Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Spatiotemporal pattern formation of membranes induced by surface molecular binding/unbinding†
Hiroshi
Noguchi
 *
*
Institute for Solid State Physics, University of Tokyo, Kashiwa, Chiba 277-8581, Japan. E-mail: noguchi@issp.u-tokyo.ac.jp
First published on 10th January 2025
Abstract
Nonequilibrium membrane pattern formation is studied using meshless membrane simulation. We consider that molecules bind to either surface of a bilayer membrane and move to the opposite leaflet by flip–flop. When binding does not modify the membrane properties and the transfer rates among the three states are cyclically symmetric, the membrane exhibits spiral-wave and homogeneous-cycling modes at high and low binding rates, respectively, as in an off-lattice cyclic Potts model. When binding changes the membrane spontaneous curvature, these spatiotemporal dynamics are coupled with microphase separation. When two symmetric membrane surfaces are in thermal equilibrium, the membrane domains form 4.8.8 tiling patterns in addition to stripe and spot patterns. In nonequilibrium conditions, moving biphasic domains and time-irreversible fluctuating patterns appear. The domains move ballistically or diffusively depending on the conditions.
1 Introduction
Various spatiotemporal patterns, such as traveling waves, have been observed in living cells.1–6 Recent experiments have revealed that the oscillation of membrane shapes can be accompanied by wave propagation.7,8 Membrane shape transformations are important processes in various functions, such as molecular transport, locomotion, and mitosis.9–15 For example, spherical buds are formed by curvature-inducing proteins such as clathrin and coat protein complexes in vesicle transports.12,13,16–19 The cell division position during mitosis is determined by the standing wave of Min proteins.7,15,20 The cell polarity of eukaryotic embryos is determined by a PAR protein pattern.1,15,21 Other membrane shape transformations can also be regulated by chemical waves.The thermal equilibrium states of lipid membranes have been extensively studied and are sufficiently understood. The morphology of liposomes and phase separation are well-understood based on the free energy, including bending energy.22–28 Curvature-inducing proteins bend the bound membrane and sense the membrane curvature (i.e., concentrate on the membranes of their preferred curvatures).29–32 The curvature dependencies of these proteins have been reproduced using mean-field theories.33–35 The assembly of these proteins produces not only spherical buds and cylindrical membrane tubes29,36–41 but also periodic patterns such as hexagonal42 and checkerboard43 arrays of curved domains and beaded-necklace-like membrane tubes.44 In contrast to membranes in equilibrium, membranes in nonequilibrium have been much less explored. The coupling of membrane deformation and reaction-diffusion dynamics has been simulated for traveling waves and Turing patterns.8,45–49 On a flat surface, nucleation and growth can induce spatiotemporal patterns, such as the homogeneous-cycling (HC) and spiral-wave (SW) modes.50–52 However, the effects of thermal fluctuations on the spatiotemporal patterns of deformable membranes have not been investigated.
This study aims to clarify the spatiotemporal patterns of membranes caused by active phase separation with nucleation and growth. We consider the binding/unbinding of molecules to/from both membrane surfaces. In our previous study,43 we found stripe, checkerboard, and kagome-lattice patterns in thermal equilibrium. In this study, we examined dynamic patterns in nonequilibrium conditions. Nonequilibrium conditions can be generated by the differences in binding chemical potentials with the two surfaces and active flip or flop by proteins (flippase or floppase, respectively). We observed various patterns, such as spiral waves, moving biphasic domains, and time-irreversible fluctuations.
The simulation model and method are described in Section 2. The results are presented and discussed in Section 3. The binding/unbinding of molecules with zero and finite spontaneous curvatures are described in Sections 3.1 and 3.2, respectively. Finally, a summary is presented in Section 4.
2 Simulation model and method
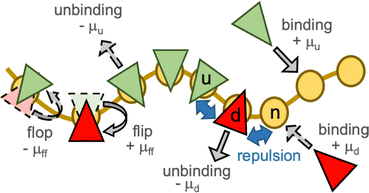
In a meshless membrane model, membrane particles self-assemble into a one-layer sheet in a fluid phase.53–56 Proteins or surfactant molecules bind to and unbind from the membrane. Here, each membrane particle is a binding site and has three states: unbound membrane (bare, denoted by “n”), bound to the upper surface of the membrane (denoted by “u”), bound to the lower surface of the membrane (denoted by “d”), as shown in Fig. 1. We call these membrane particles unbound and bound particles, respectively. The particle diameter σ is considered to be the size of a binding site, so that it is varied from σ = 5 to 100 nm depending on binding molecules. The molecules bind to the upper and lower membrane surfaces with binding chemical potentials μu and μd, respectively. The chemical potential for the molecular flip from the upper to the lower surface is μff, while that for flop from the lower to the upper surface is −μff. In thermal equilibrium, μd = μu + μff. The different binding chemical potentials (μu ≠ μd) can be generated by the concentration difference between the upper and the lower solutions. Flip–flop is considered to occur spontaneously or actively via an enzyme through ATP hydrolysis.The position and orientational vectors of the i-th particle are ri and ui, respectively. The membrane particles interact with each other via a potential U = Urep + Uatt + Ubend + Utilt + Upp. The potential Urep is an excluded volume interaction with diameter σ for all pairs of particles. The solvent is implicitly accounted for by an effective attractive potential as follows:
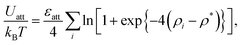 | (1) |
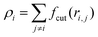 , ρ* is the characteristic density with ρ* = 7, εatt = 8 and kBT is the thermal energy, as in our previous studies.42,43fcut(r) is a C∞ cutoff function54 and ri,j = |ri,j|, with ri,j = ri − rj:
, ρ* is the characteristic density with ρ* = 7, εatt = 8 and kBT is the thermal energy, as in our previous studies.42,43fcut(r) is a C∞ cutoff function54 and ri,j = |ri,j|, with ri,j = ri − rj: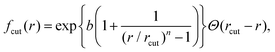 | (2) |
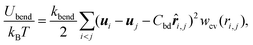 | (3) |
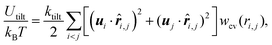 | (4) |
![[r with combining circumflex]](https://www.rsc.org/images/entities/b_i_char_0072_0302.gif) i,j = ri,j/ri,j and wcv(ri,j) is a weight function. The spontaneous curvature is given by C0 = Cbd/2σ.55
i,j = ri,j/ri,j and wcv(ri,j) is a weight function. The spontaneous curvature is given by C0 = Cbd/2σ.55
The repulsive interactions of different states (u, d, and n) are represented by
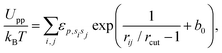 | (5) |
The position ri and orientation ui of membrane particles are updated by underdamped Langevin equations, which are integrated by the leapfrog algorithm58,59 with Δt = 0.002τ. The time unit is τ = σ2/D0, where D0 is the diffusion coefficient of the free membrane particles. Two types of states [binding/unbinding (u vs. n and d vs. n) and flip–flop (u vs. d)] are stochastically switched by a Metropolis Monte Carlo (MC) procedure with the acceptance rate pacpt:
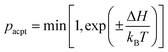 | (6) |
A membrane consisting of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 membrane particles is simulated under periodic boundary conditions. The NγT ensemble is used, where N is the total number of particles and γ is the surface tension, so that the projected area of the square membrane thermally fluctuates and can be varied by the membrane deformation.60,61 Unless otherwise specified, we use a tension of γ = 0.5 kBT/σ2, which is sufficiently large to prevent the budding of a bound domain.42,43 This tension corresponds to an average tension ≈0.02 mN m−1, which is well below the usual lysis tension (1–25 mN m−1),62–64 (σ ≈ 10 nm and kBT ≈ 4 × 10−21 J). For the unbound particles, C0 = 0 and kbend = ktilt = 10 (corresponding bending rigidity κn/kBT = 16.1 ± 1) are used. The saddle-splay modulus
600 membrane particles is simulated under periodic boundary conditions. The NγT ensemble is used, where N is the total number of particles and γ is the surface tension, so that the projected area of the square membrane thermally fluctuates and can be varied by the membrane deformation.60,61 Unless otherwise specified, we use a tension of γ = 0.5 kBT/σ2, which is sufficiently large to prevent the budding of a bound domain.42,43 This tension corresponds to an average tension ≈0.02 mN m−1, which is well below the usual lysis tension (1–25 mN m−1),62–64 (σ ≈ 10 nm and kBT ≈ 4 × 10−21 J). For the unbound particles, C0 = 0 and kbend = ktilt = 10 (corresponding bending rigidity κn/kBT = 16.1 ± 1) are used. The saddle-splay modulus ![[small kappa, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0cb.gif) is proportional to κ, as
is proportional to κ, as ![[small kappa, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0cb.gif) /κ = −0.9 ± 0.1.65 These are typical values for lipid membranes.66–68
/κ = −0.9 ± 0.1.65 These are typical values for lipid membranes.66–68
For the bound states, we examined two types of conditions: (1) no or slight changes of the membrane properties with a zero spontaneous curvature and (2) generation of a finite spontaneous curvature. In the first case, cyclically symmetric and quasi-symmetric conditions are considered. The bound particles have no spontaneous curvature (C0 = 0) and identical or slightly high bending rigidities, κp. We use κp/κn = 1–1.6 (kbend = ktilt = 10–15), since the bending rigidity linearly depends on kbend = ktilt as κp/kBT = 1.83 kbend − 2.2.55 The other parameters are symmetrically set as μu = −μd = μff = μ and εp,αβ = 3 for α,β ∈ {u, d, n}, and the MC rates are Γbind = Γflip = ΓMC. We use ΓMC = 0.5 unless otherwise specified. This model is an off-lattice version of the three-state cyclic Potts model.50,51 Here, the particles (flipping sites) laterally diffuse, and their heights vertically fluctuate, unlike those in the lattice Potts models. Note that this cyclic condition can be alternatively realized by the binding only on one leaflet of the bilayer and chemical reaction instead of the flip–flop.50
In the second case, the binding of curvature-inducing molecules (proteins or surfactants) is considered. The molecule bound to the upper and lower surfaces induces positive and negative spontaneous curvatures of C0σ = ±0.1, respectively, and their bending rigidities are high as κu/kBT = κd/kBT = 144 ± 7 (kbend = ktilt = 80). We use εp,ud = 3, εp,un = εp,dn = 2, Γbind = 1, and Γflip = 0.01. This parameter set is identical to that in our previous study,43 except for the repulsion strength (εp,un = εp,dn = 0 in ref. 43) for bound and unbound particles. In ref. 43, we obtained only steady spatial patterns in nonequilibrium. A sufficiently large repulsion to form three types of domains is key to obtaining spatiotemporal patterns. The equilibrium membrane behaviors of membranes without molecular binding, with binding to the upper surface, and binding to both surfaces are described in ref. 42, 43 and 55, respectively.
The mean cluster sizes are calculated to characterize various phases. Two membrane particles of each state are considered to belong to the same cluster when the inter-particle distance is less than ratt. The mean size of the clusters is given by 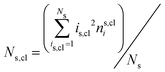 , where nis,cl is the number of clusters of size is,cl and Ns is the total number of each state (s ∈ {u, d, n}). The mean cluster size of each state is normalized by the mean total number as χs = 〈Ns,cl〉/〈Ns〉. A large percolated cluster results in χ ≃ 1. The vertical span of the membrane is calculated from the membrane height variance as
, where nis,cl is the number of clusters of size is,cl and Ns is the total number of each state (s ∈ {u, d, n}). The mean cluster size of each state is normalized by the mean total number as χs = 〈Ns,cl〉/〈Ns〉. A large percolated cluster results in χ ≃ 1. The vertical span of the membrane is calculated from the membrane height variance as 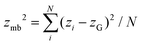 , where
, where 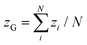 . The statistical errors are calculated from three or more independent runs.
. The statistical errors are calculated from three or more independent runs.
3 Simulation results
3.1 Membrane with zero-spontaneous-curvature
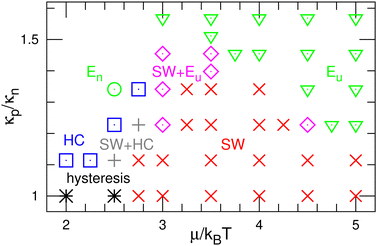
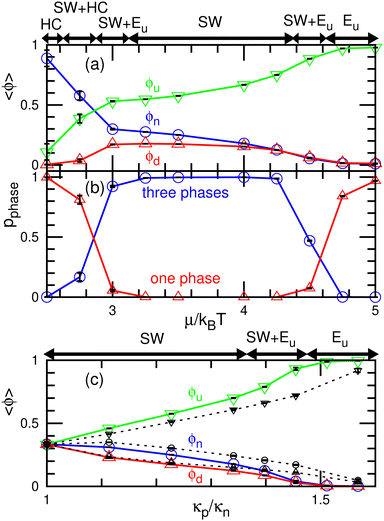
First, we describe the pattern formation of membranes with a zero spontaneous curvature. The cyclic dynamics reported in the square-lattice Potts model50,51 are also obtained in this off-lattice model (see Fig. 2–5). Three dynamic modes (SW, HC, and steady one-phase mode (Es)) and their temporal coexistence are obtained (see the phase diagram shown in Fig. 2). The temporal coexistence of SW and HC (SW + HC) is shown in Fig. 3, where “+” represents the temporal coexistence. In SW mode, the boundary of the u and d phases (or the d and n and the n and u phases) moves in the direction of the u phase (or d and n phases, respectively), and the contact point of the three phases becomes the center of a spiral wave (see the upper three snapshots in Fig. 3(a) and Movie S1, ESI†). In HC mode, the membrane is dominantly occupied by one of the three phases for most of the period, and the dominant phases change cyclically as s = n → u → d → n. These phase changes are caused by nucleation and growth, as shown in the lower three snapshots in Fig. 3(a).The mean densities 〈ϕ〉 of the three states are identical (=1/3) in the symmetric condition (κp/κn = 1). At low μ, either the HC or SW mode appears depending on the initial state owing to hysteresis; the SW mode only appears at high μ (see the bottom of Fig. 2). This phenomenon corresponds to the discontinuous transition obtained in large systems (N > 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) of the lattice Potts model.50 We also confirmed that this transition occurs in tensionless membranes (γ = 0).
000) of the lattice Potts model.50 We also confirmed that this transition occurs in tensionless membranes (γ = 0).
As κp increases, 〈ϕu〉 increases. Subsequently, the u domain covers the whole membrane (Eu mode) (see Fig. 4 and 5(c), and Movie S2, ESI†). This Es mode is similar to a homogeneous phase in thermal equilibrium. This change is caused by the mechanism reported for the lattice Potts model.51 At high κp, the unbinding is promoted since the unbound membrane has a larger undulation than the bound membrane (i.e, the unbinding gains an entropy). Hence, the duration of the d-dominant phase is reduced. This enhancement of the unbinding also suppresses the nucleation of d domains in the u-dominant phase, since small d domains disappear through the two-step process of d → n → u.51 Consequently, the duration of the Eu mode increases. When the MC rates of binding/unbinding/flip–flop are reduced to ΓMC = 0.005, the effects of κp are weakened, since the membrane can relax to the preferred projected area for the local bending rigidity (compare the dashed and solid lines in Fig. 5(c)).
The modes are determined according to the manner used in ref. 50 and 51 as follows. When the probability of three-phase coexistence (〈ϕu〉 > 0.05, 〈ϕd〉 > 0.05, and 〈ϕn〉 > 0.05) is greater than 0.05, the SW mode exists (see the blue lines with circles in Fig. 5(b)). When that of the one-phase state (〈ϕs〉 > 0.95 with s ∈ {u, d, n}) is greater than 0.05, the HC or Es mode exists. In the HC mode, all three states have a peak at ϕs ≈ 1. In the Es mode, the s state has the highest peak at ϕs ≈ 1, and at least one of the other states does not have this peak. In the upper-right region of the phase diagram (Fig. 2), the Eu mode is formed. At κp/κn = 1.23, the mode changes from HC to SW and Eu with increasing μ through the coexistence of modes (SW + HC and SW + Eu), as shown in Fig. 5. Hence, the discontinuous transition between the SW and HC modes becomes continuous at κp/κn ≃ 1.2.
Here, the SW mode is obtained only at κp/κn < 1.5 while keeping the other properties. Therefore, a spiral wave spreading over a large membrane area is difficult to obtain when the binding largely modifies the membrane properties and/or strong interactions between the bound molecules. However, biphasic domains (Fig. 4(a)) can migrate under the conditions described in Section 3.2.2.
3.2 Binding/unbinding of curvature-inducing molecules
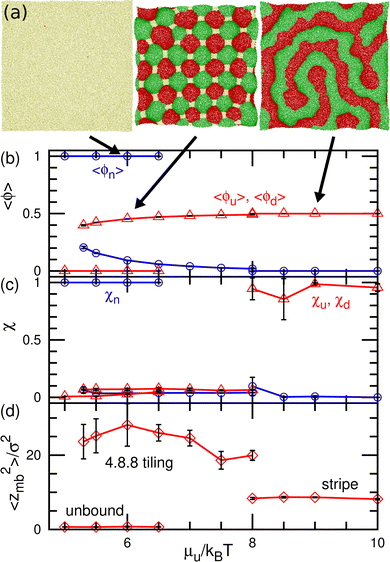
At medium strengths of the binding potential (5.3 ≤ μu/kBT ≤ 8), the u and d domains form a square lattice, and the vertices are stabilized by the n domains (see the middle snapshot in Fig. 6(a)). Owing to the symmetry, an even number of these bound domains surround the vertex. Since we added repulsion between the bound and unbound domains, the n domains at the vertices are larger than those in our previous study.43 Thus, the bound (u and d) domains are shaped as octagons and the n domain is shaped as a square. When the bound domains form a regular octagon, this domain pattern corresponds to the semi-regular tessellation 4.8.8, which consists of regular octagons and squares; this pattern is one of eight tilings to cover a flat plane by two or more regular polygons. Hence, we call this state 4.8.8 tiling. Since the u and d domains bend in the positive and negative directions, respectively, the bound membrane exhibits a bumped stripe or spherical-cap shape (see Fig. 6(d)).
The transitions between the unbound and 4.8.8 tiling phases are discontinuous, and the metastable states exist around the transition point (see Fig. 6(b)–(d)). When the binding potential exceeds μu/kBT = 8, the tiling membrane is ruptured from the boundaries of the n domains. This is due to the addition of the repulsion between the bound and unbound particles in the present study, since the discontinuous transition is obtained without rupture in the absence of this repulsion.43 In asymmetric conditions (μu ≠ μd and μd = μu + μff), kagome-lattice domain patterns reported in ref. 43 is also obtained in the present system.
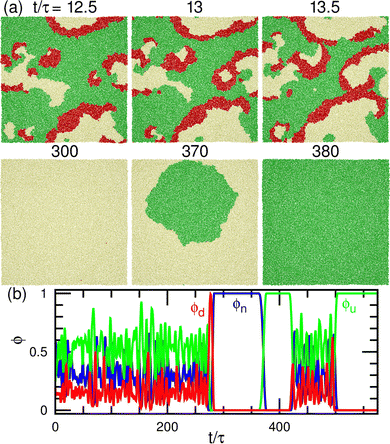
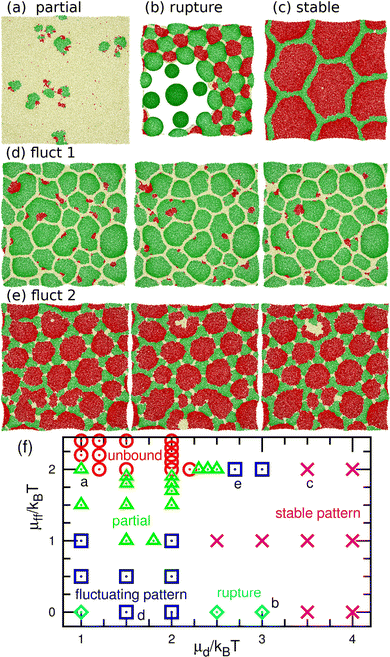
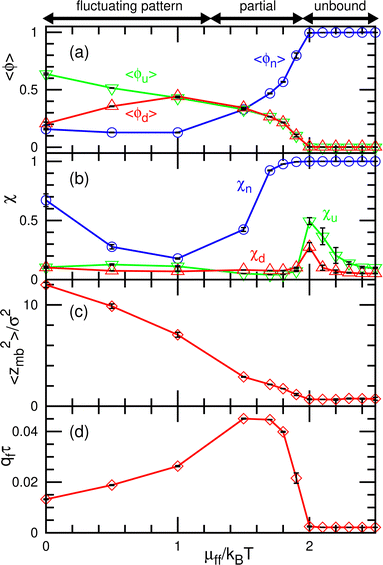
The dynamic phase diagram is shown in Fig. 7. At a relatively large μd (i.e., a small difference between μd and μu), stable patterns are formed, similar to those reported in our previous study.43 A hexagonal pattern consisting of large hexagonal d domains and the percolated hexagonal network of a u domain is formed at μd/kBT ≃ 4 and μff/kBT ≃ 2, as shown in Fig. 7(c). Hence, the d domains have a large area fraction 〈ϕd〉 ≃ 0.8, the normalized mean cluster size of the domain is χu ≈ 1, and the bumped shape causes a large vertical span zmb (see Fig. 8(a)–(c)). At a low flip potential μff ≃ 0, the 4.8.8 tiling occurs. As μd decreases, the membrane is ruptured, leading to vesicle formation, as shown in Fig. 7(b).
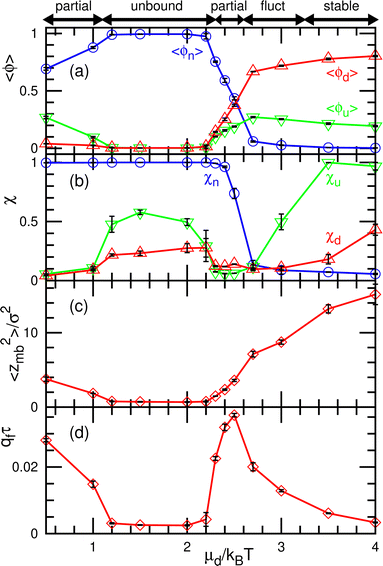
At a slightly lower μd than that in the stable-pattern region, it is found that the domain patterns exhibit time-irreversible fluctuations (called fluctuating patterns). At μd/kBT = 1.5 and μff = 0, the u and n domains form a hexagonal pattern, but a small d domain moves through a u domain, accompanied by a narrow n domain behind it (see Fig. 7(d) and Movie S3, ESI†). The backward process is negligible owing to the cyclic relation of the chemical potentials. This time-irreversible motion of d domains generates frequent changes in local patterns. At μd/kBT ≃ 3 and μff/kBT = 2, an n domain appears inside of a d domain and spreads, but the resultant large n domain is rapidly replaced by u and d domains (see Fig. 7(e) and Movie S4, ESI†). In both cases, the domain-spreading process obtained in the flat membrane (Section 3.1) occurs only locally in the separated space owing to the microphase separation. Hence, the wave propagation is restricted to the inside of the domains but frequently modifies the local domain patterns. In the fluctuating patterns, the percolation of network domains is often broken so that the membranes have low values of χu (see Fig. 8(b) and 9(b)). In the pattern fluctuations, the domain size largely fluctuates (see Fig. 7(d)), and at μd/kBT = 1 and μff = 0 the formation of large spherical buds results in membrane rupture (the diamond at the bottom left in Fig. 7(f)).
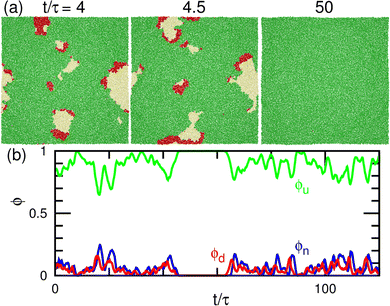
As μd further decreases at μff/kBT = 2, most of the membrane area is unbound, and isolated biphasic (u and d) domains appear (see Fig. 7(a) and 10(a) and Movies S5 and S6, ESI†). We distinguish whether the membrane is unbound or partially forms domains (called partial pattern) by the area fraction of the unbound state: unbound membranes at 〈ϕn〉 > 0.95 and partial patterns at 〈ϕn〉 ≤0.95 (see Fig. 8(a) and 9(a)). Biphasic domains are occasionally formed in the unbound membranes, whereas they appear most of the time in the partial patterns. The molecular transfer from the upper to the lower solution occurs more frequently in the partial and fluctuating patterns (see Fig. 8(d) and 9(d)).
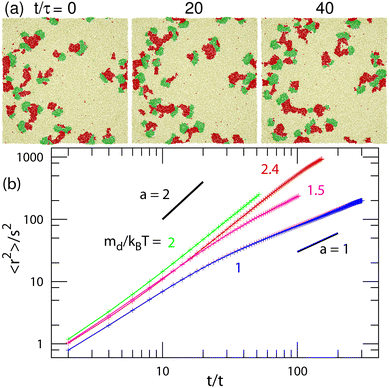
Last, we analyze the motion of the biphasic domains in the regions of the unbound membrane and partial pattern. The domain consisting of two (u and d) domains with equal size moves ballistically in the direction of the u domain, as shown in Fig. 10(a) and Movie S5 (ESI†). Conversely, when the d domains are much smaller than the u domain, the domain moves diffusively (see Fig. 7(a) and Movie S6, ESI†). This is due to frequent changes in the positions of the d domains. These moving biphasic domains have a narrower size distribution than those in the flat membrane (see Fig. 11) and thus, have sufficiently long lifetimes for quantitative analysis.
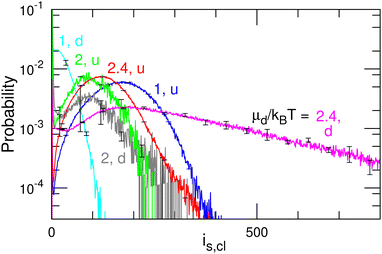 | ||
| Fig. 11 Size distribution of the u and d domains for μd/kBT = 1, 2, and 2.4 at μu/kBT = 10 and μff/kBT = 2 (corresponding to the data shown in Fig. 10). The d domains become larger with increasing μd. | ||
Fig. 10(b) shows the mean squared displacement 〈r(t)2〉 of the domain center for a u domain of cluster size iu,cl > 50. The displacement of each cluster is calculated every 2τ if both differences of the displacement and cluster size from those of the previous step are small (Δr2 < (5σ)2 and Δiu,cl < 50) in order to exclude coalesced and divided clusters. Ballistic and diffusive motions exhibit 〈r2〉 ∝ tα with exponents of α = 2 and 1, respectively. For μd/kBT ≥ 2, α = 2 is obtained, i.e., it is ballistic. The biphasic domain has a maximum velocity when it consists of u and d domains with equal size at μd/kBT = 2 (see Fig. 10(b) and 11). For μd/kBT = 1.5, the exponent changes from α = 2 to 1 at t/τ ≃ 30, such that the ballistic domain motion becomes diffusive. For μd/kBT = 1, α ∼ 1 even at t/τ < 30. The division and disappearance of d domains connecting the u domain change the moving direction so that the motion becomes diffusive. A low exponent α < 1 at t/τ ≃ 100 for μd/kBT = 1 may suggest that fast-moving domains coalesce or divide earlier than slow-moving domains. The number of remaining domains decreases exponentially over time.
4 Summary
We have studied the spatiotemporal patterns of membranes with three states. When the three states are identical but separate from each other, the membrane exhibits spiral waves of domains of three states at the cyclic flipping condition as an off-lattice cyclic Potts model. At low flipping energies, the cycling of three homogeneous phases also occurs depending on the initial state. When the bending rigidities of the three states are different, the temporal coexistence of these two modes and the non-cyclic one-phase mode also appear, since the membrane undulation modifies the stability of the states.Next, we considered that molecules (e.g., surfactants and proteins) with positive spontaneous curvatures bind to and unbind from two surfaces of the membranes and move between the two surfaces by flip–flop. In thermal equilibrium conditions, strip and 4.8.8 tiling patterns are formed. In nonequilibrium conditions, the hexagonal patterns exhibit time-irreversible fluctuations in which small domains move ballistically in the large domains of other states, and the domain patterns largely fluctuate. Moreover, biphasic domains move ballistically or diffusively depending on the conditions. These moving biphasic domains have a narrow size distribution and longer lifetime than those in the flat membrane.
In the present system, a nonequilibrium condition can be generated by the difference in chemical potential between the upper and lower solutions. Since the chemical potential can be varied by the concentration (Δμ = ΔρkBT in dilute solutions), it is easily set up in experiments. Experimentally, Miele and co-workers reported the shape transitions of the vesicles induced by the binding, unbinding, and flip–flop of surfactant molecules from the outer to the inner solutions.69,70 Although the membrane is homogeneous in their experiments, phase separation can be induced by using two types of surfactants, in which the hydrophobic segments consist of alkyl or fluoroalkyl chains because these chains can exhibit phase separation.71,72 Thus, the dynamic patterns obtained in the present study can be experimentally examined.
Data availability
Data for this article, including simulation data are available at ISSP Data Repository (ISSP-DR) at https://isspns-gitlab.issp.u-tokyo.ac.jp/hiroshi.noguchi/memb_pattern_2bind.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Jean-Baptiste Fournier for stimulating discussion. This work was supported by JSPS KAKENHI Grant Number JP24K06973.Notes and references
- A. Merino-Salomóna, L. Babla and P. Schwille, Curr. Opin. Cell Biol., 2021, 72, 106–115 CrossRef PubMed.
- M. Wu and J. Liu, Curr. Opin. Cell Biol., 2021, 68, 45–54 CrossRef CAS PubMed.
- C. Beta, L. Edelstein-Keshet, N. Gov and A. Yochelis, eLife, 2023, 12, e87181 CrossRef CAS PubMed.
- H. Noguchi, ChemSystemsChem, 2024 DOI:10.1002/syst.202400042.
- D. Taniguchi, S. Ishihara, T. Oonuki, M. Honda-Kitahara, K. Kaneko and S. Sawai, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 5016–5021 CrossRef CAS PubMed.
- M. L. Verge-Serandour and K. Alim, Annu. Rev. Condens. Matter Phys., 2024, 15, 263–289 CrossRef.
- T. Litschel, B. Ramm, R. Maas, M. Heymann and P. Schwille, Angew. Chem., 2018, 57, 16286–16290 CrossRef CAS PubMed.
- Z. Wu, M. Su, C. Tong, M. Wu and J. Liu, Nat. Commun., 2018, 9, 136 CrossRef PubMed.
- H. T. McMahon and J. L. Gallop, Nature, 2005, 438, 590–596 CrossRef CAS PubMed.
- J. Zimmerberg and M. M. Kozlov, Nat. Rev. Mol. Cell Biol., 2006, 7, 9–19 CrossRef CAS PubMed.
- S. Suetsugu, S. Kurisu and T. Takenawa, Physiol. Rev., 2014, 94, 1219–1248 CrossRef CAS PubMed.
- M. Kaksonen and A. Roux, Nat. Rev. Mol. Cell Biol., 2018, 19, 313–326 CrossRef CAS PubMed.
- J. Béthune and F. T. Wieland, Annu. Rev. Biophys., 2018, 47, 63–83 CrossRef PubMed.
- T. Svitkina, Cold Spring Harbor Perspect. Biol., 2018, 10, a018267 CrossRef PubMed.
- J. Lutkenhaus, Trends Microbiol., 2012, 20, 411–418 CrossRef CAS PubMed.
- L. Johannes, R. G. Parton, P. Bassereau and S. Mayor, Nat. Rev. Mol. Cell Biol., 2015, 16, 311–321 CrossRef CAS PubMed.
- F. Brandizzi and C. Barlowe, Nat. Rev. Mol. Cell Biol., 2013, 14, 382–392 CrossRef CAS PubMed.
- J. H. Hurley, E. Boura, L.-A. Carlson and B. Różycki, Cell, 2010, 143, 875–887 CrossRef CAS PubMed.
- H. T. McMahon and E. Boucrot, Nat. Rev. Mol. Cell Biol., 2011, 12, 517–533 CrossRef CAS PubMed.
- S. Takada, N. Yoshinaga, N. Doi and K. Fujiwara, Sci. Adv., 2022, 8, eabm8460 CrossRef CAS PubMed.
- C. Hoege and A. A. Hyman, Nat. Rev. Mol. Cell Biol., 2013, 14, 315–322 CrossRef CAS PubMed.
- Structure and Dynamics of Membranes, ed. R. Lipowsky and E. Sackmann, Elsevier Science, Amsterdam, 1995 Search PubMed.
- U. Seifert, Adv. Phys., 1997, 46, 13–137 CrossRef CAS.
- S. Svetina and B. Žekš, Adv. Colloid Interface Sci., 2014, 208, 189–196 CrossRef CAS PubMed.
- A. R. Honnerkamp-Smith, S. L. Veatch and S. L. Keller, Biochim. Biophys. Acta, Biomembr., 2009, 1788, 53–63 CrossRef PubMed.
- T. Baumgart, S. T. Hess and W. W. Webb, Nature, 2003, 425, 821–824 CrossRef CAS PubMed.
- S. Veatch and S. L. Keller, Biophys. J., 2003, 85, 3074–3083 CrossRef CAS PubMed.
- M. Yanagisawa, M. Imai and T. Taniguchi, Phys. Rev. Lett., 2008, 100, 148102 CrossRef PubMed.
- T. Baumgart, B. R. Capraro, C. Zhu and S. L. Das, Annu. Rev. Phys. Chem., 2011, 62, 483–506 CrossRef CAS PubMed.
- C. Has and S. L. Das, Biochim. Biophys. Acta, Gen. Subj., 2021, 1865, 129971 CrossRef CAS PubMed.
- C. Prévost, H. Zhao, J. Manzi, E. Lemichez, P. Lappalainen, A. Callan-Jones and P. Bassereau, Nat. Commun., 2015, 6, 8529 CrossRef PubMed.
- F.-C. Tsai, M. Simunovic, B. Sorre, A. Bertin, J. Manzi, A. Callan-Jones and P. Bassereau, Soft Matter, 2021, 17, 4254–4265 RSC.
- H. Noguchi, Int. J. Mod. Phys. B, 2022, 36, 2230002 CrossRef CAS.
- H. Noguchi, N. Walani and M. Arroyo, Soft Matter, 2023, 19, 5300–5310 RSC.
- H. Noguchi, Phys. Rev. E, 2024, 109, 024403 CrossRef CAS PubMed.
- C. Mim and V. M. Unger, Trends Biochem. Sci., 2012, 37, 526–533 CrossRef CAS PubMed.
- A. Frost, R. Perera, A. Roux, K. Spasov, O. Destaing, E. H. Egelman, P. De Camilli and V. M. Unger, Cell, 2008, 132, 807–817 CrossRef CAS PubMed.
- J. Adam, N. Basnet and N. Mizuno, Sci. Rep., 2015, 5, 15452 CrossRef CAS PubMed.
- N. Ramakrishnan, R. P. Bradley, R. W. Tourdot and R. Radhakrishnan, J. Phys.: Condens. Matter, 2018, 30, 273001 CrossRef CAS PubMed.
- H. Noguchi, Sci. Rep., 2016, 6, 20935 CrossRef CAS PubMed.
- H. Noguchi, J. Chem. Phys., 2022, 157, 034901 CrossRef CAS PubMed.
- Q. Goutaland, F. van Wijland, J.-B. Fournier and H. Noguchi, Soft Matter, 2021, 17, 5560–5573 RSC.
- H. Noguchi, Soft Matter, 2023, 19, 679–688 RSC.
- H. Noguchi, Soft Matter, 2021, 17, 10469–10478 RSC.
- B. Peleg, A. Disanza, G. Scita and N. Gov, PLoS One, 2011, 6, e18635 CrossRef CAS PubMed.
- N. Tamemoto and H. Noguchi, Sci. Rep., 2020, 10, 19582 CrossRef CAS PubMed.
- N. Tamemoto and H. Noguchi, Soft Matter, 2021, 17, 6589–6596 RSC.
- N. Tamemoto and H. Noguchi, Phys. Rev. E, 2022, 106, 024403 CrossRef CAS PubMed.
- H. Noguchi, Sci. Rep., 2023, 13, 6207 CrossRef CAS PubMed.
- H. Noguchi, F. van Wijland and J.-B. Fournier, J. Chem. Phys., 2024, 161, 025101 CrossRef CAS PubMed.
- H. Noguchi and J.-B. Fournier, New J. Phys., 2024, 26, 093043 Search PubMed.
- H. Noguchi, Sci. Rep., 2025, 15, 674 Search PubMed.
- H. Noguchi, J. Phys. Soc. Jpn., 2009, 78, 041007 CrossRef.
- H. Noguchi and G. Gompper, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2006, 73, 021903 CrossRef PubMed.
- H. Shiba and H. Noguchi, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2011, 84, 031926 CrossRef PubMed.
- H. Noguchi, Soft Matter, 2019, 15, 8741–8748 RSC.
- H. Noguchi, Soft Matter, 2012, 8, 8926–8935 RSC.
- M. P. Allen and D. J. Tildesley, Computer Simulation of Liquids, Clarendon Press, Oxford, 1987 Search PubMed.
- H. Noguchi, J. Chem. Phys., 2011, 134, 055101 CrossRef PubMed.
- S. E. Feller, Y. Zhang, R. W. Pastor and B. R. Brooks, J. Chem. Phys., 1995, 103, 4613–4621 CrossRef CAS.
- H. Noguchi, Soft Matter, 2012, 8, 3146–3153 RSC.
- E. A. Evans and F. Ludwig, J. Phys.: Condens. Matter, 2000, 12, A315–A320 CrossRef CAS.
- E. A. Evans, V. Heinrich, F. Ludwig and W. Rawicz, Biophys. J., 2003, 85, 2342–2350 CrossRef CAS PubMed.
- H. V. Ly and M. L. Longo, Biophys. J., 2004, 87, 1013–1033 CrossRef CAS PubMed.
- H. Noguchi, J. Chem. Phys., 2019, 151, 094903 CrossRef PubMed.
- M. A. S. Karal, M. M. Billah, M. Ahmed and M. K. Ahamed, Soft Matter, 2023, 19, 8285–8304 RSC.
- R. Dimova, Adv. Colloid Interface Sci., 2014, 208, 225–234 CrossRef CAS PubMed.
- M. Hu, J. J. Briguglio and M. Deserno, Biophys. J., 2012, 102, 1403–1410 CrossRef CAS PubMed.
- Y. Miele, Z. Medveczky, G. Holló, B. Tegze, I. Derényi, Z. Hórvölgyi, E. Altamura, I. Lagzi and F. Rossi, Chem. Sci., 2020, 11, 3228–3235 RSC.
- G. Holló, Y. Miele, F. Rossi and I. Lagzi, Phys. Chem. Chem. Phys., 2021, 23, 4262–4270 RSC.
- Q. Xiao, J. D. Rubien, Z. Wang, E. H. Reed, D. A. Hammer, D. Sahoo, P. A. Heiney, S. S. Yadavalli, M. Goulian, S. E. Wilner, T. Baumgart, S. A. Vinogradov, M. L. Klein and V. Percec, J. Am. Chem. Soc., 2016, 138, 12655–12663 CrossRef CAS PubMed.
- R. Wakabayashi, R. Imatani, M. Katsuya, Y. Higuchi, H. Noguchi, N. Kamiya and M. Goto, Chem. Commun., 2022, 58, 585–588 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sm01277a |
| This journal is © The Royal Society of Chemistry 2025 |