgutkar and J. S. Daniels, Carboxylic Acids and their Bioisosteres, Royal Society of Chemistry, Cambridge, 2010, pp. 99–167 Search PubMed; (c) P. J. Hajduk, M. Bures, J. Praestgaard and S. W. Fesik, J. Med. Chem., 2000, 43, 3443–3447 CrossRef CAS PubMed.
- (a) H. Pajouhesh and G. R. Lenz, NeuroRx, 2005, 2, 541–553 CrossRef PubMed; (b) M. Fung, A. Thornton and K. Mybeck, Drug Inf. J., 2001, 35, 293–317 CrossRef.
- (a) P. Lassalas, B. Gay, C. Lasfargeas, M. J. James, V. Tran, K. G. Vijayendran, K. R. Brunden, M. C. Kozlowski, C. J. Thomas, A. B. Smith III, D. M. Huryn and C. Ballatore, J. Med. Chem., 2016, 59, 3183–3203 CrossRef CAS PubMed; (b) C. Ballatore, D. M. Huryn and A. B. Smith III, ChemMedChem, 2013, 8, 385–395 CrossRef CAS PubMed; (c) C. Ballatore, J. H. Soper, F. Piscitelli, M. James, L. Huang, O. Atasoylu, D. M. Huryn, J. Q. Trojanowski, V. M. Lee, K. R. Brunden and A. B. Smith III, J. Med. Chem., 2011, 54, 6969–6983 CrossRef CAS PubMed; (d) A. Hall, M. Chatzopoulou and J. Frost, Bioorg. Med. Chem., 2024, 104, 117653 CrossRef CAS PubMed.
- (a) G. D. D'Agostino, S. N. Chaudhari and A. S. Devlin, Nat. Chem. Biol., 2024, 20, 410–421 CrossRef PubMed; (b) S. Hemmerich, Protein Sulfation, in Handbook of Neurochemistry and Molecular Neurobiology: Neural Protein Metabolism and Function, A. Lajtha and N. Banik, Springer US, Boston, MA, USA, 2007, pp. 283–302 Search PubMed; (c) G. J. Mulder, Sulfation of Drugs and Related Compounds, CRC Press, Boca Raton, USA, 1981 Search PubMed.
- (a) K. A. Schug and W. Lindner, Chem. Rev., 2005, 105, 67–113 CrossRef CAS PubMed; (b) J. Yang, T. Zhao, J. Fan, H. Zou, G. Lan, F. Guo, Y. Shi, H. Ke, H. Yu, Z. Yue, X. Wang, Y. Bai, S. Li, Y. Liu, X. Wang, Y. Chen, Y. Li and X. Lei, Cell, 2024, 187, 7164–7182 CrossRef CAS PubMed; (c) Z. Xu, Y. Liu, J. Liu, W. Ma, Z. Zhang, D. G. Chapla, L. Wen, K. W. Moremen, W. Yi and T. Li, Nat. Chem., 2024, 16, 881–892 CrossRef CAS PubMed; (d) B. B. Zhang, K. Harrison, Y. Zhong, J. W. C. Maxwell, D. J. Ford, L. P. Calvey, S. S. So, F. C. Peterson, B. F. Volkman, M. J. Stone, R. P. Bhusal, S. S. Kulkarni and R. J. Payne, J. Am. Chem. Soc., 2024, 146, 34253–34259 CrossRef CAS PubMed; (e) L. Wang, A. W. Sorum, B.-S. Huang, M. K. Kern, G. Su, N. Pawar, X. Huang, J. Liu, N. L. B. Pohl and L. C. Hsieh-Wilson, Nat. Chem., 2023, 15, 1108–1117 Search PubMed; (f) T. Gao, J. Yan, C.-C. Liu, A. S. Palma, Z. Guo, M. Xiao, X. Chen, X. Liang, W. Chai and H. Cao, J. Am. Chem. Soc., 2019, 141, 19351–119359 CrossRef CAS PubMed.
- (a) F. C. Kauffman, Drug Metab. Rev., 2004, 36, 823–843 CrossRef CAS PubMed; (b) G. M. Pacifici and K. Allegaert, Curr. Ther. Res., 2015, 77, 24–30 CrossRef CAS PubMed; (c) A. Hayashi, S. Terasaka, Y. Nukada, A. Kameyama, M. Yamane, R. Shioi, M. Iwashita, K. Hashizume and O. Morita, J. Agric. Food Chem., 2022, 70, 8264–8273 CrossRef CAS PubMed.
- Calculated pKa, log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4 and Papp values were obtained with ChemAxon, https://chemaxon.com/calculators-and-predictors.
D7.4 and Papp values were obtained with ChemAxon, https://chemaxon.com/calculators-and-predictors. - (a) S. B. Beil, T. Q. Chen, N. E. Intermaggio and D. W. C. MacMillan, Acc. Chem. Res., 2022, 55, 3481–3494 Search PubMed; (b) G. Laudadio, M. D. Palkowitz, T. E.-H. Ewing and P. S. Baran, ACS Med. Chem. Lett., 2022, 13, 1413–1420 CrossRef CAS PubMed; (c) N. Rodríguez and L. J. Goossen, Chem. Soc. Rev., 2011, 40, 5030–5048 RSC; (d) J. Xuan, Z. Zhang and W. Xiao, Angew. Chem., Int. Ed., 2015, 54, 15632–15641 CrossRef CAS PubMed; (e) P. J. Moon and R. J. Lundgren, ACS Catal., 2020, 10, 1742–1753 CrossRef CAS; (f) Z. Zeng, A. Feceu, N. Sivendran and L. J. Gooßen, Adv. Synth. Catal., 2021, 363, 2678–2722 CrossRef CAS; (g) A. Varenikov, E. Shapiro and M. Gandelman, Chem. Rev., 2021, 121, 412–484 CrossRef CAS PubMed; (h) C.-L. Ji, Y.-N. Lu, S. Xia, C. Zhu, C. Zhu, W. Li and J. Xie, Angew. Chem., Int. Ed., 2025, 64, e202423113 CrossRef CAS PubMed; (i) S. Mondal, S. Mandal, S. Mondal, S. P. Midya and P. Ghosh, Chem. Commun., 2024, 60, 9645–9658 RSC.
- (a) Z. Wang, L. Zhu, F. Yin, Z. Su, Z. Li and C. Li, J. Am. Chem. Soc., 2012, 134, 4258–4263 CrossRef CAS PubMed; (b) F. Yin, Z. Wang, Z. Li and C. Li, J. Am. Chem. Soc., 2012, 134, 10401–10404 CrossRef CAS PubMed; (c) X. Liu, Z. Wang, X. Cheng and C. Li, J. Am. Chem. Soc., 2012, 134, 14330–14333 CrossRef CAS PubMed; (d) C. Liu, X. Wang, Z. Li, L. Cui and C. Li, J. Am. Chem. Soc., 2015, 137, 9820–9823 CrossRef CAS PubMed; (e) L. Cui, H. Chen, C. Liu and C. Li, Org. Lett., 2016, 18, 2188–2191 CrossRef CAS PubMed; (f) X. Tan, T. Song, Z. Wang, H. Chen, L. Cui and C. Li, Org. Lett., 2017, 19, 1634–1637 CrossRef CAS PubMed; (g) X. Tan, Z. Liu, H. Shen, P. Zhang, Z. Zhang and C. Li, J. Am. Chem. Soc., 2017, 139, 12430–12433 CrossRef CAS PubMed; (h) Y. Dong, Z. Wang and C. Li, Nat. Commun., 2017, 8, 277 CrossRef PubMed; (i) D. Kong, P. J. Moon, O. Bsharat and R. J. Lundgren, Angew. Chem., Int. Ed., 2020, 59, 1313–1319 CrossRef CAS PubMed; (j) V. T. Nguyen, V. D. Nguyen, G. C. Haug, N. T. H. Vuong, H. T. Dang, H. D. Arman and O. V. Larionov, Angew. Chem., Int. Ed., 2020, 59, 7921–7927 CrossRef CAS PubMed; (k) R. Narobe, K. Murugesan, S. Schmid and B. König, ACS Catal., 2022, 12, 809–817 CrossRef CAS; (l) Q. Y. Li, S. N. Gockel, G. A. Lutovsky, K. S. DeGlopper, N. J. Baldwin, M. W. Bundesmann, J. W. Tucker, S. W. Bagley and T. P. Yoon, Nat. Chem., 2022, 14, 94–99 CrossRef CAS PubMed; (m) S. Shibutani, T. Kodo, M. Takeda, K. Nagao, N. Tokunaga, Y. Sasaki and H. Ohmiy, J. Am. Chem. Soc., 2020, 142, 1211–1216 Search PubMed; (n) R. Mao, J. Balon and X. Hu, Angew. Chem., Int. Ed., 2018, 57, 13624–13628 CrossRef CAS PubMed; (o) J. Xiang, M. Shang, Y. Kawamata, H. Lundberg, S. H. Reisberg, M. Chen, P. Mykhailiuk, G. Beutner, M. R. Collins, A. Davies, M. D. Bel, G. M. Gallego, J. E. Spangler, J. Starr, S. Yang, D. G. Blackmond and P. S. Baran, Nature, 2019, 573, 398–403 CrossRef CAS PubMed; (p) Y. Miyake, K. Nakajima and Y. Nishibayashi, Chem. Commun., 2013, 49, 7854–7856 RSC; (q) S. B. Lang, K. M. O'Nele and J. A. Tunge, J. Am. Chem. Soc., 2014, 136, 13606–13609 Search PubMed; (r) L. Capaldo, L. Buzzetti, D. Merli, M. Fagnoni and D. Ravelli, J. Org. Chem., 2016, 81, 7102–7109 CrossRef CAS PubMed; (s) S. Mondal, S. Mondal, S. P. Midya, S. Das, S. Mondal and P. Ghosh, Org. Lett., 2023, 25, 184–189 CrossRef CAS PubMed.
- (a) C. P. Hoiberg and R. O. Mumma, J. Am. Chem. Soc., 1969, 91, 4273–4278 CrossRef CAS; (b) T. Nakahara, M. Waki, H. Uchimura, M. Hirano, J. S. Kim, T. Matsumoto, K. Nakamura, K. Ishibashi, H. Hirano and A. Shiraishi, Anal. Biochem., 1986, 154, 194–199 CrossRef CAS PubMed.
- (a) S. Ogo, Y. Takebe, K. Uehara, T. Yamazaki, H. Nakai, Y. Watanabe and S. Fukuzumi, Organometallics, 2006, 25, 331–338 CrossRef CAS; (b) K. Uehara, S. Fukuzumi and S. Ogo, J. Organomet. Chem., 2007, 692, 499–504 CrossRef CAS; (c) O. Planas, V. Peciukenas and J. Cornella, J. Am. Chem. Soc., 2020, 142, 11382–11387 CrossRef CAS PubMed; (d) T. D. Lohrey, A. Q. Cusumano, W. A. Goddard III and B. M. Stoltz, ACS Catal., 2021, 11, 10208–10222 CrossRef CAS PubMed.
- (a) G. C. Fu, ACS Cent. Sci., 2017, 3, 692–700 CrossRef CAS PubMed; (b) C. Chen and G. C. Fu, Nature, 2023, 618, 301–307 Search PubMed.
- (a) L. Tebben and A. Studer, Angew. Chem., Int. Ed., 2011, 50, 5034–5068 CrossRef CAS PubMed; (b) J. E. Nutting, M. R. Rafiee and S. S. Stahl, Chem. Rev., 2018, 118, 4834–4885 Search PubMed; (c) J. C. Siu, G. S. Sauer, A. Saha, R. L. Macey, N. Fu, T. Chauvire, K. M. Lancaster and S. Lin, J. Am. Chem. Soc., 2018, 140, 12511–12520 CrossRef CAS PubMed.
- Z. Lu, M. Ju, Y. Wang, J. M. Meinhardt, J. I. Martinez Alvarado, E. Villemure, J. A. Terrett and S. Lin, Nature, 2023, 619, 514–520 CrossRef CAS PubMed.
- Á. M. Martínez, D. Hayrapetyan, T. Lingen, M. Dyga and L. J. Gooßen, Nat. Commun., 2020, 11, 4407 Search PubMed.
- (a) D. L. Golden, C. Zhang, S.-J. Chen, A. Vasilopoulos, I. A. Guzei and S. S. Stahl, J. Am. Chem. Soc., 2023, 145, 9434–9440 CrossRef CAS PubMed; (b) A. Vasilopoulos, S. W. Krska and S. S. Stahl, Science, 2021, 372, 398–402 Search PubMed; (c) B. Qian, S. Chen, T. Wang, X. Zhang and H. Bao, J. Am. Chem. Soc., 2017, 139, 13076–13082 CrossRef CAS PubMed; (d) M. Locklear and P. H. Dussault, Eur. J. Org. Chem., 2020, 4814–4840 Search PubMed.
- A. Dragan, T. M. Kubczyk, J. H. Rowley, S. Sproules and N. C. O. Tomkinson, Org. Lett., 2015, 17, 2618–2621 CrossRef CAS PubMed.
- (a) J. Börgel, L. Tanwar, F. Berger and T. Ritter, J. Am. Chem. Soc., 2018, 140, 16026–16031 CrossRef PubMed; (b) L. Tanwar, J. Börgel and T. Ritter, J. Am. Chem. Soc., 2019, 141, 17983–17988 CrossRef CAS PubMed.
- (a) Z. Xia, Z. Ye, T. Deng, Z. Tan, C. Song and J. Li, Angew. Chem., Int. Ed., 2025, 64, e202413847 CrossRef CAS PubMed; (b) Z. Zhao, Q. Yu, Z. Xia, Z. Ye, X. Huang, C. Song and J. Li, Nat. Synth., 2024, 3, 1529–1537 CrossRef CAS.
- (a) F. Minisci and A. Citterio, Acc. Chem. Res., 1983, 16, 27–32 CrossRef CAS; (b) S. Mandal, T. Bera, G. Dubey, J. Saha and J. K. Laha, ACS Catal., 2018, 8, 5085–5144 CrossRef CAS; (c) S. Yue, Y. Zheng, C. Song, J. Li, CCS Chem., 2025, 7, 1297–1304 Search PubMed.
- (a) J. Alshehri and A. Jones, Essays Biochem., 2024, 68, 449–466 CrossRef CAS PubMed; (b) B. K. Villuri and U. R. Desai, Chem.–Eur. J., 2024, 30, e202402268 CrossRef PubMed; (c) E. Chapman, M. D. Best, S. R. Hanson and C.-H. Wong, Angew. Chem., Int. Ed., 2004, 43, 3526–3548 CrossRef CAS PubMed; (d) X. Tang, K. Eitel, L. Kaysser, A. Kulik, S. Grond and B. Gust, Nat. Chem. Biol., 2013, 9, 610–615 CrossRef CAS PubMed; (e) C. Liu, C. Yang, S. Hwang, S. L. Ferraro, J. P. Flynn and J. Niu, Angew. Chem., Int. Ed., 2020, 59, 18435–18441 CrossRef CAS PubMed; (f) L. J. Ingram and S. D. Taylor, Angew. Chem., Int. Ed., 2006, 45, 3503–3506 CrossRef CAS PubMed.
- (a) Q. Yu, D. Zhou, Y. Liu, X. Huang, C. Song, J. Ma and J. Li, Org. Lett., 2023, 25, 47–52 CrossRef CAS PubMed; (b) Q. Yu, D. Zhou, P. Yu, C. Song, Z. Tan and J. Li, Org. Lett., 2024, 26, 5856–5861 CrossRef CAS PubMed.
- (a) N. C. Deno and M. S. Newman, J. Am. Chem. Soc., 1950, 72, 3852–3856 CrossRef CAS; (b) H. Bi, R. Mass and G. Just, Steroids, 1992, 57, 306–312 CrossRef CAS PubMed.
- W. S. Trahanovsky, J. Cramer and D. W. Brixius, J. Am. Chem. Soc., 1974, 96, 1077–1081 CrossRef CAS.
- (a) P. J. Malinowski, M. Derzsi, Z. Mazej, Z. Jagličić, B. Gaweł, W. Łasocha and W. Grochala, Angew. Chem., Int. Ed., 2010, 49, 1683–1686 CrossRef CAS PubMed; (b) P. J. Leszczyński, A. K. Budniak, M. Grzeszkiewicz, J. Gawraczyński, Ł. Dobrzycki, P. J. Malinowski, T. Jaroń, M. K. Cyrański, P. Szareka, Z. Mazej and W. Grochala, J. Fluor. Chem, 2019, 218, 105–110 CrossRef; (c) M. J. Jadwiszczak, P. J. Leszczyński, Z. Mazej, P. J. Malinowski, E. K. Nawrocka, K. Kazimierczuk, P. Kwiatkowski, P. Połczyński, M. Grochowska-Tatarczak, K. J. Fijalkowski, J. Sadło and W. Grochala, Org. Chem. Front., 2023, 10, 5637–5643 RSC; (d) F. Wang, P. Xu, F. Cong and P. Tang, Chem. Sci., 2018, 9, 8836–8841 RSC; (e) H. Yang, F. Wang, X. Jiang, Y. Zhou, X. Xu and P. Tang, Angew. Chem., Int. Ed., 2018, 57, 13266–13270 CrossRef CAS PubMed.
- T. Elkoush, C. L. Mak, D. W. Paley and M. G. Campbell, ACS Catal., 2020, 10, 4820–4826 CrossRef CAS.
- N. R. Patel and R. A. Flowers II, J. Org. Chem., 2015, 80, 5834–5841 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2255772. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc03129j |
| This journal is © The Royal Society of Chemistry 2025 |