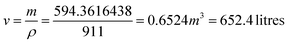Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Optimization and techno-economic evaluation of an integrated process route for the synthesis of vinyl chloride monomer
Joseph
Akintola
a,
Regina
Patinvoh
a,
Odunlami
Moradeyo
b,
Joseph
Akpan
 *c,
Gabriel
Umoh
d,
Ekpotu
Wilson
e,
Queen
Moses
f,
Philemon
Udom
g and
Edose
Osagie
h
*c,
Gabriel
Umoh
d,
Ekpotu
Wilson
e,
Queen
Moses
f,
Philemon
Udom
g and
Edose
Osagie
h
aDepartment of Chemical Engineering, Lagos State University, Ojo, 102101Nigeria
bDepartment of Chemical Engineering, Lagos State University of Science and Technology, 104101, Ikorodu, Lagos, Nigeria
cDepartment of Industrial Engineering, Durban University of Technology, Durban, 4001, South Africa. E-mail: josephsamuelakpan@gmail.com
dCENTEXIA Technical Services Limited, Lagos, 106104, Nigeria
eFaculty of Engineering and Applied Science, Memorial University of Newfoundland, St. John, NL A1B 3X7, Canada
fSchool of Engineering and Physical Sciences, Heriot-Watt University, Scotland, UK EH14 4AS, UK
gAOS Orwell Limited, Port Harcourt, PMB 029, Nigeria
hTotal Energies, Lagos, 106104, Nigeria
First published on 19th December 2024
Abstract
Vinyl chloride gas is a clear and non-irritating compound, often condensed into a liquid state for storage and transportation purposes. Its primary utilization revolves around the manufacturing of polyvinyl chloride (PVC), a material that constitutes around 12% of global plastic consumption. This study examines integrated production process routes for VC through simulation, optimization, and techno-economic analyses, combining the process routes of ethylene dichloride and vinylation in a single process route. The steady-state simulation is performed and analyzed statistically using fit regression while subjecting the simulation results to the linear model, quadratic model, and cubic model. Assessing the fitness of the model, the cubic model was found to give the best prediction and fitness of the simulation results owing to its R2 value of 98.13%, compared to 98.02% and 75.19% of quadratic and linear models. Performing energy optimization (i.e., minimization) via the pinch analysis reveals that the process route performs excellently well in minimizing energy consumption with total energy savings of 6.916 × 106 W, resulting in 56.34% savings of the actual value of $112.58 million per year. A hypothetical vinyl chloride processing plant's net present value was also assessed, and a sensitivity analysis was conducted to demonstrate the impact of interest rate fluctuations. This demonstrates that an increase in interest rates led to a decrease in net present value. Using a total capital investment and annual production cost summed up to $2.331 millions and annual revenue of $ 0.651 million, resulting in a payback period and internal rate of return values of 3.58 years and 27.94%, respectively, compared with the 6 years and 27% reported in the literature. Therefore, this study's integrated approach and the techno-economic evaluation of the vinyl chloride production process route indicate a promising choice for a sustainable large-scale VCM production plant set-up.
Sustainability spotlightThis study introduces a unique path for producing Vinyl Chloride Monomer (VCM) by integrating the ethylene dichloride and vinylation processes. The objective of the strategy is to decrease the presence of detrimental chlorinated hydrocarbons (CHCs) and improve energy efficiency. The study is in line with the United Nations Sustainable Development Goals (SDGs) 9, which focuses on industry, innovation, and infrastructure; 12, which emphasises responsible consumption and production; and 13, which addresses climate action. The simulation findings validate the practicality of the process, demonstrating a significant relationship between the rate at which feedstock is supplied and the synthesis of VCM. We anticipate this study could improve the technique for widespread sustainable industrial usage and investigation into alternative feedstocks and catalysts. |
1 Introduction
Polyvinyl chloride (PVC) is produced using some dangerous chemicals, much like many other materials. Such production procedures are expected to be strictly governed by effectively managing the dangers.1,2 Simultaneously, PVC is one of the most recyclable polymers, and due to its superior mechanical improvement, chemical inertness, and stability, PVC has grown to be the second-largest general plastic in terms of usage globally.1 PVC production capacity increased globally by 5.8% per year although it had been rising by more than 10% annually since 2005 in some countries.1,3 Building materials made with PVC are light, strong, and easy to maintain. They can withstand sunlight, harsh chemicals, and the effects of weather very well. PVC is also a flame-retardant plastic, and much research has gone into making it even more fire-resistant. PVC manufacture generates a significant amount of chlorinated hydrocarbons (CHCs), which is an increasing concern. Vinyl chloride itself is not a useful substance but serves primarily as a precursor to polyvinyl chloride polymers, making it a commodity chemical.1 To reduce or completely prevent the release of CHCs from plastics into the environment,4 vinyl chloride monomer (VCM) provides a sustainable option.5VCM is a colourless, non-irritating gas under normal pressure and temperature. It typically has no smell lower than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg m−3 (3900 ppm); however, persons with increased sensitivity may notice a pleasant smell between 200 and 500 mg m−3. It readily liquefies under pressure, making liquid storage or transportation common.6 When kept out of sunlight and away from oxygen, vinyl chloride is very unlikely to react with other chemicals. It separates into acetylene and hydrochloride at temperatures above 400 °C.7
000 mg m−3 (3900 ppm); however, persons with increased sensitivity may notice a pleasant smell between 200 and 500 mg m−3. It readily liquefies under pressure, making liquid storage or transportation common.6 When kept out of sunlight and away from oxygen, vinyl chloride is very unlikely to react with other chemicals. It separates into acetylene and hydrochloride at temperatures above 400 °C.7
Currently, the predominant method for VCM production involves chlorinating ethylene to yield dichloroethane, commonly known as Ethylene Dichloride (EDC). Subsequently, EDC undergoes thermal cracking to produce VCM.8 Dehydrochlorination of EDC is achieved through pyrolysis in cracking furnaces, typically at temperatures ranging between 500 and 550 °C. This process aims to prevent the formation of by-products and attain yields ranging from 95% to 99%.8,9
Unlike conventional hydrocarbons, chlorinated hydrocarbons (CHCs) exhibit heightened resistance to biodegradation, largely attributed to the robustness of the C–Cl bond. Consequently, synthetic CHCs increasingly contaminate the environment. Nevertheless, the production of VCM remains integral to PVC manufacturing.2,5,7
Although the ethylene chlorination process yields a high product output, it also generates a notable number of undesirable by-products. Furthermore, due to its fast material flow, this leads to substantial operational inefficiencies. The reactor coils become encrusted with a solid carbonaceous substance known as coke, requiring periodic plant shutdowns for removal. Moreover, gaseous by-products such as butadiene and chloroprene pose challenges in subsequent distillation columns.9 Therefore, there is a need to explore integrated process solutions. Studies have reported the development of integrated and novel processes due to their significant advantages. Polak et al.,10 used a hybrid model to describe the impact of raw material variations and process parameter changes on the product quantity and quality of the flow chemistry process. The obtained optimal conditions were in agreement with expectations and underlined the potential for hybrid model-based process development. Also, Zendehboudi et al.11 presented a systematic review of the applications of hybrid models in chemical, petroleum, and energy system processes. They concluded that hybrid models can be successfully utilized in the production of high-quality materials, scale-up, and optimization in terms of performance, economic, and environmental prospects. Also, Mohamadi-Baghmolaei et al.12 used a hybridized amine scrubbing process to optimize the performance of a gas-sweetening plant. Their findings revealed that adjusting the amine blend composition can significantly reduce energy loss, CO2 emissions, and operational costs while improving the overall efficiency of the plant.
The VCM technology was among the first to utilize novel production processes and advancements recommended by process simulators in the past, mostly to address issues with risk, safety, and pollution.9 Dry et al.5 presented the design for a vinyl chloride facility situated in Taft, LA, capable of producing 6.4 billion pounds annually. They established the techno-economic feasibility of the VCM production process using the oxychlorination process, HCl oxidation process, and balanced process. Heat integration of the process was assessed and found to have good energy recovery. A 99.8 mol% of final product (i.e., VCM) purity was achieved.
Dimian & Bildea13 introduced two VCM production processes: one employing a balanced approach utilizing oxychlorination and the other incorporating chlorine recovery through HCl oxidation. The researchers inferred that by incorporating initiators, some of which are generated and reused within the process, the pyrolysis yield could be improved. Dattani et al.7 designed a clean production process route for VCM using chlorination followed by thermal cracking and vinylation. The vinylation reaction was conducted to make use of the by-product, HCl, produced during the pyrolysis reaction, resulting in no liquid waste being generated through this method. The authors argued that this pathway was more environmentally sustainable than other pathways in terms of waste reduction and energy conservation (50% reduction).
Based on the balanced process, Giovanni R.14 created a model to mimic the operation of a vinyl chloride plant. The design options that the author offered were examined and shown to be an ideal process configuration that was fairly similar to the actual procedure. By comparing the simulation findings with publicly available plant and unit operations data from the literature, the accuracy of the results was confirmed. Karasek et al.15 undertook research where two methods of VCM production were contrasted: one involving the direct chlorination of ethylene and the other employing oxychlorination processes. The oxychlorination process showed improved environmental impact, utility cost, and profitability in comparison with direct chlorination.
Furthermore, an integrated process method for the production of vinyl chloride monomer from acetylene was simulated by Akintola et al.6 MINITAB 17.0 was used to experiment with Factorial Design. Artificial Neural Networks and Fit Regression were used to model the replies. The findings from the simulation and modelling revealed that the Artificial Neural Network model outperformed the Factorial Design Method model in predicting and analyzing process routes. Subsequently, additionally, Akintola et al.16 presented a computer simulation of the synthesis of VC from acetylene. Using the central composite design of the response surface approach, second-order regression models were created to forecast the statistical association between the values of the actual predictors and the dependent variables. The best conversions (%) of acetylene and HCl were found to be 89.0238% and 93.18%, respectively, at flow rates of 100 kg mole per h for acetylene and 95.312 kg mole per h for HCl. The best yield of vinyl chloride was found to be 45.7696%.
Additionally, Hickman et al.17 developed and scaled up a novel process for producing VCM directly from ethane using LaOCl/LaCl3 catalysts. The findings indicated that direct conversion of ethane to VCM can be a more economical and flexible alternative to the traditional ethylene-based method. Medrano-García et al.18 evaluated the environmental potential and economics of recently developed catalytic ethane chlorination technologies for VCM synthesis. The research showed that ethane-based routes can reduce production costs by up to 56% and, by 2050, cut the carbon footprint by 58%, assuming future decarbonization trends.
Present-day VCM facilities are consequently recognized as some of the most environmentally friendly and secure operations within the chemical process industries,19 for instance manufacturing VCM from natural gas compared to oil,20 and other low-carbon technologies towards limiting greenhouse gas emissions.21 Notwithstanding, the global developments in VCM and PVC, along with elevated raw material and labor expenses have continually been a challenge, hence, necessitating the need for economic analyses of VCM production,22 towards finding a better production route. Opportunities for optimization and development still exist because of the unique characteristics of the VCM process, which requires a trustworthy and validated model of the process in order to achieve practicable and useable outcomes to improve performance of plants. Therefore, this study focuses on the optimization and techno-economic analysis of the synthesis of VCM, which is a hybrid approach, given the benefits that it comes with.23 In order to accurately describe the actual characteristics of the important unit operations, the study develops a VCM plant economic model from the production process perspectives.
2 Methodology
2.1 Process selection
The production of VCM from ethylene, oxygen and chlorine via the ethylene dichloride” process had earlier been presented in the previous work of Dry et al.,5 and from acetylene and hydrogen chloride via “vinylation” process had earlier been presented in the previous works of akintola et al.6,16 The simulation work in this study combines the ethylene dichloride and vinylation processes in a single process route by reacting fresh feed of acetylene with the HCl produced from the ethylene dichloride method. The synthesis process uses ethylene, chlorine, oxygen and acetylene as the feed material, and a sequence of unit operations, such as splitting, mixing, heating, reaction, cooling, and separation, was followed. Therefore, in this study, the process flow diagram for the combined process route is illustrated in Fig. 1. For the simulation of vinyl chloride monomer synthesis, the Peng–Robinson (PR) model is typically chosen over SRK and NRTL because it provides more accurate results for both polar and non-polar compounds in high-pressure systems, better handles the non-ideal behavior of gases and hydrocarbons and offers superior accuracy in predicting vapor–liquid equilibrium. Although NRTL is useful for liquid–liquid interactions and SRK has some application in gas-phase hydrocarbon systems, PR is a more versatile and reliable option for producing VCM under a wider range of situations.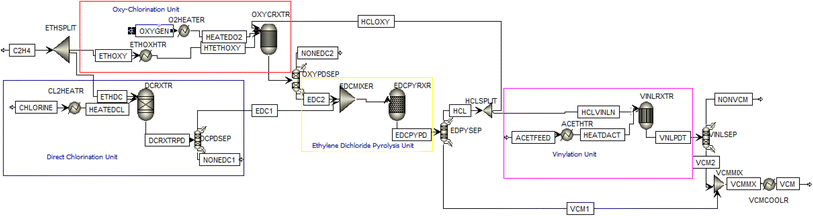 | ||
| Fig. 1 Process flow diagram for the synthesis of vinyl chloride monomer via combined ethylene dichloride and vinylation processes. | ||
2.2 Simulation basis
The stoichiometric reaction in the balanced and vinylation process defines the components used in the simulation, which are added to the component list: ethylene, chlorine, hydrogen chloride, oxygen, and acetylene. Peng–Robinson (PR) was selected as the fluid package in the simulation-based environment. Peng–Robinson was chosen in this process simulation due to its versatility in handling hydrocarbons and inorganic components.For simplicity of the model, a stoichiometric reactor was used. Here, the stoichiometry of reactants took the value of negative values, while that of products took positive values. Within the reaction set environment, ethylene was selected as the reactant, while acetone and hydrogen were selected as the products. For convergence of the global reaction set, ethylene was selected as the base component, as it is the only reactant decomposing in the reaction.
After the simulation basis was completed, the main simulation process began by switching to a “simulation environment” where all necessary equipment was added.
2.3 Process simulation
Aspen Plus 8.8 was used for the simulation. Ethylene is fed into a splitter, where the flow is diverted into both the oxychlorination reactor line and the direct chlorination line. The ethylene and oxygen are heated in separate heaters before being fed into the oxychlorination to form ethylene dichloride. Oxychlorination is employed in the manufacture of vinyl chloride as it utilizes hydrochloric acid (HCl), a significant by-product of vinyl chloride production. Through a reaction between ethylene and chlorine, ethylene dichloride is formed. The products of the oxychlorination and direct chlorination are mixed in a Mixer (EDC Mixer) and fed into an ethylene dichloride synthesis reactor (EDCPYRXTR). VCM is produced with HCl as a by-product.The various reaction stoichiometry at conversion of 0.98 for the direct chlorination, oxychlorination reaction, and ethylene dichloride cracking as given in eqn (1)–(3):
| CH2CH2 + Cl2 → ClCH2CH2Cl | (1) |
| CH2CH2 + 2HCl + 1/2O2 → ClCH2CH2Cl + O2 | (2) |
| ClCH2CH2Cl → CH2CHCl + HCl | (3) |
The VCM is separated from HCl in a flash separator (VNLSEP). The HCl produced is further reacted with a fresh feed of acetylene in a vinylation reactor (VNLRXTR) to produce vinyl chloride. The VCMs synthesized from these processes are mixed in a Vinyl Chloride Mixer (VCMMIC), as presented in Fig. 1. Table 1 shows the equipment design parameters used for achieving a converged simulated process route. This gives the actual process conditions in the simulation environment, which could be used in a real-time process for developing a pilot plant as well as a scaled-up plant.
| Splitter | Component | Split fraction | Stream order |
|---|---|---|---|
| ETH SPLIT | ETHOXY | 0.5 | 1 |
| ETH DC | 0.5 | 2 | |
| HCL SPLIT | HCL OXY | 0.4 | 1 |
| HCL VINLN | 0.6 | 2 |
| EDC MIXER | Inlet temperature (°C) | Outlet temp. (°C) | Pressure (bar) |
|---|---|---|---|
| 85.6733 | 85.6733 | 1 |
| Heat exchange equipment | Inlet temperature (°C) | Outlet temperature (°C) | Pressure (bar) | Heat duty (Gcal h−1) |
|---|---|---|---|---|
| Chlorine heater | 25 | 80 | 1 | 0.045585 |
| ETHOX HTR | 25 | 200 | 1 | 0.216555 |
| O2 HTR | 25 | 200 | 1 | 0.125487 |
| ACET HTR | 25 | 200 | 1 | 0.136524 |
| VCM COOLER | 200 | 25 | 1 | −0.469726 |
| SEPARATOR | Inlet temperature (°C) | Outlet temperature (°C) | Heat duty (Gcal h−1) | Pressure (bar) |
|---|---|---|---|---|
| DCP SEP | 80 | 80 | −0.71916903 | 2 |
| OXY SEP | 200 | 200 | −0.000317424 | 2 |
| EDPY SEP | 200 | 200 | −0.000137115 | 2 |
| VINYL SEP | 200 | 200 | −3.1228 × 10−6 | 2 |
| REACTOR | Inlet temperature (°C) | Outlet temperature (°C) | Pressure (bar) | Heat duty (Gcal h−1) |
|---|---|---|---|---|
| DC RXTR | 25 | 80 | 1 | −3.85958 |
| OXYC RXTR | 200 | 200 | 1 | −1.24541 |
| EDC PYRXTR | 85.7 | 200 | 1 | 2.71537 |
| VINYL RXTR | 200 | 200 | 1 | −1.73808 |
The simulation parameters for the production of VCM are carefully chosen to optimize reaction conditions, energy efficiency, and process safety. The split fractions are tailored to balance the distribution of reactants, ensuring optimal stoichiometric ratios and maximizing product yield. For instance, the 50–50 split for ethylene oxychlorination products and the 40–60 split for hydrochloric acid (HCl) used helps maintain efficient reactant use in downstream processes. Temperature settings are crucial for driving reaction kinetics and phase transitions. Raising reactants like ethylene, oxygen, and acetylene to 200 °C ensures activation for efficient oxychlorination, while cooling VCM to 25 °C facilitates condensation for product recovery. The heat duties are carefully managed, with significant heat removal for exothermic reactions (e.g., direct chlorination) and heat addition for endothermic processes like EDC pyrolysis, ensuring stable reaction environments and energy-efficient operations. Operating most units at 1 bar simplifies equipment design, reduces mechanical stress, and enhances safety, while higher pressures in separators (e.g., 2 bar) improve separation efficiency by promoting phase changes. The process integrates heat efficiently across units, minimizing energy consumption and maintaining a balance between thermal energy inputs and outputs.
Hence, these parameters ensure that the VCM production process is both energy-efficient and reliable, with optimal temperature, pressure, and heat management to enhance yield, safety, and operational stability.
2.4 Flow rate determination
It has been reported by Akintola et al.6 that polyvinyl chloride is 12% of the total plastic consumption. Hence, PVC consumption is calculated as:PVCconsumption = 0.12 × 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 903 903![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 = 228 000 = 228![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 kg per year 360 kg per year | (4) |
Also, PVC is made from approximately 95% VCM and some other additives. Hence, VCM consumption is calculated as:
VCMconsumption = 0.95 × 228![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 = 216 360 = 216![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 942 kg per year 942 kg per year | (5) |
| 1 kmol of VCM = 62.5 kg kmol−1 |
Hence, the flow rate in kmol h−1 is calculated as:
VCM molar flow rate = 62.5 kg kmol−1 × 216![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 942 kg per year = 3471.072 kmol per year 942 kg per year = 3471.072 kmol per year | (6) |
2.5 Sensitivity analysis
Sensitivity analysis evaluates how alterations in input parameters impact a model or system's output. It systematically varies individual factors while keeping others unchanged, enabling the quantification of uncertainty effects. This process aids decision-making by pinpointing crucial variables and their influence on outcomes, such as in financial models or engineering designs.2.6 Pinch analysis
Pinch analysis involves a methodical approach to reduce the energy usage of chemical processes. It does this by determining achievable energy targets through thermodynamic principles and then optimizing heat recovery systems, energy supply techniques, and process operations to meet these targets. The energy optimization used in this work is the pinch analysis, performed with Aspen Energy Analyzer 8.8.2.7 Techno-economic analysis
Process economics is the comprehensive economic evaluation of the process route to determine the economic viability of the process. The process economics was evaluated using the Aspen Process Economic Analyzer (APEA) and contains the equipment cost, utilities cost, installation cost, cooling water, electricity, and steam. To evaluate the practicality of the envisioned scenarios, an economic examination is conducted. This involves calculating the total capital investment (TCI) and annual production cost (APC) for the established facilities. The study utilizes Net Present Value (NPV) and Simple Payback Period (SPB) as key profitability indicators with the pricing basis, location, interest rate, and equity/debt ratio employed with the following rationale.• Pricing basis – the pricing basis is the mechanism used to determine the costs of raw materials, utilities, and finished products in the chemical process. This can be determined using market pricing, historical data, or contract rates. Pricing used is usually location-specific to ensure accuracy.
• Location – the geographic location of the factory has an impact on labor, energy, and logistics expenses. It also influences regulatory and environmental compliance costs, which have a considerable impact on profitability.24 Proximity to raw supplies and markets is critical for assessing overall economic feasibility.
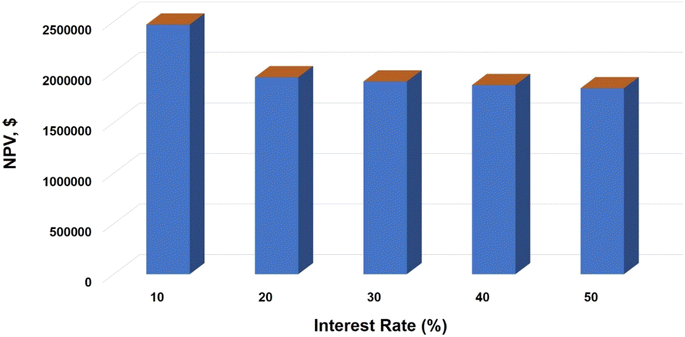
• Interest rate – the interest rate represents the cost of borrowing funds for the project. It influences the calculation of capital costs, operating expenses, and total profitability.24 Higher interest rates raise project finance costs, lowering the net present value.
• Equity/debt ratio – this ratio indicates the proportion of funding derived from shareholder equity vs. borrowed capital. A larger debt ratio boosts financial leverage while simultaneously increasing project risk due to interest payments.25 The optimal ratio balances risk and return on investment.
3 Results and discussion
Results of the conservation of the chemical system analyzed in the process flow of the balanced process clearly reveal that for the simulated steady-state process route, the mass entering the system is approximately equal mass leaving the system.Appendix A is the material and energy stream flow for the synthesis of 3474.392 kmol per year (220![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 752 kg per year) of VCM as it aligns with the recent VCM consumption in Nigeria. This approach has helped to formulate the required product, indicating a VCM total flow rate of 220
752 kg per year) of VCM as it aligns with the recent VCM consumption in Nigeria. This approach has helped to formulate the required product, indicating a VCM total flow rate of 220![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 752 kg per year from a feed rate of 246
752 kg per year from a feed rate of 246![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 269.1196 kg per year, with acetylene ethylene, chlorine and oxygen contributing 34
269.1196 kg per year, with acetylene ethylene, chlorine and oxygen contributing 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 619.92 kg per year, 110
619.92 kg per year, 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 348.7 kg per year, 69
348.7 kg per year, 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 828.5 kg per year and 31
828.5 kg per year and 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 472 kg per year, respectively.
472 kg per year, respectively.
The heat energy, presented as heat flow, describes the quantity of heat energy available, using variables such as heat, measured in kcal h−1, or the temperature measured in °C.
From the simulation result, hence, this study has justified the earlier comments of Fadayini et al.26 that computer-based simulation is a state in which a particular set of conditions is generated artificially in order to investigate a process that could really exist in reality. The final composition of the VCM stream was found to be 1.00. Hence, the product stream reveals high-purity VCM, implying that the stream is described majorly in terms of VCM products.
3.1 Statistical modeling of the ethylene dichloride process route
MINITAB 17.0 (Pen, USA) was employed to utilize linear, quadratic, and cubic regression models to analyze the statistical relationship between various independent variables and the VCM product flow rate. The simulation involved fitting first-, second-, and third-degree polynomial models, with each predicting variable's impact on the VCM flow rate (kmol per year) evaluated using analysis of variance at a confidence level of 95% and a 5% level of significance, as previously detailed by Akintola et al.6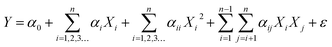 | (7) |
The significant level of the model at 75.19%, 98.02% and 98.13% for the VCM flow rate as shown by the ANOVA of the polynomial regression model for linear, quadratic, and cubic, respectively, in eqn (7). Hence, this reveals that the cubic model gives the best prediction of the response variable (VCM flow rate) owing to its highest R2 value of 98.13%, compared to 98.02% and 75.19% of quadratic and linear models, respectively. R2 indicates the extent to which variations in VCM flow rate are explained by its correlation with the flow rates of ethylene, acetylene, and oxygen. The adjusted R2 specifically accounts for the number of predictive variables in the statistical model, offering a more reliable measure. Therefore, R2 serves as an unbiased indicator of model performance.
A significance level of 0.05 was employed for testing the null hypothesis of each coefficient, reflecting the fit of the models to the experimental data. Hence, the statistical model elucidates the relationship and significance, suggesting that the coefficient has no effect Fadayini et al.26
A summary of statistical analysis and modelling, utilizing the Lack-of-Fit Test, elucidated the suitability of linear, quadratic, and cubic models. These models were developed to predict the VCM Flow rate (kmol per year), with ethylene, chlorine, and oxygen flow rates represented as A, B, and C, respectively. Consequently, the equations for first (linear), second (quadratic), and third (cubic) regression are provided as:
| Y = −367.1 + 0.29906A + 0.5578B + 0.0010C | (8) |
| Y = −46.7 + 0.1992A + 0.5070B + 0.0004C − 0.000065A2 − 0.000348B − 0.000001C2 + 0.000362AB | (9) |
| Y = −20.2 + 0.2340A + 0.3392B + 0.0013C − 0.000082A2 − 0.000240B2 − 0.00000C2 + 0.000395AB − 0.000001AC − 0.000001BC | (10) |
Fig. 2A and B are the surface plot and contour plot, respectively, for VCM flow rates. The plots depict how strongly the independent variables (ethylene, chlorine, and oxygen) correlate with the response variables. Therefore, without a real-time plant, one could analyze the predicted VCM flow rate based on the plots at the current flow rates of the feed materials.
3.2 Sensitivity analysis of the vinylation route
Sensitivity analysis offers insights into how much a design parameter needs to be adjusted to reach a specific quality threshold. Here, Fig. 3 describes the influence of the base component (acetylene) assessed on the product (VCM) flow rate. The proportional change was observed within a specified acetylene flow rate of 130 kmol per year to 1300 kmol per year, and a constant flow rate of VCM is observed as the acetylene flow rate exceeds 1300 kmol per year. Hence indicating no further synthesis of VCM, with an optimum yield of 98.7%.The integrated (combined) process route yield of 98.7% indicates considerable increases due to improved catalyst selectivity, reactor optimization, and energy integration while minimizing byproducts such as hydrogen chloride.18,27 This yield outperforms previous studies by utilizing improved heat recovery and process intensification approaches.14,18,28,29 Thus, the integrated approach highlights the impact of modern process design on enhancing yield and overall efficiency.
3.3 Pinch analysis results
Results of the Pinch analysis show that the process route performs excellently well in minimizing energy with total energy savings of 6.916 × 106, resulting in 56.34% of the actual, having the graphical representation of the actual and target in Fig. 4. During the Pinch analysis, various heat exchange parameters are considered, including the load, area, number of shells, log mean temperature difference (LMTD), fouling factor, total heat transfer coefficient, and temperature difference for both cold and hot streams. Assessing the LMTD in each of the heat exchange equipment, it was observed that an appreciable temperature difference was achieved from the pinch analysis, hence indicating the good performance of the equipment for pinch analysis.Pinch analysis is a commonly used process engineering methodology that maximizes heat recovery and minimizes energy use in order to optimize energy efficiency. The analysis in this particular process yielded notable energy savings of 6.916 × 106 units, which translates to a 56.34% decrease in energy usage relative to the actual energy consumption. The significant cost savings demonstrate how well pinch analysis works to simplify heat integration and recover energy internally. Understanding the heat exchange network and locating the pinch point—the point at which more external heating or cooling is needed—are crucial to the analysis.
Pinch analysis lowers the requirement for external utilities by efficiently recovering heat within the operation. The point at which additional heat recovery is most restricted is known as the pinch point, which is represented by the minimal temperature differential between the hot and cold streams. The system needs external heating above the pinch, and surplus heat needs to be evacuated below the limit. A number of criteria, including the heat load, fouling factors, log mean temperature difference (LMTD), and total heat transfer coefficient (U-value), were crucial in this procedure to optimize the heat exchanger network. The system ensured optimum heat recovery by adjusting the heat load, which is the quantity of heat transmitted between streams.
To make sure that there were enough temperature differences to promote efficient heat transfer across the heat exchangers, LMTD was carefully taken into account. A careful balance was maintained to prevent excessive equipment sizes, yet larger LMTD values typically translate to smaller heat exchanger areas and reduced costs. In the investigation, the fouling factor and the overall heat transfer coefficient (U-value) were also crucial variables. The efficacy of heat transmission between exchanger surfaces is measured by the U-value, which was maximized in this instance by choosing the right materials and stream flow rates. The slow accumulation of deposits on heat exchanger surfaces, which over time can lower efficiency, is explained by the fouling factor. The procedure guarantees long-term performance without the need for frequent maintenance by building the system with suitable fouling allowances.
Another crucial component of the investigation was comparing the target and actual energy usage. The results are shown graphically (as seen in Fig. 4), which highlights the energy savings that the pinch analysis generated. The difference between the target and actual energy consumption demonstrates how well the heat recovery network has been optimized. The system minimizes external energy inputs by optimizing internal heat recovery through process alignment with pinch limitations.
Notable energy savings using pinch analysis ultimately depend on a thorough evaluation of the heat exchange equipment's functioning and accurate heat load targeting. The process design demonstrated the significance of energy integration in chemical engineering processes by lowering energy consumption, capital expenses, and environmental effects.
To achieve zero carbon emissions from the actual 12.27 MW total utilities and the target of 0.5357 MW, the process need to replace using fossil fuels for utilities such as electricity and heat. These utilities emit carbon dioxide unless completely replaced by renewable or carbon-neutral sources. The figures most likely represent reduced emissions, but ultimate eradication (real zero) necessitates a shift to entirely renewable energy sources, maybe via carbon capture technology. Without such modifications, even reducing utility consumption at net-zero Carbon or the least emissions would be difficult under exisiting energy usage.
4 Techno-economic evaluation and implications
Table 2 provides an overview of the cumulative capital expenses for the formulated scenarios. Each scenario aims to yield an annual output of 220![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 752 kilograms (equivalent to 220.752 tons) of VCM. Fixed capital encompasses both direct and indirect costs, as in Tables 2 and 3. Direct costs (DC) include expenses related to purchased equipment, equipment installation, piping (including insulation), instrumentation and control systems, electrical equipment, process and administrative buildings, maintenance, site preparation, service facilities, waste treatment, fire control systems, land, and more. Indirect costs (IDC) cover aspects such as engineering and supervision, construction expenses, contractor fees, and contingencies. Variable costs consist of raw materials and labour. The total capital investment (TCI) is calculated by adding the fixed capital investment (CFCI), working capital cost (CWC), and land cost (CL) together:14
752 kilograms (equivalent to 220.752 tons) of VCM. Fixed capital encompasses both direct and indirect costs, as in Tables 2 and 3. Direct costs (DC) include expenses related to purchased equipment, equipment installation, piping (including insulation), instrumentation and control systems, electrical equipment, process and administrative buildings, maintenance, site preparation, service facilities, waste treatment, fire control systems, land, and more. Indirect costs (IDC) cover aspects such as engineering and supervision, construction expenses, contractor fees, and contingencies. Variable costs consist of raw materials and labour. The total capital investment (TCI) is calculated by adding the fixed capital investment (CFCI), working capital cost (CWC), and land cost (CL) together:14| TCI = CFCI + CWC + CL | (11) |
| CFCI = DC + IDC | (12) |
| VC = f(labour, commision, delivery costs) | (13) |
| Labour cost = 0.3 × working capital + raw materials | (14) |
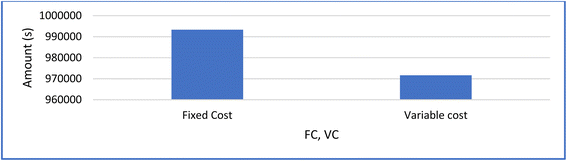
| Direct cost | 886![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 871 871 |
| Indirect Cost | 106![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 424.52 424.52 |
| Variable cost | 971![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 667.6865 667.6865 |
| Fixed Cost | 993![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 295.52 295.52 |
| Equipment | Cost ($) | Equipment | Cost ($) |
|---|---|---|---|
| Accessories | 397![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
Other purchased accessories (1) | 397![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
| ETHSPLIT | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Equipment sizing (2) = 0.2 × (1) | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 540 540 |
| VCMCOOLR | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
Piping (3) = 0.3 × (1) | 119![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 310 310 |
| HCLSPLIT | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Instrumentation (4) = 0.2 × (1) | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 540 540 |
| EDCPYRXR | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
Electrical (5) = 0.1 × (1) | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 770 770 |
| ETHOXHTR | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
Insulation (6) = 0.03 × (1) | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 931 931 |
| OXYPDSEP | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
Buildings (7) = 0.3 × (1) | 119![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 310 310 |
| VINLSEP | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
Yard (8) = 0.1 × (1) | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 770 770 |
| OXYCRXTR | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Total plan direct cost (TPDC) (9) = (1) + (2) + … + (8) | 886![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 871 871 |
| O2HEATER | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
G and a overheads (10) = 0.02 × (9) | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 737.42 737.42 |
| EDCMIXER | 500 | Contract fee (11) = 0.03 × (9) | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 606.13 606.13 |
| CL2HEATR | 8600 | Contingencies (12) = 0.07 × (9) | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 080.97 080.97 |
| VCMMIX | 500 | Fixed capital cost (13) = (9) + (10) + (11) + (12) | 993![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 295.52 295.52 |
| DCRXTR | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Working capital (14) = 0.05 × (13) | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 664.776 664.776 |
| ACETHTR | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
— | — |
| DCPDSEP | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
— | — |
| VINLRXTR | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | — |
| EDPYSEP | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
— | — |
| Purchased equip | 397![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
Total capital investment cost (TCI) (15) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 042 042![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 960.296 960.296 |
• DC RXTR, OXYC RXTR, and EDCPYR RXTR, each with a capacity of 1000 Liters, were estimated based on vendor price lists ($30,000) from Weihai Global Chemical Machinery Mfg Co. Ltd.30
• VINYL RXTR—For the VINYL RXTR, which processes the VCM component, the cost (C) estimation was determined using eqn (15) adopted from Sinnott R. and Towler G.'s previous works.31
| C = a + bSn | (15) |
VCM consumption = 216![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 942 kg per year 942 kg per year |
Assuming the plant is to run for 365 days, VCM consumption:
| ρVCM = 911 kg m−3 |
a = 53
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, b = 28
000, b = 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, s = 0.6524, taking allowance between 10–15%, s = 0.6524 × 15% = 0.75026
000, s = 0.6524, taking allowance between 10–15%, s = 0.6524 × 15% = 0.75026C = 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 + 28 000 + 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000(0.75026)0.8 000(0.75026)0.8 |
C = 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 249.869 ≈ $75 249.869 ≈ $75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
To calculate the direct expenses, we rely on the bare module costs (BMC) as this research tool helps design necessary facilities based on simulated thermodynamic conditions and then estimates their costs. It encompasses expenses for setup, piping, instrumentation, electrical components, insulation, buildings, and yard, as well as costs for facilities demanding premium materials, such as those accounted for in Aspen Plus software. Ziyai et al.32 describe the expenses linked with the contract, general and administrative overheads (G and A Overheads), and the provisions for uncertainties concerning the indirect fixed capital investment cost, which are determined in the following manner:
| Contract fee = 3% of the total plan direct cost |
| G & A overheads = 2% of the total plan direct cost |
| TCI = 7% of the total plan direct cost |
Furthermore, the working capital cost is considered as 15% of the fixed capital cost.33 Other cost assumptions, such as utilities, labor, equipment setting, instrumentation, piping, electrical, building, yards, etc., follow the same assumptions of the work by Ziyai M. et al..32 The graphical representation of the equipment cost, and total capital investment costs are presented in Fig. 5A and B, respectively. A total cost of $397,700 and $1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 042
042![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 960.296 are calculated for the purchase of equipment and capital investment, respectively. The results show that the TCI is approximately three times the purchased equipment cost.
960.296 are calculated for the purchase of equipment and capital investment, respectively. The results show that the TCI is approximately three times the purchased equipment cost.
Additionally, the financial viability of the VCM plant was assessed. Table 4 presents the total revenue per year from the VCM production plant. The cost in dollars ($) of 1 kg of Vinyl Chloride has been reported by Olivia.34 Similarly, the net present value of the plant was also evaluated. Sensitivity analysis was done to show how variations in interest rates affect the NPV. This reveals a decrease in NPV with an increase in interest rate (Fig. 6). This is in good agreement with the previous work of Ziyai et al.32
| TCI + APC | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 331 331![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 842.853 842.853 |
| VCM flow rate (kmol per year) | 3474.392 |
| VCM flow rate (kg per year) | 217![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 149.5 149.5 |
| 1 kg ($ per kg) VCM | 3 |
| VCM cost ($ per year) = Cfn | 651![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 448.5 448.5 |
Furthermore, the payback period and internal return on investment were both evaluated. The payback period was calculated as a ratio of total investment to total cash flow. Results revealed that payback for an investment in the VCM plant would be achieved in 3.579 years with a corresponding internal rate of return (IRR) of 27.93%, as compared with a payback period of 6 years and 27% IRR as reported in the literature for VCM production-friendly locations.22
The integrated VC production process detailed in this study significantly contributes to sustainability goals by addressing both environmental and social impacts. One major environmental benefit of the proposed process lies in its energy efficiency. Through pinch analysis, the process achieved a 56.34% reduction in energy consumption, leading to savings of 6.916 × 106 W. This contributes directly to reducing greenhouse gas emissions associated with energy use, aligning with global sustainability goals such as the Paris Agreement, which emphasizes energy efficiency as a key strategy for climate change mitigation.
Moreover, by eliminating the need for independent, energy-intensive stages, the combination of vinylation and EDC into a single process route reduces the environmental impact. By doing this, the amount of resources used and the emissions produced during intermediate storage and transit are decreased. Utilizing sophisticated simulation approaches, such as cubic modeling (which has an R2 of 98.13%), guarantees process design optimization and leads to a reduction in waste and byproducts, which further promotes cleaner manufacturing processes. From the standpoint of social sustainability, the process's viability, and scalability present financial advantages, especially in developing nations where the manufacture of PVC is essential to the construction of infrastructure.
Due to its favorable techno-economic metrics, which include an internal rate of return (IRR) of 27.94% and a payback period of 3.58 years, the process is attractive to industry investment, which may result in the creation of jobs and local economic growth. Reduced energy costs also translate into more inexpensive PVC production, which benefits consumers indirectly and promotes longer-term, more sustainable industrial practices ($112.5 million per year savings). As a result, by creating job opportunities in PVC-dependent businesses, fostering industrial resilience, and boosting local economies, this approach supports social sustainability as well as environmental goals through energy reduction and emissions control.
The integrated VC production process presented in this paper makes a major contribution to sustainability goals, especially in terms of energy efficiency, emissions reduction, and social-economic advantages. However, there are some significant recommendations for improving the process and directing future research and practice. First, more research into renewable energy sources for process heating and cooling is required. While the present pinch study reveals significant energy savings, incorporating renewable energy sources, such as solar or waste heat recovery, might further lower the process's carbon footprint. Studies have demonstrated that using solar energy in chemical processes can save money while also benefiting the environment. Future study could focus on determining the viability of hybrid systems that integrate pinch analysis and renewable energy technology.
Second, improvements in waste management and emissions reduction are required to connect the process with circular economy principles. For example, future research could look at the possibility of recycling by-products or unreacted materials in the manufacturing of vinyl chloride. This would eliminate waste, lower material prices, and increase overall sustainability. Emerging technologies, like as carbon capture and storage (CCS) and bio-based feedstocks, could potentially be investigated to make the process more ecologically friendly, hence, conducting a life cycle assessment (LCAs) of the integrated process with CCS to provide a more comprehensive understanding of the environmental impact, from raw material acquisition to the end of the product's life. This approach would allow for a holistic evaluation of how sustainable the process is compared to traditional VC production methods.
In terms of actual application, a recommendation is to develop a pilot-scale demonstrations to ensure that the method is scalable. Although the techno-economic research indicates that scaling up is feasible, pilot projects would enable real-world testing of the integrated process under a variety of operational scenarios. Furthermore, these pilot programs may assist detect any unexpected technical obstacles, resulting in faster transitions to full-scale industrial operations.
Finally, future research should focus on improving catalyst systems employed in the vinyl chloride synthesis process. Catalysts are essential for increasing reaction efficiency, lowering energy consumption, and reducing the creation of unwanted byproducts. Investigating more effective or sustainable catalyst alternatives may improve the overall efficiency and sustainability of the process.
5 Conclusion
This study explored the optimization and techno-economic evaluation of vinyl chloride synthesis using a hybrid methodology. The study presents an economic model for a VCM plant, integrating the production processes of ethylene dichloride and vinylation into a unified process route. The synthesis process uses ethylene, chlorine, oxygen, and acetylene as feed materials, followed by a series of unit operations including splitting, mixing, heating, reaction, cooling, and separation.The Peng–Robinson model is selected for its adaptability in managing hydrocarbons and inorganic substances. A stoichiometric reactor is used for simplicity, with ethylene chosen as the reactant. The primary simulation process initiates by transitioning to a “simulation environment,” where all requisite equipment is incorporated. Sensitivity analysis assesses the effects of variations in input parameters on the output of a model or system, facilitating decision-making by identifying key variables and their impact on results. Pinch analysis is introduced to minimize energy consumption in chemical processes by establishing attainable energy targets based on thermodynamic principles and enhancing heat recovery systems, energy supply methods, and process operations to achieve these targets.
The techno-economic analysis utilizes the Aspen Process Economic Analyzer, encompassing equipment costs, utilities costs, installation costs, cooling water, electricity, and steam. NPV and payback period are used as primary indicators of profitability. The research highlights the importance of energy integration in chemical engineering processes, resulting in reduced energy consumption, lower capital costs, and diminished environmental impacts. Achieving zero carbon emissions necessitates the cessation of fossil fuel use for utilities like electricity and heat, as these sources emit carbon dioxide unless entirely substituted with renewable or carbon-neutral alternatives.
Subsequently, the financial viability of VCM plant is assessed, focusing on annual total revenue and net present value. The integrated VC production process offers financial benefits, particularly in developing countries where PVC is crucial for infrastructure development. The favorable techno-economic metrics, such as an internal rate of return of 27.94% and a payback period of 3.58 years, make it appealing for industry investment, fostering job creation and local economic growth. Lower energy costs lead to decreased PVC production expenses, indirectly benefiting consumers and fostering sustainable industrial practices over the long term.
Data availability
The data used are included in the article; further inquiries, as applicable, can be directed to the corresponding author.Author contributions
Joseph Akintola: conceptualization, software and investigation, methodology, formal analysis, writing – original draft. Regina Patinvo and Odunlami Moradeyo: resources, validation, writing – review & editing, and project administration. Joseph Akpan: methodology, writing – original draft, formal analysis, writing – review & editing, visualization, and project administration. Gabriel Umoh and, Ekpotu Wilson: resources, visualization, and writing – review & editing. Queen Moses, Philemon Udom, and Edose Osagie: data curation, investigation, and writing – review & editing.Conflicts of interest
There is no competing interest among the authors.Appendix A
Material and energy stream results| ACETFEED | C2H4 | CHLORINE | DCRXTRPD | EDC | EDC1 | EDC2 | EDCPYPD | ETHDC | ETHOXY | |
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature K | 298.1 | 298.1 | 298.1 | 353.1 | 358.8 | 353.1 | 473.1 | 473.1 | 298.1 | 298.1 |
| Pressure N per sqm | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Vapor frac | 1 | 1 | 1 | 1 | 0.248 | 0 | 1 | 1 | 1 | 1 |
| Mole flow kmol per year | 1329.49 | 3934 | 1967 | 2163.7 | 2215.811 | 1770.3 | 445.511 | 4387.306 | 1967 | 1967 |
| Mass flow kg s−1 | 0.001 | 0.003 | 0.004 | 0.006 | 0.007 | 0.006 | 0.001 | 0.007 | 0.002 | 0.002 |
| Volume flow cum/s | 0.001 | 0.003 | 0.002 | 0.002 | 0.001 | 0 | 0.001 | 0.005 | 0.002 | 0.002 |
| Enthalpy Gcal h−1 | 0.008 | 0.006 | 0 | −0.006 | −0.009 | −0.008 | −0.001 | −0.003 | 0.003 | 0.003 |
| Mole flow kmol per year | ||||||||||
| ETHYLENE | 0 | 3934 | 0 | 196.7 | 0 | 0 | 0 | 0 | 1967 | 1967 |
| HCL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2171.495 | 0 | 0 |
| OXYGEN | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| EDC | 0 | 0 | 0 | 1770.3 | 2215.811 | 1770.3 | 445.511 | 44.316 | 0 | 0 |
| H2O | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| VINYL-01 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2171.495 | 0 | 0 |
| CHLORINE | 0 | 0 | 1967 | 196.7 | 0 | 0 | 0 | 0 | 0 | 0 |
| ACETYLENE | 1329.49 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| HCL | HCLOXY | HCLVINLN | HEATDACT | HEATEDCL | HEATEDO2 | HTETHOXY | NONEDC1 | NONEDC2 | |
|---|---|---|---|---|---|---|---|---|---|
| Temperature K | 473.1 | 473.1 | 473.1 | 473.1 | 353.1 | 473.1 | 473.1 | 353.1 | 473.1 |
| Pressure N per sqm | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Vapor frac | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Mole flow kmol per year | 2215.811 | 886.324 | 1329.487 | 1329.49 | 1967 | 1967 | 1967 | 393.4 | 3733.137 |
| Mass flow kg s−1 | 0.003 | 0.001 | 0.002 | 0.001 | 0.004 | 0.002 | 0.002 | 0.001 | 0.003 |
| Volume flow cum/s | 0.003 | 0.001 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0 | 0.005 |
| Enthalpy Gcal h−1 | −0.005 | −0.002 | −0.003 | 0.009 | 0 | 0 | 0.003 | 0 | 0 |
| Mole flow kmol per year | |||||||||
| ETHYLENE | 0 | 0 | 0 | 0 | 0 | 0 | 1967 | 196.7 | 1539.216 |
| HCL | 2171.495 | 868.598 | 1302.897 | 0 | 0 | 0 | 0 | 0 | 13.029 |
| OXYGEN | 0 | 0 | 0 | 0 | 0 | 1967 | 0 | 0 | 1753.108 |
| EDC | 44.316 | 17.726 | 26.59 | 0 | 0 | 0 | 0 | 0 | 0 |
| H2O | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 427.784 |
| VINYL-01 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CHLORINE | 0 | 0 | 0 | 0 | 1967 | 0 | 0 | 196.7 | 0 |
| ACETYLENE | 0 | 0 | 0 | 1329.49 | 0 | 0 | 0 | 0 | 0 |
| NONVCM | OXYCRXPD | OXYGEN | VCM | VCM1 | VCM2 | VCMMX | VNLPDT | |
|---|---|---|---|---|---|---|---|---|
| Temperature K | 473.1 | 473.1 | 298.1 | 298.1 | 473.1 | 473.1 | 473.1 | 473.1 |
| Pressure N/sqm | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Vapor frac | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Mole flow kmol per year | 53.183 | 4178.648 | 1967 | 3474.392 | 2171.495 | 1302.897 | 3474.392 | 1356.08 |
| Mass flow kg s−1 | 0 | 0.005 | 0.002 | 0.007 | 0.004 | 0.003 | 0.007 | 0.003 |
| Volume flow cum/sec | 0 | 0.005 | 0.002 | 0.003 | 0.003 | 0.002 | 0.004 | 0.002 |
| Enthalpy Gcal h−1 | 0 | −0.001 | 0 | 0.003 | 0.002 | 0.001 | 0.004 | 0.001 |
| Mole flow kmol per year | ||||||||
| ETHYLENE | 0 | 1539.216 | 0 | 0 | 0 | 0 | 0 | 0 |
| HCL | 0 | 13.029 | 0 | 0 | 0 | 0 | 0 | 0 |
| OXYGEN | 0 | 1753.108 | 1967 | 0 | 0 | 0 | 0 | 0 |
| EDC | 26.59 | 445.511 | 0 | 0 | 0 | 0 | 0 | 26.59 |
| H2O | 0 | 427.784 | 0 | 0 | 0 | 0 | 0 | 0 |
| VINYL-01 | 0 | 0 | 0 | 3474.392 | 2171.495 | 1302.897 | 3474.392 | 1302.897 |
| CHLORINE | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ACETYLENE | 26.593 | 0 | 0 | 0 | 0 | 0 | 0 | 26.593 |
Raw material cost (ChemAnalyst;35 Focus Technology Co. Ltd,34,36) (Fig. 7 and 8).
| Raw material costing | ACETFEED | C2H4 | CHLORINE |
|---|---|---|---|
| Mole flow kmol per year | 1329.49 | 3934 | 1967 |
| Mass flow | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 619.92 619.92 |
110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 348.7 348.7 |
69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 828.5 828.5 |
| Cost per kg ($) | 12 | 4.5 | 0.641 |
| Total cost of each raw material | 415![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 439 439 |
496![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 569.2 569.2 |
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760.069 760.069 |
| Total raw material cost | 956![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 768.3 768.3 |
References
- S. S. Moore, W. F. Carroll, R. W. Johnson, R. A. Paradis, R. Krock and D. DeCaria, Polyvinyl Chloride, in Applied Plastics Engineering Handbook, Elsevier, 2024, pp. 75–95 Search PubMed.
- Chapter 3 Vinyl chloride and polyvinyl chloride, Regul. Toxicol. Pharmacol., 1994, 20, 1, S126–S159, DOI:10.1016/S0273-2300(05)80019-4.
- K. Zhou, J. Jia, X. Li, X. Pang, C. Li and J. Zhou, et al., Continuous vinyl chloride monomer production by acetylene hydrochlorination on Hg-free bismuth catalyst: From lab-scale catalyst characterization, catalytic evaluation to a pilot-scale trial by circulating regeneration in coupled fluidized beds, Fuel Process. Technol., 2013, 108, 12–18 CrossRef CAS.
- O. J. Otorkpa and C. O. Otorkpa, Health effects of microplastics and nanoplastics: review of published case reports, Environ. Anal. Health Toxicol., 2024, 39(2), e2024020 CrossRef PubMed.
- J. Dry, B. Lawson, P. Le, I. Osisanya, D. Patel and A. Shelton, Vinyl Chloride Production Capstone Design Project Spring, University of Okhlahoma, 2003, pp. 1–9 Search PubMed.
- J. T. Akintola, A. I. Ayoola, Y. T. Abdulkareem, O. E. Akintola and C. O. Etisioro, Simulation and Modeling of an Integrated Process Route for the Synthesis of Vinyl Chloride Monomer from Acetylene: Factorial Design Method and Artificial Neural Network, Int. J. Adv. Sci. Res. Eng., 2020, 06(12), 83–93 Search PubMed.
- J. Dattani, D. Devani and O. Sahu, Cleaner Production of Vinyl Chloride Monomer (VCM), Int. J. Sci. Eng. Res., 2013, 4(5), 1–5 Search PubMed , available from: http://www.ijser.org.
- T. L. Lohr, J. R. Lockemeyer, S. D. Bishopp, A. H. Motagamwala, G. J. Wells and T. Wermink, Ethylene Oxide: A Catalyst and Process Development Success Story, Ind. Eng. Chem. Res., 2024, 63(34), 18221–18240 CrossRef CAS.
- C. Li, G. Hu, W. Zhong, W. He, W. Du and F. Qian, Coke Deposition Influence Based on a Run Length Simulation of a 1,2-Dichloroethane Cracker, Ind. Eng. Chem. Res., 2013, 52(49), 17501–17516 CrossRef CAS.
- J. Polak, M. von Stosch, M. Sokolov, L. Piccioni, A. Streit and B. Schenkel, et al., Hybrid modeling supported development of an industrial small-molecule flow chemistry process, Comput. Chem. Eng., 2023, 170, 108127 CrossRef CAS.
- S. Zendehboudi, N. Rezaei and A. Lohi, Applications of hybrid models in chemical, petroleum, and energy systems: A systematic review, Appl. Energy, 2018, 228, 2539–2566 CrossRef CAS.
- M. Mohamadi-Baghmolaei, A. Hajizadeh, P. Zahedizadeh, R. Azin and S. Zendehboudi, Evaluation of hybridized performance of amine scrubbing plant based on exergy, energy, environmental, and economic prospects: A gas sweetening plant case study, Energy, 2021, 214, 118715 CrossRef CAS.
- A. C. Dimian and C. S. Bildea, Chemical Process Design, Wiley, 2008 Search PubMed.
- R. Giovanni, Modelling of an industrial plant for vinyl chloride production [Internet]. [Padova]: Universit`a Degli Studi di Padova, 2015, [cited 2024 Oct 24]. Available from: https://thesis.unipd.it/retrieve/06ca9d3f-eff6-4d39-8d3d-a1b4678732a9/GIOVANNI_ROSSANESE_1144780_assignsubmission_file_Rossanese_Giovanni_1080770.pdf Search PubMed.
- L. Karasek, B. Kirkpatrick, A. Lipsey and K. Siahpush, Vinyl Chloride Production CHEN 4520 Chemical Process Synthesis, 2016 Search PubMed.
- J. T. Akintola, M. O. Odunlami, O. E. Akintola and Y. T. Abdulkareem, Statistical Analysis and Optimization of the Synthesis of Vinyl Chloride from Acetylene via Simulation, Int. J. Recent Technol. Eng., 2020, 9(8), 1–7 Search PubMed , Available from: www.ijltemas.in.
- D. A. Hickman, M. E. Jones, Z. R. Jovanovic, M. M. Olken, S. G. Podkolzin and E. E. Stangland, et al., Reactor Scale-up for Fluidized Bed Conversion of Ethane to Vinyl Chloride, Ind. Eng. Chem. Res., 2010, 49(21), 10674–10681 CrossRef CAS.
- J. D. Medrano-García, V. Giulimondi, A. Ceruti, G. Zichittella, J. Pérez-Ramírez and G. Guillén-Gosálbez, Economic and Environmental Competitiveness of Ethane-Based Technologies for Vinyl Chloride Synthesis, ACS Sustain. Chem. Eng., 2023, 11(35), 13062–13069 CrossRef PubMed.
- For Toxic Substances A, Registry D, Toxicological Profile for Vinyl Chloride, 2024 Search PubMed.
- G. Zichittella, A. Ceruti, G. Guillén-Gosálbez and J. Pérez-Ramírez, Catalyst: A step forward for PVC manufacture from natural gas, Chem, 2022, 8(4), 883–885 CAS.
- S. O. Akpasi, J. S. Akpan, U. O. Amune, A. A. Olaseinde and S. L. Kiambi, Methane Advances: Trends and Summary from Selected Studies, Methane, 2024, 3(2), 1–44 CrossRef.
- F. Benyahia, The VCM Process Economics: Global and Raw Material Impacts, in Proceedings of the 1st Annual Gas Processing Symposium, Elsevier, 2009, pp. 415–422 Search PubMed.
- A. M. Schweidtmann, D. Zhang and M. von Stosch, A review and perspective on hybrid modeling methodologies, Digit. Chem. Eng., 2024, 10, 100136 CrossRef.
- G. Towler and R. Sinnott, in Chemical Engineering Design Principles, Practice and Economics of Plant and Process Design, Elsevier, Heidelberg, London, 2008, Available from: http://elsevier.com Search PubMed.
- R. Smith, Chemical Process: Design and Integration, Wiley, West Sussex, 2011 Search PubMed.
- J. T. Akintola and O. M. Fadayini, Simulation and Synthesis of Toluene FIIRO & FIIRO, Available from: https://www.researchgate.net/publication/367965621.
- X. Ma, H. Wei and Z. Luo, Development and prospect of the acetylene production chain based on the process systems engineering: a focus on the polyvinyl chloride production, Rev. Chem. Eng., 2024, 40(8), 917–949 CrossRef.
- A. R. Karjavov and N. Fayzullaev, Determination of technological parameters of producing vinylchloride from acetylene, AIP Conf. Proc., 2024, 3045, 060045 CrossRef CAS.
- J. D. Medrano-García, V. Giulimondi, A. Ceruti, G. Zichittella, J. Pérez-Ramírez and G. Guillén-Gosálbez, Economic and Environmental Competitiveness of Ethane-Based Technologies for Vinyl Chloride Synthesis, ACS Sustain. Chem. Eng., 2023, 11(35), 13062–13069 CrossRef PubMed.
- Weihai Global Chemical Machinery Mfg Co. Ltd, https://www.alibaba.com/product-detail/5000L-30000L-liters-Factory-Price-Stainless_1600590380741.html?spm=a2706.7843667.0.0.69673efeB6zgfU.2024.5000L-30000LlitersFactoryPriceStainlessSteelHigh-PressureContinuousStirredTankMixingReactor.
- R. Sinnott and G. Towler, Chemical Engineering Design. SI, Elsevier, 2020 Search PubMed.
- M. R. Ziyai, M. Mehrpooya, M. Aghbashlo, M. Omid, A. S. Alsagri and M. Tabatabaei, Techno-economic comparison of three biodiesel production scenarios enhanced by glycerol supercritical water reforming process, Int. J. Hydrogen Energy, 2019, 44(33), 17845–17862 CrossRef CAS.
- S. Lee, D. Posarac and N. Ellis, Process simulation and economic analysis of biodiesel production processes using fresh and waste vegetable oil and supercritical methanol, Chem. Eng. Res. Des., 2011, 89(12), 2626–2642 CrossRef CAS.
- Focus Technology Co. L, 2024. China Factory Supplier Vinyl Chloride Resin CMP-45 with Best Price, https://cnsunman.en.made-in-china.com/product/qZxmOMIEgHhQ/China-China-Factory-Supplier-Vinyl-Chloride-Resin-CMP-45-with-Best-Price.html.
- ChemAnalyst, Liquid Chlorine Price Trend and Forecast, 2024, https://www.chemanalyst.com/Pricing-data/liquid-chlorine-45.
- Focus Technology Co. L. https://taiyugas.en.made-in-china.com/product/awAfrxsvsUTe/China-Wholesale-99-95-Ethylene-C2h4-Gas-Price.html.2024.Wholesale99.95%EthyleneC2h4GasPrice.
| This journal is © The Royal Society of Chemistry 2025 |