Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Optimization and characterization of biocrude produced from hydrothermal liquefaction of food waste
Kshanaprava
Dhalsamant
 and
Ajay K.
Dalai
and
Ajay K.
Dalai
 *
*
Catalysis and Chemical Reaction Engineering Laboratories, Department of Chemical and Biological Engineering, College of Engineering, University of Saskatchewan, Saskatoon, SK, Canada S7N 5A9. E-mail: akd983@mail.usask.ca; Tel: +1-306-546-0742
First published on 7th March 2025
Abstract
This study investigates the valorization of restaurant-derived food waste into biocrude using hydrothermal liquefaction (HTL). The selected feedstocks, including carrot, parsnip, and other vegetables, were evaluated for their physicochemical properties, showing low ash (9.1–22.0 wt%) and fixed carbon content (5.3–18.4 wt%) with high moisture levels (79–95% wet basis), suitable for HTL without additional drying. Carrot emerged as the optimal feedstock due to its elevated carbon (44.9 wt%), hydrogen (7.8 wt%), cellulose (15.3 wt%), and hemicellulose (4.1 wt%) content. Reaction parameters optimized via response surface methodology (280 °C, 1500 psi, 42 minutes) yielded 18.8 wt% biocrude with a carbon recovery of 55.9–72.8%. Quality analyses such as gas chromatography-mass spectrometry and Fourier-transform infrared spectroscopy highlighted the complex composition of biocrude, including esters, hydrocarbons, and oxygenated compounds, confirming its potential for biofuel applications. Solvent optimization experiments demonstrated that methanol was the most effective, yielding 19.6 wt% biocrude. Additionally, methanol actively participated in the extraction process by promoting esterification, generating methyl esters, as evidenced in gas chromatography-mass spectrometry analysis. These reactions enhance product yield and quality by forming bioactive compounds like methyl esters, which improve the bio-oil stability and calorific value. Despite high oxygen content (20.7 wt%), the biocrude properties can be upgraded via deoxygenation techniques, paving the way for its use as a sustainable transportation fuel. This research underscores hydrothermal liquefaction as an effective approach to manage food waste while addressing global energy challenges through renewable bioenergy production. By integrating statistical optimization and comprehensive characterization, this study contributes to advancing biofuel technology and sustainable energy solutions.
1. Introduction
In 2018, the overall output of fruits and vegetables in Canada amounted to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 048
048![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 143 metric tons, with a corresponding food waste of 90
143 metric tons, with a corresponding food waste of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 tons (∼3%).1 The production of fruits and vegetables in Canada experienced a notable increase, reaching a total of 3
600 tons (∼3%).1 The production of fruits and vegetables in Canada experienced a notable increase, reaching a total of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 208
208![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 388 metric tons in the year 2022.2 Consequently, it is expected that the amount of food waste has also risen in recent years, aligning with previous trends and the growth of the population. The statistics of fruit and vegetable production and waste in Canada are given in Tables 1 and 2. Furthermore, the category of food waste includes residual food products within the food industry, restaurants, and kitchens, as well as rotten food items originating from grocery stores or landfills. This form of waste significantly contributes to the phenomenon of global warming through the emission of greenhouse gases. Food waste (FW) refers to food that has not fulfilled its intended function.3,4 The phenomenon of global food waste is exhibiting a concurrent rise with the growth of the global population and the escalation of food consumption.5 In recent times, there has been a growing international focus on energy security, environmental sustainability, and the increasing output of waste. As a result, there has been a notable increase in research endeavors aimed at discovering alternative and renewable energy sources. Food waste affects food security and the economy of a country by affecting the improper use of investment and nutrition. Therefore, the utilization of food waste to produce biofuels as a means of generating green energy presents an alternative strategy for addressing the challenges. An area that is currently receiving significant attention is the process of valorizing food waste, which is a widespread problem that has extensive economic, environmental, and social consequences.5 Food waste not only places a burden on landfills and contributes to the release of greenhouse gases, but it also results in the wastage of valuable resources that are inherent within it. Simultaneously, the transportation industry continues to largely depend on fossil fuels, hence intensifying the depletion of limited resources and aggravating environmental issues. The utilization of biofuel obtained from biomass has promising prospects as a feasible cornerstone for the establishment of sustainable energy sources.6
388 metric tons in the year 2022.2 Consequently, it is expected that the amount of food waste has also risen in recent years, aligning with previous trends and the growth of the population. The statistics of fruit and vegetable production and waste in Canada are given in Tables 1 and 2. Furthermore, the category of food waste includes residual food products within the food industry, restaurants, and kitchens, as well as rotten food items originating from grocery stores or landfills. This form of waste significantly contributes to the phenomenon of global warming through the emission of greenhouse gases. Food waste (FW) refers to food that has not fulfilled its intended function.3,4 The phenomenon of global food waste is exhibiting a concurrent rise with the growth of the global population and the escalation of food consumption.5 In recent times, there has been a growing international focus on energy security, environmental sustainability, and the increasing output of waste. As a result, there has been a notable increase in research endeavors aimed at discovering alternative and renewable energy sources. Food waste affects food security and the economy of a country by affecting the improper use of investment and nutrition. Therefore, the utilization of food waste to produce biofuels as a means of generating green energy presents an alternative strategy for addressing the challenges. An area that is currently receiving significant attention is the process of valorizing food waste, which is a widespread problem that has extensive economic, environmental, and social consequences.5 Food waste not only places a burden on landfills and contributes to the release of greenhouse gases, but it also results in the wastage of valuable resources that are inherent within it. Simultaneously, the transportation industry continues to largely depend on fossil fuels, hence intensifying the depletion of limited resources and aggravating environmental issues. The utilization of biofuel obtained from biomass has promising prospects as a feasible cornerstone for the establishment of sustainable energy sources.6
| Rank | Vegetables | Fruits | Western Canada vegetables | |||
|---|---|---|---|---|---|---|
| Canada (metric ton) | Canada (metric ton) | Manitoba (ton) | Saskatchewan (ton) | Alberta (ton) | British Columbia (ton) | |
| 1 | Tomato (528![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 938) 938) |
Apple (414![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 494) 494) |
Carrot (6842) | Cabbage (1474) | Onion (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 806) 806) |
Cabbage (8793) |
| 2 | Carrot (348![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 135) 135) |
Cranberry (228![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 565) 565) |
Onion (4562) | Carrot (1076) | Sweet corn (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 932) 932) |
Carrot (7552) |
| 3 | Onion (287![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 499) 499) |
Blueberry (195![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 892) 892) |
Cabbage (3172) | Pumpkin (341) | Pumpkin (4463) | Sweet corn (6865) |
| 4 | Sweet corn (201![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 028) 028) |
Grape (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 116) 116) |
Cauliflower (2775) | Sweet corn (156) | Cabbage (4377) | Pumpkin (6727) |
| Year | Production (metric ton) | Loss (ton) | Year | Production (metric ton) | Loss (ton) |
|---|---|---|---|---|---|
| 2011 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 916 916![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 296 296 |
51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 358 358 |
2017 | — | — |
| 2012 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 968 968![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 533 533 |
49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 758 758 |
2018 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 138 138![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 185 185 |
90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
| 2013 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 991 991![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 266 266 |
51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 273 273 |
2019 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 160 160![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 390 390 |
— |
| 2014 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 027 027![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 260 260 |
83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 130 130 |
2020 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 089 089![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 499 499 |
— |
| 2015 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 048 048![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 398 398 |
87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 415 415 |
2021 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 074 074![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 509 509 |
— |
| 2016 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 268 268![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 389 389 |
88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 415 415 |
2022 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 208 208![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 388 388 |
— |
In response to these interconnected issues, there has been an increasing focus on the conversion of food waste into biocrude, a versatile intermediary substance that has the potential to function as a sustainable substitute for traditional transportation fuels.7,8 Biocrude, which is derived from the hydrothermal liquefaction (HTL) process, offers a potential answer by facilitating the recycling of biological waste materials and the production of sustainable energy sources.9 Generally, HTL is preferred for biocrude production from food waste as it uses the water already present in the food, therefore it does not need drying of feedstock prior to reaction.5,10 This study undertakes a thorough investigation into the process of converting food waste into biocrude and evaluates its potential as a viable transportation fuel through further characterization.
The biochemical content of food waste has a considerable influence on the results of HTL. The presence of a large amount of lipids in food waste increases the liquid yield in the HTL process, which in turn leads to higher production of biocrude.5 The protein and carbohydrate levels of a product also influence its quality, as they affect the composition of the biocrude and biochar product. Carbohydrate rich biomass leads to low biocrude yield, and high biochar yield.11 Protein rich feedstocks give high ammonia content in the aqueous phase. The formation of amines from proteins occurs at lower temperatures, which accounts for the increased biocrude production from beef at lower temperatures of 280–300 °C.12 Most of the biocrude derived from chicken is produced by the Maillard process, which is more favorable at elevated temperatures of 280–360 °C.13 Gaining a comprehensive understanding of these complex interactions is essential for maximizing the efficiency of HTL operations and achieving optimal conversion of food waste into useful bioenergy resources.5,11 This research not only tackles waste management concerns but also supports the overarching objective of sustainable energy production by utilizing various biomass feedstocks.
A study used hydrothermal carbonization (HTC) and HTL on an industrial feedstock with a moisture content of 53 wt%, including sweeteners, nuts, eggs, fish products, dairy products, meat, poultry, fresh and processed vegetables and fruits, and grain products.14 Elevated temperatures diminish liquid production, increase gas generation, and decrease charcoal formation. However, biochar carbonization rises with temperature. According to Pecchi et al.,14 lipid hydrolysis begins at 220 °C but accelerates at temperatures above 250 °C. Thus, a low-temperature HTL process reduces energy consumption, enabling full lipid breakdown and long-chain fatty acid conversion.
Anaerobic digestion (AD) and composting are carried out for conversion of food wastes for biomethane and fertilizer, respectively.15,16 However, the benefits of HTL compared to AD are notably reduced processing duration (days/weeks versus minutes/hours), decreased reactor size, and rapid initiation period. This process is useful for liquid fuels and biochar production. HTL obviates the expensive prerequisites for pretreatment, in contrast to AD.17
Statistical and mathematical optimization techniques, like the Response Surface Method (RSM), have been employed to optimize the response parameters in HTL.18 RSM is a robust statistical technique used to fit multiple regression models to output data obtained from a simulation model. Its primary objective is to determine the optimal parameter settings that yield the best results. This method is commonly employed in situations where various independent variables influence dependent variables. For example, Xu et al.19 investigated how various operating parameters affected the HTL of sewage waste from municipal areas and reported that temperature is the most important process factor in the HTL process.
The conversion of biocrude from food waste serves the dual purpose of resolving waste management challenges and responding to the pressing demand for cleaner energy alternatives. The adoption of this comprehensive strategy has the potential to make a substantial impact on the mitigation of carbon emissions, mitigation of strain on waste disposal sites, and establishment of sustainable energy routes. Nevertheless, to effectively incorporate biocrude into the transportation industry, it is imperative to possess a comprehensive understanding of its chemical composition, physicochemical features, and combustion attributes. The objective of this study is to address the existing gap in knowledge by providing a comprehensive understanding of the potential of biocrude as a sustainable and environmentally friendly fuel for transportation purposes.
In the forthcoming sections of this scholarly article, we will explore the approaches utilized in the generation of biocrude from food waste via the process of HTL. Additionally, a comprehensive examination will be conducted on the physicochemical characteristics of the biocrude that is generated, with particular emphasis on its suitability as a fuel for transportation purposes. The anticipated results of this study are poised to make valuable contributions to the scholarly discourse surrounding bioenergy and waste valorization. Additionally, they are expected to provide valuable insights to policymakers and stakeholders regarding the potential advantages and obstacles entailed in the integration of biocrude into the current transportation infrastructure.
The novelty of this study is related to the HTL of food waste for a high biocrude yield by optimizing reaction parameters. In addition to thorough screening of food waste to produce high yields of biocrude using HTL technology, the RSM technique was used to bring out the different combinations of reaction parameters for finding out the important parameters affecting biocrude production. Additionally, an optimization study of solvent identification and recovery was undertaken by using various solvents for filtration to yield a higher quality of biocrude. The solvent selection was based on the solvents with low to high polarity (methanol, acetone, ethyl acetate, ethanol, dichloromethane, toluene, and hexane). Studying the impact of different solvents on the process allows for the optimization of product yield and quality, providing novelty on the sustainable production of bio-crude from food waste using customized HTL techniques. Additionally, different quality analyses (gas chromatography-mass spectrometry (GC-MS) and inductively coupled plasma-optical emission spectrometry (ICP-OES) techniques) were performed with new analytical tools to gain an in-depth understanding of the biocrude properties including composition. The outcomes of this study have the potential to not only redefine the discourse surrounding sustainable waste management but also accelerate the transition towards cleaner and more sustainable energy sources within the transportation industry.
2. Materials and methods
2.1. Feedstocks and chemicals
The feedstock utilized for HTL reactions in this investigation consisted of food waste obtained from the restaurant located at the University of Saskatchewan in Saskatoon, Canada. The feedstock obtained as food wastes included beetroot, brussels sprouts, cabbage, carrot, celery, corn, onion, parsnip, pumpkin, and tomato. The samples were collected, sorted, and made into a slurry with the help of an industrial grinder (Cgoldenwall, 2500 W, Canada).2.2. Feedstock characterization
For the proximate analysis of the samples, the moisture content of the fresh food wastes was estimated by the ASTM D3137 method. Similarly, the volatile matter content and ash of the dry mass were calculated by the ASTM D3175 and D3174 methods, respectively. The volatile matter content was calculated from eqn (1), and the fixed carbon content was determined by the method of subtraction as given in eqn (2). | (1) |
| fixed carbon content (wt%) = 100 − [moisture content (wt%) + ash content (wt%) + volatile] matter content (wt%) | (2) |
The modified Van Soest method was employed for detergent fibre analysis using an Ankom 200 Fibre Analyzer (ANKOM Technology, Macedon, NY). The acid detergent lignin (ADL), acid detergent fiber (ADF), and neutral detergent fiber (NDF) data were collected using the Ankom 200 Method, 8, 5, and 6 respectively.20 The extraction of fibre components, such as lignin, cellulose, hemicellulose, ash, and extractives, was performed using NDF, ADF, and ADL solutions.7 The examination of food wastes' bio-composition was conducted in two sequential processes. The initial stage involved the separation of the extractives from the biomass through a series of solvent extractions utilizing hexane, ethanol, and water in a Soxhlet system. During the second phase, the biomass stripped of extractives was examined to determine its fibre (or polysaccharide) and lignin composition.21 The biomass was subjected to gravimetric analysis using the Van Soest method to determine the quantities of cellulose, hemicellulose, and lignin. In this procedure, the preliminary estimation of NDF, which consists of cellulose, hemicellulose, and lignin, was conducted by combining 1 g of biomass with 100 mL of a neutral detergent solution, 0.5 g of sodium sulfite, and 1 g alpha-amylase in a round bottom flask and the mixture was then refluxed for 1 hour. For making 1 liter of neutral detergent solution, distilled water (0.99 L), sodium lauryl sulfate (30 g), ethylenediaminetetraacetic acid (EDTA) disodium salt (18.61 g), sodium borate decahydrate (Na2B4O7·10H2O) (reagent grade) (6.81 g), sodium phosphate dibasic (Na2HPO4) anhydrous (reagent grade) (4.56 g), and triethylene glycol (reagent grade) (10 ml) were mixed. From this mixture of neutral detergent, 100 ml was taken for analysis. Following the reflux process, the reaction mixture was passed through a pre-weighed crucible employing filtration. The crucible containing the NDF was placed in a hot air oven at 105 °C for 8 hours. Upon reaching a lower temperature, the crucible was measured in terms of weight, and the NDF was determined using the subsequent equations (eqn (3)–(8)):
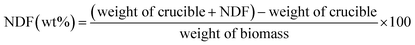 | (3) |
To estimate the ADF, the solid residue obtained from the NDF analysis was treated with an acid detergent solution consisting of 20 g cetrimonium bromide in 1000 mL of 1 N H2SO4. The mixture was then heated under reflux for 1 hour. Following the completion of the ADF estimating process, the response was halted. The ADF mixture underwent filtration, and then was transferred into a crucible and subjected to drying at 105 °C for 8 hours. The ADF was computed using the subsequent formula:
 | (4) |
| hemicellulose (wt%) = NDF (wt%) − ADF (wt%) | (5) |
 | (6) |
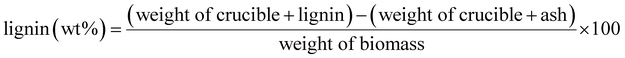 | (7) |
| extractives (wt%) = 100 − [cellulose (wt%) + hemicellose (wt%) + lignin (wt%) + ash (wt%)] | (8) |
A CHNSO analyzer (PerkinElmer Elementar, Vario EL III, Elementar Americas Inc., NJ) was utilized to conduct the ultimate analysis. The final composition, comprising carbon (C), hydrogen (H), nitrogen (N), and sulphur (S), was determined, while the oxygen content was determined by difference.
2.3. Hydrothermal liquefaction conversion in the reactor system
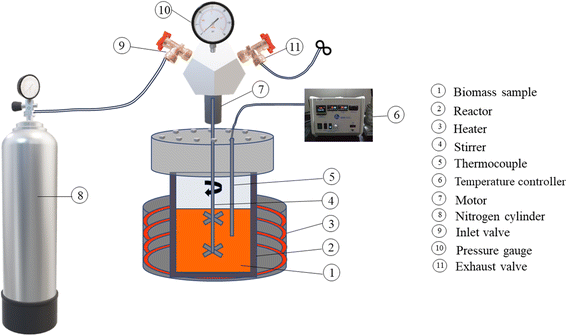
The food waste paste was used as feedstock for the HTL reactor as shown in Fig. 1. 500 g of feedstock was placed in a 1 L reactor (4848, PARR Instrument Company, US), at different sets of temperature, pressure, and time at 550 rpm rotor speed. The inherent moisture present in the feedstocks served as the reaction medium during the hydrothermal liquefaction (HTL) process, with no additional water added. The experiments were conducted under hydrothermal conditions facilitating thermochemical reactions such as hydrolysis and depolymerization. These conditions align with the standard criteria for HTL, where water acts as a reaction medium, solvent, and catalyst. These results confirm the hydrothermal nature of the process and distinguish it from simple liquefaction.Following the conclusion of the reaction, the reactor underwent a cooling process, and the slurry from the reactor was vacuum filtered for separating the aqueous phase from the biochar + biocrude mixture. Then the biochar + biocrude mixture was treated with acetone solvent (Fisher Scientific, Edmonton, Canada) with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratio and heated to extract the biocrude from the biocrude + biochar mix. After the heat treatment, the mixture was vacuum filtered to separate the biochar from the biocrude + solvent mix. Further, acetone was separated from the biocrude using a rotary evaporator (BUCHI Waterbath, B-480, Switzerland). The process flow of production of biocrude from the feedstock is given in Fig. 2.
10 ratio and heated to extract the biocrude from the biocrude + biochar mix. After the heat treatment, the mixture was vacuum filtered to separate the biochar from the biocrude + solvent mix. Further, acetone was separated from the biocrude using a rotary evaporator (BUCHI Waterbath, B-480, Switzerland). The process flow of production of biocrude from the feedstock is given in Fig. 2.
 | ||
| Fig. 2 Flow chart of different steps involved in the conversion of food waste into biocrude through hydrothermal liquefaction (HTL). | ||
2.4. Design of experiments
The Design-Expert program (version 11.0) was utilized to conduct the tests with a superior design. The Central Composite Design (CCD) procedure was applied to construct the experiments aimed at optimizing process parameters, including temperature (ranging from 280 to 320 °C), pressure (ranging from 1500 to 1900 psi), and reaction time (spanning from 15 to 45 minutes). The present study investigates the impact of these parameters on the biocrude yield (%) and oxygen content (%) of the biocrude during HTL reaction. To examine the individual and combined effects of these parameters, a series of experiments were conducted using CCD. The dependability of the model is contingent upon several factors, including F-test value, p-value, adjusted R2, R2, and forecasted R2. The experiments were conducted in duplicate. The number of tests for the CCD and biocrude yield was calculated from eqn (9) (ref. 22) and eqn (10),23 respectively. The oxygen content was obtained from the elemental analysis of the biocrude.| N = 2n + 2n + k | (9) |
Similarly, the biocrude yield was obtained from eqn (8).
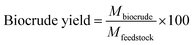 | (10) |
2.5. Quality analyses
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) ratio.
1 (v/v) ratio.
3. Results and discussion
The physicochemical analysis of the food wastes was analyzed as discussed in the Materials and methods. The proximate analysis of the samples is given in Table 3, and the fiber and ultimate analysis of the feedstock is shown in Table 4.| Food wastes | Moisture content (% wt, fresh) | Moisture content (wt%, oven dried) | Ash content (wt%) | Volatile matter content (wt%) | Fixed carbon content (wt%) |
|---|---|---|---|---|---|
| Beetroot | 86.0 ± 0.2 | 0.9 ± 0.1 | 10.1 ± 0.7 | 77.6 ± 0.8 | 11.4 ± 0.1 |
| Brussels sprout | 82.0 ± 0.1 | 0.6 ± 0.1 | 22.0 ± 0.6 | 69.4 ± 0.9 | 8.0 ± 0.4 |
| Cabbage | 90.8 ± 0.2 | 1.3 ± 0.3 | 18.2 ± 0.3 | 68.7 ± 0.3 | 11.8 ± 0.6 |
| Carrot | 90.0 ± 0.3 | 0.4 ± 0.0 | 9.7 ± 0.9 | 84.6 ± 0.3 | 5.3 ± 0.3 |
| Celery | 93.0 ± 0.7 | 0.5 ± 0.1 | 12.6 ± 0.9 | 79.3 ± 0.9 | 7.6 ± 0.5 |
| Corn | 79.5 ± 0.4 | 1.2 ± 0.1 | 9.8 ± 0.8 | 72.0 ± 0.7 | 17.0 ± 0.3 |
| Onion | 89.4 ± 0.3 | 1.4 ± 0.4 | 10.1 ± 0.7 | 79.6 ± 0.8 | 9.0 ± 0.7 |
| Parsnip | 80.0 ± 0.3 | 0.8 ± 0.0 | 9.8 ± 0.7 | 74.8 ± 0.3 | 14.7 ± 0.9 |
| Pumpkin | 79.0 ± 0.1 | 0.6 ± 0.1 | 10.4 ± 0.2 | 79.4 ± 0.6 | 9.6 ± 0.2 |
| Tomato | 95.4 ± 0.3 | 1.1 ± 0.2 | 9.1 ± 0.2 | 71.3 ± 0.8 | 18.4 ± 0.1 |
| Food waste (dry basis) | Fiber analysis | C (wt%) | H (wt%) | N (wt%) | S (wt%) | Ash (wt%) | O (wt%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Lignin (wt%) | Cellulose (wt%) | Hemicellulose (wt%) | Extractives (wt%) | |||||||
| Beetroot | 7.0 ± 0.7 | 8.4 ± 0.5 | 13.1 ± 0.1 | 61.4 ± 0.3 | 42.6 | 5.6 | 2.4 | 0.2 | 10.0 | 39.1 |
| Brussels sprout | 5.7 ± 0.0 | 11.7 ± 0.4 | 7.6 ± 0.3 | 53.0 ± 0.6 | 41.2 | 6.0 | 4.7 | 0.7 | 22.0 | 25.4 |
| Cabbage | 5.2 ± 0.2 | 12.4 ± 0.4 | 2.4 ± 0.1 | 61.8 ± 0.2 | 41.6 | 5.7 | 2.1 | 0.6 | 18.2 | 31.7 |
| Carrot | 8.1 ± 0.2 | 15.3 ± 0.2 | 4.1 ± 0.3 | 62.8 ± 0.1 | 44.9 | 7.8 | 1.0 | 0.1 | 9.7 | 36.5 |
| Celery | 7.7 ± 0.1 | 11.5 ± 0.3 | 5.0 ± 0.4 | 63.2 ± 0.1 | 42.3 | 5.7 | 4.3 | 0.4 | 12.6 | 34.7 |
| Corn | 7.9 ± 0.2 | 5.6 ± 0.3 | 21.2 ± 0.3 | 55.4 ± 0.1 | 42.6 | 7.4 | 2.1 | 0.1 | 9.8 | 38.0 |
| Onion | 3.8 ± 0.1 | 6.0 ± 0.1 | 1.7 ± 0.2 | 78.4 ± 0.1 | 42.5 | 6.4 | 1.0 | 0.5 | 10.1 | 39.5 |
| Parsnip | 8.2 ± 0.2 | 11.9 ± 0.3 | 0.5 ± 0.2 | 69.7 ± 0.1 | 43.1 | 5.7 | 1.6 | 0.3 | 9.8 | 39.6 |
| Pumpkin | 10.6 ± 0.1 | 18.6 ± 0.3 | 5.9 ± 0.7 | 54.5 ± 0.1 | 41.7 | 6.3 | 2.7 | 0.3 | 10.4 | 38.7 |
| Tomato | 10.1 ± 0.1 | 11.5 ± 0.2 | 3.3 ± 0.3 | 66.0 ± 0.1 | 41.5 | 5.5 | 2.2 | 0.2 | 9.1 | 41.5 |
As can be seen from Table 3, the food wastes collected from restaurants have a high moisture content, which serves as the reaction medium for the hydrothermal liquefaction of the food wastes. However, the feedstock for the HTL reaction was chosen based on the vegetable having low ash content, and low fixed carbon content as the focus is to get high biocrude yield.24 Similarly, feedstocks having high lignin, cellulose, carbon, and hydrogen content were preferred for the HTL reaction as they contribute towards the high biocrude yield.25 As can be seen from the fiber and ultimate analysis from Table 4, the carbon and hydrogen contents in carrots are higher than the rest of the vegetables, therefore, carrots was chosen as the feedstock for HTL.26 However, each vegetable analyzed in this study has the potential to produce biocrude to some extent, which will be accessed in future studies.
3.1. Mass balance of hydrothermal liquefaction of food waste
A set of 20 experimental runs were conducted based on the design model as shown in Table 5. A mass balance was carried out for the 20 runs of experiments, and it was observed that the maximum amount of biocrude (18.8 wt%) and lowest biochar yield (21.6 wt%) were for the reaction parameters 280 °C, 1500 psi, and 45 minutes. The oxygen content of the biocrude was in the range of 11.1–23.3 wt%, showing the need for upgradation.27,28| Run | Factor 1 | Factor 2 | Factor 3 | Mass balance | Oxygen content (wt%) (response 2) | |||
|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Pressure (psi) | Time (min) | Biocrude yield (wt%) (response 1) | Aqueous phase (wt%) | Biochar (wt%) | Gas (wt%) | ||
| 1 | 280 | 1700 | 30 | 17.8 | 2.8 | 26.6 | 52.8 | 19.3 |
| 2 | 320 | 1500 | 15 | 14.6 | 1.9 | 33.0 | 50.5 | 14.3 |
| 3 | 320 | 1900 | 15 | 14.2 | 2.3 | 32.6 | 50.9 | 11.1 |
| 4 | 300 | 1700 | 30 | 16.0 | 2.6 | 30.6 | 50.8 | 15.3 |
| 5 | 300 | 1700 | 30 | 16.2 | 2.8 | 32.2 | 48.8 | 16.3 |
| 6 | 300 | 1900 | 30 | 15.4 | 2.2 | 32.4 | 50.0 | 16.1 |
| 7 | 280 | 1500 | 45 | 18.8 | 2.8 | 21.6 | 56.8 | 17.7 |
| 8 | 300 | 1700 | 30 | 16.4 | 2.2 | 32.8 | 48.6 | 16.1 |
| 9 | 280 | 1900 | 45 | 18.4 | 2.7 | 23.8 | 55.1 | 23.3 |
| 10 | 300 | 1700 | 30 | 16.6 | 2.8 | 32.8 | 47.8 | 15.5 |
| 11 | 280 | 1500 | 15 | 16.6 | 2.8 | 25.6 | 55.0 | 17.5 |
| 12 | 320 | 1900 | 45 | 14.6 | 2.8 | 32.0 | 50.6 | 15.2 |
| 13 | 300 | 1500 | 30 | 16.4 | 2.9 | 29.4 | 51.3 | 16.2 |
| 14 | 300 | 1700 | 30 | 16.2 | 2.8 | 31.6 | 49.4 | 15.3 |
| 15 | 320 | 1500 | 45 | 14.8 | 2.7 | 34.8 | 47.7 | 12.2 |
| 16 | 300 | 1700 | 45 | 15.6 | 2.5 | 29.4 | 52.5 | 18.5 |
| 17 | 320 | 1700 | 30 | 14.6 | 2.3 | 34.0 | 49.1 | 13.7 |
| 18 | 280 | 1900 | 15 | 16.2 | 2.3 | 28.4 | 53.1 | 18.3 |
| 19 | 300 | 1700 | 15 | 14.6 | 2.4 | 29.8 | 53.2 | 16.1 |
| 20 | 300 | 1700 | 30 | 16.2 | 2.6 | 31.8 | 49.4 | 15.7 |
3.2. Statistical analysis of the model
According to the preceding description, a total of twenty sets of experiments were carried out, and the replies were evaluated with regards to the acceptability of the regression model based on various coefficients. The optimization of the process variables was conducted using the reaction model generated by the Design-Expert program. The experimental data was analyzed using various models, including two-factor interaction, linear, quadratic, and cubic models, in order to determine the regression equation. This study examines the correlation between biocrude yields and oxygen content in response to several experimental parameters, including temperature, pressure, and reaction time. The rationality of the model was determined using mathematical properties such as the sequential model sum of squares and the model summary.29,30As previously mentioned in the preceding paragraph, the verification of the model's appropriateness was conducted by examining the model summary statistics, which are provided in Table 6. The comparison between the biocrude yields and oxygen content predicted by the obtained regression model and the corresponding experimental values is presented in Table 5. The acceptability of the statistical model was significantly influenced by two criteria, namely a high F-test value and a low p-value (probability value), as indicated in Table 7.31,32 The F-test value from Table 7 was found to be 118.8 for biocrude yield, and 40.4 for oxygen content, whereas the p-value was <0.0001 for both of them, which evidenced the significant acceptability of the model. A model is considered statistically significant when the p-value is less than 0.05 and the F-test value is higher. Another crucial factor considered in assessing the quality of the model was the presence of a lack of fit. In a regression model, it is imperative for the lack of fit to be statistically small in order to deem the model acceptable.33 In the present study, the calculated p-values for the model were determined to be 0.06 and 0.07, indicating statistical significance. It is important to acknowledge that in the context of lack of fit, the p-value should exceed 0.05.
| Source | Sequential p-value | Std. dev. | Lack of fit p-value | R 2 | Adjusted R2 | Predicted R2 | Remark |
|---|---|---|---|---|---|---|---|
| Response 1: biocrude yield | |||||||
| Linear | <0.0001 | 0.471 | 0.001 | 0.881 | 0.858 | 0.773 | |
| 2FI | 0.0160 | 0.356 | 0.002 | 0.945 | 0.919 | 0.922 | |
| Quadratic | 0.0003 | 0.166 | 0.057 | 0.991 | 0.982 | 0.962 | Suggested |
| Cubic | 0.9531 | 0.204 | 0.006 | 0.992 | 0.973 | −7.317 | Aliased |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Response 2: oxygen content | |||||||
| Linear | <0.0001 | 1.3300 | 0.004 | 0.788 | 0.738 | 0.529 | |
| 2FI | 0.0002 | 0.7110 | 0.070 | 0.949 | 0.926 | 0.886 | Suggested |
| Quadratic | 0.3735 | 0.6984 | 0.061 | 0.962 | 0.928 | 0.787 | |
| Cubic | 0.6196 | 0.7445 | 0.014 | 0.974 | 0.919 | −22.266 | Aliased |
| Source | Sum of squares | Degree of freedom | Mean square | F-Value | p-Value | Remark |
|---|---|---|---|---|---|---|
| Response 1: biocrude yield (ANOVA for the quadratic model) | ||||||
| Model | 29.47 | 9 | 3.27 | 118.79 | <0.0001 | Significant |
| A: temperature (°C) | 21.90 | 1 | 21.90 | 794.74 | <0.0001 | |
| B: pressure (psi) | 0.44 | 1 | 0.44 | 16.00 | 0.0025 | |
| C: time (min) | 3.84 | 1 | 3.84 | 139.47 | <0.0001 | |
| AB | 0.0012 | 1 | 0.0012 | 0.05 | 0.8356 | |
| AC | 1.90 | 1 | 1.90 | 68.98 | <0.0001 | |
| BC | 0.0012 | 1 | 0.0012 | 0.05 | 0.8356 | |
| A 2 | 0.47 | 1 | 0.47 | 17.07 | 0.0020 | |
| B 2 | 0.07 | 1 | 0.07 | 2.67 | 0.1332 | |
| C 2 | 1.30 | 1 | 1.30 | 47.00 | <0.0001 | |
| Residual | 0.28 | 10 | 0.03 | |||
| Lack of fit | 0.23 | 5 | 0.05 | 4.70 | 0.0573 | Not significant |
| Pure error | 0.05 | 5 | 0.01 | |||
| Corrected total | 29.74 | 19 | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Response 2: oxygen content (ANOVA for the 2FI model) | ||||||
| Model | 122.63 | 6 | 20.44 | 40.44 | <0.0001 | Significant |
| A: temperature | 87.78 | 1 | 87.78 | 173.66 | <0.0001 | |
| B: pressure | 3.64 | 1 | 3.64 | 7.20 | 0.0188 | |
| C: time | 9.30 | 1 | 9.30 | 18.40 | 0.0009 | |
| AB | 5.49 | 1 | 5.49 | 10.86 | 0.0058 | |
| AC | 1.23 | 1 | 1.23 | 2.43 | 0.1432 | |
| BC | 15.20 | 1 | 15.20 | 30.07 | 0.0001 | |
| Residual | 6.57 | 13 | 0.51 | |||
| Lack of fit | 5.69 | 8 | 0.71 | 4.04 | 0.0703 | Not significant |
| Pure error | 0.88 | 5 | 0.18 | |||
| Cor total | 129.21 | 19 | ||||
The impact of model summary statistics (Table 6) on the adequacy of the mathematical model is considerable, as it is influenced by the values of R2 (coefficient of determination), anticipated R2, and adjusted R2.31,32 According to the table, the quadratic model and two-factor interactive model were suitable for biocrude yield and oxygen content, respectively under the attained experimental results. There was a notable concurrence observed between the adjusted R2 values (0.9, 0.9) and the corresponding anticipated R2 values (0.9, 0.9). There is minute difference between the two values when considering significant numbers (<0.02 and <0.04, respectively). Therefore, based on the aforementioned features such as the R2 values, F-test value, and p-value, it can be concluded that the regression model developed for the present experiments has a high level of significance and may be regarded as one of the most robust mathematical models. Following the assessment of the model's importance, a regression equation was derived and presented in eqn (11) and (12). To assess the validity of the equation, two experimental trials were conducted using randomly selected values for temperature, pressure, and reaction time. Moreover, the biocrude yield and oxygen content, as determined through experimental estimation, exhibited a satisfactory level of concurrence with the anticipated value.
| Biocrude yield (%) = +65.71 − 0.34T − 0.007P + 0.34t + 3.13 × 10−6TP − 0.0008Tt + 4.17 × 10−6Pt + 0.0005T2 + 1.6 × 10−6P2 − 0.0015t2 | (11) |
| Oxygen content (%) = −40.37 + 0.24T + 0.05P − 0.33t − 0.0002TP − 0.001Tt + 0.0005Pt | (12) |
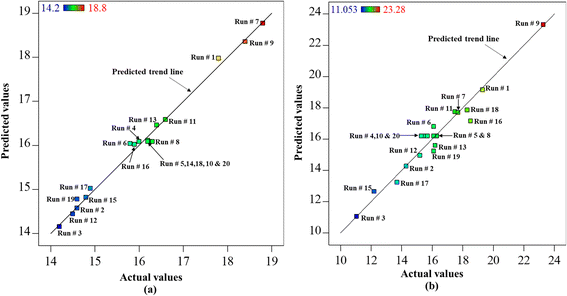
A further crucial component of the model pertains to the examination of variance, commonly referred to as analysis of variance (ANOVA), as demonstrated in Table 7. The ANOVA test table encompasses various statistical terminology, including the F-test value, p-value, degree of freedom, and sum of squares, which have been previously elucidated. The independent variables in the model are deemed significant if P ≤ 0.05. A higher F value suggests that the variables or model have a stronger statistical significance.34 The regression model in Table 7 exhibited a significantly higher F-test value and a p-value less than 0.05, providing evidence to support the acceptability of the model. In addition, the p-value for each factor in the model (p-value < 0.05) indicates the level of confidence in the factor's contribution to the significance of the regression model.35 The data shown provides compelling evidence that temperature significantly influences the yield of biocrude and oxygen content. This is supported by the p-value, which was determined to be less than 0.0001 for both factors. The aforementioned fact has been corroborated by the findings of our experimental study. The correlation between the experimental yield and anticipated yield of biocrude, as well as the oxygen content, was depicted in the parity plot (Fig. 3), demonstrating a reasonable level of agreement. The linear trend shown in the plot depicts the relationship between biocrude output and oxygen content. It is evident that, with the exception of a few experimental trials, the majority of the acquired outcomes are positioned either directly on or in close proximity to the projected trend line indicating that the model predicts experimental data reliably.
3.3. Variation of responses as per parameters
The regression model provides a comprehensive depiction of the impacts of many experimental factors, specifically process parameters, on the responses, namely biocrude yield and oxygen content. Furthermore, it elucidates the interplay among different variables and their influence on the outcome. The relevance of the factors mostly relies on the p-values associated with them, which should be below 0.05. According to the ANOVA table (Table 7), the factors of temperature (A), pressure (B), and time (C), as well as their respective squares (A2, B2, and C2), exhibited p-values below 0.05. Notably, temperature emerged as the most influential factor.Fig. 4 illustrates the impact of temperature, pressure, and duration on the production of biocrude. The data points depicted in Fig. 4(a) were obtained under varying temperatures ranging from 280 to 320 °C, while maintaining a constant pressure of 1700 psi and a fixed duration of 30 minutes. Based on the data presented in the figure, it can be observed that the biocrude yield exhibited an initial increase up to 18.8% at a specified temperature of 280 °C, beyond which a subsequent decline in yield was observed. This finding suggests that the impact of reaction temperature on the liberation of biomass macromolecules was more significant at lower temperatures compared to higher temperature circumstances. The potential cause of this phenomenon may be attributed to the relatively low concentration of solid particles present in the feedstock.36 The decrease in biocrude yield with increase in temperature could be because of polymerization of the molecules to form solids, which justifies the high biochar yield with high temperature from Table 5. As per the literature, the biocrude yield from food waste increased with temperatures up to 300 °C and decreased with further increase in temperature due to decarbonylation, dehydration, and decarboxylation of the initially formed water-soluble molecules.37 However, the pressure does not seem to affect the biocrude yield but the yield decreases slightly with increment in pressure as per Fig. 4(b). This may be due to the formation of more gaseous compounds with high pressure due to depolymerization. Similarly, Fig. 4(c) shows that the biocrude yield increases with increment in time; however, the yield is maximum (18.8%) at time of 42 minutes, and after that the yield is constant. The biocrude yield seemed to be increasing with reaction time at lower temperature than higher temperature. Similar results were also observed in the literature.38 During the process of biomass hydrolysis, various intermediate byproducts such as ethers, esters, ketones, and aldehydes are formed. The extent to which these byproducts undergo depolymerization into chemicals (at higher temperatures) or polymerization into unwanted byproducts (at lower temperatures) tends to increase with longer reaction times.39,40
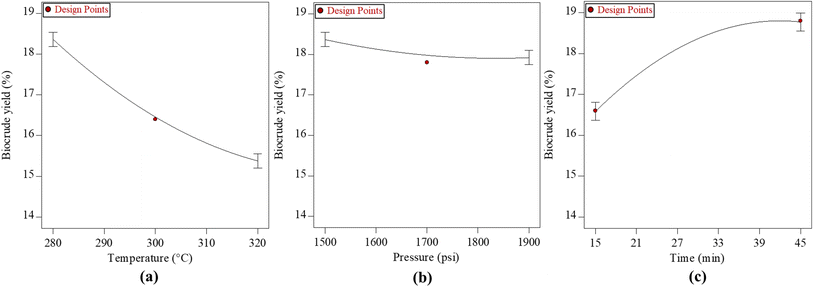 | ||
| Fig. 4 Effects of (a) temperature at 1700 psi pressure, 30 min time; (b) pressure at 300 °C temperature, 30 min time; and (c) time at 300 °C temperature, 1700 psi pressure on biocrude yield. | ||
Fig. 5 represents the effects of temperature (a), pressure (b), and reaction time (c) on the oxygen content. It can be seen from the graphs that the temperature and pressure affect the oxygen content, while time does not. This could be due to the transfer of oxygen to aqueous and gaseous phases under higher temperature and pressure.
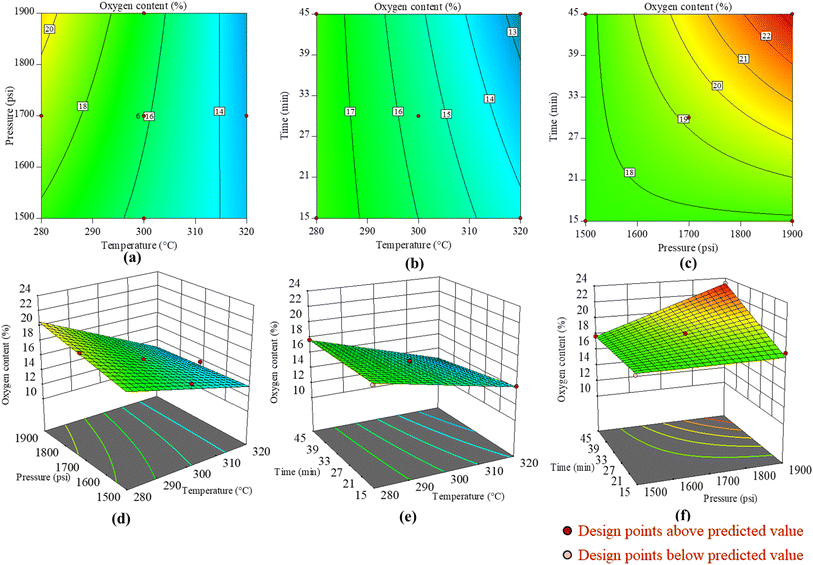
For easy understanding of the reader, Fig. 6 depicts the 2D and 3D contour plots demonstrating the collaborative impacts of temperature, pressure, and reaction time on the biocrude yield during HTL of food waste. It is perceptible from Fig. 6(a) and (d) that at a constant time with the temperature progress, the yield of biocrude reduced; however, with elevation of pressure it remains almost constant. The decrease in yield could be due to the polymerization of the molecules to form solids to form biochar at higher temperature, and depolymerization at higher pressure to form a gaseous product.36 Similarly, in Fig. 6(b) and (e) the effect of time and temperature at a constant pressure of 1700 psi on the biocrude yield is shown. It is perceivable that with increasing time, the yield increases while it decreases with temperature elevation. This phenomenon may be attributed to the extended duration of reaction time, which enables the byproducts to undergo additional depolymerization into chemical compounds at elevated temperatures or polymerization into unwanted byproducts at lower temperature.41,42 Similarly, Fig. 6(c) and (f) present the effects of time and pressure on the biocrude yield. It can be observed that the pressure did not affect the yield, however the yield increased with time. The impact of temperature and time on the yield is more pronounced compared to that of pressure.
In a similar way, Fig. 7 depicts the 2D and 3D contour plots representing the collaborative impacts of temperature, pressure, and reaction time on the oxygen content of the biocrude. The effects of pressure and temperature at a constant time of 30 min on the oxygen content are presented in Fig. 7(a) and (d). The oxygen content of the biocrude decreases with increase in temperature; this may be due to the production of more aqueous solution as the oxygenated compounds shift to the aqueous or gaseous phase at high temperature with time. However, there is a slight increase in oxygen content in the biocrude with increase in pressure. Fig. 7(b) and (e) show the effects of reaction time and temperature on the oxygen content. It can be perceived that with an increase in time and temperature, the oxygen content decreases in the biocrude, maybe because of the shift of oxygenated compounds to the aqueous or gaseous phase. Similarly, Fig. 7(c) and (f) depict the combined effects of reaction time and pressure on the oxygen content of the biocrude. It can be observed that the increment of oxygen content with increment of time and pressure may be due to polymerization of the oxygenated compound at high pressure and time.32 A similar type of result was also reported by Pattnaik et al.32 for showing the effects of temperature and reaction time on total reducing sugar yields during subcritical water hydrolysis of phragmites.
Following the validation of the regression model utilizing multiple parameters, this study elucidates the interactions among distinct components and their respective effects on the yield of biocrude. The ultimate phase of the procedure was the retrieval of the optimization criteria from the statistical model that was built. The experimental study determined that the most favorable conditions for achieving the largest biocrude yields and minimizing the oxygen content during HTL of carrot waste were a temperature of 280 °C, a pressure of 1500 psi, and a reaction time of 42 minutes (Fig. 8). In addition to the optimized process conditions, the anticipated biocrude output and oxygen content were determined to be 18.8% and 17.7%, correspondingly. The biocrude yield was evaluated by conducting an optimization run with the implementation of these settings. The experimental error, as determined by the calculation using eqn (13), was observed to be 3.1%.
 | (13) |
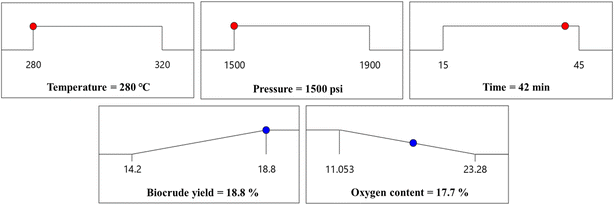 | ||
| Fig. 8 Experimental boundaries set for each of the process parameters and the responses (biocrude yield and oxygen content) together with their optimum estimates. | ||
All the feedstocks undergo HTL under the optimized conditions and fitted with eqn (9) and (10) to verify the model. The biocrude yield, oxygen content, and their percent errors obtained from HTL of the feedstocks operated at optimum conditions (280 °C, 1500 psi, and 45 minutes) are shown in Table 8. As the errors are insignificant, it validates the model and establishes that it works for these food wastes.
| Feedstock | Biocrude | Oxygen content | ||
|---|---|---|---|---|
| Yield (wt%) | Percent error (%) | Yield (wt%) | Percent error (%) | |
| Beetroot | 18.3 | 2.7 | 18.8 | 5.9 |
| Brussels sprouts | 17.9 | 5.0 | 19.5 | 9.2 |
| Cabbage | 18.2 | 3.3 | 18.4 | 3.8 |
| Carrot | 19.4 | 3.1 | 18.3 | 3.3 |
| Celery | 17.8 | 5.6 | 19.2 | 7.8 |
| Corn | 17.9 | 5.0 | 19.5 | 9.2 |
| Onion | 18.0 | 4.4 | 18.7 | 5.3 |
| Parsnip | 18.4 | 2.2 | 17.3 | 2.3 |
| Pumpkin | 17.9 | 5.0 | 18.8 | 5.9 |
| Tomato | 17.2 | 9.3 | 16.8 | 5.4 |
3.4. Solvent optimization and quality analyses
By using optimized parameters, HTL experiments were carried out to optimize the solvent of filtration for yielding maximum biocrude. For this purpose, solvents such as methanol, acetone, ethyl acetate, ethanol, dichloromethane, toluene, and hexane (Fisher Scientific, Edmonton, Canada) were selected. All the experiments were conducted in duplicate.| Sl. no. | Solvents | Polarity index | Biocrude yield (wt%) | C (wt%) | H (wt%) | N (wt%) | S (wt%) | O (wt%) | HHV (MJ kg−1) | C recovery (%) | Viscosity (cP) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Methanol | 5.1 | 19.6 | 70.0 | 7.8 | 1.4 | 0.2 | 20.7 | 30.8 | 55.9 | 1215 |
| 2 | Acetone | 5.1 | 18.8 | 73.5 | 7.2 | 1.3 | 0.3 | 17.7 | 31.3 | 63.7 | 1136 |
| 3 | Ethyl acetate | 4.4 | 18.6 | 74.4 | 7.7 | 1.4 | 0.1 | 16.5 | 32.1 | 65.7 | 1023 |
| 4 | Ethanol | 4.3 | 17.4 | 71.3 | 7.4 | 1.7 | 0.1 | 19.5 | 30.7 | 58.8 | 886 |
| 5 | Dichloromethane | 3.1 | 11.6 | 75.6 | 8.0 | 1.4 | 0.2 | 14.8 | 33.0 | 68.4 | 674 |
| 6 | Toluene | 2.4 | 10.2 | 77.6 | 8.4 | 1.0 | 0.2 | 12.8 | 36.4 | 72.8 | 515 |
| 7 | Hexane | 0.1 | 0 | — | — | — | — | — | — | — | — |
The results from Table 9 have shown that 55.9–72.8% of carbon has been recovered from carrot waste when it is converted to biocrude. A similar type of result has been reported for biocrude obtained from HTL of human feces by Lu et al.43
In the temperature range of 200–350 °C, a significant weight loss of 50–55% is observed, dominated by the decomposition of hemicellulose and cellulose, along with contributions from proteins and lipids. This stage is pivotal for understanding the breakdown of macromolecules under hydrothermal conditions. In HTL, hemicellulose and cellulose hydrolyze into sugars and further degrade into smaller volatile compounds, which serve as precursors for bio-oil formation.44,45,48 Proteins contribute to the nitrogen content in the resulting bio-crude, while lipids enhance the yield and quality of the oil fraction, aligning with previous findings on the role of biochemical composition in biomass liquefaction.46–48 In the higher temperature range of 350–500 °C, a further weight loss of approximately 20–25% is observed. While this phase includes the thermal degradation of lignin, which accounts for 8.1% of the carrot waste, the remaining weight loss in this range is attributed to the slow decomposition of other components such as proteins, residual carbohydrates, and complex organic substances such as pectins and phenolic compounds. These components, though in smaller amounts, degrade over a broad temperature range and contribute to the overall weight loss. Additionally, any unconverted lipids and the more thermally resistant fractions of hemicellulose and cellulose might also degrade in this stage.45,48,49 This phase underscores the complexity of carrot waste and its potential for producing bio-oil rich in diverse organic fractions, suitable for industrial applications.46,49
Above 500 °C, the residual material, representing 15–20% of the initial weight, consists of inorganic minerals and ash. The mineral content, including calcium, potassium, and other trace elements, is relevant for designing catalysts or addressing potential challenges such as fouling during HTL.45,46,48 The integration of TGA insights with HTL studies suggests that carrot waste's thermal characteristics make it a suitable feedstock for hydrothermal processing, offering a sustainable pathway for bioenergy and bioproducts.46,49
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching (alkenes), C
C stretching (alkenes), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching (esters, aldehydes, ketones, amides), C
O stretching (esters, aldehydes, ketones, amides), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching (unsaturated compounds), –CH stretching (aliphatic bending groups), C–H deformation in CH2 groups, C–O–C stretching (esters, ethers, other phenolic compounds), C–O stretching (carbohydrates), C–O (alcohols), C–H in plane benching (aromatic compounds), and –C
C stretching (unsaturated compounds), –CH stretching (aliphatic bending groups), C–H deformation in CH2 groups, C–O–C stretching (esters, ethers, other phenolic compounds), C–O stretching (carbohydrates), C–O (alcohols), C–H in plane benching (aromatic compounds), and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bending (inorganic carbonates), respectively.50,51 Similarly, the food waste bands at 3251, 2919, 2360, 1593, and 1024 cm−1 represented the O–H stretching (polymeric O–H, waster impurities), C–H stretching, (alkanes), C
O bending (inorganic carbonates), respectively.50,51 Similarly, the food waste bands at 3251, 2919, 2360, 1593, and 1024 cm−1 represented the O–H stretching (polymeric O–H, waster impurities), C–H stretching, (alkanes), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C conjugated and C
C conjugated and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C stretching, C
C stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching (alkenes), and C–O stretching (primary, secondary, tertiary alcohol), respectively.50,52
C stretching (alkenes), and C–O stretching (primary, secondary, tertiary alcohol), respectively.50,52
| Retention time | Compound name | Formula | % composition |
|---|---|---|---|
| Methanol | |||
| 4.81 | 4-(2-Methoxyethyl)phenol | C9H12O2 | 17.1 |
| 6.5 | 1-Buten-3-one, 1-(2-carboxy-4,4-dimethylcyclobutenyl)- | C11H14O3 | 2.2 |
| 6.82 | Ribitol, 1,3:2,4-di-O-benzylidene- | C19H20O5 | 5.3 |
| 7.17 | Benzoic acid, 2-hydroxy-5-nitro- | C7H5NO5 | 0.9 |
| 7.53 | Benzene, 1,1′-dodecylidenebis-4-methyl- | C26H38 | 1.1 |
| 7.99 | Morpholine, 3-(4,5-dihydroxy)phenyl- | C10H13NO3 | 3.9 |
| 8.79 | Phenol | C6H6O | 5.3 |
| 8.88 | 2-Vinylfuran | C6H6O | 9.6 |
| 9.84 | Oxirane-2-carboxylic acid, 3-(3,4,5-trimethoxyphenyl)-, methyl ester | C13H16O6 | 0.2 |
| 10.08 | Phthalic acid, 4-methoxyphenyl phenyl ester | C21H16O5 | 0.9 |
| 10.42 | 1,2-Nonadecanediol | C19H40O2 | 0.8 |
| 11.45 | 1-Octene, 3,7-dimethyl- | C10H20 | 1.5 |
| 11.6 | 1,3-Dioxane, 4-(hexadecyloxy)-2-pentadecyl- | C35H70O3 | 0.1 |
| 13.39 | 9-Hexadecenoic acid | C16H30O2 | 3.7 |
| 15.07 | 11-Tricosene | C23H46 | 1.6 |
| 17.5 | Hexadecanoic acid, methyl ester | C17H34O2 | 6.1 |
| 18.63 | 9-Octadecenoic acid (Z)-, methyl ester | C19H36O2 | 3.8 |
| 18.78 | Heptadecanoic acid, 16-methyl-, methyl ester | C19H38O2 | 2.1 |
| 21.09 | Phthalic acid, di(2-propylpentyl) ester | C24H38O4 | 9.5 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Acetone | |||
| 4.81 | 3-Penten-2-one, 4-methyl- | C6H10O | 6.5 |
| 5.53 | Oxalic acid, isohexyl neopentyl ester | C13H24O4 | 0.8 |
| 5.75 | 1-Hexadecanol, 2-methyl- | C17H36O | 0.5 |
| 5.9 | Heptane, 3,5-dimethyl- | C9H20 | 3.6 |
| 6.1 | 2-Pentanone, 4-hydroxy-4-methyl- | C6H12O2 | 49.9 |
| 6.43 | Heptane, 2,4-dimethyl- | C9H20 | 4.1 |
| 6.49 | Heptane, 3,4-dimethyl- | C9H20 | 1.8 |
| 6.62 | Octane, 4-methyl- | C9H20 | 3.7 |
| 6.66 | Octane, 2-methyl- | C9H20 | 1.6 |
| 6.75 | Heptane, 3-ethyl- | C9H20 | 1.0 |
| 6.8 | Heptane, 2,5-dimethyl- | C9H20 | 4.1 |
| 8.85 | Phenol | C6H6O | 17.0 |
| 10.29 | Paromomycin | C23H45N5O14 | 1.3 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Ethyl acetate | |||
| 8.86 | Phenol | C6H6O | 78.5 |
| 9.12 | 7-Oxabicyclo[4.1.0]heptane, 2-methylene- | C7H10O | 2.6 |
| 10.3 | Paromomycin | C23H45N5O14 | 6.7 |
| 12.35 | Gibberellic acid | C19H22O6 | 0.5 |
| 13.13 | Naphthalene, 1,2,3,4-tetrahydro-1,1,6-trimethyl- | C13H18 | 2.7 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Ethanol | |||
| 5.21 | 1(3H)-Isobenzofuranone | C8H6O2 | 44.3 |
| 7.6 | Benzoic acid, 2-methoxy-4-methyl-3-nitro- | C10H11NO5 | 36.4 |
| 8.99 | Benzoic acid, 4-(1,3-dioxolan-2-yl)-, methyl ester | C11H12O4 | 3.5 |
| 9 | Phenol | C6H6O | 1.2 |
| 17.94 | Hexadecanoic acid, ethyl ester | C18H36O2 | 0.7 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Dichloromethane | |||
| 8.83 | Phenol | C6H6O | 9.1 |
| 10.34 | 2,4-Heptadiene, 2,4-dimethyl- | C9H16 | 2.6 |
| 11.5 | 2(3H)-Naphthalenone, 4,4a,5,6,7,8-hexahydro-1-methoxy- | C11H16O2 | 3.1 |
| 12.34 | Phenol, 4-ethyl-2-methoxy- | C9H12O2 | 5.6 |
| 13.14 | Naphthalene, 1,2,3,4-tetrahydro-1,1,6-trimethyl- | C13H18 | 17.8 |
| 13.19 | Ethanone, 1-(1-hydroxy-2,6,6-trimethyl-2,4-cyclohexadien-1-yl)- | C11H16O2 | 1.9 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Toluene | |||
| 7.43 | 1,5-Heptadien-3-yne | C7H8 | 49.8 |
| 7.5 | 1-Undecene | C11H22 | 1.1 |
| 7.54 | Cyclohexane, 1,3-dimethyl-, trans- | C8H16 | 1.0 |
| 7.56 | Cyclopentane, propyl- | C8H16 | 0.3 |
| 7.6 | Cyclopentane, 1-ethyl-2-methyl-, cis- | C8H16 | 0.8 |
| 7.65 | Cyclooctane | C8H16 | 1.5 |
| 7.67 | Cyclohexane, ethyl- | C8H16 | 1.1 |
| 7.84 | Ethylbenzene | C8H10 | 8.2 |
| 7.92 | p-Xylene | C8H10 | 6.6 |
The GC-MS results of biocrude obtained from HTL reveal a complex composition of hydrocarbons (for example, 1,5-heptadien-3-yne; ethylbenzene; 1,2,3,4-tetrahydro-1,1,6-trimethyl-naphthalene, etc.), carboxylic acids (9-hexadecenoic acid), ketones (1(3H)-isobenzofuranone), alcohols (phenol; 1,2-nonadecanediol), esters (isohexyl oxalic acid neopentyl ester); (2-methoxy-4-methyl-3-nitro-benzoic acid methyl ester), and other organic compounds as shown Table 10. The detailed GC-MS analysis allows for identifying specific biofuel components, aiding in optimizing HTL processes for enhanced bioenergy production from food waste. As seen from Table 10, many of the compounds that are absent in biocrude derived using DCM solvent are present in biocrude derived using methanol. This confirms that non-polar solvents like DCM extract them during distillation and leave the biocrude free from them.53 It is also observed that the cyclic hydrocarbons (1,3-dimethyl-, trans-cyclohexane; cyclooctane), non-cyclic hydrocarbons (1,5-heptadien-3-yne; 1-undecene), and aromatic hydrocarbons (ethylbenzene; p-xylene) are the major groups in biocrude extracted using toluene, whereas phenolic derivatives (phenol; 4-(2-methoxyethyl)phenol), carboxylic acids/derivatives (2-hydroxy-5-nitro-benzoic acid); 9-hexadecenoic acid; phthalic acid ester derivatives, and other ester derivatives, ketones (1-(2-carboxy-4,4-dimethylcyclobutenyl, 1-buten-3-one)), alcohols (derivatized ribitol; 1,2-nonadecanediol), lower amount of hydrocarbons (1,1′-dodecylidenebis-4-methyl-benzene; 3,7-dimethyl-1-octene; 11-tricosene), and furanic compound (2-vinylfuran) are the main groups of biocrude recovered using methanol.54,55 The product profiles for methanol, acetone, ethyl acetate, and ethanol are almost the same because of their comparable high polarity.56 However, the major portion of acetone extracted biocrude contains 4-hydroxy-4-methyl-2-pentanone and phenol; ethyl acetate extracted biocrude contains phenol; and ethanol extracted biocrude contains 1(3H)-isobenzofuranone, and 2-methoxy-4-methyl-3-nitro-, methyl ester benzoic acid.55
4. Conclusions
This research has demonstrated the promising potential of valorizing food waste to produce biocrude. The reaction parameters were optimized at a temperature of 280 °C, pressure of 1500 psi, and reaction time of 42 minutes for treating wet samples. The R2 value was 0.9. The independent variables in the design expert model were significant as P ≤ 0.05. A higher F value suggested that the variables or model have a stronger statistical significance. The HTL process was proven to be efficient in breaking down complex organic molecules into a liquid fuel product (biocrude). The ultimate analysis of the biocrude showed C (70.0 wt%), H (7.8 wt%), N (1.4 wt%), S (0.2 wt%), and O (20.7 wt%) values. The oxygen content of the biocrude is high; however, it can be eliminated upon upgradation in future work, such as hydrodeoxygenation, catalytic cracking, decarboxylation, esterification, etc. The characterized biocrude exhibits properties that make it a viable candidate for blending with conventional fossil fuels or as a standalone transportation fuel. Its HHV (30.8 MJ kg−1) is found to meet relevant standards and specifications for transportation fuel applications, with viscosity ranging from 515–1215 cP. This high viscosity is because it contains a high heteroatom content, mainly O and H, and this is the reason why it is more viscous at room temperature. Therefore, future work includes the biocrude to be upgraded in terms of hydrotreatment to be blended with conventional fuel for its use as a transportation fuel. Additionally, the biocrude obtained has 55.9–72.8% carbon recovery potential. The biocrude yield varies with the selection of solvents and their polarity. The solvents with higher polarity yield more biocrude. The use of biocrude derived from food waste can contribute significantly to reducing greenhouse gas emissions and dependence on finite fossil fuel resources. This aligns with the global sustainability goals of mitigating climate change and achieving a more circular economy. While the study has demonstrated significant progress, challenges such as scale-up, economic viability, and technology optimization remain to be studied. Future research should focus on addressing these challenges to facilitate the practical implementation of food waste-derived biocrude as a transportation fuel.Data availability
The authors confirm that the data supporting the findings of this study are available within the article. Raw data that support the findings of this study are available from the corresponding author, upon reasonable request.Author contributions
Kshanaprava Dhalsamant: conceptualization; data curation; formal analysis; investigation; methodology; project administration; resources, software, validation; writing – original draft; writing – review & editing. Ajay Kumar Dalai: funding acquisition, resources; supervision, writing – review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors acknowledge the funding from the Canada Research Chair (CRC) Program and Natural Sciences and Engineering Research Council of Canada (NSERC) for this research.References
- FAO, Food and Agriculture Organizations of the United Nations, https://www.fao.org/platform-food-loss-waste/flw-data/en/, accessed 19 October 2023 Search PubMed.
- StatCan, Statistics Canada, https://www150.statcan.gc.ca/n1/en/subjects/agriculture_and_food/food, accessed 19 October 2023 Search PubMed.
- US EPA, Sustainable management of food basics, United States Environ. Prot. Agency, 2015 Search PubMed.
- USDA, Food Waste FAQs, USDA, https://www.usda.gov/foodwaste/faqs Search PubMed.
- K. Dhalsamant, P. Tirumareddy, V. B. Borugadda and A. K. Dalai, Bioresour. Technol. Rep., 2023, 24, 101595 CrossRef CAS.
- M. Govarthanan, S. Manikandan, R. Subbaiya, R. Y. Krishnan, S. Srinivasan, N. Karmegam and W. Kim, Fuel, 2022, 312, 122928 CrossRef CAS.
- F. Pattnaik, S. Nanda, V. Kumar, S. Naik and A. K. Dalai, Fuel, 2022, 311, 122618 CrossRef CAS.
- S. Jain, Sustain. Energy Technol. Assessments, 2023, 58, 103380 CrossRef.
- P. Ranganathan, Sustain. Energy Technol. Assessments, 2023, 57, 103164 CrossRef.
- R. Y. Krishnan, S. Manikandan, R. Subbaiya, W. Kim, N. Karmegam and M. Govarthanan, Sustain. Energy Technol. Assessments, 2022, 52, 102211 CrossRef.
- A. Aierzhati, M. J. Stablein, N. E. Wu, C. T. Kuo, B. Si, X. Kang and Y. Zhang, Bioresour. Technol., 2019, 284, 139–147 CrossRef CAS PubMed.
- S. Changi, M. Zhu and P. E. Savage, ChemSusChem, 2012, 5, 1743–1757 CrossRef CAS PubMed.
- Y. Fan, U. Hornung, N. Dahmen and A. Kruse, Biomass Convers. Biorefin., 2018, 8, 909–923 CrossRef CAS.
- M. Pecchi, M. Baratieri, A. R. Maag and J. L. Goldfarb, Waste Manage., 2023, 168, 281–289 CrossRef CAS PubMed.
- S. Wainaina, M. K. Awasthi, S. Sarsaiya, H. Chen, E. Singh, A. Kumar, B. Ravindran, S. K. Awasthi, T. Liu, Y. Duan, S. Kumar, Z. Zhang and M. J. Taherzadeh, Bioresour. Technol., 2020, 301, 122778 CrossRef CAS PubMed.
- A. Le Pera, M. Sellaro, E. Bencivenni and F. D'Amico, Waste Manage., 2022, 139, 341–351 CrossRef CAS PubMed.
- W. M. L. K. Abeyratne, H. Bayat, H. M. K. Delanka-Pedige, Y. Zhang, C. E. Brewer and N. Nirmalakhandan, J. Environ. Chem. Eng., 2023, 11, 109628 CrossRef CAS.
- D. Yu, J. Guo, J. Meng and T. Sun, Chemosphere, 2023, 328, 138606 CrossRef CAS PubMed.
- Z. X. Xu, J. H. Cheng, Z. X. He, Q. Wang, Y. W. Shao and X. Hu, Bioresour. Technol., 2019, 278, 311–317 CrossRef CAS PubMed.
- O. Fadele, I. N. A. Oguocha, A. G. Odeshi, M. Soleimani and L. G. Tabil, Cellulose, 2019, 26, 9463–9482 CrossRef CAS.
- F. Pattnaik, S. Nanda, V. Kumar, S. Naik and A. K. Dalai, Fuel, 2022, 311, 122618 CrossRef CAS.
- I. H. Cho and K. D. Zoh, Dyes Pigm., 2007, 75, 533–543 CrossRef CAS.
- A. Matayeva, D. Bianchi, S. Chiaberge, F. Cavani and F. Basile, Fuel, 2019, 240, 169–178 CrossRef CAS.
- H. F. Khalekuzzaman, M. Fayshal and M. A. Adnan, J. Cleaner Prod., 2024, 436, 140593 CrossRef.
- A. Mathanker, D. Pudasainee, A. Kumar and R. Gupta, Fuel, 2020, 271, 117534 CrossRef CAS.
- P. W. Simon, A. Nijabat, S. Bibi, M. Ajmal, S. Nawaz, M. Z. Sajid, S. Ullah Khan Leghari, M. Mahmood-ur-Rehman, N. Huma Naveed and A. Ali, Nat. Prod. Res., 2023, 1–10 Search PubMed.
- R. Ghadge, N. Nagwani, N. Saxena, S. Dasgupta and A. Sapre, Energy Convers. Manage.:X, 2022, 14, 100223 CAS.
- T. Shan Ahamed, S. Anto, T. Mathimani, K. Brindhadevi and A. Pugazhendhi, Fuel, 2021, 287, 119329 CrossRef CAS.
- M. Mohan, R. Timung, N. N. Deshavath, T. Banerjee, V. V. Goud and V. V. Dasu, RSC Adv., 2015, 5, 103265–103275 RSC.
- G. J. Swamy, A. Sangamithra and V. Chandrasekar, Dyes Pigm., 2014, 111, 64–74 CrossRef CAS.
- J. A. Okolie, S. Nanda, A. K. Dalai and J. A. Kozinski, Int. J. Hydrogen Energy, 2020, 45, 18275–18288 CrossRef CAS.
- F. Pattnaik, S. Nanda, V. Kumar, S. Naik and A. K. Dalai, Biomass Bioenergy, 2021, 145, 105965 CrossRef CAS.
- K. Manorach, A. Poonsrisawat, N. Viriya-Empikul and N. Laosiripojana, Optimization of Sub-critical Water Pretreatment for Enzymatic Hydrolysis of Sugarcane Bagasse, Elsevier B.V., 2015, vol. 79 Search PubMed.
- S. Masoumi and A. K. Dalai, J. Cleaner Prod., 2020, 263, 121427 CrossRef CAS.
- A. Purnomo, Y. A. W. Yudiantoro, J. N. Putro, A. T. Nugraha, W. Irawaty and S. Ismadji, Int. J. Ind. Chem., 2016, 7, 29–37 CrossRef CAS.
- J. Nallasivam, B. E. Eboibi, A. Isdepsky, M. Lavanya, S. Bhaskar and S. Chinnasamy, Biomass Convers. Biorefin., 2022, 12, 3827–3841 CrossRef CAS.
- B. Motavaf and P. E. Savage, ACS ES&T Eng., 2021, 1, 363–374 Search PubMed.
- B. Zhang, J. Chen, S. Kandasamy and Z. He, Energy, 2020, 193, 116645 CrossRef CAS.
- G. Zhu, X. Zhu, Q. Fan and X. Wan, J. Anal. Appl. Pyrolysis, 2011, 90, 182–186 CrossRef CAS.
- R. Lin, J. Cheng, L. Ding, W. Song, F. Qi, J. Zhou and K. Cen, Bioresour. Technol., 2015, 186, 8–14 CrossRef CAS PubMed.
- F. Bayat, S. Osfouri and R. Azin, Biomass Bioenergy, 2023, 178, 106963 Search PubMed.
- Z. Zhu, L. Rosendahl, S. Sohail and G. Chen, Sci. Total Environ., 2018, 630, 560–569 CrossRef CAS PubMed.
- J. Lu, J. Zhang, Z. Zhu, Y. Zhang, Y. Zhao, R. Li, J. Watson, B. Li and Z. Liu, Energy Convers. Manage., 2017, 134, 340–346 CrossRef CAS.
- S. Elkhalifa, P. Parthasarathy, H. R. Mackey, T. Al-Ansari, O. Elhassan, S. Mansour and G. McKay, Biomass Convers. Biorefin., 2022, 1–18 Search PubMed.
- J. S. Teh, Y. H. Teoh, H. G. How and F. Sher, Processes, 2021, 9(9), 1610 CrossRef CAS.
- S. Elkhalifa, S. Mariyam, H. R. Mackey, T. Al-Ansari, G. McKay and P. Parthasarathy, Energies, 2022, 15(17), 6277 CrossRef CAS.
- Y. S. Tanai, Food Waste Energy Analysis: Characterizing Energy Content as a Function of Proximate Analysis Factors, 2016, p. 2 Search PubMed.
- S. Mahadevan Subramanya and P. E. Savage, ACS Sustain. Chem. Eng., 2021, 9, 13874–13882 CrossRef CAS.
- J. Ni, L. Qian, Y. Wang, B. Zhang, H. Gu, Y. Hu and Q. Wang, Fuel, 2022, 327, 125135 CrossRef CAS.
- M. Mecozzi, M. Pietroletti, M. Scarpiniti, R. Acquistucci and M. E. Conti, Environ. Monit. Assess., 2012, 184, 6025–6036 CrossRef PubMed.
- X. Yang, H. Lyu, K. Chen, X. Zhu, S. Zhang and J. Chen, BioResources, 2014, 9, 5219–5233 Search PubMed.
- C. Z. A. Ahmad, R. Hamidin, N. Ali and U. F. M. Abidin, J. Mech. Eng. Sci., 2016, 7, 1–23 Search PubMed.
- D. Xu and P. E. Savage, Algal Res., 2014, 6, 1–7 CrossRef.
- H. Jahromi, T. Rahman, P. Roy and S. Adhikari, Energy Convers. Manage., 2022, 263, 115719 Search PubMed.
- J. Zhang and Y. Zhang, Energy Fuels, 2014, 28, 5178–5183 CrossRef CAS.
- J. A. Joseph, S. Akkermans and J. F. M. Van Impe, ACS Omega, 2022, 7, 24121–24133 CrossRef CAS PubMed.
- F. Naaz, A. Bhattacharya, K. K. Pant and A. Malik, Process Saf. Environ. Prot., 2021, 145, 141–149 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |