Open Access Article
Open Access ArticleImprovement of performance to form syngas utilizing water and CO2 over a particulate-Cu0.8Ag0.2GaS2-based photocathode by surface co-modification with ZnS and Ag†
Tomoaki
Takayama‡
 a,
Akihide
Iwase§
a,
Akihide
Iwase§
 a and
Akihiko
Kudo
a and
Akihiko
Kudo
 *ab
*ab
aDepartment of Applied Chemistry, Faculty of Science, Tokyo University of Science, 1-3 Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan. E-mail: a-kudo@rs.tus.ac.jp
bResearch Institute of Science and Technology, Carbon Value Research Center, Tokyo University of Science, Noda-shi, Chiba-ken 278-8510, Japan
First published on 27th January 2025
Abstract
Surface of a particulate-Cu0.8Ag0.2GaS2-based photocathode was co-modified with ZnS and Ag, resulting in improvement in the performance of the Cu0.8Ag0.2GaS2 photocathode for syngas (H2 + CO) formation through photoelectrochemical H2O and CO2 reduction under visible light in an aqueous electrolyte. Bubbles of the syngas were visually observed over the developed Ag and ZnS-co-modified Cu0.8Ag0.2GaS2 photocathode at 0 V vs. RHE at the applied potential using a 300 W Xe-arc lamp (λ > 420 nm). Based on various control experiments and characterization studies, the following two crucial factors have arisen: (1) formation of a (ZnS)–(Cu0.8Ag0.2GaS2) solid-solution near the surface of Cu0.8Ag0.2GaS2 particles was vital for enhancing the separation of the photogenerated carriers, (2) the Ag cocatalyst loaded on the solid-solution worked as an active site for photoelectrochemical CO2 reduction. Moreover, artificial photosynthetic syngas formation using water as an electron donor under simulated sunlight without any external bias was demonstrated by combining the developed Ag/ZnS/Cu0.8Ag0.2GaS2 photocathode with a CoOx-loaded BiVO4 photoanode.
1 Introduction
Photoelectrochemical systems which consist of a photocathode and a photoanode have been extensively studied not only for water splitting1,2 but also for CO2 reduction3–5 using water as an electron donor. This is because these systems possess a potential to produce H2 and CO as reduction products of water and CO2 separately from O2 as an oxidation product of water. The mixture of H2 and CO is called syngas that can be used as a raw chemical for liquid fuels, lower olefines and aromatics synthesis.6 In this context, it is important to explore highly active photocathodes for syngas formation in terms of utilization of CO2 and solar energy.From the viewpoint of the construction of highly efficient photoelectrochemical cells,1,7 metal sulfide photocathodes are attractive because of their narrow band gaps and positive onset potentials for the cathodic photocurrent.8 In particular, particulate-metal-sulfide-based photocathodes are advantageous because they can easily be fabricated as compared with a thin film photocathode using a vacuum process. We have reported that a particulate solid-solution of CuGaS2 and AgGaS2 (e.g., Cu0.8Ag0.2GaS2) provides higher photocathodic performance than that of pristine CuGaS2 in photoelectrochemical water reduction to form hydrogen under visible light.9 Moreover, the report10 that a particulate-(CuGa)0.5ZnS2-based photocathode is active for syngas formation under visible light motivated us to use a particulate-Cu0.8Ag0.2GaS2-based photocathode for syngas formation. However, the photoelectrochemical performance of such a particulate-based photocathode is generally not yet enough to build photoelectrochemical cells that work under solar light if modifications such as loading cocatalysts are not performed. Therefore, it is important to discover a surface modification method being effective for improving the performance of Cu0.8Ag0.2GaS2 in order to apply Cu0.8Ag0.2GaS2 to a photocathode for syngas formation utilizing water and CO2 under sunlight.
Various surface modifications of photocathodes have been investigated to develop highly active photocathodes. To date, Cu2ZnSnS4,11,12 Cu2ZnGeS4,13 CuInS2,14 Cu(In,Ga)Se2,15p-InP,16,17p-ZnTe,18p-Si,19,20 N-doped Ta2O5,16 CuFeO2,21 Cu2O,22 CuGaO2,23 and p-Fe2O3 (ref. 24) have emerged to showcase their performances for CO2 reduction to form CO and HCOOH in aqueous solutions under visible light irradiation, which have been improved by surface modifications. Specifically, n-type semiconductors, metal complexes, or metal nanoparticles have been investigated as the modifications. Furthermore, judging from the progress in photoelectrochemical water splitting,1,8 co-modification of metal sulfide photocathodes with n-type semiconductors and metallic cocatalysts is expected to bring highly efficient photocathodes for CO2 reduction. This is because co-modifications with n-type semiconductors and noble cocatalysts are frequently employed to enhance the separation of photogenerated electron–hole pairs and introduce active sites for water reduction.
Herein, we focused on the combination of ZnS with metallic cocatalysts as a co-modification. This is because ZnS is an n-type semiconductor being effective in improvement of syngas formation on the Cu2ZnGeS4 thin film photocathode13 of which the crystal structure is similar to the chalcopyrite structure of Cu0.8Ag0.2GaS2. ZnS is expected to be easily formed on the surfaces of particulate-metal-sulfide-based photocathodes by a chemical deposition method.25 Additionally, the toxicity of ZnS is lower than that of CdS, which is another typical n-type semiconductor. We first investigated the effects of modification conditions to deposit ZnS over particulate-Cu0.8Ag0.2GaS2-based photocathodes on their photocathodic performances for CO2 reduction. Sequentially, we investigated additional modifications of the ZnS-modified photocathodes with various cocatalysts. Then, we discovered a co-modification for fabricating an active photocathode for CO2 reduction to form syngas under simulated solar light, resulting in artificial photosynthetic syngas formation upon combining the developed photocathode with a CoOx-loaded BiVO4 photoanode.
2 Experimental methods
2.1 Preparation and characterization of a particulate-Cu0.8Ag0.2GaS2-based photocathode
A particulate Cu0.8Ag0.2GaS2 photocatalyst was prepared by a solid-state reaction according to a previous report.9 Starting materials Cu2S (Kojundo; 99%), Ag2S (Rare Metallic; 99.9%), and Ga2S3 (Kojundo; 99.99%) were ground using an agate mortar. A 10% excess amount of Ga2S3 was added to the starting materials. This Ga2S3-rich condition is effective in improving performance of Cu0.8Ag0.2GaS2.9 The mixture sealed in a quartz ampoule tube after evacuation (<0.1 Pa) was heated at 1073 K for 10 h to obtain Cu0.8Ag0.2GaS2 powder. The obtained powder was identified to be an almost single-phase by powder X-ray diffraction (Rigaku; Miniflex, X-ray source; Cu Kα). Diffuse reflectance spectra of powder and photocathode samples were obtained using a UV-vis-NIR spectrometer (Jasco; UbestV-570) with an integrating sphere, and were converted to absorbance spectra from the reflection by the Kubelka–Munk function. As the reference samples, (ZnS)2x–(Cu0.8Ag0.2GaS2)x solid-solutions were prepared by the same procedure above. ZnS (Sigma-Aldrich, 99.99%) was used as the starting material.A pristine particulate-Cu0.8Ag0.2GaS2-based photocathode was prepared by a drop-casting method. The Cu0.8Ag0.2GaS2 powder was dispersed in ethanol (2–8 mg mL−1). This suspension was dripped on a FTO substrate (Asahi Glass; F-doped SnO2) and thereby Cu0.8Ag0.2GaS2 powder was accumulated on the substrate with 5 mg cm−2. After drying up the ethanol at room temperature, the accumulated powder was annealed at 773 K for 2 h in N2 gas to obtain a pristine particulate-Cu0.8Ag0.2GaS2-based photocathode.
Modification of the pristine particulate-Cu0.8Ag0.2GaS2-based photocathode (denoted as CAGS) with ZnS was conducted by a chemical bath deposition method according to the literature.25 The notation of the photocathodes prepared under different conditions is summarized in Table 1. The detailed procedures of the experiments are explained as follows. ZnSO4·7H2O (Wako; 99.5%), thiourea (Wako; 98.0%) and citric acid (Wako; 98.0%) employed as the starting materials were dissolved in 270 mL of water at 353 K at concentrations of 0.033, 0.066 and 0.088 mol L−1, respectively. After adding 30 mL of 25% of an aqueous ammonia solution (Wako) into the aqueous solution, a pristine particulate-Cu0.8Ag0.2GaS2-based photocathode was immediately immersed into the final mixture for 10 minutes to deposit ZnS on the surface of the pristine photocathode. The ZnS-modified particulate-Cu0.8Ag0.2GaS2-based photocathode was dried at room temperature and sequentially was annealed at 473 or 773 K for 2 h in N2 gas. These are denoted as ZnS(473)–CAGS and ZnS(773)–CAGS. Then, the ZnS-modified particulate-Cu0.8Ag0.2GaS2-based photocathode with non-annealing is denoted as ZnS(NA)–CAGS. A ZnSO4-adsorbed particulate-Cu0.8Ag0.2GaS2-based photocathode was prepared by dipping the pristine photocathode into an aqueous solution dissolving only ZnSO4, followed by annealing at 773 K for 2 h under a N2 gas. The obtained sample is denoted as ZnSO4(773)–CAGS. The ZnSO4-adsorbed Cu0.8Ag0.2GaS2 photocathode with non-annealing is denoted as ZnSO4(NA)–CAGS. A CAGS photocathode was dipped into the mixture of thiourea, citric acid and an aqueous ammonia solution (namely, without ZnSO4) and then was annealed at 773 K for 2 h under N2 gas. The obtained sample is denoted as ZnSO4-w/o-CAGS. If a Cu0.8Ag0.2GaS2 photocathode was immersed in an aqueous solution containing either thiourea, citric acid or an aqueous ammonia solution followed by annealing at 773 K for 2 h, the obtained photoelectrodes are denoted as thiourea(773)–CAGS, citric acid(773)–CAGS and ammonia(773)–CAGS, respectively.
| Entry | Sample notation | Conditions for ZnS deposition | Cathodic photocurrent density/μA cm−2 | ||||
|---|---|---|---|---|---|---|---|
| Citric acid | Ammonia | Thiourea | ZnSO4 | Anneal | |||
| a Deposition time: 10 min. Photoelectrochemical measurement conditions; electrolyte: 0.1 mol L−1 of an aqueous K2SO4 solution with a phosphate buffer (pH 7) saturated with 1 atm of N2 gas, light source: a 300 W Xe-arc lamp with a cut-off filter (λ > 420 nm), applied potential: 0 V vs. RHE. | |||||||
| 1 | CAGS | No | No | No | No | None | 130 |
| 2 | ZnS(NA)–CAGS | Yes | Yes | Yes | Yes | None | 200 |
| 3 | ZnS(473)–CAGS | Yes | Yes | Yes | Yes | 473 K | 650 |
| 4 | ZnS(773)–CAGS | Yes | Yes | Yes | Yes | 773 K | 1850 |
| 5 | Citric acid(773)–CAGS | Yes | No | No | No | 773 K | 130 |
| 6 | Ammonia(773)–CAGS | No | Yes | No | No | 773 K | 200 |
| 7 | Thiourea(773)–CAGS | No | No | Yes | No | 773 K | 13 |
| 8 | ZnSO4-w/o-CAGS | Yes | Yes | Yes | No | 773 K | 220 |
| 9 | ZnSO4(773)–CAGS | No | No | No | Yes | 773 K | 400 |
Cocatalysts were loaded on the surfaces of photocathodes. AgNO3, Cu(NO3)2, HAuCl4, H2PtCl6, RuCl3 and RhCl3 were employed as the cocatalyst sources. An aqueous solution containing either one of the cocatalyst sources was dripped on a photocathode. The amount of the loaded cocatalyst was 100 nmol cm−2. After drying off the aqueous solvent at room temperature, the photocathode was annealed at 773 K for 2 h in N2 gas. Since it has been reported that RuCl3 loaded on the surface of the Cu0.8Ag0.2GaS2 photocathode through annealing in N2 gas is reduced to metallic Ru by cathodic photocurrent during photoelectrochemical H2 formation in an aqueous solution,9 various metal cocatalyst sources were loaded on the ZnS/Cu0.8Ag0.2GaS2 photocathode through the same procedure as that of the literature9 and could be reduced to the metallic state during the CO2 reduction in our experiments. If necessary, the photocathode was immersed in a mixture of water and acetone (the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as volumes) for about 30 seconds before dropping the aqueous solution containing the cocatalyst source in order to remove its water-repellency. Photocathode samples were analyzed using an X-ray photoelectron spectrometer (JEOL; JSP-9010MC; Mg anode). The binding energies of X-ray photoelectron spectra were corrected with the C 1s (284.3 eV) contamination peak on metallic Au foil. The binding energy of the Au foil was corrected using the reference datum of Au foil (Au 4f7/2: 84.0 eV)26 after a contamination and the oxide phase on the surface of the Au foil was carefully removed by Ar etching.
1 as volumes) for about 30 seconds before dropping the aqueous solution containing the cocatalyst source in order to remove its water-repellency. Photocathode samples were analyzed using an X-ray photoelectron spectrometer (JEOL; JSP-9010MC; Mg anode). The binding energies of X-ray photoelectron spectra were corrected with the C 1s (284.3 eV) contamination peak on metallic Au foil. The binding energy of the Au foil was corrected using the reference datum of Au foil (Au 4f7/2: 84.0 eV)26 after a contamination and the oxide phase on the surface of the Au foil was carefully removed by Ar etching.
2.2 Photoelectrochemical measurement
Photoelectrochemical properties were evaluated using a potentiostat (Hokuto Denko; HSV-110). An H-type glass cell that consists of a working electrode cell and a counter electrode cell with a Nafion membrane (Dupont) to separate the compartment was used. A Pt wire and a saturated Ag/AgCl electrode (DKK-TOA) were used as counter and reference electrodes, respectively. 0.1 mol L−1 of K2SO4 aq. with a phosphate buffer (each 0.025 mol L−1 of KH2PO4 aq. and Na2HPO4 aq.) (pH 7) and 0.1 mol L−1 of KHCO3 aq. were employed as aqueous electrolytes, respectively. The electrolyte in the H-type glass cell was saturated with 1 atm of Ar, N2 or CO2 before measurements. pH of the aqueous KHCO3 solution saturated with CO2 was ca. 7. A 300 W Xe-arc lamp was employed as a light source. The wavelength of the irradiation light was controlled by a cut-off filter, an NIR-absorbing filter and band-pass filters. A solar simulator was employed as a light source of simulated sunlight (AM1.5G). The photocathode was irradiated with the light from the FTO side. The gaseous products were determined using gas chromatographs (Shimadzu; TCD, MS-5A, Ar and He carriers, detection of H2 and O2; FID with a methanizer, MS-13X, N2 carrier, detection of CO). Formic acid was analyzed using an ion-chromatograph (TOSOH, IC-2000). An isotope experiment was conducted using 13CO2 to confirm the carbon source of the product of the CO2 reduction. 13CO of the reduction product of 13CO2 was analyzed using GC-MS (Shimadzu; GCMS-QP2010 Plus, RESTEK; RT-Msieve 5A). For photoelectrochemical measurement using a two-electrode-type cell, the photocathode (3.3 cm2) was irradiated from the FTO side with the simulated sunlight through a photoanode (1.0 cm2), resulting in that the total irradiation area was 3.3 cm2.3 Results and discussion
3.1 Surface modification with ZnS
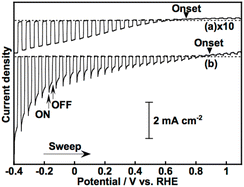
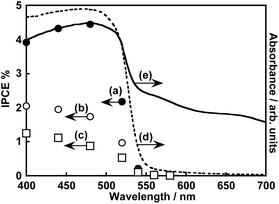
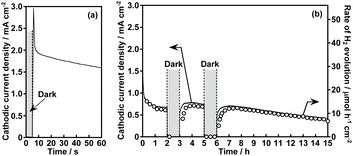
Fig. 1 shows the effect of modification with ZnS on the photoelectrochemical properties of a particulate-Cu0.8Ag0.2GaS2-based photocathode in an aqueous K2SO4 solution with a phosphate buffer (pH 7) saturated with 1 atm of N2 gas under visible light irradiation. A pristine particulate-Cu0.8Ag0.2GaS2-based photocathode gave a cathodic photocurrent under visible light irradiation as observed in a previous report (Fig. 1(a)).9 The cathodic photocurrent was increased by modification with ZnS on the surface of the particulate-Cu0.8Ag0.2GaS2-based photocathode by chemical bath deposition (CBD) (Fig. 1(b)). The onset potentials of the linear sweep voltammograms were affected by the difference in the CBD conditions (Fig. S1†), though it was difficult to find the relationship between the onset positions and the CBD conditions. Onsets of action spectra of the ZnS/Cu0.8Ag0.2GaS2 photocathodes almost agreed with the absorption edge of pristine Cu0.8Ag0.2GaS2 powder (Fig. 2), indicating that the cathodic photocurrent was generated by the band gap excitation of Cu0.8Ag0.2GaS2. Fig. 3 shows the time course of the photoelectrochemical H2 formation using the ZnS/Cu0.8Ag0.2GaS2 photocathode. Although the photocurrent density was fairly high in the initial period (Fig. 3(a)), the value decreased with reaction time (Fig. 3(b)). This deactivation in the initial period could be attributed to the elution of the ZnS similar to the CdS deposited on chalcogenide photocathodes.12,27,28 The observed photocurrent of the ZnS/Cu0.8Ag0.2GaS2 in the later period almost corresponded to the H2 evolution rates under visible light, indicating that the faradaic efficiency was almost 100% (Fig. 3(b)). In order to clarify the experimental factor affecting the H2 formation rate over a ZnS-modified Cu0.8Ag0.2GaS2 photocathode, control experiments were performed (Table 1). The annealing procedure after the ZnS modification with CBD was effective in improving the photocurrent density, while the photocurrent density of the pristine one was slightly enhanced by ZnS modification without annealing (Table 1, entries 1–4). Among them, annealing at 773 K was the most effective here. This is because, considering the heat resistance of FTO glass substrates, 773 K is close to the limit temperature. In contrast, significant improvement was not observed in the absence of ZnSO4 in the starting materials for CBD with annealing at 773 K (Table 1, entries 5–8). The photocurrent density of a ZnSO4-modified Cu0.8Ag0.2GaS2 photocathode was larger than that of the pristine one (Table 1, entry 9). Thus, the cathodic photocurrent of Cu0.8Ag0.2GaS2 was significantly improved when all of ZnSO4, thiourea, citric acid and ammonia were used for CDB with annealing at 773 K.The surface configuration of the ZnS/Cu0.8Ag0.2GaS2 photocathode was examined using X-ray photoelectron spectra (XPS), Auger spectra and additional control experiments. Auger spectra indicated that the deposited ZnS partly included ZnO and Zn(OH)2 (Fig. S2†). The ZnO and Zn(OH)2 still existed at the surface even after annealing at 473 K. Such auger spectra disappeared after annealing at 773 K. Moreover, intensities of Zn 2p XPS peaks decreased with the increase in the annealing temperature, and annealing at 773 K drastically decreased the intensities (Fig. S3†). These auger and XPS measurements indicated the following two points; (i) deposited ZnS including ZnO and Zn(OH)2 existed at the surface of ZnS(473)–CAGS, (ii) deposited Zn species were diffused in the surface of Cu0.8Ag0.2GaS2 particles in ZnS(773)–CAGS. In other words, an annealing at 773 K is crucial to diffuse the ZnS deposited on the Cu0.8Ag0.2GaS2 particles into the neighbourhood in the surface of the Cu0.8Ag0.2GaS2 particles as summarized in Fig. 4(A). Specifically, the surface of the Cu0.8Ag0.2GaS2 particles was covered with ZnS either of particles or film (Fig. 4(A)(a) and (b)). Afterwards, the ZnS was diffused into the Cu0.8Ag0.2GaS2 particles by annealing at 773 K, though almost all of the ZnS existed in the neighbourhood in the surface (Fig. 4(A)(c)). This diffusion made the solid-solution of (ZnS)–(Cu0.8Ag0.2GaS2) in the neighbourhood in the surface. The concentration of the diffused ZnS was gradually diluted from the surface to the bulk. Therefore, a crucial factor of the improvement was formation of the (ZnS)–(Cu0.8Ag0.2GaS2) solid-solution in the neighbourhood in the particulate Cu0.8Ag0.2GaS2 surface. This crucial factor could provide the following effects. The surface incorporated with ZnS could work as an active site for photoelectrochemical water reduction, since ZnS is a well-known active photocatalyst for H2 formation without loading of cocatalysts.29–32 Another aspect of the improvement may lie in the gradient of the band structure as shown in Fig. 4(B). The band gap of powdered Cu0.8Ag0.2GaS2 was narrowed by forming the solid-solution of powdered (ZnS)–(Cu0.8Ag0.2GaS2) (Fig. S4†). The same phenomenon to narrow the band gaps by homogeneously mixing with ZnS has been reported in the case of the (ZnS)–(CuGaS2) solid-solution (namely, no Ag constituent).33 These narrowed band gaps could be explained by the increase in the symmetries of MS4 tetrahedrons (M = Zn, Cu, and Ga), implying that the conduction band minimum and valence band maximum both are shifted downwardly.33 Again, XRD spectra of the (ZnS)–(Cu0.8Ag0.2GaS2) (Fig. S4†) show that the MS4 tetrahedrons (M = Zn, Cu, Ag, and Ga) tended to be close to the symmetrical tetrahedrons, judging from the peak splitting. Therefore, the conduction band minimum and valence band maximum of powdered (ZnS)–(Cu0.8Ag0.2GaS2) might be located at more positive potentials than those of Cu0.8Ag0.2GaS2 (Fig. 4(B)(a)). As for the structural information, no morphological changes in a series of ZnS-modified Cu0.8Ag0.2GaS2 were observed using a scanning electron microscope (SEM), though it was difficult to clarify the structural configurations of the deposited ZnS in the observations using a SEM equipped with an energy dispersive X-ray spectrometer (SEM-EDS) (Fig. S5†). Although the quantitative consistency of the ZnS ratios between the photocathode and the powders was unclear, the aforementioned effect to alter the band positions implied that the conduction band minimum and valence band maximum of the neighbourhood in the surface gradually shifted downwardly due to the concentration gradient of the solid-solution of ZnS(773)–Cu0.8Ag0.2GaS2 (Fig. 4(B)(b)). Assuming that the absorption coefficient of (ZnS)–(Cu0.8Ag0.2GaS2) is similar to that of CuGaS2 in the visible light region (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–50
000–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1),34 the ZnS-modified Cu0.8Ag0.2GaS2 particles near FTO were mainly photoexcited. This is because the transmittance of incident photons would be at least 0.01% when the diameters of ZnS(773)-modified Cu0.8Ag0.2GaS2 particles were ca. 2 μm (Fig. S5†). Indeed, we reported that CuGaS2 particles being far from FTO have little contribution to the photocurrents of particulate-CuGaS2-based photocathodes.35,36 Finally, the photogenerated electrons migrated to the surface region by the band bending of the ZnS-modified Cu0.8Ag0.2GaS2 particles, resulting in enhancement of the separation of the photogenerated carriers (Fig. 4(B)(b)). These two aspects could be the crucial factors for the improvement by the ZnS modification.
000 cm−1),34 the ZnS-modified Cu0.8Ag0.2GaS2 particles near FTO were mainly photoexcited. This is because the transmittance of incident photons would be at least 0.01% when the diameters of ZnS(773)-modified Cu0.8Ag0.2GaS2 particles were ca. 2 μm (Fig. S5†). Indeed, we reported that CuGaS2 particles being far from FTO have little contribution to the photocurrents of particulate-CuGaS2-based photocathodes.35,36 Finally, the photogenerated electrons migrated to the surface region by the band bending of the ZnS-modified Cu0.8Ag0.2GaS2 particles, resulting in enhancement of the separation of the photogenerated carriers (Fig. 4(B)(b)). These two aspects could be the crucial factors for the improvement by the ZnS modification.
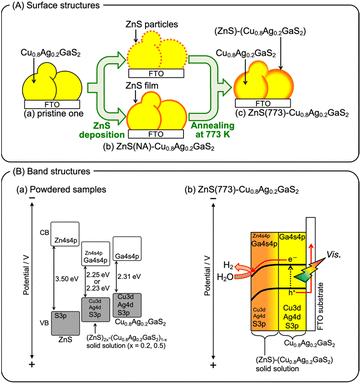 | ||
| Fig. 4 (A) Proposed surface structures of the particulate-Cu0.8Ag0.2GaS2-based photocathode, and (B) proposed band structures of (ZnS)2x–(Cu0.8Ag0.2GaS2)x powders and the ZnS(773)–Cu0.8Ag0.2GaS2 photocathode. The band gaps were estimated from the adsorption edges shown in Fig. S4.† | ||
3.2 Effect of cocatalysts loaded over ZnS/Cu0.8Ag0.2GaS2 photocathodes on CO2 reduction performances
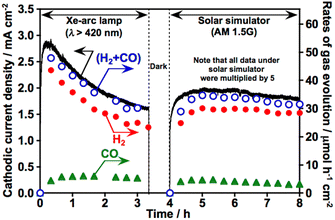
Table 2 shows the application of the ZnS(773)–Cu0.8Ag0.2GaS2 photocathode (denoted as ZnS/Cu0.8Ag0.2GaS2, hereafter) to CO2 reduction under visible light and the effects of additional modification of ZnS/Cu0.8Ag0.2GaS2 photocathodes with various cocatalysts. In this measurement, an aqueous KHCO3 solution saturated with 1 atm of CO2 gas (pH was ca. 7) was used as an electrolyte and 0 V vs. RHE was constantly applied to the photocathodes. Again, the pristine particulate-Cu0.8Ag0.2GaS2-based photocathode gave a cathodic photocurrent in the aqueous KHCO3 electrolyte (Table 2, entry 1). Partial cathodic photocurrent densities (Jpartial) for H2 and CO formations were certainly estimated. The surface modification with ZnS was effective for improving both Jpartial and FECO (Table 2, entry 2). Considering the literature about photoelectrochemical CO2 reduction to form CO over surfaces of (CuGa)0.5ZnS2 (ref. 10) and ZnS-modified Cu2ZnGeS4 (ref. 13) photocathodes, the surface of ZnS/Cu0.8Ag0.2GaS2 seems to be effective for CO formation. No cathodic photocurrents for H2 and CO formation were observed under darkness (Table 2, entry 3). In an aqueous K2SO4 solution with a phosphate buffer (pH 7) saturated with 1 atm of N2 gas under visible light irradiation instead of the KHCO3 electrolyte, photocurrent density only for H2 formation was observed (Table 2, entry 4). Various cocatalysts were further loaded on the surfaces of the ZnS/Cu0.8Ag0.2GaS2 photocathodes (Table 2, entries 5–13). Noble metal cocatalysts of Pt, Rh and Ru were not effective for CO2 reduction (Table 2, entries 5–7). Although the faradaic efficiencies of Pt- and Ru-loaded ones were beyond 100%, this is most likely because of experimental errors associated with usage of a syringe (1 mL) to extract the gaseous products. Au, Cu and Ag cocatalysts improved the partial current densities not only for H2 formation but also for CO formation (Table 2, entries 8–10). The Ag-loaded ZnS/Cu0.8Ag0.2GaS2 (Ag/ZnS/Cu0.8Ag0.2GaS2) photocathode showed the highest partial cathodic photocurrent densities for H2 and CO formation. This sufficient performance was only obtained when the surface was modified with both ZnS and Ag accompanied by annealing (Table 2, entries 10–13). Considering the following three insights: (1) RuCl3 loaded on the surface of the Cu0.8Ag0.2GaS2 photocathode through annealing in N2 gas is reduced to metallic Ru by cathodic photocurrent during photoelectrochemical H2 formation in an aqueous solution,9 (2) In2S3 modified on a Cu2ZnSnS4 photocathode is reduced to metallic In during the photoelectrochemical reduction reaction,12 (3) powdered Ag-containing metal sulfide photocatalysts with high apparent quantum yield for H2 formation may reduce silver sulfide to metallic Ag during H2 formation in an aqueous solution,37,38 it was suggested that the cathodic photocurrent was partly used to reduce the silver species loaded on the surface to metallic silver at the beginning stage. This metallic silver could be electrochemically active for CO2 reduction as reported in the literature about an Ag metal electrode.39,40 The bubbles mixed with H2 and CO gases were visually generated on the Ag/ZnS/Cu0.8Ag0.2GaS2 photocathode accompanied by O2 production on a Pt counter electrode when 0 V vs. RHE was applied to the photocathode under visible light irradiation (see an attached movie file). The Ag/ZnS/Cu0.8Ag0.2GaS2 photocathode was active even under simulated sunlight (Fig. 5). An isotope experiment using 13CO2 was conducted to confirm the carbon source of CO obtained. An aqueous K2SO4 solution saturated with 1 atm of 13CO2 was employed as the electrolyte (Fig. S6†). Not 12CO but 13CO was obtained as the reduction product of 13CO2 over the Ag/ZnS/Cu0.8Ag0.2GaS2 photocathode, indicating that the carbon source of the obtained CO was a CO2 molecule. When Ag, Cu and Au cocatalysts were employed, a small amount of formic acid was detected but it was difficult to quantify the formic acid. Thus, co-modification with ZnS and Ag using annealing has arisen as a new effective technique for improving the performance of the particulate-Cu0.8Ag0.2GaS2-based photocathode for the CO2 reduction to form CO accompanied by H2 formation in an aqueous solution under simulated sunlight.| Entry | Cocatalyst | Annealingb | ZnS | Light | Gas | J partial/μA cm−2 | FECO % | FETotal % | |
|---|---|---|---|---|---|---|---|---|---|
| H2 | CO | ||||||||
| a Electrolyte: 0.1 mol L−1 of an aqueous KHCO3 solution saturated with 1 atm of CO2 gas (pH was ca. 7), light source: a 300 W Xe-arc lamp with a cut-off filter (λ > 420 nm), applied potential: 0 V vs. RHE, reactor: a closed H-type glass cell (namely, a batch-type reactor). Jpartial indicates an average of a partial cathodic photocurrent density for 3 h. For quantification of the gaseous products, a syringe (1 mL) was used to extract the gas. b Annealing was performed under a N2 atmosphere for loading cocatalysts. FECO = (sum of the number of electrons consumed for CO formation)/(sum of the number of electrons passing through the outer circuit) × 100. FEtotal = (sum of the number of electrons consumed for H2 and CO formation)/(sum of the number of electrons passing through the outer circuit) × 100. | |||||||||
| 1 | None | — | No | Yes | CO2 | 10 | 0.3 | 3 | 98 |
| 2 | None | — | Yes | Yes | CO2 | 430 | 70 | 14 | 95 |
| 3 | None | — | Yes | No | CO2 | 0 | 0 | — | — |
| 4 | None | — | Yes | Yes | N2 | 1200 | 0 | 0 | 97 |
| 5 | Pt | 773 K | Yes | Yes | CO2 | 790 | 10 | 1 | 117 |
| 6 | Rh | 773 K | Yes | Yes | CO2 | 420 | 30 | 7 | 100 |
| 7 | Ru | 773 K | Yes | Yes | CO2 | 90 | 5 | 5 | 127 |
| 8 | Au | 773 K | Yes | Yes | CO2 | 890 | 200 | 17 | 100 |
| 9 | Cu | 773 K | Yes | Yes | CO2 | 620 | 200 | 24 | 92 |
| 10 | Ag | 773 K | Yes | Yes | CO2 | 1600 | 330 | 17 | 100 |
| 11 | Ag | 773 K | No | Yes | CO2 | 190 | 5 | 3 | 96 |
| 12 | Ag | None | No | Yes | CO2 | 60 | 1 | 2 | 98 |
| 13 | Ag | None | Yes | Yes | CO2 | 130 | 7 | 5 | 91 |
3.3 Photoelectrochemical syngas formation using water as an electron doner under simulated sunlight
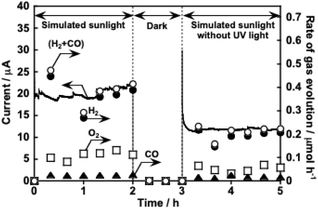
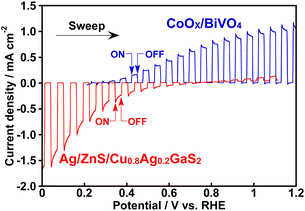
Fig. 6 shows CO2 reduction using a photoelectrochemical cell consisting of a Ag/ZnS/Cu0.8Ag0.2GaS2 photocathode and a CoOx/BiVO4 photoanode41 in an aqueous KHCO3 solution saturated with 1 atm of CO2 gas. This photoelectrochemical cell of which the structure was partly a tandem-type gave photocurrent under simulated sunlight irradiation without any external bias. This is due to an overlap between onset potentials of the photocathode and the photoanode in the CO2-saturated KHCO3 electrolyte, as shown in Fig. 7. Considering that the absorption coefficient of BiVO4 at 420 nm is 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1,42 1% of the incident photons could arrive at the Ag/ZnS/Cu0.8Ag0.2GaS2 photocathode when the thickness of our BiVO4 was regarded to be similar to the literature (ca. 300 nm).41 The faradaic efficiency for CO formation using the Ag/ZnS/Cu0.8Ag0.2GaS2 photocathode was ca. 20% regardless of the decrease in the incident photons and change in the applied potentials (Table S1†). Therefore, the cell of which the structure was partly a tandem-type would hardly affect the selectivity for syngas formation. By paying attention to Fig. 6 again, observed photocurrent almost corresponded to the summation of CO and H2 formation in the cathode cell, indicating that the photocurrent was used for syngas formation through photoelectrochemical reduction of water and CO2. Most importantly, O2 of the oxidation product of water was certainly obtained in the anode cell, whereas the rate of O2 formation was slightly less than the stoichiometry due to an experimental error with the lower photocurrent. The photocurrent was continuously observed even under visible light included in simulated sunlight without any external bias. This indicated that this cell possessed an ability to utilize the visible light of sunlight. Thus, we successfully demonstrated solar syngas formation through the reduction of water and CO2 separately from O2 of the oxidation product of water upon constructing the photoelectrochemical cell consisting of the developed Ag/ZnS/Cu0.8Ag0.2GaS2 photocathode and the CoOx/BiVO4 photoanode.
000 cm−1,42 1% of the incident photons could arrive at the Ag/ZnS/Cu0.8Ag0.2GaS2 photocathode when the thickness of our BiVO4 was regarded to be similar to the literature (ca. 300 nm).41 The faradaic efficiency for CO formation using the Ag/ZnS/Cu0.8Ag0.2GaS2 photocathode was ca. 20% regardless of the decrease in the incident photons and change in the applied potentials (Table S1†). Therefore, the cell of which the structure was partly a tandem-type would hardly affect the selectivity for syngas formation. By paying attention to Fig. 6 again, observed photocurrent almost corresponded to the summation of CO and H2 formation in the cathode cell, indicating that the photocurrent was used for syngas formation through photoelectrochemical reduction of water and CO2. Most importantly, O2 of the oxidation product of water was certainly obtained in the anode cell, whereas the rate of O2 formation was slightly less than the stoichiometry due to an experimental error with the lower photocurrent. The photocurrent was continuously observed even under visible light included in simulated sunlight without any external bias. This indicated that this cell possessed an ability to utilize the visible light of sunlight. Thus, we successfully demonstrated solar syngas formation through the reduction of water and CO2 separately from O2 of the oxidation product of water upon constructing the photoelectrochemical cell consisting of the developed Ag/ZnS/Cu0.8Ag0.2GaS2 photocathode and the CoOx/BiVO4 photoanode.
4 Conclusions
A co-modification with ZnS and Ag has been developed for improvement in H2O and CO2 reduction over a particulate-Cu0.8Ag0.2GaS2-based photocathode to form H2 and CO under visible light in an aqueous electrolyte. The faradaic efficiency and the partial cathodic photocurrent density for CO formation over the Ag/ZnS/Cu0.8Ag0.2GaS2 photocathode achieved approximately 20% and 300 μA cm−2, respectively, at 0 V vs. RHE. The Ag/ZnS/Cu0.8Ag0.2GaS2 photocathode was successfully combined with a CoOx/BiVO4 photoanode for constructing a photoelectrochemical cell. This photoelectrochemical cell gave steady photocurrent under simulated sunlight irradiation without any external bias, resulting in syngas (H2 and CO) formation using water as an electron donor. The knowledge in this work is expected to contribute to development of new particulate-photocatalyst-based photocathodes for highly efficient syngas production under sunlight.Data availability
The data supporting this article have been included as ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research (MEXT KAKENHI Grant Number: 17H06440, 17H06433 and 23H00248) and Grant-in-Aid for Young Scientists (B) (16K17948) from the Ministry of Education, Culture, Sports, Science and Technology in Japan.Notes and references
- T. Hisatomi, J. Kubota and K. Domen, Chem. Soc. Rev., 2014, 43, 7520 RSC.
- H. Kaneko, T. Minegishi and K. Domen, Chem.–Eur. J., 2018, 24, 5697 CrossRef CAS PubMed.
- J. L. White, M. F. Baruch, J. E. Pander III, Y. Hu, I. C. Fortmeyer, J. E. Park, T. Zhang, K. Liao, J. Gu, Y. Yan, T. W. Shaw, E. Abelev and A. B. Bocarsly, Chem. Rev., 2015, 115, 12888 CrossRef CAS PubMed.
- A. Dey, D. Maiti and G. K. Lahiri, Asian J. Org. Chem., 2017, 6, 1519 CrossRef CAS.
- V. Kumaravel, J. Bartlett and S. C. Pillai, ACS Energy Lett., 2020, 5, 486 CrossRef CAS.
- W. Zhou, K. Cheng, J. Kang, C. Zhou, V. Subramanian, Q. Zhan and Y. Wang, Chem. Soc. Rev., 2019, 48, 3193 RSC.
- T. Morikawa, S. Sato, K. Sekizawa, T. M. Suzuki and T. Arai, Acc. Chem. Res., 2022, 55, 933 CrossRef CAS PubMed.
- W. Septina, Gunawan, S. Ikeda, T. Harada, M. Higashi, R. Abe and M. Matsumura, J. Phys. Chem. C, 2015, 119, 8576 CrossRef CAS.
- H. Kaga, Y. Tsutsui, A. Nagane, A. Iwase and A. Kudo, J. Mater. Chem. A, 2015, 3, 21815 RSC.
- S. Yoshino, A. Iwase, Y. Yamaguchi, T. M. Suzuki, T. Morikawa and A. Kudo, J. Am. Chem. Soc., 2022, 144, 2323 CrossRef CAS PubMed.
- T. Arai, S. Tajima, S. Sato, K. Uemura, T. Morikawa and T. Kajino, Chem. Commun., 2011, 47, 12664 RSC.
- S. Kamimura, Y. Sasaki, M. Kanaya, T. Tsubota and T. Ohno, RSC Adv., 2016, 6, 112594 RSC.
- S. Ikeda, S. Fujikawa, T. Harada, H. T. Nguyen, S. Nakanishi, T. Takayama, A. Iwase and A. Kudo, ACS Appl. Energy Mater., 2019, 2, 6911 CrossRef CAS.
- T. Takashima, Y. Fujishiro and H. Irie, Catalysts, 2020, 10, 949 CrossRef CAS.
- P. B. Pati, R. Wang, E. Boutin, S. Diring, S. Jobic, N. Barreau, F. Odobel and M. Robert, Nat. Commun., 2020, 11, 3499 CrossRef CAS PubMed.
- S. Sato, T. Arai, T. Morikawa, K. Uemura, T. M. Suzuki, H. Tanaka and T. Kajino, J. Am. Chem. Soc., 2011, 133, 15240 CrossRef CAS PubMed.
- T. Arai, S. Sato, T. Kajino and T. Morikawa, Energy Environ. Sci., 2013, 6, 1274 RSC.
- Y. J. Jang, I. Jeong, J. Lee, J. Lee, M. J. Ko and J. S. Lee, ACS Nano, 2016, 10, 6980 CrossRef CAS PubMed.
- R. Hinogami, Y. Nakamura, S. Yae and Y. Nakato, J. Phys. Chem. B, 1998, 102, 974 CrossRef CAS.
- S. Roy, M. Miller, J. Warnan, J. J. Leung, C. D. Sahm and E. Reisner, ACS Catal., 2021, 11, 1868 CrossRef CAS.
- J. Gu, A. Wuttig, J. W. Krizan, Y. Hu, Z. M. Detweiler, R. J. Cava and A. B. Bocarsly, J. Phys. Chem. C, 2013, 117, 12415 CrossRef CAS.
- M. Xia, L. Pan, Y. Liu, J. Gao, J. Li, M. Mensi, K. Sivula, S. M. Zakeeruddin, D. Ren and M. Grätzel, J. Am. Chem. Soc., 2023, 145, 27939 CrossRef CAS PubMed.
- H. Kumagai, G. Sahara, K. Maeda, M. Higashi, R. Abe and O. Ishitani, Chem. Sci., 2017, 8, 4242 RSC.
- K. Sekizawa, S. Sato, T. Arai and T. Morikawa, ACS Catal., 2018, 8, 1405 CrossRef CAS.
- W. L. Liu, C. S. Yang, S. H. Hsieh, W. J. Chen and C. L. Fern, Appl. Surf. Sci., 2013, 264, 213 CrossRef CAS.
- M. P. Seah, Surf. Interface Anal., 1989, 14, 488 CrossRef CAS.
- L. Zhang, T. Minegishi, J. Kubota and K. Domen, Phys. Chem. Chem. Phys., 2014, 16, 6167 RSC.
- J. Zhao, T. Minegishi, L. Zhang, M. Zhong, M. Gunawan, M. Nakabayashi, G. Ma, T. Hisatomi, M. Katayama, S. Ikeda, N. Shibata, T. Yamada and K. Domen, Angew. Chem., Int. Ed., 2014, 53, 11808 CrossRef CAS PubMed.
- S. Yanagida, T. Azuma and H. Sakurai, Chem. Lett., 1982, 1069 CrossRef CAS.
- J.-F. Reber and K. Meier, J. Phys. Chem., 1984, 88, 5903 CrossRef CAS.
- N. Zeug, J. Bücheler and H. Kisch, J. Am. Chem. Soc., 1985, 107, 1459 CrossRef CAS.
- H. Kisch and J. Bücheler, Bull. Chem. Soc. Jpn., 1990, 63, 2378 CrossRef CAS.
- T. Kato, Y. Hakari, S. Ikeda, Q. Jia, A. Iwase and K. Kudo, J. Phys. Chem. Lett., 2015, 6, 1042 CrossRef CAS PubMed.
- R. Long, N. J. English and O. V. Prezhdo, J. Am. Chem. Soc., 2012, 134, 14238 CrossRef CAS PubMed.
- A. Iwase, Y. H. Ng, R. Amal and A. Kudo, J. Mater. Chem. A, 2015, 3, 8566 RSC.
- T. Takayama, A. Iwase and A. Kudo, ACS Appl. Mater. Interfaces, 2024, 16, 36423 CrossRef CAS PubMed.
- I. Tsuji, Y. Shimodaira, H. Kato, H. Kobayashi and A. Kudo, Chem. Mater., 2010, 22, 1402 CrossRef CAS.
- H. Kaga, K. Saito and A. Kudo, Chem. Commun., 2010, 46, 3779 RSC.
- Y. Hori, H. Wakebe, T. Tsukamoto and O. Koga, Electrochim. Acta, 1994, 39, 1833 CrossRef CAS.
- T. Hatsukade, K. P. Kuhl, E. R. Cave, D. N. Abram and T. F. Jaramillo, Phys. Chem. Chem. Phys., 2014, 16, 13814 RSC.
- Q. Jia, K. Iwashina and A. Kudo, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 11564 CrossRef CAS PubMed.
- I. Grigioni, A. Polo, M. V. Dozzi, K. G. Stamplecoskie, D. H. Jara, P. V. Kamat and E. Selli, ACS Appl. Energy Mater., 2022, 5, 13142 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: X-ray photoelectron spectroscopic measurements. See DOI: https://doi.org/10.1039/d4se01738b |
| ‡ Present address: His current affiliation is Graduate School of Science and Technology, Division of Materials Science, Nara Institute of Science and Technology, 8916-5 Takayama, Ikoma, Nara 630-0192, Japan. |
| § Present address: His current affiliation is Department of Applied Chemistry, School of Science and Technology, Meiji University, Kanagawa 214-8571, Japan. |
| This journal is © The Royal Society of Chemistry 2025 |