DOI:
10.1039/D4SM01083C
(Paper)
Soft Matter, 2025,
21, 1269-1285
Flow environment affects nutrient transport in soft plant roots
Received
12th September 2024
, Accepted 9th January 2025
First published on 10th January 2025
Abstract
This work estimates Michaelis–Menten kinetics parameters for nutrient transport under varying flow rates in the soft roots of Indian mustard (Brassica juncea) using a plant fluidic device. To find the metallic components within the roots, inductively coupled plasma mass spectrometry (ICP-MS) analysis was performed. The flow rate-dependent metabolic changes were examined using Raman spectral analysis. In addition, three-dimensional numerical simulations were conducted to assess mechanical stresses resulting from the concentration difference that enhances osmotic pressure and flow loading at the root–liquid interface. Convection, the primary mode of nutrient transport in flowing media, was observed to reduce nutrient uptake at higher flow rates. In contrast, diffusion became more prevalent in areas where the complex root structure restricted the flow field. The concentration gradient between the upstream and downstream regions of the root caused nutrient diffusion from downstream to upstream. As seen, an increase in flow rate resulted in a decrease in root length due to the reduction of advantageous metabolites, which led to lower average mechanical stress and osmotic pressure loading. Additionally, osmotic pressure at the root–liquid interface was found to increase over time. Numerical simulations revealed that the average internal mechanical stress was substantially greater when osmotic pressure was considered. This emphasizes the importance of accounting for osmotic pressure when assessing mechanical stress in roots. This study uses a fluidic device that replicates hydroponic conditions for the first time in order to evaluate the convection-dependent Michaelis–Menten kinetics of nutrient uptake in plant roots.
1. Introduction
Numerous, ionic and non-ionic nutrients significantly contribute to plant growth and development.1–5 Plants usually require sixteen key components for their growth that they obtain from the soil and atmosphere.6 For example, potassium ions activate enzymes that enhance metabolic processes, support photosynthesis, and facilitate the transfer of photoassimilates.6 Similarly, other nutrients such as magnesium,7 calcium,8 iron,9 manganese,10 and zinc,11 to name a few, play prominent roles in plant growth and yield. Plant roots absorb these nutrients from the soil. However, soil contamination and a decline in soil nutrient quality owing to the widespread use of chemical fertilizers and pesticides are negatively impacting the quality of plants grown through traditional farming methods.12,13 In this context, hydroponics offers a viable alternative for achieving high crop yields with minimal resources and less human input compared to traditional farming. It is increasingly recognized as a sustainable approach to agriculture.14–16
Hydroponics involves growing plants without soil, typically in water channels filled with a nutrient-rich solution from which the roots absorb dissolved nutrients.17,18 In these channels, flow is facilitated by pumps, and nutrient transport is governed by both advection and diffusion. The transport mechanism of nutrient species in such systems is determined by the Peclet number (Pe),19 which can be estimated as follows: Pe = (UavgH)/Di,20–25 where Uavg, H and Di are average flow velocity in the hydroponic system, characteristic height of the channel and ionic diffusion coefficient of the nutrient species, respectively.26 Considering the typical order of Uavg, H and Di as ∼10−4 m s−1, ∼10−3 m and ∼10−9 m2 s−1,6–11,17,18 the diffusive Peclet number is found to be greater than unity. This indicates that advection is the main transport mechanism in these systems. To the best of our knowledge, convection-dominated nutrient transport has not been extensively described, suggesting that further research is necessary.
A number of numerical studies, usually for root–soil systems, have recently explored the underlying mechanisms of the nutrient uptake on the root surface, taking into account its temporal variations. The study regarding the absorption of alkali cations by barley roots revealed that nutrient absorption involves the interaction of ions with metabolically derived binding groups or sites.27 In the reactions, the resulting ion-binding compound is unstable and decomposes spontaneously to form free compounds. This process resembles enzyme activity, where the ion acts as the enzyme and the binding complex functions as the substrate. However, unlike enzymatic reactions, where the enzyme chemically alters the substrate, the ion-binding interaction facilitates the transport of ions from the medium into the root cells. This mechanism can be accurately represented by Michaelis–Menten kinetics, particularly when no inhibitor is present. Previous studies have effectively demonstrated the uptake mechanism for potassium and other nutrients in the soil system using Michaelis–Menten kinetics.28–32
The nutrient-rich liquid flowing across the channel in hydroponic systems generates significant mechanical stress at the root–liquid interface, which ultimately affects the plant growth. Changes in flow conditions, such as continuous flow, no-flow, and flow stagnation, result in distinct types of root development and nitrogen uptake. For instance, continuous flow conditions have been associated with an increased number of cortical cells.33 Consequently, optimizing the flow rate in hydroponic systems is crucial.34 Recently, it has been revealed that nitrogen uptake in roots increases with flow rate up to a certain point beyond which it becomes less pronounced. The maximum root length is achieved only until the critical flow rate is reached, after which root growth declines.35 The decline is attributed to the mechanical stress caused by excess flow rate beyond the critical limit. In addition to the stress from flow loading, the root surface is also significantly affected by mechanical stress from osmotic pressure induced by concentration differences.36 The osmotic pressure (Po) generated due to the ionic nutrient concentration difference is given by Po = RTΔci,37,38 where, R, T and Δci are the universal gas constant, absolute temperature and concentration difference of the ith ionic species at the root–nutrient liquid interface, respectively. Accordingly, for a typical concentration differential of 10 mol m−3 at the root surface, the resulting osmotic pressure is estimated to be in the order of ∼104 Pa. Such high osmotic pressure can impact plant growth by inducing significant mechanical stress, which affects metabolic reactions.39
A thorough review of the literature demonstrates that nutrient uptake kinetic parameters are well understood, particularly for soil–root systems. However, convection and flow-induced stress are not considered when evaluating the Michaelis–Menten kinetics parameters for such a system. The existence of convective strength of ionic nutrient transport can alter the uptake phenomena, especially when the diffusive Peclet number exceeds unity. Concurrently, in hydroponic systems, the flow rates can reach up to the order of a few to a thousand times of mL h−1,34 resulting in a very high diffusive Peclet number (≫1). Despite this, nutrient uptake phenomena and Michaelis–Menten kinetic parameters for flowing nutrient media have not been extensively characterized. As a result, the current study focuses on assessing the Michaelis–Menten kinetics of nutrients within a fluidic device that simulates hydroponic conditions. Furthermore, the mechanical stress caused by flow loading and osmotic pressure induced by the concentration differences in the nutrient solution has been estimated by the use of three-dimensional numerical modelling.
2. Experimental procedure and setup
2.1. Root nutrient uptake fluidic device (RNUFD)
Consistent with microfluidic technology, we develop phytofluidic devices that would aid in the plant's growth and uptake of nutrients. A schematic representation of the Root nutrient uptake fluidic device (RNUFD) is shown in Fig. 1(a). RNUFD is fabricated with certain modifications to the methods adopted before in our research group,33,35 employing a vinyl-terminated polydimethylsiloxane (PDMS) substrate (SYLGARDTM 184 Silicone Elastomer from Sigma Aldrich®, USA). The device is baked in a hot air oven (IKONTM® Instruments, India) at 45 °C for 10 hours. Then, the device is taken out of the mold. The phytofluidic device measures 50 mm in length, 1 mm in width, and 10 mm in height. To fabricate the phytofluidic device (RNUFD), we here use the wire-drawing method,33,35 while a brass wire with a circular cross-section of diameter 1 mm is used to develop the horizontal cylindrical channel for root growth. Pipette tips are used to create the port to hold the plant vertically to give the root access to the flow channel.33
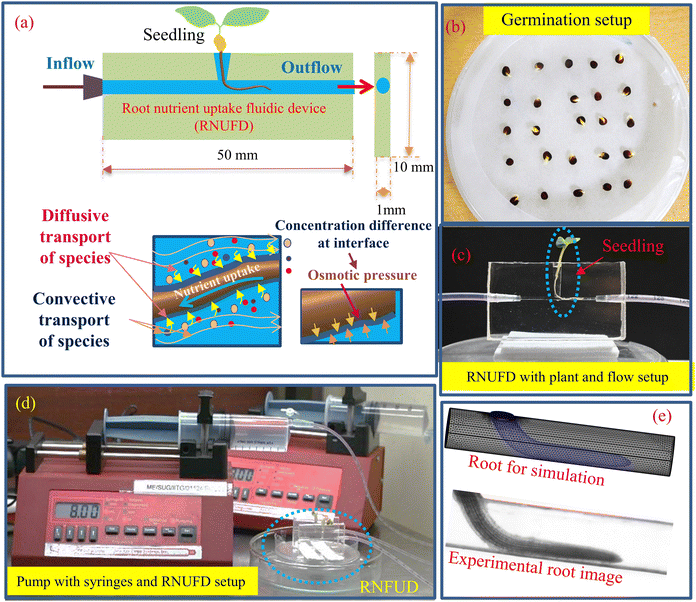 |
| | Fig. 1 (a) Schematic diagram of root nutrient uptake fluidic device (RNUFD) with inflow and outflow of the fluid direction and placement of a plant root in the device. (b) Germination arrangement of Brassica juncea seeds with a 5 × 5 pattern in a Petri plate. (c) Flow setup of RNUFD with plant and feeding tubes augmented in the device. (d) Pump setup with syringes placed in the pump providing flow to the device using feeding pipes, and (e) root geometry made for numerical simulation based on the experimental root image. | |
2.2. Preparation of seeds for germination
In a laminar airflow cabinet, Brassica juncea (Indian mustard) seeds obtained from ICAR-IARI, Regional Station, Karnal, Haryana, India are taken and placed therein in a beaker. B. juncea was chosen for the study as it is the third-largest source of comestible oils globally and accounts 32.4% of the total oilseed crop production in India.5 In addition to contributing to food security, the oil of B. juncea possesses anti-fungal, anti-bacterial, and anti-carcinogenic properties, offering numerous health benefits.5 Therefore, it is imperative for a country like India to increase the productivity and yield of B. juncea. For this reason, we have specifically focused on B. juncea in the current study. Afterwards, seeds are surface sterilized for two minutes using a pipette with a volume of one microliter in a 4% sodium hypochlorite solution (w/v). These seeds are known to exhibit superior characteristics compared to average seeds in terms of germination yield.40 This is because sodium hypochlorite acts as a chemical sterilizing agent, promoting a higher rate of germination while preventing pathogenic contamination during the early seedling stage. The seeds are then thoroughly rinsed twice with distilled water for five minutes. The excess moisture is removed from the seeds by placing them on blotting paper. Following the arrangement of the seeds in a 5 × 5 pattern as illustrated in Fig. 1(b) on moist filter papers (Whitman®, Grade 1) that are placed on the plate base, the plates are then made airtight with laboratory film (Parafilm M®). Subsequently, the seed plates are put in a germination chamber for up to 48 h in a dark cycle that is set to conditions of 26 °C and 75% relative humidity. The chamber is also subjected to a 12 h light cycle photoperiod during the experiment, with a 3000-lux light intensity supplied by a configuration of white fluorescent lamps.
2.3. Flow setup
The seeds are left for two days for germination and geminated seeds are then inserted into the RNUFD's vertical plant port (cf.Fig. 1(a)–(c)). We ensure that the root suitably enters inside the channel to facilitate proper growth with flow. Fig. 1(c) depicts the plant root positioning inside the device and the flow direction along the growing root. Fig. 1(d) shows the setup of mechanical syringes (NIPRO®, India) of 60 mL volume fixed to the mechanical syringe pump (New Era Pump System®, Inc., USA). The syringes are filled with MS media (Murashige & Skoog Medium, PT025, HIMEDIA®) and further connected with feeding pipes with a length of 200 mm and a diameter of 2.7 mm. The pipes are inserted into the fluidic device to enable flow through the phytofluidic channel. The nutrient flow was provided to the device for a continuous period of 12 h with flow rates varying from 2 mL h−1 to 10 mL h−1. We perform an experiment in a sterilized closed growth chamber with environmental conditions maintained at 26 °C with 75% relative humidity under a 12 h light cycle. We consider three replicas for each flow rate condition to ensure uniformity and accuracy of the experimental outcome.
3. Soft root structure–nutrient flow interactions: description of transport equations
3.1. Fluid flow
The flow field of the nutrient solution can be described by the continuity and momentum equations as follows:33,35| | 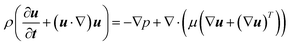 | (2) |
Here, u (≡ uî + vĵ + w![[k with combining circumflex]](https://www.rsc.org/images/entities/b_i_char_006b_0302.gif) ), p, ρ and μ are velocity vector, pressure, liquid density and dynamic viscosity, respectively.
), p, ρ and μ are velocity vector, pressure, liquid density and dynamic viscosity, respectively.
The average velocity (uinlet = Uavgî), calculated using the experimental flow rate, was specified at the inlet of the fluidic device. The gauge pressure (pgauge) was set to zero at the outlet and no-slip (u = 0) conditions were applied to the walls of the fluidic device and the root surface.
3.2. Nutrient species transport
The equation governing the nutrient flow in the phytofluidic channel as well as inside the root can be written as given below:41| | 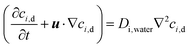 | (3a) |
| | 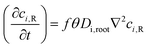 | (3b) |
Here, ci,d, ci,R, Di,water and Di,root are the ionic concentration of the ith nutrient species in flowing MS media solution in the fluidic device (sub-script “d”) and root (sub-script “R”), respectively; ionic diffusion coefficient in flowing nutrient and inside the root, respectively.
For eqn (3), the interfacial boundary condition for nutrient transport inside the root is given by the flux balance in terms of Michaelis–Menten kinetics and expressed as follows:41
| | 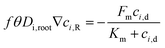 | (4) |
In
eqn (4),
θ is the volumetric water content in the root and estimated as 0.32 by measuring the dry and fresh weight of the root after germination. The term
f is the impedance factor accounting for the resistance of ion transport inside the porous root as compared to the void system. This impedance can be measured by taking the ratio of equivalent diffusion coefficient of species in the root (considered as a porous media) to water. We have used the Millington and Quirk model to estimate the equivalent diffusion coefficient, which is expressed as
Di,root =
Di,waterεp4/3, where
Di,root,
Di,water, and
εp are the diffusion coefficient of species in porous root, in water and porosity of the root, respectively. We calculate the diffusion coefficients by using the Nernst–Einstein equation,
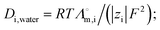 18
18 where
R,
T,
F,
zi, and
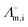
are the universal gas constant (8.314 J K
−1 mol
−1), temperature, Faradays constant (= 9.6485 × 10
4 C mol
−1), valency of nutrient ion and molar conductivity of ionic species in water at
T = 298 K (experimental condition).
42 Hence, the diffusion coefficients of nutrient species are taken as 1.96 × 10
−9, 7.05 × 10
−10, 1.32 × 10
−9, 7.19 × 10
−10, 6.88 × 10
−10, and 7.15 × 10
−10 m
2 s
−1 for K
+, Mg
2+, Ca
2+, Fe
2+, Mn
2+ and Zn
2+, respectively.
18 The porosity of the root is estimated as 0.43 using the cross-sectional SEM image of the root. Note that the calculated value conforms to the reported value as well.
43 Hence, the impedance factor is calculated as 0.325. Moreover,
Fm and
Km are the maximum influx into the root and Michaelis–Menten constant, respectively. We have elaborated on these parameters in the forthcoming section.
Furthermore, the greater flow rates in hydroponic systems result in a convection-dominated transport of ionic nutrients across the root surface. The ratio of convective to diffusive transport rates is quantified by the Peclet number (Pe = (UavgH)/Di,water) for ionic transport. Consequently, the Peclet number exceeds unity, indicating convection-dominant transport of nutrient species at higher flow-rates. In light of this, we determined that the Peclet number varies from 707 to 3538 for the taken range of flow rates with the order of the nutrient diffusion coefficient as 10−9 m2 s−1.18 As a result, we may state that Pe ≫ 1 reflects the highly convection-dominated ionic transport mechanism at the root surface.
Furthermore, the Michaelis–Menten kinetic parameters (Fm, Km) depend on both concentration of the root domain and concentration of the flowing nutrient, as expressed in eqn (4). Thus, from eqn (4), it is clear that the MS media concentration (ci,d) is strongly dependent on the flow field (u) because of the interplay between nutrient convective and diffusive transport mechanisms [see eqn (3a)]. As the flow field (u) enhances with increasing Uavg, accordingly, the change in ci,d significantly impacts the values of the Michaelis–Menten kinetic parameters (Fm, Km) [eqn (4)] at higher Pe (≫1) due to convection dominated ionic species transport.
Using inductively coupled plasma-mass spectrometry (ICP-MS), we determined the initial concentration (ci,d,initial) and the inlet concentration of nutrient species (ci,d,inlet) within the fluidic channel, as measured in MS media. Similarly, before inserting in RNUFD, the initial concentration of nutrient species within the root (ci,R,initial) was obtained from the ICP-MS analysis of the root after germination.
3.3. Structural deformation of the solid plant root
The internal mechanical stress that develops in the plant root during flow loading can be calculated using the following differential equation:33,35| | 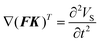 | (5) |
Here, F and K are the displacement gradient and second-Piola–Kirchhoff stress, respectively; and F can be represented in terms of the displacement field (VS) as follows:where I is the identity matrix. Moreover, the expression of K is given below:| | K = 2μL![[small epsilon, Greek, macron]](https://www.rsc.org/images/entities/b_i_char_e0c6.gif) + λLtr( + λLtr(![[small epsilon, Greek, macron]](https://www.rsc.org/images/entities/b_i_char_e0c6.gif) )I )I | (7) |
Here, ![[small epsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c6.gif) is the Lagrange–Green strain and expressed as
is the Lagrange–Green strain and expressed as ![[small epsilon, Greek, macron]](https://www.rsc.org/images/entities/b_i_char_e0c6.gif) = 0.5(F(F)T − I). The first and second Lamé parameters appearing in eqn (7) are as follows:33,35
= 0.5(F(F)T − I). The first and second Lamé parameters appearing in eqn (7) are as follows:33,35| | | λL = νE/(1 + ν)(2ν − 1), μL = E/2(1 + ν) | (8) |
where, E (= 4.16 N mm−2) and ν (= 0.49)33,35 are modulus of elasticity and Poisson's ratio of the plant root, respectively. We can represent the Cauchy stress tensor inside the root by the following relation:33,35| | 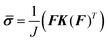 | (9) |
In eqn (9), J stands for the Jacobian of F.
The fluid flow and structural deformation of soft-solid plant roots are considered two-way coupled in this study, implying that they mutually influence each other. We applied interfacial boundary conditions at the interface between the soft-solid root and the nutrient medium, enabling the simultaneous solution of the flow field and structural deformation. This interfacial condition is expressed as follows:33,35
| | ![[small sigma, Greek, macron]](https://www.rsc.org/images/entities/b_i_char_e0d2.gif) ·nR,Γ = (−pI + μ(∇u + (∇u)T))·nd,Γ ·nR,Γ = (−pI + μ(∇u + (∇u)T))·nd,Γ | (10a) |
Furthermore, the following expression is used to translate the root deformation to the fluid velocity at the root wall:
| | 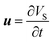 | (10b) |
where
n is the unit vector normal to the root–liquid interface,
Γ; R and d represent the soft-solid root and fluid (nutrient solution constituted by MS media) domains, respectively. We applied a fixed constrain at the extended part of the root for solving the structural equation.
Moreover, the mechanical loading due to the osmotic pressure generated at the root–liquid interface as a result of the nutrient concentration difference can be estimated by imposing another interfacial boundary condition as written below:44–47
| | 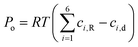 | (11) |
4. Nutrient concentration analysis in roots after a 12-hour uptake period in the RNUFD using inductively coupled plasma-mass spectrometry (ICP-MS), and metabolite analysis using a laser-based Raman experiment
Inductively coupled plasma mass spectrometry (ICP-MS) is used to measure the amount of metallic ionic nutrient species, such as magnesium, zinc, iron, etc., present in biological root materials.48 It comprises a sample introduction system, plasma converter, ion lenses, interface and detector. The sample is first formed into a fine aerosol mist by a nebulizer present in the sample introduction system. A plasma converter then converts the elements present in the sample aerosol into ions. Subsequently, ion lenses are used to focus the ions and separate them. A high-temperature ionized gas then atomizes the sample. Finally, the ions are separated according to their mass–charge ratio and their concentrations are measured at the detector.49
After the plants are removed from the RNUFD, they were kept for drying to remove moisture in a hot air oven (IKONTM® Instruments, India) for 24 h. The dried roots are then crushed finely in a mortar. 100 mg (nearly 9 to 10 plant root sample for the same set) of each root sample is taken for ICP-MS analysis. Subsequently, the samples are taken for digestion, where they are mixed with 9 mL and 1 mL acidic mixture of HNO3 and H2O2, respectively. After digestion, the samples are then diluted to 50 mL by mixing with distilled water. From the diluted solution, 10 mL are taken from the samples for syringe filtration. Finally, the filtered specimens for each sample are taken for three-times ICP-MS analysis to detect the metal ion nutrient concentration in the root.
A laser-based Raman analysis system was used to examine the metabolic changes in roots under varying MS media concentrations, and under no-flow and flow-rate conditions. Following twelve hours of experimentation, intact root samples were directly analyzed using the laser Raman apparatus, employing a 50× magnification lens to detect the corresponding Raman peaks at different flow rates for the 4.4 g L−1 MS media case. Moreover, crushed root samples were analyzed for variable MS media content under no-flow and 2 mL h−1 flow conditions to enhance the detection of organic compounds within the roots using Raman analysis. The laser micro-Raman system (HORIBA Jobin Yvon Model LabRAM HR) was employed for metabolite analysis.
5. Numerical methodology and grid independency test
As illustrated in Fig. 1(e), we consider a three-dimensional computational domain using the CAD module from the actual root geometry obtained from experiments through image analysis using ImageJ® software. The domain has been discretized into smaller subdomains using tetrahedral elements. In the numerical framework employed in this endeavor, the Yeoh mesh smoothing method in COMSOL Multiphysics was implemented to account for the deformation domain, considering the influence of fluid–solid (root) interaction. The shape functions used for pressure, velocity fields, solid deformation, and concentrations are linear, which allows the differential equation to be transformed into a system of linear algebraic equations. The local matrix for the transport variables is produced for every element. We used a segregated solver to estimate all the variables (u, p, VS, x, y, z, ci,d, ci,R). Further details of the numerical simulation and the coupling of variables are provided in Appendix A. In addition, a grid independence test has been performed to determine the concentration of a species inside the root after uptake by changing the mesh type, as indicated in Table 1. We consider mesh system M4 for all numerical simulations performed in this work, since there is less than one percent error in the calculation of average species concentration between the highly refined mesh system M5 and M4.
Table 1 Grid independency test by estimating the average concentration of K+ in the root when Q = 2 mL h−1
| Mesh type |
Number of elements |
Average concentration of species inside the root (mol m−3) |
% Error in concentration with respect to M5 |
| M1 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 146 146 |
61.081 |
−4.12 |
| M2 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 074 074 |
59.687 |
−1.752 |
| M3 |
63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 878 878 |
59.142 |
−0.823 |
| M4 |
168![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 323 323 |
58.712 |
−0.09 |
| M5 |
319![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 841 841 |
58.659 |
0 |
6. Results and discussion
In this work, the primary aim is to estimate the Michaelis–Menten kinetics constants pertaining to the soft plant root–nutrient flow interactions environment in the convection-dominated regime. By performing the experiment in the RNUFD and conducting three-dimensional numerical simulations, we systematically calculated the flow rate dependent kinetic constants (Fm, Km) for six important nutrient species, namely potassium, magnesium, calcium, iron, manganese and zinc. Furthermore, the concentration field of ionic species has been predicted from the three-dimensional numerical simulations. Based on the experimental findings and morphological study of the roots, we have estimated the mechanical stress inside the plant root by considering the osmotic pressure generated at the root–liquid interface. Moreover, the flow rate is varied from 2 mL h−1 to 10 mL h−1 in the setup. It may be mentioned here that, as evident from the existing literature,34 the chosen window of flow rate has not been considered until this endeavour.
6.1. Calculation of Michaelis–Menten kinetics parameters
We have estimated the metallic nutrient uptake concentration in the plant root by performing ICP-MS analysis after 12 hours of insertion in the RNUFD under no flow conditions. The surface area of the root is determined by imaging analysis of its morphological structure. Hence, the flux of ionic species can be calculated for different sets of MS-media concentrations (0 g L−1, 2.2 g L−1, 4.4 g L−1, 8.8 g L−1). Michaelis–Menten kinetics constants (Fm, Km) are evaluated using curve fitting in MATLAB®, as shown in Fig. 2. Also, to estimate the Michaelis–Menten constants in fluidic conditions, we have measured nutrient concentrations for certain flow rates with the MS-media concentration set to 4.4 g L−1. As it is known that the Lineweaver–Burk plot (1/flux vs. 1/concentration) is used to determine kinetics constants which follow a straight line, we have shifted the Lineweaver–Burk plot for the no flow condition to the point obtained for a given flow rate. Therefore, we can estimate the constants from the vertical and horizontal intercepts of the plot (1/Fm, Km/Fm) at the different flow rates. This procedure is shown in Fig. 3 for Q = 2 mL h−1, while the same procedure is followed for all other flow rates as well. By estimating Km and Fm for all the flow rates and calculating six nutrient components by curve fitting, we have estimated the flow rate dependent functions for Km and Fm of all the nutrients (see Fig. 4 and 5) and they are expressed in eqn (12)–(23). Due to the decrease in nutrient uptake, a negative trend for the maximum nutrient intake flux has been identified with increasing the flow rate. This can be explained by the shorter residence time for nutrient species at higher flow rate at the interface, attributed primarily to the convection dominated species transport in flowing media.| | | Fm,potassium = 2.7925 × 102 × Q2 − 2.1264 × 10−3 × Q + 2.5641 × 10−7 | (12) |
| | | Fm,magnesium = −7.7625 × 10−10 × Q2 − 6.9475 × 10−9 × Q + 2.01 × 10−7 | (13) |
| | | Fm,calcium = 2.0387 × 10−10 × Q2 − 3.4077 × 10−9 × Q + 1.76 × 10−8 | (14) |
| | | Fm,iron = 3.8937 × 10−11 × Q2 + 1.2287 × 10−10 × Q + 3.59 × 10−9 | (15) |
| | | Fm,manganese = −7.475 × 10−12 × Q2 − 3.005 × 10−11 × Q + 1.43 × 10−9 | (16) |
| | | Fm,zinc = −3.5 × 10−12 × Q2 − 2.5 × 10−11 × Q + 7.81 × 10−10 | (17) |
| | | Km,potassium = 0.0215 × Q2 − 1.6545 × Q + 19.9744 | (18) |
| | | Km,magnesium = −0.0062 × Q2 − 0.0553 × Q + 1.6104 | (19) |
| | | Km,calcium = 0.0046 × Q2 − 0.0776 × Q + 0.4 | (20) |
| | | Km,iron = −5.4357 × 10−4 × Q2 + 0.0018 × Q + 0.49 | (21) |
| | | Km,manganese = −5.4351 × 10−4 × Q2 + 0.0019 × Q + 0.1 | (22) |
| | | Km,zinc = −3.3948 × 10−5 × Q2 − 4.6233 × 10−5 × Q + 0.008 | (23) |
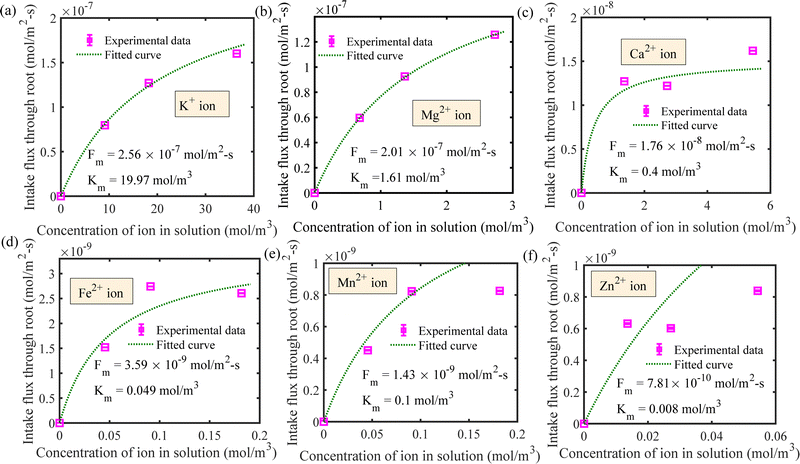 |
| | Fig. 2 Determination of Michaelis–Menten kinetics constants for the following ionic species for the no flow condition for (a) potassium, (b) magnesium, (c) calcium, (d) iron, (e) manganese and (f) zinc ions. | |
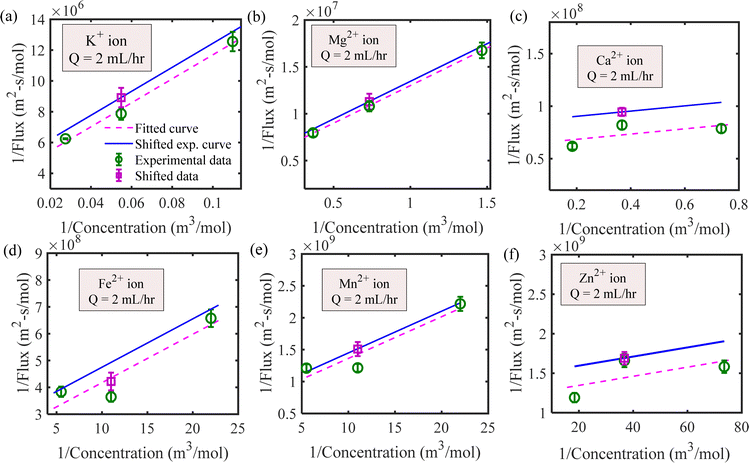 |
| | Fig. 3 Determination of the Michaelis–Menten constants for Q = 2 mL h−1 by shifting the no flow Lineweaver–Burk plot for (a) potassium, (b) magnesium, (c) calcium, (d) iron, (e) manganese and (f) zinc ions. | |
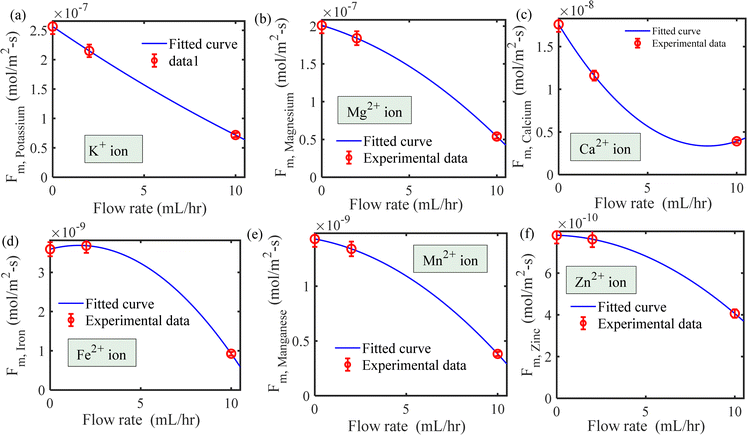 |
| | Fig. 4 Maximum influx into the root as a function of flow rate for (a) potassium, (b) magnesium, (c) calcium, (d) iron, (e) manganese and (f) zinc ions. | |
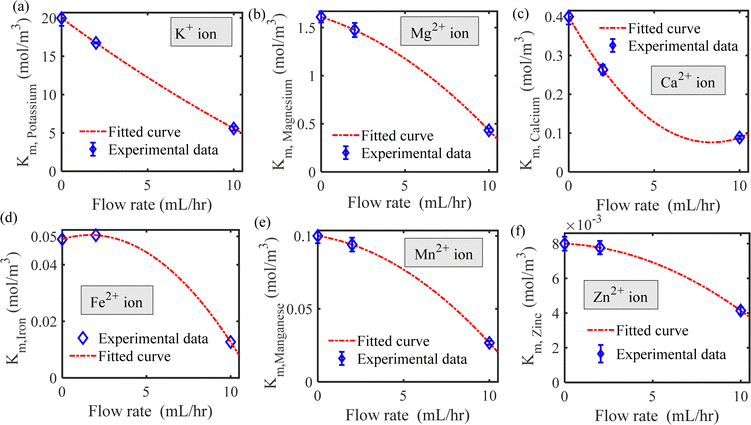 |
| | Fig. 5 Michaelis–Menten constant as a function of flow rate for (a) potassium, (b) magnesium, (c) calcium, (d) iron, (e) manganese and (f) zinc ions. | |
6.2. Numerical prediction
From the experimental results, it is found that the maximum concentration accumulated was for K+ ions followed by Mg2+, Ca2+, Fe2+, Mn2+, and Zn2+, as shown in Fig. 6(a). The K+ ion is essential for plant growth and has importance in photosynthesis and plant metabolism.6 Therefore, the root absorbs this specific ionic species in a large fraction.
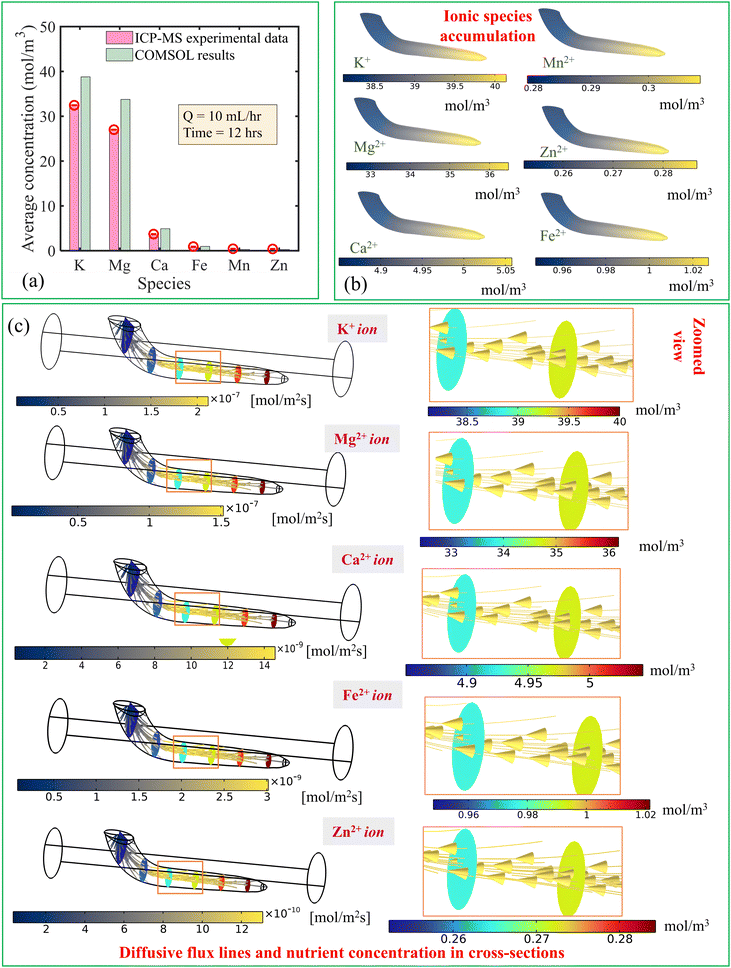 |
| | Fig. 6 (a) Comparison of the averaged concentration at the end of 12 hours obtained from numerical simulation with the results obtained by ICP-MS when the flow rate is 10 mL h−1. (b) Contours of species accumulated inside the root at the end of 12 hours when the flow rate is 10 mL h−1. (c) Diffusion mechanism (diffusive flux of species) of nutrient species along with cross-sectional concentration variation inside the root for Q = 10 mL h−1. The zoomed-in view of diffusive flux and cross-sectional view of the concentration is also depicted in the right side. | |
By performing the three-dimensional transient numerical simulations using a coupled flow field, concentration field and solid deformation field environments, we have estimated the average concentration of the species in Fig. 6(a) for Q = 10 mL h−1 after 12 hours. We find nearly identical physical patterns from numerical analysis comparable with the ICP-MS data. Furthermore, the geometry of the root chosen for the numerical simulation was based on the best results from experiments, considering repeatability. However, due to the biological variability, the standard deviation in root length across sample sets remains evident [see upcoming paragraph]. Additionally, we collected approximately 10–12 root samples for each set to perform the ICP-MS experiment, aiming to estimate the root nutrient concentration. Therefore, using numerical simulations, we observed a slight overestimation in nutrient content due to the experimental standard variation in root geometry. Nonetheless, the numerical tool proved invaluable in understanding the internal physical phenomena of nutrient transport by illustrating the diffusive fluxes and flow-induced stress distribution.
The local concentration field of six nutrient ionic species is shown in Fig. 6(b) for Q = 10 mL h−1 at the end of the 12-hour uptake period. It can be observed that nutrient concentration is higher at the downstream part of the root. We attribute this observation to the penetration of species in the direction of flow toward the root surface aligning with the streamlines. Therefore, the accumulation of ionic species is predicted to be due to the axial movement of the species triggered by the convection-dominated transport of nutrient media in the regime of Pe ≫ 1.
The internal concentration difference between the upstream and downstream parts of the root allows an interior diffusive flux to establish from the root-tip toward the shoot side [see zoomed-in view of the root in the right side of Fig. 6(c)]. We show the interior diffusive flux patterns, as shown in Fig. 6(c). It is observed that the intensity of diffusive flux is higher inside the root, leading to a higher internal concentration gradient along the length [see the cross-sectional distribution of concentration in root in Fig. 6(c)]. The higher concentration gradient of the K+ ion offers its higher flux whereas the lower concentration gradient of Zn2+ is responsible for lower diffusive flux.
Our numerical model is also able to predict the minimal concentration change inside the flowing nutrient media. The contours of convective flux lines, convective flux intensity and diffusive flux in the domain of flowing nutrient solution are depicted in Fig. 7. It is observed that the convective flux lines follow the streamlines for the considered range of flow rate and the order of the diffusive coefficient becomes 10−9 m2 s−1. This observation is attributed to the convection-dominated flow (Pe ≫ 1). As seen from Fig. 7, in the following two locations, i.e., where the root enters into the channel and the zone occupied by it in the proximity to the channel walls, the flow field is relatively weaker due to the flow resistance offered by the obstructions. Therefore, a relatively lower convective strength of ionic species transport is witnessed in these locations. Hence, the larger residence time at these locations results in more nutrient uptake by the root and a relatively larger depletion in nutrient concentration in the medium. As seen from Fig. 7, we find that diffusive flux at those locations becomes higher. Additionally, the complex structure of the root enables dominancy of both convection and diffusion modes of species transport in the nutrient medium, attributed to the geometrical configurations of the root modulated complex flow patterns. Also, due to the high diffusive Peclet number, the intensity of diffusive flux is found to be very low compared to the convective flux.
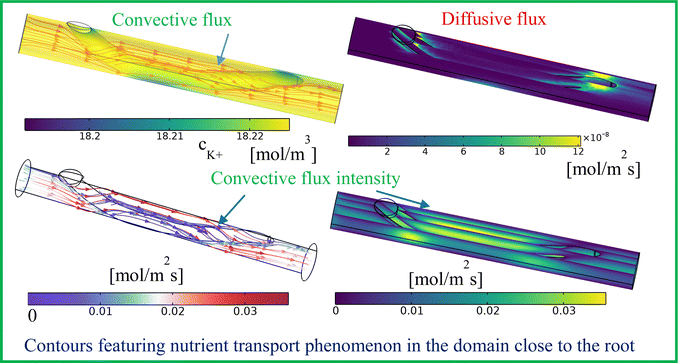 |
| | Fig. 7 Convective flux lines and intensity along with concentration with diffusive flux in the channel flowing with nutrient-rich medium depicting uptake of K+ ions into the root for Q = 2 mL h−1. | |
In Fig. 8(a), we depict the root morphology obtained from the experiments. We have measured the mean root length for three replicas for a given flow rate, as illustrated in Fig. 8(b). We observed that, in contrast to the no-flow condition, root length is greater at lower flow rates. According to the Raman analysis discussed in the following paragraph, this is attributed to minimal activation of metabolites necessary for plant development under no-flow conditions, due to the absence of a convection effect. It is observed that the root length decreases with an increase in flow rate. This observation can be attributed to the increased axially projected flow inertia relative to the root on its frontal surface in the vertical plane with increase in flow rate. Therefore, the roots undergo more physiological changes to withstand this incipient flow resistance rather than facilitating their development.50 As seen from Fig. 9, the intensity of desirable metabolites, as determined by Raman intensity (described in the upcoming section), decreases with an increase in flow rate from 2 mL h−1 to 10 mL h−1 owing to the flow rate-induced mechanical stress developing within the root.
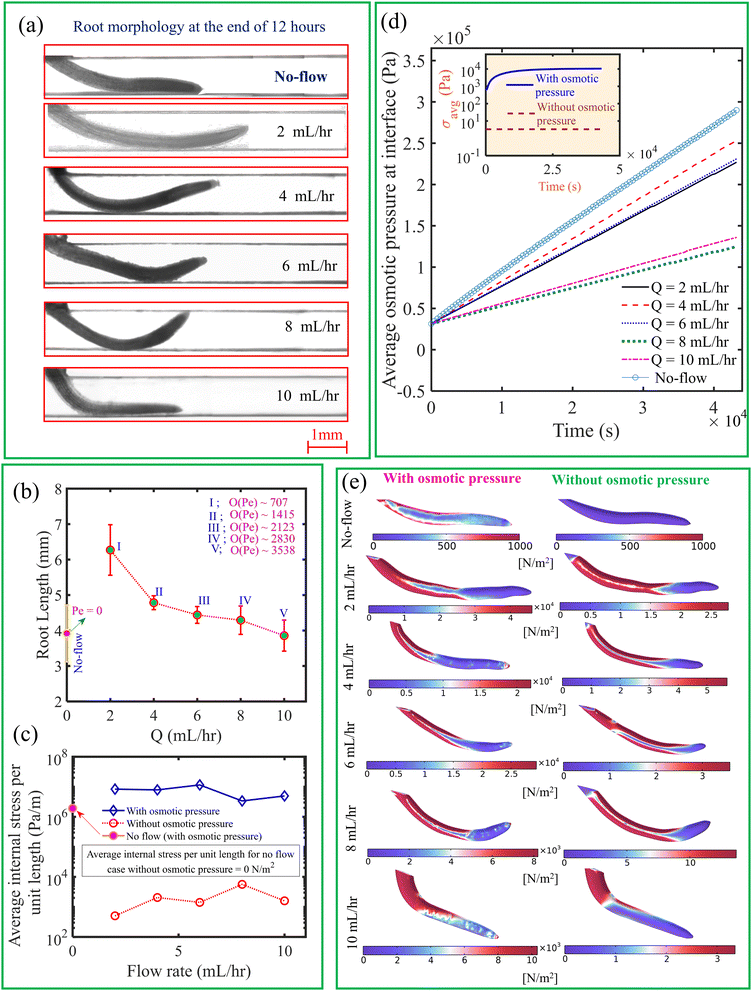 |
| | Fig. 8 (a) Variation in root morphology and length under different flow rates, including the no-flow case. (b) Root length as a function of flow rate, with the order of the diffusive Peclet number also indicated. (c) Variation in average internal stress as a function of flow rate, with and without considering osmotic pressure when Q = 10 mL h−1. (d) Temporal variation in average osmotic pressure at the interface under different flow rates. The inset subfigure represents the temporal change in average internal mechanical stress with and without osmotic pressure for Q = 10 mL h−1. (e) Comparison of average internal mechanical stress for different flow rates, with and without osmotic pressure. | |
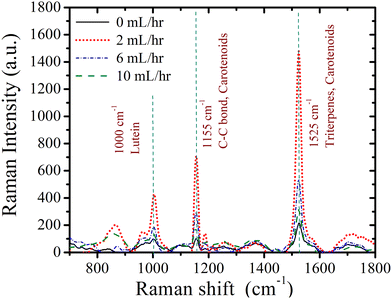 |
| | Fig. 9 Raman intensity of metabolic components in intact root samples at different flow rates under full-strength media. | |
Furthermore, we show in Fig. 8(c), the average internal mechanical stress developed in the root with osmotic pressure accounting for the concentration difference at the root–liquid interface. Note that the mechanical stress is calculated from numerical simulations. The average internal mechanical stress is obtained in the order of ∼104 Pa, which is very high as compared to the value obtained with zero osmotic pressure. An increase in concentration difference at the interface enhances average osmotic pressure over time as represented in Fig. 8(d) for different flow rates. As demonstrated in Fig. 8(e), the generated internal mechanical stress field has been identified to be substantially reliant on the alteration of the root geometry achieved through changes in flow rate. Additionally, when considering osmotic pressure in comparison to zero osmotic pressure, the local change in concentration difference permits the development of a unique form of internal mechanical stress field. Accordingly, the development of osmotic pressure also gives rise to mechanical stress over time as shown in the inset of Fig. 8(d). Therefore, we cannot underestimate the effects of osmotic pressure while estimating the stresses and deformation of the root subjected to nutrient flow. We also observe that the average internal mechanical stress nearly decreases with an increase in flow rate when osmotic pressure is considered. This is due to the fact that the reduction in root length [see Fig. 8(b)] simultaneously decreases the surface area responsible for nutrient uptake as well. Therefore, a reduction in root-surface area, which occurs mainly due to shear stress, normal pressure along with osmotic pressure, and the average internal mechanical stress developed at higher flow rates. Furthermore, we found from Fig. 8(c) that, when the impact of the osmotic pressure is considered, the average internal stress within the root is lower in the no-flow situation. This is attributed to the absence of flow-induced stress, as no flow-related load is exerted on the root. Osmotic pressure is higher at the root surface in the no-flow situation due to the increased residence time of nutrient species at the root surface, as illustrated in Fig. 8(d). As depicted in the upper left corner of Fig. 8(e), the existence of osmotic pressure at the root surface generates internal stress even in the absence of flow. Furthermore, in the no-flow condition, the internal stress is zero when osmotic pressure is not considered. Therefore, while predicting internal stress during nutrient transport, the influence of osmotic pressure is taken into account.
We performed a Raman intensity study on intact roots after 12 hours to observe metabolic changes under mechanical stress induced by flow. The impact of flow rate on metabolites is illustrated in Fig. 9, showing Raman intensity changes for lutein, C–C bonds, carotenoids, and triterpenes. We first examined lutein's Raman peak (1000 cm−1), a key component that controls the plant's structural stability.51–53 The intensity of lutein decreases as the flow rate increases and is lowest under no-flow conditions. This suggests that the absence of mechanical stress in the no-flow scenario hinders the activation of metabolites responsible for structural stabilization, resulting in the lowest lutein intensity. Conversely, the reduction in lutein intensity with increasing flow rate may reflect the plant's adaptation to higher mechanical stress [see Fig. 8(e)] by strengthening structural stability. Next, we analyzed the Raman intensity of carotenoids (1155 cm−1, associated with the vibrational mode of the C–C bond), which are critical for light absorption during photosynthesis and free radical defense.51–53 Carotenoid intensity is also lowest under no-flow conditions, indicating insufficient activation of these processes without flow. As flow rate increases, carotenoid intensity decreases further, potentially due to heightened mechanical stress diminishing the metabolite's activity. We then investigated the Raman intensity of triterpenes (1525 cm−1), which indicate growth, resistance to water loss, and defense against diseases and physical damage.51–53 In the no-flow condition, the absence of convection limits plant growth, leading to minimal triterpene intensity. This aligns with the observation that root length is significantly shorter in no-flow conditions compared to low flow rates [see Fig. 8(a)]. The reduction in triterpene intensity with increasing flow rates follows a similar trend as lutein and carotenoids. Finally, the Raman peak at 1525 cm−1 also reflects carotenoid intensity.51–53 This reinforces the trends observed, where increased mechanical stress diminishes the activity of key metabolites required for structural stability, growth, and defense.
Myo-inositol (an organic compound) has the highest vitamin fraction (100 mg L−1 in 4.4 g L−1 MS media solution), according to the product information of Murashige and Skoog Medium (MS),54,55 product code: PT025. This vitamin plays a key role in regulating plant growth, phosphorus storage, cell communication, stress response, and cell wall biosynthesis. Since myo-inositol undergoes several reactions within the plant, as detailed by Loewus and Pushpalatha,56 it is challenging to identify it in its original form after being absorbed by the plant root. Dhaliwal et al.57 reviewed the transformation of organic materials in this context. In our study, with a flow rate of 2 mL h−1 and a 4.4 g L−1 MS media solution, we measured the Raman intensity of the organic substance, aliphatic compounds (1382 cm−1), while varying the MS media content under no flow conditions, as shown in Fig. 10(a). Because of its hydrophobic nature, aliphatic compounds provide a water-tight environment that helps plants toadapt to drought conditions.57–59 Aliphatic polyester polymers typically have long-chain lengths between C16 and C32 and are composed of ω-hydroxyacids, α,ω-dicarboxylic acids (diacids; DAs), carboxylic acids, and primary alcohols.57–59 The increase in aliphatic content is linked to a decrease in hydraulic conductivity for water–solute movement.57–59
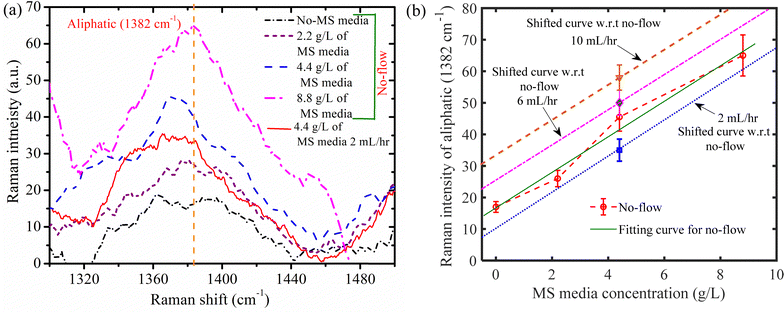 |
| | Fig. 10 (a) Raman intensity curve (generated after baseline correction and smoothing) for aliphatic (1382 cm−1) at different concentrations of MS media solution in a crushed root nutrient uptake fluidic device (RNUFD) under no-flow and 2 mL h−1 conditions. (b) Variation of Raman intensity corresponding to aliphatic (1382 cm−1) for different MS media concentrations. The fitting line for the no-flow case is generated by conducting curve fitting in MATLAB. Based on the fitted line for the no-flow case, shifted lines with the same slope are generated using the experimental data points obtained for different flow rates under a 4.4 g L−1 MS media solution. | |
The Raman intensity corresponding to the aliphatic content for the no-flow scenario is found to increase with higher MS medium concentrations, as illustrated in Fig. 10(a). This suggests that at higher MS medium concentrations, the root's ability to absorb water declines. This may be due to a higher concentration of ionic solution at the root surface, which reduces the concentration gradient and thus hinders water absorption. Furthermore, we found that when the MS medium concentration is 4.4 g L−1, the aliphatic Raman intensity is lower for the 2 mL h−1 compared to the no-flow case (Fig. 10(a)). This difference is likely due to the increased enzymatic activity induced by convection, which enhances the plant's ability to absorb water33,35 resulting in a decrease in the aliphatic Raman intensity.
Additionally, we present the corresponding Raman intensity in Fig. 10(b), where we vary the MS media concentration for the no-flow condition to demonstrate the flow rate dependency of the aliphatic content with changes in MS media concentration. Curve fitting in MATLAB was performed to provide the fitting line for the no-flow situation. The experimental data points for various flow rates under the 4.4 g L−1 MS medium solution were then used to generate shifted lines with the same slope, based on the fitted line for the no-flow condition. We observed that the Raman intensity of the aliphatic content increases as the flow rate is raised from 2 mL h−1 to 10 mL h−1. This can be attributed to the mechanical stress induced by higher flow rates and the shorter residence times of species, which lowers both water and nutrient uptake. Therefore, in Fig. 10(b), the “drought stress”-like situation is attained by the increase in Raman intensity as the flow rate is increased from 2 mL h−1 to 10 mL h−1.
7. Conclusion
In order to examine the nutrient uptake mechanism and root growth behavior of B. juncea roots under varied flow rates ranging from 2 mL h−1 to 10 mL h−1, we employed a root nutrient uptake fluidic device (RNUFD) fabricated from PDMS. We additionally took into account the no-flow case with different concentrations of MS media. The Michaelis–Menten kinetics parameters for nutrient uptake under each flowing condition were determined using the Lineweaver–Burk plot, based on measurements of root length and ICP-MS analysis. The metabolic regulation under flow rate-induced mechanical stress was examined using Raman analysis for both intact and crushed roots. Additionally, CAD modeling of the experimentally derived root geometry was employed alongside three-dimensional numerical modeling using a finite-element method-based numerical solver. We estimated the flow-induced internal mechanical stress within the root using the fluid–structure interface (FSI) framework in the numerical model. Furthermore, the numerical modeling incorporated osmotic pressure effects caused by differences in nutrient concentration at the root surface when calculating internal mechanical stress. Consequently, the numerical simulation coupled the flow field, root structural deformation, root nutrient concentration, and nutrient concentration within the fluidic device. These coupled equations were solved using a segregated solver approach.
We found that nutrient uptake and maximal nutrient flux values decreased with increasing flow rates. The regions of the root surface exposed to the nutrient domain, where convective nutrient transfer dominated, experienced the highest degree of nutrient transfer. Conversely, in localized areas with more complex root systems, flow resistance caused by obstructions led to a predominance of diffusion-driven nutrient transfer. Nutrient species were observed to diffuse from the downstream to the upstream portion of the root, as axial penetration by convection-dominated transport caused nutrient accumulation in the downstream region. Increasing the flow rate also reduced the root length. The results of the Raman spectral analysis showed that convection-driven flow has significant impacts on the metabolites, such as triterpenes and carotenoids. Also, the Raman analysis revealed that the beneficial metabolites were enhanced at lower flow rates, resulting in a longer root compared to the no-flow case. However, at higher flow rates, the increased Raman intensity of aliphatic content reduced root water absorption, resulting in a “drought stress”-like condition at extremely high flow rates. Furthermore, as the surface area subjected to flow loading and osmotic pressure decreased, average internal mechanical stress mildly decreased with the flow rate increment. The highest average internal mechanical stress [∼O (104 Pa)] was predicted when osmotic pressure was considered in the mathematical modelling. This highlights that neglecting interfacial osmotic pressure significantly underestimates internal mechanical stress under flowing conditions. The insights gained from this research offer valuable understanding of flow-dependent nutrient uptake, which is relevant to hydroponic systems and may benefit high-yield hydroponic crop cultivation.
Author contributions
Sumit Kumar Mehta: conceptualization, methodology, software, investigation, writing – original draft, visualization, experiments. Anirudha Talukdar: conceptualization, methodology, software, experiments, formal analysis, writing – original draft. Suraj Panja: conceptualization, methodology, formal analysis, visualization, writing – original draft, experiments. Jinmay Kalita: formal analysis, visualization, writing – original draft, experiments. Pranab Kumar Mondal: conceptualization, formal analysis, visualization, supervision, project administration, funding acquisition, writing – review & editing, resources. Somchai Wongwises: conceptualization, formal analysis, visualization, supervision, project administration, funding acquisition, writing – review & editing, resources.
Data availability
The authors declare that all data used in the work are available in this paper.
Conflicts of interest
The authors declare no competing financial interest.
Appendices
A. Flowchart for estimation of coupled variables in transient numerical simulation
All the simulations pertaining to this work were performed considering the deforming mesh and the two-way coupled fluid structure interactions. Eqn (10a) and (10b) were used to describe the fluid–soft-solid root interaction. The two-way coupling of fluid–soft-solid root interaction works because of the following interface conditions and mesh coordinate deformation via Yeoh mesh smoothing. The structural deformation of the soft-solid roots occurs due to flow loading provided by the flow generated pressure and viscous forces [i.e.![[small sigma, Greek, macron]](https://www.rsc.org/images/entities/b_i_char_e0d2.gif) ·nR,Γ = (−pI + μ(∇u + (∇u)T))·nd,Γ] of nutrient media. Whereas, the root deformation rate
·nR,Γ = (−pI + μ(∇u + (∇u)T))·nd,Γ] of nutrient media. Whereas, the root deformation rate 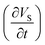 at the root wall is used to update the nutrient flow velocity
at the root wall is used to update the nutrient flow velocity 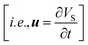 . This step combines with the update of the mesh coordinates (x, y, z) by solid root deformation for a specific iteration loop at any time instant (t), as illustrated in Fig. 11.
. This step combines with the update of the mesh coordinates (x, y, z) by solid root deformation for a specific iteration loop at any time instant (t), as illustrated in Fig. 11.
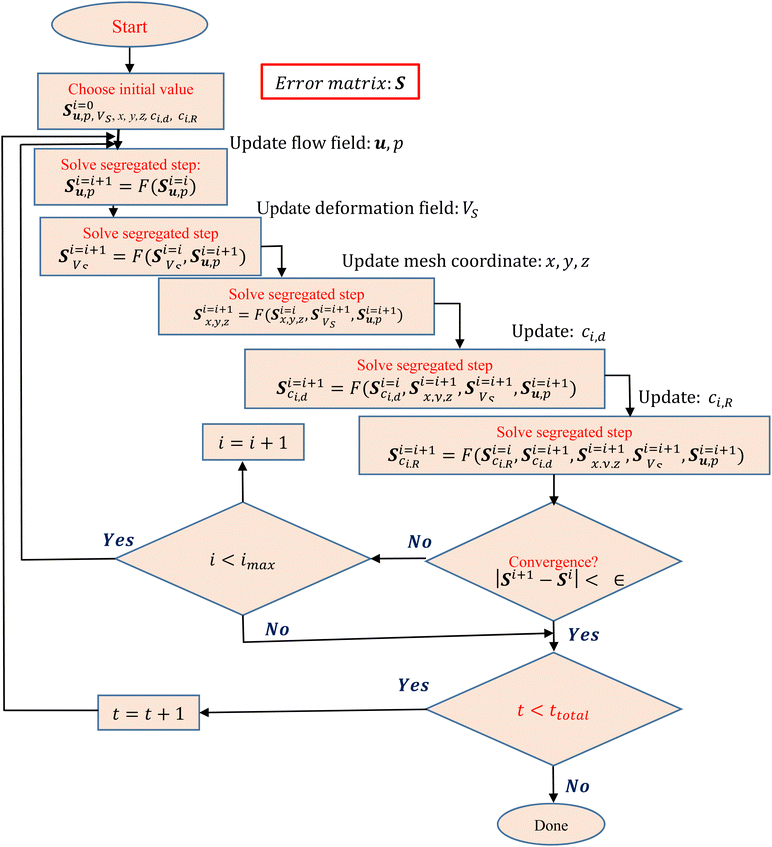 |
| | Fig. 11 Flowchart for the solution of all variables (u, p, VS, x, y, z, ci,d, ci,R) in the transient numerical simulation. | |
The segregated type of solver is employed in this study to couple the fluid soft-solid root structure interactions. First, as shown in Fig. 11, we set an initial value for each variable. The segregated kind of solver is then used to solve the flow (u, p), deformation (Vs), and updated mesh coordinate (x, y, z) because of the root structural deformation under flow loading for Yeoh mesh smoothing, nutrient concentration in the fluidic channel (ci,d) and root nutrient concentration (ci,R) fields, as shown in the flowchart in Fig. 11. The implementation of the segregated type solver minimized the required computational storage for the solution of all coupled variables step-by-step at any time instant (t) of the chosen three-dimensional, highly non-linear type formulation.
Solving the error matrix, S, as shown in Fig. 11, makes it evident that all variables are interconnected in the loop denoted by index “i”. The iteration of the following time step will begin after the convergence criteria is determined to be ∈ after performing the required number of iterations for a given time instant loop. The solution for every variable was obtained after the result for every time instant has converged.
B. Potassium uptake and flow-induced internal mechanical stress
In Fig. 9, the lower intensity of the Raman peak of lutein (1000 cm−1, controls the plant's structural stability51–53), carotenoids (1155 cm−1, which are critical for light absorption during photosynthesis and free radical defense.51–53) and triterpenes (1525 cm−1, which indicate growth, resistance to water loss, and defence against diseases and physical damage51–53) at no flow condition results in a weaker metabolic activity in the developing root. These observations, following the results reported by Wang et al.60 and Zu et al.,61 underscore that the lack of media flow (when roots grow in a quiescent nutrient media) permits accumulation of larger K+ concentration in the root, as a potential response to overcome the abiotic stress-like state. Consequently, pertaining to the no-flow case of this analysis, the K+ concentration becomes higher as witnessed in Fig. 12 (see left column of Fig. 12).
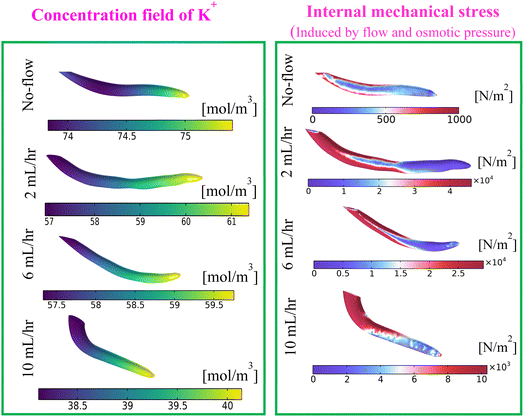 |
| | Fig. 12 Contours of K+ concentration (left) and internal mechanical stress (right) at different flow rates at the end of 12 h for full strength media concentration (4.4 g L−1). | |
Furthermore, Fig. 9 shows a higher metabolite Raman intensity for the flow rate of 2 mL h−1. The adequate flow-induced mechanical stress becomes an index that promotes the intended metabolite activities required for plant growth. It is because of the flow-induced stress (sort of abiotic stress), the maxima K+ concentration in the root becomes lower than that of the no-flow scenario (see Fig. 12, left). However, as witnessed in Fig. 9, the metabolite intensity decreases for the higher flow rate (>2 mL h−1) and its value at higher flow rates becomes almost equal to that of the no-flow case. Therefore, at higher flow rates, the increased flow velocity led to the development of higher internal mechanical stress (see Fig. 12, right), and reduced K+ concentration. This situation becomes crucial for plant cells to produce energy,62 hence impeding plant growth. From the foregoing discussion it turns out that the nutrient flow rate has a significant impact on the Michaelis–Menten parameters for K+ (see Fig. 4(a) and 5(a)). Following the aforementioned explanations, we can deduce that flow-rate dependent metabolic changes, as shown in the Raman intensity plot [see Fig. 9], influence the intake of all nutrients by the soft plant roots. Evidently, the Michaelis–Menten parameters (Fm, Km) for all other metallic nutrient (apart from K+) intake were shown to be significantly flow rate dependent, as illustrated in Fig. 4(b)–(f) and 5(b)–(f).
Acknowledgements
P. K. M. gratefully acknowledges the financial support provided by the SERB (DST), India, through project no. CRG/2022/000762. S. K. M. acknowledges the financial support provided by the National Post-Doctoral Fellowship (N-PDF), SERB, with Reference no. PDF/2023/002072. S. P. acknowledges the financial support provided by the Institute Post-Doctoral Fellowship (IITG/R&D/IPDF/2023-24\20231006P638), Indian Institute of Technology Guwahati. S. W. Acknowledges National Science and Technology Development Agency (NSTDA) under the research chair grant, and the Thailand Science Research and Innovation (TSRI) under fundamental fund 2025. We would like to express our gratitude to the Central Instruments Facility (CIF) of IIT Guwahati for providing instrumental facilities. S. K. M. acknowledges KMUTT for financial support to perform experiments. P. K. M. wishes to acknowledge KMUTT for the visiting professorship. The authors are grateful to the anonymous reviewers for their insightful comments, which led to improvement of the manuscript.
References
- E. Sparr, C. Åberg, P. Nilsson and H. Wennerström, Soft Matter, 2009, 5, 3225–3233 RSC.
- S. Liu, H. Liu, S. Feng, M. Lin, F. Xu and T. J. Lu, Soft Matter, 2017, 13, 2919–2927 RSC.
- W. D. C. C. Wijerathne, C. M. Rathnayaka, H. C. P. Karunasena, W. Senadeera, E. Sauret, I. W. Turner and Y. T. Gu, Soft Matter, 2019, 15, 901–916 RSC.
- H. C. P. Karunasena, W. Senadeera, R. J. Brown and Y. T. Gu, Soft Matter, 2014, 10, 5249–5268 RSC.
- S. Sinha, G. Sinam, R. K. Mishra and S. Mallick, Ecotoxicol. Environ. Saf., 2010, 73, 1352–1361 CrossRef CAS PubMed.
- J. Silva and R. Uchida, Plant Nutr. Manag. Hawaii's Soils, Approaches Trop. Subtrop. Agric., 2000, 31–55 CAS.
- S. R. Wilkinson, R. M. Welch, H. F. Mayland and D. L. Grunes, Met. Ions Biol. Syst., 1990, 26, 33–56 CAS.
- K. Thor, Front. Plant Sci., 2019, 10, 440 CrossRef PubMed.
- G. R. Rout and S. Sahoo, Rev. Agric. Sci., 2015, 3, 1–24 CrossRef.
- S. Alejandro, S. Höller, B. Meier and E. Peiter, Front. Plant Sci., 2020, 11, 1–23 CrossRef PubMed.
- B. Hafeez, Y. M. Khanif and M. Saleem, Am. J. Exp. Agric., 2013, 3, 374–391 CAS.
- R. A. Hassanein, H. A. Hashem, M. H. El-deep, A. Shouman, R. A. Hassanein, H. A. Hashem, M. H. El-deep and A. Shouman, J. Stress Physiol. Biochem., 2013, 9, 145–162 Search PubMed.
- Z. H. Wang, S. X. Li and S. Malhi, J. Sci. Food Agric., 2008, 88, 7–23 CrossRef CAS.
- S. A. A. Abusin and B. W. Mandikiana, Global Food Secur., 2020, 25, 100349 CrossRef.
- D. T. Armanda, J. B. Guinée and A. Tukker, Global Food Secur., 2019, 22, 13–24 CrossRef.
- M. S. Gumisiriza, P. A. Ndakidemi, Z. Nampijja and E. R. Mbega, Sci. Afr., 2023, 20, e01643 Search PubMed.
- A. Fussy and J. Papenbrock, Plants, 2024, 11, 1153 CrossRef PubMed.
-
P. Vanýsek, CRC handbook of chemistry and physics, 1993, vol. 94, pp. 74–76 Search PubMed.
- H. S. Gaikwad, G. Kumar and P. K. Mondal, Soft Matter, 2020, 16, 6304–6316 RSC.
- S. K. Mehta, P. Bhushan, P. K. Mondal and S. Wongwises, Phys. Fluids, 2024, 36, 73106 CrossRef CAS.
- S. K. Mehta and P. K. Mondal, Phys. Rev. Fluids, 2024, 9, 23301 CrossRef.
- S. K. Mehta and P. K. Mondal, Langmuir, 2023, 39, 16797–16806 CrossRef CAS PubMed.
- S. K. Mehta, R. Kakati, A. Rahman, P. K. Mondal and S. Wongwises, Phys. Fluids, 2023, 35, 92015 CrossRef CAS.
- D. Kumar, S. K. Mehta and P. K. Mondal, Phys. Fluids, 2023, 35, 72019 CrossRef CAS.
- S. K. Mehta and P. K. Mondal, Electrophoresis, 2023, 44, 1629–1636 CrossRef CAS PubMed.
- D. Pandey, P. K. Mondal and S. Wongwises, Soft Matter, 2023, 19, 1152–1163 RSC.
- E. Epstein and C. E. Hagen, Plant Physiol., 1952, 27, 457–474 CrossRef CAS PubMed.
- B. Seeling and N. Claassen, Z. Pflanzenernährung Bodenkd., 1990, 153, 301–303 CrossRef CAS.
- K. C. J. Van Rees, Newzeal. J. For. Sci., 1994, 24, 226–233 CAS.
- A. Yakirevich, S. Sorek and M. Silberbush, Transp. Porous Media, 1994, 14, 123–141 CrossRef CAS.
- P. de Willigen, N. E. Nielsen, N. Claassen and A. M. Castrignanò, Root Methods, 2000, 509–543 Search PubMed.
- D. Massa, N. S. Mattson and H. J. Lieth, Plant Soil, 2009, 318, 101–115 CrossRef CAS.
- P. Padhi, S. K. Mehta, K. Agarwal and P. K. Mondal, Phys. Fluids, 2024, 36, 043602 CrossRef CAS.
- B. Baiyin, K. Tagawa, M. Yamada, X. Wang, S. Yamada, Y. Shao, P. An, S. Yamamoto and Y. Ibaraki, Plants, 2021, 10(9), 1840 CrossRef CAS PubMed.
- K. Agarwal, S. K. Mehta and P. K. Mondal, Lab Chip, 2024, 24, 3775–3789 RSC.
- V. H. Blackman, New Phytol., 1921, 20, 106–115 CrossRef CAS.
- J. Q. Chu, W. P. Jiao and J. J. Xu, Sci. China, Ser. G: Phys., Mech. Astron., 2008, 51, 184–205 CrossRef.
- A. G. Bengough, C. Croser and J. Pritchard, Plant Soil, 1997, 189, 155–164 CrossRef CAS.
- X. He, J. Zhu and C. Yang, Soft Matter, 2022, 18, 5177–5184 RSC.
- D. K. Yadava, S. C. Giri, S. Vasudev, A. K. Yadav, B. Dass, R. S. Raje and M. Vignesh, Indian J. Agric. Sci., 2010, 80(9), 761–765 Search PubMed.
- D. Leitner, A. Schnepf, S. Klepsch and T. Roose, Plant Biosyst., 2010, 144, 443–447 CrossRef.
-
D. R. Lide, CRC handbook of chemistry and physics, CRC Press, 2004, vol. 85 Search PubMed.
- E. J. W. Visser and G. M. Bögemann, Plant Soil, 2003, 253, 81–90 CrossRef CAS.
- C. Gillespie and S. T. Milner, Soft Matter, 2020, 16, 9816–9821 RSC.
- R. Höhler, J. Seknagi and A. Kraynik, Soft Matter, 2021, 17, 6995–7003 RSC.
- J. Rodenburg, M. Dijkstra and R. van Roij, Soft Matter, 2017, 13, 8957–8963 RSC.
- M. Ullner, K. Qamhieh and B. Cabane, Soft Matter, 2018, 14, 5832–5846 RSC.
- V. Mihaylova, V. Lyubomirova and R. Djingova, Int. J. Environ. Anal. Chem., 2013, 93, 1441–1456 CrossRef CAS.
- S. C. Wilschefski and M. R. Baxter, Clin. Biochem. Rev., 2019, 40, 115–133 Search PubMed.
- M. S. Justamante, M. Mhimdi, M. Molina-Pérez, A. Albacete, M. Á. Moreno, I. Mataix and J. M. Pérez-Pérez, Plants, 2022, 11(7), 913 CrossRef CAS PubMed.
- S. Panja, S. K. Mehta, J. Kalita, M. K. Prasad and P. K. Mondal, Phys. Fluids, 2024, 36, 111914 CrossRef CAS.
- R. Morey, C. Farber, B. McCutchen, M. D. Burow, C. Simpson, D. Kurouski and J. Cason, Plant Direct, 2021, 5(8), e342 CrossRef CAS PubMed.
- L. Sanchez, A. Ermolenkov, S. Biswas, E. M. Septiningsih and D. Kurouski, Front. Plant Sci., 2020, 11, 1–8 CrossRef PubMed.
- A. Hodge, G. Berta, C. Doussan, F. Merchan and M. Crespi, Plant Root Growth, Architecture Function, Plant Soil, 2009, 321, 153–187 CrossRef CAS.
- L. R. Band, S. Úbeda-Tomás, R. J. Dyson, A. M. Middleton, T. C. Hodgman, M. R. Owen, O. E. Jensen, M. J. Bennett and J. R. King, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 7577–7582 CrossRef CAS PubMed.
- F. A. Loewus and P. P. N. Murthy, Plant Sci., 2000, 150, 1–19 CrossRef CAS.
- S. S. Dhaliwal, R. K. Naresh, A. Mandal, R. Singh and M. K. Dhaliwal, Environ. Sustainable Indic., 2019, 1–2, 100007 Search PubMed.
- K. Lorenz, R. Lal, C. M. Preston and K. G. J. Nierop, Geoderma, 2007, 142, 1–10 CrossRef CAS.
- K. Ranathunge and L. Schreiber, J. Exp. Bot., 2011, 62, 1961–1974 CrossRef CAS PubMed.
- M. Wang, Q. Zheng, Q. Shen and S. Guo, Int. J. Mol. Sci., 2013, 14, 7370–7390 CrossRef CAS PubMed.
- X. Xu, X. Du, F. Wang, J. Sha, Q. Chen, G. Tian, Z. Zhu, S. Ge and Y. Jiang, Front. Plant Sci., 2020, 11, 1–13, DOI:10.3389/fpls.2020.00904.
- V. De Col, P. Fuchs, T. Nietzel, M. Elsässer, C. P. Voon, A. Candeo, I. Seeliger, M. D. Fricker, C. Grefen, I. M. Møller, A. Bassi, B. L. Lim, M. Zancani, A. J. Meyer, A. Costa, S. Wagner and M. Schwarzländer, eLife, 2017, 6, e26770 CrossRef PubMed.
Footnote |
| † These authors have equal contributions. |
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  a,
Anirudha
Talukdar†
b,
Suraj
Panja†
a,
Anirudha
Talukdar†
b,
Suraj
Panja†
 ac,
Jinmay
Kalita
a,
Somchai
Wongwises
d and
Pranab Kumar
Mondal
ac,
Jinmay
Kalita
a,
Somchai
Wongwises
d and
Pranab Kumar
Mondal
 *ae
*ae
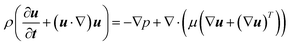
![[k with combining circumflex]](https://www.rsc.org/images/entities/b_i_char_006b_0302.gif) ), p, ρ and μ are velocity vector, pressure, liquid density and dynamic viscosity, respectively.
), p, ρ and μ are velocity vector, pressure, liquid density and dynamic viscosity, respectively.
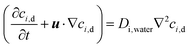
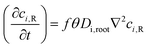
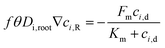
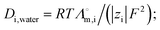 18 where R, T, F, zi, and
18 where R, T, F, zi, and 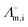 are the universal gas constant (8.314 J K−1 mol−1), temperature, Faradays constant (= 9.6485 × 104 C mol−1), valency of nutrient ion and molar conductivity of ionic species in water at T = 298 K (experimental condition).42 Hence, the diffusion coefficients of nutrient species are taken as 1.96 × 10−9, 7.05 × 10−10, 1.32 × 10−9, 7.19 × 10−10, 6.88 × 10−10, and 7.15 × 10−10 m2 s−1 for K+, Mg2+, Ca2+, Fe2+, Mn2+ and Zn2+, respectively.18 The porosity of the root is estimated as 0.43 using the cross-sectional SEM image of the root. Note that the calculated value conforms to the reported value as well.43 Hence, the impedance factor is calculated as 0.325. Moreover, Fm and Km are the maximum influx into the root and Michaelis–Menten constant, respectively. We have elaborated on these parameters in the forthcoming section.
are the universal gas constant (8.314 J K−1 mol−1), temperature, Faradays constant (= 9.6485 × 104 C mol−1), valency of nutrient ion and molar conductivity of ionic species in water at T = 298 K (experimental condition).42 Hence, the diffusion coefficients of nutrient species are taken as 1.96 × 10−9, 7.05 × 10−10, 1.32 × 10−9, 7.19 × 10−10, 6.88 × 10−10, and 7.15 × 10−10 m2 s−1 for K+, Mg2+, Ca2+, Fe2+, Mn2+ and Zn2+, respectively.18 The porosity of the root is estimated as 0.43 using the cross-sectional SEM image of the root. Note that the calculated value conforms to the reported value as well.43 Hence, the impedance factor is calculated as 0.325. Moreover, Fm and Km are the maximum influx into the root and Michaelis–Menten constant, respectively. We have elaborated on these parameters in the forthcoming section.
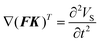
![[small epsilon, Greek, macron]](https://www.rsc.org/images/entities/b_i_char_e0c6.gif) + λLtr(
+ λLtr(![[small epsilon, Greek, macron]](https://www.rsc.org/images/entities/b_i_char_e0c6.gif) )I
)I![[small epsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c6.gif) is the Lagrange–Green strain and expressed as
is the Lagrange–Green strain and expressed as ![[small epsilon, Greek, macron]](https://www.rsc.org/images/entities/b_i_char_e0c6.gif) = 0.5(F(F)T − I). The first and second Lamé parameters appearing in eqn (7) are as follows:33,35
= 0.5(F(F)T − I). The first and second Lamé parameters appearing in eqn (7) are as follows:33,35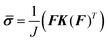
![[small sigma, Greek, macron]](https://www.rsc.org/images/entities/b_i_char_e0d2.gif) ·nR,Γ = (−pI + μ(∇u + (∇u)T))·nd,Γ
·nR,Γ = (−pI + μ(∇u + (∇u)T))·nd,Γ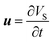
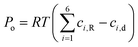
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 146
146![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 074
074![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 878
878![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 323
323![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 841
841



![[small sigma, Greek, macron]](https://www.rsc.org/images/entities/b_i_char_e0d2.gif) ·nR,Γ = (−pI + μ(∇u + (∇u)T))·nd,Γ] of nutrient media. Whereas, the root deformation rate
·nR,Γ = (−pI + μ(∇u + (∇u)T))·nd,Γ] of nutrient media. Whereas, the root deformation rate  at the root wall is used to update the nutrient flow velocity
at the root wall is used to update the nutrient flow velocity  . This step combines with the update of the mesh coordinates (x, y, z) by solid root deformation for a specific iteration loop at any time instant (t), as illustrated in Fig. 11.
. This step combines with the update of the mesh coordinates (x, y, z) by solid root deformation for a specific iteration loop at any time instant (t), as illustrated in Fig. 11.








