Open Access Article
Open Access ArticleJUNO-Checked – a live cell electrochemical biosensor for sperm function diagnostics†
Kushagr Punyaniab,
Ingela Liljeqvist Solticc,
Maria Liljandera,
Panchami Pradeepkumara,
Carolin Psotaa,
Frida Lundblanda,
Tore Duvolda,
Donogh FitzGeraldd,
Jaime Castillo-León *a and
Jae Shin*a
*a and
Jae Shin*a
aSpermosens AB, Medicon Village, Scheeletorget 1, 22381 Lund, Sweden. E-mail: jc@spermosens.com; shinjaeyen@gmail.com
bNestedBio AB, Medicon Village, 223 81, Lund, Sweden
cReproduktionsmottagning, Skåne University Hospital, 205 02, Malmö, Sweden
dFlexMedical Solutions Ltd, Eliburn Ind Park, Livingston EH54 6GQ, UK
First published on 20th March 2025
Abstract
Conventional diagnosis for male-factor infertility primarily comprises standard microscopic semen analysis, including sperm count or concentration, motility, and morphology of sperm. Unfortunately, this standard analysis offers limited predictive value for pregnancy or the success of assisted reproduction treatments. There is an urgent need for diagnostic tools that effectively probe sperm function. This study discloses a novel diagnostic tool, JUNO-Checked, comprising an electrochemical biosensor for sperm function inspired by the molecular mechanisms of fertilization, utilizing immobilized JUNO protein of oocyte origin. We show that the JUNO-Checked biosensor can quantify sperm binding to the JUNO functionalized biosensor, represented here as the JUNOScore. This score provides a unique insight into sperm function that is inaccessible through standard microscopic semen analysis. Our results underscore the novelty and potential utility of the JUNO-Checked biosensor in clinical settings for diagnostics and personalized assisted reproduction treatments.
Introduction
The limitations of standard semen analysis have long-constrained diagnosis and laboratory assessment of male-factor infertility. Since 1980, the World Health Organization (WHO) has provided guidelines for standard semen analysis that take sperm count or concentration, motility, and morphology into account and have remained unchanged for several decades.1 While informative, standard semen analysis is known to have limited value for the prognosis of fertility outcomes for natural pregnancy or success of assisted reproduction treatments (ART).2,3 Given the alarming rate at which sperm count and concentration have fallen globally in the last five decades, and even more so in recent years,4,5 there is a pressing need for the incorporation of sperm function analysis in laboratory diagnosis of male-factor infertility, as well as personalization of ART.6,7Advanced tests for sperm function analysis have long existed within academic environments, primarily focusing on sperm DNA integrity, acrosome status, and the ability of sperm to bind to human or animal oocytes.8–10 Recently, an electrochemical diagnostic device that probes the redox potential of seminal plasma to quantify the level of oxidative stress has also been described and commercialized as the MiOXSYS system.11 However, clinical implementation of these diagnostic methods is contentious due to conflicting clinical evidence and lack of standardization.8,12–14
The last decade has seen increasing evidence and expert consensus for the implementation of sperm function tests, especially the analysis of sperm DNA fragmentation in clinical practice for both ART and pregnancy loss.15–17 Despite these advances, sperm function tests that can effectively assess the ability of sperm to bind to and fertilize the oocyte have mainly existed in academic literature,6,18 including the hemizona assay, which has shown promising results for fertility outcomes in meta-analyses.8 Since sperm function tests assess the fertilization competence of sperm, they have the potential to help create personalized ART regimens by assisting with a reasonable selection of the appropriate treatments among intra uterine insemination (IUI), standard IVF (stIVF) and/or intra cytoplasmic sperm injection (ICSI).18
To develop a sperm function diagnostic tool, we resorted to inspiration from the molecular mechanisms underlying human fertilization. A functional sperm–oocyte interaction requires sperm to undergo an acrosome reaction, wherein the acrosome membrane, an anterior organelle in the sperm-head, fuses with the sperm plasma membrane. Subsequently, during sperm–egg interaction, the sperm IZUMO protein superfamily, that relocates to the sperm head outer membrane prior to fertilization,19,20 is critically involved in sperm oocyte fusion.21 With the discovery of oocyte membrane protein JUNO as the binding partner of sperm IZUMO1, it was shown that JUNO IZUMO1 pairing is the critical step for fertilization in rodents22 and humans.23
Further, independent research groups have reported the involvement of the IZUMO1 dysregulation and phosphorylation in malefactor infertility24,25 thereby underscoring the JUNO sperm-binding as the ideal sperm-function test to assess the fertilization competence of sperms. It is important to note that sperm binding to JUNO has been shown to be a good predictor for bovine fertility, a model animal to study fertility.26
In the current work, we disclose a JUNO-containing electrochemical biosensor for sperm function assessment, coined the JUNO-Checked biosensor (Fig. 1).27 The JUNO-Checked biosensor can detect IZUMO1 in solution, as well as through a semi-automated analysis, to evaluate sperm–JUNO binding as a semi-quantitative JUNOScore. While lab-on-a-chip assays for analysis of seminal plasma11 or semen concentration and motility28 have been previously described, to the best of our knowledge, JUNO-Checked is the first live-cell electrochemical biosensor that analyses the binding of motile mammalian cells to a gatekeeper membrane protein. Clinical semen samples were also tested with the JUNO-Checked biosensor, which aims to provide complementary information to standard semen analysis for personalized ART treatments.
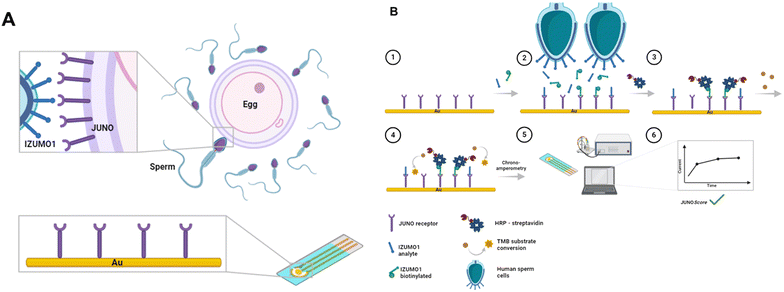 | ||
| Fig. 1 Design of the JUNO-Checked biosensor and assay: A. schematic representation of the sperm IZUMO1 and oocyte JUNO interaction during the fertilization process; B. JUNO-Checked biosensor assay design wherein1 the biosensor comprises of a gold transducer and surface-immobilized recombinant human JUNO as the biorecognition element.2 The JUNO-Checked biosensor is incubated with the competing agent – biotinylated-IZUMO1 (biotin–IZUMO1), and the analyte of interest – IZUMO1 or human sperms. After incubation,3 streptavidin–horseradish peroxidase (strep–HRP) conjugate is allowed to detect the remaining competing agent, followed by4 addition of and reaction with the HRP substrate, 3,3′,5,5′-tetramethylbenzidine (TMB).5 The electrochemical readout is enabled via chronoamperometry with a potentiostat setup, and6 the binding is visualized as the relative displacement of the competing agent in terms of current values or JUNOScore. | ||
Materials and methods
Materials
Dithio-bis(succinimidyl propionate) (DSP), tris(2-carboxyethyl)phosphine (TCEP), dimethyl sulfoxide (DMSO), phosphate buffered saline, 2-(N-morpholino)ethanesulfonic acid (MES buffer), N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) (HEPES buffer), sodium chloride (NaCl), casein hydrolysate, α,α-trehalose and 3,3′,5,5′-tetramethylbenzidine (TMB) were purchased from Merck Life Science UK Limited or Merck Life Science AB, and were of at least MQ200 quality grade. Recombinant human proteins from mammalian hosts, including JUNO, IZUMO1, and biotinylated-IZUMO1 (biotin–IZUMO1), and thin-film gold three-electrode sensors were purchased from FlexMedical Solutions Ltd (UK). Invitrogen's horseradish peroxidase-conjugated streptavidin (strep–HRP), Fujifilm irvine scientific's continuous single culture complete with gentamycin and HSA (CSCM-C) were purchased from Thermo Fisher Scientific (UK), Novovitae (Denmark), respectively.Development of JUNO-Checked biosensor
Bare gold sensors were functionalized by creating a self-assembled monolayer with DSP and TCEP. Briefly, stock solutions of DSP in DMSO and TCEP in MES were diluted in a solution containing 10% DMSO and 90% MES buffer. The final solution containing 1 mM DSP and 2 mM TCEP was added to the electrodes to fully cover the sensor surface, followed by 1 h incubation, rinsing with MES buffer and tapping dry. 5 μg mL−1 JUNO, in a solution containing 10 mM HEPES and 140 mM NaCl, was incubated for 1 h, followed by rinsing with HEPES buffer. Finally, the biosensors were blocked using 1 mg mL−1 hydrolyzed casein dissolved in PBS for 1 h, then rinsing with PBS. All incubations were at room temperature unless specified. Lastly, the drying buffer (50 mM Tris, 100 mM glycine, 500 mM NaCl, pH 7.5, 15% trehalose) was added to the electrode surface and dried at 40 °C for two hours. This drying buffer was used to maintain both the structure and function of the sensor and to allow for the stability of the active proteins for an extended period.A direct enzymatic assay was performed with biotin–IZUMO1, strep–HRP, and TMB to assess the functionality of the JUNO-Checked biosensor. To this end, biosensors were washed with 2 mL PBS and gently tapped dry. 70 μL of the biotin–IZUMO1 were added to the electrode surface and incubated for 1 h. Various concentrations of biotin–IZUMO were tested (250, 500, and 1000 ng mL−1). Upon rinsing with 1 mL PBS, 70 μL strep–HRP was added and incubated for one hour. After the incubation, the electrodes were washed with PBS, and 70 μL TMB was added and incubated in the dark for 20 min.
The biosensors were connected to the MultiPalmsSens potentiostat (PalmSens, The Netherlands) to perform a chronoamperometric analysis for 60 s at an applied potential of −0.2 V.
Clinical sample collection, analysis and processing
This study exclusively used leftover sperm samples from donors who had already provided informed consent for their samples to be used in research. Malmö Reproductive Medicine Center managed the consent process following national regulations and ethical guidelines.The samples were fully anonymized at the point of collection, with no direct or indirect identifiers and no possibility of re-identification. This distinction is critical, as it determines the ethical and regulatory requirements for human biological material research.
According to the Swedish Ethical Review Authority (Etikprövningsmyndigheten), studies using fully anonymized human samples without connection to the donor exist and do not require formal ethical approval. Furthermore, all samples were handled strictly in line with national and institutional guidelines, used solely for the intended analysis, and immediately discarded thereafter under standard protocols for biological material.
Leftover, anonymized semen samples were received after standard diagnostic analysis from healthy volunteers among patients visiting the Andrology Lab, Reproduktionsmedicinskt centrum, Skåne University Hospital, Malmö. Only volunteers aged 23–45 years, in good general health, and free from any known reproductive or sexual health disorders, were included in the study. Further, semen samples that qualified the following parameters were included: successful liquefaction between 15–60 min, under rocking at 37 °C; total semen ejaculate volume between 1–8 mL; sperm concentration ≥16 × 106 mL−1; progressive motility ≥30%; and leftover semen sample volume ≥500 μL. The liquefied semen samples were received and used immediately.
Sperm concentration and motility were assessed upon receipt of a liquefied semen sample and after simple washing. Briefly, 3 μL of semen or sperm sample was added to a Counting Chamber SCA (Microptic S.L., Spain) and was analyzed using a Nikon Ci-L microscope at 10× magnification under positive phase contrast, in conjunction with SCA Morphology module (Microptic S.L., Spain).
Liquefied leftover semen samples were processed with simple washing to remove the seminal plasma as described elsewhere.29 Briefly, 500 μL liquefied leftover semen samples were washed twice by diluting in 500 μL CSCM-C at 37 °C, followed by centrifugation at 500g for 10 min. The pellet was finally resuspended in 500 μL CSCM-C. The washed sample was then assessed for concentration and motility as described earlier, followed by adjusting the concentration of the sperm samples to 5, 10, 15, and 20 × 106 mL−1 in CSCM-C.
IZUMO1 detection and sperm binding assay on JUNO-Checked sensor
For the competitive chronoamperometric assay on the JUNO-Checked biosensors, IZUMO1 analyte at 50, 125, 250, 500, or 1000 ng mL−1 concentrations in PBS, or washed sperm samples at the desired concentration were used as the analyte solutions. As described earlier, the biosensors were washed to remove the drying buffer. The analyte solutions were dispensed to JUNO-Checked biosensors and incubated for 30 min at 37 °C, followed by washing with 2 mL of PBS and gently tapping dry. A fixed concentration of 125 ng mL−1 biotin–IZUMO1 was added to the sensors as a competing agent and incubated for 60 min. The sensors were flushed twice with 1 mL PBS and gently tapped dry. 70 μL strep–HRP was dispensed on the electrode surface, followed by 60 min incubation and washing twice with 1 mL PBS. Finally, 70 μL TMB was added to the electrodes and incubated in the dark for 20 min. All incubations were at room temperature unless specified. Chronoamperometric analysis at an applied potential of −0.2 V was performed with the MultiPalmsSens potentiostat (PalmSens, The Netherlands).Results and discussion
Development of the JUNO-Checked sensor
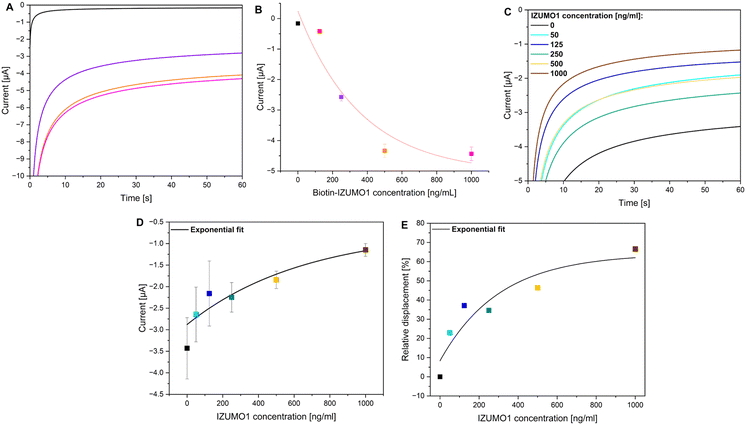
The JUNO-Checked biosensor is intended to assess the fertilization competence of sperms in a clinical setting, i.e., the test should measure the binding of JUNO-containing biosensors to sperm cells that are alive. However, developing an assay for living cells poses technical challenges, as the assay needs to be both specific and gentle. To accomplish this, we combined chronoamperometry and a competitive enzymatic-protein-linked assay. The performance of the assay design was initially tested in a direct enzymatic chronoamperometric assay using the binding of biotin–IZUMO1 to the JUNO-Checked biosensor, where the chronoamperometric reporting was enabled through the enzymatic reaction between the cognate strep–HRP and the TMB substrate.As HRP oxidizes TMB, a reduction potential was applied during the electrochemical analysis to reduce the oxidized TMB species at the working electrode. This allowed for the detection of the oxidized TMB, thereby reflecting the amount of the cognate step-HRP bound to the biotin–IZUMO1 immobilized on the JUNO-Checked biosensor surface. As expected, the observed current in chronoamperometry steadily increased with time for each of the tested concentrations of biotin–IZUMO1, reaching a plateau of the measured current within 60 s (Fig. 2A). This behavior can be attributed to the diffusion of the oxidized TMB species to the electrode surface. Further, the magnitude of the current response value observed at t = 60 s increased exponentially (R2 = 0.99) with the increasing concentration of biotin–IZUMO1 (Fig. 2B), indicating successful binding of IZUMO1 to JUNO immobilized on the sensors.
IZUMO1 detection using the electrochemical JUNO-Checked biosensor
The performance of the chronoamperometric competitive assay on the JUNO-Checked biosensor was tested at various concentrations of the IZUMO1 analyte, wherein biotin–IZUMO1 was used as a competing agent. As before, the chronoamperometric analysis allowed for the detection of the oxidized TMB, thereby reflecting the amount of the cognate step-HRP bound to the biotin–IZUMO1 tethered on the JUNO-Checked biosensor surface. Thus, similar to the previous direct enzymatic assay, the observed current in chronoamperometry steadily increased with time for each experiment, eventually reaching a plateau (Fig. 2C).However, given the assay was designed for IZUMO1 analyte and biotin–IZUMO1 to compete for the available JUNO at the biosensor surface, with increasing concentrations of IZUMO1 analyte at a fixed biotin–IZUMO1 concentration, the magnitude of the current response value observed at t = 60 s decreased exponentially with the increasing concentration of IZUMO1 analyte (Fig. 2D). This may be attributed to the binding of IZUMO1 analyte to the immobilized JUNO on the biosensor surface, leading to effective displacement of biotin–IZUMO1 (or prevention of its binding) and consequently strep–HRP, thereby leading to a decrease in HRP–TMB redox reporting. This dose-dependent effect followed an exponential trend with R2 = 0.91 (Fig. 2D).
The binding of the IZUMO1 analyte was quantified in terms of the relative displacement of biotin–IZUMO1, measuring the relative decrease in current response value observed at t = 60 s, normalized to a null concentration of IZUMO1 (Fig. 2E). Relative displacement was found to be directly correlated with increasing concentration of IZUMO1 (R2 = 0.88), thereby demonstrating the ability of the JUNO-Checked biosensor to detect IZUMO1 in solution effectively.
Detection of human sperm cells with the JUNO-Checked biosensor
After demonstrating a dose-dependent response of the JUNO-Checked biosensor against the purified human sperm IZUMO1 protein, the biosensor's performance was tested for sperm binding in clinical semen samples. For this purpose, sperm samples were adjusted to the following concentrations: 5, 10, and 20 × 106 mL−1 (for details, see Materials and methods) and assayed with the competitive assay protocol using the biotin–IZUMO1 as the competing agent and redox reporting through the HRP–TMB reaction.Remarkably, the sensor showed similar behavior as with the IZUMO1 analyte protein. The current, measured through chronoamperometry, showed a steady increase as expected, with a plateau at approximately t = 60 s (Fig. 3A). Presumably indicating upon binding of the sperm cells to the JUNO-Checked biosensor, sperms were also observed to displace biotin–IZUMO1 and strep–HRP. Consequently, with higher concentrations of sperms, a lower magnitude of current was observed at an arbitrary time point (Fig. 3A). The electrochemical response of sperm-binding on the JUNO-Checked biosensor also showed an exponential trend for current values at t = 60 s (R2 = 0.99, Fig. 3B). These values were represented as relative displacement of biotin–IZUMO1 by the sperms by normalizing the relative decrease in sensor current response value at t = 60 s for null concentrations (Fig. 3C).
While averaged relative displacements were found to be directly correlated with the sperm concentrations, each clinical semen sample was attributed with a numerical coefficient called JUNOScore (Fig. 3D), calculated as its respective relative displacement at the fixed sperm concentration of 10 × 106 mL−1, and normalized on a scale of 0–10. Here, JUNOScores 0 and 10 correspond to a theoretically null and 100% relative displacement of biotin–IZUMO1 in the competitive assay, indicating poor and excellent sperm–JUNO binding, respectively. Interestingly, a significant spread of JUNOScores was found in different semen samples, shedding light on the heterogeneity of the binding capacity of sperms to the oocyte protein JUNO.
To investigate a relationship between standard semen analysis parameters and the JUNOScore, we have measured the concentration and motility of the sperm samples (details in Materials and methods). Surprisingly, sperm concentration (Fig. 3E) and progressive motility (Fig. 3F) did not correlate with JUNOScore, thereby elucidating the uniqueness of JUNOScore owing to the testing of JUNO-binding in the assay design. The lack of correlation between a sperm–JUNO binding dependent JUNOScore and the WHO-recommended standard semen parameters further underscores the novelty and potential utility of the JUNOScore in clinical settings as a complementary diagnostic measure to the standard semen analysis.
Conclusions
Since the publication of the first WHO manual for semen analysis in 1980, microscopic analysis of sperm concentration, motility, and morphology has been the foundation of diagnosis for male-factor infertility. While we have learned that sperm count and concentration have been declining rapidly in the last five decades, microscopic semen analysis has shown clear limitations in yielding clinically helpful information for the prognosis of pregnancy or the success of ART. Thus, there is an urgent need for improving the diagnostics for male-factor infertility.The current work has introduced a significant advancement in reproductive medicine by developing a JUNO-Checked biosensor to detect JUNO–IZUMO1 binding—a gatekeeper step in oocyte fertilization. Inspired by the specific interaction during sperm–oocyte fusion, we have demonstrated a biosensor that can detect different concentrations of IZUMO1 in solution by allowing in vitro JUNO–IZUMO1 binding. By use of clinical semen samples, we have further shown that the JUNO-Checked biosensor enables live-cell detection of motile sperms by on-chip capture and can be used for direct assessment of sperm's ability to engage in the fertilization process.
Our findings reveal that the JUNOScore, derived from electrochemical analysis of sperm–JUNO binding on the surface of the JUNO-Checked biosensor, presents unique information that does not correlate with conventional semen analysis of the ejaculate semen sample, including sperm concentration and motility. This lack of correlation underscores the potential of JUNOScore to serve as a novel diagnostic parameter, offering insights into male-factor infertility that were previously inaccessible through standard laboratory evaluations. Future studies shall address the effect of media and matrix on the performance of JUNO-Checked biosensor and JUNOScore, as well as their utility in predicting the success of fertilization in stIVF and other treatment outcomes.
In conclusion, integrating complementary biosensor technologies, like the JUNO-Checked biosensor, in the assessment of sperm-function offers a promising avenue in the field. This would pave the way for enhanced first-line diagnostic precision, shed light on idiopathic and unexplained male infertility, and facilitate personalized assisted reproduction treatments.
Data availability
The datasets supporting this article have been uploaded as part of the ESI.†Author contributions
K. P.: conceptualization, methodology, funding acquisition and writing, I. L. S.: supervision and investigation, M. L.: resources, supervision, and investigation, P. P.: investigation, F. L.: investigation, T. D.: funding acquisition resources, C. P.: formal analysis, visualization, and writing, D. F.: resources and data curation, J. C. L.: visualization, data curation, and writing; J. S.: conceptualization, investigation, data curation, and writing.Conflicts of interest
Kushagr Punyani is a founder and owns stock in Spermosens AB and Nested Bio. Maria Liljander, Panchami Pradeepkumar, Jaime Castillo-León, Frida Lunbland, Carolin Psotta, Jae Shin, and Tore Duvold have been employees or consultants at Spermosens AB and may own/have owned stocks or other financial instruments in Spermosens AB. Kush Punyani and Ingela Liljeqvist Soltic are board members of Spermosens AB.Acknowledgements
This work received Vinnova funding under the 2023-00913, 2022-01404, 2021-02367, 2021-02133, and 2019-00234. The authors thank Professor Aleksander Giwercman (Lund University and Reproduktionsmedicinskt centrum, Sweden), Dr. Emir Henic (Reproduktionsmedicinskt centrum, Sweden), Prof. Trine B. Haugen (Oslo Metropolitan University, Norway), and Prof. Claus Yding Andersen (Rigshospitalet, Denmark) for their advice in planning the study.Notes and references
- C. Wang, M. Mbizvo, M. P. Festin, L. Björndahl and I. Toskin, Evolution of the WHO Semen: processing manual from the first (1980) to the sixth edition (2021), Fertil. Steril., 2022, 117(2), 237–245 CrossRef PubMed.
- J. W. van der Steeg, P. Steures, M. J. C. Eijkemans, J. D. F. Habbema, H. PGA and J. A. M. Kremer, et al. Role of semen analysis in subfertile couples, Fertil. Steril., 2011, 95(3), 1013–1019 Search PubMed.
- J. A. M. Hamilton, M. Cissen, M. Brandes, J. M. J. Smeenk, J. P. de Bruin and J. A. M. Kremer, et al. Total motile sperm count: a better indicator for the severity of male factor infertility than the WHO sperm classification system, Hum. Reprod., 2015, 30(5), 1110–1121 CrossRef PubMed.
- H. Levine, N. Jørgensen, A. Martino-Andrade, J. Mendiola, D. Weksler-Derri and M. Jolles, et al. Temporal trends in sperm count: a systematic review and meta-regression analysis of samples collected globally in the 20th and 21st centuries, Hum. Reprod. Update, 2023, 29(2), 157–176 CrossRef PubMed.
- H. Levine, N. Jørgensen, A. Martino-Andrade, J. Mendiola, D. Weksler-Derri and I. Mindlis, et al. Temporal trends in sperm count: a systematic review and meta-regression analysis, Hum. Reprod. Update, 2017, 23(6), 646–659 CrossRef PubMed.
- C. L. R. Barratt, L. Björndahl, C. J. De Jonge, D. J. Lamb, F. Osorio Martini and R. McLachlan, et al. The diagnosis of male infertility: an analysis of the evidence to support the development of global WHO guidance—challenges and future research opportunities, Hum. Reprod. Update, 2017, 23(6), 660–680 CrossRef PubMed.
- S. Kimmins, R. A. Anderson, C. L. R. Barratt, H. M. Behre, S. R. Catford and C. J. De Jonge, et al. Frequency, morbidity and equity — the case for increased research on male fertility, Nat. Rev. Urol., 2024, 21(2), 102–124 Search PubMed.
- S. Oehninger, D. R. Franken, E. Sayed, G. Barroso and P. Kolm, Sperm function assays and their predictive value for fertilization outcome in IVF therapy: a meta-analysis, Hum. Reprod. Update, 2000, 6(2), 160–168 Search PubMed.
- J. L. Fernández, L. Muriel, M. T. Rivero, V. Goyanes, R. Vazquez and J. G. Alvarez, The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation, J. Androl., 2003, 24(1), 59–66 Search PubMed.
- R. Henkel, E. Kierspel, M. Hajimohammad, T. Stalf, C. Hoogendijk and C. Mehnert, et al. DNA fragmentation of spermatozoa and assisted reproduction technology, Reprod. BioMed. Online, 2003, 7(4), 477–484 Search PubMed.
- A. Agarwal, R. Sharma, S. Roychoudhury, S. Du Plessis and E. Sabanegh, MiOXSYS: a novel method of measuring oxidation reduction potential in semen and seminal plasma, Fertil. Steril., 2016, 106(3), 566–573.e10 CrossRef PubMed.
- A. Agarwal, N. Parekh, M. K. Panner Selvam, R. Henkel, R. Shah and S. T. Homa, et al. Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility, World J. Mens Health, 2019, 37(3), 296–312 CrossRef PubMed.
- P. Castleton, P. Gyawali, N. Mathews, S. M. Mutuku, D. J. Sharkey and N. O. McPherson, MiOXSYS(®) and OxiSperm(®) II assays appear to provide no clinical utility for determining oxidative stress in human sperm-results from repeated semen collections, Andrology, 2023, 11(8), 1566–1578 CrossRef PubMed.
- J. C. Kirkman-Brown and C. De Jonge, Sperm DNA fragmentation in miscarriage – a promising diagnostic, or a test too far?, Reprod. BioMed. Online, 2017, 34(1), 3–4 Search PubMed.
- T. Tharakan, C. Bettocchi, J. Carvalho, G. Corona, T. H. Jones and A. Kadioglu, et al. European Association of Urology Guidelines Panel on Male Sexual and Reproductive Health: A Clinical Consultation Guide on the Indications for Performing Sperm DNA Fragmentation Testing in Men with Infertility and Testicular Sperm Extraction in Nonazoospermic Men, Eur. Urol. Focus, 2022, 8(1), 339–350 Search PubMed.
- E. Jerre, M. Bungum, D. Evenson and A. Giwercman, Sperm chromatin structure assay high DNA stainability sperm as a marker of early miscarriage after intracytoplasmic sperm injection, Fertil. Steril., 2019, 112(1), 46–53.e2 CrossRef CAS.
- K. Oleszczuk, A. Giwercman and M. Bungum, Sperm chromatin structure assay in prediction of in vitro fertilization outcome, Andrology, 2016, 4(2), 290–296 Search PubMed.
- R. J. Aitken, Sperm function tests and fertility, Int. J. Androl., 2006, 29(1), 68–69 CrossRef PubMed.
- M. J. Gómez-Torres, M. Hernández-Falcó, A. López-Botella, N. Huerta-Retamal and P. Sáez-Espinosa, IZUMO1 Receptor Localization during Hyaluronic Acid Selection in Human Spermatozoa, Biomedicines, 2023, 11(11), 2872 Search PubMed.
- Y. Satouh, N. Inoue, M. Ikawa and M. Okabe, Visualization of the moment of mouse sperm-egg fusion and dynamic localization of IZUMO1, J. Cell Sci., 2012, 125(Pt 21), 4985–4990 Search PubMed.
- N. Inoue, M. Ikawa, A. Isotani and M. Okabe, The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs, Nature, 2005, 434(7030), 234–238 Search PubMed.
- E. Bianchi, B. Doe, D. Goulding and G. J. Wright, Juno is the egg Izumo receptor and is essential for mammalian fertilization, Nature, 2014, 508(7497), 483–487 Search PubMed.
- C. Jean, F. Haghighirad, Y. Zhu, M. Chalbi, A. Ziyyat and E. Rubinstein, et al. JUNO, the receptor of sperm IZUMO1, is expressed by the human oocyte and is essential for human fertilisation, Hum. Reprod., 2019, 34(1), 118–126 Search PubMed.
- S. A. M. Young, J. Aitken and M. A. Baker, Phosphorylation of Izumo1 and its role in male infertility, Asian J. Androl., 2015, 17(5), 708–710 CAS.
- L. Samanta, R. Sharma, Z. Cui and A. Agarwal, Proteomic analysis reveals dysregulated cell signaling in ejaculated spermatozoa from infertile men, Asian J. Androl., 2019, 21(2), 121–130 CAS.
- J. G. Hamze, J. M. Sánchez, E. O'Callaghan, M. McDonald, P. Bermejo-Álvarez and R. Romar, et al. JUNO protein coated beads: A potential tool to predict bovine sperm fertilizing ability, Theriogenology, 2020, 155, 168–175 CAS.
- K. Punyani, S. Srivastava and M. Takwa, Biosensor for male infertility, US Pat., 11782052, 2018 Search PubMed.
- L. Björndahl, J. Kirkman-Brown, G. Hart, S. Rattle and C. L. R. Barratt, Development of a novel home sperm test, Hum. Reprod., 2006, 21(1), 145–149 Search PubMed.
- WHO, WHO laboratory manual for the examination and processing of human semen, World Health Organization, Geneva PP – Geneva, 5th edn, 2010 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sd00009b |
| This journal is © The Royal Society of Chemistry 2025 |