Open Access Article
Open Access ArticleBreaking the heavy-atom paradigm: weak-donor-engineered triplet harvesting in BODIPY photosensitizers for immunogenic pyroptosis therapy†
Hyeong Seok Kim‡
ab,
Hyeonji Rha‡a,
Mohammad Izadyar‡ ce,
Supphachok Chanmungkalakulc,
Haiqiao Huangd,
Yi Young Kangb,
Jae-Won Kab,
Yunjie Xu*a,
Mingle Li
ce,
Supphachok Chanmungkalakulc,
Haiqiao Huangd,
Yi Young Kangb,
Jae-Won Kab,
Yunjie Xu*a,
Mingle Li *d,
Xiaogang Liu
*d,
Xiaogang Liu *c and
Jong Seung Kim
*c and
Jong Seung Kim *a
*a
aDepartment of Chemistry, Korea University, Seoul, 02841, Korea. E-mail: jongskim@korea.ac.kr; xuyunjie87@korea.ac.kr
bAdvanced Functional Polymers Research Center, Korea Research Institute of Chemical Technology Daejeon, 34114, Korea
cFluorescence Research Group, Singapore University of Technology and Design, Singapore, 487372, Singapore. E-mail: xiaogang_liu@sutd.edu.sg
dCollege of Materials Science and Engineering, Shenzhen University, Shenzhen, 518060, China. E-mail: limingle@szu.edu.cn
eResearch Center for Modeling and Computational Sciences, Faculty of Science, Ferdowsi University of Mashhad, Mashhad 9177948974, Iran
First published on 14th July 2025
Abstract
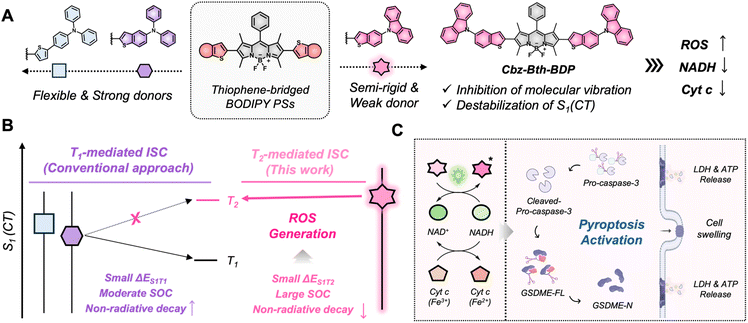
Boron-dipyrromethene (BODIPY)-based dyes emerge as promising agents for phototherapy; however, traditional methods to enhance spin–orbit coupling (SOC) through halogenation introduce dark toxicity and limit therapeutic applications. Here, we present a thiophene-bridged BODIPY functionalized scaffold with carbazole-benzothiophene (Cbz-Bth) substituents at the 2,6-positions. This design employs a weak yet semi-rigid donor to destabilize charge-transfer (CT) states, enabling T2-mediated spin–orbit charge-transfer intersystem crossing (SOCT-ISC). The resulting photosensitizer, Cbz-Bth-BDP, demonstrates effective reactive oxygen species generation and the photocatalytic transformation of biomolecules such as nicotinamide adenine dinucleotide (NADH) and cytochrome c (Cyt c). Notably, Cbz-Bth-BDP induces pyroptosis by activating gasdermin E (GSDME), leading to cell swelling and the release of intracellular content. In a 3D tumor spheroid model, Cbz-Bth-BDP significantly inhibits tumor growth by reducing adenosine triphosphate (ATP) levels. This study highlights the advantages of accessing higher excited triplet states and positions Cbz-Bth-BDP as a promising, heavy-atom-free photosensitizer for cancer treatment through pyroptosis activation.
Introduction
Boron-dipyrromethene (BODIPY)-based organic dyes are widely recognized for their phototherapeutic potential due to their ease of modification, strong absorption properties, tunable quantum yields, and excellent biocompatibility.1–5 In photodynamic therapy (PDT), a critical challenge is enhancing intersystem crossing (ISC) to efficiently populate the triplet state, thereby maximizing reactive oxygen species (ROS) production for cancer treatment.1–5 Traditional strategies to improve ISC involve incorporating heavy atoms, such as bromine or iodine, at the 2,6-positions of the BODIPY core.6–8 This enhances spin–orbit coupling (SOC) and facilitates singlet oxygen generation.6–8 However, halogenated BODIPY photosensitizers (PSs) often lead to undesirable dark toxicity, limiting their therapeutic applications in biological systems.9To mitigate these risks, non-halogenated BODIPY PSs have been explored as safer alternatives. Recent advances have focused on structural modifications to enhance ISC without heavy atoms.10–12 Twisted BODIPY helicenes with distorted symmetry, for example, promote SOC-mediated ISC and show excellent PDT performance.13–15 Similarly, rigid thiophene-fused BODIPYs, which incorporate sulfur atoms into the π-conjugated framework, enhance ISC by suppressing non-radiative decay pathways.16–18 However, despite achieving higher quantum yields for triplet-state formation, their synthetic complexity, scalability, and limited tunability present significant barriers to their widespread use in diverse photodynamic applications.
Simpler strategies have emerged, including the development of heavy-atom-free BODIPY PSs by incorporating electron-rich aryl groups19,20 or sterically hindered moieties at the meso-position of the BODIPY core.21–23 Donor–acceptor–donor (D–A–D) systems with strong donors at the β-positions have also shown promise, improving ISC efficiency and ROS generation.24 These systems rely on stable charge-transfer (CT) states that reduce the energy gap between singlet (S1) and triplet (T1) states and increase SOC to promote ISC.24,25 However, CT-state-based designs encounter inherent challenges. The rotational flexibility of substituents can result in undesirable reverse ISC and excessive non-radiative decay, compromising triplet-state quantum yields.24,25 In highly polar aqueous environments, CT states may become overly stabilized, narrowing the energy gap between the S1 (CT) and S0 states.26 This stabilization accelerates non-radiative decay (via the energy gap law), further reducing ISC efficiency. When the CT state energy drops below that of T1, ISC can be inhibited, significantly diminishing therapeutic efficacy.
To address these challenges, we introduce a novel thiophene-bridged BODIPY scaffold featuring carbazole-benzothiophene (Cbz-Bth) substituents at the 2,6-positions. Unlike conventional approaches that rely on strong donors to enhance S1–T1 transitions, our strategy employs a weak yet semi-rigid donor. This design destabilizes the CT state, facilitating efficient ISC via a T2-mediated pathway driven by a potent spin–orbit charge-transfer intersystem crossing (SOCT-ISC) mechanism. Comprehensive experimental and theoretical analyses reveal that the semi-rigid π-conjugated architecture not only narrows the S1–T2 energy gap but also optimizes ISC efficiency, leading to exceptional photocatalytic performance and robust ROS generation. Upon photoirradiation, irreversible oxidation of crucial biomolecules, e.g., nicotinamide adenine dinucleotide (NADH), and the reduction of cytochrome c (Cyt c) from ferric (Fe3+) to ferrous (Fe2+) states (Scheme 1A and B), were also observed. Remarkably, Cbz-Bth-BDP triggers pyroptosis, a highly immunogenic form of programmed cell death (Scheme 1C).27–29 These findings establish Cbz-Bth-BDP as a promising candidate for photo-controlled pyroptosis activation and offer a novel strategy for designing high-performance photosensitizers. By addressing non-radiative decay pathways and accessing higher excited triplet states, this work advances the potential of cancer phototherapy.
Results and discussion
Molecular design and synthesis of thiophene-bridged BODIPY PSs
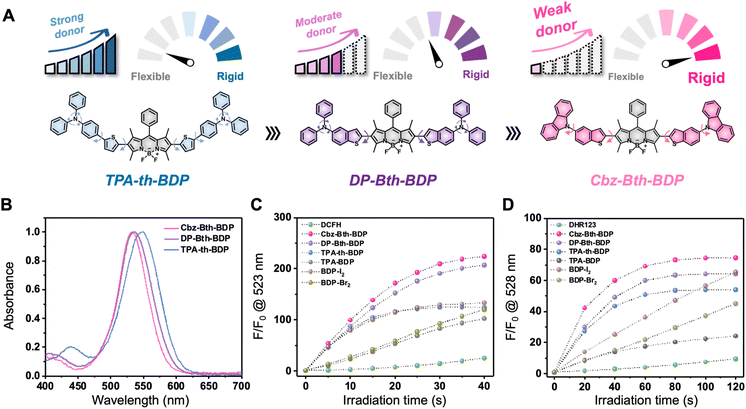
We developed a series of thiophene-bridged BODIPY PSs by replacing traditional heavy atoms with thiophene-aminophenyl donors (Scheme S1†), aiming to enhance ISC to triplet states by leveraging the thiophene bridge.30,31 The synthesis began with the coupling of borated triphenylamine with bromothiophene, followed by boration and Suzuki coupling with iodinated BODIPY to yield TPA-th-BDP. Further modifications involved conjugating carbazole and diphenylamine with benzothiophene, resulting in the final compounds, Cbz-Bth-BDP and DP-Bth-BDP (Scheme S2†). These compounds were thoroughly characterized by 1H NMR, 13C NMR, and LC-MS spectrometry, confirming their structures (Fig. S1–S17†).Optical properties
The UV-vis and fluorescence spectra of the thiophene-bridged BODIPY PSs revealed maximum absorption wavelengths at 546, 532, and 529 nm for TPA-th-BDP, DP-Bth-BDP, and Cbz-Bth-BDP, respectively, making them suitable for green-light-activated phototherapy (Table 1 and Fig. 1B). Correspondingly, their fluorescence emission maxima were observed at 623, 595, and 614 nm, respectively (Table 1 and Fig. S18–S20†). To investigate the substituents' optical properties, their ionization potential (IP) was then assessed, as lower IP values indicate stronger electron-donating effects. The electron-donating strength of the substituents followed the sequence: TPA-th (5.20 eV) > DP-Bth (5.24 eV) > Cbz-Bth (5.68 eV in water) (Fig. 1A and S22†). These findings align with the observed absorption peaks (λabs). Specifically, Cbz-Bth-BDP, with its weaker electron-donating carbazole-benzothiophene substituent, exhibited the shortest λabs, suggesting less effective intramolecular charge transfer (ICT). In contrast, TPA-th-BDP, with the strongest electron-donating substituent, exhibited the largest λabs, consistent with its lower IP value (Fig. 1B and S18–S20†).| BODIPY PSs | λexa (nm) | εb (M−1 cm−1) | λemc (nm) | ψd (%) | HOMOe (eV) | LUMOf (eV) | Egg (eV) | ΔESTh (eV) | SOCi (cm−1) | Eoxj (V) | Eredk (V) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Maximum absorption wavelength of BODIPY PSs in THF.b Molar absorptivity at the maximum absorption wavelength.c Maximum absorption wavelength of BODIP PSs in THF.d Fluorescence quantum yield in THF.e Highest occupied molecular orbital (HOMO) level determined via cyclic voltammogram (CV).f Lowest unoccupied molecular orbital (LUMO) level determined via cyclic voltammogram (CV).g Energy band gap calculated from the HOMO and LUMO levels.h Singlet–triplet energy gap derived from density functional theory (DFT) calculations.i Spin orbital coupling (SOC) value obtained from DFT calculations.j Oxidation.k Reduction potentials determined by CV. | |||||||||||
| TPA-th-BDP | 546 | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 950 950 |
623 | 27.3 | −5.24 | −3.19 | 2.05 | 0.25 | 1.29 | 0.832 | −1.22 |
| DP-th-BDP | 532 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 940 940 |
595 | 16.5 | −5.32 | −3.14 | 2.18 | 2.18 | 3.45 | 0.917 | −1.26 |
| Cbz-Bth-BDP | 529 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 320 320 |
614 | 51.7 | −5.67 | −3.45 | −3.45 | 2.22 | 3.95 | 1.26 | −0.96 |
Given the prominence of aminophenyl donors in aggregation-induced emission (AIE) chemistry,25,32,33 we investigated whether these BODIPY PSs have AIE properties in aqueous solutions with varying fractions of THF (fT). Interestingly, Cbz-Bth-BDP demonstrated significant fluorescence enhancement as the THF content increased from 0 to 99%. In contrast, under identical conditions, TPA-th-BDP only showed minimal fluorescence enhancement (Fig. S23†). DP-Bth-BDP exhibited the highest fluorescence intensity at a 10% THF fraction, although all BODIPY PSs displayed limited fluorescence in water (Fig. S23†). The fluorescence quantum yields of TPA-th-BDP, DP-Bth-BDP, and Cbz-Bth-BDP in THF solution were determined to be 27.3%, 16.5%, and 51.7%, respectively (Table 1). The superior quantum yield of Cbz-Bth-BDP can be attributed to its rigid molecular structure, which effectively suppresses non-radiative decay by restricting intramolecular motions (Fig. 1A).
ROS generation
ROS generation assays using DCFH (2,7-dichlorodihydrofluorescein) demonstrated that Cbz-Bth-BDP outperformed halogenated BODIPYs in ROS production, particularly under green-light irradiation. As depicted in Fig. 1C and S24,† Cbz-Bth-BDP showed the highest fluorescence increase at 523 nm under 530 nm irradiation (10 mW cm−2), which was much higher than that of halogenated BODIPYs (BDP-Br2 and BDP-I2), indicating the robust ROS generation and the potential for heavy-atom-free biomedical applications. In contrast, TPA-th-BDP and TPA-BDP demonstrated minimal ROS generation, suggesting lower ISC efficiency. Interestingly, Cbz-Bth-BDP and DP-Bth-BDP also displayed excellent type I PDT activities to generate superoxide radicals, as illustrated in Fig. 1D and S25.† DHR123 (dihydrorhodamine 123) assay demonstrated Cbz-Bth-BDP and DP-Bth-BDP produced more superoxide radicals than halogenated BODIPY PSs under light irradiation (100 mW cm−2), indicating Cbz-Bth-BDP may have the potential to work well under a hypoxic environment. Similarly, Cbz-Bth-BDP exhibited the highest fluorescence increase in the HPF assay (Fig. S26†), confirming efficient hydroxyl radical generation, consistent with previous reports on Fenton-like photocatalytic processes.34–36 Next, we investigated the photoinduced singlet oxygen generation by BODIPY PSs in the ABDA assay (Fig. S27†). These findings were further supported by electron paramagnetic resonance (EPR) spectroscopy using spin-trapping agents: 5,5-dimethyl-1-pyrroline N-oxide (DMPO) for superoxide radicals, 5-tert-butoxycarbonyl-5-methyl-1-pyrroline-N-oxide (BMPO) for hydroxyl radicals, and 2,2,6,6-tetramethylpiperidine (TMPO) for singlet oxygen, verifying the efficient ROS production of Cbz-Bth-BDP samples (Fig. S28†).To explore the influence of molecular aggregation on ROS generation under biologically relevant conditions, we examined the behavior of BODIPY PSs in monomeric (THF) and aggregated (distilled water) states. Aggregation is known to significantly impact the photophysical properties of photosensitizers.37 Due to their limited solubility in water, thiophene-bridged BODIPYs likely exist in an aggregated form in aqueous environments. The DPBF (1,3-diphenylisobenzofuran) assay revealed markedly faster degradation in 100% distilled water under light irradiation, indicating enhanced ROS generation in the aggregated state of Cbz-Bth-BDP (Fig. S29A†). Similarly, the DHR123 assay demonstrated a notable fluorescence increase in water, reinforcing this observation (Fig. S29B†). These findings suggest that aggregation facilitates ISC, thereby optimizing ROS production for photodynamic applications. We further tested the photostability of our thiophene-bridged BODIPY PSs in aqueous environments. The compounds were subjected to continuous irradiation at 530 nm (100 mW cm−2), and their UV-vis absorption spectra were recorded at 1-minute intervals (Fig. S30†). Compared to Rose Bengal (RB), a standard green-light photosensitizer that underwent significant photodegradation, the BODIPY derivatives exhibited remarkable spectral stability, maintaining their structural and optical integrity throughout the irradiation period. These results highlight the superior photostability of the BODIPY framework, emphasizing its potential for long-term photocatalytic applications.
DFT calculations and ISC mechanism
Enhancing the rigidity of the 2- and 6-substituents in BODIPY PSs is crucial for improving the ISC rates by reducing non-radiative decay caused by structural rotations.24,25,38 Among the studied compounds, the structural flexibility follows the order of TPA-th-BDP > DP-Bth-BDP > Cbz-Bth-BDP (Fig. 1A). The highly rigid structure of Cbz-Bth-BDP minimizes non-radiative decay, thereby facilitating efficient ISC. To investigate the mechanisms underlying ROS generation in thiophene-bridged BODIPY PSs, we performed density functional theory (DFT) calculations to examine their singlet and triplet states. Geometry optimization and frontier molecular orbital (FMO) analysis revealed significant pre-twisting (>40°) of the thiophene (th) and benzothiophene (Bth) substituents relative to the BODIPY core, disrupting π-conjugation (Fig. S31†). For Cbz-Bth-BDP, the S1 excitation corresponds to a locally excited (LE) state within the BODIPY fragment (i.e., from HOMO to LUMO), while additional molecular orbitals introduced by the substituents suggest the potential for photoinduced electron transfer (PET) in the excited state (Fig. S32†).38Comprehensive calculations of key photoexcitation and ISC states, including the Franck–Condon (FC), locally excited (LE), electron transfer (ET) singlet states, and triplet T1(LE) and T2(CT) states, demonstrated that photoexcitation primarily occurs within the BODIPY framework (Fig. 2A–C). In all three compounds, the S1(ET) state was more stable than the S1(LE) state, enabling a transition into the ET state and facilitating ISC via the SOCT-ISC mechanism. This mechanism is supported by stronger SOC between the S1(ET) and T1(LE)/T2 (CT) states compared to the S1(LE) and T1(LE) states.39,40
Among the PSs, Cbz-Bth-BDP exhibited the most efficient ISC channels (Fig. 2A). Computational analysis revealed small singlet–triplet energy gaps (ΔEST < 0.30 eV) and large SOC values (>1.00 cm−1) across all compounds (Fig. 2A–C). Notably, the S1(ET) state of Cbz-Bth-BDP was exceptionally close to T2(CT) state, with a ΔEST of only 0.02 eV (Fig. 2A), resulting in the highest SOC value of 3.95 cm−1. In contrast, DP-Bth-BDP and TPA-th-BDP showed ΔEST values of 0.22 eV and 0.26 eV (Fig. 2D), respectively, with lower SOC values (3.45 cm−1 and 1.29 cm−1) (Fig. 2E). These differences highlight the superior ISC efficiency of Cbz-Bth-BDP.
Experimental data in good solvents (e.g., aqueous solutions containing 10% DMSO; Fig. 1C and D), align closely with these computational findings, underscoring the exceptional photosensitizing performance of Cbz-Bth-BDP. While aggregation is expected to enhance intermolecular interactions and further improve ISC and ROS generation, modeling such complex aggregates exceeds the computational capacity of detailed time-dependent DFT studies. Interestingly, upon reaching the T1 state, all photosensitizers demonstrated significant electron affinity (<−4.70 eV), with Cbz-Bth-BDP showing the most negative value (Fig. 2F). These results suggest that Cbz-Bth-BDP, in particular, can effectively drive photocatalytic processes by accepting electrons from reductive biomolecules (i.e., NADH).
Photoredox catalytic activities of Cbz-Bth-BDP
In photodynamic therapy, photoredox catalysis within live cells plays a new role in inducing cell death by mediating biomolecular conversion.41 Reductive biomolecules like NADH can participate in the reductive cycling of photoexcited PSs, converting to its oxidative form (NAD+) by single electron transfer (SET) (Fig. S33†).42,43 To evaluate the photocatalytic activity of thiophene-bridged BODIPY PSs, we examined NADH oxidation under green light irradiation (530 nm, 100 mW cm−2). The absorbance of NADH at 339 nm decreased over time, with new peaks appearing at 260 nm, confirming its oxidation (Fig. 3A). Among the BODIPYs, Cbz-Bth-BDP showed the most efficient photocatalytic conversion of NADH (Fig. 3B and S34†), consistent with its superior ROS generation and electron affinity. This enhanced photocatalytic efficiency is likely facilitated by aggregation-assisted photophysical effects, including improved triplet-state harvesting.44,45Next, the cyclic voltammetry (CV) of thiophene-bridged BODIPYs was measured to demonstrate the feasibility of electron transfer for photocatalytic NADH oxidation. As shown in Fig. 3C and S35,† the redox potentials of BODIPY PSs were determined as follows: Cbz-Bth-BDP (Ered = −0.958 V), DP-Bth-BDP (Ered = −1.263 V), and TPA-th-BDP (Ered = −1.218 V). The observed electrochemical trend in reduction potential closely aligns with our earlier theoretical calculations of electron affinity (Fig. 2F). Notably, the carbazole-benzothiophene substituents on Cbz-Bth-BDP notably enhanced its reduction potential, facilitating electron transfer from biological substrates. This enables oxygen-mediated reductive cycling, enabling Cbz-Bth-BDP as a promising candidate for NADH oxidation in photodynamic therapy.
Given that NADH can serve as an electron mediator, facilitating the reduction of mitochondrial electron carriers (e.g., Cyt c) through oxygen-independent pathways, photoreduction offers a promising strategy for enhancing phototherapy.46 In our study, we observed that the photocatalytic activity of BODIPY PSs played a crucial role in modulating the absorptive changes of Cyt c. Upon photo-irradiation, the characteristic β (520 nm) and α bands (550 nm) of Cyt c (Fe2+) emerged in all BODIPY PSs, with Cbz-Bth-BDP showing the most pronounced spectral changes (Fig. 3D, S36 and S37†).
To confirm the generation of NAD radicals (NAD˙) during the NADH-mediated photoreduction of Cyt c, we conducted CYPMPO assays. NAD˙ trapping by CYPMPO inhibited Cyt c reduction, validating the radical's role in the process (Fig. 3E and S38†). Furthermore, a comparison of photoreduction in aerobic and nitrogen-purged solutions revealed greater Cyt c changes under deaerated conditions (Fig. 3F). In deaerated PBS, Cbz-Bth-BDP exhibited higher turn-over numbers (TON = 2.93) and turn-over frequency (TOF = 0.586 min−1) compared to aerobic conditions (TON = 2.27, TOF = 0.454 min−1) (Fig. S39†). These results emphasize the oxygen-independent photocatalytic activity of Cbz-Bth-BDP, showcasing its ability to disrupt cellular metabolism and its potential for phototherapy applications.
Photoexcited Cbz-Bth-BDP acts as a pyroptosis activator
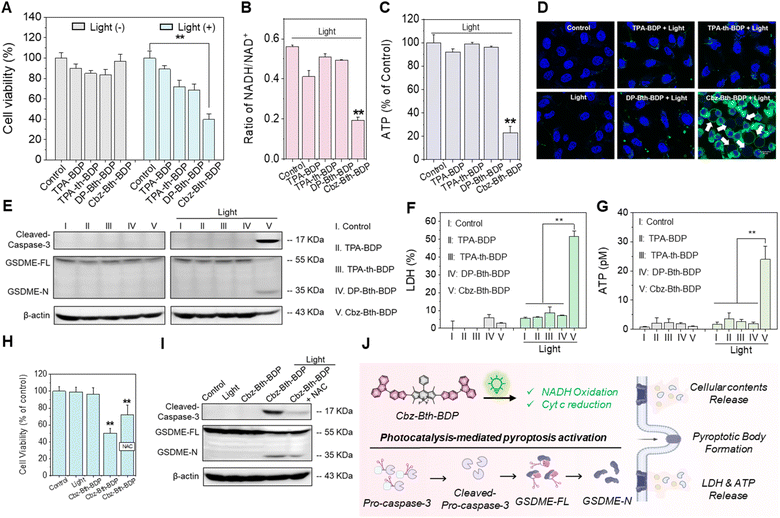
With this highly effective heavy-atom-free BODIPY-based Cbz-Bth-BDP in hand, we investigated its photocatalytic mechanism in tumor cells. Under light irradiation (530 nm, 100 mW cm−2) for 5, 10, and 15 min, Cbz-Bth-BDP induced cell death in a light-dose-dependent manner (Fig. S40†). Among all tested BODIPYs, Cbz-Bth-BDP showed the highest photocytotoxicity in MDA-MB-231 cells (Fig. 4A). Given NADH's critical role as a cofactor and redox component in tumor cells, we evaluated its interaction with Cbz-Bth-BDP. Maintaining the NADH/NAD+ balance is essential for mitochondrial function and cellular metabolism.47,48 Upon light irradiation, photoexcited Cbz-Bth-BDP led to a significant depletion of intracellular NADH levels compared to other BODIPY-based PSs (Fig. 4B). This reduction was further validated using the 3-(4,5-dimethylthiazo-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, which serves as an indirect indicator of intracellular NADH levels through MTT-formazan formation. Notably, post-irradiation, MTT-formazan formation was significantly reduced in Cbz-Bth-BDP-treated MDA-MB-231 cells relative to control groups, further validating the efficient photocatalytic conversion of NADH by Cbz-Bth-BDP (Fig. S41†). In the electron transport chain, NADH oxidation plays a crucial role in driving adenosine triphosphate (ATP) synthesis by generating a proton-motive force.49 During oxidative phosphorylation, electrons from NADH are transferred through the electron transport chain, ultimately combining with O2 to drive ATP synthesis. The oxidation of NADH to NAD+ is a key energy-yielding reaction in this process. Consistent with this, ATP levels were markedly reduced in Cbz-Bth-BDP-treated cells under light irradiation compared to other PSs (Fig. 4C). This disruption of NADH/NAD+ homeostasis by photoexcited Cbz-Bth-BDP underscores its photocatalytic impact, resulting in decreased ATP production.Upon photoirradiation, Cbz-Bth-BDP-treated cells exhibited distinct pyroptotic morphological changes, setting them apart from conventional apoptosis. To confirm these morphological changes, we performed confocal laser scanning microscopy (CLSM) with dual staining using FITC-Annexin V (green), a phosphatidylserine (PS) marker for membrane disruption, and Hoechst 33342 (blue), a nuclear stain. Unlike apoptosis, which is typically characterized by cell shrinkage, nuclear condensation, and apoptotic body formation, Cbz-Bth-BDP-treated cells exhibited robust FITC-Annexin V binding while maintaining intact nuclei, reinforcing that the observed changes were pyroptotic rather than apoptotic (Fig. 4D). Notably, other BODIPY-based photosensitizers (PSs) failed to induce these morphological changes under identical conditions (Fig. S42†), highlighting the unique ability of Cbz-Bth-BDP to trigger pyroptosis. This distinct behavior is attributed to its enhanced photocatalytic activity and efficient ROS generation, which activate the pyroptotic cascade. These findings emphasize the unique mechanism of Cbz-Bth-BDP in driving pyroptotic cell death, further distinguishing it from conventional apoptosis-inducing photosensitizers.
Pyroptosis, a proinflammatory form of programmed cell death, is driven by the gasdermin protein family, which forms membrane pores upon activation.27–29 Western blot assays confirmed that photoexcited Cbz-Bth-BDP triggered pyroptosis of MDA-MB-231 cells, evidenced by the upregulation of cleaved caspase-3 (active form of caspase 3) and GSDME-N (N-terminal fragment of gasdermin E, GSDME) (Fig. 4E and S43†). Moreover, the substantial release of lactate dehydrogenase (LDH) and ATP from Cbz-Bth-BDP-treated cells under photoirradiation (Fig. 4F and G) further provided additional evidence supporting the activation of pyroptosis. Additionally, confocal imaging of live/dead cell detection using calcein AM and propidium iodide (PI) assays supported these results. In this assay, live cells fluoresced green due to calcein AM, while dead cells emitted red fluorescence from PI staining. As shown in Fig. S44,† Cbz-Bth-BDP-treated cells displayed stronger red fluorescence after light exposure compared to other controls. Conversely, cells treated with other photosensitizers under similar conditions primarily exhibited green fluorescence. These findings position photoexcited Cbz-Bth-BDP as a highly effective and promising activator of pyroptosis among the heavy-atom-free thiophene-bridged BODIPYs.
To further evaluate the role of photocatalysis in Cbz-Bth-BDP-induced cell death, the ROS scavenger vitamin C (VC) was applied to neutralize ROS generated by Cbz-Bth-BDP. Pretreatment with VC reduced the cytotoxicity of Cbz-Bth-BDP under light irradiation compared to cells treated with Cbz-Bth-BDP alone. However, VC did not entirely prevent cell death, indicating that photocatalysis plays a significant role in Cbz-Bth-BDP-induced cytotoxicity (Fig. 4H). Western blot assays further confirmed that photoexcited Cbz-Bth-BDP could still trigger pyroptosis in the presence of VC, as evidenced by the upregulation of cleaved caspase-3 and GSDME-N (Fig. 4I and S45†). These findings underscore the critical role of the photocatalytic oxidation properties of Cbz-Bth-BDP in driving pyroptosis activation (Fig. 4J).
Photocatalytic activity of Cbz-Bth-BDP under hypoxic conditions
Photocatalytic therapy enables the direct oxidation of intracellular substrates via oxygen-independent pathways, thereby overcoming the inherent oxygen dependency in conventional phototherapy.41,43 Given the remarkable photocatalytic performance of Cbz-Bth-BDP in normoxic conditions, we further evaluated its efficacy in hypoxic environments. The cytotoxicity of Cbz-Bth-BDP against MDA-MB-231 cells was assessed using the MTT assay. As shown in Fig. 5A, a concentration of 2 μM Cbz-Bth-BDP induced significant cytotoxicity under light irradiation (530 nm, 100 mW cm−2, 10 min). In contrast, no cytotoxic effects were observed in cells treated with Cbz-Bth-BDP in the absence of light or with other PSs (TPA-BDP, TPA-th-BDP, and DP-Bth-BDP), irrespective of photoirradiation. Morphological changes in MDA-MB-231 cells after treatment with Cbz-Bth-BDP under light irradiation were further examined using the FITC-Annexin V/Hoechst 33342 staining assay. The cell membrane was stained with FITC-Annexin V, and the nucleus was stained with Hoechst 33342. After 10 min of light exposure (530 nm, 100 mW cm−2), cells treated with Cbz-Bth-BDP displayed intense green fluorescence, indicating membrane damage and pyroptotic body formation. In contrast, cells treated with other PSs displayed only blue fluorescence, signifying intact cell membranes (Fig. 5B). To confirm these findings, LDH release was quantified using the CytoTox96™, non-radioactive cytotoxicity assay. Cells treated with Cbz-Bth-BDP showed substantial LDH release upon light irradiation, confirming significant membrane damage. In comparison, minimal LDH release was detected in cells treated with other PSs, regardless of light exposure (Fig. 5C). Additionally, a calcein AM/PI assay was conducted to further evaluate cell death. As shown in Fig. 5D, cells treated with Cbz-Bth-BDP and exposed to light (530 nm, 100 mW cm−2, 10 min) exhibited strong red fluorescence, indicating effective cell death. Conversely, cells treated with other PSs under similar conditions predominantly exhibited green fluorescence. These results highlight the potent photocatalytic activity of Cbz-Bth-BDP in hypoxic conditions, demonstrating its potential as a promising candidate for antitumor therapy.3D tumor spheroid model to probe anti-cancer effects
Three-dimensional (3D) multicellular spheroids are widely recognized as a reliable model for evaluating drug sensitivity due to their structural and functional resemblance to in vivo solid tumors.50–52 Leveraging the potent photocatalytic properties of Cbz-Bth-BDP, we investigated its efficacy as an anticancer therapy using T47D breast cancer cell-derived 3D tumor spheroids (Fig. 6A). Upon treatment with Cbz-Bth-BDP and subsequent light irradiation, the spheroids exhibited significant cell death, as evidenced by confocal imaging with calcein AM (green, indicating live cells) and propidium iodide (PI, red, marking dead cells). In stark contrast, other BODIPY PSs displayed minimal cytotoxicity, regardless of photoirradiation (Fig. 6B). Additionally, photoactivated Cbz-Bth-BDP significantly inhibited the formation of T47D 3D spheroids compared to other PSs (Fig. 6C). This inhibition correlated with a substantial reduction in ATP production in the Cbz-Bth-BDP-treated group with light irradiation, highlighting its effective disruption of tumor spheroid metabolism (Fig. 6D). These findings highlight the potential of Cbz-Bth-BDP as a promising anticancer agent, demonstrating potent photocatalytic activity and significant therapeutic effects in a 3D tumor model.Conclusion
In this study, we developed Cbz-Bth-BDP, a novel heavy-atom-free photosensitizer that leverages a weak yet semi-rigid donor to achieve highly efficient ISC through a T2-mediated SOCT-ISC pathway. The incorporation of a weak donor strategically destabilizes charge transfer in a singlet state, aligning it energetically with the T2 states. This energy-level fine-tuning narrows the singlet–triplet energy gap, enhances spin–orbit coupling, and increases electron affinity, collectively boosting ISC efficiency and overall photocatalytic performance. The unique structural and photophysical properties of Cbz-Bth-BDP enable robust biological effects, including effective ROS generation, irreversible NADH oxidation, and cytochrome c transformation. These features drive pyroptosis via activating caspase 3 and GSDME, inducing cell death even under hypoxic conditions. Additionally, Cbz-Bth-BDP demonstrates significant anticancer potential by effectively inhibiting the growth of 3D tumor spheroids. While the current study establishes a strong foundation for Cbz-Bth-BDP's pyroptosis-mediated cancer immunotherapy, its in vivo application remains limited by high-energy absorption and short excitation wavelengths. Future research will focus on molecular engineering strategies to extend absorption into the red/NIR region, improve tissue penetration, and enhance biocompatibility, ultimately facilitating clinical translation. Overall, this work presents Cbz-Bth-BDP as a promising next-generation photosensitizer, offering a transformative strategy for pyroptosis-mediated cancer therapy and expanding the frontier of photocatalytic cancer immunotherapy.Data availability
Detailed synthetic schemes, experimental procedures, theoretical calculations, and other figures are provided in the ESI file.†Author contributions
Y. X., M. L., X. L., and J. S. K. conceived and supervised the study. H. S. K. contributed to the experimental design and manuscript writing. H. S. K. and H. R. performed the synthetic work and characterized the optical properties. Y. X. and H. R. carried out the anticancer mechanisms. M. I. and S. C. carried out the computational studies. H. H. and Y. Y. K. assisted with material preparation and characterization. J.-W. K. assisted with manuscript editing and revision. All authors discussed the results and provided comments on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the financial support from the National Research Foundation of Korea (CRI project no. 2018R1A3B1052702, J. S. K.; RS-2023-00241100, Y. X.). This work is also supported by the Ministry of Education, Singapore (MOE-T2EP10222-0001), Singapore University of Technology and Design (SUTD) Kickstarter Initiative (SKI 2021_03_10), National Natural Science Foundation of China (Grant No. 22308220, M. L.; No. 82203050, Y. X.), Province Key Areas Special Project for Regular Colleges and Universities (Grant No. 2024ZDZX2018, M. L.), Shenzhen University Third-Phase Project of Constructing High-Level University (Grant No. 000001032104, M. L.), Research Team Cultivation Program of Shenzhen University (Grant No. 2023QNT005, M. L.), and KRICT core project (KS2321-20). The authors are grateful for the computing service of SUTD and the National Supercomputing Centre (Singapore).References
- H. Cheng, X. Cao, S. Zhang, K. Zhang, Y. Cheng, J. Wang, J. Zhao, L. Zhou, X. Liang and J. Yoon, Adv. Mater., 2023, 35, 2207546 CrossRef CAS PubMed.
- B.-K. Liu, J. Zheng, H. Wang, L.-Y. Niu and Q.-Z. Yang, Mater. Chem. Front., 2023, 7, 5879–5890 RSC.
- J. Wang, Q. Gong, L. Jiao and E. Hao, Coord. Chem. Rev., 2023, 496, 215367 CrossRef CAS.
- E. Bassan, A. Gualandi, P. G. Cozzi and P. Ceroni, Chem. Sci., 2021, 12, 6607–6628 RSC.
- A. Kamkaew, S. H. Lim, H. B. Lee, L. V. Kiew, L. Y. Chung and K. Burgess, Chem. Soc. Rev., 2013, 42, 77–88 RSC.
- Z. Fan, K. Teng, Y. Xu, L. Niu and Q. Yang, Angew. Chem., Int. Ed., 2025, 64, e202413595 CrossRef CAS PubMed.
- M. Won, S. Koo, H. Li, J. L. Sessler, J. Y. Lee, A. Sharma and J. S. Kim, Angew. Chem., Int. Ed., 2021, 60, 3196–3204 CrossRef CAS PubMed.
- H. S. Jung, J. Han, H. Shi, S. Koo, H. Singh, H.-J. Kim, J. L. Sessler, J. Y. Lee, J.-H. Kim and J. S. Kim, J. Am. Chem. Soc., 2017, 139, 7595–7602 CrossRef CAS PubMed.
- J. Zou, Z. Yin, K. Ding, Q. Tang, J. Li, W. Si, J. Shao, Q. Zhang, W. Huang and X. Dong, ACS Appl. Mater. Interfaces, 2017, 9, 32475–32481 CrossRef CAS PubMed.
- S. Mula and M. Koli, ChemMedChem, 2024, 19, e202400041 CrossRef CAS PubMed.
- M. A. Filatov, Org. Biomol. Chem., 2020, 18, 10–27 RSC.
- X. Xiao, K. Ye, M. Imran and J. Zhao, Appl. Sci., 2022, 12, 9933 CrossRef CAS.
- X. Shi, Y. Wang, F. Qi, H. Zhang, Y. Cao, X. Xu, W. Liu and C. Li, Small, 2024, 20, 2405496 CrossRef CAS PubMed.
- W. Li, Q. Gong, Q. Wu, L. Guo, X. Guo, D. Guo, L. Jiao and E. Hao, Chem. Commun., 2023, 59, 12330–12333 RSC.
- Y. Dong, B. Dick and J. Zhao, Org. Lett., 2020, 22, 5535–5539 CrossRef CAS PubMed.
- X. Fu, Y. Man, C. Yu, Y. Sun, E. Hao, Q. Wu, A. Hu, G. Li, C.-C. Wang and J. Li, J. Org. Chem., 2024, 89, 4826–4839 CrossRef CAS PubMed.
- W. Bu, C. Yu, Y. Man, J. Li, Q. Wu, S. Gui, Y. Wei, L. Jiao and E. Hao, Chem. Commun., 2024, 60, 9809–9812 RSC.
- S. Qi, N. Kwon, Y. Yim, V.-N. Nguyen and J. Yoon, Chem. Sci., 2020, 11, 6479–6484 RSC.
- J. M. Lee, J. M. Park, H. K. Lee, H. M. Kim, J. H. Kim and J. P. Kim, Dyes Pigm., 2021, 196, 109662 CrossRef CAS.
- Y. Hou, Q. Liu and J. Zhao, Chem. Commun., 2020, 56, 1721–1724 RSC.
- P. Zhao, Z. Wang, Y. Wang, Z. Wu, Y. Guo, C. Wang, X. Fang, Z. Qu, H. Wang and G. Zhao, Dyes Pigm., 2023, 214, 111214 CrossRef CAS.
- S. Callaghan, M. A. Filatov, H. Savoie, R. W. Boyle and M. O. Senge, Photochem. Photobiol. Sci., 2019, 18, 495–504 CrossRef CAS PubMed.
- M. A. Filatov, S. Karuthedath, P. M. Polestshuk, S. Callaghan, K. J. Flanagan, M. Telitchko, T. Wiesner, F. Laquai and M. O. Senge, Phys. Chem. Chem. Phys., 2018, 20, 8016–8031 RSC.
- V. Nguyen, Y. Yim, S. Kim, B. Ryu, K. M. K. Swamy, G. Kim, N. Kwon, C. Kim, S. Park and J. Yoon, Angew. Chem., Int. Ed., 2020, 59, 8957–8962 CrossRef CAS PubMed.
- F.-Z. Xu, L. Zhu, H.-H. Han, J.-W. Zou, Y. Zang, J. Li, T. D. James, X.-P. He and C.-Y. Wang, Chem. Sci., 2022, 13, 9373–9380 RSC.
- A. Uddin, S. R. Allen, A. K. Rylski, C. J. O'Dea, J. T. Ly, T. A. Grusenmeyer, S. T. Roberts and Z. A. Page, Angew. Chem., Int. Ed., 2023, 62, e202219140 CrossRef CAS PubMed.
- K. Wang, Q. Sun, X. Zhong, M. Zeng, H. Zeng, X. Shi, Z. Li, Y. Wang, Q. Zhao, F. Shao and J. Ding, Cell, 2020, 180, 941–955 CrossRef CAS PubMed.
- M. Jiang, L. Qi, L. Li and Y. Li, Cell Death Discov., 2020, 6, 112 CrossRef CAS PubMed.
- S. Rühl, K. Shkarina, B. Demarco, R. Heilig, J. C. Santos and P. Broz, Science, 2018, 362, 956–960 CrossRef PubMed.
- X. Yang, X. Wang, X. Zhang, J. Zhang, J. W. Y. Lam, H. Sun, J. Yang, Y. Liang and B. Z. Tang, Adv. Mater., 2024, 36, 2402182 CrossRef CAS PubMed.
- P. Xiao, Z. Shen, D. Wang, Y. Pan, Y. Li, J. Gong, L. Wang, D. Wang and B. Z. Tang, Adv. Sci., 2022, 9, 2104079 CrossRef CAS PubMed.
- F. Ma, S. Zhang, J. Jiang, Y. Liu, J. Sun, J. W. Y. Lam, Z. Zhao and B. Z. Tang, Adv. Mater., 2025, 37, 2414188 CrossRef CAS PubMed.
- Z. Li, B. Z. Tang and D. Wang, Adv. Mater., 2024, 36, 2406047 CrossRef CAS PubMed.
- C. Lee, M. Park, W. C. B. Wijesinghe, S. Na, C. G. Lee, E. Hwang, G. Yoon, J. K. Lee, D.-H. Roh, Y. H. Kwon, J. Yang, S. A. Hughes, J. E. Vince, J. K. Seo, D. Min and T.-H. Kwon, Nat. Commun., 2024, 15, 4025 CrossRef CAS PubMed.
- H. Huang, S. Banerjee, K. Qiu, P. Zhang, O. Blacque, T. Malcomson, M. J. Paterson, G. J. Clarkson, M. Staniforth, V. G. Stavros, G. Gasser, H. Chao and P. J. Sadler, Nat. Chem., 2019, 11, 1041–1048 CrossRef CAS PubMed.
- Z. Zhao, Free Radic. Biol. Med., 2023, 208, 510–515 CrossRef CAS PubMed.
- Y.-F. Kang, W.-K. Chen, K.-X. Teng, L.-Y. Wang, X.-C. Xu, L.-Y. Niu, G. Cui and Q.-Z. Yang, CCS Chem., 2022, 4, 3516–3528 CrossRef CAS.
- W. Chi, J. Dai, C. Yan, D. Tan, Z. Guo and X. Liu, J. Mater. Chem. C, 2023, 11, 10205–10214 RSC.
- W. Chi, J. Chen, W. Liu, C. Wang, Q. Qi, Q. Qiao, T. M. Tan, K. Xiong, X. Liu, K. Kang, Y.-T. Chang, Z. Xu and X. Liu, J. Am. Chem. Soc., 2020, 142, 6777–6785 CrossRef CAS PubMed.
- J. T. Buck, A. M. Boudreau, A. DeCarmine, R. W. Wilson, J. Hampsey and T. Mani, Chem, 2019, 5, 138–155 CAS.
- M. Li, Y. Xu, Z. Pu, T. Xiong, H. Huang, S. Long, S. Son, L. Yu, N. Singh, Y. Tong, J. L. Sessler, X. Peng and J. S. Kim, Proc. Natl. Acad. Sci. U. S. A, 2022, 119, e2210504119 CrossRef CAS PubMed.
- Y. Xu, C. V. Chau, J. Lee, A. C. Sedgwick, L. Yu, M. Li, X. Peng, J. S. Kim and J. L. Sessler, Proc. Natl. Acad. Sci. U. S. A, 2024, 121, e2314620121 CrossRef CAS PubMed.
- M. Li, K. H. Gebremedhin, D. Ma, Z. Pu, T. Xiong, Y. Xu, J. S. Kim and X. Peng, J. Am. Chem. Soc., 2022, 144, 163–173 CrossRef CAS PubMed.
- N. Zhang, S. Trépout, H. Chen and M.-H. Li, J. Am. Chem. Soc., 2023, 145, 288–299 CrossRef CAS PubMed.
- K.-X. Teng, L.-Y. Niu, N. Xie and Q.-Z. Yang, Nat. Commun., 2022, 13, 6179 CrossRef CAS PubMed.
- L. Yu, Y. Xu, Z. Pu, H. Kang, M. Li, J. L. Sessler and J. S. Kim, J. Am. Chem. Soc., 2022, 144, 11326–11337 CrossRef CAS PubMed.
- R. P. Goodman, A. L. Markhard, H. Shah, R. Sharma, O. S. Skinner, C. B. Clish, A. Deik, A. Patgiri, Y.-H. H. Hsu, R. Masia, H. L. Noh, S. Suk, O. Goldberger, J. N. Hirschhorn, G. Yellen, J. K. Kim and V. K. Mootha, Nature, 2020, 583, 122–126 CrossRef CAS PubMed.
- C. Cantó, K. J. Menzies and J. Auwerx, Cell Metab., 2015, 22, 31–53 CrossRef PubMed.
- H. Li, S. K. Kolluri, J. Gu, M. I. Dawson, X. Cao, P. D. Hobbs, B. Lin, G. Chen, J. Lu, F. Lin, Z. Xie, J. A. Fontana, J. C. Reed and X. Zhang, Science, 2000, 289, 1159–1164 CrossRef CAS PubMed.
- S. A. Saemundsson, S. D. Curry, B. M. Bower, E. J. DeBoo, A. P. Goodwin and J. N. Cha, Biomater. Sci., 2024, 12, 4759–4769 RSC.
- E. C. Costa, A. F. Moreira, D. De Melo-Diogo, V. M. Gaspar, M. P. Carvalho and I. J. Correia, Biotechnol. Adv., 2016, 34, 1427–1441 CrossRef PubMed.
- J. Friedrich, C. Seidel, R. Ebner and L. A. Kunz-Schughart, Nat. Protoc., 2009, 4, 309–324 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc03466c |
| ‡ These authors contribute equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |