Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Peptide macrocyclisation via intramolecular interception of visible-light-mediated desulfurisation
Frances R.
Smith
a,
Declan
Meehan
a,
Rhys C.
Griffiths
a,
Harriet J.
Knowles
a,
Peiyu
Zhang
b,
Huw E. L.
Williams
c,
Andrew J.
Wilson
bd and
Nicholas J.
Mitchell
*a
aSchool of Chemistry, University of Nottingham, University Park, Nottingham, NG7 2RD, UK. E-mail: nicholas.mitchell@nottingham.ac.uk
bSchool of Chemistry, University of Leeds, Woodhouse Lane, Leeds, LS2 9JT, UK
cBiodiscovery Institute, University of Nottingham, University Park, Nottingham, NG7 2RD, UK
dSchool of Chemistry, University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK
First published on 31st January 2025
Abstract
Correction for ‘Peptide macrocyclisation via intramolecular interception of visible-light-mediated desulfurisation’ by Frances R. Smith et al., Chem. Sci., 2024, 15, 9612–9619, https://doi.org/10.1039/D3SC05865D.
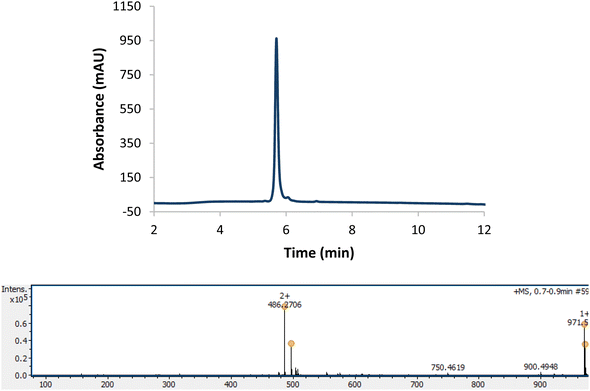
The authors regret that the incorrect analytical HPLC trace was assigned to product 53 (carba-oxytocin) in the ESI. The corrected experimental procedure, and the analytical HPLC trace and ESI-MS data for 53 (Fig. S148) have been provided here; the revised isolated yield for 53 is 35%.
The updated supplementary information file has been included with this correction article.
Fig. S148. Analytical HPLC trace and ESI MS of cyclised H-CYIQN(alG)PLG-NH2 (53); analytical gradient 10–50% B over 10 minutes, 210 nm. Calculated mass [M + H]+: 971.52, [M + 2H]2+: 486.26; observed mass [M + H]+: 971.53, [M + 2H]2+: 486.27.
Product 53 was synthesised following the optimised cyclisation protocol using H-CYIQN(alG)PLG-NH2 (52, 5 mg, 4.98 μmol). After analysis the remaining solution (4.89 μmol) was purified using semi-preparative HPLC (10–70% B over 30 minutes); the fractions containing the main products were lyophilised to yield the cyclised title compound (1.7 mg, 1.73 μmol, 35% yield) and the linear desulfurised by-product (2.5 mg, 2.54 μmol, 52% yield), both as fluffy white solids.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2025 |